Abstract
Aims
Reports of long-term outcomes of patients treated with drug-eluting stents in total coronary occlusions are limited. We analysed clinical outcomes of patients treated with the zotarolimus-eluting Resolute stent (R-ZES) implanted in coronary total occlusions versus non-occluded lesions.
Methods and results
Patients treated with R-ZES and included in four trials (RESOLUTE All Comers, RESOLUTE International, RESOLUTE China RCT, and RESOLUTE China Registry) were pooled and divided into three groups – patients with chronic total occlusions (CTO), patients with total occlusions that had occurred recently (rec-TO), and patients without total occlusions (non-TO). Clinical outcomes at five years were analysed. Of 5,487 patients treated with R-ZES in these trials, 8.0% had CTOs, 8.5% rec-TOs and 83.5% non-TOs. Patients had a mean age of 62.8 years, approximately 25% were female and 30% were diabetics. TLF was similar in the three groups at five years (TLF was 13.2%, 12.5% and 13.3% in the CTO, rec-TO and non-TO groups, respectively, p=0.96). Stent thrombosis tended to occur more frequently for rec-TO compared to CTO and non-TO patients (2.6% vs 1.2% and 1.3%, respectively, p=0.11).
Conclusions
In this large population of patients who had R-ZES implanted, five-year clinical outcomes were similar whether or not the stents were implanted in total occlusions.
Introduction
Patients with occluded coronary arteries may have clinical symptoms ranging from stable angina to acute coronary syndromes. Whereas lesions that are not totally occluded (non-TO), in addition to those occluded recently (rec-TO), rarely represent technical challenges unless containing a heavy thrombus burden, percutaneous coronary intervention (PCI) in those with chronic total occlusions (CTO) is obtained with varying technical success1. Implantation of a drug-eluting stent (DES) improves outcomes including the occurrence of restenosis in patients with coronary total occlusions2,3, despite a higher occurrence of stent strut malapposition4. We have previously reported favourable two-year outcomes in patients with both CTO and rec-TO in comparison with those with non-TO lesions treated with the Resolute™️ zotarolimus-eluting stent (R-ZES; Medtronic, Minneapolis, MN, USA)5. We found the incidence of stent thrombosis (ST) highest in patients with rec-TO, probably due to a higher level of thrombogenicity in these patients who often present with acute coronary syndromes.
The R-ZES has previously been demonstrated to be clinically safe and effective compared with other drug-eluting stents6,7,8,9. However, reports of long-term outcomes in patients with recanalised total occlusions appear to be limited, especially with the use of second-generation DES. Patients with rec-TO often have acute coronary syndrome, and from a patient population of two trials we have previously reported a slightly higher occurrence of ST in patients treated with PCI and R-ZES implantation for rec-TO when compared with both patients with CTO and those without an occlusion5. Based on the patient-level pooled data from four prospective trials and registries, our study aimed to assess the long-term safety and efficacy of second-generation R-ZES in the treatment of CTO lesions.
Results
The baseline demographic and angiographic data of the patients and procedures are presented in Table 1 and Table 2. Non-TO patients were slightly older with fewer stents implanted (especially compared to the CTO group). The majority of rec-TO patients had acute coronary syndromes (78.4%) and fewer cardiac risk factors except for diabetes. The non-TO group had significantly fewer lesions treated per patient and a significantly smaller total stent length per patient compared to the rec-TO and non-TO groups. Otherwise, no relevant differences were found among the three groups.
Table 1. Baseline characteristics.
| CTO group* (N=436) | Rec-TO group* (N=467) | Non-TO* (N=4,584) | p-value (CTO vs Non-TO) | p-value (Rec-TO vs Non-TO) | |
| Age, years | 60.8±11.2 | 60.0±11.6 | 63.3±10.9 | <0.001 | <0.001 |
| Female gender | 17.2 | 24.6 | 23.6 | 0.002 | 0.61 |
| Diabetes mellitus | 26.1 | 26.6 | 28.9 | 0.22 | 0.28 |
| Insulin-dependent | 4.6 | 4.9 | 6.2 | 0.19 | 0.29 |
| Hypertension | 64.7 | 57.6 | 68.5 | 0.10 | <0.001 |
| Hyperlipidaemia | 52.1 | 48.6 | 56.5 | 0.08 | 0.001 |
| Current smoker | 30.3 | 45.8 | 27.2 | 0.16 | <0.001 |
| Prior myocardial infarction | 37.7 | 33.5 | 29.4 | <0.001 | 0.06 |
| Prior percutaneous coronary intervention | 20.9 | 10.3 | 25.7 | 0.03 | <0.001 |
| Acute coronary syndrome | 47.0 | 78.4 | 53.3 | 0.01 | <0.001 |
| *Data presented as % or mean±standard deviation. CTO: chronic total occlusion; Rec-TO: recent total occlusion; Non-TO: no occlusion | |||||
Table 2. Angiographic characteristics.
