Background:
Icotinib is the first generation of epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) independently developed in China, which has been widely used in the treatment of advanced EGFR mutation-positive nonsmall cell lung cancer (NSCLC). The purpose of this study was to systematically evaluate the efficacy and safety of icotinib in the treatment of advanced EGFR mutation-positive NSCLC and to provide evidence-based evidence for clinical rational drug use.
Methods:
Up to September 30, 2022, the databases of PubMed, EMBASE, Cochrane Library, ClinicalTrials.gov, China National Knowledge Infrastructure, and Wanfang were searched, and the randomized controlled trials (RCTs) of icotinib (experimental group) versus gefitinib or erlotinib (control group) in the treatment of EGFR-positive advanced NSCLC were included. Two researchers independently screened the literature, extracted data, and evaluated the quality of the included literature. Revman5.4 software was used for meta-analysis.
Results:
A total of 957 patients were included in 12 studies. The results of meta-analysis showed that the objective response rate (ORR) and disease control rate (DCR) of the experimental group were better than those of the control group (relative risk (RR) = 1.29, 95% confidence interval (CI): 1.10–1.50, P = .001; RR = 1.10, 95%CI: 1.02–1.18, P = .01). There was no significant difference in progression-free survival (PFS) and overall survival between the 2 groups (P > .05). The results of stratified analysis showed that icotinib significantly improved the ORR of EGFR-positive advanced NSCLC patients compared with gefitinib (RR = 1.20, 95%CI: 1.01–1.43, P = .03), but had no significant improvement in DCR (RR = 1.08, 95%CI: 0.99–1.16, P = .07). Compared with erlotinib, icotinib significantly improved ORR and DCR (RR = 1.69, 95%CI: 1.17–2.45, P = .005; RR = 1.21, 95%CI: 1.01–1.44, P = .04). In terms of adverse events of drugs, the incidence of nausea and vomiting in the experimental group was significantly lower than that in the control group (P < .05).
Conclusion:
Icotinib is safer than gefitinib or erlotinib in the treatment of advanced EGFR-positive NSCLC and seems to bring more clinical benefits to patients. However, there is no obvious advantage in improving the survival rate of patients, and long-term follow-up clinical studies are needed to verify its efficacy.
Keywords: erlotinib, gefitinib, icotinib, meta-analysis, nonsmall cell lung cancer
1. Introduction
Lung cancer is the malignant tumor with the highest morbidity and mortality in China. 80% to 85% of lung cancer is nonsmall cell lung cancer (NSCLC).[1–3] More than 70% of the patients are advanced at the time of diagnosis and cannot undergo radical surgery.[4] For a long time, the treatment of advanced NSCLC is mainly based on standard platinum-containing dual-drug chemotherapy, but its effective rate is only 25% to 35%, the median survival time is 8 to 10 months, and the 1-year survival rate is less than 40%.[5,6] In recent years, the development and application of epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) have brought hope for the treatment of NSCLC at the bottleneck stage.[7] Several clinical studies have shown that EGFR-TKI is significantly more effective than chemotherapy in the first-line treatment of advanced EGFR-positive NSCLC and significantly prolongs progression-free survival (PFS).[8,9]
At present, the first generation of EGFR-TKI includes gefitinib, erlotinib, and icotinib.[10] Gefitinib and erlotinib were developed by AstraZeneca and Roche respectively and went public in 2003 and 2004.[11,12] Icotinib is the first self-developed small-molecule EGFR-TKI in China, and its molecular structure is similar to gefitinib and erlotinib.[13] In 2014, Icotinib was approved by the China Food and Drug Administration for first-line treatment of advanced NSCLC patients with EGFR mutations.[14] To explore the difference in efficacy and safety between icotinib and gefitinib/erlotinib, there are many related studies reported in recent years, and the conclusions are not completely consistent. Therefore, this study used meta-analysis to systematically evaluate the efficacy and safety of icotinib compared with gefitinib or erlotinib in the treatment of EGFR-positive advanced NSCLC patients, to provide an evidence-based basis for clinical rational drug use.
