Abstract
Background
Bleeding is the principal safety concern of antithrombotic therapy and occurs frequently among patients with coronary artery disease (CAD).
Aims
We aimed to evaluate the prognostic impact of bleeding on mortality compared with that of myocardial infarction (MI) in patients with CAD.
Methods
We searched Medline and Embase for studies that included patients with CAD and that reported both the association between the occurrence of bleeding and mortality, and between the occurrence of MI and mortality within the same population. Adjusted hazard ratios (HRs) for mortality associated with bleeding and MI were extracted and ratios of hazard ratios (rHRs) were pooled by using inverse variance weighted random effects meta-analyses. Early events included periprocedural or within 30-day events after revascularisation or acute coronary syndrome (ACS). Late events included spontaneous or beyond 30-day events after revascularisation or ACS.
Results
A total of 141,059 patients were included across 16 studies; 128,660 (91%) underwent percutaneous coronary intervention. Major bleeding increased the risk of mortality to the same extent as MI (rHRsbleedingvsMI 1.10, 95% CI: 0.71-1.71, p=0.668). Early bleeding was associated with a higher risk of mortality than early MI (rHRsbleedingvsMI 1.46, 95% CI: 1.13-1.89, p=0.004), although this finding was not present when only randomised trials were included. Late bleeding was prognostically comparable to late MI (rHRsbleedingvsMI 1.14, 95% CI: 0.87-1.49, p=0.358).
Conclusions
Compared with MI, major and late bleeding is associated with a similar increase in mortality, whereas early bleeding might have a stronger association with mortality.
Introduction
Bleeding is the principal safety concern in patients with coronary artery disease (CAD) receiving long-term antithrombotic therapy and the most common non-cardiac adverse event among those undergoing percutaneous coronary intervention (PCI). Single or dual antiplatelet therapy in patients with chronic atherosclerotic vascular disease carries a risk of major bleeding of 1-2% annually1,2. Moreover, in the setting of PCI, major bleeding occurs in 1-2% of patients periprocedurally and up to 4% at one year in the absence of high bleeding risk features3,4.
There is a growing body of evidence showing that bleeding has a negative prognostic impact with a heightened risk of mortality5. Several randomised trials, performed in the chronic setting, showed a decreased risk of ischaemic complications by means of more potent antiplatelet therapies without a concomitant reduction in the risk of mortality. Arguably, the excess of clinically relevant bleeding with more effective antithrombotic regimens could erode their ischaemic benefit, resulting in a shift of the risk for all-cause mortality towards the null effect. However, although the trade-off between ischaemia and bleeding is increasingly recognised6, it remains unclear whether the excess of mortality risk conferred by bleeding is of comparable magnitude to that ensuing after myocardial infarction (MI). Furthermore, whether differences between late and early events exist has not yet been determined. Therefore, we undertook a systematic review and meta-analysis to summarise the available evidence on the relative prognostic association of bleeding and MI with mortality in patients with CAD treated medically or with PCI.
Methods
SEARCH STRATEGY AND SELECTION CRITERIA
Studies had to include participants with CAD irrespective of their management (either medically or with PCI) and had to determine both the association between the occurrence of bleeding and mortality, and that between the occurrence of MI and mortality within the same population. Studies providing only the association between bleeding and mortality or, alternatively, only the association between MI and mortality were excluded. All studies had to adjust at least for age.
Studies were identified by a systematic search in Medline and Embase from each database inception to January 2021. The detailed search algorithm is provided in Supplementary Appendix 1. Reasons for exclusion were discussed and discrepancies were resolved by consensus.
The study protocol was registered in PROSPERO (CRD42020154931).
DATA ANALYSIS
Data were independently extracted by two different reviewers (A. Oliva, M. Avvedimento). We extracted adjusted hazard ratios (HRs) with 95% confidence intervals (95% CI) for the risk of mortality associated with different types of bleeding and MI, extracting HRs with 95% CI by bleeding severity (i.e., major vs minor) and setting of bleeding and MI (i.e., early vs late). Early events were defined as periprocedural events or, alternatively, as events occurring within 30 days after revascularisation or acute coronary syndrome (ACS). Late events were defined as spontaneous events or, alternatively, as events occurring beyond 30 days after revascularisation or ACS. In view of the anticipated variation among studies in the bleeding definitions used, the Bleeding Academic Research Consortium (BARC) classification took precedence over Thrombolysis In Myocardial Infarction (TIMI), followed by Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries (GUSTO), and the primary classification specified in the study’s protocol in case neither BARC, TIMI nor GUSTO classifications were used. BARC type 3 bleeding was used as a reference to define major bleeding, and other definitions were mapped accordingly. For example, TIMI minor and major bleedings were both considered major bleedings, whereas TIMI minimal bleedings were considered minor (Supplementary Table 1-Supplementary Table 3). In addition to the covariate of age, information was extracted on additional variables, if any, considered for deriving the adjusted HRs (Supplementary Table 4). Two reviewers independently assessed the risk of bias of studies using a modified version of the Newcastle-Ottawa scale score7, based on three selection criteria and three outcome criteria. We considered studies that met four or more of these Newcastle-Ottawa scale criteria to be of higher quality.
STATISTICAL ANALYSIS
The ratio of hazard ratios (rHRs) was used as a measure of the strength of the association between bleeding and MI in terms of mortality risk (Supplementary Appendix 2). An rHRsbleedingvsMI >1 indicates a stronger association of bleeding with mortality as compared with MI (i.e., higher risk of death with bleeding), whereas an rHRsbleedingvsMI <1 indicates a stronger association of MI with mortality, as compared with bleeding (i.e., higher risk of death with MI)8. HRs with 95% CI as well as their ratios were pooled with inverse variance weighted random effects meta-analysis. HRs for the associations of bleeding and MI with mortality were derived from the same participants and correlated. We therefore calculated the median design factor, defined as standard error derived after accounting for correlation within participants divided by crude standard error, in the only study that appropriately derived ratios of HRs and allowed the calculation of the corresponding crude standard error9. This median design factor of 1.041 was used to inflate the crude standard errors of rHRs of the remaining studies. We accounted for different types of bleedings and MIs, separately analysing the HRs for early and late events, and for the composite of early or late events. We also accounted for bleeding severity, analysing HRs for major bleeding or, if not available, for the composite of major or minor bleeding separately from HRs for minor bleeding events only. Sensitivity analyses were then performed by excluding outlying studies (outside the boundaries of Galbraith plots), between studies published before versus after 2015, as well as between randomised and non-randomised studies. We determined heterogeneity between trials using the I2 statistic, with estimates near 25% indicating a small, near 50% a moderate, and near 75% a large extent of heterogeneity. Studies contributing to significant heterogeneity were identified by Galbraith plots in which the estimated effect of each study was plotted against its precision10. Sensitivity analyses were then performed by excluding outlying studies (outside the boundaries of Galbraith plots). All p-values are two-sided. Analyses were performed in Stata Release 14 (StataCorp, College Station, TX, USA).
Results
We screened 5,571 unique citations and deemed 17 reports related to 16 studies eligible after full-text review, including 141,059 participants9,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25. Table 1 shows the main features of the studies selected for analysis. The definitions of bleeding and MI used in each study are reported in Supplementary Table 1 and Supplementary Table 5.
Table 1. Main features of the included studies.
