Conventional radial access has been shown to have many advantages over the transfemoral approach. The risk of potential radial artery occlusion and subsequent hand ischaemia can be reduced further by accessing the vessel distally at the anatomical snuffbox, allowing maintenance of antegrade flow to the hand by the superficial palmar arch branch. Additional potential advantages of distal radial access in comparison to the conventional radial approach at the wrist include fewer puncture-site complications and faster post-procedural haemostasis as the vessel is very superficial. Furthermore, it provides another safe, non-femoral option for vascular access. The use of ultrasound guidance enables the operator to identify important anatomical landmarks and avoid injuring adjacent structures. We provide a detailed step-by-step guide for performing distal radial access using sonographic and anatomical correlation, thereby facilitating safe access and optimising technical success.
Introduction
Transradial coronary stent placement was first described in 19931, with its advantages over femoral access being well documented since2,3,4. Distal radial access at the anatomical snuffbox has been utilised in Iran and Russia, but no publications existed in Western literature until recently5. The concept was introduced in Russia by Dr Babunashvili to facilitate retrograde radial artery recanalisation. This was followed by Dr Kaledin’s experience in over 2,500 patients which was presented in Cardio Update Europe in 2017. Dr Roghani shared his experience in 2016, utilising the technique in female patients who wear bracelets covering the forearm, limiting haemostasis. Since 2016, the global adoption of the distal radial technique has increased significantly in interventional cardiology, radiology and neurology6,7,8.
The risk of symptomatic hand ischaemia is low following conventional radial access; however, this risk can potentially be reduced further, by using the distal radial artery for access as the vessel is punctured distal to the origin of the superficial palmar arch branch. If radial occlusion occurs, the arch branch will remain patent and therefore preserve continuous antegrade flow to the hand. The length of occluded vessel should thus be limited to the segment between the access site and the origin of the superficial palmar arch. In comparison to conventional radial access, the distal radial artery is more superficial, which lends itself to even faster post-procedural haemostasis. Furthermore, the location of the puncture site allows movement at the wrist during recovery, which is otherwise limited with the conventional approach.
A small study has shown that the size of the distal radial artery at the anatomical snuffbox is not significantly different from that at the wrist. This is also reflected in the comparably high technical success rates9. With no significant calibre change, sheath downsizing is unnecessary when accessing the vessel distally. The anatomical snuffbox is defined as the triangular depression in the dorsum of the hand at the base of the thumb; it is bordered by the abductor pollicis longus and extensor pollicis brevis laterally and the extensor pollicis longus medially. The floor comprises the scaphoid and trapezium carpal bones. The contents of the anatomical snuffbox include the distal radial artery, cephalic vein and superficial branches of the radial nerve; care should therefore be taken when obtaining access. This is particularly important when only palpation, rather than ultrasound (US) guidance, is used for access. A blind puncture increases the risk of inadvertent tendon damage, while the double wall technique can cause irritation of the underlying periosteum. Double wall puncture could increase the risk of haematoma formation, caused by leakage of blood through the punctured back wall of the distal radial artery. Furthermore, if the vessel is accessed distal to the snuffbox (i.e., within the first web space), the haematoma risk increases, as there are no bones to provide support during haemostasis. In addition, a puncture distal to the extensor pollicis tendon may result in inadvertent access of the wire in a branch of the deep palmar arch. The radial pulsation may be more prominent in the latter region rather than in the snuffbox and may thus mislead the inexperienced operator. The utilisation of US allows identification of anatomical landmarks and enables accurate vessel access. The probe can be used to perform a compressibility test to confirm that the target vessel corresponds to the radial artery rather than the cephalic vein; the artery will appear pulsatile and will not collapse once pressure is applied, whereas on the contrary the vein will be readily compressible. Meticulous scanning can identify the superficial branch of the radial nerve, thus avoiding potential injury; the cutaneous branch nerve is located superficially in the snuffbox. A further benefit of US guidance is that the operator can measure the vessel size prior to puncturing, in order to determine whether it can accommodate the required procedural sheath and hardware. The purpose of this article is to provide a detailed step-by-step technical approach for performing US-guided access of the distal radial artery and discuss technical considerations specific to image-guided vascular access.
PATIENT SELECTION
Patient selection is crucial prior to embarking on radial access. Historically, the Barbeau test was recommended10; however, it has been shown to be a variable predictor for the risk of hand ischaemia11. Palpation of the radial pulse can be unreliably weak in patients with systemic shock or underlying vascular disease. Alternatively, the pulse can falsely present distally in patients with proximal radial occlusions, as a result of robust palmar collaterals. Significant benefits of US in such patient populations therefore include providing a visual target and confirming vessel patency.
