Abstract
The family of the large clostridial cytotoxins, encompassing Clostridium difficile toxins A and B as well as the lethal and hemorrhagic toxins from Clostridium sordellii, monoglucosylate the Rho GTPases by transferring a glucose moiety from the cosubstrate UDP-glucose. Here we present a new detoxification procedure to block the enzyme activity by treatment with the reactive UDP-2′,3′-dialdehyde to result in alkylation of toxin A and B. Alkylation is likely to occur in the catalytic domain, because the native cosubstrate UDP-glucose completely protected the toxins from inactivation and the alkylated toxin competes with the native toxin at the cell receptor. Alkylated toxins are good antigens resulting in antibodies recognizing only the C-terminally located receptor binding domain, whereas formaldehyde treatment resulted in antibodies recognizing both the receptor binding domain and the catalytic domain, indicating that the catalytic domain is concealed under native conditions. Antibodies against the native catalytic domain (amino acids 1 through 546) and those holotoxin antibodies recognizing the catalytic domain inhibited enzyme activity. However, only antibodies against the receptor binding domain protected intact cells from the cytotoxic activity of toxin B, whereas antibodies against the catalytic domain were protective only when inside the cell.
Pathogenic strains of Clostridium difficile coproduce toxin A and toxin B, both of which are known as causative agents of antibiotic-associated diarrhea and its severe form, pseudomembranous colitis (15, 23, 24, 31). Hemorrhagic toxin (HT) and lethal toxin (LT) from Clostridium sordellii are associated with gas gangrene in humans as well as in domestic animals (15). The cross-neutralization of C. difficile toxins by C. sordellii antitoxin was the crucial finding that led to the discovery of the C. difficile toxins (2, 28, 38) and established a close immunological relationship between the C. difficile and C. sordellii toxins. Furthermore, all of these toxins show comparable cytotoxic activities on cultured cell lines (4, 7, 11, 12, 22) and possess similar structures of the toxin molecules (3, 9, 14, 43, 44). At the amino acid level, toxin A and toxin B show homology of about 60%, whereas toxin B and LT are 90% homologous. HT has not been cloned yet. Due to these findings, these toxins have been comprised in the family of large clostridial cytotoxins.
All of these toxins have been shown to monoglucosylate small GTP-binding proteins of the Ras superfamily with UDP-glucose as a cosubstrate (13, 19–21, 40). Toxin A and toxin B from strain VPI 10463 and HT from strain VPI 9048 modify the Rho subfamily proteins Rho, Rac, and Cdc42. The glucose moiety is transferred to Thr-37 in Rho and to the equivalent Thr-35 in Rac and Cdc42. The protein substrates of LT from strain VPI 9048 are Rac and Cdc42 and the Ras subfamily members Ras, Ral, and Rap (17, 19, 36). A variant toxin B from C. difficile strain 1470 was reported to possess the same protein substrate specificity as LT (6, 39). Amino acids 1 to 546 are the minimum catalytic fragment of toxin B, which is also cytotoxic when microinjected (16). The corresponding fragment of toxin A covers amino acids 1 through 659 (10).
Reactive nucleotide diphosphate derivatives are established model compounds for study of nucleotide diphosphate sugar-binding proteins (37, 46). Indeed, the catalytic domain of toxin B, which recruits UDP-glucose as a cosubstrate, was reported to be specifically labeled with the UDP derivative 5-azidouridine 5′-diphosphoglucose (5).
Here we report on the application of the UDP derivative UDP-2′,3′-dialdehyde to inactivate the large clostridial cytotoxins and use them as antigens for the generation of antibodies. The antibodies from these inactive toxins were used to block the catalytic activity of toxin B in vitro and the cytotoxic activity in vivo and to characterize the domain structure of toxin B.
MATERIALS AND METHODS
Materials.
Recombinant Rac1, RhoA, and toxin B fragments were purified as glutathione S-transferase fusion proteins from an Escherichia coli expression system. The glutathione fusion proteins were isolated by affinity purification with glutathione-Sepharose beads (Pharmacia, Freiburg, Germany). The glutathione S-transferase carrier was cleaved off by digestion with thrombin (100 μg/ml) for 30 min at 22°C. Thrombin was removed by precipitation with benzamidine-Sepharose beads (Pharmacia). C. difficile toxin A and toxin B from strain VPI 10463, C. difficile toxin B from strain 1470, C. sordellii HT and LT from strain VPI 9048, Clostridium botulinum exoenzyme C3, and C. botulinum C2I toxin were purified as described previously (1, 13, 18, 33). UDP-[14C]glucose was obtained from BioTrend (Cologne, Germany), and [32P]NAD was obtained from NEN Life Science Products (Zaventem, Belgium). C. difficile antiserum (EX5145) was obtained from Wellcome Research Laboratories (Beckenham, United Kingdom). Briefly, to raise this serum, antigen was prepared by incubating crude culture filtrate from C. difficile strain VPI 10463, containing both toxin A and toxin B, in formaldehyde (final concentration, 0.1%) for 3 h at 37°C.
Inactivation of toxins.
C. difficile toxins A and B, C. sordellii LT, and exoenzyme C3 and C2I toxin (each at 150 μg/ml) were treated with UDP-2′,3′-dialdehyde (0.1, 0.2, and 1.0 mM) dissolved in modification buffer (20 mM Tris-HCl[pH 7.2], 150 mM NaCl) at 37°C for 3 h or as indicated, followed by reduction with 4 mM NaBH3CN. Treatment with NaBH3CN alone did not inhibit the action of the toxins. The alkylation reaction with UDP-dialdehyde was competed with 10 mM UDP or 10 mM UDP-glucose. For vaccination and competition studies with the native toxin, toxins A and B as well as LT (each at 150 μg/ml, dissolved in buffer containing 20 mM Tris-HCl [pH 7.2] and 1 M NaCl) were alkylated in the presence of 1 mM UDP-dialdehyde at 37°C for 18 h. The reaction mixture was applied to a 100-kDa-cutoff membrane (Microcon 100; Amicon) to remove the remainder of the UDP-2′,3′-dialdehyde, followed by extensive washing with phosphate-buffered saline (PBS) supplemented with 1 mM EDTA or microinjection buffer (20 mM Tris-HCl [pH 7.2], 100 mM KCl). To test the ability of inactivated toxoid to interact with the cell receptor, HeLa cells were treated with alkylated toxoid B at 37°C for 20 min, followed by incubation with native toxin B for additional 20 min. The medium was changed, and the morphology was recorded.
