Coronary access following transcatheter aortic valve replacement (TAVR) can be challenging and is increasingly relevant as TAVR expands towards lower-risk younger patients1. Prior studies have used post-implantation computed tomography (CT) scans to suggest several anatomical and device-related factors which can impact on coronary access2,3,4,5. However, the two-dimensional (2D) images and measurements may not fully capture the complex three-dimensional (3D) geometry underpinning the challenge of coronary access4.
In this image, we present a novel CT-based, 3D multi-parametric imaging approach, which was used to evaluate geometrical factors relevant for coronary access.
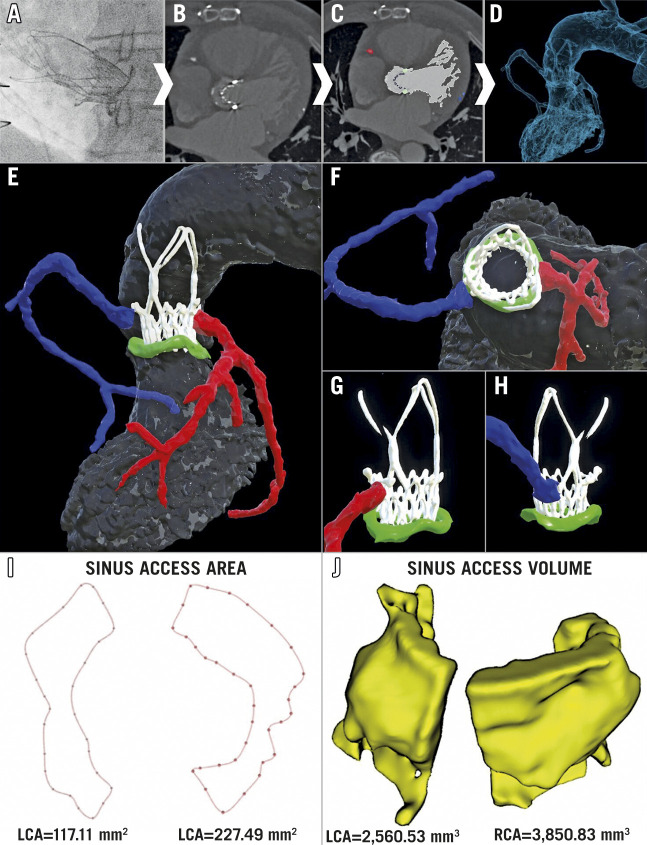
A 72-year-old male underwent ACURATE neo™️ valve (Boston Scientific, Marlborough, MA, USA) implantation to treat a degenerated Mitroflow-25 surgical bioprosthesis (Sorin Group, Saluggia, Italy) (Figure 1A). A post-procedural CT scan was performed, and the following structures were segmented: left ventricle, aortic root, coronary arteries, and surgical/transcatheter valves. Segmentation was performed using semi-automatic algorithms with additional manual correction in Slicer 3D (Figure 1B, Figure 1C). The segmentations were converted into 3D mesh structures and exported as stereolithography files to create a 3D digital model (Figure 1D, Figure 1E).
Figure 1. Multi-parametric 3D imaging evaluation of coronary access.
A post-procedural CT scan was performed and specific structures and regions of interest deemed relevant for evaluating coronary access were segmented to create a 3D digital model (A-D). Further image processing was then applied to improve the visualisation and the understanding of the geometrical relationships between the aortic sinuses (grey), ACURATE neo valve (white), surgical bioprosthesis (green), right coronary artery (blue), and left coronary artery (red) (E-H). In order to evaluate the complex 3D geometry of the sinus space, both (I) the size and morphology of the area available between the valve frame and the aortic wall to enter the sinuses (sinus access area), and (J) the volume available within the sinuses (sinus access volume) were determined for each ostium.
Key advantages of the digital model include enhanced visualisation which allows closely related structures to be easily distinguished, the ability to rotate and view the images from any 3D perspective, and the possibility of adding or removing specific structures or regions of interest to perform selective analyses (Figure 1E-Figure 1H).
Using these properties, the geometrical relationship between the coronary ostia, valve frames and surrounding sinuses was evaluated. Both coronary ostia arise below the “risk plane” for the ACURATE neo valve at the level of the upper crown (Figure 1E) and there is evidence of commissural misalignment impacting on both coronary ostia (Figure 1G, Figure 1H). The 3D visualisation highlights the importance of considering patient-specific implantation techniques to ensure commissural alignment between the implanted valve and surgical bioprosthesis or native cusps.
In this challenging scenario of low-lying coronary ostia, the sinus gap between the valve frame and coronary ostia becomes an important determinant of the challenge and feasibility of coronary access4,5. To date, this sinus gap has only been evaluated using 2D images and linear measurements1,2.
