Abstract
Aims
We aimed to evaluate the validity of the Academic Research Consortium for High Bleeding Risk (ARC-HBR) criteria for East Asian patients undergoing contemporary percutaneous coronary intervention (PCI) from the PENDULUM registry.
Methods and results
This post hoc analysis included 6,267 Japanese patients undergoing PCI between December 2015 and June 2017 enrolled in PENDULUM. The primary endpoint was the incidence of major bleeding at 12 months post index PCI. In total, 3,185 (50.8%) and 3,082 (49.2%) patients were stratified to the ARC-HBR and non-ARC-HBR groups, respectively, and almost all patients had overlapping criteria. Incidence of major bleeding was 4.2% versus 1.4% in the ARC-HBR group versus the non-ARC-HBR group (hazard ratio 3.00 [95% confidence interval: 2.11-4.27]; p<0.001). As the number of overlapping ARC-HBR criteria increased, the incidence of major bleeding also increased. In contrast, the incidence of major bleeding was 4.2% for one major criterion, 2.1% for two minor criteria. Multivariate analysis suggested that severe CKD, anticoagulant use, acute coronary syndrome, low body weight and heart failure were independent predictors of major bleeding.
Conclusions
Half of the Japanese patients who underwent PCI in the PENDULUM registry met the ARC-HBR criteria, and many patients had overlapping criteria. ARC-HBR criteria are applicable to Japanese patients undergoing contemporary PCI.
Introduction
Advances in percutaneous coronary intervention (PCI)-related technologies have allowed patients with increasingly complex medical conditions to be treated with PCI, resulting in more challenging post-PCI management1. As appropriate antiplatelet therapy is a cornerstone of PCI management, assessment of thrombotic and bleeding risk is essential2,3. The Academic Research Consortium for High Bleeding Risk (ARC-HBR) initiative aimed to define HBR in patients undergoing PCI through literature review and clinical consensus, thus enabling more consistent and higher quality clinical study data collection and reporting, and facilitating appropriate clinical practice recommendations or regulatory decisions1,4. Although not fully validated, the ARC-HBR criteria are a convenient tool for use in clinical practice because they do not require scores to be calculated and include factors that are not traditionally considered as risk factors.
Previous studies in East Asian patients have reported different risk profiles for thrombosis and bleeding compared with Western patients5. A lower dose of antiplatelet therapy was recommended for patients in Asian countries, owing to concerns with respect to a greater risk of bleeding6. However, the ARC consensus document suggests that there is a paucity of data in East Asians, stating that more research is required to elucidate the applicability of the ARC definition of HBR to Asian populations1.
The PENDULUM (Platelet rEactivity in patieNts with DrUg eLUting stent and balancing risk of bleeding and ischeMic event) registry represents contemporary PCI practice, implementing a transradial approach and proton pump inhibitors (PPIs) in daily clinical practice7. The objective of this analysis was to evaluate the applicability of ARC-HBR criteria for Japanese patients participating in the PENDULUM registry, and to explore criteria related to HBR in Japanese patients.
Methods
STUDY DESIGN AND PATIENT POPULATION
The PENDULUM registry (UMIN 000020332) was a prospective, multicentre study of Japanese patients who underwent PCI in a real-world setting. The study protocol was approved by the appropriate ethics panel at each participating centre, and the study was performed in accordance with the principles of the Declaration of Helsinki and applicable Japanese guidelines. All patients provided written informed consent.
Full details of the PENDULUM registry have been described previously7 and are also provided in Supplementary Table 1. In brief, inclusion criteria were age ≥20 years, an indication for PCI with a second-generation drug-eluting stent (DES), and receipt of an antiplatelet treatment. The type and dose of antiplatelet drug administered to patients were at the investigator’s discretion. There were no limitations placed on the treatment of any complications arising during follow-up.
ENDPOINTS
The primary endpoint for this analysis was the cumulative incidence of major bleeding at 12 months post index PCI. Major bleeding was defined as Bleeding Academic Research Consortium (BARC) types 3 and 58. The secondary endpoint was the cumulative incidence of intracranial haemorrhage (ICH). ICH was defined as a non-ischaemic stroke (e.g., cerebral haemorrhage, subarachnoid haemorrhage) with neurologic symptoms or with newly developed signs, and where the culprit lesion was detected by computed tomography or magnetic resonance imaging scans. Bleeding events were evaluated by independent assessment committees.
ANALYSIS GROUPS AND DEFINITIONS
The PENDULUM registry7 was a prospective registry that was initiated in 2015, prior to the publication of the ARC-HBR criteria in 20191. Therefore, we have integrated the prospectively collected PENDULUM data into a post hoc retrospective criterion analysis; no additional data were collected for this post hoc analysis.
For the post hoc criterion analysis, enrolled patients were retrospectively stratified into HBR and non-HBR groups according to ARC-HBR criteria. However, because the PENDULUM registry was initiated prior to the ARC-HBR publication, it did not collect data on all the specified ARC-HBR criteria. Thus, for our post hoc analysis, the HBR categories were modified (Supplementary Table 2). Scores were calculated by allocating one point for each major criterion and 0.5 points for each minor criterion. The cumulative incidence of major bleeding was calculated for each ARC-HBR criterion. In addition, for patients with a score of 0.5 to 1.5, the cumulative incidence of major bleeding was calculated for each combination of criteria.
STATISTICAL ANALYSIS
For individual ARC-HBR criteria and clinically relevant variables, the one-year cumulative incidence of major bleeding and ICH was estimated using the Kaplan-Meier method. Hazard ratios (HR) and 95% confidence intervals (CI) were generated with Cox proportional hazards regression models. Univariate and multivariate Cox proportional hazards regression models were used to identify independent predictors of major bleeding. Covariates included in the multivariate model were the ARC-HBR criteria, criteria which were considered to be clinically important, and the criteria that showed ≥4% cumulative incidence in the stratified analysis, which were not procedure-related but were clinically important. Discrimination of the bleeding risk score was assessed by C-statistics. The area under the curve as well as predictive BARC 3 or 5 bleeding probabilities were compared. All tests were two-sided with a 5% level of significance. Statistical analyses were performed using SAS, Release 9.4 (SAS Institute, Cary, NC, USA).
