Abstract
Fas-mediated gastric mucosal apoptosis is gaining attention as a cause of tissue damage due to Helicobacter pylori infection. We explored the effects of H. pylori directly, and the effects of the inflammatory environment established subsequent to H. pylori infection, on Fas-mediated apoptosis in a nontransformed gastric mucosal cell line (RGM-1). Exposure to H. pylori-activated peripheral blood mononuclear cells (PBMCs), but not H. pylori itself, induced Fas antigen (Fas Ag) expression, indicating a Fas-regulatory role for inflammatory cytokines in this system. Of various inflammatory cytokines tested, only interleukin 1β and tumor necrosis factor alpha induced Fas Ag expression, and removal of either of these from the conditioned medium abrogated the response. When exposed to Fas ligand, RGM-1 cells treated with PBMC-conditioned medium underwent massive and rapid cell death, interestingly, with a minimal effect on total cell numbers early on. Cell cycle analysis revealed a substantial increase in S phase cells among cells exposed to Fas ligand, suggesting an increase in their proliferative response. Taken together, these data indicate that the immune environment secondary to H. pylori infection plays a critical role in priming gastric mucosal cells to undergo apoptosis or to proliferate based upon their Fas Ag status.
Helicobacter pylori, first isolated from gastric biopsies in 1982 by Marshall and Warren (19), has become well recognized as the major etiologic factor in ulcer disease, chronic atrophic gastritis (19), and gastric lymphoma and carcinoma (24). The bacterium attaches, colonizes the gastric mucosa, and incites both cellular and humoral immune responses (5). Histological examination of infected tissue reveals acute and chronic inflammatory cells throughout the mucosa, with the spiral bacterium in the overlying mucus layer. The organism is not invasive, although rarely it can be found deep in crypts.
H. pylori infection is associated with elevated levels of both mucosal apoptosis (12, 15, 21, 31) and proliferation (3, 8, 15). The initiation and the regulation of the pathways that promote these paradoxical cellular responses are still unclear. Using H. pylori-infected human biopsy specimens, we previously demonstrated concomitant Fas antigen (Fas Ag) expression and gastric mucosal apoptosis, suggesting a role for Fas signaling in H. pylori-associated apoptosis (12). Fas Ag is a transmembrane receptor, which when bound specifically to its ligand (Fas L) trimerizes and initiates a cascade of events resulting in apoptosis in a variety of cell settings (2, 9, 10, 16). Interestingly, the pathway can be modulated at various points throughout, including regulation of the number of Fas receptors on the cell membrane as well as regulation of the availability of Fas L (9, 16). Fas Ag and Fas L expression have been shown to be regulated at the mRNA level in various cell types by inflammatory cytokines such as interleukin 1β (IL-1β), IL-2, tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ) (10, 12, 16, 22, 23). Although the Fas pathway has been well characterized in the immune system, less is known about the role this pathway plays in nonlymphoid tissue. Not surprisingly, H. pylori infection is also associated with increased mucosal inflammatory cytokines, including IL-1β, IL-6, IL-8, and TNF-α (6) and IFN-γ (6, 11). Production of IL-1β, IL-6, IL-8, and TNF-α has been demonstrated in H. pylori-stimulated peripheral blood mononuclear cell (PBMC) cultures, suggesting that immune cells may be the source of the mucosal cytokines found clinically (11, 18). Previously, we demonstrated the responsiveness of both gastric (KATO III) and small bowel (IEC-6) cell lines to exogenous cytokine-mediated regulation of Fas Ag mRNA (12). Furthermore, upon exposure to Fas L, these cells undergo apoptosis, confirming that the Fas pathway is intact and functional. Since cytokines have shown the capacity to induce Fas Ag expression in malignant gastric cell lines (12), we postulated that the cytokines generated during the immune response to H. pylori could prime nonmalignant gastric tissue for apoptosis by increasing mucosal expression of Fas Ag. In this scenario, Fas L, which is expressed on lymphocytes present in infected gastric tissue (12), could trigger Fas-mediated apoptosis. This study was undertaken to specifically test this prediction and to further characterize the immune regulation of Fas Ag expression in H. pylori infection. Because malignant gastric cell lines already possess a growth advantage as well as altered apoptotic sensitivity, we chose to use a nontransformed gastric cell line to address these issues. Using the RGM-1 cells as a tissue culture model, we examined individual selected components of the immune response to H. pylori for their ability to regulate gastric mucosal cell proliferation and apoptosis through Fas signaling.
MATERIALS AND METHODS
Bacterial culture and CFU determination.
H. pylori strain 43504 was obtained from the American Type Culture Collection and grown as recommended. After 4 days in culture, single colonies were picked, resuspended in 7 ml of tryptic soy broth (TSB) containing 5% fetal calf serum (FCS) in 15-ml conical tubes, and grown with mild agitation under microaerophilic conditions. Optical densities at 600 nm were measured, and CFU were determined by serial dilution plating on TSB–5% sheep blood plates. A standard curve was established based on triplicate optical density readings. Bacteria were diluted with TSB–5% FCS to a final concentration of 106 CFU/20 μl for use in cell culture.
Isolation and activation of Wistar rat PBMCs.
Ten-week-old male Wistar rats were anesthetized with an intraperitoneal injection of Ketamine (60 mg/kg) and Xylazine (7.5 mg/kg). Using a laparotomy approach, 10 ml of blood was obtained by direct aortic puncture. PBMCs were harvested using OptiPrep (Nycomed Pharma, Oslo, Norway) according to the manufacturer's protocol. Cells were washed twice in phosphate-buffered saline, resuspended in RPMI medium (Gibco BRL, Rockville, Md.)–20% FCS, and counted using a Royco automated cell counter, and 1 million cells per 100-mm-diameter dish were plated and incubated at 37°C in 5% CO2. After 4 h, 100 μl of H. pylori bacterial culture (final concentration of 106 CFU/ml as outlined above) or 100 μl of sterile bacterial medium was added to 5 ml of culture medium, and cell culture was continued for another 15 h. Medium (conditioned medium from dishes containing PBMCs and H. pylori or PBMC control medium from dishes cultured without H. pylori) was collected, pooled, filter sterilized, and used in tissue culture experiments.
