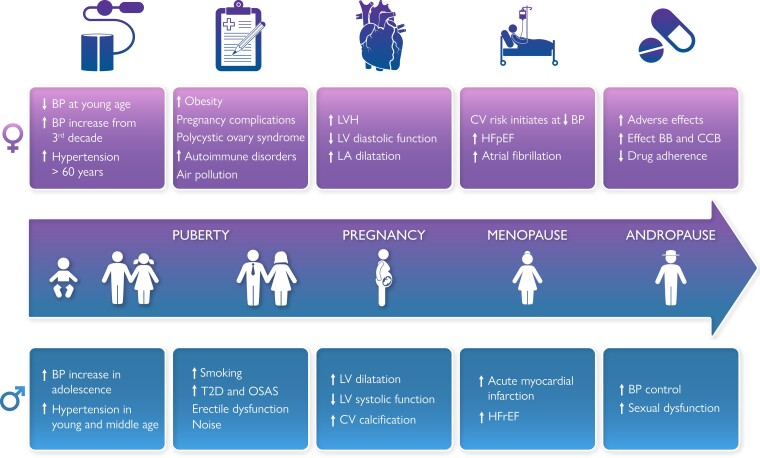
Graphical Abstract
Graphical Abstract.
Sex differences in hypertension. BP, blood pressure; CV, cardiovascular; T2D, type 2 diabetes; OSAS, obstructive sleep apnoea syndrome; LVH, left ventricular hypertrophy; LV, left ventricular; LA left atrial; HFpEF, heart failure with preserved ejection fraction; BB, beta blocker; CCB, calcium channel blocker; HFrEF, heart failure with reduced ejection fraction.
Keywords: Hypertension, Sex, Blood Pressure regulators, Hypertension-mediated organ damage, Pharmacological treatment, Adverse events, Cardiovascular disease, Sex hormones
Abstract
There is strong evidence that sex chromosomes and sex hormones influence blood pressure (BP) regulation, distribution of cardiovascular (CV) risk factors and co-morbidities differentially in females and males with essential arterial hypertension. The risk for CV disease increases at a lower BP level in females than in males, suggesting that sex-specific thresholds for diagnosis of hypertension may be reasonable. However, due to paucity of data, in particularly from specifically designed clinical trials, it is not yet known whether hypertension should be differently managed in females and males, including treatment goals and choice and dosages of antihypertensive drugs. Accordingly, this consensus document was conceived to provide a comprehensive overview of current knowledge on sex differences in essential hypertension including BP development over the life course, development of hypertension, pathophysiologic mechanisms regulating BP, interaction of BP with CV risk factors and co-morbidities, hypertension-mediated organ damage in the heart and the arteries, impact on incident CV disease, and differences in the effect of antihypertensive treatment. The consensus document also highlights areas where focused research is needed to advance sex-specific prevention and management of hypertension.
Introduction
Arterial hypertension, in particular elevated systolic blood pressure (BP), remains a major cause of reduced quality of life, cardiovascular (CV) morbidity and mortality and all-cause mortality in the world.1,2 Both BP development and BP regulation are influenced by biological effects of sex chromosomes, sex hormones and reproductive events.3 In addition, the sex difference in hypertension prevalence has been related to ethnicity, co-morbidities, socio-economic status, education and environmental pollution in middle-aged and older adults.4,5 Previous publications have documented important sex differences in hypertension related to main BP regulators, co-morbidities, CV complications and adverse effects of antihypertensive drugs.6–8 However, there is paucity in reports of sex-specific effects from clinical trials in hypertension. Recent data indicate that risk for CV complications starts at lower BP levels in females than in males, questioning current practice of using the same BP threshold for identification of hypertension in both sexes.9,10 The scope of this collaborative document is to give a comprehensive overview of current knowledge on sex differences in essential arterial hypertension, associated organ damage and CV disease (CVD) and hypertension management (Graphical Abstract), as well as identifying knowledge gaps hindering the development of sex and gender informed hypertension management.
BP development and hypertension prevalenceover the life course
BP development in the young
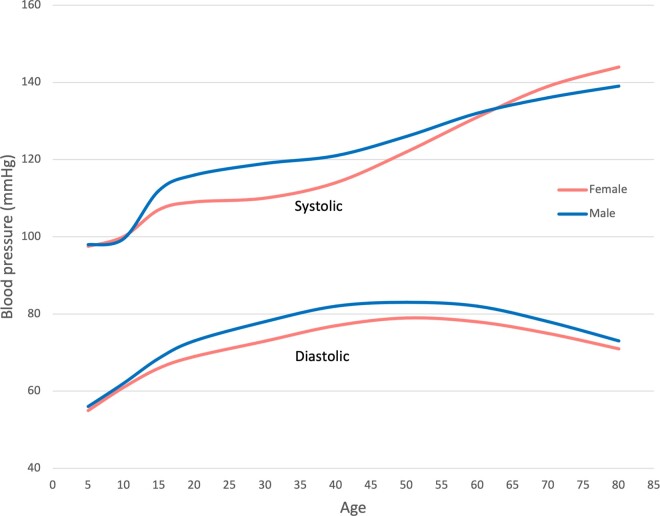
Sex differences in BP trajectories are apparent from early life and change across the life course, suggesting that early life factors may play a role in how CVD present differently in females and males.11 At age seven, both sexes have similar systolic BP, which then increases a little faster in females up to age 12, and thereafter slower in females resulting in a lower systolic BP in females than in males starting from 13 years of age (Figure 1).11 Systolic BP is 10 mmHg higher in males compared with females by the age of 18 years, a difference which slightly increases over time up to 30 years.11 Diastolic BP is higher in females at age 7, then increases similarly in both sexes up to age 12, thereafter slower in females. From age 16, diastolic BP decreases in both sexes, but faster in males.11 In the National Health and Nutrition Examination Survey (NHANES), the annual net transition rate from optimal BP (systolic BP <120 mmHg and diastolic BP <80 mmHg) to prehypertension (systolic BP 120–139 mmHg or diastolic BP 80–89 mmHg) was twice as high among males compared with females aged 8–30 years, and highest for young African American males.14 However, their findings may have been influenced by the lower BP in healthy young females, since the same BP threshold was used in both sexes.
Figure 1.
Blood pressure development in females and males during childhood, adolescence, and early adulthood. Based upon O’Keeffe et al.,11 Shen et al.,12 and Ji et al.13.
BP in midlife and beyond
The sex-specific BP trajectories from puberty to adulthood continue with different patterns during young and middle-aged adulthood.12,15 From late adolescence, males have higher levels and steeper slopes of both systolic and diastolic BP than females until early midlife, at which there is a crossover, and females have a steeper rise in BP thereafter throughout their life course (Figure 1).12,16 In subjects older than 40 years, transition from optimal BP to prehypertension was stable or decreased among males, but rapidly increased among females in NHANES.14 Diversity in the BP development during menopause transition is well demonstrated.17 An accelerated increase in systolic BP is observed in about 35%, particularly in females with early menopause and vasomotor symptoms,17 and in women with clustering of CV risk factors.18 In population-based cohort studies from Italy and the Czech Republic, BP increase during menopause transition was explained by weight gain, obesity and aging.19,20 In a pooled analysis of longitudinal individual BP measures over 43 years in >32 800 individuals in four population-based cohorts in the USA, a steeper increase in systolic, diastolic and mean BP as well as in pulse pressure was observed in females compared to males already from the third decade onwards (Figure 1).13
Globally, the age-adjusted prevalence of hypertension in adults (using systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg as definition in both sexes) was 32% in females and 34% in males in 2019, unchanged from 1990, as a result of a comparable decrease in hypertension in high-income countries and increase in low- and middle-income countries.21 However, due to the aging trend, the absolute number of patients with hypertension almost doubled during this period21 (Box 1).
Box 1 . Key messages on sex differences in BP development over the life course.
Healthy young females have lower BP than males at similar age but experience a steeper increase in BP from the third decade of life.
Better understanding of the underlying mechanisms of BP increase in midlife may provide targets for improved prevention of hypertension in both sexes.