2. Materials and Methods
2.1. Publication search
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[15] The systematic literature search was performed through PubMed, EMBASE, Cochrane Library, ClinicalTrials.gov, China National Knowledge Infrastructure, and Wanfang Database, covering all articles published up to September 30, 2022. The following keywords were used to retrieve articles: Nonsmall cell lung cancer, NSCLC, Icotinib, Gefitinib, Erlotinib. References of the retrieved publications were also screened. The search strategy for PubMed is described as follows:
#1 “Carcinoma, Non-Small-Cell Lung” [Mesh]
#2 “Non small Cell Lung Cancer” OR “Non-Small Cell Lung Cancer” OR “Non-Small Cell Lung Carcinoma” OR “Carcinoma, Non-Small Cell Lung” OR “Non Small Cell Lung Carcinoma” OR “Non-Small-Cell Lung Carcinoma” OR “Non-Small-Cell Lung Carcinomas” OR “Lung Carcinomas, Non-Small-Cell” OR “Lung Carcinoma, Non-Small-Cell” OR “Carcinomas, NonSmall-Cell Lung” OR “Carcinoma, Non Small Cell Lung” [Title/ Abstract]
#3 #1 OR #2
#4 Icotinib [Title/ Abstract]
#5 Gefitinib [Title/ Abstract]
#6 Erlotinib [Title/ Abstract]
#7 #5 OR #6
#8 #4 AND #7
#9 #3 AND #8
The Cochrane Library, EMBASE, ClinicalTrials.gov, China National Knowledge Infrastructure and Wanfang database used similar search formulae.
2.2. Literature inclusion and exclusion criteria
2.2.1. Inclusion criteria.
(1) Participants: Advanced patients with pathologically diagnosed NSCLC and unable to undergo radical surgery; Karnofsky performance status ≥ 60; expected survival time more than 3 months; at least one measurable lesion; adequate organs function (heart, liver, kidney, bone marrow, and other important organs); the EGFR gene of the patient is positive.
(2) Type of study: Randomized controlled clinical trials (RCTs)
(3) Intervention: The experimental group was treated with icotinib, and the control group was treated with gefitinib or erlotinib.
(4) Outcome indicators: Objective response rate (ORR), disease control rate (DCR), survival rate, and adverse events. The results were divided into complete response (CR), partial response (PR), stable disease, and progressive disease according to the Response Evaluation Criteria in Solid Tumors v1.1 (RECIST v1.1). The ORR was calculated as the sum of the CR and PR rates. The DCR was calculated as the sum of the CR, PR, and stable disease rates.
2.2.2. Exclusion criteria.
Non-RCTs; reviews, case reports, conference summaries, and repeated studies; the data are incomplete and the original data are not available; combined with radiotherapy or other treatment regimen during treatment.
2.3. Data extraction and literature quality evaluation
Data were independently screened, extracted, and cross-checked by 2 reviewers (DL and LY). If there is any disagreement in the process, the decision will be made through discussion or by referring to the opinions of the third reviewer (WL). The extracted data mainly include first author name, country, year of publication, TNM stage of the tumor, number of cases, age of patients, therapeutic regimens, EGFR gene status, and outcome indicators. If there is a lack of important information in the study, try to contact the first author or corresponding author by email to further obtain unpublished data. The Cochrane risk of bias tool[16] was used to evaluate the quality of each RCTs included. The risk of bias was evaluated from 7 items: selection bias (random sequence generation, allocation concealment), performance bias, detection bias, attrition bias, reporting bias, and other biases. Each item was classified as “low risk of bias,” “unclear risk of bias”, and “high risk of bias.”
2.4. Statistical analysis
The Review Manager version 5.4 software was used to perform the meta-analysis. For dichotomous data, relative risk (RR) and 95% confidence intervals (CI) were used as evaluation indexes. All p values were 2-sided, and P < .05 was considered statistically significant. The heterogeneity was tested by Q and I2 tests. When the heterogeneity exists (I2 > 50% or P < .1), the random-effects model was used for a meta-analysis, otherwise, the fixed-effects model was used. Sensitivity analysis was used to test the stability of the results and funnel plots were used to evaluate publication bias.
