Abstract
Background
Spontaneous coronary artery dissection (SCAD) is an increasingly reported but poorly understood condition. Few European data are available.
Aims
The aims of this study were to obtain European data on SCAD, determine the prevalence of fibromuscular dysplasia (FMD) and enable genetic analyses in this population.
Methods
Data from a national French registry of SCAD cases were analysed prospectively and retrospectively. Clinical and angiographic data and management strategy were collected. Major adverse cardiovascular events (MACE) were analysed after one year of follow-up. Subjects were screened for FMD and blood was collected for DNA extraction.
Results
From June 2016 to August 2018, 373 SCAD cases were confirmed by the core lab. Mean age was 51.5 years. Patients were mostly women (90.6%) and 54.7% of cases had less than two cardiovascular risk factors. At one year, 295 patients (79.1%) were treated conservatively, the MACE rate was 12.3%, and there were no cases of mortality. The recurrence rate of SCAD was 3.3%. FMD was found at ≥1 arterial site in 45.0% of cases. We also confirmed the genetic association between the PHACTR1 locus and SCAD (odds ratio=1.66, p=7.08×10–8).
Conclusions
Here we describe the DISCO registry, the largest European SCAD cohort where FMD was found in 45% of cases and the genetic association with PHACTR1 was confirmed. This nationwide cohort is a valuable resource for future clinical and genetic investigation to understand SCAD aetiology.
Introduction
Spontaneous coronary artery dissection (SCAD) is an underdiagnosed and poorly understood form of acute coronary syndrome (ACS). Long considered rare, SCAD is today recognised as an important cause of ACS and myocardial infarction with non-obstructive coronary arteries (MINOCA) in early middle-aged women1,2.
Evidence for frequent co-occurrence between SCAD and fibromuscular dysplasia (FMD) has been reported3. A genetic origin of FMD was suggested by the identification of a risk locus at the phosphatase and actin regulator 1 (PHACTR1) locus for FMD (rs9349379)4. More recently, the same locus was also reported to be associated with a genetic risk for SCAD5,6, which supports shared pathophysiological mechanisms in addition to common clinical presentations.
SCAD and FMD remain poorly understood conditions. Few European data, particularly where genetic analyses can be conducted, are available. The aim of the current study was to describe clinical, angiographic and one-year follow-up characteristics of subjects with SCAD included in a national registry in France, to assess the phenotypic prevalence of FMD in the SCAD population and to evaluate the relevance for genetic studies in this cohort.
Methods
This was an observational study (DISCO; NCT02799186) conducted in 51 French cardiology centres. The design and conduct were approved by the regional committee CPP Sud-Est 6 2016 AU-1258 (IRB approval). All subjects gave their written, informed consent to participate in the study.
Patients at least 18 years of age with a diagnosis of SCAD were included in the study. Two types of inclusion were possible: 1) retrospective with a diagnostic of SCAD made from 2010, or 2) prospective at the time of hospitalisation during which the diagnosis of SCAD was made.
Exclusion criteria included age below 18 years and patients with iatrogenic or traumatic dissection or atherosclerotic disease.
ANGIOGRAPHIC DIAGNOSIS OF SCAD
SCAD was identified as an acute or chronic coronary syndrome defined according to universal recommendations with angiographic signs suggestive of SCAD7 on the initial coronary angiography. In the context of MINOCA, a pathological cardiac magnetic resonance image could lead to the diagnosis of SCAD retrospectively by rereading the angiograms. In case of ambiguity, intravascular ultrasound (IVUS), optical coherence tomography (OCT) or repeat coronary angiography was performed at the discretion of the operator to confirm the diagnosis of SCAD. All SCAD cases were subsequently confirmed by a core lab of three operators (N. Combaret, P. Motreff, G. Souteyrand) experienced in the field of SCAD and intracoronary imaging. Confirmed SCAD was classified according to the Saw classification8. Coronary segmentation was defined according to the SYNTAX classification.
BASELINE CHARACTERISTICS
Demographic data, cardiovascular risk factors, personal history of SCAD, FMD, chronic inflammatory systemic diseases or connective tissue diseases, migraine, hormonal status, treatment and possible drug consumption were collected.
IN-HOSPITAL DATA
Functional signs, initial clinical presentation, potential triggers, ECGs, initial laboratory data and emergency treatments were collected. Initial treatment strategies were classified as: 1) conservative treatment recommended in asymptomatic patients with haemodynamic and rhythmic stability and Thrombolysis In Myocardial Infarction (TIMI) 3 flow on the affected vessel; 2) percutaneous coronary intervention (PCI) in cases of persistent ischaemia, rhythmic or haemodynamic instability and altered TIMI ≤2 flow on the target vessel; or 3) cardiac surgery (mechanical circulatory support, coronary artery bypass grafting or transplantation).
DIAGNOSIS OF FIBROMUSCULAR DYSPLASIA
Screening for FMD was carried out at the discretion of the different centres by either computed tomography (CT) scan, magnetic resonance imaging, angiography or Doppler in the cervico-cephalic, visceral and iliac arteries. The diagnosis of FMD was considered confirmed if at least one extra-cardiac location was verified by an experienced radiologist (LC) according to established criteria9. Complete screening was defined as brain to pelvis analysis.
