Abstract
Objectives:
Antimicrobial resistance (AMR) is a global priority with significant clinical and economic consequences. Multidrug-resistant (MDR) Pseudomonas aeruginosa is one of the major pathogens associated with significant morbidity and mortality. In healthcare settings, the evaluation of prevalence, microbiological characteristics, as well as mechanisms of resistance is of paramount importance to overcome associated challenges.
Methods:
Consecutive clinical specimens of P. aeruginosa were collected prospectively from 5 acute-care and specialized hospitals between October 2014 and September 2017, including microbiological, clinical characteristics and outcomes. Identification and antimicrobial susceptibility test were performed using the BD Phoenix identification and susceptibility testing system, matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF MS), and minimum inhibitory concentration (MIC) test strips. Overall, 78 selected MDR P. aeruginosa isolates were processed for whole-genome sequencing (WGS).
Results:
The overall prevalence of MDR P. aeruginosa isolates was 5.9% (525 of 8,892) and showed a decreasing trend; 95% of cases were hospital acquired and 44.8% were from respiratory samples. MDR P. aeruginosa demonstrated >86% resistance to cefepime, ciprofloxacin, meropenem, and piperacillin-tazobactam but 97.5% susceptibility to colistin. WGS revealed 29 different sequence types: 20.5% ST235, 10.3% ST357, 7.7% ST389, and 7.7% ST1284. ST233 was associated with bloodstream infections and increased 30-day mortality. All ST389 isolates were obtained from patients with cystic fibrosis. Encoded exotoxin genes were detected in 96.2% of isolates.
Conclusions:
MDR P. aeruginosa isolated from clinical specimens from Qatar has significant resistance to most agents, with a decreasing trend that should be explored further. Genomic analysis revealed the dominance of 5 main clonal clusters associated with mortality and bloodstream infections. Microbiological and genomic monitoring of MDR P. aeruginosa has enhanced our understanding of AMR in Qatar.
Over the last several decades, antimicrobial resistance (AMR) has become a global priority because of its substantial clinical and economic impact. 1 At its forefront, healthcare-associated infections (HAIs) are a major cause of morbidity and mortality in hospitals worldwide. In healthcare settings, the gram-negative ubiquitous opportunistic pathogen Pseudomonas aeruginosa is responsible for a wide range of HAIs, including respiratory, urinary, and surgical site infections as well as invasive diseases such as bacteraemia. 2,3 Emerging resistance of P. aeruginosa to different classes of antibiotics is a major concern worldwide. The success of this pathogen is partially due to the multidrug-resistant (MDR) phenotype that P. aeruginosa demonstrates, 4 which more recently has been attributed to the international spread of certain successful clones such as sequence types ST111 and ST235. 5,6 In addition to multidrug resistance, the ability to form biofilms under different conditions reduces the efficiency of the antibiotic treatments and subsequently increases the risk of chronicity. 7 Moreover, a complex interaction between epidemicity, pathogenicity, regulation, antimicrobial resistance, and the association between mechanisms of resistance and virulence contribute to the severity of P. aeruginosa–induced infections. 8
Globally, the epidemiology of MDR P. aeruginosa strains is showing regional variations, but increasing trends ranging from 15% to 30% have been observed across Europe, North America, and South America. 9 In Europe, isolates of P. aeruginosa have become increasingly resistant to standard antipseudomonal therapy, with rates of resistance to fluoroquinolones, piperacillin/tazobactam, carbapenems, ceftazidime, and aminoglycosides exceeding 10%. 10 Regionally, wide variation in the prevalence of MDR P. aeruginosa ranges from low (0%–7.3%), as in Saudi Arabia, to high (50%–75%), as in Egypt, mainly because of heterogeneity of settings and methods of data collection. 11 The main driver for the global dissemination of resistance is attributed to the spread of high-risk clones, such as ST235, ST111, and ST175, which are associated with HAIs outbreaks and transferable resistance mechanisms, especially horizontally acquired β-lactamases. 12 Furthermore, P. aeruginosa can cause severe infections due to mutations or production of potent virulence factors such as the exotoxins of the type III secretion system (ie, exoS, exoT, exoU, and exoY), which can explain poor clinical outcomes in association with MDR P. aeruginosa infections. 13
Based on local microbiological surveillance and reporting, a high prevalence of MDR Gram-negative bacteria (GNB) isolated from patients at Hamad Medical Corporation (HMC) in Qatar was noted, with P. aeruginosa being the second most prevalent (unpublished data). In 2015, 8.1% of P. aeruginosa strains from the 5 major hospitals in Qatar were MDR P. aeruginosa. 14 Detailed knowledge regarding characteristics of P. aeruginosa circulating in Qatar hospitals is lacking. In this study, we investigated the clinical impact and molecular epidemiology of MDR P. aeruginosa in Qatar over a 3-year period.
Methods
Study population
In total, 8,892 consecutive P. aeruginosa isolates were collected prospectively between October 2014 and September 2017 from various clinical specimens received by the Microbiology Division of the Department of Laboratory Medicine and Pathology (DLMP) from 5 different acute-care and specialized hospitals under HMC, Qatar, as part of the routine care. Specimens were collected from blood, tracheal aspirate, sputum, urine, wound, and corneal swabs. Using a standardized diagnostic and data collection tool, MDR P. aeruginosa samples were analyzed and then stored at −80°C pending further analysis. Clinical data were retrospectively collected from the electronic health record. Of 525 MDR P. aeruginosa isolates, 78 were selected and subjected to whole-genome sequencing (WGS).
Study setting and sample collection
During the 3 years from October 2014 to September 2017, a total of 525 MDR P. aeruginosa strains were isolated from 273 different patients hospitalized in 5 different HMC hospitals in Doha, Qatar. HMC is the main healthcare provider in the state of Qatar. It serves a population of ∼2.7 million through acute-care and specialized hospitals with a capacity of 1,868 beds and 8 intensive care units in the following facilities: Hamad General Hospital (HGH), an acute-care hospital with 603 beds, Rumaila Hospital for geriatric and long-term patients (RH) with 600 beds, the Women’s Hospital (WH) with 320 beds, the Heart Hospital (HH) with 120 beds, and the National Centre for Cancer and Research (NCCCR) with 46 beds. Patient demographic data, and data related to prior hospitalizations, antibiotic use, clinical diagnosis, risk factors, location of the patients in the hospital, and invasive applications were obtained from the electronic medical record using data-collection forms, with no direct communication between the data collector and patients, primary teams, or the infectious disease team following the patients. P. aeruginosa HAIs included signs and symptoms of the infection and species isolation as a unique pathogen for these patients as defined according to the US Center for Disease Control and Prevention (CDC) guidelines. 15 Colonization was defined as the isolation of P. aeruginosa from 2 consecutive cultures of samples from the same site with no evidence of infection. Colonization on admission was defined as the isolation of P. aeruginosa within 24 hours. This study was approved by the Research Ethics Committee at Hamad Medical Corporation, Doha, Qatar (protocol no. IRGC-01-51-033).