3. Results
3.1. Literature search and study characteristics
A total of 949 articles were retrieved, and 368 repeated articles were excluded by title, year of publication, and author information. Then after reading abstracts and full-text screening, 569 articles that did not meet the criteria were excluded and finally included 12 studies[17–28] (Fig. 1). There were 957 patients with EGFR-positive advanced NSCLC, of which 472 patients received icotinib and 485 patients received gefitinib or erlotinib.
Figure 1.
Literature screening flow chart.
The quality evaluation of the included studies is shown in Table 1. Key baseline characteristics of patients were adequately described in all included studies, as shown in Table 2. Of these, 10 RCTs were icotinib versus gefitinib, and the other 3 RCTs were icotinib versus erlotinib.
Table 1.
The methodological quality of the included studies was assessed using the Cochrane “Risk of Bias” tool.
| Study | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Other bias | |
|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | ||||||
| YK Shi 2013 | + | + | + | ? | + | + | ? |
| Y Chen 2017 | ? | ? | ? | ? | + | + | ? |
| RN Cong 2018 | ? | ? | ? | ? | + | + | ? |
| HZ Cui 2015 | ? | ? | ? | ? | + | + | ? |
| CD Ji 2017 | + | ? | ? | ? | + | + | ? |
| FP Li 2020 | ? | ? | ? | ? | + | + | ? |
| FY Li 2018 | + | ? | ? | ? | + | + | ? |
| XY Lv 2020 | + | ? | ? | ? | + | + | ? |
| F Pei 2019 | + | ? | ? | ? | + | + | ? |
| Z Song 2018 | ? | ? | ? | ? | + | + | ? |
| T Xin 2017 | ? | ? | ? | ? | + | + | ? |
| SH Xu 2020 | ? | ? | ? | ? | + | + | ? |
?= unclear risk of bias; + =low risk of bias; - =high risk of bias.
Table 2.
Basic characteristics of included literature.
| Study | TNM stage | Number of patients | Male/female | Age (yr) | Treatment scheme | Whether the subjects had EGFR mutations | Outcome indicators | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental group | Control group | Experimental group | Control group | Experimental group | Control group | Experimental group | Control group | ||||
| YK Shi 2013 | IIIB–IV | 29 | 39 | - | - | - | - | Icotinib 12 5mg po tid | Gefitinib 250 mg po qd | Yes | ①②③④ |
| Y Chen 2017 | III–IV | 47 | 47 | 31/16 | 32/15 | 61.2 ± 2.5 | 60.17 ± 2.3 | Icotinib 125 mg po tid | Gefitinib 250 mg po qd | Yes | ①② |
| RN Cong 2018 | IIIB–IV | 24 | 26 | 8/16 | 10/16 | - | - | Icotinib 125 mg po tid | Gefitinib 250 mg po qd | Yes | ①②③⑤ |
| RN Cong* 2018 | IIIB–IV | 24 | 26 | 8/16 | 12/14 | - | - | Icotinib 125 mg po tid | Erlotinib 150 mg po qd | Yes | ①②③⑤ |
| HZ Cui 2015 | IV | 28 | 28 | 18/10 | 20/8 | 58.8 ± 9.98 | 57.5 ± 11.6 | Icotinib 125 mg po tid | Gefitinib 250 mg po qd | Yes | ①②④⑤ |
| CD Ji 2017 | IIIB–IV | 45 | 45 | 31/14 | 29/16 | 55.63 ± 11.25 | 54.97 ± 10.82 | Icotinib 125 mg po tid | Gefitinib 250 mg po qd | Yes | ①② |
| FP Li 2020 | IIIB–IV | 50 | 50 | 31/19 | 29/21 | 53.8 ± 6.0 | 52.4 ± 5.7 | Icotinib 125 mg po tid | Gefitinib 250 mg po qd | Yes | ①② |
| FY Li 2018 | IV | 27 | 27 | 10/17 | 9/18 | 64.5 ± 8.4 | 65.6 ± 8.8 | Icotinib 125 mg po tid | Gefitinib 250 mg po qd | Yes | ①②⑤ |
| XY Lv 2020 | III–IV | 25 | 25 | 13/12 | 14/11 | 61.4 ± 2.1 | 61.5 ± 2.2 | Icotinib 125 mg po tid | Gefitinib 250 mg po qd | Yes | ①②⑤ |
| F Pei 2019 | III–IV | 55 | 55 | 29/26 | 31/24 | 65.07 ± 6.95 | 64.91 ± 6.93 | Icotinib 125 mg po tid | Gefitinib 250 mg po qd | Yes | ①②⑤ |
