Background:
Epstein-Barr virus (EBV) infection plays a crucial role in the progression of acquired immunodeficiency syndrome related primary central nervous system lymphoma (AR-PCNSL). This study aimed at evaluating the diagnostic value of cerebrospinal spinal fluid (CSF) EBV-deoxyribonucleic acid (DNA) for PCNSL in patients with infection of human immunodeficiency (HIV) virus through a meta-analysis of diagnostic test.
Methods:
A systematic search in PubMed, Embase, Web of Science, Wanfang, Chinese Biomedical Database and Chinese National Knowledge Infrastructure was conducted before May 10, 2022. Heterogeneity among the studies was assessed using Q test and I2 statistics. Publication bias was assessed using the Deek’s funnel plot asymmetry test. Statistical analyses were performed using Stata 16.0 software. The pooled sensitivity, specificity, positive and negative likelihood ratios (PLR and NLR), diagnostic odds ratios (DOR) and 95% confidence intervals (CI) were caculated to evaluate the diagnostic value. A symmetric receiver operating characteristic (SROC) curve and the area under the SROC curve (AUC) were constructed to evaluate the test-performance.
Results:
Twelve studies were included in the final analyses, with a total of 141 patients with AR-PCNSL and 590 controls. The pooled diagnostic values were sensitivity of 0.83 (95% CI: 0.73–0.90), specificity of 0.95 (95%CI: 0.89–0.98), PLR of 17.8 (95%CI: 6.8–46.1), NLR of 0.17 (95%CI: 0.10–0.30), DOR of 102 (95%CI: 28–379), and AUC of 0.94 (95%CI: 0.91–0.96).
Conclusion:
In summary the overall diagnostic value of CSF EBV-DNA is very high and it can be a reliable diagnostic biomarker for AR-PCNSL.
Keywords: diagnosis, Epstein-Barr virus, HIV/AIDS, meta-analysis, PCNSL, PCR
1. Introduction
Focal brain lesion (FBL) disease in patients with acquired immunodeficiency syndrome (AIDS) may be caused by various opportunistic pathogens or malignancies, including toxoplasma gondii, JC virus (JCV), cytomegalovirus, and primary central nervous system lymphoma (PCNSL), among which PCNSL is 1 of the most prevalent and major cause of morbidity and mortality. Distinguishing PCNSL from other infectious FBL diseases in human immunodeficiency virus (HIV)-infected patients is extremely significant for treatment decision making because their treatment strategies vary greatly. Histological examination of brain biopsy or resection surgery specimen is the gold standard for diagnosing PCNSL. However, its use may be limited by its invasiveness, high rate of complications,[1] and few survival benefit.[2] Early and minimally invasive diagnosis of FBL in HIV-infected patients remains a challenging clinical problem. Despite some radiographic examinations, such as computed tomography, magnetic resonance, and thallium-201 (201Tl) single-photon emission computed tomography are regularly used for its preoperative differential diagnoses. Generally, these imaging methods are insufficient for accurate diagnosis.
The strict association between acquired immunodeficiency syndrome related primary central nervous system lymphoma (AR-PCNSL) and Epstein-Barr virus (EBV) infection suggests that EBV deoxyribonucleic acid (DNA) in the cerebrospinal spinal fluid (CSF) might serve as a remarkable diagnostic marker, which can reduce the time for diagnosis and allow a minimally invasive approach. In fact, the detection of EBV-DNA in the CSF by polymerase chain reaction (PCR) has a relatively high sensitivity and specificity for AR-PCNSL in many studies.[3–6] However, the diagnostic ability from different studies varies, which may be affected by limitations such as small sample size and inter- and intraobserver variations. Considering the limitations of single center studies, we conducted this meta-analysis based on more study samples and rigorous statistics, aiming to more accurately evaluate the diagnostic efficiency of CSF EBV-DNA for PCNSL in patients with HIV infection.
2. Materials and methods
2.1. Literature search
A systematic search was conducted for relevant articles published in PubMed, Embase, Web of Science, Wanfang, Chinese National Knowledge Infrastructure (CNKI) and Chinese Biomedical Database (CBD) from inception to May 10, 2022. The following keywords were used: Epstein-Barr virus, EBV, PCR, DNA, primary central nervous system lymphoma, PCNSL, and PCL. The language was restricted in English and Chinese. This research was approved by the ethics committee of Beijing Diantan Hospital.
