Abstract
Aims
The aim of this trial was to compare the safety and efficacy of a thin-strut biodegradable polymer sirolimus-eluting cobalt-chromium stent (Orsiro) to a thick-strut biodegradable polymer biolimus-eluting stent (BioMatrix).
Methods and results
This randomised, open-label, non-inferiority trial was conducted among patients undergoing percutaneous coronary intervention. The primary endpoint was target lesion failure (TLF). Between July 2014 and September 2017, we randomly assigned 2,341 patients to BioMatrix stents (n=1,166) or Orsiro stents (n=1,175). We analysed 2,327 patients who completed 18-month follow-up. The mean patient age was 63.5 years, and 1,565 (67.3%) patients presented with acute coronary syndrome. At 18 months, 34 (2.9%) patients with BioMatrix stents and 24 (2.1%) with Orsiro stents experienced TLF (hazard ratio [HR] 0.70, upper limit of one-sided 95% confidence interval: 1.18, p for non-inferiority <0.0001). No significant differences were noted in rates of cardiac death (16 [1.4%] vs 12 [1.0%], p=0.558), target lesion-related myocardial infarction (0 [0%] vs 3 [0.3%], p=0.250), target lesion revascularisation (18 [1.6%] vs 10 [0.9%], p=0.124), or stent thrombosis (0 [0%] vs 2 [0.2%], p=0.50).
Conclusions
In patients with a high prevalence of acute coronary syndrome, Orsiro stents were not inferior to BioMatrix stents. Both showed good clinical outcomes.
Introduction
Treatment failure rates of stents after percutaneous coronary intervention (PCI) have been decreasing since the introduction of drug-eluting stents (DES)1,2,3. Advances in stent metallic structure, polymer coating, and release of antiproliferative DES agents have improved the safety and efficacy of DES. Nevertheless, stent failure remains a substantial problem, with more PCIs being performed in cases of complex lesions4,5,6.
The disappointing results of fully bioresorbable scaffolds have led to re-emerging interest in metal-based DES made of biodegradable polymers (BP) for the elution of antiproliferative agents7,8,9. The pioneer BP-DES is the BioMatrix™️ stent (Biosensors, Singapore), a biolimus-eluting stent. In the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) trial, the definite or probable stent thrombosis rate was lower with BioMatrix (3.6%) than with the CYPHER Select™ (5.2%) (Cordis, Cardinal Health, Milpitas, CA, USA)10.
The Orsiro stent (Biotronik, Bülach, Switzerland), another BP-DES, is a sirolimus-eluting stent. It showed excellent long-term outcomes including a very low rate of stent thrombosis in several studies11,12,13.
Nevertheless, few trials have compared the thin-strut Orsiro stent and the thick-strut BioMatrix/BioMatrix Flex™️ stent (Biosensors). Therefore, we conducted the BIODEGRADE study (Comparison of BIoMatrix and Orsiro Drug Eluting Stent in AngioGraphic Result in patients with Coronary Artery DiseasE), a multicentre, randomised, open-label, and parallel-arm study, to determine whether the Orsiro stent is non-inferior to the BioMatrix stent.
Methods
PATIENTS
Patients were eligible if they had chronic stable coronary artery disease or acute coronary syndromes. Inclusion and exclusion criteria as well as the characteristics of the stents are presented in detail in Supplementary Appendix 1. The study complied with the Declaration of Helsinki and was approved by the institutional review board of each participating centre. All patients provided written informed consent for trial participation before randomisation. This trial was registered with ClinicalTrials.gov (identifier: NCT02299011).
RANDOMISATION
Patients were enrolled by the investigators and randomly allocated to treatment groups after diagnostic coronary angiography and before PCI. Block randomisation by centre was used to assign patients in a 1:1 ratio to receive treatment with an Orsiro stent or a BioMatrix stent. The allocation sequence was computer-generated using a web-based randomisation program run by an independent organisation (T&W Software, Seoul, Republic of Korea).
PROCEDURES
Stents were implanted according to standard techniques. BioMatrix/BioMatrix Flex or Orsiro stents were implanted in the lesions (Supplementary Appendix 2). For multiple lesions, the assigned stent was used in at least one lesion. Other stents were used in case of device failure or in situations where the operators decided that it was in the best interest of the patient. In such cases, the patient was excluded from the per protocol (PP) analysis but was included in the intention-to-treat (ITT) analysis. Complete lesion coverage was recommended. Antithrombotic therapy was prescribed at the investigator’s discretion based on the guidelines (Supplementary Appendix 3).
OUTCOME MEASURES
The primary endpoint was target lesion failure (TLF), defined as a composite of cardiac death, target vessel-related myocardial infarction, or ischaemia-driven target lesion revascularisation within 18 months. Individual components of the primary endpoint comprised the secondary endpoints: cardiac death; myocardial infarction; ischaemia-driven target lesion revascularisation; all-cause death (cardiac and non-cardiac) and target vessel revascularisation; definite, probable, possible, and overall stent thrombosis according to the Academic Research Consortium definition; and a patient-oriented composite endpoint (all-cause death, all myocardial infarctions, or any revascularisation). The definitions of endpoints, event detection, site monitoring and event adjudication are described in Supplementary Appendix 4.
STATISTICAL ANALYSIS
The details of the rationale and methods of statistical analyses are presented in Supplementary Appendix 5. Briefly, the trial was powered for the non-inferiority of the Orsiro stent to the BioMatrix stent with respect to the primary endpoint at 18 months. We assumed an event rate of 5% in each stent group. We set a non-inferiority margin at 1.5, with a one-sided significance level of 0.05, 90% power and a 10% loss-to-follow-up rate. The calculated sample size was 1,192 in each treatment arm.