GENETIC DATA
DNA was extracted from whole blood using a chemagic™️ 360 device (PerkinElmer, Waltham, MA, USA). Genotyping was performed by direct sequencing or using the global genotyping screen array. Genetic analysis was carried out on participants with a confirmed diagnosis who met specific genetic quality criteria (DNA available, call rate per individual >95% and genetically determined European ancestry using PLINK 2.0)10. The genetic data were compared with a cohort of healthy individuals from the French Paris Prospective Study III database5,11.
FOLLOW-UP
For the prospective cohort, patients had conventional in-hospital follow-up. One-year follow-up was by consultation in person or by telephone contact. For each patient of the retrospective cohort, clinical events since initial SCAD were researched in medical history and confirmed by investigator centre. In both cohorts, major adverse cardiac events at one year (MACE; all-cause mortality, stroke, recurrence of SCAD, recurrence of infarction, revascularisation) were collected. If new angiograms were performed during follow-up, they were analysed by the same core lab (N. Combaret, G. Souteyrand, and P. Motreff) to affirm SCAD recurrence. SCAD recurrence was defined as new ACS related to SCAD but involving a different arterial segment than the original event.
STATISTICAL METHODS
Statistical analyses were performed using Stata software, Version 15 (StataCorp, College Station, TX, USA). Continuous data are presented as mean±standard deviation or median (interquartile range) according to statistical distribution. The assumption of normal distribution was tested using the Shapiro-Wilk test. To estimate the association between rs9349379 and SCAD, genotype distributions were compared between cases and controls, globally and stratified by FMD status using unconditional logistic regression under the additive genetic model (R, version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
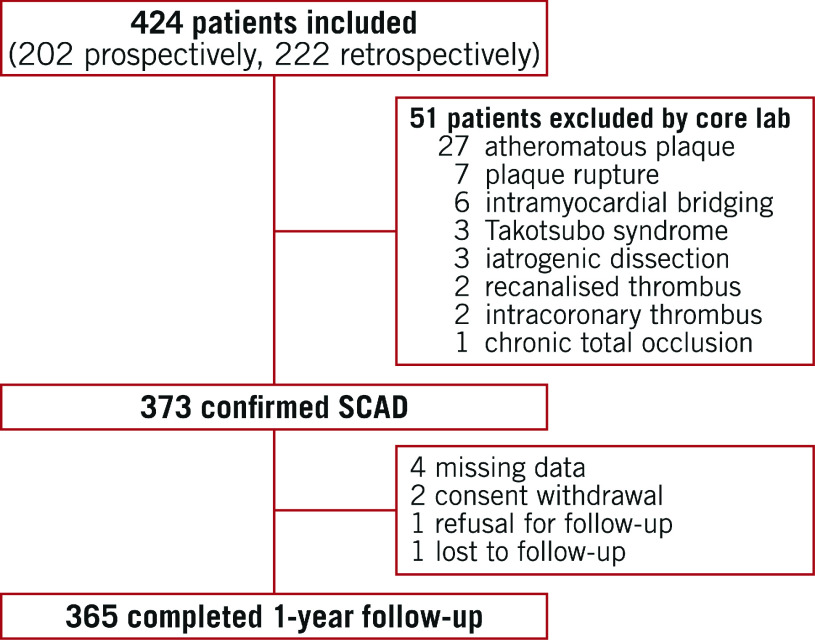
Between June 2016 and August 2018, 424 patients were included at 51 centres (202 prospectively and 222 retrospectively) and 373 SCAD were confirmed by the core lab. A flow chart of subjects is shown in Figure 1. Population characteristics are summarised in Table 1.
Figure 1.
Cohort study flow diagram.
Table 1. Population characteristics.
| Population characteristics | n=373 |
| Age, years | 51.5±10.3 |
| Female sex | 338 (90.6) |
| Smoking | 93 (24.9) |
| Dyslipidaemia | 61 (16.3) |
| Diabetes mellitus | 12 (3.2) |
| Hypertension | 119 (31.9) |
| Cardiovascular disease in family | 70 (18.8) |
| Obesity (BMI >30 kg/m2) | 58 (15.5) |
| Previous ACS | 25 (6.7) |
| Previous SCAD | 9 (2.4) |
| Stroke – TIA | 8 (2.1) |
| Previous extra coronary artery dissection | 4 (1.1) |
| History of migraine | 89/322 (27.6) |
| History of inflammatory/systemic disease | 7 (1.9) |
| No cardiovascular risk factor | 83 (22.2) |
| <2 cardiovascular risk factors | 204 (54.7) |
| Peripartum | 14/338 (4.4) |
| Contraception | 62/205 (30.2) |
| Oral contraceptive | 39/205 (19) |
| Intrauterine device | 23/205 (7.5) |
| Post menopause | 168/331 (50.7) |
| Hormone replacement therapy | 27/151 (17.9) |
| No pregnancy or birth | 23/323 (7.1) |
| <3 pregnancies | 168/323 (52) |
| ≥3 pregnancies | 132/323 (40.9) |
| ≥4 pregnancies | 63/323 (19.5) |
| Assisted reproduction technique | 18/327 (5.5) |
| Data are shown as mean±SD or n (%). ACS: acute coronary syndrome; BMI: body mass index; SCAD: spontaneous coronary artery dissection; TIA: transient ischaemic attack | |
Patients were admitted for ACS in 96.2% of cases (of which 45.6% were STEMI). Twenty-one patients (5.6%) experienced cardiac arrest during initial management. A triggering factor was found in 60.6% of cases (emotional stress in 45.7% and physical stress in 12.3%) (Supplementary Table 1).
