Abstract
Aims
Angina and no obstructive coronary artery disease (ANOCA) is common. A potential cause of angina in this patient population is a myocardial bridge (MB). We aimed to study the anatomical and haemodynamic characteristics of an MB in patients with ANOCA.
Methods and results
Using intravascular ultrasound (IVUS), we identified 184 MBs in 154 patients. We evaluated MB length, arterial compression, and halo thickness. MB muscle index (MMI) was defined as MB length×halo thickness. Haemodynamic testing of the MB was performed using an intracoronary pressure/Doppler flow wire at rest and during dobutamine stress. We defined an abnormal diastolic fractional flow reserve (dFFR) as ≤0.76 during stress. The median MB length was 22.9 mm, arterial compression 30.9%, and halo thickness 0.5 mm. The median MMI was 12.1. Endothelial and microvascular dysfunction were present in 85.4% and 22.1%, respectively. At peak dobutamine stress, 94.2% of patients had a dFFR ≤0.76 within and/or distal to the MB. MMI was associated with an abnormal dFFR.
Conclusions
In select patients with ANOCA who have an MB by IVUS, the majority have evidence of a haemodynamically significant dFFR during dobutamine stress, suggesting the MB as being a cause of their angina. A comprehensive invasive assessment of such patients during coronary angiography provides important diagnostic information that can guide management.
Introduction
Patients with angina and no obstructive coronary artery disease (ANOCA) are common in clinical practice 1, but remain a challenge with regard to diagnosis and treatment. When these patients undergo a comprehensive evaluation at the time of invasive coronary angiography (ICA), occult coronary abnormalities, including endothelial dysfunction, microvascular dysfunction, and/or a myocardial bridge (MB), are frequently found 2 3. The overall prevalence of an MB varies greatly, depending on the cohort studied and the type of imaging test performed 4. Studies utilising coronary computed tomography angiography (CCTA) have reported a prevalence of ~30% in the general population 5. Based on our previous work, the prevalence seen in patients with ANOCA is nearly double 2 6 7, raising the suspicion that MBs are a relatively common cause of angina in this patient subset.
An MB is an anatomical variant in which the coronary artery is covered by myocardium for a varying degree of length, depth, and location. MBs are well known to be missed on ICA, with only ~5% showing the classic milking effect 8. Instead, intravascular ultrasound (IVUS) identifies MBs with greater accuracy and is considered the invasive gold standard for detection of an MB 9. Traditionally, MBs were thought to be universally benign because the coronary artery fills during diastole, while compression from the bridge occurs in systole. However, early studies using IVUS and Doppler flow demonstrated that arterial compression can persist into early diastole, thus affecting flow 10. Further advances demonstrated that dobutamine rather than adenosine is most likely to evoke the dynamic compression of an MB, and that diastolic fractional flow reserve (dFFR), rather than FFR, is the preferred test for haemodynamic significance 11 12.
Here, we investigated the anatomical and functional characteristics of MBs in patients with ANOCA. Our aim was to demonstrate the application of a contemporary and comprehensive invasive evaluation in determining the extent to which haemodynamically significant MBs might contribute to angina in this challenging patient subset.
Methods
STUDY POPULATION
We prospectively enrolled patients between August 2011 and October 2018 who had persistent (>3 months) typical/atypical angina 13 and a suspected MB based on CCTA and/or the presence of focal septal buckling with apical sparing on stress echocardiography (Figure 1) 7. The CCTA and/or stress echocardiogram was ordered at the discretion of the treating physician. Patients with ongoing anginal symptoms despite maximally tolerated medical management who were referred for invasive testing were considered for inclusion in the study. Symptoms were quantified by the Seattle Angina Questionnaire. Patients underwent ICA to rule out obstructive coronary artery disease (CAD), IVUS to confirm the presence of an MB and define its anatomical characteristics, and dobutamine stress testing with intracoronary pressure and flow measurements to assess the haemodynamic significance of the MB, if present. A subset of these patients also underwent endothelial and microvascular testing (Supplementary Figure 1). The study was approved by the Stanford Institutional Review Board, and complied with the Declaration of Helsinki. Written informed consent was obtained from each patient.
Figure 1.