Sex differences in regulators of BP
The regulation of vascular function and BP differs between females and males, in particular due to sex differences related to the autonomic nervous system, the renin-angiotensin-aldosterone system (RAAS), bradykinin, nitric oxide, brain natriuretic peptides and humoral mechanisms related to sex chromosomes, sex hormones and other hormones.7,22,23
The autonomic nervous system
The autonomic nervous system plays an important role in BP regulation and contributes to modulate CVD.24 Although normotensive adult females and males share the same CV autonomic regulation, they exhibit differences in the sympatho-vagal balance and central and reflex neuronal influence on the CV system.25 Sex-dependent physiological changes related to age, menopause, obesity and physical activity might also influence neuronal hemodynamic regulation. Females have a larger increase in sympathetic nervous activity with age and obesity than males. Compared to healthy males, healthy females are characterized by lower baroreceptor reflex sensitivity and lower heart rate variability.26,27
BP effects of sex hormones
Ovarian hormones have a major role in BP regulation, endogenous oestrogen being associated with the lower BP in premenopausal females.28,29 Oestrogens modulate BP directly through non-genomic effects on vascular, renal and cardiac cells, by reducing calcium pathways and indirectly through genomic actions, controlling expression of potent vasoconstrictors, such as angiotensin II, endothelin 1 and catecholamines, and controlling the RAAS and endothelin pathway.28,30 On the contrary, testosterone is pro-hypertensive and likely contributes to the increase in CV risk observed with ageing in males and after menopause in females.28,29,31 Androgens increase BP by activating the RAAS.29 Oestrogens reduce plasma renin and angiotensin-converting enzyme (ACE) activity, and up-regulate angiotensinogen expression, leading to increased levels of angiotensin and aldosterone, and sodium retention.32 Progesterone is a potent aldosterone antagonist, which acts on the mineralocorticoid receptor to prevent sodium retention and counteracts the sodium-retaining effect of oestrogen.33 When becoming hypertensive, females tend to have lower plasma renin activity than males.34 The premenopausal cardioprotective effects of oestrogens may in part result from RAAS inhibition.35 After menopause, increased salt sensitivity is observed in females.28,36 Thus, lower oestrogen level after menopause is related to both upregulation of hormonal systems such as the RAAS and sympathetic nervous system, and to reduced vascular nitric oxide bioavailability.23 As a consequence, the synthesis of potent vasoconstrictors such as angiotensin II, endothelin-1, and catecholamines rises after menopause.37,38 Antihypertensive drug therapy in postmenopausal women may improve endothelial dysfunction caused by decreased vascular nitric oxide bioavailability and thereby reduce CV risk.39 The roles of relaxin, oxytocin, prolactin and vasopressin in the regulation of BP are less well characterized than for oestrogen, progesterone and testosterone (Box 2).
Box 2 . Key messages on sex-differences in BP regulators, CV risk factors and co-morbidities.
The activity of autonomic and endocrine BP regulators differs between sexes and may influence drug efficacy and adverse effects.
The prevalence and influence of traditional risk factors on CVD vary between females and males. Sex-specific CV risk factors are documented in both sexes. Better integration of these differences in risk assessment tools will improve CVD prevention.
Sex differences in risk factors and co-morbidities
Sex-specific CV risk factors
Important sex-specific CV risk factors have been identified.40 In males, erectile dysfunction and androgenic alopecia are associated with increased risk for hypertension as well as for CVD.41,42 Females undergo important changes in sex hormones throughout their life course that impact CV risk.18 Pregnancy-related hypertensive disorders increase the risk for chronic hypertension and CVD, even before menopause transition.43–45 Detailed European recommendations on management of peripartum hypertension were recently published.46 Women with polycystic ovary syndrome have a higher risk of hypertension, including hypertensive disorders in pregnancy.47 In transgender populations, insufficient data have been reported on the effects of gender-affirming hormone therapy on the incidence of hypertension.48
Inflammatory and autoimmune disorders are associated with increased risk for hypertension and CVD, and both the innate and adaptive immune systems are influenced by sex hormones.49 While progesterone and androgens are considered immunosuppressive, oestrogens are considered immune-stimulatory, contributing to the observed preponderance of most autoimmune diseases in females.49
Traditional CV risk factors in hypertension
Important sex differences in conventional CV risk factors have been reported in hypertension, particularly related to smoking and metabolic risk factors [obesity, type 2 diabetes (T2D) and dyslipidaemia], and to co-morbidities (obstructive sleep apnoea, renal dysfunction, and autoimmune disorders) (Table 1). Clustering of more than two metabolic risk factors is often referred to as the metabolic syndrome.60 Glucose and lipid metabolism are directly modulated by oestrogen and testosterone. Oestrogen deficiency or a relative increase in testosterone promotes insulin resistance and a pro-atherogenic lipid profile.54 Consequently, the prevalence of the metabolic syndrome is higher in males in a young hypertensive population, but higher in females in older hypertensive population.54 Dyslipidaemia is highly prevalent in hypertension, particularly among white males.56 In females, serum levels of total cholesterol, low-density lipoprotein cholesterol and apolipoprotein B increase substantially in the perimenopausal period.61 In the Women’s Health Study, the association of higher serum triglycerides with increased CVD risk attenuated with increasing age of onset.62
Table 1.
Sex differences in conventional CV risk factors and co-morbidities in hypertension
| Factor | Females | Males | Relevant references |
|---|---|---|---|
| Age | ++ | + | 13,50 |
| Obesity | ++ | + | 8,51,52 |
| Visceral obesity | + | ++ | 8,53 |
| Metabolic syndrome | + (++ after menopause) | ++ | 8,54 |
| Type 2 diabetes | + | +(+) | 55 |
| Dyslipidaemia | + | ++ | 56 |
| Smoking | + | ++ | 57 |
| Obstructive sleep apnoea | + | ++ | 58,59 |
| Autoimmune disorders | +++ | + | 49 |
| Reduced eGFR | ++ | + | 57 |
| Albuminuria | + | ++ | 57 |
| Gout | + | +++ | 7,32 |
+Common; ++more common; +++much more common vs. other sex.
Obesity is present in at least 50% of individuals with hypertension,51 and is more common in females.52 The higher fat mass in females and sex differences in adipose tissue distribution are well documented. Higher abdominal visceral adipose mass has been more strongly associated with risk of hypertension in females, and with risk of metabolic syndrome in males.53 Obesity and hypertension are both strongly associated with insulin resistance and development of T2D. The prevalence of T2D is age-dependent and higher in males than in females.55 Presence of T2D in females reduces their innate CV risk advantage, and females with T2D have a comparable risk as males for CVD.63,64 The relatively worse prognostic impact of T2D in females may be associated with different hormonal modulation of insulin sensitivity,65 sex disparities in diabetes care and greater risk factor clustering among females with T2D.66
Obstructive sleep apnoea is more prevalent in males.58 It is an independent risk factor for hypertension in both sexes, but the risk for hypertension is evident at lower sleep apnoea severity in females than in males.59 Females with hypertension more often have reduced glomerular filtration rate than males. In contrast, males with hypertension more often exhibit albuminuria, possibly due to sex differences in protein handling.57
Environmental risk factors
Air pollution contributes to risk for CVD through a number of mechanisms, including oxidative stress, systemic inflammation and vascular dysfunction that all promote hypertension.67,68 Sex differences in these relations have been little studied, but fine particulate matter (PM2.5) and sulphur dioxide have been suggested as stronger risk factors for hypertension in females and nitric dioxide and carbon monoxide stronger in males in studies from China.69,70 A post-hoc analysis within the Systolic Blood Pressure Intervention Trial (SPRINT) trial demonstrated that the effect of intensive antihypertensive drug treatment (systolic BP <120 mmHg) was greater in participants exposed to higher PM2.5 levels.71
Studies on noise around major European airports have reported aircraft noise to be associated with incident hypertension in males.72 In the UK Biobank cohort, exposure to road traffic noise above 60 dB was associated with higher systolic and diastolic BP, irrespective of antihypertensive drug treatment73 (Box 2).
Sex differences in hypertensive heart disease
Arterial hypertension causes structural and functional changes in the heart, collectively named hypertensive heart disease.50 The heart is normally smaller in females than in males from puberty onwards,74 largely due to differences in body size and composition.75 Current guidelines therefore recommend sex-specific threshold values for optimal detection of hypertensive heart disease by echocardiography.50,76
Left ventricular hypertrophy
Left ventricular (LV) hypertrophy (LVH) is the hallmark of hypertensive heart disease and a powerful prognostic marker in hypertension. Hypertensive LVH is more prevalent and less modifiable by antihypertensive treatment in females than in males.8,77,78 Persistent LVH is particularly associated with increased arterial stiffness, and higher risk of CV events and mortality during follow-up, independent of achieved BP values.79
In the Strong Heart Study, LVH was found in 36% of middle-aged females and in 23% of middle-aged males.80 During 4 years of follow-up, LVH prevalence increased despite good BP control. The lack of LVH regression was attributed to obesity and a progressive decline in renal function. The prospective Italian Campania Salute Network registry of subjects treated for hypertension demonstrated that presence of LVH off-sets the innate lower CV risk in females, and females and males with hypertension and LVH had comparable risk of CVD.81 Furthermore, during follow-up, 21% of subjects with hypertension and initial normal LV mass developed LVH, particularly females and those with obesity.82
Presence of LVH in hypertension is associated with reduced myocardial function in both sexes, whether assessed by midwall shortening or global longitudinal strain, while LV ejection fraction is usually normal. In hypertension, females exhibit higher LV myocardial function and ejection fraction than males, independent of LV geometry.83
Dilated left atrium
A dilated left atrium (LA) is another common sign of hypertensive heart disease. LA dilatation is associated with increased CVD, in particular atrial fibrillation, heart failure (HF) and ischaemic stroke.84–86 In healthy subjects, the LA is normally larger in males than in females,87 but in hypertension LA dilatation is more common in females.88,89 In older subjects with hypertension and LVH, LA dilatation was significantly more prevalent in females.88 Similarly, also in middle-aged subjects with obesity without known CVD, LA dilatation was significantly more prevalent in females than in males, and particularly associated with co-presence of hypertension and increased arterial stiffness90 (Box 3).
Box 3 . Key messages about sex-differences in hypertension mediated organ damage.
Hypertension mediated organ damage like hypertensive heart disease and arterial dysfunction show sex specific incidence, threshold values and treatment success and may develop despite treatment.
Identification of the underlying mechanisms for such development in females and males may provide targets to reduce high-risk phenotypes and progression to CVD.
