Abstract
Mycoplasma arthritidis mitogen (MAM) is a potent superantigen secreted by M. arthritidis, an agent of murine arthritis. Here we compare the abilities of MAM to induce a panel of cytokines in vitro and in vivo in BALB/c and C3H/HeJ mouse strains that differ in susceptibility to mycoplasmal arthritis. Splenocytes from both mouse strains produced high levels of all cytokines by 24 h following in vitro exposure to MAM. No differences in cytokine profiles were seen irrespective of the MAM dose. However, there were striking differences in cytokine profiles present in supernatants of splenocytes that had been collected from mice after intravenous (i.v.) injection of MAM and subsequently rechallenged with MAM in vitro. Splenocytes collected 24 and 72 h after i.v. injection of MAM and challenged in vitro with MAM showed the most marked divergence in the secreted cytokines. Type 1 cytokines were markedly elevated in C3H/HeJ cell supernatants, whereas they were depressed or remained low in BALB/c cell supernatants. In contrast, the levels of type 2 cytokines were all greatly increased in BALB/c cell cultures but were decreased or remained low in C3H/HeJ supernatants. Interleukin-12 mRNA and protein was also markedly elevated in C3H/HeJ mice, as were the levels of immunoglobulin G2a. The data indicate a major skewing in cytokine profiles to a type 1 inflammatory response in C3H/HeJ mice but to a protective type 2 response in BALB/c mice. These cytokine changes appear to be associated with the severe arthritis in C3H/HeJ mice following injection of M. arthritidis in comparison to the mild disease seen in injected BALB/c mice.
Superantigens (SAgs) are a unique class of potent immunoregulatory molecules that are produced by bacteria, viruses, and mycoplasmas (1, 5, 13, 36, 63, 70). They are presented directly to T cells in association with various class II major histocompatibility complex molecules on accessory cell surfaces, usually without the need for processing (8, 22, 27), and are recognized predominantly by T cells bearing specific Vβ chain segments of the T-cell receptor for antigen (TCR) (31, 34, 41). Since recognition is dependent upon fewer restricting elements than are required for traditional antigens, large numbers of naive T cells may be activated. This response contributes to the marked inflammation seen after in vivo administration of SAgs, which has clear implications for disease pathogenesis.
The Mycoplasma arthritidis-derived SAg, M. arthritidis mitogen (MAM), is secreted by an organism (11) that spontaneously or experimentally can induce acute and chronic forms of arthritis in rodents (18). MAM is in many respects a typical SAg (9), although it is phylogenetically unrelated to other known bacterial or viral SAgs (14). However, as for selected other SAgs (48), bridging between B cells and T cells can also lead to B-cell differentiation (20), and MAM can also directly activate macrophages and natural killer (NK) cells (3, 23, 24, 53). MAM also has a number of other unique features that differentiate it from other bacterial SAgs, including its strong preference for H-2Eα or HLA-DRα molecules rather than H-2A or HLA-DQ for presentation to T cells (10). It was recently shown that MAM, unlike other bacterial SAgs, also has contact points with the third complementarity-determining region (CDR3) of the TCR (32). Another major difference is that in proliferation assays MAM is 103- to 104-fold more effective for murine cells than are the staphylococcal SAgs in respect to the doses required to induce maximal lymphocyte proliferation (15).
Cytokine profiles elicited by microorganisms and their products play a key role in disease expression in their natural hosts. The exposure of a normal host to an infectious agent ultimately results in the acquisition of protective immunity or immunopathology. Cytokines represent the principal regulators of the immune system. The preferential activation and expansion of CD4+ T cells producing a restricted set of cytokines allow their subdivision into two major subsets: Th1 and Th2 cells (26, 46, 47). Th1 cells, which secrete interleukin-2 (IL-2), gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), are responsible for phagocyte-dependent protective immunity and tissue injury in many organ-specific autoimmune diseases. Th2 cells, which produce IL-4, IL-5, IL-10, and IL-13, are involved in the development of allergies and in defense against helminthic parasites. IL-6 is one of the earliest factors that trigger the differentiation of naive T cells into effector Th2 cells in vitro (51). Individual CD4+ T cells which may exhibit complex and quite heterogeneous patterns of cytokine production but are not characteristic of either subset have been classified as Th0 cells (35).
Cytokine responses that resemble Th1 or Th2 responses, but are not necessarily made by CD4+ T cells, are referred to as type 1 or type 2 cell-mediated immune responses, respectively. Indeed, this is a more diverse set of effector mechanisms, consisting of a large variety of cell types, including antigen-activated macrophages, IFN-α/β-activated NK cells, cytolytic CD8+ T cells, and neutralizing antibodies (often with Th1 isotype patterns). Both IL-10 and IL-4 are strong inhibitors of IFN-γ synthesis, and conversely, IFN-γ inhibits IL-10 production. This may in part explain why cell-mediated and humoral immune responses are ultimately often observed to be mutually exclusive.
IL-12 is a heterodimeric cytokine, composed of two subunits (p40 and p35), which is produced predominantly by activated monocytes/macrophages, B cells, and other accessory cell types, causes the induction of IFN-γ synthesis as well as augmentation of NK cell cytotoxicity and cytotoxic T-cell proliferation and function. Most importantly, IL-12 induces the development of Th1 cells in vitro and in vivo. IL-12 production is induced by many microbial products, including lipopolysaccharide (LPS) and lipoproteins. IL-12 is therefore a major modulator of inflammation and immune responses and is likely to play a significant role in the pathogenesis of infectious and autoimmune diseases (65).
Evidence has been obtained that lethal toxicity and dermal necrosis induced by live M. arthritidis may be influenced by MAM. Thus, inbred and congenic mice whose splenocytes are strongly activated by MAM are susceptible to these conditions, whereas mice whose lymphocytes are poorly reactive with MAM are resistant. The degree of reactivity to MAM was largely dependent upon expression of a functional H-2E major histocompatibility complex molecule which is preferentially used by MAM for presentation to T cells. However, the association of reactivity to MAM in vitro and susceptibility to arthritis was less clear, since high reactivity to MAM did not necessarily predict high susceptibility to arthritis (16).
Previous work by us had demonstrated that the intravenous (i.v.) injection of MAM into the BALB/c mouse, a strain that was fairly resistant to arthritis (17) but highly responsive to MAM in vitro, resulted in inhibition of lymphocyte proliferation in vivo (19). This anergic response was restricted to T cells bearing MAM-reactive Vβ chain segments but could be transferred to cells from naive mice by a CD4+, CD8− subset of lymphocytes (19). We also showed that MAM reduced the ability of splenocytes to secrete IL-2 in response to rechallenge with MAM but increased the production of IL-4 and IL-6. Work with other bacterial SAgs, which has also largely been conducted using the BALB/c mouse strain, has resulted in similar findings and has led to the belief that SAgs characteristically lead to a state of anergy following administration in vivo to mice and direct the cytokine profile toward a type 2 response (28).
