Abstract
Aims
Vigorous physical activity (VPA) is a time-efficient way to achieve recommended physical activity levels. There is a very limited understanding of the minimal and optimal amounts of vigorous physical activity in relation to mortality and disease incidence.
Methods and results
A prospective study in 71 893 adults [median age (IQR): 62.5 years (55.3, 67.7); 55.9% female] from the UK Biobank cohort with wrist-worn accelerometry. VPA volume (min/week) and frequency of short VPA bouts (≤2 min) were measured. The dose–response associations of VPA volume and frequency with mortality [all-cause, cardiovascular disease (CVD) and cancer], and CVD and cancer incidence were examined after excluding events occurring in the first year. During a mean post-landmark point follow-up of 5.9 years (SD ± 0.8), the adjusted 5-year absolute mortality risk was 4.17% (95% confidence interval: 3.19%, 5.13%) for no VPA, 2.12% (1.81%, 2.44%) for >0 to <10 min, 1.78% (1.53%, 2.03%) for 10 to <30 min, 1.47% (1.21%, 1.73%) for 30 to <60 min, and 1.10% (0.84%, 1.36%) for ≥60 min. The ‘optimal dose’ (nadir of the curve) was 53.6 (50.5, 56.7) min/week [hazard ratio (HR): 0.64 (0.54, 0.77)] relative to the 5th percentile reference (2.2 min/week). There was an inverse linear dose-response association of VPA with CVD mortality. The ‘minimal’ volume dose (50% of the optimal dose) was ∼15 (14.3, 16.3) min/week for all-cause [HR: 0.82 (0.75, 0.89)] and cancer [HR: 0.84 (0.74, 0.95)] mortality, and 19.2 (16.5, 21.9) min/week [HR: 0.60 (0.50, 0.72)] for CVD mortality. These associations were consistent for CVD and cancer incidence. There was an inverse linear association between VPA frequency and CVD mortality. 27 (24, 30) bouts/week was associated with the lowest all-cause mortality [HR: 0.73 (0.62, 0.87)].
Conclusion
VPA of 15–20 min/week were associated with a 16–40% lower mortality HR, with further decreases up to 50–57 min/week. These findings suggest reduced health risks may be attainable through relatively modest amounts of VPA accrued in short bouts across the week.
Keywords: Physical activity, Mortality, Cardiovascular disease, Cancer, Vigorous intensity
Structured Graphical Abstract
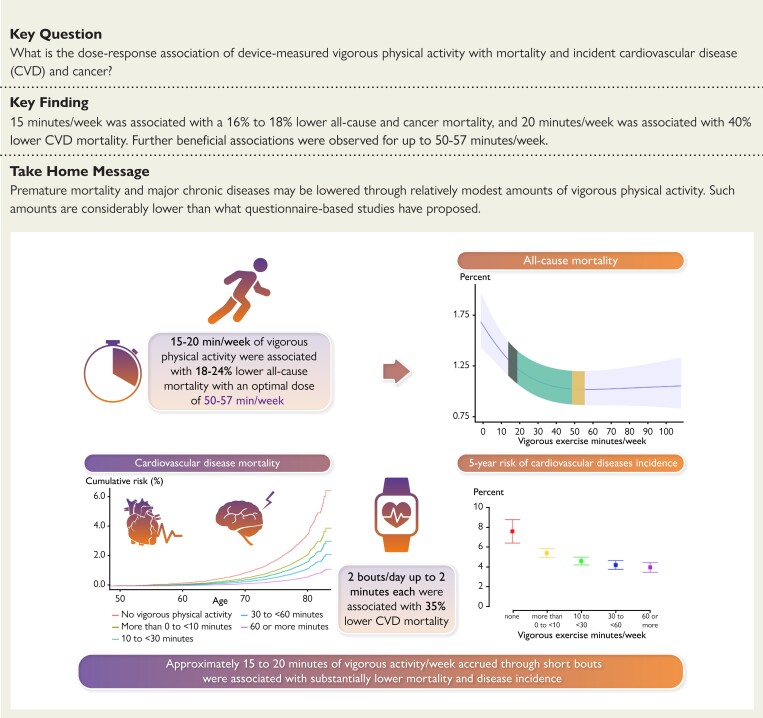
Structured Graphical Abstract.
Approximately 15–20 min of vigorous activity/week accrued through short bouts were associated with lower mortality and disease incidence. VPA = vigorous physical activity.
See the editorial comment for this article ‘The hare and the tortoise: physical activity intensity and scientific translation’, by C. E. Matthews and P. F. Saint-Maurice, https://doi.org/10.1093/eurheartj/626.
Introduction
Based on existing prospective observational evidence, the 2020 World Health Organization Physical activity and sedentary behaviour guidelines1 and the physical activity guidelines for Americans, 2nd Edition2 each recommended 150–300 min of moderate-to-vigorous physical activity (MVPA), 75–150 min of vigorous physical activity (VPA), or a combination of both a week. VPA, defined as physical activity at an energy expenditure rate of at least six metabolic equivalents (METs) is a time-efficient way to achieve recommended physical activity levels and can lead to rapid cardiorespiratory adaptations.3 For the first time, current physical activity guidelines1,2,4,5 emphasize the value of short bouts of intermittent physical activity (e.g. <5 min) for accumulating the recommended amounts. Prior studies examining the health benefits of VPA, which were limited by the inability of questionnaires to capture shorter intermittent VPA sessions lasting under 10–15 min, found that all-cause mortality (ACM) risk was lowered by approximately 10% when VPA contributed 30–50% of total MVPA time.6,7 Findings on cardiovascular disease (CVD) and cancer mortality showed similar results.8,9
Sixty to 90 min of weekly VPA accumulated through 10 to 15 min-long bouts of exercise has been shown to be associated with a 3-year extension of life expectancy and a 4% lower risk of ACM for every additional 15 min.10,11 There is limited information on how low volumes of VPA accumulated through short bouts are associated with health and mortality. Such information is pertinent to improve translation of research findings into clinical and public health interventions involving accumulation of VPA through brief episodes throughout the day.
Examining the dose-response of short and intermittent VPA bursts requires device-based measurments.9 Indeed, the World Health Organization Guidelines Development Group recently indicated the need for device-based studies to objectively assess the relationship of physical activity with mortality and disease risk as a priority for research.12 The aim of this study was to examine the dose-response association of device-measured VPA with mortality, and incident CVD and cancer in the largest accelerometry cohort of UK adults. We hypothesised inverse associations with mortality and incident CVD and cancer exist through modest amounts of VPA accrued through short bouts.
Methods
We reported this study as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline (Supplemental STROBE Statement).
Study participants
Participants were included from the UK Biobank study, a prospective cohort of 502 629 participants between 40–69 years. All participants were enrolled between 2006–10 and provided informed written consent. Ethical approval was provided by the UK National Health Service (NHS), National Research Ethics Service (Ref 11/NW/0382). Participants completed physical examinations by trained staff and touchscreen questionnaires.13 We excluded participants with prevalent CVD or cancer (ascertained through self-report, hospital admission, and cancer registry records), missing covariate data, or an event within the first 12 months after the accelerometry measurements (landmark). We considered the start of the landmark period as follow-up time onset (Supplementary material online, Figure 1).
Physical activity assessment
From 2013–15, 103,684 participants were mailed and wore an Axivity AX3 accelerometer (Newcastle upon Tyne, UK) on their dominant wrist for 24-h/day for 7 days to measure physical activity. Prior to being mailed, the AX3 accelerometers were initialized to collect data with a sampling frequency of 100 Hz and a dynamic range between ± 8 g. Participants returned the devices by mail and the data were calibrated and non-wear periods were identified according to standard procedures.14,15 Monitoring days were considered valid if wear time was greater than 16 h. To be included in analysis, participants were required to have at least four valid monitoring days, with at least one of those days being a weekend day (n = 96 459). Physical activity intensity was classified with a validated accelerometer-based activity machine learning scheme covering VPA, moderate intensity physical activity, and light intensity physical activity.16 Briefly, this activity scheme uses features in the raw acceleration signal to identify and quantify time spent in different activity types and intensities in 10 s windows. A complete description is provided in Supplementary material online, Text 1. To calculate physical activity volume, we summed time spent in each respective activity intensity band across all valid wear days. Because 96% of VPA volume occurred in bouts lasting up to 2 min, we did not carry out analyses of longer bouts.