| Study, year | Acronym | Patient population | Type of study | Mean follow-up (months) | Patients (N) | Deaths (N) | Mean age (years) | Female (%) | Diabetes (%) | ACS (%) | PCI (%) |
| Ndrepepa et al, 2008 | NSTE-ACS and elective patients undergoing PCI | RCT | 12 | 5,384 | 197 | 66.6 | 25 | 34 | 38 | 100 | |
| Mehran et al, 2009 | ACUITY | ACS patients | RCT | 12 | 13,819 | 524 | 62.6 | 30 | 28 | 100 | 56.4 |
| Montalescot et al, 2009 | STEEPLE | Patients undergoing PCI | RCT | 12 | 2,636 | 75 | 63.7 | 26 | 29 | 0 | 100 |
| Lindsey et al, 2009 | EVENT | Patients undergoing PCI | registry | 12 | 5,961 | 167 | 64.7 | 33 | 35 | 34 | 100 |
| Kim et al, 2011 | Patients undergoing PCI | registry | 36 | 3,148 | 134 | 60.5 | 29 | 27 | 14 | 100 | |
| Kikkert et al, 2013 | STEMI patients undergoing PCI | registry | 48 | 2,002 | 366 | 62.0 | 30 | 14 | 100 | 100 | |
| Stone et al, 2014 | HORIZONS- AMI | STEMI patients undergoing PCI | RCT | 36 | 3,202 | 197 | 59.9 | 23 | 16 | 100 | 100 |
| Kazi et al, 2015 | Patients undergoing PCI | registry | 53 | 32,906 | 4,048 | 64.0 | 30 | 27 | 68 | 100 | |
| Généreux et al, 2015 and Stone et al, 2013 | ADAPT-DES | Patients undergoing PCI | registry | 24 | 8,577 | 311 | 63.6 | 26 | 32 | 52 | 100 |
| Baber et al, 2016 | PARIS | Patients undergoing PCI | registry | 24 | 5,018 | 227 | 63.7 | 25 | 33 | 41 | 100 |
| Garot et al, 2017 | LEADERS FREE | High bleeding-risk patients undergoing PCI | RCT | 24 | 2,386 | 320 | 75.7 | 30 | 56 | 72 | 100 |
| Valgimigli et al, 2017 | TRACER | NSTE-ACS patients | RCT | 16 | 12,702 | 500 | 64.0 | 28 | 31 | 100 | 57.8 |
| Palmerini et al, 2017 | Patients undergoing PCI | pooled RCTs | 18 | 11,473 | 267 | 63.1 | 30 | 31 | 42 | 100 | |
| Secemsky et al, 2017 | DAPT | Patients undergoing PCI without ischaemic or bleeding events after 12 months of DAPT | RCT | 21 | 11,648 | 222 | 61.3 | 25 | 29 | 46 | 100 |
| Caneiro-Queija et al, 2018 | ACS patients | registry | 15 | 4,229 | 335 | 67.0 | 25 | 32 | 100 | 73.5 | |
| Hara et al, 2020 | GLOBAL LEADERS | Patients undergoing PCI | RCT | 24 | 15,968 | 477 | 64.5 | 23 | 19 | 47 | 100 |
Studies were published between 2008 and 2020 and had a median follow-up of 22.5 months (interquartile range [IQR] 13.5-30). Median age was 63.7 (IQR 62.3-64.7) years and 27.7% of participants were female. The proportion of participants undergoing PCI or with ACS was 91% (N=128,660) and 63% (N=88,867), respectively. Procedural characteriscs of studies are reported in Supplementary Table 6. A total of 8,367 deaths occurred in 141,059 participants, with a median risk of 3.9% across 16 studies (IQR 2.9%-7%). Bleeding and MI event rates reported across the studies are displayed in Supplementary Table 7.
MAJOR BLEEDING (±MINOR BLEEDING) vs MI
RISK OF ALL-CAUSE MORTALITY AFTER EARLY OR LATE EVENTS
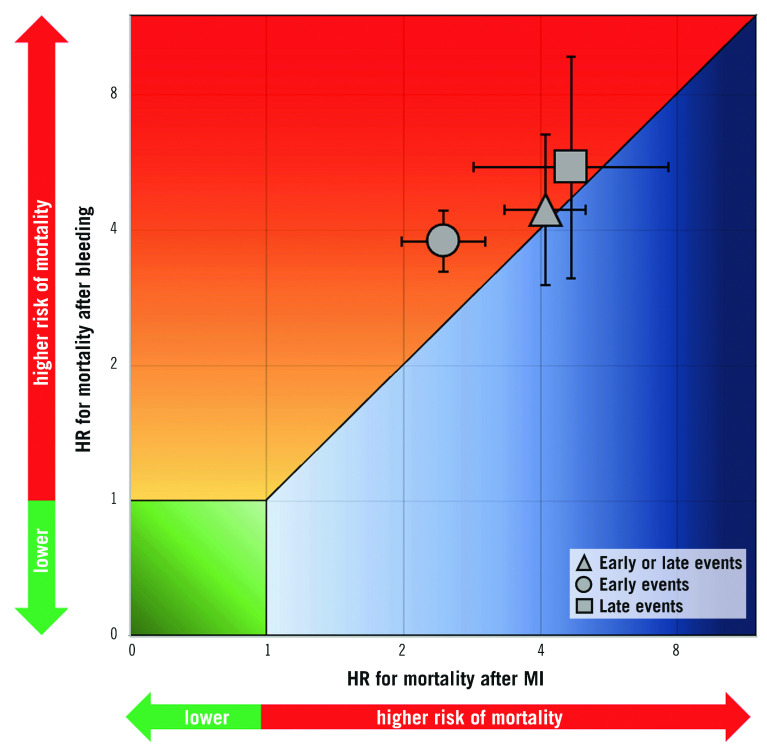
HRs for the composite of early or late events after PCI were available for eight studies (n=51,780 participants). The median risk of bleeding was 6.2% (IQR 1.9%-8.5%; 2,024 events/49,778 participants; 7 studies) and the median risk of death among participants with bleeding was 17.8% (IQR 14.6%-19%; 258 deaths/1,630 participants; 5 studies). The median risk of MI was 6.5% (IQR 3.1%-7.4%; 1,804 events/40,307 participants; 7 studies) and the median risk of death among participants with MI was 13.4% (IQR 10.4%-29.2%; 267 deaths/1,600 participants; 6 studies). The risk of all-cause mortality was significantly increased among participants with early or late bleeding (HR 4.44, 95% CI: 3.02-6.52) and MI (HR 4.10, 95% CI: 3.34-5.03). The rHRs for bleeding versus MI were not significant (rHRsbleedingvsMI 1.10, 95% CI: 0.71-1.71, p=0.668) (Central illustration, Figure 1, Figure 2). Heterogeneity was high for relative risks (I2=89% and I2=58%, for bleeding and MI, respectively) and their ratio (I2=81%). However, after the exclusion of outlying studies, the ratio of HRs remained not significant (rHRsbleedingvsMI 1.03, 95% CI: 0.80-1.32, I2=19%) (Table 2).
Central illustration.
Association between bleeding and myocardial infarction with mortality for early events, late events and early or late events.
Figure 1.
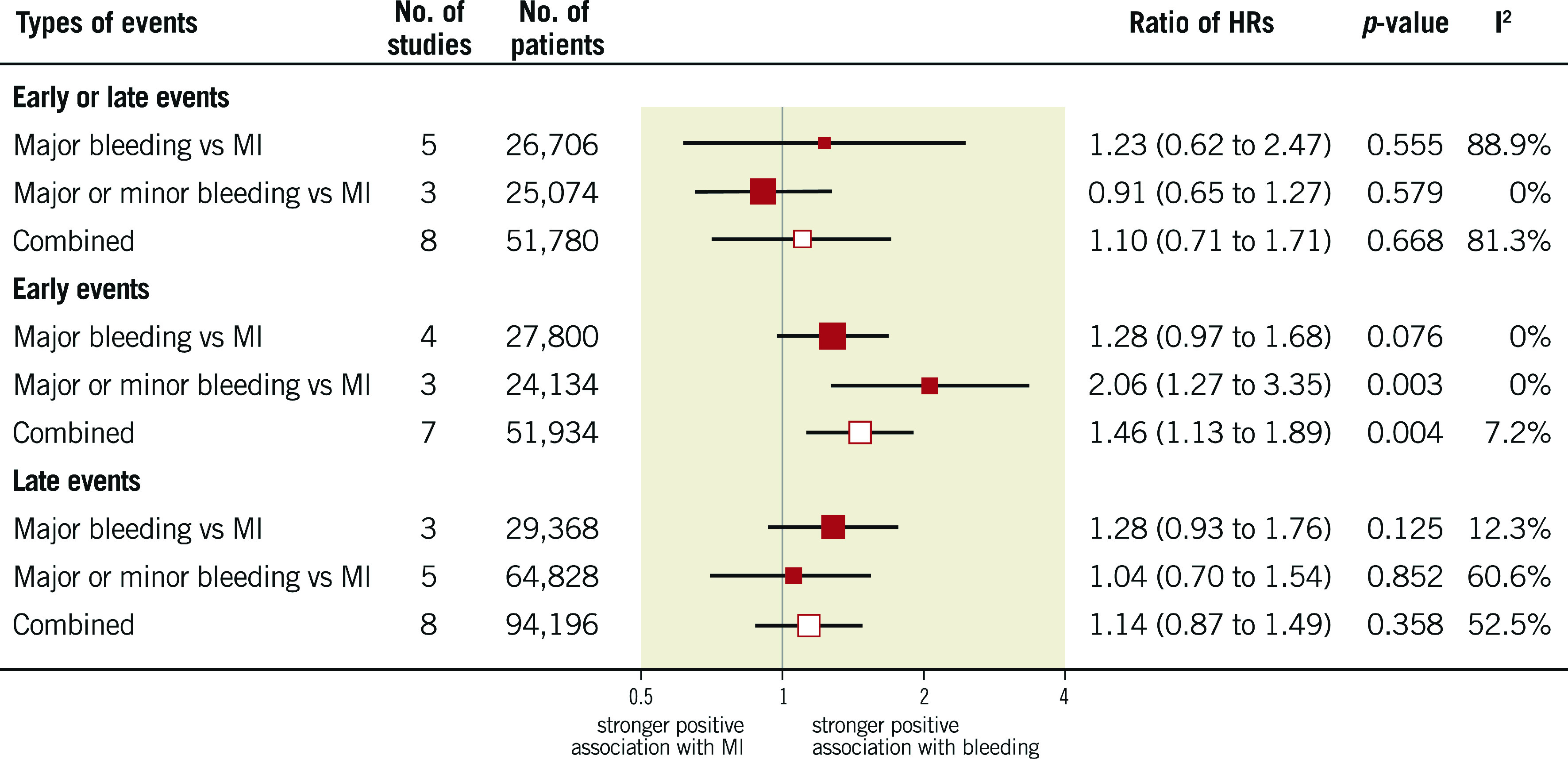
Association of bleeding and myocardial infarction with mortality in patients with coronary artery disease treated with medical therapy or percutaneous coronary intervention.
