Abstract
Background
Balloon aortic valvuloplasty (BAV) has been proposed as a therapeutic option in patients suffering from severe aortic stenosis (SAS) who need urgent non-cardiac surgery (NCS). Whether this strategy is better than medical therapy in this very specific population is unknown.
Aims
We aimed to evaluate the clinical benefit of an invasive strategy (IS) with preoperative BAV in patients with SAS requiring urgent NCS.
Methods
From 2011 to 2019, a registry conducted in two centres included 133 patients with SAS undergoing urgent NCS, of whom 93 underwent preoperative BAV (IS) and 40 a conservative strategy (CS) without BAV. All analyses were adjusted for confounding using inverse probability of treatment weighting (IPTW) (10 clinical and anatomical variables).
Results
The primary outcome was MACE at one-month follow-up after NCS including mortality, heart failure, and other cardiovascular outcomes. In patients managed conservatively, occurrence of MACE was 20.0% (n=8) and death was 10.0% (n=4) at 1 month. In patients undergoing BAV, the occurrence of MACE was 20.4% (n=19) and death was 5.4% (n=5) at 1 month. Among patients undergoing conservative management, all events were observed after NCS while, in patients undergoing BAV, 12.9% (n=12) had events between BAV and NCS including 3 deaths, and 7.5% (n=7) had events after NCS including 2 deaths. In IPTW propensity analyses, the incidence of the primary outcome (20.4% vs 20.0%; OR 0.93, 95% CI: 0.38-2.29) and three-month survival (89.2% vs 90.0%; IPTW-adjusted HR 0.90, 95% CI: 0.31-2.60) were similar in both groups.
Conclusions
Patients with SAS managed conservatively before urgent NCS are at high risk of events. A systematic invasive strategy using BAV does not provide a significant improvement in clinical outcome.
Introduction
Management of severe aortic stenosis (SAS) patients undergoing non-cardiac surgery (NCS) is a challenging and relatively frequent situation1, with no clear evidence-based strategy.
Performing elective NCS in patients with SAS has been associated with a relatively high rate of major adverse cardiac events (MACE) (18.8%) and mortality (5.9%) at one month2. In that context, an invasive strategy based on balloon aortic valvuloplasty (BAV) before NCS has been proposed as an option to reduce this risk3,4. The 2017 ESC and 2014 ESC/AHA/ACC recommendations, which are largely based on small and observational studies that are now more than three decades old5,6,7, suggest deferring the NCS whenever this is possible, while BAV can be proposed with a low level of evidence, class IIb C7.
For the specific subgroup of SAS patients requiring urgent non-elective NCS, clinical data are even more scarce8,9. The risk of performing urgent (<7 days) or emergency (<48 hrs) NCS is not well known as these high-risk surgical conditions are usually underrepresented (around 10%) in studies investigating the risk of NCS in SAS patients2,10. Besides, the utility of preoperative BAV in this particular setting is unknown. No randomised study comparing the outcomes of SAS patients undergoing urgent NCS under a conservative or invasive approach has been conducted to date and the net benefit of BAV as a preparation for urgent NCS is uncertain because of the potentially high complication rate of BAV in these conditions11,12. This explains why the practice is very wide with some centres using an invasive strategy (IS) – percutaneous BAV – in all SAS patients before urgent NCS while others do not, adopting a conservative strategy (CS) instead.
In the current study we set out to re-evaluate the outcomes of SAS patients requiring urgent non-elective NCS in the contemporary era and the potential benefit of an IS (preoperative BAV for urgent NCS) versus a CS. This was done by comparing the outcomes of SAS patients undergoing BAV prior to urgent NCS to the outcomes of SAS patients undergoing urgent NCS without prior BAV, using them as a control group.
Methods
PATIENT SELECTION
In this retrospective study, we included two centres with different strategies regarding the management of SAS patients before urgent NCS from 2011-2019, i.e., one centre with a default invasive strategy using routine BAV before NCS, and one centre with a default conservative strategy without BAV before NCS.
SAS was considered to be present at the time of surgery if documented within 12 months before surgery. SAS was defined using current transthoracic echocardiography (TTE) criteria (aortic valve area ≤1 cm², peak systolic flow velocity ≥4 m/s, mean gradient ≥40 mmHg) in conjunction with typical 2D echocardiographic appearance of severe AS13. Patients undergoing aortic valve replacement before NCS were excluded. Patients with high gradients or velocities attributable to increased cardiac output (anaemia, septic shock, etc.), as well as those with concomitant diseases that may have influenced Doppler indices of SAS (hypertrophic obstructive cardio-myopathy, subvalvular or supravalvular aortic stenosis, coarctation of the aorta, or complex congenital heart diseases) were excluded.
Baseline demographic data, type of surgical intervention, comorbidities, symptoms potentially associated with SAS (dyspnoea), and echocardiographic data just before surgery were extracted from the electronic medical records.
Regarding the BAV procedure, the size of the balloon was chosen according to the annulus measurement according to the TTE before BAV. The NuCLEUS™️ (NuMED Inc., Hopkinton, NY, USA) and Z-MED™️ (B. Braun Interventional Systems, Bethlehem, PA, USA) balloons were used for transfemoral BAV, and VACS™ II (OSYPKA AG, Rheinfelden, Germany) balloons for transradial BAV. Valvuloplasty was considered successful if a significant reduction (≥50%) of the mean transaortic gradient assessed by haemodynamic measures was obtained.
This study was approved by the institutional ethics committee at each centre.
ECHOCARDIOGRAPHY
All echocardiograms were performed as clinically indicated, and in accordance with current European and American Society of Echocardiography recommendations13. In patients with multiple echocardiograms, the study closest to the time of surgery was selected. Aortic valve parameters (valve area and valve area index, peak aortic velocity and mean aortic valve gradient), as well as left ventricular size, ejection fraction, and estimated pulmonary artery systolic pressure (based on tricuspid regurgitant velocity) were extracted from the echocardiography database.