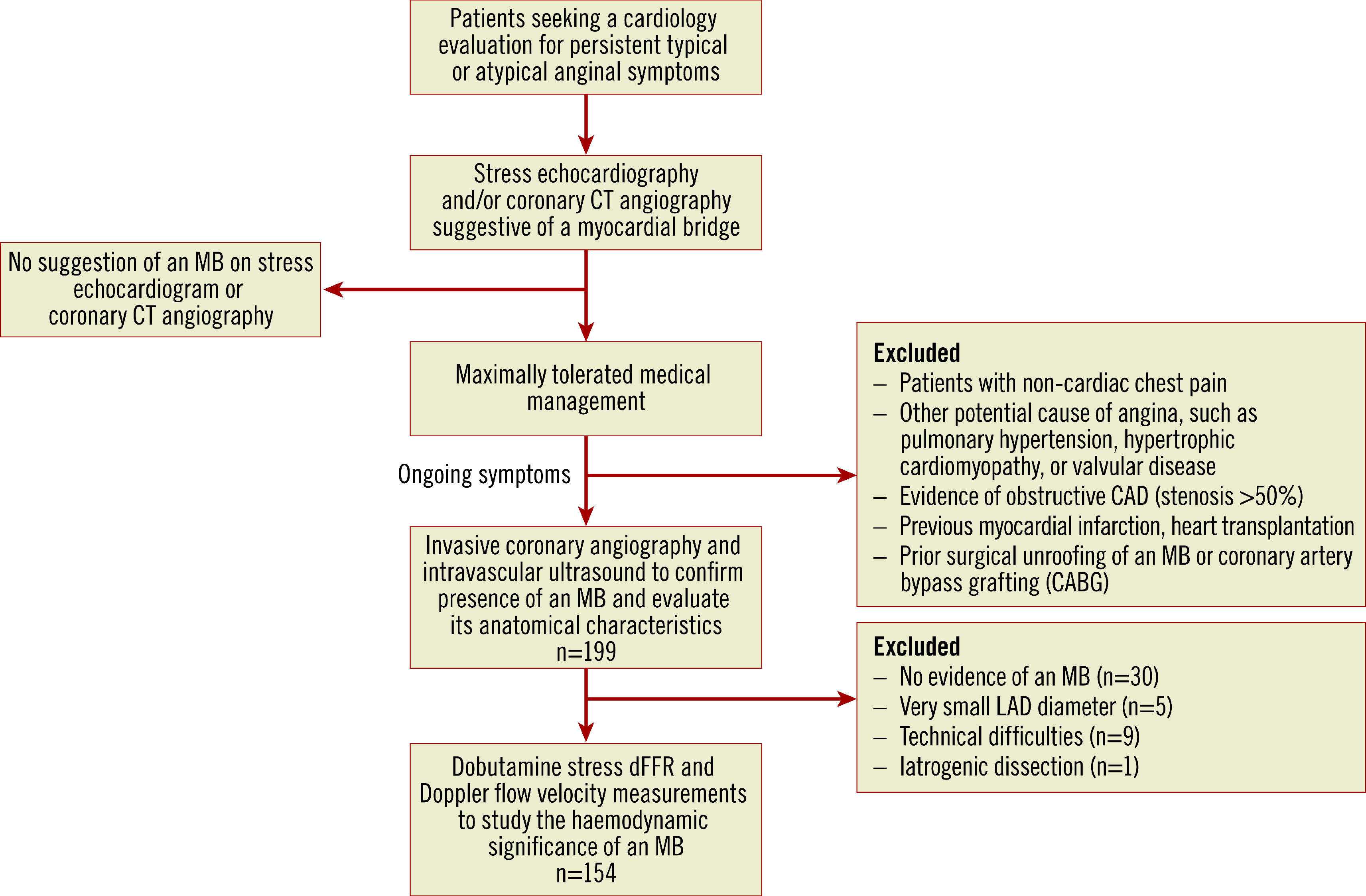
Patient selection criteria and evaluation performed. dFFR: diastolic fractional flow reserve; MB: myocardial bridge
INVASIVE CORONARY ANGIOGRAPHY
Oral negative chronotropic agents and vasodilators were discontinued two days prior to the procedure. ICA was performed in multiple standard views using a 6 Fr guiding catheter from the femoral approach.
INTRAVASCULAR ULTRASOUND
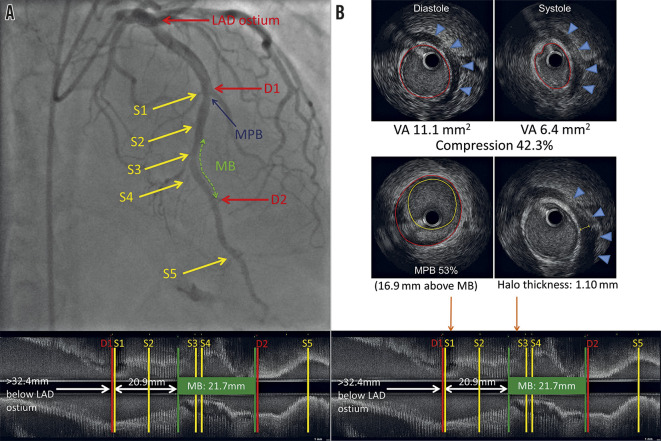
IVUS was performed using a 40-MHz mechanical IVUS catheter (Atlantis SR Pro2™; Boston Scientific, Marlborough, MA, USA), placed as far distally in the LAD as safely possible. Recordings were obtained during an automated pullback at 0.5 mm/sec; resting IVUS was also perfomed within the MB to obtain the maximal arterial compression at a single location (Figure 2A). Qualitative and quantitative evaluations were performed with echoPlaque (INDEC Systems, Santa Clara, CA, USA) by the Stanford Cardiovascular Core Analysis Laboratory. An MB was identified by the presence of an echolucent half-moon sign (halo) and/or arterial compression >10% (Figure 2B) 10.The methodology of the IVUS measurements is detailed in Supplementary Appendix 1 14.
Figure 2.
Left anterior descending artery with an MB on coronary angiography and corresponding longitudinal IVUS. A) Left anterior descending artery with an MB on coronary angiography with longitudinal IVUS. The green dotted line represents the MB. S1-S5 are septal perforators. D1-D2 are diagonal branches. The blue arrow points to the MPB (53%), located 16.9 mm proximal to the entrance of the MB. B) Arterial compression, MPB and halo thickness on IVUS. Upper panel: end-diastolic and end-systolic IVUS with 42.3% arterial compression. Middle panel: MPB of 53% and maximal MB halo thickness of 1.10 mm. Orange arrows point to the location of the MPB and maximum halo thickness on IVUS (lower panel). IVUS: intravascular ultrasound; MB: myocardial bridge; MPB: maximum plaque burden; VA: vessel area
INVASIVE HAEMODYNAMIC ASSESSMENT
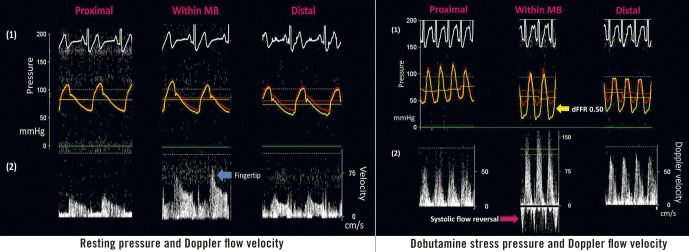
Haemodynamic measurements were made with the ComboWire® XT Pressure and Flow Wire (Volcano Corp., San Diego, CA, USA). Pressure and Doppler flow velocity waveforms were measured proximal, within, and distal to the MB at rest and at peak dobutamine stress (Figure 3). Dobutamine was given intravenously (IV) in increments of 10-20 µg/kg/min every three minutes until at least 85% of the maximal heart rate for age was achieved or a maximal dose of 50 µg/kg/min with up to 1.0 mg atropine was administered. ComboWire recordings were analysed offline on the ComboMap console. Doppler flow velocities were measured at peak flow in diastole. Using a digital calliper, Pd/Pa (coronary artery pressure/aortic pressure) was measured at rest and at peak stress in diastole 3-5 times/beat, and averaged using 2-3 beats, to get an average dFFR at rest and at peak stress. A dFFR ≤0.76 was considered haemodynamically significant 11.