The bacterial identification and antimicrobial susceptibility test (ID/AST)
The bacterial identification and initial antimicrobial susceptibility tests (ASTs) of P. aeruginosa were performed using the BD Phoenix automated identification and susceptibility testing system (Becton Dickinson, Franklin Lakes, NJ). Identification confirmed was performed using matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF MS) on the Bruker Daltonics MALDI Biotyper (Bruker Daltonics, Billerica, MA) according to the manufacturer’s instructions. The minimum inhibitory concentrations (MICs) were determined using Liofilchem MIC test strips (Liofilchem, Rosetodegli Roseto Degli Abruzzi, Italy), while Broth microdilution was used for colistin susceptibility testing (ComASP Colistin, Liofilchem, Roseto degli Abruzzi, Italy). The results were interpreted using Clinical and Laboratory Standards Institute (CLSI) reference break points. 16 The test was performed for 8 antipseudomonal drugs: gentamicin, tobramycin, amikacin, cefepime, ciprofloxacin, piperacillin-tazobactam, meropenem, and colistin. MDR P. aeruginosa isolates were defined as having in vitro nonsusceptibility to at least 1 agent from ≥ 3 antimicrobial classes. 17
Genomic assembly, multilocus sequence typing (MLST), phylogenetic tree, and antibiotic resistance genes (ARGs)
In total, 78 MDR P. aeruginosa isolates were subjected to WGS using the Illumina HiSeq 2000 system (Illumina, San Diego, CA), performed by Eurofins GATC Biotech GmbH, Konstanz, Germany. We applied the following WGS inclusion criteria: MDR P. aeruginosa isolated from blood (n = 16), cystic fibrosis patients (n = 12), isolates found to be nonsusceptible to ceftazidime/avibactam and ceftolozane-tazobactam (n = 42), and resistance to all tested antipseudomonal drugs P. aeruginosa (n = 8). The clean reads were assembled using SPAdes version 3.13.0 software (Center for Algorithmic Biotechnology, St. Petersburg, Russia). 18 MLST of MDR P. aeruginosa isolates was performed using MLST version 1.8 software (Center for Genomic Epidemiology) based on the 7 housekeeping genes (acsA, aroE, guaA, mutL, nuoD, ppsA, and trpE) as described previously. 19 Annotations generated and detection of exotoxins were performed using the PATRIC RASTtk-enabled Genome Annotation Service. 20 ARGs were predicted using the Comprehensive Antibiotic Resistance Database (CARD) version 1.2.0 (McMaster University, Hamilton, Ontario). 21
Statistical analysis
The data were analyzed in terms of frequency and percentage. The percentages over the years were calculated using the χ2 test. To test whether location, acquisition, disease severity, or the number of antibiotic treatments differed over the 3 years, we used the χ2 test or the Fisher exact test. For overall mortality, the χ2 test or the Fisher exact test was used. A statistically significant difference was considered at P < .05 and 95% confidence intervals (CIs) were calculated. Susceptibility patterns of MDR P. aeruginosa were determined using frequencies and percentages. Statistical analyses were conducted using IBM SPSS Statistics for Windows version 25.0 software (IBM, Armonk, NY). Heat maps and dendrograms were constructed to visualize and categorize the MDR P. aeruginosa based on phenotypic antibiotic resistance, presence of the main exotoxins, mucoidity, important risk factors, and clinical outcomes such as 30-day mortality. The heat maps and dendrograms were constructed in R using gplots function heatmap.2 (R package version 2 software, R Foundation for Statistical Computing, Vienna, Austria). For clustering, the Pearson correlation was used on the presence–absence matrix. 22
Results
Prevalence and distribution of MDR P. aeruginosa isolates
The study included 8,892 consecutive nonduplicate isolates of P. aeruginosa (0.6% of 1,054,672 microbiology samples) detected from October 2014 to September 2017 in clinical specimens from patients 5 HMC hospitals (Table 1). The overall prevalence of MDR P. aeruginosa was 5.9% (525 of 8,892). The overall prevalence of P. aeruginosa isolates did not change significantly over the study period: 0.84% (2,533 of 300,577) in 2015 compared to 0.73% (2,959 of 405,270) in 2017 (Table 1). The 525 MDR P. aeruginosa isolates were obtained from 273 different patients. Distributions of MDR P. aeruginosa by clinical specimen are shown in percentages for the entire study period (Table 1). Of the 525 isolates, 235 (44.76%) were collected from respiratory samples; 135 (25,71%) were from skin and soft tissue, 126 (24%) were from urine, 16 (3.05%) were from blood, and 30 (5.71%) were from other sample types (Fig. 1).
Table 1.
Prevalence of Total Samples of Pseudomonas aeruginosa Including Multidrug-Resistant Isolates Collected From Five Acute-Care and Specialized Hospitals at Hamad Medical Corporation, a Qatar, Between 2014 and 2017
| Period | Total Specimens | Total P. aeruginosa |
Total number of MDR P. aeruginosa |
Prevalence of MDR P. aeruginosa, % |
P
Value |
|---|---|---|---|---|---|
| Oct 2014–Sept 2015 | 300,577 | 2,533 | 205 | 8.1 | <.001 |
| Oct 2015–Sept 2016 | 348,825 | 3,400 | 177 | 5.2 | |
| Oct 2016–Sept 2017 | 405,270 | 2,959 | 143 | 4.8 | |
| Total | 1,054,672 | 8,892 | 525 | 5.9 |
Hamad Medical Corporation included Hamad General Hospital, Rumailah Hospital, National Center for Cancer Care and Research, Heart Hospital and Women’s Hospital.
*χ2 for trend.
Fig. 1.
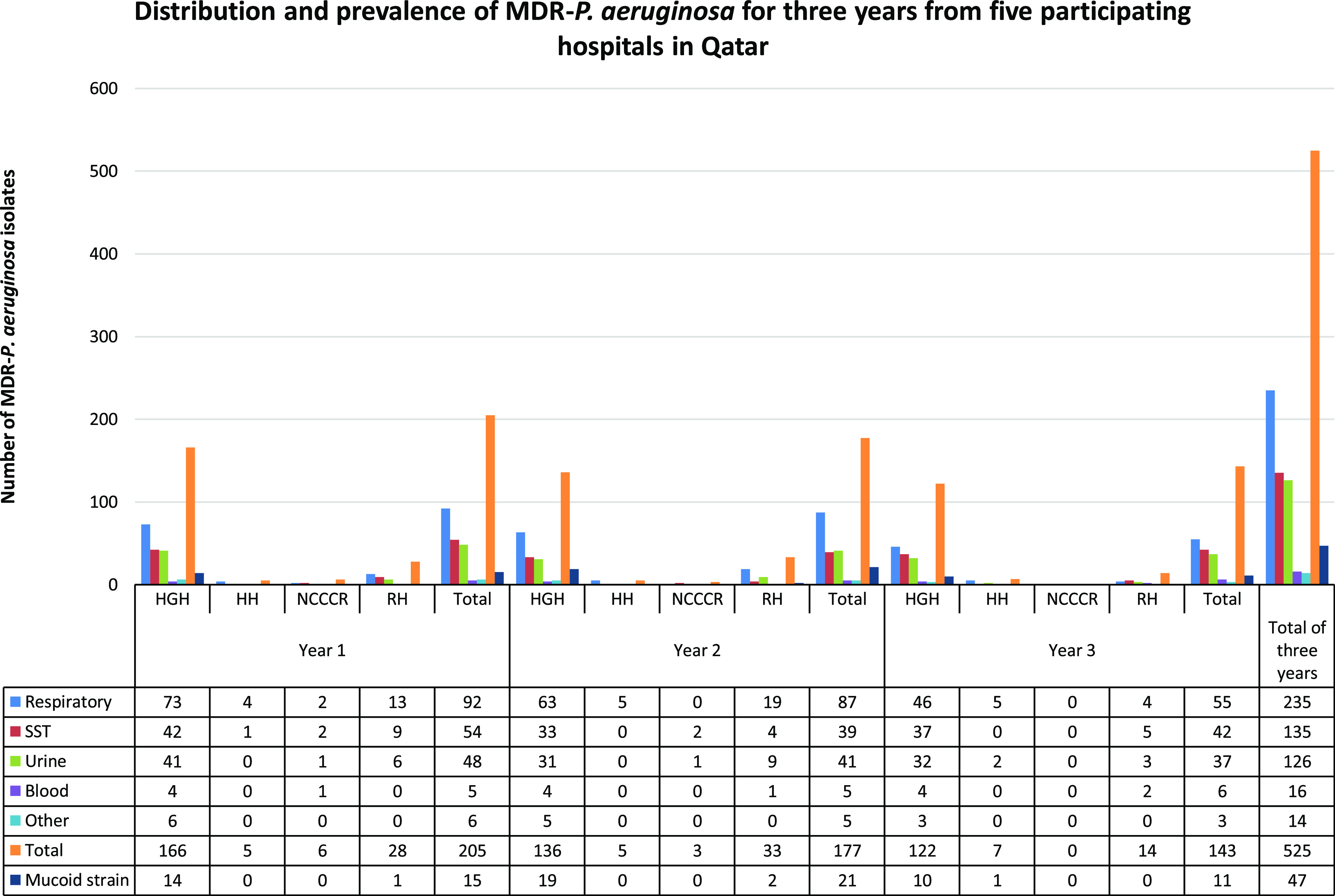
Distribution and prevalence of MDR P. aeruginosa for 3 years from 5 participating hospitals in Qatar. Note. HGH, Hamad General Hospital; RH, Rumailah Hospital; NCCCRm National Center for Cancer Care and Research; HH, Heart Hospital; SST, skin and soft-tissue (abscess, biopsy, ear, swab, tissue, and wound); Other, eye, sterile body fluid, nonsterile body fluid, and catheter tip; WH, no MDR P. aeruginosa isolates were recovered from the Women’s hospital.