Tissue culture.
RGM-1 cells, a nontransformed rat gastric cell line, were obtained from the RIKEN cell bank, Tsukuba Science City, Japan, and grown in 100-mm-diameter dishes to 70% confluence in Dulbecco's modified Eagle medium-Ham's F12 nutrient medium (DME/F12) (Gibco BRL) containing 20% FCS in 5% CO2 at 37°C. Cell monolayers were exposed to the following experimental conditions (5-ml total volume).
(i) Controls.
Controls included RPMI containing 20% FCS, DME/F12 containing 20% FCS, DME/F12 containing 20% FCS and 100 μl of H. pylori growth medium, and PBMC control medium (as described above).
(ii) Bacteria.
Monolayers were exposed to 106 CFU of H. pylori (in 20 μl of TSB containing 5% FCS) per ml of culture medium for 2, 4, 6, 8, 12, 24, 48, 72, or 96 h.
(iii) PBMC-conditioned medium.
PBMC-conditioned medium was as described above and was used undiluted or diluted to 50%, 25%, and 10% (by volume) in RPMI containing 20% FCS. Monolayers were exposed for 2, 4, 6, 8, or 24 h.
(iv) Cytokines.
Monolayers were exposed to recombinant mouse IL-1β (1 ng/ml), recombinant rat IL-2 (20 U/ml), recombinant human IL-8 (50 ng/ml), recombinant human TNF-α (300 U/ml), or recombinant rat IFN-γ (300 U/ml) (Genzyme, Cambridge, Mass.) for 2, 4, 8, 16, and 24 h. All cytokines were certified by the manufacturer to be active in the rat system.
(v) Neutralizing antibody.
Activated PBMC medium (25% by volume) was preincubated for 1 h with 24 μg of normal goat immunoglobulin G (IgG) per ml (control) or with anti-IL-1β (2 or 4 μg/ml) or anti-TNF-α (12 or 24 μg/ml) neutralizing antibodies (R&D Systems, Minneapolis, Minn.) and applied to cell monolayers for 2 h. Dilutions and exposure times were derived from prior experimental data from this lab.
ELISA analysis of IL-1β and TNF-α concentrations.
Rat TNF-α and IL-1β enzyme-linked immunosorbent assay (ELISA) detection kits (Cytoscreen; Biosource International, Camarillo, Calif.) were used according to the manufacturer's protocol. Cytokine concentrations in the undiluted conditioned medium; 50%, 25%, and 10% diluted conditioned medium; and control medium were determined and plotted against a standard curve. All samples were assayed a minimum of twice each.
Fas L treatment: measurement of apoptosis and proliferation.
RGM-1 cells were plated in 60-mm-diameter dishes to achieve 20% confluence, washed after 4 h, and refer with 1 ml of one of the following: DME/F12 containing 20% FCS (control), the control supplemented with 100 or 500 ng of Fas L (Alexis Biochemicals, San Diego, Calif.) per ml, 25% conditioned medium, or 25% conditioned medium plus 100 or 500 ng of Fas L per ml. Total cell counts were taken using the Royco cell counter. The trypan blue exclusion assay (Sigma, St. Louis, Mo.) was used as per the manufacturer's protocol to determine cell viability. Acridine orange staining (7) was performed to assess apoptosis. The cell cycle profile was determined by standard fluorescence-activated cell sorter (FACS) analysis. Briefly, cells were fixed in ice-cold 70% ethanol, treated with RNase and propidium iodide, processed (FACScan flow cytometer; Becton Dickinson, San Jose, Calif.), and analyzed using Modfit software (Becton Dickinson). Annexin staining and analysis were performed according to the manufacturer's protocol (annexin V-fluorescein isothiocyanate apoptosis detection kit; Pharmingen, San Diego, Calif.) with a FACScan flow cytometer and CellQuest software (Becton Dickinson). For each experimental time point, assays were done in quadruplicate. Cells were harvested after 2, 4, 10, 16, 20, 24, and 36 h of Fas L treatment.
[3H]thymidine incorporation studies were performed as follows. RGM-1 cells (10,000 cells per well in a 96-well plate) were grown with control medium alone or control medium plus 100 ng of Fas L per ml for 4, 12, or 30 h. Cells were harvested using a Brandel 96-well cell harvester, and incorporation was measured using a Beckman scintillation counter.
RT-PCR analysis.
Total RNA was prepared from cultured cells by using TRIzol reagent (Gibco BRL) according to the manufacturer's protocol. Reverse transcription (RT) was performed using 1.5 μg of total RNA and an oligo(dT) primer as described the SuperScript preamplification system for first-strand cDNA (Gibco BRL). Samples were treated with Escherichia coli RNase H and purified through Quick Spin columns (Boehringer Mannheim Corporation, Indianapolis, Ind.). PCR was performed using 20 ng of cDNA according to standard protocols. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used to standardize the loading quantity. All reactions were run in triplicate at two cycle intervals to assess the signal within a linear range. Each experiment was repeated a minimum of three times. Primer sequences, annealing temperatures, cycles, and product sizes were as follows: for GAPDH, TCT TCA CCA CCA TGG AGA A (sense) and ACT GTG GTC ATG AGT CCT T (antisense) primers; 52°C; 17, 19, and 21 cycles; 231-bp product; for Fas Ag, ATG CTG TGG ATC ATG GCT GTC (sense) and ATC TTG GGG GCT GTT GTG C (antisense) primers; 64°C; 23, 25, and 27 cycles; 773-bp product; and for Fas L, (i) ATG CAG CAG CCC GTG AAT TAC (sense) and CCA TAT CTG GCC AGT AGT GC (antisense) primers; 56°C; 25, 27, and 29 cycles; 237-bp product; or (ii) CCA ACA GGT CAG CTA CCC TTC ATT T (sense) and TCC CAC TCT TTC CTA CGA TCC AAA G (antisense) primers; 55°C; 27, 29, and 31 cycles; 184-bp product. PCR products were resolved on a 2.0% agarose gel precast with Sybr-Green fluorescent stain (FMC BioProducts, Rockland, Maine) and observed using a fluorescence scanner (Molecular Dynamics, Sunnyvale, Calif.). Expression levels were quantitated using Molecular Dynamics ImageQuant and are reported normalized to GAPDH levels. All oligonucleotide primers were prepared in the Molecular Resource Facility, UMDNJ, NJMS.