ULTRASOUND GUIDANCE
US guidance offers several advantages to the operator including accurate location of the puncture site, assessment of vessel size and potential tortuosity, identification of vasospasm and puncture-related complications such as haematoma or dissection. The rate of arterial spasm is lower with US-guided access and therefore technical failures are relatively rare12. Whereas both single and double wall techniques are used interchangeably for conventional radial access, the single wall technique may be preferable for distal radial access as it may decrease the risk of haematoma formation and it obviates the discomfort associated with penetration of the periosteum within the anatomical snuffbox. The single wall technique also reduces the risk of bleeding in the setting of difficult access with multiple punctures, as fewer passes are required.
A learning curve is to be expected during the initial stages of US utilisation; however, this should not deter operators from incorporating this invaluable skill in their practice. It is worth investing time to train in the basics of US, as this will decrease the clinical learning curve (Figure 1). At our institution, operators perform all types of percutaneous vascular access under US guidance. Multiple studies support the use of US guidance for conventional radial artery access versus blind with palpation as this leads to higher first pass success rates13, less time required for obtaining access and haematoma formation14. Operators with no prior US experience for conventional radial access support that this skill can be acquired and integrated into practice without significant difficulty15. We recommend that, for any operators undertaking distal radial access, the puncture is performed under guidance regardless of the level of prior radial access expertise.
Figure 1.
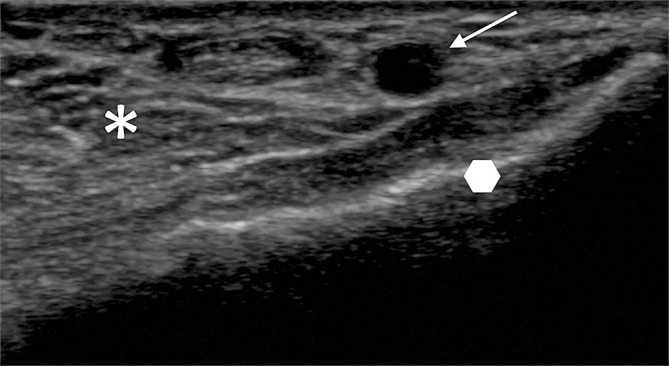
Identification of basic US anatomy at the ventral aspect of the wrist for conventional radial arterial access. The radial artery (arrow). The distal radius (polygon) is represented by a bright line with posterior shadowing. The various soft tissues on the volar aspect of the wrist are seen as different shades on the greyscale image (asterisk).
Various portable US devices are available including wireless systems (Freestyle™️; Siemens Healthineers, Erlangen, Germany), transducers connected to an android-style pad (Lumify; Philips Healthcare, Best, the Netherlands), and handheld wireless transducers that connect to any android or Apple-style pad (Clarius, Burnaby, BC, Canada). Each system has its benefits and drawbacks; choice is dependent on the service and range of clinical needs which will be fulfilled with the device.
CHOICE OF TRANSDUCER
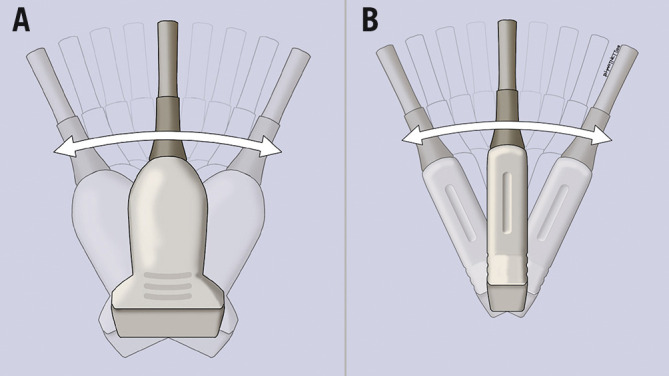
In the context of distal radial access, as the vessel is superficial, a linear (flat) transducer is the preferred option. It is often thought that a “hockey stick” type of probe would be most appropriate, as it is of ultra-high frequency (6-18 MHz) and can be easily positioned in the anatomical snuffbox because of its small size (Figure 2). However, its field of view is narrow, and some are designed with a built-in “step-off’’ which is used in musculoskeletal imaging. Because of the step-off, the needle may not be visualised for 1-2 mm initially. Therefore, given these limitations we do not recommend its use for vascular access.