Antisera.
One hundred micrograms of inactivated toxins or toxin fragment CDB1-546 (the catalytic fragment including amino acids 1 to 546) dissolved in 500 μl of PBS was mixed with 500 μl of Freund's complete adjuvant (Sigma) and homogenized by sonication. The emulsion was administered to rabbits by subcutaneous injection every third week. A maximum antitoxin titer of about 10−5 (determined by dot blot assay) was reached at about 12 weeks after the first injections, and the rabbits were bled out.
Purification of antisera.
Antisera were applied to a column of protein A/G-PLUS agarose beads (Santa Cruz) previously equilibrated with 10 mM Tris-HCl (pH 8.0). After extensive washing with 10 mM Tris-HCl (pH 8.0) at 4°C, immunoglobulin G (IgG) was eluted with 100 mM glycine (pH 2.8). Fractions (500 μl) were collected, followed by immediate neutralization with 50 μl of 1 M Tris-HCl (pH 8.0). IgG-containing fractions were pooled and dialyzed against PBS at 4°C overnight. The IgG concentration was adjusted to 1 mg/ml. The antitoxin titers (1 × 10−4 for toxin A and 3 × 10−4 for toxin B) were determined by enzyme-linked immunosorbent assay (ELISA). All of the following experiments were carried out with such purified antitoxin-IgG.
Immunoblot analysis.
Proteins were separated on polyacrylamide gels and transferred onto a nitrocellulose membrane for 2 h at 250 mA. The membrane was blocked for 1 h with 5% (wt/vol) nonfat dried milk at 24°C. Blots were incubated for 2 h with antitoxin IgG (diluted 1:5,000) in PBS containing 0.05% Tween 20, followed by incubation with a horseradish peroxidase-conjugated secondary antibody for 45 min. No cross-reactivity of the antibodies was observed with cell lysates.
Immunoprecipitation of toxin B.
Purified toxin B (1 μg) was preincubated with antitoxin (4 μg) or PBS on ice for 20 min. Antitoxin-toxin complex was precipitated by addition of protein A/G PLUS-agarose beads (30 min at 4°C). The supernatant and beads eluted with Laemmli sample buffer (containing 8 M urea) were analyzed for toxin B by immunoblotting.
ELISA.
Antibodies raised against toxin B fragments were determined by ELISA. ELISA wells were coated with recombinant toxin B fragments (50 μl [per well] of a solution containing 200 ng/ml in 0.1 M NaHCO3, pH 9.5) at room temperature for 1 h and then blocked using bovine serum albumin (1%, wt/vol), followed by the addition of anti-toxin B diluted 1:3,000 in buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, and 1% bovine serum albumin. Antitoxin IgG reactivity was detected with horseradish peroxidase-conjugated anti-rabbit IgG (1:3,000) and ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] substrate (Boehringer). The plates were read at 405 nm on a microtiter plate reader 30 min after the addition of the substrate.
Pretreatment of toxins with antitoxin.
Toxins (0.01 mg/ml) were preincubated with antitoxin IgG (1 mg/ml) or PBS as a control on ice for 15 min.
SDS-PAGE.
Sodium dodecyl sulfate-polyarcylamide gel electrophoresis (SDS-PAGE) was performed with 12.5 or 7% polyacrylamide gels. Labeled proteins were analyzed by use of a PhosphorImager SI from Molecular Dynamics (Freiburg, Germany).
Glucosylation reaction.
Recombinant Rac1 or RhoA (50 μg/ml) was incubated with toxin B (1 μg/ml) in buffer (50 mM HEPES [pH 7.2], 20 μM UDP-[14C]glucose, 0.2 mM MnCl2, 2 mM MgCl2, 100 μg of bovine serum albumin per ml) for 15 min at 37°C. The reaction was terminated by addition of Laemmli sample buffer followed by boiling at 95°C for 10 min.
Glycohydrolase activity.
Pretreated or control toxin B (10 μg/ml) was incubated in buffer (50 mM HEPES [pH 7.2], 150 mM KCl, 0.2 mM MnCl2, 40 μM UDP-[14C]glucose) for 1 h at 37°C. Samples were spotted on polyethyleneimine cellulose and run with 0.25 mM LiCl to separate UDP-[14C]glucose from [14C]glucose. Evaluation of the thin-layer chromatography (TLC) was performed with the PhosphorImager SI (Molecular Dynamics).
ADP-ribosylation reaction.
Recombinant RhoA (50 μg/ml) was ADP-ribosylated with exoenzyme C3 (1 μg/ml) in the presence of 10 μM [32P]NAD, 50 mM HEPES (pH 7.2), 2 mM MgCl2, and 1 mM dithiothreitol at 37°C for 30 min. Cytoplasmic actin from platelet cytosol was ADP-ribosylated with C2I toxin (1 μg/ml) in the presence of 10 μM [32P]NAD, 50 mM HEPES (pH 7.2), 1 mM MgCl2, and 1 mM dithiothreitol at 37°C for 20 min.
Cell culture.
HeLa cells were grown in Dulbecco's medium supplemented with 10% fetal calf serum and 4 mM glutamine-penicillin-streptomycin.
Toxin treatment of HeLa cells.
HeLa cells grown on glass coverslips were treated with control or pretreated toxin B (0.1 μg/ml of medium) or PBS as a control at 37°C. After 2 h, the cells treated with control toxin were rounded up. Cells were fixed in 4% paraformaldehyde. Images were recorded using Axiophot (Zeiss, Oberkochen, Germany).
Microinjection into HeLa cells.
HeLa cells grown on marked areas of coverslips were microinjected cytoplasmatically with 1 mg of antitoxin per ml or with microinjection buffer as a control (Micromanipulator; Eppendorf, Hamburg, Germany). After 1 h, cells were treated with toxin B (0.1 μg/ml of medium) or PBS as a control for 2 h at 37°C. Cells were fixed and photographs were taken as described above.
RESULTS AND DISCUSSION
Inactivation of toxin B.
