Abstract
Endothelial cells can influence significantly the host inflammatory response against blood-borne microbial pathogens. Previously, we found that endothelial cells respond to in vitro infection with Candida albicans by secreting interleukin 8 (IL-8) and expressing E-selectin, intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1). We have now examined the mechanisms mediating this endothelial cell response. We determined that C. albicans stimulated endothelial cells to synthesize tumor necrosis factor alpha (TNF-α), which in turn induced these infected cells to secrete IL-8 and express E-selectin by an autocrine mechanism. Expression of VCAM-1 was mediated not only by TNF-α but also by IL-1α and IL-1β, all of which were synthesized by endothelial cells in response to C. albicans. These three cytokines remained primarily cell associated rather than being secreted. Candidal induction of ICAM-1 expression was independent of TNF-α, IL-1α, and IL-1β. These observations demonstrate that different proinflammatory endothelial cell responses to C. albicans are induced by distinct mechanisms. A clear understanding of these mechanisms is important for therapeutically modulating the endothelial cell response to C. albicans and perhaps other opportunistic pathogens that disseminate hematogenously.
Candida albicans is an opportunistic pathogen that disseminates hematogenously and causes serious infections in compromised patients. The large number of patients at risk for this infection include cancer patients with neutropenia, low-birth-weight infants, and individuals who have had recent intra-abdominal surgery or extensive burns (1). The use of indwelling central venous catheters and treatment with broad-spectrum antibiotics are additional risk factors for this infection. As a result of advances in medical technology, the population at risk for hematogenously disseminated candidiasis has increased significantly in recent years, and Candida spp. is now the fourth-most-common organism to be isolated from the bloodstream of hospitalized patients (33). In spite of advances in antifungal therapy, the attributable mortality of hematogenously disseminated candidiasis is estimated to be 38% (42). Because of this unacceptably high mortality, new methods to prevent and treat this infection need to be developed. One potential strategy is to enhance the host inflammatory response to this blood-borne pathogen while it is still in the vascular compartment, before it has invaded the tissue parenchyma. Towards this end, we have been investigating the response of endothelial cells to candidal invasion in vitro.
Based on studies by ourselves and others, the following model of the events that lead to a hematogenously disseminated candidal infection has been developed. Organisms that have entered the bloodstream first adhere to the endothelial cell lining of the vasculature (15, 20, 36). This direct contact between endothelial cells and C. albicans is especially likely to occur in patients with neutropenia or neutrophil dysfunction. The adherent organisms then penetrate the endothelial cells in part by inducing their own phagocytosis (36). Induction of phagocytosis requires intact endothelial cell microfilaments and microtubules, and it does not require opsonization of the organisms by serum components (13). Once inside the endothelial cells, the organisms can injure and eventually kill these cells and thereby gain access to the deep tissues. Endothelial cell injury may also result in the exposure of the subendothelial cell matrix, which can be bound by additional organisms (21).
We have found that endothelial cells are not passive in this process but actively respond to candidal invasion by expressing leukocyte adhesion molecules, such as intercellular adhesion molecule 1 (ICAM-1), E-selectin, and vascular cell adhesion molecule 1 (VCAM-1) (12). In response to C. albicans, endothelial cells also secrete interleukin 6 (IL-6), IL-8, monocyte chemoattractant protein 1, and prostaglandins (10–12). These proinflammatory mediators likely enhance the recruitment of activated leukocytes to the site of vascular invasion, where they can aid in host defense. If a sufficient number of functional leukocytes are present, the invading organism can be killed and the infection can be aborted (8).
Because the response of endothelial cells to invasion by C. albicans is likely critical to determining the magnitude and composition of the local inflammatory response to the organism, we examined the mechanisms by which C. albicans stimulates endothelial cells to secrete IL-8 and express ICAM-1, E-selectin, and VCAM-1. Our current results indicate that this organism stimulates the synthesis of each of these different proinflammatory mediators by a discrete mechanism. The mechanisms mediating these responses are different from those mediating the response of endothelial cells to other microbial pathogens such as cytomegalovirus and Rickettsia conorii (19, 38). This multiplicity of signal transduction mechanisms likely provides endothelial cells with the important ability to selectively modulate the inflammatory response, depending on the infecting organism.
MATERIALS AND METHODS
Organisms.
C. albicans ATCC 36082, a clinical isolate, was obtained from the American Type Culture Collection (Manassas, Va.). A germination-deficient strain of C. albicans, V6, was generously provided by Helen Buckley (Temple Medical School, Philadelphia, Pa.) (3). All organisms were grown overnight on a rotating drum at 25°C in yeast nitrogen base broth (Difco Laboratories, Detroit, Mich.) supplemented with 0.5% (wt/vol) glucose as described elsewhere (11). The blastospores were harvested by centrifugation and washed twice in Dulbecco's phosphate-buffered saline (PBS; Irvine Scientific, Santa Ana, Calif.). The washed organisms were enumerated using a hemacytometer prior to use in the experiments.
Killed, germinated organisms were prepared by incubating 3 × 106 blastospores of C. albicans 36082 per ml in RPMI 1640 medium on a rotary shaker at 37°C for 90 min. Greater than 90% of the organisms produced germ tubes when grown under these conditions. The germinated organisms were then killed by exposure to 20 mM sodium periodate for 30 min at room temperature (13, 36). They were washed extensively in PBS prior to being added to the endothelial cells. An aliquot of the organisms was inoculated onto Sabouraud dextrose agar (Difco) to confirm that all of the organisms had been killed. We have shown previously that C. albicans cells killed by this method are phagocytized by endothelial cells (13).
Endothelial cells.
Human umbilical vein endothelial cells were harvested with collagenase by the method of Jaffe et al. (18). They were grown in tissue culture medium consisting of M-199 (Gibco, Grand Island, N.Y.) supplemented with 10% fetal bovine serum, 10% defined bovine calf serum (Gemini Bio-Products, Inc., Calabasas, Calif.), and 2 mM l-glutamine with penicillin and streptomycin (Irvine Scientific) (10, 13). The cells were grown in multiwell tissue culture plates (Falcon, Lincoln Park, N. J.) coated with fibronectin (Collaborative Biomedical Products, Bedford, Mass.). All experiments were performed using tightly confluent third-passage endothelial cells. By hemacytometer counts, there were approximately 5 × 104 endothelial cells per cm2 of culture surface at confluency.