Figure 2.
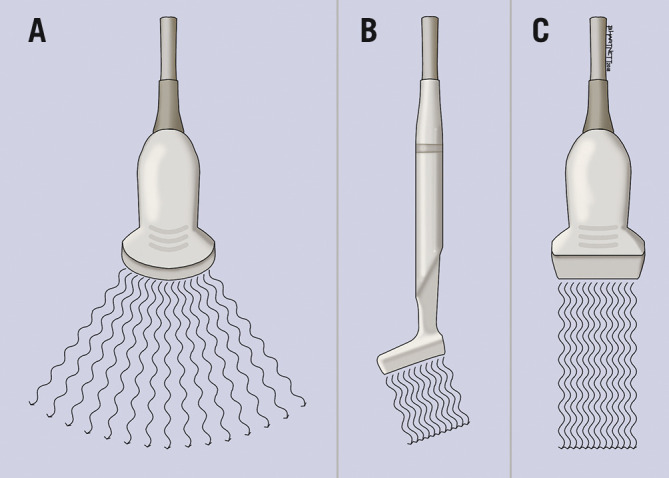
US transducer probes. A curvilinear probe (A) emits a convex beam; the “hockey stick” probe (B) provides a comparatively small field of view. The linear transducer (C) emits a parallel beam and is ideal for vascular imaging.
SCANNING TECHNIQUE
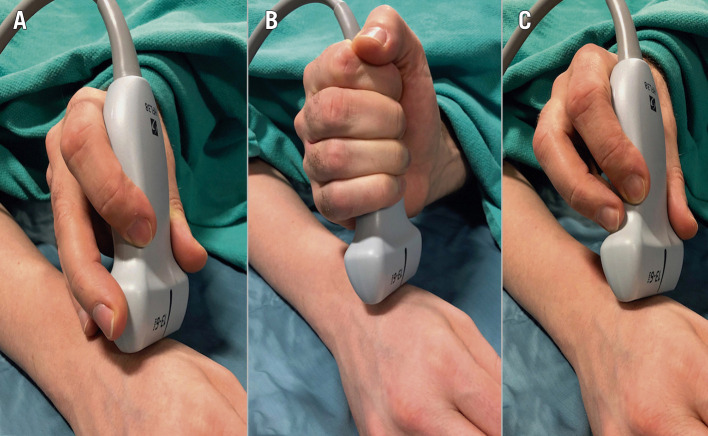
Before scanning is initiated, US gel should be applied liberally on the skin. This is essential, as air is a strong US wave reflector and will therefore prohibit visibility of structures. The gel is water-soluble and does not obstruct the needle during the puncture. US-guided vessel puncture has been a standard of care in interventional radiology with no incidents of gel obstructing standard 17G needles or micropuncture needles down to 22G. The transducer should be held lightly on the skin in a perpendicular orientation to the plane being imaged, while ensuring that good contact is maintained with the gel (Figure 3). If a high pressure is applied, the vessel will be compressed, and structures will be distorted, ultimately making the puncture more difficult if not impossible. The probe should be checked to ensure that the orientation on the screen (right to left) matches the position on the patient by gently tapping the plate and looking on the screen for the corresponding side of movement.
Figure 3.
Operator US scanning technique. A) Correct scanning technique with the operator’s hand partly resting on the patient and providing light pressure on soft tissues. B) & C) Incorrect handling of the US probe, resulting in an unstable probe position, excess compression and thus collapse of the radial artery.
Once scanning is commenced, the machine parameters can be modified in order to optimise the image obtained. Given that the distal radial artery is superficial, the depth must be adjusted such that the majority of the image demonstrates the structures in the near field (Figure 4). The focus of the image can also be altered, so that the US beam is targeted onto the specific region of interest. Adjusting the gain is very important for improving the image generated; as a rule of thumb, the higher the gain, the brighter (whiter) the image will look. Most of the US machines have an auto-gain option; however, the depth and focus need to be adjusted manually.
Figure 4.
