Abstract
Background
Most studies dichotomise indices of coronary microvascular function to assess their prognostic values.
Aims
We aimed to investigate whether coronary flow reserve (CFR) and hyperaemic microvascular resistance (HMR) as continua predict major adverse cardiovascular events (MACE), comprising all-cause death, myocardial infarction, revascularisation, and stroke in patients with ischaemia and non-obstructive coronary artery disease.
Methods
A total of 610 patients were included and followed up over a median of 8.0 years (199 individual MACE in 174 patients).
Results
Both CFR and HMR as continua predicted MACE with an odds ratio (OR) of 0.70 (per 1-unit increase, 95% confidence interval [CI]: 0.53, 0.92; p=0.01) and 1.63 (per 1 mmHg/cm/s, 95% CI: 1.20, 2.21; p=0.002), respectively. This relationship remained significant after adjustment for age and sex with an adjusted OR of 0.66 (per 1 unit increase, 95% CI: 0.49, 0.89; p=0.01) and 1.42 (per 1 mmHg/cm/s, 95% CI: 1.03, 1.94; p=0.03). HMR added prognostic value to CFR in predicting MACE (net reclassification index 0.17, 95% CI: 0.02, 0.31; p=0.03; integrated discrimination improvement 0.01, 95% CI: 0.0001, 0.02; p=0.046).
Conclusions
Both CFR and HMR as continuous variables predict future risk of MACE.
Introduction
Phenotypic identification of coronary microvascular dysfunction (CMD) was aimed at characterising the underlying pathophysiology of patients with non-obstructive coronary artery disease (CAD), which has been observed in 20-50% of patients referred for diagnostic coronary angiography1,2,3,4. Even though previous studies demonstrated the detrimental impact of CMD on quality of life and major adverse cardiovascular events (MACE)3,5,6,7, physiological assessment of coronary microvascular function to diagnose CMD has not been standardised8.
Coronary flow augmentation in response to adenosine is a measure of endothelium-independent coronary microvascular function in the absence of epicardial CAD. Coronary flow reserve (CFR) <2.5 is used to diagnose endothelium-independent CMD and was associated with myocardial perfusion abnormality and a maladaptive physiological response during exercise9. The prognostic impact of endothelium-independent CMD on MACE has also been established10,11. However, similar to blood pressure, CFR, as a continuous variable, may be a predictor of MACE12,13.
Recently, hyperaemic microvascular resistance (HMR) was postulated to stratify patients with abnormal CFR into functional or structural CMD9,14. HMR >2.0 mmHg/cm/s was associated with attenuated improvement of myocardial perfusion post revascularisation15. However, it has not yet been determined whether higher residual microvascular resistance after adenosine infusion reflects structural microvascular disturbance or pharmacological resistance against adenosine. Higher HMR was associated with recurrent chest pain in patients with non-obstructive CAD16; however, there is a lack of evidence regarding the prognostic value of continuous or dichotomised HMR measurement on future adverse clinical outcomes.
This study aimed to investigate the individual prognostic values of both continuous and dichotomised HMR and CFR as measures of coronary microvascular function in predicting MACE. Also, we aimed to examine the incremental benefit of a combined HMR and CFR measurement strategy on the assessment of risk for MACE in patients with angina and/or ischaemia and non-obstructive CAD.
Methods
STUDY POPULATION
In this observational cohort study, we enrolled patients who presented at the Mayo Clinic between 1992 and 2019 and who underwent comprehensive invasive coronary reactivity testing for evaluation of coronary microvascular function using CFR and HMR. Exclusion criteria are listed in Supplementary Appendix 1. This study was conducted in accordance with the guidelines of the Declaration of Helsinki, and the Mayo Clinic Institutional Review Board approved the study protocol. All patients provided written informed consent for participation in the current study.
CORONARY REACTIVITY TESTING
Coronary reactivity testing was performed to evaluate coronary microvascular function, as previously described17,18,19 (Supplementary Figure 1). In brief, patients without significant epicardial coronary artery stenosis (≤40% angiographic stenosis in major vessels) further proceeded with coronary reactivity testing. A Doppler guidewire (FloWire™; Volcano Therapeutics Inc, Rancho Cordova, CA, USA) was advanced within a coronary infusion catheter and positioned in the mid-left anterior descending artery (LAD). Incremental doses (18-72 μg) of adenosine were administered until maximal hyperaemia was achieved. Haemodynamic measurements were recorded at baseline resting and hyperaemic conditions. CFR was calculated as the ratio of hyperaemic flow velocity to baseline resting flow velocity. Lower CFR was defined as CFR <2.514,20. Adenosine non-responders were defined as those who had CFR during the first dose of adenosine equal to or higher than CFR during the higher dose of adenosine, indicating an abnormal dose-response relationship between the intracoronary dose of adenosine and the coronary flow velocity obtained21. Given that the difference between distal coronary pressure and aortic pressure was negligible under the condition that we only included patients with non-obstructive CAD, we calculated the ratio of the mean aortic pressure during hyperaemia and the hyperaemic flow velocity as the valid approximation of HMR (distal coronary pressure/hyperaemic flow velocity). Higher HMR was defined as HMR >2.0 mmHg/cm/s15,22 (or >2.5 mmHg/cm/s14,23). CMD was defined by the presence of lower CFR and/or higher HMR.