Figure 2.
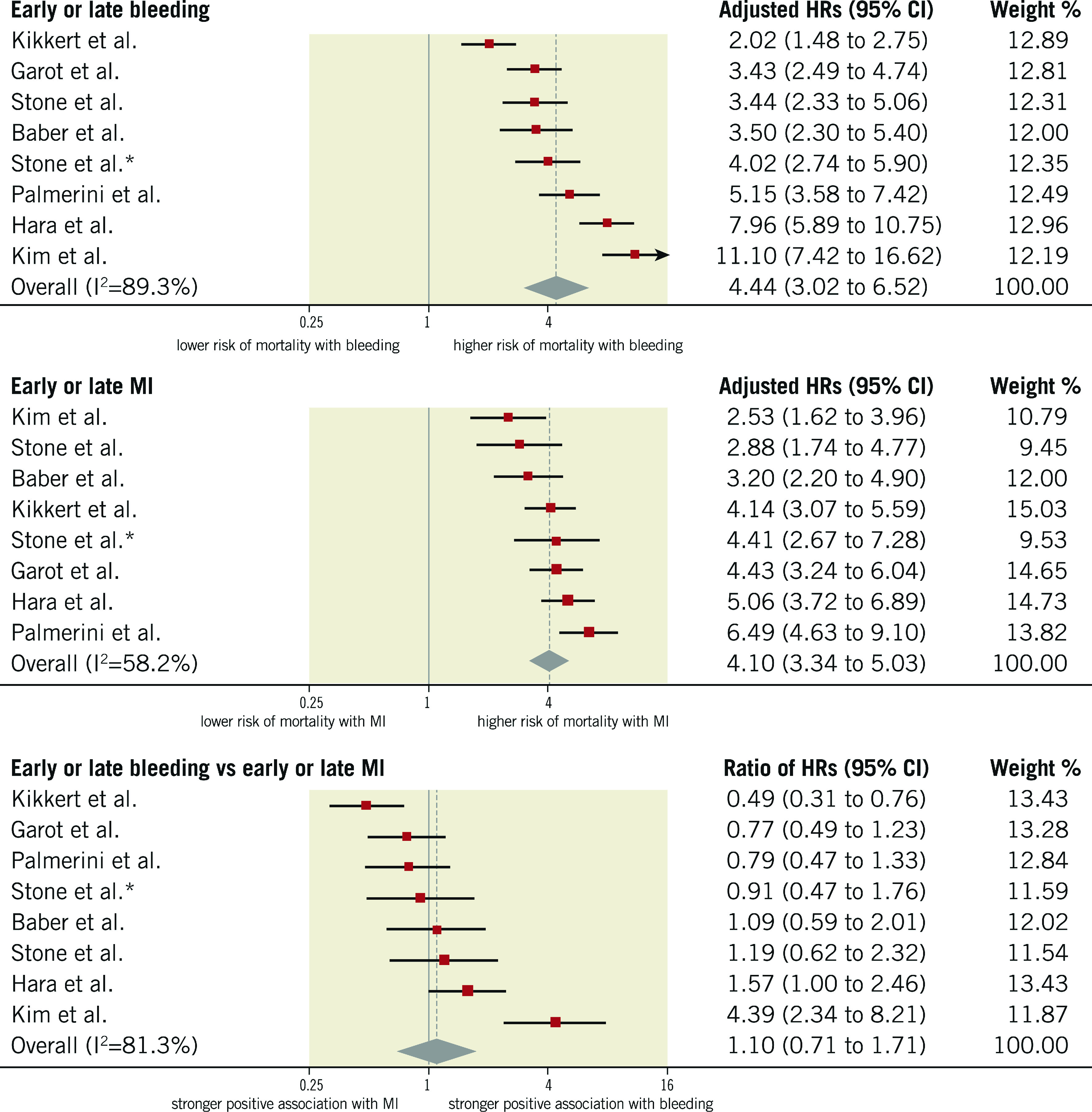
Adjusted hazard ratios for all-cause mortality for patients with early or late events. HRs for bleeding are for major bleeding. *Refers to Stone et al 2013.
Table 2. Pooled hazard ratios (HRs) with and without outlying studies.
| HR (95% CI) | I2 | No. of studies | |
| Bleeding | |||
| Early or late | |||
| Major | 4.60 (2.51-8.43) | 94% | 5 |
| Major, excluding outliers | 3.43 (2.68-4.40) | 0% | 2 |
| Major or minor | 4.25 (3.40-5.32) | 0% | 3 |
| Combined | 4.44 (3.02-6.52) | 89% | 8 |
| Combined, excluding outliers | 3.86 (3.27-4.56) | 0% | 5 |
| Minor | 2.73 (2.07-3.61) | 0% | 2 |
| Early | |||
| Major | 3.42 (2.48-4.12) | 0% | 4 |
| Major or minor | 4.65 (3.54-6.11) | 0% | 3 |
| Combined | 3.77 (3.23-4.41) | 1% | 7 |
| Late | |||
| Major | 7.92 (3.80-16.51) | 91% | 3 |
| Major, excluding outliers | 5.62 (4.37-7.22) | 0% | 2 |
| Major or minor | 4.43 (2.28-8.63) | 94% | 5 |
| Major or minor, excluding outliers | 5.62 (4.59-6.87) | 0% | 4 |
| Combined | 5.51 (3.13-9.68) | 95% | 8 |
| Combined, excluding outliers | 5.62 (4.80-6.57) | 0% | 6 |
| Minor | 2.11 (1.53-2.90) | 43% | 4 |
| Myocardial infarction | |||
| Early or late | 4.11 (3.34-5.03) | 58% | 8 |
| Early or late, excluding outliers | 4.13 (3.54-4.82) | 8% | 6 |
| Early | 2.45 (1.98-3.02) | 25% | 7 |
| Late | 4.67 (2.85-7.63) | 94% | 8 |
| Late, excluding outliers | 6.19 (4.86-7.89) | 53% | 6 |
| Bleeding vs myocardial infarction | |||
| Early or late | |||
| Major | 1.23 (0.62-2.47) | 89% | 5 |
| Major, excluding outliers | 1.13 (0.72-1.78) | 57% | 3 |
| Major or minor | 0.91 (0.65-1.27) | 0% | 3 |
| Combined | 1.10 (0.71-1.71) | 81% | 8 |
| Combined, excluding outliers | 1.03 (0.80-1.32) | 19% | 6 |
| Minor | 0.62 (0.42-0.92) | 0% | 2 |
| Early | |||
| Major | 1.28 (0.97-1.68) | 0% | 4 |
| Major or minor | 2.06 (1.27-3.35) | 0% | 3 |
| Combined | 1.46 (1.13-1.89) | 7% | 7 |
| Late | |||
| Major | 1.28 (0.93-1.76) | 12% | 3 |
| Major or minor | 1.04 (0.70-1.54) | 61% | 5 |
| Major or minor, excluding outliers | 0.86 (0.69-1.07) | 0% | 4 |
| Combined | 1.14 (0.87-1.49) | 53% | 8 |
| Combined, excluding outliers | 1.10 (0.82-1.24) | 16% | 7 |
| Minor | 0.35 (0.25-0.49) | 19% | 4 |
| Outliers were identified by visual inspection of Galbraith plots, which are reported in Supplementary Figure 2-Supplementary Figure 24. CI: confidence interval; HR: hazard ratio | |||
RISK OF ALL-CAUSE MORTALITY AFTER EARLY EVENTS
HRs for early events were available for seven studies in 51,934 participants undergoing cardiac catheterisation and/or PCI. The median risk of early bleeding was 3.5% (IQR 1.5%-4.3%; 1,439 events/49,928 participants; 6 studies with available data) and the median risk of death was 15.3% (IQR 14.4%-15.6%; 216 deaths/49,928 participants; 6 studies). The median risk of early MI was 5.2% (IQR 1.7%-5.8%; 1,836 events/49,928 participants; 6 studies) and the median risk of death was 8.6% among participants with early MI (IQR 8.1%-10.9%; 153 deaths/1,671 participants; 5 studies). The risk of all-cause mortality was increased to a greater extent after early bleeding (HR 3.77, 95% CI: 3.23- 4.41; I2=1%) than early MI (HR 2.45, 95% CI: 1.98-3.02; I2=25%), resulting in a significant rHRs (rHRsbleedingvsMI 1.46, 95% CI: 1.13-1.89, p=0.004; I2=7%) (Central illustration, Figure 2, Figure 3).