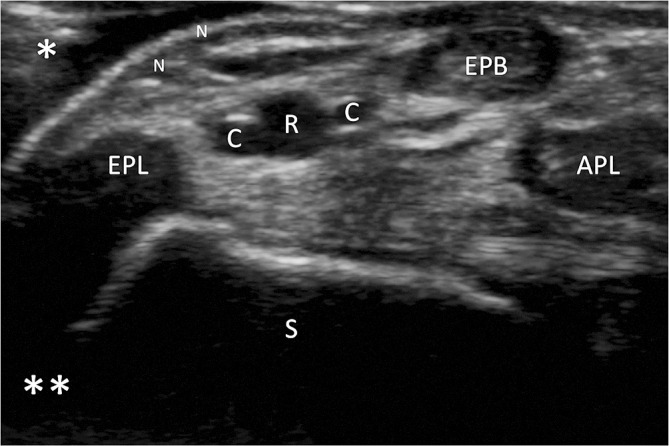
Optimisation of US image. Image demonstrating the near (single asterisk) and far (double asterisk) fields during scanning. The depth has been adjusted so that the structures in the near field comprise the majority of the image. The key anatomical structures that should be identified in the anatomical snuffbox include the distal radial artery (R), cephalic vein branches (C), extensor pollicis longus (EPL), extensor pollicis brevis (EPB), abductor pollicis longus (APL) and scaphoid (S).
ULTRASOUND ASSESSMENT
The radial artery should be scanned using an organised approach from the first dorsal web space, to the anatomical snuffbox and into the wrist at the site of conventional radial artery access. The first dorsal web space is located between the thumb and index finger and contains the first dorsal interosseous muscle. A more detailed assessment of the forearm radial artery can be performed until it is seen to join the brachial artery at the level of the elbow. This is important in order to check vessel size, patency and presence of a radial loop. It can be performed in lieu of a radial angiogram. Vessel size should be evaluated to determine equipment compatibility in the vessel. Subcutaneous injection of nitroglycerine will result in vasodilatation, making assessment of artery-catheter compatibility more accurate. A US Doppler assessment is advised to confirm vessel patency, particularly in patients where the radial artery has been accessed previously (Figure 5).
Figure 5.
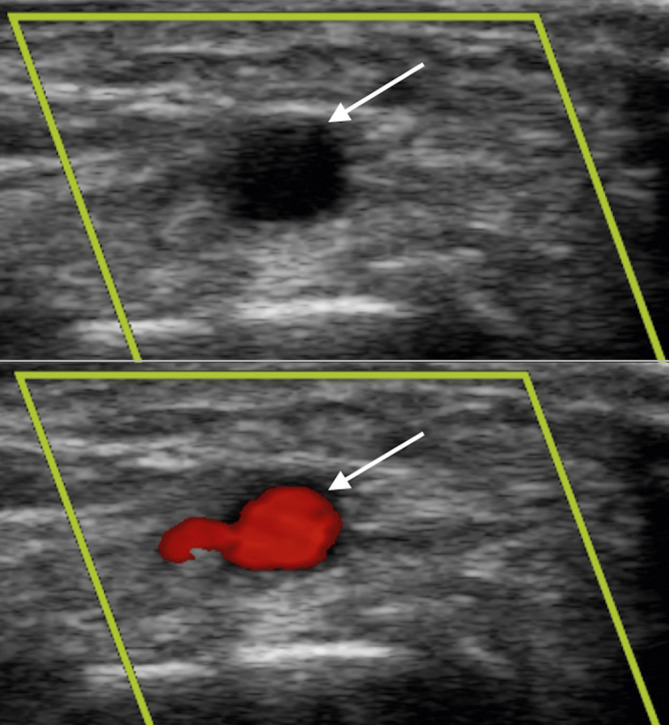
Arterial Doppler vascular assessment. US images of the distal radial artery (arrow) before and after Doppler tool is applied confirming patency.
US assessment is then performed to identify anatomical landmarks to determine a safe puncture site and avoid damaging adjacent structures. The borders of the anatomical snuffbox should be identified at this time. The tendons are seen as well-defined structures of intermediate shade on the greyscale image (Figure 6). The radial artery over the scaphoid and trapezium bones is superficial and safe to access; therefore, we recommend that the vessel is punctured at this location. Care should be taken to avoid inadvertently damaging the tendons. The superficial branch of the radial nerve is located close to the extensor pollicis longus tendon; this may be challenging to visualise given it is a very small structure.
Figure 6.
