Abstract
Using polystyrene microspheres coated with heparin or heparan sulfate, it was shown that coated microspheres specifically bound eukaryotic cells and were endocytosed by nonprofessional phagocytic cells. Coated microspheres displayed properties of binding to eukaryotic cells that were similar to those of chlamydiae, and the microspheres were competitively inhibited by chlamydial organisms. Endocytosis of heparin-coated beads resulted in the tyrosine phosphorylation of a similar set of host proteins as did endocytosis of chlamydiae; however, unlike viable chlamydial organisms, which prevent phagolysosomal fusion, endocytosed beads were trafficked to a lysosomal compartment. These findings suggest that heparin-coated beads and Chlamydia trachomatis enter eukaryotic cells by similar pathways.
Numerous bacterial pathogens enter eukaryotic cells and use this intracellular site for replication and for persistence in their human hosts. The molecular mechanism of attachment and entry in terms of the bacterial ligand and host cell receptor has been described for only a few bacteria (12). It is nevertheless apparent that microbial strategies for mammalian cell attachment and entry are intimately coupled to natural biological functions of the eukaryotic cell hosts. Thus, understanding the molecular basis of entry into nonprofessional phagocytic cells, such as epithelial cells, should provide important mechanistic insights into fundamental biological properties of both the pathogen and the eukaryotic cell.
Chlamydia trachomatis is an obligate intracellular bacterium that causes a wide spectrum of human disease affecting hundreds of millions of people (15). C. trachomatis infections are the most common cause of sexually transmitted diseases, often resulting in severe pathology in women and newborns (33). C. trachomatis is the most prevalent reported infection in the United States and may increase the risk of human immunodeficiency virus infection (22). These organisms are also the cause of trachoma, the leading cause of preventable blindness in the world (24). Chlamydiae attach to, and enter, eukaryotic epithelial cells of mucosal surfaces and grow within host cell membrane-bound vacuoles that do not fuse with lysosomes (26). Entry of chlamydiae into host cells is thought to be by receptor-mediated endocytosis (40), although little is known about the receptor on eukaryotic cells to which chlamydiae bind. Binding to the receptor is saturable (39) and sensitive to trypsin treatment of host cells (5). In addition, the host cell interaction with chlamydiae is inhibitable by exogenous heparin or heparan sulfate (21, 42).
It has previously been proposed that heparin and heparan sulfate are structural and functional analogs of the C. trachomatis LGV biovar attachment ligand, because attachment and consequent infectivity can be (i) competitively inhibited by heparin or heparan sulfate, (ii) abolished following treatment of chlamydiae with a heparan sulfate-specific lyase, and (iii) rescued by coating heparan sulfate lyase-treated chlamydiae with heparin or heparan sulfate (8, 16, 41, 42). Other glycosaminoglycans cannot restore attachment or infectivity for neutralized organisms. This central mechanism of infectivity is shared by all biovariants of the species (8); however, for some biovariant strains cell attachment, but not infectivity, is only partially inhibited by heparin (7). Thus, it is unknown if the primary function of the heparan sulfate ligand is to mediate entry of chlamydiae into epithelial cells.
To dissect the contribution of the chlamydial ligand in attachment to and entry into eukaryotic cells, polystyrene microspheres coated with heparin or heparan sulfate were employed to determine whether these molecules were necessary or sufficient to mediate attachment and entry independent of other chlamydial components. It was demonstrated that microspheres coated with heparin or heparan sulfate bound and entered eukaryotic cells. Moreover, similar eukaryotic cell proteins became tyrosine phosphorylated following uptake of C. trachomatis or heparin-coated microspheres; however, the ultimate destination of the endocytosed heparin-coated beads was different from that of viable C. trachomatis.
MATERIALS AND METHODS
Organisms.
C. trachomatis L2/434/Bu and mouse pneumonitis strains were grown in murine L929 cells for 48 h, and the infectious form of the organism, elementary bodies (EB), was purified on Renografin gradients as previously described (20). The number of inclusion-forming units (IFU) and the number of EB particles were estimated as previously described (9). IFU/EB ratios estimated using these methods averaged 1:25.
Purification of 35S-labeled heparan sulfate-like ligand.
Purified 35S-labeled chlamydial heparan sulfate-like ligand was obtained following infection of Chinese hamster ovary (CHO) 761 cells with C. trachomatis L2 as previously described (42). Briefly, at 48 h after infection the cells were washed and sonicated, and the lysate was digested with pronase, RNase, and DNase and clarified by centrifugation. The lysate was applied to a DEAE-Sepharose column (4 ml of Sepharose) and washed with 800 ml of Tris-NaCl (TBS) (50 mM Tris-Cl, 15 mM NaCl, pH 7.6). This was followed by washing with 400 ml of 0.5 M NaCl in TBS. Purified chlamydial ligand was obtained by eluting with 2 M NaCl in 50 mM Tris. The peak fractions were pooled, dialyzed extensively against water, and stored at 4°C.
Microsphere coating.
