Abstract
Background
Coronary vasomotor dysfunction can be diagnosed in a large proportion of patients with angina in the presence of non-obstructive coronary artery disease (ANOCA) using comprehensive protocols for coronary vasomotor function testing (CFT). Although consensus on diagnostic criteria for endotypes of coronary vasomotor dysfunction has been published, consensus on a standardised study testing protocol is lacking.
Aims
In this review we provide an overview of the variations in CFT used and discuss the practical principles and pitfalls of CFT.
Methods
For the purposes of this review, we assessed study protocols that evaluate coronary vasomotor response as reported in the literature. We compared these protocols regarding a number of procedural aspects and chose six examples to highlight the differences and uniqueness.
Results
Currently, numerous protocols co-exist and vary in vascular domains tested, the manner in which to test these domains (e.g., preprocedural discontinuation of medication, provocative agent, solution, infusion time, and target artery) and techniques used for measurements (e.g., Doppler vs thermodilution technique).
Conclusions
This lack of consensus on a uniform functional testing protocol hampers both a broader clinical acceptance of the concepts of coronary vasomotor dysfunction, and the widespread adoption of such testing protocols in current clinical practice. Furthermore, the endotype of coronary vasomotor dysfunction might differ among the few specialised centres that perform CFT as a result of the use of different protocols.
Introduction
Coronary vasomotor function testing (CFT) is an invasive coronary technique that aims to evaluate coronary vasomotor response in reaction to intracoronarily administered pharmacological stimuli. Endothelium-independent vasodilators, such as adenosine, are used to assess vasodilatory disorders of the coronary microvasculature, whilst endothelium-dependent vasodilators such as acetylcholine (ACh) are used to assess vasomotor disorders that result in vasoconstriction. The Coronary Vasomotor Disorders International Study (COVADIS) working group defines vasospastic angina (VSA) as coronary spasm of the epicardial artery and microvascular angina (MVA) as angina with evidence of either endothelium-dependent or independent coronary microvascular dysfunction (CMD).
Current European Society of Cardiology, American College of Cardiology/American Heart Association, and Japanese Circulation Society (JCS) guidelines emphasise that CFT should be applied in routine practice in patients with non-obstructive coronary arteries and angina or myocardial infarction (ANOCA and MINOCA, respectively)1,2,3. Although consensus papers on diagnostic criteria for MVA and VSA during CFT have been published by the COVADIS working group, consensus on a standardised CFT protocol remains to be established. Currently, a large variety of CFT protocols exists; testing is only performed routinely in a few specialised centres. This lack of consensus on a uniform functional testing protocol hampers a broader clinical acceptance of the concepts of coronary vasomotor dysfunction and the widespread adoption of CFT in clinical practice.
The purpose of this review is threefold: a) to discuss the variations in CFT protocols used in clinical practice, b) to discuss practical considerations when performing CFT, and c) to derive several principles to which a uniform protocol should adhere.
Methods
Literature search
For the purpose of this review we assessed study protocols that evaluate coronary vasomotor response as reported in the literature and evaluated the most recent papers describing this protocol. We compared these protocols regarding a number of procedural aspects and chose six examples to highlight the differences and uniqueness. References are provided for detailed descriptions of the CFT protocols we refer to.
Results
Different coronary vasomotor function protocols
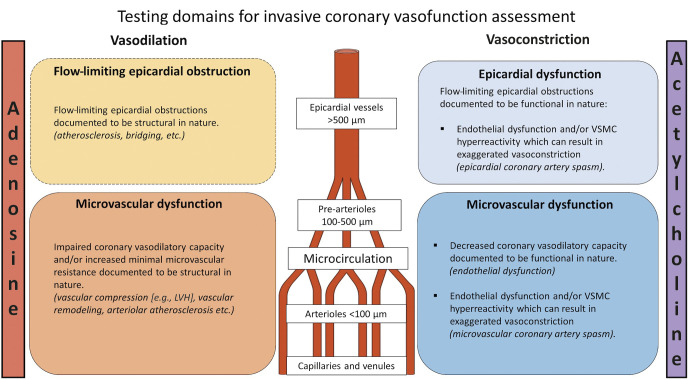
The diagnostic workup of ANOCA includes the evaluation of vascular domains (Figure 1). The pathological mechanism behind the various endotypes of coronary vasomotor dysfunction of the epicardial arteries and microcirculation can vary. Currently, the COVADIS working group define VSA as coronary spasm of the epicardial artery and MVA as angina with evidence of either endothelium-dependent or independent CMD. It is preferable to distinguish between vasodilation and vasoconstriction abnormalities of the epicardial and microvascular coronary arteries because these vascular domains require a different diagnostic and therapeutic approach (Figure 2). First, this is because disorders that result in vasoconstriction are assessed with endothelium-dependent vasodilators and can occur at either the epicardial and/or microvascular level. Second, MVA may be due to microvascular vasoconstriction and/or impaired vasodilation that is assessed using either endothelium-dependent or endothelium-independent testing, respectively.
Figure 1. Vascular testing domains.
Invasive evaluation of ANOCA includes the assessment of vasodilatory and vasoconstrictive disorders of the epicardial arteries and the microcirculation. LVH: left ventricular hypertrophy; VSMC: vascular smooth muscle cells
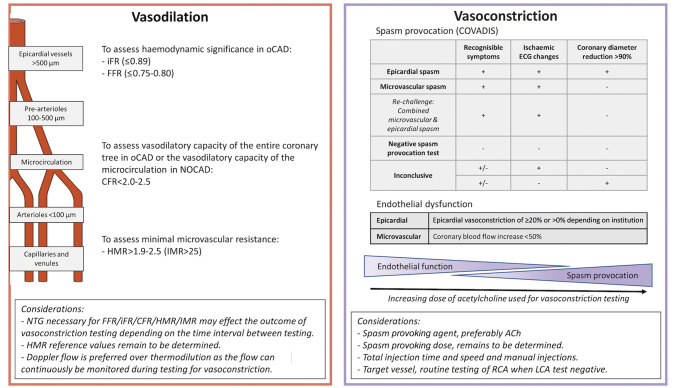
Figure 2. Current diagnostic criteria.