In the present communication, we tested the cytokine profiles in response to MAM in vitro and in vivo in mouse strains that differ in their susceptibility to the arthritogenic effects of M. arthritidis. Whereas the arthritis-resistant BALB/c mouse, whose lymphocytes are highly activated by MAM in vitro, elicits a type 2 cytokine profile in response to rechallenge with MAM in vivo, the C3H/HeJ mouse, which is susceptible to mycoplasmal arthritis (16), produces overexpression of IL-12 and a type 1 inflammatory response to MAM in vivo. Thus, MAM elicits different cytokine profiles in different strains of mice, and these profiles may predict susceptibility to mycoplasma-induced disease.
MATERIALS AND METHODS
Mice.
Female BALB/c (H-2d, H-2 Eα+) and C3H/HeJ (H-2k, H-2 Eα+) mice were obtained from Jackson Laboratory (Bar Harbor, Maine). All mice were maintained under specific-pathogen-free conditions at the Animal Resource Center of the University of Utah Health Science Center and were used at 8 to 12 weeks of age. The Animal Resource Center guarantees strict compliance with regulations established by the Animal Welfare Act.
MAM.
Native MAM (nMAM) was prepared as described previously (4). nMAM was electrophoretically homogeneous, and the leading and trailing edges of the collected peak after alkyl Superose chromatography exhibited identical N-terminal sequences by Edman degradation and showed no signs of other proteins. M. arthritidis does not contain LPS (61), and the nMAM preparations used in this study failed to induce lymphocyte proliferation in splenocytes from LPS-reactive but non-MAM-reactive SJL mice (H-2s). Similarly, nMAM failed to induce any cytokine activity in SJL splenocyte cultures in vitro (15) or in SJL mice (H.-H. Mu, A. D. Sawitzke, and B. C. Cole, unpublished observations), thus confirming the absence of LPS.
Cell culture and induction of cytokines.
Single cell suspensions of lymphoid cells were prepared from pooled spleens of at least three mice. Erythrocytes in the single cell suspensions were lysed by brief treatment with sterile 0.83% (wt/vol) ammonium chloride solution. Subsequently, the cells were washed three times and resuspended at a density of 107 cells/ml in 24-well plates (Corning Class Works, Corning, N.Y.). The culture medium consisted of RPMI 1640 supplemented with 1% Nutridoma-NS (Boehringer Mannheim Biochemicals, Indianapolis, Ind.), antibiotics, 2 mM l-glutamine, and 5 × 10−5 M 2-mercaptoethanol. For in vitro induction of cytokines, duplicate suspensions of splenocytes were activated by the addition of 0.1 to 100 ng of MAM per ml, and supernatants were collected after 24 h of incubation at 37°C in a 5% CO2 humidified incubator. Supernatants were harvested, clarified by centrifugation at 500 × g for 10 min, and stored at 4°C until assayed for specific cytokine content.
For in vivo priming studies, MAM at doses of 0.1 to 100 ng/mouse was injected i.v. into BALB/c or C3H/HeJ mice. Control mice were given an injection of phosphate-buffered saline (PBS) i.v.. After 90 min, 24 h, or 72 h, mice were bled by cardiac puncture, the sera were collected for cytokine assays, and the splenocytes were cultured in vitro in the presence or absence of MAM (0.4 ng/ml).
Determination of cytokine levels by ELISA.
Cytokines in test supernatants were quantitated in duplicate wells by a capture enzyme-linked immunosorbent assay (ELISA), using a modification of the method of Schumacher et al. (57). Briefly, 100-μl portions of cytokine-specific capture monoclonal antibodies (PharMingen, San Diego, Calif.) were added to the wells of 96-well microtest plates at a concentration of 1 to 4 μg/ml in the coating buffer solution (0.1 M NaHCO3, pH 8.2). Following extensive washing and the blocking of reactive sites on the plastic with PBS containing 20% fetal calf serum, test supernatants and twofold serial dilutions of appropriate reference cytokines (100 μl) were added. After an overnight incubation and washing, 100-μl portions of the biotinylated cytokine-specific detecting antibodies, at concentrations of 0.5 to 1 μg/ml, were also added to each well. The ELISA was developed with avidin-conjugated horseradish peroxidase and 2,2′-azino-bis(3-ethylbenzthioline-6-sulfonic acid) (ABTS) substrate (Sigma, St. Louis, Mo.). Optical density reading was performed at 405 nm with a model 3350-UV 96-well microtest plate spectrophotometer (Bio-Rad, Hercules, Calif.). Cytokine concentrations are reported in picograms per milliliter. The sensitivity for detection of these cytokines was 15 to 30 pg/ml.
RT-PCR for the identification of IL-12 p40 mRNA.
RNA was prepared by the method of Chomczynski and Sacchi (6), and reverse transcription-PCR (RT-PCR) was performed as previously described by Mu et al. (49). PCR was carried out with a DNA thermal cycler 480 (Perkin-Elmer Cetus, Emeryville, Calif.). PCR conditions were as follows: denaturation, 94°C for 1 min; annealing, 59°C for 30 s; and elongation, 72°C for 30 s. Sixteen cycles were performed for β-actin, and 28 cycles were performed for IL-12 p40. Gene-specific sequences were derived from GenBank submissions. The oligonucleotides used for these analyses have been published previously (62).
Measurement of IgG2a and IgG1 levels in serum.
Sera were obtained from mice at day 7 after injection of MAM. Immunoglobulin G2a (IgG2a) and IgG1 were determined by ELISA on microtiter plates coated with rat anti-mouse IgG2a and IgG1 (PharMingen) at a concentration of 2 μg/ml. Dilutions of serum were added to the wells, unbound sample was washed off, and the amount of bound murine immunoglobulin was detected by the addition of biotinylated IgG2a- or IgG1-specific antibodies, at concentrations of 0.5 μg/ml, to each well. The ELISA was developed and read as described above for cytokine assays. A simple linear regression analysis of the immunoglobulin titration-generated reference curve was used to extrapolate the amount of specific antibody contained in the test samples. These data are reported in micrograms per milliliter.
Induction and evaluation of arthritis induced by M. arthritidis.
M. arthritidis strain 14124P10 (29) was grown in modified Edward broth medium consisting of pleuropneumonialike organism broth (Difco Laboratories, Detroit, Mich.) supplemented with 15% heat inactivated horse serum, 2.5% yeast extract (Gibco BRL, Grand Island, N.Y.), 0.2% l-arginine HCl, and 500 U of penicillin G (16) per ml, and harvested by centrifugation at 27,000 × g for 30 minutes. After washing in Difco broth alone, the organisms were resuspended in PBS containing 5% sterile sucrose. Suspensions were frozen at −70°C, and an aliquot was thawed and quantified for CFU. Female BALB/c and C3H/HeJ mice, 8 to 10 weeks of age, were injected i.v. in groups of six with 1 × 108 or 2 × 107 cfu of M. arthritidis. Mice were examined for severity of arthritis from 3 to 28 days postinjection. Ankles and wrists were each scored from 0 to 4, and metatarsals and digits or metacarpals and digits also received combined scores of 0 to 4 per extremity. The total maximal score per mouse was 32. The results were expressed as means ± standard errors of the means (SEM).
Statistical analysis.