Figure 3.
Pressure and Doppler flow velocity at rest and at dobutamine stress. At rest, there was no significant difference in the diastolic pressures between the aorta (red tracing) and the coronary artery (yellow tracing). At peak stress, there was no significant difference in the pressures proximally, while there was a significant difference in the diastolic pressures within the MB, and a moderate difference distally.
ENDOTHELIAL FUNCTION AND CORONARY PHYSIOLOGY ASSESSMENT
A subset of the patients underwent endothelial (n=89) and microvascular (n=88) function testing, as previously described 2. Endothelial dysfunction was diagnosed if the minimal lumen diameter of an epicardial coronary artery decreased by >20% from baseline 15. Microvascular dysfunction was defined as an index of microcirculatory resistance (IMR) ≥25 16. We also measured coronary flow reserve and adenosine FFR.
STATISTICAL ANALYSIS
Normality of the data was determined using the histogram plots. Results are expressed as median (minimum-maximum value). Chi-square tests were used to assess differences between categorical variables. Mann-Whitney U tests were used to assess differences between continuous variables. Variables in Supplementary Table 1 and MB anatomical characteristics were tested for their association with a dFFR ≤0.76 in univariable logistic regression analyses. Variables with p<0.2 were included in the multivariable forward stepwise logistic regression analysis. Less significant univariables correlating significantly with other variables in the model were removed to avoid multicollinearity. A p-value <0.05 was considered statistically significant. Statistical analyses were performed using NCSS 11 (NCSS, Kaysville, UT, USA).
Results
CLINICAL CHARACTERISTICS
Supplementary Table 1 shows the clinical characteristics by sex. Typical and atypical angina was present in 119 (77.3%) and 35 (22.7%) patients, respectively. Cardiac risk factors were less prevalent than in a traditional CAD population, although patients were taking several cardiac medications for angina. Prior to invasive testing, 115 patients underwent exercise/dobutamine stress echocardiography, of whom 76 (66.1%) had reproducible chest pain/dyspnoea with stress. No traditional wall motion abnormalities were detected at rest or at stress. Focal septal buckling with apical sparing was present in 100 (86.9%) patients, and 31 (26.5%) had ST-segment depression ≥1 mm with stress. CCTA was performed in 97 patients, demonstrating an MB in 94 (96.9%).
IVUS FINDINGS
We identified 184 MBs in 154 patients, with 28 (18.2%) patients having a second distal MB. One patient had three MBs. A halo was present in 151 (98%) patients. The remaining three had arterial compression ≥10%. Multiple sex differences in MB anatomical characteristics were identified (Table 1). All patients had an atherosclerotic plaque proximal to the MB that was typically not appreciable by ICA. A higher arterial compression was associated with a greater maximum plaque burden (MPB) (Spearman rank correlation=0.18; 95% CI: 0.05-0.36; p=0.03).
Table 1. Anatomical characteristics of the MBs by sex, assessed using IVUS.
| Characteristics | Total population (n=154) | Women (n=101) | Men (n=53) | p-value |
| Halo thickness, mm | 0.50 (0.14-2.32) | 0.48 (0.17-2.2) | 0.58 (0.14-2.32) | 0.001 |
| MB length, mm | 22.9 (3.0-68.9) | 20.3 (3.0-59.7) | 25.8 (6.57-68.9) | 0.002 |
| MB muscle index | 12.1 (0.92-72.2) | 10.2 (1.6-56.9) | 16.7 (0.92-72.2) | <0.001 |
| Arterial compression, % | 30.9 (8.0-58.6) | 28.6 (8-58.6) | 36.7 (16.7-57) | <0.001 |
| Number of septal branches within the MB | 2 (0-7) | 2 (0-7) | 3 (1-7) | 0.008 |
