Abstract
The gene for natural resistance-associated macrophage protein 1 (NRAMP1) plays a dominant role in controlling the resistance of inbred mice to infection with intracellular bacteria, such as Mycobacteria, Salmonella, and Leishmania. NRAMP1 is a membrane protein with a consensus transport motif present in one of the intracellular loops. Although its functions remain unclear, recent clues suggest that NRAMP1 protein plays a potential role in ion transport, which presumably accounts for the ability of this single protein to regulate the intraphagosomal replication of several species of antigenically unrelated intracellular pathogens. Expression of NRAMP1 in mice can be induced by lipopolysaccharide (LPS) or bacterial infection; however, little is known about the mechanisms of induction. Here, we report the cloning of the full-length cDNA for porcine NRAMP1, which had over 85% identity in amino acid sequence to its congeners from humans, mice, cattle, and sheep. As for its mammalian congeners, expression of porcine NRAMP1 mRNA was cell and tissue specific and was highest in macrophages. Investigation of the molecular mechanisms by which NRAMP1 is induced showed that LPS-induced expression in macrophages, neutrophils, and peripheral blood mononuclear cells was time and dose dependent and was mediated primarily through CD14. Induction of NRAMP1 required de novo protein synthesis, and mitogen-activated protein kinases (MAPK) were essential. Blockage of either p38 or p42/44 MAPK pathways suppressed the expression of NRAMP1 to basal levels. These findings suggest that bacterial infection and proinflammatory mediators induce NRAMP1 expression via activation of MAPK pathways.
The resistance or susceptibility of inbred mouse strains to infection with intracellular pathogens, including Mycobacteria, Salmonella, and Leishmania, is controlled by the gene encoding the natural resistance-associated macrophage protein 1 (NRAMP1) (46–48). Susceptibility of inbred mice to infections is associated with a single substitution of aspartic acid for glycine at position 169 of the protein (46). Mouse NRAMP1 protein exhibits all of the hallmarks of a typical integral membrane protein, including 12 putative transmembrane (TM) domains and a consensus transport motif between TM8 and TM9 (46, 48). With more homologues identified, NRAMP1 now is established as an ancient family of highly homologous membrane proteins, because all of the above features are well conserved in its congeners in mammals, insects, plants, and several bacterial species (6, 7). Recent studies have indicated that polymorphisms of the human NRAMP1 gene are associated with an increase in susceptibility to tuberculosis and leprosy and possibly with the pathogenesis of rheumatoid arthritis (2, 16). In addition to disease resistance, several members of the NRAMP1 gene family are involved in intestinal iron transport (14, 15, 20).
NRAMP1 protein is phagocyte specific and is located on the endosomal/lysosomal compartment of the macrophage rather than the plasma membrane (19, 39). It is recruited rapidly to the phagosomal membrane upon phagocytosis. Although mechanisms by which a single NRAMP1 protein confers the resistance of mice to several species of antigenically and taxonomically unrelated intracellular bacteria remain obscure, recent clues suggest that it may regulate the intraphagosomal replication of intracellular bacteria by limiting divalent cation concentrations (16). Expression of NRAMP1 in murine macrophages is induced in response to lipopolysaccharide (LPS), gamma interferon (IFN-γ), or bacterial infection (3, 4, 17, 18). Induction of NRAMP1 by inflammatory or infectious agents may be used to better respond to exogenous insults, such as intracellular infections. However, little is known about the mechanisms and signal transduction pathways by which the NRAMP1 gene is induced in response to these stimuli.
Membrane-bound CD14 plays a central role in the initiation of signal transduction in response to LPS in monocytic cells (12, 43, 45). It is well known that one of the initial events leading to macrophage activation is protein tyrosine phosphorylation following the engagement of LPS with CD14 (9, 13, 43). Among the most prominent and best characterized tyrosine-phosphorylated proteins are mitogen-activated protein kinases (MAPKs) (9, 13, 41, 43). Activation of MAPK cascades results in the phosphorylation and activation of transcription factors, including activator protein 1, nuclear factor, interleukin-6 (IL-6), and NF-κB, which in turn transactivate a vast array of immune and inflammatory genes. To date, three major pathways mediated by MAPKs, namely, p38, p42/44 (extracellularly regulated kinase [ERK]), and c-Jun N-terminal kinase (JNK)/stress-activated protein kinase (SAPK), have been implicated in LPS-induced activation of macrophages (9, 13, 43). Binding of IL-1 and tumor necrosis factor alpha (TNF-α) to their respective receptors triggers the activation of NF-κB and AP-1, which also involves MAPKs, particularly NF-κB-inducing kinase and MAPK/ERK kinase kinase (MEKK1), a kinase of the JNK/SAPK pathway and an activator of IκB kinase (28, 31–33, 38, 49).
Here, we report the cloning of the full-length cDNA for porcine NRAMP1, which was used as a model to investigate the molecular mechanisms of NRAMP1 induction in macrophages by proinflammatory mediators. We show that LPS-induced expression of NRAMP1 is CD14 dependent and requires de novo protein synthesis. Furthermore, we provide evidence that MAPKs, particularly p38 and p42/44 (ERK1/2) MAPK, are involved in the induction of NRAMP1 expression in response to LPS, TNF-α, and IL-1β.
MATERIALS AND METHODS
Animals and reagents.
Healthy, 6- to 8-week-old crossbred pigs were obtained from the Kansas State University Swine Research Unit and housed in an environmentally controlled isolation facility at the university. The pigs were determined to be free of both clinical signs of salmonellosis and detectable Salmonella organisms in fecal cultures 1 day prior to use in our experiments. Media (RPMI 1640, Dulbecco's modified Eagle's medium, and Hanks balanced salt solution [HBSS]), antibiotics, Trizol reagent, and Platinum Taq polymerase all were purchased from Life Technologies (Rockville, Md.). Fetal bovine serum (FBS; low endotoxin) was from HyClone Laboratories (Logan, Utah). LPS from Salmonella enterica serovar Typhimurium, polymyxin B, cycloheximide, dexamethasone, and mouse immunoglobulin G2b isotype control were purchased from Sigma (St. Louis, Mo.). My4, a mouse immunoglobulin G2b monoclonal antibody (MAb) against human CD14, was from Coulter (Hialeah, Fla.). Recombinant human IL-1β and porcine TNF-α were obtained from R&D Systems (Minneapolis, Minn.) and Endogen (Woburn, Mass.), respectively. The MAPK inhibitors PD98059 and SB203580 were purchased from Calbiochem (San Diego, Calif.).