Concentrations of cytokines and immunoglobulins are shown as means ± SEM. Student's t test was performed using the Statview program (BrainPower, Inc., Calabasas, Calif.). A P value of less than 0.05 was considered significant. Analysis of variance was used to determine the significance of differences between mouse strain susceptibilities to arthritis induced by M. arthritidis.
RESULTS
Influence of mouse strain on in vitro cytokine responses to MAM.
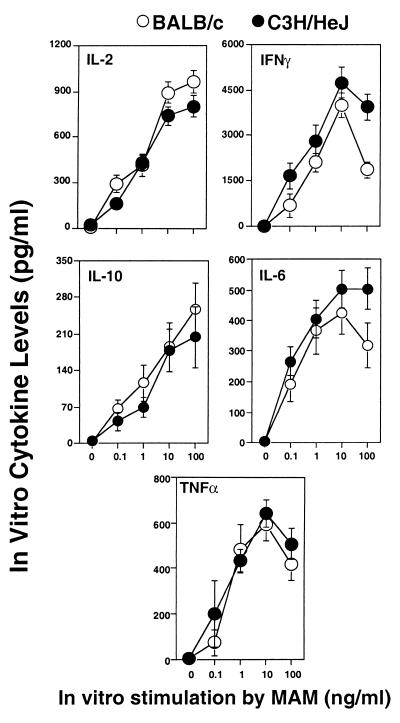
Previous studies had suggested that BALB/c mice were more resistant to arthritis induced by M. arthritidis than were C3H mice. Since both of these strains give a high proliferative response to MAM due to presence of H-2Eα and possess a full complement of MAM-reactive TCRs, dose-response experiments were conducted in vitro to compare cytokine responses to MAM. Normal splenocytes from BALB/c and C3H/HeJ mice were stimulated with MAM (0.1 to 100 ng/ml) for 24 h, and supernatants were assayed for representative type 1 (IL-2, IFN-γ, and TNF-α) and type 2 (IL-6 and IL-10) cytokines. The results given in Fig. 1 show that activated splenocytes from BALB/c and C3H/HeJ mice produce similar levels and profiles of all cytokines following 24 h of in vitro activation, irrespective of dose. Cytokines were detectable with doses of as low as 0.1 ng of MAM/ml and were maximal at 10 to 100 ng/ml. We next conducted experiments to determine whether in vivo differences between these strains in their response to MAM might relate to previous findings on susceptibility to M. arthritidis-induced arthritis.
FIG. 1.
Effect of different doses of MAM on cytokine responses in BALB/c and C3H/HeJ mice in vitro. Splenocytes (107 cells/ml) from BALB/c and C3H/HeJ strain mice were stimulated with or without various concentrations of MAM. Twenty-four hours later, culture supernatants were collected and analyzed by capture ELISA for IL-2, IFN-γ, TNF-α, IL-10, and IL-6. Data were pooled from three experiments; splenocytes from two or three mice were included in each experiment for each specific dose point, and results are expressed as means ± SEM.
Early serum cytokine levels in response to MAM in vivo.
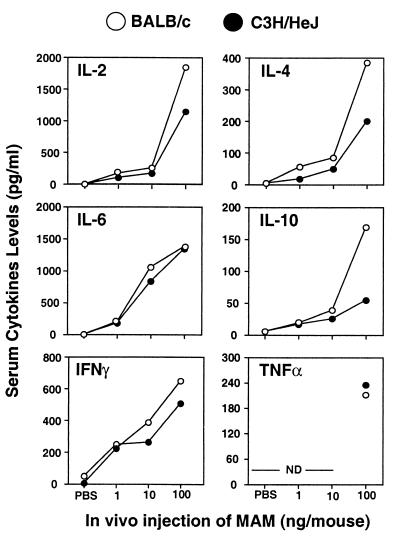
BALB/c and C3H/HeJ mice were injected i.v. with MAM at doses of 1, 10, or 100 ng or with PBS. After 90 min, the mice were exsanguinated and the sera were collected for assays. The cytokines IL-2, IL-4, IL-6, IL-10, IFN-γ, and TNF-α were assayed as described in Materials and Methods.
As expected, mice injected with PBS failed to produce significant levels of serum cytokines (Fig. 2). Furthermore, supernatants from splenocytes that were collected 90 min after injection and incubated for 24 h in the absence of inducer were also free of detectable cytokines (data not shown). However, 90 min after injection of MAM, levels of all cytokines in serum were greatly increased in a dose-dependant fashion (Fig. 2). Cytokines were detectable following injection with as little as 1 ng of MAM/mouse. Although BALB/c mice injected with 100 ng of MAM showed somewhat elevated serum IL-2, IL-4, and IL-10 levels (P < 0.05) in comparison with C3H/HeJ mice, the levels of IL-6 and IFN-γ were similar in the two strains. By 24 h, serum cytokine levels had largely subsided (data not shown).
FIG. 2.
Effect of different doses of MAM on serum cytokine profiles induced by BALB/c and C3H/HeJ mice. Mice were injected i.v. with MAM at doses of 100, 10, and 1 ng or with diluent PBS. After 90 min the mice were exsanguinated and the sera were collected for cytokine assays for IL-2, -4, -6, and -10, IFN-γ, and TNF-α. Sera from 2 or 3 mice were assayed in each experiment for each specific dose point. Similar results were seen in three repeat experiments. ND, not done.
Inducible in vitro cytokines differ in different mouse strains after in vivo exposure to MAM.
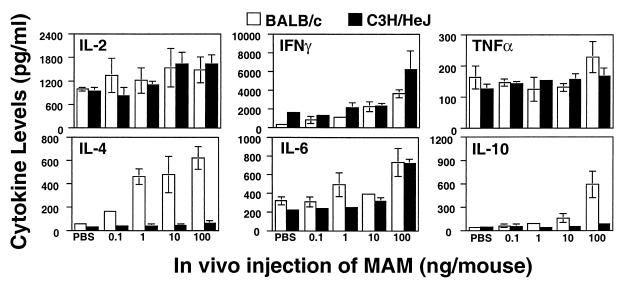
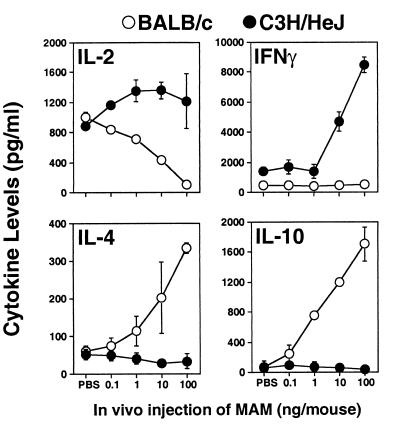
When splenocytes from MAM-injected mice were rechallenged in vitro with a second dose of MAM (0.4 ng/ml), there were striking differences in the inducible cytokine profiles between C3H/HeJ and BALB/c mice (Fig. 3 and 4). These differences appeared to be dependent upon the duration of in vivo exposure to MAM. Splenic cells taken from both C3H/HeJ and BALB/c mice injected with MAM 90 min previously and challenged in vitro showed some increase in IL-2, IFN-γ, and IL-6, but levels of TNF-α were largely unchanged compared with those in mice receiving PBS. In contrast, whereas the levels of IL-4 and IL-10 were very low in C3H/HeJ supernatants, they were markedly elevated in BALB/c supernatants (Fig. 3).