| Proximal MPB, % | 34 (10-72) | 26.9 (13.5-56.5) | 44 (10-72) | <0.001 |
| Distance of MPB from the entrance of the MB, mm | 21.9 (3.4-57.5) | 22.7 (3.4-57.5) | 20.9 (7.1-55.2) | 0.79 |
| MB: myocardial bridge; MPB: maximum plaque burden | ||||
HAEMODYNAMIC FINDINGS
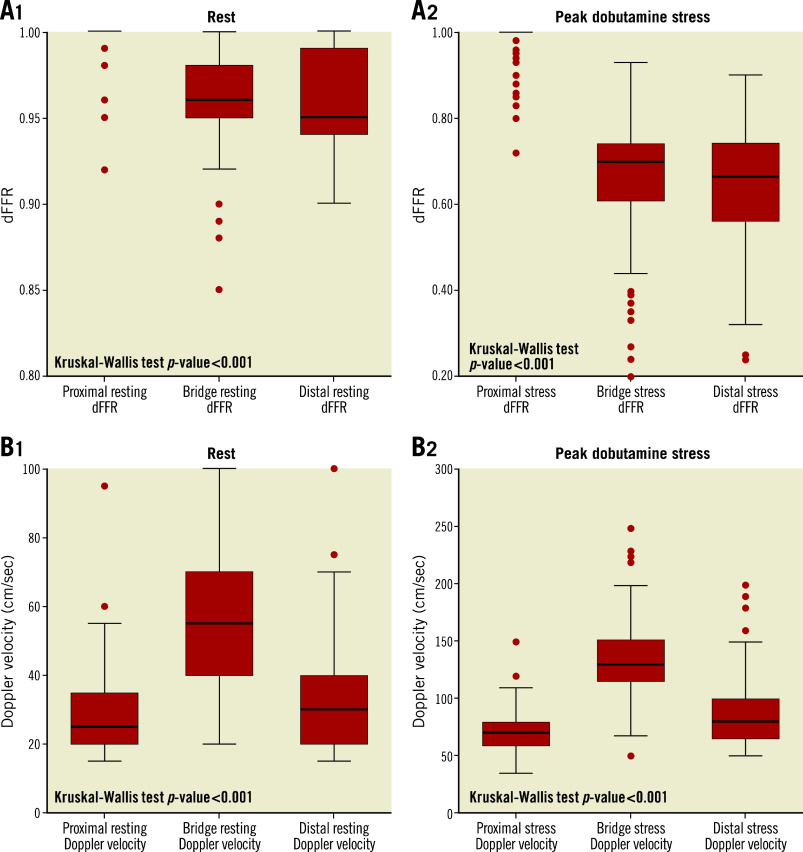
At peak dobutamine stress, 142 (92.2%) patients reported angina and 145 (94.2%) had a dFFR ≤0.76. At peak stress, dFFR did not change proximal to the MB, but decreased significantly within and distal to the MB, with 131 (85.1%) having a dFFR ≤0.76 within the MB and 97 (62.9%) having a dFFR ≤0.76 distally ( Table 2, Figure 4A). At rest, peak Doppler flow velocity was significantly higher within the MB than proximal and distal to the MB, and the characteristic “fingertip” Doppler flow pattern was observed in 136 (88.3%) patients. At stress, Doppler flow velocity increased significantly in all segments (Figure 4B), with the greatest increase within the MB. Systolic flow reversal within the MB at peak stress was seen in 108 (74.5%) patients (Figure 3). Men had significantly lower stress dFFR than women (0.63 [0.20-0.86] vs 0.70 [0.24-0.90]; p=0.02). This sex difference was not seen in Doppler flow velocity.
Table 2. dFFR and Doppler flow velocity measurements proximal, within, and distal to the MB at rest and at peak dobutamine stress.
| Rest | Stress | p-value | |
| Proximal to the MB | |||
| dFFR | 1.00 (0.92-1.00) | 1.00 (0.72-1.00) | 0.85 |
| Doppler flow velocity | 25 (15-95) | 70 (35-180) | <0.001 |
| Within the MB | |||
| dFFR | 0.96 (0.85-1.00) | 0.70 (0.20-0.85) | <0.001 |
| Doppler flow velocity | 55 (20-100) | 130 (50-250) | <0.001 |
| Distal to the MB | |||
| dFFR | 0.95 (0.80-1.00) | 0.68 (0.24-0.90) | <0.001 |
| Doppler flow velocity | 30 (15-75) | 80 (50-200) | <0.001 |
| dFFR: diastolic fractional flow reserve; MB: myocardial bridge | |||
Figure 4.
Box plot representing dFFR and Doppler flow velocity at rest and peak dobutamine stress. A) Box plots representing dFFR at rest (1) and at peak dobutamine stress (2) in all three segments. None of the patients had a dFFR ≤0.76 at rest. B) Box plots representing Doppler flow velocity at rest (1) and at peak dobutamine stress (2).
FACTORS ASSOCIATED WITH dFFR ≤0.76
Univariable correlates of a dFFR ≤0.76 (Supplementary Table 2) were included in the multivariable regression analysis. Since MB muscle index (MMI) and MB length are highly correlated (Spearman rank correlation=0.87, p<0.001), only MMI was included in the analysis. On multivariable analysis, MMI (OR 2.89 [1.05-4.99], p=0.039) and high-density lipoprotein (HDL) (0.86 [0.75-0.98], p=0.024) were associated with a dFFR ≤0.76.
MB MUSCLE INDEX AND dFFR
A receiver operating characteristic curve was plotted to establish a value of MMI that identifies a dFFR ≤0.76. MMI by IVUS was highly predictive of a dFFR ≤0.76 (area under the curve=0.90; 95% CI: 0.83-0.94; p<0.001) (Supplementary Figure 2). An MMI ≥5.05 by IVUS identified a dFFR ≤0.76 with a sensitivity of 86.0% (95% CI: 79.2-91.2%) and a specificity of 88.9% (95% CI: 51.7-99.7%). All patients with an MMI >12.2 had a dFFR ≤0.76.
ENDOTHELIAL AND MICROVASCULAR DYSFUNCTION
Supplementary Table 3 shows the details of endothelial and microvascular dysfunction testing. Endothelial dysfunction was present within the MB in 76 (85.4%) patients (Supplementary Figure 3) and 19 (22.9%) had microvascular dysfunction.
ADENOSINE FFR
The median FFR was 0.85 (0.62-0.95). FFR ≤0.8 was present in 21 (24.4%) patients. Patients with FFR ≤0.8 had a significantly higher MPB proximal to the MB (Supplementary Table 4).
