Abstract
Inflammatory bowel disease (IBD) has a global presence with rapidly increasing incidence and prevalence. Patients with IBD including those with ulcerative colitis and Crohn’s disease have a higher risk of developing colorectal cancer (CRC) compared to the general population. Risk factors for CRC in patients with IBD include long disease duration, extensive colitis, primary sclerosing cholangitis, family history of CRC, stricture, and prior dysplasia. Surveillance colonoscopy for CRC in patients with IBD should be tailored to individualized risk factors and requires careful monitoring every year to every five years. The current surveillance techniques are based on several guidelines. Chromoendoscopy with targeted biopsy is being recommended increasingly, and high-definition colonoscopy is gradually replacing standard-definition colonoscopy. However, it remains unclear whether chromoendoscopy, virtual chromoendoscopy, or white-light endoscopy has better efficiency when a high-definition scope is used. With the development of new endoscopic instruments and techniques, the paradigm of surveillance strategy has gradually changed. In this review, we discuss cutting-edge surveillance colonoscopy in patients with IBD including a review of literature.
Keywords: Colitis, ulcerative; Colonoscopy; Colorectal neoplasms; Crohn’s disease; Inflammatory bowel diseases
INTRODUCTION
Inflammatory bowel disease (IBD) has a global presence with rapidly increasing incidence and prevalence in Asia, although these remain lower than those reported in Western countries. The medical burden of this chronic inflammatory condition is gradually increasing. Due to its chronic clinical course, IBD is associated with various complications, particularly in case of long disease duration. Patients with IBD including those with ulcerative colitis (UC) and Crohn’s disease (CD) have a higher risk of colorectal cancer (CRC) than the general population. After the first case of CRC associated with UC was reported,1 many case reports were published in the 1940s and the 1950s. During this period, clinical characteristics of malignant change in UC included higher mortality and younger age at diagnosis compared to those in the general population, and CRC occurred mainly in patients with long-standing extensive colitis.2 In Korea, a case of malignant change in a patient with UC was reported for the first time in 1988.3
The dilemma regarding surveillance colonoscopy in patients with IBD is that malignant changes arising from chronic intestinal inflammation can occur without any visible, suspicious, or recognizable mucosal changes or lesions. Thus, surveillance for CRC in these patients requires more careful monitoring than that in the general population. Previous endoscopy guidelines in patients with IBD recommend that patients with left-sided or extensive UC should undergo surveillance colonoscopy with random biopsy every 1 to 2 years after 8 to 10 years of diagnosis.4 However, with developments in endoscopic instruments and techniques, the surveillance strategy paradigm is gradually changing according to an evidence-based consensus. In this review, we discuss surveillance colonoscopy in patients with IBD including a literature review.
EPIDEMIOLOGY
Patients with IBD are at an increased risk of developing CRC. In a meta-analysis published in 2001, the cumulative probability of CRC was as high as 18% at 30 years after the diagnosis of UC,5 but it was as low as 2.1% (similar to that in general population) in a 2004 population-based study in Copenhagen.6 A 30-year cohort study in Denmark showed that the overall relative risk (RR) of CRC decreased from 1.34 (95% confidence interval [CI], 1.13–1.58) in the 1980s to 0.57 (95% CI, 0.41–0.80) in the 2000s in patients with UC but did not change in patients with CD (RR, 0.85; 95% CI, 0.67–1.07).7 In patients with IBD diagnosed after age 30, a meta-analysis of nine population-based cohort studies conducted in 2013 found a tendency toward decreasing the risk of CRC in patients with IBD diagnosed after age 30, but this trend was not statistically significant.8 Nevertheless, the pooled standardized incidence ratio (SIR) of CRC in patients with IBD in 259,266 person-years (PYs) was 1.7 (95% CI, 1.2–2.2) in population-based studies.8 This decreasing risk of CRC in IBD over time might be due to the development of therapeutic drugs such as biologics and also due to surveillance colonoscopy programs. However, it could also be attributed to the age of the cohorts. High-risk patients were censored early during the follow-up and low-risk patients remained in the cohort, adding only PYs at risk to the aging cohorts. Concomitantly, sporadic CRC in the general population increased with age.8 This could explain the lower-than-expected SIR in IBD patients. For example, a population-based study showed that the risk of CRC in 7,607 patients with IBD during 198,227 PYs of follow-up did not change over time but had decreased when compared with the risk in the general population.9 To the best of our knowledge, no population-based studies in Asia have addressed the risk of CRC in IBD. However, several hospital-based studies have reported that the risk of CRC is increased in patients with long-standing IBD, a result similar to that observed in Western countries10-13
Unfortunately, CRC-related survival is lower among patients with IBD than among the general population, even after adjusting for tumor stage at diagnosis. Although the time trends for CRC-related death in patients with UC are declining, the overall adjusted hazard ratio (HR) was 1.54 (95% CI, 1.33–1.78) compared to that of the general population in a Scandinavian population-based cohort study.14 Moreover, in the CD cohort data from the same study group, CRC-related mortality was higher after adjusting for tumor stage (HR, 1.42; 95% CI, 1.16–1.75) than that of the general population.15
In a meta-analysis of five observational studies including 7,199 patients with IBD, surveillance colonoscopy for detecting CRC led to a lower cancer detection rate (odds ratio [OR], 0.58; 95% CI, 0.42–0.80; p<0.001), lower CRC-related mortality rate (OR, 0.36; 95% CI, 0.19–0.69; p=0.002), and higher early-stage CRC detection rate (OR, 5.40; 95% CI, 1.51–19.30; p=0.009) compare to the non-surveillance group.16 In a surveillance program conducted in patients with UC from the 1970s to the 2000s in the UK, the incidence rate of interval cancer steadily decreased from 2.5/1,000 PYs to 0.4/1,000 PYs (Pearson’s correlation, −0.99; p=0.007).17 Therefore, surveillance colonoscopy is needed in patients with IBD for early detection and appropriate treatment of colorectal neoplasms (CRNs).
