1 Preamble
Guidelines summarize and evaluate available evidence with the aim of assisting health professionals in proposing the best management strategies for an individual patient with a given condition. Guidelines and their recommendations should facilitate decision making of health professionals in their daily practice. However, the final decisions concerning an individual patient must be made by the responsible health professional(s) in consultation with the patient and caregiver as appropriate.
A great number of guidelines have been issued in recent years by the European Society of Cardiology (ESC) and its partners such as the European Association for Cardio-Thoracic Surgery (EACTS), as well as by other societies and organizations. Because of their impact on clinical practice, quality criteria for the development of guidelines have been established in order to make all decisions transparent to the user. The recommendations for formulating and issuing ESC Guidelines can be found on the ESC website (https://www.escardio.org/Guidelines). The ESC Guidelines represent the official position of the ESC on a given topic and are regularly updated.
In addition to the publication of Clinical Practice Guidelines, the ESC carries out the EURObservational Research Programme of international registries of cardiovascular diseases and interventions which are essential to assess diagnostic/therapeutic processes, use of resources and adherence to guidelines. These registries aim at providing a better understanding of medical practice in Europe and around the world, based on high-quality data collected during routine clinical practice.
The Members of this Task Force were selected by the ESC and EACTS, including representation from relevant ESC and EACTS sub-specialty groups, in order to represent professionals involved with the medical care of patients with this pathology. Selected experts in the field undertook a comprehensive review of the published evidence for management of a given condition according to ESC Clinical Practice Guidelines Committee (CPG). A critical evaluation of diagnostic and therapeutic procedures was performed, including assessment of the risk-benefit ratio. The level of evidence and the strength of the recommendation of particular management options were weighed and graded according to predefined scales, as outlined below.
The experts of the writing and reviewing panels provided declaration of interest forms for all relationships that might be perceived as real or potential sources of conflicts of interest. Their declarations of interest were reviewed according to the ESC declaration of interest rules and can be found on the ESC website (http://www.escardio.org/guidelines) and have been compiled in a report and published in a supplementary document simultaneously to the guidelines.
This process ensures transparency and prevents potential biases in the development and review processes. Any changes in declarations of interest that arise during the writing period were notified to the ESC and updated. The Task Force received its entire financial support from the ESC and EACTS without any involvement from the healthcare industry.
The ESC CPG supervises and coordinates the preparation of new guidelines. The Committee is also responsible for the endorsement process of these guidelines. The ESC Guidelines undergo extensive review by the CPG and external experts. After appropriate revisions the guidelines are signed-off by all the experts involved in the Task Force. The finalized document is signed-off by the CPG for publication in the European Heart Journal and the European Journal of Cardio-Thoracic Surgery. The guidelines were developed after careful consideration of the scientific and medical knowledge and the evidence available at the time of their dating.
The task of developing ESC/EACTS Guidelines also includes the creation of educational tools and implementation programmes for the recommendations including condensed pocket guideline versions, summary slides, summary cards for non-specialists and an electronic version for digital applications (smartphones, etc.). These versions are abridged and thus, for more detailed information, the user should always access to the full text version of the guidelines, which is freely available via the ESC and EACTS website and hosted on the EHJ and EJCTS website. The National Cardiac Societies of the ESC are encouraged to endorse, adopt, translate and implement all ESC Guidelines. Implementation programmes are needed because it has been shown that the outcome of disease may be favourably influenced by the thorough application of clinical recommendations.
Health professionals are encouraged to take the ESC/EACTS Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies. However, the ESC/EACTS Guidelines do not override in any way whatsoever the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient's health condition and in consultation with that patient or the patient's caregiver where appropriate and/or necessary. It is also the healthcare professional's responsibility to verify the rules and regulations applicable in each country to drugs and devices at the time of prescription.
2 Introduction
2.1 WHY DO WE NEED NEW GUIDELINES ON VALVULAR HEART DISEASE?
Since the publication of the previous version of the guidelines on the management of valvular heart disease (VHD) in 2017, new evidence has accumulated, particularly on the following topics:
Epidemiology: the incidence of the degenerative aetiology has increased in industrialized countries while, unfortunately, rheumatic heart disease is still too frequently observed in many parts of the world.1,2,3
Current practices regarding interventions and medical management have been analysed in new surveys at the national and European level.
Non-invasive evaluation using three-dimensional (3D) echocardiography, cardiac computed tomography (CCT), cardiac magnetic resonance (CMR), and biomarkers plays a more and more central role.
New definitions of severity of secondary mitral regurgitation (SMR) based on the outcomes of studies on intervention.
New evidence on anti-thrombotic therapies leading to new recommendations in patients with surgical or transcatheter bioprostheses for bridging during perioperative periods and over the long term. The recommendation for non- vitamin K antagonist oral anticoagulants (NOACs) was reinforced in patients with native valvular disease, except for significant mitral stenosis, and in those with bioprostheses.
Risk stratification for the timing of intervention. This applies mostly to (i) the evaluation of progression in asymptomatic patients based on recent longitudinal studies mostly in aortic stenosis, and (ii) interventions in high-risk patients in whom futility should be avoided. Regarding this last aspect, the role of frailty is outlined.
- Results and indication of intervention:
- The choice of the mode of intervention: current evidence reinforces the critical role of the Heart Team, which should integrate clinical, anatomical, and procedural characteristics beyond conventional scores, and informed patient’s treatment choice.
- Surgery: increasing experience and procedural safety led to expansion of indications toward earlier intervention in asymptomatic patients with aortic stenosis, aortic regurgitation or mitral regurgitation and stress the preference for valve repair when it is expected to be durable. A particular emphasis is put on the need for more comprehensive evaluation and earlier surgery in tricuspid regurgitation.
- Transcatheter techniques: (i) Concerning transcatheter aortic valve implantation (TAVI), new information from randomized studies comparing TAVI vs. surgery in low-risk patients with a follow-up of 2 years has led to a need to clarify which types of patients should be considered for each mode of intervention. (ii) Transcatheter edge-to-edge repair (TEER) is increasingly used in SMR and has been evaluated against optimal medical therapy resulting in an upgrade of the recommendation. (iii) The larger number of studies on transcatheter valve-in-valve implantation after failure of surgical bioprostheses served as a basis to upgrade its indication. (iv) Finally, the encouraging preliminary experience with transcatheter tricuspid valve interventions (TTVI) suggests a potential role of this treatment in inoperable patients, although this needs to be confirmed by further evaluation.
The new evidence described above made a revision of the recommendations necessary.
2.2 METHODOLOGY
In preparation of the 2021 VHD Guidelines, a methodology group has been created for the first time, to assist the Task Force for the collection and interpretation of the evidence supporting specific recommendations. The group was constituted of two European Society of Cardiology (ESC) and two European Association for Cardio-Thoracic Surgery (EACTS) delegates who were also members of the Task Force. Although the principle activities of the group concerned the chapter on aortic stenosis and SMR, it was not limited to these two domains. The methodology group was at disposal, upon request of the Task Force members, to resolve specific methodological issues.
2.3 CONTENT OF THESE GUIDELINES
Decision making in VHD involves accurate diagnosis, timing of intervention, risk assessment and, based on these, selection of the most suitable type of intervention. These guidelines focus on acquired VHD, are oriented towards management, and do not deal with endocarditis,4 congenital valve disease5 (including pulmonary valve disease), or recommendations concerning sports cardiology and exercise in patients with cardiovascular disease,6 as separate guidelines have been published by the ESC on these topics.
2.4 NEW FORMAT OF THE GUIDELINES
The new guidelines have been adapted to facilitate their use in clinical practice and to meet readers' demands by focusing on condensed, clearly represented recommendations. At the end of the document, key points summarize the essentials. Gaps in evidence are listed to propose topics for future research. The guideline document will be harmonized with the chapter on VHD included in the ESC Textbook of Cardiovascular Medicine (ISBN: 9780198784906). The guidelines and the textbook are complementary. Background information and detailed discussion of the data that have provided the basis for the recommendations will be found in the relevant book chapter.
2.5 HOW TO USE THESE GUIDELINES
The Committee emphasizes that many factors ultimately determine the most appropriate treatment in individual patients within a given community. These factors include the availability of diagnostic equipment, the expertise of cardiologists and surgeons, especially in the field of valve repair and percutaneous intervention, and, notably, the wishes of well-informed patients. Furthermore, owing to the lack of evidence-based data in the field of VHD, most recommendations are largely the result of expert consensus opinion. Therefore, deviations from these guidelines may be appropriate in certain clinical circumstances.
3 General comments
This section defines and discusses concepts common to all the types of VHD including the Heart Team and Heart Valve Centres, the main evaluation steps of patients presenting with VHD, as well as the most commonly associated cardiac diseases.
3.1 CONCEPTS OF HEART TEAM AND HEART VALVE CENTRE
The main purpose of Heart Valve Centres as centres of excellence in the treatment of VHD is to deliver optimal quality of care with a patient-centred approach. The main requirements of a Heart Valve Centre are presented in Table 4.
Table 4. Requirements for a Heart Valve Centre.
| Requirements |
|---|
| Centre performing heart valve procedures with institutional cardiology and cardiac surgery departments with 24 h/7-day services. |
| Heart Team: clinical cardiologist, interventional cardiologist, cardiac surgeon, imaging specialist with expertise in interventional imaging, cardiovascular anaesthesiologist. |
|
Additional specialists if required: heart failure specialist, electrophysiologist, geriatrician and other specialists (intensive care, vascular surgery, infectious disease, neurology). Dedicated nursing personnel is an important asset to the Heart Team. The Heart Team must meet on a frequent basis and work with standard operating procedures and clinical governance arrangements defined locally. A hybrid catheterization laboratory is desirable. The entire spectrum of surgical and transcatheter valve procedures should be available. High volume for hospital and individual operators. |
| Multimodality imaging including echocardiography, CCT, CMR, and nuclear medicine, as well as expertise on guidance of surgical and interventional procedures. |
| Heart Valve Clinic for outpatient and follow-up management. |
| Data review: continuous evaluation of outcomes with quality review and/or local/external audit. Education programmes targeting patient primary care, operator, diagnostic and interventional imager training and referring cardiologist. |
| CCT: cardiac computed tomography; CMR: cardiac magnetic resonance. |
This is achieved through high procedural volume in conjunction with specialized training, continuous education, and focused clinical interest. Heart Valve Centres should promote timely referral of patients with VHD for comprehensive evaluation before irreversible damage occurs.
Decisions concerning treatment and intervention should be made by an active and collaborative Heart Team with expertise in VHD, comprising clinical and interventional cardiologists, cardiac surgeons, imaging specialists with expertise in interventional imaging,7,8 cardiovascular anaesthesiologists, and other specialists if necessary (e.g. heart failure specialists or electrophysiologists). Dedicated nursing personnel with expertise in the care of patients with VHD are also an important asset to the Heart Team. The Heart Team approach is particularly advisable for the management of high-risk and asymptomatic patients, as well as in case of uncertainty or lack of strong evidence.
Heart Valve Clinics are an important component of the Heart Valve Centres, aiming to provide standardized organization of care based on guidelines. Access to Heart Valve Clinics improves outcomes.9
Physicians experienced in the management of VHD and dedicated nurses organize outpatient visits, and referral to the Heart Team, if needed. Earlier referral should be encouraged if patient’s symptoms develop or worsen before the next planned visit.10,11
Beside the whole spectrum of valvular interventions, expertise in interventional and surgical management of coronary artery disease (CAD), vascular diseases, and complications must be available.
Techniques with a steep learning curve may be performed with better results at hospitals with high procedural volume and experience. The relationship between case volume and outcomes for surgery and transcatheter interventions is complex but should not be denied.12,13,14 However, the precise numbers of procedures per individual operator or hospital required to provide high-quality care remain controversial as inequalities exist between high- and middle-income countries.15 High-volume TAVI programmes are associated with lower mortality at 30 days, particularly at hospitals with a high surgical aortic valve replacement (SAVR) volume.16,17 The data available on transcatheter mitral valve repair14,18 and, even more so, transcatheter tricuspid procedures are more limited.
Since performance does not exclusively relate to intervention volume, internal quality assessment consisting of systematic recording of procedural data and patient outcomes at the level of a given Heart Valve Centre is essential, as well as participation in national or ESC/EACTS registries.
A Heart Valve Centre should have structured and possibly combined training programmes for interventionalists, cardiac surgeons, and imaging specialists13,19,20 (https://ebcts.org/syllabus/). New techniques should be taught by competent mentors to minimize the effects of the learning curve.
Finally, Heart Valve Centres should contribute to optimizing the management of patients with VHD, provide corresponding services at the community level, and promote networks that include other medical departments, referring cardiologists and primary care physicians.
3.2 PATIENT EVALUATION
The aims of the evaluation of patients with VHD are to diagnose, quantify, and assess the mechanism of VHD, as well as its consequences.
3.2.1 CLINICAL EVALUATION
Precise evaluation of the patient's history and symptomatic status, and proper physical examination, in particular auscultation21 and search for heart failure signs, are crucial. In addition, assessment of their comorbidities and general condition require particular attention. The essential questions in the evaluation of a patient for valvular intervention are summarized in Figure 1 (Central illustration).
Figure 1 (Central illustration). Patient-centred evaluation for intervention.
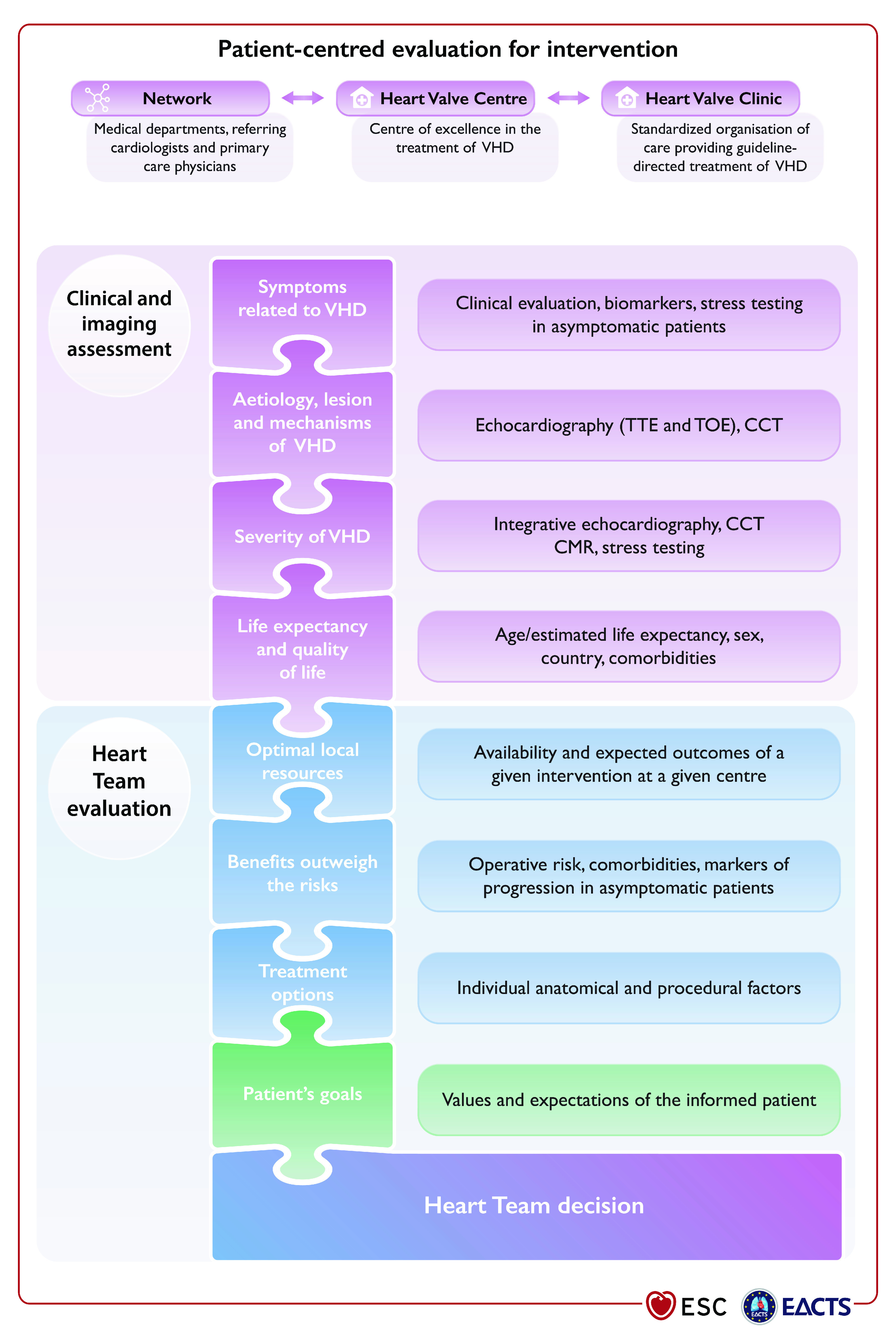
VHD: valvular heart disease; CCT: cardiac computed tomography; CMR: cardiac magnetic resonance; TOE: transoesophageal echocardiography; TTE: transthoracic echocardiography.
3.2.2 ECHOCARDIOGRAPHY
Following adequate clinical evaluation, echocardiography is the key technique used to confirm the diagnosis of VHD, as well as to assess its aetiology, mechanisms, function, severity, and prognosis. It should be performed and interpreted by properly trained imagers.22,23
Echocardiographic criteria for the definition of severe valve stenosis and regurgitation are addressed in specific documents24,25 and summarized in the specific sections of these guidelines. Echocardiography is also key to evaluating the feasibility of a specific intervention.
Indices of left ventricular (LV) enlargement and function are strong prognostic factors. Recent studies suggest that global longitudinal strain has greater prognostic value than LV ejection fraction (LVEF), although cut-off values are not uniform.26,27 Transoesophageal echocardiography (TOE) should be considered when transthoracic echocardiography (TTE) is of suboptimal quality or when thrombosis, prosthetic valve dysfunction, or endocarditis is suspected. TOE is useful when detailed functional valve anatomy is required to assess repairability. Intraprocedural TOE, preferably 3D, is used to guide transcatheter mitral and tricuspid valve procedures and to assess the immediate result of surgical valve operations. Multimodality imaging may be required in specific conditions for evaluation and/or procedural guidance in TAVI and transcatheter mitral interventions.28,29
3.2.3 OTHER NON-INVASIVE INVESTIGATIONS
3.2.3.1 Stress testing
The primary purpose of exercise testing is to unmask the objective occurrence of symptoms in patients who claim to be asymptomatic. It is especially useful for risk stratification in aortic stenosis.30 Exercise testing will also determine the level of recommended physical activity, including participation in sports. It should be emphasized that stress testing is safe and useful in asymptomatic patients with VHD. Unfortunately, the VHD II survey indicates that it is rarely performed in asymptomatic patients.1
Exercise echocardiography may identify the cardiac origin of dyspnoea. Prognostic impact has been shown mainly for aortic stenosis and mitral regurgitation.31,32
The use of stress tests to detect CAD associated with severe valvular disease is discouraged because of their low diagnostic value and potential risks in symptomatic patients with aortic stenosis.
3.2.3.2 Cardiac magnetic resonance
In patients with inadequate echocardiographic quality or discrepant results, CMR should be used to assess the severity of valvular lesions, particularly regurgitant lesions, and to assess ventricular volumes, systolic function, abnormalities of the ascending aorta, and myocardial fibrosis.33 CMR is the reference method for the evaluation of right ventricular (RV) volumes and function and is therefore particularly useful to evaluate the consequences of tricuspid regurgitation.34 It also has an incremental value for assessing the severity of aortic and mitral regurgitation.
3.2.3.3 Computed tomography
CCT may contribute to the evaluation of valve disease severity, particularly in aortic stenosis35,36 and possibly associated disease of the thoracic aorta (dilatation, calcification), as well as to evaluate the extent of MAC. CCT should be performed whenever the echocardiographic data indicate an aortic enlargement >40 mm, to clarify aortic diameter and to assess aortic morphology and configuration. CCT is essential in the pre-procedural planning of TAVI and can also be useful to assess patient-prosthesis mismatch (PPM).37 It is also a prerequisite for pre-procedural planning of mitral and tricuspid valve interventions.38 Positron emission tomography (PET)/CCT is useful in patients with a suspicion of endocarditis of a prosthetic valve.39,40
3.2.3.4 Cinefluoroscopy
Cinefluoroscopy is particularly useful for assessing the kinetics of the leaflet occluders of a mechanical prosthesis.
3.2.3.5 Biomarkers
B-type natriuretic peptide (BNP) serum levels, corrected for age and sex, are useful in asymptomatic patients and may assist selection of the appropriate time point for a given intervention,41 particularly if the level rises during follow-up. Other biomarkers have been tested, with evidence for fibrosis, inflammation, and adverse ventricular remodelling, which could improve decision making.42
3.2.3.6 Multimarkers and staging
In patients with at least moderate aortic stenosis and LVEF >50%, staging according to damage associated with aortic stenosis on LV/RV, left atrium (LA), mitral /tricuspid valve, and pulmonary circulation was predictive of excess mortality after TAVI and SAVR, and may help to identify patients who will benefit from an intervention.43,44
3.2.4 INVASIVE INVESTIGATIONS
3.2.4.1 Coronary angiography
Coronary angiography is recommended for the assessment of CAD when surgery or an intervention is planned, to determine if concomitant coronary revascularization is recommended (see recommendations for management of CAD in patients with VHD, Recommendations A).45,46 Alternatively, owing to its high negative predictive value, CCT may be used to rule out CAD in patients who are at low risk of atherosclerosis. The usefulness of fractional flow reserve or instantaneous wave-free ratio in patients with VHD is not well established, and caution is warranted in the interpretation of these measurements when VHD, and in particular aortic stenosis, is present.47,48
Recommendations A. Recommendations for management of CAD in patients with VHD.
| Recommendations | Classa | Levelb |
|---|---|---|
| Diagnosis of CAD | ||
| Coronary angiography is recommended before valve surgery in patients with severe VHD and any of the following: • History of cardiovascular disease. • Suspected myocardial ischaemia.c • LV systolic dysfunction. • In men >40 years of age and postmenopausal women. • One or more cardiovascular risk factors. |
I | C |
| Coronary angiography is recommended in the evaluation of severe SMR. | I | C |
| Coronary CT angiography should be considered as an alternative to coronary angiography before valve surgery in patients with severe VHD and low probability of CAD.d | IIa | C |
| Indications for myocardial revascularization | ||
| CABG is recommended in patients with a primary indication for aortic/mitral/tricuspid valve surgery and coronary artery diameter stenosis ≥70%.e,f | I | C |
| CABG should be considered in patients with a primary indication for aortic/mitral/tricuspid valve surgery and coronary artery diameter stenosis ≥50-70%. | IIa | C |
| PCI should be considered in patients with a primary indication to undergo TAVI and coronary artery diameter stenosis >70% in proximal segments. | IIa | C |
| PCI should be considered in patients with a primary indication to undergo transcatheter mitral valve intervention and coronary artery diameter stenosis >70% in proximal segments. | IIa | C |
| CABG: coronary artery bypass grafting; CAD: coronary artery disease; CT: computed tomography; LV: left ventricle/left ventricular; PCI: percutaneous coronary intervention; SMR: secondary mitral regurgitation; TAVI: transcatheter aortic valve implantation; VHD: valvular heart disease. aClass of recommendation. bLevel of evidence. cChest pain, abnormal non-invasive testing. dCoronary CT angiography may also be used in patients requiring emergency surgery with acute infective endocarditis with large vegetations protruding in front of a coronary ostium. eStenosis ≥50% can be considered for left main stenosis. fFFR ≤0.8 is a useful cut-off indicating the need for an intervention in patients with mitral or tricuspid diseases, but has not been validated in patients with aortic stenosis. Adapted from 45,72 | ||
3.2.4.2 Cardiac catheterization
The measurement of pressures and cardiac output or the assessment of ventricular performance and valvular regurgitation by ventricular angiography or aortography is restricted to situations where non-invasive evaluation by multimodality imaging is inconclusive or discordant with clinical findings. When elevated, pulmonary pressure is the only criterion to support the indication for surgery, and confirmation of echo data by invasive measurement is recommended. Right heart catheterization is also indicated in patients with severe tricuspid regurgitation as Doppler gradient may be impossible or underestimate the severity of pulmonary hypertension.
3.2.5 ASSESSMENT OF COMORBIDITY
The choice of specific examinations to assess comorbidity is guided by the clinical evaluation.
3.3 RISK STRATIFICATION
Risk stratification applies to any sort of intervention and is required for weighing the risk of intervention against the expected natural history of VHD and for choosing the type of intervention. Most experience relates to surgery and TAVI.
3.3.1 RISK SCORES
The Society of Thoracic Surgeons (STS) predicted risk of mortality (PROM) score (http://riskcalc.sts.org/stswebriskcalc/calculate) and the European System for Cardiac Operative Risk Evaluation II (EuroSCORE II; http://www.euroscore.org/calc.html) accurately discriminate high- and low-risk surgical patients and show good calibration to predict postoperative outcome after valvular surgery in the majority of the patients,49,50 while risk estimation may be less accurate in high-risk patients.51 The STS-PROM score is dynamic and changes over time. Of note, the risk scores have not been validated for isolated tricuspid surgical interventions.
In isolation, surgical scores have major limitations for practical use in patients undergoing transcatheter intervention because they do not include major risk factors such as frailty, as well as anatomical factors with impact on the procedure, either surgical or transcatheter [porcelain aorta, previous chest radiation, mitral annular calcification (MAC)].
New scores have been developed to estimate the risk in patients undergoing TAVI, with better accuracy and discrimination than the surgical risk scores, despite numerous limitations52,53,54 (Supplementary Table 1).
Experience with risk stratification is currently limited for other interventional procedures, such as mitral or tricuspid interventions.
3.3.2 OTHER FACTORS
Other factors should be taken into account:
• Frailty, defined as a decrease of physiologic reserve and ability to maintain homeostasis leading to an increased vulnerability to stresses and conferring an increased risk of morbidity and mortality after both surgery and TAVI.55 The assessment of frailty should not rely on a subjective approach, such as the ‘eyeball test’, but rather on a combination of different objective estimates.55,56,57,58,59 Several tools are available for assessing frailty (Supplementary Table 2,59 and Supplementary Table 3).60
• Malnutrition61 and cognitive dysfunction62 both predict poor prognosis.
• Other major organ failures (Supplementary Table 4), in particular the combination of severe lung disease,63,64 postoperative pain from sternotomy or thoracotomy and prolonged time under anaesthesia in patients undergoing SAVR via full sternotomy, may contribute to pulmonary complications. There is a positive association between the impairment of renal function and increased mortality after valvular surgery and transcatheter procedures,65 especially when the glomerular filtration rate is <30 mL/min. Liver disease, is also an important prognostic factor.66
• Anatomical aspects affecting procedural performance such as porcelain aorta or severe MAC67 (see Table 6 in section 5.1.3, and Supplementary Figure 1).
Table 6. Clinical, anatomical and procedural factors that influence the choice of treatment modality for an individual patient.
| Favours TAVI | Favours SAVR | |
|---|---|---|
| Clinical characteristics | ||
| Lower surgical risk | − | + |
| Higher surgical risk | + | − |
| Younger agea | − | + |
| Older agea | + | − |
| Previous cardiac surgery (particularly intact coronary artery bypass grafts at risk of injury during repeat sternotomy) | + | − |
| Severe frailtyb | + | − |
| Active or suspected endocarditis | − | + |
| Anatomical and procedural factors | ||
| TAVI feasible via transfemoral approach | + | − |
| Transfemoral access challenging or impossible and SAVR feasible | − | + |
| Transfemoral access challenging or impossible and SAVR inadvisable | +c | − |
| Sequelae of chest radiation | + | − |
| Porcelain aorta | + | − |
| High likelihood of severe patient-prosthesis mismatch (AVA <0.65 cm2/m2 BSA) | + | − |
| Severe chest deformation or scoliosis | + | − |
| Aortic annular dimensions unsuitable for available TAVI devices | − | + |
| Bicuspid aortic valve | − | + |
| Valve morphology unfavourable for TAVI (e.g. high risk of coronary obstruction due to low coronary ostia or heavy leaflet/LVOT calcification) | − | + |
| Thrombus in aorta or LV | − | + |
| Concomitant cardiac conditions requiring intervention | ||
| Significant multi-vessel CAD requiring surgical revascularizationd | − | + |
| Severe primary mitral valve disease | − | + |
| Severe tricuspid valve disease | − | + |
| Significant dilatation/aneurysm of the aortic root and/or ascending aorta | − | + |
| Septal hypertrophy requiring myectomy | − | + |
| AVA: aortic valve area, BSA: body surface area, CAD: coronary artery disease; ESC: European Society of Cardiology; LV: left ventricle/left ventricular; LVOT: left ventricular outflow tract; SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation. Integration of these factors provides guidance for the Heart Team decision (indications for intervention are provided in the table of recommendations on indications for intervention in symptomatic and asymptomatic aortic stenosis and recommended mode of intervention). aLife expectancy is highly dependent on absolute age and frailty, differs between men and women, and may be a better guide than age alone. There is wide variation across Europe and elsewhere in the world (http://ghdx.healthdata.org/record/ihme-data/gbd-2017-life-tables-1950-2017). bSevere frailty: >2 factors according to Katz index59 (see section 3.3 for further discussion). cVia non-transfemoral approach. d According to the 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. | ||
At the extreme of the risk spectrum, futility should be avoided. Therapeutic futility has been defined as a lack of medical efficacy, particularly when the physician judges that the therapy is unlikely to produce its intended clinical results, or lack of meaningful survival according to the personal values of the patient. Assessment of futility goes beyond survival and includes functional recovery. The futility of interventions has to be taken into consideration, particularly for transcatheter interventions.63
The high prevalence of comorbidity in the elderly makes assessment of the risk/benefit ratios of interventions more difficult, therefore the role of the Heart Team is essential in this specific population of patients (Supplementary Table 5).
3.4 PATIENT-RELATED ASPECTS
Patient-related life expectancy and expected quality of life should be considered. The patient and their family should be thoroughly informed and assisted in their decision on the best treatment option.13 A patient-centred approach would take patient-reported outcome measures and patient-reported experience measures into consideration and make these parameters part of the informed choice offered to patients.68,69
When benefit in symptom relief aligns with a patient’s goals, care is not futile. However, care is futile when no life prolongation or symptom relief is anticipated.70
3.5 LOCAL RESOURCES
Even if it is desirable that Heart Valve Centres are able to perform a large spectrum of procedures, either surgical or catheter-based, specialization and thereby expertise in specific domains will vary and should be taken into account when deciding on the orientation of the patient in specific cases, such as complex surgical valve repair or transcatheter intervention.
In addition, penetration of transcatheter interventions is heterogeneous worldwide and highly dependent on socioeconomic inequalities.15,71 Appropriate stewardship of economic resources is a fundamental responsibility of the Heart Team.
3.6 MANAGEMENT OF ASSOCIATED CONDITIONS
3.6.1 CORONARY ARTERY DISEASE
Recommendations for the management of CAD associated with VHD are provided below and are detailed in specific sections (section 5 and section 6.2) of this guideline document, as well as in other dedicated guideline documents.45,46,72,73
3.6.2 ATRIAL FIBRILLATION
Detailed recommendations on the management of patients with atrial fibrillation (AF) including management of anticoagulation are provided in specific guidelines.74 NOACs are recommended in patients with aortic stenosis, aortic regurgitation or mitral regurgitation presenting with AF75,76,77,78 as subgroup analyses of randomized controlled trials (RCTs) support the use of apixaban, dabigatran, edoxaban, and rivaroxaban. The use of NOACs is not recommended in patients who have AF associated with clinically significant mitral stenosis or those with mechanical prostheses.
Surgical ablation of AF combined with mitral valve surgery effectively reduces the incidence of AF but has no impact on adjusted short-term survival. An increased rate of pacemaker implantation has been observed after surgical ablation (9.5%, vs. 7.6% in the group with AF and no surgical ablation).79 Concomitant AF ablation should be considered in patients undergoing cardiac surgery, balancing the benefits of freedom from atrial arrhythmias with the risk factors for recurrence, such as age, LA dilatation, years in AF, renal dysfunction, and other cardiovascular risk factors. In addition, left atrial appendage (LAA) occlusion should be considered in combination with valve surgery in patients with AF and a CHA2DS2VASc score ≥2 to reduce the thromboembolic risk.80,81,82 The selected surgical technique should ensure complete occlusion of the LAA. For patients with AF and risk factors for stroke, long-term oral anticoagulation (OAC) is currently recommended, irrespective of the use of surgical ablation of AF and/or surgical LAA occlusion.
Recommendations for the management of AF in native VHD are summarized in the following table (Recommendations B). The recommendations concerning patients with valve prostheses, and the combination of anticoagulants and antiplatelet agents in patients undergoing PCI, are described in section 11 (section 11.3.2.2 and related table of recommendations for perioperative and postoperative antithrombotic management of valve replacement or repair).
Recommendations B. Recommendations on management of atrial fibrillation in patients with native VHD.
| Recommendations | Classa | Levelb |
|---|---|---|
| Anticoagulation | ||
| For stroke prevention in AF patients who are eligible for OAC, NOACs are recommended in preference to VKAs in patients with aortic stenosis, aortic and mitral regurgitation. 75,76,77,78,83,84 | I | A |
| The use of NOACs is not recommended in patients with AF and moderate to severe mitral stenosis. | III | C |
| Surgical interventions | ||
| Concomitant AF ablation should be considered in patients undergoing valve surgery, balancing the benefits of freedom from atrial arrhythmias and the risk factors for recurrence (LA dilatation, years in AF, age, renal dysfunction, and other cardiovascular risk factors). 79,85,86,87,88,89,90 | IIa | A |
| LAA occlusion should be considered to reduce the thromboembolic risk in patients, with AF and a CHA2DS2VASc score ≥2 undergoing valve surgery. 82 | IIa | B |
| AF: atrial fibrillation; LA: left atrium/left atrial; LAA: left atrial appendage; NOAC: non-vitamin K antagonist oral anticoagulant; OAC: oral anticoagulation; VKA: vitamin K antagonist. aClass of recommendation. bLevel of evidence. | ||
3.7 ENDOCARDITIS PROPHYLAXIS
Antibiotic prophylaxis should be considered for high-risk procedures in patients with prosthetic valves, including transcatheter valves, or with repairs using prosthetic material, and in patients with previous episode(s) of infective endocarditis.4 Particular attention to dental and cutaneous hygiene and strict aseptic measures during any invasive procedure are advised in this population. Antibiotic prophylaxis should be considered in dental procedures involving manipulation of the gingival or periapical region of the teeth or manipulation of the oral mucosa.4
3.8 PROPHYLAXIS FOR RHEUMATIC FEVER
Prevention of rheumatic heart disease should preferably target the first attack of acute rheumatic fever. Antibiotic treatment of group A Streptococcus infection throat is key in primary prevention. Echocardiographic screening in combination with secondary antibiotic prophylaxis in children with evidence of latent rheumatic heart disease is currently investigated to reduce its prevalence in endemic regions.91 In patients with established rheumatic heart disease, secondary long-term prophylaxis against rheumatic fever is recommended: benzathine benzyl penicillin 1.2 MUI every 3 to 4 weeks over 10 years. Lifelong prophylaxis should be considered in high-risk patients according to the severity of VHD and exposure to group A Streptococcus.92,93,94,95
4 Aortic regurgitation
Aortic regurgitation can be caused by primary disease of the aortic valve cusps and/or abnormalities of the aortic root and ascending aortic geometry. Degenerative tricuspid and bicuspid aortic regurgitation are the most common aetiologies in high-income countries, accounting for approximately two-thirds of the underlying aetiology of aortic regurgitation in the EURObservational Registry Programme Valvular Heart Disease II registry.1 Other causes include infective and rheumatic endocarditis. Acute severe aortic regurgitation is mostly caused by infective endocarditis, and less frequently by aortic dissection.
4.1 EVALUATION
4.1.1 ECHOCARDIOGRAPHY
Echocardiography is the key examination used to describe valve anatomy, quantify aortic regurgitation, evaluate its mechanisms, define the morphology of the aorta, and determine the feasibility of valve-sparing aortic surgery or valve repair.96,97 Identification of the mechanism follows the same principle such as for mitral regurgitation: normal cusps but insufficient coaptation due to dilatation of the aortic root with central jet (type 1), cusp prolapse with eccentric jet (type 2), or retraction with poor cusp tissue quality and large central or eccentric jet (type 3).96 Quantification of aortic regurgitation follows an integrated approach considering qualitative, semi-quantitative, and quantitative parameters24,98 (Table 5). New parameters obtained by 3D echocardiography and two-dimensional (2D) strain imaging as LV global longitudinal strain may be useful, particularly in patients with borderline LVEF where they may help in the decision for surgery.99 Measurement of the aortic root and ascending aorta in 2D is performed at four levels: annulus, sinuses of Valsalva, sinotubular junction, and tubular ascending aorta.100,101 Measurements are performed in the parasternal long-axis view from leading edge to leading edge at end diastole, except for the aortic annulus, which is measured in mid systole. As it will have surgical consequences, it is important to differentiate three phenotypes of the ascending aorta: aortic root aneurysms (sinuses of Valsalva >45 mm), tubular ascending aneurysm (sinuses of Valsalva <40-45 mm), and isolated aortic regurgitation (all aortic diameters <40 mm). The calculation of indexed values to account for body size has been suggested,102 in particular in patients with small stature. Anatomy of the aortic valve cusps and its suitability for valve repair should be provided by preoperative TOE if aortic valve repair or a valve-sparing surgery of the aortic root is considered. Intraoperative evaluation of the surgical result by TOE is mandatory in patients undergoing aortic valve preservation or repair.
Table 5. Echocardiographic criteria for the definition of severe aortic valve regurgitation.
| Qualitative | |
|---|---|
| Valve morphology | Abnormal/flail/large coaptation defect |
| Colour flow regurgitant jet areaa | Large in central jets, variable in eccentric jets |
| CW signal of regurgitant jet | Dense |
| Other | Holodiastolic flow reversal in descending aorta (EDV >20 cm/s) |
| Semiquantitative | |
| Vena contracta width (mm) | >6 |
| Pressure half-timeb (ms) | <200 |
| Quantitative | |
| EROA (mm2) | ≥30 |
| Regurgitant volume (mL/beat) | ≥60 |
| Enlargement of cardiac chambers | LV dilatation |
| CW: continuous wave; EDV: end-diastolic velocity; EROA: effective regurgitant orifice area; LV: left ventricle/left ventricular. aAt a Nyquist limit of 50-60 cm/s. bPressure half-time is shortened with increasing LV diastolic pressure, vasodilator therapy, and in patients with a dilated compliant aorta, or lengthened in chronic aortic regurgitation. Adapted from Lancellotti P et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2013;14:611-644. Copyright (2013) by permission of Oxford University Press on behalf of the European Society of Cardiology. | |
4.1.2 COMPUTED TOMOGRAPHY AND CARDIAC MAGNETIC RESONANCE
CMR should be used to quantify the regurgitant fraction when echocardiographic measurements are equivocal or discordant with clinical findings. In patients with aortic dilatation, CCT is recommended to assess the maximum diameter at four levels, as in echocardiography. CMR can be used for follow-up, but indication for surgery should preferably be based on CCT measurements. Different methods of aortic measurements have been reported. To improve reproducibility, it is recommended to measure diameters using the inner-inner-edge technique at end diastole on the strictly transverse plane by double oblique reconstruction perpendicular to the axis of blood flow of the corresponding segment. Maximum root diameter should be taken from sinus-to-sinus diameter rather than sinus-to-commissure diameter, as it correlates more closely to long-axis leading-edge-to-leading-edge echo maximum diameters.103,104
4.2 INDICATIONS FOR INTERVENTION
Acute aortic regurgitation may require urgent surgery. It is mainly caused by infective endocarditis and aortic dissection but may also occur after blunt chest trauma and iatrogenic complications during catheter-based cardiac interventions. Specific guidelines deal with these entities.4,101 The recommendations on indications for surgery in severe aortic regurgitation and aortic root disease may be related to symptoms, status of the LV, or dilatation of the aorta [see table of recommendations on indications for surgery in severe aortic regurgitation and aortic root or tubular ascending aortic aneurysm (irrespective of the severity of aortic regurgitation), and Figure 2].
Figure 2. Management of patients with aortic regurgitation.

BSA: body surface area; LV: left ventricle/left ventricular; LVESD: left ventricle end-systolic diameter; LVEF: left ventricular ejection fraction. aSee recommendations on indications for surgery in severe aortic regurgitation and aortic root disease for definition. bSurgery should also be considered if significant changes in LV or aortic size occur during follow-up.
In symptomatic patients, surgery is recommended irrespective of the LVEF as long as aortic regurgitation is severe and the operative risk is not prohibitive.105,106,107,108,109 Surgery is recommended in symptomatic and asymptomatic patients with severe aortic regurgitation undergoing coronary artery bypass grafting (CABG), or surgery of the ascending aorta or another valve.110,111 In asymptomatic patients with severe aortic regurgitation, impairment of LV function [LVEF ≤50% or left ventricular end-systolic diameter (LVESD) >50 mm] are associated with worse outcomes and surgery should therefore be pursued when these cut-offs are reached.107,108,112,113,114 LVESD should be related to body surface area (BSA) and a cut-off of 25 mm/m2 BSA appeared to be more appropriate, especially in patients with small body size (BSA <1.68 m2) or with large BSA who are not overweight.108,115 Some recent retrospective, non-randomized studies emphasized the role of indexed LVESD and proposed a lower cut-off value of 20 or 22 mm/m² BSA for the indexed LVESD.116,117,118 One of these studies also suggests a higher cut-off value of 55% for LVEF.118 Based on these data, low-risk surgery may be discussed in some selected asymptomatic patients with LVESD >20 mm/m2 or resting LVEF between 50% and 55%. In patients not reaching the thresholds for surgery, close follow-up is needed, and exercise testing should be liberally performed to identify borderline symptomatic patients. Progressive enlargement of the LV, or a progressive decrease in its function in asymptomatic patients not reaching the thresholds for surgery but with significant LV dilatation [left ventricular end-diastolic diameter (LVEDD) >65 mm], may also be an appropriate indicator for timing operations in asymptomatic patients.
TAVI may be considered in experienced centres for selected patients with aortic regurgitation and ineligible for SAVR.119,120
In patients with a dilated aorta, the rationale for surgery has been best defined in patients with Marfan syndrome and root dilation.121,122 Root aneurysms require root replacement, with or without preservation of the native aortic valve. In contrast, tubular ascending aortic aneurysms in the presence of normal aortic valves require only a supracommissural tube graft replacement. In patients with aortic diameters borderline indicated for aortic surgery, the family history, age, and anticipated risk of the procedure should be taken into consideration. Irrespective of the degree of aortic regurgitation and type of valve pathology, in patients with an aortic diameter ≥55 mm with tricuspid or bicuspid aortic valves, ascending aortic surgery is recommended (see recommendations on indications for surgery in severe aortic regurgitation and aortic root disease, Recommendations C) when the operative risk is not prohibitive.123,124,125 In individuals with bicuspid aortic valve, when additional risk factors or coarctation126 are present, surgery should be considered when aortic diameter is ≥50 mm.127,128,129 In all patients with Marfan syndrome, aortic surgery is recommended for a maximal aortic diameter ≥50 mm.5,121,122 When additional risk factors are present in patients with Marfan syndrome and in patients with a TGFBR1 or TGFBR2 mutation (including Loeys-Dietz syndrome), surgery should be considered at a maximal aortic diameter ≥45 mm121,130 and even earlier (aortic diameter of 40 mm or more) in women with low BSA, patients with a TGFBR2 mutation, or patients with severe extra-aortic features that appear to be at particularly high risk.130 For patients who have an indication for aortic valve surgery, an aortic diameter ≥45 mm is considered to indicate concomitant surgery of the aortic root or tubular ascending aorta. The patient's stature, the aetiology of the valvular disease (bicuspid valve), and the intraoperative shape and wall thickness of the ascending aorta should be considered for individual decisions.
Recommendations C. Recommendations on indications for surgery in (A) severe aortic regurgitation and (B) aortic root or tubular ascending aortic aneurysm (irrespective of the severity of aortic regurgitation).
| Indications for surgery | Classa | Levelb |
|---|---|---|
| A) Severe aortic regurgitation | ||
| Surgery is recommended in symptomatic patients regardless of LV function. 105,106,107,108,109 | I | B |
| Surgery is recommended in asymptomatic patients with LVESD >50mm or LVESD >25 mm/m2 BSA (in patients with small body size) or resting LVEF ≤50%. 107,108,112,114,115 | I | B |
| Surgery may be considered in asymptomatic patients with LVESD >20 mm/m2 BSA (especially in patients with small body size) or resting LVEF ≤55%, if surgery is at low risk. | IIb | C |
| Surgery is recommended in symptomatic and asymptomatic patients with severe aortic regurgitation undergoing CABG or surgery of the ascending aorta or of another valve. | I | C |
| Aortic valve repair may be considered in selected patients at experienced centres when durable results are expected. | IIb | C |
| B) Aortic root or tubular ascending aortic aneurysmc (irrespective of the severity of aortic regurgitation) | ||
| Valve-sparing aortic root replacement is recommended in young patients with aortic root dilation, if performed in experienced centres and durable results are expected. 133,134,135,136,140 | I | B |
| Ascending aortic surgery is recommended in patients with Marfan syndrome who have aortic root disease with a maximal ascending aortic diameter ≥50 mm. | I | C |
| Ascending aortic surgery should be considered in patients who have aortic root disease with maximal ascending aortic diameter: • ≥55 mm in all patients. • ≥45 mm in the presence of Marfan syndrome and additional risk factorsd or patients with a TGFBR1 or TGFBR2 mutation (including Loeys-Dietz syndrome).e • ≥50 mm in the presence of a bicuspid valve with additional risk factorsd or coarctation. |
IIa | C |
| When surgery is primarily indicated for the aortic valve, replacement of the aortic root or tubular ascending aorta should be considered when ≥45 mm.f | IIa | C |
The choice of the surgical procedure should be adapted according to the experience of the team, the presence of an aortic root aneurysm, characteristics of the cusps, life expectancy, and desired anticoagulation status.
Valve replacement is the standard procedure in the majority of patients with aortic regurgitation. Aortic valve-sparing root replacement and valve repair yield good long-term results in selected patients, with low rates of valve-related events as well as good quality of life131,132,133,134,135,136,137,138,139,140 when performed in experienced centres. Aortic valve-sparing root replacement is recommended in younger patients who have an enlargement of the aortic root with normal cusp motion, when performed by experienced surgeons.133,134,135,136,140 In selected patients, aortic valve repair132,137 or the Ross procedure138,139 may be an alternative to valve replacement, when performed by experienced surgeons.
4.3 MEDICAL THERAPY
Medical therapy, especially angiotensin-converting enzyme inhibitors (ACEI) or dihydropiridines, may provide symptomatic improvement in individuals with chronic severe aortic regurgitation in whom surgery is not feasible. The value of ACEI or dihydropiridine in delaying surgery in the presence of moderate or severe aortic regurgitation in asymptomatic patients has not been established and their use is not recommended for this indication.
In patients who undergo surgery but continue to suffer from heart failure or hypertension, ACEI, angiotensin receptor blockers (ARBs), and beta-blockers are useful.141,142
In patients with Marfan syndrome, beta-blockers remain the mainstay for medical treatment and reducing shear stress and aortic growth rate and should be considered before and after surgery.143,144,145 While ARBs did not prove to have a superior effect when compared to beta-blockers, they may be considered as an alternative in patients intolerant to beta-blockers.146,147,148 By analogy, while there are no studies that provide supporting evidence, it is common clinical practice to advise beta-blocker or ARBs in patients with bicuspid aortic valve if the aortic root and/or ascending aorta is dilated. Management of aortic regurgitation during pregnancy is discussed in section 13.
4.4 SERIAL TESTING
All asymptomatic patients with severe aortic regurgitation and normal LV function should be followed up at least every year. In patients with either a first diagnosis or with LV diameter and/or ejection fraction showing significant changes or approaching thresholds for surgery, follow-up should be continued at 3-6-month intervals. Surgery may be considered in asymptomatic patients with significant LV dilatation (LVEDD >65 mm), and with progressive enlargement in the size of LV or progressive decrease of LVEF during follow-up. Patient’s BNP levels could be of potential interest as a predictor of outcomes (particularly symptom onset and deterioration of LV function) and may be helpful in the follow-up of asymptomatic patients.149 Patients with mild-to-moderate aortic regurgitation can be seen on a yearly basis and echocardiography performed every 2 years.
If the ascending aorta is dilated (>40 mm), it is recommended to systematically perform CCT or CMR. Follow-up assessment of the aortic dimension should be performed using echocardiography and/or CMR. Any increase >3 mm should be validated by CCT angiography/CMR and compared with baseline data. After repair of the ascending aorta, Marfan patients remain at risk for dissection of the residual aorta and lifelong regular multidisciplinary follow-up at an expert centre is required.
4.5 SPECIAL PATIENT POPULATIONS
If aortic regurgitation requiring surgery is associated with severe primary and secondary mitral regurgitation, both should be treated during the same operation.
In patients with moderate aortic regurgitation who undergo CABG or mitral valve surgery, the decision to treat the aortic valve is controversial, as data show that progression of moderate aortic regurgitation is very slow in patients without aortic dilation.150 The Heart Team should decide based on the aetiology of aortic regurgitation, other clinical factors, the life expectancy of the patient, and the patient's operative risk.
The level of physical and sports activity in the presence of a dilated aorta remains a matter of clinical judgment in the absence of evidence. Current guidelines are very restrictive, particularly regarding isometric exercise, to avoid a catastrophic event.151 This approach is justified in the presence of connective tissue disease, but a more liberal approach is likely to be appropriate in other patients.
Given the familial risk of thoracic aortic aneurysms, screening and referral for genetic testing of the patient's first-degree relatives with appropriate imaging studies is indicated in patients with connective tissue disease. For patients with bicuspid valves, it is appropriate to have an echocardiographic screening of first-degree relatives.
5 Aortic stenosis
Aortic stenosis is the most common primary valve lesion requiring surgery or transcatheter intervention in Europe1 and North America. Its prevalence is rising rapidly as a consequence of the ageing population.2,152
5.1 EVALUATION
5.1.1 ECHOCARDIOGRAPHY
Echocardiography is key to confirming the diagnosis and severity of aortic stenosis, assessing valve calcification, LV function and wall thickness, detecting other valve disease or aortic pathology, and providing prognostic information.43,153,154 Assessment should be undertaken when blood pressure (BP) is well controlled to avoid the confounding flow effects of increased afterload. New echocardiographic parameters, stress imaging and CCT provide important adjunctive information when severity is uncertain (Figure 3).
Figure 3. Integrated imaging assessment of aortic stenosis.

AS: aortic stenosis; AV: aortic valve; AVA: aortic valve area; CT: computed tomography; ΔPm: mean pressure gradient; DSE: dobutamine stress echocardiography; LV: left ventricle/left ventricular; LVEF: left ventricular ejection fraction; SVi: stroke volume index; Vmax: peak transvalvular velocity. aHigh flow may be reversible in patients with anaemia, hyperthyroidism or arterio-venous fistulae, and may also be present in patients with hypertrophic obstructive cardiomyopathy. Upper limit of normal flow using pulsed Doppler echocardiography: cardiac index 4.1 L/min/m2 in men and women, SVi 54 mL/m2 in men, 51 mL/m2 in women).155 bConsider also: typical symptoms (with no other explanation), LV hypertrophy (in the absence of coexistent hypertension) or reduced LV longitudinal function (with no other cause). cDSE flow reserve: >20% increase in stroke volume in response to low-dose dobutamine. dPseudo-severe aortic stenosis: AVA >1.0 cm2 with increased flow. eThresholds for severe aortic stenosis assessed by means of CT measurement of aortic valve calcification (Agatston units): men >3000, women >1600: highly likely; men >2000, women >1200: likely; men <1600, women <800: unlikely.
Current international recommendations for the echocardiographic evaluation of patients with aortic stenosis25 depend upon measurement of mean pressure gradient (the most robust parameter), peak transvalvular velocity (Vmax), and valve area. Although valve area is the theoretically ideal measurement for assessing severity, there are numerous technical limitations. Clinical decision making in discordant cases should therefore take account of additional parameters: functional status, stroke volume, Doppler velocity index,156 degree of valve calcification, LV function, the presence or absence of LV hypertrophy, flow conditions, and the adequacy of BP control.25 Low flow is arbitrarily defined by a stroke volume index (SVi) ≤35 mL/m² –a threshold that is under current debate.155,157,158 The use of sex -specific thresholds has been recently proposed.159 Four broad categories can be defined:
High-gradient aortic stenosis [mean gradient ≥40 mmHg, peak velocity ≥4.0 m/s, valve area ≤1 cm2 (or ≤0.6 cm²/m²)]. Severe aortic stenosis can be assumed irrespective of LV function and flow conditions.
Low-flow, low-gradient aortic stenosis with reduced ejection fraction (mean gradient <40 mmHg, valve area ≤1 cm2, LVEF <50%, SVi ≤35 mL/m2). Low-dose dobutamine stress echocardiography (DSE) is recommended to distinguish between true severe and pseudo-severe aortic stenosis (increase in valve area to >1.0 cm2 with increased flow) and identify patients with no flow (or contractile) reserve.160 However, utility in elderly patients has only been evaluated in small registries.161
Low-flow, low-gradient aortic stenosis with preserved ejection fraction (mean gradient <40 mmHg, valve area ≤1 cm2, LVEF ≥50%, SVi ≤35 mL/m2). Typically encountered in hypertensive elderly subjects with small LV size and marked hypertrophy.157,162 This scenario may also result from conditions associated with low stroke volume (e.g. moderate/severe mitral regurgitation, severe tricuspid regurgitation, severe mitral stenosis, and large ventricular septal defect and severe RV dysfunction). Diagnosis of severe aortic stenosis is challenging and requires careful exclusion of measurement errors and other explanations for the echocardiographic findings,25 as well as the presence or absence of typical symptoms (with no other explanation), LV hypertrophy (in the absence of coexistent hypertension) or reduced LV longitudinal strain (with no other cause). CCT assessment of the degree of valve calcification provides important additional information [thresholds (Agatston units) for severe aortic stenosis: men >3000, women >1600 = highly likely; men >2000, women >1200 = likely; men <1600, women <800 = unlikely].35,36,163,164
Normal-flow, low-gradient aortic stenosis with preserved ejection fraction (mean gradient <40 mmHg, valve area ≤1 cm2, LVEF≥50%, SVi >35 mL/m2). These patients usually have only moderate aortic stenosis.36,165,166,167
5.1.2 ADDITIONAL DIAGNOSTIC AND PROGNOSTIC PARAMETERS
The resting Doppler velocity index (DVI, also termed ‘dimensionless index’) –the ratio of the left ventricular outflow tract (LVOT) time-velocity integral (TVI) to that of the aortic valve jet– does not require calculation of LVOT area and may assist evaluation when other parameters are equivocal (a value <0.25 suggests that severe aortic stenosis is highly likely).156 Assessment of global longitudinal strain provides additional information concerning LV function and a threshold of 15% may help to identify patients with severe asymptomatic aortic stenosis who are at higher risk of clinical deterioration or premature mortality.26,168 TOE allows evaluation of concomitant mitral valve disease and may be of value for periprocedural imaging during TAVI and SAVR.169
Natriuretic peptides predict symptom-free survival and outcome in normal and low-flow severe aortic stenosis.170,171 They can be used to arbitrate the source of symptoms in patients with multiple potential causes and identify those with high-risk asymptomatic aortic stenosis who may benefit from early intervention (section 5.2.2, Table 6 and Figure 3).