Outcome ascertainment
Participants were followed up through 31 October 2021, with deaths obtained through linkage with the NHS Digital of England and Wales or the NHS Central Register and National Records of Scotland. Inpatient hospitalization data were provided by either the Hospital Episode Statistics for England, the Patient Episode Database for Wales, or the Scottish Morbidity Record for Scotland. Cancer data linkage was obtained through national cancer registries. For England and Wales, cancer diagnosis data were provided by the Medical Research Information Service, based at the NHS Information Centre. For Scotland, cancer diagnosis data were provided by the Information Services Division, which is part of the NHS Scotland. Methods for the assessment of CVD and cancer incidence are provided in Supplementary material online, Table S1. In short, CVD was defined as diseases of the circulatory system, excluding hypertension, diseases of arteries, and lymph. Cancer was defined as neoplasms, excluding in situ, benign, uncertain, non-melanoma skin cancer, or non-well-defined cancers. Due to the nature of rolling updates for the data linkage, censoring dates varied between resources (between September 2021 and October 2021).
Covariates
Based on the directed acyclic graph presented in Supplementary material online, Figure 2, our selection of covariates included: age, sex, accelerometer wear time, light intensity physical activity minutes, moderate intensity physical activity minutes, smoking status, alcohol consumption, sleep score based on five sleep indices (morning chronotype, sleep duration, insomnia, snoring, and daytime sleepiness),17 fruit and vegetable consumption, discretionary screen-time defined as time spent watching TV or using the computer outside of work, highest attained education level, self-reported parental history of CVD and cancer, and cholesterol, blood pressure, or diabetes medication use. Complete covariate definitions are provided in Supplementary material online, Table S2.
Analysis
We tabulated mortality and disease rate per 1000 person-years, the crude risk, and age- and sex-adjusted incidence rate ratio within VPA volume groups (no VPA, >0 to <10 min/week, 10 to <30 min/week, 30 to <60 min/week, and ≥60 min/week). We calculated the dose–response absolute risk between VPA volume and each outcome using Poisson regression18 (natural splines with knots at 10th, 50th, and 90th percentiles19) to estimate the probability and the 95% confidence intervals (CIs) of an event adjusting for all covariates. Further, we examined the time-to-event dose-response associations of VPA volume, frequency (bouts/week), and the percentage contribution of VPA to total MVPA volume (%VPA) with the five outcomes. For these analyses, we calculated hazard ratios (HRs) using Cox proportional hazards (ACM) and Fine-Gray subdistribution models for CVD and cancer outcomes (treating non-CVD or cancer deaths as competing risks as appropriate) with knots at 10th, 50th, and 90th percentiles19 and age as the timescale. We also calculated the adjusted survival probability and 5-year risk. Sequential hazards modelling for VPA volume included adjustments for: sex (Model 1); Model 2 additionally adjusted for lifestyle and health factors (smoking, alcohol, sleep quality score, discretionary screen-time, diet, family history of CVD and cancer, and medication use); Model 3 additionally adjusted for physical activity variables (light and moderate intensity minutes, and accelerometer wear time), as well as mutual adjustment for volume and frequency of VPA. We present Model 3 as the main analysis. The reference was set to 2.2 min/week, equivalent to the 5th percentile of the volume distribution, one bout/week for frequency analysis, and 0.25% for %VPA analysis. Proportional hazards assumptions were assessed using Schoenfeld residuals and no violations were observed (P > 0.05). For both absolute risk and HR analyses, departure from linearity was assessed by a Wald test examining the null hypothesis that the coefficient of the second spline was equal to zero.
We calculated E-values to estimate the plausibility of bias from unmeasured confounding.20 The E-values indicate the required magnitude of the association unmeasured confounders to reduce findings to null. Additional time-to-event analyses were performed to examine associations with mortality and incident disease across lifestyle and health variable groups. To provide conservative point estimates for associations, we assessed the minimal dose, defined as the volume of VPA associated with 50% of the lowest HR (‘optimal dose’; nadir of the dose curve).21,22 We used bootstrapping with replacement (1000 iterations) to calculate CIs for the optimal and minimal dose. We fit interaction terms between VPA volume and light and moderate volume, separately. The interaction term was not significant and did not improve model fit, and therefore we do not present effect modification. To examine the possibility of reverse causation, we excluded the second year from accelerometry measurement baseline and those participants who were on CVD medication or who had self-rated poor health.
To further assess robustness of our results to alternative analytic decisions, we carried out the following sensitivity analyses: (i) we additionally adjusted analyses for (body mass index-based) obesity strata; (ii) we set the reference to zero minutes and 6.7 min/week (20th percentile of volume distribution); (iii) we included participants with less than one year of follow-up; (iv) we imputed missing data for covariates by using multiple imputation using chained equations (five imputed data sets); and (v) we assessed the dose-response of ACM with CVD and cancer deaths treated as competing risks.
We performed all analysis using R statistical software with the rms and survival packages.23,24
Results
Our analytic sample for mortality included 71 893 participants [median age (IQR): 62.5 (55.3, 67.7) years; 55.9% female; characteristics of excluded participants are shown in Supplementary material online, Table S3) followed up for an average of 5.9 ± 0.8 years (starting from the landmark period 12 months after follow-up, or 6.9 years from accelerometry measurement) with 1927 deaths (602 CVD and 1150 cancer; Supplementary material online, Table S4). Our incident CVD sample included 71 049 participants with 4567 (3965 non-fatal; Supplementary material online, Table S5) events. Our incident cancer sample included 71 070 participants with 2854 (1704 non-fatal; Supplementary material online, Table S5) events. Median VPA and %MVPA time was 16.5 (IQR = 8.3, 38.5) minutes/week and 9.0% (3.8%, 18.5%), respectively. The median frequency of VPA bouts/week lasting up to 2-minutes was 13 (5, 25). Participants wore the accelerometers for an average of 6.7 days and 22.8 h/day. Participant characteristics by VPA volume are provided in Table 1. Within each low-to-high quartile, median VPA time was 5.7, 15.7, 38.3, and 88.5 min/week, respectively.
Table 1.