The endotoxin concentrations of all tissue and fungal culture media were measured by a chromogenic Limulus amebocyte lysate test (BioWhittaker, Inc., Walkersville, Md.). The concentration of endotoxin in all media was less than 0.1 IU/ml. In addition, the presence of monocytes/macrophages in samples of endothelial cells was determined using a nonspecific esterase stain (Sigma-Aldrich Co., St. Louis, Mo.). All preparations of endothelial cells contained less than 0.1% of these cells.
Endothelial cell stimulation.
On the day of the experiment, the tissue culture medium above the endothelial cells was aspirated and replaced with fresh medium containing C. albicans. In all experiments, the ratio of C. albicans to endothelial cells was approximately 1:1.5. All incubations were for 8 h at 37°C in 5% CO2. Each experiment was repeated at least three times, using endothelial cells from different umbilical cords.
To determine whether IL-1 or tumor necrosis factor alpha (TNF-α) was required for C. albicans to stimulate endothelial cells to express leukocyte adhesion molecules or secrete IL-8, IL-1 receptor antagonist (IL-1ra; R&D Systems, Minneapolis, Minn.) and neutralizing murine monoclonal antibodies (MAbs) directed against TNF-α, IL-1α, or IL-1β (Table 1) were used. The concentration of each inhibitor was determined in titration experiments using the recombinant cytokine to which the inhibitor was directed. Each inhibitor was used at a concentration that produced greater than 95% inhibition of endothelial cell stimulation, which was measured as E-selectin expression and IL-8 secretion. IL-1ra was used at a final concentration of 300 μg/ml, and the MAbs were used at 2.5 μg/ml. In experiments where the MAbs were used, an equal concentration of murine immunoglobulin G1 (IgG1) was added to control wells of endothelial cells. All inhibitors were added to the endothelial cells immediately prior to the stimuli and were present in the medium for the duration of the experiment.
TABLE 1.
Murine MAbs used in the experiments
| Antigen | Clone | Isotype | Source |
|---|---|---|---|
| IL-1α | 4414.141 | IgG2a | R&D Systemsa |
| IL-1β | 8516.311 | IgG1 | R&D Systems |
| TNF-α | 1825.121 | IgG1 | R&D Systems |
| E-selectin | 1.2B6 | IgG1 | Biodesign Internationalb |
| ICAM-1 | 15.2 | IgG1 | Biodesign International |
| VCAM-1 | 1G11B1,1.4C3 | IgG1 | Biodesign International |
| Unspecified | 11711.11 | IgG1 | R&D Systems |
Minneapolis, Minn.
Kennebunk Port, Maine.
Because platelet-activating factor (PAF) has been reported to mediate endothelial cell expression of ICAM-1 in response to some stimuli (5, 17), the PAF receptor antagonist WEB 2086 (generously supplied by Chris Carter, Boehringer Ingelheim Pharmaceuticals, Ridgefield, Conn.) was tested for its ability to inhibit Candida-induced ICAM-1 expression. We found that PAF alone did not cause significant stimulation of endothelial cells (data not shown). Therefore, the optimal concentration of WEB 2086 was determined by its ability to inhibit platelet aggregation induced by 10 μM PAF. The extent of platelet aggregation was measured using an aggregometer by the method of Yeaman et al. (44). A WEB 2086 concentration of 50 μM was found to inhibit PAF-induced platelet aggregation completely. Thus, this concentration was used in the endothelial cell experiments. In all experiments using WEB 2086, control platelets or endothelial cells were exposed to an equal concentration of the diluent (0.1% dimethyl sulfoxide).
Preparation of conditioned media.
To obtain media conditioned by endothelial cells and/or C. albicans, 25-cm2 tissue culture flasks containing 6 ml of tissue culture medium were used. The flasks, with or without endothelial cells, were inoculated with 1.67 × 106 C. albicans. After incubation for 8 h, the conditioned media were collected, cooled on ice, and then centrifuged at 500 × g for 7 min. The supernatant fluids were then stored at −70°C for later use.
RT-PCR.
Cytokine and leukocyte adhesion molecule mRNA expression by endothelial cells was detected by reverse transcription-PCR (RT-PCR). Endothelial cells in 24-well tissue culture plates were exposed to either C. albicans or 500 μl of conditioned medium for 8 h. After the medium was aspirated and the cells were rinsed with cold Hanks balanced salt solution, the total endothelial cell RNA was extracted using guanidium isothiocyanate (6, 29). In previous experiments, we have determined that this method extracts RNA only from the endothelial cells and not from C. albicans (12). Both reverse transcription and PCR were performed in the same tube, using 10 to 200 ng of total endothelial cell RNA (29). The primers used to amplify the target mRNAs are listed in Table 2. The PCR products were separated by agarose gel electrophoresis, visualized by ethidium bromide, and quantified by densitometry. To ensure that equal amounts of RNA were amplified for each condition, the results were normalized to the expression of the constitutively expressed glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (Table 2). In preliminary experiments, the reaction conditions for each set of primers were adjusted so that the relationship between the amount of input RNA and PCR product was linear.
TABLE 2.
Oligonucleotides used to quantify target mRNAs by RT-PCR
| mRNA species | Primer sequences (5′ to 3′)a | Size of reaction product (bp) | Reference |
|---|---|---|---|
| E-selectin | CTCTGACAGAAGAAGCCAAG | 254 | 29 |
| ACTTGAGTCCACTGAAGCCA | |||
| ICAM-1 | TATGGCAACGACTCCTTCT | 238 | 29 |
| CATTCAGCGTCACCTTGG | |||
| VCAM-1 | ATGACATGCTTGAGCCAGG | 260 | 29 |
| GTGTCTCCTTCTTTGACACT | |||
| IL-8 | ATGACTTCCAAGCTGGCCGTGGCT | 289 | 26 |
| TCTCAGCCCTCTTCAAAAACATTCTC | |||
| GAPDH | CGGAGTCAACGGATTTGGTCGTAT | 306 | 43 |
| AGCCTTCTCCATGGTGGTGAAGAC |
Top sequence is the sense primer; bottom sequence is the antisense primer.
Measurement of leukocyte adhesion molecule expression and cytokine synthesis.