Cell isolation, culture, and stimulation.
Peripheral blood mononuclear cells (PBMCs), peripheral blood polymorphonuclear neutrophils (PMNs), and alveolar macrophages were isolated as previously described (26, 40, 51). Briefly, PBMCs and PMNs were obtained from heparinized venous blood by density gradient centrifugation followed by hypotonic lysis of erythrocytes. Purified cells were suspended at 2 × 106/ml in RPMI 1640 containing 10% FBS, 100 U of penicillin per ml, and 100 μg of streptomycin per ml and then seeded into six-well tissue culture plates. Following a 1-h incubation at 39°C with 5% CO2, the cells were stimulated with 0.1 μg of LPS per ml for various times before being harvested for RNA extraction. For isolation of alveolar macrophages, 400 to 500 ml of sterile HBSS per lung was infused into caudal lung lobes in 50-ml aliquots. Lavage fluid was pooled and subjected to hypotonic lysis if erythrocytes were visible. Cells were suspended at 3 × 106 to 5 × 106/ml in Dulbecco's modified Eagle's medium containing 10% FBS, 100 U of penicillin per ml, and 100 μg of streptomycin per ml and cultured at 39°C under 5% CO2 in six-well tissue culture plates. Following a 2-h incubation, nonadherent cells were removed by gently washing twice with HBSS. Adherent cells were cultured in fresh medium and used in subsequent stimulation studies by using the methods outlined in the respective figure legends.
S. enterica serovar Typhimurium infection.
Infection studies began 1 week after pigs were acclimated to the isolation facility. Pigs were gavaged with 109 CFU of S. enterica serovar Typhimurium suspended in growth medium. The bacterial strain was a primary isolate from a clinical case of salmonellosis in pigs and was confirmed to be a pure culture of S. enterica serovar Typhimurium by the National Veterinary Services Laboratory (Ames, Iowa). Clinical signs for bacterial infections were monitored throughout the experiment. Three to four pigs were euthanized at 6, 12, 24, and 48 h postinfection. Four control pigs were gavaged with growth medium alone and euthanized at 6 h. Samples of liver and spleen, collected from control and infected pigs, were homogenized in Trizol reagent and subjected to RNA isolation. All experiments were performed in accordance with guidelines of the Institutional Animal Care and Use and Biosafety Committees of Kansas State University.
3′-RACE.
The full-length cDNA sequence of porcine NRAMP1 was obtained using a 3′-rapid amplification of cDNA ends (RACE) kit (Life Technologies) as specified by the manufacturer. Briefly, 2 μg of total RNA from porcine alveolar macrophages was heat denatured for 10 min at 70°C and subjected to reverse transcription using the adapter primer 5′-GGC CAC GCG TCG ACT AGT AC (T)17-3′. The reaction was performed for 1 h at 42°C followed by 20 min at 50°C in a total volume of 20 μl containing 200 U of SuperScript II reverse transcriptase (RT), 0.5 μM adapter primer, 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 2.5 mM MgCl2, 10 mM dithiothreitol DTT, and 0.5 mM each deoxynucleoside triphosphate. After degradation of RNA template with RNase H, 1/10 of the resulting first-strand cDNA was used in a PCR with a degenerate gene-specific primer, 5′-GC(G/A) GT(C/T) C(T/A)C ATG (A/T)CA GGT GAC A-3′, and an abridged adapter primer, 5′-GGC CAC GCG TCG ACT AGT AC-3′. The gene-specific primer was designed based on the conserved NRAMP1 cDNA sequences around the start codon from humans, mice, cattle, and sheep. The PCR conditions were 94°C for 30 s, followed by 35 cycles of 94°C for 30 s, 65°C for 30 s, and 72°C for 4 min, and the final extension was at 72°C for 15 min. The reaction was performed in a 50-μl volume with 2 U of Platinum Taq polymerase, 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 2 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate, and 0.2 μM each of the gene-specific primers and the abridged adapter primer. The subsequent PCR product was isolated and cloned into the pGEM-T Easy vector (Promega, Madison, Wis.). Two independent clones with the correct size of insert were sequenced using a Thermo Sequenase 33P-labeled dideoxynucleotide terminator cycle-sequencing kit (Amersham Pharmacia Biotech, Piscataway, N.J.).
RNA isolation and semiquantitative RT-PCR.
Tissues and cells collected from two healthy pigs aged 6 to 8 weeks included lung, ileum, colon, thymus, spleen, lymph node, kidney, liver, muscle, heart, brain, testis, skin, alveolar macrophages, bone marrow, PMNs, and PBMCs. Total RNAs from porcine tissues and cells were isolated using Trizol reagent as specified by the manufacturer. A 1-μg portion of each RNA sample was used to perform first-strand cDNA synthesis under the conditions described above, except that 25 U of Moloney murine leukemia virus RT (Perkin-Elmer, Foster City, Calif.) and 10 U of RNase inhibitor (Perkin-Elmer) were used. A portion (1/10) of each resulting cDNA was used in the subsequent PCR in a 25 μl-reaction volume containing 0.1 μM each sense and antisense primer and 1.25 U of Platinum Taq polymerase as described previously (50). Primers for amplification of porcine NRAMP1, inducible nitric oxide synthase (iNOS), and β-actin were 5′-TCT GCC ATC TCT ACT ACC CTA AGG-3′ (sense) and 5′-CTA AGA AGT GCT CCC TGA GCA G-3′ (antisense), 5′-ATG TTC GAG CAC ATC TGC A-3′ (sense) and 5′-ACA TTG ATC TCC ACG ACA GCG-3′ (antisense), and 5′-GGA CTT CGA GCA GGA GAT GG-3′ (sense) and 5′-GCA CCG TGT TGG CGT AGA GG-3′ (antisense), respectively. The PCR profile was 2 min at 94°C followed by 22 cycles (for NRAMP1 amplification in PBMCs and PMNs), 26 cycles (for NRAMP1 and iNOS in livers and spleens), or 16 cycles (for β-actin) of 30 s at 94°C, 30 s at 60°C, and 1 min at 72°C. The PCR products for NRAMP1, iNOS, and β-actin were 593, 307, and 233 bp, respectively.
Hybridization probes.