Western blotting.
The protein concentration was measured with the Bio-Rad protein assay. After dilution to 1× with 0.006% bromophenol blue, proteins (50 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred (Enprotech semidry blotting systems; The WEP Company, Seattle, Wash.) onto a Polyscreen polyvinylidene difluoride transfer membrane (NEN Life Science Products, Boston, Mass.). The membrane was blocked with TBST (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.05% Tween 20) containing 5% nonfat powdered milk for 2 h at room temperature. The blot was incubated for 1 h with polyclonal anti-Fas antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.) diluted to 1:2,500, or with monoclonal anti-Fas L antibody (clone G247-4; Pharmingen) diluted to 1:800, in TBST containing 1% bovine serum albumin for 1 h at room temperature. Unbound antibody was removed by washing the membrane three times for 10 min each with TBST. Horseradish peroxidase-conjugated anti-rabbit IgG (Amersham Life Science, Arlington Heights, Ill.) or horseradish peroxidase-conjugated anti-mouse IgG (Zymed, San Francisco, Calif.) in TBST (1:5,000 or 1:10,000 dilution, respectively) was added to the blot and incubated for 45 min at room temperature. After the membrane was washed three times with TBST for 10 min each, reactive proteins were visualized with an enhanced chemiluminescence detection kit (NEN Life Science Products).
RESULTS
In order to determine the regulation of H. pylori-associated gastric mucosal Fas Ag expression, we first examined individual components of the infected mucosal milieu. Under routine culture conditions (control medium), RGM-1 cells express low, but consistently detectable, levels of Fas Ag mRNA as determined by RT-PCR. Addition of 100 μl of TBS medium alone to the normal RGM-1 culture did not alter this (Fig. 1A, panel 1). H. pylori applied to RGM-1 cells also did not change the level of Fas Ag expression at 2, 4, 6, 8, 12, 24, 48, 72, or 96 h postexposure (the 6-h point is shown in Fig. 1A, panel 2), suggesting that neither direct bacterial contact nor a secreted bacterial factor directly induces Fas Ag mRNA expression in the RGM-1 cells. However, the cell-free conditioned medium from PBMCs cocultured with H. pylori for 15 h markedly up-regulated Fas Ag expression by 2 h (Fig. 1A, panel 3), with declining levels at 4 h (Fig. 1A, panel 4) and a return to basal levels at 24 h (data not shown), suggesting that a factor or factors secreted by H. pylori-activated PBMCs, and not direct cell contact, were responsible for the up-regulation of Fas Ag in this cell line. Because H. pylori has been shown to modulate expression of a variety of cytokines in PBMCs in vitro and in H. pylori-infected tissue in vivo, we examined selected individual components (IL-1β, IL-2, IL-8, TNF-α, and IFN-γ) of the inflammatory response for their ability to up-regulate Fas Ag mRNA expression in our system. Addition of IL-1β or TNF-α resulted in a substantial increase in Fas Ag expression at 2 h (Fig. 1B, panels 2 and 5, respectively), which paralleled the increase seen with conditioned medium (Fig. 1B, panel 1). Addition of IL-2 (Fig. 1B, panel 3), IL-8 (Fig. 1B, panel 4), or IFN-γ (Fig. 1B, panel 6) alone at levels shown to induce Fas Ag expression in other systems (10, 22, 23) did not result in a significant change in Fas Ag expression in RGM-1 cells at 2 h (Fig. 1B) or at 4, 8, 16, or 24 h (data not shown).
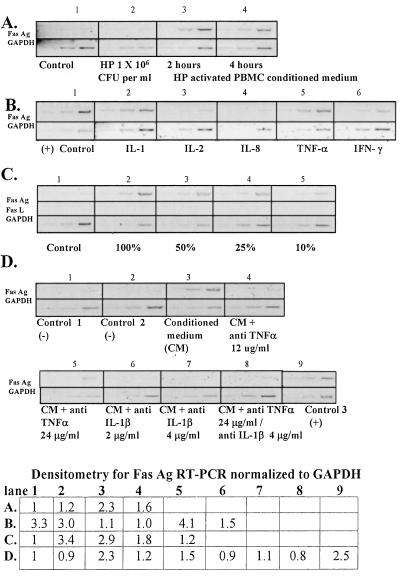
FIG. 1.
RT-PCR analysis of Fas Ag and Fas L mRNA expression in RGM-1 cells. RT-PCR data for GAPDH are shown for each experimental condition and are used to standardize the lanes. (A) Levels of Fas Ag expression in RGM-1 cells grown in control medium plus 100 μl of TSB containing 5% FCS for 4 h (panel 1), 106 H. pylori (HP) bacteria/ml for 2 h (panel 2), or filter-sterilized medium from PBMCs cocultured with 106 H. pylori bacteria/ml (conditioned medium) for 2 or 4 h (panels 3 and 4, respectively). (B) Fas Ag mRNA induction in response to conditioned medium (panel 1) or treatment with IL-1β (1 ng/ml) (panel 2), IL-2 (20 U/ml) (panel 3), IL-8 (50 ng/ml) (panel 4), TNF-α (300 U/ml) (panel 5), or IFN-γ (300 U/ml) (panel 6) in control medium for 2 h. (C) Expression patterns of Fas Ag and Fas L mRNAs in control medium (RPMI containing 20% FCS) (panel 1) or with decreasing concentrations of conditioned medium for 2 h. (D) Effects of neutralizing antibodies on Fas Ag expression in RGM-1 cells. Levels of Fas Ag in cells grown in control medium 1 (DME/F12 containing 20% FCS) (panel 1), control medium 2 (RPMI containing 20% FCS) (panel 2), conditioned medium (panel 3), and conditioned medium preincubated with one or two times the ND50 of anti-TNF-α (panels 4 and 5, respectively) or anti-IL-1β (panels 6 and 7, respectively), with two times the ND50 of both antibodies together (panel 8), or with a goat IgG control (panel 9) for 2 h are shown. (Bottom panel) Densitometric values for panels A, B, C, and D. All values are corrected for background signal and standardized to those for GAPDH. Values are reported in comparison to the normal control [control or (−)control] for samples run in the same RT-PCR and visualized on the same gel. The normal control is not shown for panel B.