Demographic profile of study population and clinical diagnosis
Overall, 395 MDR P. aeruginosa cases (75.2%) were isolated from male patients, aged between 1.5 and 90 years. Overall, 308 patients (58.7%) were aged 14–65 years and 302 patients (57.5%) were non-Qatari nationals. Of 525 cases, 501 (95.4%) were hospital acquired, and 415 (79%) were inpatients (Supplementary Material S1). Furthermore, 298 patients (56.8%) were colonized rather than infected. Sepsis was noted in 122 patients (23.2%) and septic shock occurred in 105 patients (20%). Among 525 MDR P. aeruginosa cases, 124 cases (23.6%) were treated with meropenem and 118 (22.5%) were treated with colistin (Supplementary S1). In 88 cases (16.8%), 1 antibiotic was used; in 119 cases (22.7%), a combination of 2 antibiotics was used; in 17 cases (3.2%), 3 antibiotics were used; and in 4 cases (0.8%), 4 antibiotics were used. In 56.8% of colonized cases, however, no antibiotics were used (Supplementary S1).
Comorbidity factors and clinical outcomes associated with MDR P. aeruginosa infection
The most common risk factors for patients infected with MDR P. aeruginosa (Table 2) were extensive healthcare contact (498 cases, 94.9%) and a history of exposure to antibiotics in the previous 90 days (439 cases, 83.6%) (Fig. 2). Other risk factors included presence of invasive devices (349 cases, 66.5%), history of MDR infection or colonization (329 cases, 62.7%), isolation of a prior susceptible strain of P. aeruginosa (302 cases, 57.5%), diabetes mellitus (264 cases, 50.3%), and others (Table 2).
Table 2.
Association of 30 Days Mortality With Patient Characteristics, Site of Infection and Hospital Location With Infections Secondary to MDR P. aeruginosa From Hamad Medical Corporation Between October 2014 and September 2017
| Year 1 | Year 2 | Year 3 | Sum of 3 Years | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | 30-Day Mortality | No Mortality | Total | % of 30-Day Mortality | 30-Day Mortality | No Mortality | Total | % of 30-Day Mortality | 30-Day Mortality | No Mortality | Total | % of 30-Day Mortality | 30-Day Mortality | No Mortality | Total | % of 30-Day Mortality | P Value |
| Age group | |||||||||||||||||
| Pediatric, <14 y | 0 | 12 | 12 | 0 | 0 | 10 | 10 | 0 | 1 | 13 | 14 | 7.1 | 1 | 35 | 36 | 2.8 | .069* |
| Adult, 14–65 y | 5 | 127 | 132 | 3.8 | 7 | 98 | 105 | 6.7 | 5 | 66 | 71 | 7.0 | 17 | 291 | 308 | 5.5 | |
| Geriatric, >65 y | 3 | 58 | 61 | 4.9 | 7 | 55 | 62 | 11.3 | 10 | 48 | 58 | 17.2 | 20 | 161 | 181 | 11.0 | |
| Sex | |||||||||||||||||
| Male | 7 | 147 | 154 | 4.5 | 12 | 122 | 134 | 9.0 | 9 | 98 | 107 | 8.4 | 28 | 367 | 395 | 7.1 | .818* |
| Female | 1 | 50 | 51 | 2.0 | 2 | 41 | 43 | 4.7 | 7 | 29 | 36 | 19.4 | 10 | 120 | 130 | 7.7 | |
| Location | |||||||||||||||||
| Intensive care unit | 7 | 97 | 104 | 6.7 | 11 | 55 | 66 | 16.7 | 12 | 24 | 36 | 33.3 | 30 | 176 | 206 | 14.6 | <.001* |
| Inpatient | 1 | 47 | 48 | 2.1 | 3 | 29 | 32 | 9.4 | 4 | 26 | 30 | 13.3 | 8 | 102 | 110 | 7.3 | |
| Outpatient | 0 | 53 | 53 | 0 | 0 | 79 | 79 | 0 | 0 | 77 | 77 | 0 | 0 | 209 | 209 | 0 | |
| Isolation site | |||||||||||||||||
| Respiratory | 4 | 88 | 92 | 4.3 | 8 | 79 | 87 | 9.2 | 4 | 51 | 55 | 7.3 | 16 | 218 | 234 | 6.8 | <.001 † |
| Skin and soft tissue | 2 | 52 | 54 | 3.7 | 4 | 35 | 39 | 10.3 | 7 | 35 | 42 | 16.7 | 13 | 122 | 135 | 9.6 | |
| Urine | 0 | 48 | 48 | 0 | 1 | 40 | 41 | 2.4 | 1 | 36 | 37 | 2.7 | 2 | 124 | 126 | 1.6 | |
| Blood | 2 | 3 | 5 | 40 | 1 | 4 | 5 | 20 | 2 | 4 | 6 | 33.3 | 5 | 11 | 16 | 31.3 | |
| Sterile body fluid and others | 0 | 6 | 6 | 0 | 0 | 5 | 5 | 0 | 2 | 1 | 3 | 66.7 | 2 | 12 | 14 | 14.3 | |
| Common associated underlying conditions | |||||||||||||||||
| Extensive healthcare contact a | 8 | 182 | 190 | 4.2 | 14 | 156 | 170 | 8.2 | 16 | 122 | 138 | 11.6 | 38 | 460 | 498 | 7.6 | |
| History of antibiotic exposure within 90 d | 8 | 167 | 175 | 4.6 | 13 | 131 | 144 | 9 | 16 | 104 | 120 | 13.3 | 37 | 402 | 439 | 8.4 | |
| Invasive device b | 8 | 134 | 142 | 5.6 | 14 | 116 | 130 | 10.8 | 15 | 62 | 77 | 19.5 | 37 | 312 | 349 | 10.6 | |
| Diabetes mellitus | 6 | 91 | 97 | 6.2 | 9 | 72 | 81 | 11.1 | 13 | 73 | 86 | 15.1 | 28 | 236 | 264 | 10.6 | |
| History of MDR infection or colonization within prior 90 d | 4 | 120 | 124 | 3.2 | 7 | 105 | 112 | 6.3 | 12 | 81 | 93 | 12.9 | 23 | 306 | 329 | 7 | |
| Isolation of prior susceptible P. aeruginosa | 5 | 122 | 127 | 3.9 | 9 | 94 | 103 | 8.7 | 8 | 64 | 72 | 11.1 | 22 | 280 | 302 | 7.3 | |
| Coinfection with other microorganism c | 1 | 51 | 52 | 1.9 | 5 | 61 | 66 | 7.6 | 8 | 39 | 47 | 17 | 14 | 151 | 165 | 8.5 | |
| Malignancy | 1 | 34 | 35 | 2.9 | 3 | 20 | 23 | 13 | 2 | 14 | 16 | 12.5 | 6 | 68 | 74 | 8.1 | |
| End-stage renal disease | 2 | 26 | 28 | 7.1 | 3 | 20 | 23 | 13 | 5 | 26 | 31 | 16.1 | 10 | 72 | 82 | 12.2 | |
| Renal stones | 0 | 20 | 20 | 0 | 0 | 14 | 14 | 0 | 2 | 17 | 19 | 10.5 | 2 | 51 | 53 | 3.8 | |
| Heart failure | 4 | 18 | 22 | 18.2 | 2 | 9 | 11 | 18.2 | 3 | 16 | 19 | 15.8 | 9 | 43 | 52 | 17.3 | |
| Cystic fibrosis | 0 | 12 | 12 | 0 | 0 | 6 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 18 | 18 | 0 | |
| Chronic obstructive pulmonary disease | 2 | 13 | 15 | 13.3 | 2 | 19 | 21 | 9.5 | 0 | 7 | 7 | 0 | 4 | 39 | 43 | 9.3 | |
| Chronic lung disease | 1 | 13 | 14 | 7.1 | 4 | 44 | 48 | 8.3 | 1 | 15 | 16 | 6.3 | 6 | 72 | 78 | 7.7 | |
| Post transplantation | 0 | 7 | 7 | 0 | 0 | 4 | 4 | 0 | 1 | 1 | 2 | 50 | 1 | 12 | 13 | 7.7 | |
| Chronic liver disease | 0 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 100 | 1 | 4 | 5 | 20 | |
| Neutropenic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | |
| Total | 8 | 197 | 205 | 3.9 | 14 | 163 | 177 | 7.9 | 16 | 127 | 143 | 11.2 | 38 | 487 | 525 | 7.2 | |
Extensive healthcare contact involves regular visits to outpatient medical facilities, regular home visit by home care teams, hospitalization within the preceding 90 days, or residency in a long-term care facility.
Invasive devices involves exposure to breast implant, central line, colostomy bag, cardiac resynchronization therapy implantable cardioverter defibrillator, double J stent (ureteral stent), external ventricular drain, external fixation of the pelvis, Foley catheter, internal drain, inferior vena cava filter, mechanical ventilator, nephrostomy, nasogastric tube, peritoneal dialysis catheter, permanent pacemaker, right upper abdomen drain, suprapubic catheter, surgical drain, tibial artery stent, tracheotomy tube and ventriculo-peritoneal shunt.