Figure 3.
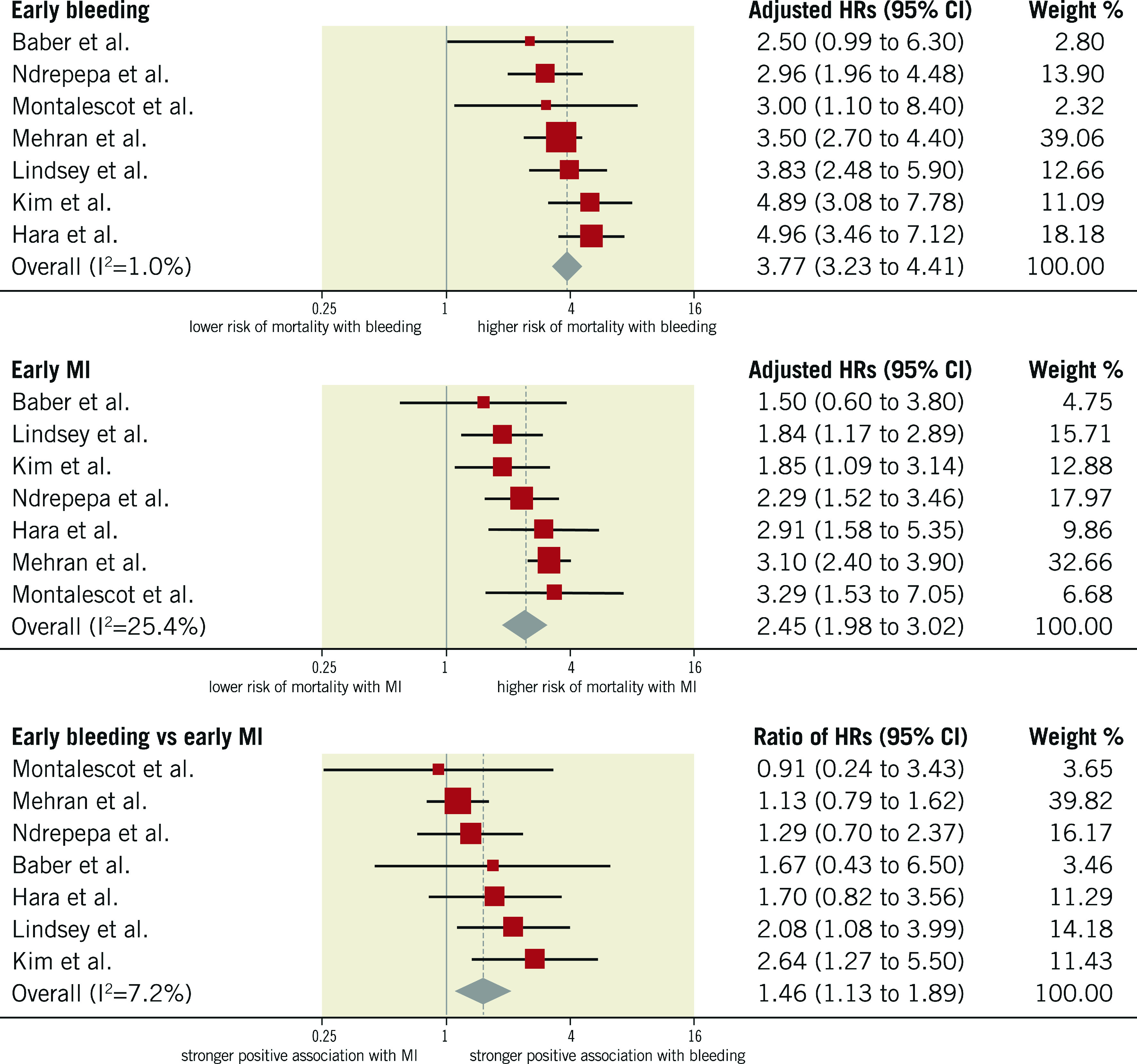
Adjusted hazard ratios for all-cause mortality for patients with early events. HRs for bleeding are for major bleeding.
RISK OF ALL-CAUSE MORTALITY AFTER LATE EVENTS
HRs for late events were available for eight studies in 94,196 participants. The median risk of late bleeding was 3.1% (IQR 2.2%-5.8%; 3,261 events/94,196 participants; 8 studies) and the median risk of death among participants with late bleeding was 17.6% (IQR 13.4%-22.1%; 557 deaths/3,261 participants; 8 studies). The median risk of late MI was 2.9% (IQR 2.5%-4.7%; 3,150 events/94,196 participants; 8 studies) and the median risk of death among participants with late MI was 17.2% (IQR 10.9%-21.3%; 571 deaths/2,724 participants; 6 studies). As shown in Figure 4, the relative risk of all-cause mortality was significantly increased after late bleeding (HR 5.51, 95% CI: 3.13-9.68; I2=95%) and late MI (HR 4.67, 95% CI: 2.85-7.63; I2=94%). The rHRs was not significant (rHRsbleedingvsMI 1.14, 95% CI: 0.87-1.49, p=0.358; I2=53%) (Central illustration, Figure 2, Figure 4), suggesting a similar impact of the two events on mortality. The rHRs remained similar after the exclusion of the studies that were identified as outliers at visual inspection of Galbraith plots (rHRsbleedingvsMI 1.10, 95% CI: 0.82-1.24; I2=16%) (Table 2).
Figure 4.
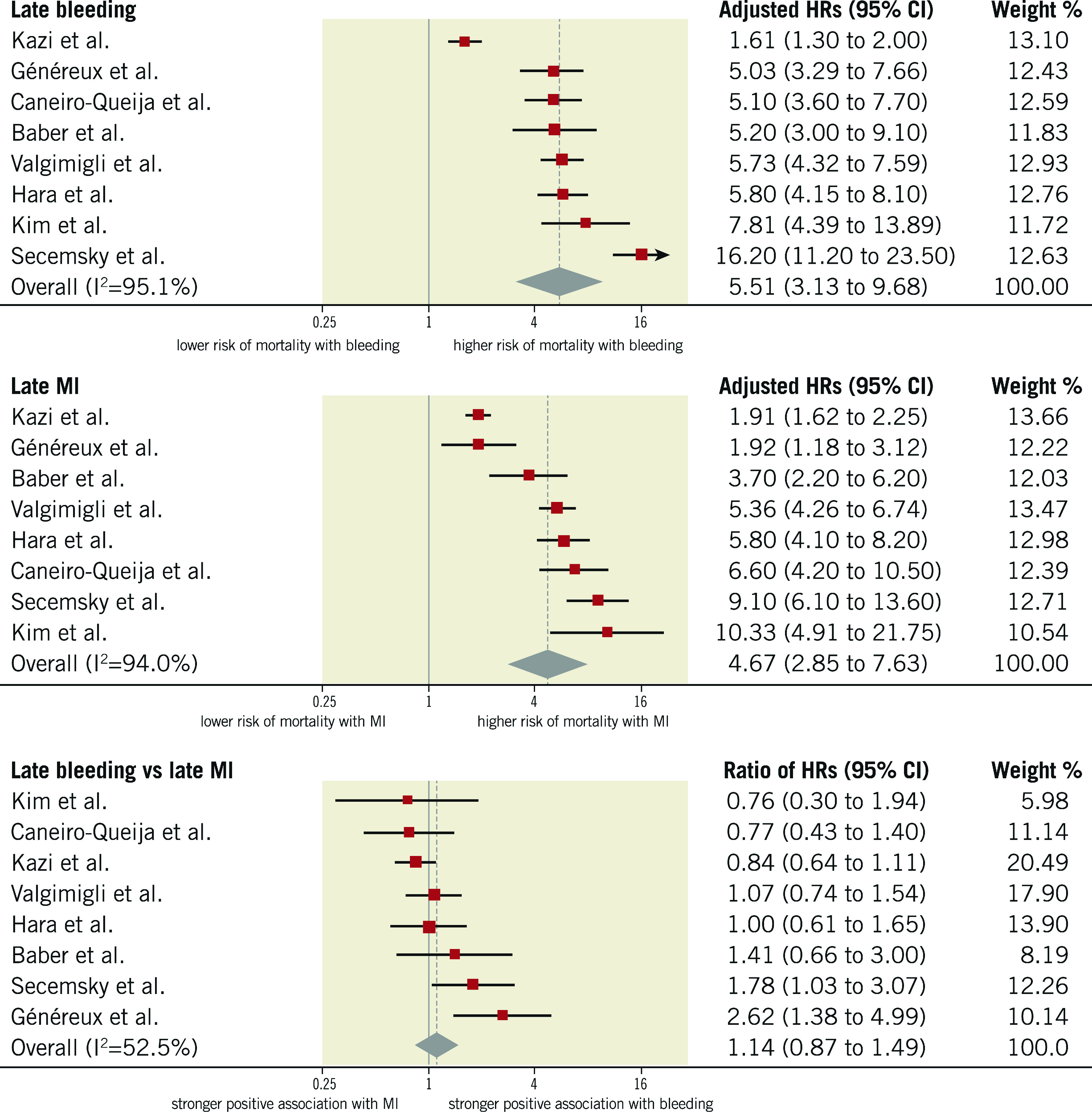
Adjusted hazard ratios for all-cause mortality for patients with late events. HRs for bleeding are for major bleeding.