Carboxylate-modified polystyrene microspheres, either 0.289 or 0.8 μm in diameter (Seradyn Inc., Indianapolis, Ind.), were used in cell attachment and entry assays. Separate 50-μl aliquots of 0.289-μm-diameter microspheres were washed three times in 1 ml of TBS by Microfuge centrifugation. The microspheres were then suspended in 1 ml of TBS containing 1 mg of protamine chloride (Sigma Chemical Co., St. Louis, Mo.) per ml and incubated at 25°C for 1 h. The microspheres were washed three times and suspended in 450 μl of TBS, and either 50 μCi of [3H]heparin (New England Nuclear, Boston, Mass.) (500 μg/ml) or heparan sulfate, chondroitin sulfate (Sigma Chemical Co.), or purified 35S-labeled chlamydial heparan sulfate-like ligand (250 μg/ml) was added and incubated at 25°C for 30 min. Uncoated microspheres were incubated for 30 min in buffer alone. After the microspheres were washed three times with TBS, nonspecific binding sites were blocked using 5% bovine serum albumin TBS at 25°C for 30 min. Coated and blocked microspheres were then suspended in 500 μl of unsupplemented RPMI 1640. The microspheres were diluted 1/500 in RPMI (approximately 108 beads/ml), and 200 μl of the bead suspension was added to wells of 24-well tissue culture plates for cell binding experiments.
Attachment and entry assays.
Microspheres were allowed to interact for various times with HeLa 229 cells either at 4°C to evaluate attachment only or at 37°C to evaluate attachment and cellular entry. HeLa 229 cells were plated at a density of 1.6 × 105 cells per well in 24-well tissue culture plates and incubated at 37°C overnight. On the following day coated microspheres were added to triplicate wells with or without competitive inhibitors. The cells were incubated at 4°C for 2 h and washed three times with 1 ml of TBS. Competitive inhibition assays consisted of mixing coated microspheres with various concentrations of exogenous glycosaminoglycans of C. trachomatis organisms and adding this mixture to cell monolayers. Attachment and uptake were separately estimated using trypsin treatment of the HeLa 229 cell monolayers to release attached but not internalized microspheres as previously described for determining chlamydial attachment (5). Trypsinized cells were transferred to centrifuge tubes and washed and pelleted by centrifugation at 1,200 × g. Sodium dodecyl sulfate (SDS) (1 ml of a 2% solution) was added to each tube, and the amount of cell association was determined by scintillation counting.
Electron microscopy.
For transmission electron microscopy, HeLa 229 cells were grown overnight in 35-mm-diameter polystyrene tissue culture dishes. The cells were washed three times with 2 ml of unsupplemented RPMI 1640 at 37°C. Uncoated or heparan sulfate-coated microspheres (500 μl) were added to each dish and incubated at 37°C for various lengths of time. For transmission electron microscopy, the cells were fixed in 1% paraformaldehyde–3% glutaraldehyde–0.1 M sodium cacodylate-HCl (pH 7.4) at 25°C for 2 hours and then fixed overnight at 4°C in 1.5% glutaraldehyde–0.1 M sodium cacodylate-HCl (pH 7.4) containing 1% sucrose. Specimens were postfixed with 1% osmium tetroxide–0.1 M sodium cacodylate-HCl (pH 7.6) containing 5% sucrose for 1 h at 4°C and subsequently stained in uranyl acetate (0.5% uranyl acetate in acetate-Veronal buffer, pH 6.0) containing 4% sucrose for 1 h at 25°C. The cells were dehydrated sequentially with increasing concentrations of ethanol (70, 95, and 100%). The cells were scraped from the dish with a rubber policeman and embedded in Epox 812. Thin sections were stained with 2% uranyl acetate and Reynolds lead citrate and were viewed in a Zeiss 10C/CR transmission electron microscope.
Detection of tyrosine-phosphorylated proteins.
Subconfluent monolayers of HeLa cells containing 2 × 106 cells in 35-mm plates-diameter were mock infected or infected for 1 h at 37°C with 5 × 106 IFU of a purified preparation of the mouse pneumonitis strain of C. trachomatis or heparin-coated beads in sucrose-phosphate-glutamate buffer diluted in serum-free DME-16 containing 100 μg of cycloheximide per ml in the presence or absence of heparin (1 mg/ml). After the media were removed, cells were washed in phosphate-buffered saline (PBS) and lysed in buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.1% SDS, 1% [wt/vol] Triton X-100, 0.5% deoxycholate, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 1 mM NaF, 1 mM aprotinin) for 15 min at 4°C. Proteins in the soluble fraction were separated on SDS–7% polyacrylamide gels. The proteins were transferred to nitrocellulose membranes and immunoblotted with the mouse monoclonal antiphosphotyrosine antibody 4G10 (Upstate Biotechnology Incorporated, Lake Placid, N.Y.) at a dilution of 1:1,000 in TBS containing 0.1% Tween 20. A 1:1,000 dilution of goat anti-mouse secondary antibody conjugated to horseradish peroxidase was used as a secondary antibody, and the immunoblot was processed using an ECL kit (Amersham, Arlington Heights, Ill.).
Immunofluorescence.