Angiographic characteristics are summarised in Supplementary Table 1.
The majority of patients received conservative initial management; PCI was performed in 58 patients (15.5%). PCI failure was reported in 10.3% of cases (inability to progress a wire or TIMI 0 at the end of the procedure) and a complication in 13.8% (worsening of dissection or haematoma progression).
The median length of hospital stay was 5 (3-8) days. During hospitalisation, 6.2% of patients received an emergency angiographic control and 2.7% received emergency PCI (Table 2, Figure 2). Fifty-four patients (14.5%) underwent scheduled control angiography with a median delay of 5 days (4-7); there was angiographic improvement in 66.7% of cases. Seven iatrogenic coronary dissections (1.9%) were reported during hospitalisation (5 in the initial phase and 2 during controls) (Table 2). No in-hospital deaths were reported.
Table 2. Management and in-hospital outcomes.
| Initial treatment strategy | n=373 | |
| Conservative treatment | 314 (84.2) | |
| PCI | 58 (15.5) | |
| CABG | 0 (0) | |
| LVAD | 1 (0.3) | |
| Indication for PCI | n=58 | |
| Haemodynamic instability | 5 (8.6) | |
| Persistent chest pain | 14 (24.1) | |
| TIMI 0 flow | 21 (37.9) | |
| Combined criteria | 10 (17.2) | |
| Other | 3 (5.2) | |
| NA | 4 (6.9) | |
| PCI procedure details | n=57 | |
| Wiring only | 3 (5.3) | |
| Balloon angioplasty only | 17 (29.8) | |
| Cutting balloon only | 1 (1.7) | |
| Cutting balloon before stenting | 2 (3.5) | |
| Stent | 36 (63.1) | |
| BMS | 2 (5.5) | |
| DES | 33 (91.7) | |
| BRS | 1 (2.7) | |
| PCI results | n=55 | |
| Final TIMI flow | 0 | 6 (10.9) |
| 1 | 2 (3.6) | |
| 2 | 1 (1.8) | |
| 3 | 46 (83.6) | |
| PCI failure | 6 (10.3) | |
| PCI complications | 8 (13.8) | |
| Haematoma progression | 1 (1.7) | |
| Dissection | 6 (10.3) | |
| Other | 1 (1.7) | |
| Unplanned angiographic control | 23 (6.2) | |
| NSTEMI | 12 (52.2) | |
| STEMI | 9 (39.1) | |
| Cardiogenic shock | 2 (8.7) | |
| Unplanned revascularisation | 10 (2.7) | |
| Planned angiographic control | 54 (14.5) | |
| Improvement | 36 (66.7) | |
| Complete angiographic healing | 4 (7.4) | |
| PCI results (cont'd) | ||
| Stability | 9 (16.7) | |
| Worsening | 9 (16.7) | |
| Planned angiographic control delay, days | 5 (4-7) | |
| Iatrogenic dissection | 7 (1.9) | |
| During initial angiography | 5 (71.4) | |
| During angiographic control | 2 (28.6) | |
| In-hospital outcomes | n=373 | |
| MACE | 20 (5.4) | |
| Death | 0 (0) | |
| Reinfarction | 20 (5.4) | |
| SCAD recurrence (de novo) | 0 (0) | |
| SCAD extension | 10 (2.7) | |
| Revascularisation | 10 (2.7) | |
| Stroke | 0 (0) | |
| Follow-up and 1-year outcomes | ||
| Planned angiographic control after discharge | n=200 | |
| Improvement | 184/198 (93) | |
| Complete angiographic healing | 114/198 (57.6) | |
| Stability | 8/198 (4) | |
| Worsening | 6/198 (3) | |
| Planned angiographic control delay, days | 56 (36-84.5) | |
| Iatrogenic dissection during angiographic control | 0 (0) | |
| At 1 year | n=365 | |
| MACE | 45 (12.3) | |
| Death | 0 (0) | |
| Recurrent myocardial infarction | 28 (7.7) | |
| SCAD recurrence (de novo) | 12 (3.3) | |
| SCAD extension | 15 (4.1) | |
| Other | 1 (0.3) | |
| Revascularisation | 31 (8.5) | |
| Stroke | 2 (0.6) | |
| Left ventricular ejection fraction, % (n=155) | 60 (56-65) | |
| Data are shown as n (%) or median (Q1-Q3). BMS: bare metal stent; BRS: bioresorbable scaffold; CABG: coronary artery bypass graft; DES: drug-eluting stent; LVAD: left ventricular assist device; MACE: major adverse cardiac events; NA: not available; NSTEMI: non-ST-elevation myocardial infarction; PCI: percutaneous coronary intervention; SCAD: spontaneous coronary artery dissection; STEMI: ST-elevation myocardial infarction; TIMI: Thrombolysis In Myocardial Infarction | ||
Figure 2.