Results
PATIENT POPULATION
A total of 6,267 patients were enrolled in the PENDULUM registry, of whom 3,185 (50.8%) were in the HBR group and 3,082 (49.2%) were in the non-HBR group (Figure 1A, Table 1). The overall mean age was 70 years, and 37.1% were ≥75 years old. The HBR group included more patients who were older (≥75 years), had diabetes, and did not present with acute coronary syndrome (Figure 1B, Table 1). Baseline laboratory parameters are described in Supplementary Table 3. Dual antiplatelet therapy (DAPT) was continued for 12 months after index PCI in 72.4% of HBR patients and in 84.1% of non-HBR patients (p<0.001). Details of DAPT adherence over time are provided in Supplementary Figure 1. Except for some factors (age ≥75 years, moderate anaemia, and moderate chronic kidney disease), the proportion of patients with a single ARC-HBR criterion was only approximately 10% for each criterion; almost all patients had overlapping ARC-HBR criteria (Supplementary Figure 2).
Figure 1.
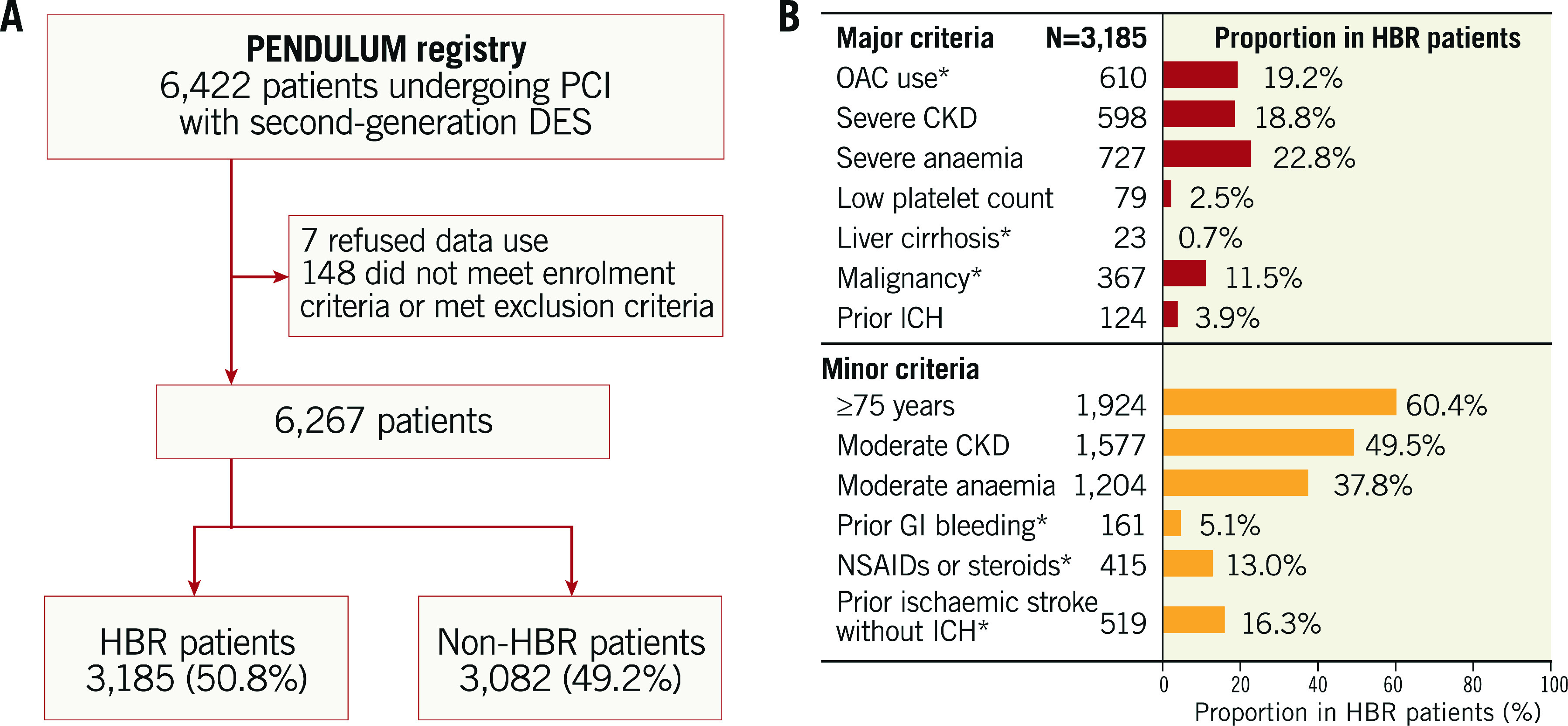
Patient disposition (A) and proportion of HBR patients by each ARC-HBR criterion (B). *Modified from the original ARC-HBR criteria. ARC: Academic Research Consortium; CKD: chronic kidney disease; DES: drug-eluting stent; HBR: high bleeding risk; NSAID: non-steroidal anti-inflammatory drug; OAC: oral anticoagulant; PCI: percutaneous coronary intervention
Table 1. Baseline demographic and clinical characteristics.
