Abstract
The intraerythrocytic stage of the simian malaria parasite Plasmodium coatneyi (CDC strain) was intravenously inoculated into two species of macaques with different susceptibilities to infection with this parasite, including four Japanese macaques (Macaca fuscata) and three cynomolgus macaques (M. fascicularis). The Japanese macaques infected with P. coatneyi developed severe clinical manifestations similar to those of severe human malaria and eventually became moribund, while the infected cynomolgus macaques, natural hosts of the parasite, exhibited no severe manifestation of disease except anemia and finally recovered from the infection. In the infected Japanese macaques, peripheral CD4+ and CD8+ T-cell populations were markedly decreased and fragmentation of chromosomal DNA in peripheral blood mononuclear cells was detected during the terminal period of infection, suggesting that apoptotic cell death was responsible at least in part for the T lymphocytopenia. Furthermore, soluble Fas ligand levels in sera of the infected Japanese macaques increased gradually to a markedly high level of 28.83 ± 10.56 pg/ml (n = 4) when the animals became moribund. On the other hand, none of the infected cynomolgus monkeys exhibited either T lymphocytopenia or elevated soluble Fas ligand level. These findings suggest that differences in immune response between the two species of macaque tested accounted for the contrasting outcomes after infection with the same isolate of malarial parasite, and in particular that a profound T lymphocytopenia due to Fas-derived apoptosis played a role in the fatal course of malaria in the infected Japanese macaques.
Plasmodium falciparum infection leads to severe signs and symptoms and a wide variety of clinical consequences, but not every patient becomes seriously ill or dies (28). The outcome of infection is influenced by individual susceptibility, parasite virulence, and a number of environmental factors, and recent intensive studies on the factors responsible for resistance to malarial infection have elucidated some effects of host genetic background on outcome of infection as well (8, 16).
P. coatneyi, a simian malarial parasite, causes mild infection in its natural host, the cynomolgus macaque (Macaca fascicularis) (4), but more serious infection in experimental hosts such as the Japanese macaque (M. fuscata) (11) and rhesus macaque (M. mulatta) (1). In the latter two species, infected monkeys develop a fulminating acute infection with pronounced parasitemia and became moribund with severe manifestations. Furthermore, they exhibit histopathological findings typical of cerebral malaria: sequestration of parasitized red blood cells and cytoadherence-associated knobs on parasitized red cells to endothelial cells were found in cerebral microvessels and capillaries of major organs in these monkeys. Japanese macaques and rhesus macaques infected with P. coatneyi can thus be used as powerful primate models for the pathophysiological study of severe human malaria.
The Japanese macaque belongs to the genus Macaca, the same genus as the rhesus macaque and cynomolgus macaque, and is found on three of the four major islands of Japan (15), where natural infection with simian malaria has never been reported. On the other hand, the cynomolgus macaque is widely distributed throughout Southeast Asian countries (15), where various species of simian malaria are endemic and the monkey is a natural host of malarial parasites such as P. coatneyi, P. cynomolgi, P. inui, and P. knowlesi (5). As previously proposed (29), one important factor maintaining the species integrity of cynomolgus macaques may be ecological isolation due to genetic resistance to certain species of simian malarial infection.
In the present study, we used two species of macaques differing in susceptibility to P. coatneyi infection, Japanese macaques and cynomolgus macaques, as models nonresistant and resistant to malarial infection, respectively. The aim of this study was to clarify immunological features in infected monkeys with different susceptibilities to the parasite. The infected Japanese macaques exhibited severe peripheral T lymphocytopenia and a markedly high serum level of soluble Fas ligand (sFasL) when they became moribund, whereas the infected cynomolgus macaques exhibited none of these changes.
We report here factors possibly determining the differences in outcome between two species of macaques with different susceptibilities to malaria infection and the applicability of the P. coatneyi-infected Japanese macaque to the pathophysiological study of severe human malaria.
MATERIALS AND METHODS
Experimental animals.