Discussion
We present the most comprehensive invasive anatomical and haemodynamic assessment of MBs as a potential cause of ANOCA. The salient findings of our study are the following. 1) Among patients with ANOCA and evidence of an MB, the MB is often haemodynamically significant. 2) Anatomical characteristics of an MB by IVUS, particularly MMI, can be used to identify the haemodynamic significance of an MB. 3) An atherosclerotic plaque, which is generally not appreciated on ICA, appears to be universally present proximal to an MB. 4) There are several sex differences in the anatomical characteristics of MBs by IVUS, which translate to sex differences with haemodynamic significance. 5) Other occult coronary abnormalities, especially endothelial dysfunction, are frequently present in patients with an MB.
ANOCA is a common, but poorly understood condition that can be challenging for patients and providers, and puts an economic strain on the healthcare system 17. Most diagnostic tests are designed to rule out obstructive CAD and are insufficient in this patient population; therapies are available, but understudied 18 19. A further discussion of stress testing and CCTA in this patient population can be found in Supplementary Appendix 2 6 7 18 20.
Still, the evidence that occult coronary abnormalities, including endothelial and microvascular dysfunction, are a common finding in these patients has been mounting for years 2, and diagnostic testing has continued to evolve 11 16. Using routine IVUS, we have shown that 58% of patients with ANOCA have an MB, which is higher than expected in the general population 2. However, the presence of an MB does not mean that it is causing symptoms, making it important to distinguish MBs that are haemodynamically significant from those that are simply incidental. In the current study, we demonstrated that, in a select group of patients with an MB and persistent angina despite maximally tolerated medical therapy, 94.2% of MBs are haemodynamically significant. This has implications for guiding therapy (Supplementary Appendix 3) 21 22 23.
Our study confirms previous findings that adenosine FFR is insufficient for determining whether or not an MB is haemodynamically significant. In patients who underwent both mean adenosine FFR and dobutamine dFFR, 91.2% had a dFFR ≤0.76, while only 24.4% had an FFR ≤0.80, and only 15.1% had an FFR ≤0.76. This discrepancy is based on the fact that an MB is a dynamic stenosis rather than a fixed stenosis, primarily affecting diastole rather than both systole and diastole (Supplementary Appendix 4) 11 12. In addition, while dobutamine has been shown to be equivalent to adenosine for a fixed stenosis, the use of dobutamine rather than adenosine has been shown to be important in evaluating an MB 12 24. Still, in our study, nearly a quarter of patients had an abnormal mean adenosine FFR, suggesting that MBs are often narrowed enough to act as a fixed stenosis. This may be due to the inability of the bridged segment ever to recover fully during diastole and/or the smaller size of the bridged segment due to negative remodelling 25. In addition, progression of MPB proximal to the MB may contribute to flow limitations within the artery. Instantaneous wave-free ratio (iFR), a diastole-only index, has also been shown to be superior to FFR in MB haemodynamic assessment 26, although validation of iFR against stress dFFR for MB assessment is needed.
We again showed that patients are more likely to have a significant dFFR within, rather than distal to, an MB 27. Consistent with this, Doppler flow velocity increases significantly within the MB compared with distally, and the higher the Doppler flow velocity, the lower the dFFR within the MB at stress. Our data highlight the importance of measuring dFFR within the MB, as well as distal to it. They also reassure operators who are unable to pass a pressure wire distal to the MB that a measurement within the MB is sufficient over 90% of the time.
Because MBs are often underappreciated on ICA 9, intravascular imaging is important, not only for identification or confirmation of an MB, but also for anatomical characteristics that might suggest haemodynamic significance. Specifically, we have demonstrated that MMI by IVUS is associated with a dFFR ≤0.76. It is presumed that longer MBs with greater halo thickness exert more compressive effects, thus leading to an increased flow velocity and reduced perfusion pressure within the MB.
It has been shown that the vessel within an MB is generally free of any atherosclerotic plaque, while the vessel proximal to an MB is at increased risk for atherosclerosis 28. Our study adds to this by demonstrating that an atherosclerotic plaque is universally found proximal to an MB in patients with ANOCA. The high detection of plaque in our study is probably due to the use of intravascular imaging, as we have previously shown that these plaques are commonly missed on CCTA 6. This development of proximal plaque is thought to be due to flow disturbances, specifically dynamic retrograde flow, created by compression of the bridged artery 10 14 29. This is supported by our finding of a direct correlation between arterial compression and MPB proximal to the MB. This identification of subclinical atherosclerosis in patients with angina and an MB has implications for prevention, specifically raising the question of whether a statin and aspirin should be considered in such patients. It has previously been shown that the plaque proximal to an MB may increase the risk of a myocardial infarction 14.
More women than men have ANOCA, but it has been suggested that MBs are more common in men than in women 30. These data are notably older and based on findings during ICA, which may have confounded the results. Not only were men more likely to undergo ICA, but we show here that MBs in men are longer and deeper, and have more arterial compression, which probably makes them more angiographically apparent. In our study, more women than men had an MB, but our study is comprised of more women. Therefore, we can draw no conclusions with regard to the prevalence of MBs, either in the general population or between women and men. However, it is notable that, among women and men presenting with ANOCA, there are more severe anatomical characteristics among men, which may translate to a sex difference in haemodynamic significance.
Finally, it is important to note that other occult coronary abnormalities are often seen in patients with an MB, especially endothelial dysfunction. We found that 87.3% of patients had endothelial dysfunction within their MB, similar to a previous study 31. Microvascular dysfunction may also be present and, as expected, any of these occult coronary abnormalities may occur in isolation or in various combinations 2. As such, a comprehensive invasive assessment during ICA, which includes endothelial and microvascular function testing, IVUS for detection of an MB and subclinical atherosclerosis, and haemodynamic testing of the MB, if present, is necessary for the full evaluation of patients with ANOCA. This thorough diagnostic evaluation can guide therapy, and may ultimately result in an improvement in patients’ symptoms and quality of life 32.