We constructed survival curves showing cumulative incidence rates based on time to events, accounting for the competing risk of death (in cases of death not included in the outcome). Patients who received the BioMatrix stent were deemed to be the reference group for overall and subgroup analyses. We calculated rate ratios for TLF at the 18-month follow-up for pre-specified patient subgroups (based on baseline demographic and clinical characteristics). We regarded a two-sided p-value of <0.05 as indicating statistical significance. Statistical analyses were performed using R, version 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
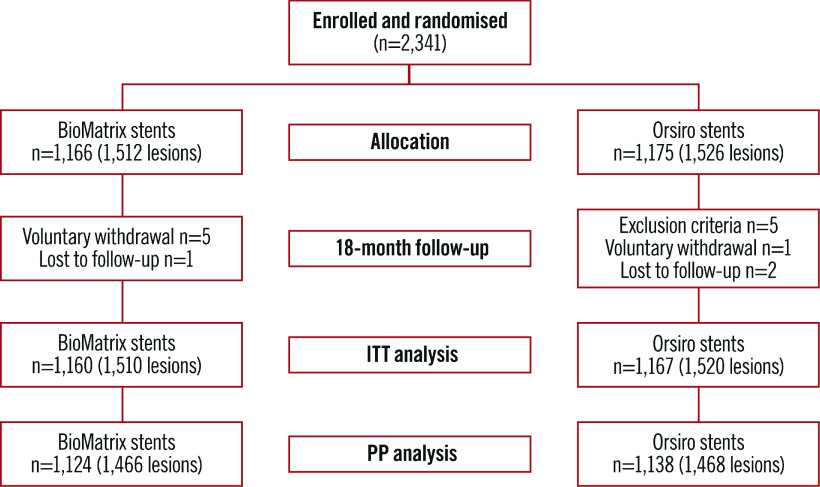
Between July 2014 and September 2017, 2,341 patients were randomly assigned to receive either the BioMatrix stent (1,166 patients [1,512 lesions]) or the Orsiro stent (1,175 patients [1,526 lesions]) (Figure 1) in the participating centres (Supplementary Table 1, Supplementary Appendix 6). Six patients withdrew consent during follow-up, three patients were lost to follow-up at one year, and five patients were excluded because they were found to meet the exclusion criteria after enrolment. Complete follow-up data were available for 2,327 patients (99.4%) for ITT analysis and 2,262 patients for PP analysis. Baseline patient characteristics are summarised in Table 1 and did not differ significantly between the two groups. Lesion and procedure characteristics did not differ significantly between the two stent groups except for the maximal pressure, which was lower in the BioMatrix stent group (10.25±3.71 atm vs 11.39±3.50 atm; p<0.001) (Table 2). A high proportion of patients in both of the groups had acute coronary syndromes, multivessel disease, and complex lesions (Table 2).
Figure 1.
Study flow chart.
Table 1. Baseline characteristics of the study population.
| BioMatrix (n=1,160) | Orsiro (n=1,167) | p-value | ||
| Age, years | 63.6±11.1 | 63.4±10.7 | 0.583 | |
| Men | 838 (72.2) | 835 (71.6) | 0.746 | |
| Body mass index, kg/m2 | 25.1±3.3 | 25.1±3.3 | 0.644 | |
| Diabetes mellitus | 393 (33.9) | 384 (32.9) | 0.650 | |
| Arterial hypertension | 706 (60.9) | 685 (58.7) | 0.307 | |
| Current smoker | 306 (26.4) | 324 (27.8) | 0.327 | |
| Dyslipidaemia | 625 (53.9) | 609 (52.2) | 0.437 | |
| Previous percutaneous coronary intervention | 147 (12.7) | 135 (11.6) | 0.452 | |
| Previous coronary artery bypass grafting | 10 (0.9) | 8 (0.7) | 0.803 | |
| Previous myocardial infarction | 56 (4.8) | 60 (5.1) | 0.801 | |
| Previous cerebrovascular accident | 83 (7.2) | 78 (6.7) | 0.710 | |
| Atrial fibrillation | 47 (4.1) | 35 (3.0) | 0.389 | |
| Clinical diagnosis for percutaneous coronary intervention | Silent ischaemia | 65 (5.6) | 55 (4.7) | 0.448 |
| Stable angina | 313 (27.0) | 328 (28.1) | ||
| Unstable angina | 424 (36.6) | 424 (36.4) | ||
| Non-ST-segment elevation myocardial infarction | 257 (22.2) | 238 (20.4) | ||
| ST-segment elevation myocardial infarction | 101 (8.7) | 121 (10.4) | ||
| IVUS/OCT-guided | 273 (23.5) | 265 (22.7) | 0.672 | |
| Total stent length per patient lesion, mm | <35 | 747 (64.4) | 719 (61.6) | 0.177 |
| ≥35 | 413 (35.6) | 448 (38.4) | ||
| Min. stent diameter per patient, mm | <3 | 541 (46.6) | 531 (45.5) | 0.611 |
| ≥3 | 619 (53.4) | 636 (54.5) | ||
| Target lesion per patient | 1 | 848 (73.1) | 854 (73.2) | 0.590 |
| 2 | 236 (20.3) | 246 (21.1) | ||
| 3 | 62 (5.3) | 59 (5.1) | ||
| >3 | 14 (1.2) | 8 (0.7) | ||
| Number per patient | 1.30 | 1.30 | ||
| Medication at discharge | ||||
| Aspirin | 1,147 (99.0) | 1,155 (99.0) | >0.999 | |
| Clopidogrel | 961 (82.9) | 961 (82.4) | 0.759 | |
| Ticagrelor | 140 (12.1) | 152 (13.0) | 0.532 | |
| Prasugrel | 43 (3.7) | 39 (3.3) | 0.709 | |
| Renin-angiotensin system inhibitors | 738 (63.7) | 737 (63.2) | 0.827 | |
| Beta-blocker | 720 (62.2) | 744 (63.8) | 0.457 | |
| Statin | 1,096 (94.6) | 1,099 (94.2) | 0.750 | |
Table 2. Lesion and procedure characteristics.