RISK STRATIFICATION
The aforementioned epidemiological cohort studies have shown that the incidence of CRC in patients with IBD gradually decreases with time, and this tendency is expected to become more prominent in the future with the development of biologics and small-molecule drugs. Therefore, it is important to select and stratify patient-related risk factors for CRN in IBD to formulate surveillance strategies.
Patients with IBD generally have an increased risk of CRC with increasing disease duration. In a Hungarian population-based study, long disease duration, extensive colitis, and primary sclerosing cholangitis (PSC) in UC were suggested as risk factors for CRC.18 In a prospective cohort study of 19,486 patients with IBD (60% with CD) in France, extensive colitis in UC led to a higher incidence of CRC than left-sided colitis, while proctitis did not increase the incidence of CRC.19 The incidence rate of CRC was 2.2 times higher in patients with IBD than that in the general population (95% CI, 1.5–3.0; p<0.001), especially in patients with a disease duration of >10 years (7.0-fold risk; 95% CI, 4.4–10.5; p<0.001). However, there was no significant difference in short-term disease duration despite extensive colitis (p=0.84).19 A 30-year Danish cohort study also revealed that the incidence of CRC was higher in patients having young-onset UC (before 40 years of age) with a long disease duration compared to that in individuals with onset after 40 years of age. Similar findings were observed in patients with young-onset CD.7 In this cohort, patients having UC with PSC showed a higher incidence rate of CRC than those without PSC (RR, 9.13; 95% CI, 4.52–18.5). Moreover, the incidence of CRC in patients with UC gradually increased with longer disease duration and was 50% higher than that in the general population after 13 years.7
Another meta-analysis showed that extensive colitis in UC (SIR, 6.9; 11,164 PYs; 95% CI, 1.9–11.9) and young-onset IBD before the age of 30 years (SIR, 8.2; 46,623 PYs; 95% CI, 1.8–14.6) were significant risk factors for CRC.8 The risk of CRC increased at a disease duration beyond 10 years with a cumulative risk of 4.5% and a CRC-related risk of 2.4/1,000 PYs (95% CI, 0.8–7.2) over a disease duration of 20 years.8
The necessary interval, follow-up duration, and surveillance method may depend on the risk factors of CRC. A recent meta-analysis classified risk factors of CRC into three categories: extensive colitis as a strong risk factor; low-grade dysplasia, stricture, PSC, family history of CRC, post-inflammatory polyps, and IBD type (UC) as moderate risk factors; and colonic surgery, male sex, and age as low risk factors.20 The 2007 European Crohn’s and Colitis Organisation (ECCO) guidelines made a similar proposal for risk factor stratification of CRC. Recently, various risk assessment tools have been developed. One study described a “cumulative inflammatory burden” point, which was defined as the sum of the average scores between each pair of surveillance episodes multiplied by the surveillance interval in years. This point was significantly associated with the incidence of CRN (HR, 2.1 per 10-point increase; 95% CI, 1.4–3.0; p<0.001).21 Surveillance colonoscopy should be considered based on risk factor stratification in patients with IBD, especially in those with long-standing IBD.