HeLa cell monolayers were grown on 12-mm-diameter coverslips and incubated with heparin-coated beads for 4 h. The monolayers were fixed and stained by the pH shift method (2) with minor modifications (36). Briefly, the monolayers were washed twice with 80 mM HEPES–5 mM EDTA–2 mM MgCl2, incubated with low-pH fixative (4% paraformaldehyde in 80 mM HEPES–5 mM EDTA–2 mM MgCl2, pH 6.5) for 5 min, and then incubated with high-pH fixative (4% paraformaldehyde in 100 mM Na borate, pH 11.0) for 10 min. Primary antibody (AC-17, an anti-LAMP-1 antibody; American Type Culture Collection) diluted 1:100 in PBS-saponin was added and left for 1 h at 37°C. The monolayers were washed four times with PBS-saponin and incubated with the secondary goat anti-mouse antibody (Zymed, South San Francisco, Calif.) diluted 1:200 and 0.2 mg of propidium iodide (Sigma Chemical Co.) per ml for 1 h at 37°C. Excess antibody was removed by washing twice with PBS-fish skin gelatin-saponin, once with PBS, once with 0.1% Triton X-100 in PBS, and once with PBS. The monolayers were postfixed with 2% paraformaldehyde in 100 mM Na cacodylate for 15 min, washed once with PBS, and mounted using Anti-Fade mounting medium (Molecular Bioprobes, Eugene, Oreg.). The samples were examined under an epifluorescence microscope.
RESULTS
Heparan sulfate-mediated attachment.
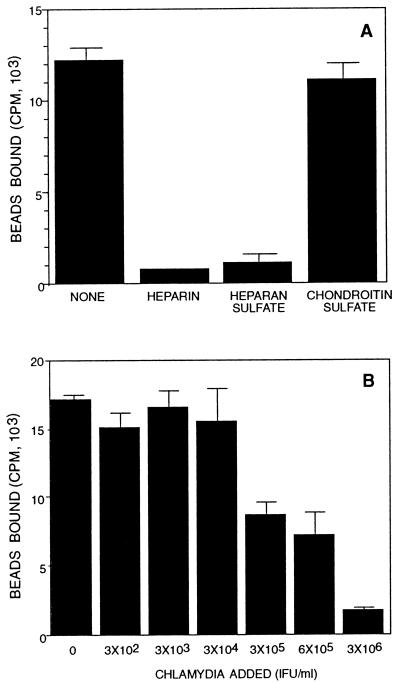
It was initially observed by light microscopy and scanning electron microscopy using 0.8-μm-diameter beads that microspheres coated with heparin or heparan sulfate bound eukaryotic cells, whereas uncoated microspheres or microspheres coated with a structurally related molecule, chondroitin sulfate, did not bind (data not shown). Similar observations were made using 0.3-μm-diameter beads, which approximate the size of chlamydial EB. Quantitative assessments of binding using 0.3-μm-diameter [3H]heparin-coated microspheres confirmed the visual observations. Heparin-coated microspheres bound host cells, and the specificity of binding was shown by competitive inhibition with exogenous heparin or heparan sulfate but not with a related molecule, chondroitin sulfate (Fig. 1A). A similar specificity of inhibition has been shown for chlamydial organisms (42).
FIG. 1.
Inhibition of heparin-coated bead binding to HeLa 229 cells in the presence of exogenous heparin, heparan sulfate, chondroitin sulfate, or chlamydial organisms. (A) Microspheres coated with [3H]heparin were incubated with HeLa 229 cell monolayers for 2 h in the absence of glycosaminoglycan competitors or in the presence of 1 mg of heparin, heparan sulfate, or chondroitin sulfate per ml. (B) Dose-dependent competitive binding inhibition of [3H]heparin-coated microspheres to HeLa cells by chlamydial organisms. Coated microspheres were incubated with HeLa cell monolayers for 2 h in the presence of increasing concentrations of chlamydiae. Error bars indicate standard deviations.
Heparan sulfate and Chlamydia compete for the same eukaryotic cell receptor.
Since heparin- or heparan sulfate-coated microspheres bound eukaryotic cells, the hypothesis that attachment of chlamydial organisms to eukaryotic cells is mediated by a heparan sulfate-like surface moiety on chlamydiae was tested by asking if native chlamydial organisms could compete with heparin-coated microspheres. A common molecular basis of attachment to eukaryotic cells was suggested by dose-dependent competitive inhibition of microsphere binding in the presence of increasing concentrations of chlamydial organisms (Fig. 1B). The observation that chlamydial organisms competitively inhibited binding of heparin-coated microspheres to eukaryotic cells supports the proposal that chlamydiae have a heparin-like analog on their surface and employ this molecule for host cell interaction.
Heparan sulfate-mediated entry.
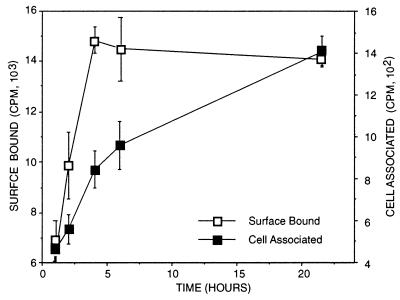
To distinguish endocytosis from binding, use was made of the observation that the eukaryotic cell receptor for chlamydiae is sensitive to trypsin. It has been shown that most surface-bound, but not internalized, chlamydiae can be eluted from eukaryotic cells after trypsin digestion (6). When [3H]heparin-coated beads were allowed to bind at 4°C to prevent uptake, it was determined that >90% of the counts were eluted following trypsin treatment (data not shown). Analogous to the kinetics of chlamydial attachment and uptake (5, 17), at 37°C, trypsin-sensitive binding of the heparin-coated beads rapidly reached steady-state levels and trypsin-resistant activity increased with time of incubation (Fig. 2). These observations suggested that the coated microspheres were taken up by the host cell.