Sex differences in arterial dysfunction
Arterial structure and function
Sex differences both in micro- and macrovascular structure and function have been documented.91 Sex- and age-specific threshold values for diagnosis of arterial dysfunction in large arteries by arterial stiffness and intima-media thickness, and in small arteries by lumen media ratio and flow-mediated vasodilatation have been published.91–94 Females have smaller aortic root dimensions than males, even after adjusting for body size.95 Sex differences in ventricular-arterial coupling and higher arterial stiffness in females, particularly in the ascending aorta, have been documented in several studies.96,97 Augmentation pressure and augmentation index are both higher in females at all ages, while carotid-femoral pulse wave velocity (PWV) does not differ by sex.94,98 Steeper increases in carotid-femoral PWV and peripheral vascular resistance are observed with aging and hypertension in females compared to males.97,98
Arterial stiffness
Arterial stiffness can be estimated from the ratio of pulse pressure to stroke volume index (PP/SVi) or more directly measured by carotid-femoral PWV. In addition to being a predictor of CVD, independent of other hypertension-mediated organ damage like LVH,99 increased PP/SVi is also an independent predictor of the transition from diastolic to isolated systolic hypertension, with a risk for transition being 30% higher in females than in males.100 This finding has been confirmed by analyzing sex differences in PWV trajectories over time, demonstrating a more rapid increase in systolic BP and higher prevalence of HF with preserved ejection fraction (HFpEF) in females than in males.13
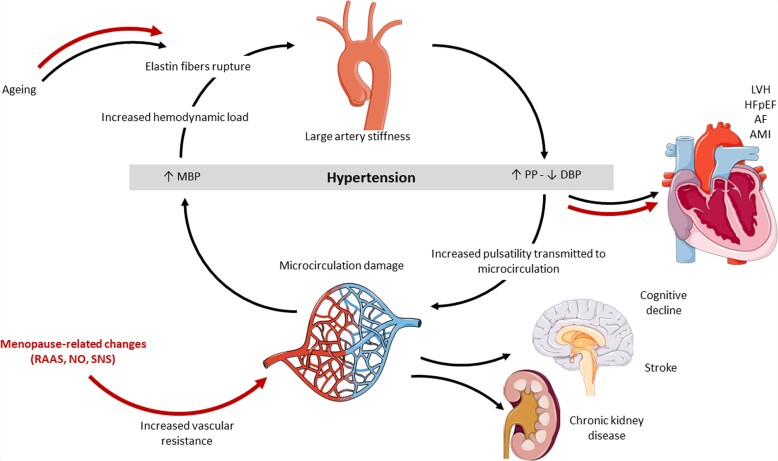
Increased arterial stiffness augments systolic BP and pulse pressure, worsening hypertension through hemodynamic load-induced elastic fibre degradation and collagen deposition in the arterial wall. Higher aortic stiffness and pressure and flow pulsatility increases the pulsatile load on the heart, promoting LVH and reduction in global longitudinal strain in the LV.101 Excessive stiffness and flow pulsatility have also been associated with microvascular lesions in high-flow organs like the brain and the kidneys, suggesting that pulsatile flow damages small arteries in these organs (Figure 2).101 Arterial stiffness is less modifiable by antihypertensive therapy in females than in males.99 Greater prognostic significance of arterial stiffness in females than in males was suggested by a study in patients with coronary artery disease,102 but this finding needs confirmation in patients without coronary artery disease (Box 3).
Figure 2.
Sex differences in development of arterial dysfunction and related complications. Red arrows indicate mechanisms that are enhanced in females. MBP, mean arterial blood pressure; PP, pulse pressure; DBP, diastolic blood pressure; LVH, left ventricular hypertrophy; HFpEF, heart failure with preserved ejection fraction; AMI, acute myocardial infarction; AF, atrial fibrillation; RAAS, renin-angiotensin-aldosterone system; NO, nitric oxide; SNS, sympathetic nervous system.
Sex differences in the association of hypertension with CVD
The association of BP with CVD
The 2019 Global Burden of Disease study report confirmed that elevated systolic BP was the most important risk factor for mortality in females worldwide and only second to smoking in males.2 The majority of these deaths are caused by CVD. Hypertension in midlife seems to be more harmful in females than in similarly aged males, with hypertension being a stronger risk factor for myocardial infarction, cognitive decline and dementia.9,103–105
Ambulatory BP recording have documented higher asleep BP in males in population-based studies, but a similar decline in BP from day-time to night-time (dipping) and prevalence of non-dipping in both sexes.106 Asleep systolic BP is a stronger predictor of all-cause mortality and CVD in females than males.106 In a study in the Italian Umbria district, non-dipping in hypertension was associated with increased risk of CVD only in females.107
Several studies have now documented that the CVD risk increases at a lower BP level in females than in males, including risk for myocardial infarction, HF and stroke.9,10,103 A case-control study of risk factors associated with myocardial infarction in 52 countries showed that hypertension was associated with greater risk of myocardial infarction in older females than males.108 Also the community based Tromso Study found higher BP a stronger risk factor for myocardial infarction in females than males.109
HF and atrial fibrillation
Females with hypertension develop more vascular and myocardial stiffness than their male counterparts at old age, contributing to their higher risk for atrial fibrillation, HFpEF and stroke.110–113 Among patients with atrial fibrillation, females have higher prevalence of hypertension than males.114,115 While some reviews have suggested that hypertension confers similar risk of atrial fibrillation in both sexes,116,117 the Tromso Study reported a stronger association of elevated systolic BP with incident atrial fibrillation in females than in males.118 In the same cohort, increased systolic BP was associated with higher risk for both paroxysmal/persistent and permanent atrial fibrillation in females, but only for paroxysmal/persistent atrial fibrillation in males.119
Hypertension is associated with high risk for HF in both sexes.120–122 Hypertension is more prevalent among female than male HF patients (50% vs. 40%) and increases the risk for HF in females by 3-fold, compared to 2-fold in males.121 Males constitute about 30% of patients with HFpEF, and females about 40% of patients with HF with reduced ejection fraction (HFrEF) by current definitions.122 Sex differences in arterial, LA and LV adaptation to pressure overload, and in micro- and macrovascular coronary disease may contribute to the higher prevalence of HFpEF in women.123 Furthermore, iron deficiency, T2D, obesity, preeclampsia, and autoimmune diseases, all common co-morbidities in females with hypertension, may contribute to higher risk of HFpEF through amplification of cardiac dysfunction and systemic inflammation.123 Hypertension may contribute to the observed higher risk of stroke in female HFrEF patients.124 In a post-hoc analysis of data from two large trials in patients with HFrEF, female patients had lower mortality despite suboptimal treatment with diuretics, anticoagulants and device therapy compared to male patients.124
Aortic valve stenosis
Both systolic and diastolic hypertension are associated with increased risk for degenerative aortic valve stenosis (AS) among older subjects.125 In AS, hypertension is particularly common in older females,126 and associated with impaired LV function and outcome in both sexes.127 In a Danish population-based study, progression of aortic valve calcification by cardiac computed tomography was particularly associated with hypertension in females, and with dyslipidaemia in males.128 The ESC recently published a consensus document on how to manage hypertension in AS.129
Stroke
Stroke is a major complication in hypertension.130 Sex differences in stroke incidence, presentation, and outcome are well documented.131,132 Females with acute stroke often report non-conventional symptoms, which may contribute to delay in diagnosis and treatment, and subsequent increased mortality and disability.133 Males have higher stroke incidence up to 85 years of age.134 However, stroke risk increases at a lower BP level in females than in males.10 A recent meta-analysis demonstrated that young women may be disproportionately at risk for ischaemic stroke.135 Among patients with ischaemic stroke, females more often present with atrial fibrillation, hypertension, obesity and T2D than males.136,137
Peripheral artery disease
Hypertension is a major risk factor for peripheral artery disease in both sexes.138 Females are usually older and more obese at the time of diagnosis. Claudication is more often reported in males than females during middle age, but this sex difference is not observed at older age.139 Among patients with critical limb ischaemia, female sex is an independent predictor for pronounced femoral-popliteal involvement and more severe and diffuse atherosclerotic disease.140 Several studies have noted sex differences in ankle-brachial index with lower values in healthy women141,142 (Box 4).
Box 4 . Key messages on sex differences in BP association with CVD.
Females with hypertension more often develop atrial fibrillation and HFpEF, while males more often develop AMI and HFrEF.
CV risk increases at a lower BP level in females than in males. Future research should explore whether different diagnostic BP threshold values or treatment targets in females and males with hypertension may improve CVD prevention.