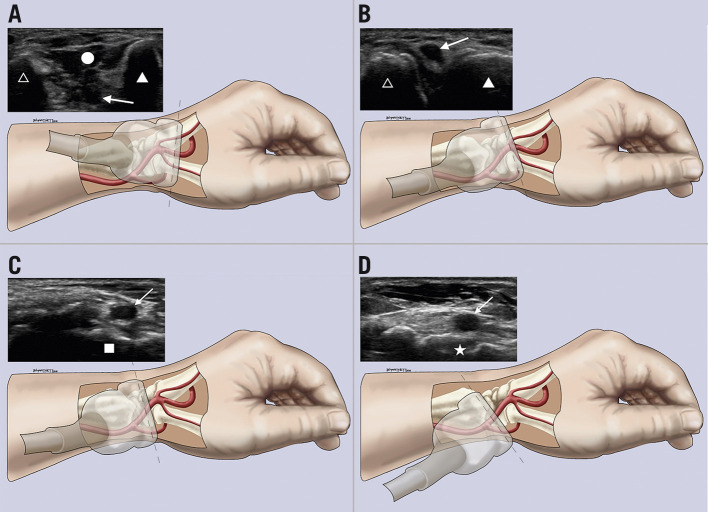
Sonographic and anatomic correlation. A) – D) Different transducer positions and their corresponding US images starting distally at the first dorsal web space (A), moving proximally to the anatomical snuffbox over the scaphoid (D). Arrow: distal radial artery; circle: first dorsal interosseous muscle; filled triangle: thumb metacarpal; unfilled triangle: index metacarpal; square: trapezium bone; star: scaphoid bone.
SUBCUTANEOUS ANAESTHESIA
Once a satisfactory puncture site has been chosen, the skin should be infiltrated with local anaesthetic solution (Figure 7). The amount varies between operators; however, for novice users liberal use of anaesthetic solution (5-10 mL) is recommended as inadvertent contact with the underlying periosteum may cause considerable pain. It is preferable to perform the deeper infiltration under US guidance in order to avoid patient discomfort; this can also assist the operator to decide the best trajectory of the needle for the puncture. Due to the superficial nature of the access site, it is important to remember that a large infusion of anaesthetic solution could be misinterpreted as a haematoma by post-procedural care staff; therefore, it is important for the operator to monitor the site after sheath insertion. A vessel that appears relatively small within the optimal area of puncture may be reassessed after US-guided infiltration of nitroglycerine. Typically, 200-400 mcg of undiluted nitroglycerine is injected on either side of the vessel with US visualisation after local anaesthesia has been given.
Figure 7.
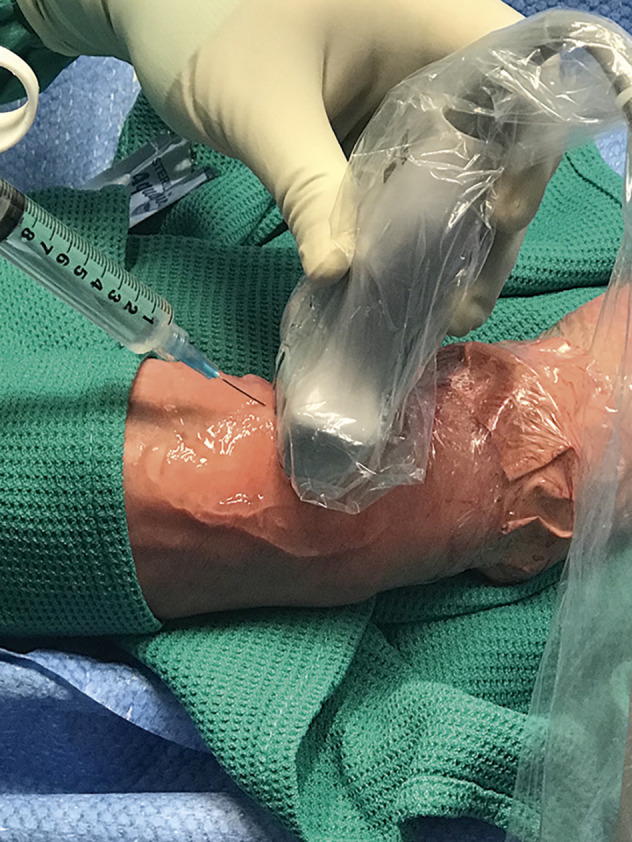
Local anaesthetic skin infiltration. The probe should be held with the operator’s non-dominant hand and skin infiltration performed using the dominant hand.