| CTO group* (N=436) | Rec-TO group* (N=467) | Non-TO* (N=4,584) | p-value (CTO vs Non-TO) | p-value (Rec-TO vs Non-TO) | |
| Reference vessel diameter, mm | 2.9±0.5 | 2.9±0.5 | 2.9±0.5 | 0.19 | 0.15 |
| Number of lesions treated/patient | 1.6±0.8 | 1.5±0.8 | 1.4±0.7 | <0.001 | 0.01 |
| Total stent length/patient, mm | 53.9±35.9 | 41.3±28.2 | 33.4±22.1 | <0.001 | <0.001 |
| Vessel location (patient level) | |||||
| Left anterior descending | 51.6 | 48.2 | 56.2 | 0.06 | <0.001 |
| Left circumflex | 27.1 | 26.6 | 27.5 | 0.86 | 0.67 |
| Right coronary artery | 50.7 | 45.0 | 30.8 | <0.001 | <0.001 |
| Left main | 0.9 | 1.5 | 2.6 | 0.03 | 0.15 |
| *Data presented as % or mean±standard deviation. CTO: chronic total occlusion; Rec-TO: recent total occlusion; Non-TO: no occlusion | |||||
Of the 6,841 patients included in the four trials, 5,487 had at least one R-ZES implanted. Among the lesions treated with R-ZES implantation, 436 (8.0%) were CTOs, 467 (8.5%) were rec-TOs, and 4,584 (83.5%) were non-TOs. Three-year follow-up data were available for 97% of patients in the RESOLUTE International trial, and five-year data were available in 98% of patients in the remaining trials.
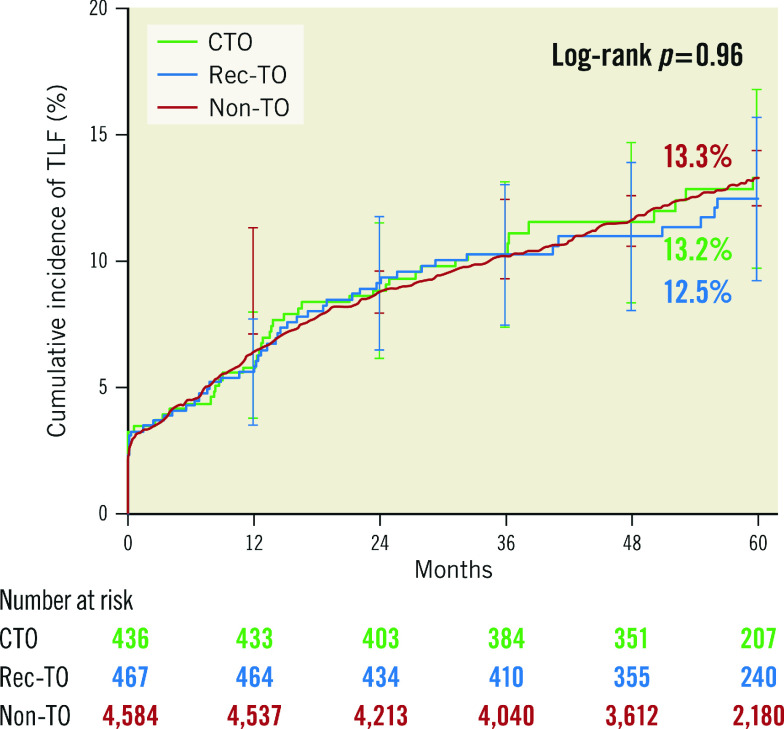
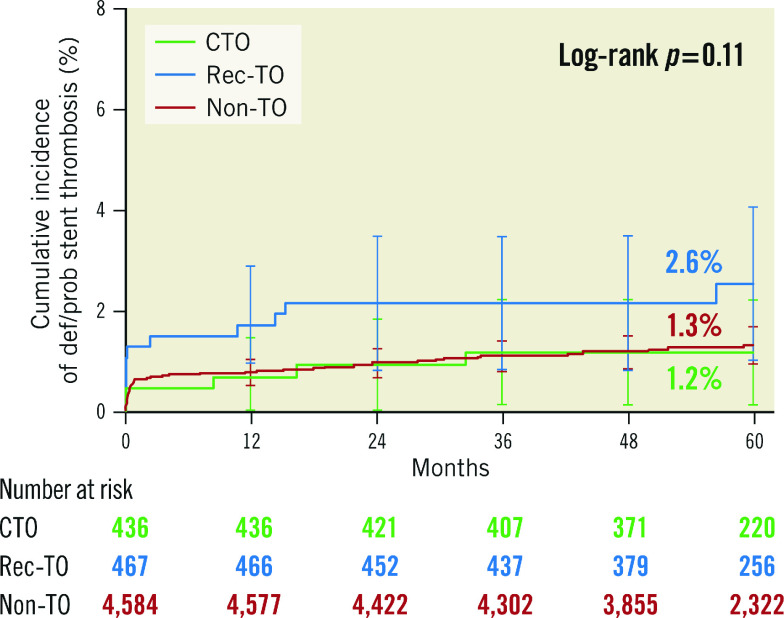
At five-year follow-up, there were no differences in the incidence of the primary endpoint (Figure 1) or its components (Figure 2) among the three groups: TLF at five years was 13.2% for CTO, 12.5% for rec-TO and 13.3% for non-TO patients (p=0.96). There was also no difference among the groups in secondary endpoints measured at five years (Table 3). Most stent thromboses occurred relatively early. The rate of ST levelled off after 12 months and was generally low, with a non-significant numerically higher incidence occurring in the patient group with recently occluded vessels (p=0.11) (Figure 3).