Sex differences in the effects of antihypertensive treatment
Adverse effects of antihypertensive drugs
Sex differences in pharmacokinetics and pharmacodynamics are well described and mostly due to differences in either drug transporters affecting absorption (i.e. P-glycoprotein) or enzymes affecting metabolism and/or clearance (i.e. cytochrome P450 activity).35 Interaction of sex hormones with enzymes involved in drug absorption and metabolism influences drug pharmacokinetics and pharmacodynamics, efficacy and adverse effects.143 Overall, females more often report adverse effects from antihypertensive drugs than males, except for mineralocorticoid receptor antagonists. In particular, females more often experience hyponatremia, hypokalemia and arrhythmia during treatment with diuretics, oedema with dihydropyridine calcium channel blockers (CCBs) and cough with ACE inhibitors (ACEI), while males more often experience gout during treatment with diuretics.7,144–147 Sexual dysfunction is almost uniquely reported in males, and particularly during beta blocker (BB) treatment.148
Efficacy of antihypertensive treatment
In the Dietary Approaches to Stop Hypertension trial, dietary sodium restriction induced a pronounced BP reduction only in females.149 Structured aerobic exercise therapy reduced BP more in males than in females in a meta-analysis of 93 trials.150
Sex differences in drug effects on BP are well described.35 In particular, females have enhanced BP reduction from treatment with BB and CCB.35 Studies on prescription of antihypertensive drugs have documented that females are more often prescribed diuretics and males more often ACEI.151,152 The Stockholm regional database including prescriptions to 292 428 subjects, found that prescription of diuretics and BB increased while prescription of ACEI, angiotensin receptors blockers (ARBs) and CCB decreased with aging in both sexes.153 In particular, treatment with BB was more common in females than males among subjects without known CVD, while treatment with ACEI or ARB was more common in males than females with HF or diabetes.153
Few ancillary analyses in clinical trials of antihypertensive drug treatment have reported sex-specific treatment results. Comparable benefits were demonstrated for females and males in the Nordic Diltiazem Study, the Treatment of Mild Hypertension Study and the Losartan Intervention For Endpoint Reduction in Hypertension Study.,144,154,155 However, sex differences in antihypertensive drug effects were reported in the Hypertension Optimal Treatment study,156 the Second Australian National BP Study,157 and in the Valsartan Antihypertensive Long-term Use Evaluation trial (Table 2).158 In the SPRINT trial, the number of females included was too low to draw conclusions on the benefit of intensive BP control in elderly females,159 and two subgroup analyses reported contrasting results.160,161 Still, SPRINT changed the American definition of hypertension and recommendations for BP management in both sexes.162
Table 2.
Overview of clinical trials in hypertension reporting results stratified by sex
| Paper | Trial name (Reference) | Number of participants | % Females | Results stratified by sex |
|---|---|---|---|---|
| Studies that documented comparable benefits of study treatment in both sexes | ||||
| Kjeldsen et al. Influence of age, sex and blood pressure on the principal endpoints of the Nordic Diltiazem Study | The Nordic Diltiazem Study154 | 10 876 | 51 | Similar treatment effect in both sexes. |
| Lewis et al. Efficacy and tolerance of antihypertensive treatment in men and women with stage 1 diastolic hypertension. Results of the Treatment of Mild Hypertension Study | Treatment of Mild Hypertension Study144 | 902 | 38 | Similar treatment effect in both sexes. |
| Os et al. Effects of losartan in women with hypertension and left ventricular hypertrophy: results from the Losartan Intervention for Endpoint Reduction in Hypertension Study | Losartan Intervention For Endpoint reduction in hypertension155 | 9193 | 54 | Similar treatment effect in both sexes. In the losartan group, females had better reduction in the primary endpoint, all-cause mortality and new-onset diabetes. |
| Studies that documented sex differences in benefits of study treatment | ||||
| Kjeldsen et al. Influence of gender on prevention of myocardial infarction by antihypertensives and acetylsalicylic acid: the HOT study | Hypertension Optimal Treatment156 | 18 790 | 47 | Achieving target diastolic BP <80 mmHg reduced myocardial infarction in women but not in men. Acetylsalicylic acid reduced incident myocardial infarction in men, but not in women. |
| Wing et al. A comparison of outcomes with angiotensin converting enzyme inhibitors and diuretics for hypertension in the elderly | Second Australian National Blood Pressure Study157 | 6083 | 51 | The benefit of ACEI treatment was only demonstrated in males. |
| Zanchetti et al. Outcomes in subgroups of hypertensive patients treated with regimens based on valsartan and amlodipine: An analysis of findings from the VALUE trial | Valsartan Antihypertensive Long-term Use Evaluation trial158 | 15 245 | 42 | Amlodipine lowered BP and reduced the primary endpoint (composite of cardiac mortality and morbidity) better in females than in males. Valsartan reduced the secondary endpoint of hospitalization for heart failure better in males. |
BP, blood pressure; ACEI, angiotensin-converting enzyme inhibitor.
A meta-analysis by the BP Lowering Treatment Trialists’ Collaboration including 31 randomized trials published before 2006, and a total of 103 268 men and 87 349 women, found comparable reductions in BP and incidence of CV events in both sexes for treatments based on ACEI, ARB, CCB, diuretics or BB.163 Similarly, another network meta-analysis based on 40 trials and 152 379 patents documented that no class of medication (ACEI, ARB, CCB or BB) was significantly better than thiazides as first-line therapy for any outcome (all-cause mortality, CV mortality, HF or stroke) in females or males analyzed separately.164 No interaction analyses between sex and drug class effects were presented.
BP awareness and control
BP control is necessary for optimal CV prevention in hypertension. Awareness of hypertension has traditionally been higher in females than in males.165–167
Studies suggest that males treated for hypertension achieve better BP control than females. In the Multi-Ethnic Study of Atherosclerosis these sex disparities increased with age and were largest in participants older than 75 years.168 Elderly females also have lower BP control rates vs. middle-aged and young females.7 Whether this is due to biological factors, inadequate treatment (physicians inertia, patient non-adherence, inappropriate drug choice), higher prevalence of CV organ damage or other co-morbidities is unknown.7 Depression and dis-satisfaction with the health care provider are known factors particularly associated with non-adherence in older females, but not in males.169 A recent data analysis from the Canadian Health Measures Survey demonstrated a particular decline in hypertension treatment and control rates in females over the period 2007–17.170 Typically, these females had lower socioeconomic status and used more than three antihypertensive drugs, both factors associated with drug non-adherence in previous research.171 In the Swedish Primary Care CV Database, BP control was not achieved to the same extent in females as in males with hypertension managed in primary health care, independent of co-morbidities.152
In small studies of patients with resistant hypertension, those with drug non-adherence included a higher proportion of females.172,173 However, sex-specific analyses were not reported (Box 5).
Box 5 . Key messages on sex-differences in the effect of antihypertensive treatment.
Sex-differences in efficacy and adverse effects of antihypertensive drugs are well described. These differences should be better communicated to health care providers to promote optimal antihypertensive drug treatment.
In general, males treated for hypertension achieve better BP control than females. Future research should target underlying causes for this difference, including patient related factors, health care related factors, socio-demographic factors and drug related factors to provide sex-specific advice for optimalization of antihypertensive drug therapy.
Conclusions
Our knowledge about sex differences in hypertension has been substantially advanced over the past decades, but much of this knowledge awaits clinical adoption. Better implementation of sex differences in BP development, regulation, and CV risk factors in prevention tools is likely to improve CVD prevention, in particular in females. Better communication of known sex differences in efficacy and adverse effects of antihypertensive drugs to health care providers may optimize treatment and improve patient adherence.
However, important knowledge gaps remain related to prevention of organ damage and CVD in hypertension. Hypertension-mediated organ damage show sex-specific incidence, threshold value and treatment success. Identification of the underlying mechanisms for CV organ damage development in females and males may provide new strategies for prevention of high-risk phenotypes and progression to CVD. Finally, future clinical studies should explore whether using sex-specific BP threshold values and treatment targets in hypertension may improve CVD prevention.
Authors contribution
E.G. and G.d.S. contributed to conception of this scientific statement; E.G., I.S., S.B., R.M.B., A.S.M., G.P. and G.d.S. drafted individual sections of the manuscript; S.B. drafted the graphical abstract; S.B. and R.M.B. drafted the figures; all authors contributed to revision of the manuscript content and approved the final version.
Contributor Information
Eva Gerdts, Center for Research on Cardiac Disease in Women, University of Bergen, Bergen, Norway.
Isabella Sudano, University Hospital Zurich University Heart Center, Cardiology, University Hospital and University of Zurich, Zurich, Switzerland.
Sofie Brouwers, Department of Cardiology, Cardiovascular Center Aalst, OLV Clinic Aalst, Aalst, Belgium; Department of Experimental Pharmacology, Faculty of Medicine and Pharmacy, Vrije Universiteit Brussel, Brussels, Belgium.
Claudio Borghi, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy.
Rosa Maria Bruno, Université de Paris Cité, Inserm, PARCC, Paris, France; Service de Pharamcologie, AP-HP, Hôpital Européen Georges Pompidou, Paris, France.
Claudio Ceconi, University of Cardiologia, ASST Garda, Desenzano del Garda, Italy.
Véronique Cornelissen, Department of Rehabilitation Sciences, KU Leuven, Leuven, Belgium.
François Diévart, Clinique Villette, Dunkerque, France.
Marc Ferrini, Department of Cardiology and Vascular Pathology, CH Saint Joseph and Saint Luc, Lyon, France.
Thomas Kahan, Karolinska Institutet, Department of Clinical Sciences, Danderyd Hospital, Division of Cardiovascular Medicine, Stockholm, Sweden.
Maja-Lisa Løchen, Department of Community Medicine, UiT The Arctic University of Norway, Tromsø, Norway.
Angela H E M Maas, Department of Cardiology, Radboudumc, Nijmegen, The Netherlands.
Felix Mahfoud, Department of Internal Medicine III, Cardiology, Angiology and Intensive Care Medicine, Saarland University Hospital, Homburg/Saar, Germany.