Exercise testing may unmask symptoms and is recommended for risk stratification of asymptomatic patients with severe aortic stenosis.172 Exercise echocardiography provides additional prognostic information by assessing the increase in mean pressure gradient and change in LV function.173
CCT provides information concerning the anatomy of the aortic root and ascending aorta, and the extent and distribution of valve and vascular calcification, and feasibility of vascular access.174 Quantification of valve calcification predicts disease progression and clinical events164 and may be useful when combined with geometric assessment of valve area in assessing the severity of aortic stenosis in patients with low valve gradient.35,36,163,164
Myocardial fibrosis is a major driver of LV decompensation in aortic stenosis (regardless of the presence or absence of CAD), which can be detected and quantified using CMR. Amyloidosis is also frequently associated with aortic stenosis in elderly patients (incidence 9-15%).175 When cardiac amyloidosis is clinically suspected, based on symptoms (neuropathy and hematologic data), diphosphonate scintigraphy and/or CMR should be considered. Both entities persist following valve intervention and are associated with poor long-term prognosis.176,177,178,179
Coronary angiography is essential prior to TAVI and SAVR to determine the potential need for concomitant revascularization (see section 3.2.4.1 and section 5.5). Retrograde LV catheterization is not recommended unless there are symptoms and signs of severe aortic stenosis and non-invasive investigations are inconclusive.
5.1.3 TAVI DIAGNOSTIC WORKUP
Prior to TAVI, CCT is the preferred imaging tool to assess: (i) aortic valve anatomy, (ii) annular size and shape, (iii) extent and distribution of valve and vascular calcification, (iv) risk of coronary ostial obstruction, (v) aortic root dimensions, (vi) optimal fluoroscopic projections for valve deployment, and (vii) feasibility of vascular access (femoral, subclavian, axillary, carotid, transcaval or transapical). Adverse anatomical findings may suggest that SAVR is a better treatment option (Table 6). TOE is more operator-dependent but may be considered when CCT is difficult to interpret or relatively contraindicated (e.g. chronic renal failure).
5.2 INDICATIONS FOR INTERVENTION (SAVR OR TAVI)
Indications for aortic valve intervention are summarized in the table of recommendations on indications for intervention in symptomatic and asymptomatic aortic stenosis and recommended mode of intervention and in Figure 4.
Figure 4. Management of patients with severe aortic stenosis.

BP: blood pressure; EuroSCORE: European System for Cardiac Operative Risk Evaluation; LVEF: left ventricular ejection fraction; SAVR: surgical aortic valve replacement; STS-PROM: Society of Thoracic Surgeons – predicted risk of mortality; TAVI: transcatheter aortic valve implantation; TF: transfemoral. aSee Figure 3: Integrated imaging assessment of aortic stenosis. bProhibitive risk is defined in Supplementary Table 5. cHeart Team assessment based upon careful evaluation of clinical, anatomical, and procedural factors (see Table 6 and table on Recommendations on indications for intervention in symptomatic and asymptomatic aortic stenosis and recommended mode of intervention). The Heart Team recommendation should be discussed with the patient who can then make an informed treatment choice. dAdverse features according to clinical, imaging (echocardiography/CT), and/or biomarker assessment. eSTS-PROM: http://riskcalc.sts.org/stswebriskcalc/#/calculate, EuroSCORE II: http://www.euroscore.org/calc.html. fIf suitable for procedure according to clinical, anatomical, and procedural factors (Table 6).
5.2.1 SYMPTOMATIC AORTIC STENOSIS
Symptomatic severe aortic stenosis has dismal prognosis and early intervention is strongly recommended in all patients. The only exceptions are for those in whom intervention is unlikely to improve quality of life or survival (due to severe comorbidities) or for those with concomitant conditions associated with survival <1 year (e.g. malignancy) (section 3).
Intervention is recommended in symptomatic patients with high-gradient aortic stenosis, regardless of LVEF. However, management of patients with low-gradient aortic stenosis is more challenging:
LV function usually improves after intervention in patients with low-flow, low-gradient aortic stenosis, when reduced ejection fraction is predominantly caused by excessive afterload.32,180 Conversely, improvement is uncertain if the primary cause of reduced ejection fraction is scarring due to myocardial infarction or cardiomyopathy. Intervention is recommended when severe aortic stenosis is confirmed by stress echocardiography (true severe aortic stenosis; Figure 3),32 while patients with pseudo-severe aortic stenosis should receive conventional heart failure treatment.142,181 The presence or absence of flow reserve (increase in stroke volume ≥20%) d [oe]s not appear to influence prognosis in contemporary series of patients undergoing TAVI or SAVR,182,183,184 and although those with no flow reserve show increased procedural mortality, both modes of intervention improve ejection fraction and clinical outcomes.32,180,182 Decision making for such patients should take account of comorbidities, degree of valve calcification, extent of CAD, and feasibility of revascularization.
Data concerning the natural history of low-flow, low-gradient aortic stenosis and preserved ejection fraction, and outcomes after SAVR and TAVI remain controversial.162,165,167 Intervention should only be considered in those with symptoms and significant valve obstruction (see table of recommendations on indications for intervention in symptomatic and asymptomatic aortic stenosis and recommended mode of intervention and Figure 4).
The prognosis of patients with normal-flow, low-gradient aortic stenosis and preserved ejection fraction is similar to that of moderate aortic stenosis –regular clinical and echocardiographic surveillance is recommended.165,166,185
5.2.2 ASYMPTOMATIC AORTIC STENOSIS
Intervention is recommended in asymptomatic patients with severe aortic stenosis and impaired LV function of no other cause,9 and those who are asymptomatic during normal activities but develop symptoms during exercise testing.172,186 Management of asymptomatic severe aortic stenosis is otherwise controversial and the decision to intervene requires careful assessment of the benefits and risks in an individual patient.
In the absence of adverse prognostic features, watchful waiting has generally been recommended with prompt intervention at symptom onset.187 Data from a single RCT have shown significant reduction in the primary endpoint (death during or within 30 days of surgery or cardiovascular death during the entire follow-up period) following early SAVR compared with conservative management [1% vs. 15%; hazard ratio 0.09; 95% confidence interval (CI), 0.01-0.67; P = 0.003].188 However, subjects were selected per inclusion criteria (median age 64 years, minimal comorbidities, low operative risk) and follow-up in the conservative group was limited. Further randomized trials [EARLY TAVR (NCT03042104), AVATAR (NCT02436655), EASY-AS (NCT04204915), EVOLVED (NCT03094143)] will help determine future recommendations.
Predictors of symptom development and adverse outcomes in asymptomatic patients include clinical characteristics (older age, atherosclerotic risk factors), echocardiographic parameters (valve calcification, peak jet velocity189,190), LVEF, rate of haemodynamic progression,189 increase in mean gradient >20 mmHg with exercise,172 severe LV hypertrophy,191 indexed stroke volume,158 LA volume,192 LV global longitudinal strain,26,168,193 and abnormal biomarker levels (natriuretic peptides, troponin, and fetuin-A).170,171,194,195 Early intervention may be considered in asymptomatic patients with severe aortic stenosis and one or more of these predictors if procedural risk is low (although application of TAVI in this setting has yet to be formally evaluated) (Table 6 and Figure 4). Otherwise, watchful waiting is a safer and more appropriate strategy.
5.2.3 THE MODE OF INTERVENTION
Use of SAVR and TAVI as complementary treatment options has allowed a substantial increase in the overall number of patients with aortic stenosis undergoing surgical or transcatheter intervention in the past decade.196 RCTs have assessed the two modes of intervention across the spectrum of surgical risk in predominantly elderly patients and a detailed appraisal of the evidence base is provided in Supplementary Section 5. In brief, these trials used surgical risk scores to govern patient selection and demonstrate that TAVI is superior to medical therapy in extreme-risk patients,197 and non-inferior to SAVR in high-198,199,200,201 and intermediate-risk patients at follow-up extending to 5 years.202,203,204,205,206,207,208 The more recent PARTNER 3 and Evolut Low Risk trials demonstrate that TAVI is non-inferior to SAVR in low-risk patients at 2-year follow-up.209,210,211,212 Importantly, patients in the low-risk trials were predominantly male and relatively elderly (e.g. PARTNER 3: mean age 73.4 years, <70 years 24%, 70-75 years 36%, >75 years 40%, >80 years 13%) whilst those with low-flow aortic stenosis or adverse anatomical characteristics for either procedure (including bicuspid aortic valves or complex coronary disease) were excluded.
Rates of vascular complications, pacemaker implantation, and paravalvular regurgitation are consistently higher after TAVI, whereas severe bleeding, acute kidney injury, and new-onset AF are more frequent after SAVR. Although the likelihood of paravalvular regurgitation has been reduced with newer transcatheter heart valve designs, pacemaker implantation (and new-onset left bundle branch block) may have long-term consequences213,214,215 and further refinements are required. Most patients undergoing TAVI have a swift recovery, short hospital stay, and rapidly return to normal activities.216,217 Despite these benefits, there is wide variation in worldwide access to the procedure as a result of high device costs and differing levels of healthcare resources.71,218,219
The Task Force has attempted to address the gaps in evidence and provide recommendations concerning the indications for intervention and mode of treatment (Recommendations on indications for intervention in symptomatic and asymptomatic aortic stenosis and recommended mode of intervention, Recommendations D, Figure 4) that are guided by the RCT findings and compatible with real-world Heart Team decision making for individual patients (many of whom fall outside the RCT inclusion criteria). Aortic stenosis is a heterogeneous condition and selection of the most appropriate mode of intervention should be carefully considered by the Heart Team for all patients, accounting for individual age and estimated life expectancy, comorbidities (including frailty and overall quality of life, section 3), anatomical and procedural characteristics (Table 6), the relative risks of SAVR and TAVI and their long-term outcomes, prosthetic heart valve durability, feasibility of transfemoral TAVI, and local experience and outcome data. These factors should be discussed with the patient and their family to allow informed treatment choice.
Recommendations D. Recommendations on indications for intervention<sup>a</sup> in symptomatic (A) and asymptomatic (B) aortic stenosis and recommended mode of intervention (C).
| A) Symptomatic aortic stenosis | Classb | Levelc |
|---|---|---|
| Intervention is recommended in symptomatic patients with severe, high-gradient aortic stenosis [mean gradient ≥40 mmHg, peak velocity ≥4.0 m/s, and valve area ≤1.0 cm2 (or ≤0.6 cm2/m2)] . 235,236 | I | B |
| Intervention is recommended in symptomatic patients with severe low-flow (SVi ≤35 mL/m2), low-gradient (<40 mmHg) aortic stenosis with reduced ejection fraction (<50%), and evidence of flow (contractile) reserve. 32,237 | I | B |
| Intervention should be considered in symptomatic patients with low-flow, low-gradient (<40 mmHg) aortic stenosis with normal ejection fraction after careful confirmation that the aortic stenosis is severed (Figure 3). | IIa | C |
| Intervention should be considered in symptomatic patients with low-flow, low-gradient severe aortic stenosis and reduced ejection fraction without flow (contractile) reserve, particularly when CCT calcium scoring confirms severe aortic stenosis. | IIa | C |
| Intervention is not recommended in patients with severe comorbidities when the intervention is unlikely to improve quality of life or prolong survival >1 year. | III | C |
| B) Asymptomatic patients with severe aortic stenosis | ||
| Intervention is recommended in asymptomatic patients with severe aortic stenosis and systolic LV dysfunction (LVEF <50%) without another cause. 9,238,239 | I | B |
| Intervention is recommended in asymptomatic patients with severe aortic stenosis and demonstrable symptoms on exercise testing. | I | C |
| Intervention should be considered in asymptomatic patients with severe aortic stenosis and systolic LV dysfunction (LVEF <55%) without another cause. 9,240,241 | IIa | B |
| Intervention should be considered in asymptomatic patients with severe aortic stenosis and a sustained fall in BP (>20 mmHg) during exercise testing. | IIa | C |
| Intervention should be considered in asymptomatic patients with LVEF >55% and a normal exercise test if the procedural risk is low and one of the following parameters is present: • Very severe aortic stenosis (mean gradient ≥60 mmHg or Vmax >5 m/s). 9,242 • Severe valve calcification (ideally assessed by CCT) and Vmax progression ≥0.3 m/s/year. 164,189,243 • Markedly elevated BNP levels (>3- age- and sex-corrected normal range) confirmed by repeated measurements and without other explanation. 163,171 |
IIa | B |
| C) Mode of intervention | ||
| Aortic valve interventions must be performed in Heart Valve Centres that declare their local expertise and outcomes data, have active interventional cardiology and cardiac surgical programmes on site, and a structured collaborative Heart Team approach. | I | C |
| The choice between surgical and transcatheter intervention must be based upon careful evaluation of clinical, anatomical, and procedural factors by the Heart Team, weighing the risks and benefits of each approach for an individual patient. The Heart Team recommendation should be discussed with the patient who can then make an informed treatment choice. | I | C |
| SAVR is recommended in younger patients who are low risk for surgery (<75 yearse and STSPROM/ EuroSCORE II <4%)e,f, or in patients who are operable and unsuitable for transfemoral TAVI. 244 | I | B |
| TAVI is recommended in older patients (≥75 years), or in those who are high risk (STSPROM/ EuroSCORE IIf >8%) or unsuitable for surgery. 197,198,199,200,201,202,203,204,205,206,245 | I | A |
| SAVR or TAVI are recommended for remaining patients according to individual clinical, anatomical, and procedural characteristics. 202,203,204,205,207,209,210,212 f,g | I | B |
| Non-transfemoral TAVI may be considered in patients who are inoperable and unsuitable for transfemoral TAVI. | IIb | C |
| Balloon aortic valvotomy may be considered as a bridge to SAVR or TAVI in haemodynamically unstable patients and (if feasible) in those with severe aortic stenosis who require urgent highrisk NCS (Figure 11). | IIb | C |
| D) Concomitant aortic valve surgery at the time of other cardiac/ascending aorta surgery | ||
| SAVR is recommended in patients with severe aortic stenosis undergoing CABG or surgical intervention on the ascending aorta or another valve. | I | C |
| SAVR should be considered in patients with moderate aortic stenosish undergoing CABG or surgical intervention on the ascending aorta or another valve after Heart Team discussion. | IIa | C |
| BNP: B-type natriuretic peptide; BP: blood pressure; CABG: coronary artery bypass grafting; CCT: cardiac computed tomography; EuroSCORE: European System for Cardiac Operative Risk Evaluation; LV: left ventricle/left ventricular; LVEF: left ventricular ejection fraction; NCS: non-cardiac surgery; SAVR: surgical aortic valve replacement; STS-PROM: Society of Thoracic Surgeons - predicted risk of mortality; SVi: stroke volume index; TAVI: transcatheter aortic valve implantation; Vmax: peak transvalvular velocity. aSAVR or TAVI. bClass of recommendation. cLevel of evidence. dExplanations other than severe aortic stenosis for a small valve area but low gradient despite preserved LVEF are frequent and must be carefully excluded (Figure 3). eSTS-PROM: http://riskcalc.sts.org/stswebriskcalc/#/calculate, EuroSCORE II: http://www.euroscore.org/calc.html. fIf suitable for surgery (see Table 6). gIf suitable for transfemoral TAVI (see Table 6). hModerate aortic stenosis is defined as a valve area of 1.0-1.5 cm2 (or mean aortic gradient of 25-40 mmHg) in normal flow conditions – clinical assessment is essential to determine whether SAVR is appropriate for an individual patient. | ||
The interplay between estimated life expectancy and prosthetic heart valve durability is a key consideration in these discussions. Age is a surrogate for life expectancy but had no impact on the outcomes of the low-risk RCTs at 1-2 year follow-up. Life expectancy varies widely across the world and is highly dependent on absolute age, sex, frailty, and the presence of comorbidities (http://ghdx.healthdata.org/record/ihme-data/gbd-2017-life-tables-1950-2017); it may be a better guide than age alone but is difficult to determine in individual patients. Although some (now abandoned) surgical bioprosthetic designs have failed early, the durability of contemporary surgical bioprosthetic valves beyond 10 years is well established.220 Conversely, registry data provide some reassurance concerning the long-term durability of TAVI devices up to 8 years but largely relate to older high-/intermediate-risk patients,221,222,223,224 whereas information concerning durability in low-risk patients is currently limited to 2-year follow-up. Data comparing the durability of transcatheter heart valves and surgical bioprostheses directly remain limited. Rates of aortic valve re-intervention were higher after TAVI using a balloon-expandable valve compared to SAVR at 5-year follow-up in the PARTNER 2A trial (3.2% vs. 0.8%; hazard ratio, 3.3; 95% CI, 1.3-8.1),206 whereas rates of structural valve deterioration (SVD) were not statistically different following SAVR and TAVI using the third generation SAPIEN 3 device in a parallel observational registry over the same time frame.225
Valve-in-valve TAVI is an established treatment option for surgical bioprosthetic valve deterioration but may not be appropriate or feasible in all patients due to the increased likelihood of PPM in patients with a small aortic root (or undersized original prosthesis), incompatible surgical valve designs associated with increased risk of coronary occlusion, or difficult vascular access; re-do SAVR should also be considered in these settings.226,227,228 Favourable short-term outcomes of redo-TAVI have been demonstrated in selected older patients with transcatheter heart valve deterioration,229 despite theoretical concerns relating to maintained coronary access.230
A bicuspid aortic valve is more frequent in younger patients with aortic stenosis. While several registries have reported excellent outcomes of TAVI in patients with a bicuspid valve who were unsuitable for surgery,231,232,233 SAVR remains more appropriate in patients with aortic stenosis affecting a bicuspid valve and in those with associated disease (e.g. aortic root dilatation, complex coronary disease, or severe mitral regurgitation) requiring a surgical approach.
In summary, prosthetic heart valve durability is a key consideration in younger patients (<75 years) at low surgical risk and SAVR (if feasible) is therefore the preferred treatment option. Conversely, durability is a lower priority in older patients (≥75 years), or those who are inoperable or high risk for surgery, and TAVI is preferred in these groups (particularly if feasible via transfemoral approach). The Heart Team should make tailored recommendations for remaining patients based upon their individual characteristics (Table 6). This guidance should be re-addressed when further data concerning the long-term durability of TAVI become available.
Balloon aortic valvuloplasty (BAV) may be considered as a bridge to TAVI or SAVR in patients with decompensated aortic stenosis and (when feasible) in those with severe aortic stenosis who require urgent high-risk non-cardiac surgery (NCS) (section 12). The procedure carries significant risk of complications234 and should only be undertaken after Heart Team discussion.
5.3 MEDICAL THERAPY
No medical therapies influence the natural history of aortic stenosis. Statins (which demonstrated favourable effects in pre-clinical studies) do not affect disease progression246 and clinical trials targeting calcium metabolic pathways are ongoing. Patients with heart failure who are unsuitable (or waiting) for SAVR or TAVI should be medically treated according to ESC heart failure Guidelines.247 ACEI are safe in aortic stenosis (provided that BP is monitored carefully) and may have beneficial myocardial effects before the onset of symptoms, and after TAVI and SAVR.248,249,250 Coexisting hypertension should be treated to avoid additional afterload, although medication (particularly vasodilators) should be titrated to avoid symptomatic hypotension.
Antithrombotic therapy after TAVI is discussed in section 11.
5.4 SERIAL TESTING
Rate of progression of aortic stenosis varies widely. Asymptomatic patients, their family and medical carers need careful education, with emphasis on the importance of regular follow-up (ideally in a Heart Valve Clinic9) and prompt reporting of symptoms. Those with severe aortic stenosis should be followed up every 6 months (at least) to allow earliest symptom detection (using exercise testing if symptoms are doubtful) and any change in echocardiographic parameters (particularly LVEF). Measurement of natriuretic peptides may be considered.
Several studies suggest that the prognosis of moderate degenerative aortic stenosis is worse than previously considered251,252,253,254 (particularly if there is significant valve calcification) and these patients should be re-evaluated at least annually. Younger patients with mild aortic stenosis and no significant calcification may be followed up every 2-3 years.
5.5 SPECIAL PATIENT POPULATIONS
Women with aortic stenosis have higher mortality than men, resulting from late diagnosis and initial specialist assessment followed by less frequent and delayed referral for intervention.255,256,257 Measures are needed to improve this situation and ensure that both sexes receive equivalent care.
CAD and aortic stenosis frequently coexist and the combination confers higher risk of clinical events, therefore the need to consider revascularization in conjunction with aortic valve intervention is common. The impact of coronary revascularization in patients with silent CAD accompanying aortic stenosis is unclear and further studies are warranted in this context (section 3). Both simultaneous SAVR and CABG, and SAVR late after CABG, carry a higher procedural risk than isolated SAVR. Nevertheless, retrospective data indicate that patients with moderate aortic stenosis, in whom CABG is indicated, benefit from concomitant SAVR. Patients aged <70 years with mean gradient progression ≥5 mmHg/year benefit from SAVR at the time of CABG once baseline peak gradient exceeds 30 mmHg.258 Decisions for individual patients should take into account haemodynamic data, rate of progression, extent of leaflet calcification, life expectancy, and associated comorbidities, as well as the individual risk of concomitant SAVR or deferred TAVI.244
Percutaneous coronary intervention (PCI) and TAVI may be undertaken as combined or staged procedures according to the clinical situation, pattern of CAD, and extent of myocardium at risk.259 In the SURTAVI trial, there was no significant difference in the primary endpoint (all-cause mortality or stroke at 2-year follow-up) in intermediate-risk patients with severe aortic stenosis and non-complex CAD (SYNTAX score <22) undergoing either TAVI and PCI or SAVR and CABG [16.0% (95% CI, 11.1-22.9) vs. 14% (95% CI, 9.2-21.1); P = 0.62].260 Assessing the clinical value of systematic PCI in TAVI patients with significant associated CAD is the objective of ongoing RCTs. Patients with severe symptomatic aortic stenosis and diffuse CAD unsuitable for revascularization should receive optimal medical therapy and undergo SAVR or TAVI according to individual characteristics.
Severity of mitral regurgitation accompanying severe aortic stenosis may be overestimated as a result of elevated LV pressures and careful quantification is required. In patients with severe primary mitral regurgitation (PMR), mitral valve surgery is required at the time of SAVR. In patients with severe SMR, surgery may also be considered in the presence of significant annular dilatation and marked LV enlargement. In high-risk or inoperable patients with severe aortic stenosis and severe mitral regurgitation, combined (or more often sequential) TAVI and TEER may be feasible, but there is insufficient experience to allow robust recommendations.261,262,263 In patients with severe PMR, TEER should be considered early if the patient remains symptomatic and mitral regurgitation is still severe after TAVI. In patients with severe SMR, TAVI should be followed by careful clinical and echocardiographic reassessment to determine whether further mitral intervention is required.264
Section 4 provides recommendations for the management of aneurysm/dilatation of the ascending aorta accompanying aortic stenosis. Assessment and management of congenital aortic stenosis is addressed in ESC Guidelines on adult congenital heart disease.265
6 Mitral regurgitation
Mitral regurgitation is the second-most frequent VHD in Europe.1,3 The underlying mechanism (primary or secondary) determines the therapeutic approach.
6.1 PRIMARY MITRAL REGURGITATION
Primary lesion of one or more components of the mitral valve apparatus characterizes PMR. Degenerative aetiology (fibroelastic deficiency and Barlow disease) is most frequent in Western countries.1,2,266 In low-income countries, rheumatic aetiology is the most frequent cause of mitral regurgitation.267 Endocarditis can cause PMR and is addressed in the corresponding ESC Guidelines.4
6.1.1 EVALUATION
Echocardiography is the first choice of imaging technique to grade PMR (Table 7). An integrative approach including qualitative, semi-quantitative, and quantitative measures of mitral regurgitation (besides quantification of LV and LA dimensions) is recommended.24,268 Routinely measured effective regurgitant orifice area (EROA) is strongly associated with all-cause mortality, and compared with the general population an excess mortality appears for an EROA ≥20 mm2 and steadily increases beyond 40 mm2.269 Evaluation of the specific lesion leading to mitral regurgitation has prognostic implications266,270 and is fundamental to determine the feasibility of surgical and transcatheter valve repair271,272,273 (Supplementary Figure 1). Three-dimensional TOE provides an ‘en face’ view of the mitral leaflets resembling the surgical inspection of the valve, thereby facilitating Heart Team discussion.24,268 In addition, 3D echocardiography has shown better agreement with CMR in quantifying the regurgitant volume than 2D echocardiography, particularly in eccentric, multiple and late-systolic regurgitant jets.274,275,276,277 When various echocardiographic parameters used to grade mitral regurgitation are inconsistent, CMR is a valid alternative to quantify the regurgitant volume and is the reference standard to quantify LV and LA volumes.278 In addition, quantification of mitral regurgitation with CMR has shown prognostic implications.277 Finally, preliminary data show that myocardial fibrosis assessed with CMR is frequent in PMR and has been associated with sudden cardiac death and ventricular arrhythmias.279
Table 7. Severe mitral regurgitation criteria based on 2D echocardiography.
| Primary mitral regurgitation | Secondary mitral regurgitation | |
|---|---|---|
| Qualitative | ||
| Mitral valve morphology | Flail leaflet, ruptured papillary muscle, severe retraction, large perforation | Normal leaflets but with severe tenting, poor leaflet coaptation |
| Colour flow jet area | Large central jet (>50% of LA) or eccentric wall impinging jet of variable size | Large central jet (>50% of LA) or eccentric wall impinging jet of variable size |
| Flow convergence | Large throughout systole | Large throughout systole |
| Continuous wave Doppler jet | Holosystolic/dense/triangular | Holosystolic/dense/triangular |
| Semiquantitative | ||
| Vena contracta width (mm) | ≥7 (≥8 mm for biplane) | ≥7 (≥8 mm for biplane) |
| Pulmonary vein flow | Systolic flow reversal | Systolic flow reversal |
| Mitral inflow | E-wave dominant (>1.2 m/s) | E-wave dominant (>1.2 m/s) |
| TVI mitral/TVI aortic | >1.4 | >1.4 |
| Quantitative | ||
| EROA (2D PISA, mm2) | ≥40 mm2 | ≥40 mm2 (may be ≥30 mm2 if elliptical regurgitant orifice area) |
| Regurgitant volume (mL/beat) | ≥60 mL | ≥60 mL (may be ≥45 mL if low flow conditions) |
| Regurgitant fraction (%) | ≥50% | ≥50% |
| Structural | ||
| Left ventricle | Dilated (ESD ≥40 mm) | Dilated |
| Left atrium | Dilated (diameter ≥55 mm or volume ≥60 mL/m2) | Dilated |
| 2D: two-dimensional; ESD: endsystolic diameter; EROA: effective regurgitant orifice area; LA: left atrium; PMR: primary mitral regurgitation; SMR: secondary mitral regurgitation; PISA: proximal isovelocity surface area; TVI: time-velocity integral. Adapted from Lancellotti P et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2013;14:611-644. Copyright (2013) by permission of Oxford University Press on behalf of the European Society of Cardiology. Reproduced from Zoghbi WA et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303-371. Copyright (2017), with permission from the American Society of Echocardiography. | ||
Exercise echocardiography permits evaluation of changes in mitral regurgitant volume and pulmonary pressures during peak exercise and is particularly helpful in patients with discordant symptoms and regurgitation grade at rest.280,281 In asymptomatic patients with severe PMR and non-dilated LV and LA, low BNP values are associated with low mortality and can be useful during follow-up.41,282
LV dimensions and ejection fraction are considered to guide the management of patients with severe PMR. However, there is cumulative evidence showing that LV global longitudinal strain has incremental prognostic value in patients treated with surgical repair.283,284 Recently, the Mitral Regurgitation International Database (MIDA) score has been proposed to estimate the risk of all-cause mortality in patients with severe PMR due to flail leaflet, who are under medical treatment or surgically treated.285 Among the variables included in the score, LA diameter ≥55 mm and LVESD ≥40 mm are new thresholds that have been included in the current recommendations.
Right heart catheterization is systematically used to confirm pulmonary hypertension diagnosed by echocardiography when this is the only criterion to refer the patient for surgery.
6.1.2 INDICATIONS FOR INTERVENTION
Urgent surgery is indicated in patients with acute severe mitral regurgitation. In the case of papillary muscle rupture as the underlying disease, valve replacement is generally required.
Indications for surgery in severe chronic PMR are shown in the following table of recommendations (Recommendations E) and in Figure 5. Surgery is recommended in patients with symptomatic severe PMR and acceptable surgical risk according to the Heart Team decision. The presence of LVEF ≤60%, LVESD ≥40 mm,285,286 LA volume ≥60 mL/m2 or diameter ≥55 mm,287,288 systolic pulmonary arterial pressure (SPAP) >50 mmHg,289 and AF290,291 have been associated with worse outcomes and are considered triggers for intervention regardless of symptomatic status.292 In the absence of these criteria, watchful waiting is a safe strategy in asymptomatic patients with severe PMR and ideally should be performed in a Heart Valve Clinic.
Recommendations E. Recommendations on indications for intervention in severe primary mitral regurgitation.
| Recommendations | Classa | Levelb |
|---|---|---|
| Mitral valve repair is the recommended surgical technique when the results are expected to be durable. 293,294,295,296 | I | B |
| Surgery is recommended in symptomatic patients who are operable and not high risk. 293,294,295,296 | I | B |
| Surgery is recommended in asymptomatic patients with LV dysfunction (LVESD ≥40 mm and/or LVEF ≤60%). 277,286,292 | I | B |
| Surgery should be considered in asymptomatic patients with preserved LV function (LVESD <40 mm and LVEF >60%) and AF secondary to mitral regurgitation or pulmonary hypertensionc (SPAP at rest >50 mmHg). 285,289 | IIa | B |
| Surgical mitral valve repair should be considered in low-risk asymptomatic patients with LVEF >60%, LVESD <40 mmd and significant LA dilatation (volume index ≥60 mL/m2 or diameter ≥55 mm) when performed in a Heart Valve Centre and a durable repair is likely. 285,288 | IIa | B |
| TEER may be considered in symptomatic patients who fulfil the echocardiographic criteria of eligibility, are judged inoperable or at high surgical risk by the Heart Team and for whom the procedure is not considered futile. 299,300,301,302 | IIb | B |
| AF: atrial fibrillation; LA: left atrium/left atrial; LV: left ventricle/left ventricular; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; SPAP: systolic pulmonary arterial pressure; TEER: transcatheter edge-to-edge repair. aClass of recommendations. bLevel of evidence. cIf an elevated SPAP is the only indication for surgery, the value should be confirmed by invasive measurement. dCut-offs refer to average-size adults and may require adaptations in patients with unusually small or large stature. | ||
Figure 5. Management of patients with severe chronic primary mitral regurgitation.

AF: atrial fibrillation; HF: heart failure; LA: left atrium/ left atrial; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; SPAP: systolic pulmonary arterial pressure; TEER: transcatheter edge-to-edge repair. aLA dilatation: volume index ≥60 mL/m2 or diameter ≥55 mm at sinus rhythm. bExtended heart failure treatment includes the following: CRT; ventricular assist devices; heart transplantation.247
When surgery is considered, mitral valve repair is the surgical intervention of first choice when the results are expected to be durable according to the Heart Team evaluation since it is associated with better survival compared to mitral valve replacement.293,294 PMR due to segmental valve prolapse can be repaired with a low risk of recurrence and reoperation.294,295,296 The reparability of rheumatic lesions, extensive valve prolapse and to a greater extent leaflet calcification or extensive annular calcification is more challenging.297,298 Patients requiring a predictably complex repair should undergo surgery in experienced repair centres with high repair rates, low operative mortality, and a record of durable results. When repair is not feasible, mitral valve replacement with preservation of the subvalvular apparatus is favoured.
Transcatheter mitral valve implantation for severe PMR is a safe alternative in patients with contraindications for surgery or high operative risk.299,300,301,302 TEER is the most evidenced, while the safety and efficacy of other techniques have been demonstrated in smaller series.303,304,305,306 The efficacy of more recent TEER system iterations307 will be investigated in high-risk (MITRA-HR study NCT03271762)308 and intermediate-risk patients (REPAIR-MR study NCT04198870).
6.1.3 MEDICAL THERAPY
In acute mitral regurgitation, nitrates and diuretics are used to reduce filling pressures. Sodium nitroprusside reduces afterload and regurgitant fraction. Inotropic agents and an intra-aortic balloon pump are of use in hypotension and haemodynamic instability.
In chronic PMR with preserved LVEF, there is no evidence to support the prophylactic use of vasodilators. In patients with overt heart failure, medical treatment as per current heart failure guidelines applies.247
6.1.4 SERIAL TESTING
Asymptomatic patients with severe mitral regurgitation and LVEF >60% should be followed clinically and by echocardiography every 6 months, ideally in the setting of a Heart Valve Centre.309 Measurement of BNP levels, exercise echocardiography, electrocardiogram-Holter monitoring and CMR are useful complementary diagnostic and risk stratification tools.268 The association between PMR, sudden cardiac death and ventricular arrhythmias remains controversial.310,311,312 The presence of mitral annulus disjunction (abnormal atrial displacement of the hinge point of the mitral valve away from the ventricular myocardium) has been also associated with increased risk of ventricular arrhythmias.310,311,313 Interestingly, the majority of these patients did not have severe mitral regurgitation. In asymptomatic patients with severe PMR and progressive increase of LV size (LVESD approaching 40 mm) or decrease of LVEF on serial studies, surgical mitral valve repair should be discussed. Asymptomatic patients with moderate mitral regurgitation and preserved LV function can be followed on a yearly basis and echocardiography should be performed every 1-2 years. After intervention, serial follow-up focuses on evaluation of symptomatic status, presence of arrhythmic events, assessment of valve function,314 and recurrence of mitral regurgitation. After surgical mitral valve repair, high-volume centres have reported good durability with a recurrence rate of moderate or severe mitral regurgitation of 12.5% at 20 years of follow-up.296 After transcatheter mitral valve repair, the currently reported rates of residual moderate and severe mitral regurgitation (23-30%) would suggest that yearly echocardiogram is appropriate.14,300,301
6.1.5 SPECIAL POPULATIONS
Sex differences in terms of prevalence of underlying aetiology of PMR and management have been reported.298,315,316 Despite the reduction in the prevalence of rheumatic disease in Western countries, women still have higher rates of rheumatic mitral regurgitation than men and emerging aetiologies such as radiation heart disease are also more frequent in women.297 These aetiologies are often characterized by severe calcification of the mitral valve apparatus and associated with mitral stenosis precluding durable repair. Women with PMR referred for surgical treatment received mitral valve repair at a similar rate to men.316 However, women more frequently present with post-operative heart failure, probably related to a later referral and more advanced disease as compared to men.
6.2 SECONDARY MITRAL REGURGITATION
In SMR, the valve leaflets and chordae are structurally normal and mitral regurgitation results from an imbalance between closing and tethering forces secondary to alterations in LV and LA geometry.317,318 It is most commonly seen in dilated or ischaemic cardiomyopathies, both in severely dilated LV with markedly depressed LV function or after an isolated infero-basal myocardial infarction leading to posterior leaflet tethering, despite almost normal LV size and ejection fraction. SMR may also arise as a consequence of LA enlargement and mitral annular dilatation in patients with longstanding AF, in whom LVEF is usually normal and LV dilatation less pronounced (so called ‘atrial functional mitral regurgitation’).319
6.2.1 EVALUATION
The echocardiographic criteria to define severe SMR do not differ from those used in PMR and an integrative approach should be used (Table 7).24,268 However, it should be acknowledged that when quantifying EROA and regurgitant volume in SMR, lower thresholds may be applied to define severe SMR. In heart failure patients, the total forward LV stroke volume is lower and that may lead to lower estimated regurgitant volume (<60 mL/beat). Calculation of regurgitant fraction in those circumstances could account for lower flows and has shown prognostic implications.320 In addition, the crescent shape of the regurgitant orifice, characteristic of SMR, may lead to underestimation of the vena contracta width and of the EROA. An EROA ≥30 mm2 by 2D proximal isovelocity surface area (PISA) likely corresponds to severe SMR. In contrast, whether an EROA ≥20 mm2 defines severe SMR remains controversial. In heart failure patients, even mild mitral regurgitation is associated with poor prognosis321 and evidence that surgical or transcatheter treatment of moderate SMR does not seem to improve patient outcomes322,323 supports the change in definition of severe SMR. Caution is required, therefore, when labelling severe SMR based solely on prognostic implications. Other factors such as the extent of myocardial scar, as assessed with CMR, have been associated with poor prognosis.324 In addition, LVEF has been shown to be misleading in patients with severe SMR, while LV global longitudinal strain has been shown to have incremental prognostic value.325,326 The use of 3D echocardiography, CMR and exercise echocardiography may help to identify patients with severe mitral regurgitation when 2D echocardiography at rest is inconclusive.24,268
6.2.2 MEDICAL THERAPY
Optimal medical therapy in line with the guidelines for the management of heart failure247 should be the first and essential step in the management of all patients with SMR and should include replacement of ACEI or ARB with sacubitril/valsartan, sodium-glucose co-transporter 2 inhibitors and/or ivabradine, whenever indicated.247,327 Indications for cardiac resynchronization therapy (CRT) should be evaluated in accordance with related guidelines.247 If symptoms persist after optimization of conventional heart failure therapy, options for mitral valve intervention should be promptly evaluated before further deterioration of LV systolic function or cardiac remodelling occur.
6.2.3 INDICATIONS FOR INTERVENTION
Chronic SMR is associated with impaired prognosis321,328 and its interventional management is complex (see recommendations on indications for mitral valve intervention in chronic severe SMR (Recommendations F), and Figure 6). The detailed analysis of the available level of evidence made by the methodology group of the task force is available in Supplementary Section 5. The importance of decision making by a multidisciplinary Heart Team needs to be emphasized in this setting. The Heart Team, including a heart failure specialist, should optimize guideline-directed medical therapy (GDMT) and consider the indications of electrophysiological, transcatheter and surgical interventions, their priority and order of implementation.
Recommendations F. Recommendations on indications for mitral valve intervention in chronic severe secondary mitral regurgitationa.
| Recommendations | Classb | Levelc |
|---|---|---|
| Valve surgery/intervention is recommended only in patients with severe SMR who remain symptomatic despite GDMT (including CRT if indicated) and has to be decided by a structured collaborative Heart Team. 247,323,336,337 | I | B |
| Patients with concomitant coronary artery or other cardiac disease requiring treatment | ||
| Valve surgery is recommended in patients undergoing CABG or other cardiac surgery. 329,330,333 | I | B |
| In symptomatic patients, who are judged not appropriate for surgery by the Heart Team on the basis of their individual characteristics,d PCI (and/or TAVI) possibly followed by TEER (in case of persisting severe SMR) should be considered. | IIa | C |
| Patients without concomitant coronary artery or other cardiac disease requiring treatment | ||
| TEER should be considered in selected symptomatic patients, not eligible for surgery and fulfilling criteria suggesting an increased chance of responding to the treatment. 337,338,356,357 e | IIa | B |
| Valve surgery may be considered in symptomatic patients judged appropriate for surgery by the Heart Team. | IIb | C |
| In high-risk symptomatic patients not eligible for surgery and not fulfilling the criteria suggesting an increased chance of responding to TEER, the Heart Team may consider in selected cases a TEER procedure or other transcatheter valve therapy if applicable, after careful evaluation for ventricular assist device or heart transplant.e | IIb | C |
| 2D: two-dimensional; CABG: coronary artery bypass grafting; CRT: cardiac resynchronization therapy; EROA: effective regurgitation orifice area; GDMT: guideline-directed medical therapy; LVEF: left ventricular ejection fraction; SMR: secondary mitral regurgitation; PCI: percutaneous coronary intervention; SMR: secondary mitral regurgitation; TAVI: transcatheter aortic valve implantation; TEER: transcatheter edge-to-edge repair. aSee Table 7 for SMR quantification (an EROA ≥30 mm2 by 2D proximal isovelocity surface area corresponds likely to severe SMR). Quantification of SMR must always be performed under optimal guidelines-directed medical treatment. bClass of recommendation. cLevel of evidence. dLVEF, predicted surgical risk, amount of myocardial viability, coronary anatomy/target vessels, type of concomitant procedure needed, TEER eligibility, likelihood of durable surgical repair, need of surgical mitral replacement, local expertise. eCOAPT criteria (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation): see Supplementary Table 7. | ||
Figure 6. Management of patients with chronic severe secondary mitral regurgitation.

CAD: coronary artery disease; CABG: coronary artery bypass grafting; CRT: cardiac resynchronization therapy; ESC: European Society of Cardiology; GDMT: guideline-directed medical therapy; HF: heart failure; HTx: heart transplantation; LVAD: left ventricular assist devices; LV: left ventricle/left ventricular; LVEF: left ventricular ejection fraction; MV: mitral valve; PCI: percutaneous coronary intervention; RV: right ventricle/right ventricular; SMR: secondary mitral regurgitation; TAVI: transcatheter aortic valve implantation; TEER: transcatheter edge-to-edge repair. aLVEF, predicted surgical risk, amount of myocardial viability, coronary anatomy/target vessels, type of concomitant procedure needed, TEER eligibility, likelihood of durable surgical repair, need of surgical mitral replacement, local expertise. bParticularly when concomitant tricuspid valve surgery is needed. cCOAPT criteria (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation): see Supplementary Table 7.
The evidence supporting surgical intervention remains limited. Mitral valve surgery is recommended in patients with severe SMR undergoing CABG or other cardiac surgery.329,330 The surgical approach has to be tailored to the individual patient.247,331 In selected patients without advanced LV remodelling, mitral valve repair with an undersized complete rigid ring restores valve competence, improves symptoms, and results in reverse LV remodelling.331 Additional valvular/subvalvular techniques or chordal sparing valve replacement may be considered in patients with echocardiographic predictors of repair failure.332 Valve replacement avoids recurrence of mitral regurgitation, although this does not translate into better LV reverse remodelling or survival.333 Indications for isolated mitral valve surgery in SMR are particularly restrictive, owing to significant procedural risk, high rates of recurrent mitral regurgitation, and the absence of proven survival benefit.333,334,335 In patients with atrial functional mitral regurgitation, LVEF is usually normal, LV dilatation less pronounced and mitral annular dilatation represents the main mechanism of mitral regurgitation. This subgroup may be more effectively treated by ring annuloplasty often associated with ablation of AF but evidence is still limited.319
TEER with the MitraClip system is a minimal-invasive treatment option for SMR. Two RCTs (COAPT and MITRA-FR)323,336,337 have evaluated its safety and efficacy in patients with symptomatic heart failure and severe SMR persisting despite medical therapy, who were considered either ineligible or not appropriate for surgery by the Heart Team (Supplementary Table 7). The results indicate that the procedure is safe and effectively reduces SMR up to 3 years.338 However, in the MITRA-FR trial,323,336 MitraClip implantation had no impact on the primary endpoint of all-cause mortality or heart failure hospitalization at 12 months and 2 years compared to GDMT alone. In the COAPT trial,337 MitraClip implantation substantially reduced the primary endpoint of cumulative hospitalizations for heart failure, as well as several pre-specified secondary endpoints, including all-cause mortality at 2 years.
Subanalyses of the COAPT trial confirm the positive response to TEER in several patient subgroups;339,340,341,342,343 conversely, the effect of the interventional treatment was neutral throughout all subgroups in an echocardiographic subanalyses of the MITRA-FR trial.344
The conflicting results of these two trials have generated considerable discussion. These diverging results might be partially explained by effect size of the trials, differences in trial design, patient selection, echocardiographic assessment of SMR severity, use of medical therapy, and technical factors. Patients in COAPT demonstrated greater severity of SMR (EROA 41±15 mm2 vs. 31±10 mm2) and less LV dilatation (mean indexed LV end-diastolic volume 101±34 mL/m2 vs. 135±35 mL/m2) than those enrolled in MITRA-FR. Perhaps reflecting greater severity of SMR in relation to LV dimensions (‘disproportionate’ mitral regurgitation), patients in COAPT were overall more likely to benefit from TEER in terms of reduced mortality and heart failure hospitalization.345
Additional studies are needed to identify patients who will benefit the most from TEER.
Therefore, TEER should be considered in selected patients with severe SMR fulfilling the COAPT inclusion criteria,346,347,348 who receive optimal medical therapy supervised by a heart failure specialist and are as close as possible to the patients actually enrolled in the study. Optimization of the procedural result should also be pursued. In addition, TEER may be considered only in selected cases when the COAPT criteria are not fulfilled with the aim of improving symptoms and quality of life.349,350,351,352,353 In patients with less severe SMR (EROA <30 mm2) and advanced LV dilatation/dysfunction, the prognostic benefit of MitraClip remains unproven.323,354,355 Patients with end-stage LV and/or RV failure and no option for revascularization may be better served by cardiac transplantation or LV assist device implantation. Valve intervention is generally not an option when LVEF is <15%.247
The management of moderate ischaemic SMR in patients undergoing CABG remains an object of debate.322,330 Surgery is more likely to be considered if myocardial viability is present and if comorbidity is low. Exercise-induced dyspnoea and a large increase in mitral regurgitation severity and SPAP favour combined surgery.
Transcatheter mitral valve repair systems other than TEER, as well as transcatheter mitral valve replacement devices, are currently the subject of intense investigation but clinical data are still limited.
7 Mitral stenosis
Aetiology of mitral stenosis is mostly rheumatic or degenerative. Rheumatic fever is the most common cause of mitral stenosis worldwide. Its prevalence has greatly decreased in industrialized countries, but it remains a significant healthcare problem in developing countries and affects young patients.2,267,358 Degenerative mitral stenosis related to MAC is a distinct pathology and its prevalence significantly increases with age.359,360 Both types of mitral stenosis are more frequent in females.361 In rare cases, mitral stenosis due to valve rigidity but without commissural fusion, may be related to chest radiation, carcinoid heart disease, or inherited metabolic diseases.
7.1 RHEUMATIC MITRAL STENOSIS
7.1.1 EVALUATION
Clinically significant mitral stenosis is defined by a mitral valve area (MVA) ≤1.5 cm2. Commissural fusion with thickening of the posterior leaflet is the most important mechanism of stenosis. Echocardiography is the preferred method for diagnosis, assessment of severity, and haemodynamic consequences of mitral stenosis. Valve area using 2D planimetry is the reference measurement of mitral stenosis severity, whereas mean transvalvular gradient and pulmonary pressures reflect its consequences and have a prognostic role.362 3D-TTE planimetry may have an additional diagnostic value. TTE usually provides sufficient information for routine management. Scoring systems have been developed to help assess suitability for percutaneous mitral commissurotomy (PMC; Supplementary Table 8),363,364,365 TOE should be performed to exclude LA thrombus before PMC or after an embolic episode, and to obtain detailed information on mitral anatomy (commissural zones and subvalvular apparatus) before intervention when TTE is suboptimal. Stress testing is indicated in patients with no symptoms or symptoms equivocal or discordant with the severity of mitral stenosis. Exercise echocardiography may provide objective information by assessing changes in mitral gradient and pulmonary artery pressure and is superior to DSE. Echocardiography plays an important role in the periprocedural monitoring of PMC and follow-up.
7.1.2 INDICATIONS FOR INTERVENTION
The type of treatment (PMC or surgery), as well as its timing, should be decided based on clinical characteristics, anatomy of valve and subvalvular apparatus, and local expertise.366,367,368,369 In general, indication for intervention should be limited to patients with clinically significant (moderate-to-severe) rheumatic mitral stenosis (valve area ≤1.5 cm2) in whom PMC has had a significant impact on its management. In Western countries where incidence of rheumatic fever and number of PMC is low, this treatment should be restricted to expert operators in specialized centres to improve safety and procedural success rate.366 Efforts should be made to increase availability of PMC in developing countries where access to treatment is limited due to economic reasons.267 PMC should be considered as an initial treatment for selected patients with mild to moderate calcification or impaired subvalvular apparatus, but who have otherwise favourable clinical characteristics.360
The management of clinically significant rheumatic mitral stenosis is summarized in Figure 7 and the indications and contraindications for PMC are provided in the table of recommendations below (Recommendations G), and Table 8.
Figure 7. Management of clinically significant rheumatic mitral stenosis (MVA ≤1.5 cm2).

AF: atrial fibrillation; LA: left atrium/left atrial; MVA: mitral valve area; NCS: non-cardiac surgery; PMC: percutaneous mitral commissurotomy. aHigh thromboembolic risk: history of systemic embolism, dense spontaneous contrast in the LA, new-onset AF. High-risk of haemodynamic decompensation: systolic pulmonary pressure >50 mmHg at rest, need for major NCS, desire for pregnancy. bSurgical commissurotomy may be considered by experienced surgical teams in patients with contraindications to PMC. cSee recommendations on indications for PMC and mitral valve surgery in clinically significant mitral stenosis in section 7.2. dSurgery if symptoms occur for a low level of exercise and operative risk is low.
Table 8. Contraindications for percutaneous mitral commissurotomy in rheumatic mitral stenosisa.
| Contraindications |
|---|
| MVA >1.5 cm2 a |
| LA thrombus |
| More than mild mitral regurgitation |
| Severe or bi-commissural calcification |
| Absence of commissural fusion |
| Severe concomitant aortic valve disease, or severe combined tricuspid stenosis and regurgitation requiring surgery |
| Concomitant CAD requiring bypass surgery |
| CAD: coronary artery disease; LA: left atrium/left atrial; MVA: mitral valve area; PMC: percutaneous mitral commissurotomy. aPMC may be considered in patients with valve area >1.5 cm2 with symptoms that cannot be explained by another cause and if the anatomy is favourable. |
7.1.3 MEDICAL THERAPY
Diuretics, beta-blockers, digoxin, non-dihydropyridine calcium channel blockers and ivabradine can improve symptoms. Anticoagulation with vitamin K antagonist (VKA) with a target international normalized ratio (INR) between 2 and 3 is indicated in patients with AF. Patients with moderate-to-severe mitral stenosis and AF should be kept on VKA and not receive NOACs. Currently there is no solid evidence to support the use of NOACs in this setting370 and a randomized clinical trial is underway (INVICTUS VKA NCT 02832544). Neither cardioversion nor catheter pulmonary vein isolation are indicated before intervention in patients with significant mitral stenosis, as they do not durably restore sinus rhythm. If AF is of recent onset and the LA is only moderately enlarged, cardioversion should be performed soon after successful intervention, it should also be considered in patients with less than severe mitral stenosis. Amiodarone is most effective in maintaining the sinus rhythm after cardioversion. In patients in sinus rhythm, OAC is recommended when there has been a history of systemic embolism or a thrombus is present in the LA and should also be considered when TOE shows dense spontaneous echocardiographic contrast or an enlarged LA (M-mode diameter >50 mm or LA volume >60 mL/m2).
7.1.4 SERIAL TESTING
Asymptomatic patients with clinically significant mitral stenosis should be followed up yearly by clinical and echocardiographic examinations; and at longer intervals (2-3 years) in case of moderate stenosis. Follow-up of patients after successful PMC is similar to that of asymptomatic patients and should be more frequent if asymptomatic restenosis occurs.
7.1.5 SPECIAL PATIENT POPULATIONS
When symptomatic restenosis occurs after surgical commissurotomy or PMC, re-intervention in most cases requires valve replacement, but PMC can be proposed in selected candidates with favourable characteristics if the predominant mechanism is commissural refusion.369
In patients with severe rheumatic mitral stenosis combined with severe aortic valve disease, surgery is preferable when it is not contraindicated. The management of patients in whom surgery is contraindicated is difficult and requires a comprehensive and individualized evaluation by the Heart Team. In cases with severe mitral stenosis associated with moderate aortic valve disease, PMC can be performed to postpone the surgical treatment of both valves. In patients with severe tricuspid regurgitation, PMC may be considered in selected patients with sinus rhythm, moderate atrial enlargement, and severe functional tricuspid regurgitation secondary to pulmonary hypertension. In other cases, surgery on both valves is preferred.371
In the elderly population with rheumatic mitral stenosis when surgery is high risk, PMC is a useful option, even if as palliative care.364,367,368 Treatment of patients with low-gradient severe mitral stenosis (MVA ≤1.5 cm2, mean gradient <10 mmHg) is difficult, as these patients are older and have less optimal anatomy.372
7.2 DEGENERATIVE MITRAL STENOSIS WITH MITRAL ANNULAR CALCIFICATION
MAC is a distinct entity that differs from rheumatic mitral stenosis. Usually, these patients are elderly and may have significant comorbidities including disease of other valves. Overall, the prognosis is poor due to high-risk profile and technical anatomic challenges resulting from the presence of annular calcification.373 Between 9% and 15% of the general population may have MAC, with higher frequency in elderly patients (40%).67,374,375,376 Furthermore, almost half of patients with aortic stenosis undergoing TAVI have MAC, and the disease is severe in 9.5% of cases.359,377 Severe MAC may result in mitral stenosis (more frequently) or mitral regurgitation, or both.
7.2.1 EVALUATION
In patients with degenerative mitral stenosis and MAC, the echocardiographic evaluation of the disease severity is difficult and the usual parameters lack validation. Planimetry is less reliable due to diffuse calcium and irregular orifice. The mean transmitral gradient has been shown to have prognostic value.378 For the evaluation of severity, it is necessary to take into account the abnormalities of LA and LV compliance before indicating an intervention. If an intervention is planned, echocardiography is used for initial evaluation and CCT is necessary to assess the degree and location of calcification and to evaluate the feasibility of an intervention.379
7.2.2 INDICATIONS FOR INTERVENTION
Treatment options, including transcatheter and surgical approaches, are high-risk procedures and evidence from randomized trials is lacking. Even if the procedure is done successfully and the transvalvular gradient is reduced, due to low compliance of the LA and LV the mean atrial pressure may remain elevated.
In elderly patients with degenerative mitral stenosis and MAC, surgery is technically challenging and high risk.380 As there is no commissural fusion, degenerative mitral stenosis is not amenable to PMC.359 In symptomatic inoperable patients with suitable anatomy, preliminary experience showed that transcatheter mitral valve implantation (in mitral position, using an inverted balloon-expandable TAVI prosthesis), is feasible in selected patients with severe mitral stenosis, when performed by experienced operators after careful pre-planning using multimodality imaging.379 The largest case series reported to date included only 116 patients.381 However, operative mortality is high, in particular due to the risk of LVOT obstruction and mid-term results are less favourable compared to mitral valve-in-valve procedures.382,383 The most recent case series show that results are improving owing to better patient selection and the use of different accesses, as well as concomitant or preventive measures such as alcohol septal ablation384 or laceration/resection of the anterior leaflet.385,386,387
Recently, a preliminary case series suggested that transcatheter mitral valve replacement using a dedicated prosthesis is feasible and can result in symptom improvement.388
8 Tricuspid regurgitation
Moderate or severe tricuspid regurgitation is observed in 0.55% of the general population and its prevalence increases with age, affecting about 4% of the patients aged 75 years or more.389 Aetiology is secondary in ≥90% of cases, due to pressure and/or volume overload mediated RV dilatation or enlarged right atrium and tricuspid annulus due to chronic AF. Secondary tricuspid regurgitation is associated with left-sided valvular or myocardial dysfunction in most cases, whereas it is isolated in 8.1% of subjects and independently related to mortality.389 Secondary tricuspid regurgitation may also develop late after left-sided valve surgery.390,391
Causes of primary tricuspid regurgitation include infective endocarditis [especially in intravenous (i.v.) drug addicts], rheumatic heart disease, carcinoid syndrome, myxomatous disease, endomyocardial fibrosis, congenital valve dysplasia (e.g. Ebstein’s anomaly), thoracic trauma, and iatrogenic valve damage.