Figure 1.
Cumulative incidence to five years for target lesion failure. CTO: chronic total occlusion; Non-TO: not totally occluded; Rec-TO: recent total occlusion; TLF: target lesion failure
Figure 2.
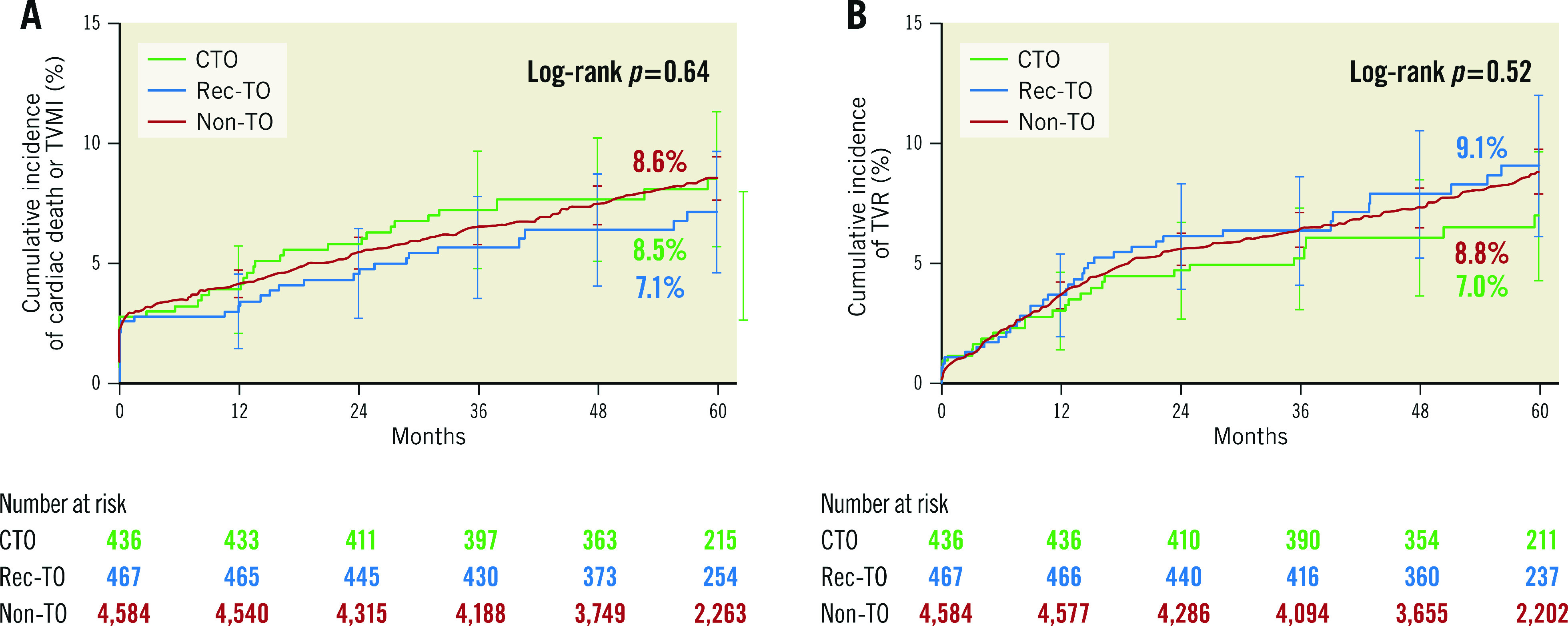
Cumulative incidence to five years for cardiac death or TVMI, and TVR. A) Cardiac death or target vessel myocardial infarction. B) Target vessel revascularisation. CTO: chronic total occlusion; Non-TO: not totally occluded; Rec-TO: recent total occlusion
Table 3. Clinical outcomes to 5 years.
| CTO group* (N=436) | Rec-TO group* (N=467) | Non-TO* (N=4,584) | p-value (CTO vs Rec-TO vs Non-TO) | |
| Target lesion failure | 13.2 (51) | 12.5 (53) | 13.3 (535) | 0.96 |
| Cardiac death | 5.3 (21) | 5.1 (19) | 4.8 (179) | 0.64 |
| Target vessel myocardial infarction | 4.2 (17) | 3.2 (14) | 4.3 (185) | 0.54 |
| Target lesion revascularisation | 5.8 (21) | 6.9 (29) | 6.5 (254) | 0.70 |
| Target vessel revascularisation | 7.0 (26) | 9.1 (36) | 8.8 (343) | 0.52 |
| Stent thrombosis (definite/probable) | 1.2 (5) | 2.6 (11) | 1.3 (55) | 0.11 |
| *Data presented as percentage of cumulative incidence of events calculated by Kaplan-Meier method with log-rank p-values (# of events). CTO: chronic total occlusion; Rec-TO: recent total occlusion; Non-TO: no occlusion | ||||
Figure 3.
Cumulative incidence to five years for stent thrombosis (definite/probable). CTO: chronic total occlusion; Non-TO: not totally occluded; Rec-TO: recent total occlusion
Methods
STUDY DESIGNS AND POPULATIONS
Our data are derived from the RESOLUTE All Comers trial, the RESOLUTE International trial, the RESOLUTE China Randomized Controlled trial (RCT) and the RESOLUTE China Registry, all registered with ClinicalTrials.gov (numbers NCT00617084, NCT00752128, NCT01334268 and NCT01243749, respectively). All trials evaluated clinical outcomes of patients with significant coronary artery disease treated with PCI including R-ZES implantation with annual clinical follow-up.