| Z Song 2018 | IIIB–IV | 38 | 38 | 20/18 | 17/21 | 62.19 ± 10.14 | 61.88 ± 10.03 | Icotinib 125 mg po tid | Erlotinib 150 mg po qd | Yes | ①② |
| T Xin 2017 | III–IV | 40 | 40 | 23/17 | 25/15 | 52.6 ± 11.3 | 50.1 ± 10.9 | Icotinib 125 mg po tid | Erlotinib 150 mg po qd | Yes | ①②③④⑤ |
| SH Xu 2020 | IIIB–IV | 40 | 39 | 28/12 | 30/9 | 61.3 ± 5.1 | 61.5 ± 5.2 | Icotinib 125 mg po tid | Gefitinib 250 mg po qd | Yes | ①② |
In the study of RN Cong*, patients were randomly divided into 3 groups and were treated with icotinib, erlotinib, and gefitinib, respectively. Therefore, it can be regarded as 2 independent RCTs (icotinib vs. erlotinib, icotinib vs. gefitinib). po = Oral administration; qd = one time per day; tid = 3 times per day; ① objective response rate; ② disease control rate; ③ progression-free survival; ④ overall survival; ⑤ adverse events.
3.2. ORR
A total of 13 RCTs in 12 studies[17–28] provided ORR data, and the results of the heterogeneity test showed that the heterogeneity among the studies was small (P = .88, I2 = 0%). Fixed-effects model analysis showed that the ORR of patients with EGFR-positive advanced NSCLC in the experimental group was significantly higher than that in the control group (RR = 1.29, 95%CI: 1.10–1.50, P = .001). See Fig. 2.
Figure 2.
ORR forest plot of experimental group versus control group.
3.3. DCR
A total of 13 RCTs in 12 studies[17–28] provided DCR data, and the results of the heterogeneity test showed that the heterogeneity among the studies was small (P = .76, I2 = 0%). Fixed-effects model analysis showed that the DCR of patients with EGFR-positive advanced NSCLC in the experimental group was significantly higher than that in the control group (RR = 1.10, 95%CI: 1.02–1.18, P = .01). See Fig. 3.
Figure 3.
DCR forest plot of experimental group versus control group.
3.4. PFS
A total of 4 RCTs in 3 studies[17,19,27] provided 1-year and 2-year PFS data. Based on the heterogeneity test results (P = .81, I2 = 0%; P = .47, I2 = 0%). The results of fixed-effects model analysis showed that there was no significant difference in PFS between the experimental group and the control group (RR = 1.36, 95%CI: 0.92–2.00, P = .13; RR = 2.22, 95%CI: 0.89–5.51, P = .09). See Figs. 4 and 5.
Figure 4.
1-year PFS rate forest plot of experimental group versus control group.
Figure 5.
2-year PFS rate forest plot of experimental group versus control group.
3.5. Overall survival (OS)
Three studies[17,20,27] reported 1-year and 2-year OS data. Based on the heterogeneity test results (P = .28, I2 = 22%; P = .69, I2 = 0%). The results of fixed-effects model analysis showed that there was no significant difference in OS between the experimental group and the control group (RR = 1.23, 95%CI: 1.00–1.52, P = .06; RR = 1.34, 95%CI: 0.87–2.08, P = .19). See Figs. 6 and 7.
Figure 6.
1-year OS rate forest plot of experimental group versus control group.
Figure 7.
2-year OS rate forest plot of experimental group versus control group.
3.6. Adverse events
Compared with the control group, the incidence of nausea and vomiting was significantly lower in the experimental group (P < .05), and there was no significant difference in the incidence of leukopenia, rash, diarrhea, and liver function damage between the 2 groups (P > .05). See Table 3.
Table 3.
