Abstract
Background
Accumulating studies demonstrated that patients with coronavirus disease 2019(COVID-19) could develop a variety of neurological manifestations and long-term neurological sequelae, which may be different from the strains. At the peak of the Omicron variant outbreak in Shanghai, China, no relevant epidemiological data about neurological manifestations associated with this strain was reported.
Objective
To investigate neurological manifestations and related clinical features in patients with mild to moderate COVID-19 patients with Omicron variant.
Methods
A self-designed clinical information registration form was used to gather the neurological manifestations of mild to moderate COVID-19 patients admitted to a designated hospital in Shanghai from April 18, 2022 to June 1, 2022. Demographics, clinical presentations, laboratory findings, treatments and clinical outcomes were compared between patients with and without neurological manifestations.
Results
One hundred sixty-nine(48.1 %) of 351 patients diagnosed with mild to moderate COVID-19 exhibited neurological manifestations, the most common of which were fatigue/weakness(25.1 %) and myalgia(20.7 %), whereas acute cerebrovascular disease(0.9 %), impaired consciousness(0.6 %) and seizure(0.6 %) were rare. Younger age(p = 0.001), female gender(p = 0.026) and without anticoagulant medication(p = 0.042) were associated with increasing proportions of neurological manifestations as revealed by multivariate logistic regressions. Patients with neurological manifestations had lower creatine kinase and myoglobin levels, as well as higher proportion of patchy shadowing on chest scan. Vaccination status, clinical classification of COVID-19 and clinical outcomes were similar between the two groups.
Conclusions
Nearly half of the involved patients have neurological manifestations which were relatively subjective and closely associated with younger age, female gender and without anticoagulation. Patients with neurologic manifestations may be accompanied by increased lung patchy shadowing.
Keywords: COVID-19, Omicron, Neurological manifestations, SARS-CoV-2, Subjective symptoms
Introduction
Since the first case of coronavirus disease 2019(COVID-2019) was identified in December 2019 in Hubei province of China, the respiratory virus has swept all over the world. COVID-2019 is a highly contagious disease with asymptomatic infection during the incubation period, and can be transmitted through respiratory droplets, contact and aerosols. To date, multiple variants of severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) have emerged, including Alpha, Beta, Gamma, Zeta, Delta, Lambda and Omicron. The Omicron variant was first reported by South Africa on November 24, 2021, and quickly spread across many countries, including China. Since early 2022, the fast spread of the SARS-CoV-2 Omicron variant has fueled a surge in newly-diagnosed cases across China, with the majority occurring in Shanghai. According to the Shanghai Municipal Health Commission, as of May 4, 2022, more than 600,000 people have been infected, most of them with the Omicron BA.2 variant [1].
The primary target of SARS-CoV-2 is respiratory system, resulting in the most common clinical symptoms such as cough, dyspnea and fever [2]. Since the initial clinical descriptions of COVID-19, there has been accumulating evidence of the potential neurologic involvement of SARS-CoV-2, which is demonstrated by symptoms like fatigue/weakness, olfactory dysfunction, myalgia, headache and cerebrovascular events [3], [4]. The pathogenesis of neurological manifestation after COVID-19 is elusive. SARS-CoV-2 is known to have neurotropic properties. The SARS-CoV virus's spike protein S1 allows it to connect to the cell membrane by engaging with the host angiotensin-converting enzyme 2(ACE2) receptor. The nervous system has been shown to express ACE2 receptors, making it a possible target for COVID-19. Furthermore, there are various plausible mechanisms such as Blood-Brain Barrier(BBB) damage, direct nerve infection, hypoxia produced by micro-thrombus, and immune-mediated by cytokine storm.
Previous studies have indicated that although Omicron evolves towards being more transmissible, its pathogenicity and clinical severity appear to be weakened compared with previous strains [5], [6]. The frequency of neurological manifestations associated with COVID-19 had quite a large variation around the world during the pandemic. Mao et al. [7] first reported that 36.4 % of hospitalized COVID-19 patients with the Alpha strain in Wuhan, China had neurological manifestations which were related to the severity of infection. At the same time, Yan et al. reported that 30.3 % of non-critically ill patients in Fangcang Shelter Hospital had neurological manifestations [8]. Similar studies, however, were reported during the pandemic all over the world with a frequency variation of 34.7–69.3 % from 2019 to 2021 [9], [10], [11]. It is still debatable whether the variations are related to the different strains or the severity of the infection. Nonetheless, little is known about the neurological manifestations of patients infected with the Omicron variant, particularly in Shanghai, China.
According to China's zero-COVID policy, symptomatic patients should be treated in designated hospitals with more medical resources and attention in order to avoid exacerbation [12]. Since severe/critical cases were usually transferred to Intensive Care Unit (ICU) where it was hard to finish the anamnestic interview. In the present study, we aimed to investigate the incidence and clinical characteristics of neurological manifestations of mild to moderate COVID-19 patients hospitalized in general wards in our designated hospital. In addition, inflammatory and coagulation indicators in blood associated with the occurrence of neurological manifestations were explored.
Materials and methods
Patients
The study was designed as a cross-sectional study carried out in patients affected by COVID-19 during the current Omicron wave in Shanghai. All patients were hospitalized in general wards of designated hospital for COVID-19 in Shanghai Ninth People's Hospital between 18 April 2022 and 01 June 2022. Eligible patients affected by the mild to moderate respiratory form of COVID-19 were defined as positive results for SARS-CoV-2 on real-time reverse-transcriptase-polymerase-chain-reaction(RT-PCR) assay of nasal and pharyngeal swab specimens and had to meet the following inclusion criteria: be over 18-years-old, conscious, cognitively and mentally conserved, and linguistically competent to respond to the anamnestic interview at discharge and one-month follow-up. Patients were consecutive enrolled in our general wards. Drop outs happened when the patients needed to be transferred to ICU.