NON-ELECTIVE NON-CARDIAC SURGERY (NCS)
Surgical interventions were classified according to current ESC/ACC/AHA guidelines into low-, intermediate-, and high-risk5. Patients undergoing low-risk (transurethral resection of the prostate, superficial, eye, breast surgery; reported cardiac risk <1%), intermediate-risk (intraperitoneal and intrathoracic surgery, carotid endarterectomy, head and neck surgery, orthopaedic surgery, prostate surgery; reported cardiac risk 1-5%) and high-risk procedures (aortic and other major vascular surgery, peripheral vascular surgery; reported risk >5%)5 under general or locoregional anaesthesia were included. Semi-urgent NCS included patients who were operated within 2 to 7 days and emergency NCS those who were operated on within 48 hours. Ambulatory, ophthalmological and percutaneous interventions were excluded.
CLINICAL ENDPOINTS
We compared the outcomes after NCS of an IS versus a CS of management of SAS patients. The primary endpoint (MACE) was a composite of one-month mortality, heart failure, myocardial infarction, stroke/transient ischaemic attack (TIA), new atrial fibrillation, acute kidney injury (AKI; rise of >twofold of baseline creatinine and/or <0.5 ml/kg/hr urine output), and life-threatening bleeding (hypovolaemic shock or severe hypotension requiring vasopressors or surgery or packed red blood cell [RBC] transfusion ≥4 units) after NCS. The secondary outcome included predictive factors of one-month MACE. Other analyses included three-month survival after NCS. All medical files were carefully reviewed and, in case of doubt, clinical events were adjudicated by a medical committee of two physicians.
STATISTICAL ANALYSIS
Quantitative variables are expressed as means (±standard deviation) in the case of normal distribution or medians (interquartile range) otherwise. Categorical variables are expressed as numbers (percentage). Normality of distributions was assessed using histograms and the Shapiro-Wilk test. Patients were divided into two groups according to the strategy used. Baseline characteristics were described according to the two study groups, and the magnitude of the between-group differences in pre-specified confounders was assessed by calculating the absolute standardised difference; an absolute standardised difference >10% was interpreted as a meaningful difference14. Between-group comparisons in surgical procedure characteristics, and association of potential predictors of the primary clinical endpoint (one-month MACE) were performed using the chi-square test or Fisher’s exact test in case of expected cell frequencies <5. Comparison in outcomes between the two study groups was carried out using a logistic regression model for MACE, a linear regression model for length of hospital stay (after log-transformation values to satisfy the residual normality) and a Cox proportional hazards model for three-month all-cause mortality; effect sizes and their 95% confidence intervals (CIs) were derived from regression models using patients treated with a CS (without preoperative BAV) as the control group. In order to take into account the pre-specified confounders, comparisons in outcomes were further performed by using the pre-inverse probability of treatment weighting (IPTW) propensity score method (using stabilised inverse propensity score weighting in regression models). The propensity score was estimated using a multivariable logistic regression model, with study groups as the dependent variable and pre-specified confounders as covariates (Table 1)15. Statistical testing was conducted at the two-tailed α-level of 0.05. Data were analysed by J. Labreuche, using SAS software version 9.4. (SAS Institute, Cary, NC, USA).
Table 1. Main baseline characteristics (pre-specified confounders) in aortic stenosis patients before urgent NCS.
| Characteristics | (n=133) | |||
| Severe AS invasive strategy (n=93) | Conservative strategy (n=40) | ASD, % | ||
| Patient characteristics | ||||
| Age, years | 79.9±9.5 | 83.0±8.0 | 35.4 | |
| Male gender | 38 (40.9) | 19 (47.5) | 13.4 | |
| Surgery period >2015 | 45 (48.4) | 19 (47.5) | 1.8 | |
| ASA score | 2 | 31 (33.3) | 13 (32.5) | 5.0 |
| 3 | 37 (39.8) | 15 (37.5) | ||
| 4 | 25 (26.9) | 12 (30.0) | ||
| STS score (Nov. 2018 %) | 3.0±1.3 | 3.2±1.4 | 10.9 | |
| TTE characteristics* | ||||
| LVE fraction, % | 56.6±12.2 | 59.2±9.3 | 23.7 | |
| AVA, cm² | 0.72±0.15 | 0.77±0.22 | 24.8 | |
| Mean transaortic gradient, mmHg | 45.0±13.6 | 42.3±8.1 | 24.1 | |
| Aortic maximal velocity, m/s | 4.2±0.6 | 4.1±0.4 | 15.6 | |
| Surgery characteristics | ||||
| Non-cardiac surgery risk** | Low | 21 (22.6) | 9 (22.5) | 29.1 |
| Intermediate | 57 (61.3) | 28 (70.0) | ||
| High | 15 (16.1) | 3 (7.5) | ||
| Values are expressed as numbers (%) unless otherwise indicated. These variables were used for the IPTW score. *Evaluated by TTE before BAV and before non-cardiac surgery. **According to ESC 2014 and 2017 recommendations. ASA: American Society of Anesthesiologists; ASD: absolute standardised difference; AVA: aortic valve area; BAV: balloon aortic valvuloplasty; LVE: left ventricular ejection; SAS: severe aortic stenosis; TTE: transthoracic echocardiography | ||||
Results
STUDY POPULATION
(I) SAS PATIENTS UNDERGOING A CS BEFORE NCS
As shown in the Central illustration, from 2011 to 2019 we identified 40 patients with SAS (aortic valve area 0.77±0.22 cm2) undergoing a CS without BAV before NCS.
Central illustration.

Study flow chart.