Next, we determined the effect of conditioned medium on Fas Ag up-regulation over a range of dilutions to verify whether the cytokine levels reached in our experimental culture setup were similar to those found in vivo (5, 11, 18). Serial dilutions of conditioned medium were tested for levels of IL-1β and TNF-α by using ELISA and for their ability to induce Fas Ag expression in RGM-1 cells. Medium from control conditions had TNF-α and IL-1β levels below the detectable limits of the assays used (TNF-α levels of <4 pg/ml and IL-1β levels of <3 pg/ml as per the manufacturer's specifications) and also failed to alter Fas Ag mRNA levels in the RGM-1 cells (Fig. 1A, C, and D, panels 1, and Fig. 1D, panel 2), even after 3 days of exposure (data not shown). Undiluted conditioned medium contained TNF-α at ≥1,000 pg/ml and IL-1β at 16.5 ± 2.0 pg/ml, as determined by rat-specific ELISA, and induced Fas Ag expression (Fig. 1C, panel 2). Conditioned medium diluted to 50% (TNF-α, 500 ± 12.8 pg/ml; IL-1β, 7.8 ± 0.8 pg/ml) and 25% (TNF-α, 360 ± 7.9 pg/ml; IL-1β, 5.0 ± 2.4 pg/ml) of the original concentration showed decreasing ability to increase Fas Ag mRNA expression (Fig. 1C, panels 3 and 4, respectively), although it did so at substantially above basal levels. At the 10% dilution (TNF-α, 118 pg/ml; IL-1β, undetectable) the level of Fas Ag expression was only marginally distinguishable (1.2-fold) from baseline levels (Fig. 1C, panel 5). Based on these results, we determined that the 25% dilution of conditioned medium was the lowest dilution capable of inducing a detectable response. None of the experimental or control conditions induced Fas L expression (Fig. 1C).
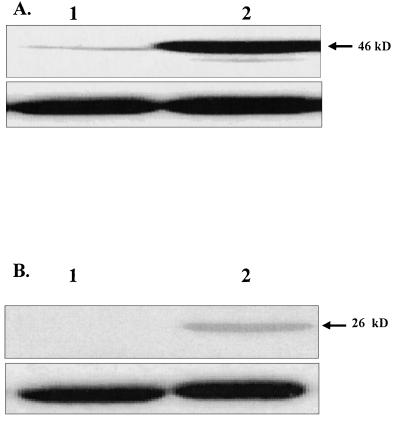
Further, to determine if IL-1β and TNF-α act alone or in combination to induce Fas Ag expression, we utilized neutralizing antibodies to effectively decrease the functional cytokine level to below that which was determined to induce Fas Ag expression in our system. The 50% neutralizing dose (ND50) was determined for both anti-IL-1β and anti-TNF-α, based on ELISA results. At one and two times the ND50, each antibody abrogated the Fas Ag response (Fig. 1D, panels 4, 5, 6, and 7) induced by conditioned medium (Fig. 1D, panel 3) to that of control levels (Fig. 1D, panels 1 and 2), as did the combination of the two (Fig. 1D, panel 8). Preincubation with the control IgG (goat IgG) had no effect on the conditioned medium-induced up-regulation of Fas Ag (Fig. 1D, panel 9). This suggests that both cytokines may act together in vivo to induce a mucosal Fas Ag response. The Fas apoptotic pathway is regulated in the immune system primarily through the regulation of Fas Ag and L mRNA levels (16). However, since little is known about this pathway in nonimmune tissue such as gastric mucosal cells, we verified whether increased Fas receptor protein levels were indeed produced and that the pathway was functional with the addition of ligand. To confirm that the conditioned medium-induced up-regulation of Fas Ag mRNA is not limited by transcriptional regulation, we tested the protein levels by using standard Western immunoblotting techniques. Even the lowest dilution (25% conditioned medium) with a detectable change in mRNA expression brought about a marked increase in the Fas protein expression (Fig. 2A), with a single band detected at 46 kDa, which represents the membrane-bound form of Fas receptor. In addition to surface Fas receptors, cellular responses resulting from activation-induced Fas signaling require the presence of Fas L in the system. In the absence of detectable Fas L expression in RGM-1 cells (Fig. 1D), we tested whether activated PBMCs themselves will produce Fas L in this system. Using standard Western blot analysis, we confirmed that H. pylori-activated (but not unactivated) PBMCs express surface Fas L (Fig. 2B).
FIG. 2.
Western blot analysis of Fas L and Fas Ag protein expression. (A) Fifty micrograms of protein from total cell extracts of RGM-1 cells grown in control medium (lane 1) or 25% conditioned medium (lane 2) for 15 h was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane, and assayed for Fas Ag by using anti-Fas antibody, followed by enhanced chemiluminescence detection. (B) Detection of Fas L in freshly isolated PBMCs (lane 1) and H. pylori-activated (for 15 h) PBMCs (lane 2) using anti-Fas L antibody, followed by enhanced chemiluminescence detection. Detection of β-actin was used to verify equal concentrations of protein and is shown for each blot.