Data collection
The medical records and extracted data on general characteristics, laboratory tests, chest computed tomography(CT) reports, treatment, prognosis, and RT-PCR dynamic detection of SARS-CoV-2 during hospitalization were recorded. Data regarding treatment and prognosis was updated until 01 June 2022. At the time of admission or during hospitalization, patients with COVID-19 fulfilling the entry criteria for the study underwent an anamnestic interview to investigate the presence of symptoms. The interview consisted of items including personal information, measured anthropometric parameters, vaccination status, and medical history. All symptoms were mainly subjectively expressed by patients and were further classified into nervous system(NS) symptoms, typical symptoms and gastrointestinal symptoms according to the previous publications. To be more specific, fatigue/weakness, headache, dizziness, emotional disorder, impaired consciousness, acute cerebrovascular disease and seizure were defined as central nervous system(CNS) symptoms; myalgia, taste impairment, smell impairment, vision impairment and neuralgia were defined as peripheral nervous system(PNS) symptoms. Symptoms like fatigue/weakness, myalgia and headache varied widely between studies due to their subjective nature and self-reporting. We selected to include them in the neurological manifestations based on the systematic review and meta-analysis [4]. These symptoms were also considered as persistent neurological manifestations in long COVID-19 syndrome according to recent review [13]. All anamnestic interviews were collected by clinicians from the general wards where patients were hospitalized, and reviewed and confirmed by two trained neurologists.
Chest CT scans were assessed in all of the patients. The clinical severity of COVID-19 was classified by reports of chest CT according to the latest version of the guidelines [12]. In addition, we classified and summarized the specific lesions described in the CT report.
Blood samples from most patients on admission were collected. Routine blood biochemistry including total white blood cell(WBC), lymphocyte and monocyte count, percentages of neutrophil, hemoglobin, platelet count, C-reactive protein(CRP), total bilirubin(TBil) and albumin; coagulation function including D-dimer, prothrombin time(PT), fibrinogen and activated partial thromboplastin time(APTT); myocardial enzymes including Creatine kinase(CK), lactate dehydrogenase and myohemoglobin.
Test reports and treatments were available in the medical history system. After patients' discharge, complications and prognosis(duration of positive to negative, treatment to negative and hospitalization) of each patient were summarized according to the discharge diagnosis along with the medical history and laboratory tests. The discharge criteria for patients were that the two consecutive RT-PCR test results, which were performed with an interval of at least 24 h, in both N gene and ORF gene of SARS-CoV-2 were over 35 Cycle threshold(Ct).
One month after discharge, all patients received a phone call from the clinical researchers to ask questions pertaining to clinical symptoms, existing comorbidities, and issues related to mobility, self-care, and the ability to perform everyday activities.
Statistical analysis
Statistical Package for the Social Sciences for Windows(SPSS version 26.0; IBM Corp, Armonk, NY, USA) was used to perform the statistical analyses. Categorical data was expressed as absolute frequencies and percentages where appropriate. Continuous variables were expressed as the mean±SD or medians(interquartile ranges [IQR], Q1-Q3). Categorical data was compared using Pearson's chi-square test or Fisher's exact test. Mann-Whitney U test and logistic regression were used to compare the differences for continuous variables between groups. Multivariate analysis was carried out using binary logistic regressions(significant variables from univariate analyses and confounding factors were entered into the logistic regression analysis model). Odds ratios and 95 % confidence intervals were estimated. A p-value (two-sided) less than 0.05 was considered significant.
Results
Demographic and clinical characteristics
Patients were consecutively enrolled in our general wards. Nine cases were classified as severe/critical COVID-19, and they were transferred to ICU and dropped out in the present study. One hundred and sixty-nine(48.1 %) out of 351 patients with a confirmed SARS-CoV2 infection had neurological manifestations. The demographic and clinical characteristics were shown in Table 1. Demographically, COVID-19 patients with neurological manifestations were younger(67.4 vs 73.7, p<0.001). Female(60.9 % vs 48.9 %, p = 0.025) made up the majority of patients with neurological manifestations. Although the comorbidities(76.9 % vs 88.5 %, p = 0.005), vaccination status(46.7 % vs 34.1 %, p = 0.017), treatment of anticoagulation(11.2 % vs 22.2 %, p = 0.007) and nutritional support(24.9 % vs 35.2 %, p = 0.037) had significant difference in univariate analysis, there were only three independent relevant factors for neurological manifestations: younger age(p = 0.001), female gender(p = 0.026), and without anticoagulant medication(p = 0.042) after multivariate regression. There was no statistically significant difference in the severity of COVID-19 and clinical outcomes between the two groups(Table 1).
Table 1.
Demographic and clinical characteristics in mild to moderate patients with SARS-CoV-2 Omicron based on neurological manifestations.