FIG. 2.
HeLa 229 cell attachment and uptake of [3H]heparin-coated microspheres determined by measuring the amounts of cell surface-bound (trypsin-sensitive) and cell-associated (trypsin-resistant) radioactivity at different times following inoculation and incubation at 37°C. Error bars indicate standard deviations.
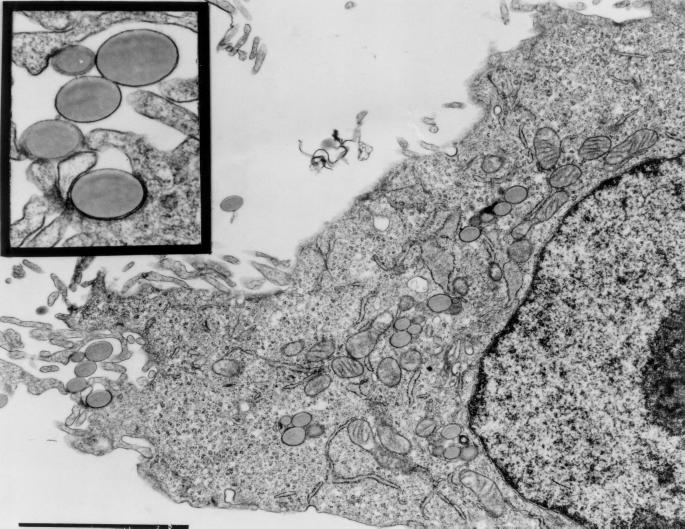
Intracellular localization of heparin-coated microspheres was confirmed by electron microscopy. Heparan sulfate-coated microspheres were observed in close apposition to the eukaryotic cell membrane typically associated with an electron-dense band on the cytoplasmic face of the membrane, and coated microspheres entered cells and appeared to be confined within a membrane-bound vacuole (Fig. 3). Uncoated beads or beads coated with chondroitin sulfate were not observed to be endocytosed by cells (data not shown).
FIG. 3.
Transmission electron microscopy of HeLa 229 cells following incubation at 37°C for 4 h with heparan sulfate-coated polystyrene microspheres. The inset shows a higher magnification of the area immediately below the inset. Bar, 1 μm.
Native-ligand-mediated attachment and entry.
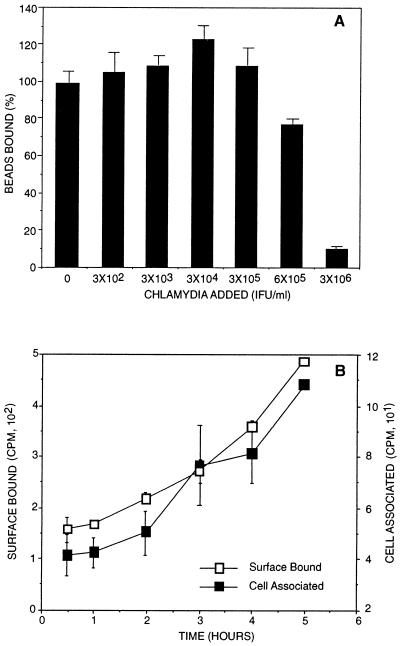
While the use of analogs for the chlamydial adhesion ligand provides a highly defined model system for heparan sulfate-host cell interactions, this model for chlamydia-host cell interactions was tested using microspheres coated with purified chlamydial heparan sulfate-like ligand to determine whether the native chlamydial molecule is sufficient to mediate binding and entry of microspheres to eukaryotic cells. CHO 761 cells, which do not produce glycosaminoglycans, were infected with chlamydiae, and the natural chlamydial ligand was isolated (42). When the purified 35S-labeled heparan sulfate-like ligand was used to coat microspheres, these microspheres bound host cells and binding was competitively inhibited by chlamydial organisms (Fig. 4A). Microspheres coated with the chlamydial heparan sulfate-like ligand also entered host cells in a time-dependent manner (Fig. 4B). Thus, like heparin- or heparan sulfate-coated microspheres, the isolated chlamydial heparan sulfate-like ligand was capable of mediating attachment and entry of microspheres into eukaryotic cells.
FIG. 4.
Binding of 35S-labeled chlamydial heparan sulfate-like ligand-coated microspheres to HeLa cells. (A) Dose-dependent competitive inhibition of coated microspheres and chlamydial organisms. Coated microspheres were incubated with HeLa cell monolayers for 2 h in the presence of increasing concentrations of chlamydiae. (B) HeLa cell attachment and uptake of 35S-labeled chlamydial heparan sulfate-like ligand-coated microspheres. The amounts of cell surface-bound (trypsin-sensitive) and cell-associated (trypsin-resistant) microspheres were determined by scintillation counting at different times following inoculation and incubation at 37°C. 35S-ligand was obtained from chlamydia-infected CHO 761 cells and purified by ion-exchange chromatography as described previously (42). Error bars indicate standard deviations.
Host cell tyrosine phosphorylation following uptake.
