BACKGROUND/OBJECTIVES:
To investigate the effect of the occurrence of early hyperchloremia on death or severe disability at 180 days in patients with severe traumatic brain injury (TBI).
DESIGN:
Post hoc analysis of Resuscitation Outcomes Consortium Hypertonic Saline (ROC HS)-TBI trial.
SETTING:
A total of 114 North American emergency medical services agencies in the ROC.
PATIENTS:
A total of 991 patients with severe TBI and Glasgow Coma Scale score of less than or equal to 8.
INTERVENTIONS:
Prehospital resuscitation with single IV dose (250 cc) of 7.5% saline in 6% dextran-70, 7.5% saline (no dextran), or crystalloid.
MEASUREMENTS AND MAIN RESULTS:
Patients with increased serum chloride concentrations (110 mmol/L or greater) 24 hours after randomization were identified. Hyperchloremia was graded into one or greater than or equal to 2 occurrences in the first 24 hours. Logistic regression analyses were performed to determine the effects of hyperchloremia on: 1) death or severe disability at 180 days and 2) death within 180 days after adjusting for confounders. Compared with patients without hyperchloremia, patients with greater than or equal to 2 occurrences of hyperchloremia had significantly higher odds of death or severe disability at 180 days (odds ratio [OR], 1.81; 95% CI, 1.19–2.75) and death within 180 days (OR, 1.89; 95% CI, 1.14–3.08) after adjustment for confounders. However, the total volume of fluids administered during the first 24 hours was an independent predictor of death within 180 days; therefore, after adding an interaction term between the total volume of fluids administered during the first 24 hours and greater than or equal to 2 occurrences of hyperchloremia, patients with greater than or equal to 2 occurrences of hyperchloremia had significantly higher odds of death within 180 days (OR, 2.35; 95% CI, 1.21–4.61 d) but not of composite outcome of death or severe disability at 180 days.
CONCLUSIONS:
After modifying for the effect of the total volume of fluids administered during the first 24 hours, multiple occurrences of hyperchloremia in the first 24 hours were associated with higher odds of death within 180 days in patients with severe TBI.
Keywords: death, disability, hyperchloremia, hypertonic saline, traumatic brain injury
KEY POINTS
Questions: This study sought to investigate the effect of occurrence of early hyperchloremia on death or severe disability at 180 days in patients with severe traumatic brain injury (TBI).
Findings: In a post hoc analysis of Resuscitation Outcomes Consortium Hypertonic Saline-TBI trial, patients with greater than or equal to 2 occurrences of hyperchloremia had significantly higher odds of death within 180 days (odds ratio, 2.35).
Meanings: Hyperchloremia may be a therapeutic target for reducing the observed death in patients with TBI.
Several studies have demonstrated an association between hyperchloremia and occurrence of acute kidney injury (AKI) and short- and long-term mortality in patients admitted to critical care units (1–5). There has been recent interest in evaluating the effect of hyperchloremia in patients with neurologic diseases (6–10). New-onset hyperchloremia has been associated with both death and death or disability in patients with ischemic stroke and intracerebral hemorrhage (ICH) (6–9). Hyperchloremia is also associated with occurrence of AKI in patients with subarachnoid hemorrhage and AKI is associated with higher mortality (10). There has been additional emphasis on the relationship between hyperchloremia and various outcome measures among patients receiving hypertonic saline due to the high-chloride load (11, 12). Hypertonic saline treatment was associated with hyperchloremia and AKI in patients with various intracranial lesions including stroke (9, 12). High rates of in-hospital mortality were observed in patients with ICH who developed moderate hyperchloremia during treatment with continuous IV infusion of 3% hypertonic saline (11). Identification of any relationship has potential therapeutic implications due to availability of balanced crystalloids with low chloride content for IV administration (13–15). We performed this analysis to determine the occurrence of hyperchloremia in patients with traumatic brain injury (TBI) and identify the association between hyperchloremia and death or disability at 180 days.
METHODS
We analyzed the data collected as part of the Resuscitation Outcomes Consortium Hypertonic Saline (ROC HS)-TBI trial (ClinicalTrials.gov number: NCT00316004). The details regarding the trial have been published previously (16–18). The analysis was approved by the University of Missouri Institutional Review Board (“Early Hyperchloremia in Traumatic Brain Injury Resuscitation,” No. 2076062, approved October 21, 2021), and all procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975. Briefly, a multicenter, double-blind, randomized, placebo-controlled clinical trial involving 114 North American emergency medical services agencies, in the ROC, was conducted between May 2006 and May 2009. The study sought to determine whether prehospital administration of 7.5% hypertonic saline/dextran, compared with current standard therapy with normal saline, as an initial resuscitation IV fluid, improves survival following severe TBI.
Patient Population
Patients were included in the trial based on the following: blunt mechanism of injury, age greater than or equal to 15 years, and Glasgow Coma Scale (GCS) score of less than or equal to 8. Patients with systolic blood pressure of less than or equal to 70 mm Hg or less or of 71 to 90 mm Hg with a concomitant heart rate of greater than or equal to 108 per minute. Out-of-hospital cardiopulmonary resuscitation, administration of more than 2,000 mL of crystalloid or any amount of colloid or blood products prior to enrollment, severe hypothermia (< 28°C), drowning, asphyxia due to hanging, burns on more than 20% of total body surface area, isolated penetrating head injury, inability to obtain IV access, more than 4 hours between receipt of dispatch call to study intervention, prisoner status, and interfacility transfer were excluded in the trial. We included patients with at least two measurements of serum chloride concentrations in the first 24 hours of randomization in our analysis. We excluded patients with missing values for age, randomization group, Injury Severity Score (ISS) (19), Marshall head CT grade (20), GCS, or 6-month Extended Glasgow Outcome Scale (GOSE).