Four Japanese macaques (J-8, J-9, J-10, and J-11) and three cynomolgus macaques (CY-1, CY-2, and CY-3) were used in this study in accordance with the guidelines for use of experimental animals authorized by the Japanese Association for Laboratory Animal Science. All monkeys were bred and grown in animal facilities in a malaria-free environment in Japan and were 2 years old when used. Each animal was kept in an individual cage in a controlled environment at 25 to 29°C and fed commercial food pellets supplemented with fresh fruits. At the time of infection, all animals were clinically healthy and used without splenectomy.
Parasite and infection.
P. coatneyi (CDC strain) was used in this study. This strain was also used in previous studies and proved infective in macaques of both species employed in this study (11, 14). Each macaque was intravenously inoculated with 108 blood-stage parasites that had been obtained from another Japanese macaque infected with the parasite and cryopreserved in liquid nitrogen until use.
Sample collection.
After inoculation, daily clinical follow-up of the monkeys was performed; no antimalarial treatment was given during the course of infection. For hematological examination and flow cytometric analyses, venous blood samples were obtained every 3 or 4 days or more frequently after infection. When the infected macaques became moribund with high parasitemia, they were anesthetized with an intramuscular injection of ketamine (15 mg/kg of body weight) and then autopsied. Thin blood films were prepared from blood obtained through earprick. Following Giemsa staining, parasitemia was counted in a total of 104 erythrocytes under an optical microscope.
Flow cytometric analyses.
One or two-color immunophenotyping was performed with monoclonal antibodies (MAbs) conjugated to fluorescein isothiocyanate (FITC) or phycoerythrin (PE): FITC-conjugated anti-human CD2, FITC-conjugated anti-human CD4 (Nichirei, Tokyo, Japan), FITC- or PE-conjugated anti-human CD20, FITC- or PE-conjugated anti-human CD8 (Becton Dickinson, San Jose, Calif.), and PE-conjugated anti-human CD95 (Pharmingen, San Diego, Calif.). These MAbs had been screened in advance for reactivity against lymphocytes of Macaca spp., including Japanese and cynomolgus macaques. Blood samples were treated and stained with each MAb according to the manufacturer's specifications. Briefly, 100 μl of sample blood was mixed with 20 μl of MAb reagent and incubated for 15 min at room temperature. Cells were treated with buffer (FACS lysing solution; Becton Dickinson), washed twice with phosphate-buffered saline, resuspended in phosphate-buffered saline, and analyzed using FACScalibur (Becton Dickinson). Analyses of the fluorescence intensities were performed with CellQuest software (Becton Dickinson). Samples were analyzed by setting appropriate forward and side scatter gates around the lymphocyte population. The number of peripheral leukocytes and their differential count were determined simultaneously. The absolute count of lymphocytes for each subset was derived from the number of circulating lymphocytes and the proportion of MAb-positive cells in flow cytometric analyses. Fas-positive rates of CD2+, CD4+, and CD8+ lymphocytes were determined in two-color immunostaining using PE-labeled anti-CD95 MAb and FITC-labeled anti-CD2, anti-CD4, or anti-CD8 MoAb simultaneously.
DNA fragmentation assay.
To detect DNA fragmentation, which is characteristic of cells undergoing apoptosis, fresh peripheral blood mononuclear cells (PBMCs) were obtained from the infected Japanese macaques and cynomolgus macaques, and PBMCs from an uninfected Japanese macaque were used as a negative control. PBMCs were separated from whole venous blood by Ficoll-Paque PLUS (Pharmacia Biotech AB, Uppsala, Sweden) density gradient, and 106 cells were treated as described previously (18) prior to isolation of chromosomal DNA. The DNA was then subjected to electrophoresis in a 0.75% agarose gel and visualized with ethidium bromide.
Detection of sFasL in serum.