The surface expression of E-selectin, ICAM-1, and VCAM-1 by endothelial cells grown in 96-well tissue culture plates was determined by whole-cell enzyme-linked immunosorbent assay (ELISA) by the method of Noel et al. (32). The MAbs used in the ELISAs are listed in Table 1. In experiments where endothelial cell IL-8 release was investigated, the cells were grown in 48-well tissue culture plates. After the cells were stimulated with C. albicans, the media were collected, centrifuged at 500 × g for 5 min at 4°C to remove cells and debris, and then stored at −70°C. At a later time, the concentration of IL-8 in the medium was measured by ELISA (R&D Systems).
The effect of C. albicans on the amount of secreted and endothelial cell-associated IL-1α, IL-1β, and TNF-α was determined using endothelial cells grown in six-well tissue culture plates. Following exposure to C. albicans, the media were collected and processed as described for the IL-8 experiments. The endothelial cells were then rinsed with ice-cold Hanks balanced salt solution and solubilized in PBS containing 100 mM Igepal CA-630 (Sigma-Aldrich), 10 mM NaN3, and 10 mM phenylmethylsulfonyl fluoride (14). The concentrations of the different cytokines in the media and lysates were measured by ELISA (R&D Systems).
Measurement of endothelial cell injury.
The extent of endothelial cell injury caused by C. albicans in the presence of the different inhibitors was determined by chromium release assay as previously described (11, 13). The incubation time, concentration of the inhibitors, and ratio of organisms to endothelial cells were the same as in the endothelial cell stimulation experiments.
Statistical analysis.
The response of the endothelial cells to the different conditions was evaluated by analysis of variance with the Bonferroni correction for multiple comparisons. P values of ≤0.05 were considered to be significant.
RESULTS
Effects of medium conditioned by Candida-infected endothelial cells on uninfected endothelial cells.
First, we investigated whether C. albicans stimulated endothelial cells to secrete IL-8 and express leukocyte adhesion molecules via a soluble factor. These immunomodulators were chosen for study because they likely contribute to the mixed suppurative granulomatous response seen at sites of hematogenously disseminated candidal infection in humans (7). Endothelial cells were infected with C. albicans for 8 h. During this incubation, the C. albicans blastospores germinated and their hyphae penetrated the endothelial cells. At the end of the incubation period, the conditioned medium above these infected endothelial cells was collected and stored. At a later time it was added to uninfected endothelial cells for an additional 8 h.
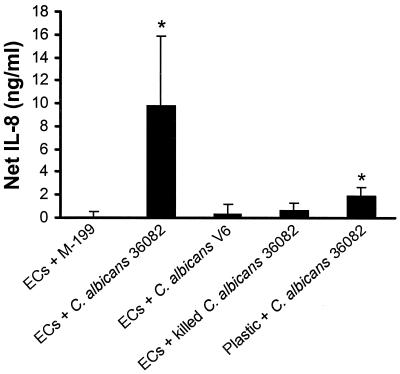
We found that medium conditioned by endothelial cells infected with live, wild-type C. albicans stimulated uninfected endothelial cells to secrete IL-8 (Fig. 1). This conditioned medium did not significantly stimulate uninfected endothelial cells to express ICAM-1, VCAM-1, or E-selectin (data not shown). The conditioned medium stimulated IL-8 secretion only when it was obtained from endothelial cells exposed to live, germinating C. albicans; media conditioned by endothelial cells exposed to either killed C. albicans or the live, nongerminating mutant (V6) were not stimulatory. These findings are consistent with our previous observations that direct contact with killed organisms or nongerminating mutants do not stimulate endothelial cells to accumulate mRNA for IL-8 (12).
FIG. 1.
Medium conditioned by endothelial cells plus C. albicans stimulates uninfected endothelial cells to secrete IL-8. Endothelial cells (EC) were incubated with media conditioned by the indicated stimuli for 8 h. The net concentration of IL-8 was calculated by subtracting the concentration of IL-8 in the conditioned medium before it was added to the endothelial cells from the concentration of IL-8 in the medium after it had been exposed to the endothelial cells for 8 h. The IL-8 concentrations were measured by ELISA. Results are mean ± standard deviations of three separate experiments. ∗, P ≤ 0.01 compared to medium conditioned by endothelial cells alone.
When C. albicans cells were incubated on bare plastic in the absence of endothelial cells, their growth and germination were similar to those of organisms grown in the presence of endothelial cells. However, medium conditioned by these organisms induced minimal IL-8 secretion when it was added to uninfected endothelial cells (Fig. 1). These findings suggest that the endothelial cells were likely the source of majority of stimulatory factor in the conditioned medium. However, we cannot completely rule out the possibility that growth of C. albicans in the presence of endothelial cells induces the organism to release a substance that contributes to the stimulation of uninfected endothelial cells.
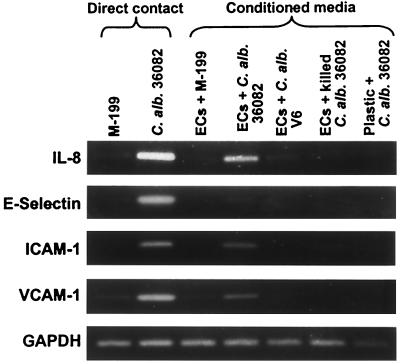
We also analyzed the endothelial cell response by RT-PCR, to determine the effects of the conditioned media on mRNA accumulation. Consistent with the above results, we found that medium conditioned by endothelial cells exposed to live, germinating C. albicans stimulated the accumulation of IL-8 mRNA (Fig. 2). However, this medium also induced the accumulation of mRNA for ICAM-1 and VCAM-1 but not E-selectin. These findings suggested that stimulation of the expression of these leukocyte adhesion molecules by C. albicans occurred by a mechanism that either was independent of a soluble factor or required an additional constituent.
FIG. 2.
Effects of conditioned media on endothelial cell mRNA expression. Endothelial cells were exposed to the indicated conditions for 8 h, after which mRNA expression was analyzed by RT-PCR. Results are representative of one of three individual experiments. Abbreviations: C. alb., C. albicans; ECs, endothelial cells; M-199, endothelial cell culture medium.
Endothelial cells synthesize IL-1α, IL-1β, and TNF-α in response to C. albicans.