CLINICAL ASSESSMENT AND FOLLOW-UP
Clinical history, laboratory data, and current medications were collected from a detailed chart review by investigators (A. Ahmad and F. Sebaali) who were blinded to the results of coronary reactivity testing. Patients were followed up for MACE, including all-cause mortality, myocardial infarction, revascularisation for stable angina (percutaneous coronary intervention or coronary artery bypass grafting), and stroke, via a standardised questionnaire sent out at one time. Self-reported MACE on the questionnaire were independently adjudicated and confirmed in patients whose medical charts were available.
STATISTICAL ANALYSIS
Detailed statistical methods are described in Supplementary Appendix 2.
Results
BASELINE CHARACTERISTICS
We enrolled 806 patients with available outcome data by questionnaire and excluded 196 patients because of the lack of CFR or HMR measurements, leaving a total of 610 patients in the analyses (Supplementary Figure 2). Distribution of CFR and HMR and the correlation between them are shown in Supplementary Figure 3. Baseline characteristics are summarised in Table 1 using CFR of 2.5 and HMR of 2.0 mmHg/cm/s as cut-offs. Of 610 patients (mean age 54.1±12.0 years, 30% male), 212 patients (35%) had lower CFR, 115 patients (19%) had higher HMR, and 57 patients (9%) had both lower CFR and higher HMR. Patients with lower CFR were older and more likely to be female. Patients with higher HMR were also older; however, the sex proportion was similar to patients with lower HMR. Furthermore, patients with higher HMR were more likely to have hypertension and impaired renal function. Comparison of baseline characteristics between patients with and without CMD (defined by the presence of CFR <2.5 and/or HMR >2.0) is summarised in Supplementary Table 1. Patients with CMD (N=270, 44%) were older and more likely to be female. Patients with CMD were more likely to have hypertension, higher HbA1c and total cholesterol levels, and decreased renal function.
Table 1. Baseline characteristics comparing patients with normal versus abnormal CFR/HMR.
| All patients N=610 | CFR ≥2.5 N=398 | CFR <2.5 N=212 | p-value | HMR ≤2.0 N=495 | HMR >2.0 N=115 | p-value | ||
| Age, years | 54.1±12.0 | 52.5±11.8 | 57.0±11.9 | <0.0001 | 52.9±12.1 | 59.1±10.5 | <0.0001 | |
| Male sex, n (%) | 180 (30) | 148 (37) | 32 (15) | <0.0001 | 142 (29) | 38 (33) | 0.36 | |
| Body mass index, kg/m2 | 28.1 (24.5, 32.8) | 28.4 (24.9, 33.3) | 27.1 (24.0, 31.9) | 0.03 | 28.1 (24.6, 32.9) | 27.5 (24.2, 32.4) | 0.41 | |
| Smoking status, n (%) | Never smoked | 331 (54) | 215 (54) | 116 (55) | 0.57 | 263 (53) | 68 (59) | 0.43 |
| Former smoker | 229 (38) | 147 (37) | 82 (39) | 189 (38) | 40 (35) | |||
| Current smoker | 50 (8) | 36 (9) | 14 (7) | 43 (9) | 7 (6) | |||
| Diabetes, n (%) | 70 (11) | 42 (11) | 28 (13) | 0.33 | 61 (12) | 9 (8) | 0.17 | |
| Hypertension, n (%) | 282 (46) | 173 (43) | 109 (51) | 0.06 | 216 (44) | 66 (57) | 0.01 | |
| Dyslipidaemia, n (%) | 362 (59) | 228 (57) | 134 (63) | 0.16 | 292 (59) | 70 (61) | 0.71 | |
| Systolic BP, mmHg | 127±18 | 127±19 | 127±18 | 0.96 | 125±17 | 133±22 | 0.001 | |
| Diastolic BP, mmHg | 76±10 | 76±10 | 75±9 | 0.10 | 75±10 | 78±11 | 0.04 | |