RESOLUTE All Comers is a randomised multicentre trial primarily evaluating clinical outcomes up to five years after implantation of R-ZES versus an everolimus-eluting stent in an unselected cohort of patients representing a variety of coronary artery disease (n=2,292)5,10. A fraction of the patients (20%) had a 13-month coronary angiography performed. The RESOLUTE International trial is a worldwide multicentre observational registry of clinical outcomes of unselected patients three years after implantation of R-ZES (n=2,349)11,12. RESOLUTE China RCT is a randomised trial comparing five-year clinical outcomes of an all-comer Chinese patient population, who have either an R-ZES or a paclitaxel-eluting stent implanted (n=400). The study included coronary angiography at nine months13. RESOLUTE China Registry is a multicentre observational registry study of clinical outcomes from unselected patients who have an R-ZES implanted and are followed for five years (n=1,800)14.
PROCEDURES
Patients were treated with dual antiplatelet therapy for at least six months according to current international guidelines. PCI was performed in accordance with local standard techniques aiming to cover (≥2 mm on each side of) the culprit lesion from healthy-to-healthy vessel with a stent with a final residual diameter of <25% by post-dilatation using non-compliant balloons.
All patients were included after written consent, and the local ethics committees approved the respective studies, that were all conducted in accordance with the Declaration of Helsinki.
ENDPOINTS AND DEFINITIONS
CTO was defined as a total occlusion with Thrombolysis In Myocardial Infarction (TIMI) grade 0 blood flow with estimated duration of at least three months prior to the index procedure. Rec-TO was defined as a recently (<3 months prior to PCI) occluded lesion with TIMI 0 blood flow. The RESOLUTE All Comers study did not collect the timing of CTO, so patients with a total occlusion were included in the CTO group if they had no recent myocardial infarction and in the rec-TO group if they had a recent myocardial infarction. Patients in all studies who were not categorised as CTO or rec-TO were considered non-TO.
The primary endpoint of the present analysis was target lesion failure (TLF), defined as the composite of cardiac death, target vessel myocardial infarction (TVMI) and target lesion revascularisation (TLR) recorded at follow-up. Secondary endpoints included the components of the primary endpoint and the rate of definite and probable stent thrombosis. Independent site monitoring was performed in all studies, and clinical events committees adjudicated the endpoints.
Deaths were considered cardiac unless an unequivocal non-cardiac cause was documented. Target vessel MI was defined as an MI clearly not located in a non-target vessel, and TLR and target vessel revascularisation as clinically driven PCI or coronary artery bypass grafting of the culprit lesion or vessel. Definite and probable stent thromboses were adjudicated in accordance with the Academic Research Consortium15.
STATISTICAL ANALYSIS
Data from patients who had an R-ZES implanted in the two randomised trials and data from all patients included in the two registries were pooled. The pooled data were divided into three groups according to the presence and duration of a total coronary occlusion.
Categorical data among groups were compared using the chi-square test, and continuous variables with the Student’s t-test. Survival curves were created using Kaplan-Meier estimates, and the log-rank test was used for comparisons between groups. A two-tailed p-value <0.05 was considered significant.
Discussion
Patients treated with R-ZES in their recanalised CTO lesions had low five-year rates of the primary endpoint of target vessel failure (TVF), despite longer stent length, smaller stent diameter and higher mean number of implanted stents and, as an unexpected finding, we were able to report almost identical TVF rates in patients with CTO and non-TO. Treatment of CTOs with R-ZES was safe with a very low five-year rate of ST, that was almost identical to that of patients without CTO. The rates of cardiac death and TVMI were also low and did not differ significantly in patients with CTO versus patients with non-TO. Probably due to the thrombogenic environment, rec-TO patients, mostly in the setting of acute coronary syndrome (3/4 of the patients in this group), had low albeit numerically higher rates of ST compared to patients in the CTO and non-TO groups.
Although the safety of PCI treatment including implantation of DES in patients with acute occlusion of a large coronary artery presenting with ST-segment elevation myocardial infarction has been investigated in long-term follow-up trials16,17, treatment of patients with coronary occlusions of longer duration remains a matter of debate. Only a small number of randomised comparisons of clinical outcomes between patients treated with optimal medical therapy versus CTO revascularisation have been performed18,19,20.
Randomised studies comparing DES and bare metal stent implantation in recently occluded coronary arteries or chronic total coronary occlusions are also scarce2,21,22, as the initial pivotal trials of DES did not include patients with complex lesions. Because of the high rate of restenosis in patients who had a bare metal stent implanted, especially in total occlusions, the use of DES in recanalised total occlusions would be expected to provide a considerable advantage by reducing the risk of restenosis and the need for repeat revascularisation. Later registries including first- and second-generation DES have confirmed the safety and efficacy of stents in recanalising total coronary occlusions.