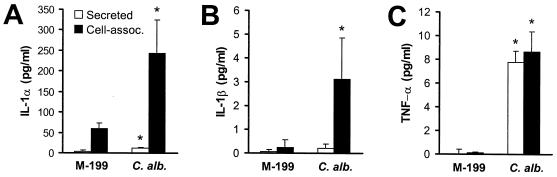
The conditioned medium experiments suggested that the stimulation of certain endothelial cell responses by C. albicans likely occurs by an indirect or feedback mechanism. We therefore investigated whether endothelial cells respond to infection with C. albicans by synthesizing IL-1α, IL-1β, and TNF-α. These cytokines were examined because they are both synthesized by endothelial cells and able to stimulate endothelial cells to secrete IL-8 and express leukocyte adhesion molecules (9, 22, 23, 27, 31, 37). We found that C. albicans induced a significant increase in the synthesis of IL-1α, IL-1β, and TNF-α (Fig. 3). The majority of IL-1α synthesized in response to C. albicans was cell associated, although there was a 2.9-fold increase in the concentration of IL-1α in the medium (Fig. 3A). C. albicans induced a small but significant increase in cell-associated IL-1β (Fig. 3B). IL-1β was not detected in the medium. In contrast to IL-1α and IL-1β, 47% of the TNF-α synthesized by the endothelial cells in response to C. albicans was secreted into the medium, while the remainder was cell associated (Fig. 3C).
FIG. 3.
C. albicans stimulates endothelial cells to synthesize IL-1α, IL-1β, and TNF-α. Endothelial cells were exposed to either medium alone or live C. albicans 36082 (C. alb.) for 8 h, after which the concentrations of the indicated cytokines in the media and whole-cell lysates were determined by ELISA. Data are the mean ± standard deviations of three independent experiments. ∗, P < 0.001 compared to uninfected endothelial cells.
Anti-TNF-α MAb and IL-1ra had different effects on various endothelial cell responses.
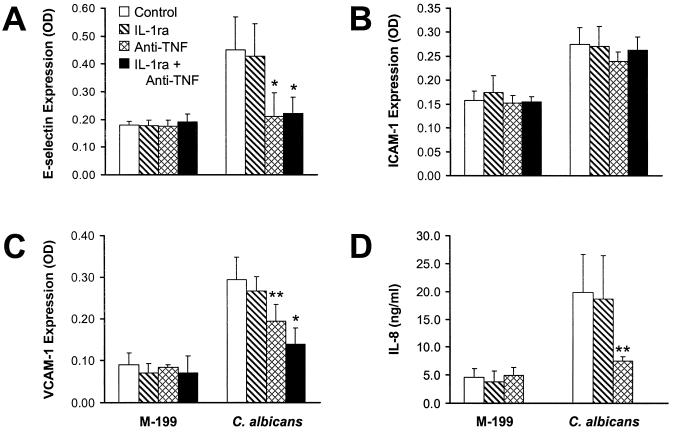
Next, we used specific inhibitors to determine the role of endothelial cell-derived TNF-α, IL-1α, and IL-1β in the induction of IL-8 secretion and leukocyte adhesion molecule expression. Inhibiting TNF-α with a MAb reduced the expression of E-selectin to basal levels (Fig. 4A). The anti-TNF-α MAb also inhibited Candida-induced VCAM-1 expression by 46% and secretion of IL-8 by 70% (Fig. 4C and D). However, neutralizing TNF-α had no effect on the expression of ICAM-1 by endothelial cells infected with C. albicans (Fig. 4B).
FIG. 4.
Effects of inhibiting IL-1 and/or TNF-α on endothelial cell stimulation by C. albicans. Endothelial cells were exposed to medium or live C. albicans 36082 in the presence of IL-1ra and/or an anti-TNF-α MAb for 8 h. Surface expression of E-selectin (A), ICAM-1 (B), and VCAM-1 (C) and the concentration of IL-8 in the media (D) were measured by ELISA. Data are the mean ± standard deviation of at least three independent experiments. ∗ and ∗∗, P < 0.001 and P ≤ 0.01 compared to endothelial cells exposed to C. albicans in the absence of inhibitors. OD, optical density.
IL-1ra, which inhibits both IL-1α and IL-1β, did not alter significantly the levels of ICAM-1, E-selectin, or VCAM-1 expression in response to C. albicans (Fig. 4). This inhibitor also had no consistent effect on Candida-induced IL-8 secretion.
Combining IL-1ra with the anti-TNF-α MAb decreased candidal stimulation of VCAM-1 expression by 67% (Fig. 4C). This combination had no detectable effect on ICAM-1 expression (Fig. 4B). It did not reduce the level of expression of E-selectin expression further than that inhibited by the anti-TNF-α MAb alone, because the latter inhibitor reduced E-selectin expression to basal levels (Fig. 4A). Finally, the effect of IL-1ra and the anti-TNF-α MAb on IL-8 secretion was not determined because it was unlikely that we could detect significantly greater inhibition than was caused by the anti-TNF-α MAb alone (Fig. 4D).
The above findings suggest that C. albicans stimulates IL-8 secretion and E-selectin expression by a mechanism that requires TNF-α. VCAM-1 expression is induced by the combination of TNF-α and IL-1.
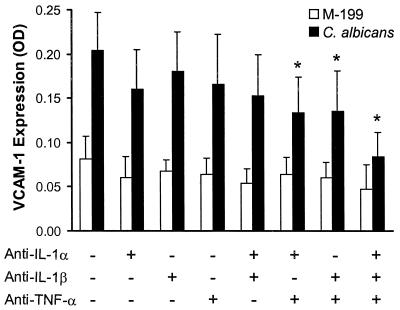
Candidal induction of VCAM-1 expression is mediated by the combination of IL-1α, IL-1β, and TNF-α.
Because IL-1ra inhibits both IL-1α and IL-1β, we used neutralizing MAbs directed against each of these cytokines to determine their roles in inducing VCAM-1 expression. Neither of these antibodies alone significantly inhibited Candida-induced VCAM-1 expression (Fig. 5). The addition of anti-TNF-α MAb to either the anti-IL-1α MAb or the anti-IL-1β MAb inhibited VCAM-1 expression by 44 and 39%, respectively. Finally, the combination of MAbs directed against TNF-α, IL-1α, and IL-1β resulted in a 70% inhibition of VCAM-1 expression. Thus, both IL-1α, and IL-1β as well as TNF-α participate in the stimulation of VCAM-1 expression by C. albicans.