Trial Intervention
Patients were randomized to a single IV dose (250 cc) of 7.5% saline in 6% dextran-70, 7.5% saline (no dextran), or crystalloid as the initial fluid for prehospital resuscitation. The concurrent trauma resuscitation, transfusions, ICU insulin infusion/blood glucose control, sedation/analgesia protocol for mechanical ventilation, mechanical ventilation, venous thromboembolism prophylaxis, ventilator-associated pneumonia management, and management of severe TBI were based on existing resuscitation and critical care management guidelines to standardize management across all participating sites.
Definition of Hyperchloremia
All in-hospital serum sodium and chloride concentrations in the first 24 hours after randomization were tracked. Patients with an increased serum chloride concentration at any recording in first 24 hours were identified. Hyperchloremia was defined by serum chloride concentrations of 110 mmol/L or greater consistent with previous studies (3–6, 21). We further categorized hyperchloremia into one occurrence or greater than or equal to 2 occurrences. We also calculated the area under the curve (AUC) to provide a summary of serum chloride concentrations over the 24-hour period. The larger the AUC value, the greater the serum chloride concentrations over the 24 hours.
Data Analyzed
The initial data, including demographics, prehospital time, and volume of fluid administered; trial intervention administered; and emergency department (ED) hemodynamic and laboratory variables, were analyzed. Furthermore, in-hospital interventions based on a daily review of the medical records and results of diagnostic studies were analyzed including total volume of fluids administered during the first 24 hours since 911 call. Renal dysfunction was classified using Multiple Organ Dysfunction scoring systems based on highest serum creatinine concentrations during hospitalization as follows: less than or equal to 100 mmol/L = 0; less than 201 mmol/L = 1; less than 351 mmol/L = 2; less than or equal to 500 mmol/L = 3; and otherwise = 4 (22). The GOSE at 1 month and 180 days post-randomization were obtained by using a previously validated (inter-rater reliability weighted kappa values of 0.85) method of structured interview by phone (23). If the patient was unable to respond to the interview, information was acquired from a family member or caregiver (23–25). The raters filled out the structured GOSE interview questionnaires based on response to 24 questions and provided an overall GOSE score for each patient as previously described (23). Vegetative state (GOSE 2) based on level of consciousness was assigned to patients who remained unresponsive and speechless, and severe disability based on function in home and outside home, was assigned as lower severe disability (GOSE 3) if patient was unable to look after themselves for 8 hours, and upper severe disability (GOSE 4) was unable to look after themselves for 24 hours or unable to shop or unable to travel. Additional assessments of neurologic outcome included the Disability Rating Score at 1 month (24). The primary outcome was death, vegetative state, or severe disability at 180 days post-randomization based on GOSE of less than or equal to 4. The secondary outcome was death within 180 days post-randomization.
Statistical Analysis
The complete dataset from ROC HS-TBI trial was used, and formal sample size calculations were not performed. Categorical and continuous data were presented as mean with sd and percentages. Means and frequencies were compared using one-way analysis of variance and the chi-square (26) method, respectively. We evaluated the association between the two strata of hyperchloremia (one occurrence or ≥ 2 occurrences) with the patients’ demographic and clinical and laboratory characteristics. Ordinal logistic regression analysis and F tests were used to identify any differences in the distribution of GOSE grades between patients without hyperchloremia and those with one occurrence or those with greater than or equal to 2 occurrences of hyperchloremia.
We evaluated the effect of hyperchloremia on the odds of death, vegetative state, or severe disability at 180 days post-randomization (27) using stepwise logistic regression analysis. We adjusted for age (continuous variable), admission GCS score (continuous variable), and CT scan classification (Marshall grades II, III, IV, mass lesion, and other entered as a categorical variable) (28–30). Because our study cohort also included polytrauma patients, we also adjusted for ISS (continuous variable) in the model (31). A second multivariate analysis was performed using death within 180 days post-randomization as the dependent variable and the model was adjusted for the same potential confounding factors as mentioned above. A p value below 0.05 was defined as an entry criterion in each of the stepwise regressions. A p value below 0.05 was considered significant. We repeated the above-mentioned two analyses after entering the total volume of fluids administered in the first 24 hours (continuous variable) and an interaction term between the total volume of fluids administered during the first 24 hours and greater than or equal to 2 occurrences of hyperchloremia.
We performed additional logistic regression analyses to determine the effect of AUC of chloride concentrations and baseline (admission) hyperchloremia on the odds of 1) death, vegetative state, or severe disability at 180 days and 2) death within 180 days after adjusting for the above-mentioned variables.
We performed all analyses using the R software (R Core Team 2021, R Foundation for Statistical Computing, Vienna, Austria [https://www.R-project.org/]).
RESULTS
Among the 991 patients analyzed, the mean number (± sd) of serum chloride concentration measurements was 3.9 ± 1.9 over a mean period (± sd) of 16.4 ± 7.5 hours since ED arrival. Hyperchloremia in the first 24 hours was seen in 164 patients with one occurrence and 580 with greater than or equal to 2 occurrences, respectively (Table 1). The proportions of patients who received hypertonic saline and hypertonic saline/dextran were significantly higher among patients with hyperchloremia. The proportions of patients with ISS greater than 26 were significantly higher among patients with hyperchloremia. The proportion of patients who Marshall grade of diffuse injury I was significantly lower among patients with greater than or equal to 2 occurrences of hyperchloremia. The total out-of-hospital time interval was shorter among patients with hyperchloremia but larger amounts of prehospital fluids were administered in patients with hyperchloremia. The first systolic blood pressure in ED was lower among patients with hyperchloremia. Patients with hyperchloremia were more likely to have lower hemoglobin and platelet counts and more likely to have higher international normalized ratio (INR) and partial thromboplastin time (PTT) in the ED.
TABLE 1.