Summary of definitions used in current CFT and of considerations when performing CFT. ACh: acetylcholine; ANOCA: angina and non-obstructive coronary arteries; CFR: coronary flow reserve; CFT: coronary vasomotor function testing; FFR: factional flow reserve; HMR: hyperaemic microcirculatory resistance; iFR: instantaneous wave-free ratio; IMR: index of microcirculatory resistance; LCA: left coronary artery; NOCAD: non-obstructive coronary artery disease; NTG: nitroglycerine; oCAD: obstructive coronary artery disease; RCA: right coronary artery
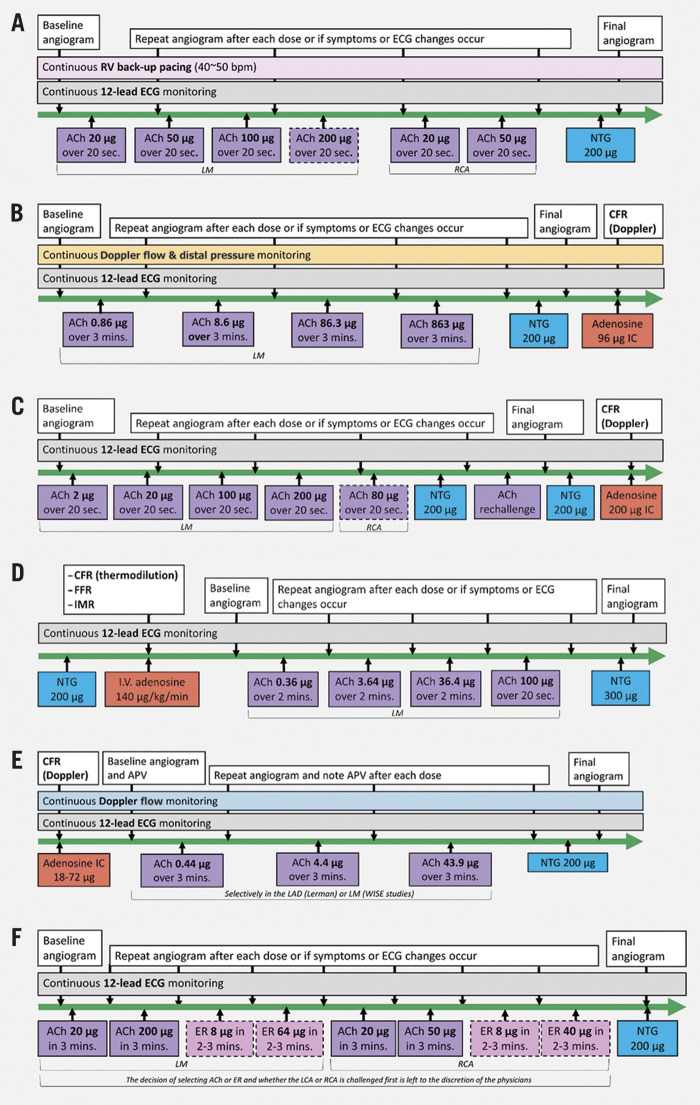
The different CFT protocols that have been considered in this review are summarised in the Central illustration. Testing for vasoconstrictive properties is performed as a vasospasm provocation test, commonly using intracoronary an ACh injection that achieves a high blood concentration of ACh. At relatively lower concentrations of ACh in normal individuals, the effects on the endothelium prevail, resulting in vasodilatation of the epicardial coronary arteries and the resistance vessels. If the endothelium is diseased, the extent of vasodilation can be reduced, absent, or some vasoconstriction may ensue. This response can be quantified by measuring the changes in coronary blood flow and coronary epicardial diameter as a measure of endothelial function.
Central illustration. Overview of different coronary vasomotor function testing protocols.
ACh: acetylcholine; APV: average peak velocity; CFR: coronary flow reserve; ECG: electrocardiogram; ER: ergonovine; FFR: factional flow reserve; IC: intracoronary; IMR: index of microcirculatory resistance; IV: intravenous; LAD: left anterior descending artery; LCA: left coronary artery; LM: left main; NTG: nitroglycerine; RCA: right coronary artery; RV: right ventricular
The CFT protocols presented vary in the vascular domains tested, the order in which these are tested, as well as in the procedural aspects and techniques to test these domains. Considerations regarding these different approaches will be discussed further.
Practical considerations for vasomotor function testing
Pharmacological agents used for coronary vasoconstriction testing
Various endogenous and pharmacological stimulatory substances have been described for coronary vasoconstriction testing, although the most widely used in clinical practice is ACh and to a lesser extent ergonovine (ER). Only the Japanese Cardiac Society provides standardised protocols in its guidelines for using ACh or ER2. Outside Japan, the coronary vasoconstriction testing protocols are centre-specific and mainly use ACh, except for the UCSC protocol, in which ER and ACh are used interchangeably4,5.