|
n (%) or mean (SD) or median[IQR] |
p- value | Multivariate regressiona |
||||
|---|---|---|---|---|---|---|
| Total |
With neurological manifestations |
Without neurological manifestations |
B (95 %CI) | p-value | ||
| (n=351) | (n=169) | (n=182) | ||||
| Characteristics | ||||||
| Age, y | 70.6(15.1) | 67.4(15.7) | 73.7(14.0) | <0.001*** | -0.026(0.959, 0.989) | 0.001** |
| Gender | ||||||
| Female | 192(54.7) | 103(60.9) | 89(48.9) | 0.025* | 0.498(1.060, 2.555) | 0.026* |
| Male | 159(45.3) | 66(39.1) | 93(51.1) | |||
| BMI | 23.1(3.4) | 22.9(3.4) | 23.2(3.4) | 0.673 | ||
| BMI>24 | 126(36.8) | 59(34.9) | 67(38.7) | 0.587 | ||
| Comorbidities | ||||||
| Any | 291(84.6) | 130(76.9) | 161(88.5) | 0.005** | -0.420(0.320, 1.350) | 0.253 |
| Hypertension | 186(53.0) | 91(53.8) | 95(52.2) | 0.831 | ||
| Diabetes | 67(19.1) | 25(14.8) | 42(23.1) | 0.057 | -0.286(0.405, 1.396) | 0.366 |
| Cardiac disease | 109(31.1) | 53(31.4) | 56(30.8) | 0.909 | ||
| Cerebrovascular disease | 73(20.8) | 36(21.3) | 37(20.3) | 0.895 | ||
| Chronic lung disease | 51(14.5) | 30(17.8) | 21(11.5) | 0.129 | ||
| Comorbidities ≥ 2 | 201(57.3) | 88(52.1) | 113(62.1) | 0.067 | 0.168(0.677, 2.068) | 0.554 |
| Vaccinated | 141(40.2) | 79(46.7) | 62(34.1) | 0.017* | 0.277(0.721, 2.413) | 0.369 |
| Booster injection | 77(22.1) | 46(27.4) | 31(17.1) | 0.028* | -0.180(0.414, 1.685) | 0.615 |
| Degree of severity | ||||||
| Mild | 286(81.5) | 132(78.1) | 154(84.6) | 0.131 | ||
| Moderate | 65(18.5) | 37(21.9) | 28(15.4) | |||
| Treatment | ||||||
| Traditional Chinese Medicine | 328(93.4) | 161(95.3) | 167(91.8) | 0.202 | ||
| Antibiotics | 78(22.3) | 38(22.5) | 40(22.1) | 1.000 | ||
| Anticoagulation | 59(16.9) | 19(11.2) | 40(22.2) | 0.007** | 0.634(0.288, 0.978) | 0.042* |
| Glucocorticoid | 27(7.7) | 9(5.3) | 18(10.0) | 0.113 | ||
| Thymosin | 73(20.9) | 32(18.9) | 41(22.7) | 0.431 | ||
| Paxlovid | 221(63.0) | 100(59.2) | 121(66.5) | 0.184 | ||
| Nutritional support | 106(30.2) | 42(24.9) | 64(35.2) | 0.037* | -0.141(0.514, 1.465) | 0.597 |
| Clinical outcomes | ||||||
| Onset of positive to negative, d | 9(7, 12,3) | 9(7, 13) | 9(7, 12) | 0.381 | ||
| Onset of treatment to negative, d | 6(2, 8) | 6(2, 8) | 6(3, 8) | 0.364 | ||
| Length of hospitalization, d | 8(5, 11) | 8.5(5, 11) | 8(5, 11) | 0.752 | ||
| Follow-up | ||||||
| Residual abnormalities | 25(7.1) | 16(9.5) | 9(4.9) | 0.145 | ||
Abbreviation: BMI, body mass index; y, year; d, day.
Note:*, p < 0.05; **, p < 0.01; ***, p < 0.001; a, Significant variables(p < 0.1) from univariate analyses(comorbidities, diabetes, vaccinated, booster injection, anticoagulation and nutritional support) were entered into the logistic regression analysis model.
Clinical symptoms
In comparison to the typical symptoms(301, 85.8 %), such as fever(157, 44.9 %), cough(253, 72.1 %), expectoration(196, 55.8 %), sore throat(132, 37.6 %), nasal obstruction(58, 16.5 %), and runny nose(107, 30.5 %), NS symptoms were the second most commonly symptoms(169, 48.1 %). Fatigue/weakness(88, 25.1 %) was the most common NS symptom. Other CNS(135, 38.5 %) symptoms included headache(48, 13.7 %), dizziness(47, 13.4 %), emotional disorder(17, 4.9 %), acute cerebrovascular disease(3, 0.9 %), impaired consciousness(2, 0.6 %) and seizure(2, 0.6 %). 105 patients(29.9 %) suffered PNS symptoms such as myalgia(73, 20.7 %), taste impairment(20, 5.7 %), smell impairment(22, 6.3 %), vision impairment(19, 5.4 %) and neuralgia(1, 0.4 %). Of these 315 patients, 100 patients(28.5 %) were found to present with gastrointestinal symptoms, including diarrhea(44, 12.5 %), abdominal pain(13, 3.7 %), nausea(22, 6.3 %), vomiting(14, 4.0 %), and poor appetite(66, 18.8 %). Clinical symptoms are shown in Table 2.
Table 2.
Symptoms of Patients with SARS-CoV-2 Omicron(n = 351).
| Symptoms | n (%) |
|---|---|
| Typical symptoms | 301(85.8) |
| Fever | 157(44.9) |
| Cough | 253(72.1) |
| Expectoration | 196(55.8) |
| Sore throat | 132(37.6) |
| Nasal obstruction | 58(16.5) |
| Runny nose | 107(30.5) |
| Gastrointestinal symptoms | 100(28.5) |
| Diarrhea | 44(12.5) |
| Abdominal pain | 13(3.7) |
| Nausea | 22(6.3) |
| Vomiting | 14(4.0) |
| Poor appetite | 66(18.8) |
| NS symptoms | |
| Any | 169(48.1) |
| CNS | 135(38.5) |
| Headache | 48(13.7) |
| Dizziness | 47(13.4) |
| Impaired consciousness | 2(0.6) |
| Acute cerebrovascular disease | 3(0.9) |
| Emotional Disorder | 17(4.9) |
| Seizure | 2(0.6) |
| Fatigue/weakness | 88(25.1) |
| PNS | 105(29.9) |
| Taste impairment | 20(5.7) |
| Smell impairment | 22(6.3) |
| Vision impairment | 19(5.4) |
| Neuralgia | 1(0.4) |
| Myalgia | 73(20.7) |
Abbreviation: NS, nervous system; CNS, central nervous system; PNS, peripheral nervous system.