Baseline Characteristics of Patients With Severe Traumatic Brain Injury According to Strata Defined by Presence or Absence of Hyperchloremia
| Variables | No Hyperchloremia | Single Occurrence of Hyperchloremia | Two or More Occurrences of Hyperchloremia |
|---|---|---|---|
| Overall, n (%) | 247 (24.9) | 164 (16.5) | 580 (58.5) |
| Age, mean ± sda | 42.5 ± 19.3 | 35.9 ± 16.8 | 37.9 ± 18.0 |
| Gender | |||
| Men | 190 (76.9) | 128 (78.0) | 416 (71.7) |
| Women | 57 (23.1) | 36 (22.0) | 164 (28.3) |
| Race/ethnicity | |||
| Asian | 6 (2.4) | 5 (3.0) | 19 (3.3) |
| African American | 27 (10.9) | 15 (9.1) | 42 (7.2) |
| Whitea | 142 (57.5) | 100 (61.0) | 275 (47.4) |
| Other | 2 (0.8) | 1 (0.6) | 12 (2.1) |
| Hispanic or Latinoa | 25 (10.1) | 22 (13.4) | 40 (6.9) |
| Randomization group | |||
| Hypertonic saline/dextrana | 17 (6.9) | 35 (21.3) | 215 (37.1) |
| Hypertonic salinea | 19 (7.7) | 47 (28.7) | 209 (36.0) |
| Normal salinea | 211 (85.4) | 82 (50.0) | 156 (26.9) |
| Clinical severity | |||
| Out-of-hospital GCS‚ median (range) | 3 (3-8) | 3 (3-7) | 3 (3-6) |
| Out-of-hospital GCS > 5 | 84 (34.0) | 50 (30.5) | 163 (28.1) |
| Out-of-hospital GCS ≤ 5 | 163 (66.0) | 114 (69.5) | 417 (71.9) |
| ISS, median (range) | 25 (14-30) | 27 (17-38) | 31 (24-42) |
| ISS > 26 | 92 (37.2) | 82 (50.0) | 362 (62.4) |
| ISS ≤ 26 | 155 (62.8) | 82 (50.0) | 218 (37.6) |
| Marshall score based on initial head CT scan | |||
| Diffuse injury Ia | 88 (35.6) | 50 (30.5) | 130 (22.4) |
| Diffuse injury II | 88 (35.6) | 66 (40.2) | 218 (37.6) |
| Diffuse injury III | 27 (10.9) | 19 (11.6) | 93 (16.0) |
| Diffuse injury IV | 5 (2.0) | 3 (1.8) | 33 (5.7) |
| Mass lesion | 37 (15.0) | 24 (14.6) | 96 (16.6) |
| Others | 2 (0.8) | 2 (1.2) | 10 (1.7) |
| Out-of-hospital variables | |||
| Out-of-hospital advanced airway | 152 (61.5) | 108 (65.9) | 372 (64.1) |
| Time from dispatch call to fluid administration, min (mean ± sd) a | 32.0 ± 17.4 | 33.8 ± 25.7 | 36.3 ± 22.2 |
| Total out-of-hospital time, min (mean ± sd)a | 17,648.8 ± 15,089.6 | 15,014.6 ± 15,366.8 | 8,044.6 ± 12,279.0 |
| Prehospital fluids (L, mean ± sd)a | 0.8 ± 0.5 | 1.0 ± 0.7 | 1.0 ± 0.8 |
| Administration of >2 L of crystalloid or mannitol | 0 (0) | 0 (0) | 1 (0.2) |
| Emergency department variables | |||
| Systolic blood pressure (mm Hg, mean ± sd)a | 144.7 ± 31.5 | 135.2 ± 31.1 | 132.8 ± 35.2 |
| Hemoglobin (g/dL, mean ± sd)a | 13.5 ± 1.9 | 12.6 ± 2.1 | 11.7 ± 2.3 |
| International normalized ratio (mean ± sd)a | 1.2 ± 0.5 | 1.3 ± 0.6 | 1.3 ± 0.5 |
| Prothromboplastin time (mean ± sd)a | 27.0 ± 6.1 | 28.8 ± 15.6 | 32.9 ± 21.2 |
| Platelet counts (mean ± sd)a | 249.6 ± 77.5 | 239.1 ± 75.9 | 230.7 ± 69.4 |
| Serum creatinine (mean ± sd)a | 71.4 ± 36.3 | 84.4 ± 76.7 | 76.9 ± 31.2 |
| First pH (mean ± sd)a | 7.3 ± 0.1 | 7.3 ± 0.1 | 7.3 ± 0.1 |
| Highest lactate 0 to 12 hr (mean ± sd) | 3.9 ± 3.2 | 4.5 ± 3.5 | 4.3 ± 2.5 |
| Intubationa | 218 (88.3) | 145 (88.4) | 554 (95.5) |
GCS = Glasgow Coma Scale, ISS = Injury Severity Score.
aSignificant at p = 0.05 (Student t test and χ2 tests with Bonferroni correction for continuous and categorical variables, respectively).
The mean total volume of IV fluids administered during the first 24 hours was significantly higher in patients with hyperchloremia (Table 2). During hospitalization, patients with hyperchloremia were more likely to develop single or greater than or equal to 2 occurrences of hypernatremia. Patients with hyperchloremia were more likely to require IV mannitol and undergo ventriculostomy or craniotomy during the first 5 days of admission. The mean worse renal organ dysfunction scores and the total duration of ICU stay were higher among patients with hyperchloremia.
TABLE 2.