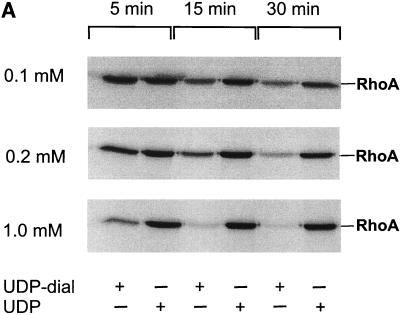
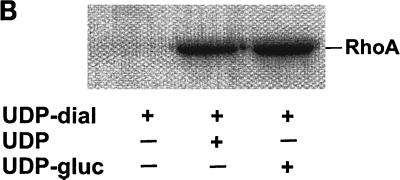
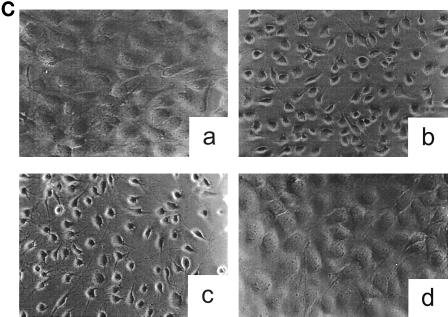
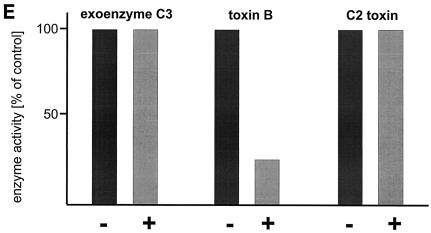
Toxin B catalyzes monoglucosylation of Rho GTPases by using UDP-glucose as a cosubstrate. The transfer of the glucose moiety is inhibited by a surplus of UDP. Therefore, we applied a reactive UDP derivative, the UDP-2′,3′-dialdehyde, which is proposed to react with free amino residues, e.g., from lysine. UDP-dialdehyde inactivated the glucosyltransferase activity of toxin B in a time- and concentration-dependent manner (Fig. 1A). Inactivation of toxin B is mediated through binding of UDP-dialdehyde to the catalytic domain, because UDP as well as the original substrate UDP-glucose fully prevented the UDP-dialdehyde-mediated inhibition of the glucosyltransferase activity (Fig. 1B). Furthermore, UDP-dialdehyde-inactivated toxin B did not exhibit cytotoxicity on HeLa cells (Fig. 1C), an effect which was fully prevented by UDP (Fig. 1C). To test whether the alkylated toxoid retained its native conformation, competition with native toxin B at the cell receptor was performed. To this end, HeLa cells were preincubated with or without a 100-fold excess of alkylated toxoid, followed by a challenge with native toxin B. As shown in Fig. 1D, toxoid-preincubated cells were protected from toxin B-induced cytotoxicity, whereas HeLa cells in the absence of the toxoid were not protected. These findings suggest that toxin B was inactivated through specific alkylation of the catalytic pocket and not through nonspecific modification of accessible alkylation sites causing denaturation of the toxin. To test whether the effect of UDP-dialdehyde is specific for glucosyltransferases, the influence of UDP-dialdehyde on the ADP-ribosyltransferase activities of exoenzyme C3 and C2 toxin, both from C. botulinum, was tested. As shown in Fig. 1E, neither C3 exoenzyme nor C2I toxin activity was changed by UDP-dialdehyde. Thus, the treatment of the large clostridial cytotoxins with UDP-dialdehyde is a specific and mild detoxification procedure which seems to preserve the overall structure of these toxins in a native state.
FIG. 1.
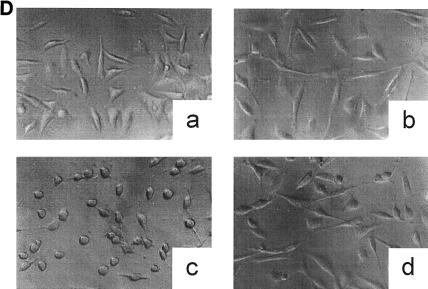
Inactivation of toxin B by treatment with UDP-dialdehyde. (A) Time course and concentration dependence of the inactivation of toxin B. Toxin B (50 μg/ml) was incubated with UDP-2′,3′-dialdehyde (UDP-dial) (concentrations as indicated) or UDP (10 mM) for the indicated times. After 1:50 dilution, toxin B-catalyzed 14C glucosylation of RhoA was tested as described in Materials and Methods. PhosphorImager data from SDS–12.5% PAGE are shown. (B) UDP and UDP-glucose (UDP-gluc) inhibit UDP-2′,3′-dialdehyde effects. Toxin B (3 μM) was treated with 1 mM UDP-2′,3′-dialdehyde in the presence of 10 mM UDP, 10 mM UDP-glucose, or buffer for 3 h. After 1:50 dilution, toxin B-catalyzed 14C glucosylation of RhoA was tested. PhosphorImager data are shown. (C) UDP inhibits UDP-2′,3′-dialdehyde effects on the cytotoxicity of toxin B. Toxin B (50 μg/ml) was treated with 1 mM UDP-2′,3′-dialdehyde (c and d) or buffer (b) in the presence of 10 mM UDP (c) or buffer (b and d) for 3 h. The remainder of the UDP-dialdehyde was removed as described in Materials and Methods. HeLa cells were incubated with 0.37 nM (0.1-μg/ml) pretreated toxin B (b to d) or PBS (a) for 90 min (phase-contrast micrographs of fixed cells are shown). a, control cells; b, untreated toxin B; c, toxin B treated with UDP-dialdehyde in the presence of UDP; d, toxin B treated with UDP-dialdehyde. (D) Competition of alkylated toxoid B with native toxin B. HeLa cells were treated with buffer (a), with 5 μg of alkylated toxoid per ml (b), with 0.05 μg of native toxin per ml (c), or with 5 μg of alkylated toxoid per ml plus 0.05 μg of native toxin per ml (d) at 37°C for 40 min. The medium was changed, and the cells were incubated for an additional 1 h. Phase-contrast micrographs of fixed cells are shown. (E) Influence of UDP-dialdehyde on other enzymatically active bacterial toxins. Exoenzyme C3 and C2I toxin from C. botulinum as well as toxin B were treated with 1 mM UDP-dialdehyde (+) or buffer (−) as described above. Thereafter, ADP-ribosyltransferase (for C21 and C3) or glucosyltransferase (for toxin B) activity was tested as described in Materials and Methods. PhosphorImager data were quantified using ImageQuant (Molecular Dynamics).