(II) SAS PATIENTS UNDERGOING AN IS (BAV) PROCEDURE BEFORE NCS
We also identified 93 patients with SAS (aortic valve area 0.72±0.15 cm2) treated with preoperative BAV before NCS (Central illustration). BAV was performed 4 (2-11) days before NCS.
BASELINE CHARACTERISTICS
Baseline characteristics and the comorbidities of the different groups are presented in Table 1 (main pre-specified confounders for IPTW score) and Table 2.
Table 2. Baseline characteristics (considered as non-specified confounders) in aortic stenosis patients.
| Invasive strategy (n=93) | Conservative strategy (n=40) | ||
| Patient characteristics | |||
| Hypertension | 72 (77) | 30 (75) | |
| Diabetes mellitus | 27 (29) | 12 (30) | |
| Obese (BMI >30) | 13 (14) | 5 (12) | |
| Iliofemoral artery disease | 21 (23) | 15 (37) | |
| Coronary disease | 33 (35) | 12 (30) | |
| Previous CABG | 2 (2) | 2 (0.5) | |
| Active cancer | 33 (35) | 3 (7) | |
| Renal dysfunction, n (%) | 24 (26) | 10 (25) | |
| COPD | 13 (14) | 6 (15) | |
| Atrial fibrillation | 29 (31) | 12 (32) | |
| Prior stroke/TIA | 11 (12) | 9 (22) | |
| Preoperative pacemaker | 8 (9) | 4 (1) | |
| Dyspnoea III-IV before NCS | 72 (77) | 12 (30) | |
| Medication at the time of surgery | |||
| Anticoagulant | 24 (25) | 10 (25) | |
| Antiplatelet | 60 (64) | 34 (85) | |
| Statins | 56 (60) | 27 (67) | |
| CEI/ARAII | 45 (48) | 19 (47) | |
| β-blockers | 25 (27) | 10 (25) | |
| Other TTE characteristics before surgery | |||
| LVE fraction, % | 57.5±12.1* | 59.2±9.3 | |
| AVA, cm2 | 0.91±0.21* | 0.77±0.22 | |
| Mean transaortic gradient, mmHg | 32.4±11.4* | 42.3±8.1 | |
| Aortic maximal velocity, m/s | 3.6±0.6* | 4.1±0.4 | |
| Bicuspid aortic valve | 7 (7) | 4 (10) | |
| Left ventricle volume, mL, mean±SD | 100.6±28.4* | 105.4±30.2 | |
| Mitral regurgitation | 31 (33)* | 14 (35) | |
| Left atrium volume, mL, mean±SD | 45.7±17.9* | 44.8±19.3 | |
| Sdti, cm/s, mean±SD | 10.8±2.1* | 11.1±3.0 | |
| Systolic PAP, mmHg, mean±SD | 35.5±11.1* | 33.5±10.7 | |
| Surgery characteristics | |||
| Timing of surgery | Emergency (<48 hrs) | 61 (65) | 22 (55) |
| Semi-urgent (2-7 days) | 32 (35) | 18 (45) | |
| General anaesthesia | 84 (90) | 33 (83) | |
| Values are expressed as numbers (%) unless otherwise indicated. Renal dysfunction defined as GFR ≤60 ml/min/m². * Evaluated by TTE after BAV and before non-cardiac surgery. ARAII: angiotensin II receptor antagonist; AVA: aortic valve area; BAV: balloon aortic valvuloplasty; CABG: coronary artery bypass graft; CEI: converting enzyme inhibitors; COPD: chronic obstructive pulmonary disease; IQR: interquartile range; LVE: left ventricular ejection; PAP: pulmonary artery pressure; SAS: severe aortic stenosis; SD: standard deviation; Sdti: tricuspid lateral annular systolic velocity; TIA: transient ischaemic attack; TTE: transthoracic echocardiography | |||
When comparing TTE characteristics in both groups at baseline (Table 1), there was no significant difference in mean aortic gradient (45.0±13.6 mmHg vs 42.3±8.1 mmHg; p=0.24) or maximal velocity (4.2±0.6 m/s vs 4.1±0.4 m/s; p=0.45), or aortic valve area (0.72±0.15 cm2 vs 0.77±0.22 cm2; p=0.16).
After IPTW using the propensity score method, the between-group differences in the main confounders (Table 1) were reduced, as shown in Supplementary Figure 1.
BAV PROCEDURE AND OUTCOMES IN THE INVASIVE STRATEGY GROUP BEFORE URGENT NCS
Mean balloon size was 21.6±1.7 mm and mean number of inflations was 1.6±0.6. Five procedures (5.3%) were performed through the radial artery. Complications (n=12, 12.9%) of BAV included: 2 (2.1%) periprocedural deaths due to cardiac arrest after crossing the valve for BAV, 1 (1.0%) death following a major stroke, 3 (3.2%) patients requiring a permanent pacemaker implantation after the procedure, 4 (4.3%) patients who had a clinical haematoma at the femoral puncture site without the need of transfusion, 1 (1.0%) patient who presented a transient (<24 hrs) hemiplegia after the BAV, and 1 (1.0%) patient who had a homolateral acute limb ischaemia requiring an urgent reperfusion. BAV was successful in most SAS cases (70% had a significant reduction [≥50%] of the mean transaortic gradient assessed by haemodynamic measures) with a significant reduction of the transaortic gradient (45.0±13.6 mmHg versus 32.4±11.4, p<0.001), the aortic maximal velocity (4.2±0.6 m/s versus 3.6±0.6, p<0.001), and the AVA (0.72±0.15 cm2 versus 0.91±0.2, p<0.001), as evaluated by TTE. Finally, of the 93 patients undergoing BAV, 90 underwent NCS.
NON-ELECTIVE NON-CARDIAC SURGERY
The two periods of NCS (before or after 2015), timing of surgery (emergency or semi-urgent) and type of anaesthesia (general or not) were well balanced in the two groups (Table 2). Details of the NCS procedures are shown in Table 3.