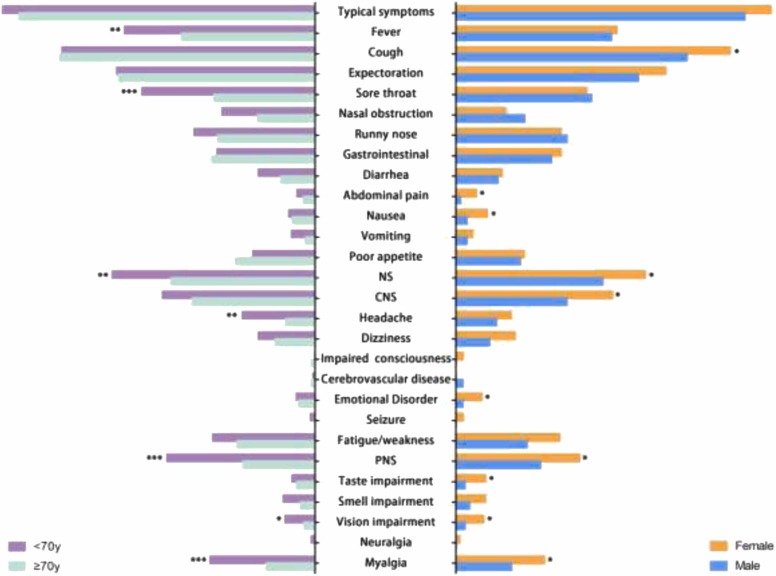
Subgroup analysis revealed that clinical symptoms were closely associated with age and gender( Fig. 1). In particular, PNS symptoms including myalgia and visual impairment were more prevalent in the female group and under the age of 70. Besides that, headache was more common in younger patients, whereas emotional disorders and taste impairment were more common in female patients. For other symptoms, younger patients were more likely to experience fever and sore throat, while female patients were more prone to experience cough, abdominal pain and nausea. Meanwhile, age and gender could also impact the clinical outcomes showed in Fig. S1. Despite having more symptoms, older patients still required longer hospital stays. Additional symptoms may have an impact on female patients' hospitalization.
Fig. 1.
Symptoms of mild to moderate Patients with SARS-CoV-2 Omicron stratified by sex and age. Abbreviation: NS, neurological symptoms; CNS, central nervous symptoms; PNS, peripheral nervous symptoms Note: The age categories were divided based on the average age of enrollment.; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Laboratory findings
When performing univariate regression, blood tests showed that participants with neurological manifestations had higher albumin(40 vs. 39, p = 0.008) and somewhat lower CRP(3.86 vs. 4.76, p = 0.013), CK(81.5 vs. 95.5, p = 0.005) and myohemoglobin (24.3 vs. 36.35, p < 0.001). However, after multivariate analysis eliminated confounding variables, only CK(p = 0.039) and myohemoglobin(p = 0.006) appeared to be decreased in the neurological manifestations group( Table 3). The findings appeared to be at odds with PNS symptoms like myalgia related to muscular damage.
Table 3.
Laboratory and chest findings on admission of the mild to moderate Patients with SARS-CoV-2 Omicron based on neurological manifestations.
| Items | With neurological manifestations |
Without neurological manifestations |
Univariate regression |
Multivariate regressiona |
||
|---|---|---|---|---|---|---|
| ( n = 169) | ( n = 182) | B (95 %CI) | p-value | B (95 %CI) | p-value | |
| Blood test, median(25th, 75th) | ||||||
| WBC count, *10^9/L | 5.20(3.83, 6.30) | 5.10(4.00, 6.20) | -0.004(0.887, 1.118) | 0.947 | -0.006(0.880, 1.124) | 0.929 |
| Monocyte cell count, *10^9/L | 0.52(0.39, 0.68) | 0.49(0.41, 0.68) | -0.196(0.391, 1.729) | 0.605 | -0.022(0.452, 2.119) | 0.956 |
| Lymphocyte count, *10^9/L | 1.35(1.00, 1.80) | 1.30(1.00, 2.00) | 0.274(0.904, 1.913) | 0.152 | 0.056(0.708, 1.580) | 0.786 |
| Neutrophil, % | 58.25(49.93, 66.58) | 59.40(41.86, 68.40) | -0.005(0.979, 1.011) | 0.534 | 0.000(0.983, 1.017) | 0.982 |
| Hemoglobin, g/L | 131.00(123.25, 142.00) | 128.00(116.00, 141.00) | 0.012(0.999, 1.024) | 0.062 | 0.012(0.997, 1.026) | 0.114 |
| Platelet count, *10^9/L | 189.00(154.00, 231.50) | 174.00(138.00, 230.00) | 0.003(1.000, 1.006) | 0.081 | 0.001(0.998, 1.004) | 0.552 |
| C-reactive protein, mg/L | 3.86(1.69, 7.49) | 4.76(1.61, 15.46) | -0.024(0.958, 0.995) | 0.013* | -0.016(0.996, 1.002) | 0.083 |
| TBil, mmol/L | 10.40(8.25, 13.85) | 10.00(8.00, 12.90) | 0.013(0.997, 1.052) | 0.476 | 0.028(0.988, 1.070) | 0.174 |
| Albumin, g/L | 40.00(38.00, 43.00) | 39.00(37.00, 43.00) | 0.071(1.019,1.131) | 0.008** | 0.032(0.974, 1.094) | 0.286 |