Limitations
This is a relatively small, single-centre study. Patients had a high suspicion of an MB based on non-invasive testing and also had persistent angina despite medical therapy, so these results would not be applicable to all patients with an MB, nor would we advocate invasive testing in all patients. All investigations were performed in the LAD, potentially limiting the applicability of the findings in other vessels, although MBs mostly (70-98%) involve the LAD. A dFFR ≤0.76 was used for haemodynamic significance of an MB based on a previous study 11, but this cut-off value has not been validated, and whether it represents haemodynamic significance of an MB is unknown. Our study lacked a control group comprised of asymptomatic patients with an MB. However, we could not ethically justify invasive testing with its inherent risks in asymptomatic individuals. We did not include outcome data here as it will be reported in a separate study.
Conclusions
Haemodynamically significant myocardial bridging is an important diagnosis among patients with ANOCA. For those with persistent angina despite medical therapy, a comprehensive invasive assessment, including MB testing with IVUS and dobutamine dFFR, can provide diagnostic information that is expected to guide therapy and improve clinical outcomes.
Impact on daily practice
A myocardial bridge (MB), while often overlooked, is a potential cause of angina in patients with angina and no obstructive CAD. Most MBs are not appreciated by invasive coronary angiography, but can be identified with stress echocardiography (focal septal buckling with apical sparing), CCTA, and/or intravascular ultrasound (IVUS). A comprehensive invasive assessment, including MB testing with IVUS and dobutamine diastolic fractional flow reserve (dFFR), can provide important diagnostic information that may guide therapy and improve patients’ symptoms and quality of life.
Supplementary data
Detailed methodology of IVUS measurements.
Accuracy of non-invasive tests for identifying an MB.
Therapy for a symptomatic myocardial bridge.
Discrepancy between adenosine FFR and dobutamine dFFR with myocardial bridging.
Comprehensive invasive assessment during ICA to identify occult coronary abnormalities as a cause of ANOCA.
ROC to calculate the value of MB muscle index that predicts the presence of a dFFR ≤0.76.
Demonstrative example of endothelial dysfunction within an MB shown on a still frame of a coronary angiogram.
Clinical characteristics of patients with an MB who underwent invasive assessment.
Univariable logistic regression analysis to identify variables associated with any dFFR ≤0.76.
Endothelial function testing and coronary physiology measurements in a subset of patients.
Anatomical characteristics of MB in patients with FFR ≤0.8 versus those with FFR >0.8.
Acknowledgments
Acknowledgements
This work was supported in part by a generous gift from the Ron and Sanne Higgins Women's Heart Health Fund.
Conflict of interest statement
V.S. Pargaonkar reports other from Gilead Sciences Inc., outside the submitted work. J.A. Tremmel reports personal fees from Boston Scientific, Abbott, and Philips, outside the submitted work. The other authors have no conflicts of interest to declare.
Abbreviations
- Ach
acetylcholine
- ANOCA
angina and no obstructive coronary artery disease
- CCTA
coronary computed tomography angiography
- dFFR
diastolic fractional flow reserve
- ICA
invasive coronary angiography
- IMR
index of microcirculatory resistance
- IVUS
intravascular ultrasound
- LAD
left anterior descending artery
- MB
myocardial bridge
- MMI
myocardial bridge muscle index
- MPB
maximum plaque burden
Contributor Information
Vedant Pargaonkar, Division of Cardiovascular Medicine, Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA.
Takumi Kimura, Division of Cardiovascular Medicine, Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA.
Ryo Kameda, Division of Cardiovascular Medicine, Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA.
Shigemitsu Tanaka, Division of Cardiovascular Medicine, Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA.
Ryotaro Yamada, Division of Cardiovascular Medicine, Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA.
Jonathan Schwartz, Division of Cardiovascular Medicine, Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA.
Leor Perl, Division of Cardiovascular Medicine, Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA.
Ian Rogers, Division of Cardiovascular Medicine, Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA.
Yasuhiro Honda, Division of Cardiovascular Medicine, Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA.
Peter Fitzgerald, Division of Cardiovascular Medicine, Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA.
Ingela Schnittger, Division of Cardiovascular Medicine, Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA.
Jennifer Tremmel, Division of Cardiovascular Medicine, Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA.