The cDNA probes for NRAMP1, iNOS, and β-actin each were prepared by ligating the respective PCR product generated with the above primers into the pGEM-T Easy vector. A 452-bp glyceraldehyde 3-phosphate dehydrogenase (GAPDH) cDNA, obtained by PCR amplification with the sense primer 5′-ACC ACA GTC CAT GCC ATC AC-3′ and antisense primer 5′-TCC ACC ACC CTG TTG CTG-3′, also was ligated into the pGEM-T Easy vector. Plasmid DNA was purified, partially sequenced to confirm the correct insert, and then digested with EcoRI. The insert was isolated, randomly labeled with 32P, and used as a hybridization probe in Southern and Northern analyses.
Southern and Northern blot analyses.
Southern and Northern analyses were performed as previously described with slight modifications (51). For Southern analysis, 10 μl of RT-PCR product was separated on 1.5% agarose gels and subjected to denaturation and overnight capillary transfer onto positively charged nylon membranes (Boehringer Mannheim, Indianapolis, Ind.). For Northern analysis, 20 μg of total RNA from tissues or 5 μg of total RNA from alveolar macrophages was heat denatured, fractionated on 1.2% agarose–formaldehyde gels, and blotted onto positively charged nylon membranes (Boehringer Mannheim). Southern and Northern blots were prehybridized for 40 min at 60°C in ExpressHyb hybridization solution (Clontech, Palo Alto, Calif.) and hybridized for 2 h under the same conditions with 32P-labeled cDNA probes. Posthybridization washes were performed twice for 10 min at 42°C with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 sodium citrate) and then twice for 20 min at 50°C with 0.1 × SSC–0.5% sodium dodecyl sulfate. Blots were exposed to Kodak X-OMAT films (Eastman Kodak, Rochester, N.Y.) with intensifying screens at −70°C. In some cases, Northern blots were stripped of the NRAMP1 cDNA probe after exposure and then probed with the 32P-labeled GAPDH cDNA. The intensity of the hybridization signal was quantified from autoradiographs by using UN-SCAN-IT Gel Automated Digitizing System (Silk Scientific Corp., Orem, Utah). Pixel values of each specific band were recorded from digitized images. In some cases, expression levels of NRAMP1 were normalized to the expression of GAPDH or β-actin the housekeeping gene by calculating the ratios of pixel values of NRAMP1 over those of the housekeeping gene.
Nucleotide sequence accession number.
The porcine NRAMP1 cDNA has been deposited under GenBank accession no. AF132037.
RESULTS
Cloning of the cDNA for porcine NRAMP1.
To determine the possible expression of NRAMP1 homologue(s) in pigs, we synthesized a degenerate gene-specific primer based on the conserved region around the start codon of NRAMP1 cDNA sequences from humans, mice, cattle, and sheep. As expected, a 2.0-kb product was obtained by 3′-RACE and cloned into a T/A vector. Two independent clones were sequenced and gave identical sequences (shown in Fig. 1A). This porcine NRAMP1 cDNA is 2,077 bp in size. It contains an entire open reading frame encoding a 538-amino-acid protein with a glycine residue at position 169, similar to the resistant allele of mouse NRAMP1 (46). The predicted porcine homologue is a typical NRAMP1, which is composed of 12 TM domains as revealed by its hydrophobicity profile (data not shown). In addition, a putative N-terminal proline- and serine-rich Src homology 3 (SH3)-binding domain, four phosphorylation sites for protein kinase C (PKC), and two N-linked glycosylation sites were observed in this protein (Fig. 1B). The consensus transport motif also was highly conserved in the intercellular loop between TM8 and TM9. Comparison of the sequence of porcine NRAMP1 protein showed over 85% identity to each of its congeners from humans, mice, cattle, and sheep, suggesting that they belong to the same family of highly homologous membrane transport proteins and probably serve similar functions (Fig. 1B). Another cDNA for porcine NRAMP1 was isolated by screening a porcine spleen cDNA library (44). However, it showed a total of 12 nucleotide differences compared to our sequence. Five nonconservative mutations also were observed in the coding region, including substitutions of serine, glycine, threonine, glycine, and aspartic acid for threonine, aspartic acid, asparagine, arginine, and asparagine at positions 2, 6, 137, 164, and 185, respectively, in the previously reported sequence (44).
FIG. 1.
Nucleotide sequence of the cDNA for porcine NRAMP1 and a comparison of its predicted amino acid sequence with those of other NRAMP1 proteins. (A) cDNA and predicted 538-amino-acid sequences of porcine NRAMP1. The polyadenylation signal is underlined. The glycine-to-aspartic-acid substitution at position 169, associated with susceptibility of mice, is circled. (B) Comparison of deduced porcine NRAMP1 with its congeners from humans, mice, and cattle. Identical residues throughout the four species are shown in bold type, and the 12 conservative putative transmembrane domains are underlined. The conserved consensus transport motif, proline- and serine-rich putative SH3-binding domain, PKC phosphorylation sites (S/T-X-R/K), and N-linked glycosylation sites (N-X-S/T) are boxed.
Cell- and tissue-specific expression of porcine NRAMP1.
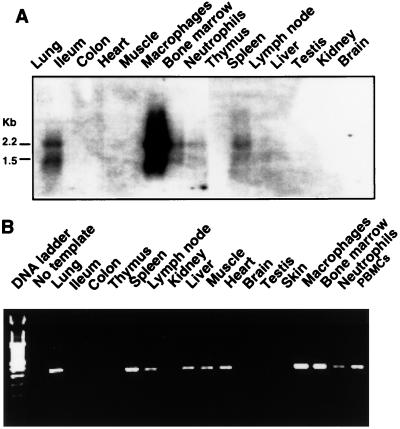
Northern blot analysis revealed a highly cell- and tissue-specific expression pattern of porcine NRAMP1; the mRNA was expressed most abundantly in alveolar macrophages and to a lesser extent in lung, spleen, bone marrow, and PMNs (Fig. 2A). More sensitive RT-PCR demonstrated that NRAMP1 also was expressed in PBMCs, liver, lymph node, heart, and muscle (Fig. 2B). Normalization of the hybridization signals to GAPDH (data not shown) indicated that the abundance of NRAMP1 expression in porcine tissues is in the following order: alveolar macrophages > PBMCs > lung > spleen > bone marrow > PMNs > liver > lymph node, heart, and muscle. The results confirmed that NRAMP1 was indeed a phagocyte-specific gene and that its expression appeared to be associated with tissues that have substantial populations of phagocytes. Interestingly, two NRAMP1 mRNA species, of ∼2.2 and 1.5 kb, were coexpressed in these tissues. The size of the larger species (2.2 kb) was consistent with the 2,077-bp size of our cloned cDNA, suggesting that the cDNA reported here is near full length. The origin of the shorter species is not clear, but it could be derived from alternate mRNA splicing.