Generation of antitoxin antisera.
Toxins A and B and LT are highly toxic to rabbits even when dissolved in Freund's adjuvant. UDP-dialdehyde-inactivated holotoxins offered the opportunity to generate antibodies to nondenatured toxoid. The catalytic domain of toxin B (CDB1-546), covering amino acids 1 through 546, is nontoxic to animals because of its failure to enter intact cells and was therefore used as an antigen without previous treatment with UDP-dialdehyde.
Recognition of the large clostridial cytotoxins.
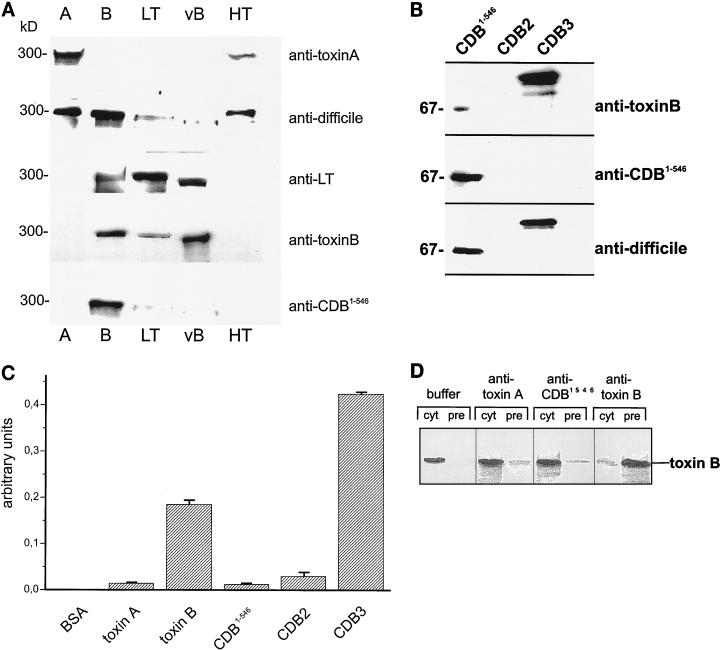
Anti-toxin A recognized only toxin A and, weakly, HT but did not recognize the other members of the cytotoxin family (Fig. 2A). Anti-toxin B cross-reacted with toxin B, the variant toxin B, and, weakly, LT but not with toxin A and HT (Fig. 2A). Anti-LT toxin recognized LT, the variant toxin B and weakly, toxin B (Fig. 2A). The horse antiserum generated against formaldehyde-treated crude preparation of toxin A and B holotoxin (Wellcome) (anti-C. difficile) recognized toxins A and B and HT (Fig. 2A). The antibody against the catalytic domain of toxin B, CDB1-546, cross-reacted exclusively with toxin B (Fig. 2A).
FIG. 2.
Immunoanalysis. (A) Immunoblot of large clostridial cytotoxins. Purified toxin A, toxin B, LT, the variant toxin B from strain 1470 (vB), and HT were separated by SDS–7% PAGE, electroblotted onto nitrocellulose, and probed with the antitoxin IgG as indicated on the right. ECL of the immunoblots is shown. (B) Immunoanalysis of toxin B fragments. Toxin B fragments (CDB1-546, CDB2 [amino acids 901 to 1750], and CDB3 [amino acids 1751 to 2366]) were separated by SDS-PAGE, electroblotted onto nitrocellulose, and probed with the indicated antitoxin IgG. ECL of the immunoblots is shown. (C) ELISA of holotoxins and toxin fragments with an antibody raised against holotoxin B. The indicated antigens (10 ng of each) were absorbed and incubated with antitoxin B IgG (1:3000), and bound antibody was visualized as described in Materials and Methods. BSA, bovine serum albumin. Error bars indicate standard deviations. (D) Immunoprecipitation of toxin B. Toxin B was incubated with anti-toxin A, anti-CDB1-546, anti-toxin B, or PBS for 20 min on ice, followed by the addition of protein A/G-agarose. Supernatants (cyt) and precipitated proteins (pre) were analyzed with anti-toxin B. ECL of the immunoblots is shown.
For toxin B, we tested which part of the toxin was recognized by the antibodies. Toxin B was divided into three fragments which were separately expressed. CDB1-546, covering amino acids 1 through 546, contains the catalytic domain; CDB2 (amino acids 901 through 1750) harbors the putative transmembrane domain; and CDB3 (amino acids 1751 through 2366) is thought to be the receptor binding domain. As expected, anti-CDB1-546 recognized only the catalytic domain, CDB1-546 (Fig. 2B). Anti-toxin B cross-reacted strongly with CDB3 and faintly with CDB1-546, whereas the anti-C. difficile antibody recognized CDB1-546 as well as CDB3 (Fig. 2B). Surprisingly, none of the antibodies recognized CDB2, the intermediary domain in toxin B (Fig. 2B). To test whether conformational rather than linear epitopes were recognized by the holotoxin antibody, an ELISA was applied. As shown in Fig. 2C, the antibody raised against holotoxin B recognized only the holotoxin and the receptor binding domain CDB3 but not the N-terminal (CDB1-546) or intermediary (CDB2) part. The ELISA fully corroborated the data found with the immunoblot analysis. From these data it can be concluded that the receptor binding domain (CDB3), harboring repetitive peptide domains, is the most antigenic part of the toxin. Under native conditions, the catalytic domain (CDB1-546) and the intermediary part (CDB2) were poorly antigenic, because they are likely to be covered by the most antigenic CDB3.
Immunoprecipitation of toxin B.
In contrast to anti-CDB1-546, only anti-toxin B was able to immunoprecipitate toxin B (Fig. 2D). This finding underlines that antibodies directed to the C-terminal part of toxin B are superior in binding to holotoxin B in comparison to antibodies towards the N-terminal domain. Thus, antibodies to the native C-terminal part are a prerequisite for immunoprecipitation.
Inhibition of enzyme activity of toxin B.