Table 3. Surgical procedures according to 2014 ESC recommendations.
| Severe AS (n=133) | p-value | ||
| Invasive strategy (n=93) | Conservative strategy (n=40) | ||
| High-risk surgery | 15 (16.1) | 3 (7.5) | 0.18 |
| Aortic and major vascular surgery | 5 (5.3) | 2 (5.0) | 0.93 |
| Pneumonectomy | 2 (2.1) | 0 (0.0) | 0.35 |
| Major digestive surgery* | 8 (8.6) | 1 (2.5) | 0.20 |
| Intermediate-risk surgery | 57 (61.3) | 28 (70.0) | 0.34 |
| Major orthopaedic surgery | 21 (22.5) | 9 (22.5) | 0.99 |
| Major urological/renal surgery | 6 (6.4) | 2 (5.0) | 0.75 |
| Major neurological surgery | 4 (4.3) | 1 (2.5) | 0.62 |
| Major gynaecologic surgery | 3 (3.2) | 1 (2.5) | 0.82 |
| Minor vascular surgery** | 5 (5.3) | 7 (17.5) | 0.03 |
| Intraperitoneal surgery# | 18 (19.3) | 8 (20.0) | 0.93 |
| Low-risk surgery | 21 (22.6) | 9 (22.5) | 0.99 |
| Superficial surgery | 2 (2.1) | 2 (5.0) | 0.38 |
| Minor orthopaedic surgery | 14 (15.0) | 6 (15.0) | 0.99 |
| Minor gynaecologic surgery | 3 (3.2) | 1 (2.5) | 0.82 |
| Minor urological surgery | 2 (2.1) | 0 (0.0) | 0.35 |
| *Duodena-pancreatic, liver, bile duct, perforated bowel surgery or oesophagectomy. **Carotid symptomatic, endovascular aneurysm, peripheral arterial surgery. #Splenectomy, hiatal hernia repair, cholecystectomy. | |||
OUTCOMES AFTER NON-ELECTIVE NON-CARDIAC SURGERY
(I) PRIMARY ENDPOINT
The rate of MACE at one month was 20.4% in the IS group and 20.0% in the CS group, unadjusted analysis odds ratio (OR) 1.03, 95% CI: 0.40-2.59, and IPTW-adjusted analysis OR 0.93, 95% CI: 0.38-2.29. Details of individual events included in MACE are available in Table 4. Reasons for death in the CS group were limb ischaemia (n=1) and multiple organ failure (n=1) after vascular surgery, cardiac arrest (asystole) after hip repair, and critical sepsis (n=1) after abdominal surgery. Other causes of death in the IS group after NCS included 1 mitral endocarditis after acute gonarthritis, and 1 digestive cancer.
Table 4. One-month outcomes after urgent NCS in patients with SAS after IPTW propensity-score analyses.
| Outcomes | Severe AS (n=133) | Unadjusted analysis | IPTW-adjusted analysis | |||
| Invasive strategy (n=93) | Conservative strategy (n=40) | Effect size (95% CI) | p-value | Effect size (95% CI) | p-value | |
| Composite cardiovascular outcome (MACE) | 19 (20.4)*# | 8 (20.0) | 1.03 (0.40 to 2.59)¶ | 0.96 | 0.93 (0.38 to 2.29)¶ | 0.88 |
| Heart failure | 8 (8.6) | 4 (10.0) | ||||
| Myocardial infarction | 1 (1.1) | 1 (2.5) | ||||
| New atrial fibrillation | 7 (7.5) | 2 (5.0) | ||||
| Life-threatening bleeding | 3 (3.2) | 0 (0.0) | ||||
| Stroke/TIA | 1 (1.1)# | 0 (0.0) | ||||
| Acute kidney injury | 5 (5.4) | 5 (12.5) | ||||
| Mortality | 5 (5.4)* | 4 (10.0) | ||||
| Length of hospital stay, days, median (IQR) | 6 (4 to 9) | 8 (4 to 16) | −0.20 (−0.53 to 0.12) | 0.22 | −0.29 (−0.62 to 0.04) | 0.082 |
| The propensity score was estimated using a multivariable logistic regression model, with study groups as the dependent variable and pre-specified confounders as covariates (age, sex, surgery period, ASA score, STS score, LVEF, AVA, mean transaortic gradient, maximal velocity, NCS risk). ¶Odds ratio calculated using unweighted (unadjusted analysis) and weighted (IPTW-adjusted analysis) logistic regression models using conservative strategy group as reference. *Including 3 deaths after BAV before NCS. #Including 1 TIA after BAV before NCS. AR: aortic regurgitation; BAV: balloon aortic valvuloplasty; CI: confidence interval; MACE: major adverse cardiovascular events; NCS: non-cardiac surgery; SAS: severe aortic stenosis; TIA: transient ischaemic attack | ||||||
(II) PREDICTIVE FACTORS FOR ONE-MONTH MACE
In the global cohort, univariate predictive factors of the primary endpoint included ASA score ≥3 (28.3% vs 4.7%; p=0.001) and preoperative pulmonary hypertension >35 mmHg (33.0% vs 14.7%; p=0.007).
In the IS group, univariate predictive factors of the primary endpoint included iliofemoral artery disease (38% vs 15%; p=0.02), ASA score ≥3 (29.1% vs 3.6%; p=0.003) and preoperative pulmonary hypertension >35 mmHg (37.2% vs 11.8%; p=0.003).
(III) OTHER OUTCOMES
Among events occurring at one month, heart failure, life-threatening bleeding, stroke/TIA and AKI occurred at the same rate in the two groups (Table 4). Regarding length of hospital stay for NCS, an IS was associated with a non-significant shorter duration (median 6 days; IQR 4 to 9) than a CS (median 8 days; IQR 4 to 16) (Table 4). In the overall cohort, 34 (25.5%) patients had a trans-catheter aortic valve implantation (TAVI) procedure after NCS (IS 31.2% vs CS SAS 12.5%) with a median delay of 103 days (52; 200). No patient had surgical aortic valve replacement.