FIG. 3.
Cytokines induced by cells from MAM-injected mice at 90 min. Splenocytes (107 cells/ml) from mice injected with MAM 90 min previously (0.1 to 100 ng of MAM/mouse) were rechallenged in vitro with a second dose of MAM (0.4 ng/ml) for an additional 24 h, and inducible cytokine profiles in C3H/HeJ and BALB/c mice were examined. IL-2, IFN-γ, TNF-α, and IL-4, -6, and -10 were analyzed by ELISA. Data were pooled from three experiments; splenocytes from two or three mice were included in each experiment for each specific dose point, and results are expressed as means ± SEM.
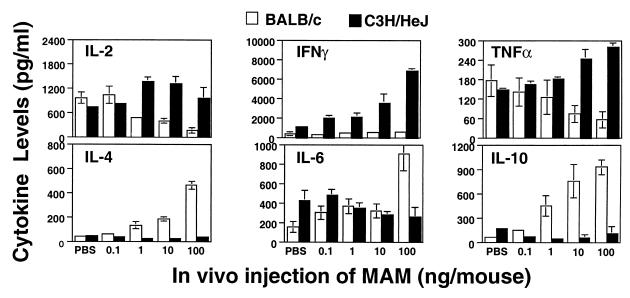
FIG. 4.
Cytokines induced by cells from MAM-injected mice at 24 h. Splenocytes (107 cells/ml) from mice injected with MAM 24 h previously (0.1 to 100 ng of MAM/mouse) were rechallenged in vitro with a second dose of MAM (0.4 ng/ml) for an additional 24 h, and inducible cytokine profiles in C3H/HeJ and BALB/c mice were examined. IL-2, IFN-γ, TNF-α, and IL-4, -6, and -10 were analyzed by ELISA. Data were pooled from three experiments; splenocytes from two or three mice were included in each experiment for each specific dose point, and results are expressed as means ± SEM.
The most striking divergence in the abilities of BALB/c and C3H/HeJ splenocytes to respond to MAM upon in vitro challenge occurred when the splenocytes were harvested after 24 h (Fig. 4) or 72 h (Fig. 5) of exposure to MAM. Thus, with increasing in vivo MAM doses, IL-2, IFN-γ, and TNF-α levels in C3H/HeJ cell supernatants were all elevated, whereas they were markedly depressed or showed no increase in BALB/c supernatants. In contrast, levels of IL-4, IL-6, and IL-10 were all markedly increased in BALB/c cell cultures but were decreased or remained low in C3H/HeJ supernatants (P ≤ 0.05). Doses of as low as 1 ng of MAM/mouse were usually sufficient to induce a profound change in inducible cytokine profiles, but these changes were optimal with the higher doses of MAM. The results indicate that MAM commits the cytokine profile to a type 1-like response in C3H/HeJ mice but to a type 2-like response in BALB/c mice.
FIG. 5.
Cytokines induced by cells from MAM-injected mice at 72 h. Splenocytes (107 cells/ml) from mice injected with MAM 72 h previously (0.1 to 100 ng of MAM/mouse) were rechallenged in vitro with a second dose of MAM (0.4 ng/ml) for an additional 24 h, and inducible cytokine profiles in C3H/HeJ and BALB/c mice were examined. IL-2, IFN-γ, and IL-4 and -10 were analyzed by ELISA. Data were pooled from two experiments; splenocytes from two or three mice were included in each experiment for each specific dose point, and results are expressed as means ± SEM.
Elevation of IL-12 p40 product in serum and gene expression in splenocytes in MAM-injected C3H/HeJ mice.
A large number of reports have documented a key role for IL-12 in the induction of type 1 immune responses (2, 33, 40, 65). In view of the described observations, we tested whether there were differences in IL-12 mRNA expression or serum IL-12 protein in C3H/HeJ versus BALB/c mice after injection of MAM.
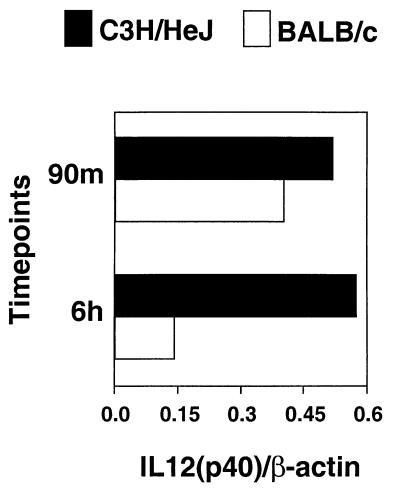
Spleens were obtained from C3H/HeJ and BALB/c mice at 90 min and 6 h after injection of MAM (10 ng/mouse). Spleen fragments were immediately frozen in liquid nitrogen, and mRNA was prepared for RT-PCR analysis. Six hours after injection of 10 ng of MAM/mouse, much less IL-12 p40 mRNA was detectable, as analyzed by RT-PCR, in splenocytes of BALB/c mice than in samples obtained from C3H/HeJ mice receiving same dose of MAM (P < 0.04) (Fig. 6). Additionally, 90 min after injection of MAM (10 ng/mouse), serum IL-12 levels, as determined by ELISA for IL-12 p40, were greatly elevated in both BALB/c and C3H/HeJ mice (P = 0.1). However, 12 h after injection of MAM, serum IL-12 p40 remained high in C3H/HeJ mice injected with 10 ng of MAM/mouse as compared to IL-12 levels in BALB/c mice receiving the same treatment (P < 0.05) (Table 1).
FIG. 6.
In vivo IL-12 expression induced by MAM in BALB/c and C3H/HeJ mice. RT-PCR was performed with mRNA isolated from homogenized spleens obtained from mice 90 min and 6 h after injection with 10 ng of MAM/mouse or with PBS (not shown). Ratios of IL-12 p40 to β-actin densitometric values are included. The PCR data are representative of two experiments.
TABLE 1.
Serum IL-12 p40 induced by MAM in BALB/c and C3H/HeJ micea
| Strain | Level of IL-12 (pg/ml) in serum at b:
|
|
|---|---|---|
| 90 min | 12 h | |
| BALB/c | 588 ± 105 | 185 ± 33 |
| C3H/HeJ | 756 ± 86 | 761 ± 72 |
| P value | 0.093 | 0.021 |
Sera were collected from two or three mice at 90 min and 12 h after injection of MAM (10 ng/mouse) in vivo. IL-12 p40 in sera was detected by ELISA as described in Materials and Methods.
Data for each specific time point were pooled from three experiments and are expressed as means ± SEM.
MAM enhances the IgG2a response in C3H/HeJ mice but not BALB/c mice.
Cytokines play a very important role in immunoglobulin isotype selection in vitro and in vivo (25). In the mouse, IL-4 preferentially augments IgG1 and IgE antibodies, whereas IFN-γ preferentially induces switching to IgG2a and IgG3 antibodies.
