Abstract
Secreted toxins play important roles in the pathogenesis of bacterial infections. In this study, we examined the presence of secreted cytotoxic factors of coagulase-negative staphylococci (CoNS) from bovine clinical and subclinical mastitis. A 34- to 36-kDa protein with cell-rounding cytotoxic activity was found in many CoNS strains, especially in Staphylococcus chromogenes strains. The protein caused cell detachment and cell rounding in several cell lines, including HEp-2, Int 407, CHO-K1, and Y-1 cells. Native protein recovered from nondenatured polyacrylamide gel electrophoresis showed both cytotoxic activity and casein hydrolysis activity. The purified protein had a pH optimal at 7.2 to 7.5 and a pI of 5.1 and was heat labile. The proteolytic activity could be inhibited by zinc and metal specific inhibitors such as 1,10-phenanthroline and EDTA, indicating that it is a metalloprotease. Protein mass analysis and peptide sequencing indicated that the protein is a novel metalloprotease. Different bacterial strains expressed variable levels of 34- to 36-kDa protease, which may provide an indication of strain virulence.
Coagulase-negative staphylococcus (CoNS) has been considered a minor pathogen of bovine mastitis; however, many studies recently have shown the importance of CoNS infection in the bovine mammary gland. Several studies indicated that CoNS is the most frequently recovered isolate from mastitis samples, especially in first lactation and unbred heifers (16, 18, 26, 30). CoNS also caused mastitis problems in other species such as milking goats (11). CoNS infections usually are more mild than Staphylococcus aureus (one coagulase-positive Staphylococcus sp.) which is a major and contagious pathogen of bovine mastitis. The somatic cell counts of CoNS-infected cows are generally two- to threefold higher than that of uninfected cows (18, 30). CoNS-infected mammary tissues exhibited greater leukocyte infiltration and increased connective tissue stroma over an uninfected control (37). One study showed that CoNS infections caused an 8.7% loss in milk production from a 305-day milk yield total (36).
Even though more researchers have realized the importance of CoNS intramammary infections, the virulence factors of CoNS remain poorly understood. The virulence factors of staphylococci have been studied most extensively in the species of S. aureus. Secreted toxins play very important roles in the pathogenesis of S. aureus (9, 10, 41). S. aureus strains isolated from bovine mastitis have been shown to express alpha, beta, gamma, and delta toxins, leukocidins, enterotoxin, and coagulase (8, 25). CoNS strains also produce several toxins and enzymes that could contribute to virulence, such as hemolysin, leucocidin, lipase, proteases, and DNase (17, 34, 39). Many CoNS strains isolated from mastitis samples had higher protease, DNase, and lecitinase activity than that of CoNS from normal cows (19). However, the roles of these enzymes on the pathogenesis of CoNS are unclear. A delta-like toxin from CoNS strains causes membrane bleb formation or rounding and refractility of the individual cells of L929 mouse skin fibroblasts (15), and it also damages the monolayer of human foreskin (34). Culture supernatants from a number of Staphylococcus hyicus (one CoNS species) isolates of porcine origin cause toxic effects to murine fibroblast and porcine keratinocyte cells in culture, and the cytotoxin has certain properties in common with the delta-like toxin (1). A metalloprotease, which may contribute to the pathogenesis of S. hyicus in piglet epidermitis, has been purified and cloned (2). No study has ever been published describing the possible cytotoxic activity of bovine CoNS species.
In this study, we focused on the cytotoxicity of CoNS strains that caused clinical and subclinical mastitis. Staphylococcus chromogenes species of CoNS can cause more severe infections than the average of other CoNS species. One study reported that there was no significant difference in inflammation parameters between an S. aureus infection and an S. chromogenes infection (27). Thus, we were especially interested in the virulence factors of S. chromogenes.
MATERIALS AND METHODS
Bacteria.
A total of 26 strains of S. chromogenes, 8 strains of S. aureus, 7 strains of S. hyicus, and 16 strains of other CoNS were used in this study. Several standard strains from the American Type Culture Collection (ATCC) were included: S. chromogenes ATCC 10530, S. aureus ATCC 12600, S. hyicus ATCC 2996, S. epidermidis ATCC 29851 and ATCC 2449, S. xylosis ATCC 2738, S. simulans ATCC 2705, and S. hominans ATCC 3474. All bacterial isolates except ATCC standard strains were recovered from clinical or subclinical mastitis milk samples submitted to the Pennsylvania State University Animal Diagnostic Lab. Bacterial species were identified with Staph-Trac and Biolog databases. Coagulase was tested by using the rabbit plasma tube test. Bacteria were frozen at −70°C in Trypticase soy broth (TSB) (Difco BBL) with 25% glycerol. All bacteria were subcultured no more than three times before they were frozen.
Tissue cells.
HEp-2 (human larynx epidermoid carcinoma cell), Int 407 (human embryonic intestine cell), CHO-K1 (Chinese hamster ovary cell), and Y-1 (mouse adrenal tumor cell) cell lines were used to study the cytotoxic effects of bacterial supernatants. Cell lines were obtained from the ATCC, Rockville, Md. HEp-2 cells were maintained in minimal essential medium (MEM; Gibco BRL) supplemented with 10% fetal bovine serum. Int 407 cells were maintained in basal essential medium (BEM; Gibco BRL) supplemented with 10% fetal bovine serum. CHO-K1 cells were maintained in Ham F-12 medium (Gibco BRL) supplemented with 10% fetal bovine serum. Y-1 cells were maintained in Ham F-10 medium (Gibco BRL) supplemented with 15% horse serum and 2.5% fetal bovine serum. All cell media were supplemented with 1× nonessential amino acids (Sigma), 2 mM l-glutamine (Sigma), 20 mM HEPES, and 100 U of penicillin G and 50 μg of streptomycin per ml (Sigma). The cultures were kept at 37°C in a humidified atmosphere of 95% air and 5% CO2. Media were changed two or three times per week, and the cells were passaged by trypsinization after monolayer formation.
Preparation of bacterial supernatants.