FIG. 2.
Tissue- and cell-specific expression of porcine NRAMP1 mRNA. (A) Northern analysis of porcine NRAMP1 expression. Total RNA was extracted from various tissues and cells, and 20 μg/sample was blotted onto nylon membranes. The blots were hybridized with 32P-labeled NRAMP1 cDNA probes and then washed at high stringency. Two mRNA species of ∼2.2 and 1.5 kb were revealed. (B) RT-PCR of porcine NRAMP1 expression. A 1-μg sample of total RNA from each sample was subjected to RT-PCR, and 1/10 of each of the 35-cycle-amplified products was separated by electrophoresis on 1.5% agarose gels stained with ethidium bromide. The expected product was 307 bp. Data are representative of two independent experiments with similar results.
Induction of NRAMP1 expression by LPS.
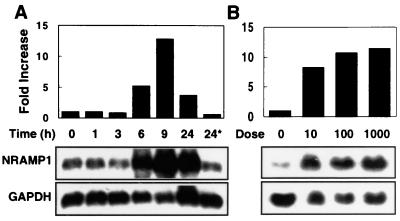
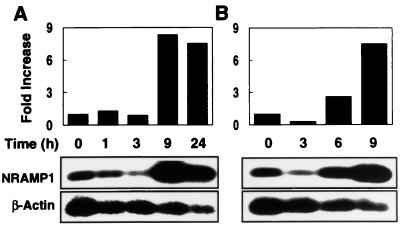
To investigate the inducibility of the porcine NRAMP1 gene, we treated alveolar macrophages, PBMCs, and PMNs with several proinflammatory mediators including LPS, TNF-α, and IL-1β. Cells were harvested after treatment, and total RNA was isolated and analyzed by Northern blotting (for alveolar macrophages) or semiquantitative RT-PCR (For PBMCs and PMNs) for the level of NRAMP1 expression. In these experiments, we observed a clear time- and dose-dependent induction of NRAMP1. Upregulation of NRAMP1 occurred after stimulation for 6 h with 0.1 μg of LPS per ml and peaked at more than 10-fold at 9 h; expression was reduced upon further incubation with LPS for 24 h (Fig. 3A). The induction of NRAMP1 also was highly sensitive to LPS; as little as 10 ng of LPS per ml induced a level of expression near the maximum induced by 1 μg of LPS per ml (Fig. 3B). LPS-induced NRAMP1 expression also occurred in two other NRAMP1-producing phagocytes, namely, PBMCs and PMNs. As shown in Fig. 4, PBMCs and PMNs treated with 0.1 μg of LPS per ml dramatically increased NRAMP1 expression, with peak induction occurring at 9 h.
FIG. 3.
Time- and dose-dependent induction of porcine NRAMP1 in alveolar macrophages in response to LPS stimulation. Alveolar macrophages were incubated for 2 h at 39°C under 5% CO2, and nonadherent cells were removed by gently washing twice with HBSS. Adherent cells were cultured in the medium stimulated with 100 ng of LPS per ml before being harvested at the time points indicated (A) or stimulated with increasing concentrations of LPS (10, 100, and 1,000 ng/ml) for 16 h before being harvested (B). Total RNA was isolated, and 5 μg/sample was subjected to Northern analysis. The right-hand lane in panel A represents cells that were incubated for 24 h in medium without LPS. After detection with NRAMP1, blots were stripped of the probe and then hybridized with the GAPDH cDNA probe. The intensity of hybridization signals was quantified from autoradiographs, and then pixel values of NRAMP1 were normalized to those of GAPDH. Data are representative of two independent experiments with similar results.
FIG. 4.
Time-dependent induction of porcine NRAMP1 in PBMCs (A) and PMNs (B) in response to LPS stimulation. Cells were stimulated with 0.1 μg of LPS per ml in medium at 39°C under 5% CO2 and then harvested for RNA isolation at the times indicated. A 1-μg portion of total RNA from each sample was subjected to semiquantitative RT-PCR followed by Southern analysis as described in Materials and Methods. The intensity of hybridization signals was quantified from autoradiographs, and then pixel values of NRAMP1 were normalized to those of β-actin. Data are representative of two independent experiments with similar results.
NRAMP1 is upregulated during infection with S. enterica serovar Typhimurium.
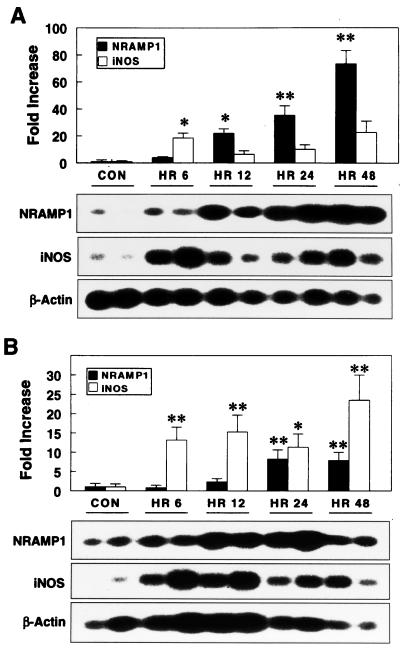
Because NRAMP1 expression could be modulated in vitro by proinflammatory mediators, we sought to determine the possible induction of NRAMP1 in vivo during the onset of disease. Healthy 6- to 8-week-old pigs were infected experimentally with 109 CFU of S. enterica serovar Typhimurium. Typical clinical signs of salmonellosis, including fever and diarrhea, were observed by 12 h following oral challenge, but they were not observed in control pigs. As shown in Fig. 5, S. enterica serovar Typhimurium infection robustly enhanced NRAMP1 expression in both liver and spleen, with a gradual increase over 2 days. A prominent increase of NRAMP1 expression occurred at 12 h, and expression peaked at 48 h postinfection.
FIG. 5.