Healthy coronary endothelium responds to ACh by releasing a number of endothelium-dependent relaxation factors (mainly nitric oxide [NO]). Simultaneously, ACh has a direct vasoconstrictor effect on the smooth muscle cells that is attenuated or even reversed by the vasodilator effect of healthy endothelium. In contrast, ER acts directly only on smooth muscle cells mainly by activation of serotonergic (5-HT2) receptors to produce vasoconstriction. ACh can only be administered intracoronarily due to its short half-life which makes non-invasive testing impossible unlike ER. However, intravenous administration of ER may result in prolonged simultaneous vasospasm of the right coronary artery (RCA) and left coronary artery (LCA) which may be difficult to reverse without intracoronary injection of nitroglycerine (NTG). The preference to perform the test intracoronarily is because NTG can be applied directly into the coronary artery. Most importantly, the advantage of ACh is the large body of evidence that it allows the identification of vasoconstriction abnormalities of the microvessels whereas for ER this is incidental6.
Abnormal vasoconstriction in reaction to ACh or ER may occur either when the coronary endothelial function is impaired, as is frequently encountered in the early stage of atherosclerosis, or when there is vascular smooth muscle hyperreactivity in the presence of intact endothelium, or both7. Epicardial coronary arteries, the coronary microvasculature, or both can be involved in the pathophysiological mechanisms of vasomotor function disorders in ANOCA or (M)INOCA and may contribute, solely or in combination, to a supply-demand mismatch in symptomatic patients.
Sensitivity and specificity
Validation studies have demonstrated high sensitivity and specificity for both the ER (91 and 97%, respectively) and ACh (90 and 99%, respectively) protocols for the diagnosis of coronary vasoconstriction in patients with spontaneous angina and non-obstructed coronary arteries8,9. The maximum dose of ACh used in this study was 100 μg per 20 seconds, which was also used in the CorMicA study combined with stratified medical therapy. All patients in the validation study had recorded spontaneous ST deviations associated with angina; a patient group with overt high disease burden that would nowadays not need to undergo spasm provocation testing according to COVADIS. Some institutes, such as the Robert-Bosch-Krankenhaus (RBK) and some Japanese institutes10, have therefore adopted the use of higher doses (200 μg per 20 seconds) in order to diagnose patients with lower disease burden.
Injection time and doses
As presented in Table 1, protocols testing for coronary vasoconstriction disorders show a wide variation in the injection time and doses of ACh. At one end of the spectrum, the Japanese Circulation Society (JCS) protocol recommends a 20-second intracoronary injection of incremental ACh doses (up to 200 μg) in an effort to provoke spasm. At the other end, endothelial function testing protocols are characterised by incremental 3-minute infusions of low-dose ACh (up to 44 μg) focusing on the assessment of the mainly endothelium-dependent changes in coronary blood flow (CBF)11,12 (Figure 1). Others combine the two techniques to provoke spasm, such as the CorMicA and RBK protocols where low-dose infusion is followed by a high-dose bolus injection of ACh (up to 100 and 200 μg, respectively). Finally, in the Academic Medical Centre (AMC) protocol as described by Piek et al13, infusion of low-dose ACh is followed by a final high dose of ACh (864 μg) that is also administered for up to 3 minutes until vasospasm is provoked. These differences in time and dosages may affect sensitivity and specificity of the test itself to some extent. Studies by Sueda et al have demonstrated that, by increasing the maximal dose of ACh from 100 to 200 μg, injected in 20 seconds, the number of positive tests will increase and multivessel spasm will be diagnosed more frequently10,14. Similarly, patients in whom a positive ACh provocation test was achieved using the 20-second protocol were re-tested with the same total dose of ACh injected over 3 minutes15. Again, spasm could be provoked more frequently using the 20-second injection compared to the 3-minute infusion (73.3% vs 33.3%, p<0.01).
Table 1. Acetylcholine doses per protocol.
| Protocol | Dose | Solution | Injection rate | Injection technique | Total dose | Theoretical comparison: corrected LAD dose*** | |||
|---|---|---|---|---|---|---|---|---|---|
| Vessel | No. | μg/ml | ml/min | in 20 sec | in 2 min | in 3 min | μg/(first) 20 sec | ||
| JCS | LM/RCA** | 1 | 4 | 15 | manual | 20 | 10.0 | ||
| LM/RCA** | 2 | 10 | 15 | 50 | 25.0 | ||||
| LM | 3 | 20 | 15 | 100 | 50.0 | ||||
| LM | (4) | 40 | 15 | 200 | 100.0 | ||||
| Endothelial function | LAD (Mayo Clinic) | 1 | 0.15 | 1 | pump</p> | 0.44 | 0.05/0.02 | ||
| LM (WISE) | 2 | 1.46 | 1 | 4.39 | 0.49/0.24 | ||||
| 3 | 14.62 | 1 | 43.86 | 4.87/2.44 | |||||
| AMC | LM/RCA** | 1 | 0.21 | 1.37 | pump</p> | 0.86 | 0.05 | ||
| 2 | 2.11 | 1.37 | 8.63 | 0.48 | |||||
| 3 | 21.05 | 1.37 | 86.33 | 4.8 | |||||
| 4 | 210.50 | 1.37 | 863.26 | 48.0 | |||||
| RBK | LM | 1 | 0.36 | 18 | manual | 2.16 | 1.1 | ||
| 2 | 3.60 | 18 | 21.6 | 10.8 | |||||
| 3 | 18 | 16.5 | 99 | 49.5 | |||||
| 4 | 18 | 33.00 | 198 | 99.0 | |||||
| RCA** | 1 | 17.78 | 13.5 | 80 | |||||
| CorMicA | LM | 1 | 0.18 | 1 | pump</p> | 0.364 | 0.03 | ||
| 2 | 1.82 | 1 | 3.64 | 0.3 | |||||
| 3 | 18.20 | 1 | 36.4 | 3.0 | |||||
| 4 | 18.20 | 16.5 | 100.1 | 50.1 | |||||
| RCA | 1 | 18.20 | 8.25 | 50.05 | |||||
| UCSC | LM or RCA | 1 | 1 | 6.67 | manual | 20 | 1.1 | ||
| LM | 2 | 10 | 6.67 | 200 | 11.1 | ||||
| RCA | 2.5 | 6.67 | 50 | ||||||
| Numbers in bold are actual doses given per protocol, all other values are calculated for comparison. **RCA testing if LAD negative or in patients with suspicion for RCA vasospasm on ECG at clinical presentation. ***Dose corrected for catheter position and assuming that half of the blood flow of the LCA goes into the LAD. LAD: left anterior descending; LM: left main; min: minutes; ml: millilitres; No: number; RCA: right coronary artery; sec: seconds | |||||||||
Comparing protocols based on the peak dose of ACh per injection may not be appropriate as ACh has a very short half-life. To account for this fact, we provide a comparison of the separate dosages given in certain periods of time between these protocols in Table 1. For instance, a 200 μg administration in 20 seconds (RBK and JCS protocols) will reach a 9 times higher blood concentration of ACh compared to an administration of the same dose in a slow infusion over 3 minutes (Catholic University of the Sacred Heart protocol)4.