Next, to test the functionality of the Fas Ag up-regulation, recombinant ligand was added to the cell culture system. Fas L has been shown to induce cell death in a dose- and time-dependent fashion in a variety of cell types under various experimental conditions. We tested low and high doses of Fas L (values were chosen based on the manufacturer's recommendations) to test for the effect on RGM-1 cells expressing Fas Ag. Control cell populations showed normal monolayer morphology, without floating cells. (Fig. 3A, top panel). Addition of Fas L induced minimal but progressive changes, with a small fraction of adherent cells rounding up, followed by the appearance of increasing numbers of floating cells. These changes were both time and dose dependent. Addition of 100 ng of Fas L per ml resulted in minimal changes, with the appearance of <2% floating cells after 16 h. When the Fas L concentration was increased to 500 ng/ml, the number of floating cells approached 12% at 16 h. We believe that addition of ligand to the control culture resulted in a small but measurable increase in cell death because of activation through basal levels of Fas receptor. Monolayers exposed to conditioned medium alone also showed morphologic changes comparable to those of the control cells treated with Fas L. Although we did not measure this, we speculate that the conditioned medium contains low levels of soluble Fas L (shown to be released from PBMCs in response to activation [30]) and would be expected to induce the observed changes. In contrast, Fas L added to monolayers previously exposed to PBMC-conditioned medium, and expressing the most Fas Ag, showed dramatic changes (Fig. 3A, bottom panel). Rounding up of adherent cells, along with detachment and blebbing, occurred as early as 3 h after the addition of ligand, with maximal changes noted at 20 h (500 ng/ml) and 36 h (100 ng/ml), respectively. Upon addition of 500 ng of ligand per ml, fewer than 10% of the monolayer cells remained adherent at 20 h. Total cell counts with the percentage of viable cells determined by trypan blue exclusion assay confirmed the extent of cell death in cells exposed to 500 ng of Fas L per ml (Fig. 3B).
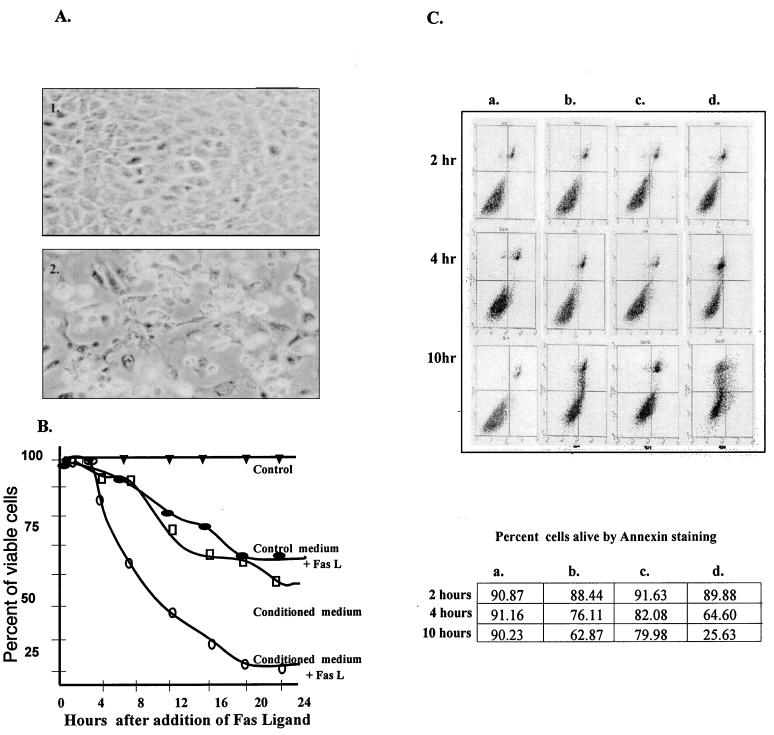
FIG. 3.
Induction of cell death by exogenous Fas L. (A) Morphology of RGM-1 cells grown in control medium (panel 1) or conditioned medium (panel 2) containing Fas L (500 ng/ml) for 16 h and photographed using a phase-contrast microscope. (B) Quantitation of loss of cell viability. Total cell counts were obtained by the trypan blue exclusion technique at 4-h intervals for a period of 24 h. Total (floating and adherent) cells were pooled, washed, and resuspended in medium containing trypan blue. Viable (colorless) and dead (blue) cells were counted using a hemocytometer. A minimum of 500 cells per sample were counted. Experiments were done in quadruplicate. (C) Quantitation of apoptosis by using the annexin staining technique. RGM-1 cells were grown in control medium (a), control medium plus Fas L (500 ng/ml) (b), conditioned medium (c), or conditioned medium plus Fas L (500 ng/ml) (d) for 2, 4, or 10 h, and the percentages of nonapoptotic, nonnecrotic cells (i.e., live cells) were calculated using 10,000 cells for each data point.
To verify whether the observed loss of viability was due to apoptosis, we employed two assays that detect different but definite stages of apoptosis. Acridine orange nuclear staining, which allows morphologic determination of apoptosis, was performed on aliquots of cultures treated with 500 ng of ligand per ml. Clumping, margination, and fragmentation of chromatin, as determined by fluorescence microscopy, were considered evidence for apoptotic cell death. Control cultures had <0.2% apoptotic cells detected at 4, 16, or 20 h. With the addition of ligand control cultures had 2.9% (4 h), 7.2% (16 h), and 10.5% (20 h) apoptotic cells. Cells preincubated with conditioned medium prior to the addition of ligand showed 16.8% (4 h), 24.6% (16 h), and 42% (20 h) apoptotic cells. To verify the disparity between the quantitation of loss of viability and acridine orange staining (which distinguishes later stages of apoptosis), we used annexin staining, a technique widely used to detect both early and late changes of apoptosis. Early in apoptosis, phosphatidylserine is translocated from the inner to the outer leaf of the cell membrane. Annexin V shows high-affinity binding to phosphatidylserine in the presence of Ca2+ and is useful for detecting early stages of apoptosis, prior to nuclear condensation or morphologic changes. Quantitation of apoptosis by using annexin staining revealed a close correlation with the trypan blue viability results (Fig. 3c). A progressive increase in the rate of apoptosis was observed in RGM-1 cells treated with Fas L (Fig. 3C, column b) or conditioned medium with exogenous Fas L (Fig. 3C, column b), while virtually no increase was observed in control cells (Fig. 3C, column a). Comparison of low-abundance Fas Ag (control medium [Fig. 3C, column b]) to high-abundance Fas Ag (conditioned medium [Fig. 3C, column d]) showed increased susceptibility to Fas L-induced apoptosis related to the quantity of surface receptor. A difference in the baseline cell viabilities determined with trypan blue and annexin was probably because of membrane damage to the cells during harvesting, which may have partially permeabilized a percentage of cells, resulting in overestimation of cell death with annexin staining.