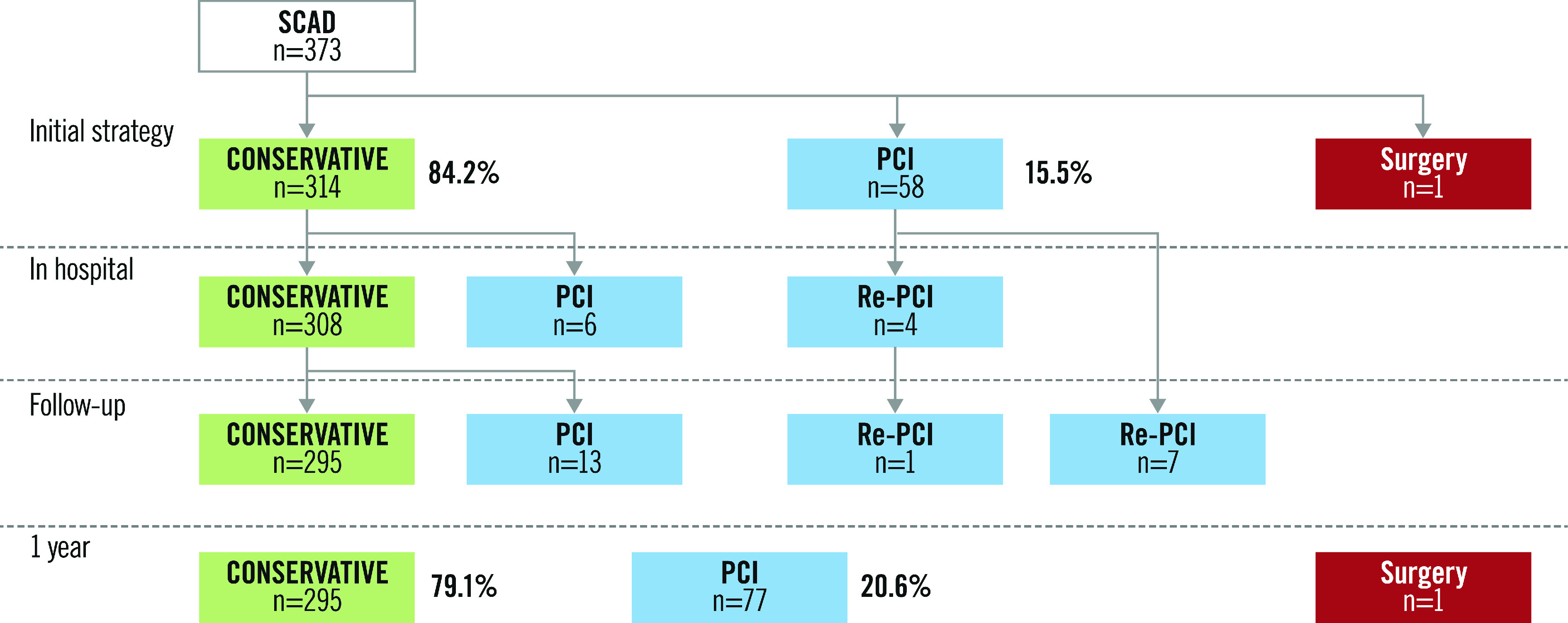
Management flow diagram.
Scheduled control angiography post discharge was performed in 200 subjects (65.9%), with a median time of 56 days (35-84.5) post discharge. Improvement was noted in 93% of cases and complete recovery in 57.6% of cases. No iatrogenic dissection occurred.
Intracoronary imaging was used during initial angiography in 68 patients (18.2%), in the first angiographic control (if performed) in 6.2% and in the second angiographic control (if performed) in 1.3% of cases. No complication was reported with the use of intracoronary imaging.
The rate of MACE at one year was 12.3% (45 events among 365 subjects). There were no deaths. Recurrence of SCAD was recorded in 3.3% of subjects and 31 patients (8.5%) required revascularisation (Table 2). At one year, 295 patients (79.1%) received a conservative treatment strategy (Figure 2). PCI was performed in 77 patients (20.6%). In the retrospective cohort, mean follow-up was 641 days. In the PCI group, STEMI was more frequent (69.0% vs 41.1% in the conservative group; p<0.01), as was TIMI ≤1 flow (55.3% vs 20.6% in the conservative group; p<0.01). Finally, in the PCI group, the MACE rate was statistically more frequent than in the conservative group (10.2% vs 22.4%, p<0.01) with higher creatine phosphokinase (CPK) peaks (986 vs 325 U/L, p<0.01) (Supplementary Table 2).
Radiological screening for FMD at at least one arterial site was carried out in 340 patients (91.1%). A complete screening was performed in 277 patients (74.3%). FMD was found at at least one arterial site in 45.0% of subjects. The FMD was isolated in 62.1% of cases, most often in renal (28.2%) and carotid (21.9%) arteries. The findings are summarised in Table 3. Among the male population (n=35), 65.7% had FMD.