MINOR BLEEDING vs MI
The median rate of minor late bleeding was 4.7% (IQR 3%-7.1%; 1,523 events/33,597 participants; 4 studies) and the median risk of death among participants with minor late bleeding was 6.3% (IQR 4.6%-8.4%; 73 deaths/1,164 participants; 3 studies). As displayed in Figure 5, the risk of mortality for participants with late minor bleeding (HR 2.11, 95% CI: 1.53-2.90; I2=43%) was lower than for those experiencing late MI (HR 5.78, 95% CI: 4.18-7.99; I2=65%), resulting in a significant rHRs (rHRsbleedingvsMI 0.35, 95% CI: 0.25-0.49, p<0.001; I2=19%). For the only two studies providing data on the composite of late or early minor bleeding, the rHRs was 0.62 (95% CI: 0.42-0.92).
Figure 5.
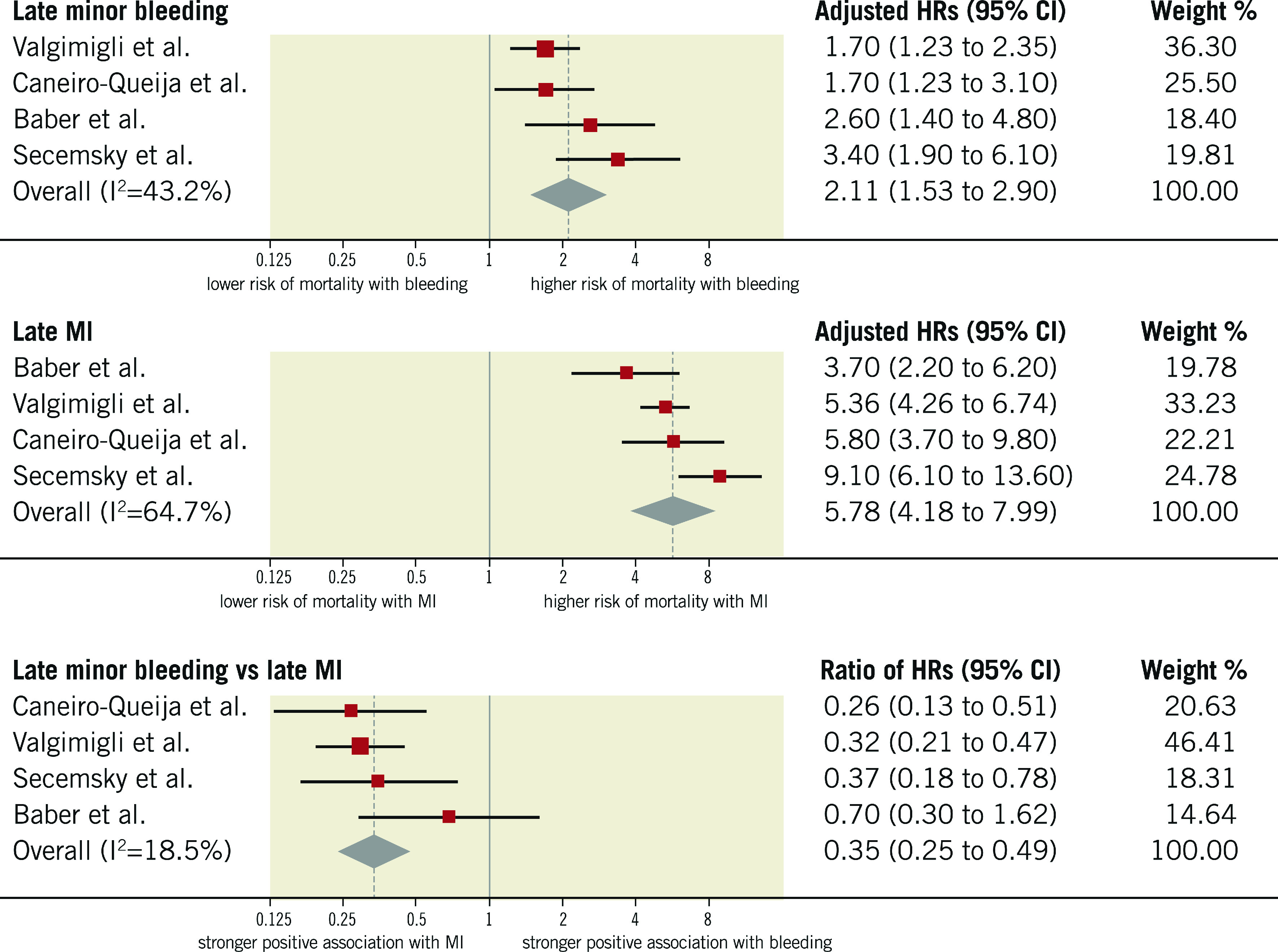
Adjusted hazard ratios for all-cause mortality for patients with late minor bleeding. HRs for bleeding are for minor bleeding.
SENSITIVITY ANALYSES, QUALITY OF STUDIES AND RISK OF BIAS
As shown in Table 2, rHRs for all-cause mortality associated with bleeding versus MI were largely consistent after the exclusion of outlying studies, identified by visual inspection of Galbraith plots (Supplementary Figure 1-Supplementary Figure 24). Results were also consistent between studies published before versus after 2015, the median publication year (Supplementary Table 8). Randomised and non-randomised studies yielded consistent findings, although there was a significant interaction for early events due to a lesser impact of early MI on mortality in non-randomised studies (Supplementary Table 9). All included studies were of high quality according to the Newcastle-Ottawa scale (Supplementary Table 10).
Discussion
In the present systematic review and meta-analysis of 16 studies, including 141,059 patients with CAD, we found strong evidence for a heightened risk of mortality associated with bleeding and MI events. However, there was a differential effect in the relative association between bleeding and MI according to the timing and severity of events: 1) early bleeding had a greater impact on mortality than early MI; 2) the risk of mortality after late bleeding was comparable to the risk of mortality after late MI; 3) the risk of mortality associated with bleeding and MI was comparable for major bleeding, whereas minor bleeding was prognostically less relevant than MI.
Bleeding after PCI is increasingly considered as important as MI. The trend in recent trials investigating antithrombotic therapies after PCI/ACS is to power for superiority with respect to bleeding reduction and only non-inferiority with respect to ischaemic/thrombotic endpoints. However, bleeding continues to represent the most common non-cardiac adverse event after PCI and is associated with increased morbidity and mortality, prolonged hospitalisation, and incremental costs26. Among bleeding avoidance strategies, the choice of vascular access plays a critical role. Indeed, in randomised and large observational studies, radial compared with femoral access showed a strong reduction in the risk of vascular and bleeding complications, affording a parallel decrease by 20-30% in the relative risk of all-cause mortality27,28. In a nested case-control analysis of the MATRIX trial comparing patients who died from causes other than bleeding with 1,370 matched control patients, actionable bleeding was associated with a threefold increase in the risk of mortality, therefore suggesting a link between bleeding prevention and mortality benefit related to radial access29. Similarly, in a trial of patients with chronic CAD and atrial fibrillation, a less intense antithrombotic regimen with rivaroxaban instead of rivaroxaban plus a single antiplatelet agent was associated with a 60% and 55% relative decrease in the risk of major bleeding and death, respectively30. A variety of mechanisms may help to explain the trade-off between bleeding and ischaemia. Discontinuation of antithrombotic therapy or the use of less effective drugs in patients with bleeding complications may result in an increased risk of ischaemic complications. Anaemia can directly affect oxygen delivery to the myocardium and induce a prothrombotic status by triggering erythropoietin release. Transfusions that are frequently required to correct anaemia in the setting of bleeding have been associated with poorer outcomes31. Also, major bleeding may increase the risk of mortality in patients suffering from trauma or malignancies.