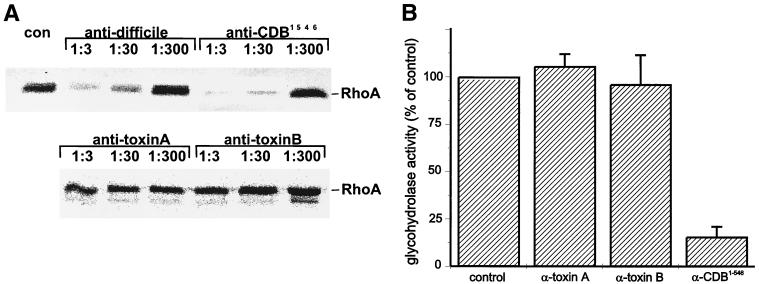
Monoglucosylation of Rac1 by toxin B was inhibited by those toxin B antibodies which recognized the catalytic domain in the immunoblot analysis. As shown in Fig. 3A, anti-C. difficile and anti-CDB1-546 inhibited the glucosylation reaction in a concentration-dependent manner. In addition to that of recombinant Rac1, glucosylation of cellular Rho, Rac, and Cdc42 also was inhibited (data not shown), indicating that inhibition of the enzyme activity is not restricted to one protein substrate but is general. Anti-toxin B, which cross-reacted very weakly with the catalytic domain, and anti-toxin A were incapable of inhibiting the enzyme activity of toxin B.
FIG. 3.
Inhibition of enzyme activity of toxin B by antitoxin. (A) Toxin B was incubated with the indicated dilutions of antitoxin IgG (anti-C. difficile, anti-CDB1-546, anti-toxin A, or anti-toxin B) or buffer (con) for 15 min on ice, followed by toxin B-catalyzed 14C glucosylation of Rac1 as described in Materials and Methods. PhosphorImager data from SDS–12.5% PAGE are shown. (B) Toxin B was incubated with antitoxin IgG (anti-toxin A, anti-toxin B, or anti-CDB1-546), or buffer (control) for 15 min on ice, followed by toxin B-catalyzed glycohydrolase reaction to cleave UDP-glucose into UDP and glucose as described in Materials and Methods. UDP-glucose and cleaved products were separated by TLC, and TLC results were quantified with ImageQuant (Molecular Dynamics). Error bars indicate standard deviations.
In addition to the glucosyltransferase activity, toxin B exhibits glycohydrolase activity to hydrolytically cleave UDP-glucose into UDP and glucose in the absence of the protein substrate. This glycohydrolase activity was inhibited only by those antibodies which also blocked the transferase activity (Fig. 3B). Thus, the catalytic domain itself or the binding site for Rho and UDP-glucose is sufficient antigenic to produce functional antibodies which inhibit the enzyme activity.
Prevention of the cytotoxic effects.
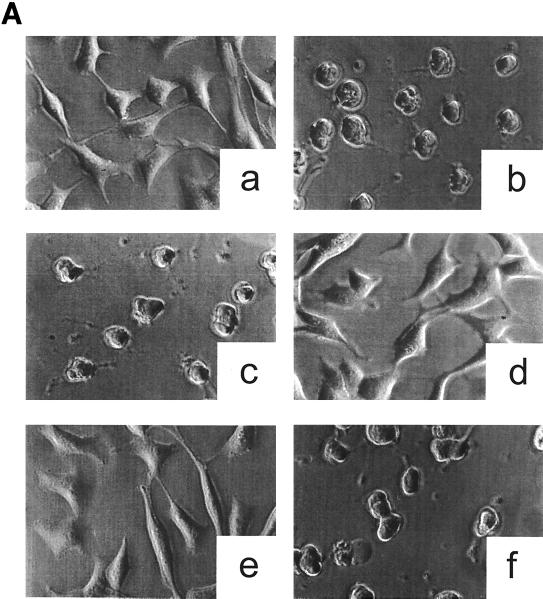
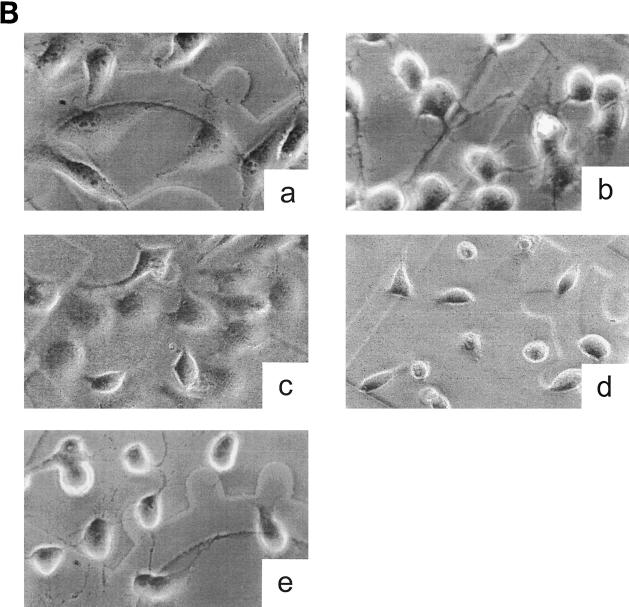
The antibody recognizing the putative receptor binding domain as well as the antibody inhibiting the enzyme activity should protect the cells from the cytotoxic attack by toxin B. As shown in Fig. 4A, anti-toxin B and anti-C. difficile prevented the cytotoxic effect when preincubated with toxin B. The cross-reactivity of anti-toxin B with LT as well as with the variant toxin B (Fig. 2A) was also functional; anti-toxin B also protected cells against the cytotoxicity of LT and the variant toxin B (data not shown). The protection lasted for more than 24 h, whereas in the absence of the antibodies cytotoxic effects were observed after 90 min, indicating high-affinity binding of the antibodies. By contrast, anti-CDB1-546, which recognizes only the catalytic domain and inhibits enzyme activity, did not protect. However, microinjection of the antibody into cells did protect the cells from intoxication by toxin B, whereas microinjection of anti-toxin B had no effects (Fig. 4B). This discrepancy is likely due to removal of the antibody anti-CDB1-546 from toxin B when toxin B reaches the acidic endosomal compartments during uptake. When anti-CDB1-546 is in the cytosol, it completely protects the cells by inhibiting glucosyltransferase activity. Again, this finding corroborates the notion that toxin B acts cytotoxically on cells through its inherent glucosyltransferase activity. Anti-toxin B and anti-C. difficile bind to the C-terminal part of the receptor binding domain to prevent interaction of toxin B with its membrane receptor.