| BioMatrix (n=1,510) | Orsiro (n=1,520) | p-value | ||
| Target vessel location | LM (%) | 57 (3.8) | 43 (2.8) | 0.175 |
| LAD (%) | 775 (51.3) | 733 (48.2) | 0.095 | |
| LCX (%) | 345 (22.9) | 340 (22.4) | 0.786 | |
| RCA (%) | 386 (25.6) | 441 (29.0) | 0.037 | |
| Lesion type | A | 106 (7.0) | 118 (7.8) | 0.682 |
| B1 | 395 (26.2) | 413 (27.2) | ||
| B2 | 385 (25.5) | 389 (25.6) | ||
| C | 624 (41.3) | 600 (39.5) | ||
| Chronic total occlusion | 72 (4.8) | 93 (6.1) | 0.119 | |
| Bifurcation lesion | 219 (14.5) | 321 (15.2) | 0.627 | |
| Lesion length, mm | 21.8±10.1 | 21.8±10.2 | 0.934 | |
| Pre-stent reference vessel diameter, mm | 2.50±0.71 | 2.50±0.74 | 0.916 | |
| Direct stenting | 174 (11.5) | 172 (11.3) | 0.842 | |
| Number of stents per patient | 1.45±0.74 | 1.47±0.76 | 0.477 | |
| Number of stents per lesion | 1.11±0.34 | 1.13±0.37 | 0.205 | |
| Total stent length per patient lesion | 34.7±21.1 | 35.4±21.6 | 0.470 | |
| Total stent length per lesion | 26.67±12.2 | 27.1±12.8 | 0.294 | |
| Sum of stent length per lesion, mm | <35 | 1,227 (81.3) | 1,192 (78.4) | 0.057 |
| ≥35 | 283 (18.7) | 328 (21.6) | ||
| Average of stent diameter, mm | 3.00±0.41 | 3.03±0.44 | 0.071 | |
| Min. stent diameter per lesion, mm | <3 | 664 (44.0) | 643 (42.3) | 0.372 |
| ≥3 | 846 (56.0) | 877 (57.7) | ||
| Maximal pressure, atm | 10.1±3.7 | 11.4±3.5 | <0.001 | |
| Minimal lumen diameter, mm | 0.58±0.39 | 0.59±0.42 | 0.384 | |
| Diameter stenosis, % | 77.7±14.1 | 77.7±14.3 | 0.970 | |
| Post-stent minimal lumen diameter, mm | 2.66±0.56 | 2.68±0.57 | 0.327 | |
| Post-stent reference diameter, mm | 3.06±0.61 | 3.07±0.06 | 0.706 | |
| Post-stent diameter stenosis, % | 13.5±7.1 | 13.2±7.3 | 0.228 | |
| Acute gain, mm | 2.08±0.62 | 2.09±0.61 | 0.826 | |
| Delivery failure | 0 (0) | 1 (0.07) | 0.319 | |
| IVUS/OCT-guided | 346 (22.9) | 345 (22.7) | 0.921 | |
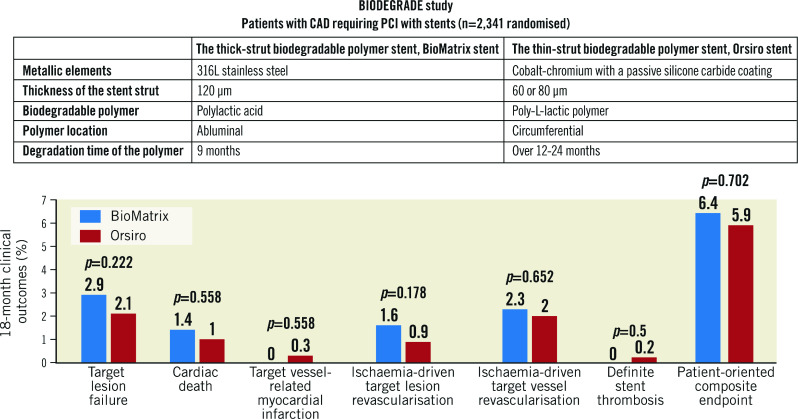
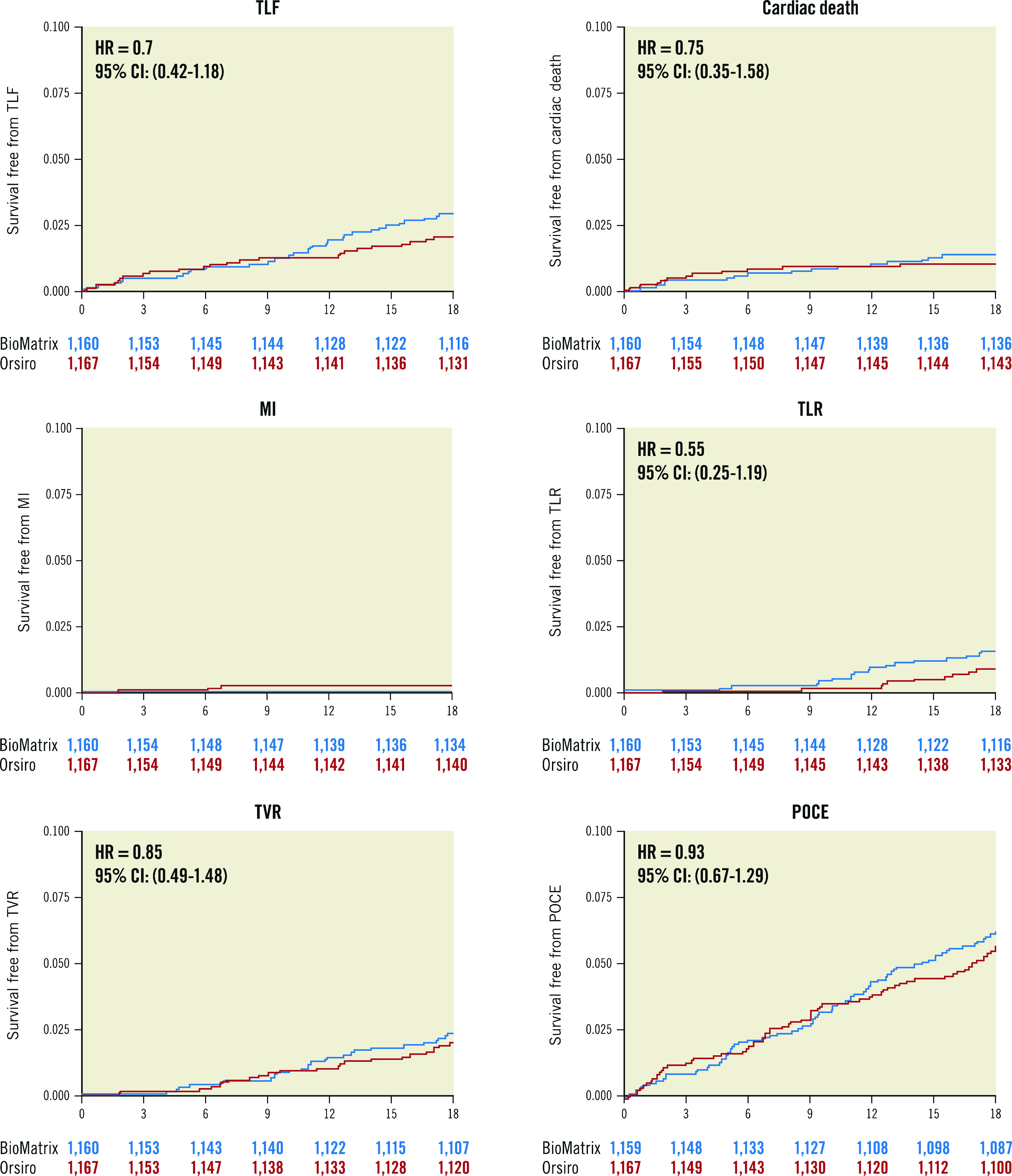
At 18 months, the primary endpoint, TLF, occurred in 34 (2.9%) patients in the BioMatrix group and 24 (2.1%) patients in the Orsiro group (Figure 2, Table 3). The non-inferiority of the Orsiro stent was confirmed with a risk ratio of 0.70 and an upper limit of the one-sided 95% confidence interval (CI) of 1.17 (p<0.001 in one-sided non-inferiority test). No significant differences were noted in the rates of cardiac death, target vessel-related myocardial infarction, ischaemia-driven target lesion revascularisation, and the patient-oriented composite endpoint (Figure 2, Table 3). Only two (0.2%) patients in the Orsiro stent group had late definite stent thrombosis (one patient at 60 days and the other at 270 days after index PCI). The two patients were taking dual antiplatelet therapy with aspirin and clopidogrel.