In contrast, with the 3D models the sinus gap can be visually inspected from any angle and a more detailed analysis of its size and morphology can be obtained (Figure 1I, Figure 1J, Supplementary Figure 1). The sinus access area (SAA) determines how much space is available for a catheter to enter into the aortic sinuses, whilst the sinus access volume (SAV) reflects the room available for catheter manipulation inside the sinus. These measurements can directly be applied to evaluate the sinus space when coronary ostia arise below the risk plane for other low-profile valves and, if adapted, may still be relevant for higher-profile valve frames.
The SAA and SAV measurements provide further detail beyond previously reported measurements and, in combination with the 3D visualisation and segmentation properties of the digital models, may provide greater insights into the geometrical factors underlying the challenge of coronary access. This multi-parametric imaging approach may aid interventionists in understanding which catheter shapes, sizes and cannulation techniques should be considered for cannulation of challenging coronary ostia (Supplementary Figure 1).
Supplementary data
Measurement of sinus space.
Acknowledgments
Acknowledgements
The authors wish to acknowledge Alessandra Laricchia, MD, Katarzyna Kotarba, MSc, Bernhard Reimers, MD, and Ghada W. Mikhail, MD, FRCP.
Conflict of interest statement
A. Mangieri has received a fee from Concept Medical, is part of the advisory board of Boston Scientific and has received an institutional grant from Boston Scientific. D. Dudek is a scientific advisory board member of Boston Scientific. W-K. Kim declares personal fees from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, Meril Life Sciences, and Shockwave Medical. The other authors have no conflicts of interest to declare.
Contributor Information
Arif A. Khokhar, Interventional Cardiology Unit, GVM Care & Research Maria Cecilia Hospital, Cotignola, Italy.
Francesco Ponticelli, Interventional Cardiology Unit, GVM Care & Research Maria Cecilia Hospital, Cotignola, Italy.
Adriana Zlahoda-Huzior, AGH University of Science and Technology, Department of AGH Department of Measurement & Electronics, Krakow, Poland.
Rossella Ruggiero, Interventional Cardiology Unit, GVM Care & Research Maria Cecilia Hospital, Cotignola, Italy.
Won-Keun Kim, Department of Cardiology and Cardiac Surgery, Kerckhoff Heart Center, Bad Nauheim, Germany.
Antonio Mangieri, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy; Cardio Center, Humanitas Research Hospital, IRCCS, Rozzano, Milan, Italy.
Antonio Colombo, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy; Cardio Center, Humanitas Research Hospital, IRCCS, Rozzano, Milan, Italy.
Dariusz Dudek, Institute of Cardiology, Jagiellonian University Medical College, Krakow, Poland.
Francesco Giannini, Interventional Cardiology Unit, GVM Care & Research Maria Cecilia Hospital, Cotignola, Italy.
References
- Barbanti M, Costa G, Picci A, Criscione E, Reddavid C, Valvo R, Todaro D, Deste W, Condorelli A, Scalia M, Licciardello A, Politi G, De Luca G, Strazzieri O, Motta S, Garretto V, Veroux P, Giaquinta A, Giuffrida A, Sgroi C, Leon MB, Webb JG, Tamburino C. Coronary Cannulation After Transcatheter Aortic Valve Replacement: The RE-ACCESS Study. JACC Cardiovasc Interv. 2020;13:2542–55. doi: 10.1016/j.jcin.2020.07.006. [DOI] [PubMed] [Google Scholar]
- Ochiai T, Chakravarty T, Yoon SH, Kaewkes D, Flint N, Patel V, Mahani S, Tiwana R, Sekhon N, Nakamura M, Cheng W, Makkar R. Coronary Access After TAVR. JACC Cardiovasc Interv. 2020;13:693–705. doi: 10.1016/j.jcin.2020.01.216. [DOI] [PubMed] [Google Scholar]
- De Backer, Landes U, Fuchs A, Yoon SH, Mathiassen ON, Sedaghat A, Kim WK, Pilgrim T, Buzzatti N, Ruile P, El Sabbagh, Barbanti M, Fiorina C, Nombela-Franco L, Steinvil A, Finkelstein A, Montorfano M, Maurovich-Horvat P, Kofoed KF, Blanke P, Bunc M, Neumann FJ, Latib A, Windecker S, Sinning JM, Norgaard BL, Makkar R, Webb JG, Søndergaard L. Coronary Access After TAVR-in-TAVR as Evaluated by Multidetector Computed Tomography. JACC Cardiovasc Interv. 2020;13:2528–38. doi: 10.1016/j.jcin.2020.06.016. [DOI] [PubMed] [Google Scholar]
- Tang GHL, Zaid S. Coronary re-access after redo TAVI: a proposed classification to simplify evaluation. EuroIntervention. 2020;16:e960–2. doi: 10.4244/EIJV16I12A176. [DOI] [PubMed] [Google Scholar]
- Tarantini G, Fabris T, Nai Fovino. TAVR-in-TAVR and coronary access: the importance of preprocedural planning. EuroIntervention. 2020;16:e129–32. doi: 10.4244/EIJ-D-19-01094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Measurement of sinus space.