FIG. 5.
Candida-induced VCAM-1 expression requires IL-1α, IL-1β, and TNF-α. Endothelial cells were infected with live C. albicans 36082 in the presence of MAbs directed against the indicated cytokines for 8 h. VCAM-1 expression was determined by cell surface ELISA. Results are the mean ± standard deviation of four separate experiments. ∗ P < 0.001 compared to endothelial cells exposed to C. albicans in the presence of control mouse IgG1. OD, optical density.
ICAM-1 expression is induced by a mechanism that is independent of PAF.
The above experiments demonstrated that neither IL-1 nor TNF-α was involved in stimulating ICAM-1 expression. Therefore, we examined the possible role of PAF (5, 17). When the PAF receptor antagonist WEB 2086 was added to the endothelial cells and C. albicans, there was no reduction in ICAM-1 expression (data not shown). To explore the possible role of PAF further, we added exogenous PAF, both alone and in combination with C. albicans, to endothelial cells. We found that PAF had no effect on ICAM-1 expression (data not shown). Therefore, C. albicans induces endothelial cells to express ICAM-1 by a mechanism that is independent of PAF.
Inhibiting IL-1, TNF-α, and PAF had no detectable effect on endothelial cell injury caused by C. albicans.
We have observed that endothelial cell injury and stimulation by C. albicans are closely associated. Both processes require that endothelial cells phagocytize live, germinating C. albicans and they follow parallel time courses (11–13). Therefore, we examined whether IL-1, PAF, or TNF-α mediated endothelial cell injury caused by C. albicans. Using a chromium release assay, we found that IL-1ra, WEB 2086, or the anti-TNF-α MAb did not significantly reduce the extent of endothelial cell injury caused by C. albicans (data not shown). These inhibitors also had no effect on candidal hyphal formation. These results indicate that IL-1, PAF, and TNF-α do not appear to play a major role in endothelial cell injury induced by C. albicans.
DISCUSSION
Endothelial cells can synthesize a broad array of cytokines and leukocyte adhesion molecules in response to microbial pathogens. These endothelial cell products have the potential to markedly influence the host inflammatory reaction by regulating the type and number of leukocytes that accumulate at the infection site. The inflammatory reaction to hematogenously disseminated candidiasis is a mixed suppurative granulomatous response consisting of both neutrophils and mononuclear cells (7). We therefore investigated the mechanisms by which endothelial cells synthesize ICAM-1, E-selectin, VCAM-1, and IL-8 in response to C. albicans, because collectively these immunomodulators can recruit both neutrophils and mononuclear cells.
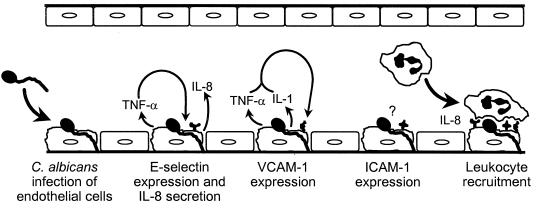
Our results demonstrate that C. albicans stimulates different endothelial cell responses by distinctly different mechanisms (Fig. 6). The induction of E-selectin expression and IL-8 secretion by C. albicans is mediated by TNF-α, whereas the expression of VCAM-1 requires the combination of endothelial cell-derived TNF-α, IL-1α and IL-1β. Alternatively, the expression of ICAM-1 is induced by a mechanism that is independent of these three cytokines and PAF. It is possible that some other factor, such as arachidonic acid or a lipoxygenase metabolite, may mediate the expression of this leukocyte adhesion molecule by an autocrine mechanism (2, 40). It is also conceivable that endothelial cell expression of ICAM-1 is induced directly by C. albicans.
FIG. 6.
Schematic diagram of the different mechanisms by which C. albicans stimulates endothelial cells to secrete IL-8 and express E-selectin, ICAM-1, and VCAM-1.
An important finding was that the mechanisms underlying the endothelial cell response to C. albicans appear to differ from those mediating the response to other microorganisms. For example, Span et al. (38) discovered that IL-1 mediates E-selectin expression in endothelial cells infected with cytomegalovirus. In contrast, we found that TNF-α mediates E-selectin expression in response to C. albicans.
While E-selectin expression can be induced by two different mechanisms, namely, IL-1 and TNF-α, endothelial cell secretion of IL-8 by can be stimulated via three different pathways, depending on the microorganism. R. conorii induces the synthesis of this cytokine by a feedback mechanism that requires IL-1α (19). C. albicans induces IL-8 secretion by stimulating endothelial cells to synthesize TNF-α. Finally, Borrelia burgdorferi stimulates IL-8 secretion by a mechanism that is independent of both IL-1 and TNF-α (4). This diversity of signaling pathways likely enables endothelial cells to respond to different microbial pathogens with specific and perhaps unique patterns of immunomodulators. The patterns of immunomodulators induced by two different organisms could potentially differ both in terms of the types of immunomodulators that are produced and the amount of each immunomodulator.
Several investigators have confirmed that endothelial cells can synthesize TNF-α, IL-1α, and IL-1β (9, 19, 22, 23, 27, 31, 37). We found that virtually all of the IL-1α and IL-1β synthesized in response to C. albicans was cell associated and very little was secreted into the medium. Approximately half of the TNF-α was also cell associated. We interpret these findings to indicate that TNF-α and IL-1 likely induce a localized response that is limited to the cells that synthesize these cytokines and possibly to adjacent cells. This conclusion is supported by our previous experiments in which we used indirect immunofluorescence to detect leukocyte adhesion molecule expression by endothelial cells infected with C. albicans. In this prior study, we observed leukocyte adhesion molecule expression only on endothelial cells that were either in direct contact with an organism or immediately adjacent to an infected endothelial cell (12).
Almost 50% of the TNF-α that was synthesized by endothelial cells was secreted into the medium. Therefore, it is likely that TNF-α was one of the factors in the medium conditioned by Candida-infected endothelial cells that induced uninfected endothelial cells to secrete IL-8. In support of this hypothesis, we have found that incubating this conditioned medium with an anti-TNF-α MAb causes at least a 60% reduction in its ability to stimulate endothelial cells to secrete IL-8 (A. S. Orozco and S. G. Filler, unpublished data).