Figure 2.
Clinical outcomes during the 18-month follow-up period. MI: target vessel-related myocardial infarction; POCE: patient-oriented composite endpoint; TLF: target lesion failure; TLR: ischaemia-driven target lesion revascularisation; TVR: ischaemia-driven target vessel revascularisation
Table 3. Clinical outcomes.
| BioMatrix (n=1,160) | Orsiro (n=1,167) | p-value | HR (95% CI) | |
| Target lesion failure | 34 (2.9) | 24 (2.1) | 0.222 | 0.70 (0.42-1.18) |
| Death | ||||
| All-cause death | 26 (2.2) | 24 (2.1) | 0.869 | 0.92 (0.53-1.60) |
| Cardiac death | 16 (1.4) | 12 (1.0) | 0.558 | 0.75 (0.35-1.58) |
| Non-cardiac death | 10 (0.9) | 12 (1.0) | 0.841 | 1.20 (0.52-2.77) |
| Target vessel-related myocardial infarction | 0 (0.0) | 3 (0.3) | 0.250 | – |
| Any myocardial infarction | 2 (0.2) | 9 (0.8) | 0.071 | 4.49 (0.97-20.80) |
| Ischaemia-driven target lesion revascularisation | 18 (1.6) | 10 (0.9) | 0.178 | 0.55 (0.25-1.19) |
| Ischaemia-driven target vessel revascularisation | 27 (2.3) | 23 (2.0) | 0.652 | 0.85 (0.49-1.48) |
| Any repeat revascularisation | 47 (4.1) | 43 (3.7) | 0.725 | 0.91 (0.60-1.37) |
| Stent thrombosis* | 0 (0.0) | 2 (0.2) | 0.500 | – |
| Patient-oriented composite endpoint | 74 (6.4) | 69 (5.9) | 0.702 | 0.93 (0.67-1.29) |
| Bleeding | 28 (2.4) | 27 (2.3) | 0.979 | 0.96 (0.56-1.63) |
| *Stent thrombosis: 1st case, definite late stent thrombosis leading to myocardial infarction; 2nd case, definite late stent thrombosis causing unstable angina. | ||||
The results on a PP basis were not different from the ITT analyses (Supplementary Table 2). We also analysed the cumulative incidence rate of TLF and a competing risk of non-cardiac death to avoid a possible upward biased estimate of the true cumulative incidence rate. In the analysis considering the competing risk, the primary endpoint was not different between the stent groups (Supplementary Figure 1).
In various subgroup analyses, the findings for TLF were consistent across the pre-specified subgroups except for diabetes mellitus (Figure 3). In patients without diabetes mellitus, TLF occurred less frequently in the Orsiro stent group than in the BioMatrix stent group (hazard ratio [HR] 0.42, 95% CI: 0.20-0.89, p=0.024, p for interaction=0.041), which was driven mainly by lower ischaemia-driven target lesion revascularisation (HR 0.15, 95% CI: 0.03-0.66, p=0.012).
Figure 3.