Comparison of the incidence of adverse events between experimental group and control group.
| Adverse events | Number of studies | Heterogeneity | RR 95%CI | P |
|---|---|---|---|---|
| Diarrhoea | 6 | P = .66, I2 = 0% | 0.78 (0.51–1.19) | .24 |
| Rash | 5 | P = .49, I2 = 0% | 0.69 (0.42–1.15) | .15 |
| Nausea and vomiting | 3 | P = .96, I2 = 0% | 0.48 (0.29–0.81) | .006 |
| liver function damage | 4 | P = .78, I2 = 0% | 1.07 (0.58–1.97) | .84 |
| Leukopenia | 2 | P = .35, I2 = 0% | 0.88 (0.59–1.32) | .54 |
CI = confidence interval; RR = relative risk.
3.7. Results of stratified analysis
A total of 13 RCTs in 12 studies, of which 10 RCTs were icotinib versus gefitinib, and the other 3 RCTs were icotinib versus erlotinib. The results of stratified analysis showed that icotinib significantly improved the ORR of EGFR-positive advanced NSCLC patients compared with gefitinib (RR = 1.20, 95%CI: 1.01–1.43, P = .03), but had no significant improvement in DCR (RR = 1.08, 95%CI: 0.99–1.16, P = .07). Compared with erlotinib, icotinib significantly improved ORR and DCR (RR = 1.69, 95%CI: 1.17–2.45, P = .005; RR = 1.21, 95%CI: 1.01–1.44, P = .04). In terms of increasing the rate of PFS and OS in EGFR-positive advanced NSCLC patients, the efficacy of icotinib was similar to that of gefitinib and erlotinib (P > .05). See Table 4.
Table 4.
Results of stratified analysis.
| Indicator | Intervention | Number of RCTs | Heterogeneity | RR 95%CI | P |
|---|---|---|---|---|---|
| ORR | I vs. G | 10 | P = .96, I2 = 0% | 1.20 (1.01–1.43) | .03 |
| I vs. E | 3 | P = .66, I2 = 0% | 1.69 (1.17–2.45) | .005 | |
| DCR | I vs. G | 10 | P = .78, I2 = 0% | 1.08 (0.99–1.16) | .07 |
| I vs. E | 3 | P = .64, I2 = 0% | 1.21 (1.01–1.44) | .04 | |
| 1-yr PFS | I vs. G | 2 | P = .96, I2 = 0% | 1.47 (0.82–2.64) | .19 |
| I vs. E | 2 | P = .37, I2 = 0% | 1.27 (0.75–2.1) | .38 | |
| 2-yr PFS | I vs. G | 2 | P = .76, I2 = 0% | 2.93 (0.80–10.70) | .10 |
| I vs. E | 2 | P = .17, I2 = 46% | 1.65 (0.45–6.12) | .45 | |
| 1-yr OS | I vs. G | 2 | P = .38, I2 = 0% | 1.11 (0.86–1.42) | .42 |
| I vs. E | 1 | - | 1.47 (1.00–2.16) | .05 | |
| 2-yr OS | I vs. G | 2 | P = .85, I2 = 0% | 1.21 (0.75–1.94) | .43 |
| I vs. E | 1 | - | 2.00 (0.65–6.11) | .22 |
I = icotinib, G = gefitinib, E = erlotinib, ORR = objective response rate, DCR = disease control rate, PFS = progression-free survival, OS = overall survival.
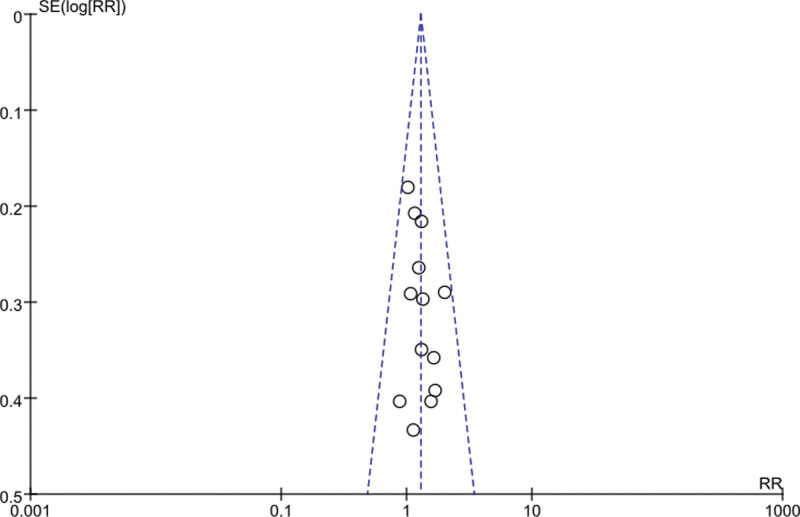
3.8. Sensitivity analysis and publication bias
Sensitivity analysis was performed for each meta-analysis, and each included study was excluded one by one before effect size was pooled. The RR values and 95%CI obtained did not change significantly, indicating that the results were stable. The funnel plots with ORR and DCR as indicators were basically symmetric, suggesting no significant publication bias. See Figs. 8 and 9.