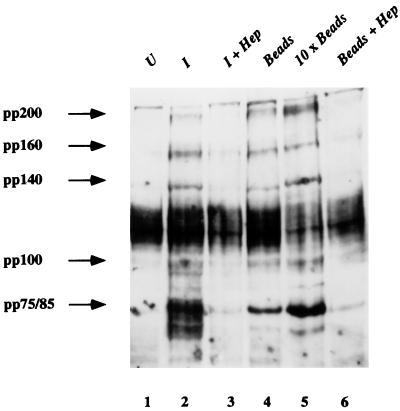
It has been demonstrated that uptake of C. trachomatis results in the tyrosine phosphorylation of several eukaryotic cell proteins (4). This finding has been generalized to the species, as a similar set of proteins are phosphorylated following uptake of the mouse pneumonitis biovar of C. trachomatis (13). Uptake of heparin-coated beads resulted in changes in host protein tyrosine phosphorylation similar to those observed following infection of cells with live C. trachomatis (Fig. 5). The amount of tyrosine phosphorylation induction was dependent upon the number of beads added and, like for chlamydiae, tyrosine phosphorylation was prevented by competitive inhibition using exogenous heparin (Fig. 5) but not by use of chondroitin sulfate (data not shown). These findings demonstrate that free heparin or heparan sulfate is not sufficient to induce host protein tyrosine phosphorylation and suggest that heparan sulfate or heparin must be presented to the cell on a matrix to induce detectable signal transduction upon uptake. The only discernible difference in the pattern of host protein tyrosine phosphorylation involved the pp75-85 complex. Upon endocytosis of the heparin-coated beads, fewer bands were apparent in this complex than were present upon endocytosis of C. trachomatis. The observation that C. trachomatis and heparin-coated beads induce the tyrosine phosphorylation of analogous series of proteins suggests that the attachment and uptake of chlamydiae and coated beads elicit common signal transduction events.
FIG. 5.
Chlamydiae and heparin-coated beads induce similar changes in host protein tyrosine phosphorylation. Immunoblot analysis of lysates from mock-infected cells (lane 1), cells infected with C. trachomatis (MoPn) for 2 h (lane 2), cells pretreated with heparin (1 mg/ml) for 1 h and then infected with chlamydial organisms for 2 h in the presence of exogenous heparin (lane 3), cells to which heparin-coated beads were added and left for 2 h (lane 4), cells to which 10-fold more heparin beads were added and left for 2 h (lane 5), or cells pretreated with heparin and then incubated with heparin-coated beads for 2 h in the presence of exogenous heparin (lane 6) that were reacted with a mouse monoclonal antibody to phosphotyrosine (4G10) as previously described (13). Molecular masses in kilodaltons are indicated on the left. Part of this figure has been reproduced from a previous publication (13).
Intracellular fate of heparin-coated beads.
One of the applications of the microsphere model of chlamydial invasion is in determining attributes defined by the pathway of entry versus attributes requiring chlamydial activities independent of the mechanisms of entry. C. trachomatis survives intracellularly by inhibiting fusion with lysosomes (26). Using antibodies to protein markers characteristic of various endosomal compartments, the intracellular location of endocytosed heparin-coated beads was evaluated by indirect immunofluorescence. In contrast to the case for live chlamydiae (36), the lysosomal marker LAMP-1 colocalized with the internalized beads, whereas the early endosome markers transferrin receptor and transferrin and the late endosome marker mannose-6-phosphate receptor did not colocalize (data not shown). Thus, entry by a heparin receptor-mediated pathway is not sufficient to specify the ultimate intracellular destination of the organism.
DISCUSSION
One emerging theme in regard to microbial attachment to eukaryotic cells involves interaction with heparan sulfate proteoglycans on host cells, as recently shown for Bordetella pertussis (25) and Borrelia burgdorferi (23) cellular adhesion. In addition, heparan sulfate proteoglycans on eukaryotic cells are bound by several pathogens to facilitate microbial invasion, including human immunodeficiency virus (30), herpes simplex virus (35), cytomegalovirus (10), Leishmania donovani (27), Trypanosoma cruzi (29), and Plasmodium circumsporozoites (14). While chlamydiae bind exogenous heparin (9) and host cell proteoglycans can be expected to contribute to chlamydial adhesion, unlike for these other microbial systems, it has been shown that C. trachomatis attachment to and invasion of eukaryotic cells are primarily mediated by a polyanionic heparan sulfate-like glycosaminoglycan on the chlamydial surface (7, 8, 42). Two other microbial pathogens have recently been shown to mediate invasion of eukaryotic host cells by using heparin or heparan sulfate polysaccharides on the surface of the microorganism. Toxoplasma gondii, a protozoan parasite, binds exogenous sulfated polysaccharides to its surface, which promotes interaction with host cells through a ternary complex consisting of a parasite surface lectin, sulfated polysaccharide, and an unknown host cell molecule (28). The Opa surface protein of Neisseria gonorrhoeae binds sulfated polysaccharides that also promote host cell invasion (38). For N. gonorrhoeae the model has been extended to include the binding of proteins, such as fibronectin, to the sulfated polysaccharide on the gonococcus that in turn mediates invasion through integrin receptors (37). Because attachment and entry are sequential steps for infectivity, previous studies with chlamydiae cannot unequivocally differentiate between the possibilities that (i) the heparan sulfate-like ligand mediates only attachment and other chlamydial components are necessary for entry or (ii) this single ligand is sufficient for attachment as well as entry into eukaryotic cells.