Although the conditioned medium stimulated endothelial cells to secrete IL-8, it did not induce the expression of any of the leukocyte adhesion molecules. In preliminary work, we performed titration experiments in which we determined the concentration of recombinant TNF-α required to produce the same level of endothelial cell stimulation as was induced by C. albicans. We found that the concentration of TNF-α required to induce IL-8 secretion was eightfold lower than that required to stimulate leukocyte adhesion molecule expression. Therefore, it is likely that the concentration of TNF-α in the conditioned medium was sufficient to induce endothelial cells to secrete IL-8 (Fig. 1) but not high enough to stimulate leukocyte adhesion molecule expression. An alternative explanation for this finding is that soluble TNF-α binds to a different receptor than does TNF-α that is membrane bound, and different TNF-α receptors may induce different endothelial cell responses. For example, it is known that in murine microvascular endothelial cells, stimulation of ICAM-1 expression by soluble TNF-α requires both TNF receptor 1 and TNF receptor 2, whereas stimulation by membrane-bound TNF-α requires only TNF receptor 2 (25).
Even though the conditioned medium did not induce the expression of leukocyte adhesion molecules on the endothelial cell surface, it stimulated the accumulation of ICAM-1 and VCAM-1 mRNAs, as determined by RT-PCR (Fig. 2). It is possible that other factors known to suppress the surface expression of ICAM-1 and VCAM-1, such as hydroxy- or hydroxyperoxy-eicosatetraenoic acid, were present in the conditioned medium (16). Alternatively, it is conceivable that the low concentration of TNF-α in the conditioned medium was adequate to induce mRNA accumulation, but insufficient to stimulate protein expression.
Media conditioned by endothelial cells exposed to either killed or nongerminating organisms did not activate uninfected endothelial cells to express leukocyte adhesion molecules or secrete IL-8. This finding is in agreement with our earlier results that only live, germinating organisms can stimulate endothelial cells (11, 12). In these and previous experiments, the organisms were killed using periodate, which significantly alters the surface carbohydrates of C. albicans. Also, all experiments were performed in the absence of complement, which likely coats the surface of C. albicans in vivo. It is possible that different results might have been obtained if the organisms had been killed by a different method or if complement had been present in the medium.
It is known that both blastospores of C. albicans and killed organisms are able to stimulate monocytes to release proinflammatory cytokines (1, 14, 35). Therefore, the finding that the killed organisms and live, nongerminating mutants did not stimulate endothelial cells strongly suggests that the TNF-α in the medium was produced by the endothelial cells and not by any contaminating monocytes.
Our in vitro results suggesting the importance of TNF-α in mediating the endothelial cell response to C. albicans are consistent with in vivo studies that demonstrate the pivotal role of this cytokine in host defense against this organism. For example, mice treated with anti-TNF-α antibodies or that lack the gene for either TNF-α or TNF receptor 1 have increased mortality and a higher tissue burden of organisms following intravenous inoculation with C. albicans (24, 28, 30, 39). Also, studies with mice that are deficient in TNF-α suggest that this cytokine is important for an organized granulomatous response (28). Although TNF-α affects the function of many types of host cells, it is possible that one of the reasons the absence of TNF-α impairs host defense is that this cytokine is required for endothelial cells to synthesize sufficient quantities of crucial leukocyte adhesion molecules and cytokines at sites of infection.
It is known that TNF-α by itself can contribute to the induction of endothelial cell injury in the absence of infectious agents (34, 41). Although infection with C. albicans stimulated endothelial cells to synthesize TNF-α, inhibiting this cytokine did not reduce the extent of endothelial cell injury caused by C. albicans. Therefore, candidal injury of endothelial cells is unlikely to be mediated by TNF-α. One possible explanation for this result is that the amount of TNF-α synthesized by the endothelial cells in response to C. albicans was below the threshold required to induce injury (41). Our finding that inhibition of PAF and IL-1 did not reduce the amount of Candida-induced endothelial cell injury suggests that this process is also independent of these immunomodulators.
In conclusion, endothelial cells respond to infection with C. albicans by expressing leukocyte adhesion molecules and secreting IL-8. These different proinflammatory responses are mediated by different mechanisms. E-selectin expression and IL-8 secretion are induced by an autocrine mechanism that requires TNF-α. Expression of VCAM-1 is mediated by the combined activities of endothelial cell-derived TNF-α, IL-1α, and IL-1β. ICAM-1 expression is stimulated by a mechanism that is independent of these three cytokines, as well as PAF. Moreover, the mechanisms that regulate the endothelial cell reaction to C. albicans appear to differ from those mediating similar responses to other microbial pathogens. Therefore, even though two different microorganisms may elicit the same endothelial cell response, such as the expression of a specific leukocyte adhesion molecule, the mechanism by which this reaction is induced may be unique. This diversity of signaling pathways likely enables the endothelial cell to respond to different microbial pathogens with a specific and perhaps unique array of proinflammatory mediators.
In future studies, these in vitro investigations must be extended by adding other cell types, such as neutrophils, to the system. Furthermore, these in vitro experiments will serve as a foundation for animal studies to determine the roles of TNF-α, IL-1α, and IL-1β in the autocrine stimulation of leukocyte adhesion molecule expression in vivo.
ACKNOWLEDGMENTS
We thank the nurses at Harbor-UCLA Medical Center for collecting umbilical cords, Michael Mador and Toshiko Lamkin for help with tissue culture, and Trang Phan and Gregg Filler for technical assistance. We also appreciate the helpful advice and discussion of John E. Edwards, Jr., Bett J. Eng, and Michael R. Yeaman.
This work was supported by Public Health Service grants R01 AI19990, P01 AI37194, R29 AI040636, and MO1 RR00425 from the National Institutes of Health and grant 1081-GI3 from the American Heart Association, Greater Los Angeles Affiliate.