Serum sFasL levels in the infected monkeys were measured using a commercially available human sFasL-measuring kit (MBL, Nagoya, Japan), which is a sandwich enzyme-linked immunosorbent assay system using two anti-human sFasL hamster MAbs, 4H9 and 4A5 (20). The cross-reactivity of monkey sFasL with these two MAbs had previously been demonstrated using recombinant cynomolgus monkey sFasL (rCyFasL) that we had developed (Y. Kirii et al., submitted for publication). The measuring procedure was basically as specified by the manufacturer except that rCyFasL was used as a standard. One hundred microliters of either sample serum or standard solution containing 5 to 1,000 pg of rCyFasL per ml was added to two wells of primary antibody (4H9)-coated ELISA plate. After reaction at room temperature for 1 h, the plate was washed five times with washing solution. Then 100 μl of diluted peroxidase-labeled secondary antibody (4A5) was added to each well and reacted at room temperature for additional 1 h. After another five washes, 100 μl of peroxidase substrate solution was added and allowed to incubate at 37°C for 30 min. Finally, acid solution was added to each well to terminate the enzyme reaction before the optical density of each well was measured at 450 nm by a dual-wavelength plate reader (THERMOmax, Molecular Devices, Sunnyvale, Calif.). The correlation between optical densities and standard rCyFasL contents ranging from 5 to 1,000 pg/ml was always significant (r > 0.9, p < 0.01). Serum sFasL content was determined by reference to the standard curve obtained from the reactivity of the rCyFasL.
RESULTS
Clinical findings and parasitemia.
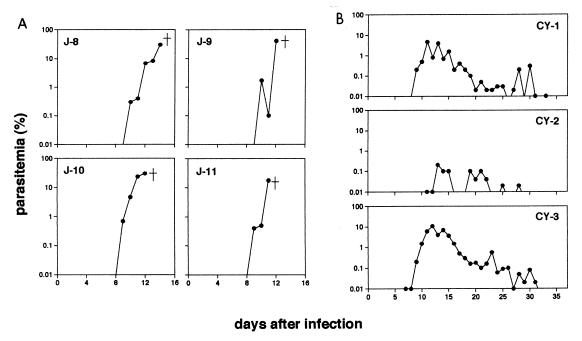
In this study, two species of Macaca monkeys, Japanese macaques and cynomolgus macaques, had quite different consequences of infection with the same isolate of the malarial parasite P. coatneyi: the infection was fatal to the Japanese macaques, whereas the cynomolgus macaques persisted with the infection and eventually survived. The infected Japanese macaques were frequently anorectic, listless, and occasionally depressed, and they finally became lethargic and severely withdrawn just before autopsy on days 14 (J-8), 12 (J-9), 12 (J-10), and 11 (J-11) after infection. The parasite was first detected in the peripheral blood of the infected Japanese macaques about 7 days after infection; parasite densities then increased sharply to around 30% within 2 weeks after infection (Fig. 1A). Maximum parasitemias were 30.6% (J-8), 41.0% (J-9), 29.7% (J-10), and 17.6% (J-11). On the other hand, the cynomolgus macaques exhibited no severe manifestation except anemia (the lowest hematocrits were 18.0% [CY-1], 34.0% [CY-2], and 11.0% [CY-3]). The prepatent period in the infected cynomolgus macaques was about 7 days; parasitemia then increased, peaked at around 5%, and subsequently decreased to less than 0.01% (Fig. 1B) within 35 days after infection. Maximum parasitemias were 4.8% (CY-1), 0.2% (CY-2), and 11.0% (CY-3).
FIG. 1.
Course of P. coatneyi parasitemia in four Japanese macaques (A) and three cynomolgus macaques (B) after infection with parasitized red blood cells.
Kinetics of lymphocyte subsets.