The rate of ST in this analysis was low, considering the fact that all studies contributing to the pooled results included unselected patient populations. We have recently reported favourable outcomes of patients who had R-ZES implanted in all-comer patients and in patients with total coronary occlusions5,7. In patients with total occlusions, the rate of definite and probable ST was higher in patients with recent occlusions, interpreted as a consequence of an increased thrombogenic nature of these lesions. This trend is seen in the present trial, although it did not reach statistical difference (Figure 3).
The present study describes long-term outcomes of patients with a variety of clinical conditions treated with R-ZES and compares patient outcome in those treated for non-occluded versus occluded target lesions. Both the level of TLF and the occurrence of ST reported in our study, especially late ST, are considerably lower than long-term reports after implantation of first-generation DES in all-comer populations23,24. This reduction may reflect that the excessive inhibition of neointimal growth seen after implantation of first-generation stents causing delayed vascular healing was modulated by introduction of new DES. The balance between complete neointimal stent strut coverage and hyperplasia has been optimised over the years, using stents with thinner struts and altered drugs and drug release characteristics. So, while previous studies of first-generation DES have reported a constant occurrence of ST up to 10 years after PCI, the levelling off of ST over time, as demonstrated in the present study, may represent an effect of the above-described combination of both an improved stent design including altered drug kinetics and thinner stent struts in comparison with the original DES25,26. The impact of a biodegradable polymer, originally created to reduce the risk of ST, is still a matter of debate, because long-term follow-up of patient cohorts with these stents implanted is still outstanding27.
There is recent evidence of a lack of association between interrupted dual antiplatelet therapy and development of ST with the use of newer DES28,29. However, because of the very low level of ST observed in the present study, we are unable to draw any conclusion about an association or lack of connection between ST and interrupted antiplatelet therapy. Indeed, shortening of the duration of dual antiplatelet therapy seems safer after implantation of newer DES compared with first-generation DES.
Limitations
The current analysis was not pre-specified. The RESOLUTE International trial followed patients up to three years, whereas the other three trials followed patients to five years. All patients in the RESOLUTE China RCT and 20% of those in RESOLUTE All Comers had angiographic follow-up at 9-13 months.
Because we only report clinical outcomes in patients with recanalised vessels, our clinical outcome results cannot be extrapolated to a general population of patients with CTO. On the other hand, the total stent length in our CTO patients (median length 42 mm) indicates that the lesions were not simple to treat.
Our results originate from pooled data from two randomised trials and two registries, all of which had independent monitoring, that was probably most thorough in the randomised trials, especially with regard to screening of patients for eligibility. Enrolment in the RESOLUTE All Comers trial was close to 50% of all patients treated with PCI at the participating centres; we expect this percentage to have been even higher in the remaining three studies, especially the registries. So, patients were not entirely treated by experienced operators. Still, a certain selection bias towards a favourable outcome in the overall enrolment cannot be ruled out, and our results should be interpreted accordingly. In addition, more research is warranted to focus on the impact of zotarolimus versus other -limus drugs on the beneficial outcome in patients with occluded coronary arteries treated with PCI.
As previously mentioned, incomplete revascularisation of patients with CTO is associated with an increase in future events, especially new or repeat revascularisations. We were not able to report the more patient-oriented endpoint of all-cause mortality, or any MI and revascularisation, known to occur approximately twice as often as our primary endpoint of TLF.
Conclusions
Long-term TLF was similar in patients with both rec-TO and CTO lesions compared with patients with non-TO lesions, suggesting that R-ZES implantation is safe and effective in patients with total occlusions.
Impact on daily practice
Our results indicate that patients who have their occluded coronary artery recanalised and the Resolute zotarolimus-eluting stent implanted have the same long-term clinical outcome compared with those who have this stent implanted in non-occluded vessels. Whether this applies to all new-generation coronary stents is unknown.
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Acknowledgments
Acknowledgements
The authors thank Sandeep Brar, MD, and Lisa Bousquette, MS, for contributions to study management, and Beth Ferri, PhD, CMPP, for editorial support, all from Medtronic.
Funding
The trials pooled in this post hoc analysis were funded by Medtronic.
Conflict of interest statement
H. Kelbæk has received research grant support from Medtronic Inc. R. Yeh has received grant support/research contract/consultant fee/honoraria/speaker’s bureau from Abbott Vascular, Abiomed, Boston Scientific Corporation, Medtronic Inc, Asahi Intecc and Teleflex. F.-J. Neumann reports grants from Medtronic, during the conduct of the study, personal fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim and Daiichi Sankyo, grants and personal fees from Pfizer, Biotronik, Edwards Lifesciences, and Bayer Healthcare, grants from Medtronic, Abbott Vascular, GlaxoSmithKline, and Boston Scientific, and personal fees from Novartis, Ferrer, and The Medicines Company, outside the submitted work. P.W. Serruys has received consultant fee/honoraria/speaker’s bureau from Abbott Vascular, Biosensors, Medtronic, Micell Technologies, Sino Medical Sciences, Philips Volcano, HeartFlow, Xeltis, and Sahayanand Medical Technologies (SMT), outside the submitted work. S. Windecker receives research and educational grants to his institution from Abbott, Amgen, BMS, Bayer, Biotronik, Boston Scientific, CSL Behring, Edwards Lifesciences, Medtronic, Sinomed and Polares. M. Liu is an employee of Medtronic Inc and owns Medtronic stock. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.