Figure 8.
Funnel plot of ORR.
Figure 9.
Funnel plot of DCR.
4. Discussion
Although gefitinib, erlotinib, and icotinib are all quinazoline family complexes with similar core structures. However, gefitinib and erlotinib have half-lives of 40 to 44 hours[29] and 18.9 hours,[30] respectively, and are administered orally once daily. Icotinib has a half-life of 6–8 hours and needs to be taken orally 3 times daily.[31] In addition, gefitinib and erlotinib pharmacokinetic data were obtained from Western NSCLC patients,[8,9] while icotinib pharmacokinetic data were obtained from the Chinese population.[32] Therefore, head-to-head clinical studies are needed to confirm the efficacy of icotinib in advanced NSCLC. The results of the ICOGEN study[17] showed that icotinib was noninferior to gefitinib in the treatment of advanced NSCLC, but the vast majority of patients enrolled in this study were EGFR-negative and only 17% were EGFR-positive.[17] To further confirm the efficacy and safety of icotinib in the treatment of EGFR-positive advanced NSCLC patients, there are many related studies in recent years, and the conclusions are not completely consistent. Therefore, this study used meta-analysis to systematically evaluate the efficacy and safety of icotinib compared with gefitinib or erlotinib in the treatment of EGFR-positive advanced NSCLC patients, to provide an evidence-based basis for clinical rational drug use.
A total of 12 studies including 13 RCTs were included in our systematic review, with a total of 957 EGFR-positive advanced NSCLC patients. The results of the meta-analysis showed that the ORR and DCR of the experimental group were better than those of the control group. There was no significant difference in PFS and OS between the 2 groups. The incidence of nausea and vomiting in the experimental group was significantly lower than that in the control group, and the difference was statistically significant. Further stratified analysis showed that there was no significant difference in DCR between icotinib and gefitinib, but the ORR of icotinib was significantly better than that of gefitinib. The ORR and DCR of icotinib were significantly better than erlotinib, and the differences were statistically significant. There was no significant difference between icotinib and gefitinib/erlotinib in improving PFS and OS rates in EGFR-positive advanced NSCLC patients. This suggests that icotinib is safer than gefitinib or erlotinib for EGFR-positive advanced NSCLC, and seems to bring more clinical benefits to patients.
Compared with the previous systematic review published by Liu et al,[33] our meta-analysis included more literature published from 2016 to 2022, excluding non-RCTs studies, and the 13 RCTs included were all head-to-head clinical studies. Importantly, our study subjects were EGFR-positive patients, excluding patients with EGFR-negative or unclear EGFR status who had an uncertain benefit from targeted therapy. The patients included in our study were all Asian, mainly the Chinese population, so the results of this study have strong reference significance for the Chinese population. However, our meta-analysis also has some limitations. First, gefitinib and erlotinib were included in the control group because they belong to the same class of drugs. However, after stratified analysis, there were only 3 RCTs of icotinib versus erlotinib, and the strength of evidence was relatively limited. Second, few studies provide long-term survival data such as OS and PFS. Third, due to the inconsistency or absence of the original data provided by the included study, it is impossible to further analyze the impact of different EGFR mutation subtypes on the efficacy of treatment. Fourth, Most of the RCTs included did not clearly explain random sequence generation, allocation concealment, and blinding. Therefore, this result still needs to be further verified by a large sample size and high-quality RCTs.