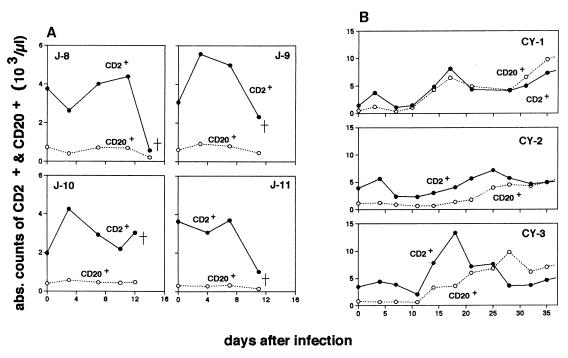
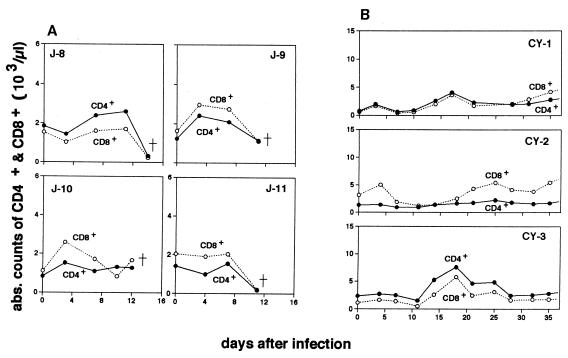
Absolute counts for each lymphocyte subset at different time points in the infected macaques are shown in Fig. 2 and 3. Of four P. coatneyi-infected Japanese macaques, three (J-8, J-9, and J-11) had a markedly reduced number of CD2+ lymphocytes when they were severely ill during the rapid increase in parasitemia (Fig. 2A). None of the infected cynomolgus macaques exhibited reduced number of CD2+ lymphocytes (Fig. 2B). The cynomolgus macaques instead exhibited a gradual increase in CD2+ lymphocyte population with one or two peaks just after infection and/or during the period in which the peak of the parasitemia was observed. In the infected Japanese macaques, differentiation of T-cell subsets by CD4 and CD8 markers revealed that the populations of both subsets had kinetics similar to those of CD2+ lymphocytes (Fig. 3A) and thus that both subtypes of lymphocytes were involved in T lymphocytopenia.
FIG. 2.
Absolute numbers of peripheral CD2+ (●) and CD20+ (○) lymphocytes in four Japanese macaques (A) and three cynomolgus macaques (B) after infection with P. coatneyi.
FIG. 3.
Kinetics of peripheral CD4+ (●) and CD8+ (○) lymphocytes in four Japanese macaques (A) and three cynomolgus macaques (B) after infection with P. coatneyi.
Involvement of apoptotic cell death in T lymphocytopenia.
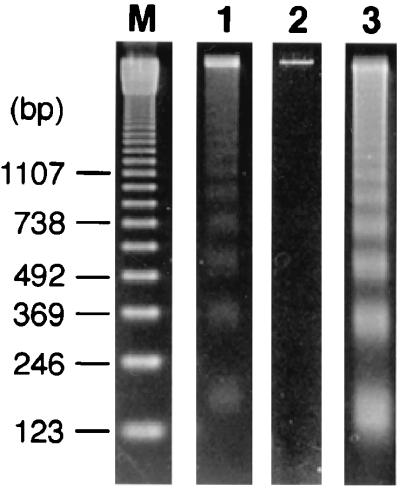
We hypothesized that T lymphocytopenia in P. coatneyi-infected Japanese macaques was caused by apoptosis of T cells on the basis of findings of previous studies of human malarial infection (24, 25). To confirm this hypothesis, chromosomal DNA was prepared from fresh PBMCs of monkeys with or without P. coatneyi infection and analyzed by agarose gel electrophoresis. As shown in Fig. 4, DNA fragmentation occurred only in PBMCs obtained from the P. coatneyi-infected Japanese macaques when they became moribund, not in an uninfected control monkey. In contrast, no ladder pattern of DNA fragments was observed on electrophoresis using the samples from the infected cynomolgus macaques on days 7, 14, and 21 after infection (data not shown).
FIG. 4.