2.2. Selection criteria
Studies that met the following criteria were included: All the AR-PCNSL patients must be diagnosed through the gold standard (histological examination); Studies that provided diagnostic value of CSF EBV-DNA for PCNSL; Studies that presented sufficient data to allow calculation of the diagnostic value (true positive, false positive, false negative, and true negative). Duplicate publications, studies with duplicate patients data, studies without qualified data, and other types of research such as letters, reviews, case reports, and editorials were excluded.
2.3. Data extraction
For each study included in this meta-analysis, the following information was extracted: the first author, year of publication, region, sample size (separately for PCNSL and control individuals), and 4 main data (true positive, false positive, false negative, true negative). Data of non-histologically diagnosed PCNSL,[7] CNS involved system non-Hodgkin lymphoma[7] or system non-Hodgkin lymphoma without CNS involved[4,8,9] were removed from this research. During the study selection process, 2 review authors (DX and LT) independently extracted the information, and cross checked the data. Discrepancies were discussed and arbitrated by a third author (FE) to achieve consensus.
2.4. Quality evaluation
Two researchers independently assessed the methodological quality of each included study using the revised quality assessment of diagnostic accuracy studies (QUADAS-2) criteria. Four key domains (patient selection, index test, reference standard, and flow and timing) were evaluated in terms of risk of bias (“high”, “unclear” or “low”) based on the answers to relevant questions. In addition, the first 3 domains (patient selection, index test, and reference standard) were assessed for concerns about applicability.[10] Any disputes were settled by a third investigator.
2.5. Statistical analysis
Stata 16.0 software (Stata Corp, College Station, TX) was used to perform all the statistical analyses. Heterogeneity among studies was assessed using the Q test and I2 statistic. An I2 > 50% and P < .1 indicated the existence of heterogeneity.[11] Publication bias was assessed based on Deek’s funnel plot asymmetry test. The bivariate mixed-effects regression model developed by van Houwelingen[12] was used to calculate the pooled sensitivity, specificity, positive and negative likelihood ratio (PLR and NLR), diagnostic odds ratio and their 95% confidence intervals (CI). A symmetric receiver operating characteristic (SROC) curve was constructed for the overall analysis. The area under the SROC curve (AUC) was calculated to evaluate the accuracy of the test. We also conducted a subgroup analysis based on the PCR method and highly active antiretroviral therapy (HARRT) therapy using univariate meta-regression. Taking the year 1996[13] as the boundary, we divide all studies into 2 subgroups: the pre-HARRT era and the post-HARRT era. Finally, a Fagan plots diagram was constructed to show the relationship between prior probability, likelihood ratio, and posterior test probability. P < .05 was considered to be statistically significant. Graphs were produced by MIDAS module for the STATA and QUADAS-2 module for RevMan 5.4.
3. Results
3.1. Study selection and characteristics
The selection flow of the literature search was presented in Figure 1. The initial search yielded 215 records. 77 duplicate records were removed, and 115 were excluded after reviewing their titles and abstracts. With further work on full-text screening of the remaining 23 articles, we removed 11 records for the following reasons: 7 lacked sufficient data, 2 lacked a control cohort and 2 had duplicated patient data.[14,15] Finally, 12 articles[3–9,16–20] were included in the ultimate meta-analysis. A search of the reference lists of the identified articles and previous systematic reviews[21] did not identify any more relevant articles.
Figure 1.
Flow diagram of studies selection process.
In Table 1, we summarized the characteristics of the 12 studies included in this meta-analysis. Across the 12 studies, the publication year ranged from 1995 to 2013. Most studies (8 of 12) were conducted in Europe, while 2 in the USA and 2 in Japan, involving 141 PCNSL patients and 590 control patients. The sample size of patients with AR-PCNSL in different studies varied from 2 to 36. The control cohort included patients with toxoplasmosis, progressive multifocal leukoencephalopathy, cytomegalovirus encephalitis, cryptococcal meningitis, tuberculoma, neurosyphilis, HIV encephalitis and other AIDS-associated neurological disorders. A qualitative method of nest PCR or general PCR was applied in 10 of the 12 included studies to detect EBV-DNA in CSF, while the other 2 used a quantitative assay of real-time (RT) PCR. Participants from 7 studies were in the pre-HARRT era, while the other 5 were in the post-HARRT era. The sensitivity of CSF EBV-DNA for diagnosing PCNSL ranged from 62.5% to 100.0%, and the specificity ranged from 76.2% to 100.0%.