In-Hospital and 30-Day Variables of Interest in Patients With Severe Traumatic Brain Injury According to Strata Defined by Presence or Absence of Hyperchloremia
| Variables | No Hyperchloremia | Single Occurrence of Hyperchloremia | Two or More Occurrences of Hyperchloremia |
|---|---|---|---|
| Overall, n (%) | 247 (24.9) | 164 (16.5) | 580 (58.5) |
| Fluids and electrolytes (first 24 hr) | |||
| Total fluids administered in first 24 hr (L, mean ± sd)a | 6.1 ± 4.4 | 8.5 ± 7.6 | 10.2 ± 7.9 |
| Single episode of hypernatremiaab | 8 (3.2) | 30 (18.3) | 95 (16.4) |
| Two or more episodes of hypernatremiaab | 19 (7.7) | 39 (23.8) | 358 (61.7) |
| In-hospital interventions | |||
| Mechanical ventilation | 106 (42.9) | 85 (51.8) | 383 (66.0) |
| Hyperventilation in first 5 d | 4 (1.6) | 3 (1.8) | 15 (2.6) |
| Ventriculostomy in first 5 da | 22 (8.9) | 24 (14.6) | 105 (18.1) |
| Craniotomy first 5 da | 25 (10.1) | 18 (11.0) | 108 (18.6) |
| Additional hypertonic fluids in first 5 d | 24 (9.7) | 23 (14.0) | 92 (15.9) |
| Mannitol use in first 5 da | 44 (17.8) | 39 (23.8) | 170 (29.3) |
| Autologous blood transfusions in first 24 hr (mL, mean ± sd) | 16.3 ± 228.5 | 34.8 ± 222.8 | 25.4 ± 253.3 |
| In-hospital events | |||
| Serious adverse events | 54 (21.9) | 42 (25.6) | 163 (28.1) |
| Serious adverse events related to intervention | 1 (0.4) | 2 (1.2) | 17 (2.9) |
| Worse renal organ dysfunction score (mean ± sd)a | 0.1 ± 0.5 | 0.3 ± 0.8 | 0.2 ± 0.6 |
| Acute respiratory distress syndrome | 17 (6.9) | 13 (7.9) | 62 (10.7) |
| Total ICU d (mean ± sd)a | 7.2 ± 9.6 | 7.9 ± 9.4 | 11.4 ± 16.2 |
| Post hospitalization events | |||
| Death within 30 da | 49 (19.8) | 39 (23.8) | 181 (31.2) |
| Death, vegetative state, or disability at 30 da | 118 (47.8) | 75 (45.7) | 320 (55.2) |
| Disability rating scale at 30 d (mean ± sd)a | 12.0 ± 12.6 | 13.4 ± 13.1 | 17.4 ± 12.8 |
aSignificant at p = 0.05 (Student t test and χ2 tests with Bonferroni correction for continuous and categorical variables, respectively).
bHypernatremia is defined by serum sodium > 145 mmol/L.
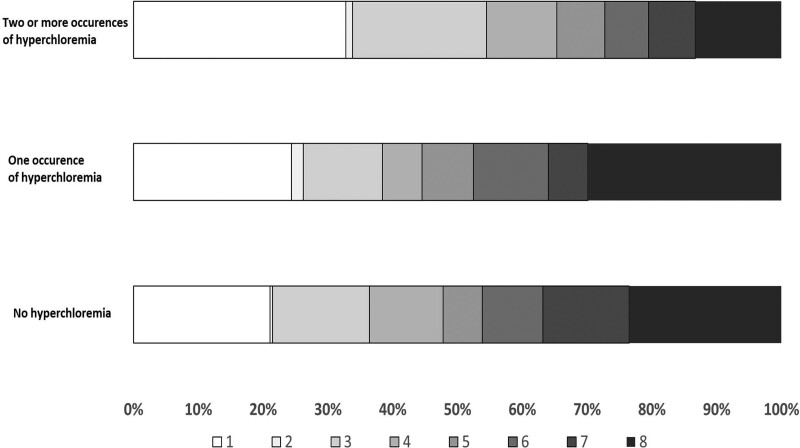
There was a higher rate of death, vegetative state, or severe disability at 180 days in patients with one occurrence (44.5%) or greater than or equal to 2 occurrences (65.3%) compared with those with no hyperchloremia (47.8%) (Table 3). There was a higher rate of death within 180 days in patients with one occurrence (24.4%) or greater than or equal to 2 occurrences (32.8%) compared with those with no hyperchloremia (21.1%). The distribution of GOSE scores at 6 months was significantly different in patients with greater than or equal to 2 occurrences (odds ratio [OR], 0.85; 95% CI, 0.8–0.9) of hyperchloremia but not with one occurrence of hyperchloremia (OR, 1; 95% CI, 0.9–1.1) from those with no occurrence (Fig. 1).
TABLE 3.
Effect of Hyperchloremia on Death or Severe Disability and Death in Patients With Traumatic Brain Injury in Multivariate Analysis
| Strata Defined Presence or Absence of Hyperchloremia | Rates of Death, Vegetative State, or Severe Disability at 180 d, n (%) | OR(95% CI) | Rates of Death Within 180 d, n (%) | OR (95% CI) |
|---|---|---|---|---|
| Model 1a | ||||
| No hyperchloremia | 118 (47.8) | Reference | 52 (21.1) | Reference |
| Single occurrence of hyperchloremia | 73 (44.5) | 0.90 (0.55–1.46) | 40 (24.4) | 1.53 (0.84–2.77) |
| Two or more occurrences of hyperchloremia | 379 (65.3) | 1.81 (1.19–2.75) | 190 (32.8) | 1.89 (1.14–3.08) |
| Model 2b | ||||
| No hyperchloremia | 118 (47.8) | Reference | 52 (21.1) | Reference |
| Single occurrence of hyperchloremia | 73 (44.5) | 0.77 (0.46–1.28) | 40 (24.4) | 1.12 (0.59–2.11) |
| Two or more occurrences of hyperchloremia | 379 (65.3) | 1.62 (0.89–2.96) | 190 (32.8) | 2.35 (1.21–4.61) |
OR = odds ratio.
aModel 1 adjusted for age, admission Glasgow Coma Scale score, randomized group, CT scan classification (Marshall grades), and Injury Severity Score.
bModel 2 adjusted for age, admission Glasgow Coma Scale score, randomized group, CT scan classification (Marshall grades), Injury Severity Score, and total fluids administered in first 24 hr (continuous variable) and an interaction term between total fluids administered in first 24 hr and hyperchloremia.