Atrial fibrillation induces annular remodelling even in the absence of left-heart disease.392 Cardiac implantable electronic device-lead implantation leads to progressive tricuspid regurgitation in 20-30% of the patients393,394,395 and predicts its progression over time.396
In patients with heart failure and reduced LVEF, secondary tricuspid regurgitation is a very frequent finding and is an independent predictor of clinical outcomes.397
8.1 EVALUATION
Tricuspid regurgitation should be evaluated first by echocardiography. In primary tricuspid regurgitation, specific abnormalities of the valve can be identified. In secondary tricuspid regurgitation, annular dilatation, along with RV and right atrium dimensions, as well as RV function should be measured, owing to their prognostic relevance.398 In experienced laboratories, RV strain27 and/or 3D measurements of RV volumes399,400 may be considered to overcome the existing limitations of conventional RV function indices.102 When available, CMR is the preferred method to assess the RV400 due to its high accuracy and reproducibility.401
Echocardiographic evaluation of tricuspid regurgitation severity is based on an integrative approach considering multiple qualitative and quantitative parameters (Table 9). Due to the non-circular and non-planar shape of the regurgitant orifice, biplane vena contracta width should be considered in addition to the conventional 2D measurement.402 Similarly, underestimation of tricuspid regurgitation severity by the PISA method may occur.403 In case of inconsistent findings, the 3D vena contracta area may be evaluated, although diverging cut-offs have been reported.402,404,405,406 Recently, a new grading scheme including two additional grades (‘massive’ and ‘torrential’) has been proposed407 and used in clinical studies on transcatheter interventions.408,409 Studies showed an incremental prognostic value of the two additional grades (massive and torrential) in terms of mortality and rehospitalization for heart failure in patients with advanced disease.410,411,412
Table 9. Echocardiographic criteria for grading severity of tricuspid regurgitation.
| Qualitative | |
|---|---|
| Tricuspid valve morphology | Abnormal/flail |
| Colour flow regurgitant jet | Very large central jet or eccentric wall impinging jeta |
| CW signal of regurgitant jet | Dense/triangular with early peaking |
| Semiquantitative | |
| Vena contracta width (mm) | >7a,b |
| PISA radius (mm) | >9c |
| Hepatic vein flowc | Systolic flow reversal |
| Tricuspid inflow | E-wave dominant ≥1 m/sd |
| Quantitative | |
| EROA (mm2) | ≥40 |
| Regurgitant volume (mL/beat) | ≥45 |
| Enlargement of cardiac chambers/vessels | RV, RA, inferior vena cava |
| CW: continuous wave; EROA: effective regurgitant orifice area; PISA: proximal isovelocity surface area; RA: right atrium/right atrial; RV: right ventricle/right ventricular; TR: tricuspid regurgitation. aAt a Nyquist limit of 50-60 cm/s. bPreferably biplane. cBaseline Nyquist limit shift of 28 cm/s. dIn the absence of other causes of elevated RA pressure. | |
Alternatively, calculation of the tricuspid regurgitant volume by CMR using RV volumetry may be helpful.
Importantly, estimation of pulmonary pressures using Doppler gradient may be impossible or might underestimate the severity of pulmonary hypertension in the presence of severe tricuspid regurgitation, justifying cardiac catheterization to evaluate pulmonary vascular resistances.413
8.2 INDICATIONS FOR INTERVENTION
Severe tricuspid regurgitation is associated with impaired survival389,414,415,416 and worsening heart failure.397,417 In clinical practice, tricuspid valve interventions are underused and often initiated too late.418,419,420 Appropriate timing of intervention is crucial to avoid irreversible RV damage and organ failure with subsequent increased surgical risk421,422 (see table of recommendations on indications for intervention in tricuspid valve disease in section 9 (Recommendations H) and Figure 8).
Recommendations H. Recommendations on indications for intervention in tricuspid valve disease.
| Recommendations | Classa | Levelb |
|---|---|---|
| Recommendations on tricuspid stenosis | ||
| Surgery is recommended in symptomatic patients with severe tricuspid stenosis.c | I | C |
| Surgery is recommended in patients with severe tricuspid stenosis undergoing left-sided valve intervention.d | I | C |
| Recommendations on primary tricuspid regurgitation | ||
| Surgery is recommended in patients with severe primary tricuspid regurgitation undergoing leftsided valve surgery. | I | C |
| Surgery is recommended in symptomatic patients with isolated severe primary tricuspid regurgitation without severe RV dysfunction. | I | C |
| Surgery should be considered in patients with moderate primary tricuspid regurgitation undergoing left-sided valve surgery. | IIa | C |
| Surgery should be considered in asymptomatic or mildly symptomatic patients with isolated severe primary tricuspid regurgitation and RV dilatation who are appropriate for surgery. | IIa | C |
| Recommendations on secondary tricuspid regurgitation | ||
| Surgery is recommended in patients with severe secondary tricuspid regurgitation undergoing left-sided valve surgery. 423,424,425,426,427 | I | B |
| Surgery should be considered in patients with mild or moderate secondary tricuspid regurgitation with a dilated annulus (≥40 mm or >21 mm/m2 by 2D echocardiography) undergoing left-sided valve surgery. 423,425,426,427 | IIa | B |
| Surgery should be considered in patients with severe secondary tricuspid regurgitation (with or without previous left-sided surgery) who are symptomatic or have RV dilatation, in the absence of severe RV or LV dysfunction and severe pulmonary vascular disease/hypertension. 418,433 e | IIa | B |
| Transcatheter treatment of symptomatic secondary severe tricuspid regurgitation may be considered in inoperable patients at a Heart Valve Centre with expertise in the treatment of tricuspid valve disease.f | IIb | C |
| 2D: two-dimensional; LV: left ventricle/left ventricular; PMC: percutaneous mitral commissurotomy; RV: right ventricle/right ventricular. aClass of recommendation. bLevel of evidence. cPercutaneous balloon valvuloplasty can be attempted as a first approach if tricuspid stenosis is isolated. dPercutaneous balloon valvuloplasty can be attempted if PMC can be performed on the mitral valve. eIn patients with previous surgery recurrent left-sided valve dysfunction needs to be excluded. fTranscatheter treatment can be performed according to Heart Team at experienced valve centres in anatomically eligible patients in whom improvement of quality of life or survival can be expected. | ||
Figure 8. Management of tricuspid regurgitation.

LV: left ventricle/left ventricular; RV: right ventricle/right ventricular; TA: tricuspid annulus; TR: tricuspid regurgitation; TV: tricuspid valve. aThe Heart Team with expertise in the treatment of tricuspid valve disease evaluates anatomical eligibility for transcatheter therapy including jet location, coaptation gap, leaflet tethering, potential interference with pacing lead. bReplacement when repair is not feasible.
Surgery is recommended in symptomatic patients with severe primary tricuspid regurgitation. In selected asymptomatic or mildly symptomatic patients who are appropriate for surgery, an intervention should also be considered when RV dilatation or declining RV function is observed. However, exact thresholds have not yet been defined.
According to observational data, tricuspid valve repair should be performed liberally during left-sided surgery in patients with secondary tricuspid regurgitation. Indeed, it does not increase operative risk, but promotes reverse remodelling of the RV and improves functional status when annular dilatation is present, even in the absence of severe tricuspid regurgitation.423,424,425,426,427
The benefit of surgical correction of isolated secondary tricuspid regurgitation compared to medical treatment is not well established428 and the procedure has a non-negligible risk of periprocedural mortality and morbidity when patients present late.429,430,431,432 However, in carefully selected candidates, surgery can be performed safely with good long-term survival.418,433 It should therefore be considered early in selected symptomatic patients appropriate for surgery, as well as in those with no or mild symptoms, RV dilatation and severe tricuspid regurgitation. Although a tricuspid annular pulmonary systolic excursion (TAPSE) <17 mm has been associated with worse prognosis in patients with secondary tricuspid regurgitation,398,434 thresholds for severe RV dysfunction making intervention futile have not yet been defined.
Reoperation on the tricuspid valve in new-onset or worsening secondary tricuspid regurgitation after left-sided surgery carries a high procedural risk, possibly due to late referral and subsequent poor clinical condition.435 To improve prognosis, treatment of severe tricuspid regurgitation in this challenging scenario should be considered even in asymptomatic patients if there are signs of RV dilatation or decline in RV function (after exclusion of left-sided valve dysfunction, severe RV or LV dysfunction and severe pulmonary vascular disease/hypertension).
Whenever possible, annuloplasty with prosthetic rings is preferable to valve replacement,423,430,436 which should only be considered when the tricuspid valve leaflets are tethered and the annulus severely dilated. In presence of a cardiac implantable electronic device lead, the technique used should be adapted to the patient’s condition and the surgeon’s experience.437
TTVI are under clinical development. Early registry and study data demonstrated the feasibility to reduce tricuspid regurgitation using various systems, enabling either leaflet approximation [,408,438-440] direct annuloplasty,409,441 or valve replacement,442,443,444 with subsequent symptomatic and haemodynamic improvement.445,446 In a propensity-score-matched study comparing medical treatment to TTVI, all-cause mortality and rehospitalizations at 1 year were lower among the patients who received the interventional treatment.447 Several RCTs will investigate the efficacy of TTVI against medical treatment.
Therefore, TTVI may be considered by the Heart Team at experienced Heart Valve Centres in symptomatic, inoperable, anatomically eligible patients in whom symptomatic or prognostic improvement can be expected. For detailed anatomical evaluation, TOE and CCT may be preferred owing to higher spatial resolution.448,449
8.3 MEDICAL THERAPY
Diuretics are useful in the presence of right heart failure. To counterbalance the activation of the renin-angiotensin-aldosterone system associated with hepatic congestion, the addition of an aldosterone antagonist may be considered.247 Dedicated treatment of pulmonary hypertension is indicated in specific cases. Although data are limited, rhythm control may help to decrease tricuspid regurgitation and contain annular dilatation in patients with chronic AF.450 Importantly, in the absence of advanced RV dysfunction or severe pulmonary hypertension, none of the above-mentioned therapies should delay referral for surgery or transcatheter therapy.
9 Tricuspid stenosis
Tricuspid stenosis is often combined with tricuspid regurgitation and most frequently of rheumatic origin. It is therefore usually associated with left-sided valve lesions, particularly mitral stenosis. Other causes are rare, including congenital, carcinoid and drug-induced valve diseases, Whipple’s disease, endocarditis, and large right atrial tumour.
9.1 EVALUATION
Echocardiography provides the most useful information. Tricuspid stenosis is often overlooked and requires careful evaluation. Echocardiographic evaluation of valve anatomy and subvalvular apparatus is important to assess valve reparability. No generally accepted grading of tricuspid stenosis severity exists, but a mean echocardiographic transvalvular gradient ≥5 mmHg at normal heart rate is considered indicative of significant tricuspid stenosis.362
9.2 INDICATIONS FOR INTERVENTION
Intervention on the tricuspid valve is usually performed concomitantly during procedures for left-sided valve disease in patients who are symptomatic despite medical therapy. Although the lack of pliable leaflet tissue is a main limitation for valve repair, the choice between repair and replacement depends on anatomy and surgical expertise. Owing to satisfactory long-term durability, biological prostheses are usually preferred over mechanical valves, which have a high risk of thrombosis.451
Percutaneous tricuspid balloon valvuloplasty has been performed in a limited number of cases, either alone or in combination with PMC. It frequently induces significant regurgitation and long-term results are lacking.452 It can be considered in rare cases with anatomically suitable valves, when tricuspid stenosis is isolated or additional mitral stenosis can also be treated interventionally (see recommendations on indications for PMC and mitral valve surgery in clinically significant mitral stenosis in section 7).
9.3 MEDICAL THERAPY
Diuretics are useful in the presence of heart failure symptoms but are of limited long-term efficacy.
10 Combined and multiple-valve diseases
Significant stenosis and regurgitation can be found on the same valve. Disease of multiple valves may be encountered in several conditions, particularly in rheumatic and congenital heart disease, but also less frequently in degenerative valve disease. There is a lack of data on combined or multiple-valve disease.453,454,455,456,457,458,459,460 This does not allow for evidence-based recommendations. The general principles for the management of combined or multiple-valve disease are as follows:
When either stenosis or regurgitation is predominant, management follows the recommendations concerning the predominant VHD. When the severity of both stenosis and regurgitation is balanced, indications for interventions should be based on symptoms and objective consequences rather than on the indices of severity of stenosis or regurgitation.453,454,455,456 In this setting, Doppler pressure gradient reflects the global haemodynamic burden (stenosis and regurgitation) of the valve lesion.453
Besides the separate assessment of each valve lesion, it is necessary to consider the interaction between the different valve lesions. As an illustration, associated mitral regurgitation may lead to underestimation of the severity of aortic stenosis, as decreased stroke volume due to mitral regurgitation lowers the flow across the aortic valve and hence the aortic gradient.453 This underlines the need to combine different measurements, including assessment of valve areas, if possible using methods that are less dependent on loading conditions, such as planimetry.457
Indications for intervention are based on global assessment of the consequences of the different valve lesions (i.e. symptoms or presence of LV dilatation or dysfunction). Intervention can be considered for non-severe multiple lesions associated with symptoms or leading to LV impairment.453
The decision to intervene on multiple valves should take into account the age, comorbidities, and risk of combined procedures, and should be made by the Heart Team after precise and comprehensive evaluation of valve lesions and their interactions with each other.453,461 The risk of combined intervention should be weighed against the evolution of untreated valve disease and the inherent risk of subsequent intervention.
The choice of surgical technique/interventional procedure should take into account the presence of the other VHD.453,458,459,461
When interventional procedures are considered, staged procedures may be preferable in cases with aortic stenosis and mitral regurgitation (see section 5.5). Improved 1-year survival after combined transcatheter treatment of mitral and tricuspid regurgitation has been reported compared to mitral regurgitation alone.263 PMC may delay surgery, in situations such as severe mitral stenosis associated with moderate aortic regurgitation.
The management of specific associations of VHD is detailed in the individual sections of this document.
11 Prosthetic valves
11.1 CHOICE OF PROSTHETIC VALVE
Factors for valve selection are the patient’s life expectancy, lifestyle, and environmental factors, bleeding and thromboembolic risks related to anticoagulation, potential for surgical or transcatheter re-intervention, and, importantly, informed patient preference. Generally, biological heart valves (BHVs) should be preferred in patients with shorter anticipated survival or comorbidities that may lead to further surgical procedures, and those who are at increased risk for bleeding complications. Thromboembolic complications are less frequent in pregnant women with BHVs.
In a nationwide observational study, patients aged 45 to 54 with surgical aortic BHV implantation and those aged 40 to 70 years with surgical mitral BHV implantation had a significantly higher 15-year mortality than those with a mechanical heart valve (MHV). An analysis of patients 55 to 64 years of age showed no difference in mortality between aortic BHV and MHV prosthesis.462 However, an earlier systematic review463 and a recent meta-analysis464 of studies comparing aortic MHVs and BHVs showed a significant mortality reduction with MHVs in patients ≤60 and in those 50-70 years of age, respectively. All these studies are limited by their predominantly observational nature and missing information on the type of prostheses implanted. There is no new high-quality evidence supporting a decrease in the established age cut-off for prosthesis selection.
The best aortic valve substitute for younger adults remains unclear. In appropriately selected patients, replacement of the aortic valve using an autograft may be performed, with long-term survival rates and valve-related reoperation that are comparable to those achieved with a MHV, but high expertise in aortic root surgery is required.465 Strategies for patients with small aortic annulus include root enlargement and use of stentless valves. Although the use of sutureless and rapid-deployment aortic valves may reduce invasiveness, cross-clamp and cardiopulmonary bypass times, and potentially lower perioperative complications of SAVR, there is a lack of a large-scale randomized comparison on both short- and long-term safety, efficacy, and haemodynamic performance of this approach against conventional aortic valve replacement, which remains the gold standard of procedure.
See Recommendations for prosthetic valve selection (Recommendations I).
Recommendations I. Recommendations for prosthetic valve selection.
| Recommendations | Classa | Levelb |
|---|---|---|
| Mechanical prostheses | ||
| A mechanical prosthesis is recommended according to the desire of the informed patient and if there are no contraindications to longterm anticoagulation.c | I | C |
| A mechanical prosthesis is recommended in patients at risk of accelerated SVD.d | I | C |
| A mechanical prosthesis should be considered in patients already on anticoagulation because of a mechanical prosthesis in another valve position. | IIa | C |
| A mechanical prosthesis should be considered in patients aged <60 years for prostheses in the aortic position and aged <65 years for prostheses in the mitral position. 462,464 e | IIa | B |
| A mechanical prosthesis should be considered in patients with a reasonable life expectancy for whom future redo valve surgery or TAVI (if appropriate) would be at high risk.f | IIa | C |
| A mechanical prosthesis may be considered in patients already on long-term anticoagulation due to the high risk for thromboembolism.f | IIb | C |
| Biological prostheses | ||
| A bioprosthesis is recommended according to the desire of the informed patient. | I | C |
| A bioprosthesis is recommended when goodquality anticoagulation is unlikely (adherence problems, not readily available), contraindicated because of high bleeding risk (previous major bleed, comorbidities, unwillingness, adherence problems, lifestyle, occupation) and in those patients whose life expectancy is lower than the presumed durability of the bioprosthesis.g | I | C |
| A bioprosthesis is recommended in case of reoperation for mechanical valve thrombosis despite good long-term anticoagulant control. | I | C |
| A bioprosthesis should be considered in patients for whom there is a low likelihood and/or a low operative risk of future redo valve surgery. | IIa | C |
| A bioprosthesis should be considered in young women contemplating pregnancy. | IIa | C |
| A bioprosthesis should be considered in patients aged >65 years for a prosthesis in the aortic position or aged >70 years in a mitral position. | IIa | C |
| A bioprosthesis may be considered in patients already on long-term NOACs due to the high risk for thromboembolism. 466,467,468,469 f | IIb | B |
| AF: atrial fibrillation; NOAC: non-vitamin K antagonist oral anticoagulant; SVD: structural valve deterioration; TAVI: transcatheter aortic valve implantation. aClass of recommendation. bLevel of evidence. cIncreased bleeding risk because of comorbidities, adherence concerns or geographic, lifestyle or occupational conditions. dYoung age (<40 years), hyperparathyroidism, haemodialysis. eIn patients 60-65 years of age who should receive an aortic prosthesis and those between 65 and 70 years of age in the case of mitral prosthesis, both valves are acceptable and the choice requires careful analysis of factors other than age. fRisk factors for thromboembolism are AF, previous unprovoked proximal deep venous thromboembolism and/or symptomatic pulmonary embolism, hypercoagulable state, antiphospholipid antibody. gLife expectancy should be estimated at >10 years according to age, sex, comorbidities, and country-specific life expectancy. | ||
11.2 BASELINE ASSESSMENT AND FOLLOW-UP
All patients with prosthetic valves require lifelong follow-up to detect early deterioration in prosthetic function or ventricular function, or progressive disease of another heart valve.314 Clinical assessment should be performed yearly or as soon as possible if new cardiac symptoms occur. TTE should be performed if any new symptoms occur or if complications are suspected. After transcatheter, as well as surgical implantation of a BHV, echocardiography, including measurement of transprosthetic gradients, should be performed within 30 days after valve implantation (i.e. baseline), at 1 year, and annually thereafter.470 TOE should be considered if TTE is of poor quality and in all cases of suspected prosthetic dysfunction (especially if the prosthesis is in the mitral position) or endocarditis.314,471 Cinefluoroscopy for MHVs and CCT scanning provide useful additional information if valve thrombus or pannus are suspected to impair valve function.314
11.3 ANTITHROMBOTIC MANAGEMENT
11.3.1 MECHANICAL PROSTHESES
11.3.1.1 Postoperative anticoagulation management
MHVs require lifelong treatment with VKA guided by the INR [.472,473] NOACs currently have no role in patients with MHVs.474 Treatment with VKA should be started on the first postoperative day in combination with bridging therapy [with therapeutic doses of either unfractionated heparin (UFH) or off-label use of low-molecular-weight heparin (LMWH)] until therapeutic INR is achieved.475 Similar safety and efficacy outcomes have been reported following bridging with either UFH or LMWH.476 Once a stable therapeutic INR is reached for ≥24 h, bridging can be discontinued. The postoperative risk of thromboembolism peaks about 1 month after implantation, but risks are substantially increased up to 6 months.477,478 Long-term prevention of valve thrombosis and thromboembolism after MHV implantation involves effective antithrombotic medication and risk factor modification for thromboembolism.479
11.3.1.2 Target international normalized ratio
Target INR should be based upon prosthesis thrombogenicity and patient-related risk factors (Table 10).479 It is recommended to target a median INR value rather than a range to avoid considering extreme values in the target range as a valid target INR. High INR variability is a strong independent predictor of adverse events after valve replacement. Although some studies have supported lowering a target INR for aortic MHVs,480,481 further evaluation in larger cohorts is warranted before updating current recommendations. The use of self-monitoring INR is associated with a lower rate of VKA-related complications in all ages.482 In a trial of lower intensity warfarin plus aspirin (INR 1.5-2.0) or standard warfarin plus aspirin (INR 2.0-3.0) after implantation of the On-X MHV in the aortic position, the similar safety of the two approaches was partly attributed to use of home INR monitoring and high degree of adherence among patients.481 Patient’s education plays an important role for achieving stable anticoagulation in the therapeutic range. Effective management of patients with unstable INR requires frequent in-clinic testing and dose titration. Because of the lack of good-quality evidence, pharmacogenetic testing cannot be recommended to guide the dosing of VKAs.
Table 10. Target international normalized ratio for mechanical prostheses.
| Prosthesis thrombogenicity | Patient-related risk factorsa | |
|---|---|---|
| None | ≥1 risk factor | |
| Lowb | 2.5 | 3.0 |
| Mediumc | 3.0 | 3.5 |
| Highd | 3.5 | 4.0 |
| AF: atrial fibrillation; LVEF: left ventricular ejection fraction. aMitral or tricuspid valve replacement; previous thromboembolism; AF; mitral stenosis of any degree; LVEF <35%. bCarbomedics, Medtronic Hall, ATS, Medtronic Open-Pivot, St Jude Medical, Sorin Bicarbon. cOther bileaflet valves with insufficient data. dLillehei-Kaster, Omniscience, Starr-Edwards (ball-cage), Bjork-Shiley and other tilting-disc valves. | ||
11.3.1.3 Management of vitamin K antagonist (VKA) overdose and bleeding
Bleeding increases exponentially with INR >4.5.483 In case of major and/or life-threatening bleeding and in patients who need to undergo urgent surgery, the VKA should be discontinued and 10 mg vitamin K should be administrated by slow i.v. infusion and repeated every 12 h if needed. Until the anticoagulation effect is reversed, administration of prothrombin complex concentration (PCC) and/or fresh frozen plasma (FFP) therapy should be initiated according to body weight and pre-treatment INR. The efficacy should be monitored by re-check of INR at 30 min and every 4-6 h until normalization. The optimal time to restart anticoagulation should be discussed in relation to location of the bleeding event and interventions performed to stop bleeding and/or to treat an underlying cause.484
In the absence of bleeding, the use of PCC and/or FFP therapy is not recommended and the decision to start vitamin K should be individualized. In asymptomatic patients with INR >10, the VKA must be stopped and oral vitamin K (2.5-5 mg) prescribed, while the INR must be monitored on a daily base for 2 weeks. Multiple RCTs in patients with INR between 4.5 and 10 suggest no difference in bleeding events with vitamin K vs. placebo.483,485 Therefore, in such patients, warfarin should be stopped temporarily, and a small dose of oral vitamin K (1-2 mg) can be considered on an individual basis balancing between the risks. Finally, asymptomatic patients with INR <4.5 require careful down-titration and/or skipping one or more doses. In all patients with MHVs, VKAs must be resumed once the INR achieves the therapeutic range or is slightly elevated.
11.3.1.4 Combination of oral anticoagulation (OAC) with antiplatelet drugs
The addition of low-dose (75-100 mg) acetylsalicylic acid (ASA) to VKA may reduce the incidence of thromboembolism at the cost of bleeding.477 Therefore, addition of antiplatelets to VKAs should be reserved for patients at very high risk of thromboembolism where advantages clearly outweigh the risks.486,487 In patients with thromboembolism despite adequate INR, low dose (75-100 mg) ASA should be added to VKAs. Management of oral antithrombotic therapy in patients with CAD is summarized in Supplementary Figure 2.
11.3.1.5 Interruption of anticoagulant therapy for planned invasive procedures
In patients with MHVs, preoperative bridging with UFH or LMWH before surgery imposes a risk of perioperative bleeding while interrupting anticoagulation results in an increased risk of thromboembolism.488 Therefore, anticoagulation in patients with MHVs undergoing elective NCS requires careful management by multidisciplinary consensus.478,489 For minor surgical procedures (e.g. dental, cataract, skin incision) in which blood loss is usually small and easily controlled, it is recommended that OAC is not interrupted. Major surgeries require temporary interruption and therapeutic bridging with either UFH or LMWH, aiming for an INR <1.5 (Supplementary Figure 3). Fondaparinux should not be routinely used for bridging, but may have a role in patients with history of heparin-induced thrombocytopenia.490
11.3.2 BIOPROSTHESES
11.3.2.1 Patients with no baseline indication to oral anticoagulation (OAC)
Surgical bioprostheses: The optimal antithrombotic strategy early after surgical implantation of an aortic BHV remains controversial due to lack of high-quality evidence. Multiple observational studies support the use of VKAs to reduce the risk of thromboembolism.491,492,493 A small randomized trial found that VKA for 3 months significantly increased major bleeding compared with ASA, without reducing the rate of deaths or thromboembolic events, but the statistical power was low for demonstrating a thrombotic benefit.494 VKA for 3 months should be considered in all patients with a mitral or tricuspid BHV and ASA or VKA should be considered for 3 months after surgical implantation of an aortic bioprosthesis.
Transcatheter bioprostheses: A meta-analysis of three small RCTs showed a significant increase in major or life-threatening bleeding with dual antiplatelet therapy (DAPT) over ASA at 30 days, with no difference in ischaemic outcomes.495 Consistently, the more recent POPular TAVI trial (cohort A) found reduced bleeding and the composite of bleeding or thromboembolic events with ASA compared with DAPT.496 A randomized trial was halted prematurely due to safety concerns with a rivaroxaban-based regimen as compared with DAPT, including a higher risk of death or thromboembolic complications and a higher risk of bleeding.497 There is a lack of data on the management of antithrombotic therapy after implantation of transcatheter mitral BHVs (e.g. valve-in-valve or valve-in-ring) for which 3 months of VKA is commonly prescribed.498
11.3.2.2 Patients with baseline indication to oral anticoagulation (OAC)
Surgical bioprostheses: OAC is recommended lifelong for patients with surgical BHVs who have other indications for anticoagulation. The evidence supporting the use of NOACs in preference to VKA has increased since the publication of the 2017 VHD Guidelines. In the RIVER trial, including patients with AF and a BHV in the mitral position, the NOAC rivaroxaban was non-inferior to warfarin with respect to a net benefit endpoint at 12 months.499 The benefit of NOAC was consistent among subgroups. However, only 20% of patients were enrolled in the trial before the third postoperative month, which raises a note of caution and calls for additional data in this particular subgroup. In the small ENAVLE trial (N = 220), including patients with and without AF, edoxaban was non-inferior to warfarin for preventing thromboembolism and the occurrence of major bleeding in the first 3 months after aortic or mitral surgical bioprosthetic valve implantation or repair, which warrants confirmation in larger investigations.500
Transcatheter bioprostheses: In the POPular TAVI trial (cohort B), the incidence of bleeding over a period of 1 month or 1 year was lower with OAC than with OAC plus clopidogrel.501 OAC alone was non-inferior to OAC plus clopidogrel with respect to ischaemic events, but the non-inferiority margin was large. An observational study suggested that there is a higher risk of ischaemic events at 1 year with NOACs compared with VKAs, after adjustment for potential confounders.502 Randomized trials comparing NOACs vs. VKAs are ongoing (NCT02943785, NCT02664649). Data on the management of antithrombotic therapy after transcatheter mitral or tricuspid valve implantation are scant.498
11.3.3 VALVE REPAIR
Observational data suggest comparable risk of thromboembolism with ASA or VKAs following mitral valve repair,503 but randomized data are lacking. The high incidence of new-onset AF and its recurrence, the thrombogenic tendency of the non-endothelialized repair components, and a relatively high rate of patients who are resistant to ASA establish VKAs as a preferable option for the initial period (e.g. 3 months). However, the potential for bleeding complications in the postoperative phase dictates careful patient selection.
The management of antithrombotic treatment after prosthetic valve implantation or valve repair is summarized in the table of recommendations for management of antithrombotic therapy after prosthetic valve implantation or valve repair (Recommendations J) and in Figure 9.
Recommendations J. Recommendations for management of antithrombotic therapy after prosthetic valve implantation or valve repair in the perioperative and postoperative periods.
| Recommendations | Classa | Levelb |
|---|---|---|
| Management of antithrombotic therapy in the perioperative period | ||
| It is recommended that VKAs are timely discontinued prior to elective surgery to aim for an INR <1.5.c | I | C |
| Bridging of OAC, when interruption is needed, is recommended in patients with any of the following indications: • Mechanical prosthetic heart valve. • AF with significant mitral stenosis. • AF with a CHA2DS2-VASc score ≥3 for women or 2 for men.d • Acute thrombotic event within the previous 4 weeks. • High acute thromboembolic risk.e |
I | C |
| Therapeutic doses of either UFH or subcutaneous LMWH are recommended for bridging. 476,504 | I | B |
| In patients with MHVs, it is recommended to (re)-initiate the VKA on the first postoperative day. | I | C |
| In patients who have undergone valve surgery with an indication for postoperative therapeutic bridging, it is recommended to start either UFH or LMWH 12-24 h after surgery. | I | C |
| In patients undergoing surgery, it is recommended that aspirin therapy, if indicated, is maintained during the periprocedural period. | I | C |
| In patients treated with DAPT after recent PCI (within 1 month) who need to undergo heart valve surgery in the absence of an indication for OAC, it is recommended to resume the P2Y12 inhibitor postoperatively, as soon as there is no concern over bleeding. | I | C |
| In patients treated with DAPT after recent PCI (within 1 month) who need to undergo heart valve surgery in the absence of an indication for OAC, bridging P2Y12 inhibitors with short-acting glycoprotein IIb/IIIa inhibitors or cangrelor may be considered. | IIb | C |
| Patients with an indication to concomitant antiplatelet therapy | ||
| After uncomplicated PCI or ACS in patients requiring long-term OAC, early cessation (≤1 week) of aspirin and continuation of dual therapy with OAC and a P2Y12 inhibitor (preferably clopidogrel) for up to 6 months (or up to 12 months in ACS) is recommended if the risk of stent thrombosis is low or if concerns about bleeding risk prevail over concerns about risk of stent thrombosis, irrespective of the type of stent used. 505,506,507,508,509 | I | B |
| Discontinuation of antiplatelet treatment in patients treated with an OAC is recommended after 12 months. 74,510,511,512 | I | B |
| After uncomplicated PCI or ACS in patients requiring both OAC and antiplatelet therapy, triple therapy with aspirin, clopidogrel and OAC for longer than 1 week should be considered when the risk of stent thrombosis outweighs the risk of bleeding, with the total duration (≤1 month) decided according to assessment of these risks and clearly specified at hospital discharge. | IIa | C |
| In patients treated with a VKA (e.g. MHVs), clopidogrel alone should be considered in selected patients (e.g. HAS-BLED ≥3 or ARC-HBR met and low risk of stent thrombosis) for up to 12 months. 512,513 | IIa | B |
| In patients requiring aspirin and/or clopidogrel in addition to VKA, the dose intensity of VKA should be considered and carefully regulated with a target INR in the lower part of the recommended target range and a time in the therapeutic range >65-70%. 505,514 | IIa | B |
| Surgical valve replacement | ||
| OAC using a VKA is recommended lifelong for all patients with an MHV prosthesis. 472,473 | I | B |
| For patients with a VKA, INR self-management is recommended provided appropriate training and quality control are performed. 482 | I | B |
| OAC is recommended for patients undergoing implantation of a surgical BHV who have other indications for anticoagulation.f | I | C |
| NOACs should be considered over VKA after 3 months following surgical implantation of a BHV in patients with AF. 74,499,500,515,516,517,518 | IIa | B |
| In patients with no baseline indications for OAC, low-dose aspirin (75-100 mg/day) or OAC using a VKA should be considered for the first 3 months after surgical implantation of an aortic BHV. 491,494 | IIa | B |
| In patients with no baseline indications for OAC, OAC using a VKA should be considered for the first 3 months after surgical implantation of a bioprosthesis in the mitral or tricuspid position. 519,520 | IIa | B |
| The addition of low-dose aspirin (75-100 mg/day) to VKA may be considered in selected patients with MHVs in case of concomitant atherosclerotic disease and low risk of bleeding. | IIb | C |
| The addition of low-dose aspirin (75-100 mg/day) to VKA should be considered after thromboembolism despite an adequate INR. |
IIa | C |
| NOACs may be considered over VKA within 3 months following surgical implantation of a BHV in mitral position in patients with AF. 499 | IIb | C |
| NOACs are not recommended in patients with a mechanical valve prosthesis. 474 | III | B |
| Surgical valve repair | ||
| OAC with VKA should be considered during the first 3 months after mitral and tricuspid repair. | IIa | C |
| SAPT with low-dose ASA (75-100 mg/day) should be considered for the first 3 months after valve-sparing aortic surgery when there are no other baseline indications to OAC. | IIa | C |
| Transcatheter aortic valve implantation | ||
| OAC is recommended lifelong for TAVI patients who have other indications for OAC. 501 f | I | B |
| Lifelong SAPT is recommended after TAVI in patients with no baseline indication for OAC. 495,496,521 | I | A |
| Routine use OAC is not recommended after TAVI in patients with no baseline indication for OAC. 497 | III | B |
| ACS: acute coronary syndrome; AF: atrial fibrillation; ARC-HBR: Academic Research Consortium - high bleeding risk; ASA: acetylsalicylic acid; BHV: biologic al heart valve; DAPT: dual antiplatelet therapy; INR: internation al normalized ratio; LMWH: low-molecular-weight heparin; LV: left ventricle/left ventricular; PCI: percutaneous coronary intervention; MHV: mechanical heart valve; NOAC: non-vitamin K antagonist oral anticoagulant; OAC: oral anticoagulation; SAPT: single antiplatelet therapy; TAVI: transcatheter aortic valve implantation; UFH: unfractionated heparin; VKA: vitamin K antagonist. aClass of recommendation. bLevel of evidence. c≤5 days for warfarin and ≤3 days for acenocoumarol. dCHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 (2 points), diabetes, prior stroke (2 points) - vascular disease, age 65-74, sex category (female). eLV apex thrombus, antithrombin 3 deficit and proteins C and/or S deficit. fAF, venous thromboembolism, hypercoagulable state or, with alesser degree of evidence, severely impaired LV dysfunction (ejection fraction <35%). | ||
Figure 9. Antithrombotic therapy for valve prostheses.

AF: atrial fibrillation; ASA: acetylsalicylic acid; CAD: coronary artery disease; DAPT: dual antiplatelet therapy; INR: international normalized ratio; LMWH: low-molecular-weight heparin; LV: left ventricle/left ventricular; MHV: mechanical heart valve; MVR: mitral valve replacement or repair; OAC: oral anticoagulation; SAPT: single antiplatelet therapy; SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation; TVR: tricuspid valve replacement or repair; UFH: unfractionated heparin; VKA: vitamin K antagonist. Colour coding corresponds to class of recommendation.
11.4 MANAGEMENT OF PROSTHETIC VALVE DYSFUNCTION AND COMPLICATIONS
11.4.1 STRUCTURAL VALVE DETERIORATION
Definitions of SVD and bioprosthetic valve failure (BVF) were standardized by recent consensus.470,522 The comparative durability of TAVI and SAVR BHVs must be ascertained at longer term. Reversible causes of BVF (e.g. endocarditis, thrombosis) should be excluded, and considerations on timing of dysfunction (e.g. for BHV obstruction, mismatch in early phases, thrombosis in later phases) and location of malfunction (e.g. endocarditis or SVD in case of central regurgitation, endocarditis or anatomical/technical factors in case of paravalvular regurgitation) may reveal the most plausible underlying cause and guide clinical decision making.
Percutaneous balloon interventions should be avoided in the treatment of stenotic left-sided bioprostheses. Transcatheter valve-in-valve implantation is an option for treating degenerated BHVs in patients with increased surgical risk.227,523,524,525 Redo-TAVI is a safe and feasible option in selected patients, but the risk of PPM in small valves and that of coronary occlusion, as well the possibility for future access to the coronary arteries need to be considered.229,526,527,528 Experience is mostly in aortic BHVs and remains limited for BHVs in the mitral position and even more so in the tricuspid position529,530,531,532 for which valve-in-valve procedures may be reasonable in patients at increased surgical risk.531,533 Valve-in-ring mitral procedures are also acceptable in selected candidates, while the role of valve-in-ring tricuspid procedures remains uncertain. It is necessary for the Heart Team to discuss every patient and choose the best individualized approach. Careful pre-procedural planning is needed to minimize the risk of coronary artery obstruction and enable future coronary re-access in aortic BHV re-interventions if necessary. For mitral re-interventions the risk of LVOT obstruction should be carefully evaluated.534
11.4.2 NON-STRUCTURAL VALVE DYSFUNCTION
11.4.2.1 Patient-prosthesis mismatch
Patient-prosthesis mismatch (PPM) significantly decreases long-term survival, correlates with SVD and increases readmission rates for both heart failure and reoperation.535,536,537 Efforts to prevent PPM should receive more emphasis to improve long-term survival after either SAVR or TAVI.538
11.4.2.2 Paravalvular leak and haemolysis
Blood tests for haemolysis should be part of routine follow-up after valve replacement. Diagnosis of haemolytic anaemia requires TOE to detect paravalvular leaks for prostheses in the mitral position if TTE is not contributory. Reoperation is recommended if the paravalvular leak is related to endocarditis or causes haemolysis requiring repeated blood transfusions or leading to severe symptoms. Transcatheter closure of a paravalvular leak is feasible, but experience is limited and there is presently no conclusive evidence to show consistent efficacy.539 Transcatheter closure of paravalvular leaks should be considered for anatomically suitable paravalvular leaks in candidates selected by the Heart Team.540 Medical therapy (including iron supplementation, beta-blockers, and erythropoietin) is indicated in patients with severe haemolytic anaemia when contraindications to surgical or transcatheter closure are present.540
11.4.3 ENDOCARDITIS
The management of patients with endocarditis should follow the relevant guidelines.4
11.4.4 THROMBOSIS
11.4.4.1 General comments
Obstructive valve thrombosis should be suspected promptly in any patient with any type of prosthetic valve who presents with recent dyspnoea or an embolic event. The diagnosis should be confirmed by TTE and TOE, cinefluoroscopy, or CCT if promptly available.268,314 Valve thrombosis occurs mainly in MHVs. However, cases of thrombosis of BHVs have also been reported after surgery or transcatheter valve implantation.541 Thrombus on BHVs can present as hypo-attenuated leaflet thickening (HALT) with relatively normal leaflet motion, HALT with reduced leaflet motion but normal gradients, and clinical valve thrombosis with elevated gradients. Distinguishing between thrombus and pannus by means of CCT is important to guide decision making.
11.4.4.2 Valve thrombosis
The management of MHVs thrombosis is high risk, whatever the option taken. Fibrinolysis carries risks of bleeding, systemic embolism, and recurrent thrombosis.542 Emergency valve replacement is recommended for obstructive prosthetic valve thrombosis in critically ill patients without a contraindication to surgery. Management of non-obstructive thrombosis of an MHV depends mainly on the occurrence of a thromboembolic event and the size of the thrombus. Surgery should be considered for a large (>10 mm) non-obstructive prosthetic valve thrombus that is complicated by embolism or persists despite optimal anticoagulation.543 Fibrinolysis may be considered if surgery is not an option or is very high risk for the treatment of thrombosis of right-sided prostheses, but carries a risk of bleeding and thromboembolism. Anticoagulation using a VKA and/or UFH is the first-line treatment of BHV thrombosis. Because BHV thrombosis is associated with recurrence and early prosthetic degeneration, indefinite anticoagulation should be considered after a confirmed episode, but this strategy must be balanced against an increased risk of bleeding544,545 (Figure 10).
Figure 10. Management of left-sided obstructive and non-obstructive mechanical prosthetic thrombosis.
ASA: acetylsalicylic acid; CCT: cardiac computed tomography; i.v.: intravenous; TOE: transoesophageal echocardiography; TE: thromboembolism; TTE: transthoracic echocardiography; UFH: unfractionated heparin. Risk and benefits of both treatments should be individualized. The presence of a first-generation prosthesis is an incentive to surgery. aRefer to recommendations for the imaging assessment of prosthetic heart valves. Evaluation generally includes TTE plus TOE or CCT and occasionally fluoroscopy.
11.4.4.3 Subclinical leaflet thrombosis
HALT is detected by CCT in 12.4% and 32.4% of TAVI patients on OAC or DAPT at 3 months, respectively.546 The clinical significance of these findings is uncertain. Selective use of oral anticoagulants in patients with confirmed HALT and restricted leaflet motion with elevated gradients should be considered.
11.4.5 HEART FAILURE
Heart failure after valve surgery should lead to a quick search for SVD or PPM, deterioration of repair, LV dysfunction, or progression of another valve disease. Non-valvular-related causes such as CAD, hypertension, or sustained arrhythmias should also be considered. The management of patients with heart failure should follow the relevant guidelines and consensus documents.142,247
See Recommendations on management of prosthetic valve dysfunction (Recommendations K).
Recommendations K. Recommendations on management of prosthetic valve dysfunction.
| Recommendations | Classa | Levelb |
|---|---|---|
| Mechanical prosthetic thrombosis | ||
| Urgent or emergency valve replacement is recommended for obstructive thrombosis in critically ill patients without serious comorbidity. 542 | I | B |
| Fibrinolysis (using recombinant tissue plasminogen activator 10 mg bolus + 90 mg in 90 min with UFH or streptokinase 1 500 000 U in 60 min without UFH) should be considered when surgery is not available or is very high risk, or for thrombosis of right-sided prostheses. 542 | IIa | B |
| Surgery should be considered for large (>10 mm) non-obstructive prosthetic thrombus complicated by embolism. | IIa | C |
| Bioprosthetic thrombosis | ||
| Anticoagulation using a VKA and/or UFH is recommended in bioprosthetic valve thrombosis before considering re-intervention. | I | C |
| Anticoagulation should be considered in patients with leaflet thickening and reduced leaflet motion leading to elevated gradients, at least until resolution. 541,546 | IIa | B |
| Haemolysis and paravalvular leak | ||
| Reoperation is recommended if a paravalvular leak is related to endocarditis or causes haemolysis requiring repeated blood transfusions or leading to severe heart failure symptoms. | I | C |
| Transcatheter closure should be considered for suitable paravalvular leaks with clinically significant regurgitation and/or haemolysis in patients at high or prohibitive surgical risk. 547 | IIa | B |
| Decision on transcatheter or surgical closure of clinically significant paravalvular leaks should be considered based on patient risk status, leak morphology, and local expertise. | IIa | C |
| Bioprosthetic failure | ||
| Reoperation is recommended in symptomatic patients with a significant increase in transprosthetic gradient (after exclusion of valve thrombosis) or severe regurgitation. | I | C |
| Transcatheter, transfemoral valve-in-valve implantation in the aortic position should be considered by the Heart Team depending on anatomic considerations, features of the prosthesis, and in patients who are at high operative risk or inoperable. 529 | IIa | B |
| Transcatheter valve-in-valve implantation in the mitral and tricuspid position may be considered in selected patients at high risk for surgical reintervention. 382,531,532 | IIb | B |
| Reoperation should be considered in asymptomatic patients with significant prosthetic dysfunction if reoperation is low risk. | IIa | C |
| UFH: unfractionated heparin; VKA: vitamin K antagonist. aClass of recommendation. bLevel of evidence. | ||
12 Management during non-cardiac surgery
Cardiovascular morbidity and mortality are increased in patients with VHD who undergo NCS. Symptomatic severe aortic stenosis or mitral stenosis may require valve replacement or percutaneous intervention before NCS. A detailed description of recommendations in the setting is available in specific ESC Guidelines.489
12.1 PREOPERATIVE EVALUATION
Patient and surgical specific factors dictate the strategy.489,548,549 The cardiologist provides recommendations on pre- and perioperative management, surveillance, and continuation of chronic cardiovascular medical treatment. Echocardiography should be performed in any patient with VHD requiring NCS. Determination of functional capacity is a pivotal step in preoperative risk assessment, measured either by ability to perform activities in daily life or by exercise test. The decision for management should be taken after multidisciplinary discussion involving cardiologists, surgeons, and cardiac anaesthesiologists, as well as the team who will be in charge of NCS.
Patients receiving anticoagulation treatment should be managed as discussed in section 11.
12.2 SPECIFIC VALVE LESIONS
12.2.1 AORTIC STENOSIS
In patients with severe aortic stenosis, urgent NCS should be performed under careful haemodynamic monitoring. In case of high risk of NCS, balloon valvuloplasty may be considered before NCS.549 Management related to elective NCS depends on the presence of symptoms and the type of surgery.489,549,550,551,552,553 In symptomatic patients, aortic valve procedure should be considered before NCS. The type of procedure, TAVI or SAVR, is decided by the Heart Team. In asymptomatic patients, elective NCS, if at low to moderate risk, can be performed safely, albeit with a risk of worsening heart failure.489,552,553 If NCS implies large volume shifts, aortic valve procedure (TAVI or SAVR) should be considered first according to the Heart Team’s decision (Figure 11).
Figure 11. Management of non-cardiac surgery (NCS) in patients with severe aortic stenosis.
AV: aortic valve; BAV: balloon aortic valvuloplasty; NCS: non cardiac surgery; SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation.
12.2.2 MITRAL STENOSIS
NCS can be performed safely in patients with non-significant mitral stenosis (valve area >1.5 cm2) and in asymptomatic patients with significant mitral stenosis and an SPAP <50 mmHg. In symptomatic patients or in patients with SPAP >50 mmHg, correction of mitral stenosis, by means of PMC whenever possible, should be attempted before NCS if it is high risk.
12.2.3 AORTIC AND MITRAL REGURGITATION
NCS can be performed safely in asymptomatic patients with severe mitral regurgitation or aortic regurgitation and preserved LV function. The presence of symptoms or LV dysfunction should lead to consideration of valvular surgery, but this is seldom needed before NCS. If LV dysfunction is severe (ejection fraction <30%) and/or SPAP is >50/60 mmHg, NCS should be performed only if strictly necessary and after optimization of medical therapy for heart failure.
12.3 PERIOPERATIVE MONITORING
Heart rate control (particularly in mitral stenosis) and careful fluid management (particularly in aortic stenosis) are needed. TOE monitoring may be considered.
13 Management during pregnancy
Detailed guidelines on the management of cardiovascular disease during pregnancy are available in another document.554 The decision for management before and during pregnancy should be taken after multidisciplinary discussion in the pregnancy Heart Team involving cardiologists, cardiac surgeons, obstetricians, neonatologists, and anaesthesiologists.
13.1 MANAGEMENT BEFORE PREGNANCY
Valve disease should be evaluated before pregnancy and treated if necessary.554,555
Pregnancy should be discouraged, and intervention should be recommended before pregnancy in the following cases:
Patients with mitral stenosis and a valve area <1.5 cm² (especially if <1.0 cm²).554,556
All symptomatic patients with severe AS or asymptomatic patients with impaired LV function (LVEF <50%) or an abnormal exercise test should be counselled against pregnancy, and surgery should be performed pre-pregnancy.554,557
Women with Marfan syndrome and an aortic diameter >45 mm should be strongly discouraged from becoming pregnant without prior aortic repair because of the high risk of aortic dissection. Although an aortic diameter <40 mm is rarely associated with aortic dissection, a completely safe diameter does not exist. With an aortic diameter between 40 and 45 mm, previous aortic growth and family history are important for advising pregnancy with or without aortic repair.558 Although the actual risk of dissection is not well documented in the setting of bicuspid valves, counselling against pregnancy is recommended in the setting of aortic diameters >50 mm (>27 mm² BSA).559 Finally, an aortic diameter >25 mm/m² BSA in Turner syndrome and all patients with vascular Ehlers-Danlos syndrome are also contraindications for pregnancy.
In women considering pregnancy and requiring heart valve replacement, it is recommended to choose the prosthesis in consultation with a pregnancy Heart Team.554,560
Pregnancy in women with a mechanical valve, especially in the mitral position, is associated with a high risk of maternal and foetal complications,554,561 which should be carefully discussed with the patient and family.
13.2 MANAGEMENT DURING PREGNANCY
13.2.1 PATIENTS WITH NATIVE VALVE DISEASE
Moderate or severe mitral stenosis with a valve area <1.5 cm² in pregnant women is usually poorly tolerated. PMC should be considered in severely symptomatic patients [New York Heart Association (NYHA) class III-IV] and/or those with SPAP >50 mmHg despite optimal therapy. PMC should preferably be performed after the 20th week of pregnancy in experienced centres.554
In patients who are severely symptomatic despite medical therapy, BAV for severe aortic stenosis can be undertaken by an experienced operator.557 TAVI is a promising alternative, but experience during pregnancy is very limited.554
Surgery under cardiopulmonary bypass is associated with a foetal mortality rate of 15-56%562 and should be restricted to the rare conditions that threaten the mother’s life if transcatheter intervention is not possible or has failed. Valve replacement should be considered after early delivery by caesarean section.
Caesarean section is recommended for patients with severe mitral or aortic stenosis, ascending aortic diameter >45 mm, severe pulmonary hypertension, or if delivery starts while treated with a VKA or <2 weeks after discontinuation of a VKA.
13.2.2 MECHANICAL PROSTHESIS
It is recommended to manage pregnancy in patients with MHV in a centre with a pregnancy Heart Team.554
Therapeutic anticoagulation during pregnancy is of utmost importance to avoid complications in these patients, keeping in mind that no anticoagulation regimen is ideal and management will require a careful balance between maternal and foetal risks.
In patients requiring <5 mg/day warfarin, oral anticoagulants throughout pregnancy and a change to UFH before delivery is favoured. In patients requiring higher doses, switching to LMWH during the first trimester with strict anti-Xa monitoring (therapeutic range 0.8-1.2 IU/mL, aortic valve prosthesis; and 1.0-1.2 IU/mL, mitral and right sided valve prosthesis) and the use of oral anticoagulants afterwards is favoured with a change to UFH before delivery.554
14 Key messages
GENERAL COMMENTS
1. Precise evaluation of the patient’s history and symptomatic status, as well as proper physical examination, are crucial for the diagnosis and management of VHD.
2. Echocardiography is the key technique to diagnose VHD and assess its severity and prognosis. Other non-invasive investigations such as CMR, CCT, fluoroscopy, and biomarkers provide important additional information in selected patients. Stress testing should be widely used in asymptomatic patients. Invasive investigation, beyond preoperative coronary angiography, is restricted to situations where non-invasive evaluation is inconclusive.
3. Decision making in elderly patients requires the integration of multiple parameters, including estimation of life expectancy and anticipated quality of life, evaluation of comorbidities, and general condition (including frailty).
4. Decision making in asymptomatic patients weighs the risk of intervention against the expected natural history of VHD. Stress testing should be liberally performed.
5. Informed patient’s expectations and values are an important part of the decision-making process.
6. Interventions (surgery or transcatheter) are indicated in symptomatic patients (spontaneous or exercise induced) in the absence of futility. In selected asymptomatic patients, presence of predictors of rapid symptom progression justifies early intervention when procedural risk is low.
7. Heart Valve Centres with multidisciplinary Heart Teams, Heart Valve Clinics, comprehensive equipment, and sufficient volumes of procedures are required to deliver high-quality care and provide adequate training.
8. Careful follow-up of symptomatic status, LV/RV size, and function is mandatory in asymptomatic patients with severe VHD if an intervention is not yet indicated.
9. In patients with AF, NOACs are contraindicated in patients with clinically significant mitral stenosis or mechanical valves. For stroke prevention in patients who are eligible for OAC, NOACs are recommended in preference to VKAs in patients with aortic stenosis, aortic and mitral regurgitation, or aortic bioprostheses >3 months after implantation.
AORTIC REGURGITATION
10. The evaluation of aortic regurgitation requires careful assessment of potentially associated aortic dilatation to guide the timing and type of surgery.
AORTIC STENOSIS
11. Diagnosis of severe aortic stenosis requires integrative evaluation of pressure gradients (the most robust measurements), AVA, extent of valve calcification, flow conditions, and LV function.
12. Selection of the most appropriate mode of intervention by the Heart Team should take into account clinical characteristics (age and estimated life expectancy, general condition), anatomical characteristics, the relative risks of SAVR and TAVI, the feasibility of transfemoral TAVI, local experience and outcome data, as well as informed patient preference.
MITRAL REGURGITATION
13. Regarding imaging, routine quantification of EROA is an important part of the integrative evaluation for quantification and risk stratification in patients with PMR. 3D transoesophageal echocardiography is more accurate than 2D echocardiography for defining the underlying mechanism of PMR. CMR is useful when echocardiographic evaluation of severe PMR grade is inconclusive.
14. Surgical mitral valve repair is the preferred method of treatment in PMR if a durable repair can be achieved. TEER is a safe but less efficacious alternative that may be considered in patients with contraindications for surgery or high operative risk.
15. In patients with severe SMR, GDMT (including CRT if indicated) should be the first step. If the patient remains symptomatic: mitral surgery is recommended concomitantly in patients with an indication for CABG or other cardiac surgery. Isolated valve surgery may be considered in selected patients. TEER should be considered in patients not eligible for surgery and fulfilling criteria indicating an increased chance of responding to the treatment. Circulatory support devices, cardiac transplantation, or palliative care should be considered as an alternative in patients with end-stage LV and/or RV failure.
MITRAL STENOSIS
16. PMC is currently the standard of care in patients with severe rheumatic mitral stenosis and favourable valve anatomy.
17. Decision making as to the type of intervention used in patients with unfavourable anatomy is still a matter of debate and must take into account the multifactorial nature of predicting the results of PMC.
TRICUSPID REGURGITATION
18. Relevant tricuspid regurgitation requires early intervention to avoid secondary damage of the RV.
19. Tricuspid regurgitation should be liberally treated at the time of left-sided valve surgery. Isolated surgery of severe secondary tricuspid regurgitation (with or without previous left-sided valve surgery) requires comprehensive assessment of the underlying disease, pulmonary haemodynamics, and RV function.
PROSTHETIC VALVES
20. The choice between a mechanical prosthesis and a bioprosthesis should be patient-centred and multifactorial based on patient characteristics, the indication for lifelong anticoagulation, the potential and risks of a re-intervention, and the informed patient preference.
21. Clinical assessment of prosthetic valves should be performed yearly and as soon as possible if new cardiac symptoms occur.
15 Gaps in evidence
Important gaps in evidence exist in the following aspects of VHD:
GENERAL COMMENTS
1. Prognostic value of CMR-derived indices in patients with aortic regurgitation, aortic stenosis, and mitral regurgitation.
2. Tools for risk stratification for the decision for intervention (including the avoidance of futile interventions) and the choice of the type of intervention (TAVI vs. SAVR for aortic stenosis, repair vs. replacement for mitral and aortic regurgitation).
3. In asymptomatic patients with aortic regurgitation, aortic stenosis, and mitral regurgitation, identification and evaluation of earlier markers of LV dysfunction (biomarkers, imaging, multimodality) as well as longitudinal and translational studies on progression.
4. Gender issues regarding pathophysiology, indications, and timing of treatment.
5. Minimum volumes of procedures that are required to achieve optimal results of intervention.
6. Safety and efficacy of NOACs in patients with surgical or transcatheter bioprostheses in the first 3 months after implantation.