Table 1.
Characteristics and diagnostic performance of CSF EBV-DNA in PCNSL patients from the included studies.
| NO | Authorref | Year | Region | Sample size PCNSL | Sample size Others | Study design | Analytic method | Amplified Gene | TP | FP | TN | FN | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Arribas[16] | 1995 | USA | 6 | 16 | Retrospective | PCR | BamHI-W and EBNA1 | 5 | 1 | 15 | 1 | 0.833 | 0.938 |
| 2 | Yanagisawa[9] | 2013 | Japan | 8 | 63 | Retrospective | RT-PCR | BNRF1 | 5 | 13 | 50 | 3 | 0.625 | 0.794 |
| 3 | Tachikawa[3] | 1999 | Japan | 5 | 12 | Retrospective | nested PCR | EBNA1 | 5 | 0 | 12 | 0 | 1.000 | 1.000 |
| 4 | Hirsch[17] | 1998 | Switzer- land |
9 | 18 | Retrospective | nested PCR | EBNA1 | 6 | 0 | 18 | 3 | 0.667 | 1.000 |
| 5 | Deluca[4] | 1995 | Italy | 8 | 26 | Prospective | nested PCR | EBNA1 | 7 | 0 | 26 | 1 | 0.875 | 1.000 |
| 6 | Bossolasco[7] | 2002 | Italy | 13 | 16 | Retrospective | RT-PCR | LMP-1 | 9 | 2 | 14 | 4 | 0.692 | 0.875 |
| 7 | Brink[5] | 1998 | UK | 7 | 96 | Prospective | nested PCR | NA | 7 | 9 | 87 | 0 | 1.000 | 0.906 |
| 8 | Wang[8] | 2007 | UK | 4 | 60 | Retrospective | nested PCR | EBNA1 | 3 | 7 | 53 | 1 | 0.750 | 0.883 |
| 9 | Ivers[18] | 2004 | USA | 2 | 21 | Retrospective | PCR | BamH1W | 2 | 5 | 16 | 0 | 1.000 | 0.762 |
| 10 | Antinori[14] | 1999 | Italy | 13 | 18 | Prospective | nested PCR | EBNA1 | 11 | 0 | 18 | 2 | 0.846 | 1.000 |
| 11 | Cinque[6] | 1996 | Italy | 36 | 183 | Prospective and Retrospective | nested PCR | EBNA1 | 35 | 3 | 180 | 1 | 0.972 | 0.984 |
| 12 | Cingolani[20] | 1998 | Italy | 30 | 61 | Prospective | nested PCR | EBNA1 | 24 | 0 | 61 | 6 | 0.800 | 1.000 |
CSF = cerebrospinal spinal fluid, DNA = deoxyribonucleic acid, EBV = Epstein-Barr virus, FN = false negative, FP = false positive, PCNSL = primary central nervous system lymphoma, TN = true negative, TP = true positive.