Table 3. Screening and presence of fibromuscular dysplasia.
| n=373 | ||
| No screening performed | 33 (8.8) | |
| Incomplete screening (at least one location screened) | 63 (16.9) | |
| Complete screening | 277 (74.3) | |
| Screening modality | n=340 | |
| Computed tomographic angiography only | 237 (69.7) | |
| Magnetic resonance angiography only | 37 (10.9) | |
| Angiography during catheterisation only | 7 (2.1) | |
| Ultrasound only | 3 (0.8) | |
| Multimodal | 56 (16.5) | |
| Presence of FMD | n=340 | |
| FMD | 153 (45) | |
| One localisation | 95 (27.9) | |
| Multifocal FMD | 58 (17.1) | |
| FMD prevalence and complete screening | n=277 | |
| FMD | 131 (47.3) | |
| FMD localisation | Carotid | 63/288 (21.9) |
| Vertebral | 20/289 (6.9) | |
| Renal | 93/330 (28.2) | |
| Iliac | 47/325 (14.5) | |
| Visceral | 16/263 (6.1) | |
| Data are shown as n (%). FMD: fibromuscular dysplasia | ||
Genetic analysis was performed in 313 SCAD patients (83.9%), among whom 140 presented with FMD. All were compared to 3,468 controls from the Paris Prospective Study III (PPS3) (Supplementary Table 3). The PHACTR1 A allele was associated with increased risk of SCAD overall (OR=1.66, 95% confidence interval [CI]: 1.38-1.99, p=7.08 ×10-8). The increased risk estimate was higher when we analysed SCAD patients with FMD (OR=1.96, 95% CI: 1.49-1.99, p=2.31 ×10-6), compared to the sample of SCAD without FMD (OR=1.59, 95% CI: 1.24-2.06, p=3.79 ×10-4). We note that all estimates of OR were significant with overlapping 95% CI (Supplementary Table 3).
Discussion
The DISCO study is the first European multicentre analysis of SCAD patients and one of the largest in the world. In this large population of SCAD patients, the rate of FMD was 45%. There was also a significant association between variations in the PHACTR1 locus and the risk of SCAD, and a trend towards a higher estimate among patients with FMD.
SCAD PRESENTATION AND FOLLOW-UP
The population characteristics were comparable to SCAD populations previously described2,12, with a large predominance of women aged around 50 years with few cardiovascular risk factors. We also confirm a rate of more than 50% post-menopausal women (62.3% in the Canadian cohort12) and a relatively low rate (4.4%) of pregnancy-associated SCAD (<5% in a previous study13). It is interesting to note that 40% of patients in our cohort reported at least three pregnancies and 19.5% at least four, which is higher than the rates (<10%) previously reported12.
The high rate of cardiac arrest during initial SCAD management demonstrates the risk associated with this pathology (multivessel or proximal coronary artery involvement). It is likely that a substantial number of sudden deaths in younger women may be related to SCAD. Apart from pre-hospital mortality, the prognosis is favourable whether patients are treated medically or by PCI, with a MACE rate of 12.3%, mainly explained by SCAD recurrences (3.3%) and revascularisations (8.5%). These results seem to be more favourable than those recently published by Gad et al14 with a significant rate of readmission at 30 days (12.3% with half during the first week) following hospitalisation for SCAD with most readmissions due to cardiac causes.
ANGIOGRAPHIC DATA AND MANAGEMENT
Angiographic analysis identified single-vessel involvement in almost all of our cases, similar to what has been found in other cohorts12. There was a strong predominance of haematomas (type 2 in Saw's classification). No intimal rupture was found in four out of five cases. Type 2 and type 3 SCAD are the most difficult to recognise by angiography, often confused with a developing atheroma or a coronary spasm; they can be underdiagnosed in case of distal involvement. The angiographic signs described7 must be systematically sought, especially in the absence of atheroma. In case of ambiguity, IVUS or OCT may prove useful to confirm the diagnosis (in the initial phase or during systematic control). In our series, there was no increased risk of complications with intravascular imaging. On the other hand, in this context of arterial fragility, if the angiographic diagnosis is certain or if the disease is presumed to be distal, intracoronary imaging is certainly not recommended.
A conservative management strategy should be favoured when possible15. In our series, >80% were treated medically in the initial phase and almost all had a favourable clinical outcome at one year. Almost all patients with scheduled coronary angiography showed spontaneous improvement, and complete healing was reported in more than half of the cases at 56 days versus less than a tenth during in-hospital control. These data indicate that control angiography in order to evaluate healing is not useful when performed too early. Similar conclusions were drawn in the study of Hassan et al16, who reported complete healing in 95% of cases with a repeat angiogram after ≥30 days. We recommend avoiding in-hospital planned angiographic control in case of non-extensive SCAD and favourable in-hospital evolution.
In patients with SCAD, PCI is associated with a high risk of complications, as seen in our cohort and in published studies17. In addition to the risk of procedural failure, the current cohort had a relevant rate of angiographic aggravation by propagation of the haematoma or secondary iatrogenic dissection. The interventional cardiologist should be aware of certain precautions, e.g., avoidance of deep catheter engagement, non-coaxial positioning of the catheter tip, and strong contrast agent injection2,15.
FIBROMUSCULAR DYSPLASIA
The screening for FMD confirmed an enhanced prevalence of FMD in the SCAD population. At 45%, this is comparable to rates in the latest studies18,19. The predominance of cervico-cephalic, renal and iliac localisations in our cohort was in agreement with that which has been reported in North American cohorts18. In the male population (n=35), the rate of FMD was higher (65.7%) than in female subjects, even though this condition is recognised as rare in men (data from the American registry found only 9% of the 447 patients with FMD to be male and French data found 16% in a population of 394 patients20).