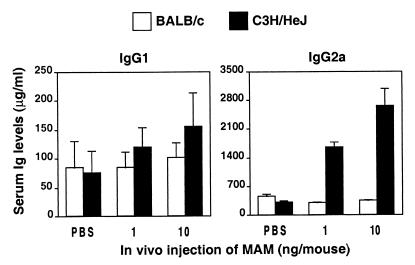
Hence, IgG2a was used as a marker for type 1 responses induced by MAM, while the titers of IgG1 reflect type 2 responses. The expression of IgG isotypes (Fig. 7) in both mouse strains after injection of MAM was assessed. Total IgG1 and IgG2a concentrations were measured over a period of 33 days after injection of MAM. Serum IgG1 levels increased slightly in response to MAM, but there was no significant difference between the two mouse strains. However, the total IgG2a response of mice injected with MAM was significantly higher in C3H/HeJ mice than in BALB/c mice from day 7 (Fig. 7), and this difference was present through day 33 (data not shown), when the experiment was terminated.
FIG. 7.
MAM greatly enhances IgG2a responses in C3H/HeJ mice but not BALB/c mice. Serum samples were collected from mice of both strains 7 days after injection with 1 or 10 ng of MAM/mouse or with PBS. IgG2a and IgG1 isotypes in serum samples from BALB/c mice and C3H/HeJ mice were measured by ELISA. The results shown are pooled data from two separate experiments, each consisting of two or three animals per group. Values are expressed as means ± SEM.
Susceptibility of BALB/c and C3H/HeJ mice to arthritis induced by M. arthritidis.
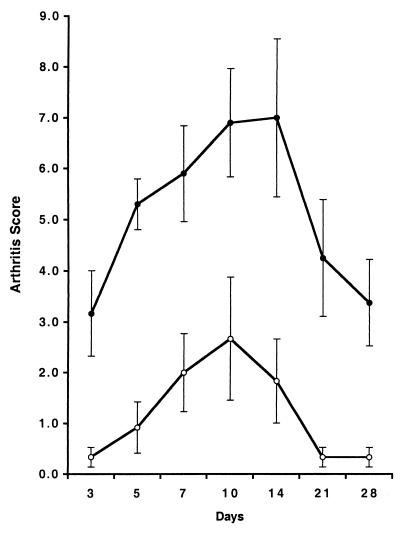
Because of the potential genetic drift of mouse strains tested many years previously for susceptibility to arthritis induced by mycoplasmas, we repeated the earlier observations using BALB/c and C3H/HeJ mice of the same age and source as those that were used for determination of the cytokine responses to MAM. Arthritis scores obtained after an i.v. injection of 108 CFU of M. arthritidis/mouse over a 1-month period are shown in Fig. 8. Disease severity was significantly greater at all time periods in C3H/HeJ mice than in BALB/c mice (P ≤ 0.02), although the incidence of arthritis was 100% in both groups. Disease onset was also more rapid and persisted longer in the C3H/HeJ group. Mice given lower doses of organisms (2 × 107/mouse) exhibited similar differences in disease severity (data not shown), with only 33% of BALB/c mice developing arthritis (mean maximum score of 0.3) and a 100% incidence in the C3H/HeJ group (mean maximum score of 4.5). Thus, C3H/HeJ mice remain significantly more susceptible to arthritis induced by M. arthritidis than BALB/c mice.
FIG. 8.
Susceptibility of BALB/c and C3H/HeJ mice to M. arthritidis. Mice were injected i.v. with 108 CFU of M. arthritidis and scored for arthritis as described in Materials and Methods through 28 days. Mean scores and SEM for BALB/c (○) and C3H/HeJ (●) mice are shown. C3H/HeJ mice were significantly more susceptible at all time periods (P < 0.002).
DISCUSSION
SAgs are known to modify host immune systems and are increasingly thought to play a role in both infectious and autoimmune diseases (38, 56, 64). Three main findings have arisen from the present studies. First, in vitro cytokine profiles induced by the mycoplasma SAg MAM in splenocyte cultures from different strains of mice do not predict their in vivo effects. Second, the in vivo cytokine profile in response to MAM differs in different strains of mice, and the patterns exhibit a distinct time dependency. Third, there is an association between susceptibility to arthritis induced by M. arthritidis and the type of cytokine profile elicited in vivo by its SAg MAM. Thus, the arthritis-susceptible C3H/HeJ mouse produces a type 1 inflammatory cytokine response to MAM, whereas the arthritis-resistant BALB/c mouse produces an arthritis-protective type 2 profile in response to MAM.
We and others (3, 7, 52, 53) have previously shown that MAM induces diverse cell types to produce a wide variety of cytokines in vitro. In the present studies, both type 1 (IL-2, IFN-γ, and TNF-α) and type 2 (IL-4, IL-6, and IL-10) cytokines were induced in 24-h murine splenic cell cultures by using MAM concentrations from 0.1 to 100 ng/ml. Neither the cytokine profiles nor the relative levels of each cytokine produced differed between BALB/c and C3H/HeJ mice. Maximum levels were obtained with 10 to 100 ng of MAM per ml, and 50% of the maximal response occurred at about 1 ng/ml. These results were in striking contrast to the data from subsequent in vivo studies.
Importantly, we observed a pronounced time-dependent polarization of the cytokine profiles in C3H/HeJ versus BALB/c mice in response to the in vivo administration of a single dose of MAM of as low as 1 ng/mouse. The change in profiles with time likely reflects the change from an early innate to an adaptive immune response. Cytokines were rapidly produced and measurable in sera reflecting the early macrophage, NK cell, and B- and T-cell responses. The two mouse strains were not markedly different in this early response, although levels of the type 2 cytokines IL-4 and IL-10 were somewhat higher in the sera from BALB/c mice. Splenocytes removed from BALB/c mice at 90 min and challenged in vitro with MAM were also beginning to show an increase in type 2 cytokines, but type 1 cytokines IL-2, IFN-γ, and TNF-α did not differ from the response seen with cells from C3H/HeJ mice. Following 24 and 72 h of in vivo exposure to MAM, splenocytes from the two mouse strains exhibited totally divergent cytokine profiles following rechallenge with MAM in vitro. BALB/c cells secreted elevated levels of type 2 cytokines and depressed levels of type 1 cytokines, whereas the reverse was true for C3H/HeJ cells. The early finding of elevated IL-12 in the sera and IL-12 mRNA in splenocytes from MAM-injected C3H/HeJ mice suggests an important role for this cytokine in mediating the profile differences observed in the adaptive responses. IL-12 is known to promote clonal expansion of Th1 cells (65), and the decreased level of this cytokine in BALB/c cells may at least contribute to the overall type 2 profile seen in this mouse strain in response to MAM. The persisting increased levels of IgG2a in the sera of C3H/HeJ mice are also consistent with the development of a type 1 cytokine profile in these mice.