Bacteria were first grown on 5% washed sheep blood TSB agar plates at 37°C for 16 h, and then one clone was selected and inoculated into 5-ml B-broth tubes (10 g of casein hydrolate, 5 g of yeast extract, 5 g of NaCl, 1 g KH2PO4, and 1 g of glucose in 1 liter of distilled deionized water). Bacteria were grown at 37°C at 180 rpm with shaking for 16 to 18 h. Bacterial densities reached approximately 1.5 ± 0.05 at 578 nm (Beckman DU 650). Bacteria were then pelleted (Beckman J2-MI) at 7,000 × g for 15 min at 4°C, and the pH of supernatants was adjusted to 7.2 ± 0.05 with NaOH or HCl. All supernatants were filtered with a 0.2-μm (pore size) filter (ValuPrep; VWR) and stored at 4°C.
Cytotoxic effect assay.
HEp-2, CHO-K1, Int 407, and Y-1 cells were seeded in 48-well cell culture plates in 500 μl of the appropriate cell culture medium at concentrations of 105 cells/ml and then incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2 for 24 h to form a monolayer. Media were removed. Bacterial supernatants were serially diluted in cell culture media to 1:2.5, 1:5, and 1:10 levels and added to the cell monolayers. Normal B-broth (noninoculated) was also serially diluted as a negative control. After 12, 24, and 36 h of incubation, the cells were examined microscopically for morphological changes. Cell viability was determined by trypan blue dye exclusion. The highest dilution at which all of the cells exhibited cytopathic effects was recorded as the cytotoxic level.
For heat treatment, bacterial supernatants and the B-broth control were treated at 65°C for 30 min and then diluted at a 1:2.5 dilution and added to the cell monolayers. After 12, 24, and 36 h, the cells were examined as described above.
Immunoblotting.
Polyclonal rabbit antisera were raised against the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)-electroeluted 34- to 36-kDa protein from one of the S. chromogenes strains (690LR). Briefly, 1 liter of overnight-grown bacterial supernatant was concentrated 300 to 500 times by using a dialysis Stir Cell (Amicon; 10-kDa cutoff). SDS-PAGE (12%) was used to separate the secreted proteins, and then the band of interest was cut and recovered from the gel via electroelution (Bio-Rad model 422). The eluted protein was dialyzed against Tris buffer (50 mM Tris-HCl, pH 7.4) and then used to immunize rabbits. At day 1, 5 ml of blood was taken as the baseline (control serum), and 200 μg of purified 34- to 36-kDa protein was injected subcutaneously with Freund complete adjuvant. At days 21 and 44, second and third injections (200 μg of purified protein with Freund incomplete adjuvant) were performed subcutaneously. Antibody titiers were measured by enzyme-linked immunosorbent assay on serum collected at day 58.
SDS-PAGE gels were subsequently blotted using a semidry blotter system (Bio-Rad). Immobilon P membranes (Millipore) were blocked for 2 h with 5% bovine serum albumin in TBS (0.02 M Tris-HCl, 0.9% saline; pH 7.2) and then incubated with primary antibody (rabbit anti-toxin, 1:5,000 dilution) in antibody incubation solution (0.1% bovine serum albumin in TBS supplemented with 0.05% Tween 20). Alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G antibody (Sigma) was diluted 1:5,000 in antibody incubation solution and incubated with the membrane for 1 h. The phosphatase reaction was developed in 25 ml of Tris-HCl (0.1 M) with 1 ml of each 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium according to the instructions of the manufacturer (KPL).
Purification of native protein.
The native, functional protein was purified from preparative 8% nondenatured PAGE gels (22) by electroelution as described above at 4°C. Both gel and sample buffer are free of SDS. The eluted protein was dialyzed against Tris-HCl buffer with 2 mM CaCl2 (Millipore, 10-kDa cutoff). The purity of the protein was analyzed by SDS-PAGE.
Protease assay.
Protease activity was measured by using a modified method described by Ayora and Götz (2) with azocasein as the substrate. A 125-μl aliquot of 2% azocasein solution in 2 mM CaCl2–40 mM Tris-HCl (pH 7.8) was incubated with 75 μl of enzyme solution (purified protein or bacterial supernatants) for 45 min at 37°C. The reaction was stopped by adding 600 μl of 10% trichloroacetic acid. After incubation for 10 min at room temperature, the mixture was centrifuged for 5 min at 12,500 rpm (Fisher model 235C), and 600 μl of the supernatant was transferred to a tube containing 500 μl of 1 M NaOH. The absorbance was measured at 440 nm (Beckman DU650).
Molecular weight determination and protein mass analysis.
The molecular weight of the cell-rounding toxin was determined by comparing its migration rate in SDS-PAGE with a low-range protein molecular mass standard (Bio-Rad). For protein analysis, the protein band was directly cut from 12% SDS-PAGE and sent to the Protein Chemistry Core Facility at Columbia University. In-gel digestion was completed with trypsin, and peptides were extracted from the gel pieces. Matrix-assisted laser disorption ionization (MALDI) mass spectroscopic analysis was performed by using a PerSeptive Voyager PE-RP mass spectrometer.
RESULTS
Cytotoxic assay.
HEp-2 cells were used to study the cytotoxic effects of all bacterial strains. Two cytotoxic effects—cell rounding and lysis—were primarily observed. After incubation with some bacterial supernatants for 20 to 24 h, HEp-2 cells exhibited spindle-shaped changes at a high dilution level (1:5) but were rounded at a low dilution level (1:2.5) (Fig. 1). The cell-rounding cytotoxic effect was found in most of the S. chromogenes strains (24 of 26), and it was very uncommon among other CoNS species (Table 1). Different strains had variable cytotoxic levels, and 67% of the S. chromogenes strains had a cytotoxic effect at a 1:5 dilution. Cell lysis was observed after HEp-2 cells were incubated with supernatants of most S. aureus (6 of 8) and some CoNS strains (3 of 49) for 2 to 4 h. None of the S. chromogenes strains showed cytolytic effect. CHO-K1, Int 407, and Y-1 cell lines were also used to study the cell-rounding toxin. Bacterial supernatants which showed toxic effects on HEp-2 cells caused the exact same morphological changes in the Int 407 cell line (Fig. 2). However, when CHO-K1 and Y-1 cells were incubated with bacterial supernatants, cell rounding was observed but spindle-shaped changes were not seen (Fig. 3 and Fig. 4). The cell-rounding capabilities were lost in all these positive strains after bacterial supernatants were treated at 65°C for 30 min, indicating the heat-labile property of this toxin. Conversely, heat treatment did not inactivate the cell lysis toxins of S. aureus. Because the cell lysis activity was not decreased after heat treatment, it may be attributed by other toxins rather than alpha and beta toxins, which are normally inactivated at 56°C for 30 min (40).