References
- Ouellette ML, Löffler AI, Beller GA, Workman VK, Holland E, Bourque JM. Clinical Characteristics, Sex Differences, and Outcomes in Patients With Normal or Near-Normal Coronary Arteries, Non-Obstructive or Obstructive Coronary Artery Disease. J Am Heart Assoc. 2020;7:e007965. doi: 10.1161/JAHA.117.007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, Lim HS, Fearon WF, Yong AS, Yamada R, Tanaka S, Lee DP, Yeung AC, Tremmel JA. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–1060. doi: 10.1161/CIRCULATIONAHA.114.012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ WISE Investigators. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–41. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- Tarantini G, Migliore F, Cademartiri F, Fraccaro C, Iliceto S. Left Anterior Descending Artery Myocardial Bridging: A Clinical Approach. J Am Coll Cardiol. 2016;68:2887–99. doi: 10.1016/j.jacc.2016.09.973. [DOI] [PubMed] [Google Scholar]
- La Grutta L, Runza G, Lo Re G, Galia M, Alaimo V, Grassedonio E, Bartolotta TV, Malago R, Tedeschi C, Cademartiri F, De Maria M, Cardinale AE, Lagalla R, Midiri M. Prevalence of myocardial bridging and correlation with coronary atherosclerosis studied with 64-slice CT coronary angiography. Radiol Med. 2009;114:1024–36. doi: 10.1007/s11547-009-0446-y. [DOI] [PubMed] [Google Scholar]
- Forsdahl SH, Rogers IS, Schnittger I, Tanaka S, Kimura T, Pargaonkar VS, Chan FP, Fleischmann D, Tremmel JA, Becker HC. Myocardial Bridges on Coronary Computed Tomography Angiography - Correlation With Intravascular Ultrasound and Fractional Flow Reserve. Circ J. 2017;81:1894–900. doi: 10.1253/circj.CJ-17-0284. [DOI] [PubMed] [Google Scholar]
- Pargaonkar VS, Rogers IS, Su J, Forsdahl SH, Kameda R, Schreiber D, Chan FP, Becker HC, Fleischmann D, Tremmel JA, Schnittger I. Accuracy of a novel stress echocardiography pattern for myocardial bridging in patients with angina and no obstructive coronary artery disease - A retrospective and prospective cohort study. Int J Cardiol. 2020;311:107–13. doi: 10.1016/j.ijcard.2020.02.006. [DOI] [PubMed] [Google Scholar]
- Soran O, Pamir G, Erol C, Kocakavak C, Sabah I. The incidence and significance of myocardial bridge in a prospectively defined population of patients undergoing coronary angiography for chest pain. Tokai J Exp Clin Med. 2000;25:57–60. [PubMed] [Google Scholar]
- Tsujita K, Dani L, Moses JW, Stone GW, Collins MB, Kreps EM, Lansky AJ, Kirtane AJ, Mehran R, Dangas GD, Biro S, Maehara A, Dasgupta N, Franklin-Bond T, Oviedo C, Yang J, Liu J, Castellanos C, Kubo T, Doi H, Mintz GS, Leon MB. Comparison of angiographic and intravascular ultrasonic detection of myocardial bridging of the left anterior descending coronary artery. Am J Cardiol. 2008;102:1608–13. doi: 10.1016/j.amjcard.2008.07.054. [DOI] [PubMed] [Google Scholar]
- Ge J, Jeremias A, Rupp A, Abels M, Baumgart D, Liu F, Haude M, Görge G, von Birgelen C, Sack S, Erbel R. New signs characteristic of myocardial bridging demonstrated by intracoronary ultrasound and Doppler. Eur Heart J. 1999;20:1707–16. doi: 10.1053/euhj.1999.1661. [DOI] [PubMed] [Google Scholar]
- Escaned J, Cortés J, Flores A, Goicolea J, Alfonso F, Hernandez R, Fernández-Ortiz A, Sabate M, Banuelos C, Macaya C. Importance of diastolic fractional flow reserve and dobutamine challenge in physiologic assessment of myocardial bridging. J Am Coll Cardiol. 2003;42:226–33. doi: 10.1016/S0735-1097(03)00588-6. [DOI] [PubMed] [Google Scholar]
- Hakeem A, Cilingiroglu M, Leesar MA. Hemodynamic and intravascular ultrasound assessment of myocardial bridging: fractional flow reserve paradox with dobutamine versus adenosine. Catheter Cardiovasc Interv. 2010;75:229–36. doi: 10.1002/ccd.22237. [DOI] [PubMed] [Google Scholar]
- Diamond GA. A clinically relevant classification of chest discomfort. J Am Coll Cardiol. 1983;1:574–5. doi: 10.1016/S0735-1097(83)80093-X. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Takimoto T, Ito K, Nishimura C, Shimada K, Shimokawa R, Yanagida-Iida M, Yamada K, Satoh H, Taki K, Shinagawa T, Komatsu A, Sugiura H, Akasaka Y, Kawahara Y, Ebihara Y, Morinaga S, Ogata K, Tanaka M, Niino H, Yamazaki K, Ogawa T, Fujiwara M, Suzuki K, Ishii T. Anatomic properties of myocardial bridge predisposing to myocardial infarction. Circulation. 2009;120:376–83. doi: 10.1161/CIRCULATIONAHA.108.820720. [DOI] [PubMed] [Google Scholar]
- Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.CIR.101.9.948. [DOI] [PubMed] [Google Scholar]
- Ng MKC, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006;113:2054–61. doi: 10.1161/CIRCULATIONAHA.105.603522. [DOI] [PubMed] [Google Scholar]
- Shaw LJ, Merz CN, Pepine CJ, Reis SE, Bittner V, Kip KE, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Sopko G Women’s Ischemia Syndrome Evaluation (WISE) Investigators. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health--National Heart, Lung, and Blood Institute--sponsored Women’s Ischemia Syndrome Evaluation. Circulation. 2006;114:894–904. doi: 10.1161/CIRCULATIONAHA.105.609990. [DOI] [PubMed] [Google Scholar]