Abbreviations
- CTO
chronic total occlusions
- DES
drug-eluting stent
- non-TO
not totally occluded
- PCI
percutaneous coronary intervention
- R-ZES
Resolute zotarolimus-eluting stent
- RCT
randomised controlled trial
- rec-TO
recently totally occluded
- ST
stent thrombosis
- TLF
target lesion failure
- TLR
target lesion revascularisation
- TVF
target vessel failure
- TVMI
target vessel myocardial infarction
Contributor Information
Henning Kelbæk, Department of Cardiology, Zealand University Hospital, Roskilde, Denmark.
Robert W. Yeh, Smith Center for Outcomes Research in Cardiology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Thomas Engstrøm, Department of Cardiology, The Heart Centre, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
Franz-Josef Neumann, Department of Cardiology and Angiology II, University Heart Center, Bad Krozingen, Germany.
Patrick Serruys, Imperial College London, London, United Kingdom; Department of Cardiology, National University of Ireland, Galway, Ireland.
Stephan Windecker, Department of Interventional Cardiology, Bern University Hospital, Bern, Switzerland.
Jorge A. Belardi, Division of Cardiology, Instituto Cardiovascular de Buenos Aires, Buenos Aires, Argentina.
Shubin Qiao, Fu Wai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, Beijing, China.
Bo Xu, Fu Wai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, Beijing, China.
Minglei Liu, Coronary and Structural Heart, Medtronic PLC, Santa Rosa, CA, USA.
Sigmund Silber, Department of Cardiology, Isar Heart Center, Munich, Germany.
References
- Brilakis ES, Burke MN. Chronic Total Occlusion Percutaneous Coronary Intervention. Circ Cardiovasc Interv. 2018;11:e007362. doi: 10.1161/CIRCINTERVENTIONS.118.007362. [DOI] [PubMed] [Google Scholar]
- Kelbæk H, Helqvist S, Thuesen L, Klovgaard L, Jorgensen E, Saunamäki K, Krusell LR, Botker HE, Engstrom T, Jensen GV SCANDSTENT investigators. Sirolimus versus bare metal stent implantation in patients with total coronary occlusions: subgroup analysis of the Stenting Coronary Arteries in Non-Stress/Benestent Disease (SCANDSTENT) trial. Am Heart J. 2006;152:882–6. doi: 10.1016/j.ahj.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Rubartelli P, Petronio AS, Guiducci V, Sganzerla P, Bolognese L, Galli M, Sheiban I, Chirillo F, Ramondo A, Bellotti Gruppo Italiano di Studio sullo Stent nelle Occlusioni Coronariche II GISE Investigators. Comparison of sirolimus-eluting and bare metal stent for treatment of patients with total coronary occlusions: results of the GISSOC II-GISE multicentre randomized trial. Eur Heart J. 2010;31:2014–20. doi: 10.1093/eurheartj/ehq199. [DOI] [PubMed] [Google Scholar]
- Jia H, Hu S, Liu H, Zhu Y, Zhe CY, Li L, Mustafina I, Hou J, Zhang S, Yu B. Chronic total occlusion is associated with a higher incidence of malapposition and uncovered stent struts: OCT findings at 6 months following DES implantation. Catheter Cardiovasc Interv. 2017;89:582–91. doi: 10.1002/ccd.26969. [DOI] [PubMed] [Google Scholar]
- Kelbæk H, Holmvang L, Richardt G, Eberli FR, Stella P, Buszman PE, Neumann FJ, Serruys PW, Windecker S, Widimský P, Belardi JA, Silber S. Clinical results with the Resolute zotarolimus-eluting stent in total coronary occlusions. EuroIntervention. 2015;11:650–7. doi: 10.4244/EIJY14M07_14. [DOI] [PubMed] [Google Scholar]
- Raungaard B, Jensen LO, Tilsted HH, Christiansen EH, Maeng M, Terkelsen CJ, Krusell LR, Kaltoft A, Kristensen SD, Botker HE, Thuesen L, Aaroe J, Jensen SE, Villadsen AB, Thayssen P, Veien KT, Hansen KN, Junker A, Madsen M, Ravkilde J and Lassen JF. Zotarolimus-eluting durable-polymer-coated stent versus a biolimus-eluting biodegradable-polymer-coated stent in unselected patients undergoing percutaneous coronary intervention (SORT OUT VI): a randomised non-inferiority trial. Lancet. 2015;385:1527–35. doi: 10.1016/S0140-6736(14)61794-3. [DOI] [PubMed] [Google Scholar]
- Serruys PW, Silber S, Garg S, van Geuns RJ, Richardt G, Buszman PE, Kelbaek H, van Boven AJ, Hofma SH, Linke A, Klauss V, Wijns W, Macaya C, Garot P, DiMario C, Manoharan G, Kornowski R, Ischinger T, Bartorelli A, Ronden J, Bressers M, Gobbens P, Negoita M, van Leeuwen F and Windecker S. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med. 2010;363:136–46. doi: 10.1056/NEJMoa1004130. [DOI] [PubMed] [Google Scholar]