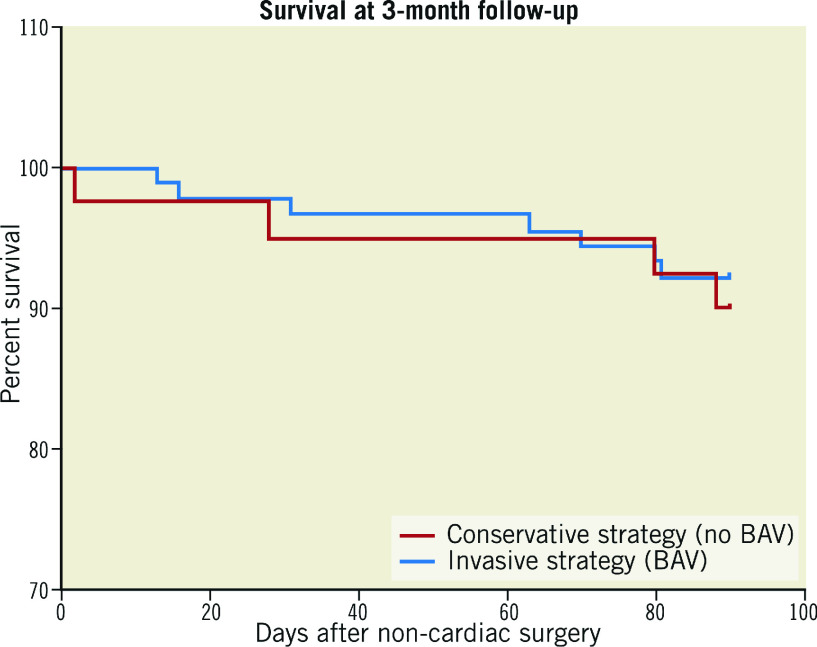
As shown in Figure 1, there was no difference in 3-month survival between CS and IS (89.2% vs 90.0%) with an IPTW-adjusted HR of 0.90 (95% CI: 0.31-2.60).
Figure 1.
Three-month survival after NCS in patients with an invasive (BAV) or conservative (without BAV) strategy. BAV: balloon aortic valvuloplasty; NCS: non-cardiac surgery
Among subgroups of interest, there was no difference in 1-month MACE (26.1% vs 23.4%; p=0.82) or 1-month mortality (15.2% vs 8.9%; p=0.39) between CS and IS before emergency NCS (<48 hrs).
There was also no difference in 1-month MACE (33.9% vs 40.0%; p=0.82) or 1-month mortality (33.5% vs 20.8%; p=0.61) between CS and IS before high-risk NCS (aortic and other major vascular surgery, peripheral vascular surgery; reported risk >5%).
Discussion
The best preoperative management of SAS patients before urgent NCS is unknown. This study is the first (i) to provide a large set of SAS patients undergoing urgent NCS, and to include a large proportion (>60%) of emergent (<48 hrs) NCS, and (ii) to compare a conservative versus an invasive strategy before urgent NCS using IPTW analysis. Overall, it reflects the reality of managing old patients with SAS who suffer, for example, from hip fracture which requires emergency surgery to preserve their autonomy8.
The main findings from this IPTW analysis are the following. (i) Patients with SAS managed conservatively before NCS are at high risk of events, namely high 1-month MACE (20.0%) and 1-month mortality (10.0%). (ii) Performing BAV in such a population is not “benign” and is associated with 3.2% mortality and 9.6% non-fatal complications at 7 days. (iii) While “immediate” 1-month mortality after NCS might be lower in “survivors” of the invasive strategy, overall, 1-month MACE and 3-month survival are similar in SAS patients treated with or without BAV. (iv) An ASA score ≥3 or preoperative pulmonary hypertension >35 mmHg seems to impact on prognosis after NCS.
Some studies have previously described the outcomes of AS patients versus non-AS patients undergoing NCS (Table 5). Patients with AS undergoing NCS have not been shown to be at increased risk of mortality, but have significantly higher rates of adverse cardiovascular events compared to patients without AS16. In particular, those with symptomatic SAS have more MACE (acute myocardial infarction, acute heart failure, arrhythmia) than those with asymptomatic SAS (36% vs 16%, respectively) and higher mortality rates than those with moderate AS (16% vs 4%)17.
Table 5. Summary of studies with AS patients and non-cardiac surgery.
| Study | N | Urgent non-elective NCS (n, %) | Preop BAV (n) | Type of NCS | NCS requiring GA (%) | NCS within 7 days | ASA score | Risk score of NCS* | Comparative group | Ref. |
| Hayes et al. – Mayo Clin Proc (1989) | 15 | 9 (60%) | 15 (100%) | Miscellaneous | 60% | 80% | NA | NA | No comparison | 4 |
| Leibowitz et al. Gerontology (2009) | 32 | 32 (100%) | 0 (0%) | Hip fracture | 30% | 100% | NA | NA | Matched control comparison without AS | 9 |
| Calleja et al. AJC (2010) | 30 | 3 (10%) | 0 (0%) | Miscellaneous | 73% | NA | NA | Intermediate-low | Matched control comparison without AS | 10 |
| Tashiro et al. EHJ (2014) | 256 | 24 (10%) | 0 (0%) | Miscellaneous | NA | NA | NA | High-intermediate | Matched control comparison without AS | 2 |
| Keswani et al. Injury (2016) | 65 | 65 (100%) | 0 (0%) | Hip fracture | 60% | 100% | ASA 3,4 100% | NA | Matched control comparison without AS | 8 |
| MacIntyre et al. Anaesth Intensive Care (2018) | 147 | 30% | 0 (0%) | Miscellaneous | NA | NA | ASA 4= 18-37% | High: 4-15% | Comparison moderate vs severe AS (no propensity) | 17 |
| Debry et al. EuroIntervention (2021) | 133 | 133 (100%) | 93 (70%) | Miscellaneous | 90% | 100% | ASA 4= 15-24% | High: 10-15% | IPTW comparison between invasive (BAV) and conservative strategy | – |
| *According to ESC 2014 recommendations. AS: aortic stenosis; ASA: American Society of Anesthesiologists; BAV: balloon aortic valvuloplasty; GA: general anaesthesia; NA: not available; NCS: non-cardiac surgery | ||||||||||
We report higher 1-month mortality (10.0%) and MACE (20.0%) rates with a CS after urgent NCS than Tashiro et al from the Mayo Clinic, but the latter explored only asymptomatic patients with AS after scheduled NCS (1-month mortality 3.3% and MACE 12%)2.