Agarose gel electrophoresis of chromosomal DNA isolated from PBMCs of Japanese macaques with (lane 1) or without (lane 2) P. coatneyi infection. A sample from a parasite-infected Japanese macaque (J-10) was obtained when the animal developed severe signs of disease (12 days after infection). The positive control DNA sample was isolated from PBMCs after incubation with an apoptosis-inducing concentration (50 ng/ml) of recombinant sFasL for 24 h (lane 3). Lane M shows DNA molecular markers.
Rates of Fas expression of lymphocyte subsets.
Since Fas antigen is a cell surface protein that mediates apoptosis, the Fas-positive rate of each lymphocyte subset was determined before and after infection with P. coatneyi (Table 1). In both species of macaques, Fas positivity of CD2+ cells decreased on 11 to 14 days after P. coatneyi infection, which was more pronounced in the infected Japanese macaques when they became severely ill. In addition, the Fas expression rate of CD4+ and CD8+ cells also decreased in only Japanese macaques when they showed severe manifestations.
TABLE 1.
Fas expression rates of each lymphocyte subset in Japanese macaques and cynomolgus macaques before and after infection with P. coatneyi
| Animal | Day after infection | Rate of Fas positivity (%)
|
||
|---|---|---|---|---|
| CD2+ | CD4+ | CD8+ | ||
| J-10 | 0 | 86.3 | 65.1 | 81.9 |
| 7 | 83.2 | 61.4 | 85.3 | |
| 12 (severe illness) | 58.5 | 46.4 | 66.4 | |
| J-11 | 0 | 75.2 | 48.4 | 89.2 |
| 7 | 68.9 | 51.1 | 86.4 | |
| 11 (severe illness) | 58.7 | 45.3 | 71.2 | |
| CY-1 | 0 | 47.5 | 30.6 | 67.8 |
| 7 | 52.8 | 41.3 | 63.9 | |
| 14 | 50.7 | 47.1 | 57.9 | |
| CY-2 | 0 | 79.5 | 29.9 | 94.6 |
| 7 | 81.2 | 41.7 | 93.1 | |
| 14 | 65.7 | 56.0 | 84.6 | |
| CY-3 | 0 | 33.3 | 24.7 | 50.1 |
| 7 | 44.0 | 38.3 | 66.4 | |
| 14 | 28.7 | 21.7 | 36.1 | |
sFasL concentration in serum.
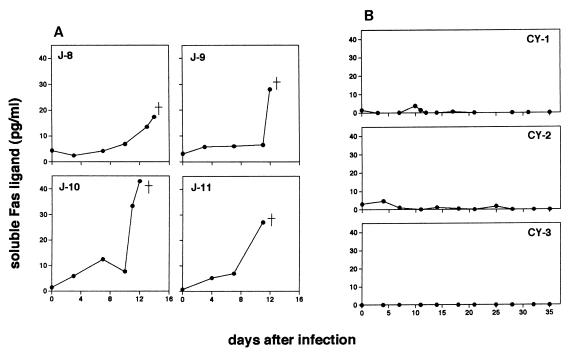
We subsequently measured sFasL concentration in serum obtained from the infected monkeys. As clearly shown in Fig. 5A, the infected Japanese macaques had markedly increased levels of sFasL, and this elevation was simultaneous with the period of T lymphocytopenia. The Japanese macaques had high maximum levels of sFasL (17.38 pg/ml in J-8, 28.00 pg/ml in J-9, 42.94 pg/ml in J-10, and 27.00 pg/ml in J-11) at the time of autopsy. In the cynomolgus macaques, which were free of T lymphocytopenia, the concentration of serum sFasL remained much lower than those in the infected Japanese macaques throughout the course of infection (the highest concentrations were 3.69 pg/ml in CY-1, 7.11 pg/ml in CY-2, and 0.06 pg/ml in CY-3 [Fig. 5B]).
FIG. 5.
Time course of serum sFasL levels in four Japanese macaques (A) and three cynomolgus macaques (B) after infection with P. coatneyi.
DISCUSSION
Host genetic factors including immune response which govern susceptibility to malaria infection are well documented (8, 16). In the present study, two species of Macaca monkeys exhibited markedly different outcomes of infection with the same isolate of malaria parasite: infection was lethal to Japanese macaques but not to cynomolgus macaques. These results were consistent with those described in previous reports (4, 11).