| D-dimer, mg/L | 0.36(0.19, 0.73)) | 0.46(0.25, 0.90) | -0.202(0.639, 1.044) | 0.106 | -0.053(0.729, 1.238) | 0.699 |
| PT, s | 10.80(10.40, 11.30) | 11.00(10.50, 11.40) | -0.023(0.920, 1.038) | 0.449 | -0.014(0.941, 1.033) | 0.558 |
| Fibrinogen, g/L | 2.92(2.53, 3.52) | 3.10(2.62, 3.68) | -0.021(0.922, 1.040) | 0.492 | 0.022(0.985, 1.092) | 0.506 |
| APTT, s | 28.50(26.70, 30.20) | 28.60(26.50, 30.80) | -0.021(0.922, 1.040) | 0.492 | 0.022(0.958, 1.092) | 0.506 |
| Creatine kinase, U/L | 81.50(57.25, 108.75) | 95.50(64.00, 139.00) | -0.004(0.994, 0.999) | 0.005** | -0.003(0.995, 1.000) | 0.039* |
| Lactate dehydrogenase, U/L | 203.00(175.5, 229.75) | 210.50(188.75, 238.00) | -0.006(0.988, 1.000) | 0.058 | -0.005(0.989, 1.002) | 0.146 |
| Myohemoglobin, μg/L | 24.30(16.55, 35.70) | 36.35(24.10, 54.08) | -0.023(0.967, 0.988) | <0.001*** | -0.016(0.974, 0.996) | 0.006** |
| Chest CT findings,n(%) | ||||||
| Bilateral lung involvement | 69(40.8) | 72(39.6) | 0.053(0.688, 1.616) | 0.809 | 0.272(0.827, 2.083) | 0.249 |
| Patchy shadowing | 84(49.7) | 79(43.4) | 0.253(0.846, 1.962) | 0.238 | 0.497(1.048, 2.577) | 0.030* |
| Effusion shadowing | 3(1.8) | 17(9.3) | -1.741(0.050, 0.610) | 0.006** | -1.268(0.077, 1.025) | 0.054 |
| Ground-glass opacity | 10(5.9) | 12(6.6) | -0.115(0.375, 2.120) | 0.794 | -0.270(0.310, 1.879) | 0.557 |
| Interstitial abnormalities | 13(7.7) | 18(9.9) | -0.275(0.360, 1.601) | 0.469 | -0.025(0.450, 2.113) | 0.949 |
| Consolidation | 3(1.8) | 8(4.4) | -0.934(0.103, 1.507) | 0.173 | -0.540(0.142, 2.390) | 0.453 |
Abbreviation: WBC, White blood cell; TBil, total bilirubin; PT, prothrombin time; APTT, activated partial thromboplastin time.
Note: CI, confidence interval; *, p < 0.05; **, p < 0.01; ***, p < 0.001; a, confounding factors(age, gender and anticoagulation) from Table 1 were entered into the logistic regression analysis model.
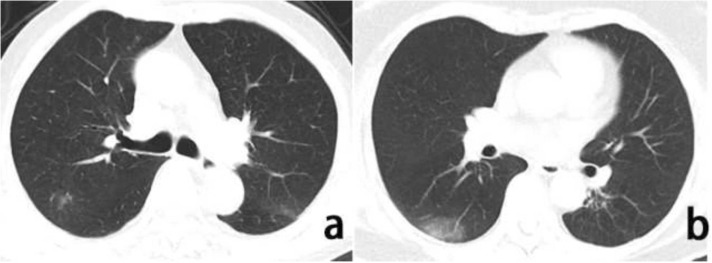
In a univariate analysis of the chest CT results, the proportion of effusion shadowing(1.8 % vs. 9.3 %, p = 0.006) was increased in neurological manifestations group. Patchy shadowing ( Fig. 2a) on chest CT was still an independent associated factor of patients with neurological manifestations as revealed in multivariate analysis (p = 0.030) (Table 3). The typical CT sign, ground-glass opacity (Fig. 2b) showed no difference between the two groups.
Fig. 2.
Chest CT images of a COVID-19 patient with neurological manifestations. Note: Axis chest CT scan showed atypical patchy shadowing(a) and patchy ground-glass opacity consistent with typical moderate COVID-19(b).
Discussion
In the research of COVID-19, neurological manifestations have always been a hot topic. According to our knowledge, this is the first study to be conducted in China on the epidemiology of neurological symptoms following Omicron variant infection. 351 patients with the mild to moderate COVID-19 Omicron variant enrolled in a designated hospital revealed that: (1) nearly half of COVID-19 patients infected with the Omicron strain had neurological manifestations; (2) younger age, female gender and anticoagulants medication had an impact on the presence of neurological manifestations; (3) mismatched blood test results with the symptoms indicated most neurological symptoms were highly subjective and unspecific; and (4) patients with neurologic manifestations may be accompanied by increased lung patchy shadowing.