No randomised study comparing the outcomes of SAS patients undergoing urgent NCS under a conservative or invasive approach has been conducted to date. The IS reduced the “immediate” 1-month mortality rate after NCS (3.2% vs 10.0%; p=0.04) but not if we take into account the mortality induced by the BAV itself (5.4% and 10.0%; p=0.33). Partly because of the insufficient “haemodynamic result” and the complications linked to the IS, both strategies have similar 1-month MACE after NCS. While we confirm that using a routine IS for SAS patients is not recommended, it may be beneficial in selected patients.
Asymptomatic SAS patients or patients requiring low- to intermediate-risk NCS could be managed conservatively10. As the Tashiro study reminds us, urgent and scheduled NCS do not have the same morbidity, as emergency NCS alone is also a strong predictor of 30-day mortality2. Published reports indicate that, on the basis of TTE, adverse events during NCS occurred primarily in AS patients with an AVA ≤0.7 cm² and a mean gradient ≥50 mmHg9. In our study, because of a small cohort and the presence of severe AS in both groups, we were not able to identify anatomical aortic criteria that would encourage us to perform BAV before NCS. However, preoperative pulmonary hypertension >35 mmHg and an ASA score ≥3 are associated with higher short-term MACE and mortality rates. An IS in patients with these criteria could be discussed to improve prognosis after NCS.
The first way to decrease morbimortality after NCS may be to reduce the morbidity related to the BAV procedure. Using a smaller unilateral18 or bilateral Glidesheath Slender™ (Terumo Corp., Tokyo, Japan)19, transradial access for BAV is safe and feasible. BAV with low-profile compliant balloons20, without pacemaker back-up21, or with pacing on the left ventricular guidewire22 has also recently been described.
The second way is to improve the haemodynamic result of the BAV procedure. In our study, 30% of the patients did not experience a significant improvement of the haemodynamic parameters following BAV. In addition, in the remaining 70% with some improvement, the mean residual gradient was 30.0±4.0 mmHg. The best means to achieve a consistent haemodynamic improvement, and possibly to decrease morbimortality after NCS, is to perform direct TAVI before NCS. However, TAVI can be technically difficult to perform in the specific setting of urgent NCS because it requires a dedicated technical platform with on-site multislice computed tomography (CT) scanning facilities and an available catheterisation laboratory. It requires at least a 14 Fr vascular access, larger than for a transradial BAV (9 Fr), and it may be associated with more complications. Performing TAVI before NCS can also carry a very high risk of endocarditis, in particular when the surgery is associated with bacteraemia (e.g., urgent digestive surgery, or septic orthopaedic surgery). In addition, in our study, only 25% of the global SAS cohort had a TAVI procedure within three months after urgent NCS. This highlights that this population is not a typical TAVI population, as it includes a combination of patients with frailty and multiple comorbidities including cancer and disabilities, which in the end may cause the TAVI procedure to be postponed or even cancelled. On the other hand, studies have reported that cancer patients with severe AS who underwent aortic valve replacement had improved survival, regardless of cancer status23. When compared to aortic surgery, TAVI under local anaesthesia is less invasive and may avoid the possibility of cancer dissemination due to extracorporeal circulation for patients with malignancy.
Large-scale prospective cohort studies are needed to clarify the above points and delineate the role of direct TAVI in this population. For the time being, a case-by-case multidisciplinary Heart Team discussion remains the best option to choose the optimal strategy in those SAS patients requiring urgent NCS.
Limitations
The limitations to this study are inherent to the non-randomised design. The present findings are derived from observational analyses, which are subject to well-known limitations. The main limitation is the potential for confounding by measured or unmeasured variables, which cannot be ruled out, even after IPTW adjustment. In particular, we could not exclude a residual bias related to age or NCS risk since both remained not completely balanced in IPTW-adjusted analysis, as well as to other patient characteristics not included in the propensity score calculation. No formal sample size calculation was carried out; we therefore caution that we could not exclude a lack of adequate statistical power to detect the between-group differences. In an a posteriori power calculation, our study sample size (93 patients with an IS and 40 patients with a CS) allows, with 80% power, detecting with a type-1 error of 5%, an OR of MACE at one month of 3.2 (or 0.19 for protective effect) for patients with an IS versus a CS. These calculations were done by considering the observed rate of MACE at one month in the conservative group (20%), 80% power and a two-sided type-1 error of 5%.
Conclusions
Patients with SAS managed conservatively before urgent NCS are at high risk of events. A systematic invasive strategy using BAV does not provide a significant improvement in clinical outcome.
Impact on daily practice
SAS in patients requiring urgent non-elective NCS (including 62% emergency surgery performed <48 hrs) presents a high risk of mortality and clinical events. Our study suggests that the performance of BAV before NCS does not provide enough safety and haemodynamic benefit to be performed in a systematic fashion. The indication for BAV needs to be discussed on an individual basis. Large-scale prospective cohort studies are needed to delineate the role of a minimally invasive BAV procedure or direct TAVI in selected high-risk patients before NCS.