Studies of lymphocyte kinetics revealed that profound T lymphocytopenia occurred in the P. coatneyi-infected Japanese macaques, whereas the infected cynomolgus macaques were free of T lymphocytopenia. T lymphocytopenia is also a well-known finding for human patients with acute severe malaria (3, 7, 9, 26), but little is known concerning the changes in T-cell populations following infection with falciparum malaria. Our study of lymphocyte kinetics using a primate model of severe malaria revealed that T-cell numbers decreased most markedly when the infected Japanese macaques became moribund, suggesting that T lymphocytopenia was intimately associated with severe signs of disease.
Apoptosis is considered a possible mechanism of T lymphocytopenia in human malaria, since patients with acute P. falciparum infection have higher in vitro percentages of lymphocyte apoptosis than do healthy individuals (24, 25). To determine whether the T lymphocytopenia observed in our study was also due to apoptosis, we examined fragmentation of chromosomal DNA, one of the characteristics of cells undergoing apoptotic death. We found DNA fragmentation in PBMCs of the infected Japanese macaques suffering from severe disease but not in those of the infected cynomolgus macaques. These findings support our hypothesis that apoptotic T-cell death was, at least in part, responsible for peripheral T lymphocytopenia in the infected Japanese macaques.
Fas is a membrane protein which mediates apoptosis when it is cross-linked with its ligand. Fas-derived apoptosis plays roles in physiological immune regulatory mechanisms (2, 6, 10), but it can also be harmful (12) and even lethal (21). To examine whether the apoptosis in the infected Japanese macaques was mediated by Fas and its ligand, we measured both Fas expression rates on the surface of lymphocytes and serum sFasL concentration. We found marked elevation of serum sFasL in nonresistant Japanese macaques, which was simultaneous with the decrease in the T-cell population. Interestingly, the resistant cynomolgus macaques, in which T lymphocytopenia was absent, had outcomes very different from those of the nonresistant Japanese macaques: in the former, the serum level of sFasL remained very low throughout the course of infection. In addition, we found that the Fas-positive CD2+, CD4+, and CD8+ cell counts markedly decreased just after the elevation of serum sFasL concentration only in the infected Japanese macaques. These results strongly suggest that Fas/FasL-mediated apoptosis involves the elimination of peripheral T cells, resulting in severe lymphocytopenia and manifestations of disease in the infected Japanese macaques. The difference in immune responses including the function of FasL-producing cells during the course of malaria infection might explain the difference in susceptibility between Japanese macaques and cynomolgus macaques.
As described for other infectious diseases (13, 19, 23, 27), falciparum malaria infection also induces host immune dysfunction including a decreased number of circulating T cells (3, 7, 9, 26) and in vitro depression of proliferative response of PBMCs to parasite antigens (17, 22), which may be responsible for the severe disease of the patients with falciparum malaria. In fact, T lymphocytopenia is also considered one of the typical features of human acute falciparum malaria, and the degree of T lymphocytopenia is correlated with disease severity: it is higher in patients with severe malaria and those with cerebral malaria than in patients with uncomplicated malaria (9). Although the pathogenic process of severe malaria is considered multifactorial, our findings suggest the additional possibility that malarial infection induces a high level of sFasL in the periphery of nonresistant hosts, which causes T lymphocytopenia and subsequent immune dysfunction and severe disease. These immunopathological changes in severe malaria should be taken into account in the design and analysis of cellular investigations and the clinical treatment of patients with severe manifestations of falciparum malaria.
ACKNOWLEDGMENTS
We are grateful to W. E. Collins, Centers for Disease Control and Prevention, for providing the parasite stock. We thank Shin Nippon Biomedical Laboratories, Ltd.; the New Drug Discovery Research Laboratory, Kanebo Ltd.; and Yasuko Nonaka for technical support.