Based upon the ability to rescue chlamydial attachment and infectivity of heparan sulfate lyase-treated organisms with exogenous heparin (7, 8, 42), we reasoned that the potential role of this pathway in cellular attachment and/or entry of chlamydiae could be dissected by modeling this interaction using polystyrene microspheres coated with functional and structural analogs of chlamydial heparan sulfate-like ligand. As genetic manipulations cannot be conducted with Chlamydia (3), this approach provides one of the few experimental means to evaluate the role of the heparan sulfate-like ligand in attachment and invasion independent of other chlamydial activities. The use of functional analogs mitigates potential confounding effects from unidentified chlamydial components that might copurify with the chlamydial heparan sulfate-like ligand. The functional evaluation of native chlamydial heparan sulfate-like ligand demonstrated that the natural ligand has the capacity to mediate the functions ascribed to it from the results of studies with analogs.
Microspheres coated with heparin or heparan sulfate attached to eukaryotic cells and, by assessments of trypsin-resistant cell association and visualization by electron microscopy, coated microspheres were found to enter eukaryotic cells. Attachment and entry of coated microspheres were competitively inhibited by chlamydial organisms, thus showing that inhibition of chlamydial organisms by heparin (7, 21) and inhibition of heparin binding to eukaryotic cells by chlamydiae are reciprocal. The ability of chlamydial organisms to competitively inhibit the interaction of heparin-coated microspheres that display only heparin strongly suggests that native chlamydiae functionally interact with eukaryotic cells by means of a sulfated glycosaminoglycan-like molecule associated with the surface of the organism. This model of attachment and entry is remarkably similar to the interactions observed for C. trachomatis with eukaryotic cells in that the kinetics of attachment and entry for coated microspheres are analogous to those observed for chlamydiae (5, 17, 39). This was so despite the fact that physicochemical binding of the chlamydial heparan sulfate-like ligand or functional analogs to microspheres is undoubtedly different than their specific association with chlamydial organisms.
Using either the native chlamydial molecule or functional analogs as surface ligands on polystyrene microspheres, it was shown that a heparan sulfate-like ligand alone was sufficient for C. trachomatis-specific attachment to mammalian cells. The pattern of tyrosine-phosphorylated proteins of cells infected with chlamydiae differs from those proteins phosphorylated following entry of other intracellular bacterial pathogens, such as Yersinia (1), Salmonella (31), Shigella (11), and enteropathogenic Escherichia coli (32), yet was similar to the pattern elicited following uptake of heparin-coated microspheres. These findings suggest that endocytosis mediated by receptor engagement by heparin or heparan sulfate triggers intracellular signal transduction events and is associated with the receptor-specific endocytosis process. Furthermore, these data demonstrate that the paramount role for the chlamydial heparan sulfate-like ligand is in invasion of chlamydiae into host cells. It is likely that this pathogen exploits the presence and natural function of an uncharacterized eukaryotic cell receptor to promote entry into eukaryotic cells.
Based upon the observations that (i) heparin-coated (and chlamydial ligand-coated) microspheres bound and entered cells, (ii) binding was competitive with chlamydial organisms, and (iii) entry of coated microspheres elicited a pattern of tyrosine-phosphorylated proteins similar to that elicited by chlamydial uptake, we conclude that chlamydial attachment and entry can be coupled events mediated by a single molecular component resembling heparan sulfate, a ubiquitous host cell ligand (19). These data strongly support the hypothesis that chlamydiae display a heparan sulfate-like glycosaminoglycan (GAG) on their surfaces that is necessary and sufficient for mediating chlamydial invasion of eukaryotic host cells. The demonstration that sulfated polysaccharides such as heparin attached to a solid support mediate entry into eukaryotic cells suggests that other pathogens capable of binding sulfated polysaccharides also could enter cells by this pathway.
Once chlamydiae enter eukaryotic cells, their survival depends upon inhibition of lysosomal fusion with vacuoles harboring chlamydiae. The heparin bead model of chlamydial entry was used to determine which events after uptake may be predefined by the pathway of entry and which events require other chlamydia-specific attributes. Using antibodies to well-defined markers of the various components of the endocytic pathway, it was shown that the heparin-coated beads enter a lysosomal compartment within 4 h. In contrast, viable C. trachomatis organisms remain in a nonlysosomal compartment (18, 36). Thus, a heparin-like molecule is sufficient to mediate attachment, entry, and signal transduction, but additional chlamydial components are necessary to engineer survival within the host cell by preventing fusion with lysosomes. This is consistent with the demonstration that inhibition of chlamydial protein synthesis during host cell uptake results in chlamydial vacuoles fusing with lysosomes (34).
This bead model should be useful for cell biology studies to provide new information about the heparan sulfate pathway and provide a unique opportunity to elucidate this fundamental cellular process. Heparan sulfate is one of a family of structurally related glycosaminoglycans that, as cell membrane proteoglycans or free glycans, have been implicated in a wide variety of biological effects (19). However, the precise functions and mechanism of cellular uptake of heparan sulfate are unknown. Capitalizing on the known association of chlamydiae with mucosal cells, heparin- or heparan sulfate-coated microspheres may be a useful vehicle for targeted delivery of subunit vaccines and chemotherapeutic agents to mucosal cells of humans.