GENETIC DATA
Our registry is the first to perform systematic DNA collection from SCAD patients. We have previously reported significant association between rs9349379, a common variant at the PHACTR1 locus, and SCAD in a sub-sample of 189 patients from the DISCO cohort5. Turley et al recently revealed five replicated risk loci and positional candidate genes for SCAD, including this PHACTR1 locus. Once again, this association was confirmed in the current analysis that included all eligible samples in the current series. The association was not related to the phenotypic status of FMD. The OR was slightly higher in the presence of FMD, which is the opposite to what was reported in a Mayo Clinic registry5. This difference can be explained by sampling fluctuations due to the relatively small sizes of the two cohorts where the analysis was available. This is further supported by the overlap of the confidence intervals for estimates of the ORs with SCAD in subjects with and without FMD. A further reason for the disparities may be differences in FMD screening between the two registries in terms of completeness and imaging modalities. Overall, our results confirm that the genetic association between SCAD and the PHACTR1 locus is not fully explained by the association with FMD4.
Limitations
The DISCO registry has limitations in terms of data quality and completeness, especially in FMD screening. As patients were enrolled both prospectively and retrospectively, and the registry included not all interventional cardiology centres in France, the data represent a selection and it is not possible to draw conclusions on the incidence of SCAD in the French population. Moreover, the frequent favourable evolution of the disease does not exclude the possibility of missed diagnosis. Concerning genetic analysis, the prevalence of FMD in the control group from the PPS3 cohort is not known. This may represent a bias, even if the prevalence of FMD in this cohort is estimated to be the same as in the general population.
Finally, prolonged follow-up remains necessary to establish long-term recurrence rates.
Conclusions
The DISCO registry is the largest European SCAD cohort and the first European resource with prolonged follow-up where genetic analysis can be performed. The work confirmed in a European cohort the epidemiological characteristics of SCAD, which mainly affects middle-aged women with few or no cardiovascular risk factors. Conservative management, when possible, was safe with no deaths at one year. FMD was found in 45% of SCAD patients, with predominant involvement of the cervico-cephalic and renal arteries. Finally, we confirmed the genetic association between variations in the PHACTR1 locus and the occurrence of SCAD, with or without the presence of FMD. Longer-term follow-up data and further systematic genetic analysis will be needed to confirm these findings.
Impact on daily practice
Strong links between spontaneous coronary artery dissection (SCAD) and fibromuscular dysplasia (FMD) are now confirmed in North American and European cohorts. Genetic associations between these two pathologies are described and some genetic loci such as PHACTR1 seem to be involved. This nationwide SCAD cohort will allow long-term follow-up to determine the prognosis and recurrence rate of this pathology. Systematic blood extraction in all patients will permit further genetic analysis to confirm the first results published with some high-risk loci and provide a better understanding of the common genetic mechanisms between SCAD and FMD.
Appendix. Study collaborators
Céline Lambert, PhD; Délégation Recherche Clinique et Innovations, CHU Clermont-Ferrand, Clermont-Ferrand, France. Christian Spaulding, MD, PhD; Department of Cardiology, European Hospital Georges Pompidou, APHP, Sudden Cardiac Death Expert Center, INSERM U 971, Paris Descartes University, Paris, France. Hakim Benamer, MD; Cardiology Department, Institut Cardiovasculaire Paris Sud, Ramsay Générale de Santé, Massy, France. Xavier Jeunemaitre, PhD, Adrien Georges, PhD; Université de Paris, PARCC, INSERM, Paris, France. Didier Bresson, MD; Department of Cardiology, ICU, Emile Muller Hospital, Mulhouse, France. Lionel Mangin, MD; Department of Cardiology, Centre Hospitalier Annecy-Genevois, Metz-Tessy, France.
Supplementary data
List of all investigators of the DISCO registry.
Presenting characteristics.
Follow-up and MACE rate in Conservative group versus PCI group.
Association analysis between rs9349379 (PHACTR1) and SCAD stratified according to the presence of fibromuscular dysplasia in the DISCO study.
Acknowledgments
Acknowledgements
The authors would like to thank the following: the French Society of Cardiology and the French Coronary Atheroma and Interventional Cardiology Group for their support; the Clinical Research Associates of the Clermont-Ferrand University Hospital (Elodie Chazot, Carole Bellanger, Laurie Cubizolles, Aurélie Thalamy, Ouarda Lamallem and Marie-Ange Cartalade, and Quentin Liabot, medicine resident in the cardiology department) for data analyses; all the investigator sites (Supplementary Appendix 1). The authors would also like to acknowledge the contribution of the European Global Screening Array Consortium. We thank J.P. Empana and X. Jouven, PIs of the PPS3.
Funding
This study was supported by the Fondation Coeur et Recherche. The genetic study was supported by a European Research Council grant (ERC-Stg-ROSALIND-716628).
Conflict of interest statement
The authors/study collaborators have no conflicts of interest to declare in relation to this work.