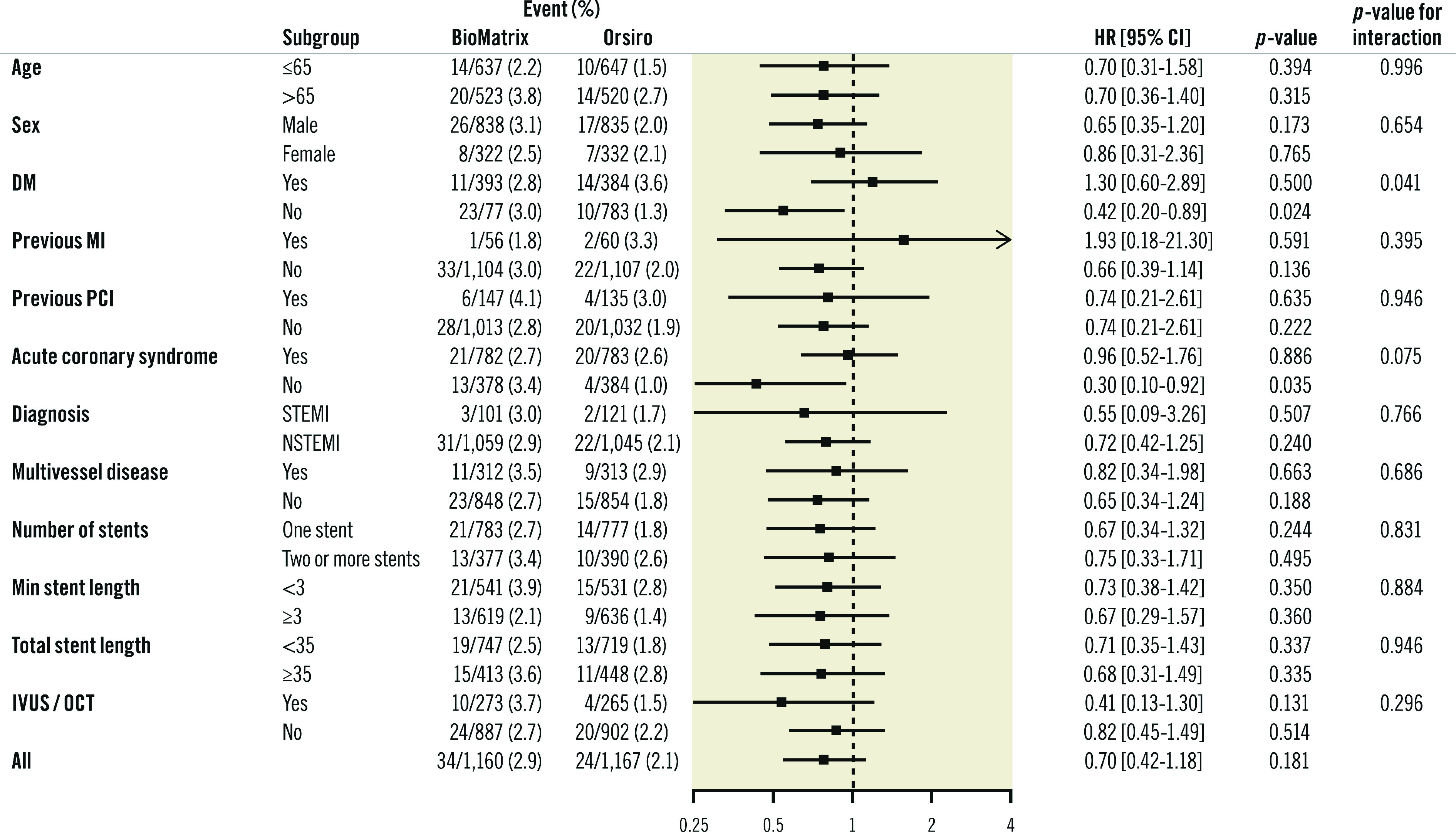
Subgroup analysis. DM: diabetes mellitus; MI: myocardial infarction; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; RR: risk ratio; STEMI: ST-segment elevation myocardial infarction
Discussion
In this BIODEGRADE study, the biodegradable polymer thin-strut Orsiro stent was non-inferior to the biodegradable polymer thick-strut BioMatrix stent for TLF at 18 months. All secondary outcomes including cardiac mortality, target vessel-related myocardial infarction, target lesion revascularisation, and stent thrombosis did not differ between the two groups.
The BIODEGRADE study was designed to confirm the non-inferiority of the Orsiro stent to the BioMatrix stent, which is currently the leading BP-DES. Compared with the BioMatrix stent, the sirolimus-eluting Orsiro stent has improved stent design with hypothetical benefit: a silicone carbide coating, thinner stent struts (60-80 μm vs 120 μm), slower drug release (12 weeks vs four weeks), and more delayed polymer degradation (after 12-24 months vs 6-9 months) than the BioMatrix stent. The thin-strut Orsiro stent recently showed better efficacy than the second-generation thick-strut DES12,13,14. Definite stent thrombosis was absent in the Orsiro stent12, and the 12-month TLF rate was lower with the Orsiro stent than with the XIENCE stent (Abbott Vascular, Santa Clara, CA, USA). In addition, patients with the Orsiro stent in small coronary vessels showed notably less three-year TLF than those with second-generation zotarolimus-eluting stents14. These studies compared the thin-strut BP-DES to the second-generation DES with durable polymer in which BP-DES showed superiority. However, the trials comparing thinner DES and the thicker BioMatrix stent did not show superiority of the thin-strut DES15. Similarly, in our study, we compared two BP-DES with different strut thicknesses and found that there was no difference in the outcomes. We think that the durability of the polymer may have a more important role in the inferior clinical outcome than has the thickness. However, we need more data to conclude the thickness hypothesis.
While we were recruiting patients, the result of SORT-OUT VII was reported, i.e., that the Orsiro stent had better efficacy than the Nobori™ stent (Terumo, Tokyo, Japan)16. The one-year TLF rate was 3.8% (48 patients) in the Orsiro group and 4.6% (58 patients) in the Nobori group, and that of definite stent thrombosis was 0.4% (5 patients) in the Orsiro group and 1.2% (15 patients) in the Nobori group (p=0.034). Nobori and BioMatrix stents are similar in all aspects, except for the presence of an ultra-thin non-degradable parylene coating between the stent and the polymer on the Nobori stent. The Nobori stent, unlike the BioMatrix stent, failed to show non-inferiority to the CYPHER™ stent (Cordis) within one year in the SORT OUT V trial17. In the COMPARE II trial, definite or probable stent thrombosis was higher in the Nobori stent group than in the second-generation XIENCE stent group18. However, the thick-strut BioMatrix stent showed no difference with respect to TLF and stent thrombosis compared to the thin-strut everolimus-eluting SYNERGY™️ stent (Boston Scientific, Marlborough, MA, USA) with biodegradable polymers15. It is not certain whether the parylene coating influences the stent thrombosis. It is certain, however, that the Nobori and BioMatrix stents showed different clinical outcomes.
We observed less ischaemia-driven target lesion revascularisation in Orsiro stent-treated patients without diabetes mellitus. The five-year outcomes of the BIOFLOW-II study revealed an interaction with the stents and diabetes mellitus: the Orsiro stent was associated with a significantly higher target lesion revascularisation than the XIENCE stent in patients with diabetes mellitus12. However, there was no interaction with diabetes mellitus in the SORT OUT VII study16. It may be interesting to elucidate whether the Orsiro stent performs better in patients without diabetes mellitus. However, this is only a hypothesis-generating finding, and could have been an incidental finding.