Participant descriptive characteristics by quartiles of vigorous physical activity volume (min/week)
| Vigorous physical activity (minutes/week) | ||||||
|---|---|---|---|---|---|---|
| Total | None | 1 to <10 | ≥10 to <30 | ≥30 to <60 | ≥60 | |
| n (%) | 71 893 (100.0) | 2532 (3.5) | 18 333 (25.5) | 27 031 (37.6) | 14 070 (19.6) | 9927 (13.8) |
| Follow-up, yearsa | 5.9 (0.8) | 5.8 (1.1) | 5.9 (0.9) | 5.9 (0.8) | 5.9 (0.8) | 5.9 (0.7) |
| Age, years, median (IQR) | 62.5 (55.3, 67.7) | 67.8 (62.9, 71.6) | 65.2 (58.7, 69.4) | 62.7 (55.7, 67.7) | 60.3 (53.4, 66.2) | 57.3 (51.4, 63.9) |
| Male sex, n (%) | 31 678 (44.1) | 805 (31.8) | 6640 (36.2) | 11 678 (43.2) | 6899 (49.0) | 5656 (57.0) |
| Ethnicity, n (%) | ||||||
| White | 69 568 (96.8) | 2465 (97.4) | 17 764 (96.9) | 26 231 (97.0) | 13 562 (96.4) | 9546 (96.2) |
| Asian | 825 (1.1) | 25 (1.0) | 205 (1.1) | 297 (1.1) | 168 (1.2) | 130 (1.3) |
| Black | 579 (0.8) | 11 (0.4) | 128 (0.7) | 193 (0.7) | 144 (1.0) | 103 (1.0) |
| Mixed | 387 (0.5) | 10 (0.4) | 94 (0.5) | 128 (0.5) | 92 (0.7) | 63 (0.6) |
| Other | 534 (0.7) | 21 (0.8) | 142 (0.8) | 182 (0.7) | 104 (0.7) | 85 (0.9) |
|
Smoking
history, n (%) |
||||||
| Never | 41 159 (57.3) | 1308 (51.7) | 10 136 (55.3) | 15 338 (56.7) | 8273 (58.8) | 6104 (61.5) |
| Previous | 25 781 (35.9) | 262 (10.3) | 1474 (8.0) | 1816 (6.7) | 870 (6.2) | 531 (5.3) |
| Current | 4953 (6.9) | 962 (38.0) | 6723 (36.7) | 9877 (36.5) | 4927 (35.0) | 3292 (33.2) |
| Alcohol intakeb | 4.2 (1.2) | 3.9 (1.2) | 4.1 (1.2) | 4.2 (1.1) | 4.3 (1.1) | 4.3 (1.1) |
| Sleep scorec, n (%) | ||||||
| 0 | 79 (0.1) | 7 (0.3) | 29 (0.2) | 24 (0.1) | 15 (0.1) | 4 (0.0) |
| 1 | 1328 (1.8) | 79 (3.1) | 429 (2.3) | 495 (1.8) | 211 (1.5) | 114 (1.1) |
| 2 | 6973 (9.7) | 324 (12.8) | 2060 (11.2) | 2628 (9.7) | 1225 (8.7) | 736 (7.4) |
| 3 | 19 178 (26.7) | 761 (30.1) | 5342 (29.1) | 7306 (27.0) | 3491 (24.8) | 2278 (22.9) |
| 4 | 27 382 (38.1) | 897 (35.4) | 6701 (36.6) | 10 301 (38.1) | 5544 (39.4) | 3939 (39.7) |
| 5 | 16 953 (23.6) | 464 (18.3) | 3772 (20.6) | 6277 (23.2) | 3584 (25.5) | 2856 (28.8) |
| Discretionary screen-timed | 4.6 (2.2) | 5.0 (2.4) | 4.9 (2.3) | 4.6 (2.2) | 4.4 (2.2) | 4.2 (2.2) |
| Education, n (%) | ||||||
| College/University | 31 529 (43.9) | 1033 (40.8) | 7568 (41.3) | 11 610 (43.0) | 6336 (45.0) | 4982 (50.2) |
| A/AS levels | 9639 (13.4) | 318 (12.6) | 2409 (13.1) | 3701 (13.7) | 1870 (13.3) | 1341 (13.5) |
| O levels | 14 684 (20.4) | 514 (20.3) | 3804 (20.7) | 5692 (21.1) | 2921 (20.8) | 1753 (17.7) |
| CSE | 2843 (3.9) | 66 (2.6) | 685 (3.7) | 1046 (3.9) | 589 (4.2) | 457 (4.6) |
| NVQ/HND/HNC | 3861 (5.4) | 147 (5.8) | 992 (5.4) | 1441 (5.3) | 758 (5.4) | 523 (5.3) |
| Other | 9337 (12.9) | 454 (17.9) | 2875 (15.7) | 3541 (13.1) | 1596 (11.3) | 871 (8.8) |
| Diete | 8.1 (4.3) | 8.0 (4.1) | 8.0 (4.2) | 8.1 (4.4) | 8.0 (4.2) | 8.3 (4.5) |
| Family history of CVD, n (%) | 39 337 (54.7) | 1519 (60.0) | 10 744 (58.6) | 14 842 (54.9) | 7338 (52.2) | 4894 (49.3) |
| Family history of cancer, n (%) | 17 915 (24.9) | 667 (26.3) | 4732 (25.8) | 6833 (25.3) | 3388 (24.1) | 2295 (23.1) |
| Medication, n (%) | ||||||
| Cholesterol | 10 052 (14.0) | 664 (26.2) | 3522 (19.2) | 3747 (13.9) | 1425 (10.1) | 694 (7.0) |
| Blood pressure | 11 748 (16.3) | 832 (32.9) | 4189 (22.8) | 4360 (16.1) | 1581 (11.2) | 786 (7.9) |
| Diabetes | 458 (0.6) | 37 (1.5) | 177 (1.0) | 142 (0.5) | 63 (0.4) | 39 (0.4) |
| Wear-time, days | 6.7 (0.7) | 6.7 (0.9) | 6.7 (0.7) | 6.7 (0.7) | 6.7 (0.6) | 6.7 (0.6) |
| Total activity, median (IQR) | 854.1 (643.8, 1144.7) | 500.1 (333.5, 696.0) | 677.3 (498.5, 878.3) | 848.0 (668.5, 1089.0) | 1012.3 (763.2, 1286.2) | 1215.5 (928.3, 1518.8) |
| Light activity, median (IQR) | 530.7 (341.5, 793.0) | 336.6 (190.5, 500.0) | 430.5 (256.5, 639.8) | 526.2 (355.3, 788.8) | 614.3 (428.3, 892.3) | 671.3 (476.3, 971.0) |
| Moderate activity, median (IQR) | 199.2 (106.8, 379.3) | 97.4 (36.3, 196.0) | 154.50 (68.7, 306.5) | 196.00 (104.7, 367.3) | 235.00 (144.0, 423.1) | 397.67 (196.0, 515.6) |
| Vigorous activity, median (IQR) | 16.5 (8.3, 38.5) | - | 5.7 (3.7, 7.7) | 15.7 (11.2, 20.7) | 38.3 (32.5, 46.5) | 88.5 (71.8, 96.2) |
| %VPA, median (IQR) | 8.8 (3.8, 18.5) | - | 3.4 (1.6, 7.4) | 8.1 (4.6, 14.7) | 14.6 (8.7, 22.4) | 19.9 (11.6, 25.6) |
| Vigorous bouts (up to 2 minutes), median (IQR) | 13 (5, 25) | - | 3 (1, 5) | 13 (9, 17) | 29 (23, 35) | 49 (34, 64) |
Values represent mean (SD), unless specified otherwise.
Landmark period 12 months after primary exposure measurement.
Units/week (1 unit = 8 grams of pure ethanol).
Sleep scores were determined using an established method (Huang B-H et al. BJSM 2021). In brief, participants were categorized by how many healthy sleep characteristics (morning chronotype, adequate sleep duration (7–8 hr/d), never or rare insomnia, never or rare snoring, and infrequent daytime sleepiness) they displayed.
Discretionary screen-time composed of time spent/day watching TV and using a computer.
Fruit and vegetable servings/day.
Mortality and disease incidence risk
Tables 2 and 3present the crude event rates per 1000 person-years, crude risk, and sex and age adjusted incidence rate ratios for mortality and disease incidence by VPA volume groups. Compared to participants with zero minutes of VPA, the incidence rate ratio among participants with 10 to 30 min/week was approximately one-third for all-cause [0.35 (95% CI: 0.30, 0.42)] and CVD mortality [0.34 (0.26, 0.46)]. The rate was about one-half for 10–30 min/week for CVD [0.58 (0.50, 0.67)] and cancer incidence [0.44 (0.34, 0.56)].
Table 2.
Mortality and disease incidence event rates per 1000 person-yearsa
| Vigorous physical activity (min/week)b | ||||||
|---|---|---|---|---|---|---|
| Events | None | >0 to <10 | ≥10 to <30 | ≥30 to <60 | ≥60 | |
| All-cause mortality | 1927 | 13.4 (11.7, 15.4) | 5.5 (5.1, 5.9) | 3.8 (3.6, 4.1) | 2.6 (2.3, 3.0) | 1.8 (1.4, 2.1) |
| CVD mortality | 602 | 4.4 (3.4, 5.6) | 1.9 (1.7, 2.2) | 1.2 (1.0, 1.4) | 0.7 (0.5, 0.9) | 0.3 (0.2, 0.5) |
| Cancer mortality | 1150 | 5.5 (4.4, 6.9) | 2.6 (2.3, 2.9) | 1.9 (1.7, 2.1) | 1.4 (1.2, 1.7) | 1.0 (0.8, 1.4) |
| CVD incidence | 4567 | 22.5 (20.1, 25.1) | 14.5 (13.9, 15.2) | 10.8 (10.3, 11.3) | 8.8 (8.2, 9.4) | 7.4 (6.7, 8.1) |
| Cancer incidence | 2854 | 13.2 (11.4, 15.2) | 8.0 (7.5, 8.5) | 6.1 (5.8, 6.5) | 5.2 (4.7, 5.7) | 3.9 (3.4, 4.5) |
Unadjusted estimates.