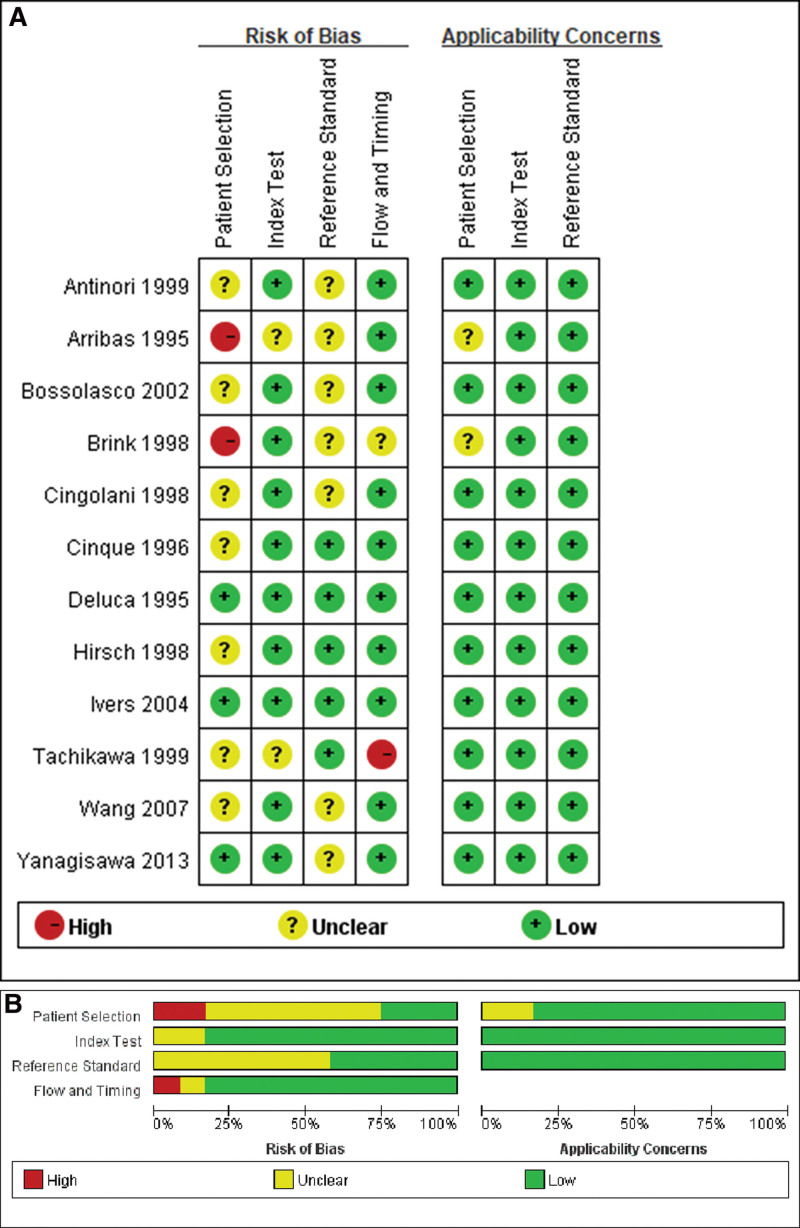
3.2. Quality assessment
The methodological quality of the included studies using QUADAS-2 criteria is summarized in Figure 2 (the risk of bias and applicability concerns summary [A] and graph [B]). The overall quality of the available literature is moderate. Sample selection was heterogeneous for several reasons. First, the control patients varied from separate studies, including patients without mass effect such as HIV encephalopathy, CNS vasculitis, various types of encephalitis, retinitis, and AIDS dementia. And not all patients in the control group were histologically diagnosed. In addition, many patients underwent biopsy diagnosis only after the absence of clinical or radiologic response to the 2-week empiric therapy, resulting in an increased incidence of PCNSL in these patients.
Figure 2.
Methodological quality of the included studies using the QUADAS-2 checklist. (A) Risk of bias and applicability concerns summary. (B) Risk of bias and applicability concerns graph. QUADAS-2 = quality assessment of diagnostic accuracy studies.
3.3. Heterogeneity and publication bias
Heterogeneity test among studies with an I2 = 20% and Q = 2.49(P = .144) indicated the absence of heterogeneity, which was also visualized in the Galbraith graph and bivariate boxplot (Figure S1, Supplemental Digital Content, http://links.lww.com/MD/H923), A and B. Nevertheless, we identified publication bias by Deeks’ regression test of asymmetry (P < .001, Figure S2, Supplemental Digital Content, http://links.lww.com/MD/H924).