ACKNOWLEDGMENTS
We sincerely thank M. Chiappino and V. Powers for their contributions to these studies. We also thank J. Esko (University of Alabama) for providing the GAG-deficient CHO 761 cells.
This work was supported by National Institutes of Health grants AI32943, EY07757, and AI24436 and The Lucille Markey Charitable Trust (to J.N.E.). J.N.E. is a Lucille Markey Biomedical Scholar.
REFERENCES
- 1.Andersson K, Carballeira N, Magnusson K E, Persson C, Stendahl O, Wolf-Watz H, Fallman M. YopH of Yersinia pseudotuberculosis interrupts early phosphotyrosine signalling associated with phagocytosis. Mol Microbiol. 1996;20:1057–1069. doi: 10.1111/j.1365-2958.1996.tb02546.x. [DOI] [PubMed] [Google Scholar]
- 2.Bacallao R, Stelzer E H. Preservation of biological specimens for observation in a confocal fluorescence microscope and operational principals of confocal fluorescence microscopy. Methods Cell Biol. 1989;31:437–452. doi: 10.1016/s0091-679x(08)61621-0. [DOI] [PubMed] [Google Scholar]
- 3.Beatty P R, Stephens R S. Identification of Chlamydia trachomatis antigens by use of murine T-cell lines. Infect Immun. 1992;60:4598–603. doi: 10.1128/iai.60.11.4598-4603.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birkelund S, Johnsen H, Christiansen G. Chlamydia trachomatis serovar L2 induces protein tyrosine phosphorylation during uptake by HeLa cells. Infect Immun. 1994;62:4900–4908. doi: 10.1128/iai.62.11.4900-4908.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrne G I. Kinetics of phagocytosis of Chlamydia psittaci by mouse fibroblasts (L cells): separation of the attachment and ingestion stages. Infect Immun. 1978;19:607–612. doi: 10.1128/iai.19.2.607-612.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne G I, Moulder J W. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect Immun. 1978;19:598–606. doi: 10.1128/iai.19.2.598-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J C, Stephens R S. Chlamydia trachomatis glycosaminoglycan-dependent and independent attachment to eukaryotic cells. Microb Pathog. 1997;22:23–30. doi: 10.1006/mpat.1996.0087. [DOI] [PubMed] [Google Scholar]
- 8.Chen J C, Stephens R S. Trachoma and LGV biovars of Chlamydia trachomatis share the same glycosaminoglycan-dependent mechanism for infection of eukaryotic cells. Mol Microbiol. 1994;11:501–507. doi: 10.1111/j.1365-2958.1994.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen J C, Zhang J P, Stephens R S. Structural requirements of heparin binding to Chlamydia trachomatis. J Biol Chem. 1996;271:11134–11140. [PubMed] [Google Scholar]
- 10.Compton T, Nowlin D M, Cooper N R. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology. 1993;193:834–841. doi: 10.1006/viro.1993.1192. [DOI] [PubMed] [Google Scholar]
- 11.Dehio C, Prevost M C, Sansonetti P J. Invasion of epithelial cells by Shigella flexneri induces tyrosine phosphorylation of cortactin by a pp60c-src-mediated signalling pathway. EMBO J. 1995;14:2471–2482. doi: 10.1002/j.1460-2075.1995.tb07244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falkow S, Isberg R R, Portnoy D A. The interaction of bacteria with mammalian cells. Annu Rev Cell Biol. 1992;8:333–363. doi: 10.1146/annurev.cb.08.110192.002001. [DOI] [PubMed] [Google Scholar]
- 13.Fawaz F S, van Ooij C, Homola E, Mutka S C, Engel J N. Infection with Chlamydia trachomatis alters the tyrosine phosphorylation and localization of several host cell proteins including cortactin. Infect Immun. 1997;65:5301–5308. doi: 10.1128/iai.65.12.5301-5308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frevert U, Sinnis P, Cerami C, Shreffler W, Takacs B, Nussenzweig V. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. J Exp Med. 1993;177:1287–1298. doi: 10.1084/jem.177.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grayston J T, Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975;132:87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez-Martin C B, Ojcius D M, Hsia R C, Hellio R, Baviol P M, Dautry-Varsat A. Heparin-mediated inhibition of Chlamydia psittaci adherence to HeLa cells. Microb Pathog. 1997;22:47–57. doi: 10.1006/mpat.1996.0090. [DOI] [PubMed] [Google Scholar]
- 17.Hatch T P, Vance D W, Jr, Al-Hossainy E. Attachment of Chlamydia psittaci to formaldehyde-fixed and unfixed L cells. J Gen Microbiol. 1981;125:273–283. doi: 10.1099/00221287-125-2-273. [DOI] [PubMed] [Google Scholar]
- 18.Heinzen R A, Scidmore M A, Rockey D D, Hackstadt T. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect Immun. 1996;64:796–809. doi: 10.1128/iai.64.3.796-809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson R L, Busch S J, Cardin A D. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev. 1991;71:481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- 20.Koehler J E, Birkelund S, Stephens R S. Overexpression and surface localization of the Chlamydia trachomatis major outer membrane protein in Escherichia coli. Mol Microbiol. 1992;6:1087–1094. doi: 10.1111/j.1365-2958.1992.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuo C C, Grayston T. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect Immun. 1976;13:1103–1109. doi: 10.1128/iai.13.4.1103-1109.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laga M, Nzila N, Goeman J. The interrelationship of sexually transmitted diseases and HIV infection: implications for the control of both epidemics in Africa. AIDS. 1991;5(Suppl 1):S55–S63. [PubMed] [Google Scholar]