Study limitations
First, the observed 18-month TLF rate was 2.9% for the control group (the BioMatrix stents), which was lower than the assumed 5% used in the study power calculation. If we had assumed an expected event rate of 2.9% instead of 5%, the statistical power of this study to detect non-inferiority would have been as low as 76%. Second, despite the all-comer nature of the study population with wide inclusion criteria and very few exclusion criteria, the event rates were very low. There was also follow-up loss, which may raise the question of underreporting. However, this limitation may not have biased the results because the follow-up loss was negligible (only 0.6%) and evenly distributed between the treatment groups, and periodic monitoring and data audits were thoroughly performed during this trial. One possible reason for the low event rates may be the selection bias during screening of eligible patients because we excluded patients with cardiogenic shock with Killip class IV, symptomatic heart failure, or non-cardiac comorbid conditions that may result in life expectancy <1 year or protocol non-compliance. We did not measure post-PCI cardiac enzyme routinely in the study, which may have led to the lower incidence of events such as periprocedural myocardial infarction. There might also be an ethnic or genetic factor, as trials conducted in East Asian populations have consistently reported lower event rates19,20. Finally, our primary endpoint might be limited by the relatively short follow-up period. Therefore, we will assess the TLF at three years, as previously set out in the design of the trial.
Conclusions
The Orsiro stent was not inferior to the BioMatrix stent in patients with minimum exclusion criteria and a high proportion of acute coronary syndromes. Both BP-DES showed good clinical outcomes.
Impact on daily practice
In this trial, we randomly assigned 1,175 and 1,166 patients to treatment with thin-strut sirolimus-eluting stents (Orsiro), and treatment with thick-strut biolimus-eluting stents (BioMatrix), respectively. Both the thin-strut Orsiro stents and the thick-strut BioMatrix stents showed good intermediate-term clinical outcomes. The stent design of biodegradable polymer may have limited effects on outcomes.
Appendix. Study collaborators
Nam-Ho Lee, MD; Hallym University Kangnam Sacred Heart Hospital, Seoul, Republic of Korea. Kyung-Joo Ahn, MD; KEPCO Medical Center, Seoul, Republic of Korea. Hun-Sik Park, MD; Kyoungpook National University Hospital, Daegu, Republic of Korea. Dae-Gyun Park, MD; Hallym University Kangdong Sacred Heart Hospital, Seoul, Republic of Korea. Doo-Il Kim, MD; Inje University Paik Hospital, Pusan, Republic of Korea. Hee-Yeol Kim, MD; The Catholic University of St. Mary’s Hospital, Bucheon, Republic of Korea. Sang-Uk Lym, MD; Cha University Bundang Cha Medical Center, Seongnam, Republic of Korea. Yong Sung Seo, MD; Myongji Hospital, Goyangsi, Republic of Korea. Ki-Yuk Chang, MD; The Catholic University of St. Mary’s Hospital, Seoul, Republic of Korea. Tae-Jun Cha, MD; Kosin University Gospel Hospital, Pusan, Republic of Korea. Byeong-Hee Hwang, MD; The Catholic University of St Paul’s Hospital, Seoul, Republic of Korea. Byung-Ryul Cho, MD; Kangwon National University Hospital, Chuncheon, Republic of Korea. Byoung-Eun Park, MD; Dankook University Hospital, Cheonan, Republic of Korea. Hee-Kyung Jeon, MD; The Catholic University of St. Mary’s Hospital, Uijeongbu, Republic of Korea. Jong-Seon Park, MD; Yongnam University Medical Center, Daegu, Republic of Korea.
Supplementary data
Inclusion and exclusion criteria.
The characteristics of the stents.
Antithrombotic therapy.
The definition of endpoints, event detection, site monitoring and event adjudication.
Statistical analysis.
A list of centres and investigators.
The cumulative incidence rate of the primary endpoint and a competing risk, non-cardiac death.
The number of patients per centre.
The results on a per-protocol basis.
Visual summary.
Comparison of the BioMatrix and Orsiro stents: summary of 18-month outcomes. Aetiopathology of debris captured by cerebral embolic protection filters during TAVI, including risk factors for greater amounts or larger particles of debris.
Acknowledgments
Funding
This research was supported by grants from the Korean Health Technology R&D Project, Ministry of Health and Welfare (HI17C1799, HI18C0493, and HI19C0655) and grants from BIOTRONIK Korea, Seoul, Republic of Korea, and DIO Korea, Seoul, Republic of Korea.
Conflict of interest statement
The authors/study collaborators have no conflicts of interest to declare.
Abbreviations
- BP
biodegradable polymer
- CI
confidence interval
- DES
drug-eluting stent(s)
- PCI
percutaneous coronary intervention
- TLF
target lesion failure
Contributor Information
Chang-Hwan Yoon, Seoul National University Bundang Hospital, Seongnam, Rebublic of Korea.
Young Choi, Sejong General Hospital, Bucheon, Republic of Korea.
Jin Park, Seoul National University Bundang Hospital, Seongnam, Rebublic of Korea.
Si-Hyuck Kang, Seoul National University Bundang Hospital, Seongnam, Rebublic of Korea.
Sun-Hwa Kim, Seoul National University Bundang Hospital, Seongnam, Rebublic of Korea.
Jung-Won Suh, Seoul National University Bundang Hospital, Seongnam, Rebublic of Korea.
Young-Seok Cho, Seoul National University Bundang Hospital, Seongnam, Rebublic of Korea.
Tae-Jin Youn, Seoul National University Bundang Hospital, Seongnam, Rebublic of Korea.
Myeong-Kon Kim, Catholic Kwandong University International St. Mary’s Hospital, Incheon, Republic of Korea.
Kwang Cha, Pusan National University Hospital, Pusan, Republic of Korea.
Seung-Hwan Lee, Wonju Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea.
Bum-Kee Hong, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea.
Seung-Woon Rha, Korea University Guro Hospital, Seoul, Republic of Korea.
Woong-Chol Kang, Gachon University Gil Medical Center, Incheon, Republic of Korea.
Jae-Hwan Lee, Chungnam National University Hospital, Daejeon, Republic of Korea.