3.4. Pooled diagnostic values
The fixed effect model was used to pool the sensitivity and specificity because the I2 values were no more than 50%. The overall pooled sensitivity and specificity were 0.83 (95%CI: 0.73–0.90) and 0.95 (95%CI: 0.89–0.98) (Fig. 3). The pooled PLR was 17.8 (95%CI: 6.8–46.1), NLR was 0.17 (95%CI: 0.10–0.30), and diagnostic odds ratios was 102 (95%CI: 28–379). The overall SROC curve with an AUC of 0.94 (95%CI: 0.91–0.96) was presented in Figure 4. In Fagan plots diagram (Fig. 5), the prior probability was 20%, the post-test probability was 82% for PLR, and 4% for NLR. Scatter matrix of the likelihood ratio (Fig. 6) showed that the integrated LRP and LRN of the pooled studies fell into the first quadrant (LRP > 10, LRN > 0.1).
Figure 3.
Forest plot of pooled sensitivity and specificity of CSF EBV-DNA for AR-PCNSL. AR-PCNSL = acquired immunodeficiency syndrome related primary central nervous system lymphoma, CSF = cerebrospinal spinal fluid, DNA = deoxyribonucleic acid, EBV = Epstein-Barr virus.
Figure 4.
Symmetric receiver operator characteristic curve with 95% confidence contour and 95% prediction contour for the pooled accuracy of CSF EBV-DNA for AR-PCNSL. AR-PCNSL = acquired immunodeficiency syndrome related primary central nervous system lymphoma, CSF = cerebrospinal spinal fluid, DNA = deoxyribonucleic acid, EBV = Epstein-Barr virus.
Figure 5.
Fagan plots diagram of the overall diagnostic value of CSF EBV-DNA for AR-PCNSL. AR-PCNSL = acquired immunodeficiency syndrome related primary central nervous system lymphoma, CSF = cerebrospinal spinal fluid, DNA = deoxyribonucleic acid, EBV = Epstein-Barr virus.
Figure 6.
Scatter matrix of the likelihood ratio.
3.5. Subgroup analyses
Subgroup analyses based on the PCR method and HARRT therapy were performed (Fig. 7), which showed that both factors were sources of heterogeneity in this meta-analysis. The sensitivity and specificity with the use of qualitative method (nest PCR) were 0.89 (95%CI: 0.81–0.97) and 0.97 (95%CI: 0.94–1.00) while the sensitivity and specificity with the use of quantitative method (real-time PCR) were 0.67 (95%CI: 0.44–0.90) and 0.84 (95%CI: 0.59–1.00) independently. The sensitivity and specificity of pre-HARRT era were 0.89 (95%CI: 0.81–0.97) and 0.98 (95%CI: 0.96–1.00) while the sensitivity and specificity of post-HARRT era were 0.76 (95%CI: 0.61–0.92) and 0.87 (95%CI: 0.77–0.97) independently.
Figure 7.
Subgroup analyses based on the PCR method and the therapy of HARRT. HARRT = highly active antiretroviral therapy.
4. Discussion
Patients with HIV infection have a significantly increased risk of PCNSL, which is 3600 times higher than that of the general population.[22] With the advent of the HARRT era, the incidence of AIDS-related tumors including AR-PCNSL has decreased. However, it remains 1 of the most common AIDS-related malignancies, with a prevalence of 20.6% in HIV-positive patients with FBL.[23] EBV infection plays a crucial role in the progression of AR-PCNSL. EBV is a gamma herpesvirus that infects more than 90% of the world’s population as a latent asymptomatic infection of B-lymphocytes. In immunosuppressed hosts, EBV-infected lymphocytes may proliferate without regulation, resulting in malignant lymphomas.[24] The most likely etiology is that the ineffective immunoregulation of EBV induced oncogenic protein expression, subsequent loss of apoptosis and increased proliferation of lymphocytes, and ultimately resulted in malignant lymphomas. Nevertheless, there is no difference in the plasma viral load between AR-PCNSL patients and the control groups.[7] However, the detection of EBV-DNA in the CSF of HIV-infected individuals has been reported as a reliable marker for diagnosing AR-PCNSL in numerous studies.[3–6] In this study, a meta-analysis was conducted to assess the diagnostic significance of CSF EBV-DNA in patients with AR-PCNSL. Our meta-analysis showed that the sensitivity and specificity of EBV-DNA detection in CSF could be as high as 0.83 (0.73–0.90) and 0.95 (0.89-0.98) for PCNSL diagnosis, with an AUC of 0.94, indicating a very high level of overall accuracy. The scatter matrix of the likelihood ratio showed that a positive CSF EBV-DNA detection is very supportive for PCNSL, and negative results do not exclude PCNSL.