INITIATION OF SURVEILLANCE COLONOSCOPY
In the 2002 British Society of Gastroenterology (BSG) and 2003 American Gastroenterology Association (AGA) guidelines, surveillance colonoscopy is recommended at 8 to 10 years after the onset of symptoms for extensive colitis or CD and after 15 to 20 years for left-sided colitis.22,23 However, while conducting surveillance according to these guidelines, one study showed that 17% and 22% of the patients having IBD and CRC were diagnosed with CRC within 8 and 15 years from the onset of symptoms or diagnosis, respectively.22 Therefore, in the 2019 BSG and ECCO guidelines, initiation of surveillance is recommended 8 years after the onset of symptoms in patients with colonic involvement. However, in patients with concurrent PSC, it is recommended to start the surveillance at the time of diagnosis regardless of disease activity, extent, or duration.24,25 The 2019 American College of Gastroenterology (ACG) guidelines for UC also recommend surveillance colonoscopy to assess dysplasia in patients with UC except in cases wherein the disease is limited to the rectum at 8 years after the diagnosis.26 The 2018 ACG guidelines for CD are very similar to the ECCO guidelines, and routine surveillance for small bowel CD is not recommended.27 The 2021 AGA clinical practice update retains the recommendations from the 2019 BSG and ECCO guidelines regarding the timing to start the screening.28
TECHNIQUES FOR SURVEILLANCE
In the 2019 BSG and ECCO guidelines for endoscopy techniques, similar to the 2015 Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients: International Consensus Recommendations (SCENIC) international consensus, high-definition (HD) rather than standard-definition (SD) endoscopy and chromoendoscopy (CE) with methylene blue or indigo carmine rather than white-light endoscopy (WLE) are strongly recommended with a moderate quality of evidence.24,25,29 HD WLE can be an alternate to CE, since special equipment and longer procedure times are required for CE. The 2019 ACG guidelines for UC have suggested HD plus either WLE with narrow-band imaging (NBI) or CE.26 In contrast, the same organization suggested against the use of NBI in CD.27 If invisible low-grade dysplasia is observed, CE and random biopsies are recommended within 3 months. If invisible high-grade dysplasia or carcinoma is observed, urgent repeat CE is recommended to determine the possibility of endoscopic resection or to assess synchronous dysplasia.24 Similar recommendations are made in the 2019 ACG guidelines for UC and in the 2021 AGA clinical practice update, suggesting CE as the first examination after UC-associated dysplasia is detected.26,28
SD CE vs. SD WLE
SD CE was superior to SD WLE in the detection of dysplasia in several randomized controlled trials (RCTs). In the first RCT in 2003, the SD CE group exhibited a greater incidence of intraepithelial neoplasia than the SD WLE group among patients with long-standing (≥8 years) UC (38.1% [32/84] vs. 12.3% [10/81]; p=0.003).30 The 2015 SCENIC international consensus also recommended SD CE over SD WLE.29 In this consensus, analyses of eight studies (two RCTs, four prospective tandem studies, and two retrospective studies) revealed that the dysplasia detection rate was 1.8-fold higher in SD CE with targeted biopsies than in SD WLE with random biopsies. A recent meta-analysis including three RCTs showed the overall superiority of SD CE over SD WLE (RR, 2.12; 95% CI, 1.15–3.91).31 Currently, when SD endoscopy is used for surveillance, major guidelines recommend SD CE rather than SD WLE.24-26
Although current guidelines recommend CE for surveillance, the need for special equipment, longer procedure time, and requirement of specialized training must be considered when compared with what is needed for WLE. CE techniques were as follows: (1) for lesion detection, two ampules of indigo carmine (0.8%, 5-mL ampule) plus 250 mL saline (0.03%) or one ampule of methylene blue (1%, 10-mL ampule) plus 240 mL saline (0.04%) and (2) for lesion characterization and delineation of borders, one ampule of indigo carmine plus 25 mL saline (0.13%) or one ampule of methylene blue plus 40 mL saline (0.2%) (Fig. 1).29
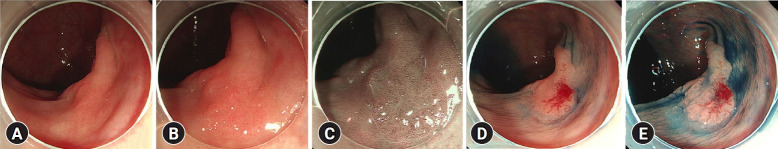
Fig. 1.
Endoscopic images of non-polypoid dysplasia. (A) Standard-definition white-light endoscopy. (B) High-definition white-light endoscopy. (C) High-definition narrow-band imaging. (D) Standard-definition chromoendoscopy using indigo carmine at a low concentration (0.04%). (E) Standard-definition chromoendoscopy using indigo carmine at a high concentration (0.2%).
SD vs. HD WLE
A retrospective study of patients having long-standing IBD (>7 years) compared 106 cases of SD WLE with 209 cases of HD WLE.32 The number of dysplastic lesions was 11 (6 on targeted biopsy) in the SD WLE group and 32 (27 on targeted biopsy) in the HD WLE group. The adjusted prevalence ratios of HD WLE compared with those of SD WLE for the detection of dysplasia and for any dysplasia detected with targeted biopsy were 2.21 (95% CI, 1.09–4.45; p=0.03) and 2.99 (95% CI, 1.16–7.77; p=0.02), respectively. HD WLE has a resolution of more than 1 million pixels per image, allowing a higher adenoma detection rate compared to SD WLE in the general population.33 Although there have been no RCTs in patients with IBD, we acknowledge that HD WLE is likely to improve the adenoma detection rate when compared with SD WLE.
HD CE vs. HD WLE
It is important to consider whether CE can improve the detection rate of dysplasia when compared with WLE, even when HD endoscopy is used. Thus, it is important to compare HD CE and HD WLE, since HD WLE is the current standard in endoscopic practice.