Figure 1.
The Extended Glasgow Outcome Scale score distribution at 180 d post-randomization in patients enrolled in Resuscitation Outcomes Consortium Hypertonic Saline-Traumatic Brain Injury trial according to groups defined by occurrence of hyperchloremia.
In model 1, patients with greater than or equal to 2 occurrences of hyperchloremia had significantly higher odds of death, vegetative state, or severe disability at 180 days (OR, 1.81; 95% CI, 1.19–2.75; Table 3) and death within 180 days (OR, 1.89; 95% CI, 1.14–3.08) compared with patients without hyperchloremia, after adjustment for age, randomization group, ISS, Marshall head CT grade, and GCS. In model 2, after entering total volume of fluids administered during first 24 hours and the interaction term between total volume of fluids administered during the first 24 hours and greater than or equal to 2 occurrences of hyperchloremia, patients with greater than or equal to 2 occurrences of hyperchloremia compared with patients without hyperchloremia, had significantly higher odds of death within 180 days (OR, 2.35; 95% CI, 1.21–4.61). Patients who received higher total volume of fluids during the first 24 hours had higher odds of death at 180 days (OR, 2.35; 95% CI, 1.21–4.61) but not death, vegetative state, or severe disability at 180 days (OR, 1.62; 95% CI, 0.89–2.96). The interaction term between total volume of fluids administered during the first 24 hours and greater than or equal to 2 occurrences of hyperchloremia was significant in the analysis of death within 180 days (p = 0.02) but not significant in the analysis of death, vegetative state, or severe disability at 180 days (p = 0.43).
There was a significant association between AUC of serum chloride concentrations and death within 180 days (OR, 0.999; 95% CI, 0.999–1.000; p = 0.026). There was no association between AUC of serum chloride concentrations and death, vegetative state, or severe disability at 180 days (OR, 1.000; 95% CI, 0.999–1.000; p = 0.996). There was no association between baseline hyperchloremia and death within 180 days (OR, 0.998; 95% CI, 0.97–1.03; p = 0.872) and death, vegetative state, or severe disability at 180 days (OR, 0.98; 95% CI, 0.95–1.01; p = 0.113).
DISCUSSION
Patients with TBI who had greater than or equal to 2 occurrences of hyperchloremia had higher odds of death, vegetative state, or severe disability at 180 days (as defined by GOSE) and death within 180 days. The association was independent of other known predictors of outcome including age, admission GCS score, CT scan classification (Marshall grades), and ISS (28–31). The total volume of fluids administered during the first 24 hours was higher in patients with hyperchloremia with an interaction identified between the two variables as discussed in the subsequent paragraph. Patients with hyperchloremia had higher proportions of patients who received hypertonic saline and hypertonic saline/dextran and also received larger volumes of prehospital fluids and total fluids administered during the first 24 hours. The patients who developed hyperchloremia had lower systolic blood pressure, lower hemoglobin, and platelet counts and more likely to have higher INR and PTT in the ED. Patients with hyperchloremia were more likely to require IV mannitol and undergo ventriculostomy or craniotomy in the first 5 days of admission. The mean worse renal organ dysfunction scores were higher among patients with hyperchloremia. The greater severity of TBI, as reflected by these variables, may be a contributor to the higher rates of death and death, vegetative state, or severe disability observed among patients with greater than or equal to 2 occurrences of hyperchloremia.
In our analysis, when the total volume of fluids administered with first 24 hours was entered in the multivariate model, the association between greater than or equal to 2 occurrences of hyperchloremia and death, vegetative state, or severe disability lost statistical significance but the association with death within 180 days increased (from OR of 1.89 to 2.35). The total volume of fluids administered during the first 24 hours was an independent predictor of death within 180 days. There was a significant interaction between greater than or equal to 2 occurrences of hyperchloremia and the total volume of fluids administered during the first 24 hours in the multivariate analysis. The complex relationship between volume of total fluids administered and serum chloride concentrations were also identified in the isotonic Solution Administration Logistical Testing (SALT) study (32). In the SALT study (32), patients who received normal saline (compared with those who received lower chloride content crystalloid solution) had higher serum chloride concentrations, higher peak serum creatinine concentrations, greater occurrence of AKI, and more frequent renal replacement treatments compared with those patients. However, the relationship was only seen in patients who received larger volumes of crystalloids but not in those who received smaller volumes. Like other trials (32, 33), we were unable to further discern the complex relationship between chloride load, serum chloride concentrations, total fluid administered, and intravascular fluid volume.
Our data add to existing body of evidence in the relationship between hyperchloremia and death or disability observed in other neurologic diseases such as ischemic stroke and ICH (6, 7, 10), particularly among those receiving IV hypertonic saline (11, 12). There is ambiguity regarding whether hyperchloremia was directly contributing to death or death, vegetative state, or severe disability in patients with TBI or simply a manifestation of more severe neurologic and systemic involvement after TBI and did not contribute directly to death, vegetative state, or severe disability. In theory, hyperchloremia may induce metabolic acidosis, which can result in intracellular and extracellular acidosis in the brain leading to cytotoxicity via activation of enzymes and alteration in ionic movements (34, 35) and dysfunction of aquaporin-4 receptor leading to edema formation (36) and neural excitotoxicity (37). Hyperchloremia may also augment pro-inflammatory response (38, 39) and modification of neutrophil and myeloperoxidase activity (40). We also studied the effect of iso-osmolar doses (5.5 mOsm/kg) of IV mannitol (1 g/kg), 3% sodium chloride (5.3 mL/kg), or 23.4% sodium chloride (0.7 mL/kg) on blood-brain barrier permeability in a canine model of ICH, 2 hours after the introduction of hematoma (41). Significant blood-brain barrier disruption was slightly higher after 2 hours of administration of 23.4% sodium chloride compared with both mannitol and 3% sodium chloride. Hyperchloremia has been associated with AKI in patients with ischemic stroke and ICH (7, 9, 10). The mean worse renal organ dysfunction scores were higher among patients with hyperchloremia in the current analysis. High IV chloride loads can result in reduction of renal blood flow, glomerular filtration rates, and renal cortical tissue perfusion in experimental models and clinical studies (42, 43). Occurrence of AKI has been associated with higher rates of mortality among TBI patients (44) and maybe contributing to higher mortality associated with hyperchloremia. There are two aspects of our findings that may suggest a bystander effect rather than a cause-effect relationship between hyperchloremia and outcomes in severe TBI patients. First, the exposure to hyperchloremia was short (in the first 24 hr) and second, the relationship was not seen with death, vegetative state, or severe disability (but only death) at 180 days. However, in the absence of more in-depth analysis involving several variables that were not available, no definitive conclusions can be derived.