In conclusion, icotinib is safer than gefitinib or erlotinib in the treatment of advanced EGFR-positive NSCLC and seems to bring more clinical benefits to patients. However, there is no obvious advantage in improving the survival rate of patients, and long-term follow-up clinical studies are needed to verify its efficacy.
Author contributions
Conceptualization: Dailong Li.
Data curation: Dailong Li, Ling Yao, Lu Xu.
Formal analysis: Dailong Li, Ling Yao, Wanqiang Li, Yuan Che.
Methodology: Ling Yao, Dailong Li, Lu Xu, Yuan Che.
Software: Dailong Li, Ling Yao, Lu Xu.
Supervision: Dailong Li, Yuan Che.
Validation: Dailong Li, Wanqiang Li.
Writing—original draft: Dailong Li.
Writing—review and editing: Dailong Li, Ling Yao, Lu Xu, Yuan Che.
Abbreviations:
- CI =
- confidence interval
- CR =
- complete response
- DCR =
- disease control rate
- EGFR-TKI =
- epidermal growth factor receptor-tyrosine kinase inhibitor
- NSCLC =
- nonsmall cell lung cancer
- ORR =
- objective response rate
- OS =
- overall survival
- PD =
- progressive disease
- PFS =
- progression-free survival
- PR =
- partial response
- RCTs =
- randomized controlled trials
- RR =
- risk ratio
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
DL and LY contributed equally to this work.
The author reports no conflicts of interest in this work.
Ethical approval was not necessary, because this article is a meta-analysis and it does not involve the participation of ethics committee.
How to cite this article: Li D, Yao L, Xu L, Li W, Che Y. Efficacy and safety of icotinib in the treatment of advanced EGFR mutation-positive nonsmall cell lung cancer: A meta-analysis of randomized controlled trials. Medicine 2022;101:48(e32164).
Contributor Information
Dailong Li, Email: 649427819@qq.com.
Ling Yao, Email: 919068099@qq.com.
Lu Xu, Email: xlnick@qq.com.
Wanqiang Li, Email: 649427819@qq.com.
References
- [1].Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- [2].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [3].Reck M, Heigener DF, Mok T, et al. Management of non-small-cell lung cancer: recent developments. Lancet. 2013;382:709–19. [DOI] [PubMed] [Google Scholar]
- [4].Li D, Li W, Pang Y, et al. The effect of adjuvant chemoradiotherapy on survival after R0 resection for stage III-N2 nonsmall cell lung cancer: a meta-analysis. Medicine (Baltim). 2022;101:e29580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hanna NH, Robinson AG, Temin S, et al. Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2021;39:1040–91. [DOI] [PubMed] [Google Scholar]
- [6].Alexander M, Kim SY, Cheng H. Update 2020: management of non-small cell lung cancer. Lung. 2020;198:897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Greenhalgh J, Dwan K, Boland A, et al. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst Rev. 2016;5:CD010383. [DOI] [PubMed] [Google Scholar]
- [8].Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29:2866–74. [DOI] [PubMed] [Google Scholar]
- [9].Rosell R, Carcereny E, Gervais R, et al.; Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. [DOI] [PubMed] [Google Scholar]
- [10].Wu Q, Luo W, Li W, et al. First-generation EGFR-TKI plus chemotherapy versus EGFR-TKI alone as first-line treatment in advanced NSCLC With EGFR activating mutation: a systematic review and meta-analysis of randomized controlled trials. Front Oncol. 2021;11:598265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhao C, Han SY, Li PP. Pharmacokinetics of gefitinib: roles of drug metabolizing enzymes and transporters. Curr Drug Deliv. 2017;14:282–8. [DOI] [PubMed] [Google Scholar]
- [12].Bonomi P. Erlotinib: a new therapeutic approach for non-small cell lung cancer. Expert Opin Investig Drugs. 2003;12:1395–401. [DOI] [PubMed] [Google Scholar]