REFERENCES
- 1.Aybay C, Imir T. Tumor necrosis factor (TNF) induction from monocyte/macrophages by Candida species. Immunobiology. 1996;196:363–374. doi: 10.1016/S0171-2985(96)80059-3. [DOI] [PubMed] [Google Scholar]
- 2.Barry O P, Praticò D, Savani R C, FitzGerald G A. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Investig. 1998;102:136–144. doi: 10.1172/JCI2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckley H R, Price M R, Daneo-Moore L. Isolation of a variant of Candida albicans. Infect Immun. 1982;37:1209–1217. doi: 10.1128/iai.37.3.1209-1217.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns M J, Sellati T J, Teng E I, Furie M B. Production of interleukin-8 (IL-8) by cultured endothelial cells in response to Borrelia burgdorferi occurs independently of secreted IL-1 and tumor necrosis factor alpha and is required for subsequent transendothelial migration of neutrophils. Infect Immun. 1997;65:1217–1222. doi: 10.1128/iai.65.4.1217-1222.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chihara J, Maruyama I, Yasuba H, Yasukawa A, Yamamoto T, Kurachi D, Mouri T, Seguchi M, Nakajima S. Possible induction of intercellular adhesion molecule-1 (ICAM-1) expression on endothelial cells by platelet-activating factor (PAF) J Lipid Mediat. 1992;5:159–162. [PubMed] [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–169. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Edwards J E., Jr . Candida species. In: Mandell G L, Bennett J E, Dolin R, editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 4th ed. New York, N. Y.: Churchill Livingstone Inc.; 1995. pp. 2289–2306. [Google Scholar]
- 8.Edwards J E, Jr, Rotrosen D, Fontaine J W, Haudenschild C C, Diamond R D. Neutrophil-mediated protection of cultured human vascular endothelial cells from damage by growing Candida albicans hyphae. Blood. 1987;69:1450–1457. [PubMed] [Google Scholar]
- 9.Eissner G, Kohlhuber F, Grell M, Ueffing M, Scheurich P, Hieke A, Multhoff G, Bornkamm G W, Holler E. Critical involvement of transmembrane tumor necrosis factor-α in endothelial programmed cell death mediated by ionizing radiation and bacterial endotoxin. Blood. 1995;86:4184–4193. [PubMed] [Google Scholar]
- 10.Filler S G, Ibe B O, Ibrahim A S, Ghannoum M A, Raj J U, Edwards J E., Jr Mechanisms by which Candida albicans induces endothelial cell prostaglandin synthesis. Infect Immun. 1994;62:1064–1069. doi: 10.1128/iai.62.3.1064-1069.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filler S G, Ibe B O, Luckett P M, Raj J U, Edwards J E., Jr Candida stimulates endothelial cell eicosanoid production. J Infect Dis. 1991;164:928–935. doi: 10.1093/infdis/164.5.928. [DOI] [PubMed] [Google Scholar]
- 12.Filler S G, Pfunder A S, Spellberg B J, Spellberg J P, Edwards J E., Jr Candida albicans stimulates cytokine production and leukocyte adhesion molecule expression by endothelial cells. Infect Immun. 1996;64:2609–2617. doi: 10.1128/iai.64.7.2609-2617.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filler S G, Swerdloff J N, Hobbs C, Luckett P M. Penetration and damage of endothelial cells by Candida albicans. Infect Immun. 1995;63:976–983. doi: 10.1128/iai.63.3.976-983.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghezzi M C, Raponi G, Angeletti S, Mancini C. Serum-mediated enhancement of TNF-alpha release by human monocytes stimulated with the yeast form of Candida albicans. J Infect Dis. 1998;178:1743–1749. doi: 10.1086/314484. [DOI] [PubMed] [Google Scholar]
- 15.Hostetter M K. Adhesins and ligands involved in the interaction of Candida spp. with epithelial and endothelial surfaces. Clin Microbiol Rev. 1994;7:29–42. doi: 10.1128/cmr.7.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Z H, Bates E J, Ferrante J V, Hii C S, Poulos A, Robinson B S, Ferrante A. Inhibition of stimulus-induced endothelial cell intercellular adhesion molecule-1, E-selectin, and vascular cellular adhesion molecule-1 expression by arachidonic acid and its hydroxy and hydroperoxy derivatives. Circ Res. 1997;80:149–158. doi: 10.1161/01.res.80.2.149. [DOI] [PubMed] [Google Scholar]
- 17.Ishizuka T, Suzuki K, Kawakami M, Kawaguchi Y, Hidaka T, Matsuki Y, Nakamura H. DP-1904, a specific inhibitor of thromboxane A2 synthesizing enzyme, suppresses ICAM-1 expression by stimulated vascular endothelial cells. Eur J Pharmacol. 1994;262:113–123. doi: 10.1016/0014-2999(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 18.Jaffe E A, Nachman R L, Becker C G, Ninick C R. Culture of human endothelial cells derived from umbilical veins: identification by morphologic and immunologic criteria. J Clin Investig. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplanski G, Teysseire N, Farnarier C, Kaplanski S, Lissitzky J, Durand J, Soubeyrand J, Dinarello C A, Bongard P. IL-6 and IL-8 production from cultured human endothelial cells stimulated by infections with Rickettsia conorii via a cell-associated IL-1α-dependent pathway. J Clin Investig. 1995;96:2839–2844. doi: 10.1172/JCI118354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klotz S A. Fungal adherence to the vascular compartment: a critical step in the pathogenesis of disseminated candidiasis. Clin Infect Dis. 1992;14:340–347. doi: 10.1093/clinids/14.1.340. [DOI] [PubMed] [Google Scholar]
- 21.Klotz S A, Maca R D. Endothelial cell contraction increases Candida adherence to exposed extracellular matrix. Infect Immun. 1988;56:2495–2498. doi: 10.1128/iai.56.9.2495-2498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurt-Jones E A, Fiers W, Pober J S. Membrane interleukin 1 induction on human endothelial cells and dermal fibroblasts. J Immunol. 1987;139:2317–2324. [PubMed] [Google Scholar]
- 23.Libby P, Ordovas J M, Auger K R, Robbins A H, Birinyi L K, Dinarello C A. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986;124:179–185. [PMC free article] [PubMed] [Google Scholar]