- 23.Leong J M, Morrissey P E, Ortega-Barria E, Pereira M E, Coburn J. Hemagglutination and proteoglycan binding by the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:874–883. doi: 10.1128/iai.63.3.874-883.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mabey D C, Bailey R L, Ward M E, Whittle H C. A longitudinal study of trachoma in a Gambian village: implications concerning the pathogenesis of chlamydial infection. Epidemiol Infect. 1992;108:343–351. doi: 10.1017/s0950268800049815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menozzi F D, Gantiez C, Locht C. Interaction of the Bordetella pertussis filamentous hemagglutinin with heparin. FEMS Microbiol Lett. 1991;62:59–64. doi: 10.1111/j.1574-6968.1991.tb04417.x. [DOI] [PubMed] [Google Scholar]
- 26.Moulder J W. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991;55:143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukhopadhyay N K, Shome K, Saha A K, Hassell J R, Glew R H. Heparin binds to Leishmania donovani promastigotes and inhibits protein phosphorylation. Biochem J. 1989;264:517–525. doi: 10.1042/bj2640517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortega-Barria E, Boothroyd J C. A Toxoplasma lectin-like activity specific for sulfated polysaccharides is involved in host cell infection. J Biol Chem. 1999;274:1267–1276. doi: 10.1074/jbc.274.3.1267. [DOI] [PubMed] [Google Scholar]
- 29.Ortega-Barria E, Pereira M E. A novel T. cruzi heparin-binding protein promotes fibroblast adhesion and penetration of engineered bacteria and trypanosomes into mammalian cells. Cell. 1991;67:411–421. doi: 10.1016/0092-8674(91)90192-2. [DOI] [PubMed] [Google Scholar]
- 30.Patel M, Yanagishita M, Roderiquez G, Bou-Habib D C, Oravecz T, Hascall V C, Norcross M A. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res Hum Retroviruses. 1993;9:167–174. doi: 10.1089/aid.1993.9.167. [DOI] [PubMed] [Google Scholar]
- 31.Rosenshine I, Ruschkowski S, Foubister V, Finlay B B. Salmonella typhimurium invasion of epithelial cells: role of induced host cell tyrosine protein phosphorylation. Infect Immun. 1994;62:4969–4974. doi: 10.1128/iai.62.11.4969-4974.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenshine I, Ruschkowski S, Stein M, Reinscheid D J, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 33.Schachter J. Chlamydial infections. West J Med. 1990;153:523–534. [PMC free article] [PubMed] [Google Scholar]
- 34.Scidmore M A, Rockey D D, Fischer E R, Heinzen R A, Hackstadt T. Vesicular interactions of the Chlamydia trachomatis inclusion are determined by chlamydial early protein synthesis rather than route of entry. Infect Immun. 1996;64:5366–5372. doi: 10.1128/iai.64.12.5366-5372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shieh M T, WuDunn D, Montgomery R I, Esko J D, Spear P G. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992;116:1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Ooij C, Apodaca G, Engel J. Characterization of the Chlamydia trachomatis vacuole and its interaction with the host endocytic pathway in HeLa cells. Infect Immun. 1997;65:758–766. doi: 10.1128/iai.65.2.758-766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Putten J P M, Duensing T D, Cole R L. Entry of OpaA+ gonococci into HEp-2 cells requires concerted action of glycosaminoglycans, fibronectin and integrin receptors. Mol Microbiol. 1998;29:369–379. doi: 10.1046/j.1365-2958.1998.00951.x. [DOI] [PubMed] [Google Scholar]
- 38.van Putten J P M, Hayes S F, Duensing T D. Natural proteoglycan receptor analogs determine the dynamics of Opa adhesin-mediated gonococcal infection of Chang epithelial cells. Infect Immun. 1997;65:5028–5034. doi: 10.1128/iai.65.12.5028-5034.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vretou E, Goswami P C, Bose S K. Adherence of multiple serovars of Chlamydia trachomatis to a common receptor on HeLa and McCoy cells is mediated by thermolabile protein(s) J Gen Microbiol. 1989;135:3229–3237. doi: 10.1099/00221287-135-12-3229. [DOI] [PubMed] [Google Scholar]
- 40.Wyrick P B, Choong J, Davis C H, Knight S T, Royal M O, Maslow A S, Bagnell C R. Entry of genital Chlamydia trachomatis into polarized human epithelial cells. Infect Immun. 1989;57:2378–2389. doi: 10.1128/iai.57.8.2378-2389.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaretzky F R, Pearce-Pratt R, Phillips D M. Sulfated polyanions block Chlamydia trachomatis infection of cervix-derived human epithelia. Infect Immun. 1995;63:3520–3526. doi: 10.1128/iai.63.9.3520-3526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J P, Stephens R S. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell. 1992;69:861–869. doi: 10.1016/0092-8674(92)90296-o. [DOI] [PubMed] [Google Scholar]