Coinfection are associated with the following organisms: Achromoba xylosoxidans, Bacteroid fragilis, Bacteroid vugatus, Escherichia coli, Enterococcus fecalis, Candida glaberata, Candida spp, Candida tropicalis, Citrobacter froundi, Enterobacter cloacae, Klebsiella pneumonia, K. oxytoca, Proteus mirabilis, methicillin-resistant Staphylococcus aureus, methicillin-sensitive Staphylococcus aureus, Serratia marcescenes, Stenotrophomonas maltophilia, Streptococcus group C.
Pearson χ2 test of association.
Fisher exact test.
Fig. 2.
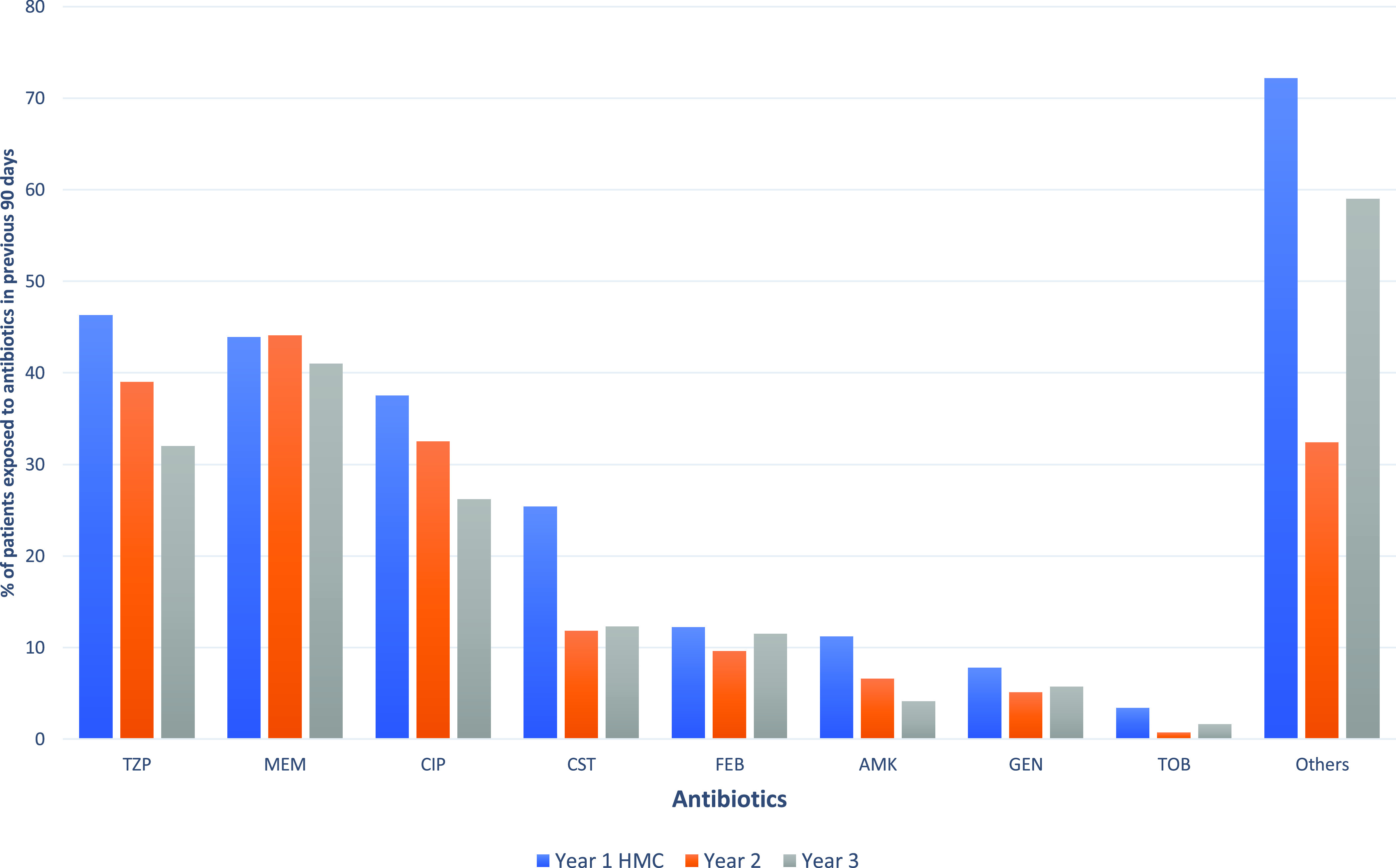
Patient history of previous antibiotic exposure during the prior 90 days from 525 cases with MDR P. aeruginosa isolated from Hamad Medical Corporation between October 2014 and September 2017. Antibiotics: AMK, amikacin; CIP, ciprofloxacin; CST, colistin; FEP, cefepime; GEN, gentamicin; MEM, meropenem; TZP, piperacillin/tazobactam; TOB, tobramycin. Others include the following antibiotics: azithromycin, ceftazidime, ceftriaxone, cefuroxime, cefixime, clarithromycin, clindamycin, cloxacillin, doxycyclin, ertapenem, erythromycin, fluroquinolone, levofloxacin, linezolid, meropenem, metronidazole, minocycline, moxifloxacin, nitrofurantoin, rifampcin, septrin, teicoplanin, tigecycline and vancomycin.
We analyzed the clinical outcomes of patients infected with MDR P. aeruginosa. Overall, 38 (7.2%) of these 525 patients died within 30 days after infection. The mortality rate increased from 3.9% in the first year to 11.2 in the third year, and it was significantly higher in critical care patients compared to other patients (P < .001). When clinical outcomes were compared based on isolation of MDR P. aeruginosa from different infection sites, we detected a significant association between mortality rate and bacteremia over the 3-year study period of 31.3% (P < .001) (Table 2).
Antimicrobial susceptibility patterns of MDR P. aeruginosa isolates
Over the 3-year study period, the antimicrobial susceptibility results revealed that 97.9% of the clinical isolates were resistant to cefepime, followed by ciprofloxacin (89.5%), meropenem (88.6%), and piperacillin-tazobactam (87%). In addition, the clinical isolates showed less resistance to aminoglycosides such as gentamicin (68%), amikacin (54.9%), and tobramycin (52.8%). However, the isolates were highly susceptible to colistin (2.5% resistance), as shown in the cumulative MIC distribution of antipseudomonal agents (Table 3).
Table 3.