One RCT conducted in China showed that HD CE with targeted biopsy was superior to HD WLE with targeted biopsy in the diagnosis of dysplasia in patients having UC with a disease duration of ≥6 years (9.7% [14/145] vs. 1.9% [3/154]; p=0.004).34 This study also showed that HD CE with targeted biopsy and HD WLE with random biopsy had similar diagnosis rates for dysplasia (9.7% [14/145] vs. 8.1% [12/148]; p=0.642).34 In contrast, a Canadian RCT with targeted biopsy showed no significant difference in the diagnosis rates between HD CE and HD WLE (17.8% [16/90] vs. 18.9% [17/90]; p=0.91) in patients having long-standing IBD (>8 years).35 Another Swedish RCT including 32 random biopsies and additional targeted biopsies showed superior diagnostic efficacy of HD CE over HD WLE in extensive and long-standing UC or colonic CD (11.2% [17/152] vs. 4.6% [7/153], p=0.032).36 Another RCT in Korea showed no difference between HD CE with targeted biopsy and HD WLE with random biopsy (3.9% [4/102] vs. 5.6% [6/108]; p=0.749) in the detection of colitis-associated dysplasia among patients with UC (duration ≥8 years).37 Interestingly, the withdrawal time was similar between the groups in this study, although the total number of biopsies was greater in HD WLE than in HD CE. The aforementioned four RCTs are summarized in Table 1.34-37 In a meta-analysis of these four published RCTs, HD CE showed a similar detection rate for dysplastic lesions when compared with HD WLE (RR, 1.39; 95% CI, 0.95–2.04) (Fig. 2).
Table 1.
Summary of four randomized controlled trials for surveillance colonoscopy comparing HD CE vs. HD WLE in patients with IBD
| Study | Country | HD CE | HD WLE | Dysplasia detection rates |
|---|---|---|---|---|
| Iacucci et al. (2018)35 | Canada (single-center) | 90 Patients with IBD, targeted biopsy | 90 Patients with IBD, targeted biopsy | HD CE vs. HD WLE: 17.8% (16/90) vs. 18.9% (17/90), p=0.91 |
| Yang et al. (2019)37 | Korea (multi-center) | 108 Patients with UC, targeted biopsy | 102 Patients with UC, random biopsy | HD CE vs. HD WLE: 3.9% (4/102) vs. 5.6% (6/108), p=0.749 |
| Alexandersson et al. (2020)36 | Sweden (single-center) | 152 Patients with IBD, random biopsy | 153 Patients with IBD, random biopsy | HD CE vs. HD WLE: 11.2% (17/152) vs 4.6% (7/153), p=0.032 |
| Wan et al. (2020)34 | China (multi-center) | 39 Patients with UC, targeted biopsy | 43 Patients with UC, targeted biopsy | HD CE vs. HD WLE with targeted biopsy: 9.7% (14/145) vs. 1.9% (3/154), p=0.004 |
| 40 Patients with UC, random biopsy | HD CE vs. HD WLE with random biopsy: 9.7% (14/145) vs. 8.1% (12/148), p=0.642 |
HD, high-definition; CE, chromoendoscopy; WLE, white-light endoscopy; IBD, inflammatory bowel disease; UC, ulcerative colitis.
Fig. 2.
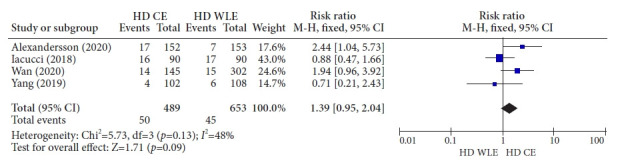
Forest plot for randomized controlled trials comparing high-definition chromoendoscopy and high-definition white-light endoscopy. HD, high-definition; CE, chromoendoscopy; WLE, white-light endoscopy; M-H, Mantel-Haenszel; CI, confidence interval.
HD CE vs. HD virtual CE
When HD endoscopy was used in a prospective randomized crossover tandem study with targeted biopsy, detection of true-positive lesions was similar between CE and NBI (5.8% [12/208] vs. 7.3% [10/136], p=0.644) among 60 patients with IBD (duration ≥8 years).38 However, NBI exhibited a higher trend of missed lesions on second inspection compared to CE (31.8% [7/22] vs. 13.6% [3/22]; p=0.2).38 In another tandem study, HD NBI followed by HD CE by two blinded endoscopists, and there was no significant trend in favor of HD CE over HD NBI for the detection of dysplasia (23 dysplastic lesions in 11 patients vs. 20 dysplastic lesions in ten patients; p=0.18) among 44 patients with long-standing IBD.39 Based on these results, the 2015 SCENIC international consensus and the 2017 ECCO guidelines suggested against the use of HD NBI in place of HD CE.24,29
Recently, an RCT including 131 patients with long-standing UC compared HD CE and HD NBI and found no differences between them in terms of detection of colitis-associated dysplasia (21.2% [14/66] vs. 21.5% [14/65], p=0.964).40 A meta-analysis including 4 RCTs showed no significant difference between CE and NBI (risk difference, 0.06; 95% CI, −0.08 to 0.21).41 In addition to NBI, i-Scan and flexible spectral imaging color enhancement were also used as types of virtual CE (VCE). There was no difference between HD VCE with i-Scan and HD WLE in the detection of neoplasia.42 Similar findings were observed while comparing HD VCE with i-Scan and HD CE.43 Diagnostic accuracy for dysplasia exhibited by HD VCE with flexible spectral imaging color enhancement was at least as good as that exhibited by HD CE.44 Summarizing the results of the aforementioned studies, HD VCE has offered no benefit in colonoscopy surveillance over HD WLE or HD CE to date. However, considering the shorter procedure time and no additional requirement of dyes or equipment, HD VCE is a promising technique for surveillance colonoscopy.