| Characteristics | Total (N=6,267) | ARC-HBR (n=3,185) | Non-ARC-HBR (n=3,082) | p-value (ARC-HBR vs non-ARC-HBR) | |
| Age, years | 70.0 (10.7) | 74.9 (9.2) | 65.0 (9.9) | <0.001 | |
| ≥75 | 2,324 (37.1) | 1,924 (60.4) | 400 (13.0) | <0.001 | |
| Sex, male | 4,909 (78.3) | 2,332 (73.2) | 2,577 (83.6) | <0.001 | |
| Body weight, kg | 64.0 (12.6) | 60.8 (12.0) | 67.2 (12.4) | <0.001 | |
| ≤50 | 794 (12.7) | 583 (18.3) | 211 (6.8) | <0.001 | |
| Body mass index, kg/m² | 24.2 (3.6) | 23.7 (3.6) | 24.8 (3.6) | <0.001 | |
| Hypertension | 5,186 (82.8) | 2,773 (87.1) | 2,413 (78.3) | <0.001 | |
| Hyperlipidaemia | 4,919 (78.5) | 2,402 (75.4) | 2,517 (81.7) | <0.001 | |
| Diabetes mellitus | 2,767 (44.2) | 1,515 (47.6) | 1,252 (40.6) | <0.001 | |
| Current smoker | 1,327 (21.2) | 449 (14.1) | 878 (28.5) | <0.001 | |
| Heart failurea | 850 (13.6) | 642 (20.2) | 208 (6.7) | 0.056 | |
| Peripheral artery disease | 421 (6.7) | 324 (10.2) | 97 (3.1) | <0.001 | |
| Atrial fibrillation | 538 (8.6) | 477 (15.0) | 61 (2.0) | <0.001 | |
| Malignancy | 367 (5.9) | 367 (11.5) | 0 (0.0) | <0.001 | |
| History of myocardial infarction | 1,575 (25.1) | 825 (25.9) | 750 (24.3) | 0.128 | |
| History of PCI | 2,567 (41.0) | 1,361 (42.7) | 1,206 (39.1) | <0.05 | |
| History of coronary artery bypass grafting | 265 (4.2) | 179 (5.6) | 86 (2.8) | <0.001 | |
| History of ischaemic stroke | 655 (10.5) | 557 (17.5) | 98 (3.2) | <0.001 | |
| History of transient ischaemic attack | 80 (1.3) | 55 (1.7) | 25 (0.8) | <0.001 | |
| History of ICH | 124 (2.0) | 124 (3.9) | 0 (0.0) | <0.001 | |
| History of gastrointestinal bleeding | 183 (2.9) | 161 (5.1) | 22 (0.7) | <0.001 | |
| Clinical presentation | Non-ACS | 4,252 (67.8) | 2,262 (71.0) | 1,990 (64.6) | <0.001 |
| ACS | 2,015 (32.2) | 923 (29.0) | 1,092 (35.4) | ||
| Unstable angina | 790 (12.6) | 387 (12.2) | 403 (13.1) | 0.270 | |
| Non-STEMI | 323 (5.2) | 165 (5.2) | 158 (5.1) | <0.05 | |
| STEMI | 908 (14.5) | 373 (11.7) | 535 (17.4) | ||
| Medication at discharge | Thienopyridine | 6,195 (98.9) | 3,129 (98.2) | 3,066 (99.5) | <0.001 |
| Clopidogrel | 2,213 (35.3) | 1,333 (41.9) | 880 (28.6) | <0.001 | |
| Prasugrel | 3,921 (62.6) | 1,756 (55.1) | 2,165 (70.2) | ||
| Aspirin | 6,143 (98.0) | 3,092 (97.1) | 3,051 (99.0) | <0.001 | |
| OAC | 610 (9.7) | 610 (19.2) | 0 (0.0) | <0.001 | |