NRAMP1 is upregulated in liver (A) and spleen (B) of pigs during infection with S. enterica serovar Typhimurium. Pigs were gavaged with 109 CFU of S. enterica serovar Typhimurium, and then livers and spleens of three to four pigs were sampled for RNA isolation at 6, 12, 24, and 48 h postinfection. Control pigs were mock challenged with sterile growth medium at 0 h. Semiquantitative RT-PCR and Southern analysis were performed to detect the expression of NRAMP1, iNOS, and β-actin. The intensity of hybridization signals was quantified from autoradiographs, and then pixel values of NRAMP1 and iNOS were normalized to those of β-actin. Values are means and standard errors of the mean of three to four pigs for each time point (∗, P < 0.05; ∗∗, P < 0.01), and blots are representative of two replicates of three to four pigs for each time point.
Mycobacterium-resistant mice have a greater capacity to produce nitric oxide (NO) than do susceptible mice following macrophage activation (36, 37), and transfection of the resistant allele into susceptible macrophages results in enhanced NO generation and l-arginine uptake (1). To evaluate the interplay between porcine NRAMP1 and iNOS, we analyzed the expression of both mediators following infection with S. enterica serovar Typhimurium. Interestingly, the kinetics of iNOS induction were distinct from those detected for NRAMP1. In both liver and spleen, expression of iNOS had two peaks postinfection; the first occurred between 6 and 12 h, and the second occurred between 24 and 48 h (Fig. 5). The first peak of iNOS expression preceded and the second peak followed a substantial increase of NRAMP1, which occurred between 12 and 24 h postinfection. These findings suggest that the first peak of iNOS gene expression was induced independent of NRAMP1 expression; however, the enhanced NRAMP1 synthesis might have given rise to the second wave of iNOS expression. We also investigated the expression of various cytokines, including IL-1β, IL-6, IL-10, IL-12p40, IL-18, TNF-α, and IFN-γ, in liver and spleen following infection. However, none of these cytokines resembled NRAMP1 in kinetics of induction (G. Zhang, C. R. Ross, and F. Blecha, unpublished data), suggesting that distinct transcriptional activation mechanisms are involved in the induction of NRAMP1 gene in response to infection.
LPS-induced expression of NRAMP1 is dependent upon CD14.
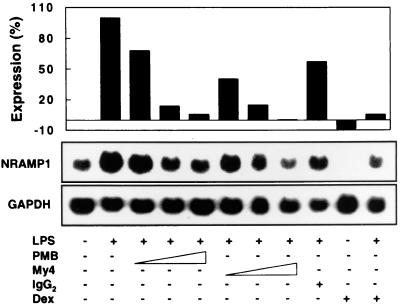
To study the involvement of CD14 in the upregulation of NRAMP1 by LPS, alveolar macrophages were incubated for 16 h with LPS in the presence of different concentrations of PMB and an anti-CD14 MAb, My4. Northern analysis revealed that LPS-induced expression of NRAMP1 was dose-dependently inhibited by increasing concentrations of PMB and My4 (Fig. 6). PMB at 10 μg/ml suppressed NRAMP1 expression by 95%, and My4 at 10 μg/ml diminished the expression of NRAMP1 to basal levels. This inhibition was specific, because the isotype control had only a minimal effect on NRAMP1 expression (Fig. 6). Interestingly, dexamethasone completely abolished the basal expression of NRAMP1, and this effect was reversed partially by coincubation of cells with LPS and dexamethasone (Fig. 6).
FIG. 6.
LPS-induced expression of NRAMP1 is CD14 dependent. Adherent alveolar macrophages were incubated for 16 h at 37°C with 5% CO2 in medium supplemented with different stimuli as indicated. LPS was used at 0.1 μg/ml, and polymyxin B (PMB) (0.1, 1, and 10 μg/ml) or My4 (0.2, 2, 10 μg/ml) was added simultaneously with LPS. Dexamethasone (Dex) was used at 10 μg/ml and added at the same time as LPS. Total RNA was isolated, and 5 μg/sample was subjected to Northern analysis. After detection with NRAMP1, blots were stripped of the probe and then hybridized with the GAPDH cDNA probe. The intensity of hybridization signals was quantified from autoradiographs, and then pixel values of NRAMP1 were normalized to those of GAPDH. Results are expressed as a percentage of NRAMP1 expression according to the formula (pixels of each sample − pixels of the sample without LPS treatment) × 100%/(pixels of the sample treated only with LPS − pixels of the sample without LPS treatment). Data are representative of two independent experiments with similar results.
To determine if LPS could lead directly to the transcriptional activation of NRAMP1, we preincubated macrophages for 30 min in medium supplemented with a protein synthesis inhibitor, cycloheximide, at 10 μg/ml and then stimulated the cells for 16 h with 0.1 μg of LPS per ml. The results showed that cycloheximide abrogated the NRAMP1 expression in response to LPS (data not shown), suggesting that LPS does not lead directly to NRAMP1 gene transcription but that de novo protein synthesis is required.
Both p38 and p42/44 MAPK pathways are involved in NRAMP1 induction by LPS, TNF-α, and IL-1β.
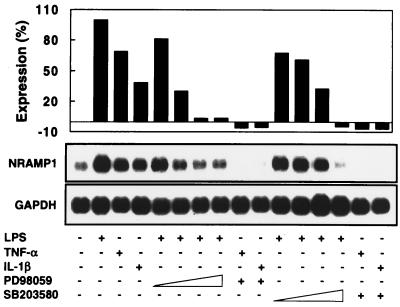
Similar to the inducing effect of LPS, stimulation of alveolar macrophages with 10 ng of TNF-α or IL-1β per ml also robustly enhanced the expression of NRAMP1 (Fig. 7). In addition, the induction of NRAMP1 by TNF-α and IL-1β was additive, because simultaneous treatment of cells with both cytokines resulted in further increased levels of NRAMP1 mRNA (data not shown). To investigate the potential involvement of MAPK pathways in the induction of NRAMP1 expression, alveolar macrophages were pretreated for 30 min with PD98059 and SB203580, specific inhibitors of p42/44 and p38 MAPK pathways (8, 10, 29), respectively, before stimulation for 16 h with LPS, TNF-α, and IL-1β. As shown in Fig. 7, both PD98059 and SB203580 inhibited NRAMP1 expression in a dose-dependent fashion, with 50 μM being sufficient to reduce LPS-induced NRAMP1 expression to basal levels. NRAMP1 expression induced by TNF-α or IL-1β was sensitive to PD98059 and SB203580 treatments because both drugs completely abolished the background expression of NRAMP1 (Fig. 7). These results suggest that both p42/44 and p38 MAPK-mediated pathways are critical in the induction of NRAMP1 by inflammatory agents. The viability of alveolar macrophages was not affected by 100 μM PD98059 or 50 μM SD203580 during 16 h of incubation as measured by trypan blue dye exclusion.