- von Birgelen C, Basalus MW, Tandjung K, van Houwelingen KG, Stoel MG, Louwerenburg JH, Linssen GC, Saïd SA, Kleijne MA, Sen H, Löwik MM, van der Palen J, Verhorst PM and de Man FH. A randomized controlled trial in second-generation zotarolimus-eluting Resolute stents versus everolimus-eluting Xience V stents in real-world patients: the TWENTE trial. J Am Coll Cardiol. 2012;59:1350–61. doi: 10.1016/j.jacc.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Birgelen C, Sen H, Lam MK, Danse PW, Jessurun GA, Hautvast RW, Houwelingen GK, Schramm AR, Gin RM, Louwerenburg JW, Man FH, Stoel MG, Löwik MM, Linssen GC, Saïd SA, Nienhuis MB, Verhorst PM, Basalus MW, Doggen CJ and Tandjung K. Third-generation zotarolimus-eluting and everolimus-eluting stents in all-comer patients requiring a percutaneous coronary intervention (DUTCH PEERS): a randomised, single-blind, multicentre, non-inferiority trial. Lancet. 2014;383:413–23. doi: 10.1016/S0140-6736(13)62037-1. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Serruys PW, Silber S, Kelbaek H, Richardt G, Morel MA, Negoita M, Buszman PE, Windecker S. Comparison of zotarolimus-and everolimus-eluting coronary stents: Final 5-year report of the RESOLUTE all-comers trial. Circ Cardiovasc Interv. 2015;8:e002230. doi: 10.1161/CIRCINTERVENTIONS.114.002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardi JA, Widimský P, Neumann FJ, Mauri L, Albertal M RESOLUTE International Investigators. Real-world safety and effectiveness outcomes of a zotarolimus-eluting stent: final 3-year report of the RESOLUTE International study. J Interv Cardiol. 2013;26:515–23. doi: 10.1111/joic.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann FJ, Widimský P, Belardi JA. One-year outcomes of patients with the zotarolimus-eluting coronary stent: RESOLUTE International Registry. EuroIntervention. 2012;7:1181–8. doi: 10.4244/EIJV7I10A189. [DOI] [PubMed] [Google Scholar]
- Xu B, Yang Y, Yuan Z, Du Z, Wong SC, Genereux P, Lu China RCT Investigators. Zotarolimus- and paclitaxel-eluting stents in an all-comer population in China: the RESOLUTE China randomized controlled trial. JACC Cardiovasc Interv. 2013;6:664–70. doi: 10.1016/j.jcin.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Qiao S, Chen L, Chen S, Wang W, Zhu G. One-year outcomes from an all-comers Chinese population of patients implanted with the Resolute zotarolimus-eluting stent. Am J Cardiol. 2014;113:613–20. doi: 10.1016/j.amjcard.2013.10.042. [DOI] [PubMed] [Google Scholar]
- Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- Holmvang L, Kelbæk H, Kaltoft A, Thuesen L, Lassen JF, Clemmensen P, Klovgaard L, Engstrom T, Botker HE, Saunamäki K, Krusell LR, Jorgensen E, Tilsted HH, Christiansen EH, Ravkilde J, Kober L, Kofoed KF, Terkelsen CJ, Helqvist S. Long-term outcome after drug-eluting versus bare-metal stent implantation in patients with ST-segment elevation myocardial infarction: 5 years follow-up from the randomized DEDICATION trial (Drug Elution and Distal Protection in Acute Myocardial Infarction). JACC Cardiovasc Interv. 2013;6:548–53. doi: 10.1016/j.jcin.2012.12.129. [DOI] [PubMed] [Google Scholar]
- Sabate M, Brugaletta S, Cequier A, Iñiguez A, Serra A, Jiménez-Quevedo P, Mainar V, Campo G, Tespili M, den Heijer P, Bethencourt A, Vazquez N, van Es GA, Backx B, Valgimigli M and Serruys PW. Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. Lancet. 2016;387:357–66. doi: 10.1016/S0140-6736(15)00548-6. [DOI] [PubMed] [Google Scholar]
- Galassi AR, Tomasello SD, Reifart N, Werner GS, Sianos G, Bonnier H, Sievert H, Ehladad S, Bufe A, Shofer J, Gershlick A, Hildick-Smith D, Escaned J, Erglis A, Sheiban I, Thuesen L, Serra A, Christiansen E, Buettner A, Costanzo L, Barrano G, Di Mario C. In-hospital outcomes of percutaneous coronary intervention in patients with chronic total occlusion: insights from the ERCTO (European Registry of Chronic Total Occlusion) registry. EuroIntervention. 2011;7:472–9. doi: 10.4244/EIJV7I4A77. [DOI] [PubMed] [Google Scholar]
- Lee SW, Lee PH, Ahn JM, Park DW, Yun SC, Han S, Kang H, Kang SJ, Kim YH, Lee CW, Park SW, Hur SH, Rha SW, Her SH, Choi SW, Lee BK, Lee NH, Lee JY, Cheong SS, Kim MH, Ahn YK, Lim SW, Lee SG, Hiremath S, Santoso T, Udayachalerm W, Cheng JJ, Cohen DJ, Muramatsu T, Tsuchikane E, Asakura Y, Park SJ. Randomized Trial Evaluating Percutaneous Coronary Intervention for the Treatment of Chronic Total Occlusion. Circulation. 2019;139:1674–83. doi: 10.1161/CIRCULATIONAHA.118.031313. [DOI] [PubMed] [Google Scholar]