Cumulative MIC Distribution for Antipseudomonal Agents Against Clinical Isolates of MDR P. aeruginosa Between October 2014 and September 2017 From Four Different Hospitals at Hamad Medical Corporation, Qatar
| No. (Cumulative %) of Isolates Inhibited at an MIC (mg/L) | MIC range | Total number of isolates | No. of resistant isolates (%) | Total resistance over three years (%) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotic | Year/MIC | ≤0.75 | 1 | 1.5 | 2 | 3 | 4 | 6 | 8 | 12 | 16 | 24 | 32 | 48 | 64 | 96 | 128 | 192 | 256 | MIC50 | MIC90 | ||||
| FEP | Year 1 | 0 (0) | 0 (0) | 1 (0.5) | 1 (1) | 0 (1) | 1 (1.5) | 0 (1.5) | 4 (3.4) | 13 (9.8) | 16 (17.6) | 19 (26.8) | 18 (35.6) | 17 (44) | 13 (50.2) | 8 (54.1) | 8 (58) | 8 (62) | 78 (100) | 64 | 256 | 1.5-256 | 205 | 198 (96.6) | 513 (97.7) |
| Year 2 | 0 | 0 | 0 | 0 | 1 (0.6) | 0 (0.6) | 1 (1.1) | 0 (1.1) | 8 (5.6) | 13 (13) | 12 (19.8) | 19 (30.5) | 13 (37.9) | 14 (45.8) | 15 (54.2) | 6 (57.6) | 5 (60.5) | 70 (100) | 64 | 256 | 3-256 | 177 | 175 (98.9) | ||
| Year 3 | 0 | 1 (0.7) | 0 (0.7) | 1 (0.7) | 2 (0.7) | 3 (0.7) | 1 (1.4) | 1 (2.1) | 6 (6.3) | 14 (16.1) | 6 (20.3) | 12 (28.7) | 5 (32.2) | 13 (41.3) | 5 (44.8) | 2 (46.2) | 3 (48.3) | 74 (100) | 256 | 256 | 1-256 | 143 | 140 (97.9) | ||
| GEN | Year 1 | 7 (3.4) | 7 (6.8) | 8 (10.7) | 10 (15.6) | 13 (22) | 9 (26.3) | 12 (32.2) | 17 (40.5) | 1 (41) | 6 (44) | 6 (46.8) | 4 (48.8) | 5 (51.2) | 2 (52.2) | 0 (52.2) | 0 (52.2) | 3 (53.7) | 95 (100) | 48 | 256 | 0.25-256 | 205 | 150 (73.2) | 356 (67.8) |
| Year 2 | 8 (4.5) | 7 (8,5) | 10 (14.1) | 10 (19.8) | 13 (27.1) | 17 (36.7) | 9 (41.8) | 8 (46.3) | 3 (48) | 8 (52.5) | 2 (53.7) | 2 (54.8) | 1 (55.4) | 2 (56.5) | 0 (56.5) | 4 (58.8) | 0 (58.8) | 73 (100) | 16 | 256 | 0.38-256 | 177 | 112 (63.3) | ||
| Year 3 | 11 (7.7) | 12 (16.1) | 9 (22.4) | 11 (30.1) | 4 (32.9) | 2 (34.3) | 5 (37.8) | 1 (28.5) | 4 (41.3) | 3 (43.4) | 3 (45.5) | 2 (46.9) | 1 (47.6) | 0 (47.6) | 2 (49) | 0 (49) | 0 (49) | 73 (100) | 256 | 256 | 0.5-256 | 143 | 94 (65.7) | ||
| TZP | Year 1 | 0 (0) | 2 (1) | 0 (1) | 1 (1.5) | 0 (1.5) | 1 (2) | 3 (3.4) | 3 (4.9) | 3 (6.3) | 7 (9.8) | 10 (14.1) | 12 (20) | 12 (26.3) | 12 (32.2) | 9 (36.6) | 4 (38.5) | 8 (42.9) | 118 (100) | 256 | 256 | 1-256 | 205 | 186 (90.7) | 457 (87) |
| Year 2 | 1 (0.6) | 0 (0.6) | 0 (0.6) | 3 (2.3) | 0 (2.3) | 2 (3.4) | 1 (4) | 1 (4.5) | 6 (7.9) | 8 (12.4) | 8 (16.9) | 10 (22.6) | 7 (26.6) | 7 (30.5) | 2 (31.6) | 7 (35.6) | 1 (36.2) | 113 (100) | 256 | 256 | 0.75-256 | 177 | 155 (87.6) | ||
| Year 3 | 2 (1.4) | 0 (1.4) | 0 (1.4) | 0 (1.4) | 2 (2.8) | 2 (4.2) | 6 (8.4) | 0 (8.4) | 4 (11.2) | 11 (18.9) | 7 (23.8) | 13 (32.9) | 9 (39.2) | 9 (45.5) | 8 (51) | 8 (56.6) | 3 (58.7) | 59 (100) | 96 | 256 | 0.38-256 | 143 | 116 (81.1) | ||
| AMK | Year 1 | 1 (0.5) | 1 (1) | 2 (2) | 2 (2.9) | 8 (6.8) | 12 (12.7) | 8 (16.7) | 19 (25.9) | 15 (33.2) | 16 (41) | 13 (47.3) | 8 (51.2) | 9 (55.6) | 6 (58.5) | 8 (62.4) | 8 (66.3) | 2 (67.3) | 67 (100) | 32 | 256 | 0.75-256 | 205 | 119 (58.0) | 287 (54.7) |
| Year 2 | 0 | 0 | 1 (0.6) | 5 (3.4) | 7 (7.3) | 15 (15.8) | 10 (21.5) | 11 (27.7) | 14 (35.6) | 10 (41.2) | 15 (49.7) | 8 (54.2) | 9 (59.3) | 8 (63.8) | 6 (67.2) | 7 (71.2) | 1 (71.8) | 50 (100) | 32 | 256 | 2-256 | 177 | 104 (58.8) | ||
| Year 3 | 1 (0.7) | 1 (1.4) | 3 (3.5) | 8 (9.1) | 17 (21) | 12 (29.4) | 12 (37.8) | 9 (44.1) | 7 (49) | 9 (55.2) | 9 (61.5) | 3 (63.6) | 2 (65) | 3 (67.1) | 1 (67.8) | 2 (69.2) | 2 (70.6) | 42 (100) | 16 | 256 | 1-256 | 143 | 64 (44.8) | ||
| TOB | Year 1 | 13 (6.3) | 20 (16.1) | 27 (29.3) | 18 (38) | 7 (41.4) | 7 (44.9) | 2 (45.9) | 0 (45.9) | 4 (47.8) | 1 (48.3) | 12 (54.1) | 13 (60.5) | 9 (64.9) | 5 (67.3) | 4 (69.3) | 5 (71.7) | 1 (72.2) | 57 (100) | 24 | 256 | 0.38-256 | 0 | 112 (54.6) | 277 (52.8) |
| Year 2 | 26 (14.7) | 19 (25.4) | 24 (39) | 14 (46.9) | 5 (49.7) | 6 (53.1) | 2 (54.2) | 1 (54.8) | 0 (54.8) | 6 (58.2) | 3 (59.9) | 8 (64.4) | 13 (71.8) | 5 (74.6) | 1 (75.1) | 4 (77.4) | 1 (78) | 39 (100) | 4 | 256 | 83 (46.9) | 177 | 83 (46.9) | ||
| Year 3 | 43 (30.1) | 7 (35) | 8 (40.6) | 2 (42) | 0 (42) | 1 (42.7) | 4 (45.5) | 2 (46.9) | 6 (51) | 3 (53.1) | 7 (58) | 2 (59.4) | 3 (61.5) | 5 (65) | 4 (67.8) | 4 (70.6) | 0 (70.6) | 42 (100) | 12 | 256 | 82 (57.3) | 143 | 82 (57.3) | ||
| CST | Year 1 | 43 (21) | 38 (39) | 82 (79.5) | 35 (96.6) | 3 (98) | 2 (99) | 1 (99.5) | 1 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 1.5 | 2 | 0.19-8 | 205 | 7 (3.4) | 13 (2.5) |
| Year 2 | 30 (16.9) | 28 (32.8) | 76 (75.7) | 40 (98.3) | 0 (98.3) | 1)98.9) | 1 (99.4) | 1 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 1.5 | 2 | 0.125-8 | 177 | 3 (1.7) | ||
| Year 3 | 20 (14) | 40 (42) | 63 (86) | 17 (97.9) | 1 (98.6) | 0 (98.6) | 1 (99.3) | 1 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 1.5 | 2 | 0.125-9 | 143 | 3 (2.1) | ||
| MEM | Year 1 | 8 (3.9) | 1 (4.4) | 5 (6.8) | 6 (9.8) | 4 (11.7) | 4 (13.7) | 4 (15.6) | 9 (20) | 4 (22) | 3 (23.4) | 1 (23.9) | 156 (100) | 32 | 32 | 0.125-32 | 205 | 185 (90.2) | 465 (88.6) | ||||||
| Year 2 | 10 (5.60 | 3 (7.4) | 7 (11.3) | 1 (11.9) | 3 (13.6) | 7 (17.5) | 7 (21.5) | 3 (23.2) | 4 (25.4) | 2 (26.6) | 2 (27.7) | 128 (100) | Not applicable | 32 | 32 | 0.064-32 | 177 | 156 (88.1) | |||||||
| Year 3 | 3 (2.1) | 4 (4.9) | 5 (8.4) | 7 (13.3) | 2 (14.7) | 3 (16.8) | 0 (16.8) | 0 (16.8) | 0 (16.8) | 2 (18.2) | 1 (18.9) | 116 (100) | 32 | 32 | 0.19-32 | 143 | 124 (86.7) | ||||||||
| CIP | Year 1 | 12 (5.9) | 5 (8.3) | 5 (10.7) | 7 (14.1) | 9 (18.5) | 14 (25.4) | 6 (28.3) | 6 (31.2) | 6 (34.1) | 2 (35.1) | 3 (36.6) | 130 (100) | 32 | 32 | 0.125-32 | 205 | 187 (91.2) | 463 (88.2) | ||||||
| Year 2 | 17 (9.6) | 5 (12.4) | 12 (19.2) | 7 (23.2) | 9 (28.2) | 8 (32.8) | 7 (36.7) | 5 (39.5) | 3 (41.2) | 5 (44.1) | 0 (44.1) | 99 (100) | Not applicable | 32 | 32 | 0.094-32 | 177 | 155 (87.6) | |||||||
| Year 3 | 16 (11.2) | 6 (15.4) | 5 (18.9) | 7 (22.4) | 8 (29.4) | 8 (35) | 6 (39.2) | 6 (43.4) | 3 (45.5) | 3 (47.6) | 2 (49) | 73 (100) | 32 | 32 | 0.125-32 | 143 | 121 (84.6) | ||||||||
Note. MIC, minimum inhibitory concentration; AMK, amikacin; CIP, ciprofloxacin; C/T, ceftolozane-tazobactam; CZA, ceftazidime-avibactam; FEP, cefepime; GEN, gentamicin; MDR, multidrug resistant; MEM, meropenem; TZP, piperacillin-tazobactam. No shading indicates susceptible; gray shading indicates nonsusceptible.