Our study showed 48.1 % patient with neurological manifestations, which is higher than the study in Wuhan with the frequency of 30.3 %−36.5 % [7], [8] back in 2019. In the USA and Europe, similar series with an overall frequency of 34.7 %−69.3 % [9], [10], [11]. These contrasting results could be related to the definition of neurological manifestations, the different severity of infection and the variants of SARS-CoV-2. Definitions of neurological symptoms have varied considerably during the pandemic. The early studies of COVID-19 always focus on severe neurological impairments, such as cerebrovascular events, impaired consciousness and epilepsy, as neurological manifestations because patients had more severe symptoms during the first wave of the pandemic. However, as the virus mutates, concern about non-specific neurological symptoms like fatigue/weakness, myalgia, headaches, and dizziness have been increasing in recent studies of COVID-19 related neurological manifestations [4]. Based on all these studies, our study was designed as a cross-sectional study. To fully collect all relevant symptoms and detect neurologic manifestations that may otherwise be missed throughout the entire hospitalization period, an anamnestic interview was prospectively designed as a Yes-or-No question, which could be a reason that our data showed an outstanding increase of non-specific neurological symptoms. Non-specific neurological symptoms like fatigue/weakness and myalgia are common symptoms of any viral or bacterial infection that make researchers consider them as systemic symptoms at the beginning of the pandemic. But recently studies show that they are also common COVID-19 sequelas even after 6-months follow-up which challenge the theory that they are caused by acute viral infection and inflammation [13], indicating they may be the results of the virus's effect on the muscles and mental health. Some studies showed that COVID-19-associated inflammation might lead to neurotransmitter impairment, possibly representing the basis of fatigue and explaining mental disorder [14]. But the pathology of long COVID syndrome is still unclear. And the timing of risk for acute, subacute, and long-term neurologic manifestations remains unknown, implying that all clinical data collected at different time points should be consistent in order to obtain comparable data. Meanwhile, the severe neurological impairments such as impaired consciousness and acute cerebrovascular disease were decreased compared with the data back in Wuhan, which was consistent with the reduced rate of severe patients and pathogenicity of the virus strain [15], [16]. According to the research published on Nature [17], Omicron replication is ACE2 dependent and the binding of the spike of Omicron spike to ACE2 is enhanced compared with that of the WT virus which indicate Omicron is shown to have little effect on receptor binding affinity, but the efficiency of entry into host cells is reduced in cells expressing the TMPRSS2 protease. This mutation increased the ability of Omicron to enter the body, leading to increased infectivity and transmission and a new wave of infection around the world [18], [19]. ACE2 is widely distributed in the nervous system [20], and Omicron's reduced dependence on TMPRSS2 makes virus more easily to entry into the nervous system. Thus, compared to studies in the early stage of the pandemic, the proportion of neurological manifestations reported is getting higher.
Our study suggests that both age and gender may have an impact on the development of neurological manifestations in mild to moderate patients. Study from Liotta EM et al. [21]. suggested that younger patients (mean age of 57.9), without or had a long time from COVID-19 onset to hospitalization, were more likely to present any neurologic manifestations than older people (mean age 62.9 years). While in most studies, encephalopathy such as altered mental status and cerebrovascular events, the most frequent CNS manifestation in COVID-19, had been manifested more likely in older patients [22], [23]. However, the majority of these studies included severe patients, indicating that neurological symptoms were more likely to occur in such people. Previous research had shown that neurological symptoms [24], [25], particularly the PNS, were inversely correlated with age in mild to moderate patients, which was what we focused on. With regard to the role of gender on resistance and disease severity, there may be some gender differences in the neuropathological events caused by COVID-19, even though multiple publications found that women are at lower risk of COVID-19 severity than males in various cohorts [26], [27]. In COVID-19-positive females compared to males, chemosensory dysfunctions and subjective neurological symptoms are more prevalent [28], [29]. Although these studies have shown that gender does affect the infection of SARS-CoV-2, there are still no consensual studies that can explain this difference. Some [30], [31] believe that women acquire stronger immune responses than men, which results in faster pathogen clearance but also contributes to their increased susceptibility to strong symptoms.
The only treatment in our trial that appeared to be related to the onset of neurological symptoms was anticoagulant medication. Patients with COVID-19 frequently have a pro-coagulative condition as a result of complement cascade hyper-activation, cytokine storm, and endothelial dysfunction brought on by the virus [32]. Diffuse microvascular thrombi are frequently seen in several organs, which appear to be directly connected to the severity of the condition [33]. After review, the majority of the patients in our study who used anticoagulants were high-risk patients, and the sample size was small. Once the confounding element of age, which was most likely to change the results, was eliminated, anticoagulation still had impact on the development of neurological symptoms. Theoretical support is still lacking in mild cases, though.
In our investigation, the most of the blood tests results were within normal ranges. Inflammatory and coagulation indicators did not differ between groups. However, previous researches [3], [7], [8], [9], [10], [11] have revealed neurological manifestations that can be related to both indicators. Surprisingly, the indicators that could directly represent muscular injury also showed a negative connection between groups, indicating that symptoms like myalgia were highly subjective and had little bearing on the outcome of the disease. According to Liguori’s research [34], COVID-19 infection could cause subjective neurological symptoms, and women were more likely to experience these symptoms than men. This outcome seems to be consistent with what we discovered. But in fact, although the neurological symptoms in this study were classified as subjective symptoms, no further evidence indicated whether their occurrence was related to an objective infection or injury in nervous system. As shown in a discovery published in Nature [35], SARS-CoV-2 is linked to alterations in brain structure even in cases of mild to moderate infection. The SARS-CoV-2 can cross the Blood-Brain Barrier(BBB) and cause pathological changes including hypoxia, ischemia, micro-bleeds, and inflammation [36], [37]. These changes in the structure of the nervous system are insidious and persistent, but can result in a variety of non-specific neurological manifestations. These changes in brain could lead to the persistent neurological manifestations in long COVID syndrome as well, for the most common symptoms in COVID-19 sequelae were also highly subjective like fatigue and brain fog [13]. Further research is still needed to determine whether neurological manifestations in the early stages of infection can signal these modifications.