7. Patient education for shared decision making and timely evaluation.
8. Systematic epidemiological data addressing the burden of rheumatic heart disease.
9. Advocacy of VHD.
AORTIC REGURGITATION
10. Potential differences in the risk of aortic complications depending on subtypes of aortic aneurysms (site and morphology), as well as in patients with bicuspid aortic valves.
11. Further evaluation of surgical aortic valve repair.
AORTIC STENOSIS
12. Pathophysiology of progression and novel therapeutic targets for medical treatment.
13. Further research to evaluate the role of intervention:
• a. Long-term durability of transcatheter heart valves in comparison with surgical bioprostheses.
• b. Role of intervention (SAVR or TAVI) in asymptomatic patients.
• c. Role of TAVI in younger low-risk patients, patients with aortic stenosis affecting bicuspid valves, and patients with moderate aortic stenosis and LV impairment.
• d. Results of re-intervention (valve or coronary) after TAVI or SAVR.
• e. The role of revascularization in patients with severe aortic stenosis and asymptomatic concomitant CAD.
MITRAL REGURGITATION
14. Association between PMR and sudden cardiac death and ventricular arrhythmias.
15. Role of genetic testing to mitral valve prolapse.
16. Further evaluation of the role of intervention:
• a. Long-term results of transcatheter intervention.
• b. Indications of transcatheter intervention in patients with severe PMR at lower surgical risk.
• c. Potential impact of mitral valve intervention (surgery and catheter intervention) on survival in patients with SMR.
• d. Selection of criteria to identify responders to TEER for SMR (severity criteria, concept of ‘disproportionate mitral regurgitation’).
• e. The role of newer transcatheter treatment options (annuloplasty, combined repair techniques, valve replacement).
MITRAL STENOSIS
17. Scores predicting the results and complications of PMC, particularly that of severe mitral regurgitation.
18. Role of transcatheter mitral valve implantation in high-risk patients, particularly in patients with severe degenerative mitral stenosis and MAC.
TRICUSPID REGURGITATION
19. Quantification of tricuspid regurgitation severity and evaluation of RV function.
20. Further research to evaluate the role of intervention:
• a. Criteria for optimal timing of surgery in primary tricuspid regurgitation.
• b. Evidence on the clinical impact, timing, and treatment modality of isolated severe secondary tricuspid regurgitation.
• c. Criteria for concomitant tricuspid valve surgery at the time of left-sided surgery in patients without severe tricuspid regurgitation.
• d. Results and indications of transcatheter tricuspid valve treatment.
COMBINED AND MULTI-VALVE DISEASES
21. Further evaluation of the impact on outcomes and modalities of transcatheter intervention to better define the indications for intervention.
PREGNANCY
22. Optimal management of pregnant women with MHVs regarding antithrombotic regimens.
NON-CARDIAC SURGERY
23. Evaluation of the role of ‘urgent TAVI’ in the management of patients with severe aortic stenosis undergoing NCS.
16 To Do and Not To Do
See Recommendations L.
Recommendations L. Recommendations.
| Recommendations | Classa | Levelb |
|---|---|---|
| Recommendations for management of CAD in patients with VHD | ||
| Diagnosis of CAD | ||
| Coronary angiography is recommended before valve surgery in patients with severe VHD and any of the following: • History of cardiovascular disease. • Suspected myocardial ischaemia. • LV systolic dysfunction. • In men >40 years of age and postmenopausal women. • One or more cardiovascular risk factors. |
I | C |
| Coronary angiography is recommended in the evaluation of severe SMR. | I | C |
| Indications for myocardial revascularization | ||
| CABG is recommended in patients with a primary indication for aortic/mitral/tricuspid valve surgery and coronary artery diameter stenosis ≥70%. | I | C |
| Recommendations on management of atrial fibrillation in patients with native VHD | ||
| Anticoagulation | ||
| For stroke prevention in AF patients who are eligible for OAC, NOACs are recommended in preference to VKAs in patients with aortic stenosis, aortic and mitral regurgitation. | I | A |
| The use of NOACs is not recommended in patients with AF and moderate to severe mitral stenosis. | III | C |
| Recommendations on indications for surgery in (A) severe aortic regurgitation and (B) aortic root or tubular ascending aortic aneurysm (irrespective of the severity of aortic regurgitation) | ||
| A) Severe aortic regurgitation | ||
| Surgery is recommended in symptomatic patients regardless of LV function. | I | B |
| Surgery is recommended in asymptomatic patients with LVESD >50mm or LVESD >25 mm/m2 BSA (in patients with small body size) or resting LVEF ≤50%. | I | B |
| Surgery is recommended in symptomatic and asymptomatic patients with severe aortic regurgitation undergoing CABG or surgery of the ascending aorta or of another valve. | I | C |
| B) Aortic root or tubular ascending aortic aneurysm (irrespective of the severity of aortic regurgitation) | ||
| Valve-sparing aortic root replacement is recommended in young patients with aortic root dilation, if performed in experienced centres and durable results are expected. | I | B |
| Ascending aortic surgery is indicated in patients with Marfan syndrome who have aortic root disease with a maximal ascending aortic diameter ≥50 mm. | I | C |
| Recommendations on indications for intervention in symptomatic (A) and asymptomatic (B) aortic stenosis and recommended mode of intervention (C) | ||
| A) Symptomatic aortic stenosis | ||
| Intervention is recommended in symptomatic patients with severe, high-gradient aortic stenosis [mean gradient ≥40 mmHg, peak velocity ≥4.0 m/s and valve area ≤1.0 cm2 (or ≤0.6 cm2/m2)] . | I | B |
| Intervention is recommended in symptomatic patients with severe low-flow (SVi ≤35 mL/m2), low-gradient (<40 mmHg) aortic stenosis with reduced ejection fraction (<50%) and evidence of flow (contractile) reserve. | I | B |
| Intervention is not recommended in patients with severe comorbidities when the intervention is unlikely to improve quality of life or prolong survival >1 year. | III | C |
| B) Asymptomatic patients with severe aortic stenosis | ||
| Intervention is recommended in asymptomatic patients with severe aortic stenosis and systolic LV dysfunction (LVEF <50%) without another cause. | I | B |
| Intervention is recommended in asymptomatic patients with severe aortic stenosis and demonstrable symptoms on exercise testing. | I | C |
| C) Mode of intervention | ||
| Aortic valve interventions must be performed in Heart Valve Centres that declare their local expertise and outcomes data, have active interventional cardiology and cardiac surgical programmes on site, and a structured collaborative Heart Team approach. | I | C |
| The choice between surgical and transcatheter intervention must be based upon careful evaluation of clinical, anatomical, and procedural factors by the Heart Team, weighing the risks and benefits of each approach for an individual patient. The Heart Team recommendation should be discussed with the patient who can then make an informed treatment choice. | I | C |
| SAVR is recommended in younger patients who are low risk for surgery (<75 years and STS-PROM/ EuroSCORE II <4%), or in patients who are operable and unsuitable for transfemoral TAVI. | I | B |
| TAVI is recommended in older patients (≥75 years), or in those who are high risk (STS-PROM/EuroSCORE II >8%) or unsuitable for surgery. | I | A |
| SAVR or TAVI are recommended for remaining patients according to individual clinical, anatomical, and procedural characteristics. | I | B |
| D) Concomitant aortic valve surgery at the time of other cardiac/ascending aorta surgery | ||
| SAVR is recommended in patients with severe aortic stenosis undergoing CABG or surgical intervention on the ascending aorta or another valve. | I | C |
| Recommendations on indications for intervention in severe primary mitral regurgitation | ||
| Mitral valve repair is the recommended surgical technique when the results are expected to be durable. | I | B |
| Surgery is recommended in symptomatic patients who are operable and not high risk. | I | B |
| Surgery is recommended in asymptomatic patients with LV dysfunction (LVESD ≥40 mm and/or LVEF ≤60%). | I | B |
| Recommendations on indications for mitral valve intervention in chronic severe secondary mitral regurgitation | ||
| Valve surgery/intervention is recommended only in patients with severe SMR who remain symptomatic despite GDMT (including CRT if indicated) and has to be decided by a structured collaborative Heart Team. | I | B |
| Patients with concomitant coronary artery or other cardiac disease requiring treatment | ||
| Valve surgery is recommended in patients undergoing CABG or other cardiac surgery. | I | B |
| Recommendations on indications for percutaneous mitral commissurotomy and mitral valve surgery in clinically significant (moderate or severe) mitral stenosis (valve area ≤1.5 cm2) | ||
| PMC is recommended in symptomatic patients without unfavourable characteristics for PMC. | I | B |
| PMC is recommended in any symptomatic patients with a contraindication or a high risk for surgery. | I | C |
| Mitral valve surgery is recommended in symptomatic patients who are not suitable for PMC in the absence of futility. | I | C |
| Recommendations on indications for intervention in tricuspid valve disease | ||
| Recommendations on tricuspid stenosis | ||
| Surgery is recommended in symptomatic patients with severe tricuspid stenosis. | I | C |
| Surgery is recommended in patients with severe tricuspid stenosis undergoing left-sided valve intervention. | I | C |
| Recommendations on primary tricuspid regurgitation | ||
| Surgery is recommended in patients with severe primary tricuspid regurgitation undergoing left-sided valve surgery. | I | C |
| Surgery is recommended in symptomatic patients with isolated severe primary tricuspid regurgitation without severe RV dysfunction. | I | C |
| Recommendations on secondary tricuspid regurgitation | ||
| Surgery is recommended in patients with severe secondary tricuspid regurgitation undergoing left-sided valve surgery. | I | B |
| Recommendations for prosthetic valve selection | ||
| Mechanical prostheses | ||
| A mechanical prosthesis is recommended according to the desire of the informed patient and if there are no contraindications to long-term anticoagulation. | I | C |
| A mechanical prosthesis is recommended in patients at risk of accelerated SVD. | I | C |
| Biological prostheses | ||
| A bioprosthesis is recommended according to the desire of the informed patient. | I | C |
| A bioprosthesis is recommended when good-quality anticoagulation is unlikely (adherence problems, not readily available), contraindicated because of high bleeding risk (previous major bleed, comorbidities, unwillingness, adherence problems, lifestyle, occupation), and in those patients whose life expectancy is lower than the presumed durability of the bioprosthesis. | I | C |
| A bioprosthesis is recommended in case of reoperation for mechanical valve thrombosis despite good long-term anticoagulant control. | I | C |
| Recommendations for perioperative and postoperative antithrombotic management of valve replacement or repair | ||
| Management of antithrombotic therapy in the perioperative period | ||
| It is recommended that VKAs are timely discontinued prior to elective surgery to aim for an INR <1.5. | I | C |
| Bridging of OAC, when interruption is needed, is recommended in patients with any of the following indications: • Mechanical prosthetic heart valve. • AF with significant mitral stenosis. • AF with a CHA2DS2-VASc score ≥3 for women or 2 for men. • Acute thrombotic event within the previous 4 weeks. • High acute thromboembolic risk. |
I | C |
| Therapeutic doses of either UFH or subcutaneous LMWH are recommended for bridging. | I | B |
| In patients with MHVs, it is recommended to (re)-initiate the VKA on the first postoperative day. | I | C |
| In patients who have undergone valve surgery with an indication for postoperative therapeutic bridging, it is recommended to start either UFH or LMWH 12-24 h after surgery. | I | C |
| In patients undergoing surgery, it is recommended that aspirin therapy, if indicated, is maintained during the periprocedural period. | I | C |
| In patients treated with DAPT after recent PCI (within 1 month) who need to undergo heart valve surgery in the absence of an indication for OAC, it is recommended to resume the P2Y12 inhibitor postoperatively as soon as there is no concern over bleeding. | I | C |
| Patients with an indication to concomitant antiplatelet therapy | ||
| After uncomplicated PCI or ACS in patients requiring long-term OAC, early cessation (≤1 week) of aspirin and continuation of dual therapy with OAC and a P2Y12 inhibitor (preferably clopidogrel) for up to 6 months (or up to 12 months in ACS) is recommended if the risk of stent thrombosis is low or if concerns about bleeding risk prevail over concerns about risk of stent thrombosis, irrespective of the type of stent used. | I | B |
| Discontinuation of antiplatelet treatment in patients treated with an OAC is recommended after 12 months. | I | B |
| Surgical valve replacement | ||
| OAC using a VKA is recommended lifelong for all patients with a MHV prosthesis. | I | B |
| For patients with a VKA, INR self-management is recommended provided appropriate training and quality control are performed. | I | B |
| OAC is recommended for patients undergoing implantation of a surgical BHV who have other indications for anticoagulation. | I | C |
| NOACs are not recommended in patients with an MHV. | III | B |
| Transcatheter aortic valve implantation | ||
| OAC is recommended lifelong for TAVI patients who have other indications for anticoagulation. | I | B |
| Lifelong SAPT is recommended after TAVI in patients with no baseline indication for OAC. | I | A |
| Routine use of OAC is not recommended after TAVI in patients who have no baseline indication for OAC. | III | B |
| Recommendations on management of prosthetic valve dysfunction | ||
| Mechanical prosthetic thrombosis | ||
| Urgent or emergency valve replacement is recommended for obstructive thrombosis in critically ill patients without serious comorbidity. | I | B |
| Bioprosthetic thrombosis | ||
| Anticoagulation using a VKA and/or UFH is recommended in bioprosthetic valve thrombosis before considering reintervention. | I | C |
| Haemolysis and paravalvular leak | ||
| Reoperation is recommended if a paravalvular leak is related to endocarditis or causes haemolysis requiring repeated blood transfusions or leading to severe heart failure symptoms. | I | C |
| Bioprosthetic failure | ||
| Reoperation is recommended in symptomatic patients with a significant increase in transprosthetic gradient (after exclusion of valve thrombosis) or severe regurgitation. | I | C |
| ACS: acute coronary syndrome; AF: atrial fibrillation; BHV: biological heart valve; BSA: body surface area; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CRT: cardiac resynchronization therapy; DAPT: dual antiplatelet therapy; EuroSCORE: European System for Cardiac Operative Risk Evaluation; GDMT: guidelinedirected medical therapy; h: hours; INR: international normalized ratio; LMWH: low-molecular-weight heparin; LV: left ventricle/left ventricular; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; MHV: mechanical heart valve; MR: mitral regurgitation; NOAC: non-vitamin K antagonist oral anticoagulant; OAC: oral anticoagulation; PCI: percutaneous coronary intervention; PMC: percutaneous mitral commissurotomy; RV: right ventricle/right ventricular; SAPT: single antiplatelet therapy; SAVR: surgical aortic valve replacement; SMR: secondary mitral regurgitation; STS-PROM: Society of Thoracic Surgeons – predicted risk of mortality; SVD: structural valve deterioration; SVi: stroke volume index; TAVI: transcatheter aortic valve implantation; UFH: unfractionated heparin; VHD: valvular heart disease; VKA: vitamin K antagonist. | ||
Appendix
Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)
Authors/Task Force Members: Alec Vahanian* (ESC Chairperson) (France), Friedhelm Beyersdorf*1 (EACTS Chairperson) (Germany), Fabien Praz (ESC Task Force Coordinator) (Switzerland), Milan Milojevic1 (EACTS Task Force Coordinator) (Serbia), Stephan Baldus (Germany), Johann Bauersachs (Germany), Davide Capodanno (Italy), Lenard Conradi1 (Germany), Michele De Bonis1 (Italy), Ruggero De Paulis1 (Italy), Victoria Delgado (Netherlands), Nick Freemantle1 (United Kingdom), Martine Gilard (France), Kristina H. Haugaa (Norway), Anders Jeppsson1 (Sweden), Peter Jüni (Canada), Luc Pierard (Belgium), Bernard D. Prendergast (United Kingdom), J. Rafael Sádaba1 (Spain), Christophe Tribouilloy (France), Wojtek Wojakowski (Poland), ESC/EACTS Scientific Document Group
1Representing the European Association for Cardio-Thoracic Surgery (EACTS)
Author/Task Force Member affiliations: listed in Author information.
ESC Clinical Practice Guidelines Committee (CPG): listed in the Appendix.
EACTS Council: listed in the Appendix.
Associations: Association for Acute CardioVascular Care (ACVC), European Association of Cardiovascular Imaging (EACVI), European Association of Percutaneous Cardiovascular Interventions (EAPCI), European Heart Rhythm Association (EHRA), Heart Failure Association (HFA).
Councils: Council on Valvular Heart Disease.
Working Groups: Cardiovascular Surgery, Thrombosis.
Vahanian A, Beyersdorf F, Praz F, et al, 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS), European Heart Journal, 2021; doi:10.1093/eurheartj/ehab395. Reprinted by permission of Oxford University Press on behalf of the European Society of Cardiology.
This publication comprises a reprint of the official full text version of the "2021 ESC/EACTS Guidelines for the management of valvular heart disease" ("ESC Guidelines") originally published in English in the European Heart Journal by Oxford University Press under licence from the European Society of Cardiology ("ESC").
This publication is for personal and educational use only. No commercial use is authorized. No part of this publication or the original ESC Guidelines from which it is derived may be translated or reproduced in any form without written permission from the ESC. Permission may be obtained upon submission of a written request to Oxford University Press, the publisher of the European Heart Journal and the party authorized to handle such permissions by the ESC. Merck Group has obtained permission to publish this reprint and to distribute it to health professionals globally.
All rights reserved; no part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise without the prior written permission of Oxford University Press or its licensee Oxford Publishing Limited ("OPL").
For permissions, please contact journals.permissions@oup.com
The ESC Guidelines represent the views of the ESC and were produced after careful consideration of the scientific and medical knowledge and the evidence available at the time of their dating.
The ESC is not responsible in the event of any contradiction, discrepancy and/or ambiguity between the ESC Guidelines and any other official recommendations or guidelines issued by the relevant public health authorities, in particular in relation to good use of health care or therapeutic strategies. Health professionals are encouraged to take the ESC Guidelines fully into account when exercising their clinical judgment as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies. However, the ESC Guidelines do not override in any way whatsoever the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient's health condition and in consultation with that patient and the patient's caregiver where appropriate and/or necessary. Nor do the ESC Guidelines exempt health professionals from taking careful and full consideration of the relevant official updated recommendations or guidelines issued by the competent public health authorities in order to manage each patient's case in light of the scientifically accepted data pursuant to their respective ethical and professional obligations. It is also the health professional's responsibility to verify the applicable rules and regulations relating to drugs and medical devices at the time of prescription.
Document Reviewers: Franz-Josef Neumann (ESC Review Coordinator) (Germany), Patrick Myers1 (EACTS Review Coordinator) (Switzerland), Magdy Abdelhamid (Egypt), Stephan Achenbach (Germany), Riccardo Asteggiano (Italy), Fabio Barili1 (Italy), Michael A. Borger (Germany), Thierry Carrel1 (Switzerland), Jean-Philippe Collet (France), Dan Foldager (Denmark), Gilbert Habib (France), Christian Hassager (Denmark), Alar Irs1 (Estonia), Bernard Iung (France), Marjan Jahangiri1 (United Kingdom), Hugo A. Katus (Germany), Konstantinos C. Koskinas (Switzerland), Steffen Massberg (Germany), Christian E. Mueller (Switzerland), Jens Cosedis Nielsen (Denmark), Philippe Pibarot (Canada), Amina Rakisheva (Kazakhstan), Marco Roffi (Switzerland), Andrea Rubboli (Italy), Evgeny Shlyakhto (Russia), Matthias Siepe1 (Germany), Marta Sitges (Spain), Lars Sondergaard (Denmark), Miguel Sousa-Uva1 (Portugal), Guiseppe Tarantini (Italy), Jose Luis Zamorano (Spain)
1Representing the European Association for Cardio-Thoracic Surgery (EACTS)
All experts involved in the development of these guidelines have submitted declarations of interest.
These have been compiled in a report and published in a supplementary document simultaneously to the guidelines. The report is also available on the ESC website www.escardio.org/guidelines.
For the Supplementary Data which include background information and detailed discussion of the data that have provided the basis for the guidelines see European Heart Journal online.
ESC subspecialty communities having participated in the development of this document:
This article has been co-published with permission in the European Heart Journal and European Journal of Cardio-Thoracic Surgery. VC the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery 2021. All rights reserved. The articles are identical except for minor stylistic and spelling differences in keeping with each journal’s style. Either citation can be used when citing this article. For permissions, please email journals.permissions@oup.com.
Supplementary data
Cardiovascular and non-cardiovascular factors linked with transcatheter aortic valve implantation-related futility featured within the PARTNER and FRANCE 2 transcatheter aortic valve implantation-risk score models.
Katz Index of Independence in Activities of Daily Living.
Essential frailty toolset in older adults undergoing aortic valve replacement.
Medical comorbidities and factors predicting poorer outcomes post transcatheter aortic valve implantation.
Integrated approach for estimating transcatheter aortic valve implantation-specific risk and futility.
Risk-of-bias judgments for all evaluated trials.
Main inclusion/exclusion criteria suggesting an increased chance of responding to TEER in patients with SMR.
Echocardiographic scores used for assessing the feasibility of percutaneous mitral commissurotomy: Wilkins score, Cormier score, and Echo score ‘Revisited’.
Criteria for patients selection for MitraClip procedure.
Peri- and post-procedural management of antithrombotic therapy in patients with indication to OAC and ACS/PCI.
Management of OAC in patients with an indication for preoperative bridging.
Table 1. Classes of recommendations.

Table 2. Levels of evidence.
Table 3. What is new.
| New or Revised | Recommendations in 2017 version | Class | Recommendations in 2021 version | Class |
|---|---|---|---|---|
| Section 3. Management of atrial fibrillation in patients with native VHD | ||||
| Revised | Surgical excision or external clipping of the LAA may be considered in patients undergoing valve surgery. | IIb | LAA occlusion should be considered to reduce the thromboembolic risk in patients with AF and a CHA2DS2VASc score ≥2 undergoing valve surgery. | IIa |
| Revised | NOACs should be considered as an alternative to VKAs in patients with aortic stenosis, aortic regurgitation and mitral regurgitation presenting with AF. | IIa | For stroke prevention in AF patients who are eligible for OAC, NOACs are recommended in preference to VKAs in patients with aortic stenosis, aortic and mitral regurgitation. | I |
| Section 4. Recommendations on indications for surgery in severe aortic regurgitation | ||||
| Revised | Surgery is indicated in asymptomatic patients with resting ejection fraction ≤50%. | I | Surgery is recommended in asymptomatic patients with LVESD >50 mm or LVESD >25 mm/m2 BSA (in patients with small body size) or resting LVEF ≤50%. | I |
| Surgery should be considered in asymptomatic patients with resting ejection fraction >50% with severe LV dilatation: LVEDD >70 mm or LVESD >50 mm (or LVESD >25 mm/m2 BSA in patients with small body size). | IIa | |||
| New | Surgery may be considered in asymptomatic patients with LVESD >20 mm/m2 BSA (especially in patients with small body size) or resting LVEF ≤55%, if surgery at low-risk. | IIb | ||
| Revised | Heart Team discussion is recommended in selected patients in whom aortic valve repair may be a feasible alternative to valve replacement. | I | Aortic valve repair may be considered in selected patients at experienced centres when durable results are expected. | IIb |
| Section 4. Recommendations on indications for surgery in aortic root or tubular ascending aortic aneurysm (irrespective of the severity of aortic regurgitation) | ||||
| Revised | Aortic valve repair, using the reimplantation or remodelling with aortic annuloplasty technique, is recommended in young patients with aortic root dilation and tricuspid aortic valves, when performed by experienced surgeons. | I | Valve-sparing aortic root replacement is recommended in young patients with aortic root dilation, if performed in experienced centres and durable results are expected. | I |
| Section 5. Recommendations on indications for intervention in symptomatic and asymptomatic aortic stenosis | ||||
| Symptomatic aortic stenosis | ||||
| Revised | Intervention is indicated in symptomatic patients with severe, high-gradient aortic stenosis (mean gradient ≥40 mmHg or peak velocity ≥4.0 m/s). | I | Intervention is recommended in symptomatic patients with severe, high-gradient aortic stenosis [mean gradient ≥40 mmHg, peak velocity ≥4.0 m/s and valve area ≤1.0 cm2 (or ≤0.6 cm2/m2)]. | I |
| Asymptomatic patients with severe aortic stenosis | ||||
| New | Intervention should be considered in asymptomatic patients with severe aortic stenosis and systolic LV dysfunction (LVEF <55%) without another cause. | IIa | ||
| Revised | SAVR should be considered in asymptomatic patients with normal ejection fraction and none of the above-mentioned exercise test abnormalities if the surgical risk is low and one of the following findings is present: • Very severe aortic stenosis defined by a Vmax >5.5 m/s. • Severe valve calcification and a rate of Vmax progression ≥0.3 m/s/year. • Markedly elevated BNP levels (>3x age- and sex-corrected normal range) confirmed by repeated measurements without other explanations. • Severe pulmonary hypertension (systolic pulmonary artery pressure at rest >60 mmHg confirmed by invasive measurement) without other explanation. |
IIa | Intervention should be considered in asymptomatic patients with LVEF >55% and a normal exercise test if the procedural risk is low and one of the following parameters is present: • Very severe aortic stenosis (mean gradient ≥60 mmHg or Vmax ≥5 m/s). • Severe valve calcification (ideally assessed by CCT) and Vmax progression ≥0.3 m/s/year. • Markedly elevated BNP levels (>3x age- and sex-corrected normal range) confirmed by repeated measurements and without other explanation. |
IIa |
| Section 5. Recommended mode of intervention in patients with aortic stenosis | ||||
| Revised | The choice for intervention must be based on careful individual evaluation of technical suitability and weighing of risks and benefits of each modality. In addition, the local expertise and outcomes data for the given intervention must be taken into account. | I | The choice between surgical and transcatheter intervention must be based upon careful evaluation of clinical, anatomical and procedural factors by the Heart Team, weighing the risks and benefits of each approach for an individual patient. The Heart Team recommendation should be discussed with the patient who can then make an informed treatment choice. | I |
| Revised | SAVR is recommended in patients at low surgical risk (STS or EuroSCORE II <4% or logistic EuroSCORE I <10%, and no other risk factors not included in these scores, such as frailty, porcelain aorta, sequelae of chest radiation). | I | SAVR is recommended in younger patients who are low risk for surgery (<75 years and STS-PROM/ EuroSCORE II <4%) or in patients who are operable and unsuitable for transfemoral TAVI. | I |
| Revised | TAVI is recommended in patients who are not suitable for SAVR as assessed by the Heart Team. | I | TAVI is recommended in older patients (≥75 years), or in those who are high-risk (STS-PROM/ EuroSCORE II >8%) or unsuitable for surgery. | I |
| Revised | In patients who are at increased surgical risk (STS or EuroSCORE II ≥4% or logistic EuroSCORE I ≥10%, or other risk factors not included in these scores such as frailty, porcelain aorta, sequelae of chest radiation), the decision between SAVR and TAVI should be made by the Heart Team according to the individual patient characteristics, with TAVI being favoured in elderly patients suitable for transfemoral access. | I | SAVR or TAVI are recommended for remaining patients according to individual clinical, anatomical and procedural characteristics. | I |
| New | Non-transfemoral TAVI may be considered in patients who are inoperable for SAVR and unsuitable for transfemoral TAVI. | IIb | ||
| Section 6. Indications for intervention in severe primary mitral regurgitation | ||||
| Revised | Surgery is indicated in asymptomatic patients with LV dysfunction (LVESD ≥45 mm and/or LVEF ≤60%). | I | Surgery is recommended in asymptomatic patients with LV dysfunction (LVESD ≥40 mm and/or LVEF ≤60%). | I |
| Revised | Surgery should be considered in asymptomatic patients with preserved LV function (LVESD <45 mm and LVEF >60%) and AF secondary to mitral regurgitation or pulmonary hypertension (SPAP at rest >50 mmHg). | IIa | Surgery should be considered in asymptomatic patients with preserved LV function (LVESD <40 mm and LVEF >60%) and AF secondary to mitral regurgitation or pulmonary hypertension (SPAP at rest >50 mmHg). | IIa |
| Revised | Surgery should be considered in asymptomatic patients with preserved LVEF (>60%) and LVESD 40-44 mm when a durable repair is likely, surgical risk is low, the repair is performed in a Heart Valve Centre and at least one of the following findings is present: • flail leaflet or; • presence of significant LA dilatation (volume index ≥60 mL/m2 BSA) in sinus rhythm. |
IIa | Surgical mitral valve repair should be considered in low-risk asymptomatic patients with LVEF >60%, LVESD <40 mm and significant LA dilatation (volume index ≥60 mL/m2 or diameter ≥55 mm) when performed in a Heart Valve Centre and a durable repair is likely. | IIa |
| Section 6. Indications for mitral valve intervention in chronic severe secondary mitral regurgitation | ||||
| New | Valve surgery/intervention is recommended only in patients with severe SMR who remain symptomatic despite GDMT (including CRT if indicated) and has to be decided by a structured collaborative Heart Team. | I | ||
| Patients with concomitant coronary artery or other cardiac disease requiring treatment | ||||
| New | In symptomatic patients, who are judged not appropriate for surgery by the Heart Team on the basis of their individual characteristics, PCI (and/or TAVI) possibly followed by TEER (in case of persisting severe SMR) should be considered. | IIa | ||
| Revised | Surgery is indicated in patients with severe SMR undergoing CABG and LVEF >30%. | I | Valve surgery is recommended in patients under- going CABG or other cardiac surgery. |
I |
| Patients without concomitant coronary artery or other cardiac disease requiring treatment | ||||
| Revised | When revascularization is not indicated and surgical risk is not low, a percutaneous edge-to-edge procedure may be considered in patients with severe secondary mitral regurgitation and LVEF >30% who remain symptomatic despite optimal medical management (including CRT if indicated) and who have a suitable valve morphology by echocardiography, avoiding futility. | IIb | TEER should be considered in selected symptomatic patients, not eligible for surgery and fulfilling criteria suggesting an increased chance of responding to the therapy. | IIa |
| Revised | In patients with severe SMR and LVEF <30% who remain symptomatic despite optimal medical management (including CRT if indicated) and who have no option for revascularization, the Heart Team may consider a percutaneous edge-to-edge procedure or valve surgery after careful evaluation for a ventricular assist device or heart transplant according to individual patient characteristics. | IIb | In high-risk symptomatic patients not eligible for surgery and not fulfilling the criteria suggesting an increased chance of responding to TEER, the Heart Team may consider in selected cases a TEER procedure or other trans-catheter valve therapy if applicable, after careful evaluation for ventricular assist device or heart transplant. | IIb |
| Section 8. Indications for intervention in primary tricuspid regurgitation | ||||
| Revised | Surgery should be considered in asymptomatic or mildly symptomatic patients with severe isolated primary tricuspid regurgitation and progressive RV dilatation or deterioration of RV function. | IIa | Surgery should be considered in asymptomatic or mildly symptomatic patients with isolated severe primary tricuspid regurgitation and RV dilatation who are appropriate for surgery. | IIa |
| Revised | After previous left-sided surgery and in absence of recurrent left-sided valve dysfunction, surgery should be considered in patients with severe tricuspid regurgitation who are symptomatic or have progressive RV dilatation/dysfunction, in the absence of severe RV or LV dysfunction and severe pulmonary vascular disease/hypertension. | IIa | Surgery should be considered in patients with severe secondary tricuspid regurgitation (with or without previous left-sided surgery) who are symptomatic or have RV dilatation, in the absence of severe RV or LV dysfunction and severe pulmonary vascular disease/hypertension. | IIa |
| New | Transcatheter treatment of symptomatic secondary severe tricuspid regurgitation may be considered in inoperable patients at a Heart Valve Centre with expertise in the treatment of tricuspid valve disease. | IIb | ||
| Section 11. Recommendations for prosthetic valve selection | ||||
| New | A bioprosthesis may be considered in patients already on long-term NOACs due to the high risk for thromboembolism. | IIb | ||
| Revised | A bioprosthesis should be considered in those (patients) whose life expectancy is lower than the presumed durability of the bioprosthesis. | IIa | A bioprosthesis is recommended when good-quality anticoagulation is unlikely (adherence problems, not readily available), contraindicated because of high bleeding risk (previous major bleed, comorbidities, unwillingness, adherence problems, life-style, occupation) and in those patients whose life expectancy is lower than the presumed durability of the bioprosthesis. | I |
| Section 11. Recommendations for perioperative and postoperative antithrombotic management of valve replacement or repair | ||||
| Management of antithrombotic therapy in the perioperative period | ||||
| New | Bridging of OAC, when interruption is needed, is recommended in patients with any of the following indication: • Mechanical prosthetic heart valve. • AF with significant mitral stenosis. • AF with a CHA2DS2-VASc score ≥3 for women or 2 for men. • Acute thrombotic event within the previous 4 weeks. • High acute thromboembolic risk. |
I | ||
| New | It is recommended that VKAs are timely discontinued prior to elective surgery to aim for an INR <1.5. | I | ||
| New | In patients undergoing surgery, it is recommended that aspirin therapy, if indicated, is maintained during the periprocedural period. | I | ||
| New | In patients who have undergone valve surgery with an indication for postoperative therapeutic bridging, it is recommended to start either UFH or LMWH 12-24 hours after surgery. | I | ||
| New | In patients with MHVs, it is recommended to (re)-initiate VKAs on the first postoperative day. | I | ||
| New | In patients treated with DAPT after recent PCI (within 1 month) who need to undergo heart valve surgery, in the absence of an indication for OAC, it is recommended to resume the P2Y12 inhibitor postoperatively, as soon as there is no concern over bleeding. | I | ||
| New | In patients treated with DAPT after recent PCI (within 1 month) who need to undergo heart valve surgery, in the absence of an indication for OAC, bridging P2Y12 inhibitors with glycoprotein IIb/IIIa inhibitors or cangrelor may be considered. | IIb | ||
| Patients with an indication to concomitant antiplatelet therapy | ||||
| Revised | In patients undergoing an uncomplicated PCI dual therapy comprising VKA and clopidogrel (75 mg/day) should be considered as an alternative to 1-month triple antithrombotic therapy in patients in whom the bleeding risk outweighs the ischaemic risk. | IIa | After uncomplicated PCI or ACS in patients requiring long -term OAC, early cessation (≤1 week) of aspirin and continuation of dual therapy with OAC and a P2Y12 inhibitor (preferably clopidogrel) for up to 6 months (or up to 12 months in ACS) is recommended if the risk of stent thrombosis is low or if concerns about bleeding risk prevail over concerns about risk of stent thrombosis, irrespective of the type of stent used. | I |
| New | Discontinuation of antiplatelet treatment in patients treated with an OAC is recommended after 12 months. | I | ||
| New | In patients treated with a VKA (e.g. MHVs), clopidogrel alone should be considered in selected patients (e.g. HAS-BLED ≥3 or ARC-HBR met and low risk of stent thrombosis) for up to 12 months. | IIa | ||
| New | In patients requiring aspirin and/or clopidogrel in addition to VKA, the dose intensity of VKA should be considered and carefully regulated with a target INR in the lower part of the recommended target range and a time in the therapeutic range >65-70%. | IIa | ||
| New | After uncomplicated PCI or ACS in patients requiring both OAC and antiplatelet therapy, triple therapy with aspirin, clopidogrel and OAC for longer than 1 week should be considered when the risk of stent thrombosis outweighs the risk of bleeding, with a total duration (≤1 month) decided according to assessment of these risks and clearly specified at hospital discharge. | IIa | ||
| Surgical valve replacement | ||||
| New | NOACs should be considered over VKA after 3 months following surgical implantation of a BHV, in patients with AF. | IIa | ||
| New | In patients with no baseline indications for OAC, low-dose aspirin (75-100 mg/day) or OAC using a VKA should be considered for the first 3 months after surgical implantation of an aortic BHV. | IIa | ||
| New | NOACs may be considered over VKA within 3 months following surgical implantation of a BHV in mitral position in patients with AF. | IIb | ||
| Transcatheter Aortic Valve Implantation | ||||
| New | OAC is recommended lifelong for TAVI patients who have other indications for OAC. | I | ||
| Revised | SAPT may be considered after TAVI in the case of high bleeding risk. | IIb | Lifelong SAPT is recommended after TAVI in patients with no baseline indication for OAC. | I |
| New | Routine use of OAC is not recommended after TAVI in patients with no baseline indication for OAC. | III | ||
| Section 11. Recommendations on management of prosthetic valve dysfunction | ||||
| Haemolysis and paravalvular leak | ||||
| New | Decision on transcatheter or surgical closure of clinically significant paravalvular leaks should be considered based on patient risk status, leak morphology, and local expertise. | IIa | ||
| Bioprosthetic thrombosis | ||||
| New | Anticoagulation should be considered in patients with leaflet thickening and reduced leaflet motion leading to elevated gradients, at least until resolution. | IIa | ||
| Bioprosthetic failure | ||||
| New | Transcatheter valve-in-valve implantation in the mitral and tricuspid position may be considered in selected patients at high-risk for surgical re-intervention. | IIb | ||
| ACS: acute coronary syndrome; AF: atrial fibrillation; ARC-HBR: Academic Research Consortium - high bleeding risk; BHV: biological heart valve; BNP: B-type natriuretic peptide; BSA: body surface area; CABG: Coronary artery bypass grafting; CCT: cardiac computed tomography; CRT: cardiac resynchronization therapy; DAPT: dual anti-platelet therapy; EuroSCORE: European System for Cardiac Operative Risk Evaluation; GDMT: guideline-directed medical therapy; INR: international normalized ratio; LA: left atrium/left atrial; LAA: left atrial appendage; LMWH: low-molecular-weight heparin; LV: left ventricle/left ventricular; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: Left ventricular end-systolic diameter; MHV: mechanical heart valve; NOAC: non-vitamin K antagonist oral anticoagulant; OAC: oral anticoagulation; PCI: percutaneous coronary intervention; RV: right ventricle/right ventricular; SAPT: single antiplatelet therapy; SAVR: surgical aortic valve replacement; SMR: secondary mitral regurgitation; SPAP: systolic pulmonary arterial pressure; STS-PROM: Society of Thoracic Surgeons - predicted risk of mortality; TAVI: transcatheter aortic valve implantation; TEER: transcatheter edge-to-edge repair; UFH: unfractionated heparin; VHD: valvular heart disease; VKA: vitamin K antagonist; Vmax: peak transvalvular velocity. | ||||
Recommendations G. Recommendations on indications for percutaneous mitral commissurotomy and mitral valve surgery in clinically significant (moderate or severe) mitral stenosis (valve area ≤1.5 cm2).
| Recommendations | Classa | Levelb |
|---|---|---|
| PMC is recommended in symptomatic patients without unfavourable characteristicsc for PMC. 360,363,364,365,367 | I | B |
| PMC is recommended in any symptomatic patients with a contraindication or a high risk for surgery. | I | C |
| Mitral valve surgery is recommended in symptomatic patients who are not suitable for PMC in the absence of futility. | I | C |
| PMC should be considered as initial treatment in symptomatic patients with suboptimal anatomy but no unfavourable clinical characteristics for PMC.c | IIa | C |
| PMC should be considered in asymptomatic patients without unfavourable clinical and anatomical characteristicsc for PMC and: • High thromboembolic risk (history of systemic embolism, dense spontaneous contrast in the LA, new-onset or paroxysmal AF), and/or • High risk of haemodynamic decompensation (systolic pulmonary pressure >50 mmHg at rest, need for major NCS, desire for pregnancy). |
IIa | C |
| AF: atrial fibrillation; LA: left atrium/left atrial; MVA: mitral valve area; NCS: non-cardiac surgery; PMC: percutaneous mitral commissurotomy. aClass of recommendation. bLevel of evidence. cUnfavourable characteristics for PMC can be defined by the presence of several of the following characteristics. Clinical characteristics: old age, history of commissurotomy, New York Heart Association class IV, permanent AF, severe pulmonary hypertension. Anatomical characteristics: echocardiographic score >8, Cormier score 3 (calcification of mitral valve of any extent as assessed by fluoroscopy), very small MVA, severe tricuspid regurgitation. For the definition of scores, see Supplementary Table 8. | ||
References
- Iung B, Delgado V, Rosenhek R, Price S, Prendergast B, Wendler O, De Bonis, Tribouilloy C, Evangelista A, Bogachev-Prokophiev A, Apor A, Ince H, Laroche C, Popescu BA, Pierard L, Haude M, Hindricks G, Ruschitzka F, Windecker S, Bax JJ, Maggioni A, Vahanian A, EORP VHD. Contemporary presentation and management of valvular heart disease: The EURObservational Research Programme Valvular Heart Disease II Survey. Circulation. 2019;140:1156–69. doi: 10.1161/CIRCULATIONAHA.119.041080. [DOI] [PubMed] [Google Scholar]
- Yadgir S, Johnson CO, Aboyans V, Adebayo OM, Adedoyin RA, Afarideh M, Alahdab F, Alashi A, Alipour V, Arabloo J, Azari S, Barthelemy CM, Benziger CP, Berman AE, Bijani A, Carrero JJ, Carvalho F, Daryani A, Duraes AR, Esteghamati A, Farid TA, Farzadfar F, Fernandes E, Filip I, Gad MM, Hamidi S, Hay SI, Ilesanmi OS, Naghibi Irvani, Jurisson M, Kasaeian A, Kengne AP, Khan AR, Kisa A, Kisa S, Kolte D, Manafi N, Manafi A, Mensah GA, Mirrakhimov EM, Mohammad Y, Mokdad AH, Negoi RI, Thi Nguyen, Nguyen TH, Nixon MR, Otto CM, Patel S, Pilgrim T, Radfar A, Rawaf DL, Rawaf S, Rawasia WF, Rezapour A, Roever L, Saad AM, Saadatagah S, Senthilkumaran S, Sliwa K, Tesfay BE, Tran BX, Ullah I, Vaduganathan M, Vasankari TJ, Wolfe CDA, Yonemoto N, Roth GA, Global Burden. Global, regional, and national burden of calcific aortic valve and degenerative mitral valve diseases, 1990-2017. Circulation. 2020;141:1670–80. doi: 10.1161/CIRCULATIONAHA.119.043391. [DOI] [PubMed] [Google Scholar]
- Cahill TJ, Prothero A, Wilson J, Kennedy A, Brubert J, Masters M, Newton JD, Dawkins S, Enriquez-Sarano M, Prendergast BD, Myerson SG. Community prevalence, mechanisms and outcome of mitral or tricuspid regurgitation. Heart. 2021 doi: 10.1136/heartjnl-2020-318482. [DOI] [PubMed] [Google Scholar]
- Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti, Dulgheru R, El Khoury, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas, Vilacosta I, Zamorano JL ESC Scientific Document Group. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075–128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- Baumgartner H, De Backer, Babu-Narayan SV, Budts W, Chessa M, Diller GP, Lung B, Kluin J, Lang IM, Meijboom F, Moons P, Mulder BJM, Oechslin E, Roos-Hesselink JW, Schwerzmann M, Sondergaard L, Zeppenfeld K ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42:563–645. doi: 10.1093/eurheartj/ehaa554. [DOI] [PubMed] [Google Scholar]
- Pelliccia A, Sharma S, Gati S, Back M, Borjesson M, Caselli S, Collet JP, Corrado D, Drezner JA, Halle M, Hansen D, Heidbuchel H, Myers J, Niebauer J, Papadakis M, Piepoli MF, Prescott E, Roos-Hesselink JW, Graham Stuart, Taylor RS, Thompson PD, Tiberi M, Vanhees L, Wilhelm M, ESC Scientific. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42:17–96. doi: 10.1093/eurheartj/ehaa605. [DOI] [PubMed] [Google Scholar]
- Agricola E, Ancona F, Brochet E, Donal E, Dweck M, Faletra F, Lancellotti P, Mahmoud-Elsayed H, Marsan NA, Maurovich-Hovart P, Monaghan M, Ribeiro J, Sade LE, Swaans M, Von Bardeleben, Wunderlich N, Zamorano JL, Popescu BA, Cosyns B, Edvardsen T Reviewers: This document was reviewed by members of the 2018-2020 EACVI Scientific Documents Committee. The structural heart disease interventional imager rationale, skills and training: a position paper of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2021;22:471–9. doi: 10.1093/ehjci/jeab005. [DOI] [PubMed] [Google Scholar]
- Hahn RT, Mahmood F, Kodali S, Lang R, Monaghan M, Gillam LD, Swaminathan M, Bonow RO, von Bardeleben, Bax JJ, Grayburn P, Zoghbi WA, Sengupta PP, Chandrashekhar Y, Little SH. Core Competencies in Echocardiography for Imaging Structural Heart Disease Interventions: An Expert Consensus Statement. JACC Cardiovasc Imaging. 2019;12:2560–70. doi: 10.1016/j.jcmg.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancellotti P, Magne J, Dulgheru R, Clavel MA, Donal E, Vannan MA, Chambers J, Rosenhek R, Habib G, Lloyd G, Nistri S, Garbi M, Marchetta S, Fattouch K, Coisne A, Montaigne D, Modine T, Davin L, Gach O, Radermecker M, Liu S, Gillam L, Rossi A, Galli E, Ilardi F, Tastet L, Capoulade R, Zilberszac R, Vollema EM, Delgado V, Cosyns B, Lafitte S, Bernard A, Pierard LA, Bax JJ, Pibarot P, Oury C. Outcomes of patients with asymptomatic aortic stenosis followed up in heart valve clinics. JAMA Cardiol. 2018;3:1060–8. doi: 10.1001/jamacardio.2018.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancellotti P, Rosenhek R, Pibarot P, Iung B, Otto CM, Tornos P, Donal E, Prendergast B, Magne J, La Canna, Pierard LA, Maurer G. ESC Working Group on Valvular Heart Disease position paper–heart valve clinics: organization, structure, and experiences. Eur Heart J. 2013;34:1597–606. doi: 10.1093/eurheartj/ehs443. [DOI] [PubMed] [Google Scholar]
- Chambers JB, Prendergast B, Iung B, Rosenhek R, Zamorano JL, Pierard LA, Modine T, Falk V, Kappetein AP, Pibarot P, Sundt T, Baumgartner H, Bax JJ, Lancellotti P. Standards defining a ‘Heart Valve Centre’: ESC Working Group on Valvular Heart Disease and European Association for Cardiothoracic Surgery Viewpoint. Eur Heart J. 2017;38:2177–83. doi: 10.1093/eurheartj/ehx370. [DOI] [PubMed] [Google Scholar]
- Badheka AO, Patel NJ, Panaich SS, Patel SV, Jhamnani S, Singh V, Pant S, Patel N, Patel N, Arora S, Thakkar B, Manvar S, Dhoble A, Patel A, Savani C, Patel J, Chothani A, Savani GT, Deshmukh A, Grines CL, Curtis J, Mangi AA, Cleman M, Forrest JK. Effect of hospital volume on outcomes of transcatheter aortic valve implantation. Am J Cardiol. 2015;116:587–94. doi: 10.1016/j.amjcard.2015.05.019. [DOI] [PubMed] [Google Scholar]
- Nishimura RA, O’Gara PT, Bavaria JE, Brindis RG, Carroll JD, Kavinsky CJ, Lindman BR, Linderbaum JA, Little SH, Mack MJ, Mauri L, Miranda WR, Shahian DM, Sundt TM. 2019 AATS/ACC/ASE/SCAI/STS Expert Consensus Systems of Care Document: a proposal to optimize care for patients with valvular heart disease: a joint report of the American Association for Thoracic Surgery, American College of Cardiology, American Society of Echocardiography, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2019;73:2609–35. doi: 10.1016/j.jacc.2018.10.007. [DOI] [PubMed] [Google Scholar]
- Chhatriwalla AK, Vemulapalli S, Szerlip M, Kodali S, Hahn RT, Saxon JT, Mack MJ, Ailawadi G, Rymer J, Manandhar P, Kosinski AS, Sorajja P. Operator experience and outcomes of transcatheter mitral valve repair in the United States. J Am Coll Cardiol. 2019;74:2955–65. doi: 10.1016/j.jacc.2019.09.014. [DOI] [PubMed] [Google Scholar]
- Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, Mossialos EA, Maggioni AP, Kazakiewicz D, May HT, De Smedt, Flather M, Zuhlke L, Beltrame JF, Huculeci R, Tavazzi L, Hindricks G, Bax J, Casadei B, Achenbach S, Wright L, Vardas P, European Society. European Society of Cardiology: cardiovascular disease statistics 2019. Eur Heart J. 2020;41:12–85. doi: 10.1093/eurheartj/ehz859. [DOI] [PubMed] [Google Scholar]
- Vemulapalli S, Carroll JD, Mack MJ, Li Z, Dai D, Kosinski AS, Kumbhani DJ, Ruiz CE, Thourani VH, Hanzel G, Gleason TG, Herrmann HC, Brindis RG, Bavaria JE. Procedural volume and outcomes for transcatheter aortic-valve replacement. N Engl J Med. 2019;380:2541–50. doi: 10.1056/NEJMsa1901109. [DOI] [PubMed] [Google Scholar]
- Mao J, Redberg RF, Carroll JD, Marinac-Dabic D, Laschinger J, Thourani V, Mack M, Sedrakyan A. Association between hospital surgical aortic valve replacement volume and transcatheter aortic valve replacement outcomes. JAMA Cardiol. 2018;3:1070–8. doi: 10.1001/jamacardio.2018.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonow RO, O’Gara PT, Adams DH, Badhwar V, Bavaria JE, Elmariah S, Hung JW, Lindenfeld J, Morris A, Satpathy R, Whisenant B, Woo YJ. 2019 AATS/ACC/SCAI/STS Expert Consensus Systems of Care Document: operator and institutional recommendations and requirements for transcatheter mitral valve intervention: a joint report of the American Association for Thoracic Surgery, the American College of Cardiology, the Society for Cardiovascular Angiography and Interventions, and The Society of Thoracic Surgeons. J Am Coll Cardiol. 2020;76:96–117. doi: 10.1016/j.jacc.2019.12.002. [DOI] [PubMed] [Google Scholar]
- Garbi M, Chambers J, Pierard L, Maisano F, Lancellotti P. Heart Valve Specialist Core Syllabus: a learning framework for continuous medical education on valvular heart disease. European Society of Cardiology. 2021. Jun 1, [20 July 2021]. https://www.escardio.org/Councils/Council-on-Valvular-Heart-Disease/heart-valve-specialist-core-syllabus.