A key question is why the cytokine profiles induced by MAM in vivo differ in C3H/HeJ versus BALB/c mice. Different mouse strains are known to elicit diverse cytokine responses to various microbial products. Whereas infection by Listeria monocytogenes results in a type 1 response in susceptible C3H/HeJ mice, a type 2 response is seen in resistant BALB/c mice (33). Leishmania major likewise elicits a type 2 response in BALB/c mice but a type 1 response in C57BL/6 mice (59). Evidence that the BALB/c mouse is not a “type 2 mouse” per se comes from the observation that whole Bordetella pertussis organisms elicit a type 1 response in this strain, while pertussis toxin results in a mixed response (44, 49, 60). In addition, Mycobacterium bovis bacillus Calmette Guérin (BCG) also induces a predominantly type 1 cytokine response in BALB/c mice (37, 66). Although it could be argued that contaminating LPS might be responsible for the differences in response between the LPS-resistant C3H/HeJ and LPS-susceptible BALB/c mice, it should be noted that M. arthritidis does not possess LPS (61) and that all nMAM preparations used here failed to induce a cytokine response in splenocyte cultures from MAM-refractory mouse strains. Differences in genetic background affecting such factors as glucocorticoid hormones (21, 42) can also profoundly affect cytokine profiles. Thus, these hormones are down-regulated in BALB/c mice infected with L. monocytogenes, changing the cytokine profile to a type 1 response, whereas up-regulation of glucocorticoids in the infected C3H/HeJ mouse promotes a type 2 cytokine response (30). For MAM, the pathways leading to differential cytokine expression remain to be identified.
What are the implications of our observations for understanding the role of SAgs in disease, particularly that induced by M. arthritidis? The striking difference in cytokine profiles elicited by MAM in arthritis-resistant versus susceptible mouse strains suggests that the type 1 inflammatory cytokine profile seen with MAM in vivo in the arthritis-susceptible C3H/HeJ mouse may also be influencing M. arthritidis-induced joint disease. Arthritis in this strain was more severe and of longer duration than that in the BALB/c mouse, which produced a type 2 protective cytokine profile in response to injection with MAM. Although the mechanism(s) determining the susceptibility or resistance to M. arthritidis-induced disease in different mouse strains is not fully understood, it is well known that type 1 cytokines such as IL-2, IFN-γ, and TNF-α can be associated with acute inflammation. Pathologic reactions may also result from defective cross-regulation by IL-4, IL-10, or other cytokines that normally inhibit type 1 effector functions (58, 68).
In the present study, doses of MAM (10 to 100ng/mouse) which induce a maximal cytokine response in vivo did not induce a significant toxic effect, and clinical arthritis failed to result from i.v. injection of these doses. The apparent inability of MAM alone to cause arthritis and the production of MAM by avirulent strains of M. arthritidis (14) indicate that other factors also influence disease expression. Our present finding that two highly MAM-responsive mouse strains exhibit different degrees of susceptibility to M. arthritidis supports this view. Rat arthritis induced by M. arthritidis is in part dependent upon the expression of certain adhesins (69), and the virulence of arthritogenic strains is also associated in rats with a newly discovered lysogenic virus (67). Membranes of the organism contain leukocyte-activating components distinct from MAM (B. C. Cole et al., unpublished observations). Recently, potent lipoprotein macrophage-activating components have been identified in Mycoplasma fermentans (50). It is likely, therefore, that an interplay between these components and MAM as well as the host genetic factors determines the ultimate outcome of infection with this organism.
An excessive or uncontrolled type 1 cytokine response is known to occur in a number of autoimmune diseases, such as rheumatoid arthritis, insulin-dependent diabetes mellitus, and multiple sclerosis (39, 45, 54). There is increasing evidence for a role of SAgs in triggering these conditions (56). In fact, studies from our laboratory have clearly established that MAM can trigger and cause flares in collagen-induced arthritis of mice (12), and type 1 cytokines are known to be elevated in this model of arthritis as well (43). Our present finding that MAM also induces a similar type 1 cytokine response in mouse strains that are susceptible to mycoplasma-induced arthritis combined with our recent finding of antibodies to MAM in the sera from rheumatoid arthritis patients (55) further points to the value of the M. arthritidis model for human rheumatoid arthritis.
Although much of the work on the mechanisms of SAg action has been conducted with mice using the staphylococcal SAgs, particularly staphylococcal enterotoxin B (SEB), several findings presented here and elsewhere lead us to believe that the MAM SAg provides certain advantages for these studies. First, MAM is produced by an organism that naturally infects rodents, resulting in a variety of syndromes, including arthritis. Also, MAM is much more potent for murine cells than are the staphylococcal SAgs, and on the basis of the present and previous data (4), amounts of MAM which might be expected to be produced in the host by the organisms have been shown to profoundly affect immune functions in vivo. It is attractive to speculate that changes in the cytokine profiles as seen in the C3H/HeJ and BALB/c mice may play a significant role not only in the disease manifestations of M. arthritidis arthritis but also in other natural diseases for which SAg involvement has been hypothesized. Our current studies are directed toward further defining the interaction of MAM with the murine immune system with a view to eventually understanding how SAgs determine polarization of the adaptive immune response.
ACKNOWLEDGMENTS
We thank William Sewell and Janice Weiss for their critical and useful comments on this manuscript. We thank Tamara Knappenberger and Xiao-Dong Li for their technical assistance.
We acknowledge the support of the DNA synthesis facility by grant CA42014 from the National Cancer Institute. This work was supported by grant AR-02255 from the National Institute of Arthritis and Musculoskeletal Diseases, by grant AI-12103 from the National Institute of Allergy and Infectious Diseases, by a grant from the Nora Eccles Treadwell Foundation, and by a grant from the Vallois Egbert Foundation. B.C.C. is the recipient of the Nora Eccles Harrison Chair in Rheumatology.