Pulmonary infection is an important marker of the severity of COVID-19 infection. Ground-glass opacities are characteristic CT signs of the lungs. In our study, there was no statistical difference between the two groups in terms of disease severity and the presentation of typical pulmonary CT signs. Atypical patchy shadows, however, were more common in patients with neurological manifestations. After searching the literatures, this was found for the first time in COVID-19 patients that lacks generalizability and theoretical justification. Whereas it is concerning that the Post-COVID-19 Syndrome is generally accompanied by atypical pulmonary symptoms like cough and non-specific neurological manifestations like fatigue [38], [39], [40]. Song et al. reviewed studies showed that cough could often be accompanied by chronic fatigue, cognitive impairment, or pain collection of long-term effects referred to as the post-COVID syndrome or long COVID, which persisted for weeks or months after SARS-CoV-2 infection. They suggested that neurotropism, neuroinflammation, and neuroimmunomodulation were all involved in pulmonary symptoms [41]. But there are still gaps in understanding of the mechanisms. More epidemiological and fundamental science research is required to explain the connection.
Our research represents the first cross-sectional survey of COVID-19-related neurological manifestations during the Omicron wave in Shanghai, China. In contrast to earlier studies, ours utilized a prospective design to fully collect all relevant data throughout the entire hospitalization period as well as the short-term follow-up of mild to moderate patients. The neurologists performed a face-to-face assessment of neurological manifestations, which ensured the reliability and professionalism of diagnosis. Nevertheless, we have a few limitations. This study is only a single-center study with a limited sample size. It was challenging to consistently assess the psychological status of the patients in order to identify subjective symptoms in such situations due to the personal protective equipment (PPE). No adequate neuropsychiatric scales were used in our study to formally categorize the non-specific neurological symptoms. Non-specific symptoms such as fatigue could not necessarily be CNS mediated.and it was hard to distinguish between mental/cognitive and physical fatigue in the acute phase of infection that could also make the results rather subjective. Furthermore, patients could not receive additional auxillary examinations like magnetic resonance imaging (MRI) and electroencephalography(EEG) since they were at the peak of the pandemic.
Conclusions
The epidemiological information and clinical traits of neurological manifestations connected to Omicron are expanded by our study based on the Chinese population. Nearly half of COVID-19 patients infected with the Omicron strain have neurological manifestations, mostly in young women. The clinical symptoms and the results from blood test do not correspond, indicating that the majority of neurological symptoms are highly subjective. Subjects with neurological manifestations may be accompanied by increased lung patchy shadowing. More mechanism research and long-term follow-up of neurological manifestations in COVID-19 are warranted in the future.
Funding statement
This research was supported by grants from 200 talent project from Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (No. 20161422 to J-RL) , Natural Science Foundation Project from the Shanghai Municipal Science and Technology Commission (No. 22ZR1436900 to J-RL), Clinical Research Program of Ninth People’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine (JYLJ202003 to WC) and Project of Biobank from Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (YBKB202120 to WC).
Ethics statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China (2022-T130-2).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
The authors would like to thank all the participants for their kind understanding and cooperation which made this study possible.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jiph.2022.12.005.
Appendix A. Supplementary material
Supplementary material.
.
References
- 1.Zhang X., Zhang W., Chen S. Shanghai's life-saving efforts against the current Omicron wave of the COVID-19 pandemic. Lancet. 2022;399(10340):2011–2012. doi: 10.1016/S0140-6736(22)00838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang H., Zhou P., Wei Y., Yue H, Wang Y, Hu M, et al. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med. 2020;172(9):629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collantes M.E.V., Espiritu A.I., Sy M.C.C., Anlacan VMM, Jamora RDG. Neurological manifestations in COVID-19 infection: a systematic review and meta-analysis. Can J Neurol Sci. 2021;48(1):66–76. doi: 10.1017/cjn.2020.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Misra S., Kolappa K., Prasad M., Radhakrishnan D, Thakur KT, Solomon T, et al. Frequency of neurologic manifestations in COVID-19: a systematic review and meta-analysis. Neurology. 2021;97(23):e2269–e2281. doi: 10.1212/WNL.0000000000012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian D., Sun Y., Xu H., Ye Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J Med Virol. 2022;94(6):2376–2383. doi: 10.1002/jmv.27643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meo S.A., Meo A.S., Al-Jassir F.F., Klonoff D.C. Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics. Eur Rev Med Pharm Sci. 2021;25(24):8012–8018. doi: 10.26355/eurrev_202112_27652. [DOI] [PubMed] [Google Scholar]
- 7.Mao L., Jin H., Wang M., Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019. Wuhan. China JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan N., Xu Z., Mei B., Gao Y, Lv D, Zhang J. Neurological implications of non-critically Ill patients with coronavirus disease 2019 in a Fangcang Shelter Hospital in Wuhan, China. Front Neurol. 2020;11:895. doi: 10.3389/fneur.2020.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flores-Silva F.D., García-Grimshaw M., Valdés-Ferrer S.I., Vigueras-Hernández AP, Domínguez-Moreno R, Tristán-Samaniego DP, et al. Neurologic manifestations in hospitalized patients with COVID-19 in Mexico City. PLoS One. 2021;16(4) doi: 10.1371/journal.pone.0247433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á, Layos-Romero A, García-García J, et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95(8):e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luigetti M., Iorio R., Bentivoglio A.R., Tricoli L, Riso V, Marotta J, et al. Assessment of neurological manifestations in hospitalized patients with COVID-19. Eur J Neurol. 2020;27(11):2322–2328. doi: 10.1111/ene.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang, Haijuan Diagnosis and Treatment Protocol for COVID-19 Patients (Tentative 9th Version). Infectious Diseases & Immunity: July 2022 - Volume 2 - Issue 3 - p 135–144. http://doi.org/10.1097/ID9.0000000000000059. [DOI] [PMC free article] [PubMed]