Prevalence distribution, and clustering of the MDR P. aeruginosa
The genomic data analysis showed that the 78 MDR P. aeruginosa isolates belonged to 29 different sequence types: 16 ST235 (20.5%), 8 ST357 (10.3%), 6 ST389 (7.7%), 6 ST1284 (7.7%), 6 ST233 (7.7%), and others (36 of 75, 46.2%) (Table 4). Moreover, 9 of 16 ST235 isolates and 2 of 3 ST308 isolates were obtained from patients with urinary tract infections (UTIs) who attended the outpatient department of the acute-care hospital at HGH (P < .01). In addition, 5 of 8 ST357 isolates were isolated from the inpatient department at HGH. All 6 ST389 isolates were from cystic fibrosis patients. All 4 ST274 isolates were isolated from patients with respiratory tract infections, and 3 of 5 ST233 isolates were isolated from patients with bloodstream infections. The association of isolation of different sequence types with infection sites and locations is depicted in Table 4.
Table 4.
The Prevalence and Distribution of Sequence Types of 78 MDR P. aeruginosa Isolates Associated With Site of Infection, Location, and Clinical and Microbiological Characteristics
| Sequence Type | Isolates, No. (%) | Clinical Diagnosis | Acquisition | Isolation Site | Location | Hospital | Mucoidity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infection | Colonization | Hospital | Community | Respiratory | Skin & Soft Tissue | Urine | Blood | Catheter Tip | OPD | IPD | ICU | HGH | RH | NCCCR | HH | Non-mucoid | Mucoid | ||
| ST235 | 16 (20.5) | 10 | 6 | 16 | 0 | 2 | 4 | 9 | 0 | 1 | 9 | 5 | 2 | 16 | 0 | 0 | 1 | 15 | 1 |
| ST357 | 8 (10.3) | 6 | 2 | 7 | 1 | 1 | 2 | 3 | 2 | 0 | 2 | 5 | 1 | 8 | 0 | 0 | 0 | 8 | 0 |
| ST389 | 6 (7.7) | 3 | 3 | 6 | 0 | 6 | 0 | 0 | 0 | 0 | 5 | 1 | 0 | 6 | 0 | 0 | 0 | 6 | 0 |
| ST1284 | 6 (7.7) | 4 | 2 | 6 | 0 | 0 | 3 | 2 | 1 | 0 | 2 | 3 | 1 | 5 | 1 | 0 | 0 | 5 | 1 |
| ST233 | 5 (6.4) | 2 | 3 | 5 | 0 | 1 | 1 | 0 | 3 | 0 | 0 | 4 | 1 | 4 | 1 | 0 | 0 | 4 | 1 |
| ST274 | 4 (5.1) | 3 | 1 | 4 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 4 | 0 | 0 | 0 | 3 | 1 |
| ST244 | 3 (3.8) | 1 | 2 | 2 | 1 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 2 | 3 | 0 | 0 | 0 | 3 | 0 |
| ST308 | 3 (3.8) | 1 | 2 | 3 | 0 | 1 | 0 | 2 | 0 | 0 | 2 | 1 | 0 | 3 | 0 | 0 | 0 | 1 | 2 |
| ST823 | 3 (3.8) | 2 | 1 | 3 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 2 | 0 | 1 | 0 | 3 | 0 |
| ST2819 | 2 (2.6) | 1 | 1 | 2 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 2 |
| ST17 | 1 (1.3) | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| ST27 | 1 (1.3) | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 |
| ST179 | 1 (1.3) | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| ST252 | 1 (1.3) | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| ST253 | 1 (1.3) | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| ST292 | 1 (1.3) | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| ST310 | 1 (1.3) | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| ST313 | 1 (1.3) | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| ST348 | 1 (1.3) | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| ST381 | 1 (1.3) | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| ST446 | 1 (1.3) | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| ST560 | 1 (1.3) | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| ST598 | 1 (1.3) | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| ST639 | 1 (1.3) | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| ST664 | 1 (1.3) | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| ST699 | 1 (1.3) | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| ST773 | 1 (1.3) | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| ST1076 | 1 (1.3) | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| ST2178 | 1 (1.3) | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| ST3022 | 1 (1.3) | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| ST3043 | 1 (1.3) | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| ST-Unknown | 1 (1.3) | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Total (%) | 78 (100) | 45 (57.7) | 33 (42.3) | 76 (97.4) | 2 (2.6) | 29 (37.2) | 16 (20.5) | 16 (20.5) | 16 (20.5) | 1 (1.3) | 24 (30.8) | 39 (50) | 15 (19.2) | 71 (91) | 5 (6.4) | 1 (1.3) | 1 (1.3) | 67 (85.9) | 11 (14.1) |
Note. HGH, Hamad General Hospital; RH, Rumailah Hospital; NCCCR, National Center for Cancer Care and Research; HH, Heart Hospital; OPD, outpatient department; IPD, inpatient department; ICU, intensive care unit.
Based on phenotypic and genotypic characteristics, important risk factors, and clinical outcomes such as 30-day mortality, we detected 5 clusters (Fig. 3). Cluster analysis of the 78 MDR P. aeruginosa showed that cluster 5 was associated with bloodstream infections as well as 30-day mortality (Fig. 3). For the 4 main P. aeruginosa–encoded exotoxin genes of the type III secretion system, exoS was detected in 31 (39.7%) of 78 specimens, exoU was detected in 44 specimens (56.4%), exoY was detected in 75 specimens (96.2%), and exoT was detected in 76 specimens (97.4%) (Fig. 3).
Fig. 3.
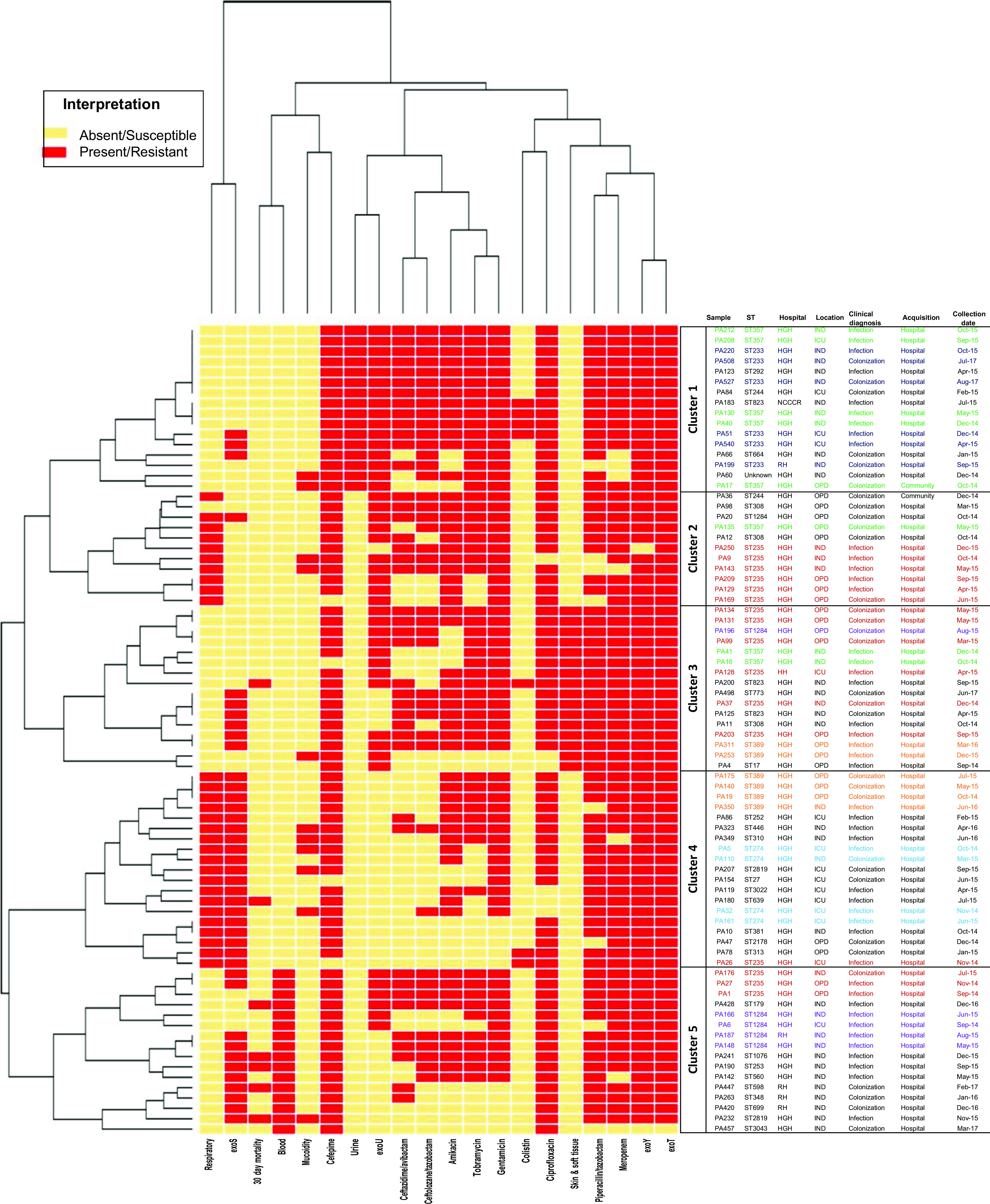
Heat maps and dendograms constructed using the presence or absence of main P. aeruginosa–encoded exotoxin, phenotypic resistance to tested antibiotics and important risk factors associated with 78 MDR P. aeruginosa isolates collected from Qatar between October 2014 and September 2017. Note. OPD, outpatient department; IPD, inpatient department; ICU, intensive care unit.