Groupings are based on quartiles of vigorous physical activity volume with zero minutes/week as its own group.
CVD included ICD-10 codes: I0, I11, I13, I20-I51, I60-I69.
Cancer included ICD-10 codes: C0-C9, excluding basal and squamous cell carcinoma.
Table 3.
Crude risk, and sex and age adjusted incidence rate ratio by vigorous physical activity groups
| Vigorous activity (min/week)a | Crude risk (%) | Incidence rate ratio | |
|---|---|---|---|
| All-cause mortality | |||
| None | 10.23 (8.77, 11.70) | Reference | |
| >0 to <10 | 4.02 (3.78 4.35) | 0.45 (0.39, 0.54) | |
| ≥10 to <30 | 2.66 (2.49, 2.82) | 0.35 (0.30, 0.42) | |
| ≥30 to <60 | 1.82 (1.63, 2.01) | 0.27 (0.22, 0.33) | |
| ≥60 | 1.23 (1.03, 1.42) | 0.20 (0.16, 0.26) | |
| Cardiovascular disease mortality | |||
| None | 3.47 (2.58, 4.36) | Reference | |
| >0 to <10 | 1.41 (1.27, 1.54) | 0.51 (0.39, 0.67) | |
| ≥10 to <30 | 0.82 (0.73, 0.91) | 0.34 (0.26, 0.46) | |
| ≥30 to <60 | 0.47 (0.37, 0.57) | 0.22 (0.15, 0.31) | |
| ≥60 | 0.21 (0.12, 0.29) | 0.11 (0.06, 0.19) | |
| Cancer mortality | |||
| None | 4.94 (3.89, 5.99) | Reference | |
| >0 to <10 | 2.21 (2.04, 2.39) | 0.55 (0.43, 0.71) | |
| ≥10 to <30 | 1.62 (1.49, 1.75) | 0.44 (0.34, 0.56) | |
| ≥30 to <60 | 1.21 (1.06, 1.37) | 0.37 (0.28, 0.50) | |
| ≥60 | 0.90 (0.73, 1.06) | 0.31 (0.22, 0.44) | |
| Cardiovascular disease incidence | |||
| None | 15.52 (13.77, 17.27) | Reference | |
| >0 to <10 | 9.78 (9.43, 10.13) | 0.73 (0.64, 0.84) | |
| ≥10 to <30 | 7.21 (6.94, 7.48) | 0.58 (0.50, 0.67) | |
| ≥30 to <60 | 5.92 (5.58, 6.25) | 0.50 (0.43, 0.58) | |
| ≥60 | 5.05 (4.64, 5.45) | 0.47 (0.39, 0.55) | |
| Cancer incidence | |||
| None | 5.80 (4.36, 7.24) | Reference | |
| >0 to <10 | 3.77 (3.49, 4.04) | 0.53 (0.42, 0.68) | |
| ≥10 to <30 | 2.31 (2.10, 2.52) | 0.44 (0.34, 0.56) | |
| ≥30 to <60 | 1.68 (1.41, 1.94) | 0.40 (0.30, 0.53) | |
| ≥60 | 0.80 (0.51, 1.09) | 0.38 (0.25, 0.56) |
Groupings are based on quartiles of vigorous activity volume with zero minutes/week as its own group.
CVD included ICD-10 codes: I0, I11, I13, I20-I51, I60-I69.
Cancer included ICD-10 codes: C0-C9, excluding basal cell carcinoma and squamous cell carcinoma.
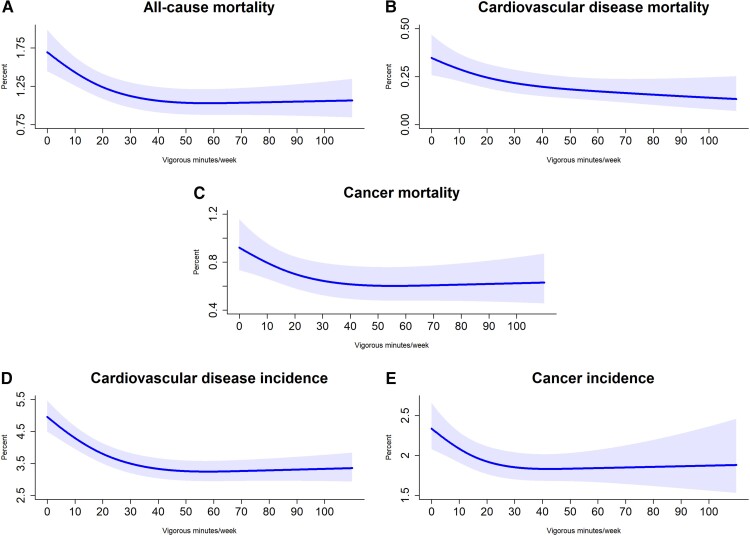
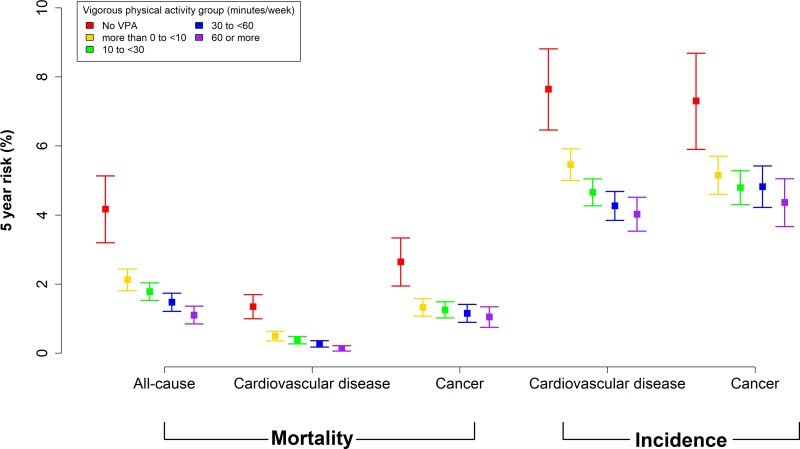
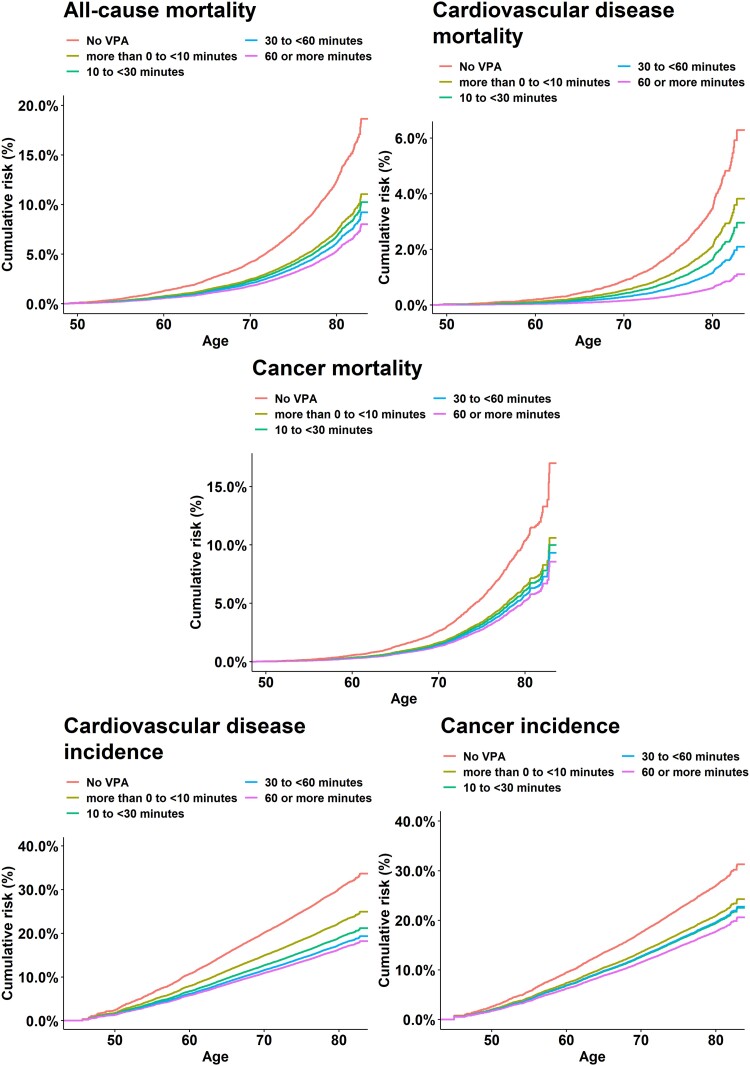
Figures 1–3 show the adjusted absolute risk, adjusted 5-year risk, and adjusted survival curves. Participants with zero minutes of VPA had an absolute risk of 1.69% (1.45%, 1.99%) (5-year risk = 4.17% (3.19%, 5.13%) for all-cause mortality. In comparison, 10 to <30 min/week of VPA was associated with a risk of 1.35% (1.18%, 1.55%) [5-year risk = 1.78% (1.53%, 2.03%)], 30 to <60 min/week had a risk of 1.06% (0.90%, 1.24%) [5-year risk = 1.47% (1.21%, 1.73%)], and ≥60 min/week had a risk of 1.05% (0.87%, 1.27%) [5-year risk = 1.10% (0.84%, 1.36%)]. For CVD incidence, corresponding results were 4.96% (4.50%, 5.47%) [5-year risk = 7.64% (6.46%, 8.81%), 4.08% (3.75%, 4.45%)] [5-year risk = 4.65% (4.26%, 5.04%)], 3.32% (3.02%, 3.65%) [5-year risk = 4.26% (3.84%, 4.68%)], and 3.29% (2.95%, 3.68%) [5-year risk = 4.02% (3.53%, 4.51%)], respectively. For cancer incidence, they were 2.34% (2.08%, 2.66%) [5-year risk = 7.30% (5.90%, 8.68%)], 1.94% (1.78%, 2.13%) [5-year risk = 4.79% (4.30%, 5.28%)], 1.84% (1.68%, 2.03%) [5-year risk = 4.82% (4.22%, 5.42%)], and 1.86% (1.60%, 2.24%) [5-year risk = 4.36% (3.67%, 5.05%)]. Supplementary material online, Table S6 presents the absolute risk estimates in five minute increments for all mortality and disease incidence outcomes.