Although the pathomechanism leading to the development of AR-PCNSL is unclear, it is well established that there is a strong association between EBV infection and AR-PCNSL. All of these studies showed that CSF examination for EBV-DNA is a recommended diagnostic test for PCNSL. Nonetheless, some factors can influence the accuracy of this test. With the advent of new PCR technology, EBV-DNA load can be tested quantitatively. In 2 of the included studies, the real-time PCR assay was used to detect EBV-DNA quantitatively with a cutoff of 100 and 200 copies/mL independently. Unexpectedly, in our subgroup analysis according to the method of EBV detection, the sensitivity and specificity of studies with the quantitative method were lower than those with the qualitative method (P = .02 and P = .15 respectedly). Decreasing cutoff value will increase its sensitivity, but the specificity will be worse at the same time. Corcoran[25] found that using a cutoff of 10,000 copies/mL can increase the specificity and positive predictive value (PPV) for diagnosing PCNSL compared with qualitative EBV-DNA detection. HARRT, another factor that can influence the accuracy of the test, was found in our study to decrease both sensitivity (P = .02) and specificity (P < .001), which was similar to the previous studies.[18,26] One of the reasons for this decline seems to be the lower incidence of PCNSL in the post-HAART era compared to that in the pre-HAART era. The use of ganciclovir is also associated with lower or undetectable EBV-DNA load in the CSF of patients with AR-PCNSL. In a study, EBV-DNA load was found to be significantly lower for ganciclovir-treated patients, compared with untreated patients (median value, 2.15 vs 4.16 log copies/mL).[27] We did not analyze this factor in the present study, as none of the 12 included articles mentioned the treatment of ganciclovir neither in the PCNSL patients or the control cohort.
To the best of our knowledge, this is the first meta-analysis to cover the largest sample size pooled from rigorously screened studies to evaluate the diagnostic value of CSF EBV-DNA in patients with AR-PCNSL. However, these results should be noted due to certain limitations. First, publication bias and the complicated control types in this study may contribute to heterogeneity and then affect the accuracy of the pooled results. Second, most of the included populations were Italian, which could lead to population selection bias. Third, nucleic acid extraction techniques, DNA primers, and amplification technologies vary across different research centers, including the inconsistent cutoff values. Therefore, a prospective and multi-centered research should be conducted to further evaluate the diagnostic value of CSF EBV-DNA in AR-PCNSL.
5. Conclusion
The detection of EBV-DNA from the CSF by PCR is an extremely sensitive and specific diagnostic method for AR-PCNSL as a possible alternative to brain biopsy, and should be routinely evaluated in HIV-FBL patients before their empiric anti-Toxoplasma therapy. A positive CSF EBV-DNA detection is very supportive for AR-PCNSL, and negative results do not exclude PCNSL. In summary, this meta-analysis indicated that CSF EBV-DNA is a reliable diagnostic biomarker for AR-PCNSL.
Acknowledgements
This study was funded by Seedling Plan of Beijing Ditan Hospital Research Fund (DTYM-202106). The authors would like to acknowledge all the authors for their contributions to this study. DX and FE were responsible for data analysis. DX, LT, and CS helped design the studies. LB, GH, WJ, LH, ZX, and WF participated in the collection of the literature. DX, LT, and FE assessed the methodological quality of each included study. FE supervised this study. DX is the primary author of the manuscript. All authors contributed to the review and development of this manuscript.
Author contributions
Conceptualization: Enshan Feng.
Data curation: Haili Gao, Jianbo Wang, Xinmei Zheng.
Formal analysis: Hongxing Liu.
Investigation: Peiliang Li.
Methodology: Fang Wang, Shichao Chen.
Resources: Bo Liang, Xinmei Zheng.
Software: Xinghuan Ding.
Validation: Enshan Feng.
Visualization: Shichao Chen, Enshan Feng.
Writing – original draft: Xinghuan Ding.
Writing – review & editing: Xinghuan Ding, Tingyu Liang.