Random vs. targeted biopsy
Traditionally, random biopsy of 32 samples from all four quadrants at every 10 cm from the cecum to the rectum, with additional biopsies of suspicious areas has been recommended. However, the efficacy of random biopsies remains controversial. Although CE with random biopsies is superior to WLE with random biopsies for the detection of dysplasia (OR, 8.9; 95% CI, 3.4–23),45 even HD CE with random biopsy has a low detection rate of only 0.2% per biopsy (68/31,865) and 1.2% per colonoscopy (12/1,000) among patients with long-standing IBD.46 In the same study, random biopsies showed efficacy only in patients having PSC, history of dysplasia, or chronic or severe inflammatory features such as post-inflammatory polyps, stricture, and tubular or shortened colons.46 Recently, targeted biopsy has been suggested increasingly in surveillance strategies. An RCT compared targeted and random biopsies in 246 patients having UC for >7 years.47 HD endoscopy or CE was not mandatory in this study and targeted biopsies were performed if dysplasia was suspected, even in the random biopsy group. The detection rate was 11.4% (13/114) for targeted biopsies and 9.3% (10/107) for random biopsies (p=0.617). The random group had a longer procedure time and a greater number of biopsy samples. Notably, dysplasia was observed only at sites where inflammation or scarring was present in the random biopsy group, and an additional targeted biopsy detected 40% of these lesions. Although targeted biopsy requires training, it is noteworthy that most of the dysplastic lesions can be detected using endoscopy, especially using CE. Therefore, random biopsies of the entire colon may not be appropriate for all patients with IBD. In the 2019 BSG and ECCO guidelines, targeted biopsy is recommended over random biopsy.24,25 According to the 2021 SCENIC update, targeted biopsy was acceptable regardless of the modality (WLE, CE, or VCE) while using an HD scope.48,49 Random biopsy might be an option only under specific conditions with high-risk features due to its low detection yield rate, longer procedure time, and higher cost.
INTERVAL FOR SURVEILLANCE COLONOSCOPY
Recently, all major guidelines have suggested optimizing the surveillance interval according to CRC risk stratification. Particularly, the 2019 ECCO and BSG guidelines classified the surveillance interval into a high-risk group (1-year interval), an intermediate-risk group (2 to 3-year interval), and a low-risk group (5-year interval) among patients with colonic involvement (Table 2).24,25 In these guidelines, the risk factors were as follows: (1) high-risk group: PSC, first-degree relatives with age at CRC diagnosis <50 years, extensive colitis with moderate to severe inflammation, and stricture or dysplasia within 5 years; (2) moderate-risk group: post-inflammatory polyps, first-degree relatives with a history of CRC and age at CRC diagnosis ≥50 years, and extensive colitis with mild to moderate inflammation; and (3) low-risk group: any factors not included in the high-risk and moderate-risk groups. In the 2019 ACG guidelines for UC, surveillance is recommended broadly at 1 to 3-year intervals based on combined risk factors for CRC.26 The 2021 AGA clinical practice update has guidelines similar to the 2019 ECCO and BSG guidelines for the interval of surveillance colonoscopy.28
Table 2.
Modified colonoscopy surveillance interval according to risk stratification in patients with IBD24,25
| Risk level | High risk | Moderate risk | Low risk |
|---|---|---|---|
| Surveillance interval | Every year | Every 2–3 years | Every 5 years |
| Risk factors | ● Extensive colitis with moderate to severe endoscopic and/or histological inflammation | ● Extensive colitis with mild endoscopic and/or histological inflammation | ● Colitis affecting less than 50% of the colon |
| ● CRC in a first-degree relative diagnosed before 50 years of age | ● CRC in a first-degree relative diagnosed after 50 years of age | ||
| ● Concurrent PSC | ● Post-inflammatory polyps | ||
| ● Stricture within 5 years | |||
| ● Dysplasia within 5 years |
IBD, inflammatory bowel disease; CRC, colorectal cancer; PSC, primary sclerosing cholangitis.
UNMET NEEDS IN SURVEILLANCE
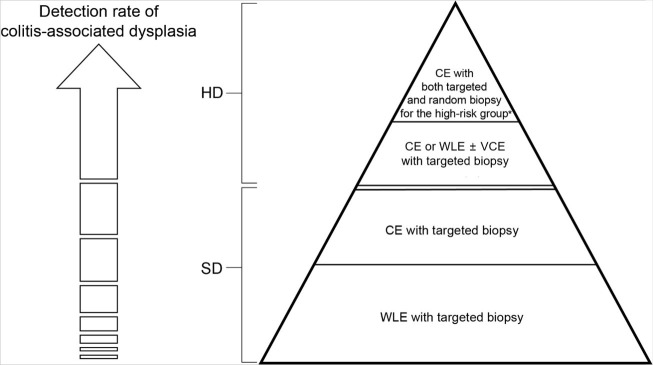
The surveillance strategies recommended in the current guidelines are based on relatively limited data and evidence. In terms of cost-effectiveness, these surveillance strategies require resource-intensive practice. Moreover, they lack the support of well-designed individualized prospective studies. In addition, such strategies do not exclude the occurrence of interval cancer due to missed lesions despite adequate procedural quality and appropriate surveillance intervals. For example, one retrospective study involving 1,273 patients with IBD and having a follow-up duration of 5.3 (±3.0) years showed that approximately 30% of the CRC cases were those of interval cancer even though the surveillance had adequate quality and appropriate intervals according to the guidelines.50 Another retrospective study involving 2,801 patients with IBD and 25,391 PYs of follow-up revealed that 54.5% (6/11) of the patients with UC and 33.3% (3/9) of the patients with CD who were diagnosed with CRC had potentially preventable post-colonoscopy CRCs and missed lesions were believed to be the only etiology.51 Notably, CRNs may be missed even after adequate surveillance, and strategies to minimize missed lesions in surveillance are still being developed. The proposed positioning of techniques for surveillance colonoscopy in patients is shown in Figure 3.