| Proton pump inhibitor | 5,295 (84.5) | 2,679 (84.1) | 2,616 (84.9) | 0.402 | |
| NSAIDs except aspirin | 334 (5.3) | 259 (8.1) | 75 (2.4) | <0.001 | |
| Steroids | 250 (4.0) | 198 (6.2) | 52 (1.7) | <0.001 | |
| Data are presented as n (%) or mean (SD). a Heart failure was defined as either hospitalisation or having a treatment history. ACS: acute coronary syndrome; ARC: Academic Research Consortium; HBR: high bleeding risk; ICH: intracranial haemorrhage; NSAIDs: non-steroidal anti-inflammatory drugs; OAC: oral anticoagulant; PCI: percutaneous coronary intervention; SD: standard deviation; STEMI: ST-elevation myocardial infarction | |||||
CLINICAL OUTCOME AT 12 MONTHS
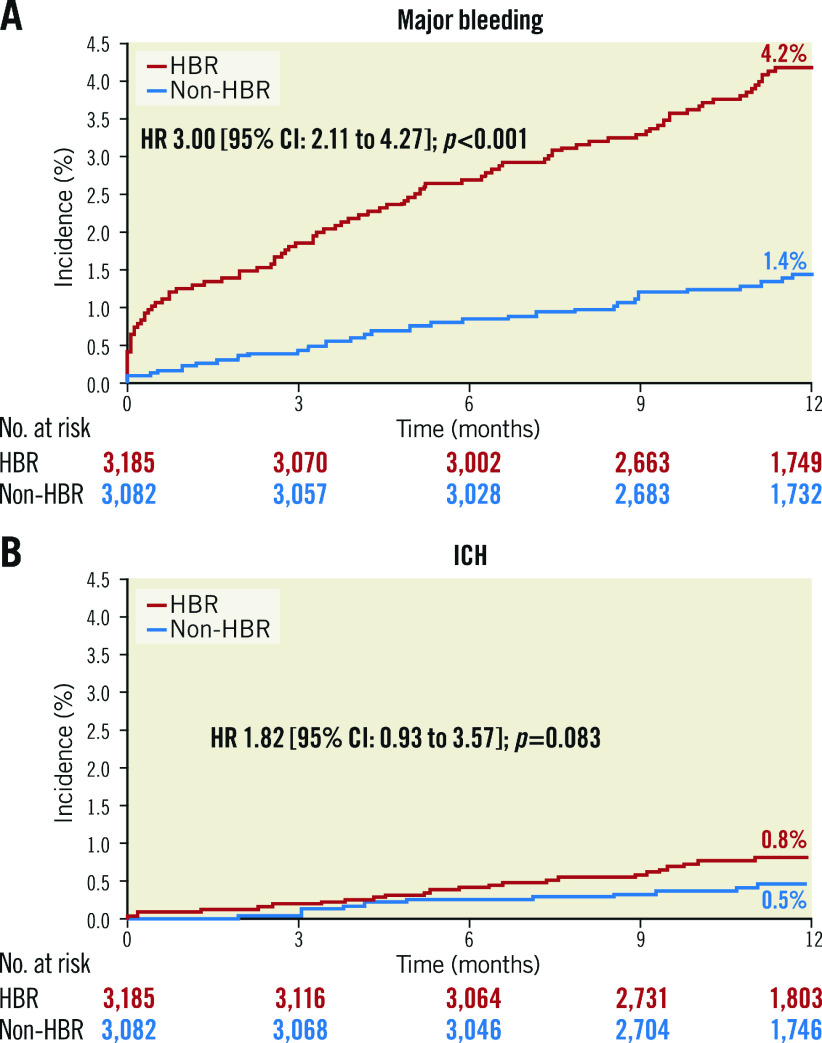
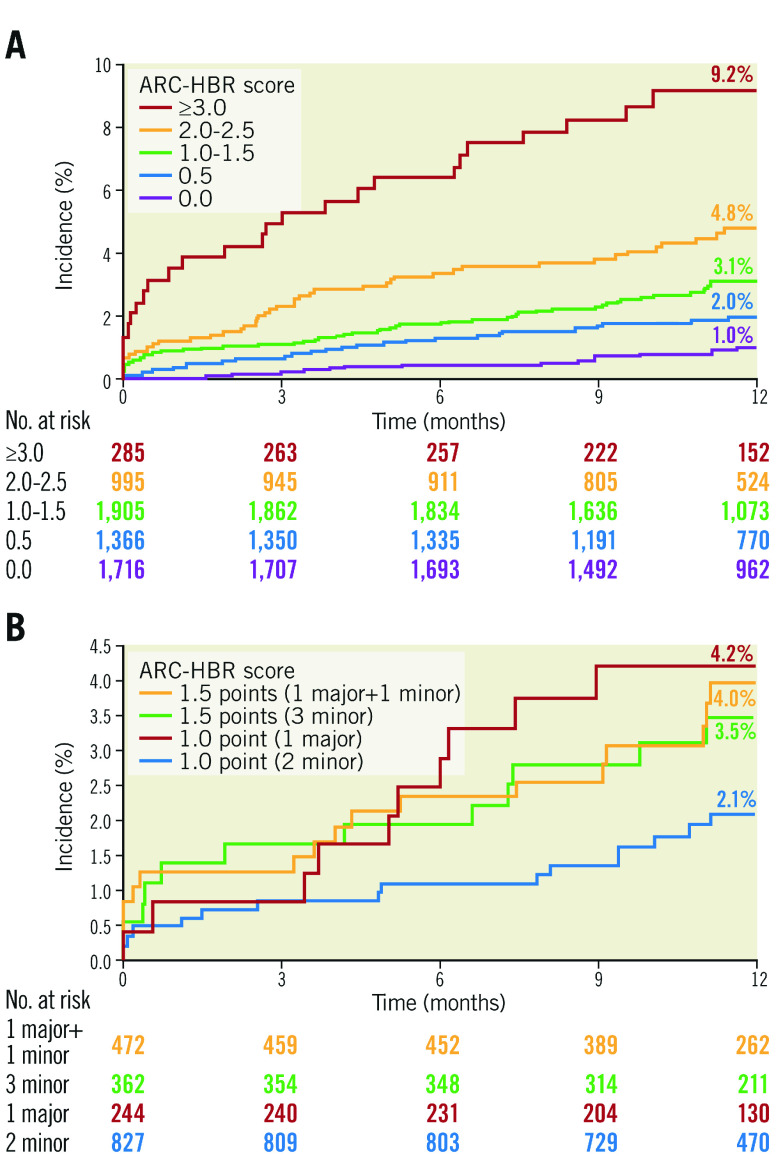
The cumulative incidence of major bleeding at 12 months post PCI was significantly higher in HBR patients than non-HBR patients (4.2% vs 1.4%; HR 3.00 [95% CI: 2.11 to 4.27]; p<0.001) (Figure 2A), as was the incidence of ICH (0.8% vs 0.5%; HR 1.82 [95% CI: 0.93 to 3.57]; p=0.083) (Figure 2B). As the number of overlapping ARC-HBR criteria increased, the cumulative incidence of major bleeding also increased. In contrast, the incidence of major bleeding was 4.2% for one major criterion, 2.1% for two minor criteria (Figure 3).
Figure 2.
Cumulative incidence of major bleeding (A) and ICH (B) by ARC-HBR status. CI: confidence interval; HBR: high bleeding risk; HR: hazard ratio; ICH: intracranial haemorrhage
Figure 3.
Cumulative incidence of major bleeding by ARC-HBR score categories; 0 to ≥3.0 points (A), and 1 to 1.5 points (B). ARC: Academic Research Consortium; HBR: high bleeding risk
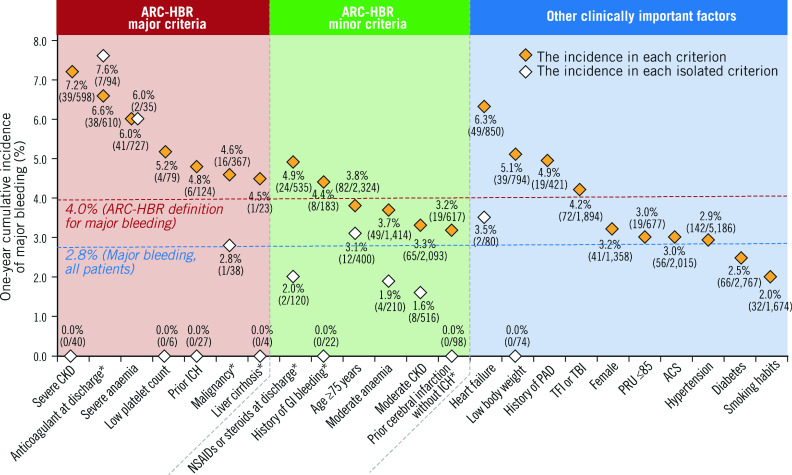
When the cumulative incidence of major bleeding was stratified by ARC-HBR criteria (Figure 4), all of the ARC-HBR major criteria were associated with an incidence of major bleeding of ≥4%; however, among patients who fulfilled a single criterion, the incidence of major bleeding was >4% with anticoagulant use or severe anaemia (major criteria), <4% in those with any of the other major criteria, and <4% for each minor criterion (Figure 4, Supplementary Table 4). The cumulative incidence of ICH stratified by ARC-HBR criteria is shown in Supplementary Figure 3. The adjusted cumulative incidences of major bleeding stratified by ARC-HBR criteria and other clinically important factors are shown in Supplementary Figure 4.