Conversely, interventions proven effective in preventing ischaemic complications at the expense of increased major bleeding have not necessarily translated into a mortality benefit. In a patient-level meta-analysis of 48,817 patients, extended clopidogrel therapy on a background of aspirin significantly reduced the risk of ischaemic events and increased the risk of major bleeding, including fatal events, with no effect on mortality32. Along the same lines, in a meta-analysis of 10 trials, prolongation (≥12 months) of dual antiplatelet therapy after PCI yielded increased risks of major bleeding and all-cause mortality despite reduced risks of MI and stent thrombosis6. This observation, however, was largely influenced by a single randomised trial with a higher risk of non-cardiovascular fatalities among patients randomised to prolonged thienopyridine treatment, principally due to cancer-related deaths in the absence of clinically evident bleeding liability33. Collectively, these results might be reconciled in view of the comparable prognostic impact of late bleeding and MI on mortality as supported by the present study.
Another principal finding of our meta-analysis is that early bleeding showed a greater impact on mortality than early MI. Again, this may explain the mortality benefit observed with radial access that is attributable to the prevention of access-site bleeding and also the decrease in major cardiovascular events reported by trials using safer anticoagulant strategies among patients undergoing PCI34. At the same time, it is noteworthy that early MI presented a weaker association with mortality with a pooled risk estimate similar to minor bleeding (HR 2.45 vs 2.11, respectively).
The present study may also have implications for the design of future randomised clinical trials. Given the equipoise between MI and major bleeding in terms of mortality, a net clinical outcome (or net clinical benefit) might be used as the primary endpoint in order to assess the risk-benefit ratio of a given intervention thoroughly and also increase the total number of endpoint events, resulting in more statistical precision and more accurate trials. Finally, by providing a quantitative description of the mortality risk associated with MI and different types of bleeding, the findings of this study may serve as the basis for the estimation of weighting factors in weighted composite endpoint methodologies, in which non-fatal events are accounted for differently based on their relative severity35.
Study limitations
The results of this study should be interpreted in view of several limitations. First, bleeding and MI definitions were different across studies and this represented a source of heterogeneity, which unfortunately was present for most of the outcomes. Moreover, early events included both periprocedural and within 30-day post-PCI events. Similarly, late events included both spontaneous and after 30-day post-PCI events. However, associations remained largely unchanged after the exclusion of outlying studies. Second, although the meta-analysis showed an equipoise between major bleeding and MI in terms of subsequent risk of mortality, our findings do not prove causation of this association. Third, follow-up duration and periprocedural management, including antithrombotic therapy and vascular access, were not uniform across studies. Fourth, the included studies were published over a long period (from 2008 to 2020). However, the results remained largely consistent between newer versus older studies. Fifth, not all the included studies contributed to the analyses which might have affected the precision of pooled risk estimates. Sixth, although all studies had to provide HRs adjusted at least for age, there was substantial variation in the covariates used. Seventh, we used Galbraith plots to identify outlying studies visually, although they have limited value when the number of studies is low.
Conclusions
Our analysis showed that major and late bleedings were associated with a similar increase in the risk of all-cause mortality compared with MI. In the setting of PCI, early bleeding might have a stronger association with mortality than MI, emphasising the importance of bleeding avoidance strategies in perioperative management of patients undergoing percutaneous myocardial revascularisation.
Impact on daily pratice
Major and late bleedings should be considered prognostically equivalent to MI, given the similar association with mortality. Early bleeding has an even stronger association with mortality than early MI, emphasising the importance of bleeding avoidance strategies among patients undergoing PCI.
Supplementary data
Search strategy.
Methods.
Flow chart for the systematic review and meta-analysis.
Galbraith plot for studies reporting HRs for the composite of late or early major bleeding.
Galbraith plot for studies reporting HRs for the composite of late or early major or minor bleeding.
Galbraith plot for studies reporting HRs for the composite of late or early major bleeding and for the composite of late or early major or minor bleeding.
Galbraith plot for studies reporting HRs for early major bleeding.
Galbraith plot for studies reporting HRs for early major or minor bleeding.
Galbraith plot for studies reporting HRs of early major bleeding and for early major or minor bleeding.
Galbraith plot for studies reporting HRs for late minor bleeding.
Galbraith plot for studies reporting HRs for late major bleeding.
Galbraith plot for studies reporting HRs for late major or minor bleeding.
Galbraith plot for studies reporting HRs for late major bleeding and for late major or minor bleeding.
Galbraith plot for studies reporting HRs for early myocardial infarction and late myocardial infarction.
Galbraith plot for studies reporting HRs for early myocardial infarction.
Galbraith plot for studies reporting HRs for late myocardial infarction.
Galbraith plot for studies in which ratio of HRs was derived for the composite of late or early major bleeding versus the composite of late or early MI.
Galbraith plot for studies in which ratio of HRs was derived for the composite of late or early major or minor bleeding versus the composite of late or early MI.
Galbraith plot for studies in which ratio of HRs was derived for the composite of late or early major and major or minor bleeding versus the composite of late or early MI.
Galbraith plot for studies in which ratio of HRs was derived for early major bleeding versus early MI.
Galbraith plot for studies in which ratio of HRs was derived for early major or minor bleeding versus early MI.
Galbraith plot for studies in which ratio of HRs was derived for the composite of early major and major or minor bleeding versus early MI.
Galbraith plot for studies in which ratio of HRs was derived for late major bleeding versus late MI.
Galbraith plot for studies in which ratio of HRs was derived for late major or minor bleeding versus late MI.
Galbraith plot for studies in which ratio of HRs was derived for the composite of late major and major or minor bleeding versus late MI.
Galbraith plot for studies in which ratio of HRs was derived for late minor bleeding versus late MI.
Bleeding definitions across studies.
Contribution of each study to the analysis of bleeding according to the timing and severity of bleeding.
Comparison across major bleeding definitions.
Covariates used for adjusted hazard ratios.
Definitions of myocardial infarction for the analysis of early events.
Definitions of myocardial infarction for the analysis of late events.
Definitions of myocardial infarction for the analysis of the composite of early or late events.
Procedural characteristics of the studies.
Bleeding and myocardial infarction events reported across studies.
Sensitivity analysis according to median publication year of the included studies (before vs after 2015).
Sensitivity analysis for randomised versus non-randomised studies.
Newcastle-Ottawa scale.
Acknowledgments
Conflict of interest statement
R. Piccolo reports personal fees from Abbott Vascular. S. Windecker reports research and educational grants to the institution from Abbott, Amgen, BMS, Bayer, Boston Scientific, Biotronik, Cardinal Health, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Johnson&Johnson, Medtronic, Querbet, Polares, Sanofi, Terumo, and Sinomed. M. Valgimigli reports grants and personal fees from Terumo, personal fees from AstraZeneca, Alvimedica/CID, Abbott Vascular, Daiichi Sankyo, Opsens, Bayer, CoreFlow, Idorsia Pharmaceuticals Ltd, Universität Basel Dept. Klinische Forschung, Vifor, Bristol Myers Squibb SA, iVascular, and Medscape. P. Jüni serves as unpaid member of the steering group of trials funded by AstraZeneca, Biotronik, Biosensors, St. Jude Medical and The Medicines Company, has received research grants to the institution from AstraZeneca, Biotronik, Biosensors International, Eli Lilly and The Medicines Company, and honoraria to the institution for participation in advisory boards and/or consulting from Amgen, Ava and Fresenius, but has not received personal payments by any pharmaceutical company or device manufacturer, and he has no other relationships or activities that could appear to have influenced the submitted work. The other authors have no conflicts of interest to declare.
Abbreviations
- ACS
acute coronary syndrome
- CAD
coronary artery disease
- HR
hazard ratio
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
Contributor Information
Raffaele Piccolo, Department of Advanced Biomedical Sciences, University of Naples Federico II, Naples, Italy.
Angelo Oliva, Department of Advanced Biomedical Sciences, University of Naples Federico II, Naples, Italy.
Marisa Avvedimento, Department of Advanced Biomedical Sciences, University of Naples Federico II, Naples, Italy.
Anna Franzone, Department of Advanced Biomedical Sciences, University of Naples Federico II, Naples, Italy.
Stephan Windecker, Department of Cardiology, Bern University Hospital, University of Bern, Bern, Switzerland.
Marco Valgimigli, Department of Cardiology, Bern University Hospital, University of Bern, Bern, Switzerland.