- Dreyfus G, Windecker S. How to shape the future of cardiology and cardiac surgery? Eur Heart J. 2020;41:3693–701. doi: 10.1093/eurheartj/ehaa707. [DOI] [PubMed] [Google Scholar]
- Iung B, Delgado V, Lazure P, Murray S, Sirnes PA, Rosenhek R, Price S, Metra M, Carrera C, De Bonis, Haude M, Hindricks G, Bax J, Vahanian A. Educational needs and application of guidelines in the management of patients with mitral regurgitation. A European mixed-methods study. Eur Heart J. 2018;39:1295–303. doi: 10.1093/eurheartj/ehx763. [DOI] [PubMed] [Google Scholar]
- Popescu BA, Andrade MJ, Badano LP, Fox KF, Flachskampf FA, Lancellotti P, Varga A, Sicari R, Evangelista A, Nihoyannopoulos P, Zamorano JL, Document R, Kasprzak JD, Roelandt JR. European Association of Echocardiography recommendations for training, competence, and quality improvement in echocardiography. Eur J Echocardiogr. 2009;10:893–905. doi: 10.1093/ejechocard/jep151. [DOI] [PubMed] [Google Scholar]
- Chambers JB, Garbi M, Nieman K, Myerson S, Pierard LA, Habib G, Zamorano JL, Edvardsen T, Lancellotti P, Cosyns B, Donal E, Dulgheru R, Galderisi M, Lombardi M, Muraru D, Kauffmann P, Cardim N, Haugaa K, Rosenhek R. Appropriateness criteria for the use of cardiovascular imaging in heart valve disease in adults: a European Association of Cardiovascular Imaging report of literature review and current practice. Eur Heart J Cardiovasc Imaging. 2017;18:489–98. doi: 10.1093/ehjci/jew309. [DOI] [PubMed] [Google Scholar]
- Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL, Scientific Document. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2013;14:611–44. doi: 10.1093/ehjci/jet105. [DOI] [PubMed] [Google Scholar]
- Baumgartner HC, Hung JC-C, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, Lancellotti P, LeFevre M, Miller F , Otto CM. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2017;18:254–75. doi: 10.1093/ehjci/jew335. [DOI] [PubMed] [Google Scholar]
- Magne J, Cosyns B, Popescu BA, Carstensen HG, Dahl J, Desai MY, Kearney L, Lancellotti P, Marwick TH, Sato K, Takeuchi M, Zito C, Casalta AC, Mohty D, Pierard L, Habib G, Donal E. Distribution and prognostic significance of left ventricular global longitudinal strain in asymptomatic significant aortic stenosis: an individual participant data meta-analysis. JACC Cardiovasc Imaging. 2019;12:84–92. doi: 10.1016/j.jcmg.2018.11.005. [DOI] [PubMed] [Google Scholar]
- Prihadi EA, van der, Dietz M, Abou R, Vollema EM, Marsan NA, Delgado V, Bax JJ. Prognostic implications of right ventricular free wall longitudinal strain in patients with significant functional tricuspid regurgitation. Circ Cardiovasc Imaging. 2019;12:e008666. doi: 10.1161/CIRCIMAGING.118.008666. [DOI] [PubMed] [Google Scholar]
- van Rosendael, van Wijngaarden, Kamperidis V, Kong WKF, Leung M, Ajmone Marsan, Delgado V, Bax JJ. Integrated imaging of echocardiography and computed tomography to grade mitral regurgitation severity in patients undergoing transcatheter aortic valve implantation. Eur Heart J. 2017;38:2221–6. doi: 10.1093/eurheartj/ehw612. [DOI] [PubMed] [Google Scholar]
- Schulz E, Tamm A, Kasper-Konig W, Beiras-Fernandez A, Vahl CF, Munzel T, von Bardeleben. Transapical implantation of a transcatheter aortic valve prosthesis into a mitral annuloplasty ring guided by real-time three-dimensional cardiac computed tomography-fluoroscopy fusion imaging. Eur Heart J. 2018;39:327–8. doi: 10.1093/eurheartj/ehx296. [DOI] [PubMed] [Google Scholar]
- Henri C, Pierard LA, Lancellotti P, Mongeon FP, Pibarot P, Basmadjian AJ. Exercise testing and stress imaging in valvular heart disease. Can J Cardiol. 2014;30:1012–26. doi: 10.1016/j.cjca.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Picano E, Pibarot P, Lancellotti P, Monin JL, Bonow RO. The emerging role of exercise testing and stress echocardiography in valvular heart disease. J Am Coll Cardiol. 2009;54:2251–60. doi: 10.1016/j.jacc.2009.07.046. [DOI] [PubMed] [Google Scholar]
- Monin JL, Quéré JP, Monchi M, Petit H, Baleynaud S, Chauvel C, Pop C, Ohlmann P, Lelguen C, Dehant P, Tribouilloy C, Guéret P. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation. 2003;108:319–24. doi: 10.1161/01.CIR.0000079171.43055.46. [DOI] [PubMed] [Google Scholar]
- Bing R, Cavalcante JL, Everett RJ, Clavel MA, Newby DE, Dweck MR. Imaging and impact of myocardial fibrosis in aortic stenosis. JACC Cardiovasc Imaging. 2019;12:283–96. doi: 10.1016/j.jcmg.2018.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College, Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, Ho VB, Jerosch-Herold M, Kramer CM, Manning WJ, Patel M, Pohost GM, Stillman AE, White RD, Woodard PK , ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55:2614–62. doi: 10.1016/j.jacc.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueff C, Serfaty JM, Cimadevilla C, Laissy JP, Himbert D, Tubach F, Duval X, Iung B, Enriquez-Sarano M, Vahanian A, Messika-Zeitoun D. Measurement of aortic valve calcification using multislice computed tomography: correlation with haemodynamic severity of aortic stenosis and clinical implication for patients with low ejection fraction. Heart. 2011;97:721–6. doi: 10.1136/hrt.2010.198853. [DOI] [PubMed] [Google Scholar]
- Clavel MA, Messika-Zeitoun D, Pibarot P, Aggarwal SR, Malouf J, Araoz PA, Michelena HI, Cueff C, Larose E, Capoulade R, Vahanian A, Enriquez-Sarano M. The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined Doppler echocardiographic and computed tomographic study. J Am Coll Cardiol. 2013;62:2329–38. doi: 10.1016/j.jacc.2013.08.1621. [DOI] [PubMed] [Google Scholar]
- Pibarot P, Magne J, Leipsic J, Cote N, Blanke P, Thourani VH, Hahn R. Imaging for predicting and assessing prosthesis-patient mismatch after aortic valve replacement. JACC Cardiovasc Imaging. 2019;12:149–62. doi: 10.1016/j.jcmg.2018.10.020. [DOI] [PubMed] [Google Scholar]
- Pulerwitz TC, Khalique OK, Leb J, Hahn RT, Nazif TM, Leon MB, George I, Vahl TP, D’Souza B, Bapat VN, Dumeer S, Kodali SK, Einstein AJ. Optimizing cardiac CT protocols for comprehensive acquisition prior to percutaneous MV and TV repair/replacement. JACC Cardiovasc Imaging. 2020;13:836–50. doi: 10.1016/j.jcmg.2019.01.041. [DOI] [PubMed] [Google Scholar]
- San S, Ravis E, Tessonier L, Philip M, Cammilleri S, Lavagna F, Norscini G, Arregle F, Martel H, Oliver L, Torras O, Renard S, Ambrosi P, Camoin L, Casalta AC, Hubert S, Casalta JP, Gouriet F, Riberi A, Avierinos JF, Lepidi H, Collart F, Raoult D, Drancourt M, Habib G. Prognostic value of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in infective endocarditis. J Am Coll Cardiol. 2019;74:1031–40. doi: 10.1016/j.jacc.2019.06.050. [DOI] [PubMed] [Google Scholar]
- Habib G, Erba PA, Iung B, Donal E, Cosyns B, Laroche C, Popescu BA, Prendergast B, Tornos P, Sadeghpour A, Oliver L, Vaskelyte JJ, Sow R, Axler O, Maggioni AP, Lancellotti P, EURO-ENDO Investigators. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Eur Heart J. 2019;40:3222–32. doi: 10.1093/eurheartj/ehz620. [DOI] [PubMed] [Google Scholar]
- Clavel MA, Tribouilloy C, Vanoverschelde JL, Pizarro R, Suri RM, Szymanski C, Lazam S, Oberti P, Michelena HI, Jaffe A, Enriquez-Sarano M. Association of B-type natriuretic peptide with survival in patients with degenerative mitral regurgitation. J Am Coll Cardiol. 2016;68:1297–307. doi: 10.1016/j.jacc.2016.06.047. [DOI] [PubMed] [Google Scholar]
- Lindman BR, Clavel MA, Abu-Alhayja’a R, Cote N, Dagenais F, Novak E, Voisine P, Poulin A, Arsenault BJ, Desmeules P, Dahou A, Taster L, Aldahoun K, Bosse Y, Mathieu P, Pibarot P. Multimarker approach to identify patients with higher mortality and rehospitalization rate after surgical aortic valve replacement for aortic stenosis. JACC Cardiovasc Interv. 2018;11:2172–81. doi: 10.1016/j.jcin.2018.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tastet L, Tribouilloy C, Marechaux S, Vollema EM, Delgado V, Salaun E, Shen M, Capoulade R, Clavel MA, Arsenault M, Bedard E, Bernier M, Beaudoin J, Narula J, Lancellotti P, Bax JJ, Genereux P, Pibarot P. Staging cardiac damage in patients with asymptomatic aortic valve stenosis. J Am Coll Cardiol. 2019;74:550–63. doi: 10.1016/j.jacc.2019.04.065. [DOI] [PubMed] [Google Scholar]
- Genereux P, Pibarot P, Redfors B, Mack MJ, Makkar RR, Jaber WA, Svensson LG, Kapadia S, Tuzcu EM, Thourani VH, Babaliaros V, Herrmann HC, Szeto WY, Cohen DJ, Lindman BR, McAndrew T, Alu MC, Douglas PS, Hahn RT, Kodali SK, Smith CR, Miller DC, Webb JG, Leon MB. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur Heart J. 2017;38:3351–8. doi: 10.1093/eurheartj/ehx381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Authors/Task Force, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541–619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- Scarsini R, Pesarini G, Zivelonghi C, Piccoli A, Ferrero V, Lunardi M, Gottin L, Zanetti C, Faggian G, Ribichini F. Physiologic evaluation of coronary lesions using instantaneous wave-free ratio (iFR) in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. EuroIntervention. 2018;13:1512–9. doi: 10.4244/EIJ-D-17-00542. [DOI] [PubMed] [Google Scholar]
- Scarsini R, Pesarini G, Zivelonghi C, Piccoli A, Ferrero V, Lunardi M, Barbierato M, Caprioglio F, Vassanelli C, Ribichini F. Coronary physiology in patients with severe aortic stenosis: comparison between fractional flow reserve and instantaneous wave-free ratio. Int J Cardiol. 2017;243:40–6. doi: 10.1016/j.ijcard.2017.05.117. [DOI] [PubMed] [Google Scholar]
- Osnabrugge RL, Speir AM, Head SJ, Fonner CE, Fonner E, Kappetein AP, Rich JB. Performance of EuroSCORE II in a large US database: implications for transcatheter aortic valve implantation. Eur J Cardiothorac Surg. 2014;46:400–8. doi: 10.1093/ejcts/ezu033. [DOI] [PubMed] [Google Scholar]
- Barili F, Pacini D, Capo A, Rasovic O, Grossi C, Alamanni F, Di Bartolomeo, Parolari A. Does EuroSCORE II perform better than its original versions? A multicentre validation study. Eur Heart J. 2013;34:22–9. doi: 10.1093/eurheartj/ehs342. [DOI] [PubMed] [Google Scholar]
- Provenchere S, Chevalier A, Ghodbane W, Bouleti C, Montravers P, Longrois D, Iung B. Is the EuroSCORE II reliable to estimate operative mortality among octogenarians? PLoS One. 2017;12:e0187056. doi: 10.1371/journal.pone.0187056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iung B, Laouenan C, Himbert D, Eltchaninoff H, Chevreul K, Donzeau-Gouge P, Fajadet J, Leprince P, Leguerrier A, Lievre M, Prat A, Teiger E, Laskar M, Vahanian A, Gilard M, FRANCE 2. Predictive factors of early mortality after transcatheter aortic valve implantation: individual risk assessment using a simple score. Heart. 2014;100:1016–23. doi: 10.1136/heartjnl-2013-305314. [DOI] [PubMed] [Google Scholar]
- Edwards FH, Cohen DJ, O’Brien SM, Peterson ED, Mack MJ, Shahian DM, Grover FL, Tuzcu EM, Thourani VH, Carroll J, Brennan JM, Brindis RG, Rumsfeld J, Holmes DR , Steering Committee of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy R. Development and validation of a risk prediction model for in-hospital mortality after transcatheter aortic valve replacement. JAMA Cardiol. 2016;1:46–52. doi: 10.1001/jamacardio.2015.0326. [DOI] [PubMed] [Google Scholar]
- Arnold SV, Reynolds MR, Lei Y, Magnuson EA, Kirtane AJ, Kodali SK, Zajarias A, Thourani VH, Green P, Rodes-Cabau J, Beohar N, Mack MJ, Leon MB, Cohen DJ, PARTNER Investigators. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation. 2014;129:2682–90. doi: 10.1161/CIRCULATIONAHA.113.007477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afilalo J. The Clinical Frailty Scale: Upgrade your eyeball test. Circulation. 2017;135:2025–7. doi: 10.1161/CIRCULATIONAHA.116.025958. [DOI] [PubMed] [Google Scholar]
- Kundi H, Popma JJ, Reynolds MR, Strom JB, Pinto DS, Valsdottir LR, Shen C, Choi E, Yeh RW. Frailty and related outcomes in patients undergoing transcatheter valve therapies in a nationwide cohort. Eur Heart J. 2019;40:2231–9. doi: 10.1093/eurheartj/ehz187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosler QP, Maltagliati AJ, Shi SM, Afilalo J, Popma JJ, Khabbaz KR, Laham RJ, Guibone K, Kim DH. A practical two-stage frailty assessment for older adults undergoing aortic valve replacement. J Am Geriatr Soc. 2019;67:2031–7. doi: 10.1111/jgs.16036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394:1376–86. doi: 10.1016/S0140-6736(19)31785-4. [DOI] [PubMed] [Google Scholar]
- Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–7. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- Afilalo J, Lauck S, Kim DH, Lefevre T, Piazza N, Lachapelle K, Martucci G, Lamy A, Labinaz M, Peterson MD, Arora RC, Noiseux N, Rassi A, Palacios IF, Genereux P, Lindman BR, Asgar AW, Kim CA, Trnkus A, Morais JA, Langlois Y, Rudski LG, Morin JF, Popma JJ, Webb JG, Perrault LP. Frailty in older adults undergoing aortic valve replacement: the FRAILTY-AVR study. J Am Coll Cardiol. 2017;70:689–700. doi: 10.1016/j.jacc.2017.06.024. [DOI] [PubMed] [Google Scholar]
- Goldfarb M, Lauck S, Webb JG, Asgar AW, Perrault LP, Piazza N, Martucci G, Lachapelle K, Noiseux N, Kim DH, Popma JJ, Lefevre T, Labinaz M, Lamy A, Peterson MD, Arora RC, Morais JA, Morin JF, Rudski LG, Afilalo J. Malnutrition and mortality in frail and non-frail older adults undergoing aortic valve replacement. Circulation. 2018;138:2202–11. doi: 10.1161/CIRCULATIONAHA.118.033887. [DOI] [PubMed] [Google Scholar]
- Yanagisawa R, Tanaka M, Yashima F, Arai T, Kohno T, Shimizu H, Fukuda K, Naganuma T, Mizutani K, Araki M, Tada N, Yamanaka F, Shirai S, Tabata M, Ueno H, Takagi K, Higashimori A, Watanabe Y, Yamamoto M, Hayashida K. Frequency and consequences of cognitive impairment in patients underwent transcatheter aortic valve implantation. Am J Cardiol. 2018;122:844–50. doi: 10.1016/j.amjcard.2018.05.026. [DOI] [PubMed] [Google Scholar]
- Puri R, Iung B, Cohen DJ, Rodes-Cabau J. TAVI or no TAVI: identifying patients unlikely to benefit from transcatheter aortic valve implantation. Eur Heart J. 2016;37:2217–25. doi: 10.1093/eurheartj/ehv756. [DOI] [PubMed] [Google Scholar]
- Gunter RL, Kilgo P, Guyton RA, Chen EP, Puskas JD, Cooper WA, Halkos ME, Lattouf OM, Babaliaros V, Myung R, Leshnower B, Thourani VH. Impact of preoperative chronic lung disease on survival after surgical aortic valve replacement. Ann Thorac Surg. 2013;96:1322–8. doi: 10.1016/j.athoracsur.2013.05.061. [DOI] [PubMed] [Google Scholar]
- Allende R, Webb JG, Munoz-Garcia AJ, de Jaegere, Tamburino C, Dager AE, Cheema A, Serra V, Amat-Santos I, Velianou JL, Barbanti M, Dvir D, Alonso-Briales JH, Nuis RJ, Faqiri E, Imme S, Benitez LM, Cucalon AM, Al Lawati, Garcia del, Lopez J, Natarajan MK, DeLarochelliere R, Urena M, Ribeiro HB, Dumont E, Nombela-Franco L, Rodes-Cabau J. Advanced chronic kidney disease in patients undergoing transcatheter aortic valve implantation: insights on clinical outcomes and prognostic markers from a large cohort of patients. Eur Heart J. 2014;35:2685–96. doi: 10.1093/eurheartj/ehu175. [DOI] [PubMed] [Google Scholar]
- Tirado-Conte G, Rodes-Cabau J, Rodriguez-Olivares R, Barbanti M, Lhermusier T, Amat-Santos I, Toggweiler S, Cheema AN, Munoz-Garcia AJ, Serra V, Giordana F, Veiga G, Jimenez-Quevedo P, Campelo-Parada F, Loretz L, Todaro D, Del Trigo, Hernandez-Garcia JM, Garcia Del, Bruno F, de la, Stella P, Tamburino C, Macaya C, Nombela-Franco L. Clinical outcomes and prognosis markers of patients with liver disease undergoing transcatheter aortic valve replacement: a propensity score-matched analysis. Circ Cardiovasc Interv. 2018;11:e005727. doi: 10.1161/CIRCINTERVENTIONS.117.005727. [DOI] [PubMed] [Google Scholar]
- Abramowitz Y, Kazuno Y, Chakravarty T, Kawamori H, Maeno Y, Anderson D, Allison Z, Mangat G, Cheng W, Gopal A, Jilaihawi H, Mack MJ, Makkar RR. Concomitant mitral annular calcification and severe aortic stenosis: prevalence, characteristics and outcome following transcatheter aortic valve replacement. Eur Heart J. 2017;38:1194–203. doi: 10.1093/eurheartj/ehw594. [DOI] [PubMed] [Google Scholar]
- Lindman BR, Arnold SV, Bagur R, Clarke L, Coylewright M, Evans F, Hung J, Lauck SB, Peschin S, Sachdev V, Tate LM, Wasfy JH, Otto CM. Priorities for patient-centered research in valvular heart disease: a report from the National Heart, Lung, and Blood Institute Working Group. J Am Heart Assoc. 2020;9:e015975. doi: 10.1161/JAHA.119.015975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejjaji V, Cohen DJ, Carroll JD, Li Z, Manandhar P, Vemulapalli S, Nelson AJ, Malik AO, Mack MJ, Spertus JA, Arnold SV. Practical application of patient-reported health status measures for transcatheter valve therapies: insights from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapies Registry. Circ Cardiovasc Qual Outcomes. 2021;14:e007187. doi: 10.1161/CIRCOUTCOMES.120.007187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JM, Cooper S, Kirkpatrick JN. Palliative care in end-stage valvular heart disease. Heart. 2017;103:1233–7. doi: 10.1136/heartjnl-2016-310538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis A, Gale CP, Flather M, Maniadakis N, Vardas P. Cardiovascular disease statistics from the European atlas: inequalities between high- and middle-income member countries of the ESC. Eur Heart J Qual Care Clin Outcomes. 2018;4:1–3. doi: 10.1093/ehjqcco/qcx045. [DOI] [PubMed] [Google Scholar]
- Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–88. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder, Van Putte, Watkins CL ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- Breithardt G, Baumgartner H, Berkowitz SD, Hellkamp AS, Piccini JP, Stevens SR, Lokhnygina Y, Patel MR, Halperin JL, Singer DE, Hankey GJ, Hacke W, Becker RC, Nessel CC, Mahaffey KW, Fox KA, Califf RM, Committee RAS, Investigators Clinical characteristics and outcomes with rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation but underlying native mitral and aortic valve disease participating in the ROCKET AF trial. Eur Heart J. 2014;35:3377–85. doi: 10.1093/eurheartj/ehu305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avezum A, Lopes RD, Schulte PJ, Lanas F, Gersh BJ, Hanna M, Pais P, Erol C, Diaz R, Bahit MC, Bartunek J, De Caterina, Goto S, Ruzyllo W, Zhu J, Granger CB, Alexander JH. Apixaban in comparison with warfarin in patients with atrial fibrillation and valvular heart disease: findings from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation. 2015;132:624–32. doi: 10.1161/CIRCULATIONAHA.114.014807. [DOI] [PubMed] [Google Scholar]
- Ezekowitz MD, Nagarakanti R, Noack H, Brueckmann M, Litherland C, Jacobs M, Clemens A, Reilly PA, Connolly SJ, Yusuf S, Wallentin L. Comparison of dabigatran and warfarin in patients with atrial fibrillation and valvular heart disease: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulant Therapy). Circulation. 2016;134:589–98. doi: 10.1161/CIRCULATIONAHA.115.020950. [DOI] [PubMed] [Google Scholar]
- De Caterina, Renda G, Carnicelli AP, Nordio F, Trevisan M, Mercuri MF, Ruff CT, Antman EM, Braunwald E, Giugliano RP. Valvular heart disease patients on edoxaban or warfarin in the ENGAGE AF-TIMI 48 trial. J Am Coll Cardiol. 2017;69:1372–82. doi: 10.1016/j.jacc.2016.12.031. [DOI] [PubMed] [Google Scholar]
- Rankin JS, Grau-Sepulveda MV, Ad N, Damiano RJ , Gillinov AM, Brennan JM, McCarthy PM, Thourani VH, Jacobs JP, Shahian DM, Badhwar V. Associations between surgical ablation and operative mortality after mitral valve procedures. Ann Thorac Surg. 2018;105:1790–6. doi: 10.1016/j.athoracsur.2017.12.035. [DOI] [PubMed] [Google Scholar]
- Tsai YC, Phan K, Munkholm-Larsen S, Tian DH, La Meir, Yan TD. Surgical left atrial appendage occlusion during cardiac surgery for patients with atrial fibrillation: a meta-analysis. Eur J Cardiothorac Surg. 2015;47:847–54. doi: 10.1093/ejcts/ezu291. [DOI] [PubMed] [Google Scholar]
- Martin Gutierrez, Castano M, Gualis J, Martinez-Comendador JM, Maiorano P, Castillo L, Laguna G. Beneficial effect of left atrial appendage closure during cardiac surgery: a meta-analysis of 280 585 patients. Eur J Cardiothorac Surg. 2020;57:252–62. doi: 10.1093/ejcts/ezz289. [DOI] [PubMed] [Google Scholar]
- Whitlock RP, Belley-Cote EP, Paparella D, Healey JS, Brady K, Sharma M, Reents W, Budera P, Baddour AJ, Fila P, Devereaux PJ, Bogachev-Prokophiev A, Boening A, Teoh KHT, Tagarakis GI, Slaughter MS, Royse AG, McGuinness S, Alings M, Punjabi PP, Mazer CD, Folkeringa RJ, Colli A, Avezum A, Nakamya J, Balasubramanian K, Vincent J, Voisine P, Lamy A, Yusuf S, Connolly SJ, LAAOS III. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med. 2021;384:2081–91. doi: 10.1056/NEJMoa2101897. [DOI] [PubMed] [Google Scholar]
- Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–62. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- Wang KL, Lip GY, Lin SJ, Chiang CE. Non-vitamin K antagonist oral anticoagulants for stroke prevention in Asian patients with nonvalvular atrial fibrillation: meta-analysis. Stroke. 2015;46:2555–61. doi: 10.1161/STROKEAHA.115.009947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badhwar V, Rankin JS, Ad N, Grau-Sepulveda M, Damiano RJ, Gillinov AM, McCarthy PM, Thourani VH, Suri RM, Jacobs JP, Cox JL. Surgical ablation of atrial fibrillation in the United States: trends and propensity matched outcomes. Ann Thorac Surg. 2017;104:493–500. doi: 10.1016/j.athoracsur.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Gillinov AM, Gelijns AC, Parides MK, DeRose JJ , Moskowitz AJ, Voisine P, Ailawadi G, Bouchard D, Smith PK, Mack MJ, Acker MA, Mullen JC, Rose EA, Chang HL, Puskas JD, Couderc JP, Gardner TJ, Varghese R, Horvath KA, Bolling SF, Michler RE, Geller NL, Ascheim DD, Miller MA, Bagiella E, Moquete EG, Williams P, Taddei-Peters WC, O’Gara PT, Blackstone EH, Argenziano M, Investigators C. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med. 2015;372:1399–409. doi: 10.1056/NEJMoa1500528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman MD, Karmali KN, Berendsen MA, Andrei AC, Kruse J, McCarthy PM, Malaisrie SC. Concomitant atrial fibrillation surgery for people undergoing cardiac surgery. Cochrane Database Syst Rev. 2016:D011814. doi: 10.1002/14651858.CD011814.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Han J, Wang Z, Yin Z, Liu Z, Jin Y, Han H. A prospective randomized trial of the cut-and-sew Maze procedure in patients undergoing surgery for rheumatic mitral valve disease. J Thorac Cardiovasc Surg. 2018;155:608–17. doi: 10.1016/j.jtcvs.2017.07.084. [DOI] [PubMed] [Google Scholar]
- Lawrance CP, Henn MC, Miller JR, Sinn LA, Schuessler RB, Maniar HS, Damiano RJ, Jr A minimally invasive Cox maze IV procedure is as effective as sternotomy while decreasing major morbidity and hospital stay. J Thorac Cardiovasc Surg. 2014;148:955–61. doi: 10.1016/j.jtcvs.2014.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimar T, Schena S, Bailey MS, Maniar HS, Schuessler RB, Cox JL, Damiano RJ, Jr The Cox-Maze procedure for lone atrial fibrillation: a single-center experience over 2 decades. Circ Arrhythm Electrophysiol. 2012;5:8–14. doi: 10.1161/CIRCEP.111.963819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Uranw S, Bastola S, Mahato R, Shrestha NR, Sherpa K, Dhungana S, Odutayo A, Gurung K, Pandey N, Agrawal K, Shah P, Rothenbuhler M, Juni P, Pilgrim T. Effectiveness of systematic echocardiographic screening for rheumatic heart disease in Nepalese schoolchildren: a cluster randomized clinical trial. JAMA Cardiol. 2021;6:420–6. doi: 10.1001/jamacardio.2020.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber MA, Baltimore RS, Eaton CB, Gewitz M, Rowley AH, Shulman ST, Taubert KA. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2009;119:1541–51. doi: 10.1161/CIRCULATIONAHA.109.191959. [DOI] [PubMed] [Google Scholar]
- Remenyi B, Carapetis J, Wyber R, Taubert K, Mayosi BM, World Heart. Position statement of the World Heart Federation on the prevention and control of rheumatic heart disease. Nat Rev Cardiol. 2013;10:284–92. doi: 10.1038/nrcardio.2013.34. [DOI] [PubMed] [Google Scholar]
- Watkins DA, Beaton AZ, Carapetis JR, Karthikeyan G, Mayosi BM, Wyber R, Yacoub MH, Zuhlke LJ. Rheumatic heart disease worldwide: JACC Scientific Expert Panel. J Am Coll Cardiol. 2018;72:1397–416. doi: 10.1016/j.jacc.2018.06.063. [DOI] [PubMed] [Google Scholar]
- Kumar RK, Antunes MJ, Beaton A, Mirabel M, Nkomo VT, Okello E, Regmi PR, Remenyi B, Sliwa-Hahnle K, Zuhlke LJ, Sable C, American Heart Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Contemporary diagnosis and management of rheumatic heart disease: implications for closing the gap: a scientific statement from the American Heart Association. Circulation. 2020;142:e337–57. doi: 10.1161/CIR.0000000000000921. [DOI] [PubMed] [Google Scholar]
- le Polain, Pouleur AC, Goffinet C, Vancraeynest D, Van Dyck, Robert A, Gerber BL, Pasquet A, El Khoury, Vanoverschelde JL. Functional anatomy of aortic regurgitation: accuracy, prediction of surgical repairability, and outcome implications of transesophageal echocardiography. Circulation. 2007;116:I264–269. doi: 10.1161/CIRCULATIONAHA.106.680074. [DOI] [PubMed] [Google Scholar]
- Lansac E, Di Centa, Raoux F, Al Attar, Acar C, Joudinaud T, Raffoul R. A lesional classification to standardize surgical management of aortic insufficiency towards valve repair. Eur J Cardiothorac Surg. 2008;33:872–8. doi: 10.1016/j.ejcts.2007.12.033. [DOI] [PubMed] [Google Scholar]
- Lancellotti P, Tribouilloy C, Hagendorff A, Moura L, Popescu BA, Agricola E, Monin JL, Pierard LA, Badano L, Zamorano JL, European Association. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 1: aortic and pulmonary regurgitation (native valve disease). Eur J Echocardiogr. 2010;11:223–44. doi: 10.1093/ejechocard/jeq030. [DOI] [PubMed] [Google Scholar]
- Alashi A, Khullar T, Mentias A, Gillinov AM, Roselli EE, Svensson LG, Popovic ZB, Griffin BP, Desai MY. Long-term outcomes after aortic valve surgery in patients with asymptomatic chronic aortic regurgitation and preserved LVEF: impact of baseline and follow-up global longitudinal strain. JACC Cardiovasc Imaging. 2020;13:12–21. doi: 10.1016/j.jcmg.2018.12.021. [DOI] [PubMed] [Google Scholar]
- Goldstein SA, Evangelista A, Abbara S, Arai A, Asch FM, Badano LP, Bolen MA, Connolly HM, Cuellar-Calabria H, Czerny M, Devereux RB, Erbel RA, Fattori R, Isselbacher EM, Lindsay JM, McCulloch M, Michelena HI, Nienaber CA, Oh JK, Pepi M, Taylor AJ, Weinsaft JW, Zamorano JL, Dietz H, Eagle K, Elefteriades J, Jondeau G, Rousseau H, Schepens M. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging: endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2015;28:119–82. doi: 10.1016/j.echo.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwöger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RS, Vrints CJ ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–70. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- Freeman LA, Young PM, Foley TA, Williamson EE, Bruce CJ, Greason KL. CT and MRI assessment of the aortic root and ascending aorta. AJR Am J Roentgenol. 2013;200:W581–592. doi: 10.2214/AJR.12.9531. [DOI] [PubMed] [Google Scholar]
- Amsallem M, Ou P, Milleron O, Henry-Feugeas MC, Detaint D, Arnoult F, Vahanian A, Jondeau G. Comparative assessment of ascending aortic aneurysms in Marfan patients using ECG-gated computerized tomographic angiography versus trans-thoracic echocardiography. Int J Cardiol. 2015;184:22–7. doi: 10.1016/j.ijcard.2015.01.086. [DOI] [PubMed] [Google Scholar]
- Chaliki HP, Mohty D, Avierinos JF, Scott CG, Schaff HV, Tajik AJ, Enriquez-Sarano M. Outcomes after aortic valve replacement in patients with severe aortic regurgitation and markedly reduced left ventricular function. Circulation. 2002;106:2687–93. doi: 10.1161/01.cir.0000038498.59829.38. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Ejiofor JI, Neely RC, McGurk S, Ivkovic V, Stevenson LW, Leacche M, Cohn LH. Aortic regurgitation with markedly reduced left ventricular function is not a contraindication for aortic valve replacement. Ann Thorac Surg. 2016;102:41–7. doi: 10.1016/j.athoracsur.2015.12.068. [DOI] [PubMed] [Google Scholar]
- Tornos P, Sambola A, Permanyer-Miralda G, Evangelista A, Gomez Z, Soler-Soler J. Long-term outcome of surgically treated aortic regurgitation: influence of guideline adherence toward early surgery. J Am Coll Cardiol. 2006;47:1012–7. doi: 10.1016/j.jacc.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Dujardin KS, Enriquez-Sarano M, Schaff HV, Bailey KR, Seward JB, Tajik AJ. Mortality and morbidity of aortic regurgitation in clinical practice. A long-term follow-up study. Circulation. 1999;99:1851–7. doi: 10.1161/01.cir.99.14.1851. [DOI] [PubMed] [Google Scholar]
- Klodas E, Enriquez-Sarano M, Tajik AJ, Mullany CJ, Bailey KR, Seward JB. Optimizing timing of surgical correction in patients with severe aortic regurgitation: role of symptoms. J Am Coll Cardiol. 1997;30:746–52. doi: 10.1016/s0735-1097(97)00205-2. [DOI] [PubMed] [Google Scholar]
- Tribouilloy CM, Enriquez-Sarano M, Schaff HV, Orszulak TA, Fett SL, Bailey KR, Tajik AJ, Frye RL. Excess mortality due to coronary artery disease after valve surgery. Secular trends in valvular regurgitation and effect of internal mammary artery bypass. Circulation. 1998;98:II108–115. [PubMed] [Google Scholar]
- Fiedler AG, Bhambhani V, Laikhter E, Picard MH, Wasfy MM, Tolis G, Melnitchouk S, Sundt TM, Wasfy JH. Aortic valve replacement associated with survival in severe regurgitation and low ejection fraction. Heart. 2018;104:835–40. doi: 10.1136/heartjnl-2017-312024. [DOI] [PubMed] [Google Scholar]
- Forman R, Firth BG, Barnard MS. Prognostic significance of preoperative left ventricular ejection fraction and valve lesion in patients with aortic valve replacement. Am J Cardiol. 1980;45:1120–5. doi: 10.1016/0002-9149(80)90468-3. [DOI] [PubMed] [Google Scholar]
- Bonow RO, Lakatos E, Maron BJ, Epstein SE. Serial long-term assessment of the natural history of asymptomatic patients with chronic aortic regurgitation and normal left ventricular systolic function. Circulation. 1991;84:1625–35. doi: 10.1161/01.cir.84.4.1625. [DOI] [PubMed] [Google Scholar]
- Bhudia SK, McCarthy PM, Kumpati GS, Helou J, Hoercher KJ, Rajeswaran J, Blackstone EH. Improved outcomes after aortic valve surgery for chronic aortic regurgitation with severe left ventricular dysfunction. J Am Coll Cardiol. 2007;49:1465–71. doi: 10.1016/j.jacc.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Sambola A, Tornos P, Ferreira-Gonzalez I, Evangelista A. Prognostic value of preoperative indexed end-systolic left ventricle diameter in the outcome after surgery in patients with chronic aortic regurgitation. Am Heart J. 2008;155:1114–20. doi: 10.1016/j.ahj.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Yang LT, Michelena HI, Scott CG, Enriquez-Sarano M, Pislaru SV, Schaff HV, Pellikka PA. Outcomes in chronic hemodynamically significant aortic regurgitation and limitations of current guidelines. J Am Coll Cardiol. 2019;73:1741–52. doi: 10.1016/j.jacc.2019.01.024. [DOI] [PubMed] [Google Scholar]
- Mentias A, Feng K, Alashi A, Rodriguez LL, Gillinov AM, Johnston DR, Sabik JF, Svensson LG, Grimm RA, Griffin BP, Desai MY. Long-term outcomes in patients with aortic regurgitation and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2016;68:2144–53. doi: 10.1016/j.jacc.2016.08.045. [DOI] [PubMed] [Google Scholar]
- de Meester, Gerber BL, Vancraeynest D, Pouleur AC, Noirhomme P, Pasquet A, de Kerchove, El Khoury, Vanoverschelde JL. Do guideline-based indications result in an outcome penalty for patients with severe aortic regurgitation? JACC Cardiovasc Imaging. 2019;12:2126–38. doi: 10.1016/j.jcmg.2018.11.022. [DOI] [PubMed] [Google Scholar]
- Sawaya FJ, Deutsch MA, Seiffert M, Yoon SH, Codner P, Wickramarachchi U, Latib A, Petronio AS, Rodes-Cabau J, Taramasso M, Spaziano M, Bosmans J, Biasco L, Mylotte D, Savontaus M, Gheeraert P, Chan J, Jorgensen TH, Sievert H, Mocetti M, Lefevre T, Maisano F, Mangieri A, Hildick-Smith D, Kornowski R, Makkar R, Bleiziffer S, Sondergaard L, De Backer. Safety and efficacy of transcatheter aortic valve replacement in the treatment of pure aortic regurgitation in native valves and failing surgical bioprostheses: results from an International Registry Study. JACC Cardiovasc Interv. 2017;10:1048–56. doi: 10.1016/j.jcin.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Schmidt T, Bleiziffer S, Schofer N, Fiorina C, Munoz-Garcia AJ, Yzeiraj E, Amat-Santos IJ, Tchetche D, Jung C, Fujita B, Mangieri A, Deutsch MA, Ubben T, Deuschl F, Kuwata S, De Biase, Williams T, Dhoble A, Kim WK, Ferrari E, Barbanti M, Vollema EM, Miceli A, Giannini C, Attizzani GF, Kong WKF, Gutierrez-Ibanes E, Jimenez Diaz, Wijeysundera HC, Kaneko H, Chakravarty T, Makar M, Sievert H, Hengstenberg C, Prendergast BD, Vincent F, Abdel-Wahab M, Nombela-Franco L, Silaschi M, Tarantini G, Butter C, Ensminger SM, Hildick-Smith D, Petronio AS, Yin WH, De Marco, Testa L, Van Mieghem, Whisenant BK, Kuck KH, Colombo A, Kar S, Moris C, Delgado V, Maisano F, Nietlispach F, Mack MJ, Schofer J, Schaefer U, Bax JJ, Frerker C, Latib A, Makkar RR. Transcatheter aortic valve replacement in pure native aortic valve regurgitation. J Am Coll Cardiol. 2017;70:2752–63. doi: 10.1016/j.jacc.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Jondeau G, Detaint D, Tubach F, Arnoult F, Milleron O, Raoux F, Delorme G, Mimoun L, Krapf L, Hamroun D, Beroud C, Roy C, Vahanian A, Boileau C. Aortic event rate in the Marfan population: a cohort study. Circulation. 2012;125:226–32. doi: 10.1161/CIRCULATIONAHA.111.054676. [DOI] [PubMed] [Google Scholar]
- Desai MY, Kalahasti V, Hutt Centeno, Chen K, Alashi A, Rivas CG, Roselli EE, Johnston DR, Griffin BP, Svensson LG. Adult patients with Marfan syndrome and ascending aortic surgery. J Am Coll Cardiol. 2019;73:733–4. doi: 10.1016/j.jacc.2018.12.010. [DOI] [PubMed] [Google Scholar]
- Borger MA, Preston M, Ivanov J, Fedak PW, Davierwala P, Armstrong S, David TE. Should the ascending aorta be replaced more frequently in patients with bicuspid aortic valve disease? J Thorac Cardiovasc Surg. 2004;128:677–83. doi: 10.1016/j.jtcvs.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Coady MA, Rizzo JA, Hammond GL, Mandapati D, Darr U, Kopf GS, Elefteriades JA. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg. 1997;113:476–91. doi: 10.1016/S0022-5223(97)70360-X. [DOI] [PubMed] [Google Scholar]
- Davies RR, Goldstein LJ, Coady MA, Tittle SL, Rizzo JA, Kopf GS, Elefteriades JA. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17–27. doi: 10.1016/s0003-4975(01)03236-2. [DOI] [PubMed] [Google Scholar]
- Oliver JM, Alonso-Gonzalez R, Gonzalez AE, Gallego P, Sanchez-Recalde A, Cuesta E, Aroca A, Lopez-Sendon JL. Risk of aortic root or ascending aorta complications in patients with bicuspid aortic valve with and without coarctation of the aorta. Am J Cardiol. 2009;104:1001–6. doi: 10.1016/j.amjcard.2009.05.045. [DOI] [PubMed] [Google Scholar]
- Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM, Eidem B, Edwards WD, Sundt TM, Enriquez-Sarano M. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–12. doi: 10.1001/jama.2011.1286. [DOI] [PubMed] [Google Scholar]
- Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT, Webb GD, Siu SC. Outcomes in adults with bicuspid aortic valves. JAMA. 2008;300:1317–25. doi: 10.1001/jama.300.11.1317. [DOI] [PubMed] [Google Scholar]
- Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74:S1877–1880. doi: 10.1016/s0003-4975(02)04147-4. [DOI] [PubMed] [Google Scholar]
- Jondeau G, Ropers J, Regalado E, Braverman A, Evangelista A, Teixedo G, De Backer, Muino-Mosquera L, Naudion S, Zordan C, Morisaki T, Morisaki H, Von Kodolitsch, Dupuis-Girod S, Morris SA, Jeremy R, Odent S, Ades LC, Bakshi M, Holman K, LeMaire S, Milleron O, Langeois M, Spentchian M, Aubart M, Boileau C, Pyeritz R, Milewicz DM, Montalcino Aortic. International Registry of patients carrying TGFBR1 or TGFBR2 mutations: results of the MAC (Montalcino Aortic Consortium ). Circ Cardiovasc Genet. 2016;9:548–58. doi: 10.1161/CIRCGENETICS.116.001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher D, Fries R, Rodionycheva S, Schmidt K, Langer F, Schafers HJ. Aortic valve repair leads to a low incidence of valve-related complications. Eur J Cardiothorac Surg. 2010;37:127–32. doi: 10.1016/j.ejcts.2009.06.021. [DOI] [PubMed] [Google Scholar]
- de Meester, Pasquet A, Gerber BL, Vancraeynest D, Noirhomme P, El Khoury, Vanoverschelde JL. Valve repair improves the outcome of surgery for chronic severe aortic regurgitation: a propensity score analysis. J Thorac Cardiovasc Surg. 2014;148:1913–20. doi: 10.1016/j.jtcvs.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Klotz S, Stock S, Sievers HH, Diwoky M, Petersen M, Stierle U, Richardt D. Survival and reoperation pattern after 20 years of experience with aortic valve-sparing root replacement in patients with tricuspid and bicuspid valves. J Thorac Cardiovasc Surg. 2018;155:1403–1411 e1401. doi: 10.1016/j.jtcvs.2017.12.039. [DOI] [PubMed] [Google Scholar]
- Elbatarny M, Tam DY, Edelman JJ, Rocha RV, Chu MWA, Peterson MD, El-Hamamsy I, Appoo JJ, Friedrich JO, Boodhwani M, Yanagawa B, Ouzounian M, Canadian Thoracic. Valve-sparing root replacement versus composite valve grafting in aortic root dilation: a meta-analysis. Ann Thorac Surg. 2020;110:296–306. doi: 10.1016/j.athoracsur.2019.11.054. [DOI] [PubMed] [Google Scholar]
- Leontyev S, Schamberger L, Davierwala PM, Von Aspern, Etz C, Lehmann S, Misfeld M, Borger MA. Early and late results after David vs Bentall procedure: a propensity matched analysis. Ann Thorac Surg. 2020;110:120–6. doi: 10.1016/j.athoracsur.2019.10.020. [DOI] [PubMed] [Google Scholar]
- Mastrobuoni S, de Kerchove, Navarra E, Watremez C, Vancraeynest D, Rubay J, Noirhomme P, El Khoury. Long-term experience with valve-sparing reimplantation technique for the treatment of aortic aneurysm and aortic regurgitation. J Thorac Cardiovasc Surg. 2019;158:14–23. doi: 10.1016/j.jtcvs.2018.10.155. [DOI] [PubMed] [Google Scholar]
- Lansac E, Di Centa, Sleilaty G, Lejeune S, Khelil N, Berrebi A, Diakov C, Mankoubi L, Malergue MC, Noghin M, Zannis K, Salvi S, Dervanian P, Debauchez M. Long-term results of external aortic ring annuloplasty for aortic valve repair. Eur J Cardiothorac Surg. 2016;50:350–60. doi: 10.1093/ejcts/ezw070. [DOI] [PubMed] [Google Scholar]
- Mazine A, Rocha RV, El-Hamamsy I, Ouzounian M, Yanagawa B, Bhatt DL, Verma S, Friedrich JO. Ross procedure vs mechanical aortic valve replacement in adults: a systematic review and meta-analysis. JAMA Cardiol. 2018;3:978–87. doi: 10.1001/jamacardio.2018.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takkenberg JJ, Klieverik LM, Schoof PH, van Suylen, van Herwerden, Zondervan PE, Roos-Hesselink JW, Eijkemans MJ, Yacoub MH, Bogers AJ. The Ross procedure: a systematic review and meta-analysis. Circulation. 2009;119:222–8. doi: 10.1161/CIRCULATIONAHA.107.726349. [DOI] [PubMed] [Google Scholar]
- Lee H, Cho YH, Sung K, Kim WS, Park KH, Jeong DS, Park PW, Lee YT. Clinical outcomes of root reimplantation and Bentall procedure: propensity score matching analysis. Ann Thorac Surg. 2018;106:539–47. doi: 10.1016/j.athoracsur.2018.02.057. [DOI] [PubMed] [Google Scholar]
- Elder DH, Wei L, Szwejkowski BR, Libianto R, Nadir A, Pauriah M, Rekhraj S, Lim TK, George J, Doney A, Pringle SD, Choy AM, Struthers AD, Lang CC. The impact of renin-angiotensin-aldosterone system blockade on heart failure outcomes and mortality in patients identified to have aortic regurgitation: a large population cohort study. J Am Coll Cardiol. 2011;58:2084–91. doi: 10.1016/j.jacc.2011.07.043. [DOI] [PubMed] [Google Scholar]
- Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, de Boer, Drexel H, Ben Gal, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJS. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:1169–86. doi: 10.1002/ejhf.1531. [DOI] [PubMed] [Google Scholar]
- Lacro RV, Dietz HC, Sleeper LA, Yetman AT, Bradley TJ, Colan SD, Pearson GD, Selamet Tierney, Levine JC, Atz AM, Benson DW, Braverman AC, Chen S, De Backer, Gelb BD, Grossfeld PD, Klein GL, Lai WW, Liou A, Loeys BL, Markham LW, Olson AK, Paridon SM, Pemberton VL, Pierpont ME, Pyeritz RE, Radojewski E, Roman MJ, Sharkey AM, Stylianou MP, Wechsler SB, Young LT, Mahony L, Pediatric Heart. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med. 2014;371:2061–71. doi: 10.1056/NEJMoa1404731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forteza A, Evangelista A, Sanchez V, Teixido-Tura G, Sanz P, Gutierrez L, Gracia T, Centeno J, Rodriguez-Palomares J, Rufilanchas JJ, Cortina J, Ferreira-Gonzalez I, Garcia-Dorado D. Efficacy of losartan vs. atenolol for the prevention of aortic dilation in Marfan syndrome: a randomized clinical trial. Eur Heart J. 2016;37:978–85. doi: 10.1093/eurheartj/ehv575. [DOI] [PubMed] [Google Scholar]
- Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med. 2008;358:2787–95. doi: 10.1056/NEJMoa0706585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milleron O, Arnoult F, Ropers J, Aegerter P, Detaint D, Delorme G, Attias D, Tubach F, Dupuis-Girod S, Plauchu H, Barthelet M, Sassolas F, Pangaud N, Naudion S, Thomas-Chabaneix J, Dulac Y, Edouard T, Wolf JE, Faivre L, Odent S, Basquin A, Habib G, Collignon P, Boileau C, Jondeau G. Marfan Sartan: a randomized, double-blind, placebo-controlled trial. Eur Heart J. 2015;36:2160–6. doi: 10.1093/eurheartj/ehv151. [DOI] [PubMed] [Google Scholar]
- Groenink M, den Hartog, Franken R, Radonic T, de Waard, Timmermans J, Scholte AJ, van den, Spijkerboer AM, Marquering HA, Zwinderman AH, Mulder BJ. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J. 2013;34:3491–500. doi: 10.1093/eurheartj/eht334. [DOI] [PubMed] [Google Scholar]
- Mullen M, Jin XY, Child A, Stuart AG, Dodd M, Aragon-Martin JA, Gaze D, Kiotsekoglou A, Yuan L, Hu J, Foley C, Van Dyck, Knight R, Clayton T, Swan L, Thomson JDR, Erdem G, Crossman D, Flather M, AIMS Investigators. Irbesartan in Marfan syndrome (AIMS): a double-blind, placebo-controlled randomised trial. Lancet. 2020;394:2263–70. doi: 10.1016/S0140-6736(19)32518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro R, Bazzino OO, Oberti PF, Falconi ML, Arias AM, Krauss JG, Cagide AM. Prospective validation of the prognostic usefulness of B-type natriuretic peptide in asymptomatic patients with chronic severe aortic regurgitation. J Am Coll Cardiol. 2011;58:1705–14. doi: 10.1016/j.jacc.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Weisenberg D, Omelchenko A, Shapira Y, Vaturi M, Monakier D, Bental T, Sagie A. Mid-term echocardiographic progression of patients with moderate aortic regurgitation: implications for aortic valve surgery. J Heart Valve Dis. 2013;22:192–4. [PubMed] [Google Scholar]
- Budts W, Pieles GE, Roos-Hesselink JW, Sanz de, D’Ascenzi F, Giannakoulas G, Muller J, Oberhoffer R, Ehringer-Schetitska D, Herceg-Cavrak V, Gabriel H, Corrado D, van Buuren, Niebauer J, Borjesson M, Caselli S, Fritsch P, Pelliccia A, Heidbuchel H, Sharma S, Stuart AG, Papadakis M. Recommendations for participation in competitive sport in adolescent and adult athletes with Congenital Heart Disease (CHD): position statement of the Sports Cardiology & Exercise Section of the European Association of Preventive Cardiology (EAPC), the European Society of Cardiology (ESC) Working Group on Adult Congenital Heart Disease and the Sports Cardiology, Physical Activity and Prevention Working Group of the Association for European Paediatric and Congenital Cardiology (AEPC ). Eur Heart J. 2020;41:4191–9. doi: 10.1093/eurheartj/ehaa501. [DOI] [PubMed] [Google Scholar]
- d’Arcy JL, Coffey S, Loudon MA, Kennedy A, Pearson-Stuttard J, Birks J, Frangou E, Farmer AJ, Mant D, Wilson J, Myerson SG, Prendergast BD. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J. 2016;37:3515–22. doi: 10.1093/eurheartj/ehw229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prihadi EA, Vollema EM, Ng ACT, Ajmone Marsan, Bax JJ, Delgado V. Determinants and prognostic implications of left ventricular mechanical dispersion in aortic stenosis. Eur Heart J Cardiovasc Imaging. 2019;20:740–8. doi: 10.1093/ehjci/jez004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilardi F, Marchetta S, Martinez C, Sprynger M, Ancion A, Manganaro R, Sugimoto T, Tsugu T, Postolache A, Piette C, Cicenia M, Esposito G, Galderisi M, Oury C, Dulgheru R, Lancellotti P. Impact of aortic stenosis on layer-specific longitudinal strain: relationship with symptoms and outcome. Eur Heart J Cardiovasc Imaging. 2020;21:408–16. doi: 10.1093/ehjci/jez215. [DOI] [PubMed] [Google Scholar]
- Rusinaru D, Bohbot Y, Djelaili F, Delpierre Q, Altes A, Serbout S, Kubala M, Marechaux S, Tribouilloy C. Normative reference values of cardiac output by pulsed-wave Doppler echocardiography in adults. Am J Cardiol. 2021;140:128–33. doi: 10.1016/j.amjcard.2020.10.046. [DOI] [PubMed] [Google Scholar]
- Rusinaru D, Malaquin D, Marechaux S, Debry N, Tribouilloy C. Relation of dimensionless index to long-term outcome in aortic stenosis with preserved LVEF. JACC Cardiovasc Imaging. 2015;8:766–75. doi: 10.1016/j.jcmg.2015.01.023. [DOI] [PubMed] [Google Scholar]
- Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–64. doi: 10.1161/CIRCULATIONAHA.106.668681. [DOI] [PubMed] [Google Scholar]
- Rusinaru D, Bohbot Y, Ringle A, Marechaux S, Diouf M, Tribouilloy C. Impact of low stroke volume on mortality in patients with severe aortic stenosis and preserved left ventricular ejection fraction. Eur Heart J. 2018;39:1992–9. doi: 10.1093/eurheartj/ehy123. [DOI] [PubMed] [Google Scholar]
- Guzzetti E, Poulin A, Annabi MS, Zhang B, Kalavrouziotis D, Couture C, Dagenais F, Pibarot P, Clavel MA. Transvalvular flow, sex, and survival after valve replacement surgery in patients with severe aortic stenosis. J Am Coll Cardiol. 2020;75:1897–1909. doi: 10.1016/j.jacc.2020.02.065. [DOI] [PubMed] [Google Scholar]
- Annabi MS, Touboul E, Dahou A, Burwash IG, Bergler-Klein J, Enriquez-Sarano M, Orwat S, Baumgartner H, Mascherbauer J, Mundigler G, Cavalcante JL, Larose E, Pibarot P, Clavel MA. Dobutamine stress echocardiography for management of low-flow, low-gradient aortic stenosis. J Am Coll Cardiol. 2018;71:475–85. doi: 10.1016/j.jacc.2017.11.052. [DOI] [PubMed] [Google Scholar]
- Ribeiro HB, Lerakis S, Gilard M, Cavalcante JL, Makkar R, Herrmann HC, Windecker S, Enriquez-Sarano M, Cheema AN, Nombela-Franco L, Amat-Santos I, Munoz-Garcia AJ, Garcia Del, Zajarias A, Lisko JC, Hayek S, Babaliaros V, Le Ven, Gleason TG, Chakravarty T, Szeto WY, Clavel MA, de Agustin, Serra V, Schindler JT, Dahou A, Puri R, Pelletier-Beaumont E, Cöté M, Pibarot P, Rodés-Cabau J. Transcatheter Aortic Valve Replacement in Patients With Low-Flow, Low-Gradient Aortic Stenosis: The TOPAS-TAVI Registry. J Am Coll Cardiol. 2018;71:1297–308. doi: 10.1016/j.jacc.2018.01.054. [DOI] [PubMed] [Google Scholar]
- Clavel MA, Dumesnil JG, Capoulade R, Mathieu P, Senechal M, Pibarot P. Outcome of patients with aortic stenosis, small valve area, and low-flow, low-gradient despite preserved left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1259–67. doi: 10.1016/j.jacc.2011.12.054. [DOI] [PubMed] [Google Scholar]
- Clavel MA, Pibarot P, Messika-Zeitoun D, Capoulade R, Malouf J, Aggarval S, Araoz PA, Michelena HI, Cueff C, Larose E, Miller JD, Vahanian A, Enriquez-Sarano M. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol. 2014;64:1202–13. doi: 10.1016/j.jacc.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawade T, Clavel MA, Tribouilloy C, Dreyfus J, Mathieu T, Tastet L, Renard C, Gun M, Jenkins WSA, Macron L, Sechrist JW, Lacomis JM, Nguyen V, Galian Gay, Cuellar Calabria, Ntalas I, Cartlidge TRG, Prendergast B, Rajani R, Evangelista A, Cavalcante JL, Newby DE, Pibarot P, Messika Zeitoun, Dweck MR. Computed tomography aortic valve calcium scoring in patients with aortic stenosis. Circ Cardiovasc Imaging. 2018;11:e007146. doi: 10.1161/CIRCIMAGING.117.007146. [DOI] [PubMed] [Google Scholar]