Figure 1.
Adjusted absolute risk estimates for mortality and disease incidence by vigorous physical activity volume (minutes/week). Adjusted for age, sex, wear time, light intensity, moderate intensity, frequency of vigorous bouts, smoking history, alcohol consumption, sleep score, diet, discretionary screen-time, education, self-reported parental history of CVD and cancer, and self-reported medication use (cholesterol, blood pressure, and diabetes). The range was capped at the 97.5 percentile to minimize the influence of sparse data. Mortality: n = 71 893; events: all-cause = 1,927, cardiovascular disease = 602, cancer = 1150. Cardiovascular disease: n = 71,049, events = 4567. Cancer: n = 71,070, events = 2854.
Figure 2.
Adjusted 5-year risk for mortality and disease incidence by vigorous physical activity volume groups. Timescale was follow-up years. Adjusted for age, sex, wear time, light intensity, moderate intensity, frequency of vigorous bouts, smoking history, alcohol consumption, sleep score, diet, discretionary screen-time, education, self-reported parental history of CVD and cancer, and self-reported medication use (cholesterol, blood pressure, and diabetes). Mortality: n = 71 893; events: all-cause = 1,927, cardiovascular disease = 602, cancer = 1150. Cardiovascular disease: n = 71,049, events = 4567. Cancer: n = 71,070, events = 2854.
Figure 3.
Adjusted survival curves for mortality and disease incidence by vigorous physical activity volume groups. Timescale was age. Adjusted for sex, wear time, light intensity, moderate intensity, frequency of vigorous bouts, smoking history, alcohol consumption, sleep score, diet, discretionary screen-time, education, self-reported parental history of CVD and cancer, and self-reported medication use (cholesterol, blood pressure, and diabetes). Mortality: n = 71 893; events: all-cause = 1,927, cardiovascular disease = 602, cancer = 1150. Cardiovascular disease: n = 71,049, events = 4567. Cancer: n = 71,070, events = 2854.
Multivariable-adjusted associations with all-cause, cardiovascular disease, and cancer mortality
Volume
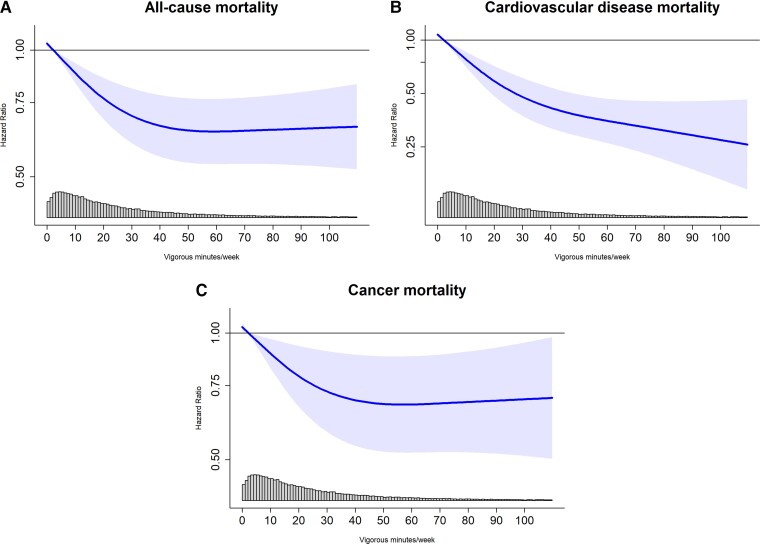
We observed a non-linear (pnon-linear < 0.01) dose–response association for VPA volume and ACM with the optimal dose (lowest HR) at 53.6 (50.5, 56.7) minutes/week [corresponding to an HR of 0.64 (0.54, 0.77)]compared with the referent 2.2 min/week (Figure 4A). The minimum dose of VPA was 14.9 [14.3, 15.4] min/week [0.82 (0.75, 0.89)] with an E-value of 1.74 (lower 95% CI 1.49). There was an inverse linear (pnon-linear = 0.42) dose-response association of VPA volume and CVD mortality (Figure 4B). The minimum dose was 19.2 (16.5, 21.9) min/week [0.60 (0.50, 0.72)] with an E-value of 2.73 (2.11). Higher VPA volume was associated with decreased cancer mortality in a non-linear (pnon-linear = 0.02) relationship with the optimal dose at 55.4 (54.0, 56.0) minutes/week [0.68 (0.52, 0.88)] (Figure 4C). The minimum dose was 15.9 (15.5, 16.3) minutes/week [0.84 (0.74, 0.95) with an E-value of 1.68 (1.29)]. Across lifestyle and health groups, we observed lower mortality HR for the minimum dose of VPA with all three mortality outcomes except for participants with a parental history of cancer (Supplementary material online, Figures 3–5). Supplementary material online, Figure 6 shows the sequential modelling results.
Figure 4.
Dose–response association between vigorous physical activity volume (minutes/week) and all-cause, cardiovascular disease, and cancer mortality. Timescale was age. Adjusted for sex, wear time, light intensity, moderate intensity, frequency of vigorous bouts, smoking history, alcohol consumption, sleep score, diet, discretionary screen-time, education, self-reported parental history of CVD and cancer, and self-reported medication use (cholesterol, blood pressure, and diabetes). The range was capped at the 97.5 percentile to minimize the influence of sparse data. Sample = 71 893; events: all-cause = 1,927, cardiovascular disease = 602, cancer = 1150; reference= 2.2 min/week. Linearity: ACM (P < 0.01); CVD (P = 0.42); cancer (P < 0.01). Nadir: ACM [53.6 min/wk; HR = 0.64 (0.54, 0.77)]; cancer [55.4 min/wk; HR = 0.68 (0.52, 0.88)].