Abbreviations
- ACS
acute coronary syndrome
- FMD
fibromuscular dysplasia
- IVUS
intravascular ultrasound
- MINOCA
myocardial infarction with non-obstructive coronary arteries
- OCT
optical coherence tomography
- PCI
percutaneous coronary intervention
- SCAD
spontaneous coronary artery dissection
- STEMI
ST-elevation myocardial infarction
Contributor Information
Nicolas Combaret, Department of Cardiology, CHU Clermont-Ferrand, CNRS, Université Clermont Auvergne, Clermont-Ferrand, France.
Edouard Gerbaud, Cardiology ICU and Interventional Cardiology, Hôpital Cardiologique du Haut Lévêque, CHU de Bordeaux, Bordeaux Cardio-Thoracic Research Centre, U1045, Bordeaux University, Pessac, France.
François Dérimay, Department of Interventional Cardiology, Cardiovascular Hospital and Claude-Bernard University, INSERM Unit 1060 CARMEN, Lyon, France.
Geraud Souteyrand, Department of Cardiology, CHU Clermont-Ferrand, CNRS, Université Clermont Auvergne, Clermont-Ferrand, France.
Lucie Cassagnes, Department of Radiology, CHU Clermont-Ferrand, CNRS, Université Clermont Auvergne, Clermont-Ferrand, France.
Sara Bouajila, Department of Cardiology, Hôpital Lariboisière, APHP, Paris, France.
Takiy Berrandou, Université de Paris, PARCC, INSERM, Paris, France.
Gregoire Rangé, Service de Cardiologie, Hôpitaux de Chartres, Le Coudray, France.
Nicolas Meneveau, Besancon University Hospital, EA3920, University of Burgundy Franche-Comté, Besancon, France.
Brahim Harbaoui, Service de cardiologie, hôpital Croix-Rousse et hôpital Lyon Sud, hospices civils de Lyon, Université Lyon1, CREATIS UMR5220, Inserm U1044, INSA-15, Lyon, France.
Benoit Lattuca, Department of Cardiology, Nîmes University Hospital, Montpellier University, Nîmes, France.
Pascal Motreff, Department of Cardiology, CHU Clermont-Ferrand, CNRS, Université Clermont Auvergne, Clermont-Ferrand, France.
References
- Mahmoud AN, Taduru SS, Mentias A, Mahtta D, Barakat AF, Saad M, Elgendy AY, Mojadidi MK, Omer M, Abuzaid A, Agarwal N, Elgendy IY, Anderson RD, Saw J. Trends of Incidence, Clinical Presentation, and In-Hospital Mortality Among Women With Acute Myocardial Infarction With or Without Spontaneous Coronary Artery Dissection: A Population-Based Analysis. JACC Cardiovasc Interv. 2018;11:80–90. doi: 10.1016/j.jcin.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, Ganesh SK, Gulati R, Lindsay ME, Mieres JH, Naderi S, Shah S, Thaler DE, Tweet MS, Wood MJ American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; Stroke Council. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement From the American Heart Association. Circulation. 2018;137:e523–57. doi: 10.1161/CIR.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelis KC, Olin JW, Kadian-Dodov D, D’Escamard V, Kovacic JC. Coronary artery manifestations of fibromuscular dysplasia. J Am Coll Cardiol. 2014;64:1033–46. doi: 10.1016/j.jacc.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiando SR, Tucker NR, Castro-Vega LJ, Katz A, D’Escamard V, Tréard C, Fraher D, Albuisson J, Kadian-Dodov D, Ye Z, Austin E, Yang ML, Hunker K, Barlassina C, Cusi D, Galan P, Empana JP, Jouven X, Gimenez-Roqueplo AP, Bruneval P, Hyun Kim ES, Olin JW, Gornik HL, Azizi M, Plouin PF, Ellinor PT, Kullo IJ, Milan DJ, Ganesh SK, Boutouyrie P, Kovacic JC, Jeunemaitre X, Bouatia-Naji N. PHACTR1 Is a Genetic Susceptibility Locus for Fibromuscular Dysplasia Supporting Its Complex Genetic Pattern of Inheritance. PLoS Genet. 2016;12:e1006367. doi: 10.1371/journal.pgen.1006367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlam D, Olson TM, Combaret N, Kovacic JC, Iismaa SE, Al-Hussaini A, O'Byrne MM, Bouajila S, Georges A, Mishra K, Braund PS, d'Escamard V, Huang S, Margaritis M, Nelson CP, de Andrade M, Kadian-Dodov D, Welch CA, Mazurkiewicz S, Jeunemaitre X CARDIoGRAMPlusC4D Study Group. Association of the PHACTR1/EDN1 Genetic Locus With Spontaneous Coronary Artery Dissection. J Am Coll Cardiol. 2019;73:58–66. doi: 10.1016/j.jacc.2018.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley TN, O’Byrne MM, Kosel ML, de Andrade M, Gulati R, Hayes SN, Tweet MS, Olson TM. Identification of Susceptibility Loci for Spontaneous Coronary Artery Dissection. JAMA Cardiol. 2020;5:929–38. doi: 10.1001/jamacardio.2020.0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motreff P, Malcles G, Combaret N, Barber-Chamoux N, Bouajila S, Pereira B, Amonchot A, Citron B, Lusson JR, Eschalier R, Souteyrand G. How and when to suspect spontaneous coronary artery dissection: Novel insights from a single-centre series on prevalence and angiographic appearance. EuroIntervention. 2017;12:e2236–43. doi: 10.4244/EIJ-D-16-00187. [DOI] [PubMed] [Google Scholar]
- Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2014;84:1115–22. doi: 10.1002/ccd.25293. [DOI] [PubMed] [Google Scholar]
- Gornik HL, Persu A, Adlam D, Aparicio LS, Azizi M, Boulanger M, Bruno RM, De Leeuw P, Fendrikova-Mahlay N, Froehlich J, Ganesh SK, Gray BH, Jamison C, Januszewicz A, Jeunemaitre X, Kadian-Dodov D, Kim ESH, Kovacic JC, Mace P, Morganti A, Sharma A, Southerland AM, Touzé E, Van der Niepen P, Wang J, Weinberg I, Wilson S, Olin JW, Plouin PF Working Group ‘Hypertension and the Kidney’ of the European Society of Hypertension (ESH) and the Society for Vascular Medicine (SVM) First international consensus on the diagnosis and management of fibromuscular dysplasia. J Hypertens. 2019;37:229–52. doi: 10.1097/HJH.0000000000002019. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Empana JP, Bean K, Guibout C, Thomas F, Bingham A, Pannier B, Boutouyrie P, Jouven X PPS3 Study Group. Paris Prospective Study III: a study of novel heart rate parameters, baroreflex sensitivity and risk of sudden death. Eur J Epidemiol. 2011;26:887–92. doi: 10.1007/s10654-011-9618-x. [DOI] [PubMed] [Google Scholar]
- Saw J, Starovoytov A, Humphries K, Sheth T, So D, Minhas K, Brass N, Lavoie A, Bishop H, Lavi S, Pearce C, Renner S, Madan M, Welsh RC, Lutchmedial S, Vijayaraghavan R, Aymong E, Har B, Ibrahim R, Gornik HL, Ganesh S, Buller C, Matteau A, Martucci G, Ko D, Mancini GBJ. Canadian spontaneous coronary artery dissection cohort study: in-hospital and 30-day outcomes. Eur Heart J. 2019;40:1188–97. doi: 10.1093/eurheartj/ehz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw J. Pregnancy-Associated Spontaneous Coronary Artery Dissection Represents an Exceptionally High-Risk Spontaneous Coronary Artery Dissection Cohort. Circ Cardiovasc Interv. 2017;10:e005119. doi: 10.1161/CIRCINTERVENTIONS.117.005119. [DOI] [PubMed] [Google Scholar]
- Gad MM, Mahmoud AN, Saad AM, Bazarbashi N, Ahuja KR, Karrthik AK, Elgendy IY, Elgendy AY, Don CW, Hira RS, Massoomi MR, Saw J, Anderson RD, Kapadia SR. Incidence, Clinical Presentation, and Causes of 30-Day Readmission Following Hospitalization With Spontaneous Coronary Artery Dissection. JACC Cardiovasc Interv. 2020;13:921–32. doi: 10.1016/j.jcin.2019.12.033. [DOI] [PubMed] [Google Scholar]
- Adlam D, Alfonso F, Maas A, Vrints C Writing Committee. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J. 2018;39:3353–68. doi: 10.1093/eurheartj/ehy080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan S, Prakash R, Starovoytov A, Saw J. Natural History of Spontaneous Coronary Artery Dissection with Spontaneous Angiographic Healing. JACC Cardiovasc Interv. 2019;12:518–27. doi: 10.1016/j.jcin.2018.12.011. [DOI] [PubMed] [Google Scholar]
- Tweet MS, Eleid MF, Best PJM, Lennon RJ, Lerman A, Rihal CS, Holmes DR Jr, Hayes SN, Gulati R. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7:777–86. doi: 10.1161/CIRCINTERVENTIONS.114.001659. [DOI] [PubMed] [Google Scholar]
- Prasad M, Tweet MS, Hayes SN, Leng S, Liang JJ, Eleid MF, Gulati R, Vrtiska TJ. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol. 2015;115:1672–7. doi: 10.1016/j.amjcard.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Henkin S, Negrotto SM, Tweet MS, Kirmani S, Deyle DR, Gulati R, Olson TM, Hayes SN. Spontaneous coronary artery dissection and its association with heritable connective tissue disorders. Heart. 2016;102:876–81. doi: 10.1136/heartjnl-2015-308645. [DOI] [PubMed] [Google Scholar]
- Plouin PF, Baguet JP, Thony F, Ormezzano O, Azarine A, Silhol F, Oppenheim C, Bouhanick B, Boyer L, Persu A, Hammer F, Gosse P, Mounier-Véhier C, Le Hello C, Jeunemaitre X, Azizi M, Amar L, Chatellier G, Mousseaux E, Touzé E ARCADIA Investigators. High Prevalence of Multiple Arterial Bed Lesions in Patients With Fibromuscular Dysplasia: The ARCADIA Registry (Assessment of Renal and Cervical Artery Dysplasia). Hypertension. 2017;70:652–8. doi: 10.1161/HYPERTENSIONAHA.117.09539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of all investigators of the DISCO registry.
Presenting characteristics.
Follow-up and MACE rate in Conservative group versus PCI group.
Association analysis between rs9349379 (PHACTR1) and SCAD stratified according to the presence of fibromuscular dysplasia in the DISCO study.