This work was supported by Grant-in-Aid for Scientific Research on Priority Areas 08281103 from the Ministry of Education, Science, Culture and Sports, Japan, and the Japan-U.S. Medical Science Programme.
REFERENCES
- 1.Aikawa M, Brown A, Smith C D, Tegoshi T, Howard R J, Hasler T H, Ito Y, Perry G, Collins W E, Webster K. A primate model for human cerebral malaria: Plasmodium coatneyi-infected rhesus monkeys. Am J Trop Med Hyg. 1992;46:391–397. doi: 10.4269/ajtmh.1992.46.391. [DOI] [PubMed] [Google Scholar]
- 2.Brunner T, Mogil R J, LaFace D, Yoo N J, Mahboubi A, Echeverri F, Martin S T, Force W R, Lynch D H, Ware C F, Green D R. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 3.Chougnet C, Tallet S, Ringwald P, Deloron P. Kinetics of lymphocyte subsets from peripheral blood during a Plasmodium falciparum attack. Clin Exp Immunol. 1992;90:405–408. doi: 10.1111/j.1365-2249.1992.tb05859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coatney G R, Collins W E, Warren M, Contacos P G. The primate malarias. Washington, D.C.: U.S. Government Printing Office; 1971. pp. 289–299. [Google Scholar]
- 5.Eyles D E. The species of simian malaria: taxonomy, morphology, life cycle, and geographical distribution of the monkey species. J Parasitol. 1963;49:866–887. [PubMed] [Google Scholar]
- 6.Green, D. R., and D. W. Scott. Activation-induced apoptosis in lymphocytes. Curr. Opin. Immunol. 6:476–487. [DOI] [PubMed]
- 7.Greenwood B M, Oduloju A J, Stratton D. Lymphocyte changes in acute malaria. Trans R Soc Trop Med Hyg. 1977;71:408–410. doi: 10.1016/0035-9203(77)90039-6. [DOI] [PubMed] [Google Scholar]
- 8.Hill A V S. Genetic susceptibility to malaria and other infectious diseases: from the MHC to the whole genome. Parasitology. 1996;112(Suppl.):S75–S84. doi: 10.1017/s003118200007668x. [DOI] [PubMed] [Google Scholar]
- 9.Hviid L, Kurtzhals J A L, Goka B Q, Oliver-Commey J O, Nkrumah F K, Theander T G. Rapid reemergence of T cells into peripheral circulation following treatment of severe and uncomplicated Plasmodium falciparum malaria. Infect Immun. 1997;65:4090–4093. doi: 10.1128/iai.65.10.4090-4093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ju S, Panka D J, Cui H, Ettinger R, El-Khatib M, Sherr D H, Stanger B Z, Marshak-Rothstein A. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 11.Kawai S, Aikawa M, Kano S, Suzuki M. A primate model for severe human malaria with cerebral involvement: Plasmodium coatneyi-infected Macaca fuscata. Am J Trop Med Hyg. 1993;48:630–636. doi: 10.4269/ajtmh.1993.48.630. [DOI] [PubMed] [Google Scholar]
- 12.Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S. Essential roles of the Fas ligand in the development of hepatitis. Nat Med. 1997;3:409–413. doi: 10.1038/nm0497-409. [DOI] [PubMed] [Google Scholar]
- 13.Large M K, Kittlesen D J, Hahn Y S. Suppression of host immune response by the core protein of hepatitis C virus: possible implications for hepatitis C virus persistence. J Immunol. 1999;162:931–938. [PubMed] [Google Scholar]
- 14.Migot-Nabias F, Ollomo B, Dubreuil G, Morelli A, Domarle O, Nabias R, Georges A J, Millet P. Plasmodium coatneyi: differential clinical and immune responses of two populations of Macaca fascicularis from different origins. Exp Parasitol. 1999;91:30–39. doi: 10.1006/expr.1999.4342. [DOI] [PubMed] [Google Scholar]