| HbA1c, % | 5.4 (5.1, 5.7) | 5.3 (5.1, 5.7) | 5.4 (5.2, 5.7) | 0.01 | 5.4 (5.1, 5.7) | 5.4 (5.2, 5.7) | 0.45 | |
| Total cholesterol, mg/dL | 183 (157, 213) | 181 (156, 212) | 187 (159, 217) | 0.17 | 184 (156, 213) | 183 (163, 220) | 0.67 | |
| LDL-C, mg/dL | 101 (78, 127) | 101 (76, 127) | 101 (80, 129) | 0.59 | 101 (78, 127) | 99 (79, 132) | 0.88 | |
| HDL-C, mg/dL | 52 (43, 65) | 50 (42, 63) | 56 (46, 69) | 0.001 | 51 (43, 65) | 52 (45, 65) | 0.58 | |
| Triglyceride, mg/dL | 107 (74, 171) | 107 (76, 176) | 105 (70, 168) | 0.35 | 107 (73, 171) | 109 (75, 172) | 0.76 | |
| eGFR, ml/min/1.73 m2 | 75.8±17.3 | 76.7±16.6 | 74.0±18.5 | 0.08 | 76.5±17.8 | 72.7±14.9 | 0.02 | |
| CFR | 2.8 (2.4, 3.2) | 3.1 (2.8, 3.5) | 2.3 (2.1, 2.4) | <0.0001 | 2.8 (2.4, 3.3) | 2.6 (2.2, 3.1) | 0.001 | |
| Baseline APV, cm/sec | 25 (19, 32) | 23 (18, 28) | 30 (23, 36) | <0.0001 | 26 (21, 33) | 19 (15, 23) | <0.0001 | |
| Hyperaemic APV, cm/sec | 65 (54, 81) | 67 (55, 82) | 61 (52, 77) | 0.01 | 71 (60, 85) | 44 (37, 51) | <0.0001 | |
| HMR, mmHg/cm/sec | 1.49 (1.22, 1.85) | 1.41 (1.20, 1.75) | 1.61 (1.29, 2.05) | <0.0001 | 1.37 (1.17, 1.63) | 2.30 (2.11, 2.71) | <0.0001 | |
| MAP, mmHg | 99±14 | 98±14 | 101±16 | 0.02 | 98±14 | 106±16 | <0.0001 | |
| APV: average peak velocity; BP: blood pressure; CFR: coronary flow reserve; eGFR: estimated glomerular filtration rate; HbA1c: haemoglobin A1c; HDL-C: high-density lipoprotein cholesterol; HMR: hyperaemic microvascular resistance; LDL-C: low-density lipoprotein cholesterol; MAP: mean aortic pressure | ||||||||
COMPOSITE MACE
The median duration from coronary reactivity testing to questionnaires filled or death was 8.0 (interquartile range, 4.7-13.4) years. Of 610 patients, 199 individual MACE events (96 all-cause death, 35 myocardial infarction, 38 revascularisations, and 30 strokes) were reported in 174 patients. We could adjudicate and confirm individual events in 160 patients (92%). The incidence of composite MACE was significantly higher in patients with higher HMR than in those with lower HMR (Table 2). Patients with CMD had a significantly higher incidence of composite MACE than those without (91 [34%] vs 83 [24%], p=0.01) (Table 3).
Table 2. Comparison of individual MACE between patients with normal versus abnormal CFR/HMR.
| All patients N=610 | CFR ≥2.5 N=398 | CFR <2.5 N=212 | p-value | HMR ≤2.0 N=495 | HMR >2.0 N=115 | p-value | |
| Composite MACE, n (%) | 174 (29) | 105 (26) | 69 (33) | 0.11 | 130 (26) | 44 (38) | 0.01 |
| Death, n (%) | 96 (16) | 60 (15) | 36 (17) | 0.54 | 70 (14) | 26 (23) | 0.02 |
| Myocardial infarction, n (%) | 35 (6) | 22 (6) | 13 (6) | 0.76 | 24 (5) | 11 (10) | 0.05 |
| Revascularisation, n (%) | 38 (6) | 24 (6) | 14 (7) | 0.78 | 28 (6) | 10 (9) | 0.22 |
| Stroke, n (%) | 30 (5) | 15 (4) | 15 (7) | 0.07 | 27 (5) | 3 (3) | 0.20 |
| CFR: coronary flow reserve; HMR: hyperaemic microvascular resistance; MACE: major adverse cardiovascular events | |||||||
Table 3. Comparison of individual MACE events between patients with and without CMD.
| All patients N=610 | CMD– N=340 | CMD+ N=270 | p-value | |
| Composite MACE, n (%) | 174 (29) | 83 (24) | 91 (34) | 0.01 |
| Death, n (%) | 96 (16) | 45 (13) | 51 (19) | 0.06 |
| Myocardial infarction, n (%) | 35 (6) | 17 (5) | 18 (7) | 0.38 |