FIG. 7.
Involvement of p38 and p42/44 MAPK in the induction of NRAMP1 by LPS, TNF-α, and IL-1β. Adherent alveolar macrophages were pretreated for 30 min with PD98059 (1, 10, 50, or 100 μM) or SD203580 (0.5, 5, 20, or 50 μM) before being stimulated with 0.1 μg of LPS per ml for another 16 h. For inhibition of TNF-α- and IL-1β-induced NRAMP1 expression, 50 μM PD98059 or 20 μM SD203580 was used to pretreat cells for 30 min before stimulation for 16 h with the two cytokines, each at 10 ng/ml. An equal volume (1:1,000) of dimethyl sulfoxide, the solvent of MAPK inhibitors, was added to each well during incubation. Total RNA then was isolated, and 5 μg/sample was subjected to Northern analysis. After detection with NRAMP1, blots were stripped of the probe and then hybridized with the GAPDH cDNA probe. Results are expressed as a percentage of NRAMP1 expression according to the formula described in the legend of Fig. 6. Data are representative of two independent experiments with similar results.
DISCUSSION
In the present study, we have cloned the full-length cDNA for porcine NRAMP1, taking advantage of conserved regions in its mammalian congeners. The encoded 538-amino-acid protein is a typical member of the NRAMP1 family, having over 85% identity to its homologues from humans, mice, cattle, and sheep. The consensus transport motif, N-terminal putative SH3-binding domain, and PKC phosphorylation sites are all highly conserved in the porcine NRAMP1 protein (Fig. 1). Subsequent studies of the tissue expression pattern of porcine NRAMP1 revealed an interspecies variation in tissue specificity. Although expression of NRAMP1 is restricted to professional phagocytes among mammalian species, the abundance of expression varies among cell types. For instance, NRAMP1 is produced most abundantly in tissue macrophages and blood monocytes in mice (46), cattle (11), and pigs (Fig. 2), whereas the human congener is expressed at the highest level in PMNs and to a lesser extent in macrophages and monocytes (5).
Sequence analysis of promoter regions of the NRAMP1 gene in mice (17), cattle (11), and humans (5) revealed the presence of consensus binding sites for NF-κB and NF–IL-6, as well as response elements for IFN-γ and granulocyte-macrophage colony-stimulating factor, indicating that NRAMP1 might be regulated in response to phagocyte activation signals such as LPS, IFN-γ, and granulocyte-macrophage colony-stimulating factor. Induction of NRAMP1 expression was indeed observed in mouse macrophages treated with these agents (3, 4, 17, 18). Here we also confirmed a clear time- and dose-dependent induction of NRAMP1 in porcine alveolar macrophages (Fig. 3), as well as in PBMCs and PMNs (Fig. 4), stimulated with LPS. In addition, a robust induction of NRAMP1 occurred in alveolar macrophages when they were treated with the proinflammatory cytokines TNF-α and IL-1β (Fig. 7) and during infection with S. enterica serovar Typhimurium. Because some porcine viruses, such as porcine reproductive and respiratory syndrome virus, cause altered molecular responses in alveolar macrophages (52) and because we did not test our experimental animals for the presence of viruses, it is possible that these viruses influenced the expression of NRAMP1. However, because control macrophage cultures, which were not stimulated with LPS, did not exhibit increased expression of NRAMP1 (Fig. 3A), the likelihood of porcine viruses influencing our findings appears minimal.
Little is known about mechanisms and signal transduction pathways by which the NRAMP1 gene is induced in response to inflammatory agents, particularly LPS, TNF-α, and IL-1β. To address these issues, we first investigated the involvement of CD14 in LPS-induced NRAMP1 expression. CD14 is the major receptor on phagocytic cells for LPS-mediating signaling, although several potential LPS receptors, including macrophage scavenger receptors (21, 22), β2 leukocyte integrins (CD11/CD18) (24), L-selectin (30), and Toll-like receptors (27, 34, 35, 50), have been identified. Here, we demonstrate that My4, a MAb against human CD14, inhibited LPS-induced expression of NRAMP1 in a dose-dependent manner, with 10 μg/ml being sufficient to suppress NRAMP1 expression to basal levels. These results indicate that LPS-induced upregulation of NRAMP1 is mediated principally through CD14 rather than other receptors. The fact that cycloheximide substantially abrogated NRAMP1 expression in response to LPS suggests that LPS does not directly induce NRAMP1 gene transcription but, rather, that de novo protein synthesis is required. This is consistent with studies of mouse NRAMP1, for which induction of expression was blocked by treatment of RAW264.7 macrophages with both LPS and cycloheximide (18). However, the identity of the newly synthesized protein(s), which leads to the direct transcriptional activation of the NRAMP1 gene, remains to be elucidated.
A number of intracellular signaling pathways are involved in the activation of monocytic cells triggered by LPS, TNF-α, and IL-1β, among which MAPK cascades are the best characterized. Therefore, we investigated the role of MAPKs in the induction of NRAMP1 in response to LPS, TNF-α, and IL-1β. PD98059 is a specific inhibitor of MEK1, the kinase that activates the p42/44 MAPK pathway (8, 10). Inhibition of MEK1 by PD98059 prevents subsequent phosphorylation of MAPK substrates for p42/44 (ERK1/2) MAPK. SB203580, a pyridinyl imidazole, is a specific inhibitor of p38 MAPK via binding to the kinase suppressing the phosphorylation of its substrates (8, 29). Both inhibitors are capable of suppressing LPS-induced production of TNF-α, IL-1β, IL-6, and several other pro- or anti-inflammatory cytokines in monocytes/macrophages (8, 29). Dose-dependent inhibition of LPS-, TNF-α, and IL-1β-induced NRAMP1 expression by PD95098 and SB203580 provides clear evidence that upregulation of NRAMP1 is mediated intracellularly through both MAPK pathways. Glucocorticoids are well known to selectively suppress activation of JNK/SAPK but not that of p38 and p42/44, which in turn leads to the inhibition of c-Jun phosphorylation and subsequent downregulation of AP-1 activity and gene transcription (23, 25, 42). Strong inhibition of LPS-induced NRAMP1 expression by the synthetic glucocorticoid dexamethasone suggests that the third major MAPK pathway, JNK/SAPK, also might be involved in transcription of the NRAMP1 gene.