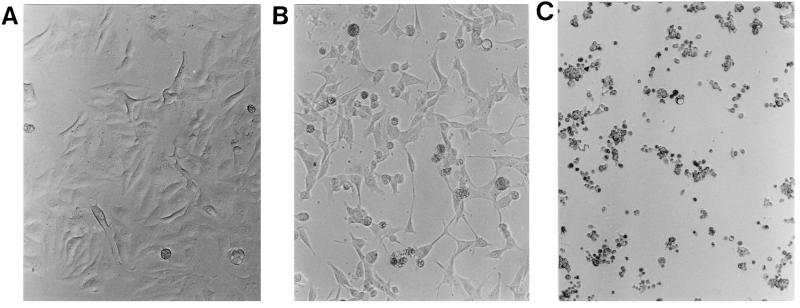
FIG. 1.
Cytotoxic effects of bacterial supernatant (S. chromogenes 690LR) on HEp-2 cells. B-broth and bacterial cultural supernatants were diluted in MEM, loaded on a HEp-2 cell monolayer, and incubated for 20 h at 37°C in 95% air and 5% CO2. (A) B-broth at a 1:2.5 dilution in MEM caused no cytopathic effects on the cultured HEp-2 cells. (B) Bacterial supernatant at a 1:5 dilution in MEM caused detachment and rounding of HEp-2 cells. (C) Bacterial supernatants at a 1:2.5 dilution in MEM caused rounding of all HEp-2 cells.
TABLE 1.
Cytotoxic assaya
| Species | Total no. | No. of isolates (% of total no.) showing:
|
|
|---|---|---|---|
| Cell lysis | Cell rounding | ||
| S. aureus | 8 | 6 (75) | 0 (0) |
| S. chromogenes | 26 | 0 (0) | 24 (92.3) |
| S. hyicus | 7 | 0 (0) | 1 (14.3) |
| Other CoNS sp. | 16 | 3 (18.8) | 1 (6.3) |
Bacteria were grown in B-broth overnight; supernatants were then diluted 1:2.5 and 1:5 in MEM and loaded onto HEp-2 cell monolayers. After 20 h of incubation at 37°C in 95% air and 5% CO2, the cells were examined microscopically for morphological changes. Cell lysis was indicated by HEp-2 cell destruction. Cell rounding indicated HEp-2 cells detached from the plastic cultural surface and floating in the cell culture medium.
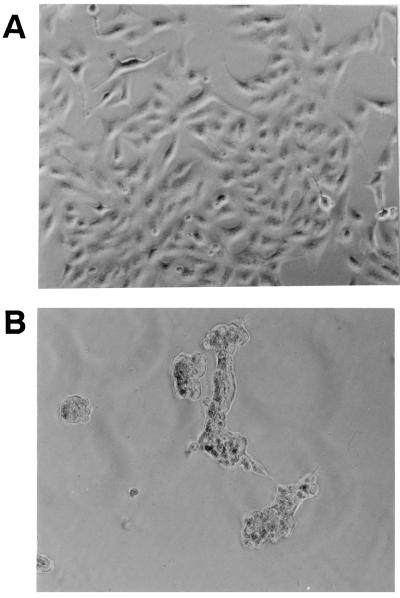
FIG. 2.
Cytotoxic effects of bacterial supernatant (S. chromogenes 690LR) on Int 407 cells. B-broth and bacterial cultural supernatants were diluted in BEM, loaded onto a Int 407 cell monolayer, and incubated for 20 h at 37°C in 95% air and 5% CO2. (A) B-broth at a 1:2.5 dilution in BEM caused no cytopathic effects on cultured Int 407 cells. (B) Bacterial supernatants at a 1:2.5 dilution in BEM caused rounding and clumping of Int 407 cells.
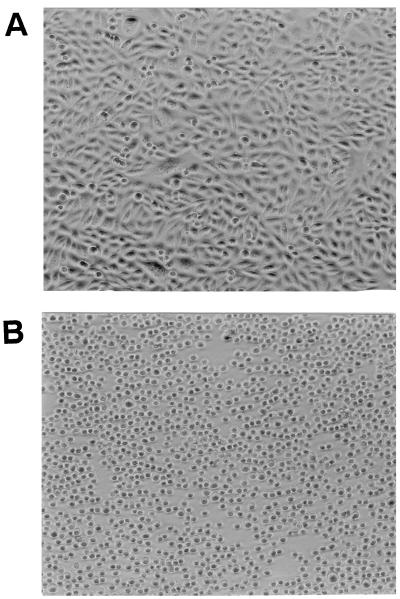
FIG. 3.
Cytotoxic effects of bacterial supernatant (S. chromogenes 690LR) on CHO-K1 cells. B-broth and bacterial cultural supernatants were diluted in Ham F-12 medium, loaded onto an Int 407 cell monolayer, and incubated for 20 h at 37°C in 95% air and 5% CO2. (A) B-broth at a 1:2.5 dilution in Ham F-12 medium caused no cytopathic effects on the cultured CHO-K1 cells. (B) Bacterial supernatants at 1:2.5 and 1:5 dilutions in Ham F-12 medium caused the rounding of CHO-K1 cells.