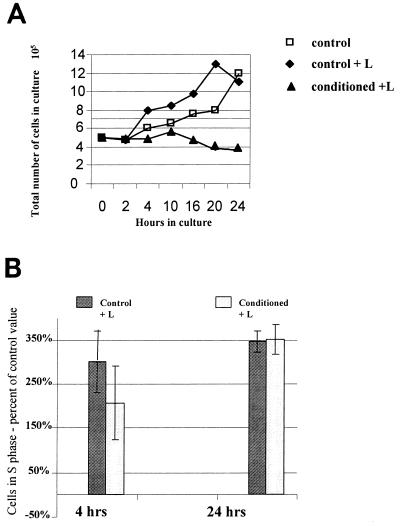
A comparison of growth properties of cells under different treatment conditions led to an interesting observation. Cells treated with Fas L consistently showed a proliferative advantage over cells grown in control medium alone, at all time points. Even in cells treated with both conditioned medium and Fas L, where more than 90% cell death was observed by multiple assays, the total cell number as determined after excluding fragmented cells was maintained with minimal effect (Fig. 4A). Taking into account the presence of more than 40% acridine-orange positive apoptotic cells at 20 h after Fas L treatment, most of which are excluded by Royco cell counts, the maintenance of a stable cell number is not plausible without a compensatory increase in cell proliferation. The growth advantage of cells exposed to control medium plus ligand, compared to those exposed to control medium alone, was modest but consistent and reproducible (Fig. 4A). [3H]thymidine studies were performed to confirm the observed increase in proliferation as a result of ligand exposure. RGM-1 cells grown in control medium showed a marked increase (three times the control value) in [3H]thymidine incorporation at 30 h, verifying the findings from standard cell counts (data not shown). These observations thus suggest a simultaneous activation of cell death and proliferation pathways under the conditions tested. To determine whether the treatment conditions that induced Fas-mediated apoptosis also enhanced the cycling of the cells, we examined the cell cycle status of RGM-1 cells treated with control medium alone, control medium with the addition of Fas L, and conditioned medium with Fas L (Fig. 4B) by using FACS techniques. The addition of ligand resulted in a substantial increase (compared to control cells without ligand) in the percentage of cells proliferating, represented by an increase in cells in the S phase of the cell cycle. A similar increase was seen in cells exposed to conditioned medium prior to addition of ligand (Fig. 4B); however, this was also accompanied by massive concomitant cell death. This also suggests that the quantity of Fas Ag protein expression plays an important role in the commitment of mucosal cells towards choosing between a proliferative response or apoptotic cell death.
FIG. 4.
Growth characteristics of RGM-1 cells exposed to Fas L. (A) Growth properties of RGM-1 cells. Cells (5 × 105) were seeded in 60-mm-diameter plates, allowed to adhere for 4 h, washed, and refed with control medium, control medium containing 100 ng of Fas L per ml, or conditioned medium containing 100 ng of Fas L per ml. Total cell counts were performed as described in Materials and Methods. The result for each time point is the average for six plates. (B) Effect of 4 and 24 h of Fas L (100 ng/ml) exposure on proliferation (S phase) in RGM-1 cells exposed to control medium or conditioned medium, expressed as a percentage of the S phase value in control cells. Results are the averages from two experiments. Error bars represent standard deviations from the mean. The change in proliferation between control levels and either experimental group was significant at a P value of <0.001.
DISCUSSION
Apoptotic cell death has been increasingly recognized as a factor in H. pylori-related mucosal injury (12, 15, 21, 28, 31), with several lines of evidence supporting the involvement of the Fas Ag-Fas L pathway (12, 14, 25). Using biopsy specimens from H. pylori-infected gastric and duodenal ulcers, we have shown that Fas Ag mRNA is up-regulated concomitant with elevated mucosal apoptosis (12). We also demonstrated sensitivity of gastric (KATO III) and small bowel (IEC-6) cell lines to cytokine-mediated up-regulation of Fas Ag mRNA and subsequent increased susceptibility to ligand-induced apoptosis, suggesting a role for the Fas pathway in the pathogenesis of H. pylori-related ulcer disease. On the other hand, Jones et al. (14) have recently shown that H. pylori is capable of directly inducing death in gastric cell culture in the absence of immune cells or their products. KATO III cells underwent necrotic cell death following prolonged culture with the bacterium, whereas AGS cells, another malignant gastric cell line, underwent apoptosis, in association with increased Fas Ag receptor expression (14). However, the factor(s) responsible for regulation of the Fas pathway and its potential role in cell death in gastric epithelial cells is largely unknown. Addressing these problems with malignant cell lines is problematic. Malignant cells often have dysregulated apoptotic, proliferative, and intracellular signaling pathways, which makes them less-than-ideal model systems to study in vivo disease progression. In order to circumvent these problems, we chose to study RGM-1 cells, a nontransformed rat gastric cell line which represents normal gastric epithelium.
In contrast to the case for AGS cells, the presence of H. pylori alone did not regulate Fas expression in RGM-1 cells; therefore, endotoxin or other secreted or surface components of the bacteria are unlikely candidates for the Fas-activating factor in this system. On the other hand, conditioned medium from H. pylori-activated PBMCs markedly increased Fas Ag expression in RGM-1 cells, prompting us to explore the possible involvement of inflammatory cytokines as an activating factor(s). The cytokines we chose to examine were those shown to be increased in clinical H. pylori infection and known to up-regulate Fas expression in other systems. H. pylori-activated PBMCs behave like “generically” activated PBMCs and produce IL-1β, IL-2, IL-6, IL-8, TNF-α, IFN-γ, and surface Fas L in addition to other immune mediators (5, 6, 11). IL-1β, IL-2, TNF-α, and IFN-γ are known inducers of Fas Ag expression in a variety of cell types and were previously shown by us to up-regulate Fas Ag expression in the KATO III and IEC-6 cell lines (12). In the RGM-1 cells, however, only the addition of IL-1β or TNF-α increased Fas Ag expression levels, which were comparable to levels induced with PBMC-conditioned medium, suggesting that these cytokines, alone or in combination, may act to regulate Fas Ag expression in H. pylori disease. Of particular interest also for us to investigate was IL-8, the cytokine which has been shown to most closely correlate with H. pylori disease severity in vivo (26, 27). Addition of IL-8 showed no effect on Fas Ag expression in the present experimental setup. Of note is that both IL-1β and TNF-α regulate IL-8 production through the JNK and mitogen-activated protein kinase pathways as well as through NF-κB (20), suggesting that IL-8 may be a marker for the presence of these cytokines rather than causing mucosal injury itself. H. pylori has also been shown to activate NF-κB directly (17). We also confirmed by ELISA that the levels of IL-1β and TNF-α present in conditioned medium represented levels that could be found in vivo (32). Neutralizing antibody experiments suggested that TNF-α and IL-1β are the predominant activators, because neutralizing either of them from the medium decreased Fas Ag mRNA expression to basal levels.