In summary, we have cloned porcine NRAMP1 cDNA and characterized tissue expression patterns and molecular mechanisms of induction of NRAMP1 by LPS, TNF-α, and IL-1β. We have shown that LPS-induced NRAMP1 induction in macrophages is primarily dependent upon CD14 and also involves newly synthesized protein(s). Furthermore, we provide evidence that at least two MAPK-mediated signaling pathways, namely, the p38 and p42/44 MAPK cascades, are involved in the induction of NRAMP1 expression in response to LPS, TNF-α, and IL-1β. The relative significance of other protein kinases, such as PKC, protein kinase A, G proteins, and ceramide-activated protein kinase, in NRAMP1 induction remains to be elucidated.
ACKNOWLEDGMENTS
We thank Steve Dritz for assistance with the challenge studies and Dani Goodband for excellent technical assistance.
This work was supported in part by U.S. Department of Agriculture National Research Initiative grants 95-37204-2141 and 98-35204-6397 (F. Blecha and C. Ross).
Footnotes
Contribution 99-426-J of the Kansas Agricultural Experiment Station.
REFERENCES
- 1.Barton C H, Whitehead S H, Blackwell J M. Nramp transfection transfers Ity/Lsh/Bcg-related pleiotropic effects on macrophage activation: influence on oxidative burst and nitric oxide pathways. Mol Med. 1995;1:267–279. [PMC free article] [PubMed] [Google Scholar]
- 2.Blackwell J M. Structure and function of the natural resistance-associated macrophage protein (Nramp1), a candidate protein for infectious and autoimmune disease susceptibility. Mol Med Today. 1996;2:205–211. doi: 10.1016/1357-4310(96)88773-9. [DOI] [PubMed] [Google Scholar]
- 3.Brown D H, Lafuse W, Zwilling B S. Cytokine-mediated activation of macrophage from Mycobacterium bovis BCG-resistant and -susceptible mice: differential effects of corticosterone on antimycobacterial activity and the expression of the Bcg gene (candidate Nramp) Infect Immun. 1995;63:2983–2988. doi: 10.1128/iai.63.8.2983-2988.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown D H, Lafuse W, Zwilling B S. Stabilized expression of mRNA is associated with mycobacterial resistance controlled by Nramp1. Infect Immun. 1997;65:597–603. doi: 10.1128/iai.65.2.597-603.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cellier M, Govoni G, Vidal S, Kwan T, Groulx N, Liu J, Sanchez F, Skamene E, Schurr E, Gros P. Human natural resistance-associated macrophage protein: cDNA cloning, chromosomal mapping, genomic organization, and tissue-specific expression. J Exp Med. 1994;180:1741–1752. doi: 10.1084/jem.180.5.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cellier M, Prive G, Belouchi A, Kwan T, Rodrigues V, Chia W, Gros P. Nramp defines a family of membrane proteins. Proc Natl Acad Sci USA. 1995;92:10089–10093. doi: 10.1073/pnas.92.22.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cellier M, Belouchi A, Gros P. Resistance to intracellular infections: comparative genomic analysis of Nramp. Trends Genet. 1996;12:201–204. doi: 10.1016/0168-9525(96)30042-5. [DOI] [PubMed] [Google Scholar]
- 8.Cohen P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 1997;7:353–361. doi: 10.1016/S0962-8924(97)01105-7. [DOI] [PubMed] [Google Scholar]
- 9.DeFranco A L, Crowley M T, Finn A, Hambleton J, Weinstein S L. The role of tyrosine kinases and MAP kinases in LPS-induced signaling. Prog Clin Biol Res. 1998;397:119–136. [PubMed] [Google Scholar]
- 10.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng J, Li Y, Hashad M, Schurr E, Gros P, Adams L G, Templeton J W. Bovine natural resistance-associated macrophage protein 1 (Nramp1) gene. Genome Res. 1996;6:956–964. doi: 10.1101/gr.6.10.956. [DOI] [PubMed] [Google Scholar]
- 12.Fenton M J, Golenbock D T. LPS-binding proteins and receptors. J Leukoc Biol. 1998;64:25–32. doi: 10.1002/jlb.64.1.25. [DOI] [PubMed] [Google Scholar]
- 13.Han J, Bohuslav J, Jiang Y, Kravchenko V V, Lee J-D, Li Z-J, Mathison J, Richter B, Tobias P, Ulevitch R J. CD14 dependent mechanisms of cell activation. Prog Clin Biol Res. 1998;397:157–168. [PubMed] [Google Scholar]
- 14.Fleming M D, Trenor C C, Su M A, Foernzler D, Beier D R, Dietrich W F, Andrews N C. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- 15.Fleming M D, Romano M A, Su M A, Garrick L M, Garrick M D, Andrews N C. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci USA. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Govoni G, Gros P. Macrophage NRAMP1 and its role in resistance to microbial infections. Inflamm Res. 1998;47:277–284. doi: 10.1007/s000110050330. [DOI] [PubMed] [Google Scholar]
- 17.Govoni G, Vidal S, Cellier M, Lapage P, Malo D, Gros P. Genomic structure, promoter sequence, and induction of expression of the mouse Nramp1 gene in macrophages. Genomics. 1995;27:9–19. doi: 10.1006/geno.1995.1002. [DOI] [PubMed] [Google Scholar]
- 18.Govoni G, Gauthier S, Billia F, Iscove N N, Gros P. Cell-specific and inducible Nramp1 gene expression in mouse macrophages in vitro and in vivo. J Leukoc Biol. 1997;62:277–286. doi: 10.1002/jlb.62.2.277. [DOI] [PubMed] [Google Scholar]
- 19.Gruenheid S, Pinner E, Desjardins M, Gros P. Natural resistance to infection with intracellular pathogens: the Nramp1 protein is recruited to the membrane of the phagosome. J Exp Med. 1997;185:717–730. doi: 10.1084/jem.185.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunshin H, Mackenzie B, Berger U B, Gunshin Y, Romero M F, Boron W F, Nussberger S, Gollan J L, Hediger M A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 21.Hampton R Y, Golenbock D T, Penman M, Krieger M, Raetz C R H. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991;352:342–344. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- 22.Haworth R, Platt N, Keshav S, Hughes D, Darley E, Susuki H, Kurihara Y, Kodama T, Gordon S. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J Exp Med. 1997;186:1431–1439. doi: 10.1084/jem.186.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirasawa N, Sato Y, Fujita Y, Mue S, Ohuchi K. Inhibition by dexamethasone of antigen-induced c-Jun N-terminal kinase activity in rat basophilic leukemia cells. J Immunol. 1998;161:4939–4943. [PubMed] [Google Scholar]