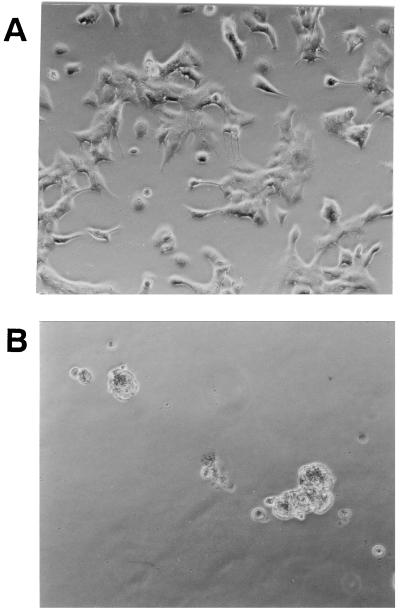
FIG. 4.
Cytotoxic effects of bacterial supernatant (S. chromogenes 690LR) on Y-1 cells. B-broth and bacterial cultural supernatants were diluted in Ham F-12 medium, loaded onto a Y-1 cell monolayer, and incubated for 20 h at 37°C in 95% air and 5% CO2. (A) B-broth at a 1:2.5 dilution in Ham F-12 medium caused no cytopathic effects on cultured Y-1 cells. (B) Bacterial supernatants at 1:2.5 and 1:5 dilutions in Ham F-12 medium caused rounding of Y-1 cells.
The cell rounding was not lethal. The viability of rounded cells was checked with trypan blue staining and monolayer formation assay. After HEp-2, Int 407, CHO-K1, and Y-1 cells were rounded for more than 24 h, trypan blue staining indicated that these rounded cells were still viable. When they were resuspended in fresh, bacterial-supernatant-free media, they could again form monolayers.
Identification and characterization of the cell-rounding toxin.
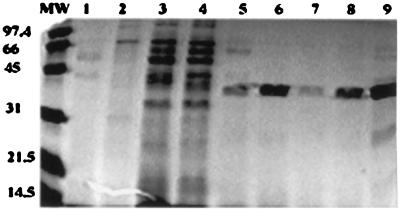
When the concentrated bacterial supernatants were separated by SDS–12% PAGE, a similar molecular size band of ca. 34 to 36 kDa was evident in all cell-rounding-positive strains but not cell-rounding-negative strains (Fig. 5). The 34- to 36-kDa protein was the dominant protein in supernatants in all the cytotoxic strains and was absent from most other CoNS and cytotoxin-negative S. chromogenes strains. The presence of the protein was positively correlated with the cell rounding, and strains expressing high levels of the protein were highly toxic. When the supernatants were treated at 65°C for 30 min, the 34- to 36-kDa band could not detected or became very weak on the SDS-PAGE and Western blots, a finding consistent with the cytotoxin assay results (Fig. 6). The 34- to 36-kDa protein might be degraded into very small fragments so that Western blots could not detect them on SDS–12% PAGE. The protein was eluted from nondenatured PAGE from two S. chromogenes strains, 690LR and 302RR, and the protein purity was checked by SDS-PAGE (Fig. 7). The purified 34- to 36-kDa protease from 690LR had a pI of about 5.1 on the isoelectric focusing gel. When different amounts of the purified protein were loaded onto a HEp-2 cell monolayer, we observed spindle-shaped cells at concentrations of 15 μg/ml and cell rounding at concentrations of 25 to 30 μg/ml after 12 h of incubation. With polyclonal antiserum (rabbit antibody against the 34- to 36-kDa protein from S. chromogenes 690LR), Western blots showed identity with the 34- to 36-kDa protein in all of the cell-rounding-positive strains but not the cell-rounding-negative strains (Fig. 8), thus indicating the structural and functional homology of the protein among different species and strains of CoNS.
FIG. 5.
Bacterial supernatants were concentrated 15 times with a 10-kDa molecular mass cutoff Centricon Plus-20 (Amicon). The concentrated supernatants (20 μl of each sample) were loaded onto SDS–12% PAGE gels. MW, low-range protein molecular mass marker (in kilodaltons) (Bio-Rad). Lanes 1 and 2, supernatants of two S. simulans strains; lanes 3 and 4, supernatants of two cytotoxin-negative S. chromogenes strains; lanes 5 to 9, supernatants of five cytotoxin-positive S. chromogenes strains. A 34- to 36-kDa protein band was only observed on cytotoxin-positive S. chromogenes strains.
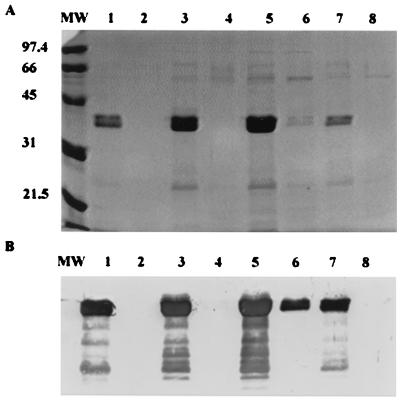
FIG. 6.
The 34- to 36-kDa protein was heat labile. The same amounts of concentrated supernatants were used for loading treated and untreated wells. For heat treatment, the supernatants were heated at 65°C for 30 min. (A) SDS-PAGE. MW, low-range protein molecular mass marker (in kilodaltons) (Bio-Rad). Lanes 1, 3, 5, and 7, untreated supernatants from S. chromogenes strains 1LR, 690LR, 302RR, and 726LR; lanes 2, 4, 6, and 8, the corresponding heat-treated supernatants. (B) Western blots. MW, low-range protein molecular mass marker (in kilodaltons) (Bio-Rad). Lanes 1, 3, 5, and 7, untreated supernatants from S. chromogenes strains 1LR, 690LR, 302RR, and 726LR; lanes 2, 4, 6, and 8, the corresponding heat-treated supernatants. The polyclonal antibody was raised in rabbits against the 34- to 36-kDa protein purified from S. chromogenes 690LR.
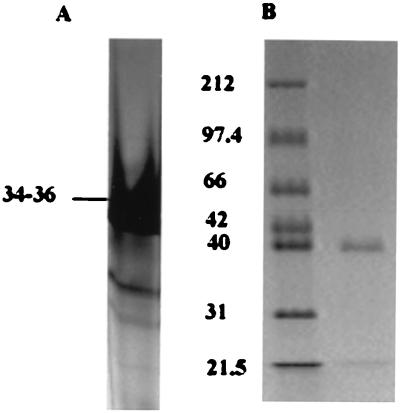
FIG. 7.