Catheter position and target vessel
The position of the catheter, through which ACh reaches the coronary artery, differs among CFT protocols. Most protocols test the complete LCA by positioning the guiding catheter in the left main (LM). In contrast, some selectively test the left anterior descending artery (LAD) for fear of multivessel or LM spasm, by placing a 2.2 Fr tracker coronary infusion catheter (SCIMED Life Systems/Boston Scientific) into the proximal LAD, such as in the CorMicA and endothelial function testing protocol. In most protocols the RCA is not routinely tested due to the transient atrioventricular block that can be observed. Only the JCS guidelines recommend routine testing of the RCA under back-up pacing with a temporary pacemaker electrode. Other protocols only test the RCA on indication if the LCA tests negative, or based on the clinical presentation. The RBK protocol describes that it is feasible to slow the manual injection speed of ACh when transient atrioventricular block occurs, as it usually resolves within seconds after reducing the speed. This approach avoids potential complications from pacemaker electrodes. In the UCSC protocol the decision as to whether the LCA or RCA is challenged first is at the discretion of the operator.
Whether ACh is administered into the blood volume of the complete LCA or selectively into that of the LAD also further complicates a theoretical comparison between dosages given per CFT protocol. Such is the case with endothelial function testing protocols, where both protocols infuse the same concentration of ACh at the same infusion rate; however, in the WISE study16, this was administered in the LCA and in studies from the Mayo Clinic, selectively into the LAD. We therefore also provide a corrected blood concentration of ACh for these protocols, assuming the averaged volumetric blood flow of a right dominant system is 80 ml/min in the LAD and 160/min in the complete LCA17 (Table 1).
Testing for non-endothelial vasodilation (intracoronary flow assessment)
Besides testing for vasoconstriction abnormalities, most protocols describe testing for vasodilation abnormalities that could cause angina in ANOCA patients as well. This is important for therapeutic purposes as MVA caused by increased vasoconstriction and/or impaired vasodilatation abnormalities requires different treatment. The pathophysiology of an impaired non-endothelial vasodilation due to a decreased hyperaemic response has been documented as structural in nature (e.g., vascular remodelling, vascular rarefaction, extramural compression, etc.)18. Administered endothelium-independent vasodilators, such as adenosine, evaluate the microvascular dilatory capacity of the coronary microcirculation and are expressed as the coronary flow reserve (CFR) and as indices of microvascular resistance (HMR/IMR)12. The dose of adenosine also shows a wide variation among the protocols and is administered either intravenously at 140 μg/kg/min, or as intracoronary bolus injections that range from 18 μg to 200 μg; the dose is a matter of debate. Studies show that adenosine-induced vasodilation is not entirely endothelium-independent19. This may hamper the assessment of non-endothelial microvascular vasodilation.
Microvascular endothelial dilatory capacity is traditionally assessed by quantifying changes in volumetric CBF in reaction to low grade ACh using Doppler flow velocity measurements and coronary diameter which requires offline QCA analysis. More recently, thermodilution has also been used to assess endothelial microvascular dilatory capacity. This technique requires nitroglycerine prior to the measurement due to the assumption of constant macrovascular volume required for reliable thermodilution. Therefore, macrovascular and microvascular endothelial dysfunction cannot be assessed simultaneously20.
Diagnostic criteria for CFT
a. Vasospasm based on COVADIS criteria
COVADIS defines a positive response for epicardial vasoconstriction to ACh testing as the test induces all of the following: (i) reproduction of the previously reported chest pain, (ii) the induction of ischaemic ECG changes (ST-segment deviation or new U-waves), and (iii) >90% vasoconstriction on angiography21. Patients fulfilling the first two criteria mentioned above yet without epicardial vasoconstriction of >90% are felt to have microvascular spasm. All protocols described in this article that perform spasm provocation adhere to these definitions. However, other criteria were previously used in large important studies to increase sensitivity and specificity of the test, such as a reduction of the necessary epicardial diameter to 75% in the ACOVA study22. Otherwise adhering to the COVADIS criteria, this study reported that 29.1% of patients undergoing spasm provocation had an unspecific reaction to ACh, meaning that they had either ECG changes or recognisable angina, but not both. Remarkably, in a large multicentre registry in Japan, a total or subtotal (>90%) coronary artery narrowing induced by provocation with ACh or ER accompanied by either ECG changes or angina was sufficient for a positive diagnosis23. Improvements in techniques and diagnostic criteria of objective markers of ischaemia could therefore increase the overall diagnostic yield of the spasm provocation. This would be useful where ECG changes are minimal or not present, such as in the case of ST-segment cancellation when concurrent ischaemia of regions supplied by the LAD and circumflex coronary artery (Cx) occurs or in patients where ECG interpretation is hampered due to pre-existing bundle branch block24, a dilemma that is especially important in the diagnosis of microvascular spasm.