Supplementary data
Absolute standardised differences between the invasive and conservative strategies before and after IPTW propensity score weighting.
Acknowledgments
Conflict of interest statement
The authors have no conflicts of interest to declare.
Abbreviations
- AKI
acute kidney injury
- AR
aortic regurgitation
- BAV
balloon aortic valvuloplasty
- CABG
coronary artery bypass graft surgery
- COPD
chronic obstructive pulmonary disease
- CS
conservative strategy
- IPTW
inverse probability of treatment weighting
- IS
invasive strategy
- LVEF
left ventricular ejection fraction
- MACE
major adverse cardiac events
- NCS
non-cardiac surgery
- SAS
severe aortic stenosis
- STS
Society of Thoracic Surgeons
- TAVI
transcatheter aortic valve implantation
- TIA
transient ischaemic attack
- TTE
transthoracic echocardiography
Contributor Information
Nicolas Debry, CHU Lille, Institut Coeur Poumon, Cardiology, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Lille, France; Groupement des Hôpitaux de l’Institut Catholique de Lille (GHICL), Cardiology Department and Heart Valve Centre, Faculté Libre de Médecine/Université Catholique de Lille, Lille, France; INSERM, U1011, Lille, France.
Alexandre Altes, Groupement des Hôpitaux de l’Institut Catholique de Lille (GHICL), Cardiology Department and Heart Valve Centre, Faculté Libre de Médecine/Université Catholique de Lille, Lille, France.
Flavien Vincent, CHU Lille, Institut Coeur Poumon, Cardiology, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Lille, France; INSERM, U1011, Lille, France; Université de Lille, Lille, France.
Cédric Delhaye, CHU Lille, Institut Coeur Poumon, Cardiology, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Lille, France.
Guillaume Schurtz, CHU Lille, Institut Coeur Poumon, Cardiology, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Lille, France.
Farid Nedjari, Groupement des Hôpitaux de l’Institut Catholique de Lille (GHICL), Cardiology Department and Heart Valve Centre, Faculté Libre de Médecine/Université Catholique de Lille, Lille, France.
Gabin Legros, Groupement des Hôpitaux de l’Institut Catholique de Lille (GHICL), Cardiology Department and Heart Valve Centre, Faculté Libre de Médecine/Université Catholique de Lille, Lille, France.
Sina Porouchani, CHU Lille, Institut Coeur Poumon, Cardiology, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Lille, France.
Augustin Coisne, INSERM, U1011, Lille, France; Université de Lille, Lille, France; CHU Lille, Department of Clinical Physiology and Echocardiography, Lille, France.
Marjorie Richardson, CHU Lille, Department of Clinical Physiology and Echocardiography, Lille, France.
Alessandro Cosenza, CHU Lille, Institut Coeur Poumon, Cardiology, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Lille, France.
Basile Verdier, CHU Lille, Institut Coeur Poumon, Cardiology, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Lille, France.
Tom Denimal, CHU Lille, Institut Coeur Poumon, Cardiology, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Lille, France.
Thibault Pamart, CHU Lille, Institut Coeur Poumon, Cardiology, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Lille, France.
Hugues Spillemaeker, CHU Lille, Institut Coeur Poumon, Cardiology, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Lille, France.
Habib Sylla, CHU Lille, Institut Coeur Poumon, Cardiology, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Lille, France.
Arnaud Sudre, CHU Lille, Institut Coeur Poumon, Cardiology, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Lille, France.
Dany Janah, CHU Lille, Institut Coeur Poumon, Cardiology, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Lille, France.
David Aouate, CHU Lille, Institut Coeur Poumon, Cardiology, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Lille, France.
Wassima Marsou, Groupement des Hôpitaux de l’Institut Catholique de Lille (GHICL), Cardiology Department and Heart Valve Centre, Faculté Libre de Médecine/Université Catholique de Lille, Lille, France.
Ludovic Appert, Groupement des Hôpitaux de l’Institut Catholique de Lille (GHICL), Cardiology Department and Heart Valve Centre, Faculté Libre de Médecine/Université Catholique de Lille, Lille, France.
Gilles Lemesle, CHU Lille, Institut Coeur Poumon, Cardiology, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Lille, France; INSERM, U1011, Lille, France; Université de Lille, Lille, France.
Julien Labreuche, Université de Lille, CHU Lille, Lille, France.
Sylvestre Maréchaux, Groupement des Hôpitaux de l’Institut Catholique de Lille (GHICL), Cardiology Department and Heart Valve Centre, Faculté Libre de Médecine/Université Catholique de Lille, Lille, France.
Eric Van Belle, CHU Lille, Institut Coeur Poumon, Cardiology, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Lille, France; INSERM, U1011, Lille, France; Université de Lille, Lille, France.