Purification of native 34- to 36-kDa protein from nondenatured PAGE. (A) Supernatant of S. chromogenes 690LR was concentrated 300-fold and separated on 8% nondenatured PAGE gels. The dominant band was easy to recognize. (B) The dominant band was cut and eluted from the gel, and the purity of the eluted protein was checked by SDS–12% PAGE.
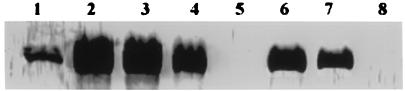
FIG. 8.
Western blots showed a specific reaction of antisera with the 34- to 36-kDa protein among different species and strains. The polyclonal antibody was raised in rabbits against the 34- to 36-kDa protein purified from S. chromogenes 690LR. Lane 1, S. chromogenes 690LR; lanes 2, 3, and 6, field isolates of S. chromogenes; lane 7, S. chromogenes ATCC 10530; lane 4, a field isolate of Staphylococcus hominis (CoNS) strain (cytotoxin positive); lanes 5 and 8, field isolates of Staphylococcus simulans (CoNS) strains (cytotoxin negative).
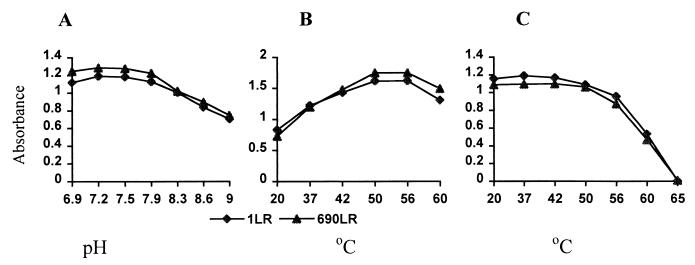
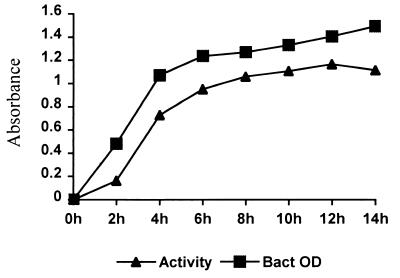
When the purified 34- to 36-kDa protein was loaded into a well within casein agar plates (Remel), it showed strong casein hydrolysis activity. The same activity was observed corresponding to the 34- to 36-kDa band by laying the nondenatured PAGE directly onto a casein agar plate (Fig. 9). The protease activity was inhibited by the metal chelator EDTA, Chelax-100, and 1,10-phenanthroline but not by the serine protease inhibitor phenylmethylsulfonyl fluoride (PMSF) (Table 2). Several divalent cations such as Mg2+, Ca2+, and Zn2+ could not restore the hydrolytic or cytotoxic activity after EDTA and Chelax-100 treatments. Protein degradation may have occurred after chelator treatment since the 34- to 36-kDa band intensity on SDS-PAGE faded. When the protease activity was measured at different pH buffer conditions, the optimal activity occurred at between pH 7.2 and 7.5 (Fig. 10A). The optimal temperature for enzymatic activity was between 50 and 55°C (Fig. 10B) as determined by measuring it at different incubation temperatures. To determine the protein thermostability, the protein was first heated at different temperatures before the protease activity was measured. The protein was thermostable at below 42°C, but the stability decreased at higher temperature (Fig. 10C). As we expected, no measurable activity remained after treatment at 65°C for 30 min. The protease activity, which reflects the production of the 34- to 36-kDa protease, from a culture of S. chromogenes 690LR in B-broth increased through the log phase and reached a maximum in the early-stationary-growth phase. The protease activity remained relative stable during the stationary phase (Fig. 11).
FIG. 9.
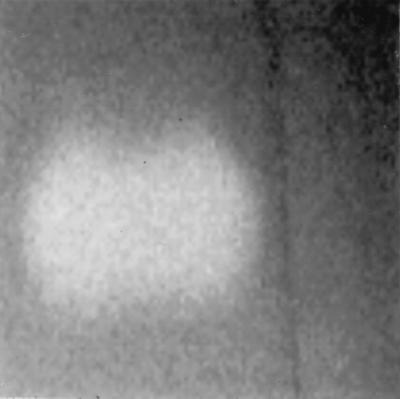
Casein hydrolysis activity of the 34- to 36-kDa protein from S. chromogenes 302RR. The white spot represents hydrolysis of casein. The crude supernatant was separated by nondenatured PAGE. After 10 min of washing in Tris-HCl buffer with 2 mM CaCl2, the gel was laid over a casein plate. The result was recorded after 2 h of incubation at 37°C.
TABLE 2.
Inhibition of the 34- to 36-kDa protein activity by various inhibitorsa
| Inhibitor | Type | Concn | Remaining activity (%) |
|---|---|---|---|
| EDTA | Metal chelator | 5 mM | 0 |
| 1,10-phenanthroline | Metal chelator | 1–2 mM | 0 |
| Chelax-100 | Metal chelator | 1 g/10 ml | 0 |
| PMSF | Serine protease inhibitor | 1–2 mM | 100 |
The purified 34- to 36-kDa protease was treated with different inhibitors for 30 min at room temperature; the protease activity was then measured at 37°C for 45 min as described in Materials and Methods. The untreated protease was used as the standard for 100% activity.
FIG. 10.
The optimal pH (A), optimal temperature (B), and thermostability (C) of the protease activity of the 34- to 36-kDa protein were studied by using the protease assay (see Materials and Methods). The same amount of crude purified protein was used for all the reactions. 1LR and 690LR were field S. chromogenes strains. (A) The protease activity was measured in 100 mM Tris-HCl buffer with different pHs, ranging from pH 6.9 to 9. (B) The protease activity was measured after 45 min of incubation at different temperatures, from 20 to 60°C. (C) The crude purified protein was first incubated at different temperatures, from 20 to 65°C, for 20 min, and the protein activity was then measured.
FIG. 11.