Sang-Hyun Kim, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Republic of Korea.
In-Ho Chae, Seoul National University Bundang Hospital, Seongnam, Rebublic of Korea.
References
- Kastrati A, Mehilli J, von Beckerath N, Dibra A, Hausleiter J, Pache J, Schühlen H, Schmitt C, Dirschinger J, Schomig A and Investigators I-DS. Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: a randomized controlled trial. JAMA. 2005;293:165–71. doi: 10.1001/jama.293.2.165. [DOI] [PubMed] [Google Scholar]
- Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnar F, Falotico R RAVEL Study Group. Randomized Study with the Sirolimus- Coated Bx Velocity Balloon-Expandable Stent in the Treatment of Patients with de Novo Native Coronary Artery Lesions. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–80. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE and Investigators S. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–23. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- Stone GW, Sabik JF, Serruys PW, Simonton CA, Généreux P, Puskas J, Kandzari DE, Morice MC, Lembo N, Brown WM, 3rd, Taggart DP, Banning A, Merkely B, Horkay F, Boonstra PW, van Boven AJ, Ungi I, Bogáts G, Mansour S, Noiseux N, Sabate M, Pomar J, Hickey M, Gershlick A, Buszman P, Bochenek A, Schampaert E, Pagé P, Dressler O, Kosmidou I, Mehran R, Pocock SJ, Kappetein AP EXCEL Trial Investigators. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N Engl J Med. 2016;375:2223–35. doi: 10.1056/NEJMoa1610227. [DOI] [PubMed] [Google Scholar]
- Mäkikallio T, Holm NR, Lindsay M, Spence MS, Erglis A, Menown IB, Trovik T, Eskola M, Romppanen H, Kellerth T, Ravkilde J, Jensen LO, Kalinauskas G, Linder RB, Pentikainen M, Hervold A, Banning A, Zaman A, Cotton J, Eriksen E, Margus S, Sørensen HT, Nielsen PH, Niemelä M, Kervinen K, Lassen JF, Maeng M, Oldroyd K, Berg G, Walsh SJ, Hanratty CG, Kumsars I, Stradins P, Steigen TK, Fröbert O, Graham AN, Endresen PC, Corbascio M, Kajander O, Trivedi U, Hartikainen J, Anttila V, Hildick-Smith D, Thuesen L, Christiansen EH NOBLE study investigators. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet. 2016;388:2743–52. doi: 10.1016/S0140-6736(16)32052-9. [DOI] [PubMed] [Google Scholar]
- Lee SW, Lee PH, Ahn JM, Park DW, Yun SC, Han S, Kang H, Kang SJ, Kim YH, Lee CW, Park SW, Hur SH, Rha SW, Her SH, Choi SW, Lee BK, Lee NH, Lee JY, Cheong SS, Kim MH, Ahn YK, Lim SW, Lee SG, Hiremath S, Santoso T, Udayachalerm W, Cheng JJ, Cohen DJ, Muramatsu T, Tsuchikane E, Asakura Y, Park SJ. Randomized Trial Evaluating Percutaneous Coronary Intervention for the Treatment of Chronic Total Occlusion. Circulation. 2019;139:1674–83. doi: 10.1161/CIRCULATIONAHA.118.031313. [DOI] [PubMed] [Google Scholar]
- Stefanini GG, Kalesan B, Serruys PW, Heg D, Buszman P, Linke A, Ischinger T, Klauss V, Eberli F, Wijns W, Morice MC, Di Mario C, Corti R, Antoni D, Sohn HY, Eerdmans P, van Es GA, Meier B, Windecker S and Juni P. Long-term clinical outcomes of biodegradable polymer biolimus-eluting stents versus durable polymer sirolimus-eluting stents in patients with coronary artery disease (LEADERS): 4 year follow-up of a randomised non-inferiority trial. Lancet. 2011;378:1940–8. doi: 10.1016/S0140-6736(11)61672-3. [DOI] [PubMed] [Google Scholar]
- Hamon M, Niculescu R, Deleanu D, Dorobantu M, Weissman NJ, Waksman R. Clinical and angiographic experience with a third-generation drug-eluting Orsiro stent in the treatment of single de novo coronary artery lesions (BIOFLOW-I): a prospective, first-in-man study. EuroIntervention. 2013;8:1006–11. doi: 10.4244/EIJV8I9A155. [DOI] [PubMed] [Google Scholar]
- Meredith IT, Verheye S, Dubois C, Dens J, Farah B, Carrié D, Walsh S, Oldroyd K, Varenne O, El-Jack S, Moreno R, Christen T, Allocco DJ. Final five-year clinical outcomes in the EVOLVE trial: a randomized evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting stent. EuroIntervention. 2018;13:2047–50. doi: 10.4244/EIJ-D-17-00529. [DOI] [PubMed] [Google Scholar]
- Serruys PW, Farooq V, Kalesan B, de Vries T, Buszman P, Linke A, Ischinger T, Klauss V, Eberli F, Wijns W, Morice MC, Di Mario C, Corti R, Antoni D, Sohn HY, Eerdmans P, Rademaker-Havinga T, van Es GA, Meier B, Juni P and Windecker S. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) randomized, noninferiority trial. JACC Cardiovasc Interv. 2013;6:777–89. doi: 10.1016/j.jcin.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Windecker S, Haude M, Neumann FJ, Stangl K, Witzenbichler B, Slagboom T, Sabate M, Goicolea J, Barragan P, Cook S, Piot C, Richardt G, Merkely B, Schneider H, Bilger J, Erne P, Waksman R, Zaugg S, Juni P and Lefevre T. Comparison of a novel biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: results of the randomized BIOFLOW-II trial. Circ Cardiovasc Interv. 2015;8:e001441. doi: 10.1161/CIRCINTERVENTIONS.114.001441. [DOI] [PubMed] [Google Scholar]