FIG. 4.
Influence of the antitoxins on the cytotoxic activity of toxin B. (A) Prevention of the cytotoxic effect of toxin B by antitoxin. Toxin B was pretreated with antitoxins or PBS for 15 min on ice. HeLa cells were incubated with PBS (a), with toxin B plus PBS (b), with toxin B plus anti-toxin A (c), with toxin B plus anti-toxin B (d), with toxin B plus anti-C. difficile (e), or with toxin B plus anti-CDB1-546 (f) for 2 h. The concentration of toxin B was 0.37 nM (0.1 μg/ml) in the cell medium. Phase-contrast micrographs of fixed cells are shown. (B) Intracellular antitoxin protects the cells from toxin B. HeLa cells were microinjected with either microinjection buffer (a and b), anti-CDB1-546 (c), anti-toxin B (d), or anti-toxin A (e). After 1 h, toxin B (b to e) (0.1 μg/ml) or PBS (a) was added to the medium, and incubation was continued for 2 h. Fixed cells were observed by phase-contrast micrography.
Toxoids are inactivated protein toxins used as vaccines which have traditionally been prepared by formaldehyde treatment. Formaldehyde inactivation is based on cross-linking of reactive lysine residues to glutamic acid, aspartic acid, or tyrosine residues (32). Furthermore, alkylation results in a loss of the positive charge of lysine residues, reducing the overall charge on the surface of the toxoid (29). Mucosal immunization with C. difficile toxoids has been shown to induce optimal protection against antibiotic-associated diarrhea and colitis caused by C. difficile in hamsters (41). However, formalin-inactivated molecules have been shown not to bind to mucosal surfaces, resulting in their being poorer mucosal immunogens than molecules that can target receptors on the mucosal surface (8). This disadvantage of the formaldehyde-detoxified immunogens has been suggested to be overcome by recombinant expression of toxin proteins inactivated by site-directed mutagenesis (25, 34, 35). However, the family of large clostridial cytotoxins are single-chain peptides with molecular masses ranging from 250 to 308 kDa, which have escaped recombinant expression so far. An alternative is the generation of the recombinant nontoxic C-terminal domains of toxins A and B, which have been reported to be good vaccine candidates (26, 45). We present a different strategy. Holotoxins A and B were detoxified by specific alkylation with UDP-dialdehyde. This new, promising approach offers the opportunity to generate immunogens derived from the whole molecule, thereby overcoming the disadvantages of formaldehyde treatment. Furthermore, the enzymatically deficient toxins can serve as excellent controls in in vivo as well as in in vitro studies to exclude toxin receptor-mediated effects.
We have worked out a new mild and specific detoxification procedure to prepare enzymatically inactive clostridial cytotoxins which are nontoxic but retain their native structure. Using such inactivated cytotoxins, we found that the most antigenic part is the C-terminal part, consisting of the repetitive peptide structures thought to be responsible for receptor binding (27, 44). This repetitive structure is very likely the reason for the excellent antigenicity of toxin B, which has also been reported for toxin A. Antibodies generated against denatured toxin A or short toxin A peptides protect well against enterotoxic activity of toxin A but protect not at all or only poorly against cytotoxic activity (30, 42). Antibodies raised against recombinant receptor binding domains of toxin A and B are neutralizing and protective in a hamster infection model to prevent C. difficile-associated diarrhea, but whether they are protective against cytotoxicity was not reported (26). This discrepancy in protective potency may be due to reduced affinity of the antibodies to the toxin, which becomes detectable in the very sensitive cytotoxicity assay but is not realized in the less sensitive hamster model or rabbit ileal loop assay. The antibodies against native toxins fully protect against cytotoxic activity, suggesting high-affinity binding. Therefore, the detoxification of the C. difficile toxins by alkylation may be a promising approach to generate a vaccine which induces the formation of protective antibodies.
In the holotoxin B the catalytic domain localized at the N-terminal part is very poorly antigenic, resulting in antibodies which do not recognize the catalytic domain. The catalytic domain CDB1-546 itself was antigenic and resulted in antibodies inhibiting enzyme activity. However, in contrast to anti-toxin B, anti-CDB1-546 was not able to immunoprecipitate holotoxin B. This apparent contradiction may be based on the binding of antibodies whose affinity is too low to allow immunoprecipitation but which still prevent the interaction of the enzyme with its substrate. From these findings, it can be concluded that the catalytic domain seems to be masked in the holotoxin by the receptor binding domain. The intermediary CDB2 domain is concealed, a finding which supports the notion that this hidden region is hydrophobic and involved in the translocation procedure of the toxin through the membrane.
In conclusion, UDP-dialdehyde treatment results in enzymatically deficient but structurally native toxins which are excellent antigens to generate antibodies against native toxins. These antibodies can be used for immunoprecipitation or mapping of functional domains of the toxins.
ACKNOWLEDGMENTS
This work was supported by Deutsche Forschungsgemeinschaft Projects Ju231/3 and SFB 388.
We thank Gerhard Wetterer for excellent technical assistance and Maria Lerm for performance of microinjection experiments.