Clinical H. pylori infection is associated with both apoptosis (ulcer disease) and increased proliferation (predisposition to malignancy). H. pylori infection activates PBMCs that contribute to a local cytokine environment capable of affecting regulation of the Fas signaling pathway. Activated lymphocytes may supply the necessary Fas L required to activate the pathway and lead to mucosal cell apoptosis. Based on these data, the Fas pathway may be responsible for (or at least contribute to) the apoptosis that accompanies H. pylori ulcer disease. Interestingly, proliferation and apoptosis coexist in H. pylori infection. It has been suggested that the proliferation seen with mucosal H. pylori infection may be a result of apoptotic tissue damage and comprises a normal healing response, while another school of thought suggests that the bacteria or bacterial products directly enhance cell growth (3, 8, 15). We present evidence in this communication to suggest, for the first time, that Fas signaling may be involved in simultaneous activation of dysregulated cell death and proliferation of RGM-1 cells. Much work, however, is needed to define the pathways that lead to these paradoxical cellular responses in H. pylori-infected gastric mucosal cells. In RGM-1 cells, H. pylori did not directly alter proliferation (J. Houghton, unpublished data). However, addition of Fas L to RGM-1 cells grown in control or PBMC-conditioned medium led to substantial increases in proliferative capacity, as demonstrated by a marked increase in the percentage of cells in the S phase of the cell cycle. Cells grown in control medium expressed this proliferative advantage as an increase in total cell numbers. Cells grown in conditioned medium (which also promoted Fas Ag induction), on the other hand, revealed both an increase in proliferation and a marked increase in cell death. These paradoxical cell responses as a result of Fas activation were confirmed by cell cycle analysis and apoptosis assays and were instrumental in maintaining the total cell number without major fluctuations over a period of time. Ligand binding to the Fas Ag receptor may initiate dual signaling programs, and the decision to pursue apoptosis or proliferation may depend upon the cell type and the magnitude of receptor aggregation (1, 9). In addition, reverse signaling through Fas L has recently been shown to induce proliferation (29). Fas L was not detected by either RT-PCR or Western blot analysis in RGM-1 cells exposed to PBMC-conditioned medium; therefore, autocrine Fas L signaling is unlikely to cause proliferation in RGM-1 cells.
H. pylori infection is almost always associated with inflammation; however, ulcer disease and gastric carcinoma occur only in a subset of patients. Within the subset of patients with disease, the natural history is one of recurrences interspersed with disease-free periods. Interindividual cytokine variations are felt to be related to polymorphisms within the cytokine genes themselves and have been demonstrated for TNF-α and IL-1β, as well as other cytokines. In addition, there appear to be genetic differences in the Fas Ag gene (4, 13) which may introduce another level of differential regulation, further complicating the issue. Little is known specifically about these genetic variations within the cytokine genes or the Fas promoter in populations at risk for different aspects of H. pylori disease. However, it is tempting to speculate that genetically determined differential responses of the Fas promoter to H. pylori infection may dictate some of the differences in disease susceptibility and presentations. In addition to or in combination with these differences, variations in the cytokine response of the individual (secondary to concomitant disease, smoking, stress, etc.) may affect the regulation of Fas Ag expression. Understanding the complex interplay of H. pylori and the immune system may help target patients at greater risk for complications of disease and enable physicians to focus intervention and therapy more specifically with these patients.
ACKNOWLEDGMENTS
J.H. is supported by NIH Physician Scientist training grant CA 64173. R.M.K. is supported by a UMDNJ Foundation award.
Special thanks go to B. Barton for assistance with FACS analysis and annexin studies.