The 34- to 36-kDa protein expression was associated with the bacterial growth phase. Quantitative analysis of the 34- to 36-kDa protein was measured by the protease activity, and the bacterial growth was monitored by reading the optical density (OD) at 578 nm.
Protein mass analysis.
The 34- to 36-kDa protein was separated on SDS-PAGE and the stained band was cut out and subjected to in-gel tryptic digestion. Peptides extracted from the gel were analyzed by MALDI mass spectrometry. The mass data were used to search the Genpept and NCBInr databases for a match. No protein matched the peptide masses entered, suggesting that the protein we had isolated was unique.
DISCUSSION
In our experiment, a cell-rounding cytotoxic activity was found in the supernatants of most S. chromogenes strains and some other CoNS strains. Bacterial supernatants caused reversible cell rounding and cell spindle-shaped changes in several cell lines, including HEp-2, CHO-K1, Int 407, and Y-1 cells. However, S. aureus supernatants and some CoNS supernatants produced cytolytic toxins that caused cell lysis. Ten or more hours were required to observe the cell-rounding activity, but cell lysis was evident within 2 to 4 h. The rounded cells were still viable after 24 to 48 h, and normal morphology was restored by the addition of fresh cell medium. The cytotoxic activity was similar to that of several other toxins. Exfoliative toxin (sET from S. aureus strains and shET from S. hyicus strains) caused cell rounding of several cell lines but was not cytolethal (33). A cytotoxic fraction obtained from isoelectric focusing of S. hyicus strains caused cell detachment, a decrease in cell size, and crenation of the cell membrane, but cell membranes were not sufficiently damaged to allow an adequate uptake of trypan blue (1). Several cell-rounding toxins have been reported (5, 13, 28, 31). Cell cytoskeletal rearrangement or damage with different mechanisms is associated with a cytotoxic cell-rounding effect (13, 21, 24, 28). Non-membrane-damaging cytotoxin from Vibrio cholerae causes cell rounding with restructuring of microfilament net work and the microtubular component by a rise in the intracellular calcium level (6). Studies have shown that thrombin could regulate the actin cytoskeleton by a Rho protein-dependent passway (23, 38). Several cytotoxic proteases can directly affect cytoskeletal architecture of cells and cause cell rounding (14, 28, 32). We have observed rearrangement of actin in fluorescein isothiocyanate phalloidin-stained rounded HEp-2 cells (data not shown), and the mechanisms of cell rounding need to be further studied. Since the 34- to 36-kDa protein is a metalloprotease, it may directly affect the cell cytoskeleton.
A 34- to 36-kDa protein was associated with the cell-rounding cytotoxity. All proteins purified from several strains had the cell-rounding cytotoxic activity. The protein expression levels were variable among different CoNS species and strains. Many S. chromogenes strains could produce high levels of the protease under the experimental conditions and may provide an indication of strain virulence. The cytotoxic effects were dose and time dependent. High doses (50 to 60 μg/ml) caused cell rounding within 6 to 8 h, but low doses (10 to 15 μg/ml) needed 24 to 48 h for cell rounding to occur. The purified protein also had protease activity and could hydrolyze casein. Metal chelators completely inhibited the protease activity, suggesting that this cell-rounding protein was a metalloprotease. Some divalent cations are important for the metalloprotease catalytic functions or protein stabilization (35). SDS-PAGE showed that the 34- to 36-kDa protein was totally degraded after treatment with Chelax-100 and EDTA at 4°C for 20 min but was stable after treatment with 1,10-phenanthroline (metal chelator with high affinity to zinc). This finding indicates that zinc plays a role in the catalytic activity and that other cations, such as Ca2+ and Mg2+, may stabilize the 34- to 36-kDa protease, which is similar to other metalloproteases (2, 3).
Several metalloproteases were already sequenced and/or characterized from different Staphylococcus species, including S. hyicus, S. epidermidis, and S. aureus (2, 3, 4, 7, 35). There was no homology or very little homology among these proteases (2, 35). The 34- to 36-kDa protease from S. chromogenes showed a unique mass spectrometer pattern, and the peptide sequence failed to match any published protease sequences. This indicated that the protein could be a novel metalloprotease. However, homology may exist between the 34- to 36-kDa protease and the metalloproteases from other Staphylococcus species, such as S. hyicus and S. epidermidis. The polyclonal antibody raised against the 34- to 36-kDa protein of S. chromogenes 690LR reacts very well with the 34-kDa metalloprotease from an ATCC S. hyicus strain of porcine origin. The S. hyicus strain of porcine origin has two proteases in its supernatant: a 41-kDa protease (ShpI) and a 34-kDa protease (ShpII). Our rabbit polyclonal antibody reacted only with the 34-kDa band. This may indicate homology between the new protease and ShpII.
The 34- to 36-kDa cell-rounding metalloprotease could be an important factor for the pathogenesis of S. chromogenes. Several bacterial proteases are known to be cytotoxic (1, 12, 21). A metalloprotease toxin of Bacillus fragilis is associated with diarrheal disease in farm animals and humans and is an important virulence factor in B. fragilis associated with diarrhea and sepsis (20, 29). Our in vitro study showed cell-rounding effects of the novel 34- to 36-kDa protease on several cell lines and indicated its cytotoxic activity. Many S. chromogenes strains produce high levels of this protease, and this is consistent with the clinical observation that S. chromogenes is more virulent than other CoNS species. The protease was produced in B-broth, skim milk, and milk whey, and our preliminary studies detected the 34- to 36-kDa protease in the milk of challenged goats, suggesting production in vivo (i.e., in the mammary gland). Casein is an important nutrient in milk, and casein hydrolysis may result in a growth advantage for the bacteria. When we challenged goats with cytotoxic-positive S. chromogenes strains, clinical and subclinical mastitis were observed in all challenged goats (unpublished data). Significant edema, mammary epithelial cell damage, and neutrophil infiltration have been seen in infected mammary tissues. We propose the 34- to 36-kDa protease causes damage to mammary epithelial and vascular endothelial cells, leading to intravascular fluid leaking and migration of neutrophils. Future research will focus on cloning and sequencing the novel protease gene and studing the in vivo function of the protease via goat challenge studies with isogenic mutant strains.