b. Endothelial dysfunction
Protocols that are aimed at evaluating endothelial function measure the changes in CBF in response to 3-minute graded infusions of ACh, where a >50% increase in CBF compared to baseline is considered normal. Depending on the protocol, epicardial diameter response can also be taken into account to define a normal (epicardial) reaction and varies from being disregarded in the equation to a >5% or even a >20% increase in epicardial diameter12,16,25. The range of achieved blood concentrations of ACh in endothelial function testing is generally lower and can overlap with the achieved concentrations in protocols that are aimed at provoking spasm (Table 1). However, it remains elusive whether patients diagnosed with endothelial dysfunction in protocols using low graded doses of ACh would be diagnosed with vasospastic angina when administered a high(er) dose of ACh and vice versa. The ACOVA study reported that at the second dose (20 μg over 3 minutes), around 8-9% had coronary vasospasm, whilst in the endothelial function testing protocols, the highest dose is twice that of the ACOVA study (44 μg over 3 minutes), and only 2.3% coronary vasospasm was reported in the WISE study and none in the study from the Mayo Clinic12,16,22.
c. Impaired microvascular dilatory function
Disorders that cause ANOCA due to abnormal vasodilation include: (i) impaired epicardial and microvascular vasodilator capacity (CFR <2.0-2.5), and/or (ii) increased microvascular resistance (IMR ≥25, HMR >1.9-2.5)26. Neither the presence nor the absence of an abnormal vasoconstriction excludes the presence of concomitant abnormal vasodilation, giving rise to a difficult-to-treat mixed type.
Order of testing
Testing for disorders of coronary vasoconstriction can be influenced by concomitant use or prior administration of (other) vasoactive medication. It is for this reason that most protocols urge the discontinuation of some if not all cardiovascular (vasoactive) medication 24 to 72 hours prior to testing (Table 2). Additionally, most protocols urge withholding the administration of all vasospastic agents including radial cocktail when radial access is used. Preferably, CFT is performed ad hoc after routine diagnostic coronary angiography (CAG), such as in the CorMicA protocol27. Whether a low dose of radial cocktail (2 ml) given through the sheath at the start of the diagnostic procedure will hamper testing for vasoconstriction abnormalities remains to be determined.
Table 2. Advice on discontinuation of medication prior to testing per protocol.
| Medication | JCS 2 | RBK 28 | WISE 16 | AMC 13 | UCSC 4 | CorMicA 27 |
|---|---|---|---|---|---|---|
| Radial cocktail | N/S | N/S | N/S | None | N/S | N/S |
| Long-acting calcium antagonists | >48 h | >24 h | >24 h | >24 h | >24 h | N/S |
| Long-acting nitrates | >48 h | >24 h | >24 h | >24 h | >24 h | N/S |
| Short-acting calcium antagonists | N/S | N/S | >24 h | N/S | >24 h | N/S |
| α-blockers | N/S | N/S | >24 h | N/S | N/S | N/S |
| β-blockers | N/S | >24 h | >24 h | >72 h | N/S | N/S |
| Loop diuretics | N/S | N/S | N/S | >12 h | N/S | N/S |
| Sublingual nitrates | N/S | allowed | >4 h | N/S | >24 h | N/S |
| Caffeine | N/S | N/S | >24 h | N/S | N/S | N/S |
| Nicotine | N/S | N/S | >4 h | N/S | N/S | N/S |
| Fasting | N/S | N/S | >12 h | N/S | N/S | N/S |
| N/S: not specified | ||||||
COVADIS has listed indications for vasospasm provocation testing20. The working group recommends excluding patients with significant epicardial stenosis, defined as >50% by visual assessment or fractional flow reserve (FFR) ≤0.80 prior to spasm provocation testing. Unfortunately, ad hoc CFT suggested by COVADIS wherein CFT is performed directly after CAG provides a new dilemma: NTG administration is necessary for FFR (or instantaneous wave-free ratio [iFR] or CFR) measurement, yet this theoretically interferes with testing for vasoconstriction abnormalities for the same reasons vasoactive medications are discontinued prior to testing in most protocols. On the other hand, whether residual spasm after provocation affects testing for vasodilatory disorders remains elusive, especially in patients who are reported to be poor NTG responders, such as most patients with microvascular spasm28. However, the disappearance of symptoms and a return to baseline average peak velocity (APV) may constitute complete alleviation of spasm. The endothelial function testing and CorMicA protocol describe such testing for vasodilation abnormalities prior to testing of vasoconstrictive responses, while only the UCSC and AMC protocols state that FFR/iFR/CFR should be routinely measured after vasoconstriction testing (Central illustration, panel E).
Re-challenge
The RBK protocol is characterised by the possibility to re-challenge vasoconstriction, in which the spasm-provoking ACh dose is re-administered after IC NTG administration (Central illustration, panel B)28. This allows guidance for targeted therapy as it can evaluate the efficacy of NTG in preventing the re-occurrence of spasm. The non-responders should not be treated with high doses of NTG. The re-challenge can also unmask concomitant microvascular spasm in patients with epicardial spasm and provide valuable information regarding the pathophysiology of vasomotor dysfunction28.