PUNCTURE
The arm is placed in a pronated position (Figure 8). Under US guidance, the radial artery is punctured with a 21G needle using a single wall puncture technique. Usually, the needle is directed from ulnar to radial, at a 30-degree angle with the radial artery in the horizontal plane and a 45- to 60-degree angle in the vertical plane. The puncture can be performed in either the longitudinal or axial plane. With the longitudinal plane, the entire length of the needle can be visualised through the subcutaneous tissue into the vessel on a single image; this is referred to as in-plane imaging as the needle is continuously positioned within the US beam. This method is more difficult to master as the operator must place the needle perfectly within the narrow US beam; however, once achieved, it allows continuous tracking of the needle. A second method, referred to as out-of-plane imaging, images the vessel in the axial (cross-sectional) plane. This allows more latitude to the operator regarding needle position; however, because the needle is perpendicular to the US beam, only the portion passing through the beam can be visualised. This method relies on the operator understanding the limitations of this technique and ensuring that the tip of the needle is always visible.
Figure 8.
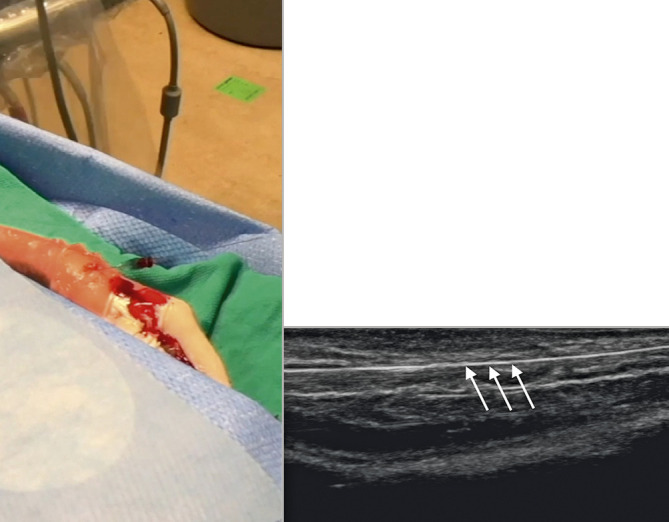
Intravascular access. The position of the needle and wire at the anatomical snuffbox. Confirmation of appropriate intravascular position of the wire can be obtained on the US image as indicated by the arrows.
A common error made by operators is simultaneous movement of the needle and the US probe. The easiest technique to ensure that the vessel is punctured in the correct position is to place the probe on the skin over the vessel at the intended entry site. The probe should remain stationary and only tilted on either its long (heel-toe movement) or short axis (toggle movement) to locate the needle tip (Figure 9). The needle trajectory can then be adjusted to ensure that it enters the vessel at the exact intended position. Moving the probe along the length of the vessel in an attempt to locate the needle tip will often result in puncturing the vessel where the needle tip is located rather than at the desired puncture site over the carpal bones. Utilising the heel-toe and toggle techniques with US-guided access is essential for accurate vessel access.
Figure 9.
Sonographic vascular assessment. A) Heel-toe movement; the US probe is tilted on its long axis. B) Toggle movement; the US probe is tilted on its short axis.
Intravascular position is confirmed when the position of the needle is satisfactory on the US image and blood is seen with a continuous trickle or steady pulsatile flow through the hub. If either of these is not present, then the needle is unlikely to be truly intravascular. It is essential for the operator not only to assess needle position on the US monitor, but also to scan the needle tip and screen constantly as the needle nears the vessel wall. It is common with early adoption to be searching the monitor for the needle tip and for there to be unwitnessed steady blood flow at the needle hub. Once in a satisfactory position, the needle should be held securely, and the US probe released. A 0.018” or 0.021” wire can be used for access.
The wire used can influence the ease of advancement through the vessel. In our experience, using a spring-coiled wire has a higher incidence of failure, whereas a stainless steel reinforced wire provides support for sheath access and tracks significantly more easily through the vessel on insertion. If any resistance is met during advancement, the needle bezel should be slowly rotated through 360° while gently probing with the wire. In some cases, the bevel may be up against the back or side wall, preventing the wire from advancing. The needle angle can also be adjusted if rotation of the bevel does not allow easy passage of the wire. If resistance is noted once the wire exits the needle, this may be due to the wire advancing into the superficial palmar branch; US can then be used to assess wire position. Furthermore, if the puncture is performed distal to the anatomical snuffbox, the wire may track into a side branch of the deep palmar arch. If these situations arise, it is worthwhile asking the patient to adduct their thumb; this will straighten the artery and therefore reduce the chances of the wire being misdirected. Routine checking of the volar aspect of the wrist with US is recommended in order to confirm the correct wire position in the forearm radial artery prior to sheath insertion (Figure 8). Alternatively, if the operator prefers, confirmation of wire position can also be performed fluoroscopically.