Anastasia S Mihailidou, Department of Cardiology and Kolling Institute, Royal North Shore Hospital, St Leonards, UK; Faculty of Medicine and Health Sciences, Macquarie University, Sydney, Australia.
Trine Moholdt, Department of Circulation and Medical Imaging, Norwegian University of Science and Technology, Trondheim, Norway.
Gianfranco Parati, Department of Cardiac, Neural and Metabolic Sciences, Instituto Auxologico Italiano, IRCCS, Milan, Italy; Department of Medicine and Surgery, University of Milano-Bicocca, Milan, Italy.
Giovanni de Simone, Hypertension Research Center and Department of Advanced Biomedical Sciences, Federico II University, Naples, Italy.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. GBD 2013 Risk Factors Collaborators, Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet 2015;386:2287–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2019 Risk Factors Collaborators . Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020;396:1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bartz D, Chitnis T, Kaiser UB, Rich-Edwards JW, Rexrode KM, Pennell PB, et al. Clinical advances in sex- and gender-informed medicine to improve the health of all: A review. JAMA Intern Med 2020;180:574–583. [DOI] [PubMed] [Google Scholar]
- 4. NCD Risk Factor Collaboration (NCD-RisC) . Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: an analysis of 123 nationally representative surveys. Lancet 2019;394:639–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang BY, Qian Z, Howard SW, Vaughn MG, Fan SJ, Liu KK, et al. Global association between ambient air pollution and blood pressure: a systematic review and meta-analysis. Environ Pollut 2018;235:576–588. [DOI] [PubMed] [Google Scholar]
- 6. Gerdts E, de Simone G. Hypertension in women: should there be a sex-specific threshold? Eur Cardiol 2021;16:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wenger NK, Arnold A, Bairey Merz CN, Cooper-DeHoff RM, Ferdinand KC, Fleg JL, et al. Hypertension across a woman’s life cycle. J Am Coll Cardiol 2018;71:1797–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med 2019;25:1657–1666. [DOI] [PubMed] [Google Scholar]
- 9. Kringeland E, Tell GS, Midtbo H, Igland J, Haugsgjerd TR, Gerdts E. Stage 1 hypertension, sex, and acute coronary syndromes during midlife: the Hordaland health study. Eur J Prev Cardiol 2022;29:147–154. [DOI] [PubMed] [Google Scholar]
- 10. Ji H, Niiranen TJ, Rader F, Henglin M, Kim A, Ebinger JE, et al. Sex differences in blood pressure associations with cardiovascular outcomes. Circulation 2021;143:761–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Keeffe LM, Simpkin AJ, Tilling K, Anderson EL, Hughes AD, Lawlor DA, et al. Sex-specific trajectories of measures of cardiovascular health during childhood and adolescence: a prospective cohort study. Atherosclerosis 2018;278:190–196. [DOI] [PubMed] [Google Scholar]
- 12. Shen W, Zhang T, Li S, Zhang H, Xi B, Shen H, et al. Race and sex differences of long-term blood pressure profiles from childhood and adult hypertension: the Bogalusa heart study. Hypertension 2017;70:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz CN, et al. Sex differences in blood pressure trajectories over the life course. JAMA Cardiol 2020;5:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hardy ST, Holliday KM, Chakladar S, Engeda JC, Allen NB, Heiss G, et al. Heterogeneity in blood pressure transitions over the life course: age-specific emergence of racial/ethnic and sex disparities in the United States. JAMA Cardiol 2017;2:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hopstock LA, Bonaa KH, Eggen AE, Grimsgaard S, Jacobsen BK, Lochen ML, et al. Longitudinal and secular trends in blood pressure among women and men in birth cohorts born between 1905 and 1977: the Tromso study 1979 to 2008. Hypertension 2015;66:496–501. [DOI] [PubMed] [Google Scholar]
- 16. Wills AK, Lawlor DA, Matthews FE, Sayer AA, Bakra E, Ben-Shlomo Y, et al. Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med 2011;8:e1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Samargandy S, Matthews KA, Brooks MM, Barinas-Mitchell E, Magnani JW, Thurston RC, et al. Trajectories of blood pressure in midlife women: does menopause matter? Circ Res 2022;130:312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maas A, Rosano G, Cifkova R, Chieffo A, van Dijken D, Hamoda H, et al. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: a consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur Heart J 2021;42:967–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Casiglia E, Tikhonoff V, Caffi S, Bascelli A, Schiavon L, Guidotti F, et al. Menopause does not affect blood pressure and risk profile, and menopausal women do not become similar to men. J Hypertens 2008;26:1983–1992. [DOI] [PubMed] [Google Scholar]
- 20. Cifkova R, Pitha J, Lejskova M, Lanska V, Zecova S. Blood pressure around the menopause: a population study. J Hypertens 2008;26:1976–1982. [DOI] [PubMed] [Google Scholar]
- 21. NCD Risk Factor Collaboration (NCD-RisC) . Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021;398:957–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension 2009;54:11–18. [DOI] [PubMed] [Google Scholar]
- 23. Yanes LL, Romero DG, Iliescu R, Zhang H, Davis D, Reckelhoff JF. Postmenopausal hypertension: role of the renin-angiotensin system. Hypertension 2010;56:359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J 2012;33:1058–1066. [DOI] [PubMed] [Google Scholar]
- 25. Philbois SV, Facioli TP, Gastaldi AC, Rodrigues JAL, Tank J, Fares TH, et al. Important differences between hypertensive middle-aged women and men in cardiovascular autonomic control-a critical appraisal. Biol Sex Differ 2021;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamidovic A, Van Hedger K, Choi SH, Flowers S, Wardle M, Childs E. Quantitative meta-analysis of heart rate variability finds reduced parasympathetic cardiac tone in women compared to men during laboratory-based social stress. Neurosci Biobehav Rev 2020;114:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sevre K, Lefrandt JD, Nordby G, Os I, Mulder M, Gans RO, et al. Autonomic function in hypertensive and normotensive subjects: the importance of gender. Hypertension 2001;37:1351–1356. [DOI] [PubMed] [Google Scholar]
- 28. Colafella KMM, Denton KM. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol 2018;14:185–201. [DOI] [PubMed] [Google Scholar]
- 29. Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension 2001;37:1199–1208. [DOI] [PubMed] [Google Scholar]
- 30. Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 1999;340:1801–1811. [DOI] [PubMed] [Google Scholar]
- 31. dos Santos RL, da Silva FB, Ribeiro RF Jr., Stefanon I. Sex hormones in the cardiovascular system. Horm Mol Biol Clin Investig 2014;18:89–103. [DOI] [PubMed] [Google Scholar]
- 32. Oparil S, Miller AP. Gender and blood pressure. J Clin Hypertens (Greenwich) 2005;7:300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mallareddy M, Hanes V, White WB. Drospirenone, a new progestogen, for postmenopausal women with hypertension. Drugs Aging 2007;24:453–466. [DOI] [PubMed] [Google Scholar]
- 34. Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res 2002;53:672–677. [DOI] [PubMed] [Google Scholar]
- 35. Tamargo J, Rosano G, Walther T, Duarte J, Niessner A, Kaski JC, et al. Gender differences in the effects of cardiovascular drugs. Eur Heart J Cardiovasc Pharmacother 2017;3:163–182. [DOI] [PubMed] [Google Scholar]
- 36. Pechere-Bertschi A, Burnier M. Gonadal steroids, salt-sensitivity and renal function. Curr Opin Nephrol Hypertens 2007;16:16–21. [DOI] [PubMed] [Google Scholar]
- 37. Barnes JN, Hart EC, Curry TB, Nicholson WT, Eisenach JH, Wallin BG, et al. Aging enhances autonomic support of blood pressure in women. Hypertension 2014;63:303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res 2002;53:688–708. [DOI] [PubMed] [Google Scholar]
- 39. Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol 2002;40:505–510. [DOI] [PubMed] [Google Scholar]
- 40. Perrino C, Ferdinandy P, Botker HE, Brundel B, Collins P, Davidson SM, et al. Improving translational research in sex-specific effects of comorbidities and risk factors in ischaemic heart disease and cardioprotection: position paper and recommendations of the ESC working group on cellular biology of the heart. Cardiovasc Res 2021;117:367–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ahouansou S, Le Toumelin P, Crickx B, Descamps V. Association of androgenetic alopecia and hypertension. Eur J Dermatol 2007;17:220–222. [DOI] [PubMed] [Google Scholar]
- 42. Shamloul R, Ghanem H. Erectile dysfunction. Lancet 2013;381:153–165. [DOI] [PubMed] [Google Scholar]
- 43. Riise HKR, Sulo G, Tell GS, Igland J, Egeland G, Nygard O, et al. Hypertensive pregnancy disorders increase the risk of maternal cardiovascular disease after adjustment for cardiovascular risk factors. Int J Cardiol 2019;282:81–87. [DOI] [PubMed] [Google Scholar]