| Revascularisation, n (%) | 38 (6) | 19 (6) | 19 (7) | 0.46 |
| Stroke, n (%) | 30 (5) | 14 (4) | 16 (6) | 0.31 |
| CFR: coronary flow reserve; HMR: hyperaemic microvascular resistance; MACE: major adverse cardiovascular events | ||||
LOGISTIC REGRESSION ANALYSIS TO PREDICT COMPOSITE MACE
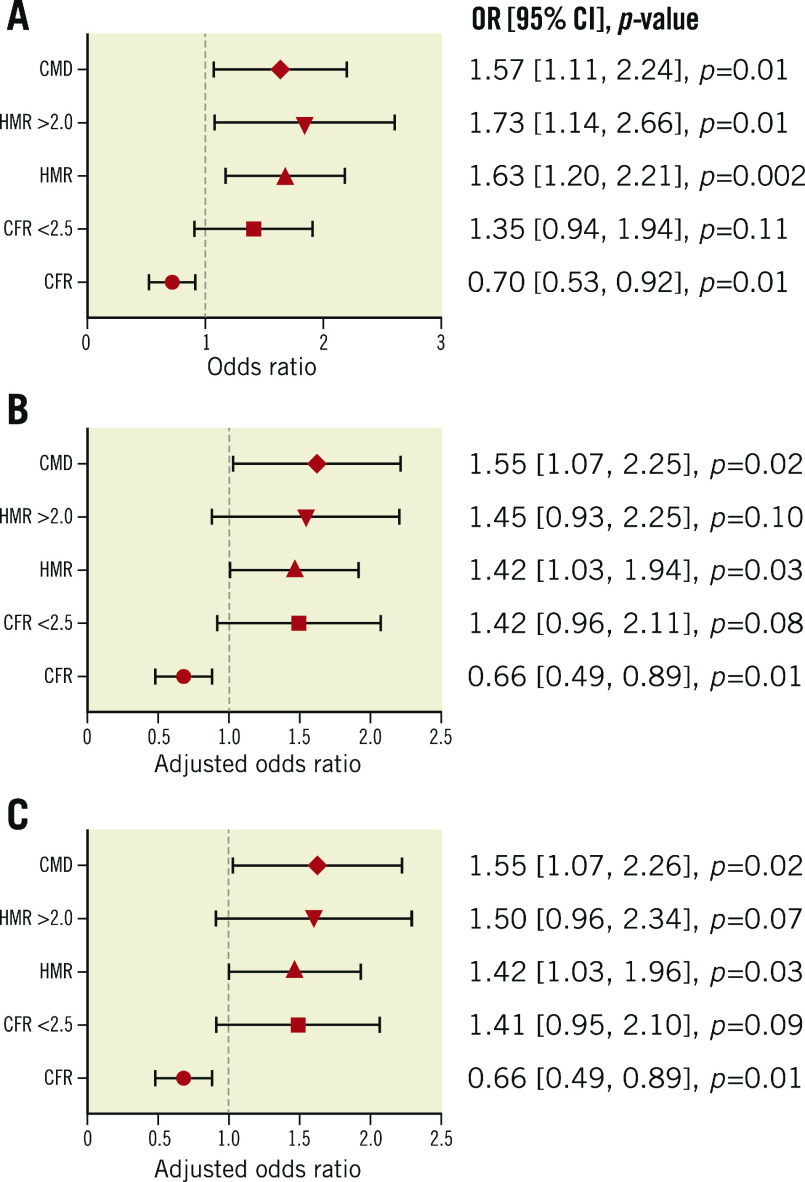
Logistic regression analysis was performed to estimate the prognostic value of individual CFR and HMR as both dichotomised and continuous variables and CMD for the risk of composite MACE. Higher HMR was significantly associated with increased risk of composite MACE as dichotomised and continuous variables, with odds ratios (OR) of 1.73 (95% confidence interval [CI]: 1.14, 2.66; p=0.01) and 1.63 (per 1 mmHg/cm/s, 95% CI: 1.20, 2.21; p=0.002), respectively (Figure 1A). Higher CFR was significantly associated with lower risk of composite MACE as a continuous variable, with an OR of 0.70 (per 1-unit increase, 95% CI: 0.53, 0.92; p=0.01) (Figure 1A). After adjustment for other covariates, higher HMR and CFR remained significantly associated with composite MACE as continuous variables (Figure 1B, Figure 1C). The discriminatory accuracy improved after adding HMR to CFR in predicting MACE (Supplementary Appendix 3, Supplementary Table 2). CMD was significantly associated with an increased risk of composite MACE (OR 1.57, 95% CI: 1.11, 2.24; p=0.01) and remained significant after adjustment for other covariates (Figure 1A-Figure 1C). When we divided patients into two groups by HMR of 2.5 mmHg/cm/s, HMR >2.5 (N=34) was significantly associated with an increased risk of composite MACE (OR 3.44, 95% CI: 1.71, 6.94; p=0.001; age- and sex-adjusted OR 3.08, 95% CI: 1.51, 6.28; p=0.002). Logistic regression analysis was also performed by dividing patients into four groups (Supplementary Appendix 4, Supplementary Figure 4A-Supplementary Figure 4D).
Figure 1.
Association between CFR, HMR, and composite MACE. Forest plots showing the association between coronary physiologic parameters (CFR and HMR), CMD, and future risk of MACE. A) Univariate. B) Multivariate (model 1: age and sex). C) Multivariate (model 2: age, sex, hypertension, dyslipidaemia, diabetes mellitus, body mass index, and estimated glomerular filtration rate). CFR: coronary flow reserve; CI: confidence interval; CMD: coronary microvascular dysfunction; HMR: hyperaemic microvascular resistance; MACE: major adverse cardiovascular events; OR: odds ratio
THE PREVALENCE OF ADENOSINE NON-RESPONDERS
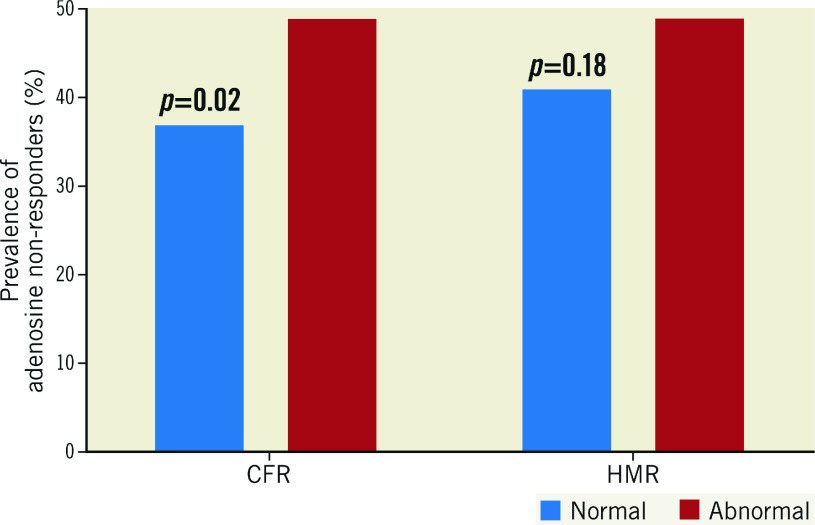
The administered doses of adenosine by which maximum CFR was achieved were not different between patients with higher versus lower CFR or lower versus higher HMR (CFR 44±15 μg vs 46±15 μg, p=0.33; HMR 45±15 μg vs 44±16 μg, p=0.75). However, the prevalence of adenosine non-responders was significantly higher in patients with lower CFR than in those with higher CFR (49% vs 37%, p=0.02), whereas no significant difference was observed between patients with higher versus lower HMR (49% vs 41%, p=0.18) (Figure 2). Adenosine responders tended to have higher average peak velocity compared to adenosine non-responders at given doses of intracoronary adenosine (Supplementary Figure 5).