- Mehrotra P, Jansen K, Flynn AW, Tan TC, Elmariah S, Picard MH, Hung J. Differential left ventricular remodelling and longitudinal function distinguishes low flow from normal-flow preserved ejection fraction low-gradient severe aortic stenosis. Eur Heart J. 2013;34:1906–14. doi: 10.1093/eurheartj/eht094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribouilloy C, Rusinaru D, Marechaux S, Castel AL, Debry N, Maizel J, Mentaverri R, Kamel S, Slama M, Levy F. Low-gradient, low-flow severe aortic stenosis with preserved left ventricular ejection fraction: characteristics, outcome, and implications for surgery. J Am Coll Cardiol. 2015;65:55–66. doi: 10.1016/j.jacc.2014.09.080. [DOI] [PubMed] [Google Scholar]
- Jander N, Minners J, Holme I, Gerdts E, Boman K, Brudi P, Chambers JB, Egstrup K, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Rossebo A, Pedersen TR, Skjaerpe T, Willenheimer R, Wachtell K, Neumann FJ, Gohlke-Barwolf C. Outcome of patients with low-gradient “severe” aortic stenosis and preserved ejection fraction. Circulation. 2011;123:887–95. doi: 10.1161/CIRCULATIONAHA.110.983510. [DOI] [PubMed] [Google Scholar]
- Vollema EM, Sugimoto T, Shen M, Tastet L, Ng ACT, Abou R, Marsan NA, Mertens B, Dulgheru R, Lancellotti P, Clavel MA, Pibarot P, Genereux P, Leon MB, Delgado V, Bax JJ. Association of left ventricular global longitudinal strain with asymptomatic severe aortic stenosis: natural course and prognostic value. JAMA Cardiol. 2018;3:839–47. doi: 10.1001/jamacardio.2018.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamorano JL, Badano LP, Bruce C, Chan KL, Goncalves A, Hahn RT, Keane MG, La Canna, Monaghan MJ, Nihoyannopoulos P, Silvestry FE, Vanoverschelde JL, Gillam LD. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. Eur Heart J. 2011;32:2189–214. doi: 10.1093/eurheartj/ehr259. [DOI] [PubMed] [Google Scholar]
- Bergler-Klein J, Klaar U, Heger M, Rosenhek R, Mundigler G, Gabriel H, Binder T, Pacher R, Maurer G, Baumgartner H. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation. 2004;109:2302–8. doi: 10.1161/01.CIR.0000126825.50903.18. [DOI] [PubMed] [Google Scholar]
- Clavel MA, Malouf J, Michelena HI, Suri RM, Jaffe AS, Mahoney DW, Enriquez-Sarano M. B-type natriuretic peptide clinical activation in aortic stenosis: impact on long-term survival. J Am Coll Cardiol. 2014;63:2016–25. doi: 10.1016/j.jacc.2014.02.581. [DOI] [PubMed] [Google Scholar]
- Rafique AM, Biner S, Ray I, Forrester JS, Tolstrup K, Siegel RJ. Meta-analysis of prognostic value of stress testing in patients with asymptomatic severe aortic stenosis. Am J Cardiol. 2009;104:972–7. doi: 10.1016/j.amjcard.2009.05.044. [DOI] [PubMed] [Google Scholar]
- Marechaux S, Hachicha Z, Bellouin A, Dumesnil JG, Meimoun P, Pasquet A, Bergeron S, Arsenault M, Le Tourneau, Ennezat PV, Pibarot P. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J. 2010;31:1390–7. doi: 10.1093/eurheartj/ehq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawade T, Sheth T, Guzzetti E, Dweck MR, Clavel MA. Why and how to measure aortic valve calcification in patients with aortic stenosis. JACC Cardiovasc Imaging. 2019;12:1835–48. doi: 10.1016/j.jcmg.2019.01.045. [DOI] [PubMed] [Google Scholar]
- Nitsche C, Scully PR, Patel KP, Kammerlander AA, Koschutnik M, Dona C, Wollenweber T, Ahmed N, Thornton GD, Kelion AD, Sabharwal N, Newton JD, Ozkor M, Kennon S, Mullen M, Lloyd G, Fontana M, Hawkins PN, Pugliese F, Menezes LJ, Moon JC, Mascherbauer J, Treibel TA. Prevalence and outcomes of concomitant aortic stenosis and cardiac amyloidosis. J Am Coll Cardiol. 2021;77:128–39. doi: 10.1016/j.jacc.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treibel TA, Lopez B, Gonzalez A, Menacho K, Schofield RS, Ravassa S, Fontana M, White SK, DiSalvo C, Roberts N, Ashworth MT, Diez J, Moon JC. Reappraising myocardial fibrosis in severe aortic stenosis: an invasive and non-invasive study in 133 patients. Eur Heart J. 2018;39:699–709. doi: 10.1093/eurheartj/ehx353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett RJ, Tastet L, Clavel MA, Chin CWL, Capoulade R, Vassiliou VS, Kwiecinski J, Gomez M, van Beek, White AC, Prasad SK, Larose E, Tuck C, Semple S, Newby DE, Pibarot P, Dweck MR. Progression of hypertrophy and myocardial fibrosis in aortic stenosis: a multicenter cardiac magnetic resonance study. Circ Cardiovasc Imaging. 2018;11:e007451. doi: 10.1161/CIRCIMAGING.117.007451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa TA, Treibel TA, Vassiliou VS, Captur G, Singh A, Chin C, Dobson LE, Pica S, Loudon M, Malley T, Rigolli M, Foley JRJ, Bijsterveld P, Law GR, Dweck MR, Myerson SG, McCann GP, Prasad SK, Moon JC, Greenwood JP. Myocardial scar and mortality in severe aortic stenosis. Circulation. 2018;138:1935–47. doi: 10.1161/CIRCULATIONAHA.117.032839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternacle J, Krapf L, Mohty D, Magne J, Nguyen A, Galat A, Gallet R, Teiger E, Cote N, Clavel MA, Tournoux F, Pibarot P, Damy T. Aortic stenosis and cardiac amyloidosis: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74:2638–51. doi: 10.1016/j.jacc.2019.09.056. [DOI] [PubMed] [Google Scholar]
- Tribouilloy C, Levy F, Rusinaru D, Gueret P, Petit-Eisenmann H, Baleynaud S, Jobic Y, Adams C, Lelong B, Pasquet A, Chauvel C, Metz D, Quere JP, Monin JL. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol. 2009;53:1865–73. doi: 10.1016/j.jacc.2009.02.026. [DOI] [PubMed] [Google Scholar]
- Fougeres E, Tribouilloy C, Monchi M, Petit-Eisenmann H, Baleynaud S, Pasquet A, Chauvel C, Metz D, Adams C, Rusinaru D, Gueret P, Monin JL. Outcomes of pseudo-severe aortic stenosis under conservative treatment. Eur Heart J. 2012;33:2426–33. doi: 10.1093/eurheartj/ehs176. [DOI] [PubMed] [Google Scholar]
- Levy F, Laurent M, Monin JL, Maillet JM, Pasquet A, Le Tourneau, Petit-Eisenmann H, Gori M, Jobic Y, Bauer F, Chauvel C, Leguerrier A, Tribouilloy C. Aortic valve replacement for low-flow/low-gradient aortic stenosis operative risk stratification and long-term outcome: a European multicenter study. J Am Coll Cardiol. 2008;51:1466–72. doi: 10.1016/j.jacc.2007.10.067. [DOI] [PubMed] [Google Scholar]
- Sato K, Sankaramangalam K, Kandregula K, Bullen JA, Kapadia SR, Krishnaswamy A, Mick S, Rodriguez LL, Grimm RA, Menon V, Desai MY, Svensson LG, Griffin BP, Popovic ZB. Contemporary outcomes in low-gradient aortic stenosis patients who underwent dobutamine stress echocardiography. J Am Heart Assoc. 2019;8:e011168. doi: 10.1161/JAHA.118.011168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes F, Lerakis S, Barbosa Ribeiro, Gilard M, Cavalcante JL, Makkar R, Herrmann HC, Windecker S, Enriquez-Sarano M, Cheema AN, Nombela-Franco L, Amat-Santos I, Munoz-Garcia AJ, Garcia Del, Zajarias A, Lisko JC, Hayek S, Babaliaros V, Le Ven, Gleason TG, Chakravarty T, Szeto W, Clavel MA, de Agustin, Serra V, Schindler JT, Dahou A, Salah-Annabi M, Pelletier-Beaumont E, Cote M, Puri R, Pibarot P, Rodes-Cabau J. Outcomes from transcatheter aortic valve replacement in patients with low-flow, low-gradient aortic stenosis and left ventricular ejection fraction less than 30%: a substudy from the TOPAS-TAVI Registry. JAMA Cardiol. 2019;4:64–70. doi: 10.1001/jamacardio.2018.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha G, Bohbot Y, Rusinaru D, Marechaux S, Tribouilloy C. Outcome of normal-flow low-gradient severe aortic stenosis with preserved left ventricular ejection fraction: a propensity-matched study. J Am Heart Assoc. 2019;8:e012301. doi: 10.1161/JAHA.119.012301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J. 2005;26:1309–13. doi: 10.1093/eurheartj/ehi250. [DOI] [PubMed] [Google Scholar]
- Genereux P, Stone GW, O’Gara PT, Marquis-Gravel G, Redfors B, Giustino G, Pibarot P, Bax JJ, Bonow RO, Leon MB. Natural history, diagnostic approaches, and therapeutic strategies for patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. 2016;67:2263–88. doi: 10.1016/j.jacc.2016.02.057. [DOI] [PubMed] [Google Scholar]
- Kang DH, Park SJ, Lee SA, Lee S, Kim DH, Kim HK, Yun SC, Hong GR, Song JM, Chung CH, Song JK, Lee JW, Park SW. Early Surgery or Conservative Care for Asymptomatic Aortic Stenosis. N Engl J Med. 2020;382:111–9. doi: 10.1056/NEJMoa1912846. [DOI] [PubMed] [Google Scholar]
- Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–7. doi: 10.1056/NEJM200008313430903. [DOI] [PubMed] [Google Scholar]
- Rosenhek R, Zilberszac R, Schemper M, Czerny M, Mundigler G, Graf S, Bergler-Klein J, Grimm M, Gabriel H, Maurer G. Natural history of very severe aortic stenosis. Circulation. 2010;121:151–6. doi: 10.1161/CIRCULATIONAHA.109.894170. [DOI] [PubMed] [Google Scholar]
- Cioffi G, Faggiano P, Vizzardi E, Tarantini L, Cramariuc D, Gerdts E, de Simone. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart. 2011;97:301–7. doi: 10.1136/hrt.2010.192997. [DOI] [PubMed] [Google Scholar]
- Rusinaru D, Bohbot Y, Kowalski C, Ringle A, Marechaux S, Tribouilloy C. Left atrial volume and mortality in patients with aortic stenosis. J Am Heart Assoc. 2017;6:e006615. doi: 10.1161/JAHA.117.006615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl JS, Videbaek L, Poulsen MK, Rudbaek TR, Pellikka PA, Moller JE. Global strain in severe aortic valve stenosis: relation to clinical outcome after aortic valve replacement. Circ Cardiovasc Imaging. 2012;5:613–20. doi: 10.1161/CIRCIMAGING.112.973834. [DOI] [PubMed] [Google Scholar]
- Chin CW, Shah AS, McAllister DA, Joanna Cowell, Alam S, Langrish JP, Strachan FE, Hunter AL, Maria Choy, Lang CC, Walker S, Boon NA, Newby DE, Mills NL, Dweck MR. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J. 2014;35:2312–21. doi: 10.1093/eurheartj/ehu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos R, Brandenburg V, Mahnken AH, Muhlenbruch G, Stanzel S, Gunther RW, Floege J, Jahnen-Dechent W, Kelm M, Kuhl HP. Association of fetuin-A levels with the progression of aortic valve calcification in non-dialyzed patients. Eur Heart J. 2009;30:2054–61. doi: 10.1093/eurheartj/ehp158. [DOI] [PubMed] [Google Scholar]
- Carroll JD, Mack MJ, Vemulapalli S, Herrmann HC, Gleason TG, Hanzel G, Deeb GM, Thourani VH, Cohen DJ, Desai N, Kirtane AJ, Fitzgerald S, Michaels J, Krohn C, Masoudi FA, Brindis RG, Bavaria JE. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2020;76:2492–516. doi: 10.1016/j.jacc.2020.09.595. [DOI] [PubMed] [Google Scholar]
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- Deeb GM, Reardon MJ, Chetcuti S, Patel HJ, Grossman PM, Yakubov SJ, Kleiman NS, Coselli JS, Gleason TG, Lee JS, Hermiller JB , Heiser J, Merhi W, Zorn GL 3rd, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Maini B, Mumtaz M, Conte J, Resar J, Aharonian V, Pfeffer T, Oh JK, Qiao H, Adams DH, Popma JJ, CoreValve USCI. 3-Year outcomes in high-risk patients who underwent surgical or transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;67:2565–74. doi: 10.1016/j.jacc.2016.03.506. [DOI] [PubMed] [Google Scholar]
- Smith CR, Leon MB, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani V, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ., Wang D, Pocock SJ PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH, Kodali SK, Makkar RR, Fontana GP, Kapadia S, Bavaria J, Hahn RT, Thourani VH, Babaliaros V, Pichard A, Herrmann HC, Brown DL, Williams M, Akin J, Davidson MJ, Svensson LG PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477–84. doi: 10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J, Kleiman NS, Chetcuti S, Heiser J, Merhi W, Zorn G, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J, Maini B, Mumtaz M, Chenoweth S, Oh JK: S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–8. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- Thyregod HG, Steinbruchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, Chang Y, Franzen OW, Engstrom T, Clemmensen P, Hansen PB, Andersen LW, Olsen PS, Sondergaard L. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the All-Comers NOTION randomized clinical trial. J Am Coll Cardiol. 2015;65:2184–94. doi: 10.1016/j.jacc.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG PARTNER 2 Investigators. Transcatheter or Surgical Aortic Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609–20. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- Thourani VH, Kodali S, Makkar RR, Herrmann HC, Williams M, Babaliaros V, Smalling R, Lim S, Malaisrie SC, Kapadia S, Szeto WY, Greason KL, Kereiakes D, Ailawadi G, Whisenant BK, Devireddy C, Leipsic J, Hahn RT, Pibarot P, Weissman NJ, Jaber WA, Cohen DJ, Suri R, Tuzcu EM, Svensson LG, Webb JG, Moses JW, Mack MJ, Miller DC, Smith CR, Alu MC, Parvataneni R, D'Agostino RB, Leon MB. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387:2218–25. doi: 10.1016/S0140-6736(16)30073-3. [DOI] [PubMed] [Google Scholar]
- Reardon MJ, Van Mieghem, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PW, Kappetein AP SURTAVI Investigators. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2017;376:1321–31. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- Makkar RR, Thourani VH, Mack MJ, Kodali SK, Kapadia S, Webb JG, Yoon SH, Trento A, Svensson LG, Herrmann HC, Szeto WY, Miller DC, Satler L, Cohen DJ, Dewey TM, Babaliaros V, Williams MR, Kereiakes DJ, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Brown DL, Fearon WF, Russo MJ, Pibarot P, Hahn RT, Jaber WA, Rogers E, Xu K, Wheeler J, Alu MC, Smith CR, Leon MB PARTNER 2 Investigators. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N Engl J Med. 2020;382:799–809. doi: 10.1056/NEJMoa1910555. [DOI] [PubMed] [Google Scholar]
- Thyregod HGH, Ihlemann N, Jørgensen TH, Nissen H, Kjeldsen BJ, Petursson P, Chang Y, Franzen OW, Engstrøm T, Clemmensen P, Hansen PB, Andersen LW, Steinbruüchel DA, Olsen PS, Søndergaard L. Five-Year Clinical and Echocardiographic Outcomes From the NOTION Randomized Clinical Trial in Patients at Lower Surgical Risk. Circulation. 2019 Feb 1. [Epub ahead of print] doi: 10.1161/CIRCULATIONAHA.118.036606. [DOI] [PubMed] [Google Scholar]
- Siontis GC, Praz F, Pilgrim T, Mavridis D, Verma S, Salanti G, Sondergaard L, Juni P, Windecker S. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of severe aortic stenosis: a meta-analysis of randomized trials. Eur Heart J. 2016;37:3503–12. doi: 10.1093/eurheartj/ehw225. [DOI] [PubMed] [Google Scholar]
- Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR PARTNER 3 Investigators. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380:1695–705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, Askew J, Sorajja P, Rovin J, Chetcuti SJ, Adams DH, Teirstein PS, Zorn GL, Forrest JK, Tchetche D, Resar J, Walton A, Piazza N, Ramlawi B, Robinson N, Petrossian G, Gleason TG, Oh JK, Boulware MJ, Qiao H, Mugglin AS, Reardon MJ Evolut Low Risk Trial Investigators. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med. 2019;380:1706–15. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- Siontis GCM, Overtchouk P, Cahill TJ, Modine T, Prendergast B, Praz F, Pilgrim T, Petrinic T, Nikolakopoulou A, Salanti G, Søndergaard L, Verma S, Jüni P, Windecker S. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: an updated meta-analysis. Eur Heart J. 2019;40:3143–53. doi: 10.1093/eurheartj/ehz275. [DOI] [PubMed] [Google Scholar]
- Leon MB, Mack MJ, Hahn RT, Thourani VH, Makkar R, Kodali SK, Alu MC, Madhavan MV, Chau KH, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Blanke P, Leipsic JA, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Herrmann HC, Szeto WY, Genereux P, Pershad A, Lu M, Webb JG, Smith CR, Pibarot P, PARTNER 3. Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol. 2021;77:1149–61. doi: 10.1016/j.jacc.2020.12.052. [DOI] [PubMed] [Google Scholar]
- Greason KL, Lahr BD, Stulak JM, Cha YM, Rea RF, Schaff HV, Dearani JA. Long-term mortality effect of early pacemaker implantation after surgical aortic valve replacement. Ann Thorac Surg. 2017;104:1259–64. doi: 10.1016/j.athoracsur.2017.01.083. [DOI] [PubMed] [Google Scholar]
- Auffret V, Puri R, Urena M, Chamandi C, Rodriguez-Gabella T, Philippon F, Rodés-Cabau J. Conduction Disturbances After Transcatheter Aortic Valve Replacement: Current Status and Future Perspectives. Circulation. 2017;136:1049–69. doi: 10.1161/CIRCULATIONAHA.117.028352. [DOI] [PubMed] [Google Scholar]
- Nazif TM, Chen S, George I, Dizon JM, Hahn RT, Crowley A, Alu MC, Babaliaros V, Thourani VH, Herrmann HC, Smalling RW, Brown DL, Mack MJ, Kapadia S, Makkar R, Webb JG, Leon MB, Kodali SK. New-onset left bundle branch block after transcatheter aortic valve replacement is associated with adverse long-term clinical outcomes in intermediate-risk patients: an analysis from the PARTNER II trial. Eur Heart J. 2019;40:2218–27. doi: 10.1093/eurheartj/ehz227. [DOI] [PubMed] [Google Scholar]
- Tam DY, Hughes A, Wijeysundera HC, Fremes SE. Cost-effectiveness of self-expandable transcatheter aortic valves in intermediate-risk patients. Ann Thorac Surg. 2018;106:676–83. doi: 10.1016/j.athoracsur.2018.03.069. [DOI] [PubMed] [Google Scholar]
- Baron SJ, Wang K, House JA, Magnuson EA, Reynolds MR, Makkar R, Herrmann HC, Kodali S, Thourani VH, Kapadia S, Svensson L, Mack MJ, Brown DL, Russo MJ, Smith CR, Webb J, Miller C, Leon MB, Cohen DJ. Cost-effectiveness of transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at intermediate risk. Circulation. 2019;139:877–88. doi: 10.1161/CIRCULATIONAHA.118.035236. [DOI] [PubMed] [Google Scholar]
- Pilgrim T, Windecker S. Expansion of transcatheter aortic valve implantation: new indications and socio-economic considerations. Eur Heart J. 2018;39:2643–5. doi: 10.1093/eurheartj/ehy228. [DOI] [PubMed] [Google Scholar]
- Barbato E, Noc M, Baumbach A, Dudek D, Bunc M, Skalidis E, Banning A, Legutko J, Witt N, Pan M, Tilsted HH, Nef H, Tarantini G, Kazakiewicz D, Huculeci R, Cook S, Magdy A, Desmet W, Cayla G, Vinereanu D, Voskuil M, Goktekin O, Vardas P, Timmis A, Haude M. Mapping interventional cardiology in Europe: the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Atlas Project. Eur Heart J. 2020;41:2579–88. doi: 10.1093/eurheartj/ehaa475. [DOI] [PubMed] [Google Scholar]
- Johnston DR, Soltesz EG, Vakil N, Rajeswaran J, Roselli EE, Sabik JF, Smedira NG, Svensson LG, Lytle BW, Blackstone EH. Long-term durability of bioprosthetic aortic valves: implications from 12,569 implants. Ann Thorac Surg. 2015;99:1239–47. doi: 10.1016/j.athoracsur.2014.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman DJ, Saraf S, MacCarthy PA, Myat A, Anderson SG, Malkin CJ, Cunnington MS, Somers K, Brennan P, Manoharan G, Parker J, Aldalati O, Brecker SJ, Dowling C, Hoole SP, Dorman S, Mullen M, Kennon S, Jerrum M, Chandrala P, Roberts DH, Tay J, Doshi SN, Ludman PF, Fairbairn TA, Crowe J, Levy RD, Banning AP, Ruparelia N, Spence MS, Hildick-Smith D. Long-term durability of transcatheter aortic valve prostheses. J Am Coll Cardiol. 2019;73:537–45. doi: 10.1016/j.jacc.2018.10.078. [DOI] [PubMed] [Google Scholar]
- Barbanti M, Costa G, Zappulla P, Todaro D, Picci A, Rapisarda G, di Simone E, Sicuso R, Buccheri S, Gulino S, Pilato G, La Spina K, D’Arrigo P, Valvo R, Indelicato A, Giannazzo D, Immè S, Tamburino C, Patanè M, Sgroi C, Giuffrida A, Trovato D, Monte IP, Deste W, Capranzano P, Capodanno D, Tamburino C. Incidence of Long-Term Structural Valve Dysfunction and Bioprosthetic Valve Failure After Transcatheter Aortic Valve Replacement. J Am Heart Assoc. 2018;7:e008440. doi: 10.1161/JAHA.117.008440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier R, Eltchaninoff H, Donzeau-Gouge P, Chevreul K, Fajadet J, Leprince P, Leguerrier A, Lievre M, Prat A, Teiger E, Lefevre T, Tchetche D, Carrie D, Himbert D, Albat B, Cribier A, Sudre A, Blanchard D, Rioufol G, Collet F, Houel R, Dos Santos, Meneveau N, Ghostine S, Manigold T, Guyon P, Cuisset T, Le Breton, Delepine S, Favereau X, Souteyrand G, Ohlmann P, Doisy V, Lognone T, Gommeaux A, Claudel JP, Bourlon F, Bertrand B, Iung B, Gilard M. Five-year clinical outcome and valve durability after transcatheter aortic valve replacement in high-risk patients. Circulation. 2018;138:2597–607. doi: 10.1161/CIRCULATIONAHA.118.036866. [DOI] [PubMed] [Google Scholar]
- Deutsch MA, Erlebach M, Burri M, Hapfelmeier A, Witt OG, Ziegelmueller JA, Wottke M, Ruge H, Krane M, Piazza N, Bleiziffer S, Lange R. Beyond the five-year horizon: long-term outcome of high-risk and inoperable patients undergoing TAVR with first-generation devices. EuroIntervention. 2018;14:41–9. doi: 10.4244/EIJ-D-17-00603. [DOI] [PubMed] [Google Scholar]
- Pibarot P, Ternacle J, Jaber WA, Salaun E, Dahou A, Asch FM, Weissman NJ, Rodriguez L, Xu K, Annabi MS, Guzzetti E, Beaudoin J, Bernier M, Leipsic J, Blanke P, Clavel MA, Rogers E, Alu MC, Douglas PS, Makkar R, Miller DC, Kapadia SR, Mack MJ, Webb JG, Kodali SK, Smith CR, Herrmann HC, Thourani VH, Leon MB, Hahn RT, PARTNER 2. Structural deterioration of transcatheter versus surgical aortic valve bioprostheses in the PARTNER-2 Trial. J Am Coll Cardiol. 2020;76:1830–43. doi: 10.1016/j.jacc.2020.08.049. [DOI] [PubMed] [Google Scholar]
- Tam DY, Vo TX, Wijeysundera HC, Dvir D, Friedrich JO, Fremes SE. Transcatheter valve-in-valve versus redo surgical aortic valve replacement for the treatment of degenerated bioprosthetic aortic valve: a systematic review and meta-analysis. Catheter Cardiovasc Interv. 2018;92:1404–11. doi: 10.1002/ccd.27686. [DOI] [PubMed] [Google Scholar]
- Deharo P, Bisson A, Herbert J, Lacour T, Etienne CS, Porto A, Theron A, Collart F, Bourguignon T, Cuisset T, Fauchier L. Transcatheter valve-in-valve aortic valve replacement as an alternative to surgical re-replacement. J Am Coll Cardiol. 2020;76:489–99. doi: 10.1016/j.jacc.2020.06.010. [DOI] [PubMed] [Google Scholar]
- Gozdek M, Raffa GM, Suwalski P, Kolodziejczak M, Anisimowicz L, Kubica J, Navarese EP, Kowalewski M, SIRIO-TAVI group. Comparative performance of transcatheter aortic valve-in-valve implantation versus conventional surgical redo aortic valve replacement in patients with degenerated aortic valve bioprostheses: systematic review and meta-analysis. Eur J Cardiothorac Surg. 2018;53:495–504. doi: 10.1093/ejcts/ezx347. [DOI] [PubMed] [Google Scholar]
- Landes U, Webb JG, De Backer, Sondergaard L, Abdel-Wahab M, Crusius L, Kim WK, Hamm C, Buzzatti N, Montorfano M, Ludwig S, Schofer N, Voigtlaender L, Guerrero M, El Sabbagh, Rodés-Cabau J, Guimaraes L, Kornowski R, Codner P, Okuno T, Pilgrim T, Fiorina C, Colombo A, Mangieri A, Eltchaninoff H, Nombela-Franco L, Van Wiechen, Van Mieghem, Tchétché D, Schoels WH, Kullmer M, Tamburino C, Sinning JM, Al-Kassou B, Perlman GY, Danenberg H, Ielasi A, Fraccaro C, Tarantini G, De Marco, Witberg G, Redwood SR, Lisko JC, Babaliaros VC, Laine M, Nerla R, Castriota F, Finkelstein A, Loewenstein I, Eitan A, Jaffe R, Ruile P, Neumann FJ, Piazza N, Alosaimi H, Sievert H, Sievert K, Russo M, Andreas M, Bunc M, Latib A, Govdfrey R, Hildick-Smith D, Sathananthan J, Hensey M, Alkhodair A, Blanke P, Leipsic J, Wood DA, Nazif TM, Kodali S, Leon MB, Barbanti M. Transcatheter Aortic Valve Replacement for Transcatheter Prosthesis Dysfunction. J Am Coll Cardiol. 2020;75:1882–93. doi: 10.1016/j.jacc.2020.02.051. [DOI] [PubMed] [Google Scholar]
- Buzzatti N, Romano V, De Backer, Soendergaard L, Rosseel L, Maurovich-Horvat P, Karady J, Merkely B, Ruggeri S, Prendergast B, De Bonis, Colombo A, Montorfano M, Latib A. Coronary access after repeated transcatheter aortic valve implantation: a glimpse into the future. JACC Cardiovasc Imaging. 2020;13:508–15. doi: 10.1016/j.jcmg.2019.06.025. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Bleiziffer S, De Backer, Delgado V, Arai T, Ziegelmueller J, Barbanti M, Sharma R, Perlman GY, Khalique OK, Holy EW, Saraf S, Deuschl F, Fujita B, Ruile P, Neumann FJ, Pache G, Takahashi M, Kaneko H, Schmidt T, Ohno Y, Schofer N, Kong WKF, Tay E, Sugiyama D, Kawamori H, Maeno Y, Abramowitz Y, Chakravarty T, Nakamura M, Kuwata S, Yong G, Kao HL, Lee M, Kim HS, Modine T, Wong SC, Bedgoni F, Testa L, Teiger E, Butter C, Ensminger SM, Schaefer U, Dvir D, Blanke P, Leipsic J, Nietlispach F, Abdel-Wahab M, Chevalier B, Tamburino C, Hildick-Smith D, Whisenant BK, Park SJ, Colombo A, Latib A, Kodali SK, Bax JJ, Sondergaard L, Webb JG, Lefevre T, Leon MB, Makkar R. Outcomes in Transcatheter Aortic Valve Replacement for Bicuspid Versus Tricuspid Aortic Valve Stenosis. J Am Coll Cardiol. 2017;69:2579–89. doi: 10.1016/j.jacc.2017.03.017. [DOI] [PubMed] [Google Scholar]
- Halim SA, Edwards FH, Dai D, Li Z, Mack MJ, Holmes DR, Tuzcu EM, Thourani VH, Harrison JK, Brennan JM. Outcomes of Transcatheter Aortic Valve Replacement in Patients With Bicuspid Aortic Valve Disease: A Report From the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circulation. 2020;141:1071–9. doi: 10.1161/CIRCULATIONAHA.119.040333. [DOI] [PubMed] [Google Scholar]
- Forrest JK, Kaple RK, Ramlawi B, Gleason TG, Meduri CU, Yakubov SJ, Jilaihawi H, Liu F, Reardon MJ. Transcatheter Aortic Valve Replacement in Bicuspid Versus Tricuspid Aortic Valves From the STS/ACC TVT Registry. JACC Cardiovasc Interv. 2020;13:1749–59. doi: 10.1016/j.jcin.2020.03.022. [DOI] [PubMed] [Google Scholar]
- Alkhouli M, Zack CJ, Sarraf M, Bashir R, Nishimura RA, Eleid MF, Nkomo VT, Sandhu GS, Gulati R, Greason KL, Holmes DR, Rihal CS. Morbidity and mortality associated with balloon aortic valvuloplasty: a national perspective. Circ Cardiovasc Interv. 2017;10 doi: 10.1161/CIRCINTERVENTIONS.116.004481. [DOI] [PubMed] [Google Scholar]
- Horstkotte D, Loogen F. The natural history of aortic valve stenosis. Eur Heart J. 1988;9 Suppl E:57–64. doi: 10.1093/eurheartj/9.suppl_e.57. [DOI] [PubMed] [Google Scholar]
- Lund O. Preoperative risk evaluation and stratification of long-term survival after valve replacement for aortic stenosis. Reasons for earlier operative intervention. Circulation. 1990;82:124–39. doi: 10.1161/01.cir.82.1.124. [DOI] [PubMed] [Google Scholar]
- Mangner N, Stachel G, Woitek F, Haussig S, Schlotter F, Hollriegel R, Adam J, Lindner A, Mohr FW, Schuler G, Kiefer P, Leontyev S, Borger MA, Thiele H, Holzhey D, Linke A. Predictors of mortality and symptomatic outcome of patients with low-flow severe aortic stenosis undergoing transcatheter aortic valve replacement. J Am Heart Assoc. 2018;7:e007977. doi: 10.1161/JAHA.117.007977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl JS, Eleid MF, Michelena HI, Scott CG, Suri RM, Schaff HV, Pellikka PA. Effect of left ventricular ejection fraction on postoperative outcome in patients with severe aortic stenosis undergoing aortic valve replacement. Circ Cardiovasc Imaging. 2015;8:e002917. doi: 10.1161/CIRCIMAGING.114.002917. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, Kitai T, Kadota K, Izumi C, Nakatsuma K, Sasa T, Watanabe H, Kuwabara Y, Makiyama T, Ono K, Shizuta S, Kato T, Saito N, Minatoya K, Kimura T, CURRENT AS. Prognostic impact of left ventricular ejection fraction in patients with severe aortic stenosis. JACC Cardiovasc Interv. 2018;11:145–57. doi: 10.1016/j.jcin.2017.08.036. [DOI] [PubMed] [Google Scholar]
- Bohbot Y, de Meester, Chadha G, Rusinaru D, Belkhir K, Trouillet C, Pasquet A, Marechaux S, Vanoverschelde JL, Tribouilloy C. Relationship between left ventricular ejection fraction and mortality in asymptomatic and minimally symptomatic patients with severe aortic stenosis. JACC Cardiovasc Imaging. 2019;12:38–48. doi: 10.1016/j.jcmg.2018.07.029. [DOI] [PubMed] [Google Scholar]
- Capoulade R, Le Ven, Clavel MA, Dumesnil JG, Dahou A, Thebault C, Arsenault M, O’Connor K, Bedard E, Beaudoin J, Senechal M, Bernier M, Pibarot P. Echocardiographic predictors of outcomes in adults with aortic stenosis. Heart. 2016;102:934–42. doi: 10.1136/heartjnl-2015-308742. [DOI] [PubMed] [Google Scholar]
- Bohbot Y, Kowalski C, Rusinaru D, Ringle A, Marechaux S, Tribouilloy C. Impact of mean transaortic pressure gradient on long-term outcome in patients with severe aortic stenosis and preserved left ventricular ejection fraction. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, Kraft CD, Miyake-Hull CY, Schwaegler RG. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation. 1997;95:2262–70. doi: 10.1161/01.cir.95.9.2262. [DOI] [PubMed] [Google Scholar]
- Thourani VH, Suri RM, Gunter RL, Sheng S, O’Brien SM, Ailawadi G, Szeto WY, Dewey TM, Guyton RA, Bavaria JE, Babaliaros V, Gammie JS, Svensson L, Williams M, Badhwar V, Mack MJ. Contemporary real-world outcomes of surgical aortic valve replacement in 141,905 low-risk, intermediate-risk, and high-risk patients. Ann Thorac Surg. 2015;99:55–61. doi: 10.1016/j.athoracsur.2014.06.050. [DOI] [PubMed] [Google Scholar]
- Gleason TG, Reardon MJ, Popma JJ, Deeb GM, Yakubov SJ, Lee JS, Kleiman NS, Chetcuti S, Hermiller JB, Heiser J, Merhi W, Zorn GL, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte JV, Mumtaz M, Oh JK, Huang J, Adams DH CoreValve U. S. Pivotal High Risk Trial Clinical Investigators. 5-Year Outcomes of Self-Expanding Transcatheter Versus Surgical Aortic Valve Replacement in High-Risk Patients. J Am Coll Cardiol. 2018;72:2687–96. doi: 10.1016/j.jacc.2018.08.2146. [DOI] [PubMed] [Google Scholar]
- Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Barwolf C, Holme I, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R, SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–56. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der, ESC Scientific. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- Bull S, Loudon M, Francis JM, Joseph J, Gerry S, Karamitsos TD, Prendergast BD, Banning AP, Neubauer S, Myerson SG. A prospective, double-blind, randomized controlled trial of the angiotensin-converting enzyme inhibitor Ramipril In Aortic Stenosis (RIAS trial). Eur Heart J Cardiovasc Imaging. 2015;16:834–41. doi: 10.1093/ehjci/jev043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai T, Saito S, Yamanaka F, Shishido K, Tanaka Y, Yamabe T, Shirai S, Tada N, Araki M, Naganuma T, Watanabe Y, Yamamoto M, Hayashida K. Renin-angiotensin system blockade therapy after transcatheter aortic valve implantation. Heart. 2018;104:644–51. doi: 10.1136/heartjnl-2017-311738. [DOI] [PubMed] [Google Scholar]
- Dahl JS, Videbaek L, Poulsen MK, Pellikka PA, Veien K, Andersen LI, Haghfelt T, Moller JE. Effect of candesartan treatment on left ventricular remodeling after aortic valve replacement for aortic stenosis. Am J Cardiol. 2010;106:713–9. doi: 10.1016/j.amjcard.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Strange G, Stewart S, Celermajer D, Prior D, Scalia GM, Marwick T, Ilton M, Joseph M, Codde J, Playford D, National Echocardiography. Poor long-term survival in patients with moderate aortic stenosis. J Am Coll Cardiol. 2019;74:1851–63. doi: 10.1016/j.jacc.2019.08.004. [DOI] [PubMed] [Google Scholar]
- van Gils, Clavel MA, Vollema EM, Hahn RT, Spitzer E, Delgado V, Nazif T, De Jaegere, Geleijnse ML, Ben-Yehuda O, Bax JJ, Leon MB, Pibarot P, Van Mieghem. Prognostic implications of moderate aortic stenosis in patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2017;69:2383–92. doi: 10.1016/j.jacc.2017.03.023. [DOI] [PubMed] [Google Scholar]
- Delesalle G, Bohbot Y, Rusinaru D, Delpierre Q, Marechaux S, Tribouilloy C. Characteristics and prognosis of patients with moderate aortic stenosis and preserved left ventricular ejection fraction. J Am Heart Assoc. 2019;8:e011036. doi: 10.1161/JAHA.118.011036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad Z, Vora AN, Dunning A, Schulte PJ, Shaw LK, Al-Enezi F, Ersboll M, McGarrah RW, Vavalle JP, Shah SH, Kisslo J, Glower D, Harrison JK, Velazquez EJ. Aortic valve surgery and survival in patients with moderate or severe aortic stenosis and left ventricular dysfunction. Eur Heart J. 2016;37:2276–86. doi: 10.1093/eurheartj/ehv701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaker Z, Badhwar V, Alqahtani F, Aljohani S, Zack CJ, Holmes DR, Rihal CS, Alkhouli M. Sex differences in the utilization and outcomes of surgical aortic valve replacement for severe aortic stenosis. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote N, Clavel MA. Sex differences in the pathophysiology, diagnosis, and management of aortic stenosis. Cardiol Clin. 2020;38:129–38. doi: 10.1016/j.ccl.2019.09.008. [DOI] [PubMed] [Google Scholar]
- Tribouilloy C, Bohbot Y, Rusinaru D, Belkhir K, Diouf M, Altes A, Delpierre Q, Serbout S, Kubala M, Levy F, Marechaux S, Enriquez Sarano. Excess mortality and undertreatment of women with severe aortic stenosis. J Am Heart Assoc. 2021;10:e018816. doi: 10.1161/JAHA.120.018816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WT, Ferguson TB, Ryan T, Landolfo CK, Peterson ED. Should coronary artery bypass graft surgery patients with mild or moderate aortic stenosis undergo concomitant aortic valve replacement? A decision analysis approach to the surgical dilemma. J Am Coll Cardiol. 2004;44:1241–7. doi: 10.1016/j.jacc.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Faroux L, Campelo-Parada F, Munoz-Garcia E, Nombela-Franco L, Fischer Q, Donaint P, Serra V, Veiga G, Gutierrez E, Vilalta V, Alperi A, Regueiro A, Asmarats L, Ribeiro HB, Matta A, Munoz-Garcia A, Armijo G, Urena M, Metz D, Rodenas-Alesina E, la de, Fernandez-Nofrerias E, Pascual I, Perez-Fuentes P, Arzamendi D, Campanha-Borges DC, Del Val, Couture T, Rodes-Cabau J. Procedural characteristics and late outcomes of percutaneous coronary intervention in the workup pre-TAVR. JACC Cardiovasc Interv. 2020;13:2601–13. doi: 10.1016/j.jcin.2020.07.009. [DOI] [PubMed] [Google Scholar]
- Sondergaard L, Popma JJ, Reardon MJ, Van Mieghem, Deeb GM, Kodali S, George I, Williams MR, Yakubov SJ, Kappetein AP, Serruys PW, Grube E, Schiltgen MB, Chang Y, Engstrom T, SURTAVI Trial. Comparison of a complete percutaneous versus surgical approach to aortic valve replacement and revascularization in patients at intermediate surgical risk: results from the randomized SURTAVI Trial. Circulation. 2019;140:1296–305. doi: 10.1161/CIRCULATIONAHA.118.039564. [DOI] [PubMed] [Google Scholar]
- Nombela-Franco L, Eltchaninoff H, Zahn R, Testa L, Leon MB, Trillo-Nouche R, D’Onofrio A, Smith CR, Webb J, Bleiziffer S, De Chiara, Gilard M, Tamburino C, Bedogni F, Barbanti M, Salizzoni S, Garcia del, Sabate M, Moreo A, Fernandez C, Ribeiro HB, Amat-Santos I, Urena M, Allende R, Garcia E, Macaya C, Dumont E, Pibarot P, Rodes-Cabau J. Clinical impact and evolution of mitral regurgitation following transcatheter aortic valve replacement: a meta-analysis. Heart. 2015;101:1395–405. doi: 10.1136/heartjnl-2014-307120. [DOI] [PubMed] [Google Scholar]
- D’Ancona G, Kische S, Senges J, Ouarrak T, Puls M, Bekeredjian R, Sievert H, Safak E, Ortak J, Oner A, Schillinger W, Ince H. Combined mitro-aortic pathology: impact of previous aortic valve replacement upon outcomes of MitraClip therapy (from the German transcatheter mitral valve interventions registry). EuroIntervention. 2017;13:475–82. doi: 10.4244/EIJ-D-17-00222. [DOI] [PubMed] [Google Scholar]
- Witberg G, Codner P, Landes U, Barbanti M, Valvo R, De Backer, Ooms JF, Sievert K, El Sabbagh, Jimenez-Quevedo P, Brennan PF, Sedaghat A, Masiero G, Werner P, Overtchouk P, Watanabe Y, Montorfano M, Bijjam VR, Hein M, Fiorina C, Arzamendi D, Rodriguez-Gabella T, Fernandez-Vazquez F, Baz JA, Laperche C, Grasso C, Branca L, Estevez-Loureiro R, Benito-Gonzalez T, Amat Santos, Ruile P, Mylotte D, Buzzatti N, Piazza N, Andreas M, Tarantini G, Sinning JM, Spence MS, Nombela-Franco L, Guerrero M, Sievert H, Sondergaard L, Van Mieghem, Tchetche D, Webb JG, Kornowski R. Transcatheter treatment of residual significant mitral regurgitation following TAVR: a multicenter registry. JACC Cardiovasc Interv. 2020;13:2782–91. doi: 10.1016/j.jcin.2020.07.014. [DOI] [PubMed] [Google Scholar]
- Khan F, Okuno T, Malebranche D, Lanz J, Praz F, Stortecky S, Windecker S, Pilgrim T. Transcatheter aortic valve replacement in patients with multivalvular heart disease. JACC Cardiovasc Interv. 2020;13:1503–14. doi: 10.1016/j.jcin.2020.03.052. [DOI] [PubMed] [Google Scholar]
- Baumgartner H, Bonhoeffer P, De Groot, de Haan, Deanfield JE, Galie N, Gatzoulis MA, Gohlke-Baerwolf C, Kaemmerer H, Kilner P, Meijboom F, Mulder BJ, Oechslin E, Oliver JM, Serraf A, Szatmari A, Thaulow E, Vouhe PR, Walma E, Task Force, Association for, ESC Committee. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010 ). Eur Heart J. 2010;31:2915–57. doi: 10.1093/eurheartj/ehq249. [DOI] [PubMed] [Google Scholar]
- Dziadzko V, Dziadzko M, Medina-Inojosa JR, Benfari G, Michelena HI, Crestanello JA, Maalouf J, Thapa P, Enriquez-Sarano M. Causes and mechanisms of isolated mitral regurgitation in the community: clinical context and outcome. Eur Heart J. 2019;40:2194–202. doi: 10.1093/eurheartj/ehz314. [DOI] [PubMed] [Google Scholar]
- Kingue S, Ba SA, Balde D, Diarra MB, Anzouan-Kacou JB, Anisubia B, Damorou JM, Ndobo P, Menanga A, Kane A, Kakou-Guikahue M, Kenfack M, Metogo B, Chelo D, Yangnigni E, Tantchou C, Bertrand E, Monsuez JJ, Working Group. The VALVAFRIC study: a registry of rheumatic heart disease in Western and Central Africa. Arch Cardiovasc Dis. 2016;109:321–9. doi: 10.1016/j.acvd.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–71. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- Antoine C, Benfari G, Michelena HI, Maalouf JF, Nkomo VT, Thapa P, Enriquez-Sarano M. Clinical outcome of degenerative mitral regurgitation. Circulation. 2018;138:1317–26. doi: 10.1161/CIRCULATIONAHA.117.033173. [DOI] [PubMed] [Google Scholar]
- Samad Z, Shaw LK, Phelan M, Glower DD, Ersboll M, Toptine JH, Alexander JH, Kisslo JA, Wang A, Mark DB, Velazquez EJ. Long-term outcomes of mitral regurgitation by type and severity. Am Heart J. 2018;203:39–48. doi: 10.1016/j.ahj.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Carpentier A. Cardiac valve surgery–the “French correction ”. J Thorac Cardiovasc Surg. 1983;86:323–37. [PubMed] [Google Scholar]
- Boekstegers P, Hausleiter J, Baldus S, von Bardeleben, Beucher H, Butter C, Franzen O, Hoffmann R, Ince H, Kuck KH, Rudolph V, Schafer U, Schillinger W, Wunderlich N, Germany Society. Percutaneous interventional mitral regurgitation treatment using the Mitra-Clip system. Clin Res Cardiol. 2014;103:85–96. doi: 10.1007/s00392-013-0614-x. [DOI] [PubMed] [Google Scholar]
- Gavazzoni M, Taramasso M, Zuber M, Russo G, Pozzoli A, Miura M, Maisano F. Conceiving MitraClip as a tool: percutaneous edge-to-edge repair in complex mitral valve anatomies. Eur Heart J Cardiovasc Imaging. 2020;21:1059–67. doi: 10.1093/ehjci/jeaa062. [DOI] [PubMed] [Google Scholar]
- Cawley PJ, Hamilton-Craig C, Owens DS, Krieger EV, Strugnell WE, Mitsumori L, D’Jang CL, Schwaegler RG, Nguyen KQ, Nguyen B, Maki JH, Otto CM. Prospective comparison of valve regurgitation quantitation by cardiac magnetic resonance imaging and transthoracic echocardiography. Circ Cardiovasc Imaging. 2013;6:48–57. doi: 10.1161/CIRCIMAGING.112.975623. [DOI] [PubMed] [Google Scholar]
- Shanks M, Siebelink HM, Delgado V, van de, Ng AC, Sieders A, Schuijf JD, Lamb HJ, Ajmone Marsan, Westenberg JJ, Kroft LJ, de Roos, Bax JJ. Quantitative assessment of mitral regurgitation: comparison between three-dimensional transesophageal echocardiography and magnetic resonance imaging. Circ Cardiovasc Imaging. 2010;3:694–700. doi: 10.1161/CIRCIMAGING.110.947176. [DOI] [PubMed] [Google Scholar]
- Uretsky S, Gillam L, Lang R, Chaudhry FA, Argulian E, Supariwala A, Gurram S, Jain K, Subero M, Jang JJ, Cohen R, Wolff SD. Discordance between echocardiography and MRI in the assessment of mitral regurgitation severity: a prospective multicenter trial. J Am Coll Cardiol. 2015;65:1078–88. doi: 10.1016/j.jacc.2014.12.047. [DOI] [PubMed] [Google Scholar]
- Penicka M, Vecera J, Mirica DC, Kotrc M, Kockova R, Van Camp. Prognostic implications of magnetic resonance-derived quantification in asymptomatic patients with organic mitral regurgitation: comparison with Doppler echocardiography-derived integrative approach. Circulation. 2018;137:1349–60. doi: 10.1161/CIRCULATIONAHA.117.029332. [DOI] [PubMed] [Google Scholar]
- Garg P, Swift AJ, Zhong L, Carlhall CJ, Ebbers T, Westenberg J, Hope MD, Bucciarelli-Ducci C, Bax JJ, Myerson SG. Assessment of mitral valve regurgitation by cardiovascular magnetic resonance imaging. Nat Rev Cardiol. 2020;17:298–312. doi: 10.1038/s41569-019-0305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitkungvan D, Nabi F, Kim RJ, Bonow RO, Khan MA, Xu J, Little SH, Quinones MA, Lawrie GM, Zoghbi WA, Shah DJ. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J Am Coll Cardiol. 2018;72:823–34. doi: 10.1016/j.jacc.2018.06.048. [DOI] [PubMed] [Google Scholar]
- Bakkestrom R, Banke A, Christensen NL, Pecini R, Irmukhamedov A, Andersen M, Borlaug BA, Moller JE. Hemodynamic characteristics in significant symptomatic and asymptomatic primary mitral valve regurgitation at rest and during exercise. Circ Cardiovasc Imaging. 2018;11:e007171. doi: 10.1161/CIRCIMAGING.117.007171. [DOI] [PubMed] [Google Scholar]
- Utsunomiya H, Hidaka T, Susawa H, Izumi K, Harada Y, Kinoshita M, Itakura K, Masada K, Kihara Y. Exercise-Stress Echocardiography and Effort Intolerance in Asymptomatic/Minimally Symptomatic Patients With Degenerative Mitral Regurgitation Combined Invasive-Noninvasive Hemodynamic Monitoring. Circ Cardiovasc Imaging. 2018;11:e007282. doi: 10.1161/CIRCIMAGING.117.007282. [DOI] [PubMed] [Google Scholar]
- Pizarro R, Bazzino OO, Oberti PF, Falconi M, Achilli F, Arias A, Krauss JG, Cagide AM. Prospective validation of the prognostic usefulness of brain natriuretic peptide in asymptomatic patients with chronic severe mitral regurgitation. J Am Coll Cardiol. 2009;54:1099–106. doi: 10.1016/j.jacc.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Hiemstra YL, Tomsic A, van Wijngaarden, Palmen M, Klautz RJM, Bax JJ, Delgado V, Ajmone Marsan. Prognostic value of global longitudinal strain and etiology after surgery for primary mitral regurgitation. JACC Cardiovasc Imaging. 2020;13:577–85. doi: 10.1016/j.jcmg.2019.03.024. [DOI] [PubMed] [Google Scholar]
- Kim HM, Cho GY, Hwang IC, Choi HM, Park JB, Yoon YE, Kim HK. Myocardial strain in prediction of outcomes after surgery for severe mitral regurgitation. JACC Cardiovasc Imaging. 2018;11:1235–44. doi: 10.1016/j.jcmg.2018.03.016. [DOI] [PubMed] [Google Scholar]
- Grigioni F, Clavel MA, Vanoverschelde JL, Tribouilloy C, Pizarro R, Huebner M, Avierinos JF, Barbieri A, Suri R, Pasquet A, Rusinaru D, Gargiulo GD, Oberti P, Theron A, Bursi F, Michelena H, Lazam S, Szymanski C, Nkomo VT, Schumacher M, Bacchi-Reggiani L, Enriquez-Sarano M, MIDA Investigators. The MIDA Mortality Risk Score: development and external validation of a prognostic model for early and late death in degenerative mitral regurgitation. Eur Heart J. 2018;39:1281–91. doi: 10.1093/eurheartj/ehx465. [DOI] [PubMed] [Google Scholar]
- Tribouilloy C, Grigioni F, Avierinos JF, Barbieri A, Rusinaru D, Szymanski C, Ferlito M, Tafanelli L, Bursi F, Trojette F, Branzi A, Habib G, Modena MG, Enriquez-Sarano M, MIDA Investigators. Survival implication of left ventricular end-systolic diameter in mitral regurgitation due to flail leaflets a long-term follow-up multicenter study. J Am Coll Cardiol. 2009;54:1961–8. doi: 10.1016/j.jacc.2009.06.047. [DOI] [PubMed] [Google Scholar]
- Essayagh B, Antoine C, Benfari G, Messika-Zeitoun D, Michelena H, Le Tourneau, Mankad S, Tribouilloy CM, Thapa P, Enriquez-Sarano M. Prognostic implications of left atrial enlargement in degenerative mitral regurgitation. J Am Coll Cardiol. 2019;74:858–70. doi: 10.1016/j.jacc.2019.06.032. [DOI] [PubMed] [Google Scholar]
- Rusinaru D, Tribouilloy C, Grigioni F, Avierinos JF, Suri RM, Barbieri A, Szymanski C, Ferlito M, Michelena H, Tafanelli L, Bursi F, Mezghani S, Branzi A, Habib G, Modena MG, Enriquez-Sarano M, Mitral Regurgitation. Left atrial size is a potent predictor of mortality in mitral regurgitation due to flail leaflets: results from a large international multicenter study. Circ Cardiovasc Imaging. 2011;4:473–81. doi: 10.1161/CIRCIMAGING.110.961011. [DOI] [PubMed] [Google Scholar]
- Barbieri A, Bursi F, Grigioni F, Tribouilloy C, Avierinos JF, Michelena HI, Rusinaru D, Szymansky C, Russo A, Suri R, Bacchi Reggiani, Branzi A, Modena MG, Enriquez-Sarano M, Mitral Regurgitation. Prognostic and therapeutic implications of pulmonary hypertension complicating degenerative mitral regurgitation due to flail leaflet: a multicenter long-term international study. Eur Heart J. 2011;32:751–9. doi: 10.1093/eurheartj/ehq294. [DOI] [PubMed] [Google Scholar]
- Grigioni F, Benfari G, Vanoverschelde JL, Tribouilloy C, Avierinos JF, Bursi F, Suri RM, Guerra F, Pasquet A, Rusinaru D, Marcelli E, Theron A, Barbieri A, Michelena H, Lazam S, Szymanski C, Nkomo VT, Capucci A, Thapa P, Enriquez-Sarano M, MIDA Investigators. Long-term implications of atrial fibrillation in patients with degenerative mitral regurgitation. J Am Coll Cardiol. 2019;73:264–74. doi: 10.1016/j.jacc.2018.10.067. [DOI] [PubMed] [Google Scholar]
- Szymanski C, Magne J, Fournier A, Rusinaru D, Touati G, Tribouilloy C. Usefulness of preoperative atrial fibrillation to predict outcome and left ventricular dysfunction after valve repair for mitral valve prolapse. Am J Cardiol. 2015;115:1448–53. doi: 10.1016/j.amjcard.2015.02.027. [DOI] [PubMed] [Google Scholar]
- Suri RM, Vanoverschelde JL, Grigioni F, Schaff HV, Tribouilloy C, Avierinos JF, Barbieri A, Pasquet A, Huebner M, Rusinaru D, Russo A, Michelena HI, Enriquez-Sarano M. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA. 2013;310:609–16. doi: 10.1001/jama.2013.8643. [DOI] [PubMed] [Google Scholar]
- Jung JC, Jang MJ, Hwang HY. Meta-analysis comparing mitral valve repair versus replacement for degenerative mitral regurgitation across all ages. Am J Cardiol. 2019;123:446–53. doi: 10.1016/j.amjcard.2018.10.024. [DOI] [PubMed] [Google Scholar]
- Lazam S, Vanoverschelde JL, Tribouilloy C, Grigioni F, Suri RM, Avierinos JF, de Meester, Barbieri A, Rusinaru D, Russo A, Pasquet A, Michelena HI, Huebner M, Maalouf J, Clavel MA, Szymanski C, Enriquez-Sarano M, MIDA Investigators. Twenty-year outcome after mitral repair versus replacement for severe degenerative mitral regurgitation: analysis of a large, prospective, multicenter, international registry. Circulation. 2017;135:410–22. doi: 10.1161/CIRCULATIONAHA.116.023340. [DOI] [PubMed] [Google Scholar]
- Chikwe J, Toyoda N, Anyanwu AC, Itagaki S, Egorova NN, Boateng P, El-Eshmawi A, Adams DH. Relation of mitral valve surgery volume to repair rate, durability, and survival. J Am Coll Cardiol. 2017 doi: 10.1016/j.jacc.2017.02.026. [DOI] [PubMed] [Google Scholar]
- David TE, David CM, Tsang W, Lafreniere-Roula M, Manlhiot C. Long-term results of mitral valve repair for regurgitation due to leaflet prolapse. J Am Coll Cardiol. 2019;74:1044–53. doi: 10.1016/j.jacc.2019.06.052. [DOI] [PubMed] [Google Scholar]
- Donnellan E, Alashi A, Johnston DR, Gillinov AM, Pettersson GB, Svensson LG, Griffin BP, Desai MY. Outcomes of patients with mediastinal radiation-associated mitral valve disease undergoing cardiac surgery. Circulation. 2019;140:1288–90. doi: 10.1161/CIRCULATIONAHA.119.040546. [DOI] [PubMed] [Google Scholar]
- Vakamudi S, Jellis C, Mick S, Wu Y, Gillinov AM, Mihaljevic T, Cosgrove DM, Svensson L, Cho L. Sex differences in the etiology of surgical mitral valve disease. Circulation. 2018;138:1749–51. doi: 10.1161/CIRCULATIONAHA.118.035789. [DOI] [PubMed] [Google Scholar]
- Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, Loghin C, Trento A, Skipper ER, Fudge T, Letsou GV, Massaro JM, Mauri L EVEREST II Investigators. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- Feldman T, Kar S, Elmariah S, Smart SC, Trento A, Siegel RJ, Apruzzese P, Fail P, Rinaldi MJ, Smalling RW, Hermiller JB, Heimansohn D, Gray WA, Grayburn PA, Mack MJ, Lim DS, Ailawadi G, Herrmann HC, Acker MA, Silvestry FE, Foster E, Wang A, Glower DD, Mauri L EVEREST II Investigators. Randomized Comparison of Percutaneous Repair and Surgery for Mitral Regurgitation: 5-Year Results of EVEREST II. J Am Coll Cardiol. 2015;66:2844–54. doi: 10.1016/j.jacc.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Buzzatti N, Van Hemelrijck, Denti P, Ruggeri S, Schiavi D, Scarfò IS, Reser D, Taramasso M, Weber A, La Canna, De Bonis, Maisano F, Alfieri O. Transcatheter or surgical repair for degenerative mitral regurgitation in elderly patients: A propensity-weighted analysis. J Thorac Cardiovasc Surg. 2019;158:86–94. doi: 10.1016/j.jtcvs.2019.01.023. [DOI] [PubMed] [Google Scholar]
- Sorajja P, Vemulapalli S, Feldman T, Mack M, Holmes DR, Stebbins A, Kar S, Thourani V, Ailawadi G. Outcomes with transcatheter mitral valve repair in the United States: an STS/ACC TVT Registry Report. J Am Coll Cardiol. 2017;70:2315–27. doi: 10.1016/j.jacc.2017.09.015. [DOI] [PubMed] [Google Scholar]
- Gammie JS, Bartus K, Gackowski A, D’Ambra MN, Szymanski P, Bilewska A, Kusmierczyk M, Kapelak B, Rzucidlo-Resil J, Moat N, Duncan A, Yadev R, Livesey S, Diprose P, Gerosa G, D’Onofrio A, Pitterello D, Denti P, La Canna, De Bonis, Alfieri O, Hung J, Kolsut P. Beating-heart mitral valve repair using a novel ePTFE cordal implantation device: a prospective trial. J Am Coll Cardiol. 2018;71:25–36. doi: 10.1016/j.jacc.2017.10.062. [DOI] [PubMed] [Google Scholar]
- Lim DS, Kar S, Spargias K, Kipperman RM, O’Neill WW, Ng MKC, Fam NP, Walters DL, Webb JG, Smith RL, Rinaldi MJ, Latib A, Cohen GN, Schafer U, Marcoff L, Vandrangi P, Verta P, Feldman TE. Transcatheter valve repair for patients with mitral regurgitation: 30-day results of the CLASP Study. JACC Cardiovasc Interv. 2019;12:1369–78. doi: 10.1016/j.jcin.2019.04.034. [DOI] [PubMed] [Google Scholar]
- Praz F, Braun D, Unterhuber M, Spirito A, Orban M, Brugger N, Brinkmann I, Spring K, Moschovitis A, Nabauer M, Blazek S, Pilgrim T, Thiele H, Lurz P, Hausleiter J, Windecker S. Edge-to-edge mitral valve repair with extended clip arms: early experience from a multicenter observational study. JACC Cardiovasc Interv. 2019;12:1356–65. doi: 10.1016/j.jcin.2019.03.023. [DOI] [PubMed] [Google Scholar]
- Praz F, Spargias K, Chrissoheris M, Büllesfeld L, Nickenig G, Deuschl F, Schueler R, Fam NP, Moss R, Makar M, Boone R, Edwards J, Moschovitis A, Kar S, Webb J, Schäfer U, Feldman T, Windecker S. Compassionate use of the PASCAL transcatheter mitral valve repair system for patients with severe mitral regurgitation: a multicentre, prospective, observational, first-in-man study. Lancet. 2017;390:773–80. doi: 10.1016/S0140-6736(17)31600-8. [DOI] [PubMed] [Google Scholar]
- Chakravarty T, Makar M, Patel D, Oakley L, Yoon SH, Stegic J, Singh S, Skaf S, Nakamura M, Makkar RR. Transcatheter Edge-to-Edge Mitral Valve Repair With the MitraClip G4 System. JACC Cardiovasc Interv. 2020;13:2402–14. doi: 10.1016/j.jcin.2020.06.053. [DOI] [PubMed] [Google Scholar]
- Piriou N, Al Habash, Donal E, Senage T, Le Tourneau, Pattier S, Guyomarch B, Roussel JC, Trochu JN, Vahanian A, Obadia JF, Iung B, Guerin P. The MITRA-HR study: design and rationale of a randomised study of MitraClip transcatheter mitral valve repair in patients with severe primary mitral regurgitation eligible for high-risk surgery. EuroIntervention. 2019;15:e329–35. doi: 10.4244/EIJ-D-18-01086. [DOI] [PubMed] [Google Scholar]
- Zilberszac R, Heinze G, Binder T, Laufer G, Gabriel H, Rosenhek R. Long-term outcome of active surveillance in severe but asymptomatic primary mitral regurgitation. JACC Cardiovasc Imaging. 2018;11:1213–21. doi: 10.1016/j.jcmg.2018.05.014. [DOI] [PubMed] [Google Scholar]
- Dejgaard LA, Skjolsvik ET, Lie OH, Ribe M, Stokke MK, Hegbom F, Scheirlynck ES, Gjertsen E, Andresen K, Helle-Valle TM, Hopp E, Edvardsen T, Haugaa KH. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol. 2018;72:1600–9. doi: 10.1016/j.jacc.2018.07.070. [DOI] [PubMed] [Google Scholar]
- Han HC, Ha FJ, Teh AW, Calafiore P, Jones EF, Johns J, Koshy AN, O’Donnell D, Hare DL, Farouque O, Lim HS. Mitral valve prolapse and sudden cardiac death: a systematic review. J Am Heart Assoc. 2018;7:e010584. doi: 10.1161/JAHA.118.010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalliah CJ, Mahajan R, Elliott AD, Haqqani H, Lau DH, Vohra JK, Morton JB, Semsarian C, Marwick T, Kalman JM, Sanders P. Mitral valve prolapse and sudden cardiac death: a systematic review and meta-analysis. Heart. 2019;105:144–51. doi: 10.1136/heartjnl-2017-312932. [DOI] [PubMed] [Google Scholar]
- Essayagh B, Sabbag A, Antoine C, Benfari G, Yang LT, Maalouf J, Asirvatham S, Michelena H, Enriquez-Sarano M. Presentation and outcome of arrhythmic mitral valve prolapse. J Am Coll Cardiol. 2020;76:637–49. doi: 10.1016/j.jacc.2020.06.029. [DOI] [PubMed] [Google Scholar]
- Lancellotti P, Pibarot P, Chambers J, Edvardsen T, Delgado V, Dulgheru R, Pepi M, Cosyns B, Dweck MR, Garbi M, Magne J, Nieman K, Rosenhek R, Bernard A, Lowenstein J, Vieira ML, Rabischoffsky A, Vyhmeister RH, Zhou X, Zhang Y, Zamorano JL, Habib G. Recommendations for the imaging assessment of prosthetic heart valves: a report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:589–90. doi: 10.1093/ehjci/jew025. [DOI] [PubMed] [Google Scholar]
- Yu M, Georges A, Tucker NR, Kyryachenko S, Toomer K, Schott JJ, Delling FN, Fernandez-Friera L, Solis J, Ellinor PT, Levine RA, Slaugenhaupt SA, Hagege AA, Dina C, Jeunemaitre X, Milan DJ, Norris RA, Bouatia-Naji N. Genome-wide association study-driven gene-set analyses, genetic, and functional follow-up suggest GLIS1 as a susceptibility gene for mitral valve prolapse. Circ Genom Precis Med. 2019;12:e002497. doi: 10.1161/CIRCGEN.119.002497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani F, Clavel MA, Michelena HI, Suri RM, Schaff HV, Enriquez-Sarano M. Comprehensive imaging in women with organic mitral regurgitation: implications for clinical outcome. JACC Cardiovasc Imaging. 2016;9:388–96. doi: 10.1016/j.jcmg.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol. 2015;65:1231–48. doi: 10.1016/j.jacc.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Bertrand PB, Schwammenthal E, Levine RA, Vandervoort PM. Exercise dynamics in secondary mitral regurgitation: pathophysiology and therapeutic implications. Circulation. 2017;135:297–314. doi: 10.1161/CIRCULATIONAHA.116.025260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deferm S, Bertrand PB, Verbrugge FH, Verhaert D, Rega F, Thomas JD, Vandervoort PM. Atrial functional mitral regurgitation: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;73:2465–76. doi: 10.1016/j.jacc.2019.02.061. [DOI] [PubMed] [Google Scholar]
- Bartko PE, Arfsten H, Heitzinger G, Pavo N, Toma A, Strunk G, Hengstenberg C, Hulsmann M, Goliasch G. A unifying concept for the quantitative assessment of secondary mitral regurgitation. J Am Coll Cardiol. 2019;73:2506–17. doi: 10.1016/j.jacc.2019.02.075. [DOI] [PubMed] [Google Scholar]
- Goliasch G, Bartko PE, Pavo N, Neuhold S, Wurm R, Mascherbauer J, Lang IM, Strunk G, Hulsmann M. Refining the prognostic impact of functional mitral regurgitation in chronic heart failure. Eur Heart J. 2018;39:39–46. doi: 10.1093/eurheartj/ehx402. [DOI] [PubMed] [Google Scholar]
- Michler RE, Smith PK, Parides MK, Ailawadi G, Thourani V, Moskowitz AJ, Acker MA, Hung JW, Chang HL, Perrault LP, Gillinov AM, Argenziano M, Bagiella E, Overbey JR, Moquete EG, Gupta LN, Miller MA, Taddei-Peters WC, Jeffries N, Weisel RD, Rose EA, Gammie JS, DeRose JJ, Puskas JD, Dagenais F, Burks SG, El-Hamamsy I, Milano CA, Atluri P, Voisine P, O’Gara PT, Gelijns AC, CTSN Two-year outcomes of surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2016;374:1932–41. doi: 10.1056/NEJMoa1602003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obadia JF, Messika-Zeitoun D, Leurent G, Lung B, Bonnet G, Piriou N, Lefèvre T, Piot C, Rouleau F, Carrié D, Nejjari M, Ohlmann P, Leclercq F, Saint Etienne, Teiger E, Leroux L, Karam N, Michel N, Gilard M, Donal E, Trochu JN, Cormier B, Armoiry X, Boutitie F, Maucort-Boulch D, Barnel C, Samson G, Guerin P, Vahanian A, Mewton N, MITRA-FR Investigators. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N Engl J Med. 2018;379:2297–306. doi: 10.1056/NEJMoa1805374. [DOI] [PubMed] [Google Scholar]
- Cavalcante JL, Kusunose K, Obuchowski NA, Jellis C, Griffin BP, Flamm SD, Kwon DH. Prognostic impact of ischemic mitral regurgitation severity and myocardial infarct quantification by cardiovascular magnetic resonance. JACC Cardiovasc Imaging. 2020;13:1489–501. doi: 10.1016/j.jcmg.2019.11.008. [DOI] [PubMed] [Google Scholar]
- Kamperidis V, Marsan NA, Delgado V, Bax JJ. Left ventricular systolic function assessment in secondary mitral regurgitation: left ventricular ejection fraction vs. speckle tracking global longitudinal strain. Eur Heart J. 2016;37:811–6. doi: 10.1093/eurheartj/ehv680. [DOI] [PubMed] [Google Scholar]
- Namazi F, van der, Hirasawa K, Kamperidis V, van Wijngaarden, Mertens B, Leon MB, Hahn RT, Stone GW, Narula J, Ajmone Marsan, Delgado V, Bax JJ. Prognostic value of left ventricular global longitudinal strain in patients with secondary mitral regurgitation. J Am Coll Cardiol. 2020;75:750–8. doi: 10.1016/j.jacc.2019.12.024. [DOI] [PubMed] [Google Scholar]
- Kang DH, Park SJ, Shin SH, Hong GR, Lee S, Kim MS, Yun SC, Song JM, Park SW, Kim JJ. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation. 2019;139:1354–65. doi: 10.1161/CIRCULATIONAHA.118.037077. [DOI] [PubMed] [Google Scholar]
- Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–64. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- Acker MA, Jessup M, Bolling SF, Oh J, Starling RC, Mann DL, Sabbah HN, Shemin R, Kirklin J, Kubo SH. Mitral valve repair in heart failure: five-year follow-up from the mitral valve replacement stratum of the Acorn randomized trial. J Thorac Cardiovasc Surg. 2011;142:569–574, 574 e561. doi: 10.1016/j.jtcvs.2010.10.051. [DOI] [PubMed] [Google Scholar]
- Deja MA, Grayburn PA, Sun B, Rao V, She L, Krejca M, Jain AR, Leng Chua, Daly R, Senni M, Mokrzycki K, Menicanti L, Oh JK, Michler R, Wrobel K, Lamy A, Velazquez EJ, Lee KL, Jones RH. Influence of mitral regurgitation repair on survival in the surgical treatment for ischemic heart failure trial. Circulation. 2012;125:2639–48. doi: 10.1161/CIRCULATIONAHA.111.072256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrus AHJ, Dekkers OM, Tops LF, Timmer E, Klautz RJM, Braun J. Impact of recurrent mitral regurgitation after mitral valve repair for functional mitral regurgitation: long-term analysis of competing outcomes. Eur Heart J. 2019;40:2206–14. doi: 10.1093/eurheartj/ehz306. [DOI] [PubMed] [Google Scholar]
- Harmel EK, Reichenspurner H, Girdauskas E. Subannular reconstruction in secondary mitral regurgitation: a meta-analysis. Heart. 2018;104:1783–90. doi: 10.1136/heartjnl-2017-312277. [DOI] [PubMed] [Google Scholar]
- Acker MA, Parides MK, Perrault LP, Moskowitz AJ, Gelijns AC, Voisine P, Smith PK, Hung JW, Blackstone EH, Puskas JD, Argenziano M, Gammie JS, Mack M, Ascheim DD, Bagiella E, Moquete EG, Ferguson TB, Horvath KA, Geller NL, Miller MA, Woo YJ, D’Alessandro DA, Ailawadi G, Dagenais F, Gardner TJ, O’Gara PT, Michler RE, Kron IL, CTSN Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370:23–32. doi: 10.1056/NEJMoa1312808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaljevic T, Lam BK, Rajeswaran J, Takagaki M, Lauer MS, Gillinov AM, Blackstone EH, Lytle BW. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol. 2007;49:2191–201. doi: 10.1016/j.jacc.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Wu AH, Aaronson KD, Bolling SF, Pagani FD, Welch K, Koelling TM. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol. 2005;45:381–7. doi: 10.1016/j.jacc.2004.09.073. [DOI] [PubMed] [Google Scholar]
- Iung B, Armoiry X, Vahanian A, Boutitie F, Mewton N, Trochu JN, Lefèvre T, Messika-Zeitoun D, Guerin P, Cormier B, Brochet E, Thibault H, Himbert D, Thivolet S, Leurent G, Bonnet G, Donal E, Piriou N, Piot C, Habib G, Rouleau F, Carrie D, Nejjari M, Ohlmann P, Saint Etienne, Leroux L, Gilard M, Samson G, Rioufol G, Maucort-Boulch D, Obadia JF, MITRA-FR Investigators. Percutaneous repair or medical treatment for secondary mitral regurgitation: outcomes at 2 years. Eur J Heart Fail. 2019;21:1619–27. doi: 10.1002/ejhf.1616. [DOI] [PubMed] [Google Scholar]
- Stone GW, Lindenfeld JA, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rionaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ COAPT Investigators. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med. 2018;379:2307–18. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- Mack MJ, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant BK, Grayburn PA, Rinaldi MJ, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Rogers JH, Marx SO, Cohen DJ, Weissman NJ, Stone GW COAPT Investigators. 3-Year Outcomes of Transcatheter Mitral Valve Repair in Patients With Heart Failure. J Am Coll Cardiol. 2021;77:1029–40. doi: 10.1016/j.jacc.2020.12.047. [DOI] [PubMed] [Google Scholar]
- Giustino G, Lindenfeld J, Abraham WT, Kar S, Lim DS, Grayburn PA, Kapadia SR, Cohen DJ, Kotinkaduwa LN, Weissman NJ, Mack MJ, Stone GW. NYHA Functional Classification and Outcomes After Transcatheter Mitral Valve Repair in Heart Failure: The COAPT Trial. JACC Cardiovasc Interv. 2020;13:2317–28. doi: 10.1016/j.jcin.2020.06.058. [DOI] [PubMed] [Google Scholar]
- Malik UI, Ambrosy AP, Ku IA, Mishell JM, Kar S, Lim DS, Whisenant BK, Cohen DJ, Arnold SV, Kotinkaduwa LN, Lindenfeld J, Abraham WT, Mack MJ, Stone GW. Baseline functional capacity and transcatheter mitral valve repair in heart failure with secondary mitral regurgitation. JACC Cardiovasc Interv. 2020;13:2331–41. doi: 10.1016/j.jcin.2020.07.030. [DOI] [PubMed] [Google Scholar]
- Kosmidou I, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant BK, Kipperman RM, Boudoulas KD, Redfors B, Shahim B, Zhang Z, Mack MJ, Stone GW. Transcatheter mitral valve repair in patients with and without cardiac resynchronization therapy: the COAPT Trial. Circ Heart Fail. 2020;13:e007293. doi: 10.1161/CIRCHEARTFAILURE.120.007293. [DOI] [PubMed] [Google Scholar]
- Lerakis S, Kini AS, Asch FM, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Weissman NJ, Rinaldi MJ, Sharma SK, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Tang GHL, Li D, Crowley A, Lindenfeld J, Abraham WT, Mack MJ, Stone GW. Outcomes of transcatheter mitral valve repair for secondary mitral regurgitation by severity of left ventricular dysfunction. EuroIntervention. 2021;17:e335–42. doi: 10.4244/EIJ-D-20-01265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz ZM, Herrmann HC, Lim DS, Kar S, Kapadia SR, Reed GW, Puri R, Krishnaswamy A, Gersh BJ, Weissman NJ, Asch FM, Grayburn PA, Kosmidou I, Redfors B, Zhang Z, Abraham WT, Lindenfeld J, Stone GW, Mack MJ. Implications of Atrial Fibrillation on the Mechanisms of Mitral Regurgitation and Response to MitraClip in the COAPT Trial. Circ Cardiovasc Interv. 2021;14:e010300. doi: 10.1161/CIRCINTERVENTIONS.120.010300. [DOI] [PubMed] [Google Scholar]
- Messika-Zeitoun D, Iung B, Armoiry X, Trochu JN, Donal E, Habib G, Brochet E, Thibault H, Piriou N, Cormier B, Tribouilloy C, Guerin P, Lefèvre T, Maucort-Boulch D, Vahanian A, Boutitie F, Obadia JF. Impact of mitral regurgitation severity and left ventricular remodeling on outcome after MitraClip implantation: results from the Mitra-FR Trial. JACC Cardiovasc Imaging. 2021;14:742–52. doi: 10.1016/j.jcmg.2020.07.021. [DOI] [PubMed] [Google Scholar]
- Grayburn PA, Sannino A, Packer M. Proportionate and disproportionate functional mitral regurgitation: a new conceptual framework that reconciles the results of the MITRA-FR and COAPT trials. JACC Cardiovasc Imaging. 2019;12:353–62. doi: 10.1016/j.jcmg.2018.11.006. [DOI] [PubMed] [Google Scholar]
- Mack MJ, Abraham WT, Lindenfeld J, Bolling SF, Feldman TE, Grayburn PA, Kapadia SR, McCarthy PM, Lim DS, Udelson JE, Zile MR, Gammie JS, Gillinov AM, Glower DD, Heimansohn DA, Suri RM, Ellis JT, Shu Y, Kar S, Weissman NJ, Stone GW. Cardiovascular outcomes assessment of the MitraClip in patients with heart failure and secondary mitral regurgitation: design and rationale of the COAPT trial. Am Heart J. 2018;205:1–11. doi: 10.1016/j.ahj.2018.07.021. [DOI] [PubMed] [Google Scholar]
- Asch FM, Grayburn PA, Siegel RJ, Kar S, Lim DS, Zaroff JG, Mishell JM, Whisenant B, Mack MJ, Lindenfeld J, Abraham WT, Stone GW, Weissman NJ COAPT Investigators. Echocardiographic Outcomes After Transcatheter Leaflet Approximation in Patients With Secondary Mitral Regurgitation: The COAPT Trial. J Am Coll Cardiol. 2019;74:2969–79. doi: 10.1016/j.jacc.2019.09.017. [DOI] [PubMed] [Google Scholar]
- Coats AJS, Anker SD, Baumbach A, Alfieri O, von Bardeleben, Bauersachs J, Bax JJ, Boveda S, Celutkiene J, Cleland JG, Dagres N, Deneke T, Farmakis D, Filippatos G, Hausleiter J, Hindricks G, Jankowska EA, Lainscak M, Leclercq C, Lund LH, McDonagh T, Mehra MR, Metra M, Mewton N, Mueller C, Mullens W, Muneretto C, Obadia JF, Ponikowski P, Praz F, Rudolph V, Ruschitzka F, Vahanian A, Windecker S, Zamorano JL, Edvardsen T, Heidbuchel H, Seferovic PM, Prendergast B. The management of secondary mitral regurgitation in patients with heart failure: a joint position statement from the Heart Failure Association (HFA), European Association of Cardiovascular Imaging (EACVI), European Heart Rhythm Association (EHRA), and European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC. Eur Heart J. 2021;42:1254–69. doi: 10.1093/eurheartj/ehab086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pibarot P, Delgado V, Bax JJ. MITRA-FR vs. COAPT: lessons from two trials with diametrically opposed results. Eur Heart J Cardiovasc Imaging. 2019;20:620–4. doi: 10.1093/ehjci/jez073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praz F, Grasso C, Taramasso M, Baumbach A, Piazza N, Tamburino C, Windecker S, Maisano F, Prendergast B. Mitral regurgitation in heart failure: time for a rethink. Eur Heart J. 2019;40:2189–93. doi: 10.1093/eurheartj/ehz222. [DOI] [PubMed] [Google Scholar]
- Adamo M, Grasso C, Capodanno D, Rubbio AP, Scandura S, Giannini C, Fiorelli F, Fiorina C, Branca L, Brambilla N, Bedogni F, Petronio AS, Curello S, Tamburino C. Five-year clinical outcomes after percutaneous edge-to-edge mitral valve repair: insights from the multicenter GRASP-IT registry. Am Heart J. 2019;217:32–41. doi: 10.1016/j.ahj.2019.06.015. [DOI] [PubMed] [Google Scholar]
- Ailawadi G, Lim DS, Mack MJ, Trento A, Kar S, Grayburn PA, Glower DD, Wang A, Foster E, Qasim A, Weissman NJ, Ellis J, Crosson L, Fan F, Kron IL, Pearson PJ, Feldman T, EVEREST II. One-year outcomes after MitraClip for functional mitral regurgitation. Circulation. 2019;139:37–47. doi: 10.1161/CIRCULATIONAHA.117.031733. [DOI] [PubMed] [Google Scholar]
- Iliadis C, Metze C, Korber MI, Baldus S, Pfister R. Impact of COAPT trial exclusion criteria in real-world patients undergoing transcatheter mitral valve repair. Int J Cardiol. 2020;316:189–94. doi: 10.1016/j.ijcard.2020.05.061. [DOI] [PubMed] [Google Scholar]
- Lindenfeld J, Abraham WT, Grayburn PA, Kar S, Asch FM, Lim DS, Nie H, Singhal P, Sundareswaran KS, Weissman NJ, Mack MJ, Stone GW, Cardiovascular Outcomes. Association of effective regurgitation orifice area to left ventricular end-diastolic volume ratio with transcatheter mitral valve repair outcomes: a secondary analysis of the COAPT Trial. JAMA Cardiol. 2021;6:427–36. doi: 10.1001/jamacardio.2020.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RG, Simard T, Kovach C, Flint K, Don C, Di Santo, Adamo M, Branca L, Valentini F, Benito-Gonzalez T, Fernandez-Vazquez F, Estevez-Loureiro R, Berardini A, Conti N, Rapezzi C, Biagini E, Parlow S, Shorr R, Levi A, Manovel A, Cardenal-Piris R, Diaz Fernandez, Shuvy M, Haberman D, Sala A, Alkhouli MA, Marini C, Bargagna M, Schiavi D, Denti P, Markovic S, Buzzatti N, Chan V, Hynes M, Mesana T, Labinaz M, Pappalardo F, Taramasso M, Hibbert B. Transcatheter mitral valve repair in cardiogenic shock and mitral regurgitation: a patient-level, multicenter analysis. JACC Cardiovasc Interv. 2021;14:1–11. doi: 10.1016/j.jcin.2020.08.037. [DOI] [PubMed] [Google Scholar]
- Adamo M, Fiorelli F, Melica B, D’Ortona R, Lupi L, Giannini C, Silva G, Fiorina C, Branca L, Chiari E, Chizzola G, Spontoni P, Espada Guerreiro, Curello S, Petronio AS, Metra M. COAPT-like profile predicts long-term outcomes in patients with secondary mitral regurgitation undergoing MitraClip implantation. JACC Cardiovasc Interv. 2021;14:15–25. doi: 10.1016/j.jcin.2020.09.050. [DOI] [PubMed] [Google Scholar]
- Iung B, Messika-Zeitoun D, Boutitie F, Trochu JN, Armoiry X, Maucort-Boulch D, Obadia JF, Vahanian A. Characteristics and outcome of COAPT-eligible patients in the MITRA-FR Trial. Circulation. 2020;142:2482–4. doi: 10.1161/CIRCULATIONAHA.120.049743. [DOI] [PubMed] [Google Scholar]
- Iung B, Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol. 2014;30:962–70. doi: 10.1016/j.cjca.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Mitral Annulus Calcification. J Am Coll Cardiol. 2015;66:1934–41. doi: 10.1016/j.jacc.2015.08.872. [DOI] [PubMed] [Google Scholar]
- Desnos C, Iung B, Himbert D, Ducrocq G, Urena M, Cormier B, Brochet E, Ou P, Vahanian A, Bouleti C. Temporal trends on percutaneous mitral commissurotomy: 30 years of experience. J Am Heart Assoc. 2019;8:e012031. doi: 10.1161/JAHA.119.012031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andell P, Li X, Martinsson A, Andersson C, Stagmo M, Zoller B, Sundquist K, Smith JG. Epidemiology of valvular heart disease in a Swedish nationwide hospital-based register study. Heart. 2017;103:1696–703. doi: 10.1136/heartjnl-2016-310894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M, EAE/ASE Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10:1–25. doi: 10.1093/ejechocard/jen303. [DOI] [PubMed] [Google Scholar]
- Bouleti C, Iung B, Laouenan C, Himbert D, Brochet E, Messika-Zeitoun D, Detaint D, Garbarz E, Cormier B, Michel PL, Mentre F, Vahanian A. Late results of percutaneous mitral commissurotomy up to 20 years: development and validation of a risk score predicting late functional results from a series of 912 patients. Circulation. 2012;125:2119–27. doi: 10.1161/CIRCULATIONAHA.111.055905. [DOI] [PubMed] [Google Scholar]
- Nunes MC, Tan TC, Elmariah S, do Lago, Margey R, Cruz-Gonzalez I, Zheng H, Handschumacher MD, Inglessis I, Palacios IF, Weyman AE, Hung J. The echo score revisited: impact of incorporating commissural morphology and leaflet displacement to the prediction of outcome for patients undergoing percutaneous mitral valvuloplasty. Circulation. 2014;129:886–95. doi: 10.1161/CIRCULATIONAHA.113.001252. [DOI] [PubMed] [Google Scholar]
- Wilkins GT, Weyman AE, Abascal VM, Block PC, Palacios IF. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988;60:299–308. doi: 10.1136/hrt.60.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badheka AO, Shah N, Ghatak A, Patel NJ, Chothani A, Mehta K, Singh V, Patel N, Grover P, Deshmukh A, Panaich SS, Savani GT, Bhalara V, Arora S, Rathod A, Desai H, Kar S, Alfonso C, Palacios IF, Grines C, Schreiber T, Rihal CS, Makkar R, Cohen MG, O’Neill W, de Marchena. Balloon mitral valvuloplasty in the United States: a 13-year perspective. Am J Med. 2014;127:1126.e1–1126.e12. doi: 10.1016/j.amjmed.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Tomai F, Gaspardone A, Versaci F, Ghini AS, Altamura L, De Luca, Gioffre G, Gioffre PA. Twenty year follow-up after successful percutaneous balloon mitral valvuloplasty in a large contemporary series of patients with mitral stenosis. Int J Cardiol. 2014;177:881–5. doi: 10.1016/j.ijcard.2014.10.040. [DOI] [PubMed] [Google Scholar]
- Bouleti C, Iung B, Himbert D, Messika-Zeitoun D, Brochet E, Garbarz E, Cormier B, Vahanian A. Relationship between valve calcification and long-term results of percutaneous mitral commissurotomy for rheumatic mitral stenosis. Circ Cardiovasc Interv. 2014;7:381–9. doi: 10.1161/CIRCINTERVENTIONS.113.000858. [DOI] [PubMed] [Google Scholar]
- Bouleti C, Iung B, Himbert D, Brochet E, Messika-Zeitoun D, Detaint D, Garbarz E, Cormier B, Vahanian A. Reinterventions after percutaneous mitral commissurotomy during long-term follow-up, up to 20 years: the role of repeat percutaneous mitral commissurotomy. Eur Heart J. 2013;34:1923–30. doi: 10.1093/eurheartj/eht097. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim SH, Myong JP, Kim YR, Kim TS, Kim JH, Jang SW, Oh YS, Lee MY, Rho TH. Outcomes of direct oral anticoagulants in patients with mitral stenosis. J Am Coll Cardiol. 2019;73:1123–31. doi: 10.1016/j.jacc.2018.12.047. [DOI] [PubMed] [Google Scholar]
- Song H, Kang DH, Kim JH, Park KM, Song JM, Choi KJ, Hong MK, Chung CH, Song JK, Lee JW, Park SW, Park SJ. Percutaneous mitral valvuloplasty versus surgical treatment in mitral stenosis with severe tricuspid regurgitation. Circulation. 2007;116:I246–250. doi: 10.1161/CIRCULATIONAHA.107.678151. [DOI] [PubMed] [Google Scholar]
- El Sabbagh, Reddy YNV, Barros-Gomes S, Borlaug BA, Miranda WR, Pislaru SV, Nishimura RA, Pellikka PA. Low-gradient severe mitral stenosis: hemodynamic profiles, clinical characteristics, and outcomes. J Am Heart Assoc. 2019;8:e010736. doi: 10.1161/JAHA.118.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N, Padang R, Scott CG, Guerrero M, Pislaru SV, Pellikka PA. The natural history of severe calcific mitral stenosis. J Am Coll Cardiol. 2020;75:3048–57. doi: 10.1016/j.jacc.2020.04.049. [DOI] [PubMed] [Google Scholar]
- Nishimura RA, Vahanian A, Eleid MF, Mack MJ. Mitral valve disease- -current management and future challenges. Lancet. 2016;387:1324–34. doi: 10.1016/S0140-6736(16)00558-4. [DOI] [PubMed] [Google Scholar]
- Barasch E, Gottdiener JS, Larsen EK, Chaves PH, Newman AB, Manolio TA. Clinical significance of calcification of the fibrous skeleton of the heart and aortosclerosis in community dwelling elderly. The Cardiovascular Health Study (CHS). Am Heart J. 2006;151:39–47. doi: 10.1016/j.ahj.2005.03.052. [DOI] [PubMed] [Google Scholar]
- Alexis SL, Malik AH, El-Eshmawi A, George I, Sengupta A, Kodali SK, Hahn RT, Khalique OK, Zaid S, Guerrero M, Bapat VN, Leon MB, Adams DH, Tang GHL. Surgical and transcatheter mitral valve replacement in mitral annular calcification: a systematic review. J Am Heart Assoc. 2021;10:e018514. doi: 10.1161/JAHA.120.018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno T, Brugger N, Asami M, Heg D, Siontis GCM, Winkel MG, Lanz J, Grani C, Huber A, Stortecky S, George I, Kodali S, Pilgrim T, Windecker S, Khalique OK, Praz F. Clinical impact of mitral calcium volume in patients undergoing transcatheter aortic valve implantation. J Cardiovasc Comput Tomogr. 2021;15:356–65. doi: 10.1016/j.jcct.2020.10.003. [DOI] [PubMed] [Google Scholar]
- Bertrand PB, Churchill TW, Yucel E, Namasivayam M, Bernard S, Nagata Y, He W, Andrews CT, Picard MH, Weyman AE, Levine RA, Hung J. Prognostic importance of the transmitral pressure gradient in mitral annular calcification with associated mitral valve dysfunction. Eur Heart J. 2020;41:4321–8. doi: 10.1093/eurheartj/ehaa819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urena M, Himbert D, Brochet E, Carrasco JL, Iung B, Nataf P, Vahanian A. Transseptal transcatheter mitral valve replacement using balloon-expandable transcatheter heart valves: a step-by-step approach. JACC Cardiovasc Interv. 2017;10:1905–19. doi: 10.1016/j.jcin.2017.06.069. [DOI] [PubMed] [Google Scholar]
- Sud K, Agarwal S, Parashar A, Raza MQ, Patel K, Min D, Rodriguez LL, Krishnaswamy A, Mick SL, Gillinov AM, Tuzcu EM, Kapadia SR. Degenerative mitral stenosis: unmet need for percutaneous interventions. Circulation. 2016;133:1594–604. doi: 10.1161/CIRCULATIONAHA.115.020185. [DOI] [PubMed] [Google Scholar]
- Guerrero M, Urena M, Himbert D, Wang DD, Eleid M, Kodali S, George I, Chakravarty T, Mathur M, Holzhey D, Pershad A, Fang HK, O'Hair D, Jones N, Mahadevan VS, Dumonteil N, Rodés-Cabau J, Piazza N, Ferrari E, Ciaburri D, Nejjari M, DeLago A, Sorajja P, Zahr F, Rajagopal V, Whisenant B, Shah PB, Sinning JM, Witkowski A, Eltchaninoff H, Dvir D, Martin B, Attizzani GF, Gaia D, Nunes NSV, Fassa AA, Kerendi F, Pavlides G, Iyer V, Kaddissi G, Witzke C, Wudel J, Mishkel G, Raybuck B, Wang C, Waksman R, Palacios I, Cribier A, Webb J, Bapat V, Reisman M, Makkar R, Leon M, Rihal C, Vahanian A, O'Neill W, Feldman T. 1-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients With Severe Mitral Annular Calcification. J Am Coll Cardiol. 2018;71:1841–53. doi: 10.1016/j.jacc.2018.02.054. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Whisenant BK, Bleiziffer S, Delgado V, Dhoble A, Schofer N, Eschenbach L, Bansal E, Murdoch DJ, Ancona M, Schmidt T, Yzeiraj E, Vincent F, Niikura H, Kim WK, Asami M, Unbehaun A, Hirji S, Fujita B, Silaschi M, Tang GHL, Kuwata S, Wong SC, Frangieh AH, Barker CM, Davies JE, Lauten A, Deuschl F, Nombela-Franco L, Rampat R, Nicz PFG, Masson JB, Wijeysundera HC, Sievert H, Blackman DJ, Gutierrez-Ibanes E, Sugiyama D, Chakravarty T, Hildick-Smith D, de Brito , Jensen C, Jung C, Smalling RW, Arnold M, Redwood S, Kasel AM, Maisano F, Treede H, Ensminger SM, Kar S, Kaneko T, Pilgrim T, Sorajja P, Van Belle E, Prendergast BD, Bapat V, Modine T, Schofer J, Frerker C, Kempfert J, Attizzani GF, Latib A, Schaefer U, Webb JG, Bax JJ, Makkar RR. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur Heart J. 2019;40:441–51. doi: 10.1093/eurheartj/ehy590. [DOI] [PubMed] [Google Scholar]
- Guerrero M, Vemulapalli S, Xiang Q, Wang DD, Eleid M, Cabalka AK, Sandhu G, Salinger M, Russell H, Greenbaum A, Kodali S, George I, Dvir D, Whisenant B, Russo MJ, Pershad A, Fang K, Coylewright M, Shah P, Babaliaros V, Khan JM, Tommaso C, Saucedo J, Kar S, Makkar R, Mack M, Holmes D, Leon M, Bapat V, Thourani VH, Rihal C, O'Neill W, Feldman T. Thirty-Day Outcomes of Transcatheter Mitral Valve Replacement for Degenerated Mitral Bioprostheses (Valve-in-Valve), Failed Surgical Rings (Valve-in-Ring), and Native Valve With Severe Mitral Annular Calcification (Valve-in-Mitral Annular Calcification) in the United States: Data From the Society of Thoracic Surgeons/American College of Cardiology/Transcatheter Valve Therapy Registry. Circ Cardiovasc Interv. 2020;13:e008425. doi: 10.1161/CIRCINTERVENTIONS.119.008425. [DOI] [PubMed] [Google Scholar]
- Wang DD, Guerrero M, Eng MH, Eleid MF, Meduri CU, Rajagopal V, Yadav PK, Fifer MA, Palacios IF, Rihal CS, Feldman TE, O’Neill WW. Alcohol septal ablation to prevent left ventricular outflow tract obstruction during transcatheter mitral valve replacement: first-in-man study. JACC Cardiovasc Interv. 2019;12:1268–79. doi: 10.1016/j.jcin.2019.02.034. [DOI] [PubMed] [Google Scholar]
- Khan JM, Babaliaros VC, Greenbaum AB, Foerst JR, Yazdani S, McCabe JM, Paone G, Eng MH, Leshnower BG, Gleason PT, Chen MY, Wang DD, Tian X, Stine AM, Rogers T, Lederman RJ. Anterior leaflet laceration to prevent ventricular outflow tract obstruction during transcatheter mitral valve replacement. J Am Coll Cardiol. 2019;73:2521–34. doi: 10.1016/j.jacc.2019.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sabbagh, Eleid MF, Foley TA, Al-Hijji MA, Daly RC, Rihal CS, Said SM. Direct transatrial implantation of balloon-expandable valve for mitral stenosis with severe annular calcifications: early experience and lessons learned. Eur J Cardiothorac Surg. 2018;53:162–9. doi: 10.1093/ejcts/ezx262. [DOI] [PubMed] [Google Scholar]
- Praz F, Khalique OK, Lee R, Veeragandham R, Russell H, Guerrero M, Islam AM, Deaton DW, Kaneko T, Kodali SK, Leon MB, Bapat V, Takayama H, Borger MA, George I. Transatrial implantation of a transcatheter heart valve for severe mitral annular calcification. J Thorac Cardiovasc Surg. 2018;156:132–42. doi: 10.1016/j.jtcvs.2018.03.016. [DOI] [PubMed] [Google Scholar]
- Sorajja P, Gössl M, Babaliaros V, Rizik D, Conradi L, Bae R, Burke RF, Schäfer U, Lisko JC, Riley RD, Guyton R, Dumonteil N, Berthoumieu P, Tchetche D, Blanke P, Cavalcante JL, Sun B. Novel Transcatheter Mitral Valve Prosthesis for Patients With Severe Mitral Annular Calcification. J Am Coll Cardiol. 2019;74:1431–40. doi: 10.1016/j.jacc.2019.07.069. [DOI] [PubMed] [Google Scholar]
- Topilsky Y, Maltais S, Medina Inojosa, Oguz D, Michelena H, Maalouf J, Mahoney DW, Enriquez-Sarano M. Burden of tricuspid regurgitation in patients diagnosed in the community setting. JACC Cardiovasc Imaging. 2019;12:433–42. doi: 10.1016/j.jcmg.2018.06.014. [DOI] [PubMed] [Google Scholar]
- Song H, Kim MJ, Chung CH, Choo SJ, Song MG, Song JM, Kang DH, Lee JW, Song JK. Factors associated with development of late significant tricuspid regurgitation after successful left-sided valve surgery. Heart. 2009;95:931–6. doi: 10.1136/hrt.2008.152793. [DOI] [PubMed] [Google Scholar]
- Kwak JJ, Kim YJ, Kim MK, Kim HK, Park JS, Kim KH, Kim KB, Ahn H, Sohn DW, Oh BH, Park YB. Development of tricuspid regurgitation late after left-sided valve surgery: a single-center experience with long-term echocardiographic examinations. Am Heart J. 2008;155:732–7. doi: 10.1016/j.ahj.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Ortiz-Leon XA, Posada-Martinez EL, Trejo-Paredes MC, Ivey-Miranda JB, Pereira J, Crandall I, DaSilva P, Bouman E, Brooks A, Gerardi C, Ugonabo I, Chen W, Houle H, Akar JG, Lin BA, McNamara RL, Lombo-Lievano B, Arias-Godinez JA, Sugeng L. Understanding tricuspid valve remodelling in atrial fibrillation using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging. 2020;21:747–55. doi: 10.1093/ehjci/jeaa058. [DOI] [PubMed] [Google Scholar]
- Kim JB, Spevack DM, Tunick PA, Bullinga JR, Kronzon I, Chinitz LA, Reynolds HR. The effect of transvenous pacemaker and implantable cardioverter defibrillator lead placement on tricuspid valve function: an observational study. J Am Soc Echocardiogr. 2008;21:284–7. doi: 10.1016/j.echo.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Höke U, Auger D, Thijssen J, Wolterbeek R, van der Velde ET, Holman ER, Schalij MJ, Bax JJ, Delgado V, Marsan NA. Significant lead-induced tricuspid regurgitation is associated with poor prognosis at long-term follow-up. Heart. 2014;100:960–8. doi: 10.1136/heartjnl-2013-304673. [DOI] [PubMed] [Google Scholar]
- Anvardeen K, Rao R, Hazra S, Hay K, Dai H, Stoyanov N, Birnie D, Dwivedi G, Chan KL. Prevalence and Significance of Tricuspid Regurgitation Post-Endocardial Lead Placement. JACC Cardiovasc Imaging. 2019;12:562–4. doi: 10.1016/j.jcmg.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Prihadi EA, van der, Gursoy E, Abou R, Mara Vollema, Hahn RT, Stone GW, Leon MB, Ajmone Marsan, Delgado V, Bax JJ. Development of significant tricuspid regurgitation over time and prognostic implications: new insights into natural history. Eur Heart J. 2018;39:3574–81. doi: 10.1093/eurheartj/ehy352. [DOI] [PubMed] [Google Scholar]
- Benfari G, Antoine C, Miller WL, Thapa P, Topilsky Y, Rossi A, Michelena HI, Pislaru S, Enriquez-Sarano M. Excess mortality associated with functional tricuspid regurgitation complicating heart failure with reduced ejection fraction. Circulation. 2019;140:196–206. doi: 10.1161/CIRCULATIONAHA.118.038946. [DOI] [PubMed] [Google Scholar]
- Dietz MF, Prihadi EA, van der, Goedemans L, Mertens BJA, Gursoy E, van Genderen, Ajmone Marsan, Delgado V, Bax JJ. Prognostic implications of right ventricular remodeling and function in patients with significant secondary tricuspid regurgitation. Circulation. 2019;140:836–45. doi: 10.1161/CIRCULATIONAHA.119.039630. [DOI] [PubMed] [Google Scholar]
- Muraru D, Badano LP, Nagata Y, Surkova E, Nabeshima Y, Genovese D, Otsuji Y, Guida V, Azzolina D, Palermo C, Takeuchi M. Development and prognostic validation of partition values to grade right ventricular dysfunction severity using 3D echocardiography. Eur Heart J Cardiovasc Imaging. 2020;21:10–21. doi: 10.1093/ehjci/jez233. [DOI] [PubMed] [Google Scholar]
- Park JB, Lee SP, Lee JH, Yoon YE, Park EA, Kim HK, Lee W, Kim YJ, Cho GY, Sohn DW. Quantification of right ventricular volume and function using single-beat three-dimensional echocardiography: a validation study with cardiac magnetic resonance. J Am Soc Echocardiogr. 2016;29:392–401. doi: 10.1016/j.echo.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J. 2004;147:218–23. doi: 10.1016/j.ahj.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Song JM, Jang MK, Choi YS, Kim YJ, Min SY, Kim DH, Kang DH, Song JK. The vena contracta in functional tricuspid regurgitation: a real-time three-dimensional color Doppler echocardiography study. J Am Soc Echocardiogr. 2011;24:663–70. doi: 10.1016/j.echo.2011.01.005. [DOI] [PubMed] [Google Scholar]
- de Agustin, Viliani D, Vieira C, Islas F, Marcos-Alberca P, Gomez de, Nunez-Gil IJ, Almeria C, Rodrigo JL, Luaces M, Garcia-Fernandez MA, Macaya C, Perez de. Proximal isovelocity surface area by single-beat three-dimensional color Doppler echocardiography applied for tricuspid regurgitation quantification. J Am Soc Echocardiogr. 2013;26:1063–72. doi: 10.1016/j.echo.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Chen TE, Kwon SH, Enriquez-Sarano M, Wong BF, Mankad SV. Three-dimensional color Doppler echocardiographic quantification of tricuspid regurgitation orifice area: comparison with conventional two-dimensional measures. J Am Soc Echocardiogr. 2013;26:1143–52. doi: 10.1016/j.echo.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Dahou A, Ong G, Hamid N, Avenatti E, Yao J, Hahn RT. Quantifying Tricuspid Regurgitation Severity: A Comparison of Proximal Isovelocity Surface Area and Novel Quantitative Doppler Methods. JACC Cardiovasc Imaging. 2019;12:560–2. doi: 10.1016/j.jcmg.2018.11.015. [DOI] [PubMed] [Google Scholar]
- Utsunomiya H, Harada Y, Susawa H, Takahari K, Ueda Y, Izumi K, Itakura K, Ikenaga H, Hidaka T, Fukuda Y, Shiota T, Kihara Y. Comprehensive evaluation of tricuspid regurgitation location and severity using vena contracta analysis: a color Doppler three-dimensional transesophageal echocardiographic study. J Am Soc Echocardiogr. 2019;32:1526–1537.e2. doi: 10.1016/j.echo.2019.07.022. [DOI] [PubMed] [Google Scholar]
- Hahn RT, Zamorano JL. The need for a new tricuspid regurgitation grading scheme. Eur Heart J Cardiovasc Imaging. 2017;18:1342–3. doi: 10.1093/ehjci/jex139. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Weber M, Lurz P, von Bardeleben, Sitges M, Sorajja P, Hausleiter J, Denti P, Trochu JN, Nabauer M, Dahou A, Hahn RT. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet. 2019;394:2002–11. doi: 10.1016/S0140-6736(19)32600-5. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Weber M, Schueler R, Hausleiter J, Nabauer M, von Bardeleben, Sotiriou E, Schafer U, Deuschl F, Kuck KH, Kreidel F, Juliard JM, Brochet E, Latib A, Agricola E, Baldus S, Friedrichs K, Vandrangi P, Verta P, Hahn RT, Maisano F. 6-Month outcomes of tricuspid valve reconstruction for patients with severe tricuspid regurgitation. J Am Coll Cardiol. 2019;73:1905–15. doi: 10.1016/j.jacc.2019.01.062. [DOI] [PubMed] [Google Scholar]
- Santoro C, Marco Del, Gonzalez-Gomez A, Monteagudo JM, Hinojar R, Lorente A, Abellas M, Vieitez JM, Garcia Martin, Casas Rojo, Ruiz S, Barrios V, Luis Moya, Jimenez-Nacher JJ, Zamorano Gomez, Fernandez-Golfin C. Mid-term outcome of severe tricuspid regurgitation: are there any differences according to mechanism and severity? Eur Heart J Cardiovasc Imaging. 2019;20:1035–42. doi: 10.1093/ehjci/jez024. [DOI] [PubMed] [Google Scholar]
- Miura M, Alessandrini H, Alkhodair A, Attinger-Toller A, Biasco L, Lurz P, Braun D, Brochet E, Connelly KA, de Bruijn, Denti P, Deuschl F, Estevez-Loureiro R, Fam N, Frerker C, Gavazzoni M, Hausleiter J, Himbert D, Ho E, Juliard JM, Kaple R, Besler C, Kodali S, Kreidel F, Kuck KH, Latib A, Lauten A, Monivas V, Mehr M, Muntane-Carol G, Nazif T, Nickenig G, Pedrazzini G, Philippon F, Pozzoli A, Praz F, Puri R, Rodes-Cabau J, Schafer U, Schofer J, Sievert H, Tang GHL, Thiele H, Rommel KP, Vahanian A, Von Bardeleben, Webb JG, Weber M, Windecker S, Winkel M, Zuber M, Leon MB, Maisano F, Hahn RT, Taramasso M, TriValve Investigators. Impact of massive or torrential tricuspid regurgitation in patients undergoing transcatheter tricuspid valve intervention. JACC Cardiovasc Interv. 2020;13:1999–2009. doi: 10.1016/j.jcin.2020.05.011. [DOI] [PubMed] [Google Scholar]
- Peri Y, Sadeh B, Sherez C, Hochstadt A, Biner S, Aviram G, Ingbir M, Nachmany I, Topaz G, Flint N, Keren G, Topilsky Y. Quantitative assessment of effective regurgitant orifice: impact on risk stratification, and cut-off for severe and torrential tricuspid regurgitation grade. Eur Heart J Cardiovasc Imaging. 2020;21:768–76. doi: 10.1093/ehjci/jez267. [DOI] [PubMed] [Google Scholar]
- Stocker TJ, Hertell H, Orban M, Braun D, Rommel KP, Ruf T, Ong G, Nabauer M, Deseive S, Fam N, von Bardeleben, Thiele H, Massberg S, Lurz P, Hausleiter J. Cardiopulmonary Hemodynamic Profile Predicts Mortality After Transcatheter Tricuspid Valve Repair in Chronic Heart Failure. JACC Cardiovasc Interv. 2021;14:29–38. doi: 10.1016/j.jcin.2020.09.033. [DOI] [PubMed] [Google Scholar]
- Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43:405–9. doi: 10.1016/j.jacc.2003.09.036. [DOI] [PubMed] [Google Scholar]
- Topilsky Y, Nkomo VT, Vatury O, Michelena HI, Letourneau T, Suri RM, Pislaru S, Park S, Mahoney DW, Biner S, Enriquez-Sarano M. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging. 2014;7:1185–94. doi: 10.1016/j.jcmg.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Chorin E, Rozenbaum Z, Topilsky Y, Konigstein M, Ziv-Baran T, Richert E, Keren G, Banai S. Tricuspid regurgitation and long-term clinical outcomes. Eur Heart J Cardiovasc Imaging. 2020;21:157–65. doi: 10.1093/ehjci/jez216. [DOI] [PubMed] [Google Scholar]
- Topilsky Y, Inojosa JM, Benfari G, Vaturi O, Maltais S, Michelena H, Mankad S, Enriquez-Sarano M. Clinical presentation and outcome of tricuspid regurgitation in patients with systolic dysfunction. Eur Heart J. 2018;39:3584–92. doi: 10.1093/eurheartj/ehy434. [DOI] [PubMed] [Google Scholar]
- Kadri AN, Menon V, Sammour YM, Gajulapalli RD, Meenakshisundaram C, Nusairat L, Mohananey D, Hernandez AV, Navia J, Krishnaswamy A, Griffin B, Rodriguez L, Harb SC, Kapadia S. Outcomes of patients with severe tricuspid regurgitation and congestive heart failure. Heart. 2019;105:1813–7. doi: 10.1136/heartjnl-2019-315004. [DOI] [PubMed] [Google Scholar]
- Stuge O, Liddicoat J. Emerging opportunities for cardiac surgeons within structural heart disease. J Thorac Cardiovasc Surg. 2006;132:1258–61. doi: 10.1016/j.jtcvs.2006.08.049. [DOI] [PubMed] [Google Scholar]
- Kilic A, Saha-Chaudhuri P, Rankin JS, Conte JV. Trends and outcomes of tricuspid valve surgery in North America: an analysis of more than 50,000 patients from the Society of Thoracic Surgeons database. Ann Thorac Surg. 2013;96:1546–52. doi: 10.1016/j.athoracsur.2013.06.031. [DOI] [PubMed] [Google Scholar]
- Dreyfus J, Ghalem N, Garbarz E, Cimadevilla C, Nataf P, Vahanian A, Caranhac G, Messika-Zeitoun D. Timing of referral of patients with severe isolated tricuspid valve regurgitation to surgeons (from a French nationwide database). Am J Cardiol. 2018;122:323–6. doi: 10.1016/j.amjcard.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Antunes MJ, Rodriguez-Palomares J, Prendergast B, De Bonis M, Rosenhek R, Al-Attar N, Barili F, Casselman F, Folliguet T, Iung B, Lancellotti P, Muneretto C, Obadia JF, Pierard L, Suwalski P, Zamorano P ESC Working Groups of Cardiovascular Surgery and Valvular Heart Disease. Management of tricuspid valve regurgitation: Position statement of the European Society of Cardiology Working Groups of Cardiovascular Surgery and Valvular Heart Disease. Eur J Cardiothorac Surg. 2017;52:1022–30. doi: 10.1093/ejcts/ezx279. [DOI] [PubMed] [Google Scholar]
- Dreyfus GD, Corbi PJ, Chan KM, Bahrami T. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg. 2005;79:127–32. doi: 10.1016/j.athoracsur.2004.06.057. [DOI] [PubMed] [Google Scholar]
- Van de, Braun J, Delgado V, Versteegh MI, Dion RA, Klautz RJ, Bax JJ. Tricuspid annuloplasty prevents right ventricular dilatation and progression of tricuspid regurgitation in patients with tricuspid annular dilatation undergoing mitral valve repair. J Thorac Cardiovasc Surg. 2011;141:1431–9. doi: 10.1016/j.jtcvs.2010.05.050. [DOI] [PubMed] [Google Scholar]
- Chikwe J, Itagaki S, Anyanwu A, Adams DH. Impact of concomitant tricuspid annuloplasty on tricuspid regurgitation, right ventricular function, and pulmonary artery hypertension after repair of mitral valve prolapse. J Am Coll Cardiol. 2015;65:1931–8. doi: 10.1016/j.jacc.2015.01.059. [DOI] [PubMed] [Google Scholar]
- Badhwar V, Rankin JS, He M, Jacobs JP, Furnary AP, Fazzalari FL, O’Brien S, Gammie JS, Shahian DM. Performing concomitant tricuspid valve repair at the time of mitral valve operations is not associated with increased operative mortality. Ann Thorac Surg. 2017;103:587–93. doi: 10.1016/j.athoracsur.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Brescia AA, Ward ST, Watt TMF, Rosenbloom LM, Baker M, Khan S, Ziese E, Romano MA, Bolling SF, Michigan Mitral. Outcomes of guideline-directed concomitant annuloplasty for functional tricuspid regurgitation. Ann Thorac Surg. 2020;109:1227–32. doi: 10.1016/j.athoracsur.2019.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell AL, Bhambhani V, Moonsamy P, Healy EW, Picard MH, Sundt TM, Wasfy JH. Surgery does not improve survival in patients with isolated severe tricuspid regurgitation. J Am Coll Cardiol. 2019;74:715–25. doi: 10.1016/j.jacc.2019.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack CJ, Fender EA, Chandrashekar P, Reddy YNV, Bennett CE, Stulak JM, Miller VM, Nishimura RA. National trends and outcomes in isolated tricuspid valve surgery. J Am Coll Cardiol. 2017;70:2953–60. doi: 10.1016/j.jacc.2017.10.039. [DOI] [PubMed] [Google Scholar]
- Dhoble A, Zhao Y, Vejpongsa P, Loghin C, Smalling RW, Estrera A, Nguyen TC. National 10-year trends and outcomes of isolated and concomitant tricuspid valve surgery. J Cardiovasc Surg (Torino) 2019;60:119–27. doi: 10.23736/S0021-9509.18.10468-X. [DOI] [PubMed] [Google Scholar]
- Alqahtani F, Berzingi CO, Aljohani S, Hijazi M, Al-Hallak A, Alkhouli M. Contemporary Trends in the Use and Outcomes of Surgical Treatment of Tricuspid Regurgitation. J Am Heart Assoc. 2017;6:e007597. doi: 10.1161/JAHA.117.007597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus J, Flagiello M, Bazire B, Eggenspieler F, Viau F, Riant E, Mbaki Y, Bohbot Y, Eyharts D, Senage T, Dubrulle H, Nicol M, Doguet F, Nguyen V, Coisne A, Le Tourneau, Lavie-Badie Y, Tribouilloy C, Donal E, Tomasi J, Habib G, Selton-Suty C, Raffoul R, Iung B, Obadia JF, Messika-Zeitoun D. Isolated tricuspid valve surgery: impact of aetiology and clinical presentation on outcomes. Eur Heart J. 2020;41:4304–17. doi: 10.1093/eurheartj/ehaa643. [DOI] [PubMed] [Google Scholar]
- Hamandi M, Smith RL, Ryan WH, Grayburn PA, Vasudevan A, George TJ, Dimaio JM, Hutcheson KA, Brinkman W, Szerlip M, Moore DO, Mack MJ. Outcomes of Isolated Tricuspid Valve Surgery Have Improved in the Modern Era. Ann Thorac Surg. 2019;108:11–5. doi: 10.1016/j.athoracsur.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Dietz MF, Prihadi EA, van der, Ajmone Marsan, Delgado V, Bax JJ. Prognostic implications of staging right heart failure in patients with significant secondary tricuspid regurgitation. JACC Heart Fail. 2020;8:627–36. doi: 10.1016/j.jchf.2020.02.008. [DOI] [PubMed] [Google Scholar]