Percent contribution of vigorous activity and frequency
There was a non-linear inverse dose–response (Supplementary material online, Figure 7) association for %VPA and all three mortality outcomes. The optimal dose was 8.4% (6.7%, 10.2%) and 8.1% (6.1%, 10.2%) for ACM [0.54 (0.46, 0.63)] and CVD [0.42 (0.31, 0.55)], respectively. Attenuation of the association for %VPA became pronounced at >11.0% for CVD mortality. For cancer mortality, there was no appreciable (rate of HR change <0.003) HR decrease beyond 15.0% [0.63 (0.45, 0.88)]. Bouts lasting up to 2 min exhibited an inverse non-linear (pnon-linear <0.01) association with all-cause and cancer mortality, with the optimal dose at 27 (24, 30) bouts/week [0.73 (0.62, 0.87)] and 31 (27, 35) bouts/week [0.60 (0.49, 0.73)], respectively. CVD mortality exhibited an inverse linear association (pnon-linear = 0.38) with a minimum frequency dose of 14 (12, 16) bouts/week [0.65 (0.53, 0.80)] (Supplementary material online, Figure 8A–C).
Multivariable-adjusted associations with cardiovascular disease, and cancer incidence
Volume
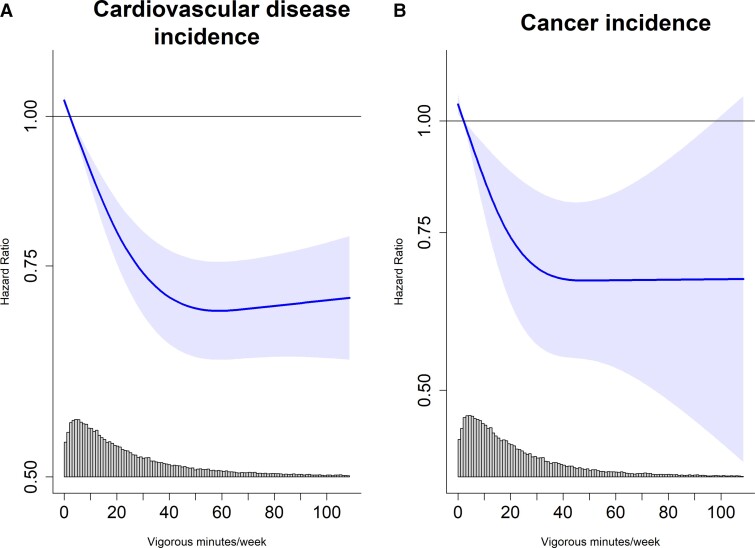
Associations for CVD and cancer incidence were non-linear (pnon-linear <0.01) with the optimal dose at 56.5 (55.4, 55.6) min/week [0.69 (0.63, 0.76)] and 46.3 (42.9, 49.7) min/week [0.67 (0.55, 0.82)] (Figure 5A–B). The minimal dose for CVD was 15.0 (14.3, 15.7) min/week [0.85 (0.81, 0.89); E-value = 1.65 (1.51)], and cancer was 12.0 (10.3, 13.7) min/week [0.83 (0.75, 0.93); E-value = 1.69 (1.36)]. Supplementary material online, Figure 9 shows the sequential modelling results.
Figure 5.
Dose–response association between vigorous physical activity volume (min/week) and incidence of cardiovascular disease (n = 71 049; events = 3730) and cancer (n = 71 070; events = 1315). Timescale was age. Adjusted for sex, wear time, light intensity physical activity, moderate intensity physical activity, frequency of vigorous bouts, smoking history, alcohol consumption, sleep score, diet, screentime, education, self-reported parental history of CVD and cancer, and self-reported medication use (cholesterol, blood pressure, and diabetes). The range was capped at the 97.5 percentile to minimize the influence of sparse data. Cardiovascular disease: n = 71,049, events = 4567. Cancer: n = 71,070, events = 2854. Reference = 2.17 min/week. Linearity: CVD (P < 0.01); cancer (P < 0.01). Nadir: CVD (56.5 min/wk; HR = 0.69 [0.63, 0.76]); cancer (46.3 min/wk; HR = 0.67 [0.55, 0.82]).
Percent contribution of vigorous activity and frequency
The dose–response curves for %VPA with CVD and cancer incidence were non-linear (pnon-linear <0.01) (Supplementary material online, Figure 10). The optimal dose for CVD and cancer was 7.1% (3.3%, 10.4%) and 9.1% (6.1%, 12.1%) corresponding to an HR of 0.66 (0.56, 0.79) and 0.61 (0.47, 0.80). The minimum frequency dose for bouts lasting up to 2 min was 10 (7, 13) bouts/week for CVD and cancer incidence corresponding to an HR of 0.84 (0.80, 0.89) and 0.83 (0.74, 0.92) (Supplementary material online, Figure 8D–E).
Sensitivity and additional analyses
Our sensitivity analyses produced similar findings. For example, excluding the first 2 years of follow-up, participants with self-rated poor health, or using CVD medication, there was an inverse linear (pnon-linear = 0.10) dose–response association for CVD mortality, and for ACM, the optimal and minimum dose was 56.0 (50.4, 61.4) min/week [0.74 (0.59, 0.92)] and 16.0 (12.8, 19.2) min/week [0.87 (0.78, 0.97)] (Supplementary material online, Figure 11). Results were robust when adjusting for obesity strata (results available upon request). Dose–response associations were consistent for VPA volume when zero min, or 6.7 min (20th percentile) was the reference (Supplementary material online, Figure 12), or when multiple imputation of covariates was applied (results available upon request). Including participants with an event in the first year of follow-up showed similar HRs as the main analysis except for CVD incidence where associations were more pronounced (Supplementary material online, Figures 13 and 14). In the ACM analyses treating CVD and cancer deaths as competing risk (Supplementary material online, Figure 15) the optimal dose for bouts/week was 24 (20, 28) corresponding to an HR of 0.50 (0.36, 0.71). The optimal dose for VPA volume was 52.2 (48.9, 55.5) min/week [0.41 (0.28, 0.61)] with a minimal dose of 12.8 (10.2, 15.4) min/week [0.70 (0.60, 0.82)].
Discussion
We observed a consistent non-linear inverse association between VPA and all-cause and cancer mortality, and a linear dose-response association for CVD mortality. The incident disease optimal and minimal dose results were broadly comparable with those from mortality, with a steep gradient for 5-year CVD incidence risk. While acknowledging that VPA guidelines were largely derived from questionnaire data, our dose–response curves for all three mortality outcomes suggested that levels well under the current recommended 75 min/week of VPA were associated with the lowest risk.
We found ∼53 min/week of VPA was associated with 36% lower ACM, with modest additional beneficial associations for more VPA. Regarding minimum dose, ∼15 min/week was associated with a 16–18% lower all-cause and cancer mortality risk, and 20 min/week was associated with a 40% lower CVD mortality risk (Structured Graphical Abstract). These findings are important from a public health and clinical perspective, given that lack of time remains the most commonly cited barrier to regular physical activity across age, sex, ethnicity, and health status.25–27
Only 20% of middle age to older adults report engaging in any VPA for at least 15 continuous minutes.28 Sustained participation in VPA leisure-time physical activity requires considerable time and often monetary commitment and can be physically challenging for people with poor fitness or established cardiovascular and cancer risk factors such as hypertension and obesity. Our results show accumulating VPA in short bouts that last up to 2 min on average four times/day was associated with substantially lower (27%) mortality risk. Although not directly assessed in this study, our findings suggest that short VPA bouts may be also embedded into regular activities of daily living and accrued intermittently throughout a week.3 The VPA volume doses we identified as potentially beneficial were consistent across age, sex, and many lifestyle and health risk factors. They are also consistent with proof of concept trials showing demonstrable effects of short VPA bouts on cardiorespiratory fitness in physically inactive adults.29,30 Findings from these trials suggest short VPA durations can stimulate the cardiorespiratory system and lead to measurable cardiovascular adaptations. This is particularly relevant for clinicians and health practitioners who provide intervention to individuals who may be unable or unwilling to engage in long blocks of sustained exercise-based VPA. The latest European Society of Cardiology guidelines identified physical activity as a modifiable risk factor that remains challenging to address, even among patients considered to be at high CVD risk.31 Encouraging participation in VPA of any length throughout the day provides additional options for adults of all ages, which might facilitate engagement, long-term adherence, and promote VPA opportunities.