Figure 4.
Cumulative incidence of major bleeding by each ARC-HBR criterion and other clinically important factors. *Modified from the original ARC-HBR criteria. ACS: acute coronary syndrome; ARC: Academic Research Consortium; CKD: chronic kidney disease; GI: gastrointestinal; HBR: high bleeding risk; ICH: intracranial haemorrhage; PAD: peripheral arterial disease; PRU: platelet reactivity unit; TBI: transbrachial intervention; TFI: transfemoral intervention
REGRESSION ANALYSIS TO IDENTIFY INDEPENDENT PREDICTORS OF MAJOR BLEEDING EVENTS
Based on the data indicating that, in addition to the ARC-HBR criteria, low body weight, heart failure, peripheral arterial disease, and non-radial approach were also associated with an incidence of major bleeding of ≥4%, univariate and multivariate analyses were used to calculate statistically significant independent predictors of major bleeding events (Table 2).
Table 2. Univariate and multivariate regression analysis of major bleeding at one year.
| Variable | Events (%) | Univariate | Multivariate | |||
| HR | 95% CI | HR | 95% CI | |||
| Age, years; ≥75 | 82 (3.5) vs 83 (2.1) | 1.714 | 1.263-2.325 | 0.991 | 0.656-1.497 | |
| Sex; male | 124 (2.5) vs 41 (3.0) | 0.828 | 0.581-1.178 | 1.292 | 0.793-2.102 | |
| Body weight, kg; ≤50 | 39 (4.9) vs 120 (2.3) | 2.260 | 1.574-3.243 | 1.849 | 1.125-3.039 | |
| Hypertension | 142 (2.7) vs 23 (2.1) | 1.272 | 0.819-1.976 | 0.980 | 0.586-1.639 | |
| Diabetes mellitus | 66 (2.4) vs 99 (2.8) | 0.846 | 0.620-1.155 | 0.772 | 0.526-1.135 | |
| Current smoker | 32 (1.9) vs 109 (2.8) | 0.694 | 0.468-1.029 | 0.756 | 0.478-1.193 | |
| Heart failure | 49 (5.8) vs 116 (2.1) | 2.813 | 2.014-3.928 | 1.871 | 1.219-2.872 | |
| Peripheral arterial disease | 19 (4.5) vs 146 (2.5) | 1.824 | 1.131-2.943 | 1.437 | 0.806-2.560 | |
| Malignancy | 16 (4.4) vs 149 (2.5) | 1.787 | 1.067-2.992 | 1.338 | 0.690-2.595 | |
| Liver cirrhosis | 1 (4.3) vs 164 (2.6) | 1.734 | 0.243-12.384 | 1.388 | 0.183-10.548 | |
| History of ischaemic stroke without ICH | 19 (3.1) vs 135 (2.5) | 1.247 | 0.772-2.016 | – | – | |
| History of ICH | 6 (4.8) vs 148 (2.5) | 1.950 | 0.862-4.410 | – | – | |
| History of ischaemic stroke or ICH | 25 (3.4) vs 129 (2.4) | 1.399 | 0.912-2.147 | 1.059 | 0.639-1.753 | |
| History of gastrointestinal bleeding | 8 (4.4) vs 142 (2.5) | 1.802 | 0.884-3.674 | 1.228 | 0.535-2.818 | |
| ACS | 56 (2.8) vs 109 (2.6) | 1.105 | 0.800-1.525 | 1.480 | 1.010-2.169 | |
| Haemoglobina, g/dL | <11 | 41 (5.6) vs 69 (1.7) | 3.388 | 2.302-4.986 | 1.756 | 1.022-3.017 |
| Male: ≥11 to <13; Female: ≥11 to <12 | 49 (3.5) vs 69 (1.7) | 2.017 | 1.398-2.909 | 1.529 | 0.984-2.377 | |
| eGFRb, mL/min/1.73 m2 | <30 | 39 (6.5) vs 59 (1.7) | 3.991 | 2.663-5.981 | 1.999 | 1.131-3.534 |
| ≥30 to <60 | 65 (3.1) vs 59 (1.7) | 1.823 | 1.282-2.594 | 1.274 | 0.827-1.961 | |
| Platelet count, ×10 4 /μL; <10 | 4 (5.1) vs 154 (2.6) | 2.129 | 0.789-5.740 | 1.339 | 0.412-4.354 | |
| PRU value for 12-48 hours after initial PCI | >208 | 66 (3.0) vs 88 (2.4) | 1.250 | 0.908-1.719 | – | – |
| >85 | 135 (2.6) vs 19 (2.8) | 0.918 | 0.568-1.484 | – | – | |
| Puncture site; except radial | 72 (3.8) vs 93 (2.1) | 1.825 | 1.342-2.482 | – | – | |
| Complex PCI | 40 (3.1) vs 125 (2.5) | 1.262 | 0.884-1.802 | – | – | |
| Anticoagulant at discharge | 38 (6.2) vs 127 (2.2) | 2.834 | 1.973-4.072 | 2.506 | 1.619-3.879 | |
| NSAIDs or steroids at discharge | 24 (4.5) vs 141 (2.5) | 1.825 | 1.184-2.813 | 1.381 | 0.772-2.471 | |
| aHazard ratio was calculated using ≥13 g/dL for male and ≥12 g/dL for female as the reference standard. bHazard ratio was calculated using ≥60 mL/min/1.73 m2 as the reference standard. ACS: acute coronary syndrome; CI: confidence interval; eGFR: estimated glomerular filtration rate; HR: hazard ratio; ICH: intracranial haemorrhage; NSAIDs: non-steroidal anti-inflammatory drugs; PCI: percutaneous coronary intervention; PRU: platelet reactivity unit | ||||||
The addition of low body weight and heart failure to the ARC-HBR criteria increased the prevalence of HBR to 57.2% overall. The group of patients who met ARC-HBR criteria and had low body weight or heart failure had a significantly higher cumulative incidence of bleeding events compared with the other patient group (for major bleeding: 4.1% vs 1.2%, HR 3.60 [95% CI: 2.41 to 5.38], p<0.001; for ICH: 0.9% vs 0.4%, HR 2.37 [95% CI: 1.12 to 5.02], p<0.05) (Supplementary Figure 5). The C-indices did not improve when low body weight and heart failure were added into the ARC-HBR criteria; however, both ARC-HBR and ARC-HBR with low body weight and heart failure had higher sensitivity (but lower specificity) in estimating the occurrence of major bleeding. The PARIS major bleeding score had higher specificity than sensitivity (Supplementary Figure 6).