An advantage of using the distal radial artery is that, if problems should arise at the initial puncture site, the operator can still attempt radial access by puncturing the vessel at the conventional location at the wrist.
SHEATH INSERTION
The need for a skin incision in the snuffbox is based on operator preference. Some operators have found the skin to be thicker than on the volar aspect of the wrist and so a small skin incision can be made to allow easy sheath insertion. From our experience, it has been noted that some thin-walled, low-profile sheaths designed specifically for conventional radial access may kink when used in the distal radial artery. This is due to the curve at the level of the radioscaphoid joint (Figure 10) and the thin wall of the sheath not providing sufficient radial force. Therefore, this should be borne in mind during procedures. Difficulty in sheath advancement despite adequate wire position may occasionally occur as a result of resistance at the skin or subcutaneous tissue or as a consequence of a vertical entry into the vessel. Once it has been confirmed that the entry was not inadvertently made through connective tissue or tendon, one may consider dilating the tissue tract with a micropuncture sheath before attempting re-advancement of the radial introducer sheath.
Figure 10.
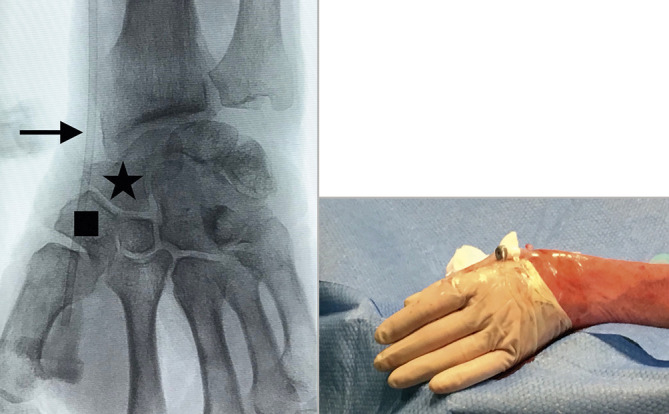
Distal radial access sheath position. Fluoroscopic image showing the position of the vascular sheath. The puncture site is over the trapezium (square), with the sheath crossing over the scaphoid (star) and radioscaphoid joint. The location which is at the highest risk of kinking is indicated by the arrow. Once the sheath is inserted, it is secured as shown in the image.
Conclusion
The use of the distal radial artery for image-guided, vascular interventions offers several advantages over conventional radial access. Concomitant use of US guidance is a powerful tool for maximising technical success and reducing puncture site-related complications. Optimal performance of this technique requires a basic understanding of the anatomy of the hand and some degree of facility with US image acquisition.
Acknowledgments
Conflict of interest statement
S. Nathan is a consultant for Medtronic, Merit Medical and Terumo Interventional Systems. F. Kiemeneij is a consultant for Merit Medical. D. Klass is a consultant for Merit Medical. The other author has no conflicts of interest to declare.
Contributor Information
Anastasia Hadjivassiliou, Department of Interventional Radiology, Vancouver General Hospital, University of British Columbia, Vancouver, BC, Canada.
Ferdinand Kiemeneij, Department of Cardiology, Tergooi Hospital, Blaricum, the Netherlands.
Sandeep Nathan, University of Chicago Medicine, Chicago, IL, USA.
Darren Klass, Department of Interventional Radiology, Vancouver General Hospital, University of British Columbia, Vancouver, BC, Canada.