Questionnaire-based studies have suggested 60–70 min/week of VPA behaviour can attenuate mortality risk by 30%.10,11,32,33 Our device-based findings suggest that a minimal dose of 20 min/week of actual VPA provides similar levels of lower mortality risk. While acknowledging that questionnaires and devices measure related but different constructs, our study suggests a ∼3:1 equivalence of VPA time captured by questionnaires and accelerometers.
Previous studies assessing %VPA reported much higher percentages (30 to 50%) were associated with 10% lower mortality relative to no VPA.6,7 These previous %VPA findings may be affected by the susceptibility of over-reporting due to social desirability bias from self-reports. By using objective device-based measures of physical activity, we found a contribution of 8% had the strongest association and lowered mortality risk by 45% to 53%. These findings suggest that relatively modest contributions of VPA relative to total MVPA are associated with substantively lower risk for mortality and incident disease, calling for promotion of even small amounts of vigorous intensity activities. These beneficial associations are more pronounced than previously reported by studies using questionnaire-based data.6–8 This may provide opportunities for improvement of CVD preventative strategies in cardio-oncology where high-intensity activity has been shown to attenuate the cardiotoxicity of cancer treatments.34,35 Whilst our results reflect associations that can be expected in the general population, the health benefits from contributions of different %VPA proportions should be considered in relation to a person’s capacity. Narrative reviews36 and meta-analyses37,38 report mixed findings on the relative contributions of moderate and vigorous activities. A recent review of physical activity intensity39 suggests that the balance of physical activity intensities needs to be determined relative to a person’s fitness and functional capacity, reflecting metabolic conditions above which physiological homeostasis is challenged and adaptations occur.
Previous device-based studies40–42 have used a lower resolution of physical activity, measured in 1 minute intervals, which may mask short VPA durations and lead to an under-estimation of VPA volume, and over-estimation of VPA volumes associated with health outcomes. Under-estimation of VPA volume would have contributed to low statistical power, making it difficult to discern associations of VPA volume and frequency with health outcomes. Using a higher resolution of physical activity measures (10 second interval), we found the majority (92%) of VPA durations lasted 1 minute or less. This is consistent with a study43 in overweight postmenopausal women that reported significant interval effects for estimated VPA time over 7 days for 10 s intervals compared with 1 min intervals and a review in children that found VPA volume decreased 4-fold when measurement intervals increased from 5 s to 1 min using wearable devices.44 Studies assessing the association between CVD incidence and physical activity intensity volume with wrist-worn devices have reported an inverse linear association.45,46 We now further focus specifically on VPA and investigate in depth not only the volume dose response but also the associations of weekly frequency and the percentage contribution of VPA to total MVPA time with mortality and incident disease risk. By using a two-step activity recognition approach that considers activity type and intensity,47,48 our study provides translation-ready VPA findings for public health guidelines and preventive care practice.
Strengths and limitations
Strengths of our study include the use accelerometers to objectively measure physical activity in the largest resource to date with linkage to prospective outcomes.49 The large sample size and long follow up allowed us to reduce the risk of reverse causality by removing participants who had an event in the first two years, prevalence of major disease, self-rated poor health, or used CVD medication. Despite the extensive precautionary measures, the potential for reverse causation may still exist caused by low activity levels due to undiagnosed or prodromal disease.50 Due to the observational design, we cannot rule out the presence of unmeasured confounding. However, our e-values indicate an unmeasured confounder would have to have a strong association between 1.65 and 2.73 with the exposure and outcome for the observed relationship to be null. The UK Biobank had a very low response rate, and participants in our sample were subject to additional selection criteria and should be considered when interpreting our results. Although, evidence suggests that this and the subsequent unrepresentativeness to the target population does not affect estimates of physical activity with mortality.51
Conclusion
Approximately 15–20 min of vigorous activity per week accrued through short bouts were associated with lower mortality, and CVD and cancer incidence. Our findings suggest premature mortality and major chronic disease may be lowered through relatively modest amounts of VPA with further decreases up to 50–57 min/week. These results may inform future physical activity recommendations and, combined with effective intervention strategies, may improve population health outcomes.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
This research has been conducted using the UK Biobank Resource, a major biomedical database, under application number 25813. The authors would like thank all the participants and professionals contributing to the UK Biobank, and Dr. Bo-Huei Huang for his assistance with an early version of this manuscript.
Contributor Information
Matthew N Ahmadi, Charles Perkins Centre, School of Health Sciences, Faculty of Medicine and Health, The University of Sydney, NSW, Australia.
Philip J Clare, Charles Perkins Centre, School of Health Sciences, Faculty of Medicine and Health, The University of Sydney, NSW, Australia; Prevention Research Collaboration, School of Public Health, Faculty of Medicine and Health, The University of Sydney, NSW, Australia; National Drug and Alcohol Research Centre, UNSW Sydney, NSW, Australia.
Peter T Katzmarzyk, Population and Public Health Sciences, Pennington Biomedical Research Center, Baton Rouge, LA, USA.
Borja del Pozo Cruz, Department of Sports Science and Clinical Biomechanics, University of Southern Denmark, Odense, Denmark.
I Min Lee, Division of Preventive Medicine, Brigham & Women’s Hospital, Harvard Medical School, Boston, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Emmanuel Stamatakis, Charles Perkins Centre, School of Health Sciences, Faculty of Medicine and Health, The University of Sydney, NSW, Australia.
Funding
This study is funded by an Australian National Health and Medical Research Council (NHMRC) Investigator Grant (APP 1194510).
Data availability
The UK Biobank data that support the findings of this study can be accessed by researchers on application (https://www.ukbiobank.ac.uk/register-apply/).
References
- 1. Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World health organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54:1451–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The physical activity guidelines for Americans. JAMA 2018;320:2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stamatakis E, Huang BH, Maher C, Thøgersen-Ntoumani C, Stathi A, Dempsey PC, et al. Untapping the health enhancing potential of vigorous intermittent lifestyle physical activity (VILPA): rationale, scoping review, and a 4-pillar research framework. Sport Med 2021;51:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ross R, Chaput JP, Giangregorio LM, Janssen I, Saunders TJ, Kho ME, et al. Canadian 24-Hour Movement guidelines for adults aged 18–64 years and adults aged 65 years or older: an integration of physical activity, sedentary behaviour, and sleep. Appl Physiol Nutr Metab 2020;45:S57–S102. [DOI] [PubMed] [Google Scholar]
- 5. Department of Health and Social Care. Physical Activity Guidelines: UK Chief Medical Officers’ Report; Department of Health and Social Care: London, UK, 2019. Available online: https://www.gov.uk/government/publications/physical-activity-guidelines-uk-ch.