Discussion
This study was the first to investigate the suitability of ARC-HBR in >6,000 Japanese patients receiving second-generation DES and enrolled in the PENDULUM real-world registry. The main findings of this study are the following: (i) approximately 50% of the Japanese patients who underwent PCI were classified as ARC-HBR patients, and the majority of HBR patients had overlapping criteria; (ii) the ARC-HBR criteria were suitable for Japanese patients in the contemporary PCI era, where patients are commonly managed with strategies such as lower doses of antiplatelet drugs6, a transradial PCI approach9, and use of PPIs; (iii) the multivariate regression analysis suggested that low body weight and heart failure are predictive factors for high bleeding risk but C-indices were not improved.
Ueki et al recently reported that approximately 40% of patients from the Bern Registry fulfilled the ARC-HBR criteria10. In the present study, 50.8% of patients (3,185/6,267) were found to have HBR. In previous reports, the proportion of HBR patients in Japan has been reported to be 43%11. Taken together, it can be assumed that the incidence of HBR is approximately 50% in Japanese daily practice. Patient characteristics were similar between our study and those in the Bern Registry; however, the proportion of patients with overlapping bleeding risk criteria was higher in our study than in the Bern Registry. Only 244 (3.9%) patients had a single major ARC-HBR criterion even though the prevalence of HBR was 51% in this study. Of 4,781 HBR patients, 799 (16.7%) had a single major ARC-HBR criterion in the Bern Registry10. This may suggest that Japanese patients are more likely to have overlapping risk factors. The elucidation of the real impact of each minor criterion warrants further study.
The incidence of major bleeding in our study was lower than that in previous studies. In the Bern Registry, the risk of BARC 3 or 5 bleeding was 6.4% in the HBR group and 1.9% in the non-HBR group10, and in the CREDO-Kyoto registry cohort-211 the risk of major bleeding was 10.4% versus 3.4%, respectively. There are several explanations as to why these differences might be possible. Firstly, the difference in patient demographics should be considered. It is well known that the incidence of major bleeding is higher in acute coronary syndrome patients. In the present study, the proportion of acute coronary syndrome patients was limited to almost 30% compared with almost 50% in the Bern Registry study. In addition, IIb/IIIa antagonist treatment is not available in Japan and the approved doses of antiplatelet regimens in Japan differ from those in other countries. Secondly, technical advances coupled with the improvement of medical management might contribute to the reduction of bleeding risk. It should be noted that the CREDO-Kyoto registry cohort-2 is almost 10 years old, and the procedures and medications used11,12 were different from those used in the present study. Additionally, the hazard risk of bleeding in HBR patients compared to non-HBR patients (three times higher) was in line with the CREDO-Kyoto registry cohort-2 and the Bern Registry.
All of the ARC-HBR major criteria were found to be associated with an incidence of major bleeding ≥4% at one year. The same observation was made in patients who met isolated major criteria of anticoagulant use at discharge or severe anaemia. In our analysis, the incidence of major bleeding in patients with ARC-HBR scores of 1-1.5 was 3.1% (i.e., lower than 4%). Our results showed that, as the number of overlapping ARC-HBR criteria increased, the cumulative incidence of major bleeding also increased in East Asian patients, which was in line with the recent findings reported by Cao et al13. The incidence of major bleeding in patients with only one major criterion was 4.2%, while that in those with two minor criteria was 2.1%, suggesting that the contribution of minor criteria to bleeding risk can be considered. The incidence of major bleeding was 7.6% and 6.0% in patients who met a single criterion of “use of anticoagulant at discharge” and “severe anaemia”, respectively. Owing to the overlap of multiple HBR criteria in this cohort, the study is underpowered to evaluate the incidence of major bleeding in patients who met each criterion alone. To adjust the confounders, Cox regression analysis was performed. Severe chronic kidney disease, anticoagulant use at discharge, acute coronary syndrome, low body weight (≤50 kg), and heart failure were found to be independent risk factors.
A history of heart failure has been reported as an independent predictor of bleeding in the PENDULUM registry and CREDO-Kyoto registry cohort-27,11. This is likely to be related to the higher mean ages of enrolled patients in the PENDULUM registry and in the CREDO-Kyoto registry cohort-2 (70.0 and 68.2 years, respectively)7,11, compared with other registries discussed in the original ARC-HBR paper1. However, as our results are based on a post hoc analysis, the findings should be interpreted with caution. Indeed, most cases that met an isolated criterion did not demonstrate an incidence of major bleeding >4% and the cumulative incidence of major bleeding was similar. Furthermore, C-statistics did not improve with the addition of these factors. Further studies are warranted to assess the clinical impact of these factors in predicting major bleeding.
When comparing the HBR and non-HBR groups, there was a statistically significant difference in DAPT duration. Whether antiplatelet drug de-escalation is effective in reducing bleeding would require hypothetical testing. Even in patients with HBR, DAPT was used in 70% of patients after 12 months, and bleeding events were observed frequently. This strongly suggests that there is room for improvement.