Supplementary Material
Abbreviations:
- AIDS =
- acquired immunodeficiency syndrome
- AR-PCNSL =
- acquired immunodeficiency syndrome related primary central nervous system lymphoma
- AUC =
- area under the SROC curve
- CI =
- confidence interval
- CSF =
- cerebrospinal spinal fluid
- DNA =
- deoxyribonucleic acid
- EBV =
- Epstein-Barr virus
- FBL =
- focal brain lesion
- HARRT =
- highly active antiretroviral therapy
- HIV =
- human immunodeficiency virus
- NLR =
- negative likelihood ratios
- PCR =
- polymerase chain reaction
- PLR =
- positive likelihood ratios
- QUADAS-2 =
- quality assessment of diagnostic accuracy studies
- SROC =
- symmetric receiver operating characteristic
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Ding X, Liang T, Liang B, Gao H, Wang J, Liu H, Wang F, Zheng X, Li P, Chen S, Feng E. Diagnostic value of EBV-DNA in CSF for PCNSL in AIDS patients with focal brain lesions: A meta-analysis of diagnostic test. Medicine 2022;101:48(e31793).
Contributor Information
Xinghuan Ding, Email: dingxinghuan0107@163.com.
Tingyu Liang, Email: 13436829433@163.com.
Bo Liang, Email: 13436829433@163.com.
Haili Gao, Email: sdgaohaili@163.com.
Jianbo Wang, Email: 49123202@qq.com.
Hongxing Liu, Email: lhxcm621@ccmu.edu.cn.
Fang Wang, Email: 49123202@qq.com.
Xinmei Zheng, Email: zhengxinmei0204@126.com.
Peiliang Li, Email: mayi12121@163.com.
Shichao Chen, Email: 56552885@qq.com.
References
- [1].Skolasky RL, Dal Pan GJ, Olivi A, et al. HIV-associated primary CNS lymorbidity and utility of brain biopsy. J Neurol Sci. 1999;163:32–8. [DOI] [PubMed] [Google Scholar]
- [2].Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017;35:2410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tachikawa N, Goto M, Hoshino Y, et al. Detection of Toxoplasma gondii, Epstein-Barr virus, and JC virus DNAs in the cerebrospinal fluid in acquired immunodeficiency syndrome patients with focal central nervous system complications. Internal Med (Tokyo, Japan). 1999;38:556–62. [DOI] [PubMed] [Google Scholar]
- [4].De Luca A, Antinori A, Cingolani A, et al. Evaluation of cerebrospinal fluid EBV-DNA and IL-10 as markers for in vivo diagnosis of AIDS-related primary central nervous system lymphoma. Br J Haematol. 1995;90:844–9. [DOI] [PubMed] [Google Scholar]
- [5].Brink NS, Sharvell Y, Howard MR, et al. Detection of Epstein-Barr virus and Kaposi’s sarcoma-associated herpesvirus DNA in CSF from persons infected with HIV who had neurological disease. J Neurol Neurosurg Psychiatry. 1998;65:191–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cinque P, Vago L, Dahl H, et al. Polymerase chain reaction on cerebrospinal fluid for diagnosis of virus-associated opportunistic diseases of the central nervous system in HIV-infected patients. AIDS. 1996;10:951–8. [DOI] [PubMed] [Google Scholar]
- [7].Bossolasco S, Cinque P, Ponzoni M, et al. Epstein-Barr virus DNA load in cerebrospinal fluid and plasma of patients with AIDS-related lymphoma. J Neurovirol. 2002;8:432–8. [DOI] [PubMed] [Google Scholar]
- [8].Wang J, Ozzard A, Nathan M, et al. The significance of Epstein-Barr virus detected in the cerebrospinal fluid of people with HIV infection. HIV Med. 2007;8:306–11. [DOI] [PubMed] [Google Scholar]
- [9].Yanagisawa K, Tanuma J, Hagiwara S, et al. Epstein-Barr viral load in cerebrospinal fluid as a diagnostic marker of central nervous system involvement of AIDS-related lymphoma. Intern Med (Tokyo, Japan). 2013;52:955–9. [DOI] [PubMed] [Google Scholar]
- [10].Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- [11].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [12].van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21:589–624. [DOI] [PubMed] [Google Scholar]