Figure 2.
Adenosine responders versus non-responders. A) Bar graphs showing the prevalence of adenosine non-responders (%). B) Dose-response relationship between intracoronary adenosine and APV. APV: average peak velocity; CFR: coronary flow reserve; HMR: hyperaemic microvascular resistance
Discussion
This study demonstrated that both continuous CFR and HMR, measures of coronary microvascular function, are predictors of long-term MACE, even after adjustment for age and sex. Dichotomised CFR <2.5 and HMR >2.0 mmHg/cm/s were not significantly associated with an increased risk of MACE individually after adjustment for age and sex; however, CMD, defined as having abnormal CFR and/or abnormal HMR, remained significantly associated with an increased risk of MACE even after adjustment. Thus, the current study underscores the importance of a comprehensive assessment of coronary microvascular physiology in the cardiac catheterisation laboratory, including coronary vasomotor response and residual microvascular resistance in response to adenosine, to stratify long-term risk in patients presenting with angina and non-obstructive CAD.
CFR AND MACE
The myocardium maintains a high oxygen extraction rate at rest, and thus depends mainly on coronary blood flow to meet its oxygen demand24,25,26. Coronary blood flow is controlled by a complex interplay between epicardial and microvascular coronary arteries27. In the absence of obstructive epicardial artery disease, CFR mainly reflects the endothelium-independent microvascular vasomotor response to pharmacologically induced increased demand, usually by adenosine infusions20. CFR can be measured using different modalities, including the gold standard invasive assessment by intracoronary Doppler flow wire or using non-invasive assessment using positron emission tomography or transthoracic echocardiography. Decreased CFR is consistently associated with an increased risk of MACE28. However, most of the previous studies dichotomised CFR to assess its prognostic value. One observational study reported that invasively measured CFR <3.0 was associated with a sixfold increased risk of all-cause death compared to CFR ≥3.0 over a mean follow-up of 8.5 years29. Another study employing invasively measured CFR also reported that patients with CFR ≤2.0 had a threefold increased risk of MACE compared to those with CFR >2.0 over five years30. In this study, dichotomised invasively measured CFR <2.5 showed a borderline significant association with an increased risk of MACE after adjustment for age and sex, whereas continuous CFR predicted the risk of MACE even after adjustment for other covariates, consistent with the previous observation showing the significant association between continuous CFR measured by positron emission tomography and MACE13. These results highlight the prognostic value of CFR as a continuous variable in predicting MACE.
HMR AND MACE
In this study, we demonstrated that HMR as a continuous variable was associated with future risk of MACE. Further, HMR might have an additional prognostic value in predicting MACE over CFR. Patients with HMR ≥2.0 mmHg/cm/s are reported to have a higher plaque burden and more diffuse CAD than those with HMR <2.0 mmHg/cm/s22. Our patients with higher HMR were more likely to have poorly controlled hypertension, which is consistent with a previous report showing that patients with HMR ≥2.0 mmHg/cm/s had more established cardiovascular risk factors (uncontrolled hypertension and diabetes mellitus), indicating the existence of structural remodelling in the microvasculature4,14. In addition to the structural remodelling, patients with higher HMR were reported to have impaired endothelium-dependent systemic vasodilation with a resultant increase in systolic blood pressure during exercise, reflecting the systemic nature of microvascular disease and indicating that an exercise-induced increase in adenosine or shear stress does not translate into vasodilation14,31. Endogenous adenosine has been shown to contribute to exercise hyperaemia based on the linear correlation between interstitial adenosine and femoral artery blood flow during lower limb exercise, which reduces 20% by theophylline, an adenosine receptor antagonist32,33. In this study, we showed that adenosine non-responders were significantly more prevalent in patients with lower CFR than in those with higher CFR, indicating that an impaired adenosine signalling pathway might be involved in the reduction in vasomotor response to adenosine. Intact endothelium is not necessary for the response to adenosine in vitro; however, there are conflicting results demonstrating that the vasodilatory effect of adenosine is inhibited by a nitric oxide inhibitor in vivo in humans34. Given that impaired shear stress-induced vasodilation is related to higher microvascular resistance at hyperaemia, our previous observation showing that coronary microvascular endothelial dysfunction was associated with low shear stress, a higher plaque burden and unstable plaque features, may partly explain why these patients with higher HMR had a higher plaque burden35,36. Increased microvascular resistance is an independent predictor of plaque instability, which in turn is related to lower fractional flow reserve, reaffirming the presence of cross-talk between the coronary microvasculature and the epicardial vessels37. Though further studies are warranted to identify the link between microvascular resistance, its relationship with epicardial vessels, and worsening clinical outcomes38, our current data show that HMR as a continuous variable independently predicts future risk of MACE and mortality with the largest data set so far.