Limitations
This analysis has some limitations. First, the post hoc nature of the analysis means that not all variables from the original ARC-HBR definitions were collected, and we were unable to address some criteria (e.g., chronic bleeding diathesis and recent major surgery or major trauma within 30 days prior to PCI). The differences in the original ARC-HBR criteria and the modified version used in our analysis mean that the data from our study may not be directly comparable to other publications assessing HBR, limiting their clinical utility. Importantly, many of the ARC-HBR criteria were not available or were markedly modified, including our definition of gastrointestinal bleeding, which did not include hospitalisations or transfusion treatment. The incidence of bleeding was >4% in patients with prior gastrointestinal bleeding; therefore, it was included as a minor criterion in our study. Second, selection bias was inevitable, because this study was an observational study and not all patients undergoing PCI at each institution could be enrolled. Third, ARC-HBR defined major bleeding incidence as ≥4%; however, because East Asian and Western patients are reported to have different risk profiles for bleeding and thrombosis5, 4% may not be a suitable cut-off for Japanese patients. This requires further study. Fourth, all patients enrolled in this study were Japanese and, thus, the results may not be completely generalisable to other East Asian populations. Fifth, we did not use a quantitative description for heart failure in our study (e.g., ejection fraction). Instead, we defined heart failure based on hospitalisation or having a treatment history. Finally, the present study focused on the risk of bleeding in Japanese patients and did not assess the risk of cardiovascular events or mortality, although we can assume that such risks are also increased in patients with HBR. Further analysis is needed to understand and manage these additional clinical risks in HBR patients.
Conclusions
In conclusion, this analysis showed that half of the Japanese patients who underwent PCI in the PENDULUM registry met ARC-HBR criteria, and many had overlapping criteria. The ARC-HBR criteria are applicable to Japanese patients undergoing contemporary PCI.
Impact on daily practice
Appropriate management strategies for patients with high bleeding risk (HBR) requiring dual antiplatelet therapy after percutaneous coronary intervention have not been fully established, particularly in East Asian patients, who have a different risk profile to Western patients. This analysis showed that the Academic Research Consortium (ARC) for HBR criteria are appropriate for estimation of bleeding risk in Japanese patients. Half of the Japanese patients who underwent PCI in the PENDULUM registry met the ARC-HBR criteria, and many patients had overlapping criteria.
Supplementary data
Proportion of patients who continued to receive DAPT over time.
Proportion of patients who fulfilled each ARC-HBR criterion.
Cumulative incidence of ICH stratified by ARC-HBR criteria and other clinically important factors.
Adjusted cumulative incidence of major bleeding stratified by ARC-HBR criteria and other clinically important factors.
Cumulative incidence of major bleeding (A) and intracranial haemorrhage (B) by ARC-HBR criteria plus low body weight and heart failure.
Receiver operating characteristic curve analysis of major bleeding (A) and intracranial haemorrhage (B) for each bleeding risk criteria category.
Full methodological details of the PENDULUM (Platelet rEactivity in patieNts with DrUg eLUting stent and balancing risk of bleeding and ischeMic event) registry study7.
High bleeding risk definitions.
Baseline laboratory parameters.
The proportion of events in each combination of criteria.
Acknowledgments
Acknowledgements
We thank Sheridan Henness, PhD, of Edanz Evidence Generation for providing medical writing support, which was funded by Daiichi Sankyo Co., Ltd.
Funding
This study was supported by Daiichi Sankyo Co., Ltd., Tokyo, Japan. Daiichi Sankyo Co., Ltd. played a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of interest statement
M. Nakamura has received grants from Daiichi Sankyo Co., Ltd., Sanofi K.K., and Bayer K.K., and other fees from Daiichi Sankyo Co., Ltd., Sanofi K.K., Terumo Corporation, and Bristol Myers Squibb K.K. K. Kadota has received personal fees from Daiichi Sankyo Co., Ltd., and Sanofi K.K. K. Nakao has received personal fees from Daiichi Sankyo Co., Ltd. Y. Nakagawa has received personal fees from Bristol Myers Squibb K.K. and Kowa Pharmaceutical Co., Ltd., and grants and personal fees from Daiichi Sankyo Co., Ltd., Bayer Yakuhin, Ltd., Sanofi K.K., Boston Scientific Corporation, and Abbott Vascular Japan Co., Ltd. J. Shite has received personal fees from Daiichi Sankyo Co., Ltd., Nipro, Abbott, and Terumo Corporation. H. Yokoi has received personal fees from Daiichi Sankyo Co., Ltd., Bayer K.K., and Sanofi K.K. K. Kozuma has received grants and personal fees from Daiichi Sankyo Co., Ltd. K. Tanabe has received personal fees from Daiichi Sankyo Co., Ltd., Sanofi K.K., AstraZeneca, Abbott Vascular Japan, Co., Ltd., Boston Scientific Corporation, and Terumo Corporation. R. Iijima has received personal fees from Daiichi Sankyo Co., Ltd. A. Harada and T. Kuroda are employees of Daiichi Sankyo Co., Ltd. Y. Murakami has received personal fees from Daiichi Sankyo Co., Ltd., SRD Co., Ltd., and Sanofi K.K.
Abbreviations
- ARC
Academic Research Consortium
- BARC
Bleeding Academic Research Consortium
- CI
confidence interval
- DAPT
dual antiplatelet therapy
- DES
drug-eluting stent
- HBR
high bleeding risk
- HR
hazard ratio
- ICH
intracranial haemorrhage
- PCI
percutaneous coronary intervention
- PPI
proton pump inhibitor
Contributor Information
Masato Nakamura, Division of Cardiovascular Medicine, Toho University Ohashi Medical Center, Tokyo, Japan.
Kazushige Kadota, Department of Cardiology, Kurashiki Central Hospital, Kurashiki, Japan.
Koichi Nakao, Division of Cardiology, Saiseikai Kumamoto Hospital Cardiovascular Center, Kumamoto, Japan.
Yoshihisa Nakagawa, Department of Cardiovascular Medicine, Shiga University of Medical Science, Otsu, Japan.
Junya Shite, Division of Cardiology, Osaka Saiseikai Nakatsu Hospital, Osaka, Japan.
Hiroyoshi Yokoi, Cardiovascular Medicine Center, Fukuoka Sanno Hospital, Fukuoka, Japan.
Ken Kozuma, Division of Cardiology, Department of Internal Medicine, Teikyo University, Tokyo, Japan.
Kengo Tanabe, Division of Cardiology, Mitsui Memorial Hospital, Tokyo, Japan.
Raisuke Iijima, Division of Cardiovascular Medicine, Toho University Ohashi Medical Center, Tokyo, Japan.
Atsushi Harada, Medical Science Department, Daiichi Sankyo Co., Ltd., Tokyo, Japan.
Takeshi Kuroda, Medical Science Department, Daiichi Sankyo Co., Ltd., Tokyo, Japan.