- 44. Honigberg MC, Zekavat SM, Aragam K, Klarin D, Bhatt DL, Scott NS, et al. Long-Term cardiovascular risk in women with hypertension during pregnancy. J Am Coll Cardiol 2019;74:2743–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sondergaard MM, Hlatky MA, Stefanick ML, Vittinghoff E, Nah G, Allison M, et al. Association of adverse pregnancy outcomes with risk of atherosclerotic cardiovascular disease in postmenopausal women. JAMA Cardiol 2020;5:1390–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cifkova R, Johnson MR, Kahan T, Brguljan J, Williams B, Coca A, et al. Peripartum management of hypertension: a position paper of the ESC Council on Hypertension and the European Society of Hypertension. Eur Heart J Cardiovasc Pharmacother 2020;6:384–393. [DOI] [PubMed] [Google Scholar]
- 47. Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update 2006;12:673–683. [DOI] [PubMed] [Google Scholar]
- 48. Connelly PJ, Marie Freel E, Perry C, Ewan J, Touyz RM, Currie G, et al. Gender-Affirming hormone therapy, vascular health and cardiovascular disease in transgender adults. Hypertension 2019;74:1266–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moulton VR. Sex hormones in acquired immunity and autoimmune disease. Front Immunol 2018;9:2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 51. de Simone G, Mancusi C, Izzo R, Losi MA, Aldo Ferrara L. Obesity and hypertensive heart disease: focus on body composition and sex differences. Diabetol Metab Syndr 2016;8:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mancusi C, Gerdts E, Losi MA, D’Amato A, Manzi MV, Canciello G, et al. Differential effect of obesity on prevalence of cardiac and carotid target organ damage in hypertension (the Campania salute network). Int J Cardiol 2017;244:260–264. [DOI] [PubMed] [Google Scholar]
- 53. Liu J, Fox CS, Hickson DA, May WD, Hairston KG, Carr JJ, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson heart study. J Clin Endocrinol Metab 2010;95:5419–5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Regitz-Zagrosek V, Lehmkuhl E, Mahmoodzadeh S. Gender aspects of the role of the metabolic syndrome as a risk factor for cardiovascular disease. Gend Med 2007;4:S162–S177. [DOI] [PubMed] [Google Scholar]
- 55. Nordstrom A, Hadrevi J, Olsson T, Franks PW, Nordstrom P. Higher prevalence of type 2 diabetes in men than in women is associated with differences in visceral fat mass. J Clin Endocrinol Metab 2016;101:3740–3746. [DOI] [PubMed] [Google Scholar]
- 56. O’Meara JG, Kardia SL, Armon JJ, Brown CA, Boerwinkle E, Turner ST. Ethnic and sex differences in the prevalence, treatment, and control of dyslipidemia among hypertensive adults in the GENOA study. Arch Intern Med 2004;164:1313–1318. [DOI] [PubMed] [Google Scholar]
- 57. Muiesan ML, Ambrosioni E, Costa FV, Leonetti G, Pessina AC, Salvetti M, et al. Sex differences in hypertension-related renal and cardiovascular diseases in Italy: the I-DEMAND study. J Hypertens 2012;30:2378–2386. [DOI] [PubMed] [Google Scholar]
- 58. Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev 2017;34:70–81. [DOI] [PubMed] [Google Scholar]
- 59. Bauters FA, Hertegonne KB, Pevernagie D, De Buyzere ML, Chirinos JA, Rietzschel ER. Sex differences in the association between arterial hypertension, blood pressure, and sleep apnea in the general population. J Clin Sleep Med 2021;17:1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005;365:1415–1428. [DOI] [PubMed] [Google Scholar]
- 61. Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol 2009;54:2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dugani SB, Moorthy MV, Li C, Demler OV, Alsheikh-Ali AA, Ridker PM, et al. Association of lipid, inflammatory, and metabolic biomarkers with age at onset for incident coronary heart disease in women. JAMA Cardiol 2021;6:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The rancho Bernardo study. JAMA 1991;265:627–631. [PubMed] [Google Scholar]
- 65. Sutton-Tyrrell K, Wildman RP, Matthews KA, Chae C, Lasley BL, Brockwell S, et al. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the study of women across the nation (SWAN). Circulation 2005;111:1242–1249. [DOI] [PubMed] [Google Scholar]
- 66. Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes Mellitus. Endocr Rev 2016;37:278–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, et al. Expert position paper on air pollution and cardiovascular disease. Eur Heart J 2015;36:83–93b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Back M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–3337. [DOI] [PubMed] [Google Scholar]
- 69. Wu Y, Ye Z, Fang Y. Spatial analysis of the effects of PM2.5 on hypertension among the middle-aged and elderly people in China. Int J Environ Health Res 2021;31:729–740. [DOI] [PubMed] [Google Scholar]
- 70. Liu YR, Dong JY, Zhai GY. Association between air pollution and hospital admissions for hypertension in Lanzhou, China. Environ Sci Pollut Res 2022;29:11976–11989. [DOI] [PubMed] [Google Scholar]
- 71. Al-Kindi SG, Brook RD, Bhatt U, Brauer M, Cushman WC, Hanson HA, et al. The benefits of intensive versus standard blood pressure treatment according to fine particulate matter air pollution exposure: a post hoc analysis of SPRINT. Hypertension 2021;77:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Evrard AS, Lefevre M, Champelovier P, Lambert J, Laumon B. Does aircraft noise exposure increase the risk of hypertension in population living near airports in France? Occup Environ Med 2017;74:123–129. [DOI] [PubMed] [Google Scholar]
- 73. Kupcikova Z, Fecht D, Ramakrishnan R, Clark C, Cai YS. Road traffic noise and cardiovascular disease risk factors in UK biobank. Eur Heart J 2021;42:2072–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. de Simone G, Devereux RB, Daniels SR, Meyer RA. Gender differences in left ventricular growth. Hypertension 1995;26:979–983. [DOI] [PubMed] [Google Scholar]
- 75. De Simone G, Devereux RB, Chinali M, Roman MJ, Barac A, Panza JA, et al. Sex differences in obesity-related changes in left ventricular morphology: the strong heart study. J Hypertens 2011;29:1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 77. Gerdts E, Okin PM, de Simone G, Cramariuc D, Wachtell K, Boman K, et al. Gender differences in left ventricular structure and function during antihypertensive treatment: the losartan intervention for endpoint reduction in hypertension study. Hypertension 2008;51:1109–1114. [DOI] [PubMed] [Google Scholar]
- 78. Okin PM, Gerdts E, Kjeldsen SE, Julius S, Edelman JM, Dahlof B, et al. Gender differences in regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy. Hypertension 2008;52:100–106. [DOI] [PubMed] [Google Scholar]
- 79. Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA 2004;292:2350–2356. [DOI] [PubMed] [Google Scholar]
- 80. de Simone G, Devereux RB, Izzo R, Girfoglio D, Lee ET, Howard BV, et al. Lack of reduction of left ventricular mass in treated hypertension: the strong heart study. J Am Heart Assoc 2013;2:e000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gerdts E, Izzo R, Mancusi C, Losi MA, Manzi MV, Canciello G, et al. Left ventricular hypertrophy offsets the sex difference in cardiovascular risk (the Campania salute network). Int J Cardiol 2018;258:257–261. [DOI] [PubMed] [Google Scholar]
- 82. Izzo R, Losi MA, Stabile E, Lonnebakken MT, Canciello G, Esposito G, et al. Development of left ventricular hypertrophy in treated hypertensive outpatients: the Campania salute network. Hypertension 2017;69:136–142. [DOI] [PubMed] [Google Scholar]
- 83. Gerdts E, Zabalgoitia M, Bjornstad H, Svendsen TL, Devereux RB. Gender differences in systolic left ventricular function in hypertensive patients with electrocardiographic left ventricular hypertrophy (the LIFE study). Am J Cardiol 2001;87:980–983. A4 [DOI] [PubMed] [Google Scholar]
- 84. Gerdts E, Wachtell K, Omvik P, Otterstad JE, Oikarinen L, Boman K, et al. Left atrial size and risk of major cardiovascular events during antihypertensive treatment: losartan intervention for endpoint reduction in hypertension trial. Hypertension 2007;49:311–316. [DOI] [PubMed] [Google Scholar]
- 85. Gupta S, Matulevicius SA, Ayers CR, Berry JD, Patel PC, Markham DW, et al. Left atrial structure and function and clinical outcomes in the general population. Eur Heart J 2013;34:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sardana M, Lessard D, Tsao CW, Parikh NI, Barton BA, Nah G, et al. Association of left atrial function Index with atrial fibrillation and cardiovascular disease: the Framingham offspring study. J Am Heart Assoc 2018;7:e008435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kuznetsova T, Haddad F, Tikhonoff V, Kloch-Badelek M, Ryabikov A, Knez J, et al. Impact and pitfalls of scaling of left ventricular and atrial structure in population-based studies. J Hypertens 2016;34:1186–1194. [DOI] [PubMed] [Google Scholar]
- 88. Gerdts E, Oikarinen L, Palmieri V, Otterstad JE, Wachtell K, Boman K, et al. Correlates of left atrial size in hypertensive patients with left ventricular hypertrophy: the losartan intervention for endpoint reduction in hypertension (LIFE) study. Hypertension 2002;39:739–743. [DOI] [PubMed] [Google Scholar]
- 89. Losi MA, Mancusi C, Midtbo H, Saeed S, de Simone G, Gerdts E. Impact of estimated left atrial volume on prognosis in patients with asymptomatic mild to moderate aortic valve stenosis. Int J Cardiol 2019;297:121–125. [DOI] [PubMed] [Google Scholar]
- 90. Halland H, Lonnebakken MT, Pristaj N, Saeed S, Midtbo H, Einarsen E, et al. Sex differences in subclinical cardiac disease in overweight and obesity (the FATCOR study). Nutr Metab Cardiovasc Dis 2018;28:1054–1060. [DOI] [PubMed] [Google Scholar]