The practical implication is how do these findings apply in the context of previous comparisons between normal saline and physiologically balanced crystalloids, such as lactated Ringer’s solution (sodium 130 mmol/L, chloride 109 mmol/L) and Plasma-Lyte A (sodium 140 mmol/L, chloride 98 mmol/L) in other patient populations. A meta-analysis of 21 studies involving 6,253 patients (13) found that high-chloride fluids were associated with a significantly higher risk of AKI, hyperchloremia, metabolic acidosis, blood transfusion volume, and mechanical ventilation time. However, no increase in mortality was observed (13). The Isotonic Solutions and Major Adverse Renal Events Trial (45) compared normal saline with balanced crystalloids (lactated Ringer’s solution or Plasma-Lyte A) in 15,802 critically ill adults. The primary endpoint was a composite of major renal adverse events within 30 days, death from any cause, new renal replacement therapy, or persistent renal dysfunction. Overall, the primary endpoint was significantly higher in patients who received normal saline, presumably due to higher chloride load (15.4% vs 14.3%). However, the primary outcome was lower (14% vs 15%) in patients with TBI treated with normal saline and higher in patients without TBI (15.5% vs 14.3%). Similarly, in the Balanced Solutions in Intensive Care Study (33), a prespecified subgroup analysis reported that patients with TBI who were treated with buffered solutions had a higher mortality compared with those treated with normal saline (31.3% vs 21.1%). The results highlighted the issue regarding osmolality, which may have to considered concurrently with the chloride load in patients with TBI. Some of the most commonly used “balanced” solutions (like lactated Ringer’s solution) are relatively hypotonic with an osmolarity of 273 mOsmol/L and a measured osmolality of 254 mOsmol/kg (46, 47), which may increase brain water content worsening cerebral edema.
Our analysis has certain limitations that should be considered. We were unable to determine whether the volume of fluids administered during the first 24 hours was driven by clinical manifestations of low intravascular volume and whether the fluid administration was adequate to replace the intravascular volume deficit or whether under-resuscitation had occurred, particularly in those patients who suffered death, vegetative state, or severe disability. The ISS, Marshall head CT grade, and GCS are not informative about important parameters such as degree of resuscitation, fluid loss, or severity of hemorrhage. We did not observe any differences in other surrogate markers of tissue perfusion and resuscitation such as serum lactate concentration or volume of autologous blood transfusions between groups defined by occurrence of hyperchloremia. The mean worse renal organ dysfunction scores were higher among patients with hyperchloremia, which could potentially be related to low intravascular volume. We were unable to determine the contribution of hypertonic saline in predisposing to hyperchloremia, which would require segregation of each of the normal saline-resuscitated patients based on normal and high serum chloride concentrations with measure of total chloride administered from additional fluids and the diluted volume (intravascular volume at the time). There was variability in the number and the timing of measurements of serum chloride concentrations between patients, which could induce a bias with patients anticipated to have hyperchloremia more likely to get multiple measurements. We used AUC to provide a more detailed perspective of serum chloride concentrations (8, 9). We could not use within-subjects regressions in which every measurement of each subject is regressed on the time axis using random effects modeling (48) to create a summary statistic because prehospital serum chloride concentrations were not measured. The data on serum chloride concentrations after the first 24 hours was not ascertained and precluded any analysis of effect of occurrence of hyperchloremia after the first 24 hours of randomization. Therefore, the total chloride burden during the ICU stay was not quantified limiting the analysis between exposure and outcome. We acknowledge the limitations of post hoc analysis apply to our analysis (49, 50) because the subgroup hypothesis, statistical plan, or the direction of effect was not prespecified (49, 50). Therefore, the results may also be contaminated by chance, differences in patients’ clinical characteristics and other concurrent interventions. While each site in the trial was encouraged to implement resuscitation and critical care management guidelines, previous studies have found variance between institutions in compliance to such guidelines (51). The trial protocol or existing guidelines at that time did not specify any specific guidelines for detection or management of hyperchloremia (16, 52, 53), so there may considerable variations in management of hyperchloremia with undetermined effect on our. Serum chloride concentration can be measured using several techniques (54) including colorimetric, coulometric, mercurimetric, and chloride-specific ion electrode methods. We cannot comment on variability in methods used between sites in ROC HS-TBI trial to determine serum chloride concentration.
CONCLUSIONS
After modifying the total volume of fluids administered during the first 24 hours, we found that multiple occurrences of hyperchloremia in the first 24 hours in patients with severe TBI were associated with higher odds of death within 180 days.