Fig. 3.
Proposed positioning of techniques for surveillance colonoscopy in patients with inflammatory bowel disease. CE, chromoendoscopy; HD, high-definition; SD, standard-definition; WLE, white-light endoscopy; VCE, virtual CE. a)Risk factors for the high-risk group include primary sclerosing cholangitis, history of dysplasia, and chronic or severe inflammatory features such as post-inflammatory polyps, stricture, and tubular/shortened bowel.
CONCLUSIONS
The risk of CRC is higher in patients with IBD involving >30% of the colon compared to that in the general population. Colonoscopic surveillance is recommended to start at 8 years after the onset of symptoms. Targeted biopsy is being increasingly recommended for surveillance. However, random biopsy should be considered in cases of invisible colitis-associated dysplasia with PSC, history of dysplasia, and chronic or severe inflammatory features. HD endoscopy has shown superiority over SD endoscopy and has been widely available in recent times. HD CE and HD WLE with or without VCE are acceptable modalities and their use may depend on the skill of the endoscopist and risk stratification in patients. VCE technology has shown promise for future CRC surveillance programs.
Footnotes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: WM; Supervision: WM; Validation: WM, SYN; Visualization: SYN; Writing–original draft: SYN; Writing–review&editing: SYN, WM.
REFERENCES
- 1.Crohn B. The sigmoidoscopic picture of chronic ulcerative colitis (non-specific) Am J Med Sci. 1925;170:220–228. [Google Scholar]
- 2.Goldgraber MB, Kirsner JB. Carcinoma of the colon in ulcerative colitis. Cancer. 1964;17:657–665. doi: 10.1002/1097-0142(196405)17:5<657::aid-cncr2820170515>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Kim KT, Lee HJ, Kang SW, et al. Malignant change of chronic ulcerative colitis: report of a case. J Korean Radiol Soc. 1988;24:1103–1106. [Google Scholar]
- 4.Leighton JA, Shen B, Baron TH, et al. ASGE guideline: endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest Endosc. 2006;63:558–565. doi: 10.1016/j.gie.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winther KV, Jess T, Langholz E, et al. Long-term risk of cancer in ulcerative colitis: a population-based cohort study from Copenhagen County. Clin Gastroenterol Hepatol. 2004;2:1088–1095. doi: 10.1016/s1542-3565(04)00543-9. [DOI] [PubMed] [Google Scholar]
- 7.Jess T, Simonsen J, Jørgensen KT, et al. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143:375–381. doi: 10.1053/j.gastro.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Lutgens MW, van Oijen MG, van der Heijden GJ, et al. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. 2013;19:789–799. doi: 10.1097/MIB.0b013e31828029c0. [DOI] [PubMed] [Google Scholar]
- 9.Soderlund S, Brandt L, Lapidus A, et al. Decreasing time-trends of colorectal cancer in a large cohort of patients with inflammatory bowel disease. Gastroenterology. 2009;136:1561–1567. doi: 10.1053/j.gastro.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 10.Gong W, Lv N, Wang B, et al. Risk of ulcerative colitis-associated colorectal cancer in China: a multi-center retrospective study. Dig Dis Sci. 2012;57:503–507. doi: 10.1007/s10620-011-1890-9. [DOI] [PubMed] [Google Scholar]
- 11.Wei SC, Shieh MJ, Chang MC, et al. Long-term follow-up of ulcerative colitis in Taiwan. J Chin Med Assoc. 2012;75:151–155. doi: 10.1016/j.jcma.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Hata K, Watanabe T, Kazama S, et al. Earlier surveillance colonoscopy programme improves survival in patients with ulcerative colitis associated colorectal cancer: results of a 23-year surveillance programme in the Japanese population. Br J Cancer. 2003;89:1232–1236. doi: 10.1038/sj.bjc.6601247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HS, Park SH, Yang SK, et al. The risk of colorectal cancer in inflammatory bowel disease: a hospital-based cohort study from Korea. Scand J Gastroenterol. 2015;50:188–196. doi: 10.3109/00365521.2014.989538. [DOI] [PubMed] [Google Scholar]
- 14.Olén O, Erichsen R, Sachs MC, et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. 2020;395:123–131. doi: 10.1016/S0140-6736(19)32545-0. [DOI] [PubMed] [Google Scholar]
- 15.Olén O, Erichsen R, Sachs MC, et al. Colorectal cancer in Crohn's disease: a Scandinavian population-based cohort study. Lancet Gastroenterol Hepatol. 2020;5:475–484. doi: 10.1016/S2468-1253(20)30005-4. [DOI] [PubMed] [Google Scholar]