- 91. Bruno RM, Grassi G, Seravalle G, Savoia C, Rizzoni D, Virdis A, et al. Age- and sex-specific reference values for Media/lumen ratio in small arteries and relationship with risk factors. Hypertension 2018;71:1193–1200. [DOI] [PubMed] [Google Scholar]
- 92. Engelen L, Ferreira I, Stehouwer CD, Boutouyrie P, Laurent S. Reference values for arterial measurements C. Reference intervals for common carotid intima-media thickness measured with echotracking: relation with risk factors. Eur Heart J 2013;34:2368–2380. [DOI] [PubMed] [Google Scholar]
- 93. Holder SM, Bruno RM, Shkredova DA, Dawson EA, Jones H, Hopkins ND, et al. Reference intervals for brachial artery flow-mediated dilation and the relation with cardiovascular risk factors. Hypertension 2021;77:1469–1480. [DOI] [PubMed] [Google Scholar]
- 94. McEniery CM Y, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR, et al. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the anglo-cardiff collaborative trial (ACCT). J Am Coll Cardiol 2005;46:1753–1760. [DOI] [PubMed] [Google Scholar]
- 95. Lonnebakken MT, Izzo R, Mancusi C, Losi MA, Stabile E, Rozza F, et al. Aortic root dimension and arterial stiffness in arterial hypertension: the Campania salute network. J Hypertens 2016;34:1109–1114. [DOI] [PubMed] [Google Scholar]
- 96. Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol 2013;61:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Campos-Arias D, De Buyzere ML, Chirinos JA, Rietzschel ER, Segers P. Longitudinal changes of input impedance, pulse wave velocity, and wave reflection in a middle-aged population: the Asklepios study. Hypertension 2021;77:1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nardin C, Maki-Petaja KM, Miles KL, Yasmin, McDonnell BJ, Cockcroft JR, et al. Cardiovascular phenotype of elevated blood pressure differs markedly between young males and females: the Enigma study. Hypertension 2018;72:1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mancusi C, Gerdts E, de Simone G, Midtbo H, Lonnebakken MT, Boman K, et al. Higher pulse pressure/stroke volume index is associated with impaired outcome in hypertensive patients with left ventricular hypertrophy the LIFE study. Blood Press 2017;26:150–155. [DOI] [PubMed] [Google Scholar]
- 100. Esposito R, Izzo R, Galderisi M, De Marco M, Stabile E, Esposito G, et al. Identification of phenotypes at risk of transition from diastolic hypertension to isolated systolic hypertension. J Hum Hypertens 2016;30:392–396. [DOI] [PubMed] [Google Scholar]
- 101. Mitchell GF. Aortic stiffness, pressure and flow pulsatility, and target organ damage. J Appl Physiol 2018;125:1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hametner B, Wassertheurer S, Mayer CC, Danninger K, Binder RK, Weber T. Aortic pulse wave velocity predicts cardiovascular events and mortality in patients undergoing coronary angiography: a comparison of invasive measurements and noninvasive estimates. Hypertension 2021;77:571–581. [DOI] [PubMed] [Google Scholar]
- 103. Millett ERC, Peters SAE, Woodward M. Sex differences in risk factors for myocardial infarction: cohort study of UK biobank participants. BMJ 2018;363:k4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gilsanz P, Mayeda ER, Glymour MM, Quesenberry CP, Mungas DM, DeCarli C, et al. Female sex, early-onset hypertension, and risk of dementia. Neurology 2017;89:1886–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Anstey KJ, Peters R, Mortby ME, Kiely KM, Eramudugolla R, Cherbuin N, et al. Association of sex differences in dementia risk factors with sex differences in memory decline in a population-based cohort spanning 20-76 years. Sci Rep 2021;11:7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Boggia J, Thijs L, Hansen TW, Li Y, Kikuya M, Bjorklund-Bodegard K, et al. Ambulatory blood pressure monitoring in 9357 subjects from 11 populations highlights missed opportunities for cardiovascular prevention in women. Hypertension 2011;57:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension 1994;24:793–801. [DOI] [PubMed] [Google Scholar]
- 108. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 109. Albrektsen G, Heuch I, Lochen ML, Thelle DS, Wilsgaard T, Njolstad I, et al. Risk of incident myocardial infarction by gender: interactions with serum lipids, blood pressure and smoking. The Tromso study 1979-2012. Atherosclerosis 2017;261:52–59. [DOI] [PubMed] [Google Scholar]
- 110. Mancusi C, Gerdts E, De Simone G, Abdelhai YM, Lonnebakken MT, Boman K, et al. Impact of isolated systolic hypertension on normalization of left ventricular structure during antihypertensive treatment (the LIFE study). Blood Press 2014;23:206–212. [DOI] [PubMed] [Google Scholar]
- 111. Regnault V, Thomas F, Safar ME, Osborne-Pellegrin M, Khalil RA, Pannier B, et al. Sex difference in cardiovascular risk: role of pulse pressure amplification. J Am Coll Cardiol 2012;59:1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Coutinho T. Arterial stiffness and its clinical implications in women. Can J Cardiol 2014;30:756–764. [DOI] [PubMed] [Google Scholar]
- 113. Lip GYH, Coca A, Kahan T, Boriani G, Manolis AS, Olsen MH, et al. Hypertension and cardiac arrhythmias: a consensus document from the European Heart Rhythm Association (EHRA) and ESC Council on Hypertension, endorsed by the Heart Rhythm Society (HRS), Asia-pacific heart rhythm society (APHRS) and sociedad latinoamericana de estimulacion Cardiaca y electrofisiologia (SOLEACE). Europace 2017;19:891–911. [DOI] [PubMed] [Google Scholar]
- 114. Gillis AM. Atrial fibrillation and ventricular arrhythmias: sex differences in electrophysiology, epidemiology, clinical presentation, and clinical outcomes. Circulation 2017;135:593–608. [DOI] [PubMed] [Google Scholar]
- 115. Ko D, Rahman F, Schnabel RB, Yin X, Benjamin EJ, Christophersen IE. Atrial fibrillation in women: epidemiology, pathophysiology, presentation, and prognosis. Nat Rev Cardiol 2016;13:321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Westerman S, Wenger N. Gender differences in atrial fibrillation: a review of epidemiology, management, and outcomes. Curr Cardiol Rev 2019;15:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kavousi M. Differences in epidemiology and risk factors for atrial fibrillation between women and men. Front Cardiovasc Med 2020;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sharashova E, Wilsgaard T, Ball J, Morseth B, Gerdts E, Hopstock LA, et al. Long-term blood pressure trajectories and incident atrial fibrillation in women and men: the Tromso study. Eur Heart J 2020;41:1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Espnes H, Ball J, Lochen ML, Wilsgaard T, Njolstad I, Mathiesen EB, et al. Sex-Specific associations between blood pressure and risk of atrial fibrillation subtypes in the Tromso study. J Clin Med 2021;10:1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kubicki DM, Xu M, Akwo EA, Dixon D, Munoz D, Blot WJ, et al. Race and sex differences in modifiable risk factors and incident heart failure. JACC Heart Fail 2020;8:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA 1996;275:1557–1562. [PubMed] [Google Scholar]
- 122. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 123. Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation 2018;138:198–205. [DOI] [PubMed] [Google Scholar]
- 124. Dewan P, Rorth R, Jhund PS, Shen L, Raparelli V, Petrie MC, et al. Differential impact of heart failure with reduced ejection fraction on men and women. J Am Coll Cardiol 2019;73:29–40. [DOI] [PubMed] [Google Scholar]
- 125. Aronow WS, Ahn C, Kronzon I, Goldman ME. Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am J Cardiol 2001;88:693–695. [DOI] [PubMed] [Google Scholar]
- 126. Cramariuc D, Rogge BP, Lonnebakken MT, Boman K, Bahlmann E, Gohlke-Barwolf C, et al. Sex differences in cardiovascular outcome during progression of aortic valve stenosis. Heart 2015;101:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rieck AE, Cramariuc D, Boman K, Gohlke-Barwolf C, Staal EM, Lonnebakken MT, et al. Hypertension in aortic stenosis: implications for left ventricular structure and cardiovascular events. Hypertension 2012;60:90–97. [DOI] [PubMed] [Google Scholar]
- 128. Diederichsen A, Lindholt JS, Moller JE, Gerke O, Rasmussen LM, Dahl JS. Sex differences in factors associated with progression of aortic valve calcification in the general population. Circ Cardiovasc Imaging 2022;15:e013165. [DOI] [PubMed] [Google Scholar]
- 129. Mancusi C, de Simone G, Brguljan Hitij J, Sudano I, Mahfoud F, Parati G, et al. Management of patients with combined arterial hypertension and aortic valve stenosis: a consensus document from the council on hypertension and council on valvular heart disease of the European Society of Cardiology, the European Association of Cardiovascular Imaging (EACVI), and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J Cardiovasc Pharmacother 2021;7:242–250. [DOI] [PubMed] [Google Scholar]
- 130. O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet 2016;388:761–775. [DOI] [PubMed] [Google Scholar]