REFERENCES
- 1.Aktories K, Rösener S, Blaschke U, Chhatwal G S. Botulinum ADP-ribosyltransferase C3. Purification of the enzyme and characterization of the ADP-ribosylation reaction in platelet membranes. Eur J Biochem. 1988;172:445–450. doi: 10.1111/j.1432-1033.1988.tb13908.x. [DOI] [PubMed] [Google Scholar]
- 2.Allo M, Silva J, Fekety R, Rifkin G D, Waskin H. Prevention of clindamycin-induced colitis in hamsters by Clostridium sordellii antitoxin. Gastroenterology. 1979;76:351–355. [PubMed] [Google Scholar]
- 3.Barroso L A, Wang S-Z, Phelps C J, Johnson J L, Wilkins T D. Nucleotide sequence of Clostridium difficile toxin B gene. Nucleic Acids Res. 1990;18:4004. doi: 10.1093/nar/18.13.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bette P, Oksche A, Mauler F, Von Eichel-Streiber C, Popoff M R, Habermann E. A comparative biochemical, pharmacological and immunological study of Clostridium novyi α-toxin, C. difficile toxin B and C. sordellii lethal toxin. Toxicon. 1991;29:877–887. doi: 10.1016/0041-0101(91)90224-f. [DOI] [PubMed] [Google Scholar]
- 5.Busch C, Hofmann F, Selzer J, Munro J, Jeckel D, Aktories K. A common motif of eukaryotic glycosyltransferases is essential for the enzyme activity of large clostridial cytotoxins. J Biol Chem. 1998;273:19566–19572. doi: 10.1074/jbc.273.31.19566. [DOI] [PubMed] [Google Scholar]
- 6.Chaves-Olarte E, Löw P, Freer E, Norlin T, Weidmann M, Von Eichel-Streiber C, Thelestam M. A novel cytotoxin from Clostridium difficile serogroup F is a functional hybrid between two other large clostridial cytotoxins. J Biol Chem. 1999;274:11046–11052. doi: 10.1074/jbc.274.16.11046. [DOI] [PubMed] [Google Scholar]
- 7.Ciesielski-Treska J, Ulrich G, Baldacini O, Monteil H, Aunis D. Phosphorylation of cellular proteins in response to treatment with Clostridium difficile toxin B and Clostridium sordellii toxin L. Eur J Cell Biol. 1991;56:68–78. [PubMed] [Google Scholar]
- 8.Cropley I, Douce G, Roberts M, Chatfield S, Pizza M, Marsili I, Rappuoli R, Dougan G. Mucosal and systemic immunogenicity of a recombinant, non ADP-ribosylating pertussis toxin: effects of formaldehyde treatment. Vaccine. 1995;13:1643–1648. doi: 10.1016/0264-410x(95)00134-m. [DOI] [PubMed] [Google Scholar]
- 9.Dove C H, Wang S Z, Price S B, Phelps C J, Lyerly D M, Wilkins T D, Johnson J L. Molecular characterization of the Clostridium difficile toxin A gene. Infect Immun. 1990;58:480–488. doi: 10.1128/iai.58.2.480-488.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faust C, Ye B, Song K-P. The enzymatic domain of Clostridium difficile toxin A is located within its N-terminal region. Biochem Biophys Res Commun. 1998;251:100–105. doi: 10.1006/bbrc.1998.9383. [DOI] [PubMed] [Google Scholar]
- 11.Fiorentini C, Arancia G, Paradisi S, Donelli G, Giuliano M, Piemonto F, Mastrantonio P. Effects of Clostridium difficile toxins A and B on cytoskeleton organization in HEp-2 cells: a comparative morphological study. Toxicon. 1989;27:1209–1218. doi: 10.1016/0041-0101(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 12.Fiorentini C, Thelestam M. Clostridium difficile toxin A and its effects on cells. Toxicon. 1991;29:543–567. doi: 10.1016/0041-0101(91)90050-2. [DOI] [PubMed] [Google Scholar]
- 13.Genth H, Hofmann F, Selzer J, Rex G, Aktories K, Just I. Difference in protein substrate specificity between hemorrhagic toxin and lethal toxin from Clostridium sordellii. Biochem Biophys Res Commun. 1996;229:370–374. doi: 10.1006/bbrc.1996.1812. [DOI] [PubMed] [Google Scholar]
- 14.Green G A, Schué V, Monteil H. Cloning and characterization of the cytotoxin L-encoding gene of Clostridium sordellii: homology with Clostridium difficile cytotoxin B. Gene. 1995;161:57–61. doi: 10.1016/0378-1119(95)00263-6. [DOI] [PubMed] [Google Scholar]
- 15.Hatheway C L. Toxigenic clostridia. Clin Microbiol Rev. 1990;3:66–98. doi: 10.1128/cmr.3.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann F, Busch C, Prepens U, Just I, Aktories K. Localization of the glucosyltransferase activity of Clostridium difficile toxin B to the N-terminal part of the holotoxin. J Biol Chem. 1997;272:11074–11078. doi: 10.1074/jbc.272.17.11074. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann F, Rex G, Aktories K, Just I. The Ras-related protein Ral is monoglucosylated by Clostridium sordellii lethal toxin. Biochem Biophys Res Commun. 1996;227:77–81. doi: 10.1006/bbrc.1996.1470. [DOI] [PubMed] [Google Scholar]
- 18.Just I, Selzer J, Hofmann F, Aktories K. Clostridium difficile toxin B as a probe for Rho GTPases. In: Aktories K, editor. Bacterial toxins—tools in cell biology and pharmacology. Weinheim, Germany: Chapman & Hall; 1997. pp. 159–168. [Google Scholar]
- 19.Just I, Selzer J, Hofmann F, Green G A, Aktories K. Inactivation of Ras by Clostridium sordellii lethal toxin-catalyzed glucosylation. J Biol Chem. 1996;271:10149–10153. doi: 10.1074/jbc.271.17.10149. [DOI] [PubMed] [Google Scholar]
- 20.Just I, Selzer J, Wilm M, Von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 21.Just I, Wilm M, Selzer J, Rex G, Von Eichel-Streiber C, Mann M, Aktories K. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J Biol Chem. 1995;270:13932–13936. doi: 10.1074/jbc.270.23.13932. [DOI] [PubMed] [Google Scholar]
- 22.Kawabe H, Hayashi H, Hayaishi O. Differential calcium effects on prostaglandin D2 generation and histamine release from isolated rat peritoneal mast cells. Biochem Biophys Res Commun. 1987;143:467–474. doi: 10.1016/0006-291x(87)91377-5. [DOI] [PubMed] [Google Scholar]
- 23.Kelly C P, LaMont J T. Clostridium difficile infection. Annu Rev Med. 1998;49:375–390. doi: 10.1146/annurev.med.49.1.375. [DOI] [PubMed] [Google Scholar]