ACKNOWLEDGMENT
This work was supported by U.S. Department of Agriculture grant 96-34163-2712.
REFERENCES
- 1.Allaker R P, Whitlock M, Lloyd D H. Cytotoxic activity of Staphylococcus hyicus. Vet Microbiol. 1991;26:161–166. doi: 10.1016/0378-1135(91)90052-h. [DOI] [PubMed] [Google Scholar]
- 2.Ayora S, Götz F. Genetic and biochemical properties of an extracellular neutral metalloprotease from Staphylococcus hyicus subsp. hyicus. Mol Gen Genet. 1994;242:421–430. doi: 10.1007/BF00281792. [DOI] [PubMed] [Google Scholar]
- 3.Ayora S, Lindgren P, Götz F. Biochemical properties of a novel metalloprotease from Staphylococcus hyicus subsp. hyicus involved in extracellular lipase processing. J Bacteriol. 1994;176:3218–3223. doi: 10.1128/jb.176.11.3218-3223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avidson S, Holme T, Lindholm B. Studies of extracellular proteolytic enzymes from Staphylococcus aureus. Biochim Biophys Acta. 1973;302:135–148. doi: 10.1016/0005-2744(73)90016-8. [DOI] [PubMed] [Google Scholar]
- 5.Ball D W, Van Tassell R L, Roberts M D, Hahn P E, Lyerly D M, Wilkins T D. Purification and characterization of alpha-toxin produced by Clostridium novyi Type A. Infect Immun. 1993;61:2912–2918. doi: 10.1128/iai.61.7.2912-2918.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basu I, Mitra R, Saha P K, Fhosh A N, Ahattacharya J, Chakrabarti M K, Takeda Y, Nair G B. Morphological and cytoskeletal changes caused by non-membrane damaging cytotoxin of Vibrio cholerae on Int 407 and HeLa cells. FEMS Microbiol Lett. 1999;179:255–263. doi: 10.1111/j.1574-6968.1999.tb08736.x. [DOI] [PubMed] [Google Scholar]
- 7.Bjorklind A, Jornvall H. Substrate specificity of three different extracellular proteolytic enzymes for Staphylococcus aureus. Biochim Biophys Acta. 1974;370:524–529. doi: 10.1016/0005-2744(74)90113-2. [DOI] [PubMed] [Google Scholar]
- 8.Bramley A J, Patel A H, O'Reilly M, Foster R, Foster T J. Roles of alpha-toxin and beta-toxin in virulence of Staphylococcus aureus for the mouse mammary gland. Infect Immun. 1989;57:2489–2494. doi: 10.1128/iai.57.8.2489-2494.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cifrian E, Guidry A J, O'Brien C N, Marquardt W W. Effect of antibodies to Staphylococcal alpha and beta toxins and Staphylococcus aureus on the cytotoxicity and adherence of the organism to bovine mammary epithelial cells. Am J Vet Res. 1996;57:1308–1311. [PubMed] [Google Scholar]
- 10.Cifrian E, Guidry A J, Bramley A J, Norcross N L, Bastida-Corcuera F D, Marquardt W W. Effect of staphylococcal beta toxin on the cytotoxicity, proliferation and adherence of Staphylococcus aureus to bovine mammary epithelial cells. Vet Microbiol. 1996;48:187–198. doi: 10.1016/0378-1135(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 11.Deinhofer M, Pernthaner A. Staphylococcus spp. as mastitis-related pathogens in goat milk. Vet Microbiol. 1995;43:161–166. doi: 10.1016/0378-1135(95)92532-g. [DOI] [PubMed] [Google Scholar]
- 12.Eke P I, Laughon B E, Rotim V O. Cytotoxic activity of crude extracts of Bacteroides gingivalis. J Med Microbiol. 1989;28:5–8. doi: 10.1099/00222615-28-1-5. [DOI] [PubMed] [Google Scholar]
- 13.Fiorentini C, Arancia G, Paradisi S, Donelli G, Giuliano M, Piemonte F, Mastrantonio P. Effects of Clostridium difficile toxins A and B on cytoskeleton organization in HEp-2 cells: a comparative morphological study. Toxicon. 1989;27:1209–1218. doi: 10.1016/0041-0101(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 14.Fiorentini C, Malorni W, Paradisi S, Giuliano M, Mastrantonio P, Donelli G. Interaction of Clostridium difficile toxin A with cultured cells: cytoskeletal changes and nuclear polarization. Infect Immun. 1990;58:2329–2336. doi: 10.1128/iai.58.7.2329-2336.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gemmell C G. Extracellular toxins and enzymes of coagulase-negative staphylococci. In: Easmon C S F, Adlam C, editors. Staphylococci and staphylococcal infections. New York, N.Y: Academic Press, Inc.; 1983. pp. 819–822. [Google Scholar]
- 16.Hammon R J, Langlois B E. Mastitis due to coagulase negative Staphylococcus species. National Mastitis Council annual meeting proceedings; 1995. pp. 56–67. [Google Scholar]
- 17.Hebert G A, Hancock G A. Synergistic hemolysis exhibited by species of Staphylococci. J Clin Microbiol. 1985;22:409–415. doi: 10.1128/jcm.22.3.409-415.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorun J. Classification of coagulase negative staphylococci isolated from bovine clinical and subclinical mastitis. Vet Microbiol. 1991;27:151–158. doi: 10.1016/0378-1135(91)90006-2. [DOI] [PubMed] [Google Scholar]
- 19.Karadzhov I, Mekhandzhiiska L, Gerganova E. Coagulase negative staphylococci isolated from the milk of cows with subclinical mastitis. Vet Med Nauki. 1981;18:51–54. [PubMed] [Google Scholar]
- 20.Kato N, Kato H, Tanaka-Bandoh K, Watanabe K, Ueno K. Detection of Bacteroides fragilis enterotoxin from extraintestinal clinical isolates of B. fragilis group organisms. Kansenshogaku Zasshi. 1995;69:903–907. doi: 10.11150/kansenshogakuzasshi1970.69.903. [DOI] [PubMed] [Google Scholar]