- Pargaonkar VS, Kobayashi Y, Kimura T, Schnittger I, Chow EKH, Froelicher VF, Rogers IS, Lee DP, Fearon WF, Yeung AC, Stefanick ML, Tremmel JA. Accuracy of non-invasive stress testing in women and men with angina in the absence of obstructive coronary artery disease. Int J Cardiol. 2019;282:7–15. doi: 10.1016/j.ijcard.2018.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, Kaski JC, Bairey Merz CN Coronary Vasomotion Disorders International Study Group (COVADIS) International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. doi: 10.1016/j.ijcard.2017.08.068. [DOI] [PubMed] [Google Scholar]
- Jubran A, Schnittger I, Tremmel J, Pargaonkar V, Rogers I, Becker HC, Yang S, Mastrodicasa D, Willemink M, Fleischmann D, Nieman K. Computed Tomographic Angiography-Based Fractional Flow Reserve Compared with Catheter-Based Dobutamine-Stress Diastolic Fractional Flow Reserve in Symptomatic Patients with a Myocardial Bridge and No Obstructive Coronary Artery Disease. Circ Cardiovasc Imaging. 2020;13:e009576. doi: 10.1161/CIRCIMAGING.119.009576. [DOI] [PubMed] [Google Scholar]
- Schwarz ER, Klues HG, vom Dahl J, Klein I, Krebs W, Hanrath P. Functional, angiographic and intracoronary Doppler flow characteristics in symptomatic patients with myocardial bridging: effect of short-term intravenous beta-blocker medication. J Am Coll Cardiol. 1996;27:1637–45. doi: 10.1016/0735-1097(96)00062-9. [DOI] [PubMed] [Google Scholar]
- Bourassa MG, Butnaru A, Lesperance J, Tardif JC. Symptomatic myocardial bridges: overview of ischemic mechanisms and current diagnostic and treatment strategies. J Am Coll Cardiol. 2003;41:351–9. doi: 10.1016/S0735-1097(02)02768-7. [DOI] [PubMed] [Google Scholar]
- Boyd JH, Pargaonkar VS, Scoville DH, Rogers IS, Kimura T, Tanaka S, Yamada R, Fischbein MP, Tremmel JA, Mitchell RS, Schnittger I. Surgical Unroofing of Hemodynamically Significant Left Anterior Descending Myocardial Bridges. Ann Thorac Surg. 2017;103:1443–50. doi: 10.1016/j.athoracsur.2016.08.035. [DOI] [PubMed] [Google Scholar]
- Bartunek J, Wijns W, Heyndrickx GR, De Bruyne B. Effects of dobutamine on coronary stenosis physiology and morphology: comparison with intracoronary adenosine. Circulation. 1999;100:243–9. doi: 10.1161/01.CIR.100.3.243. [DOI] [PubMed] [Google Scholar]
- Ge J, Erbel R, Rupprecht HJ, Koch L, Kearney P, Görge G, Haude M, Meyer J. Comparison of intravascular ultrasound and angiography in the assessment of myocardial bridging. Circulation. 1994;89:1725–32. doi: 10.1161/01.CIR.89.4.1725. [DOI] [PubMed] [Google Scholar]
- Tarantini G, Barioli A, Nai Fovino L, Fraccaro C, Masiero G, Iliceto S, Napodano M. Unmasking Myocardial Bridge-Related Ischemia by Intracoronary Functional Evaluation. Circ Cardiovasc Interv. 2018;11:e006247. doi: 10.1161/CIRCINTERVENTIONS.117.006247. [DOI] [PubMed] [Google Scholar]
- Lin S, Tremmel JA, Yamada R, Rogers IS, Yong CM, Turcott R, McConnell MV, Dash R, Schnittger I. A novel stress echocardiography pattern for myocardial bridge with invasive structural and hemodynamic correlation. J Am Heart Assoc. 2013;2:e000097. doi: 10.1161/JAHA.113.000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaura T, Nagayoshi Y, Awai K, Utsunomiya D, Kawano H, Ogawa H, Yamashita Y. Myocardial bridging is associated with coronary atherosclerosis in the segment proximal to the site of bridging. J Cardiol. 2014;63:134–9. doi: 10.1016/j.jjcc.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Yamada R, Tremmel JA, Tanaka S, Lin S, Kobayashi Y, Hollak MB, Yock PG, Fitzgerald PJ, Schnittger I, Honda Y. Functional Versus Anatomic Assessment of Myocardial Bridging by Intravascular Ultrasound: Impact of Arterial Compression on Proximal Atherosclerotic Plaque. J Am Heart Assoc. 2016;5:e001735. doi: 10.1161/JAHA.114.001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydar Y, Yazici HU, Birdane A, Nasifov M, Nadir A, Ulus T, Goktekin O, Gorenek B, Unalir A. Gender differences in the types and frequency of coronary artery anomalies. Tohoku J Exp Med. 2011;225:239–47. doi: 10.1620/tjem.225.239. [DOI] [PubMed] [Google Scholar]
- Kim JW, Seo HS, Na JO, Suh SY, Choi CU, Kim EJ, Rha SW, Park CG, Oh DJ. Myocardial bridging is related to endothelial dysfunction but not to plaque as assessed by intracoronary ultrasound. Heart. 2008;94:765–9. doi: 10.1136/hrt.2007.115980. [DOI] [PubMed] [Google Scholar]
- Ford TJ, McDade R, Oldroyd KG, Touyz RM, McConnachie A, Rush C, Collison D, Corcoran D, McCartney P, Sidik N, Yii E, McGeoch R, Stanley B, Hood S, Robertson K, Lindsay M, Shaukat A, Eteiba H, Watkins S, McEntegart M, Rocchiccioli P, Good R, Berry C. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol. 2018;72:2841–55. doi: 10.1016/j.jacc.2018.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed methodology of IVUS measurements.
Accuracy of non-invasive tests for identifying an MB.
Therapy for a symptomatic myocardial bridge.
Discrepancy between adenosine FFR and dobutamine dFFR with myocardial bridging.
Comprehensive invasive assessment during ICA to identify occult coronary abnormalities as a cause of ANOCA.
ROC to calculate the value of MB muscle index that predicts the presence of a dFFR ≤0.76.
Demonstrative example of endothelial dysfunction within an MB shown on a still frame of a coronary angiogram.
Clinical characteristics of patients with an MB who underwent invasive assessment.
Univariable logistic regression analysis to identify variables associated with any dFFR ≤0.76.
Endothelial function testing and coronary physiology measurements in a subset of patients.
Anatomical characteristics of MB in patients with FFR ≤0.8 versus those with FFR >0.8.