Limitations
Limitations of this study are discussed in detail in Supplementary Appendix 5. First, because of its retrospective observational cohort design, causal associations cannot be derived from the current study. Second, clinical outcomes were collected by questionnaires. Therefore, recall bias might have affected the results. Since coronary reactivity testing was part of clinical assessment to guide therapy, patients and attending physicians were not blinded to the measurements, potentially affecting medical therapies with resultant change in outcomes. Our lack of data regarding the specific causes of death limits our ability to assess the association between CMD and the specific cause of death meaningfully. Interestingly, reduction of CFR is reported to be independently associated with cardiovascular as well as cancer mortality39, which is consistent with our previous observation showing that abnormal peripheral microvascular vasomotor response is associated with increased risk of incident cardiovascular events and cancer40,41,42. Third, we used aortic pressure during hyperaemia for the approximation of coronary pressure to calculate HMR. Given that only patients with non-obstructive CAD were included in the study, the difference between aortic pressure and coronary pressure is negligible and calculation of HMR using mean aortic pressure is valid.
Conclusions
Lower CFR and higher HMR are associated with an increased risk of MACE as continuous variables. These findings highlight the concept that functional coronary vasomotor response to adenosine and residual hyperaemic microvascular resistance both affect long-term clinical outcomes. Future studies are required to validate these findings in different populations.
Impact on daily practice
Dichotomising physiological parameters is useful to stratify patients by cut-offs; however, optimal cut-off values may differ depending on the population and the clinical outcomes being predicted. Both CFR and HMR as continuous variables predict MACE in patients with chest pain and non-obstructive CAD and can be used to evaluate the future risk of MACE in this population.
Supplementary data
Study population (exclusion criteria).
Statistical analysis.
The discriminatory power of HMR for composite MACE.
Logistic regression analysis to predict composite MACE.
Limitations.
Doppler flow measurement.
Study flow chart.
Distribution of CFR (A) and HMR (B) and correlation between them (C).
Stepwise risk assessment of MACE.
Relationship between adenosine dose and average peak velocity.
Baseline characteristics comparing patients with and without CMD.
Discriminatory power of HMR for composite MACE.
Acknowledgments
Funding
This study was partly supported by the National Institutes of Health (NIH grant numbers DK120292 and DK122734) and the Mayo Foundation.
Conflict of interest statement
A. Lerman reports consulting for Philips. The other authors have no conflicts of interest to declare.
Abbreviations
- CFR
coronary flow reserve
- CI
confidence interval
- CMD
coronary microvascular dysfunction
- HMR
hyperaemic microvascular resistance
- MACE
major adverse cardiovascular events
- OR
odds ratio
Contributor Information
Takumi Toya, Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA; Division of Cardiology, National Defense Medical College, Tokorozawa, Saitama, Japan.
Michel T. Corban, Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA.
Ji Park, Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA.
Ali Ahmad, Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA.
Ilke Ӧzcan, Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA.
Faten Sebaali, Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA.
Jaskanwal Sara, Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA.
Rajiv Gulati, Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA.
Lilach O. Lerman, Division of Nephrology and Hypertension, Mayo Clinic, Rochester, MN, USA.
Amir Lerman, Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA.
References
- Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–95. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radico F, Cicchitti V, Zimarino M, De Caterina R. Angina pectoris and myocardial ischemia in the absence of obstructive coronary artery disease: practical considerations for diagnostic tests. JACC Cardiovasc Interv. 2014;7:453–63. doi: 10.1016/j.jcin.2014.01.157. [DOI] [PubMed] [Google Scholar]
- Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jørgensen E, Kelbæk H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–44. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas A, Prescott E, Karam N, Appelman Y, Fraccaro C, Buchanan GL, Manzo-Silberman S, Al-Lamee R, Regar E, Lansky A, Abbott JD, Badimon L, Duncker DJ, Mehran R, Capodanno D, Baumbach A. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention. 2021;16:1049–69. doi: 10.4244/EIJY20M07_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, Pepine CJ, Merz CN. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169:843–50. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Shaw LJ, Buchthal SD, Bairey Merz CN, Kim HW, Scott KN, Doyle M, Olson MB, Pepine CJ, den Hollander J, Sharaf B, Rogers WJ, Mankad S, Forder JR, Kelsey SF, Pohost GM National Institutes of Health-National Heart, Lung, and Blood Institute. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109:2993–9. doi: 10.1161/01.CIR.0000130642.79868.B2. [DOI] [PubMed] [Google Scholar]