Safety of functional testing protocols that evaluate coronary vasomotor response
One of the reasons why CFT protocols may not have been adopted widely is due to safety concerns. Several reviews of side effects during spasm provocation testing have shown that provocative testing with intracoronary administration of ACh or ER is safe. Severe side effects such as an abrupt coronary flow obstruction, which can result in cardiac arrythmias including ventricular fibrillation, occur in the same order of magnitude as described for diagnostic cardiac catheterisation29,30. The side effect most often reported is ACh-induced bradycardia, which occurs in 3.2% of patients and is always transient29. It is likely that protocols that achieve higher concentrations of ACh will encounter such side effects more frequently. Due to these concerns, the JCS CFT protocol advises the use of a temporary pacemaker electrode in the right ventricle, while the RBK protocol describes reducing the speed of injection until normal rhythm returns. The AMC protocol adopts continuous Doppler flow assessment during spasm provocation as changes in the pitch of the acoustic signal due to epicardial and/or microvascular vasospasm precede patient symptoms or ECG changes. Some avoid testing the RCA, as bradycardia most often occurs when ACh is injected into the RCA. A stepwise approach with increasing doses of ACh is recommended to yield the optimum risk versus benefit ratio. Recently, several studies have shown that an ACh provocation test can even be safely performed in ACS patients with no obstructive culprit lesions (MINOCA) on emergency CAG, and may be useful to diagnose coronary spasm in those patients31.
Discussion
Patients suffering from ANOCA remain poorly characterised, complicating their identification in clinical practice, and they therefore continue to be a clinical and therapeutical challenge. By identifying the specific endotype of coronary vasomotor dysfunction in ANOCA, the clinician can tailor the therapy accordingly. Such a stratified approach results in improvements in quality of life and angina26.
In our review we show that there is a large variation in CFT protocols among specialised centres regarding definitions, the vascular domains tested and in techniques used to assess the domains. This is felt to be expected based on local factors, although these variations could affect the sensitivity and specificity of the ACh test itself. Finding consensus on a uniform CFT protocol in trials will therefore improve the scientific progress on coronary vasomotor dysfunction and the widespread adoption of such protocols in current clinical practice. In turn, this is a prerequisite to improvement in tailored medical therapy.
Ideally, the principles to which a uniform CFT protocol should adhere should: a) include testing all vascular domains and be able to identify all possible endotypes of coronary vasomotor dysfunction, b) be able to be performed as an ad hoc procedure after CAG, c) include routine testing of the RCA when the LCA tests negative, and d) use manual injections of ACh as it is easier to prepare and more practical for ad hoc testing after CAG and the infusion rate can easily be adjusted when bradycardia occurs. Improvements in and the use of techniques and diagnostic criteria for objective markers of ischaemia will improve the diagnostic yield and overall safety of the test.
Conclusions
There is a large variation in study CFT protocols among specialised centres with regard to the vascular domains tested and in techniques used to assess the domains. Therefore, there is an unmet need for a standardised study CFT protocol for vasomotor testing in which all vascular domains can be tested in one setting and which is practical to use to ensure widespread adoption. A uniform protocol with uniform definitions of vascular domains is a prerequisite for the improvement of tailored medical therapy in patients with coronary vasomotor disorders.
Impact on daily practise
Currently, numerous study protocols for coronary vasomotor function testing co-exist and vary in vascular domains tested, the manner of testing these domains (e.g., preprocedural discontinuation of medication, provocative agent, solution, infusion time, and target artery) and techniques used for measurements (e.g., Doppler vs thermodilution technique). This lack of consensus on a uniform functional testing protocol for trials hampers both a broader clinical acceptance of the concepts of coronary vasomotor dysfunction, and the widespread adoption of such testing protocols in current clinical practice. Furthermore, the endotype of coronary vasomotor dysfunction might differ among the few specialised centres that perform coronary vasomotor function testing as a result of the use of different protocols.
Acknowledgments
Conflict of interest statement
M. Beijk receives an unrestricted grant from Johnson & Johnson. U. Sechtem, P. Ong and A. Seitz receive payments to their institution from Berthold-Leibinger-Foundation. U. Sechtem declares payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events and support for attending meetings and/or travel by Servier and Abbott. P. Ong has received honoraria for lectures at Pfizer, Bayer Healthcare and Philips/Volcano. He has received travel support from Pfizer and is on the Advisory Board of Boehringer Ingelheim. The other authors have no conflicts of interest to declare.
Abbreviations
- ACC/AHA
American College of Cardiology/American Heart Association
- ACh
acetylcholine
- AMC
Academic Medical Centre
- ANOCA
angina and non-obstructive coronary arteries
- CBF
coronary blood flow
- CFR
coronary flow reserve
- CFT
coronary vasomotor function testing
- COVADIS
Coronary Vasomotor Disorders International Study
- CVF
coronary vasomotor function
- Cx
circumflex coronary artery
- ER
ergonovine
- ESC
European Society of Cardiology
- HMR
hyperaemic microcirculatory resistance
- IMR
index of microcirculatory resistance
- INOCA
ischaemia and non-obstructive coronary arteries
- JCS
Japanese Circulation Society
- LAD
left anterior descending artery
- LM
left main coronary artery
- MVA
microvascular angina
- NTG
nitroglycerine
- RBK
Robert-Bosch-Krankenhaus
- RCA
right coronary artery
- UCSC
Catholic University of the Sacred Heart
- VSA
vasospastic angina
Contributor Information
Rutger G.T. Feenstra, Department of Clinical and Experimental Cardiology, Amsterdam UMC, Heart Center, Amsterdam Cardiovascular Sciences, Amsterdam, the Netherlands.
Andreas Seitz, Department of Cardiology, Robert-Bosch-Krankenhaus, Stuttgart, Germany.
Coen K.M. Boerhout, Department of Clinical and Experimental Cardiology, Amsterdam UMC, Heart Center, Amsterdam Cardiovascular Sciences, Amsterdam, the Netherlands.