Giovanni Esposito, Department of Advanced Biomedical Sciences, University of Naples Federico II, Naples, Italy.
Peter Jüni, Applied Health Research Centre of the Li Ka Shing Knowledge Institute, Department of Medicine, St Michael's Hospital, University of Toronto, Toronto, Ontario, Canada.
References
- Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR, Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, Rinaldi MJ, Massaro JM DAPT Study Investigators. Twelve or 30 months of dual-antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155–66. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, Bengtsson O, Oude Ophuis T, Budaj A, Theroux P, Ruda M, Hamm C, Goto S, Spinar J, Nicolau JC, Kiss RG, Murphy SA, Wiviott SD, Held P, Braunwald E, Sabatine MS PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–800. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]
- Spertus JA, Decker C, Gialde E, Jones PG, McNulty EJ, Bach R, Chhatriwalla AK. Precision medicine to improve use of bleeding avoidance strategies and reduce bleeding in patients undergoing percutaneous coronary intervention: prospective cohort study before and after implementation of personalized bleeding risks. BMJ. 2015;350:1302. doi: 10.1136/bmj.h1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, Farb A, Gibson CM, Gregson J, Haude M, James SK, Kim HS, Kimura T, Konishi A, Laschinger J, Leon MB, Magee PFA, Mitsutake Y, Mylotte D, Pocock S, Price MJ, Rao SV, Spitzer E, Stockbridge N, Valgimigli M, Varenne O, Windhoevel U, Yeh RW, Krucoff MW, Morice MC. Defining High Bleeding Risk in Patients Undergoing Percutaneous Coronary Intervention. Circulation. 2019;140:240–61. doi: 10.1161/CIRCULATIONAHA.119.040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SV. The conundrum of reducing ischemic and bleeding events after PCI. J Am Coll Cardiol. 2015;65:1421–3. doi: 10.1016/j.jacc.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Navarese EP, Andreotti F, Schulze V, Kolodziejczak M, Buffon A, Brouwer M, Costa F, Kowalewski M, Parati G, Lip GY, Kelm M, Valgimigli M. Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention with drug eluting stents: meta-analysis of randomised controlled trials. BMJ. 2015;350:h1618. doi: 10.1136/bmj.h1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells GA, Shea, O’Connell, Peterson, Welch, Losos, Tugwell The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, Ottawa Hosp Res Inst. 2000. http://ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Emdin CA, Wong CX, Hsiao AJ, Altman DG, Peters SA, Woodward M, Odutayo AA. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ. 2016;532:h7013. doi: 10.1136/bmj.h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valgimigli M, Costa F, Lokhnygina Y, Clare RM, Wallentin L, Moliterno DJ, Armstrong PW, White HD, Held C, Aylward PE, Van de Werf F, Harrington RA, Mahaffey KW, Tricoci P. Trade-off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome: lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) randomized trial. Eur Heart J. 2017;38:804–10. doi: 10.1093/eurheartj/ehw525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith RF. Graphical display of estimates having differing standard errors. Technometrics. 1988;30:271–81. doi: 10.1080/00401706.1988.10488400. [DOI] [Google Scholar]
- Ndrepepa G, Berger PB, Mehilli J, Seyfarth M, Neumann FJ, Schomig A, Kastrati A. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: Appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol. 2008;51:690–7. doi: 10.1016/j.jacc.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Mehran R, Pocock SJ, Stone GW, Clayton TC, Dangas GD, Feit F, Manoukian SV, Nikolsky E, Lansky AJ, Kirtane A, White HD, Colombo A, Ware JH, Moses JW, Ohman EM. Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non-ST-elevation acute coronary syndromes: a risk model from the ACUITY trial. Eur Heart J. 2009;30:1457–66. doi: 10.1093/eurheartj/ehp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalescot G, Gallo R, White HD, Cohen M, Steg PG, Aylward PEG, Bode C, Chiariello M, King SB, 3rd, Harrington RA, Desmet WJ, Macaya C, Steinhubl SR STEEPLE Investigators. Enoxaparin versus unfractionated heparin in elective percutaneous coronary intervention. 1-year results from the STEEPLE (SafeTy and efficacy of enoxaparin in percutaneous coronary intervention patients, an international randomized evaluation) trial. JACC Cardiovasc Interv. 2009;2:1083–91. doi: 10.1016/j.jcin.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Lindsey JB, Marso SP, Pencina M, Stolker JM, Kennedy KF, Rihal C, Barsness G, Piana RN, Goldberg SL, Cutlip DE, Kleiman NS, Cohen DJ EVENT Registry Investigators. Prognostic impact of periprocedural bleeding and myocardial infarction after percutaneous coronary intervention in unselected patients: results from the EVENT (evaluation of drug-eluting stents and ischemic events) registry. JACC Cardiovasc Interv. 2009;2:1074–82. doi: 10.1016/j.jcin.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Kim YH, Lee JY, Ahn JM, Song H, Kim WJ, Yun SC, Park DW, Kang SJ, Lee SW, Whan Lee C, Park SW, Park SJ. Impact of bleeding on subsequent early and late mortality after drug-eluting stent implantation. JACC Cardiovasc Interv. 2011;4:423–31. doi: 10.1016/j.jcin.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Kikkert WJ, Zwinderman AH, Vis MM, Baan J Jr, Koch KT, Peters RJ, de Winter RJ, Piek JJ, Tijssen JG, Henriques JP. Timing of mortality after severe bleeding and recurrent myocardial infarction in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2013;6:391–8. doi: 10.1161/CIRCINTERVENTIONS.113.000425. [DOI] [PubMed] [Google Scholar]
- Stone GW, Clayton T, Deliargyris EN, Prats J, Mehran R, Pocock SJ. Reduction in cardiac mortality with bivalirudin in patients with and without major bleeding: The HORIZONS-AMI trial (harmonizing outcomes with revascularization and stents in acute myocardial infarction). J Am Coll Cardiol. 2014;63:15–20. doi: 10.1016/j.jacc.2013.09.027. [DOI] [PubMed] [Google Scholar]
- Kazi DS, Leong TK, Chang TI, Solomon MD, Hlatky MA, Go AS. Association of spontaneous bleeding and myocardial infarction with long-term mortality after percutaneous coronary intervention. J Am Coll Cardiol. 2015;65:1411–20. doi: 10.1016/j.jacc.2015.01.047. [DOI] [PubMed] [Google Scholar]
- Généreux P, Giustino G, Witzenbichler B, Weisz G, Stuckey TD, Rinaldi MJ, Neumann FJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri E, Yadav M, Francese DP, Palmerini T, Kirtane AJ, Litherland C, Mehran R, Stone GW. Incidence, Predictors, and Impact of Post-Discharge Bleeding After Percutaneous Coronary Intervention. J Am Coll Cardiol. 2015;66:1036–45. doi: 10.1016/j.jacc.2015.06.1323. [DOI] [PubMed] [Google Scholar]
- Stone GW, Witzenbichler B, Weisz G, Rinaldi MJ, Neumann FJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri E, Gurbel PA, Xu K, Parise H, Kirtane AJ, Brodie BR, Mehran R, Stuckey TD ADAPT-DES Investigators. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet. 2013;382:614–23. doi: 10.1016/S0140-6736(13)61170-8. [DOI] [PubMed] [Google Scholar]
- Baber U, Dangas G, Chandrasekhar J, Sartori S, Steg PG, Cohen DJ, Giustino G, Ariti C, Witzenbichler B, Henry TD, Kini AS, Krucoff MW, Gibson CM, Chieffo A, Moliterno DJ, Weisz G, Colombo A, Pocock S, Mehran R. Time-Dependent Associations Between Actionable Bleeding, Coronary Thrombotic Events, and Mortality Following Percutaneous Coronary Intervention: Results From the PARIS Registry. JACC Cardiovasc Interv. 2016;9:1349–57. doi: 10.1016/j.jcin.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Garot P, Morice MC, Tresukosol D, Pocock SJ, Meredith IT, Abizaid A, Carrié D, Naber C, Iñiguez A, Talwar S, Menown IBA, Christiansen EH, Gregson J, Copt S, Hovasse T, Lurz P, Maillard L, Krackhardt F, Ong P, Byrne J, Redwood S, Windhövel U, Greene S, Stoll HP, Urban P LEADERS FREE Investigators. 2-Year Outcomes of High Bleeding Risk Patients After Polymer-Free Drug-Coated Stents. J Am Coll Cardiol. 2017;69:162–71. doi: 10.1016/j.jacc.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Palmerini T, Bacchi Reggiani L, Della Riva D, Romanello M, Feres F, Abizaid A, Gilard M, Morice MC, Valgimigli M, Hong MK, Kim BK, Jang Y, Kim HS, Park KW, Colombo A, Chieffo A, Ahn JM, Park SJ, Schüpke S, Kastrati A, Montalescot G, Steg PG, Diallo A, Vicaut E, Helft G, Biondi-Zoccai G, Xu B, Han Y, Généreux P, Bhatt DL, Stone GW. Bleeding-Related Deaths in Relation to the Duration of Dual-Antiplatelet Therapy After Coronary Stenting. J Am Coll Cardiol. 2017;69:2011–22. doi: 10.1016/j.jacc.2017.02.029. [DOI] [PubMed] [Google Scholar]