- 24.Ingalls R R, Golenbock D T. CD11c/CD18, a transmembrane signaling receptor for lipopolysaccharide. J Exp Med. 1995;181:1473–1479. doi: 10.1084/jem.181.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonat C, Rahmsdorf H J, Park K-K, Cato A C B, Gebel S, Ponta H, Herrlich P. Antitumor and antiiflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990;62:1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- 26.Kielian T L, Ross C R, McVey D S, Chapes S K, Blecha F. Lipopolysaccharide modulation of a CD14-like molecule on porcine alveolar macrophages. J Leukoc Biol. 1995;57:581–586. doi: 10.1002/jlb.57.4.581. [DOI] [PubMed] [Google Scholar]
- 27.Kirscning C J, Wesche H, Ayres T M, Rothe M. Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee F S, Hagler J, Chen Z J, Maniatis T. Activation of the IκBα kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 29.Lee J C, Young P R. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J Leukoc Biol. 1996;59:152–157. doi: 10.1002/jlb.59.2.152. [DOI] [PubMed] [Google Scholar]
- 30.Malhotra R, Bird M I. l-Selectin: a novel receptor for lipopolysaccharide and its potential role in bacterial sepsis. Bioessays. 1997;19:919–923. doi: 10.1002/bies.950191012. [DOI] [PubMed] [Google Scholar]
- 31.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 32.Martin M U, Falk W. The interleukin-1 receptor complex and interleukin-1 signal transduction. Eur Cytokine Netw. 1997;8:5–17. [PubMed] [Google Scholar]
- 33.O'Neill L A J, Greene C. Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects, and plants. J Leukoc Biol. 1998;63:650–657. [PubMed] [Google Scholar]
- 34.Poltorak A, He X, Smirnova I, Liu M Y, Huffel C V, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 35.Qureshi S T, Lariviere L, Leveque G, Clermont S, Moore K J, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radzioch D, Kramnik I, Skamene E. Molecular mechanisms of natural resistance to mycobacterial infections. Circ Shock. 1995;44:115–120. [PubMed] [Google Scholar]
- 37.Roach T I, Kiderlen A F, Blackwell J M. Role of inorganic nitrogen oxides and tumor necrosis factor-alpha in killing Leishmania donovani amastigotes in gamma interferon-lipopolysaccharide-activated macrophages from Lshs and Lshr congenic mouse strains. Infect Immun. 1991;59:3935–3944. doi: 10.1128/iai.59.11.3935-3944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulze-Osthoff K, Ferrari D, Riehemann K, Wesselborg S. Regulation of NF-κB activation by MAP kinase cascades. Immunobiology. 1997;198:35–49. doi: 10.1016/s0171-2985(97)80025-3. [DOI] [PubMed] [Google Scholar]
- 39.Searle S, Bright N A, Roach T I A, Atkinson P G P, Barton C H, Meloen R H, Blackwell J M. Localization of Nramp1 in macrophages: modulation with activation and infection. J Cell Sci. 1998;111:2855–2866. doi: 10.1242/jcs.111.19.2855. [DOI] [PubMed] [Google Scholar]
- 40.Shi J, Ross C R, Chengappa M M, Blecha F. Identification of a proline-arginine-rich antibacterial peptide from neutrophils that is analogous to PR-39, an antibacterial peptide from the small intestine. J Leukoc Biol. 1994;56:807–811. doi: 10.1002/jlb.56.6.807. [DOI] [PubMed] [Google Scholar]
- 41.Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996;8:402–411. doi: 10.1016/s0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- 42.Swantek J L, Cobb M H, Geppert T D. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-α) translation: glucocorticoids inhibit TNF-α translation by blocking JNK/SAPK. Mol Cell Biol. 1997;17:6274–6282. doi: 10.1128/mcb.17.11.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sweet M J, Hume D A. Endotoxin signal transduction in macrophages. J Leukoc Biol. 1996;60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- 44.Tuggle C K, Schmitz C B, Gingerich-Feil D. Rapid communication: cloning of a pig full-length natural resistance-associated macrophage protein (Nramp1) cDNA. J Anim Sci. 1997;75:277. doi: 10.2527/1997.751277x. [DOI] [PubMed] [Google Scholar]
- 45.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 46.Vidal S M, Malo D, Vogan K, Skamene E, Gros P. Natural resistance to infection with intracellular parasites: identification of a candidate gene for Bcg. Cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- 47.Vidal S M, Tremblay M L, Govoni G, Gauthier S, Sebastiani G, Malo D, Skamene E, Olivier M, Jothy S, Gros P. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the NRAMP1 gene. J Exp Med. 1995;182:655–666. doi: 10.1084/jem.182.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vidal S M, Gros P, Skamene E. Natural resistance to infection with intracellular parasites: molecular genetics identifies Nramp1 as the Bcg/Ity/Lsh locus. J Leukoc Biol. 1995;58:382–390. doi: 10.1002/jlb.58.4.382. [DOI] [PubMed] [Google Scholar]
- 49.Winston B W, Chan E D, Johnson G L, Riches D W H. Activation of p38mapk, MKK3, and MKK4 by TNF-α in mouse bone-marrow-derived macrophages. J Immunol. 1997;159:4491–4497. [PubMed] [Google Scholar]
- 50.Yang R-B, Mark M R, Gray A, Huang A, Xie M H, Zhang M, Goddard A, Wood W, Gurney A L, Godowski P L. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signaling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 51.Zhang G, Wu H, Shi J, Ganz T, Ross C R, Blecha F. Molecular cloning and tissue expression of porcine β-defensin-1. FEBS Lett. 1998;424:37–40. doi: 10.1016/s0014-5793(98)00134-3. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X, Shin J, Molitor T W, Schook L B, Rutherford M S. Molecular responses of macrophages to porcine reproductive and respiratory syndrome virus infection. Virology. 1999;262:152–162. doi: 10.1006/viro.1999.9914. [DOI] [PubMed] [Google Scholar]