REFERENCES
- 1.Acha-Orbea H, Palmer E. Mls—a retrovirus exploits the immune system. Immunol Today. 1991;12:356–361. doi: 10.1016/0167-5699(91)90066-3. [DOI] [PubMed] [Google Scholar]
- 2.Afonso L C, Scharton T M, Vieira L Q, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 3.al-Daccak R, Mehindate K, Hebert J, Rink L, Mecheri S, Mourad W. Mycoplasma arthritidis-derived superantigen induces proinflammatory monokine gene expression in the THP-1 human monocytic cell line. Infect Immun. 1994;62:2409–2416. doi: 10.1128/iai.62.6.2409-2416.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkin C L, Wei S, Cole B C. The Mycoplasma arthritidis superantigen MAM: purification and identification of an active peptide. Infect Immun. 1994;62:5367–5375. doi: 10.1128/iai.62.12.5367-5375.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi Y, Kappler J W, Marrack P. A superantigen encoded in the open reading frame of the 3′ long terminal repeat of mouse mammary tumour virus. Nature (London) 1991;350:203–207. doi: 10.1038/350203a0. [DOI] [PubMed] [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Cole B C, Ahmed E, Araneo B A, Shelby J, Kamerath C, Wei S, McCall S, Atkin C L. Immunomodulation in vivo by the Mycoplasma arthritidis superantigen, MAM. Clin Infect Dis. 1993;17:S163–S169. doi: 10.1093/clinids/17.supplement_1.s163. [DOI] [PubMed] [Google Scholar]
- 8.Cole B C, Araneo B A, Sullivan G J. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. IV. Murine T hybridoma cells exhibit differential accessory cell requirements for activation by M. arthritidis T cell mitogen, concanavalin A, or egg-white lysozyme. J Immunol. 1986;136:3572–3578. [PubMed] [Google Scholar]
- 9.Cole B C, Atkin C L. The Mycoplasma arthritidis T-cell mitogen, MAM: a model superantigen. Immunol Today. 1991;12:271–276. doi: 10.1016/0167-5699(91)90125-D. [DOI] [PubMed] [Google Scholar]
- 10.Cole B C, David C S, Lynch D H, Kartchner D R. The use of transfected fibroblasts and transgenic mice establishes that stimulation of T cells by the Mycoplasma arthritidis mitogen is mediated by Eα. J Immunol. 1990;144:420–424. [PubMed] [Google Scholar]
- 11.Cole B C, Daynes R A, Ward J R. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. I. Transformation is associated with an H-2-linked gene that maps to the I-E/I-C subregion. J Immunol. 1981;127:1931–1936. [PubMed] [Google Scholar]
- 12.Cole B C, Griffiths M M. Triggering and exacerbation of autoimmune arthritis by the Mycoplasma arthritidis superantigen MAM. Arthritis Rheum. 1993;36:994–1002. doi: 10.1002/art.1780360717. [DOI] [PubMed] [Google Scholar]
- 13.Cole B C, Kartchner D R, Wells D J. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. VII. Responsiveness is associated with expression of a product(s) of the Vβ8 gene family present on the T cell receptor α/β for antigen. J Immunol. 1989;142:4131–4137. [PubMed] [Google Scholar]
- 14.Cole B C, Knudtson K L, Oliphant A, Sawitzke A D, Pole A, Manohar M, Benson L S, Ahmed E, Atkin C L. The sequence of the Mycoplasma arthritidis superantigen, MAM: identification of functional domains and comparison with microbial superantigens and plant lectin mitogens. J Exp Med. 1996;183:1105–1110. doi: 10.1084/jem.183.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole B C, Sawitzke A D, Ahmed E A, Atkin C L, David C S. Allelic polymorphisms at the H-2A and HLA-DQ loci influence the response of murine lymphocytes to the Mycoplasma arthritidis superantigen MAM. Infect Immun. 1997;65:4190–4198. doi: 10.1128/iai.65.10.4190-4198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole B C, Thorpe R N, Hassell L A, Ward J R. Toxicity but not arthritogenicity of Mycoplasma arthritidis for mice associates with the haplotype expressed at the major histocompatibility complex. Infect Immun. 1983;41:1010–1015. doi: 10.1128/iai.41.3.1010-1015.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole B C, Ward J R, Golightly-Rowland L. Factors influencing the susceptibility of mice to Mycoplasma arthritidis. Infect Immun. 1973;7:218–225. doi: 10.1128/iai.7.2.218-225.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole B C, Washburn L R, Taylor-Robinson D. Mycoplasma induced arthritis. In: Razin S, Bairle M F, editors. The Mycoplasmas. Vol. 4. New York, N.Y: Academic Press; 1985. pp. 107–160. [Google Scholar]
- 19.Cole B C, Wells D J. Immunosuppressive properties of the Mycoplasma arthritidis T-cell mitogen in vivo: inhibition of proliferative responses to T-cell mitogens. Infect Immun. 1990;58:228–236. doi: 10.1128/iai.58.1.228-236.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crow M K, Zagon G, Chu Z, Ravina B, Tumang J R, Cole B C, Friedman S M. Human B cell differentiation induced by microbial superantigens: unselected peripheral blood lymphocytes secrete polyclonal immunoglobulin in response to Mycoplasma arthritidis mitogen. Autoimmunity. 1992;14:23–32. doi: 10.3109/08916939309077353. [DOI] [PubMed] [Google Scholar]
- 21.Daynes R, Araneo B, Hennebold J, Enioutina E, Mu H. Steroids as essential regulators of the mammalian immune response. J Invest Dermatol. 1995;105:14S–19S. doi: 10.1111/1523-1747.ep12315187. [DOI] [PubMed] [Google Scholar]
- 22.Dellabona P, Peccoud J, Kappler J, Marrack P, Benoist C, Mathis D. Superantigens interact with MHC class II molecules outside of the antigen groove. Cell. 1990;62:1115–1121. doi: 10.1016/0092-8674(90)90388-u. [DOI] [PubMed] [Google Scholar]
- 23.Dietz J N, Cole B C. Direct activation of the J774.1 murine macrophage cell line by Mycoplasma arthritidis. Infect Immun. 1982;37:811–819. doi: 10.1128/iai.37.2.811-819.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'Orazio J A, Cole B C, Stein-Streilein J. Mycoplasma arthritidis mitogen up-regulates human NK cell activity. Infect Immun. 1996;64:441–447. doi: 10.1128/iai.64.2.441-447.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finkelman F D, Holmes J, Katona I M, Urban J F, Jr, Beckmann M P, Park L S, Schooley K A, Coffman R L, Mosmann T R, Paul W E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 26.Fiorentino D F, Bond M W, Mosmann T R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleischer B. Stimulation of human T cells by microbial ‘superantigens’. Immunol Res. 1991;10:349–355. doi: 10.1007/BF02919720. [DOI] [PubMed] [Google Scholar]
- 28.Florquin S, Amraoui A, Goldman M. Persistent production of TH2-type cytokines and polyclonal B cell activation after chronic administration of staphylococcal enterotoxin B in mice. J Autoimmun. 1996;9:609–615. doi: 10.1006/jaut.1996.0080. [DOI] [PubMed] [Google Scholar]
- 29.Golightly-Rowland L, Cole B C, Ward J R, Wiley B B. Effect of animal passage on arthritogenic and biological properties of Mycoplasma arthritidis. Infect Immun. 1970;1:538–545. doi: 10.1128/iai.1.6.538-545.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hennebold J D, Mu H H, Poynter M E, Chen X P, Daynes R A. Active catabolism of glucocorticoids by 11 beta-hydroxysteroid dehydrogenase in vivo is a necessary requirement for natural resistance to infection with Listeria monocytogenes. Int Immunol. 1997;9:105–115. doi: 10.1093/intimm/9.1.105. [DOI] [PubMed] [Google Scholar]