Discussion
The present study is the first and the largest from the Middle East to address systematic surveillance, molecular epidemiology, microbiological and clinical characteristics, as well as clinical outcomes associated with infections secondary to MDR P. aeruginosa in a major hospital system. During the 3-year study period in Qatar, the overall prevalence of MDR P. aeruginosa was 5.6%, which is lower than reports from neighboring countries or regions. 11 The prevalence of MDR P. aeruginosa initially peaked at 8.1% in the first year of the study, then decreased throughout the study until it reached 4.8% in the third year. At the start the of the study, the upward trend in the epidemiology of GNB, including MDR P. aeruginosa, prompted the monitoring process. The significant decline in the prevalence of MDR P. aeruginosa over the study period might be attributed to the efficacy of the antimicrobial stewardship program (ASP), which targeted broad-spectrum antimicrobials, including antipseudomonal agents. This effort began around 2015 across all hospitals in Qatar to curb rising AMR rates. 23 The downward trend of MDR P. aeruginosa demonstrates a probable positive impact of the ASP, supported by the observation that the overall proportion of clinical isolates of MDR P. aeruginosa did not change over the study period. In addition, we detected a substantial decrease in the use of antipseudomonal agents, reaching 30%, with no major changes in infection control and prevention practices (Table 1).
Similarly, a Cochrane Database systemic review demonstrated the success of ASP interventions to improve antibiotic prescribing in hospital inpatients and reduce AMR. 24 Our findings agree with those from another study from the United States that investigated the relationship of carbapenem restriction in 22 university teaching hospitals and the incidence of carbapenem-resistant P. aeruginosa. 25
Regarding the isolation of MDR P. aeruginosa from different body sites, the distribution and rank order of P. aeruginosa isolates were generally in agreement with isolates obtained through the Global SENTRY Antimicrobial Surveillance Program 1997–1999. Respiratory isolates were most prevalent, followed by skin and soft-tissue, urine, and blood isolates. 26 The low proportion of MDR P. aeruginosa bloodstream isolates in this study, which belonged to 13 different sequence types, suggests that cross transmission is not a major problem in the context of MDR P. aeruginosa bacteremia in Qatar. Furthermore, 95% of MDR P. aeruginosa infections were hospital acquired, and the leading risk factors for acquisition of MDR P. aeruginosa included a prior encounter with healthcare settings, colonization with a susceptible P. aeruginosa strain, as well as exposure to antimicrobials in the preceding 90 days. These findings are in line with those of previous studies. 14
Risk factors for acquisition and clinical outcomes for MDR P. aeruginosa were not related to age or sex, whereas the 30-day mortality rate significantly increased from 3.9% in the first year to 11.2% in the third year. This rate was significantly higher for critical care patients, and 31.3% of these cases were bloodstream infections (Table 2). This finding is in accordance with previous studies. 27,28 A noticeable feature of this study was the associated comorbidities with MDR P. aeruginosa acquisition (ie, diabetes mellitus, end-stage renal disease, malignancy, heart failure, and chronic lung diseases); however, this observational correlation probably stems from the association of comorbidities with prolonged hospital stay and infection acquisition. Such associations have been observed in other AMR morbidity and mortality studies. 25,29
Genomic analysis of major clones revealed the dominance of 5 major epidemic sequence types: ST235, ST357, ST389, ST1284, and ST233 (Table 4). These notorious epidemic clones exhibit high MIC values for ceftazidime-avibactam and ceftolozane-tazobactam, and they are responsible for spreading resistance globally, including in the Middle East. 30–32 Interestingly, most of ST235 and ST308 isolates were isolated from UTIs from patients who attended the outpatient department of the same hospital at HGH, and most ST357 isolates were obtained from patients the inpatient department of the same facility, which supports clonality of HAIs. On the other hand, all ST389 isolates (all from cystic fibrosis patients) and ST274 isolates were obtained from patients with respiratory tract infections, whereas most ST233 isolates were obtained from patients with bloodstream infections. These observations suggest that specific virulence factors are associated with certain sequence types that are site specific. 33,34 The major clones, dominated by ST235 and ST357, have been associated with global epidemic dissemination and spread of MDR strains. These epidemic clones follow regional locations established at specific healthcare settings, which supports environmental endemicity and clonality of MDR P. aeruginosa (Table 4). 22 Other less frequently isolated clones, such as ST389, ST1284, and ST233, seem to be endemic to our setting. Reports of ST1284 and ST233 are rare and seem to be highly resistant, harboring carbapenem resistance genes such as bla VIM, which has also been observed in Brazil and Japan. 35,36 In contrast, none of the previously reported high-risk P. aeruginosa clones, such as ST111 and ST175, were detected in the present study. 9
Our analysis of microbiological characteristics demonstrated a significant resistance profile, reaching or exceeding 90% for primary antipseudomonal agents (Table 3). This observation is worrying because it establishes that AMR for MDR P. aeruginosa is a significant challenge with limited treatment options, particularly because other available agents, such as colistin, demonstrated the highest susceptibility rates (97.5%) at the expense of associated adverse events. 37 Probably because of significant underlying embedded regional resistance profiles, even before the introduction of novel agents such as ceftazidime-avibactam and ceftolozane-tazobactam in our clinical practice, observed susceptibility of the collection for both agents was lower than other regions at 68.8% and 62.9%, respectively. 38 In our previous study, we examined β-lactamase–mediated genes through selective genomic analysis of 75 MDR P. aeruginosa isolates, which included 42 isolates that were resistant to 1 or both agents. They all possessed class C and/or class D β-lactamases; 50% of isolates possessed class A β-lactamases; and 26.7% possessed class B β-lactamases. 39,40
Additionally, genomic analyses of virulence factors in previous studies have detected the presence of mutations in distinct genes for MDR P. aeruginosa–encoded type III exotoxins: exoS, exoT, exoU, exoY, which are known to be associated with severe infections. 13 In this study, comparative analysis of the presence or absence of P. aeruginosa virulence factors against sequence types, site of isolation, antipseudomonal agents, and clinical outcomes revealed that they are clustered within certain sequence types and are related to negative clinical outcomes, such as bloodstream infections, but are probably not related to AMR (Fig. 3).
In conclusion, our 3-year study of 525 clinical isolates of MDR P. aeruginosa is one of the largest global collections that established the initial relatively higher prevalence of resistant clinical isolates demonstrating significant AMR in Qatar, which was later ameliorated through effective surveillance, monitoring, and implementation of a successful ASP. We also observed the role of specific endemic clones that were geographically located demonstrating high-level resistance as well as being associated with specific infections such as bloodstream and exacerbation of infections in patients with cystic fibrosis probably related to certain underlying virulence factors. In the fight to control HAIs, we advocate continuous surveillance, monitoring as well as genomic analysis to understand pathogens epidemiology, microbiological characteristics as well as underlying mechanisms of virulence and resistance.