- Sapontis J, Salisbury AC, Yeh RW, Cohen DJ, Hirai T, Lombardi W, McCabe JM, Karmpaliotis D, Moses J, Nicholson WJ, Pershad A, Wyman RM, Spaedy A, Cook S, Doshi P, Federici R, Thompson CR, Marso SP, Nugent K, Gosch K, Spertus JA, Grantham JA. Early Procedural and Health Status Outcomes After Chronic Total Occlusion Angioplasty: A Report From the OPEN-CTO Registry (Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures). JACC Cardiovasc Interv. 2017;10:1523–34. doi: 10.1016/j.jcin.2017.05.065. [DOI] [PubMed] [Google Scholar]
- Colmenarez HJ, Escaned J, Fernández C, Lobo L, Cano S, del Angel JG, Alfonso F, Jimenez P, Banuelos C, Gonzalo N, Garcia E, Hernandez R, Macaya C. Efficacy and safety of drug-eluting stents in chronic total coronary occlusion recanalization: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1854–66. doi: 10.1016/j.jacc.2009.12.038. [DOI] [PubMed] [Google Scholar]
- Suttorp MJ, Laarman GJ, Rahel BM, Kelder JC, Bosschaert MA, Kiemeneij F, ten Berg JM, Bal ET, Rensing BJ, Eefting FD and Mast EG. Primary Stenting of Totally Occluded Native Coronary Arteries II (PRISON II): a randomized comparison of bare metal stent implantation with sirolimus-eluting stent implantation for the treatment of total coronary occlusions. Circulation. 2006;114:921–8. doi: 10.1161/CIRCULATIONAHA.106.613588. [DOI] [PubMed] [Google Scholar]
- Kandzari DE, Kini AS, Karmpaliotis D, Moses JW, Tummala PE, Grantham JA, Orr C, Lombardi W, Nicholson WJ, Lembo NJ, Popma JJ, Wang J, Larracas C, Rutledge DR. Safety and Effectiveness of Everolimus-Eluting Stents in Chronic Total Coronary Occlusion Revascularization: Results From the EXPERT CTO Multicenter Trial (Evaluation of the XIENCE Coronary Stent, Performance, and Technique in Chronic Total Occlusions). JACC Cardiovasc Interv. 2015;8:761–9. doi: 10.1016/j.jcin.2014.12.238. [DOI] [PubMed] [Google Scholar]
- Kandzari DE, Rao SV, Moses JW, Džavík V, Strauss BH, Kutryk MJ, Simonton CA, Garg J, Lokhnygina Y, Mancini GB, Yeoh E, Buller CE ACROSS/TOSCA-4 Investigators. Clinical and angiographic outcomes with sirolimus-eluting stents in total coronary occlusions: the ACROSS/TOSCA-4 (Approaches to Chronic Occlusions With Sirolimus-Eluting Stents/Total Occlusion Study of Coronary Arteries-4) trial. JACC Cardiovasc Interv. 2009;2:97–106. doi: 10.1016/j.jcin.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Galløe AM, Kelbæk H, Thuesen L, Hansen HS, Ravkilde J, Hansen PR, Christiansen EH, Abildgaard U, Stephansen G, Lassen JF, Engstrøm T, Jensen JS, Jeppesen JL, Bligaard N SORT OUT II Investigators. 10-Year Clinical Outcome After Randomization to Treatment by Sirolimus- or Paclitaxel-Eluting Coronary Stents. J Am Coll Cardiol. 2017;69:616–24. doi: 10.1016/j.jacc.2016.11.055. [DOI] [PubMed] [Google Scholar]
- Yamaji K, Räber L, Zanchin T, Spitzer E, Zanchin C, Pilgrim T, Stortecky S, Moschovitis A, Billinger M, Schönenberger C, Eberli F, Jüni P, Lüscher TF, Heg D, Windecker S. Ten-year clinical outcomes of first-generation drug-eluting stents: the Sirolimus-Eluting vs. Paclitaxel-Eluting Stents for Coronary Revascularization (SIRTAX) VERY LATE trial. Eur Heart J. 2016;37:3386–95. doi: 10.1093/eurheartj/ehw343. [DOI] [PubMed] [Google Scholar]
- Bangalore S. The Elusive Late Benefit of Biodegradable Polymer Drug-Eluting Stents. Circulation. 2019;139:334–6. doi: 10.1161/CIRCULATIONAHA.118.038378. [DOI] [PubMed] [Google Scholar]
- Kolandaivelu K, Swaminathan R, Gibson WJ, Kolachalama VB, Nguyen-Ehrenreich KL, Giddings VL, Coleman L, Wong GK, Edelman ER. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123:1400–9. doi: 10.1161/CIRCULATIONAHA.110.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmerini T, Sangiorgi D, Valgimigli M, Biondi-Zoccai G, Feres F, Abizaid A, Costa RA, Hong MK, Kim BK, Jang Y, Kim HS, Park KW, Mariani A, Della Riva D, Généreux P, Leon MB, Bhatt DL, Bendetto U, Rapezzi C, Stone GW. Short- versus long-term dual antiplatelet therapy after drug-eluting stent implantation: an individual patient data pairwise and network meta-analysis. J Am Coll Cardiol. 2015;65:1092–102. doi: 10.1016/j.jacc.2014.12.046. [DOI] [PubMed] [Google Scholar]