- Olson MB, Kelsey SF, Matthews K, Shaw LJ, Sharaf BL, Pohost GM, Cornell CE, McGorray SP, Vido D, Bairey Merz CN. Symptoms, myocardial ischaemia and quality of life in women: results from the NHLBI-sponsored WISE Study. Eur Heart J. 2003;24:1506–14. doi: 10.1016/S0195-668X(03)00279-3. [DOI] [PubMed] [Google Scholar]
- Widmer RJ, Samuels B, Samady H, Price MJ, Jeremias A, Anderson RD, Jaffer FA, Escaned J, Davies J, Prasad M, Grines C, Lerman A. The functional assessment of patients with non-obstructive coronary artery disease: expert review from an international microcirculation working group. EuroIntervention. 2019;14:1694–702. doi: 10.4244/EIJ-D-18-00982. [DOI] [PubMed] [Google Scholar]
- Rahman H, Ryan M, Lumley M, Modi B, McConkey H, Ellis H, Scannell C, Clapp B, Marber M, Webb A, Chiribiri A, Perera D. Coronary Microvascular Dysfunction Is Associated With Myocardial Ischemia and Abnormal Coronary Perfusion During Exercise. Circulation. 2019;140:1805–16. doi: 10.1161/CIRCULATIONAHA.119.041595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527. doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation. 2017;135:1075–92. doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj NS, Osborne MT, Gupta A, Tavakkoli A, Bravo PE, Vita T, Bibbo CF, Hainer J, Dorbala S, Blankstein R, Bhatt DL, Di Carli MF, Taqueti VR. Coronary Microvascular Dysfunction and Cardiovascular Risk in Obese Patients. J Am Coll Cardiol. 2018;72:707–17. doi: 10.1016/j.jacc.2018.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman H, Demir OM, Khan F, Ryan M, Ellis H, Mills MT, Chiribiri A, Webb A, Perera D. Physiological Stratification of Patients With Angina Due to Coronary Microvascular Dysfunction. J Am Coll Cardiol. 2020;75:2538–49. doi: 10.1016/j.jacc.2020.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorla R, Verna E, Scotti S, Ghiringhelli S, Zoli L, Provasoli S, Garancini S, De Ponti R, Salerno-Uriarte JA. Clinical role of post-angioplasty hyperemic microvascular resistances in chronic ischemic left ventricular dysfunction. J Cardiovasc Med (Hagerstown) 2017;18:332–40. doi: 10.2459/JCM.0000000000000490. [DOI] [PubMed] [Google Scholar]
- Sheikh AR, Zeitz CJ, Rajendran S, Di Fiore DP, Tavella R, Beltrame JF. Clinical and coronary haemodynamic determinants of recurrent chest pain in patients without obstructive coronary artery disease—a pilot study. Int J Cardiol. 2018;267:16–21. doi: 10.1016/j.ijcard.2018.04.077. [DOI] [PubMed] [Google Scholar]
- Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.CIR.101.9.948. [DOI] [PubMed] [Google Scholar]
- Targonski PV, Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr, Lerman A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107:2805–9. doi: 10.1161/01.CIR.0000072765.93106.EE. [DOI] [PubMed] [Google Scholar]
- Corban MT, Godo S, Burczak DR, Noseworthy PA, Toya T, Lewis BR, Lerman LO, Gulati R, Lerman A. Coronary Endothelial Dysfunction Is Associated With Increased Risk of Incident Atrial Fibrillation. J Am Heart Assoc. 2020;9:e014850. doi: 10.1161/JAHA.119.014850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of Coronary Microvascular Dysfunction Among Patients With Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc Interv. 2015;8:1445–553. doi: 10.1016/j.jcin.2015.06.017. [DOI] [PubMed] [Google Scholar]
- Adjedj J, Toth GG, Johnson NP, Pellicano M, Ferrara A, Floré V, Di Gioia G, Barbato E, Muller O, De Bruyne B. Intracoronary Adenosine: Dose-Response Relationship With Hyperemia. JACC Cardiovasc Interv. 2015;8:1422–30. doi: 10.1016/j.jcin.2015.04.028. [DOI] [PubMed] [Google Scholar]
- AlBadri A, Eshtehardi P, Hung OY, Bouchi Y, Khawaja S, Mercado K, Corban MT, Mehta PK, Shaw LJ, Samady H. Coronary Microvascular Dysfunction Is Associated With Significant Plaque Burden and Diffuse Epicardial Atherosclerotic Disease. JACC Cardiovasc Interv. 2019;12:1519–20. doi: 10.1016/j.jcin.2019.05.003. [DOI] [PubMed] [Google Scholar]
- Williams RP, de Waard GA, De Silva K, Lumley M, Asrress K, Arri S, Ellis H, Mir A, Clapp B, Chiribiri A, Plein S, Teunissen PF, Hollander MR, Marber M, Redwood S, van Royen N, Perera D. Doppler versus thermodilution-derived coronary microvascular resistance to predict coronary microvascular dysfunction in patients with acute myocardial infarction or stable angina pectoris. Am J Cardiol. 2018;121:1–8. doi: 10.1016/j.amjcard.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing RJ, Hammond MM, et al. The measurement of coronary blood flow, oxygen consumption, and efficiency of the left ventricle in man. Am Heart J. 1949;38:1–24. doi: 10.1016/0002-8703(49)90788-7. [DOI] [PubMed] [Google Scholar]