- 24.Louie A, Baltch A L, Smith R P, Franke M A, Ritz W J, Singh J K, Gordon M A. Tumor necrosis factor alpha has a protective role in a murine model of systemic candidiasis. Infect Immun. 1994;62:2761–2772. doi: 10.1128/iai.62.7.2761-2772.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucas R, Juillard P, Decoster E, Redard M, Donati Y, Giroud C, Monso-Hinard C, De Kesel T, Burrman W A, Moore M W, Dayer J-M, Fiers W, Bluethmann H, Grau G E. Crucial role of tumor necrosis factor (TNF) receptor 2 and membrane-bound TNF in experimental cerebral malaria. Eur J Immunol. 1997;27:1719–1725. doi: 10.1002/eji.1830270719. [DOI] [PubMed] [Google Scholar]
- 26.Lupetti R, Mortarini R, Panceri P, Sensi M, Anichini A. Interaction with fibronectin regulates cytokine gene expression in human melanoma cells. Int J Cancer. 1996;66:110–116. doi: 10.1002/(SICI)1097-0215(19960328)66:1<110::AID-IJC19>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 27.Marceau F, Grassi J, Frobert Y, Bergeron C, Poubelle P E. Effects of experimental conditions on the production of interleukin-1α and -1β by human endothelial cells cultured in vitro. Int J Immunopharmacol. 1992;14:525–534. doi: 10.1016/0192-0561(92)90113-y. [DOI] [PubMed] [Google Scholar]
- 28.Marino M W, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old L J. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meagher L, Mahiouz D, Sugars K, Burrows N, Norris P, Yarwood H, Becker-Andre M, Haskard D O. Measurement of mRNA for E-selectin, VCAM-1, and ICAM-1 by reverse transcription and polymerase chain reaction. J Immunol Methods. 1994;175:237–246. doi: 10.1016/0022-1759(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 30.Mencacci A, Cenci E, Del Sero G, Fe d'Ostiani C, Mosci P, Montagnoli C, Bacci A, Bistoni F, Quesniaux V F, Ryffel B, Romani L. Defective co-stimulation and impaired Th1 development in tumor necrosis factor/lymphotoxin-alpha double-deficient mice infected with Candida albicans. Int Immunol. 1998;10:37–48. doi: 10.1093/intimm/10.1.37. [DOI] [PubMed] [Google Scholar]
- 31.Molossi S, Clausell N, Rabinovitch M. Coronary artery endothelial interleukin-1β mediates enhanced fibronectin production related to post-cardiac transplant arteriopathy in piglets. Circulation. 1993;88:248–256. [PubMed] [Google Scholar]
- 32.Noel R F, Jr, Sat T T, Mendez C, Johnson M C, Pohlman T H. Activation of human endothelial cells by viable or heat-killed gram-negative bacteria requires soluble CD14. Infect Immun. 1995;63:4046–4053. doi: 10.1128/iai.63.10.4046-4053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfaller M A, Jones R N, Messer S A, Edmond M B, Wenzel R P. National surveillance of nosocomial blood stream infection due to Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn Microbiol Infect Dis. 1998;31:327–332. doi: 10.1016/s0732-8893(97)00240-x. [DOI] [PubMed] [Google Scholar]
- 34.Pober J S. Activation and injury of endothelial cells by cytokines. Pathol Biol (Paris) 1998;46:159–163. [PubMed] [Google Scholar]
- 35.Rapponi G, Ghezzi M C, Mancini C, Filadoro F. Culture filtrates and whole heat-killed Candida albicans stimulate human monocytes to release interleukin-6. Microbiologica. 1993;16:267–274. [PubMed] [Google Scholar]
- 36.Rotrosen D, Edwards J E, Jr, Gibson T R, Moore J D, Cohen A H, Green I. Adherence of Candida to cultured vascular endothelial cells: mechanisms of attachment and endothelial cell penetration. J Infect Dis. 1985;152:1264–1273. doi: 10.1093/infdis/152.6.1264. [DOI] [PubMed] [Google Scholar]
- 37.Schmid E, Müller T H, Budzinski R-M, Binder K, Pfizenmaier K. Signaling by E-selectin and ICAM-1 induces endothelial tissue factor production via autocrine secretion of platelet-activating factor and tumor necrosis factor α. J Interferon Cytokine Res. 1995;15:819–825. doi: 10.1089/jir.1995.15.819. [DOI] [PubMed] [Google Scholar]
- 38.Span A H M, Mullers W, Miltenburg A M M, Bruggeman C A. Cytomegalovirus induced PMN adherence in relation to an ELAM-1 antigen present on infected endothelial cell monolayers. Immunology. 1991;72:355–360. [PMC free article] [PubMed] [Google Scholar]
- 39.Steinshamn S, Bemelmans M H, van Tits L J, Bergh K, Buurman W A, Waage A. TNF receptors in murine Candida albicans infection: evidence for an important role of TNF receptor p55 in antifungal defense. J Immunol. 1996;157:2155–2159. [PubMed] [Google Scholar]
- 40.Sultana C, Shen Y, Rattan V, Kalra V K. Lipoxygenase metabolites induced expression of adhesion molecules and transendothelial migration of monocyte-like HL-60 cells is linked to protein kinase C activation. J Cell Physiol. 1996;167:477–487. doi: 10.1002/(SICI)1097-4652(199606)167:3<477::AID-JCP12>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 41.Toborek M, Blanc E M, Kaiser S, Mattson M P, Hennig B. Linoleic acid potentiates TNF-mediated oxidative stress, disruption of calcium homeostasis, and apoptosis of cultured vascular endothelial cells. J Lipid Res. 1997;38:2155–2167. [PubMed] [Google Scholar]
- 42.Wey S B, Mori M, Pfaller M A, Woolson R F, Wenzel R P. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch Intern Med. 1988;148:2642–2645. doi: 10.1001/archinte.148.12.2642. [DOI] [PubMed] [Google Scholar]
- 43.Wong H, Anderson W D, Cheng T, Riabowol K T. Monitoring mRNA expression by polymerase chain reaction: the “primer-dropping” method. Anal Biochem. 1994;223:251–258. doi: 10.1006/abio.1994.1581. [DOI] [PubMed] [Google Scholar]
- 44.Yeaman M R, Norman D C, Bayer A S. Staphylococcus aureus susceptibility to thrombin-induced platelet microbicidal protein is independent of platelet adherence and aggregation in vitro. Infect Immun. 1992;60:2368–2374. doi: 10.1128/iai.60.6.2368-2374.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]