Laura H. Bukkems, Department of Clinical Pharmacy, Amsterdam UMC, Amsterdam, the Netherlands.
Valérie E. Stegehuis, Department of Clinical and Experimental Cardiology, Amsterdam UMC, Heart Center, Amsterdam Cardiovascular Sciences, Amsterdam, the Netherlands.
Patty J.I. Teeuwisse, Department of Clinical Pharmacy, Amsterdam UMC, Amsterdam, the Netherlands.
Robbert J. de Winter, Department of Clinical and Experimental Cardiology, Amsterdam UMC, Heart Center, Amsterdam Cardiovascular Sciences, Amsterdam, the Netherlands.
Udo Sechtem, Department of Cardiology, Robert-Bosch-Krankenhaus, Stuttgart, Germany.
Jan J. Piek, Department of Clinical and Experimental Cardiology, Amsterdam UMC, Heart Center, Amsterdam Cardiovascular Sciences, Amsterdam, the Netherlands.
Tim P. van de Hoef, Department of Clinical and Experimental Cardiology, Amsterdam UMC, Heart Center, Amsterdam Cardiovascular Sciences, Amsterdam, the Netherlands.
Peter Ong, Department of Cardiology, Robert-Bosch-Krankenhaus, Stuttgart, Germany.
Marcel A.M. Beijk, Department of Clinical and Experimental Cardiology, Amsterdam UMC, Heart Center, Amsterdam Cardiovascular Sciences, Amsterdam, the Netherlands.
References
- Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- JCS Joint. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014;78:2779–801. doi: 10.1253/circj.cj-66-0098. [DOI] [PubMed] [Google Scholar]
- Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- Montone RA, Niccoli G, Fracassi F, Russo M, Gurgoglione F, Camma G, Lanza GA, Crea F. Patients with acute myocardial infarction and non-obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J. 2018;39:91–8. doi: 10.1093/eurheartj/ehx667. [DOI] [PubMed] [Google Scholar]
- Sueda S, Fujimoto K, Sasaki Y, Sakaue T, Yoshii T, Habara H, Kohno H. Differential incidence and morphology of provoked spasm between intracoronary acetylcholine and ergonovine testing: recommendation of supplementary use. Heart Vessels. 2019;34:745–54. doi: 10.1007/s00380-018-1299-x. [DOI] [PubMed] [Google Scholar]
- Horimoto M, Igarashi K, Takenaka T, Inoue H, Yamazaki K, Sakuragi H. Acetylcholine- and ergonovine-induced coronary microvascular spasm reflected by increased coronary vascular resistance and myocardial lactate production. Clin Cardiol. 2000;23:221–5. doi: 10.1002/clc.4960230320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert A, Seitz A, Pereyra VM, Bekeredjian R, Sechtem U, Ong P. Coronary Artery Spasm: The Interplay Between Endothelial Dysfunction and Vascular Smooth Muscle Cell Hyperreactivity. Eur Cardiol. 2020;15:e12. doi: 10.15420/ecr.2019.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heupler FA, Proudfit WL, Razavi M, Shirey EK, Greenstreet R, Sheldon WC. Ergonovine maleate provocative test for coronary arterial spasm. Am J Cardiol. 1978;41:631–40. doi: 10.1016/0002-9149(78)90810-x. [DOI] [PubMed] [Google Scholar]
- Okumura K, Yasue H, Matsuyama K, Goto K, Miyagi H, Ogawa H, Matsuyama K. Sensitivity and specificity of intracoronary injection of acetylcholine for the induction of coronary artery spasm. J Am Coll Cardiol. 1988;12:883–8. doi: 10.1016/0735-1097(88)90449-4. [DOI] [PubMed] [Google Scholar]
- Sueda S, Kohno H, Miyoshi T, Sakaue T, Sasaki Y, Habara H. Maximal acetylcholine dose of 200 μg into the left coronary artery as a spasm provocation test: comparison with 100 μg of acetylcholine. Heart Vessels. 2015;30:771–8. doi: 10.1007/s00380-014-0563-y. [DOI] [PubMed] [Google Scholar]
- Hasdai D, Gibbons RJ, Holmes DR, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–5. doi: 10.1161/01.cir.96.10.3390. [DOI] [PubMed] [Google Scholar]
- Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of Coronary Microvascular Dysfunction Among Patients With Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc Interv. 2015;8:1445–53. doi: 10.1016/j.jcin.2015.06.017. [DOI] [PubMed] [Google Scholar]
- Beijk MA, Vlastra WV, Delewi R, van de, Boekholdt SM, Sjauw KD, Piek JJ. Myocardial infarction with non-obstructive coronary arteries: a focus on vasospastic angina. Neth Heart J. 2019;27:237–45. doi: 10.1007/s12471-019-1232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueda S, Fujimoto K, Sasaki Y, Sakaue T, Kohno H. Dose maximal acetylcholine dose into the left coronary artery affect the positive provoked spasm in the left circumflex artery? Coron Artery Dis. 2019;30:547–8. doi: 10.1097/MCA.0000000000000756. [DOI] [PubMed] [Google Scholar]
- Sueda S, Kohno H. The acetylcholine administration time plays the key role for provoked spasm in the spasm provocation test. J Cardiol. 2017;70:141–6. doi: 10.1016/j.jjcc.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, Azarbal B, Petersen J, Sharaf B, Handberg E, Shufelt C, Kothawade K, Sopko G, Lerman A, Shaw L, Kelsey SF, Pepine CJ, Merz CN. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women's Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv. 2012;5:646–53. doi: 10.1016/j.jcin.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto S, Takahashi S, Coskun AU, Papafaklis MI, Takahashi A, Saito S, Stone PH, Feldman CL. Relation of distribution of coronary blood flow volume to coronary artery dominance. Am J Cardiol. 2013;111:1420–4. doi: 10.1016/j.amjcard.2013.01.290. [DOI] [PubMed] [Google Scholar]