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Breen TJ, Brueske B, Sidhu MS, et al. : Abnormal serum chloride is associated with increased mortality among unselected cardiac intensive care unit patients. PLoS One 2021; 16:e0250292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shao M, Li G, Sarvottam K, et al. : Dyschloremia is a risk factor for the development of acute kidney injury in critically ill patients. PLoS One 2016; 11:e0160322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yessayan L, Neyra JA, Canepa-Escaro F, et al. ; Acute Kidney Injury in Critical Illness Study Group: Effect of hyperchloremia on acute kidney injury in critically ill septic patients: A retrospective cohort study. BMC Nephrol 2017; 18:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neyra JA, Canepa-Escaro F, Li X, et al. ; Acute Kidney Injury in Critical Illness Study Group: Association of hyperchloremia with hospital mortality in critically ill septic patients. Crit Care Med 2015; 43:1938–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boniatti MM, Cardoso PR, Castilho RK, et al. : Is hyperchloremia associated with mortality in critically ill patients? A prospective cohort study. J Crit Care 2011; 26:175–179 [DOI] [PubMed] [Google Scholar]
- 6.Huang K, Hu Y, Wu Y, et al. : Hyperchloremia is associated with poorer outcome in critically ill stroke patients. Front Neurol 2018; 9:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh TK, Jeon YT, Sohn H, et al. : Association of perioperative hyperchloremia and hyperchloremic metabolic acidosis with acute kidney injury after craniotomy for intracranial hemorrhage. World Neurosurg 2019; 125:e1226–e1240 [DOI] [PubMed] [Google Scholar]
- 8.Qureshi AI, Huang W, Gomez FE, et al. : Early hyperchloremia and outcomes after acute ischemic stroke. J Stroke Cerebrovasc Dis 2022; 31:106523. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi AI, Huang W, Hanley DF, et al. : Early hyperchloremia is independently associated with death or disability in patients with intracerebral hemorrhage. Neurocritical Care 2022; 1:10 [DOI] [PubMed] [Google Scholar]
- 10.Sadan O, Singbartl K, Kandiah PA, et al. : Hyperchloremia is associated with acute kidney injury in patients with subarachnoid hemorrhage. Crit Care Med 2017; 45:1382–1388 [DOI] [PubMed] [Google Scholar]
- 11.Riha HM, Erdman MJ, Vandigo JE, et al. : Impact of moderate hyperchloremia on clinical outcomes in intracerebral hemorrhage patients treated with continuous infusion hypertonic saline: A pilot study. Crit Care Med 2017; 45:e947–e953 [DOI] [PubMed] [Google Scholar]
- 12.Sigmon J, May CC, Bryant A, et al. : Assessment of acute kidney injury in neurologically injured patients receiving hypertonic sodium chloride: Does chloride load matter? Ann Pharmacother 2020; 54:541–546 [DOI] [PubMed] [Google Scholar]
- 13.Krajewski ML, Raghunathan K, Paluszkiewicz SM, et al. : Meta-analysis of high- versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg 2015; 102:24–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadan O, Singbartl K, Kraft J, et al. : Low-chloride- versus high-chloride-containing hypertonic solution for the treatment of subarachnoid hemorrhage-related complications: The ACETatE (A low ChloriE hyperTonic solution for brain Edema) randomized trial. J Intensive Care 2020; 8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semler MW, Kellum JA: Balanced crystalloid solutions. Am J Respir Crit Care Med 2019; 199:952–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brasel KJ, Bulger E, Cook AJ, et al. ; Resuscitation Outcomes Consortium Investigators: Hypertonic resuscitation: Design and implementation of a prehospital intervention trial. J Am Coll Surg 2008; 206:220–232 [DOI] [PubMed] [Google Scholar]
- 17.Bulger EM, May S, Brasel KJ, et al. ; ROC Investigators: Out-of-hospital hypertonic resuscitation following severe traumatic brain injury: A randomized controlled trial. JAMA 2010; 304:1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qureshi AI, Malik AA, Adil MM, et al. : Hematoma enlargement among patients with traumatic brain injury: Analysis of a prospective multicenter clinical trial. J Vasc Interv Neurol 2015; 8:42–49 [PMC free article] [PubMed] [Google Scholar]
- 19.Walder A, Yeoman P, Turnbull A: The abbreviated injury scale as a predictor of outcome of severe head injury. Intensive Care Med 1995; 21:606–609 [DOI] [PubMed] [Google Scholar]
- 20.Marshall LF, Marshall SB, Klauber MR, et al. : The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma 1992; 9:S287–S292 [PubMed] [Google Scholar]
- 21.Kim HJ, Oh TK, Song I, et al. : Association between fluctuations in serum chloride levels and 30-day mortality among critically ill patients: A retrospective analysis. BMC Anesthesiol 2019; 19:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall JC, Cook DJ, Christou NV, et al. : Multiple organ dysfunction score: A reliable descriptor of a complex clinical outcome. Crit Care Med 1995; 23:1638–1652 [DOI] [PubMed] [Google Scholar]
- 23.Pettigrew LE, Wilson JT, Teasdale GM: Assessing disability after head injury: Improved use of the Glasgow Outcome Scale. J Neurosurg 1998; 89:939–943 [DOI] [PubMed] [Google Scholar]
- 24.Rappaport M, Hall KM, Hopkins K, et al. : Disability rating scale for severe head trauma: Coma to community. Arch Phys Med Rehabil 1982; 63:118–123 [PubMed] [Google Scholar]
- 25.Wilson JT, Pettigrew LE, Teasdale GM: Structured interviews for the glasgow outcome scale and the extended glasgow outcome scale: Guidelines for their use. J Neurotrauma 1998; 15:573–585 [DOI] [PubMed] [Google Scholar]
- 26.Chang EF, Meeker M, Holland MC: Acute traumatic intraparenchymal hemorrhage: Risk factors for progression in the early post-injury period. Neurosurgery 2006; 58:647–656; discussion 647–656 [DOI] [PubMed] [Google Scholar]