Yoshitaka Murakami, Department of Medical Statistics, School of Medicine, Toho University, Tokyo, Japan.
References
- Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, Farb A, Gibson CM, Gregson J, Haude M, James SK, Kim HS, Kimura T, Konishi A, Laschinger J, Leon MB, Magee PFA, Mitsutake Y, Mylotte D, Pocock S, Price MJ, Rao SV, Spitzer E, Stockbridge N, Valgimigli M, Varenne O, Windhoevel U, Yeh RW, Krucoff MW, Morice MC. Defining High Bleeding Risk in Patients Undergoing Percutaneous Coronary Intervention. Circulation. 2019;140:240–61. doi: 10.1161/CIRCULATIONAHA.119.040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger C, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O’Gara PT, Sabatine MS, Smith PK, Smith SC., Jr 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082–115. doi: 10.1016/j.jacc.2016.03.513. [DOI] [PubMed] [Google Scholar]
- Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–60. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- Krucoff MW, Mehran R, van Es GA, Boam AB, Cutlip DE. The academic research consortium governance charter. JACC Cardiovasc Interv. 2011;4:595–6. doi: 10.1016/j.jcin.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Levine GN, Jeong YH, Goto S, Anderson JL, Huo Y, Mega JL, Taubert K, Smith SC., Jr Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol. 2014;11:597–606. doi: 10.1038/nrcardio.2014.104. [DOI] [PubMed] [Google Scholar]
- Saito S, Isshiki T, Kimura T, Ogawa H, Yokoi H, Nanto S, Takayama M, Kitagawa K, Nishikawa M, Miyazaki S, Nakamura M. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: the PRASFIT-ACS study. Circ J. 2014;78:1684–92. doi: 10.1253/circj.CJ-13-1482. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Kadota K, Takahashi A, Kanda J, Anzai H, Ishii Y, Shibata Y, Yasaka Y, Takamisawa I, Yamaguchi J, Takeda Y, Harada A, Motohashi T, Iijima R, Uemura S, Murakami Y PENDULUM Registry Investigators*. Relationship Between Platelet Reactivity and Ischemic and Bleeding Events After Percutaneous Coronary Intervention in East Asian Patients: 1-Year Results of the PENDULUM Registry. J Am Heart Assoc. 2020;9:e015439. doi: 10.1161/JAHA.119.015439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- Fujii T, Ikari Y, Hashimoto H, Kadota K, Amano T, Uemura S, Takashima H, Nakamura M J-PCI Investigators. Post-interventional adverse event risk by vascular access site among patients with acute coronary syndrome in Japan: observational analysis with a national registry J-PCI database. Cardiovasc Interv Ther. 2019;34:297–304. doi: 10.1007/s12928-019-00582-0. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Bär S, Losdat S, Otsuka T, Zanchin C, Zanchin T, Gragnano F, Gargiulo G, Siontis GCM, Praz F, Lanz J, Hunziker L, Stortecky S, Pilgrim T, Heg D, Valgimigli M, Windecker S, Räber L. Validation of the Academic Research Consortium for High Bleeding Risk (ARC-HBR) criteria in patients undergoing percutaneous coronary intervention and comparison with contemporary bleeding risk scores. EuroIntervention. 2020;16:371–9. doi: 10.4244/EIJ-D-20-00052. [DOI] [PubMed] [Google Scholar]
- Kimura T, Morimoto T, Furukawa Y, Nakagawa Y, Kadota K, Iwabuchi M, Shizuta S, Shiomi H, Tada T, Tazaki J, Kato Y, Hayano M, Abe M, Tamura T, Shirotani M, Miki S, Matsuda M, Takahashi M, Ishii K, Tanaka M, Aoyama T, Doi O, Hattori R, Tatami R, Suwa S, Takizawa A, Takatsu Y, Takahashi M, Kato H, Takeda T, Lee JD, Nohara R, Ogawa H, Tei C, Horie M, Kambara H, Fujiwara H, Mitsudo K, Nobuyoshi M, Kita T. Long-term safety and efficacy of sirolimus-eluting stents versus bare-metal stents in real world clinical practice in Japan. Cardiovasc Interv Ther. 2011;26:234–45. doi: 10.1007/s12928-011-0065-0. [DOI] [PubMed] [Google Scholar]
- Natsuaki M, Morimoto T, Shiomi H, Yamaji K, Watanabe H, Shizuta S, Kato T, Ando K, Nakagawa Y, Furukawa Y, Tada T, Nagao K, Kadota K, Toyofuku M, Kimura T. Application of the Academic Research Consortium High Bleeding Risk Criteria in an All-Comers Registry of Percutaneous Coronary Intervention. Circ Cardiovasc Interv. 2019;12:e008307. doi: 10.1161/CIRCINTERVENTIONS.119.008307. [DOI] [PubMed] [Google Scholar]
- Cao D, Mehran R, Dangas G, Baber U, Sartori S, Chandiramani R, Stefanini GG, Angiolillo DJ, Capodanno D, Urban P, Morice MC, Krucoff M, Goel R, Roumeliotis A, Sweeny J, Sharma SK, Kini A. Validation of the Academic Research Consortium High Bleeding Risk Definition in Contemporary PCI Patients. J Am Coll Cardiol. 2020;75:2711–22. doi: 10.1016/j.jacc.2020.03.070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Proportion of patients who continued to receive DAPT over time.
Proportion of patients who fulfilled each ARC-HBR criterion.
Cumulative incidence of ICH stratified by ARC-HBR criteria and other clinically important factors.
Adjusted cumulative incidence of major bleeding stratified by ARC-HBR criteria and other clinically important factors.
Cumulative incidence of major bleeding (A) and intracranial haemorrhage (B) by ARC-HBR criteria plus low body weight and heart failure.
Receiver operating characteristic curve analysis of major bleeding (A) and intracranial haemorrhage (B) for each bleeding risk criteria category.
Full methodological details of the PENDULUM (Platelet rEactivity in patieNts with DrUg eLUting stent and balancing risk of bleeding and ischeMic event) registry study7.
High bleeding risk definitions.
Baseline laboratory parameters.
The proportion of events in each combination of criteria.