- 6. Wang Y, Nie J, Ferrari G, Rey-Lopez JP, Rezende LFM. Association of physical activity intensity with mortality: a national cohort study of 403681 US adults. JAMA Intern Med 2021;181:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gebel K, Ding D, Chey T, Stamatakis E, Brown WJ, Bauman AE. Effect of moderate to vigorous physical activity on all-cause mortality in middle-aged and older Australians. JAMA Int Med 2015;175:970–977. [DOI] [PubMed] [Google Scholar]
- 8. Rey Lopez JP, Gebel K, Chia D, Stamatakis E. Associations of vigorous physical activity with all-cause, cardiovascular and cancer mortality among 64 913 adults. BMJ Open Sport Exerc Med 2019;5:e000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pablo J, Lopez R, Sabag A, Juan MM. Do vigorous-intensity and moderate- intensity physical activities reduce mortality to the same extent ? A systematic review and meta-analysis. BMJ Open Sport Exerc Med 2020;0:e000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wen CP, Wai JPM, Tsai MK, Yang YC, Cheng TYD, Lee MC, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet 2011;378:1244–1253. [DOI] [PubMed] [Google Scholar]
- 11. Lee DC, Pate RR, Lavie CJ, Sui X, Church TS, Blair SN. Leisure-Time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol 2014;64:472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DiPietro L, Al-Ansari SS, Biddle SJH, Borodulin K, Bull FC, Buman MP, et al. Advancing the global physical activity agenda: recommendations for future research by the 2020 WHO physical activity and sedentary behavior guidelines development group. Int J Behav Nutr Phys Act 2020;17:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allen N, Sudlow C, Downey P, Peakman T, Danesh J, Elliott P, et al. UK Biobank: current status and what it means for epidemiology. Health Policy Technol 2012;1:123–126. [Google Scholar]
- 14. Sipos M, Paces P, Rohac J, Novacek P. Analyses of triaxial accelerometer calibration algorithms. IEEE Sens J 2012;12:1157–1165. [Google Scholar]
- 15. Ahmadi MN, Nathan N, Sutherland R, Wolfenden L, Trost SG. Non-wear or sleep? Evaluation of five non-wear detection algorithms for raw accelerometer data. J Sports Sci 2020;38:399–404. [DOI] [PubMed] [Google Scholar]
- 16. Pavey TG, Gilson ND, Gomersall SR, Clark B, Trost SG. Field evaluation of a random forest activity classifier for wrist-worn accelerometer data. J Sci Med Sport 2017;20:75–80. [DOI] [PubMed] [Google Scholar]
- 17. Huang BH, Duncan MJ, Cistulli PA, Nassar N, Hamer M, Stamatakis E. Sleep and physical activity in relation to all-cause, cardiovascular disease and cancer mortality risk. Br J Sports Med 2022;56:718–724. [DOI] [PubMed] [Google Scholar]
- 18. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 19. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010;29:1037–1057. [DOI] [PubMed] [Google Scholar]
- 20. Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA 2019;321:602–603. [DOI] [PubMed] [Google Scholar]
- 21. Ritz C, Baty F, Streibig JC, Gerhard D. Dose-response analysis using R. PLoS One 2015;10:e0146021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rampinelli C, De Marco P, Origgi D, Maisonneuve P, Casiraghi M, Veronesi G, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ 2017;356:j347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harrell FE Jr. RMS: Regression Modeling Strategies. R package version 4.0-0: Vanderbilt University; 2017. [Google Scholar]
- 24. Therneau T, Lumley T. R survival package. 2013.
- 25. Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults’ participation in physical activity: review and update. 2003; (January). [DOI] [PubMed]
- 26. Hoare E, Stavreski B, Jennings GL, Kingwell BA. Exploring motivation and barriers to physical activity among active and inactive Australian adults. Sports 2017;5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cerin E, Leslie E, Sugiyama T, Owen N. Perceived barriers to leisure-time physical activity in adults: an ecological perspective. J Phys Act Health 2010;7:451–459. [DOI] [PubMed] [Google Scholar]
- 28. O’Donovan G, Lee IM, Hamer M, Stamatakis E. Association of “weekend warrior” and other leisure time physical activity patterns with risks for all-cause, cardiovascular disease, and cancer mortality. JAMA Intern Med 2017;177:335–342. [DOI] [PubMed] [Google Scholar]
- 29. Jenkins EM, Nairn LN, Skelly LE, Little JP, Gibala MJ. Do stair climbing exercise “snacks” improve cardiorespiratory fitness? Appl Physiol Nutr Metab 2019;44:681–684. [DOI] [PubMed] [Google Scholar]
- 30. Allison MK, Baglole JH, Martin BJ, Macinnis MJ, Gurd BJ, Gibala MJ. Brief intense stair climbing improves cardiorespiratory fitness. Med Sci Sports Exerc 2017;49:298–307. [DOI] [PubMed] [Google Scholar]
- 31. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–3337. [DOI] [PubMed] [Google Scholar]
- 32. Chomistek AK, Cook NR, Flint AJ, Rimm EB. Vigorous-intensity leisure-time physical activity and risk of major chronic disease in men. Med Sci Sports Exerc 2012;44:1898–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leitzmann MF, Park Y, Blair A, Ballard-Barbash R, Mouw T, Hollenbeck AR, et al. Physical activity recommendations and decreased risk of mortality. Arch Intern Med 2007;167:2453–2460. [DOI] [PubMed] [Google Scholar]
- 34. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines. Eur Heart J 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 35. D’ascenzi F, Anselmi F, Fiorentini C, Mannucci R, Bonifazi M, Mondillo S. The benefits of exercise in cancer patients and the criteria for exercise prescription in cardio-oncology. Eur J Prev Cardiol 2021;28:725–735. [DOI] [PubMed] [Google Scholar]
- 36. Eijsvogels TMH, Molossi S, Lee DC, Emery MS, Thompson PD. Exercise at the extremes: the amount of exercise to reduce cardiovascular events. J Am Coll Cardiol 2016;67:316–329. [DOI] [PubMed] [Google Scholar]
- 37. Löllgen H, Böckenhoff A, Knapp G. Physical activity and all-cause mortality: an updated meta-analysis with different intensity categories. Int J Sports Med 2009;30:213–224. [DOI] [PubMed] [Google Scholar]
- 38. Samitz G, Egger M, Zwahlen M. Domains of physical activity and all-cause mortality: systematic review and dose-response meta-analysis of cohort studies. Int J Epidemiol 2011;40:1382–1400. [DOI] [PubMed] [Google Scholar]
- 39. Iannetta D, Keir DA, Fontana FY, Inglis EC, Mattu AT, Paterson DH, et al. Evaluating the accuracy of using fixed ranges of METs to categorize exertional intensity in a heterogeneous group of healthy individuals: implications for cardiorespiratory fitness and health outcomes. Sports Med 2021;51:2411–2421. [DOI] [PubMed] [Google Scholar]
- 40. Lee IM, Shiroma EJ, Kamada M, Bassett DR, Matthews CE, Buring JE. Association of step volume and intensity with all-cause mortality in older women. JAMA Intern Med 2019;179:1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ekelund U, Tarp J, Steene-johannessen J, Hansen BH, Jefferis B, Fagerland MW, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality : systematic review and harmonised meta-analysis. BMJ 2019;366:l4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saint-Maurice PF, Troiano RP, Bassett DR, Graubard BI, Carlson SA, Shiroma EJ, et al. Association of daily step count and step intensity with mortality among US adults. JAMA 2020;323:1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gabriel KP, McClain JJ, Schmid KK, Storti KL, High RR, Underwood DA, et al. Issues in accelerometer methodology: the role of epoch length on estimates of physical activity and relationships with health outcomes in overweight, post-menopausal women. Int J Behav Nutr Phys Act 2010;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trost SG, Mciver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc 2005;37:S531–S543. [DOI] [PubMed] [Google Scholar]
- 45. Ramakrishnan R, Doherty A, Smith-Byrne K, Rahimi K, Bennett D, Woodward M, et al. Accelerometer measured physical activity and the incidence of cardiovascular disease: evidence from the UK biobank cohort study. PLoS Med 2021;18:e1003487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cruz BDP, Ahmadi M, Inan-Eroglu E, Huang BH, Stamatakis E. Prospective associations of accelerometer-assessed physical activity with mortality and incidence of cardiovascular disease among adults with hypertension: the UK biobank study. J Am Heart Assoc 2022;11:e023290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Trost SG. Population-level physical activity surveillance in young people: are accelerometer-based measures ready for prime time? Int J Behav Nutr Phys Act 2020;17:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosenberger ME, Haskell WL, Albinali F, Mota S, Nawyn J, Intille S. Estimating activity and sedentary behavior from an accelerometer on the hip or wrist. Med Sci Sports Exerc 2013;45:964–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Doherty A, Jackson D, Hammerla N, Plotz T, Olivier P, Granat M, et al. Large scale population assessment of physical activity using wrist worn accelerometers: the UK biobank study. PLoS One 2017;12:e0169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tarp J, Hansen BH, Fagerland MW, Steene-johannessen J, Anderssen SA, Ekelund U. Accelerometer-measured physical activity and sedentary time in a cohort of US adults followed for up to 13 years : the influence of removing early follow-up on associations with mortality. Int J Behav Nutr Phys Act 2020;17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stamatakis E, Owen KB, Shepherd L, Drayton B, Hamer M, Bauman AE. Is cohort representativeness passé? Poststratified associations of lifestyle risk factors with mortality in the UK biobank. Epidemiology 2021;32:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The UK Biobank data that support the findings of this study can be accessed by researchers on application (https://www.ukbiobank.ac.uk/register-apply/).