- 21.Koshy S S, Montrose M H, Sears C L. Human intestinal epithelial cells swell and demonstrate actin rearrangement in response to the metalloprotease toxin of Bacteroides fragilis. Infect Immun. 1996;64:5022–5028. doi: 10.1128/iai.64.12.5022-5028.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Majumdar M, Seacholtz T M, Goldstein D, de Lanerolle P, Brown J H. Requirement for Rho-mediated myosin light chain phosphorylation in thrombin-stimulated cell rounding and its dissociation from mitogenesis. J Biol Chem. 1998;273:10099–10106. doi: 10.1074/jbc.273.17.10099. [DOI] [PubMed] [Google Scholar]
- 24.Malorni W, Fiorentini C, Paradisi S, Giuliano M, Mastrantonio P, Donelli G. Surface blebbing and cytoskeletal changes induced in vitro by toxin B Clostridium difficile: an immunochemical and ultrastructural study. Exp Mol Pathol. 1990;52:340–356. doi: 10.1016/0014-4800(90)90074-n. [DOI] [PubMed] [Google Scholar]
- 25.Matsunaga T, Kamata S, Kakiichi N, Uchida K. Characteristics of Staphylococcus aureus isolated from peracute, acute and chronic bovine mastitis. J Vet Med Sci. 1993;55:297–300. doi: 10.1292/jvms.55.297. [DOI] [PubMed] [Google Scholar]
- 26.Myllys V. Staphylococci in heifer mastitis before and after parturition. J Dairy Res. 1995;62:51–60. doi: 10.1017/s0022029900033665. [DOI] [PubMed] [Google Scholar]
- 27.Myllys V, Honkanen-Buzladki T, Virtanen H, Pyorala S, Muller H P. Effect of abrasion teat orifice epithelium on development of bovine staphylococcal mastitis. J Dairy Sci. 1994;77:446–452. doi: 10.3168/jds.S0022-0302(94)76972-1. [DOI] [PubMed] [Google Scholar]
- 28.Navarro-Garcia F, Sears C, Eslava C, Cravioto A, Nataro J P. Cytoskeletal effects induced by pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect Immun. 1999;67:2184–2192. doi: 10.1128/iai.67.5.2184-2192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obiso R J, Jr, Lyerly D M, Van Tassell R L, Wilkins T D. Proteolytic activity of the Bacteroides fragilis enterotoxin causes fluid secretion and intestinal damage in vivo. Infect Immun. 1995;63:3820–3826. doi: 10.1128/iai.63.10.3820-3826.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliver S P, Jayaro B M. National Mastitis Council annual meeting proceedings. National Mastitis Council; 1995. Coagulase negative staphylococcus species intramammary infections in heifers and cows during the nonlacting and perpartum periods; pp. 68–77. [Google Scholar]
- 31.Saha P K, Koley H, Nair G B. Purification and characterization of an extracellular secretogenic non-membrane-damaging cytotoxin produced by clinical strains of vibrio cholerae non-O1. Infect Immun. 1996;64:3101–3108. doi: 10.1128/iai.64.8.3101-3108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saidi R F, Jaeger K, Montrose M H, Wu S, Sears C L. Bacteroides fragilis toxin rearranges the actin cytoskeleton of HT29/C1 cells without direct proteolysis of actin or decrease in F-actin content. Cell Motil Cytoskeleton. 1997;37:159–165. doi: 10.1002/(SICI)1097-0169(1997)37:2<159::AID-CM8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Sato H, Kuramoto M, Tanabe T, Saito H. Susceptibility of various animals and cultured cells to exfoliative toxin produced by Staphylococcus hyicus subsp. hyicus. Vet Microbiol. 1991;28:157–169. doi: 10.1016/0378-1135(91)90090-3. [DOI] [PubMed] [Google Scholar]
- 34.Scheifele D W, Bjornson G L, Dyer R A, Dimmick J E. Delta-like toxin produced by coagulase-negative staphylococci is associated with neonatal necrotizing enterocolitis. Infect Immun. 1987;55:2268–2273. doi: 10.1128/iai.55.9.2268-2273.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teufel P, Götz F. Characterization of an extracellular metalloprotease with elastase activity from Staphylococcus epidermidis. J Bacteriol. 1993;175:4218–4224. doi: 10.1128/jb.175.13.4218-4224.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Timms L L, Schultz L H. Dynamics and significance of coagulase negative staphylococcal intramammary infections. J Dairy Sci. 1987;70:2648–2657. doi: 10.3168/jds.S0022-0302(87)80335-1. [DOI] [PubMed] [Google Scholar]
- 37.Trinidad P, Nickerson S C, Alley T K. Prevalence of intramammary infection and teat canal colonization in unbred and primigravid dairy heifers. J Dairy Sci. 1990;73:107–114. doi: 10.3168/jds.S0022-0302(90)78652-3. [DOI] [PubMed] [Google Scholar]
- 38.Vouret-Craviari V, Boquet P, Pouyssegur J, Van Obberghen-Schilling E. Regulation of the actin cytoskeleton by thrombin in human endothelial cells: role of Rho proteins in endothelial barrier function. Mol Biol Cell. 1998;9:2639–2653. doi: 10.1091/mbc.9.9.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watts J L, Owens W E. Synergistic hemolysis associated with coagulase negative staphylococci isolated from bovine mammary glands. J Clin Microbiol. 1987;25:2037–2039. doi: 10.1128/jcm.25.11.2037-2039.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vann J M, Proctor R A. Cytotoxic effects of ingested Staphylococcus aureus on bovine endothelial cells: role of S. aureus alpha-hemolysin. Microb Pathog. 1988;4:443–453. doi: 10.1016/0882-4010(88)90029-0. [DOI] [PubMed] [Google Scholar]
- 41.Zavizion B, Bramley A J, Politis I, Gilmore J, Turner J D, Patel A H, Foster T J. Effects of Staphylococcus aureus toxins on the growth of bovine mammary epithelial cells (MAC-T) in culture. J Dairy Sci. 1994;78:277–284. doi: 10.3168/jds.S0022-0302(95)76635-8. [DOI] [PubMed] [Google Scholar]