- Sechtem U, Brown D, Godo S, Lanza GA, Shimokawa H, Sidik N. Coronary microvascular dysfunction in stable ischaemic heart disease (non-obstructive coronary artery disease and obstructive coronary artery disease). Cardiovasc Res. 2020;116:771–86. doi: 10.1093/cvr/cvaa005. [DOI] [PubMed] [Google Scholar]
- Buus NH, Bøttcher M, Hermansen F, Sander M, Nielsen TT, Mulvany MJ. Influence of nitric oxide synthase and adrenergic inhibition on adenosine-induced myocardial hyperemia. Circulation. 2001;104:2305–10. doi: 10.1161/hc4401.098293. [DOI] [PubMed] [Google Scholar]
- Diez-Delhoyo F, Gutiérrez-Ibanes E, Sanz-Ruiz R, Vazquez-Alvarez ME, Gonzalez Saldivar, Rivera Juarez, Sarnago F, Martinez-Sellés M, Bermejo J, Soriano J, Elizaga J, Fernandez-Avilés F. Prevalence of Microvascular and Endothelial Dysfunction in the Nonculprit Territory in Patients With Acute Myocardial Infarction. Circ Cardiovasc Interv. 2019;12:e007257. doi: 10.1161/CIRCINTERVENTIONS.118.007257. [DOI] [PubMed] [Google Scholar]
- Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Bairey Merz Coronary Vasomotion Disorders International Study Group (COVADIS) International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565–8. doi: 10.1093/eurheartj/ehv351. [DOI] [PubMed] [Google Scholar]
- Aziz A, Hansen HS, Sechtem U, Prescott E, Ong P. Sex-Related Differences in Vasomotor Function in Patients With Angina and Unobstructed Coronary Arteries. J Am Coll Cardiol. 2017;70:2349–58. doi: 10.1016/j.jacc.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Yasuda S, Takahashi J, Tsunoda R, Ogata Y, Seki A, Sumiyoshi T, Matsui M, Goto T, Tanabe Y, Sueda S, Sato T, Ogawa S, Kubo N, Momomura S, Ogawa H, Shimokawa H Japanese Coronary Spasm Association. Clinical implications of provocation tests for coronary artery spasm: safety, arrhythmic complications, and prognostic impact: multicentre registry study of the Japanese Coronary Spasm Association. Eur Heart J. 2013;34:258–67. doi: 10.1093/eurheartj/ehs199. [DOI] [PubMed] [Google Scholar]
- Vives-Borras M, Jorge E, Amoros-Figueras G, Millan X, Arzamendi D, Cinca J. Summation and Cancellation Effects on QRS and ST-Segment Changes Induced by Simultaneous Regional Myocardial Ischemia. Front Physiol. 2018;9:275. doi: 10.3389/fphys.2018.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman H, Corcoran D, Aetesam-Ur-Rahman M, Hoole SP, Berry C, Perera D. Diagnosis of patients with angina and non-obstructive coronary disease in the catheter laboratory. Heart. 2019;105:1536–42. doi: 10.1136/heartjnl-2019-315042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas AHEM, Prescott E, Karam N, Appelman Y, Fraccaro C, Buchanan GL, Manzo-Silberman S, Al-Lamee R, Regar E, Lansky A, Abbott JD, Badimon L, Duncker DJ, Mehran R, Capodanno D, Baumbach A. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention. 2021;16:1049–69. doi: 10.4244/EIJY20M07_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, Yii E, Sidik N, McCartney P, Corcoran D, Collison D, Rush C, McConnachie A, Touyz RM, Oldroyd KG, Berry C. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J Am Coll Cardiol. 2018;72:2841–55. doi: 10.1016/j.jacc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Seitz A, Feenstra RGT, Konst RE, Martínez Pereyra, Beck S, Beijk M, van de, van Royen, Bekeredjian R, Sechtem U, Damman P, Piek JJ, Ong P. Acetylcholine Re-challenge: A First Step Towards Tailored Treatment in Patients with Coronary Artery Spasm. J Am Coll Cardiol Intv. 2022;15:65–75. doi: 10.1016/j.jcin.2021.10.003. [DOI] [PubMed] [Google Scholar]
- Ciliberti G, Seshasai SRK, Ambrosio G, Kaski JC. Safety of intracoronary provocative testing for the diagnosis of coronary artery spasm. Int J Cardiol. 2017;244:77–83. doi: 10.1016/j.ijcard.2017.05.109. [DOI] [PubMed] [Google Scholar]
- Noto TJ, Johnson LW, Krone R, Weaver WF, Clark DA, Kramer JR, Vetrovec GW. Cardiac catheterization 1990: a report of the Registry of the Society for Cardiac Angiography and Interventions (SCA&I). Cathet Cardiovasc Diagn. 1991;24:75–83. doi: 10.1002/ccd.1810240202. [DOI] [PubMed] [Google Scholar]
- Probst S, Seitz A, Martinez Pereyra, Hubert A, Becker A, Storm K, Bekeredjian R, Sechtem U, Ong P. Safety assessment and results of coronary spasm provocation testing in patients with myocardial infarction with unobstructed coronary arteries compared to patients with stable angina and unobstructed coronary arteries. Eur Heart J Acute Cardiovasc Care. 2020:2048872620932422. doi: 10.1177/2048872620932422. [DOI] [PubMed] [Google Scholar]