- 131. Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American heart association/American stroke association. Stroke 2014;45:1545–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gasbarrino K, Di Iorio D, Daskalopoulou SS. Importance of sex and gender in ischaemic stroke and carotid atherosclerotic disease. Eur Heart J 2022;43:460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lisabeth LD, Brown DL, Hughes R, Majersik JJ, Morgenstern LB. Acute stroke symptoms: comparing women and men. Stroke 2009;40:2031–2036. [DOI] [PubMed] [Google Scholar]
- 134. Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke 2009;40:1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Leppert MH, Burke JF, Lisabeth LD, Madsen TE, Kleindorfer DO, Sillau S, et al. Systematic review of sex differences in ischemic strokes among young adults: are young women disproportionately at risk? Stroke 2022;53:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wang X, Phan HT, Li J, Reeves MJ, Thrift AG, Cadilhac DA, et al. Sex differences in disease profiles, management, and outcomes among people with atrial fibrillation after ischemic stroke: aggregated and individual participant data meta-analyses. Womens Health Rep (New Rochelle) 2020;1:190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Chen MQ, Shi WR, Wang HY, Sun YX. Sex differences of combined effects between hypertension and general or central obesity on ischemic stroke in a middle-aged and elderly population. Clin Epidemiol 2021;13:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries Endorsed by: the European Stroke Organization (ESO). the task force for the diagnosis and treatment of peripheral arterial diseases of the European society of cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763–816. [DOI] [PubMed] [Google Scholar]
- 139. Frank U, Nikol S, Belch J, Boc V, Brodmann M, Carpentier PH, et al. ESVM guideline on peripheral arterial disease. Vasa 2019;48:1–79. [DOI] [PubMed] [Google Scholar]
- 140. Ortmann J, Nuesch E, Traupe T, Diehm N, Baumgartner I. Gender is an independent risk factor for distribution pattern and lesion morphology in chronic critical limb ischemia. J Vasc Surg 2012;55:98–104. [DOI] [PubMed] [Google Scholar]
- 141. Aboyans V, Criqui MH, McClelland RL, Allison MA, McDermott MM, Goff DC Jr., et al. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: the multi-ethnic study of atherosclerosis (MESA). J Vasc Surg 2007;45:319–327. [DOI] [PubMed] [Google Scholar]
- 142. Kapoor R, Ayers C, Visotcky A, Mason P, Kulinski J. Association of sex and height with a lower ankle brachial index in the general population. Vasc Med 2018;23:534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet 2009;48:143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Lewis CE, Grandits A, Flack J, McDonald R, Elmer PJ. Efficacy and tolerance of antihypertensive treatment in men and women with stage 1 diastolic hypertension. Results of the treatment of mild hypertension study. Arch Intern Med 1996;156:377–385. [PubMed] [Google Scholar]
- 145. Os I, Bratland B, Dahlof B, Gisholt K, Syvertsen JO, Tretli S. Female preponderance for lisinopril-induced cough in hypertension. Am J Hypertens 1994;7:1012–1015. [DOI] [PubMed] [Google Scholar]
- 146. Kajiwara A, Saruwatari J, Kita A, Oniki K, Yamamura M, Murase M, et al. Younger females are at greater risk of vasodilation-related adverse symptoms caused by dihydropyridine calcium channel blockers: results of a study of 11,918 Japanese patients. Clin Drug Investig 2014;34:431–435. [DOI] [PubMed] [Google Scholar]
- 147. Kloner RA, Sowers JR, DiBona GF, Gaffney M, Wein M. Sex- and age-related antihypertensive effects of amlodipine. The amlodipine cardiovascular community trial study group. Am J Cardiol 1996;77:713–722. [DOI] [PubMed] [Google Scholar]
- 148. de Simone G, Mancusi C. Erectile dysfunction and arterial hypertension: still looking for a scapegoat. Eur J Intern Med 2020;81:22–23. [DOI] [PubMed] [Google Scholar]
- 149. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. DASH-sodium collaborative research group. N Engl J Med 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 150. Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc 2013;2:e004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Keyhani S, Scobie JV, Hebert PL, McLaughlin MA. Gender disparities in blood pressure control and cardiovascular care in a national sample of ambulatory care visits. Hypertension 2008;51:1149–1155. [DOI] [PubMed] [Google Scholar]
- 152. Ljungman C, Kahan T, Schioler L, Hjerpe P, Hasselstrom J, Wettermark B, et al. Gender differences in antihypertensive drug treatment: results from the Swedish primary care cardiovascular database (SPCCD). J Am Soc Hypertens 2014;8:882–890. [DOI] [PubMed] [Google Scholar]
- 153. Wallentin F, Wettermark B, Kahan T. Drug treatment of hypertension in Sweden in relation to sex, age, and comorbidity. J Clin Hypertens (Greenwich) 2018;20:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kjeldsen SE, Hedner T, Syvertsen JO, Lund-Johansen P, Hansson L, Lanke J, et al. Influence of age, sex and blood pressure on the principal endpoints of the nordic diltiazem (NORDIL) study. J Hypertens 2002;20:1231–1237. [DOI] [PubMed] [Google Scholar]
- 155. Os I, Franco V, Kjeldsen SE, Manhem K, Devereux RB, Gerdts E, et al. Effects of losartan in women with hypertension and left ventricular hypertrophy: results from the losartan intervention for endpoint reduction in hypertension study. Hypertension 2008;51:1103–1108. [DOI] [PubMed] [Google Scholar]
- 156. Kjeldsen SE, Warnold I, Hansson L, Group HOTSGHOTS . Influence of gender on prevention of myocardial infarction by antihypertensives and acetylsalicylic acid: the HOT study. J Gend Specif Med 2000;3:35–38. [PubMed] [Google Scholar]
- 157. Wing LM, Reid CM, Ryan P, Beilin LJ, Brown MA, Jennings GL, et al. A comparison of outcomes with angiotensin-converting–enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med 2003;348:583–592. [DOI] [PubMed] [Google Scholar]
- 158. Zanchetti A, Julius S, Kjeldsen S, McInnes GT, Hua T, Weber M, et al. Outcomes in subgroups of hypertensive patients treated with regimens based on valsartan and amlodipine: an analysis of findings from the VALUE trial. J Hypertens 2006;24:2163–2168. [DOI] [PubMed] [Google Scholar]
- 159. SPRINT Research Group, Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Foy CG, Lovato LC, Vitolins MZ, Bates JT, Campbell R, Cushman WC, et al. Gender, blood pressure, and cardiovascular and renal outcomes in adults with hypertension from the systolic blood pressure intervention trial. J Hypertens 2018;36:904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ochoa-Jimenez R, Viquez-Beita K, Daluwatte C, Zusterzeel R. Sex differences of patients with systemic hypertension (from the analysis of the systolic blood pressure intervention trial [SPRINT]). Am J Cardiol 2018;122:985–993. [DOI] [PubMed] [Google Scholar]
- 162. Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged >/=75 years: A randomized clinical trial. JAMA 2016;315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Turnbull F, Woodward M, Neal B, Barzi F, Ninomiya T, Chalmers J, et al. Do men and women respond differently to blood pressure-lowering treatment? Results of prospectively designed overviews of randomized trials. Eur Heart J 2008;29:2669–2680. [DOI] [PubMed] [Google Scholar]
- 164. Reboussin DM, Allen NB, Griswold ME, Guallar E, Hong Y, Lackland DT, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation 2018;138:e595–e616. [DOI] [PubMed] [Google Scholar]
- 165. Egan BM, Zhao Y, Axon RN. US Trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA 2010;303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 166. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 2013;127:e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Lloyd-Sherlock P, Beard J, Minicuci N, Ebrahim S, Chatterji S. Hypertension among older adults in low- and middle-income countries: prevalence, awareness and control. Int J Epidemiol 2014;43:116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Osude N, Durazo-Arvizu R, Markossian T, Liu K, Michos ED, Rakotz M, et al. Age and sex disparities in hypertension control: the multi-ethnic study of atherosclerosis (MESA). Am J Prev Cardiol 2021;8:100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Holt E, Joyce C, Dornelles A, Morisky D, Webber LS, Muntner P, et al. Sex differences in barriers to antihypertensive medication adherence: findings from the cohort study of medication adherence among older adults. J Am Geriatr Soc 2013;61:558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Leung AA, Williams JVA, McAlister FA, Campbell NRC, Padwal RS, Hypertension Canada’s R, et al. Worsening hypertension awareness, treatment, and control rates in Canadian women between 2007 and 2017. Can J Cardiol 2020;36:732–739. [DOI] [PubMed] [Google Scholar]
- 171. Burnier M, Egan BM. Adherence in hypertension. Circ Res 2019;124:1124–1140. [DOI] [PubMed] [Google Scholar]
- 172. Lawson AJ, Hameed MA, Brown R, Cappuccio FP, George S, Hinton T, et al. Nonadherence to antihypertensive medications is related to pill burden in apparent treatment-resistant hypertensive individuals. J Hypertens 2020;38:1165–1173. [DOI] [PubMed] [Google Scholar]
- 173. Kulkarni S, Rao R, Goodman JDH, Connolly K, O’Shaughnessy KM. Nonadherence to antihypertensive medications amongst patients with uncontrolled hypertension: a retrospective study. Medicine (Baltimore) 2021;100:e24654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.