- 27.Weir J, Steyerberg EW, Butcher I, et al. : Does the extended glasgow outcome scale add value to the conventional glasgow outcome scale? J Neurotrauma 2012; 29:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chesnut RM, Ghajar J, Maas A, et al. : Early indicators of prognosis in severe traumatic brain injury. J Neurotrauma 2000; 17:557–627 [Google Scholar]
- 29.Murray GD, Butcher I, McHugh GS, et al. : Multivariable prognostic analysis in traumatic brain injury: Results from the IMPACT study. J Neurotrauma 2007; 24:329–337 [DOI] [PubMed] [Google Scholar]
- 30.Riley RD, Hayden JA, Steyerberg EW, et al. ; PROGRESS Group: Prognosis Research Strategy (PROGRESS) 2: Prognostic factor research. PLoS Med 2013; 10:e1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foreman BP, Caesar RR, Parks J, et al. : Usefulness of the abbreviated injury score and the injury severity score in comparison to the Glasgow Coma Scale in predicting outcome after traumatic brain injury. J Trauma 2007; 62:946–950 [DOI] [PubMed] [Google Scholar]
- 32.Semler MW, Wanderer JP, Ehrenfeld JM, et al. ; SALT Investigators * and the Pragmatic Critical Care Research Group: Balanced crystalloids versus saline in the intensive care unit. The SALT randomized trial. Am J Respir Crit Care Med 2017; 195:1362–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zampieri F, Machado F, Biondi R, et al. : BaSICS investigators and the BRICNet members: Effect of intravenous fluid treatment with a balanced solution vs 0.9% saline solution on mortality in critically ill patients: The BaSICS randomized clinical trial. JAMA 2021; 326:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gusev E, Skvortsova VI: Metabolic acidosis and ischemic damage. In: Brain Ischemia. Boston, MA, Springer, 2003, pp 95–99 [Google Scholar]
- 35.Vogh BP, Maren TH: Sodium, chloride, and bicarbonate movement from plasma to cerebrospinal fluid in cats. Am J Physiol 1975; 228:673–683 [DOI] [PubMed] [Google Scholar]
- 36.Bithal PK: Sodium bicarbonate is knocking at the door of neurocritical care unit: Should we allow its entry? J Neuroanaesthesiol Crit Care 2018; 5:75–76 [Google Scholar]
- 37.Zhao H, Cai Y, Yang Z, et al. : Acidosis leads to neurological disorders through overexciting cortical pyramidal neurons. Biochem Biophys Res Commun 2011; 415:224–228 [DOI] [PubMed] [Google Scholar]
- 38.Kellum JA, Song M, Almasri E: Hyperchloremic acidosis increases circulating inflammatory molecules in experimental sepsis. Chest 2006; 130:962–967 [DOI] [PubMed] [Google Scholar]
- 39.Kellum JA, Song M, Li J: Lactic and hydrochloric acids induce different patterns of inflammatory response in LPS-stimulated RAW 264.7 cells. Am J Physiol Regul Integr Comp Physiol 2004; 286:R686–R692 [DOI] [PubMed] [Google Scholar]
- 40.Aiken ML, Painter RG, Zhou Y, et al. : Chloride transport in functionally active phagosomes isolated from human neutrophils. Free Radic Biol Med 2012; 53:2308–2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qureshi AI, Wilson DA, Traystman RJ: Treatment of elevated intracranial pressure in experimental intracerebral hemorrhage: Comparison between mannitol and hypertonic saline. Neurosurgery 1999; 44:1055–1063; discussion 1063 [DOI] [PubMed] [Google Scholar]
- 42.Chowdhury AH, Cox EF, Francis ST, et al. : A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte(R) 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg 2012; 256:18–24 [DOI] [PubMed] [Google Scholar]
- 43.Wilcox CS: Regulation of renal blood flow by plasma chloride. J Clin Invest 1983; 71:726–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore EM, Bellomo R, Nichol A, et al. : The incidence of acute kidney injury in patients with traumatic brain injury. Ren Fail 2010; 32:1060–1065 [DOI] [PubMed] [Google Scholar]
- 45.Semler MW, Self WH, Wanderer JP, et al. ; SMART Investigators and the Pragmatic Critical Care Research Group: Balanced crystalloids versus saline in critically ill adults. N Engl J Med 2018; 378:829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams EL, Hildebrand KL, McCormick SA, et al. : The effect of intravenous lactated Ringer’s solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg 1999; 88:999–1003 [DOI] [PubMed] [Google Scholar]
- 47.Zornow MH, Todd MM, Moore SS: The acute cerebral effects of changes in plasma osmolality and oncotic pressure. Anesthesiol 1987; 67:936–941 [DOI] [PubMed] [Google Scholar]
- 48.Qureshi AI, Bliwise DL, Bliwise NG, et al. : Rate of 24-hour blood pressure decline and mortality after spontaneous intracerebral hemorrhage: A retrospective analysis with a random effects regression model. Crit Care Med 1999; 27:480–485 [DOI] [PubMed] [Google Scholar]
- 49.Hasford J, Bramlage P, Koch G, et al. : Inconsistent trial assessments by the National Institute for Health and Clinical Excellence and IQWiG: Standards for the performance and interpretation of subgroup analyses are needed. J Clin Epidemiol 2010; 63:1298–1304 [DOI] [PubMed] [Google Scholar]
- 50.Srinivas TR, Ho B, Kang J, et al. : Post hoc analyses: After the facts. Transplantation 2015; 99:17–20 [DOI] [PubMed] [Google Scholar]
- 51.Shafi S, Barnes SA, Millar D, et al. : Suboptimal compliance with evidence-based guidelines in patients with traumatic brain injuries. J Neurosurg 2014; 120:773–777 [DOI] [PubMed] [Google Scholar]
- 52.Bratton S, Chestnut R, Ghajar J, et al. : Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS. Guidelines for the management of severe traumatic brain injury. J Neurotrauma 2007; 24:S59–S6417511547 [Google Scholar]
- 53.Patel A, Vieira MM, Abraham J, et al. : Quality of the development of traumatic brain injury clinical practice guidelines: A systematic review. PLoS One 2016; 11:e0161554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker HK, Hall WD, Hurst JW: Clinical Methods: The History, Physical, and Laboratory Examinations, Buttersworth, Boston, MA (USA). 1990 [Google Scholar]