- 16.Bye WA, Ma C, Nguyen TM, et al. Strategies for detecting colorectal cancer in patients with inflammatory bowel disease: a Cochrane systematic review and meta-analysis. Am J Gastroenterol. 2018;113:1801–1809. doi: 10.1038/s41395-018-0354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi CH, Rutter MD, Askari A, et al. Forty-year analysis of colonoscopic surveillance program for neoplasia in ulcerative colitis: an updated overview. Am J Gastroenterol. 2015;110:1022–1034. doi: 10.1038/ajg.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakatos L, Mester G, Erdelyi Z, et al. Risk factors for ulcerative colitis-associated colorectal cancer in a Hungarian cohort of patients with ulcerative colitis: results of a population-based study. Inflamm Bowel Dis. 2006;12:205–211. doi: 10.1097/01.MIB.0000217770.21261.ce. [DOI] [PubMed] [Google Scholar]
- 19.Beaugerie L, Svrcek M, Seksik P, et al. Risk of colorectal high-grade dysplasia and cancer in a prospective observational cohort of patients with inflammatory bowel disease. Gastroenterology. 2013;145:166–175. doi: 10.1053/j.gastro.2013.03.044. [DOI] [PubMed] [Google Scholar]
- 20.Wijnands AM, de Jong ME, Lutgens MW, et al. Prognostic factors for advanced colorectal neoplasia in inflammatory bowel disease: systematic review and meta-analysis. Gastroenterology. 2021;160:1584–1598. doi: 10.1053/j.gastro.2020.12.036. [DOI] [PubMed] [Google Scholar]
- 21.Choi CR, Al Bakir I, Ding NJ, et al. Cumulative burden of inflammation predicts colorectal neoplasia risk in ulcerative colitis: a large single-centre study. Gut. 2019;68:414–422. doi: 10.1136/gutjnl-2017-314190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutgens MW, Vleggaar FP, Schipper ME, et al. High frequency of early colorectal cancer in inflammatory bowel disease. Gut. 2008;57:1246–1251. doi: 10.1136/gut.2007.143453. [DOI] [PubMed] [Google Scholar]
- 23.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-update based on new evidence. Gastroenterology. 2003;124:544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 24.Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144–164. doi: 10.1093/ecco-jcc/jjy113. [DOI] [PubMed] [Google Scholar]
- 25.Lamb CA, Kennedy NA, Raine T, et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114:384–413. doi: 10.14309/ajg.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 27.Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018;113:481–517. doi: 10.1038/ajg.2018.27. [DOI] [PubMed] [Google Scholar]
- 28.Murthy SK, Feuerstein JD, Nguyen GC, et al. AGA clinical practice update on endoscopic surveillance and management of colorectal dysplasia in inflammatory bowel diseases: expert review. Gastroenterology. 2021;161:1043–1051. doi: 10.1053/j.gastro.2021.05.063. [DOI] [PubMed] [Google Scholar]
- 29.Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc. 2015;81:489–501. doi: 10.1016/j.gie.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Kiesslich R, Fritsch J, Holtmann M, et al. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880–888. doi: 10.1053/gast.2003.50146. [DOI] [PubMed] [Google Scholar]
- 31.Feuerstein JD, Rakowsky S, Sattler L, et al. Meta-analysis of dye-based chromoendoscopy compared with standard-and high-definition white-light endoscopy in patients with inflammatory bowel disease at increased risk of colon cancer. Gastrointest Endosc. 2019;90:186–195. doi: 10.1016/j.gie.2019.04.219. [DOI] [PubMed] [Google Scholar]
- 32.Subramanian V, Ramappa V, Telakis E, et al. Comparison of high definition with standard white light endoscopy for detection of dysplastic lesions during surveillance colonoscopy in patients with colonic inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:350–355. doi: 10.1002/ibd.23002. [DOI] [PubMed] [Google Scholar]
- 33.Buchner AM, Shahid MW, Heckman MG, et al. High-definition colonoscopy detects colorectal polyps at a higher rate than standard white-light colonoscopy. Clin Gastroenterol Hepatol. 2010;8:364–370. doi: 10.1016/j.cgh.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Wan J, Zhang Q, Liang SH, et al. Chromoendoscopy with targeted biopsies is superior to white-light endoscopy for the long-term follow-up detection of dysplasia in ulcerative colitis patients: a multicenter randomized-controlled trial. Gastroenterol Rep (Oxf) 2020;9:14–21. doi: 10.1093/gastro/goaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iacucci M, Kaplan GG, Panaccione R, et al. A randomized trial comparing high definition colonoscopy alone with high definition dye spraying and electronic virtual chromoendoscopy for detection of colonic neoplastic lesions during IBD surveillance colonoscopy. Am J Gastroenterol. 2018;113:225–234. doi: 10.1038/ajg.2017.417. [DOI] [PubMed] [Google Scholar]