REFERENCES
- 1.Aggarwal B B, Singh S, LaPushin R, Totpal K. Fas antigen signals proliferation of normal human diploid fibroblasts and its mechanism is different from tumor necrosis factor receptor. FEBS Lett. 1995;364:5–8. doi: 10.1016/0014-5793(95)00339-b. [DOI] [PubMed] [Google Scholar]
- 2.Bennett M W, O'Connell J, O'Sullivan G C, Collins J K, Shanahan F. Fas-mediated apoptosis in autoimmune and Helicobacter pylori gastritis. Gastroenterology. 1998;114:A930. [Google Scholar]
- 3.Brenes F, Ruiz B, Correa P, Hunter F, Rhamarkrishman T, Fontham E, Shi T Y. Helicobacter pylori causes hyperproliferation of the gastric epithelium: pre and posteradication indices of proliferating cell nuclear antigen. Am J Gastroenterol. 1993;88:1870–1875. [PubMed] [Google Scholar]
- 4.Chan H, Bartos D P, Owen-Schaub L B. Activation-dependent transcriptional regulation of the human Fas promoter requires NF-κB p50-p65 recruitment. Mol Cell Biol. 1999;19:2098–2108. doi: 10.1128/mcb.19.3.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crabtree J E. Immune and inflammatory responses to Helicobacter pylori infection. Scand J Gastroenterol. 1996;215:3–10. [PubMed] [Google Scholar]
- 6.D'Elios M M, et al. T helper 1 effector cells specific for HP in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–7. [PubMed] [Google Scholar]
- 7.Dhanaraj S N, Marcus A M, Korah R M, Small M B. Characterization of c-myc-transformed rat fibroblasts resistant to apoptosis induced by growth factor deprivation. Exp Cell Res. 1996;224:52–62. doi: 10.1006/excr.1996.0110. [DOI] [PubMed] [Google Scholar]
- 8.Fan X G, Kelleher D, Fan X J, Xia H X, Keeling P W N. Helicobacter pylori increases proliferation of gastric epithelial cells. Gut. 1996;38:19–22. doi: 10.1136/gut.38.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freiberg R A, Spencer D M, Choate K A, Duh H J, Schreiber S L, Crabtree G R, Khavari P A. Fas signal transduction triggers either proliferation or apoptosis in human fibroblasts. J Invest Dermatol. 1997;108:215–219. doi: 10.1111/1523-1747.ep12334273. [DOI] [PubMed] [Google Scholar]
- 10.Giordano C, Stassi G, De Maria R, Todaro M, Richiusa P, Papoff G, Ruberti G, Bagnasco, Testi R, Gassuzzo A. Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto's thyroiditis. Science. 1997;275:960–963. doi: 10.1126/science.275.5302.960. [DOI] [PubMed] [Google Scholar]
- 11.Harris P R, Mobley H L, Perez-Perez G I, Blaser M J, Smith P D. Helicobacter pylori urease is a potent stimulus of mononuclear phagocyte activation and inflammatory cytokine production. Gastroenterology. 1996;111:419–425. doi: 10.1053/gast.1996.v111.pm8690207. [DOI] [PubMed] [Google Scholar]
- 12.Houghton J, Korah R M, Condon M R, Kim K H. Apoptosis in Helicobacter pylori-associated gastric and duodenal ulcer disease is mediated via the Fas antigen pathway. Digest Dis Sci. 1999;44:465–478. doi: 10.1023/a:1026628601284. [DOI] [PubMed] [Google Scholar]
- 13.Huang Q R, Morris D, Manolios N. Identification and characterisation of polymorphisms in the promoter region of the human APO-1/Fas (CD95) gene. Mol Immunol. 1997;34:577–582. doi: 10.1016/s0161-5890(97)00081-3. [DOI] [PubMed] [Google Scholar]
- 14.Jones N L, Day A S, Jennings H A, Sherman P M. Helicobacter pylori induces gastric epithelial cell apoptosis in association with increased Fas receptor expression. Infect Immun. 1999;67:4237–4242. doi: 10.1128/iai.67.8.4237-4242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones N L, Shannon P T, Cutz E, Yeger H, Sherman P M. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am J Pathol. 1997;151:1695–1703. [PMC free article] [PubMed] [Google Scholar]
- 16.Katsikis P D, Wunderlich E S, Smith C A, Herzenberg L A. Fas Ag stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J Exp Med. 1995;181:2029–2036. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keats S, Hitti Y S, Upton M, Kelly C P. Helicobacter pylori infection activates NF-kappa B in gastric epithelial cells. Gastroenterology. 1997;113:1099–1109. doi: 10.1053/gast.1997.v113.pm9322504. [DOI] [PubMed] [Google Scholar]
- 18.Maekawa K. Helicobacter pylori induces proinflammatory cytokines and HHC class II Ag in mouse gastric epithelial cells. J Lab Clin Med. 1997;130:442–449. doi: 10.1016/s0022-2143(97)90045-7. [DOI] [PubMed] [Google Scholar]
- 19.Marshall B J, Warren J R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;i:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto K, Hashimoto S, Gon Y, Nakayama T, Horie T. Proinflammatory cytokine-induced and chemical mediator-induced IL-8 expression in human bronchial epithelial cells through p38 mitogen-activated protein kinase-dependent pathway. J Allergy Clin Immunol. 1998;98:825–831. doi: 10.1016/S0091-6749(98)70311-2. [DOI] [PubMed] [Google Scholar]
- 21.Moss S F, Calam J, Agarwal B, Wang S, Holt P R. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oyaizu N, McCloskey T W, Than S, Pahwa S. Inhibition of CD4 crosslinking induced lymphocytes apoptosis by vesnarinone as a novel immunomodulating agent: vesnarinone inhibits Fas expression and apoptosis by blocking cytokine secretion. Blood. 1996;87:2361–2368. [PubMed] [Google Scholar]
- 23.Oyaizu N, McCloskey T W, Than S, Hu R, Kalyanaraman V S, Pahwa S. Crosslinking of CD4 molecules upregulates Fas antigen expression in lymphocytes by inducing interferon gamma and tumor necrosis factor-alpha secretion. Blood. 1994;84:2622–2631. [PubMed] [Google Scholar]
- 24.Parsonnet J, Hansen S, Rodrigues L, Gelb A B, Warnke R A, Jellum E, Orentreich N, Vogelman J H, Friedman G D. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 25.Rudi J, Kuck D, Strand S, von Herbay A, Mariani S M, Krammer P H, Krammer P H, Galle P R, Stemmel W. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric cell apoptosis. J Clin Investig. 1998;102:1506–1514. doi: 10.1172/JCI2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma S A, Tummuru M K, Blaser M J, Kerr L D. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J Immunol. 1998;160:2401–2407. [PubMed] [Google Scholar]
- 27.Sharma S A, Tummuru M K, Miller G, Blaser M J. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995;63:1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shirin H, Moss S F. Helicobacter pylori induced apoptosis. Gut. 1998;43:592–594. doi: 10.1136/gut.43.5.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki I, Fink P. Maximal proliferation of cytotoxic T lymphocytes requires reverse signaling through Fas ligand. J Exp Med. 1998;187:123–128. doi: 10.1084/jem.187.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human Fas ligand in activated lymphocytes. EMBO J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner S, Beil W, Westermann J, Logan R P H, Bock C T, Trautwein C, Bleck J S, Manns M P. Regulation of gastric epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology. 1997;113:1836–1847. doi: 10.1016/s0016-5085(97)70003-9. [DOI] [PubMed] [Google Scholar]
- 32.Yaqoob P, Newsholme E A, Calder P C. Comparison of cytokine production in cultures of whole human blood and purified mononuclear cells. Cytokine. 1998;8:600–605. doi: 10.1006/cyto.1998.0471. [DOI] [PubMed] [Google Scholar]