Acknowledgments
These findings are solely the responsibility of the authors.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/ash.2022.226.
click here to view supplementary material
Financial support
The study was funded by the Medical Research Centre at Hamad Medical Corporation, Doha, Qatar (internal research grant no. IRGC-01–51-033 to E.B.I.) by the Qatar National Research Fund (a member of the Qatar Foundation, NPRP grant no. NPRP12S-0219-190109). Support was also provided by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas) (grant no. 219-2014-837 to J. J. and B.S.).
Conflicts of interest
All authors do not have any conflict of interest to declare in relation to this manuscript.
References
- 1. O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. Review on Antimicrobial Resistance website. https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf. Published May 2016. Accessed May 12, 2022.
- 2. Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev 1993;6:428–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis 2005;41:848–854. [DOI] [PubMed] [Google Scholar]
- 4. Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 2009;22:582–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Correa A, Del Campo R, Perenguez M, et al. Dissemination of high-risk clones of extensively drug-resistant Pseudomonas aeruginosa in Colombia. Antimicrob Agents Chemother 2015;59:2421–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edelstein MV, Skleenova EN, Shevchenko OV, et al. Spread of extensively resistant VIM-2–positive ST235 Pseudomonas aeruginosa in Belarus, Kazakhstan, and Russia: a longitudinal epidemiological and clinical study. Lancet Infect Dis 2013;13:867–876. [DOI] [PubMed] [Google Scholar]
- 7. Rasamiravaka T, Labtani Q, Duez P, El Jaziri M. The formation of biofilms by Pseudomonas aeruginosa: a review of the natural and synthetic compounds interfering with control mechanisms. Biomed Res Int 2015;2015:759348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Balasubramanian D, Schneper L, Kumari H, Mathee K. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res 2013;41:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horcajada JP, Montero M, Oliver A, et al. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin Microbiol Rev 2019;32:e00031–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Surveillance of antimicrobial resistance in Europe 2018. European Centre for Disease Prevention and Control website. https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2018. Published 2019. Accessed May 11, 2021.
- 11. Al-Orphaly M, Hadi HA, Eltayeb FK, et al. Epidemiology of multidrug-resistant Pseudomonas aeruginosa in the Middle East and North Africa region. mSphere 2021;6:e00202–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oliver A, Mulet X, Lopez-Causape C, Juan C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist Update 2015;21–22:41–59. [DOI] [PubMed] [Google Scholar]
- 13. Sato H, Frank DW, Hillard CJ, et al. The mechanism of action of the Pseudomonas aeruginosa–encoded type III cytotoxin, ExoU. Embo J 2003;22:2959–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahmed MAS, Abu Jarir S, Abdel Hadid HA, et al. Emergence of multidrug- and pandrug-resistant Pseudomonas aeruginosa from five hospitals in Qatar. Infect Prev Pract 2019;1:100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Corona-Nakamura AL, Miranda-Novales MG, Leaños-Miranda B, et al. Epidemiologic study of Pseudomonas aeruginosa in critical patients and reservoirs. Arch Med Res 2001;32:238–242. [DOI] [PubMed] [Google Scholar]
- 16. Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 30 th edition. Wayne, PA: CLSI; 2020. [Google Scholar]
- 17. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268–281. [DOI] [PubMed] [Google Scholar]
- 18. Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 2012;19:455–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Larsen MV, Cosentino S, Rasmussen S, et al. Multilocus sequence typing of total-genome–sequenced bacteria. J Clin Microbiol 2012;50:1355–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brettin T, Davis JJ, Disz T, et al. RASTtk: a modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci Rep 2015;5:8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jia B, Raphenya AR, Alcock B, et al. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res 2017;45(D1):D566–D573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Warnes, G , Bolker B, Bonebakker L, et al. Gplots: Various R Programming Tools for Plotting Data. R package version 2.17.0; 2015.
- 23. Sid Ahmed MA, Abdel Hadi H, Abu Jarir S, et al. Impact of an antimicrobial stewardship programme on antimicrobial utilization and the prevalence of MDR Pseudomonas aeruginosa in an acute-care hospital in Qatar. JAC Antimicrob Resist 2020;2:dlaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pakyz AL, Oinonen M, Polk RE. Relationship of carbapenem restriction in 22 university teaching hospitals to carbapenem use and carbapenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 2009;53:1983–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Defez C, Fabbro-Peray P, Bouziges N, et al. Risk factors for multidrug-resistant Pseudomonas aeruginosa nosocomial infection. J Hosp Infect 2004;57:209–216. [DOI] [PubMed] [Google Scholar]
- 26. Gales AC, Jones RN, Turnidge J, Rennie R, Ramphal R. Characterization of Pseudomonas aeruginosa isolates: occurrence rates, antimicrobial susceptibility patterns, and molecular typing in the global SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis 2001;32 suppl 2:S146–S155. [DOI] [PubMed] [Google Scholar]
- 27. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004;39:309–317. [DOI] [PubMed] [Google Scholar]
- 28. Parkins MD, Gregson DB, Pitout JD, Ross T, Laupland KB. Population-based study of the epidemiology and the risk factors for Pseudomonas aeruginosa bloodstream infection. Infection 2010;38:25–32. [DOI] [PubMed] [Google Scholar]
- 29. Micek ST, Wunderink RG, Kollef MH, et al. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: impact of multidrug resistance. Crit Care 2015;19:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perez F, Hujer AM, Marshall SH, et al. Extensively drug-resistant Pseudomonas aeruginosa isolates containing blaVIM-2 and elements of Salmonella genomic island 2: a new genetic resistance determinant in northeast Ohio. Antimicrob Agents Chemother 2014;58:5929–5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miyoshi-Akiyama T, Tada T, Ohmagari N, et al. Emergence and spread of epidemic multidrug-resistant Pseudomonas aeruginosa . Genome Biol Evol 2017;9:3238–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abdouchakour F, Aujoulat F, Licznar-Fajardo P, et al. Intraclonal variations of resistance and phenotype in Pseudomonas aeruginosa epidemic high-risk clone ST308: a key to success within a hospital? Int J Med Microbiol 2018;308:279–289. [DOI] [PubMed] [Google Scholar]
- 33. Behzadi P, Baráth Z, Gajdács M. It’s not easy being green: a narrative review on the microbiology, virulence and therapeutic prospects of multidrug-resistant Pseudomonas aeruginosa . Antibiotics (Basel) 2021;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Francis VI, Stevenson EC, Porter SL. Two-component systems required for virulence in Pseudomonas aeruginosa . FEMS Microbiol Lett 2017;364:fnx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Balero de Paula S, Cayô R, Streling AP, et al. Detection of bla VIM-7 in an extensively drug-resistant Pseudomonas aeruginosa isolate belonging to ST1284 in Brazil. Diagn Microbiol Infect Dis 2017;89:80. [DOI] [PubMed] [Google Scholar]
- 36. del Barrio-Tofiño E, López-Causapé C, Oliver A. Pseudomonas aeruginosa epidemic high-risk clones and their association with horizontally acquired β-lactamases: 2020 update. Int J Antimicrob Agents 2020;56:106196. [DOI] [PubMed] [Google Scholar]
- 37. Spapen H, Jacobs R, Van Gorp V, Troubleyn J, Honoré PM. Renal and neurological side effects of colistin in critically ill patients. Ann Intensive Care 2011;1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sid Ahmed MA, Abdel Hadi H, Hassan AAI, et al. Evaluation of in vitro activity of ceftazidime/avibactam and ceftolozane/tazobactam against MDR Pseudomonas aeruginosa isolates from Qatar. J Antimicrob Chemother 2019;74:3497–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sid Ahmed MA, Khan FA, Sultan AA, et al. β-lactamase-mediated resistance in MDR Pseudomonas aeruginosa from Qatar. Antimicrob Resist Infect Control 2020;9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sid Ahmed MA, Khan FA, Hadi HA, Skariah S, Sultan AA, Salam A, Al Khal AL, Söderquist B, Ibrahim EB, Omrani AS, Jass J. Association of bla VIM-2, bla PDC-35, bla OXA-10, bla OXA-488 and bla VEB-9 β-Lactamase Genes with Resistance to Ceftazidime-Avibactam and Ceftolozane-Tazobactam in Multidrug-Resistant Pseudomonas aeruginosa . Antibiotics (Basel) 2022;11(2):130. doi: 10.3390/antibiotics11020130. PMID: 35203733; PMCID: PMC8868128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/ash.2022.226.
click here to view supplementary material