- 24.Kelly C P, Pothoulakis C, LaMont J T. Clostridium difficile colitis. N Engl J Med. 1994;330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 25.Killeen K P, Escuyer V, Mekalanos J J, Collier R J. Reversion of recombinant toxoids: mutations in diphtheria toxin that partially compensate for active-site deletions. Proc Natl Acad Sci USA. 1992;89:6207–6209. doi: 10.1073/pnas.89.13.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kink J A, Williams J A. Antibodies to recombinant Clostridium difficile toxins A and B are an effective treatment and prevent relapse of C. difficile-associated disease in a hamster model of infection. Infect Immun. 1998;66:2018–2025. doi: 10.1128/iai.66.5.2018-2025.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krivan H C, Clark G F, Smith D F, Wilkins T D. Cell surface binding site for Clostridium difficile enterotoxin: evidence for a glycoconjugate containing the sequence Galα1-3Galβ1-4GlcNAc. Infect Immun. 1986;53:573–581. doi: 10.1128/iai.53.3.573-581.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larson H E, Proce A B. Pseudomembranous colitis; presence of clostridial toxin. Lancet. 1977;ii:1312–1314. doi: 10.1016/s0140-6736(77)90363-4. [DOI] [PubMed] [Google Scholar]
- 29.London E. Diphtheria toxin: membrane interaction and membrane translocation. Biochim Biophys Acta. 1992;1113:25–51. doi: 10.1016/0304-4157(92)90033-7. [DOI] [PubMed] [Google Scholar]
- 30.Lyerly D M, Phelps C J, Toth J, Wilkins T D. Characterization of toxins A and B of Clostridium difficile with monoclonal antibodies. Infect Immun. 1986;54:70–76. doi: 10.1128/iai.54.1.70-76.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyerly D M, Wilkins T D. Clostridium difficile. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press, Ltd.; 1995. pp. 867–891. [Google Scholar]
- 32.Martiny-Baron G, Mehrabian M, Martinson H G. Contact-site cross-linking agents. Mol Cell Biochem. 1981;34:3–13. doi: 10.1007/BF02354846. [DOI] [PubMed] [Google Scholar]
- 33.Ohishi I, Iwasaki M, Sakaguchi G. Purification and characterization of two components of botulinum C2 toxin. Infect Immun. 1980;30:668–673. doi: 10.1128/iai.30.3.668-673.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pizza M, Domenighini M, Hol W, Gianelli V, Fontana M R, Giuliani M M, Magagnoli C, Peppolini S, Manetti R, Rappuoli R. Probing the structure-activity relationship of Escherichia coli LT-A by site-directed mutagenesis. Mol Microbiol. 1994;14:51–60. doi: 10.1111/j.1365-2958.1994.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 35.Pizza M, Fontana M R, Giuliani M M, Domenighini M, Magagnoli C, Gianelli V, Nucci D, Hol W, Manetti R, Rappuoli R. A genetically detoxified derivative of heat-labile Escherichia coli enterotoxin induces neutralizing antibodies against the A subunit. J Exp Med. 1994;180:2147–2153. doi: 10.1084/jem.180.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popoff M R, Chaves O E, Lemichez E, Von Eichel-Streiber C, Thelestam M, Chardin P, Cussac D, Chavrier P, Flatau G, Giry M, Gunzburg J, Boquet P. Ras, Rap, and Rac small GTP-binding proteins are targets for Clostridium sordellii lethal toxin glucosylation. J Biol Chem. 1996;271:10217–10224. doi: 10.1074/jbc.271.17.10217. [DOI] [PubMed] [Google Scholar]
- 37.Powell J T, Brew K. Affinity labeling of bovine colostrum galactosyltransferase with a uridine 5′-diphosphate derivative. Biochemistry. 1976;15:3499–3505. doi: 10.1021/bi00661a016. [DOI] [PubMed] [Google Scholar]
- 38.Rifkin G D, Fekety F R, Silva J, Sack R B. Antibiotic-induced colitis. Implication of a toxin neutralised by Clostridium sordellii antitoxin. Lancet. 1977;ii:1103–1106. doi: 10.1016/s0140-6736(77)90547-5. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt M, Vo M, Thiel M, Bauer B, Grannass A, Tapp E, Cool R H, De Gunzburg J, Von Eichel-Streiber C, Jakobs K H. Specific inhibition of phorbol ester-stimulated phospholipase D by Clostridium sordellii lethal toxin and Clostridium difficile toxin B-1470 in HEK-293 cells. J Biol Chem. 1998;273:7413–7422. doi: 10.1074/jbc.273.13.7413. [DOI] [PubMed] [Google Scholar]
- 40.Selzer J, Hofmann F, Rex G, Wilm M, Mann M, Just I, Aktories K. Clostridium novyi α-toxin-catalyzed incorporation of GlcNAc into Rho subfamily proteins. J Biol Chem. 1996;271:25173–25177. doi: 10.1074/jbc.271.41.25173. [DOI] [PubMed] [Google Scholar]
- 41.Torres J F, Lyerly D M, Monath T P. Evaluation of formalin-inactivated Clostridium difficile vaccines administered by parenteral and mucosal routes of immunization in hamsters. Infect Immun. 1995;63:4619–4627. doi: 10.1128/iai.63.12.4619-4627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torres J F, Monath T P. Antigenicity of amino-acid sequences from Clostridium difficile toxin B. J Med Microbiol. 1996;44:464–474. doi: 10.1099/00222615-44-6-464. [DOI] [PubMed] [Google Scholar]
- 43.Von Eichel-Streiber C, Heringdorf D M Z, Habermann E, Sartingen S. Closing in on the toxic domain through analysis of a variant Clostridium difficile cytotoxin B. Mol Microbiol. 1995;17:313–321. doi: 10.1111/j.1365-2958.1995.mmi_17020313.x. [DOI] [PubMed] [Google Scholar]
- 44.Von Eichel-Streiber C, Laufenberg-Feldmann R, Sartingen S, Schulze J, Sauerborn M. Comparative sequence analysis of the Clostridium difficile toxins A and B. Mol Gen Genet. 1992;233:260–268. doi: 10.1007/BF00587587. [DOI] [PubMed] [Google Scholar]
- 45.Ward S J, Douce G, Dougan G, Wren B W. Local and systemic neutralizing antibody responses induced by intranasal immunization with the nontoxic binding domain of toxin A from Clostridium difficile. Infect Immun. 1999;67:5124–5132. doi: 10.1128/iai.67.10.5124-5132.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yadav S, Brew K. Identification of a region of UDP-galactose: N-acetylglucosamine β4-galactosyltransferase involved in UDP-galactose binding by differential labeling. J Biol Chem. 1990;265:14163–14169. [PubMed] [Google Scholar]