- [13].Tan F, Shi Y, Wang Y, et al. Icotinib, a selective EGF receptor tyrosine kinase inhibitor, for the treatment of non-small-cell lung cancer. Future Oncol. 2015;11:385–97. [DOI] [PubMed] [Google Scholar]
- [14].Hu X, Han B, Gu A, et al. A single-arm, multicenter, safety-monitoring, phase IV study of icotinib in treating advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2014;86:207–12. [DOI] [PubMed] [Google Scholar]
- [15].Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Higgins JP, Altman DG, Gøtzsche PC, et al.; Cochrane Bias Methods Group. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol. 2013;14:953–61. [DOI] [PubMed] [Google Scholar]
- [18].Chen Y, Zhu WB. The clinical effect of molecular targeted drug Eckinib in treating EGFR mutation state with clear advanced non-small cell lung cancer. China Foreign Med Treat. 2017;33:148–9 + 185. [Google Scholar]
- [19].Cong RN, Gu F, Wang B. Efficacy of gefitinib, erlotinib and icotinib in advanced non-small cell lung cancer (NSCLC) with EGFR mutation. J Mod Oncol. 2018;02:216–9. [Google Scholar]
- [20].Cui HZ, Guan JZ, Liao GQ, et al. Efficacy of icotinib and gefitinib in advanced lung adenocarcinoma with EGFR mutation. Acad J Chin PLA Med Sch. 2015;04:326–8 + 341. [Google Scholar]
- [21].Ji CD, Yu XQ, Lai YX. Hydrochloric acid treatment for ek, EGFR mutation status clear advanced non-small cell lung cancer clinical observation. Hebei Med. 2017;01:100–2. [Google Scholar]
- [22].Li FP. Efficacy analysis of icotinib targeted therapy in epidermal growth factor gene mutated non-small cell lung cancer. Gems Health. 2020;18:30–30,32. [Google Scholar]
- [23].Li FY, Zhou L, Zhu LC. Comparison of clinical efficacy of ectini and gefitinib in the treatment of stage IV lung adenocarcinoma patients with EGFR sensitive gene mutation. Chin J Prim Med Pharm. 2018;11:1403–140. [Google Scholar]
- [24].Lv XY, Ren YC. To observe and analyze the effect of gefitinib and icotinib targeted therapy in patients with advanced non-small cell lung cancer. Cardiovasc Dis Electron J Integr Tradit Chin West Med. 2020;25:52–3. [Google Scholar]
- [25].Pei F, Jia XF, Cao YL. Analysis of the efficacy and safety of icotinib in EGFR mutated elderly non-small cell lung cancer. J Mod Oncol. 2019;27:52–5. [Google Scholar]
- [26].Song Z, Hu Y. Clinical analysis of icteninib hydrochloride in the treatment of advanced non-small cell lung cancer with definite egfr mutation status. China Foreign Med Treat. 2018;21:90–2. [Google Scholar]
- [27].Xin T, Xin XB, Han FL. Effect and safety of icotinib hydrochloride for treating advanced non-small cell lung cancer. Clin Res Pract. 2017;14:1–2 + 5. [Google Scholar]
- [28].Xu SH, Zhou DX, Du HY. Efficacy of icotinib hydrochloride in the treatment of advanced non-small cell lung cancer. Chin J Clin Oncol Rehabil. 2020;05:568–71. [Google Scholar]
- [29].Baselga J, Rischin D, Ranson M, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol. 2002;20:4292–302. [DOI] [PubMed] [Google Scholar]
- [30].Hidalgo M, Siu LL, Nemunaitis J, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001;19:3267–79. [DOI] [PubMed] [Google Scholar]
- [31].Zhao Q, Shentu J, Xu N, et al. Phase I study of icotinib hydrochloride (BPI-2009H), an oral EGFR tyrosine kinase inhibitor, in patients with advanced NSCLC and other solid tumors. Lung Cancer. 2011;73:195–202. [DOI] [PubMed] [Google Scholar]
- [32].Tan F, Shen X, Wang D, et al. Icotinib (BPI-2009H), a novel EGFR tyrosine kinase inhibitor, displays potent efficacy in preclinical studies. Lung Cancer. 2012;76:177–82. [DOI] [PubMed] [Google Scholar]
- [33].Liu Y, Zhang Y, Feng G, et al. Comparison of effectiveness and adverse effects of gefitinib, erlotinib and icotinib among patients with non-small cell lung cancer: a network meta-analysis. Exp Ther Med. 2017;14:4017–32. [DOI] [PMC free article] [PubMed] [Google Scholar]