- [13].Brandsma D, Bromberg JEC. Primary CNS lymphoma in HIV infection. Handb Clin Neurol. 2018;152:177–86. [DOI] [PubMed] [Google Scholar]
- [14].Antinori A, Larocca LM, Fassone L, et al. HHV-8/KSHV is not associated with AIDS-related primary central nervous system lymphoma. Brain Pathol (Zurich, Switzerland). 1999;9:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cinque P, Brytting M, Vago L, et al. Epstein-Barr virus DNA in cerebrospinal fluid from patients with AIDS-related primary lymphoma of the central nervous system. Lancet (London, England). 1993;342:398–401. [DOI] [PubMed] [Google Scholar]
- [16].Arribas JR, Clifford DB, Fichtenbaum CJ, et al. Detection of Epstein-Barr virus DNA in cerebrospinal fluid for diagnosis of AIDS-related central nervous system lymphoma. J Clin Microbiol. 1995;33:1580–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hirsch HH, Meylan PR, Zimmerli W, et al. HIV-1-infected patients with focal neurologic signs: diagnostic role of PCR for Toxoplasma gondii, Epstein-Barr virus, and JC virus. Clin Microbiol Inf. 1998;4:577–84. [DOI] [PubMed] [Google Scholar]
- [18].Ivers LC, Kim AY, Sax PE. Predictive value of polymerase chain reaction of cerebrospinal fluid for detection of Epstein-Barr virus to establish the diagnosis of HIV-related primary central nervous system lymphoma. Clin Inf Dis. 2004;38:1629–32. [DOI] [PubMed] [Google Scholar]
- [19].Antinori A, De Rossi G, Ammassari A, et al. Value of combined approach with thallium-201 single-photon emission computed tomography and Epstein-Barr virus DNA polymerase chain reaction in CSF for the diagnosis of AIDS-related primary CNS lymphoma. J Clin Oncol. 1999;17:554–60. [DOI] [PubMed] [Google Scholar]
- [20].Cingolani A, De Luca A, Larocca LM, et al. Minimally invasive diagnosis of acquired immunodeficiency syndrome-related primary central nervous system lymphoma. J Natl Cancer Inst. 1998;90:364–9. [DOI] [PubMed] [Google Scholar]
- [21].Wang Y, Yang J, Wen Y. Lessons from Epstein-Barr virus DNA detection in cerebrospinal fluid as a diagnostic tool for EBV-induced central nervous system dysfunction among HIV-positive patients. Biomed Pharmacother. 2022;145:112392. [DOI] [PubMed] [Google Scholar]
- [22].Goedert JJ. The epidemiology of acquired immunodeficiency syndrome malignancies. Semin Oncol. 2000;27:390–401. [PubMed] [Google Scholar]
- [23].Acosta MC, Kundro M, Viloria G, et al. The role of brain biopsy in the clinical management of HIV-related focal brain lesions. HIV Med. 2018;19:673–8. [DOI] [PubMed] [Google Scholar]
- [24].Yu GH, Montone KT, Frias-Hidvegi D, et al. Cytomorphology of primary CNS lymphoma: review of 23 cases and evidence for the role of EBV. Diagn Cytopathol. 1996;14:114–20. [DOI] [PubMed] [Google Scholar]
- [25].Corcoran C, Rebe K, van der Plas H, et al. The predictive value of cerebrospinal fluid Epstein-Barr viral load as a marker of primary central nervous system lymphoma in HIV-infected persons. J Clin Virol. 2008;42:433–6. [DOI] [PubMed] [Google Scholar]
- [26].Cinque P, Cingolani A, Bossolasco S, et al. Positive predictive value of Epstein-Barr virus DNA detection in HIV-related primary central nervous system lymphoma. Clin Inf Dis. 2004;39:1396–8. [DOI] [PubMed] [Google Scholar]
- [27].Bossolasco S, Falk KI, Ponzoni M, et al. Ganciclovir is associated with low or undetectable Epstein-Barr virus DNA load in cerebrospinal fluid of patients with HIV-related primary central nervous system lymphoma. Clin Inf Dis. 2006;42:e21–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.