References
- Alkhouli M, Zack CJ, Sarraf M, Bashir R, Nishimura RA, Eleid MF, Nkomo VT, Sandhu GS, Gulati R, Greason KL, Holmes DR, Rihal CS. Morbidity and Mortality Associated With Balloon Aortic Valvuloplasty: A National Perspective. Circ Cardiovasc Interv. 2017 doi: 10.1161/CIRCINTERVENTIONS.116.004481. [DOI] [PubMed] [Google Scholar]
- Tashiro T, Pislaru SV, Blustin JM, Nkomo VT, Abel MD, Scott CG, Pellikka PA. Perioperative risk of major non-cardiac surgery in patients with severe aortic stenosis: a reappraisal in contemporary practice. Eur Heart J. 2014;35:2372–81. doi: 10.1093/eurheartj/ehu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RB, Palacios IF, Block PC. Percutaneous aortic balloon valvuloplasty: its role in the management of patients with aortic stenosis requiring major noncardiac surgery. J Am Coll Cardiol. 1989;13:1039–41. doi: 10.1016/0735-1097(89)90258-1. [DOI] [PubMed] [Google Scholar]
- Hayes SN, Holmes DR, Nishimura RA, Reeder GS. Palliative percutaneous aortic balloon valvuloplasty before noncardiac operations and invasive diagnostic procedures. Mayo Clin Proc. 1989;64:753–7. doi: 10.1016/S0025-6196(12)61746-7. [DOI] [PubMed] [Google Scholar]
- Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, Hert SD, Ford I, González-Juanatey JR, Gorenek B, Heyndrickx GR, Hoeft A, Huber K, Iung B, Kjeldsen KP, Longrois D, Lüscher TF, Pierard L, Pocock S, Price S, Roffi M, Sirnes PA, Sousa-Uva M, Voudris V, Funck-Brentano C Authors/Task Force Members. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35:2383–431. doi: 10.1093/eurheartj/ehu282. [DOI] [PubMed] [Google Scholar]
- Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, Davila-Roman VG, Gerhard-Herman MD, Holly TA, Kane GC, Marine JE, Nelson MT, Spencer CC, Thompson A, Ting HH, Uretsky BF, Wijeysundera DN. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e278–333. doi: 10.1161/CIR.0000000000000106. [DOI] [PubMed] [Google Scholar]
- Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–91. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- Keswani A, Lovy A, Khalid M, Blaufarb I, Moucha C, Forsh D, Chen D. The effect of aortic stenosis on elderly hip fracture outcomes: A case control study. Injury. 2016;47:413–8. doi: 10.1016/j.injury.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Leibowitz D, Rivkin G, Schiffman J, Rott D, Weiss AT, Mattan Y, Kandel L. Effect of severe aortic stenosis on the outcome in elderly patients undergoing repair of hip fracture. Gerontology. 2009;55:303–6. doi: 10.1159/000209245. [DOI] [PubMed] [Google Scholar]
- Calleja AM, Dommaraju S, Gaddam R, Cha S, Khandheria BK, Chaliki HP. Cardiac risk in patients aged >75 years with asymptomatic, severe aortic stenosis undergoing noncardiac surgery. Am J Cardiol. 2010;105:1159–63. doi: 10.1016/j.amjcard.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Lieberman EB, Bashore TM, Hermiller JB, Wilson JS, Pieper KS, Keeler GP, Pierce CH, Kisslo KB, Harrison JK, Davidson CJ. Balloon aortic valvuloplasty in adults: failure of procedure to improve long-term survival. J Am Coll Cardiol. 1995;26:1522–8. doi: 10.1016/0735-1097(95)00363-0. [DOI] [PubMed] [Google Scholar]
- [No authors listed] Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation. 1991;84:2383–97. doi: 10.1161/01.CIR.84.6.2383. [DOI] [PubMed] [Google Scholar]
- Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. doi: 10.1016/j.echo.2008.11.029. [DOI] [PubMed] [Google Scholar]
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33:1057–69. doi: 10.1002/sim.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok CS, Bagur R, Rashid M, Lavi R, Cibelli M, de Belder MA, Moat N, Hildick-Smith D, Ludman P, Mamas MA. Aortic stenosis and non-cardiac surgery: A systematic review and meta-analysis. Int J Cardiol. 2017;240:145–53. doi: 10.1016/j.ijcard.2017.04.037. [DOI] [PubMed] [Google Scholar]
- MacIntyre PA, Scott M, Seigne R, Clark A, Deveer F, Minchin I. An observational study of perioperative risk associated with aortic stenosis in non-cardiac surgery. Anaesth Intensive Care. 2018;46:207–14. doi: 10.1177/0310057X1804600211. [DOI] [PubMed] [Google Scholar]
- Tumscitz C, Campo G, Tebaldi M, Gallo F, Pirani L, Biscaglia S. Safety and Feasibility of Transradial Mini-Invasive Balloon Aortic Valvuloplasty: A Pilot Study. JACC Cardiovasc Interv. 2017;10:1375–7. doi: 10.1016/j.jcin.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Vassileva A, Valsecchi O, Guagliumi G, Fiocca L, Canova PA, Cereda AF. Bilateral Slender Transradial Aortic Balloon Valvuloplasty. J Invasive Cardiol. 2018;30:E48–9. [PubMed] [Google Scholar]
- Rau EM, El-Hajjar M. Aortic valvuloplasty via the radial artery: Case reports and review of the literature. Catheter Cardiovasc Interv. 2018;92:597–600. doi: 10.1002/ccd.27021. [DOI] [PubMed] [Google Scholar]
- Rigatelli G, Dell’avvocata F, Conte L, Lanza D, Giatti S, Del Santo P, Roncon L, Faggian G. Modified balloon aortic valvuloplasty in fragile symptomatic patients unsuitable for both surgical and percutaneous valve replacement. Cardiovasc Revasc Med. 2018;19:444–7. doi: 10.1016/j.carrev.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Faurie B, Souteyrand G, Staat P, Godin M, Caussin C, Van Belle E, Mangin L, Meyer P, Dumonteil N, Abdellaoui M, Monségu J, Durand-Zaleski I, Lefevre T EASY TAVI Investigators. Left Ventricular Rapid Pacing Via the Valve Delivery Guidewire in Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2019;12:2449–59. doi: 10.1016/j.jcin.2019.09.029. [DOI] [PubMed] [Google Scholar]
- Yusuf SW, Sarfaraz A, Durand JB, Swafford J, Daher IN. Management and outcomes of severe aortic stenosis in cancer patients. Am Heart J. 2011;161:1125–32. doi: 10.1016/j.ahj.2011.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Absolute standardised differences between the invasive and conservative strategies before and after IPTW propensity score weighting.