- 15.Preston-Mafham K, Preston-Mafham R. Primates of the world. 1992. pp. 61–83. . Blandford Publishing, London, England. [Google Scholar]
- 16.Riley E M. The role of MHC- and non-MHC-associated genes in determining the human immune response to malaria antigens. Parasitology. 1996;112(Suppl.):S39–S51. [PubMed] [Google Scholar]
- 17.Riley E M, Andersson G, Otoo L N, Jepsen S, Greenwood B M. Cellular immune response to Plasmodium falciparum antigens in Gambian children during and after an acute attack of falciparum malaria. Clin Exp Immunol. 1988;73:17–22. [PMC free article] [PubMed] [Google Scholar]
- 18.Sellins K S, Cohen J J. Gene induction by γ-irradiation leads to DNA fragmentation in lymphocytes. J Immunol. 1987;139:3199–3206. [PubMed] [Google Scholar]
- 19.Sztein M B, Kierszenbaum F. Mechanisms of development of immunosuppression during Trypanosoma infections. Parasitol Today. 1993;9:424–428. doi: 10.1016/0169-4758(93)90053-i. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka M, Suda T, Haze K, Nakamura N, Sato K, Kimura F, Motoyoshi K, Mizuki M, Tagawa S, Ohga S, Hatake K, Drummond A H, Nagata S. Fas ligand in human serum. Nat Med. 1996;2:317–322. doi: 10.1038/nm0396-317. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Suda T, Yatomi T, Nakamura N, Nagata S. Lethal effect of recombinant human Fas ligand in mice pretreated with Propionibacterium acnes. J Immunol. 1997;158:2303–2309. [PubMed] [Google Scholar]
- 22.Theander T G, Bygbjerg I C, Andersen B J, Jepsen S, Kharazmi A, Odum N. Suppression of parasite specific response in Plasmodium falciparum malaria. A longitudinal study of blood mononuclear cell proliferation and subset composition. Scand J Immunol. 1986;24:73–81. doi: 10.1111/j.1365-3083.1986.tb02071.x. [DOI] [PubMed] [Google Scholar]
- 23.Tishon A, Manchester M, Scheiflinger F, Oldstone M B A. A model of measles virus-induced immunosuppression: enhanced susceptibility of neonatal human PBLs. Nat Med. 1996;2:1250–1254. doi: 10.1038/nm1196-1250. [DOI] [PubMed] [Google Scholar]
- 24.Toure-Balde A, Sarthou J L, Aribot G, Michel P, Trape J F, Rogier C, Roussilhon C. Plasmodium falciparum induces apoptosis in human mononuclear cells. Infect Immun. 1996;64:744–750. doi: 10.1128/iai.64.3.744-750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toure-Balde A, Sarthou J L, Roussilhon C. Acute Plasmodium falciparum infection is associated with increased percentages of apoptotic cells. Immunol Lett. 1995;46:59–62. doi: 10.1016/0165-2478(95)00017-y. [DOI] [PubMed] [Google Scholar]
- 26.Troye-Blomberg M, Sjöholm P E, Perlmann H, Patarroyo M E, Perlmann P. Regulation of the immune response in Plasmodium falciparum malaria. I. Non-specific proliferative responses in vitro and characterization of lymphocytes. Clin Exp Immunol. 1983;53:335–344. [PMC free article] [PubMed] [Google Scholar]
- 27.Valentine F T, Paolino A, Saito A, Holzman R S. Lymphocyte-proliferative responses to HIV antigens as a potential measure of immunological reconstitution in HIV disease. AIDS Res Hum Retroviruses. 1998;14(Suppl. 2):S161–S166. [PubMed] [Google Scholar]
- 28.Warrell D A. Clinical features of malaria. In: Gills H M, Warrell D A, editors. Bruce-Chwatt's essential malariology. 3rd ed. London, England: Edward Arnold; 1993. pp. 35–49. [Google Scholar]
- 29.Wheatley B P. Malaria as a possible selective factor in the speciation of macaques. J Mamm. 1980;61:307–311. [PubMed] [Google Scholar]