- 36.Alexandersson B, Hamad Y, Andreasson A, et al. High-definition chromoendoscopy superior to high-definition white-light endoscopy in surveillance of inflammatory bowel diseases in a randomized trial. Clin Gastroenterol Hepatol. 2020;18:2101–2107. doi: 10.1016/j.cgh.2020.04.049. [DOI] [PubMed] [Google Scholar]
- 37.Yang DH, Park SJ, Kim HS, et al. High-definition chromoendoscopy versus high-definition white light colonoscopy for neoplasia surveillance in ulcerative colitis: a randomized controlled trial. Am J Gastroenterol. 2019;114:1642–1648. doi: 10.14309/ajg.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 38.Pellisé M, López-Cerón M, Rodríguez de Miguel C, et al. Narrow-band imaging as an alternative to chromoendoscopy for the detection of dysplasia in long-standing inflammatory bowel disease: a prospective, randomized, crossover study. Gastrointest Endosc. 2011;74:840–848. doi: 10.1016/j.gie.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 39.Efthymiou M, Allen PB, Taylor AC, et al. Chromoendoscopy versus narrow band imaging for colonic surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2132–2138. doi: 10.1097/MIB.0b013e31829637b9. [DOI] [PubMed] [Google Scholar]
- 40.Bisschops R, Bessissow T, Joseph JA, et al. Chromoendoscopy versus narrow band imaging in UC: a prospective randomised controlled trial. Gut. 2018;67:1087–1094. doi: 10.1136/gutjnl-2016-313213. [DOI] [PubMed] [Google Scholar]
- 41.Resende RH, Ribeiro IB, de Moura DT, et al. Surveillance in inflammatory bowel disease: is chromoendoscopy the only way to go? A systematic review and meta-analysis of randomized clinical trials. Endosc Int Open. 2020;8:E578–E590. doi: 10.1055/a-1120-8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kandiah K, Subramaniam S, Thayalasekaran S, et al. Multicentre randomized controlled trial on virtual chromoendoscopy in the detection of neoplasia during colitis surveillance high-definition colonoscopy (the VIRTUOSO trial) Gut. 2021;70:1684–1690. doi: 10.1136/gutjnl-2020-320980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.González-Bernardo O, Riestra S, Vivas S, et al. Chromoendoscopy with indigo carmine vs virtual chromoendoscopy (iSCAN 1) for neoplasia screening in patients with inflammatory bowel disease: a prospective randomized study. Inflamm Bowel Dis. 2021;27:1256–1262. doi: 10.1093/ibd/izaa291. [DOI] [PubMed] [Google Scholar]
- 44.Gulati S, Dubois P, Carter B, et al. A randomized crossover trial of conventional vs virtual chromoendoscopy for colitis surveillance: dysplasia detection, feasibility, and patient acceptability (CONVINCE) Inflamm Bowel Dis. 2019;25:1096–1106. doi: 10.1093/ibd/izy360. [DOI] [PubMed] [Google Scholar]
- 45.Soetikno R, Subramanian V, Kaltenbach T, et al. The detection of nonpolypoid (flat and depressed) colorectal neoplasms in patients with inflammatory bowel disease. Gastroenterology. 2013;144:1349–1352. doi: 10.1053/j.gastro.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Moussata D, Allez M, Cazals-Hatem D, et al. Are random biopsies still useful for the detection of neoplasia in patients with IBD undergoing surveillance colonoscopy with chromoendoscopy? Gut. 2018;67:616–624. doi: 10.1136/gutjnl-2016-311892. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe T, Ajioka Y, Mitsuyama K, et al. Comparison of targeted vs random biopsies for surveillance of ulcerative colitis-associated colorectal cancer. Gastroenterology. 2016;151:1122–1130. doi: 10.1053/j.gastro.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Rabinowitz LG, Kumta NA, Marion JF. Beyond the SCENIC route: updates in chromoendoscopy and dysplasia screening in patients with inflammatory bowel disease. Gastrointest Endosc. 2022;95:30–37. doi: 10.1016/j.gie.2021.07.024. [DOI] [PubMed] [Google Scholar]
- 49.Kiesslich R. SCENIC update 2021: is chromoendoscopy still standard of care for inflammatory bowel disease surveillance? Gastrointest Endosc. 2022;95:38–41. doi: 10.1016/j.gie.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Mooiweer E, van der Meulen-de Jong AE, Ponsioen CY, et al. Incidence of interval colorectal cancer among inflammatory bowel disease patients undergoing regular colonoscopic surveillance. Clin Gastroenterol Hepatol. 2015;13:1656–1661. doi: 10.1016/j.cgh.2015.04.183. [DOI] [PubMed] [Google Scholar]
- 51.Wintjens DS, Bogie RM, van den Heuvel TR, et al. Incidence and classification of postcolonoscopy colorectal cancers in inflammatory bowel disease: a Dutch population-based cohort study. J Crohns Colitis. 2018;12:777–783. doi: 10.1093/ecco-jcc/jjy044. [DOI] [PubMed] [Google Scholar]