References
- Kiemeneij F, Laarman GJ. Percutaneous transradial artery approach for coronary stent implantation. Cathet Cardiovasc Diagn. 1993;30:173–8. doi: 10.1002/ccd.1810300220. [DOI] [PubMed] [Google Scholar]
- Chase AJ, Fretz EB, Warburton WP, Klinke WP, Carere RG, Pi D, Berry B, Hilton JD. Association of the arterial access site at angioplasty with transfusion and mortality: the M.O.R.T.A.L study (Mortality benefit Of Reduced Transfusion after percutaneous coronary intervention via the Arm or Leg). Heart. 2008;94:1019–25. doi: 10.1136/hrt.2007.136390. [DOI] [PubMed] [Google Scholar]
- Jolly SS, Yusuf S, Cairns J, Niemelä K, Xavier D, Widimský P, Budaj A, Niemelä M, Valentin V, Lewis BS, Avezum A, Steg PG, Rao SV, Gao P, Afzal R, Joyner CD, Chrolavicius S, Mehta SR RIVAL trial group. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–20. doi: 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- Valgimigli M, Gagnor A, Calabro P, Frigoli E, Leonardi S, Zaro T, Rubartelli P, Briguori C, Ando G, Repetto A, Limbruno U, Cortese B, Sganzerla P, Lupi A, Galli M, Colangelo S, Ierna S, Ausiello A, Presbitero P, Sardella G, Varbella F, Esposito G, Santarelli A, Tresoldi S, Nazzaro M, Zingarelli A, de Cesare N, Rigattieri S, Tosi P, Palmieri C, Brugaletta S, Rao SV, Heg D, Rothenbuhler M, Vranckx P, Jüni P MATRIX Investigators. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385:2465–76. doi: 10.1016/S0140-6736(15)60292-6. [DOI] [PubMed] [Google Scholar]
- Kiemeneij F. Left distal transradial access in the anatomical snuffbox for coronary angiography (ldTRA) and interventions (ldTRI). EuroIntervention. 2017;13:851–7. doi: 10.4244/EIJ-D-17-00079. [DOI] [PubMed] [Google Scholar]
- Kim Y, Ahn Y, Kim I, Lee DH, Kim MC, Sim DS, Hong YJ, Kim JH, Jeong MH. Feasibility of Coronary Angiography and Percutaneous Coronary Intervention via Left Snuffbox Approach. Korean Circ J. 2018;48:1120–30. doi: 10.4070/kcj.2018.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pua U, Sim JZT, Quek LHH, Kwan J, Lim GHT, Huang IKH. Feasibility Study of “Snuffbox” Radial Access for Visceral Interventions. J Vasc Interv Radiol. 2018;29:1276–80. doi: 10.1016/j.jvir.2018.05.002. [DOI] [PubMed] [Google Scholar]
- McCarthy DJ, Chen SH, Brunet MC, Shah S, Peterson E, Starke RM. Distal Radial Artery Access in the Anatomical Snuffbox for Neurointerventions: Case Report. World Neurosurg. 2019;122:355–9. doi: 10.1016/j.wneu.2018.11.030. [DOI] [PubMed] [Google Scholar]
- Hadjivassiliou A, Cardarelli-Leite L, Jalal S, Chung J, Liu D, Ho S, Klass D. Safety and Efficacy of a Truncated Deflation Algorithm for Distal Transradial Access. J Vasc Interv Radiol. 2020;31:1328–33. doi: 10.1016/j.jvir.2020.02.027. [DOI] [PubMed] [Google Scholar]
- Barbeau GR, Arsenault F, Dugas L, Simard S, Larivière MM. Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: comparison with the Allen’s test in 1010 patients. Am Heart J. 2004;147:489–93. doi: 10.1016/j.ahj.2003.10.038. [DOI] [PubMed] [Google Scholar]
- van Leeuwen MAH, Hollander MR, van der Heijden DJ, van de Ven PM, Opmeer KHM, Taverne YJHJ, Ritt MJPF, Kiemeneij F, Van Mieghem NM, van Royen N. The ACRA Anatomy Study (Assessment of Disability After Coronary Procedures Using Radial Access): A Comprehensive Anatomic and Functional Assessment of the Vasculature of the Hand and Relation to Outcome After Transradial Catheterization. Circ Cardiovasc Interv. 2017;10:e005753. doi: 10.1161/CIRCINTERVENTIONS.117.005753. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Alshammari MT, Liu DM, Chung J, Ho SGF, Legiehn GM, Machan L, Fischman AM, Patel RS, Klass D. Transradial access for interventional radiology: single centre procedural and clinical outcome analysis. Can Assoc Radiol J. 2017;68:318–27. doi: 10.1016/j.carj.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Moussa Pacha H, Alahdab F, Al-Khadra Y, Idris A, Rabbat F, Darmoch F, Soud M, Zaitoun A, Kaki A, Rao SV, Kwok CS, Mamas MA, Alraies MC. Ultrasound-guided versus palpation-guided radial artery catheterization in adult population: A systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2018;204:1–8. doi: 10.1016/j.ahj.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Tang L, Wang F, Li Y, Zhao L, Xi H, Guo Z, Li X, Gao C, Wang J, Zhou L. Ultrasound guidance for radial artery catheterization: an updated meta analysis of randomized controlled trials. PLoS One. 2014;9:e111527. doi: 10.1371/journal.pone.0111527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J, Manur R. Ultrasound-guided radial artery access by a non-ultrasound trained interventional cardiologist improved first-attempt success rates and shortened time for successful radial artery cannulation. J Invasive Cardiol. 2013;25:676–9. [PubMed] [Google Scholar]