- 13.Pinzon R.T., Wijaya V.O., Jody A.A., Nunsio PN, Buana RB. Persistent neurological manifestations in long COVID-19 syndrome: a systematic review and meta-analysis. J Infect Public Health. 2022;15(8):856–869. doi: 10.1016/j.jiph.2022.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ortelli P., Ferrazzoli D., Sebastianelli L., Engl M, Romanello R, Nardone R, et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: insights into a challenging symptom. J Neurol Sci. 2021;420:117271. doi: 10.1016/j.jns.2020.117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyberg T., Ferguson N.M., Nash S.G., Webster HH, Flaxman S, Andrews N, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menni C., Valdes A.M., Polidori L., Antonelli M, Penamakuri S, Nogal A, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399(10335):1618–1624. doi: 10.1016/S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hui K.P.Y., Ho J.C.W., Cheung M.C., Ng KC, Ching RHH, Lai KL, et al. SARS-CoV-2 omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603(7902):715–720. doi: 10.1038/s41586-022-04479-6. [DOI] [PubMed] [Google Scholar]
- 18.Karim S.S.A., Karim Q.A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398(10317):2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long B., Carius B.M., Chavez S., Liang SY, Brady WJ, Koyfman A, et al. Clinical update on COVID-19 for the emergency clinician: presentation and evaluation. Am J Emerg Med. 2022;54:46–57. doi: 10.1016/j.ajem.2022.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewanjee S., Vallamkondu J., Kalra R.S., Puvvada N, Kandimalla R, Reddy PH. Emerging COVID-19 neurological manifestations: present outlook and potential neurological challenges in COVID-19 pandemic. Mol Neurobiol. 2021;58(9):4694–4715. doi: 10.1007/s12035-021-02450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liotta E.M., Batra A., Clark J.R., Shlobin NA, Hoffman SC, Orban ZS, et al. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol. 2020;7(11):2221–2230. doi: 10.1002/acn3.51210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varatharaj A., Thomas N., Ellul M.A., Davies NWS, Pollak TA, Tenorio EL, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellul M.A., Benjamin L., Singh B., Lant S, Michael BD, Easton A, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nouchi A., Chastang J., Miyara M., Lejeune J, Soares A, Ibanez G, et al. Prevalence of hyposmia and hypogeusia in 390 COVID-19 hospitalized patients and outpatients: a cross-sectional study. Eur J Clin Microbiol Infect Dis. 2021;40(4):691–697. doi: 10.1007/s10096-020-04056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andalib S., Biller J., Di Napoli M., Moghimi N., McCullough L.D., Rubinos C.A., et al. Peripheral Nervous System Manifestations Associated with COVID-19. Curr Neurol Neurosci Rep. 2021;21(3):9. doi: 10.1007/s11910-021-01102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin J.M., Bai P., He W., Wu F, Liu XF, Han DM, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onder G., Rezza G., Brusaferro S. Case-Fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 28.Ferraro S., Tuccori M., Convertino I., Valdiserra G, Cappello E, Maggi F, et al. Olfactory and gustatory impairments in COVID-19 patients: Role in early diagnosis and interferences by concomitant drugs. Br J Clin Pharm. 2021;87(5):2186–2188. doi: 10.1111/bcp.14634. [DOI] [PubMed] [Google Scholar]
- 29.Giacomelli A., Pezzati L., Conti F., Bernacchia D, Siano M, Oreni L, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71(15):889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conti P., Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents. 2020;34(2):339–343. doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- 31.Pilotto A., Masciocchi S., Volonghi I., Crabbio M, Crabbio M, Magni E, et al. Clinical presentation and outcomes of severe acute respiratory syndrome coronavirus 2-related encephalitis: the ENCOVID multicenter study. J Infect Dis. 2021;223(1):28–37. doi: 10.1093/infdis/jiaa609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali M.A.M., Spinler S.A. COVID-19 and thrombosis: from bench to bedside. Trends Cardiovasc Med. 2021;31(3):143–160. doi: 10.1016/j.tcm.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carfora V., Spiniello G., Ricciolino R., Di Mauro M, Migliaccio MG, Mottola FF, et al. Anticoagulant treatment in COVID-19: a narrative review. J Thromb Thrombolysis. 2021;51(3):642–648. doi: 10.1007/s11239-020-02242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liguori C., Pierantozzi M., Spanetta M., Sarmati L, Cesta N, Iannetta M, et al. Subjective neurological symptoms frequently occur in patients with SARS-CoV2 infection. Brain Behav Immun. 2020;88:11–16. doi: 10.1016/j.bbi.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Douaud G., Lee S., Alfaro-Almagro F., Arthofer C, Wang C, McCarthy P, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604(7907):697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maiese A., Manetti A.C., Bosetti C., Del Duca F, La Russa R, Frati P, et al. SARS-CoV-2 and the brain: a review of the current knowledge on neuropathology in COVID-19. Brain Pathol. 2021;31(6) doi: 10.1111/bpa.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erickson M.A., Rhea E.M., Knopp R.C., Banks W.A. Interactions of SARS-CoV-2 with the Blood-Brain Barrier. Int J Mol Sci. 2021;22(5):2681. doi: 10.3390/ijms22052681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moghimi N., Di Napoli M., Biller J., Siegler JE, Shekhar R, McCullough LD, et al. The neurological manifestations of post-acute sequelae of SARS-CoV-2 infection. Curr Neurol Neurosci Rep. 2021;21(9):44. doi: 10.1007/s11910-021-01130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ceban F., Ling S., Lui L.M.W., Lee Y, Gill H, Teopiz KM, et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esendağli D., Yilmaz A., Akçay Ş., Özlü T. Post-COVID syndrome: pulmonary complications. Turk J Med Sci. 2021;51(SI-1):3359–3371. doi: 10.3906/sag-2106-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song W.J., Hui C.K.M., Hull J.H., Birring SS, McGarvey L, Mazzone SB, et al. Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respir Med. 2021;9(5):533–544. doi: 10.1016/S2213-2600(21)00125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.