- Donald KW. Exercise and heart disease; a study in regional circulation. Br Med J. 1959;1:985–94. doi: 10.1136/bmj.1.5128.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binak K, Harmanci N, Sirmaci N, Ataman N, Ogan H. Oxygen extraction rate of the myocardium at rest and on exercise in various conditions. Br Heart J. 1967;29:422–7. doi: 10.1136/hrt.29.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waard GA, Cook CM, van Royen N, Davies JE. Coronary autoregulation and assessment of stenosis severity without pharmacological vasodilation. Eur Heart J. 2018;39:4062–71. doi: 10.1093/eurheartj/ehx669. [DOI] [PubMed] [Google Scholar]
- Gdowski MA, Murthy VL, Doering M, Monroy-Gonzalez AG, Slart R, Brown DL. Association of Isolated Coronary Microvascular Dysfunction With Mortality and Major Adverse Cardiac Events: A Systematic Review and Meta-Analysis of Aggregate Data. J Am Heart Assoc. 2020;9:e014954. doi: 10.1161/JAHA.119.014954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DS, Gudapati S, Prisant LM, Weir B, diDonato-Gonzalez C, Waller JL, Houghton JL. Mortality in patients with microvascular disease. J Clin Hypertens (Greenwich) 2004;6:304–9. doi: 10.1111/j.1524-6175.2004.03254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Choi KH, Hwang D, Park J, Jung JH, Kim HY, Jung HW, Cho YK, Yoon HJ, Song YB, Hahn JY, Doh JH, Nam CW, Shin ES, Hur SH, Koo BK. Prognostic Implication of Thermodilution Coronary Flow Reserve in Patients Undergoing Fractional Flow Reserve Measurement. JACC Cardiovasc Interv. 2018;11:1423–33. doi: 10.1016/j.jcin.2018.05.005. [DOI] [PubMed] [Google Scholar]
- Martin EA, Nicholson WT, Eisenach JH, Charkoudian N, Joyner MJ. Bimodal distribution of vasodilator responsiveness to adenosine due to difference in nitric oxide contribution: implications for exercise hyperemia. J Appl Physiol (1985) 2006;101:492–9. doi: 10.1152/japplphysiol.00684.2005. [DOI] [PubMed] [Google Scholar]
- Lott ME, Hogeman CS, Vickery L, Kunselman AR, Sinoway LI, MacLean DA. Effects of dynamic exercise on mean blood velocity and muscle interstitial metabolite responses in humans. Am J Physiol Heart Circ Physiol. 2001;281:H1734–41. doi: 10.1152/ajpheart.2001.281.4.H1734. [DOI] [PubMed] [Google Scholar]
- Rådegran G, Calbet JA. Role of adenosine in exercise-induced human skeletal muscle vasodilatation. Acta Physiol Scand. 2001;171:177–85. doi: 10.1046/j.1365-201x.2001.00796.x. [DOI] [PubMed] [Google Scholar]
- Layland J, Carrick D, Lee M, Oldroyd K, Berry C. Adenosine: physiology, pharmacology, and clinical applications. JACC Cardiovasc Interv. 2014;7:581–591. doi: 10.1016/j.jcin.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Siasos G, Sara JD, Zaromytidou M, Park KH, Coskun AU, Lerman LO, Oikonomou E, Maynard CC, Fotiadis D, Stefanou K, Papafaklis M, Michalis L, Feldman C, Lerman A, Stone PH. Local Low Shear Stress and Endothelial Dysfunction in Patients With Nonobstructive Coronary Atherosclerosis. J Am Coll Cardiol. 2018;71:2092–102. doi: 10.1016/j.jacc.2018.02.073. [DOI] [PubMed] [Google Scholar]
- Godo S, Corban MT, Toya T, Gulati R, Lerman LO, Lerman A. Association of coronary microvascular endothelial dysfunction with vulnerable plaque characteristics in early coronary atherosclerosis. EuroIntervention. 2020;16:387–94. doi: 10.4244/EIJ-D-19-00265. [DOI] [PubMed] [Google Scholar]
- Usui E, Yonetsu T, Kanaji Y, Hoshino M, Yamaguchi M, Hada M, Fukuda T, Sumino Y, Ohya H, Hamaya R, Kanno Y, Yuki H, Murai T, Lee T, Hirao K, Kakuta T. Optical Coherence Tomography-Defined Plaque Vulnerability in Relation to Functional Stenosis Severity and Microvascular Dysfunction. JACC Cardiovasc Interv. 2018;11:2058–68. doi: 10.1016/j.jcin.2018.07.012. [DOI] [PubMed] [Google Scholar]
- Corban MT, Lerman LO, Lerman A. Coronary Microvasculature: Are the Small and the Mighty Cross-Talking With the Epicardial Vessels? JACC Cardiovasc Interv. 2018;11:2069–71. doi: 10.1016/j.jcin.2018.07.059. [DOI] [PubMed] [Google Scholar]
- Gaibazzi N, Picano E, Suma S, Garibaldi S, Porter TR, Botti A, Tuttolomondo D, Tedeschi A, Lorenzoni V. Coronary Flow Velocity Reserve Reduction Is Associated with Cardiovascular, Cancer, and Noncancer, Noncardiovascular Mortality. J Am Soc Echocardiogr. 2020;33:594–603. doi: 10.1016/j.echo.2020.01.007. [DOI] [PubMed] [Google Scholar]
- Toya T, Sara JD, Corban MT, Taher R, Godo S, Herrmann J, Lerman LO, Lerman A. Assessment of peripheral endothelial function predicts future risk of solid-tumor cancer. Eur J Prev Cardiol. 2020;27:608–18. doi: 10.1177/2047487319884246. [DOI] [PubMed] [Google Scholar]
- Toya T, Sara JD, Ahmad A, Nardi V, Taher R, Lerman LO, Lerman A. Incremental Prognostic Impact of Peripheral Microvascular Endothelial Dysfunction on the Development of Ischemic Stroke. J Am Heart Assoc. 2020;9:e015703. doi: 10.1161/JAHA.119.015703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–8. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study population (exclusion criteria).
Statistical analysis.
The discriminatory power of HMR for composite MACE.
Logistic regression analysis to predict composite MACE.
Limitations.
Doppler flow measurement.
Study flow chart.
Distribution of CFR (A) and HMR (B) and correlation between them (C).
Stepwise risk assessment of MACE.
Relationship between adenosine dose and average peak velocity.
Baseline characteristics comparing patients with and without CMD.
Discriminatory power of HMR for composite MACE.