- McCarthy PM, Bhudia SK, Rajeswaran J, Hoercher KJ, Lytle BW, Cosgrove DM, Blackstone EH. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg. 2004;127:674–85. doi: 10.1016/j.jtcvs.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Chang BC, Lim SH, Yi G, Hong YS, Lee S, Yoo KJ, Kang MS, Cho BK. Long-term clinical results of tricuspid valve replacement. Ann Thorac Surg. 2006;81:1317–23. doi: 10.1016/j.athoracsur.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Glikson M, Nielsen JC, Michowitz Y, Kronborg MB, Auricchio A, Barbash IM, Barrabés JA, Boriani G, Braunschweig F, Brignole M, Burri H, Coats AJS, Deharo JC, Delgado V, Diller GP, Israel CW, Keren A, Knops RE, Kotecha D, Leclercq C, Merkely B, Starck C, Thylén I, Tolosana JM ESC Scientific Document Group. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021 doi: 10.1093/eurheartj/ehab699. [DOI] [PubMed] [Google Scholar]
- Fam NP, Braun D, von Bardeleben, Nabauer M, Ruf T, Connelly KA, Ho E, Thiele H, Lurz P, Weber M, Nickenig G, Narang A, Davidson CJ, Hausleiter J. Compassionate use of the PASCAL transcatheter valve repair system for severe tricuspid regurgitation: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2019;12:2488–95. doi: 10.1016/j.jcin.2019.09.046. [DOI] [PubMed] [Google Scholar]
- Lurz P, Stephan von Bardeleben R, Weber M, Sitges M, Sorajja P, Hausleiter J, Denti P, Trochu JN, Nabauer M, Tang GHL, Biaggi P, Ying SW, Trusty PM, Dahou A, Hahn RT, Nickenig G TRILUMINATE Investigators. Transcatheter Edge-to-Edge Repair for Treatment of Tricuspid Regurgitation. J Am Coll Cardiol. 2021;77:229–39. doi: 10.1016/j.jacc.2020.11.038. [DOI] [PubMed] [Google Scholar]
- Kodali S, Hahn RT, Eleid MF, Kipperman R, Smith R, Lim DS, Gray WA, Narang A, Pislaru SV, Koulogiannis K, Grayburn P, Fowler D, Hawthorne K, Dahou A, Deo SH, Vandrangi P, Deuschl F, Mack MJ, Leon MB, Feldman T, Davidson CJ CLASP TR EFS Investigators. Feasibility Study of the Transcatheter Valve Repair System for Severe Tricuspid Regurgitation. J Am Coll Cardiol. 2021;77:345–56. doi: 10.1016/j.jacc.2020.11.047. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Weber M, Schuler R, Hausleiter J, Nabauer M, von Bardeleben, Sotiriou E, Schafer U, Deuschl F, Alessandrini H, Kreidel F, Juliard JM, Brochet E, Latib A, Montorfano M, Agricola E, Baldus S, Friedrichs KP, Deo SH, Gilmore SY, Feldman T, Hahn RT, Maisano F. Tricuspid valve repair with the Cardioband system: two-year outcomes of the multicentre, prospective TRI-REPAIR study. EuroIntervention. 2021;16:e1264–71. doi: 10.4244/EIJ-D-20-01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn RT, Kodali S, Fam N, Bapat V, Bartuś K, Rodés-Cabau J, Dagenais F, Estevez-Loureiro R, Forteza A, Kapadia S, Latib A, Maisano F, McCarthy P, Navia J, Ong G, Peterson M, Petrossian G, Pozzoli A, Reinartz M, Ricciardi MJ, Robinson N, Sievert H, Taramasso M, Agarwal V, Bèdard E, Tarantini G, Colli A. Early Multinational Experience of Transcatheter Tricuspid Valve Replacement for Treating Severe Tricuspid Regurgitation. JACC Cardiovasc Interv. 2020;13:2482–93. doi: 10.1016/j.jcin.2020.07.008. [DOI] [PubMed] [Google Scholar]
- Fam NP, von Bardeleben RS, Hensey M, Kodali SK, Smith RL, Hausleiter J, Ong G, Boone R, Ruf T, George I, Szerlip M, Nabauer M, Ali FM, Moss R, Bapat V, Schnitzler K, Kreidel F, Ye J, Deva DP, Mack MJ, Grayburn PA, Peterson MD, Leon MB, Hahn RT, Webb JG. Transfemoral Transcatheter Tricuspid Valve Replacement With the EVOQUE System: A Multicenter, Observational, First-in-Human Experience. JACC Cardiovasc Interv. 2021;14:501–11. doi: 10.1016/j.jcin.2020.11.045. [DOI] [PubMed] [Google Scholar]
- Lu FL, Ma Y, An Z, Cai CL, Li BL, Song ZG, Han L, Wang J, Qiao F, Xu ZY. First-in-Man Experience of Transcatheter Tricuspid Valve Replacement With LuX-Valve in High-Risk Tricuspid Regurgitation Patients. JACC Cardiovasc Interv. 2020;13:1614–6. doi: 10.1016/j.jcin.2020.03.026. [DOI] [PubMed] [Google Scholar]
- Rommel KP, Besler C, Noack T, Blazek S, von Roeder, Fengler K, Ender J, Gutberlet M, Desch S, Borger MA, Thiele H, Lurz P. Physiological and clinical consequences of right ventricular volume overload reduction after transcatheter treatment for tricuspid regurgitation. JACC Cardiovasc Interv. 2019;12:1423–34. doi: 10.1016/j.jcin.2019.02.042. [DOI] [PubMed] [Google Scholar]
- Montalto C, Sticchi A, Crimi G, Laricchia A, Khokhar A, Giannini F, Ferlini M, Colombo A, Latib A, Mangieri A. Functional and echocardiographic improvement after transcatheter repair for tricuspid regurgitation: a systematic review and pooled analysis. JACC Cardiovasc Interv. 2020;13:2719–29. doi: 10.1016/j.jcin.2020.08.020. [DOI] [PubMed] [Google Scholar]
- Taramasso M, Benfari G, van der, Alessandrini H, Attinger-Toller A, Biasco L, Lurz P, Braun D, Brochet E, Connelly KA, de Bruijn, Denti P, Deuschl F, Estevez-Loureiro R, Fam N, Frerker C, Gavazzoni M, Hausleiter J, Ho E, Juliard JM, Kaple R, Besler C, Kodali S, Kreidel F, Kuck KH, Latib A, Lauten A, Monivas V, Mehr M, Muntané-Carol G, Nazif T, Nickening G, Pedrazzini G, Philippon F, Pozzoli A, Praz F, Puri R, Rodés-Cabau J, Schäfer U, Schofer J, Sievert H, Tang GHL, Thiele H, Topilsky Y, Rommel KP, Delgado V, Vahanian A, Von Bardeleben, Webb JG, Weber M, Windecker S, Winkel M, Zuber M, Leon MB, Hahn RT, Bax JJ, Enriquez-Sarano M, Maisano F. Transcatheter Versus Medical Treatment of Patients With Symptomatic Severe Tricuspid Regurgitation. J Am Coll Cardiol. 2019;74:2998–3008. doi: 10.1016/j.jacc.2019.09.028. [DOI] [PubMed] [Google Scholar]
- Prihadi EA, Delgado V, Hahn RT, Leipsic J, Min JK, Bax JJ. Imaging needs in novel transcatheter tricuspid valve interventions. JACC Cardiovasc Imaging. 2018;11:736–54. doi: 10.1016/j.jcmg.2017.10.029. [DOI] [PubMed] [Google Scholar]
- Hahn RT. State-of-the-art review of echocardiographic imaging in the evaluation and treatment of functional tricuspid regurgitation. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.116.005332. [DOI] [PubMed] [Google Scholar]
- Utsunomiya H, Itabashi Y, Mihara H, Berdejo J, Kobayashi S, Siegel RJ, Shiota T. Functional tricuspid regurgitation caused by chronic atrial fibrillation: a real-time 3-dimensional transesophageal echocardiography study. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.116.004897. [DOI] [PubMed] [Google Scholar]
- Filsoufi F, Anyanwu AC, Salzberg SP, Frankel T, Cohn LH, Adams DH. Long-term outcomes of tricuspid valve replacement in the current era. Ann Thorac Surg. 2005;80:845–50. doi: 10.1016/j.athoracsur.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Yeter E, Ozlem K, Kilic H, Ramazan A, Acikel S. Tricuspid balloon valvuloplasty to treat tricuspid stenosis. J Heart Valve Dis. 2010;19:159–60. [PubMed] [Google Scholar]
- Unger P, Pibarot P, Tribouilloy C, Lancellotti P, Maisano F, Iung B, Pierard L, European Society. Multiple and mixed valvular heart diseases. Circ Cardiovasc Imaging. 2018;11:e007862. doi: 10.1161/CIRCIMAGING.118.007862. [DOI] [PubMed] [Google Scholar]
- Egbe AC, Luis SA, Padang R, Warnes CA. Outcomes in moderate mixed aortic valve disease: is it time for a paradigm shift? J Am Coll Cardiol. 2016;67:2321–9. doi: 10.1016/j.jacc.2016.03.509. [DOI] [PubMed] [Google Scholar]
- Egbe AC, Poterucha JT, Warnes CA. Mixed aortic valve disease: midterm outcome and predictors of adverse events. Eur Heart J. 2016;37:2671–8. doi: 10.1093/eurheartj/ehw079. [DOI] [PubMed] [Google Scholar]
- Zilberszac R, Gabriel H, Schemper M, Zahler D, Czerny M, Maurer G, Rosenhek R. Outcome of combined stenotic and regurgitant aortic valve disease. J Am Coll Cardiol. 2013;61:1489–95. doi: 10.1016/j.jacc.2012.11.070. [DOI] [PubMed] [Google Scholar]
- Unger P, Tribouilloy C. Aortic stenosis with other concomitant valvular disease: aortic regurgitation, mitral regurgitation, mitral stenosis, or tricuspid regurgitation. Cardiol Clin. 2020;38:33–46. doi: 10.1016/j.ccl.2019.09.002. [DOI] [PubMed] [Google Scholar]
- Philip JL, Zens T, Lozonschi L, De Oliveira, Osaki S, Kohmoto T, Akhter SA, Tang PC. Outcomes of surgical aortic valve replacement for mixed aortic valve disease. J Thorac Dis. 2018;10:4042–51. doi: 10.21037/jtd.2018.06.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine J, Kadri AN, Gajulapalli RD, Krishnaswamy A, Mick S, Perez O, Lak H, Nair RM, Montane B, Tak J, Tuzcu EM, Griffin B, Svensson LG, Harb SC, Kapadia SR. Outcomes of Transcatheter Aortic Valve Replacement in Mixed Aortic Valve Disease. JACC Cardiovasc Interv. 2019;12:2299–306. doi: 10.1016/j.jcin.2019.06.020. [DOI] [PubMed] [Google Scholar]
- Yang LT, Enriquez-Sarano M, Scott CG, Padang R, Maalouf JF, Pellikka PA, Michelena HI. Concomitant mitral regurgitation in patients with chronic aortic regurgitation. J Am Coll Cardiol. 2020;76:233–46. doi: 10.1016/j.jacc.2020.05.051. [DOI] [PubMed] [Google Scholar]
- Mehr M, Karam N, Taramasso M, Ouarrak T, Schneider S, Lurz P, von Bardeleben, Fam N, Pozzoli A, Lubos E, Boekstegers P, Schillinger W, Plicht B, Eggebrecht H, Baldus S, Senges J, Maisano F, Hausleiter J TriValve and TRAMI Investigators. Combined Tricuspid and Mitral Versus Isolated Mitral Valve Repair for Severe MR and TR: An Analysis From the TriValve and TRAMI Registries. JACC Cardiovasc Interv. 2020;13:543–50. doi: 10.1016/j.jcin.2019.10.023. [DOI] [PubMed] [Google Scholar]
- Goldstone AB, Chiu P, Baiocchi M, Lingala B, Patrick WL, Fischbein MP, Woo YJ. Mechanical or biologic prostheses for aortic-valve and mitral-valve replacement. N Engl J Med. 2017;377:1847–57. doi: 10.1056/NEJMoa1613792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head SJ, Celik M, Kappetein AP. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J. 2017;38:2183–91. doi: 10.1093/eurheartj/ehx141. [DOI] [PubMed] [Google Scholar]
- Diaz R, Hernandez-Vaquero D, Alvarez-Cabo R, Avanzas P, Silva J, Moris C, Pascual I. Long-term outcomes of mechanical versus biological aortic valve prosthesis: systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2019;158:706–714.e18. doi: 10.1016/j.jtcvs.2018.10.146. [DOI] [PubMed] [Google Scholar]
- David TE, Ouzounian M, David CM, Lafreniere-Roula M, Manlhiot C. Late results of the Ross procedure. J Thorac Cardiovasc Surg. 2019;157:201–8. doi: 10.1016/j.jtcvs.2018.06.037. [DOI] [PubMed] [Google Scholar]
- Malik AH, Yandrapalli S, Aronow WS, Panza JA, Cooper HA. Oral anticoagulants in atrial fibrillation with valvular heart disease and bioprosthetic heart valves. Heart. 2019;105:1432–6. doi: 10.1136/heartjnl-2019-314767. [DOI] [PubMed] [Google Scholar]
- Duan L, Doctor JN, Adams JL, Romley JA, Nguyen LA, An J, Lee MS. Comparison of direct oral anticoagulants versus warfarin in patients with atrial fibrillation and bioprosthetic heart valves. Am J Cardiol. 2021;146:22–8. doi: 10.1016/j.amjcard.2021.01.016. [DOI] [PubMed] [Google Scholar]
- Pasciolla S, Zizza LF, Le T, Wright K. Comparison of the efficacy and safety of direct oral anticoagulants and warfarin after bioprosthetic valve replacements. Clin Drug Investig. 2020;40:839–45. doi: 10.1007/s40261-020-00939-x. [DOI] [PubMed] [Google Scholar]
- Russo V, Carbone A, Attena E, Rago A, Mazzone C, Proietti R, Parisi V, Scotti A, Nigro G, Golino P, D’Onofrio A. Clinical benefit of direct oral anticoagulants versus vitamin K antagonists in patients with atrial fibrillation and bioprosthetic heart valves. Clin Ther. 2019;41:2549–57. doi: 10.1016/j.clinthera.2019.10.008. [DOI] [PubMed] [Google Scholar]
- Capodanno D, Petronio AS, Prendergast B, Eltchaninoff H, Vahanian A, Modine T, Lancellotti P, Sondergaard L, Ludman PF, Tamburino C, Piazza N, Hancock J, Mehilli J, Byrne RA, Baumbach A, Kappetein AP, Windecker S, Bax J, Haude M. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS ). Eur Heart J. 2017;38:3382–90. doi: 10.1093/eurheartj/ehx303. [DOI] [PubMed] [Google Scholar]
- Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA, Khandheria BK, Levine RA, Marx GR, Miller FA , Nakatani S, Quinones MA, Rakowski H, Rodriguez LL, Swaminathan M, Waggoner AD, Weissman NJ, Zabalgoitia M, American Society of Echocardiography’s G, Standards C, Task Force on Prosthetic V, American College of Cardiology Cardiovascular Imaging C, Cardiac Imaging Committee of the American Heart A, European Association of E, European Society of C, Japanese Society of E, Canadian Society of E, American College of Cardiology F, American Heart A, European Association of E, European Society of C, Japanese Society of E, Canadian Society of E. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009;22:975–1014. doi: 10.1016/j.echo.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Cannegieter SC, Rosendaal FR, Briet E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89:635–41. doi: 10.1161/01.cir.89.2.635. [DOI] [PubMed] [Google Scholar]
- Mok CK, Boey J, Wang R, Chan TK, Cheung KL, Lee PK, Chow J, Ng RP, Tse TF. Warfarin versus dipyridamole-aspirin and pentoxifylline-aspirin for the prevention of prosthetic heart valve thromboembolism: a prospective randomized clinical trial. Circulation. 1985;72:1059–63. doi: 10.1161/01.cir.72.5.1059. [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, Blatchford J, Devenny K, Friedman J, Guiver K, Harper R, Khder Y, Lobmeyer MT, Maas H, Voigt JU, Simoons ML, Van de, RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–14. doi: 10.1056/NEJMoa1300615. [DOI] [PubMed] [Google Scholar]
- Iung B, Rodes-Cabau J. The optimal management of anti-thrombotic therapy after valve replacement: certainties and uncertainties. Eur Heart J. 2014;35:2942–9. doi: 10.1093/eurheartj/ehu365. [DOI] [PubMed] [Google Scholar]
- Caldeira D, David C, Santos AT, Costa J, Pinto FJ, Ferreira JJ. Efficacy and safety of low molecular weight heparin in patients with mechanical heart valves: systematic review and meta-analysis. J Thromb Haemost. 2014;12:650–9. doi: 10.1111/jth.12544. [DOI] [PubMed] [Google Scholar]
- Laffort P, Roudaut R, Roques X, Lafitte S, Deville C, Bonnet J, Baudet E. Early and long-term (one-year) effects of the association of aspirin and oral anticoagulant on thrombi and morbidity after replacement of the mitral valve with the St. Jude medical prosthesis: a clinical and transesophageal echocardiographic study. J Am Coll Cardiol. 2000;35:739–46. doi: 10.1016/s0735-1097(99)00598-7. [DOI] [PubMed] [Google Scholar]
- Sousa-Uva M, Head SJ, Milojevic M, Collet JP, Landoni G, Castella M, Dunning J, Gudbjartsson T, Linker NJ, Sandoval E, Thielmann M, Jeppsson A, Landmesser U. 2017 EACTS Guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53:5–33. doi: 10.1093/ejcts/ezx314. [DOI] [PubMed] [Google Scholar]
- Butchart EG, Gohlke-Barwolf C, Antunes MJ, Tornos P, De Caterina, Cormier B, Prendergast B, Iung B, Bjornstad H, Leport C, Hall RJ, Vahanian A, Working Groups Thrombosis and Cardiac Rehabilitation and Exercise Physiology, European Society of Cardiology. Recommendations for the management of patients after heart valve surgery. Eur Heart J. 2005;26:2463–71. doi: 10.1093/eurheartj/ehi426. [DOI] [PubMed] [Google Scholar]
- Torella M, Torella D, Chiodini P, Franciulli M, Romano G, De Santo, De Feo, Amarelli C, Sasso FC, Salvatore T, Ellison GM, Indolfi C, Cotrufo M, Nappi G. LOWERing the INtensity of oral anticoaGulant Therapy in patients with bileaflet mechanical aortic valve replacement: results from the “LOWERING-IT” Trial. Am Heart J. 2010;160:171–8. doi: 10.1016/j.ahj.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Puskas J, Gerdisch M, Nichols D, Quinn R, Anderson C, Rhenman B, Fermin L, McGrath M, Kong B, Hughes C, Sethi G, Wait M, Martin T, Graeve A, PROACT Investigators. Reduced anticoagulation after mechanical aortic valve replacement: interim results from the prospective randomized on-X valve anticoagulation clinical trial randomized Food and Drug Administration investigational device exemption trial. J Thorac Cardiovasc Surg. 2014;147:1202–10. doi: 10.1016/j.jtcvs.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Heneghan C, Ward A, Perera R, Self-Monitoring Trialist, Bankhead C, Fuller A, Stevens R, Bradford K, Tyndel S, Alonso-Coello P, Ansell J, Beyth R, Bernardo A, Christensen TD, Cromheecke ME, Edson RG, Fitzmaurice D, Gadisseur AP, Garcia-Alamino JM, Gardiner C, Hasenkam JM, Jacobson A, Kaatz S, Kamali F, Khan TI, Knight E, Kortke H, Levi M, Matchar D, Menendez-Jandula B, Rakovac I, Schaefer C, Siebenhofer A, Souto JC, Sunderji R, Gin K, Shalansky K, Voller H, Wagner O, Zittermann A. Self-monitoring of oral anticoagulation: systematic review and meta-analysis of individual patient data. Lancet. 2012;379:322–34. doi: 10.1016/S0140-6736(11)61294-4. [DOI] [PubMed] [Google Scholar]
- Crowther MA, Ageno W, Garcia D, Wang L, Witt DM, Clark NP, Blostein MD, Kahn SR, Vesely SK, Schulman S, Kovacs MJ, Rodger MA, Wells P, Anderson D, Ginsberg J, Selby R, Siragusa S, Silingardi M, Dowd MB, Kearon C. Oral vitamin K versus placebo to correct excessive anticoagulation in patients receiving warfarin: a randomized trial. Ann Intern Med. 2009;150:293–300. doi: 10.7326/0003-4819-150-5-200903030-00005. [DOI] [PubMed] [Google Scholar]
- Halvorsen S, Storey RF, Rocca B, Sibbing D, Ten Berg, Grove EL, Weiss TW, Collet JP, Andreotti F, Gulba DC, Lip GYH, Husted S, Vilahur G, Morais J, Verheugt FWA, Lanas A, Al-Shahi Salman, Steg PG, Huber K, ESC Working. Management of antithrombotic therapy after bleeding in patients with coronary artery disease and/or atrial fibrillation: expert consensus paper of the European Society of Cardiology Working Group on Thrombosis. Eur Heart J. 2017;38:1455–62. doi: 10.1093/eurheartj/ehw454. [DOI] [PubMed] [Google Scholar]
- Lubetsky A, Yonath H, Olchovsky D, Loebstein R, Halkin H, Ezra D. Comparison of oral vs intravenous phytonadione (vitamin K1) in patients with excessive anticoagulation: a prospective randomized controlled study. Arch Intern Med. 2003;163:2469–73. doi: 10.1001/archinte.163.20.2469. [DOI] [PubMed] [Google Scholar]
- Massel DR, Little SH. Antiplatelet and anticoagulation for patients with prosthetic heart valves. Cochrane Database Syst Rev. 2013:CD003464. doi: 10.1002/14651858.CD003464.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen ML, Sorensen R, Clausen MT, Fog-Petersen ML, Raunso J, Gadsboll N, Gislason GH, Folke F, Andersen SS, Schramm TK, Abildstrom SZ, Poulsen HE, Kober L, Torp-Pedersen C. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433–41. doi: 10.1001/archinternmed.2010.271. [DOI] [PubMed] [Google Scholar]
- Douketis JD, Spyropoulos AC, Kaatz S, Becker RC, Caprini JA, Dunn AS, Garcia DA, Jacobson A, Jaffer AK, Kong DF, Schulman S, Turpie AG, Hasselblad V, Ortel TL, BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823–33. doi: 10.1056/NEJMoa1501035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen SD, Knuuti J, Saraste A, Anker S, Botker HE, Hert SD, Ford I, Gonzalez-Juanatey JR, Gorenek B, Heyndrickx GR, Hoeft A, Huber K, Iung B, Kjeldsen KP, Longrois D, Luscher TF, Pierard L, Pocock S, Price S, Roffi M, Sirnes PA, Sousa-Uva M, Voudris V, Funck-Brentano C, Authors/Task Force. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA ). Eur Heart J. 2014;35:2383–431. doi: 10.1093/eurheartj/ehu282. [DOI] [PubMed] [Google Scholar]
- Gellatly RM, Leet A, Brown KE. Fondaparinux: an effective bridging strategy in heparin-induced thrombocytopenia and mechanical circulatory support. J Heart Lung Transplant. 2014;33:118. doi: 10.1016/j.healun.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Brennan JM, Edwards FH, Zhao Y, O’Brien S, Booth ME, Dokholyan RS, Douglas PS, Peterson ED, DEcIDE AVR. Early anticoagulation of bioprosthetic aortic valves in older patients: results from the Society of Thoracic Surgeons Adult Cardiac Surgery National Database. J Am Coll Cardiol. 2012;60:971–7. doi: 10.1016/j.jacc.2012.05.029. [DOI] [PubMed] [Google Scholar]
- Merie C, Kober L, Skov Olsen, Andersson C, Gislason G, Skov Jensen, Torp-Pedersen C. Association of warfarin therapy duration after bioprosthetic aortic valve replacement with risk of mortality, thromboembolic complications, and bleeding. JAMA. 2012;308:2118–25. doi: 10.1001/jama.2012.54506. [DOI] [PubMed] [Google Scholar]
- Christersson C, James SK, Lindhagen L, Ahlsson A, Friberg O, Jeppsson A, Stahle E. Comparison of warfarin versus antiplatelet therapy after surgical bioprosthetic aortic valve replacement. Heart. 2020;106:838–44. doi: 10.1136/heartjnl-2019-315453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiq S, Steinbruchel DA, Lilleor NB, Moller CH, Lund JT, Thiis JJ, Kober L, Olsen PS. Antithrombotic therapy after bioprosthetic aortic valve implantation: warfarin versus aspirin, a randomized controlled trial. Thromb Res. 2017;150:104–10. doi: 10.1016/j.thromres.2016.11.021. [DOI] [PubMed] [Google Scholar]
- Maes F, Stabile E, Ussia GP, Tamburino C, Pucciarelli A, Masson JB, Marsal JR, Barbanti M, Cote M, Rodes-Cabau J. Meta-analysis comparing single versus dual antiplatelet therapy following transcatheter aortic valve implantation. Am J Cardiol. 2018;122:310–5. doi: 10.1016/j.amjcard.2018.04.006. [DOI] [PubMed] [Google Scholar]
- Brouwer J, Nijenhuis VJ, Delewi R, Hermanides RS, Holvoet W, Dubois CLF, Frambach P, De Bruyne, van Houwelingen, Van Der, Tousek P, van der, Buysschaert I, Schotborgh CE, Ferdinande B, van der, Roosen J, Peper J, Thielen FWF, Veenstra L, Chan Pin, Swaans MJ, Rensing B, van ‘t, Timmers L, Kelder JC, Stella PR, Baan J, Ten Berg. Aspirin with or without clopidogrel after transcatheter aortic-valve implantation. N Engl J Med. 2020;383:1447–57. doi: 10.1056/NEJMoa2017815. [DOI] [PubMed] [Google Scholar]
- Dangas GD, Tijssen JGP, Wohrle J, Sondergaard L, Gilard M, Mollmann H, Makkar RR, Herrmann HC, Giustino G, Baldus S, De Backer, Guimaraes AHC, Gullestad L, Kini A, von Lewinski, Mack M, Moreno R, Schafer U, Seeger J, Tchetche D, Thomitzek K, Valgimigli M, Vranckx P, Welsh RC, Wildgoose P, Volkl AA, Zazula A, van Amsterdam, Mehran R, Windecker S, GALILEO Investigators. A controlled trial of rivaroxaban after transcatheter aortic-valve replacement. N Engl J Med. 2020;382:120–9. doi: 10.1056/NEJMoa1911425. [DOI] [PubMed] [Google Scholar]
- Pagnesi M, Moroni F, Beneduce A, Giannini F, Colombo A, Weisz G, Latib A. Thrombotic risk and antithrombotic strategies after transcatheter mitral valve replacement. JACC Cardiovasc Interv. 2019;12:2388–401. doi: 10.1016/j.jcin.2019.07.055. [DOI] [PubMed] [Google Scholar]
- Guimaraes HP, Lopes RD, de Barros, Liporace IL, Sampaio RO, Tarasoutchi F, Hoffmann-Filho CR, de Lemos, Leiria TLL, Lamprea D, Precoma DB, Atik FA, Silveira FS, Farias FR, Barreto DO, Almeida AP, Zilli AC, de Souza, Cavalcante MA, Figueira F, Kojima FCS, Damiani L, Santos RHN, Valeis N, Campos VB, Saraiva JFK, Fonseca FH, Pinto IM, Magalhaes CC, Ferreira JFM, Alexander JH, Pavanello R, Cavalcanti AB, Berwanger O, RIVER Trial. Rivaroxaban in patients with atrial fibrillation and a bioprosthetic mitral valve. N Engl J Med. 2020;383:2117–26. doi: 10.1056/NEJMoa2029603. [DOI] [PubMed] [Google Scholar]
- Shim CY, Seo J, Kim YJ, Lee SH, De Caterina, Lee S, Hong GR Explore the Efficacy and Safety of Edoxaban in Patients after Heart Valve Repair or Bioprosthetic Valve Replacement (ENAVLE) study group. Efficacy and safety of edoxaban in patients early after surgical bioprosthetic valve implantation or valve repair: a randomized clinical trial. J Thorac Cardiovasc Surg. 2021 doi: 10.1016/j.jtcvs.2021.01.127. [DOI] [PubMed] [Google Scholar]
- Nijenhuis VJ, Brouwer J, Delewi R, Hermanides RS, Holvoet W, Dubois CLF, Frambach P, De Bruyne, van Houwelingen, Van Der, Tousek P, van der, Buysschaert I, Schotborgh CE, Ferdinande B, van der, Roosen J, Peper J, Thielen FWF, Veenstra L, Chan Pin, Swaans MJ, Rensing B, van ‘t, Timmers L, Kelder JC, Stella PR, Baan J, Ten Berg. Anticoagulation with or without clopidogrel after transcatheter aortic-valve implantation. N Engl J Med. 2020;382:1696–707. doi: 10.1056/NEJMoa1915152. [DOI] [PubMed] [Google Scholar]
- Jochheim D, Barbanti M, Capretti G, Stefanini GG, Hapfelmeier A, Zadrozny M, Baquet M, Fischer J, Theiss H, Todaro D, Chieffo A, Presbitero P, Colombo A, Massberg S, Tamburino C, Mehilli J. Oral anticoagulant type and outcomes after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12:1566–76. doi: 10.1016/j.jcin.2019.03.003. [DOI] [PubMed] [Google Scholar]
- van der, Olsthoorn JR, Heuts S, Klautz RJM, Tomsic A, Jansen EK, Vonk ABA, Sardari Nia, Klok FA, Huisman MV. Antithrombotic therapy after mitral valve repair: VKA or aspirin? J Thromb Thrombolysis. 2018;46:473–81. doi: 10.1007/s11239-018-1724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyropoulos AC, Turpie AG, Dunn AS, Kaatz S, Douketis J, Jacobson A, Petersen H, REGIMEN Investigators. Perioperative bridging therapy with unfractionated heparin or low-molecular-weight heparin in patients with mechanical prosthetic heart valves on long-term oral anticoagulants (from the REGIMEN Registry). Am J Cardiol. 2008;102:883–9. doi: 10.1016/j.amjcard.2008.05.042. [DOI] [PubMed] [Google Scholar]
- Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet, Herrman JP, Adriaenssens T, Vrolix M, Heestermans AA, Vis MM, Tijsen JG, van ’t, ten Berg WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381:1107–15. doi: 10.1016/S0140-6736(12)62177-1. [DOI] [PubMed] [Google Scholar]
- Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels, Korjian S, Daaboul Y, Lip GY, Cohen M, Husted S, Peterson ED, Fox KA. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N Engl J Med. 2016;375:2423–34. doi: 10.1056/NEJMoa1611594. [DOI] [PubMed] [Google Scholar]
- Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, Goodman SG, Windecker S, Darius H, Li J, Averkov O, Bahit MC, Berwanger O, Budaj A, Hijazi Z, Parkhomenko A, Sinnaeve P, Storey RF, Thiele H, Vinereanu D, Granger CB, Alexander JH AUGUSTUS Investigators. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N Engl J Med. 2019;380:1509–24. doi: 10.1056/NEJMoa1817083. [DOI] [PubMed] [Google Scholar]
- Fiedler KA, Maeng M, Mehilli J, Schulz-Schupke S, Byrne RA, Sibbing D, Hoppmann P, Schneider S, Fusaro M, Ott I, Kristensen SD, Ibrahim T, Massberg S, Schunkert H, Laugwitz KL, Kastrati A, Sarafoff N. Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting stent implantation: the ISAR-TRIPLE Trial. J Am Coll Cardiol. 2015;65:1619–29. doi: 10.1016/j.jacc.2015.02.050. [DOI] [PubMed] [Google Scholar]
- Vranckx P, Valgimigli M, Eckardt L, Tijssen J, Lewalter T, Gargiulo G, Batushkin V, Campo G, Lysak Z, Vakaliuk I, Milewski K, Laeis P, Reimitz PE, Smolnik R, Zierhut W, Goette A. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019;394:1335–43. doi: 10.1016/S0140-6736(19)31872-0. [DOI] [PubMed] [Google Scholar]
- Lamberts M, Gislason GH, Lip GY, Lassen JF, Olesen JB, Mikkelsen AP, Sorensen R, Kober L, Torp-Pedersen C, Hansen ML. Antiplatelet therapy for stable coronary artery disease in atrial fibrillation patients taking an oral anticoagulant: a nationwide cohort study. Circulation. 2014;129:1577–85. doi: 10.1161/CIRCULATIONAHA.113.004834. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, Miyauchi K, Hagiwara N, Kimura K, Hirayama A, Matsui K, Ogawa H, AFIRE Investigators. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. 2019;381:1103–13. doi: 10.1056/NEJMoa1904143. [DOI] [PubMed] [Google Scholar]
- Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- Dewilde WJ, Janssen PW, Kelder JC, Verheugt FW, De Smet, Adriaenssens T, Vrolix M, Brueren GB, Van Mieghem, Cornelis K, Vos J, Breet NJ, ten Berg. Uninterrupted oral anticoagulation versus bridging in patients with long-term oral anticoagulation during percutaneous coronary intervention: subgroup analysis from the WOEST trial. EuroIntervention. 2015;11:381–90. doi: 10.4244/EIJY14M06_07. [DOI] [PubMed] [Google Scholar]
- Lip GYH, Collet JP, Haude M, Byrne R, Chung EH, Fauchier L, Halvorsen S, Lau D, Lopez-Cabanillas N, Lettino M, Marin F, Obel I, Rubboli A, Storey RF, Valgimigli M, Huber K, ESC Scientific. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: a joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Europace. 2019;21:192–3. doi: 10.1093/europace/euy174. [DOI] [PubMed] [Google Scholar]
- Carnicelli AP, De Caterina, Halperin JL, Renda G, Ruff CT, Trevisan M, Nordio F, Mercuri MF, Antman E, Giugliano RP, ENGAGE AF-TIMI. Edoxaban for the prevention of thromboembolism in patients with atrial fibrillation and bioprosthetic valves. Circulation. 2017;135:1273–5. doi: 10.1161/CIRCULATIONAHA.116.026714. [DOI] [PubMed] [Google Scholar]
- Philippart R, Brunet-Bernard A, Clementy N, Bourguignon T, Mirza A, Babuty D, Angoulvant D, Lip GY, Fauchier L. Prognostic value of CHA2DS2-VASc score in patients with ‘non-valvular atrial fibrillation’ and valvular heart disease: the Loire Valley Atrial Fibrillation Project. Eur Heart J. 2015;36:1822–30. doi: 10.1093/eurheartj/ehv163. [DOI] [PubMed] [Google Scholar]
- Philippart R, Brunet-Bernard A, Clementy N, Bourguignon T, Mirza A, Angoulvant D, Babuty D, Lip GY, Fauchier L. Oral anticoagulation, stroke and thromboembolism in patients with atrial fibrillation and valve bioprosthesis. The Loire Valley Atrial Fibrillation Project. Thromb Haemost. 2016;115:1056–63. doi: 10.1160/TH16-01-0007. [DOI] [PubMed] [Google Scholar]
- Siontis KC, Yao X, Gersh BJ, Noseworthy PA. Direct oral anticoagulants in patients with atrial fibrillation and valvular heart disease other than significant mitral stenosis and mechanical valves: a meta-analysis. Circulation. 2017;135:714–6. doi: 10.1161/CIRCULATIONAHA.116.026793. [DOI] [PubMed] [Google Scholar]
- Russo A, Grigioni F, Avierinos JF, Freeman WK, Suri R, Michelena H, Brown R, Sundt TM, Enriquez-Sarano M. Thromboembolic complications after surgical correction of mitral regurgitation incidence, predictors, and clinical implications. J Am Coll Cardiol. 2008;51:1203–11. doi: 10.1016/j.jacc.2007.10.058. [DOI] [PubMed] [Google Scholar]
- Butnaru A, Shaheen J, Tzivoni D, Tauber R, Bitran D, Silberman S. Diagnosis and treatment of early bioprosthetic malfunction in the mitral valve position due to thrombus formation. Am J Cardiol. 2013;112:1439–44. doi: 10.1016/j.amjcard.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Rodes-Cabau J, Masson JB, Welsh RC, Garcia Del, Pelletier M, Webb JG, Al-Qoofi F, Genereux P, Maluenda G, Thoenes M, Paradis JM, Chamandi C, Serra V, Dumont E, Cote M. Aspirin versus aspirin plus clopidogrel as antithrombotic treatment following transcatheter aortic valve replacement with a balloon-expandable valve: the ARTE (Aspirin Versus Aspirin + Clopidogrel Following Transcatheter Aortic Valve Implantation) randomized clinical trial. JACC Cardiovasc Interv. 2017;10:1357–65. doi: 10.1016/j.jcin.2017.04.014. [DOI] [PubMed] [Google Scholar]
- Dvir D, Bourguignon T, Otto CM, Hahn RT, Rosenhek R, Webb JG, Treede H, Sarano ME, Feldman T, Wijeysundera HC, Topilsky Y, Aupart M, Reardon MJ, Mackensen GB, Szeto WY, Kornowski R, Gammie JS, Yoganathan AP, Arbel Y, Borger MA, Simonato M, Reisman M, Makkar RR, Abizaid A, McCabe JM, Dahle G, Aldea GS, Leipsic J, Pibarot P, Moat NE, Mack MJ, Kappetein AP, Leon MB, VIVID Investigators. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation. 2018;137:388–99. doi: 10.1161/CIRCULATIONAHA.117.030729. [DOI] [PubMed] [Google Scholar]
- Tam DY, Dharma C, Rocha RV, Ouzounian M, Wijeysundera HC, Austin PC, Chikwe J, Gaudino M, Fremes SE. Transcatheter ViV versus redo surgical AVR for the management of failed biological prosthesis: early and late outcomes in a propensity-matched cohort. JACC Cardiovasc Interv. 2020;13:765–74. doi: 10.1016/j.jcin.2019.10.030. [DOI] [PubMed] [Google Scholar]
- Bleiziffer S, Simonato M, Webb JG, Rodés-Cabau J, Pibarot P, Kornowski R, Windecker S, Erlebach M, Duncan A, Seiffert M, Unbehaun A, Frerker C, Conzelmann L, Wijeysundera H, Kim WK, Montorfano M, Latib A, Tchetche D, Allali A, Abdel-Wahab M, Orvin K, Stortecky S, Nissen H, Holzamer A, Urena M, Testa L, Agrifoglio M, Whisenant B, Sathananthan J, Napodano M, Landi A, Fiorina C, Zittermann A, Veulemans V, Sinning JM, Saia F, Brecker S, Presbitero P, De Backer, Søndergaard L, Bruschi G, Franco LN, Petronio AS, Barbanti M, Cerillo A, Spargias K, Schofer J, Cohen M, Muñoz-Garcia A, Finkelstein A, Adam M, Serra V, Teles RC, Champagnac D, Iadanza A, Chodor P, Eggebrecht H, Welsh R, Caixeta A, Salizzoni S, Dager A, Auffret V, Cheema A, Ubben T, Ancona M, Rudolph T, Gummert J, Tseng E, Noble S, Bunc M, Roberts D, Kass M, Gupta A, Leon MB, Dvir D. Long-term outcomes after transcatheter aortic valve implantation in failed bioprosthetic valves. Eur Heart J. 2020;41:2731–42. doi: 10.1093/eurheartj/ehaa544. [DOI] [PubMed] [Google Scholar]
- Hirji SA, Percy ED, Zogg CK, Malarczyk A, Harloff MT, Yazdchi F, Kaneko T. Comparison of in-hospital outcomes and readmissions for valve-in-valve transcatheter aortic valve replacement vs. reoperative surgical aortic valve replacement: a contemporary assessment of real-world outcomes. Eur Heart J. 2020;41:2747–55. doi: 10.1093/eurheartj/ehaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbanti M, Costa G, Picci A, Criscione E, Reddavid C, Valvo R, Todaro D, Deste W, Condorelli A, Scalia M, Licciardello A, Politi G, De Luca G, Strazzieri O, Motta S, Garretto V, Veroux P, Giaquinta A, Giuffrida A, Sgroi C, Leon MB, Webb JG, Tamburino C. Coronary Cannulation After Transcatheter Aortic Valve Replacement: The RE-ACCESS Study. JACC Cardiovasc Interv. 2020;13:2542–55. doi: 10.1016/j.jcin.2020.07.006. [DOI] [PubMed] [Google Scholar]
- De Backer, Landes U, Fuchs A, Yoon SH, Mathiassen ON, Sedaghat A, Kim WK, Pilgrim T, Buzzatti N, Ruile P, El Sabbagh, Barbanti M, Fiorina C, Nombela-Franco L, Steinvil A, Finkelstein A, Montorfano M, Maurovich-Horvat P, Kofoed KF, Blanke P, Bunc M, Neumann FJ, Latib A, Windecker S, Sinning JM, Norgaard BL, Makkar R, Webb JG, Søndergaard L. Coronary Access After TAVR-in-TAVR as Evaluated by Multidetector Computed Tomography. JACC Cardiovasc Interv. 2020;13:2528–38. doi: 10.1016/j.jcin.2020.06.016. [DOI] [PubMed] [Google Scholar]
- Jawitz OK, Gulack BC, Grau-Sepulveda MV, Matsouaka RA, Mack MJ, Holmes DR , Carroll JD, Thourani VH, Brennan JM. Reoperation after transcatheter aortic valve replacement: an analysis of the Society of Thoracic Surgeons Database. JACC Cardiovasc Interv. 2020;13:1515–25. doi: 10.1016/j.jcin.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir D, Webb JG, Bleiziffer , S Pasic, Waksman R, Kodali S, Barbanti M, Latib A, Schaefer U, Rodés-Cabau J, Treede H, Piazza N, Hildick-Smith D, Himbert D, Walther T, Hengstenberg C, Nissen H, Bekeredjian R, Presbitero P, Ferrari E, Segev A, de Weger, Windecker S, Moat NE, Napodano M, Wilbring M, Cerillo AG, Brecker S, Tchetche D, Lefèvre T, De Marco, Fiorina C, Petronio AS, Teles RC, Testa L, Laborde JC, Leon MB, Kornowski R Valve-in-Valve International Data Registry Investigators. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312:162–70. doi: 10.1001/jama.2014.7246. [DOI] [PubMed] [Google Scholar]
- Ye J, Cheung A, Yamashita M, Wood D, Peng D, Gao M, Thompson CR, Munt B, Moss RR, Blanke P, Leipsic J, Dvir D, Webb JG. Transcatheter aortic and mitral valve-in-valve implantation for failed surgical bioprosthetic valves: an 8-year single-center experience. JACC Cardiovasc Interv. 2015;8:1735–44. doi: 10.1016/j.jcin.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Simonato M, Whisenant B, Ribeiro HB, Webb JG, Kornowski R, Guerrero M, Wijeysundera H, Sondergaard L, De Backer, Villablanca P, Rihal C, Eleid M, Kempfert J, Unbehaun A, Erlebach M, Casselman F, Adam M, Montorfano M, Ancona M, Saia F, Ubben T, Meincke F, Napodano M, Codner P, Schofer J, Pelletier M, Cheung A, Shuvy M, Palma JH, Gaia DF, Duncan A, Hildick-Smith D, Veulemans V, Sinning JM, Arbel Y, Testa L, de Weger, Eltchaninoff H, Hemery T, Landes U, Tchetche D, Dumonteil N, Rodes-Cabau J, Kim WK, Spargias K, Kourkoveli P, Ben-Yehuda O, Teles RC, Barbanti M, Fiorina C, Thukkani A, Mackensen GB, Jones N, Presbitero P, Petronio AS, Allali A, Champagnac D, Bleiziffer S, Rudolph T, Iadanza A, Salizzoni S, Agrifoglio M, Nombela-Franco L, Bonaros N, Kass M, Bruschi G, Amabile N, Chhatriwalla A, Messina A, Hirji SA, Andreas M, Welsh R, Schoels W, Hellig F, Windecker S, Stortecky S, Maisano F, Stone GW, Dvir D. Transcatheter mitral valve replacement after surgical repair or replacement: comprehensive midterm evaluation of valve-in-valve and valve-in-ring implantation from the VIVID Registry. Circulation. 2021;143:104–16. doi: 10.1161/CIRCULATIONAHA.120.049088. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Yazdchi F, Alexis SL, Percy E, Premkumar A, Hirji S, Bapat VN, Bhatt DL, Kaneko T, Tang GHL. Reoperative mitral surgery versus transcatheter mitral valve replacement: a systematic review. J Am Heart Assoc. 2021;10:e019854. doi: 10.1161/JAHA.120.019854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urena M, Vahanian A, Brochet E, Ducrocq G, Iung B, Himbert D. Current indications for transcatheter mitral valve replacement using transcatheter aortic valves: valve-in-valve, valve-in-ring, and valve-in-mitral annulus calcification. Circulation. 2021;143:178–96. doi: 10.1161/CIRCULATIONAHA.120.048147. [DOI] [PubMed] [Google Scholar]
- Little SH, Bapat V, Blanke P, Guerrero M, Rajagopal V, Siegel R. Imaging guidance for transcatheter mitral valve intervention on prosthetic valves, rings, and annular calcification. JACC Cardiovasc Imaging. 2021;14:22–40. doi: 10.1016/j.jcmg.2019.10.027. [DOI] [PubMed] [Google Scholar]
- Fallon JM, DeSimone JP, Brennan JM, O’Brien S, Thibault DP, DiScipio AW, Pibarot P, Jacobs JP, Malenka DJ. The incidence and consequence of prosthesis-patient mismatch after surgical aortic valve replacement. Ann Thorac Surg. 2018;106:14–22. doi: 10.1016/j.athoracsur.2018.01.090. [DOI] [PubMed] [Google Scholar]
- Flameng W, Herregods MC, Vercalsteren M, Herijgers P, Bogaerts K, Meuris B. Prosthesis-patient mismatch predicts structural valve degeneration in bioprosthetic heart valves. Circulation. 2010;121:2123–9. doi: 10.1161/CIRCULATIONAHA.109.901272. [DOI] [PubMed] [Google Scholar]
- Zorn GL, Little SH, Tadros P, Deeb GM, Gleason TG, Heiser J, Kleiman NS, Oh JK, Popma JJ, Adams D, Huang J, Reardon MJ. Prosthesis-patient mismatch in high-risk patients with severe aortic stenosis: a randomized trial of a self-expanding prosthesis. J Thorac Cardiovasc Surg. 2016;151:1014–1022, 1023. e1–3. doi: 10.1016/j.jtcvs.2015.10.070. [DOI] [PubMed] [Google Scholar]
- Head SJ, Mokhles MM, Osnabrugge RL, Pibarot P, Mack MJ, Takkenberg JJ, Bogers AJ, Kappetein AP. The impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: a systematic review and meta-analysis of 34 observational studies comprising 27 186 patients with 133 141 patient-years. Eur Heart J. 2012;33:1518–29. doi: 10.1093/eurheartj/ehs003. [DOI] [PubMed] [Google Scholar]
- Sorajja P, Bae R, Lesser JA, Pedersen WA. Percutaneous repair of paravalvular prosthetic regurgitation: patient selection, techniques and outcomes. Heart. 2015;101:665–73. doi: 10.1136/heartjnl-2014-306270. [DOI] [PubMed] [Google Scholar]
- Ruiz CE, Hahn RT, Berrebi A, Borer JS, Cutlip DE, Fontana G, Gerosa G, Ibrahim R, Jelnin V, Jilaihawi H, Jolicoeur EM, Kliger C, Kronzon I, Leipsic J, Maisano F, Millan X, Nataf P, O’Gara PT, Pibarot P, Ramee SR, Rihal CS, Rodes-Cabau J, Sorajja P, Suri R, Swain JA, Turi ZG, Tuzcu EM, Weissman NJ, Zamorano JL, Serruys PW, Leon MB, Paravalvular Leak. Clinical trial principles and endpoint definitions for paravalvular leaks in surgical prosthesis. Eur Heart J. 2018;39:1224–45. doi: 10.1093/eurheartj/ehx211. [DOI] [PubMed] [Google Scholar]
- Chakravarty T, Sondergaard L, Friedman J, De Backer, Berman D, Kofoed KF, Jilaihawi H, Shiota T, Abramowitz Y, Jorgensen TH, Rami T, Israr S, Fontana G, de Knegt, Fuchs A, Lyden P, Trento A, Bhatt DL, Leon MB, Makkar RR, Resolve , SAVORY Investigators. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. 2017;389:2383–92. doi: 10.1016/S0140-6736(17)30757-2. [DOI] [PubMed] [Google Scholar]
- Karthikeyan G, Senguttuvan NB, Joseph J, Devasenapathy N, Bahl VK, Airan B. Urgent surgery compared with fibrinolytic therapy for the treatment of left-sided prosthetic heart valve thrombosis: a systematic review and meta-analysis of observational studies. Eur Heart J. 2013;34:1557–66. doi: 10.1093/eurheartj/ehs486. [DOI] [PubMed] [Google Scholar]
- Laplace G, Lafitte S, Labeque JN, Perron JM, Baudet E, Deville C, Roques X, Roudaut R. Clinical significance of early thrombosis after prosthetic mitral valve replacement: a postoperative monocentric study of 680 patients. J Am Coll Cardiol. 2004;43:1283–90. doi: 10.1016/j.jacc.2003.09.064. [DOI] [PubMed] [Google Scholar]
- Petrescu I, Egbe AC, Ionescu F, Nkomo VT, Greason KL, Pislaru C, Pellikka PA, Connolly HM, Pislaru SV. Long-term outcomes of anticoagulation for bioprosthetic valve thrombosis. J Am Coll Cardiol. 2020;75:857–66. doi: 10.1016/j.jacc.2019.12.037. [DOI] [PubMed] [Google Scholar]
- Sellers SL, Turner CT, Sathananthan J, Cartlidge TRG, Sin F, Bouchareb R, Mooney J, Norgaard BL, Bax JJ, Bernatchez PN, Dweck MR, Granville DJ, Newby DE, Lauck S, Webb JG, Payne GW, Pibarot P, Blanke P, Seidman MA, Leipsic JA. Transcatheter aortic heart valves: histological analysis providing insight to leaflet thickening and structural valve degeneration. JACC Cardiovasc Imaging. 2019;12:135–45. doi: 10.1016/j.jcmg.2018.06.028. [DOI] [PubMed] [Google Scholar]
- De Backer, Dangas GD, Jilaihawi H, Leipsic JA, Terkelsen CJ, Makkar R, Kini AS, Veien KT, Abdel-Wahab M, Kim WK, Balan P, Van Mieghem, Mathiassen ON, Jeger RV, Arnold M, Mehran R, Guimaraes AHC, Norgaard BL, Kofoed KF, Blanke P, Windecker S, Sondergaard L, GALILEO-4D Investigators. Reduced leaflet motion after transcatheter aortic-valve replacement. N Engl J Med. 2020;382:130–9. doi: 10.1056/NEJMoa1911426. [DOI] [PubMed] [Google Scholar]
- Alkhouli M, Rihal CS, Zack CJ, Eleid MF, Maor E, Sarraf M, Cabalka AK, Reeder GS, Hagler DJ, Maalouf JF, Nkomo VT, Schaff HV, Said SM. Transcatheter and surgical management of mitral paravalvular leak: long-term outcomes. JACC Cardiovasc Interv. 2017;10:1946–56. doi: 10.1016/j.jcin.2017.07.046. [DOI] [PubMed] [Google Scholar]
- Dakik HA, Chehab O, Eldirani M, Sbeity E, Karam C, Abou Hassan, Msheik M, Hassan H, Msheik A, Kaspar C, Makki M, Tamim H. A new index for pre-operative cardiovascular evaluation. J Am Coll Cardiol. 2019;73:3067–78. doi: 10.1016/j.jacc.2019.04.023. [DOI] [PubMed] [Google Scholar]
- Tashiro T, Pislaru SV, Blustin JM, Nkomo VT, Abel MD, Scott CG, Pellikka PA. Perioperative risk of major non-cardiac surgery in patients with severe aortic stenosis: a reappraisal in contemporary practice. Eur Heart J. 2014;35:2372–81. doi: 10.1093/eurheartj/ehu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugene M, Urena M, Abtan J, Carrasco JL, Ghodbane W, Nataf P, Vahanian A, Himbert D. Effectiveness of rescue percutaneous balloon aortic valvuloplasty in patients with severe aortic stenosis and acute heart failure. Am J Cardiol. 2018;121:746–50. doi: 10.1016/j.amjcard.2017.11.048. [DOI] [PubMed] [Google Scholar]
- Kolte D, Khera S, Vemulapalli S, Dai D, Heo S, Goldsweig AM, Aronow HD, Elmariah S, Inglessis I, Palacios IF, Thourani VH, Sharaf BL, Gordon PC, Abbott JD. Outcomes following urgent/emergent transcatheter aortic valve replacement: insights from the STS/ACC TVT Registry. JACC Cardiovasc Interv. 2018;11:1175–85. doi: 10.1016/j.jcin.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Calleja AM, Dommaraju S, Gaddam R, Cha S, Khandheria BK, Chaliki HP. Cardiac risk in patients aged >75 years with asymptomatic, severe aortic stenosis undergoing noncardiac surgery. Am J Cardiol. 2010;105:1159–63. doi: 10.1016/j.amjcard.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Tarantini G, Nai Fovino, Tellaroli P, Fabris T, Iliceto S. Asymptomatic severe aortic stenosis and noncardiac surgery. Am J Cardiol. 2016;117:486–8. doi: 10.1016/j.amjcard.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomstrom-Lundqvist C, Cifkova R, De Bonis, Iung B, Johnson MR, Kintscher U, Kranke P, Lang IM, Morais J, Pieper PG, Presbitero P, Price S, Rosano GMC, Seeland U, Simoncini T, Swan L, Warnes CA, ESC Scientific. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–241. doi: 10.1093/eurheartj/ehy340. [DOI] [PubMed] [Google Scholar]
- Roos-Hesselink J, Baris L, Johnson M, De Backer, Otto C, Marelli A, Jondeau G, Budts W, Grewal J, Sliwa K, Parsonage W, Maggioni AP, van Hagen, Vahanian A, Tavazzi L, Elkayam U, Boersma E, Hall R. Pregnancy outcomes in women with cardiovascular disease: evolving trends over 10 years in the ESC Registry Of Pregnancy And Cardiac disease (ROPAC). Eur Heart J. 2019;40:3848–55. doi: 10.1093/eurheartj/ehz136. [DOI] [PubMed] [Google Scholar]
- van Hagen, Thorne SA, Taha N, Youssef G, Elnagar A, Gabriel H, ElRakshy Y, Iung B, Johnson MR, Hall R, Roos-Hesselink JW, ROPAC Investigators. Pregnancy outcomes in women with rheumatic mitral valve disease: results from the registry of pregnancy and cardiac disease. Circulation. 2018;137:806–16. doi: 10.1161/CIRCULATIONAHA.117.032561. [DOI] [PubMed] [Google Scholar]
- Orwat S, Diller GP, van Hagen, Schmidt R, Tobler D, Greutmann M, Jonkaitiene R, Elnagar A, Johnson MR, Hall R, Roos-Hesselink JW, Baumgartner H, ROPAC Investigators. Risk of pregnancy in moderate and severe aortic stenosis: from the Multinational ROPAC Registry. J Am Coll Cardiol. 2016;68:1727–37. doi: 10.1016/j.jacc.2016.07.750. [DOI] [PubMed] [Google Scholar]
- Meijboom LJ, Vos FE, Timmermans J, Boers GH, Zwinderman AH, Mulder BJ. Pregnancy and aortic root growth in the Marfan syndrome: a prospective study. Eur Heart J. 2005;26:914–20. doi: 10.1093/eurheartj/ehi103. [DOI] [PubMed] [Google Scholar]
- McKellar SH, MacDonald RJ, Michelena HI, Connolly HM, Sundt TM. Frequency of cardiovascular events in women with a congenitally bicuspid aortic valve in a single community and effect of pregnancy on events. Am J Cardiol. 2011;107:96–9. doi: 10.1016/j.amjcard.2010.08.061. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Urena M, Chong-Nguyen C, Kikoine J, Brochet E, Abtan J, Fischer Q, Ducrocq G, Vahanian A, Iung B, Himbert D. Valve-in-valve and valve-in-ring transcatheter mitral valve implantation in young women contemplating pregnancy. Circ Cardiovasc Interv. 2020;13:e009579. doi: 10.1161/CIRCINTERVENTIONS.120.009579. [DOI] [PubMed] [Google Scholar]
- van Hagen, Roos-Hesselink JW, Ruys TP, Merz WM, Goland S, Gabriel H, Lelonek M, Trojnarska O, Al Mahmeed, Balint HO, Ashour Z, Baumgartner H, Boersma E, Johnson MR, Hall R ROPAC Investigators and the EURObservational Research Programme (EORP) Team. Pregnancy in women with a mechanical heart valve: data of the European Society of Cardiology Registry of Pregnancy and Cardiac Disease (ROPAC). Circulation. 2015;132:132–42. doi: 10.1161/CIRCULATIONAHA.115.015242. [DOI] [PubMed] [Google Scholar]
- Elassy SM, Elmidany AA, Elbawab HY. Urgent cardiac surgery during pregnancy: a continuous challenge. Ann Thorac Surg. 2014;97:1624–9. doi: 10.1016/j.athoracsur.2013.10.067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cardiovascular and non-cardiovascular factors linked with transcatheter aortic valve implantation-related futility featured within the PARTNER and FRANCE 2 transcatheter aortic valve implantation-risk score models.
Katz Index of Independence in Activities of Daily Living.
Essential frailty toolset in older adults undergoing aortic valve replacement.
Medical comorbidities and factors predicting poorer outcomes post transcatheter aortic valve implantation.
Integrated approach for estimating transcatheter aortic valve implantation-specific risk and futility.
Risk-of-bias judgments for all evaluated trials.
Main inclusion/exclusion criteria suggesting an increased chance of responding to TEER in patients with SMR.
Echocardiographic scores used for assessing the feasibility of percutaneous mitral commissurotomy: Wilkins score, Cormier score, and Echo score ‘Revisited’.
Criteria for patients selection for MitraClip procedure.
Peri- and post-procedural management of antithrombotic therapy in patients with indication to OAC and ACS/PCI.
Management of OAC in patients with an indication for preoperative bridging.