- Lefevre T, Haude M, Neumann FJ, Stangl K, Skurk C, Slagboom T, Sabate M, Goicolea J, Barragan P, Cook S, Macia JC, Windecker S. Comparison of a Novel Biodegradable Polymer Sirolimus-Eluting Stent With a Durable Polymer Everolimus-Eluting Stent: 5-Year Outcomes of the Randomized BIOFLOW-II Trial. JACC Cardiovasc Interv. 2018;11:995–1002. doi: 10.1016/j.jcin.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Kandzari DE, Mauri L, Koolen JJ, Massaro JM, Doros G, Garcia-Garcia HM, Bennett J, Roguin A, Gharib EG, Cutlip DE, Waksman R BIOFLOW V Investigators. Ultrathin, bioresorbable polymer sirolimus-eluting stents versus thin, durable polymer everolimus-eluting stents in patients undergoing coronary revascularisation (BIOFLOW V): a randomised trial. Lancet. 2017;390:1843–52. doi: 10.1016/S0140-6736(17)32249-3. [DOI] [PubMed] [Google Scholar]
- Buiten RA, Ploumen EH, Zocca P, Doggen CJM, van der Heijden LC, Kok MM, Danse PW, Schotborgh CE, Scholte M, de Man FHAF, Linssen GCM, von Birgelen C. Outcomes in Patients Treated With Thin-Strut, Very Thin-Strut, or Ultrathin-Strut Drug-Eluting Stents in Small Coronary Vessels: A Prespecified Analysis of the Randomized BIO-RESORT Trial. JAMA Cardiol. 2019;4:659–69. doi: 10.1001/jamacardio.2019.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng M, Christiansen EH, Raungaard B, Kahlert J, Terkelsen CJ, Kristensen SD, Carstensen S, Aaroe J, Jensen SE, Villadsen AB, Lassen JF, Thim T, Eftekhari A, Veien KT, Hansen KN, Junker A, Botker HE, Jensen LO SORT OUT VIII Investigators. Everolimus-Eluting Versus Biolimus-Eluting Stents With Biodegradable Polymers in Unselected Patients Undergoing Percutaneous Coronary Intervention: A Randomized Noninferiority Trial With 1-Year Follow-Up (SORT OUT VIII Trial). JACC Cardiovasc Interv. 2019;12:624–33. doi: 10.1016/j.jcin.2018.12.036. [DOI] [PubMed] [Google Scholar]
- Jensen LO, Thayssen P, Maeng M, Ravkilde J, Krusell LR, Raungaard B, Junker A, Terkelsen CJ, Veien KT, Villadsen AB, Kaltoft A, Tilsted HH, Hansen KN, Aaroe J, Kristensen SD, Hansen HS, Jensen SE, Madsen M, Botker HE, Berencsi K, Lassen JF, Christiansen EH. Randomized Comparison of a Biodegradable Polymer Ultrathin Strut Sirolimus-Eluting Stent With a Biodegradable Polymer Biolimus-Eluting Stent in Patients Treated With Percutaneous Coronary Intervention: The SORT OUT VII Trial. Circ Cardiovasc Interv. 2016;9 doi: 10.1161/CIRCINTERVENTIONS.115.003610. [DOI] [PubMed] [Google Scholar]
- Christiansen EH, Jensen LO, Thayssen P, Tilsted HH, Krusell LR, Hansen KN, Kaltoft A, Maeng M, Kristensen SD, Botker HE, Terkelsen CJ, Villadsen AB, Ravkilde J, Aaroe J, Madsen M, Thuesen L, Lassen JF and Scandinavian Organization for Randomized Trials with Clinical Outcome Vi. Biolimus-eluting biodegradable polymer-coated stent versus durable polymer-coated sirolimus-eluting stent in unselected patients receiving percutaneous coronary intervention (SORT OUT V): a randomised non-inferiority trial. Lancet. 2013;381:661–9. doi: 10.1016/S0140-6736(12)61962-X. [DOI] [PubMed] [Google Scholar]
- Vlachojannis GJ, Smits PC, Hofma SH, Togni M, Vazquez N, Valdes M, Voudris V, Slagboom T, Goy JJ, den Heijer P, van der Ent M. Biodegradable Polymer Biolimus-Eluting Stents Versus Durable Polymer Everolimus-Eluting Stents in Patients With Coronary Artery Disease: Final 5-Year Report From the COMPARE II Trial (Abluminal Biodegradable Polymer Biolimus-Eluting Stent Versus Durable Polymer Everolimus-Eluting Stent). JACC Cardiovasc Interv. 2017;10:1215–21. doi: 10.1016/j.jcin.2017.02.029. [DOI] [PubMed] [Google Scholar]
- Park KW, Chae IH, Lim DS, Han KR, Yang HM, Lee HY, Kang HJ, Koo BK, Ahn T, Yoon JH, Jeong MH, Hong TJ, Chung WY, Jo SH, Choi YJ, Hur SH, Kwon HM, Jeon DW, Kim BO, Park SH, Lee NH, Jeon HK, Gwon HC, Jang YS and Kim HS. Everolimus-eluting versus sirolimus-eluting stents in patients undergoing percutaneous coronary intervention: the EXCELLENT (Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting) randomized trial. J Am Coll Cardiol. 2011;58:1844–54. doi: 10.1016/j.jacc.2011.07.031. [DOI] [PubMed] [Google Scholar]
- Park KW, Kang SH, Kang HJ, Koo BK, Park BE, Cha KS, Rhew JY, Jeon HK, Shin ES, Oh JH, Jeong MH, Kim S, Hwang KK, Yoon JH, Lee SY, Park TH, Moon KW, Kwon HM, Hur SH, Ryu JK, Lee BR, Park YW, Chae IH, Kim HS HOST–ASSURE Investigators. A randomized comparison of platinum chromium-based everolimus-eluting stents versus cobalt chromium-based Zotarolimus-Eluting stents in all-comers receiving percutaneous coronary intervention: HOST-ASSURE (harmonizing optimal strategy for treatment of coronary artery stenosis-safety & effectiveness of drug-eluting stents & anti-platelet regimen), a randomized, controlled, noninferiority trial. J Am Coll Cardiol. 2014;63:2805–16. doi: 10.1016/j.jacc.2014.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Inclusion and exclusion criteria.
The characteristics of the stents.
Antithrombotic therapy.
The definition of endpoints, event detection, site monitoring and event adjudication.
Statistical analysis.
A list of centres and investigators.
The cumulative incidence rate of the primary endpoint and a competing risk, non-cardiac death.
The number of patients per centre.
The results on a per-protocol basis.