- 31.Herman A, Kappler J W, Marrack P, Pullen A M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- 32.Hodtsev A S, Choi Y, Spanopoulou E, Posnett D. Mycoplasma superantigen is a CDR3-dependent ligand for the T cell antigen receptor. J Exp Med. 1998;187:319–327. doi: 10.1084/jem.187.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 34.Janeway J, C A, Yagi J, Conrad P J, Katz M E, Jones B, Vroegop S, Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol Rev. 1989;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 35.Kelso A. Th1 and Th2 subsets: paradigms lost? Immunol Today. 1995;16:374–379. doi: 10.1016/0167-5699(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 36.Lafon M. Rabies virus superantigen. Res Immunol. 1993;144:209–213. doi: 10.1016/0923-2494(93)80121-e. [DOI] [PubMed] [Google Scholar]
- 37.Lagranderie M R R, Balazuc A-M, Deriaud E, Leclerc C D, Gheorghiu M. Comparison of immune responses of mice immunized with five different Mycobacterium bovis BCG vaccine strains. Infect Immun. 1996;64:1–9. doi: 10.1128/iai.64.1.1-9.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leung D Y M, Huber B T, Schlievert P M, editors. Superantigens: molecular biology, immunology, and relevance to human disease. New York, N.Y: Marcel Decker, Inc; 1997. [Google Scholar]
- 39.Liblau R, Singer S, McDevitt H. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 40.Manetti R, Parronchi P, Cuidizi M, et al. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4 producing cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 42.Mason D, MacPhee I, Antoni F. The role of the neuroendocrine system in determining genetic susceptibility to experimental allergic encephalomyelitis in the rat. Immunology. 1990;70:1. [PMC free article] [PubMed] [Google Scholar]
- 43.Mauri C, Williams R O, Walmsley M, Feldmann M. Relationship between Th1/Th2 cytokine patterns and the arthritogenic response in collagen-induced arthritis. Eur J Immunol. 1996;26:1511–1518. doi: 10.1002/eji.1830260716. [DOI] [PubMed] [Google Scholar]
- 44.Mills K H, Ryan M, Ryan E, Mahon B P. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect Immun. 1998;66:594–602. doi: 10.1128/iai.66.2.594-602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miossec P, van den Berg W. Th1/Th2 cytokine balance in arthritis. Arthritis Rheum. 1997;40:2105–2115. doi: 10.1002/art.1780401203. [DOI] [PubMed] [Google Scholar]
- 46.Mosmann T, Coffman R. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 47.Mosmann T, Coffman R. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 48.Mourad W, Scholl P, Diaz A, Geha R, Chatila T. The staphylococcal toxic shock syndrome toxin 1 triggers B cell proliferation and differentiation via major histocompatibility complex-unrestricted cognate T/B cell interaction. J Exp Med. 1989;170:2011–2022. doi: 10.1084/jem.170.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mu H-H, Sewell W A. Enhancement of interleukin-4 production of pertussis toxin. Infect Immun. 1993;61:2834–2840. doi: 10.1128/iai.61.7.2834-2840.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muhlradt P F, Kiess M, Meyer H, Sussmuth R, Jung G. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J Exp Med. 1997;185:1951–1958. doi: 10.1084/jem.185.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell R A. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–9. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rink L, Nicklas W, Alvarez Ossorio L, Koester M, Kirchner H. Differential induction of tumor necrosis factor alpha in murine and human leukocytes by Mycoplasma arthritidis-derived superantigen. Infect Immun. 1994;62:462–467. doi: 10.1128/iai.62.2.462-467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rink L, Nicklas W, Luhm J, Kruse R, Kirchner H. Induction of a proinflammatory cytokine network by Mycoplasma arthritidis-derived superantigen (MAS) J Interferon Cytokine Res. 1996;16:861–868. doi: 10.1089/jir.1996.16.861. [DOI] [PubMed] [Google Scholar]
- 54.Romagnani S. Th1 and Th2 in human diseases. Clin Immunol Immunopathol. 1996;80:225–235. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- 55.Sawitzke, A. D., K. L. Knudtson, D. E. Joyner, H.-H. Mu, and B. C. Cole.Anti-MAM antibodies in rheumatic disease: evidence for a MAM-like superantigen in rheumatoid arthritis? J. Rheumatol., in press. [PubMed]
- 56.Sawitzke A D, Mu H, Cole B. Superantigens and autoimmune disease: are they involved? Curr Opin Infect Dis. 1999;12:213–219. doi: 10.1097/00001432-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 57.Schumacher J, O'Garra S, van Kimmenade B A, Bond M, Mosmann T, Coffman R. The characterization of four monoclonal antibodies specific for mouse IL-5 and development of mouse and human IL-5 enzyme-linked immunosorbent. J Immunol. 1988;141:1576. [PubMed] [Google Scholar]
- 58.Scott B, Liblau R, Degermann S, Marconi L, Ogata L, Caton A, et al. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity. 1994;1:1–20. doi: 10.1016/1074-7613(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 59.Scott P, Pearce E, Cheever A W, Coffman R L, Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 60.Sewell W A, de Mocrloose P A, Hamilton J A, Schrader J W, Mackay I R, Vadas M A. Potentiation of delayed-type hypersensitivity by pertussigen or cyclophosphamide with release of different lymphokines. Immunology. 1987;61:483–488. [PMC free article] [PubMed] [Google Scholar]
- 61.Smith P. Membrane lipid and polysaccharide structures. In: Maniloff J, editor. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 79–91. [Google Scholar]
- 62.Spencer N F, Daynes R A. IL-12 directly stimulates expression of IL-10 by CD5+ B cells and IL-6 by both CD5+ and CD5− B cells: possible involvement in age-associated cytokine dysregulation. Int Immunol. 1997;9:745–754. doi: 10.1093/intimm/9.5.745. [DOI] [PubMed] [Google Scholar]
- 63.Tomai M A, Aelion J A, Dockter M E, Majumdar G, Spinella D G, Kotb M. T cell receptor V gene usage by human T cells stimulated with superantigen streptococcal M protein. J Exp Med. 1991;174:285–288. doi: 10.1084/jem.174.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torres B A, Johnson H M. Modulation of disease by superantigens. Curr Opin Immunol. 1998;10:465–470. doi: 10.1016/s0952-7915(98)80122-2. [DOI] [PubMed] [Google Scholar]
- 65.Trinchieri G. Interleukin 12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 66.Tsicopoulos A, Hamid Q, Varney V, Ying S, Moqbel R, Durham S R, Kay A B. Preferential messenger RNA expression of Th1-type cells (IFN-gamma+, IL-2+) in classical delayed-type (tuberculin) hypersensitivity reactions in human skin. J Immunol. 1992;148:2058–2061. [PubMed] [Google Scholar]
- 67.Voelker L, Weaver K, Ehle L, Washburn L. Association of lysogenic bacteriophage MAV1 with virulence of Mycoplasma arthritidis. Infect Immun. 1995;63:4016–4023. doi: 10.1128/iai.63.10.4016-4023.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.von Herrath M G, Guerder S, Lewicki H, Flavell R A, Oldstone M B. Coexpression of B7-1 and viral (“self”) transgenes in pancreatic beta cells can break peripheral ignorance and lead to spontaneous autoimmune diabetes. Immunity. 1995;3:727–738. doi: 10.1016/1074-7613(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 69.Washburn L R, Weaver E J. Protection of rats against Mycoplasma arthritidis-induced arthritis by active and passive immunizations with two surface antigens. Clin Diagn Lab Immunol. 1997;4:321–327. doi: 10.1128/cdli.4.3.321-327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White J, Herman A, Pullen A M, Kubo R, Kappler J W, Marrack P. The Vβ-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989;56:27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]