- Caneiro-Queija B, Abu-Assi E, Raposeiras-Roubín S, Manzano-Fernández S, Flores Blanco P, López-Cuenca Á, Cobas-Paz R, Gómez-Molina M, Rodríguez-Rodríguez JM, Calvo-Iglesias F, Valdes-Chavarri M, Iñiguez-Romo A. Differential Prognostic Impact on Mortality of Myocardial Infarction Compared With Bleeding Severity in Contemporary Acute Coronary Syndrome Patients. Rev Esp Cardiol (English Ed) 2018;71:829–36. doi: 10.1016/j.recesp.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Hara H, Takahashi K, Kogame N, Tomaniak M, Kerkmeijer LSM, Ono M, Kawashima H, Wang R, Gao C, Wykrzykowska JJ, de Winter RJ, Neumann FJ, Plante S, Lemos Neto PA, Garg S, Jüni P, Vranckx P, Windecker S, Valgimigli M, Hamm C, Steg PG, Onuma Y, Serruys PW. Impact of Bleeding and Myocardial Infarction on Mortality in All-Comer Patients Undergoing Percutaneous Coronary Intervention. Circ Cardiovasc Interv. 2020;13:e009177. doi: 10.1161/CIRCINTERVENTIONS.120.009177. [DOI] [PubMed] [Google Scholar]
- Ndrepepa G, Kastrati A. Bleeding complications in patients undergoing percutaneous coronary interventions: current status and perspective. Coron Artery Dis. 2014;25:247–57. doi: 10.1097/MCA.0000000000000096. [DOI] [PubMed] [Google Scholar]
- Piccolo R, Galasso G, Capuano E, De Luca S, Esposito G, Trimarco B, Piscione F. Transradial versus transfemoral approach in patients undergoing percutaneous coronary intervention for acute coronary syndrome. A meta-analysis and trial sequential analysis of randomized controlled trials. PLoS One. 2014;9:e96127. doi: 10.1371/journal.pone.0096127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando G, Capodanno D. Radial Versus Femoral Access in Invasively Managed Patients With Acute Coronary Syndrome: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163:932–40. doi: 10.7326/M15-1277. [DOI] [PubMed] [Google Scholar]
- Valgimigli M, Gagnor A, Calabro P, Frigoli E, Leonardi S, Zaro T, Rubartelli P, Briguori C, Ando G, Repetto A, Limbruno U, Cortese B, Sganzerla P, Lupi A, Galli M, Colangelo S, Ierna S, Ausiello A, Presbitero P, Sardella G, Varbella F, Esposito G, Santarelli A, Tresoldi S, Nazzaro M, Zingarelli A, de Cesare N, Rigattieri S, Tosi P, Palmieri C, Brugaletta S, Rao SV, Heg D, Rothenbuhler M, Vranckx P, Jüni P MATRIX Investigators. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385:2465–76. doi: 10.1016/S0140-6736(15)60292-6. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, Miyauchi K, Hagiwara N, Kimura K, Hirayama A, Matsui K, Ogawa H AFIRE Investigators. Antithrombotic Therapy for Atrial Fibrillation with Stable Coronary Disease. N Engl J Med. 2019;381:1103–13. doi: 10.1056/NEJMoa1904143. [DOI] [PubMed] [Google Scholar]
- Kwok CS, Sherwood MW, Watson SM, Nasir SB, Sperrin M, Nolan J, Kinnaird T, Kiatchoosakun S, Ludman PF, de Belder MA, Rao SV, Mamas MA. Blood transfusion after percutaneous coronary intervention and risk of subsequent adverse outcomes: a systematic review and meta-analysis. JACC Cardiovasc Interv. 2015;8:436–46. doi: 10.1016/j.jcin.2014.09.026. [DOI] [PubMed] [Google Scholar]
- Elmariah S, Doros G, Benavente OR, Bhatt DL, Connolly SJ, Yusuf S, Steinhubl SR, Liu Y, Hsieh WH, Yeh RW, Mauri L. Impact of Clopidogrel Therapy on Mortality and Cancer in Patients with Cardiovascular and Cerebrovascular Disease: A Patient-Level Meta-Analysis. Circ Cardiovasc Interv. 2018;11:e005795. doi: 10.1161/CIRCINTERVENTIONS.117.005795. [DOI] [PubMed] [Google Scholar]
- Mauri L, Elmariah S, Yeh RW, Cutlip DE, Steg PG, Windecker S, Wiviott SD, Cohen DJ, Massaro JM, D’Agostino RB, Sr, Braunwald E, Kereiakes DJ DAPT Study Investigators. Causes of late mortality with dual antiplatelet therapy after coronary stents. Eur Heart J. 2016;37:378–85. doi: 10.1093/eurheartj/ehv614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangalore S, Toklu B, Kotwal A, Volodarskiy A, Sharma S, Kirtane AJ, Feit F. Anticoagulant therapy during primary percutaneous coronary intervention for acute myocardial infarction: a meta-analysis of randomized trials in the era of stents and P2Y12 inhibitors. BMJ. 2014;349:g6419. doi: 10.1136/bmj.g6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakal JA, Roe MT, Ohman EM, Goodman SG, Fox KA, Zheng Y, Westerhout CM, Hochman JS, Lokhnygina Y, Brown EB, Armstrong PW. Applying novel methods to assess clinical outcomes: insights from the TRILOGY ACS trial. Eur Heart J. 2015;36:385–92a. doi: 10.1093/eurheartj/ehu262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy.
Methods.
Flow chart for the systematic review and meta-analysis.
Galbraith plot for studies reporting HRs for the composite of late or early major bleeding.
Galbraith plot for studies reporting HRs for the composite of late or early major or minor bleeding.
Galbraith plot for studies reporting HRs for the composite of late or early major bleeding and for the composite of late or early major or minor bleeding.
Galbraith plot for studies reporting HRs for early major bleeding.
Galbraith plot for studies reporting HRs for early major or minor bleeding.
Galbraith plot for studies reporting HRs of early major bleeding and for early major or minor bleeding.
Galbraith plot for studies reporting HRs for late minor bleeding.
Galbraith plot for studies reporting HRs for late major bleeding.
Galbraith plot for studies reporting HRs for late major or minor bleeding.
Galbraith plot for studies reporting HRs for late major bleeding and for late major or minor bleeding.
Galbraith plot for studies reporting HRs for early myocardial infarction and late myocardial infarction.
Galbraith plot for studies reporting HRs for early myocardial infarction.
Galbraith plot for studies reporting HRs for late myocardial infarction.
Galbraith plot for studies in which ratio of HRs was derived for the composite of late or early major bleeding versus the composite of late or early MI.
Galbraith plot for studies in which ratio of HRs was derived for the composite of late or early major or minor bleeding versus the composite of late or early MI.
Galbraith plot for studies in which ratio of HRs was derived for the composite of late or early major and major or minor bleeding versus the composite of late or early MI.
Galbraith plot for studies in which ratio of HRs was derived for early major bleeding versus early MI.
Galbraith plot for studies in which ratio of HRs was derived for early major or minor bleeding versus early MI.
Galbraith plot for studies in which ratio of HRs was derived for the composite of early major and major or minor bleeding versus early MI.
Galbraith plot for studies in which ratio of HRs was derived for late major bleeding versus late MI.
Galbraith plot for studies in which ratio of HRs was derived for late major or minor bleeding versus late MI.
Galbraith plot for studies in which ratio of HRs was derived for the composite of late major and major or minor bleeding versus late MI.
Galbraith plot for studies in which ratio of HRs was derived for late minor bleeding versus late MI.
Bleeding definitions across studies.
Contribution of each study to the analysis of bleeding according to the timing and severity of bleeding.
Comparison across major bleeding definitions.
Covariates used for adjusted hazard ratios.
Definitions of myocardial infarction for the analysis of early events.
Definitions of myocardial infarction for the analysis of late events.
Definitions of myocardial infarction for the analysis of the composite of early or late events.
Procedural characteristics of the studies.
Bleeding and myocardial infarction events reported across studies.
Sensitivity analysis according to median publication year of the included studies (before vs after 2015).
Sensitivity analysis for randomised versus non-randomised studies.
Newcastle-Ottawa scale.