Abstract
Immunotherapy (IO) has altered the therapeutic landscape for multiple cancers. There are emerging data from retrospective studies on a subset of patients who do not benefit from IO, instead experiencing rapid progression with dramatic acceleration of disease trajectory, termed ‘hyperprogressive disease’ (HPD). The incidence of HPD ranges from 4% to 29% from the studies reported. Biological basis and mechanisms of HPD are currently being elucidated, with one theory involving the Fc region of antibodies. Another group has shown EGFR and MDM2/MDM4 amplifications in patients with HPD. This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease. Thus, prospective studies are urgently needed to confirm the underlying biology, predict patients who are susceptible to HPD, and determine the modality of therapy post progression.
Hyperprogressive Disease: A Provocative Phenomenon in the Era of Immunotherapy
The treatment landscape for patients with cancer continues to evolve at a rapid pace. The latest addition to the clinical artillery is the revamping of IO with the advent of immune checkpoint inhibitors (ICI) (see Glossary). As an increasing number of patients are treated with these agents, there are emerging data that a subset of patients do not benefit from IO, instead experiencing rapid progression with dramatic acceleration of disease trajectory, termed HPD) (Figure 1). One research group defined HPD as a tumor growth rate (TGR) that was at least twofold greater during ICI therapy than immediately before IO during traditional chemotherapy [1]. Others define HPD as a >50% increase in tumor burden with a <2-month ‘time to treatment failure’ (TTF) and doubling of pace progression [2]. Still other groups have defined HPD as disease progression of >50% at the time of the first evaluation from before treatment [3]. Although these definitions differ slightly, the underpinnings are all the same: expansive growth or change in the rate of tumor progression that is grossly different from baseline causing a detrimental effect on the patient. Several preclinical studies have hypothesized mechanisms, but a clearcut biological underpinning of this phenomenon remains elusive. Thus, prospective studies are needed to recognize this phenomenon, and to predict patients who are susceptible to HPD. Herein, we review the concept of HPD based on currently available evidence.
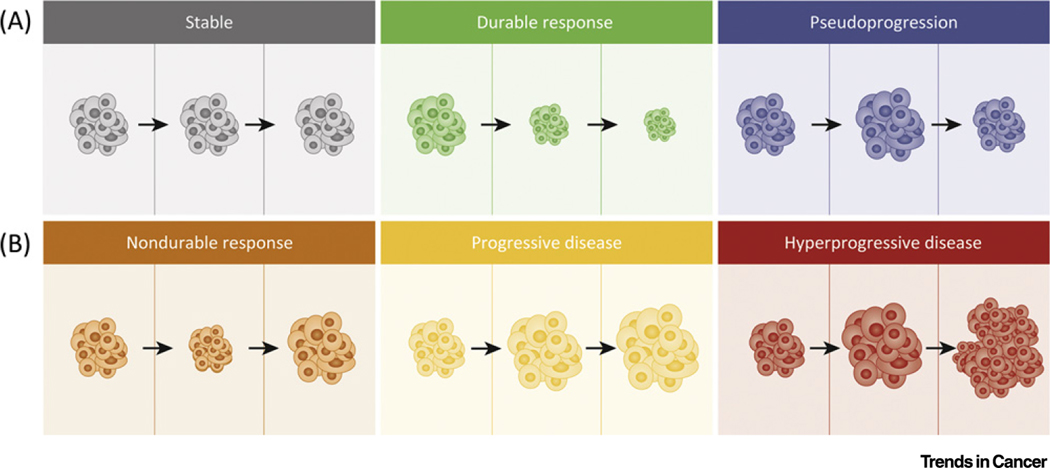
Figure 1. Potential Outcomes of Immunotherapy.
(A) Gray shading indicates stable disease, where changes in tumor are either minimal or nonexistent; green shading indicates a durable response where the tumor shrinks and is sustained over time; blue shading indicates pseudoprogression, where initial enlargement of the tumor is seen on imaging with subsequent shrinkage on subsequent scans. (B) Orange shading indicates a nondurable response, where initially shrinkage in the tumor is seen, regarded as a response; however, after time, the tumor begins to progress; yellow shading indicates progressive disease where the tumor enlarges on subsequent scans; and red shading shows hyperprogressive disease, where tumor growth becomes expansive on imaging.
Hyperprogressive Disease Series and Genomic Correlations
The correlation between HPD and ICI has been shown across multiple tumor types at multiple centers worldwide. Multiple studies have shown the impact HPD has on progression-free survival (PFS) and overall survival (OS) as well as change in rate of tumor progression. The clinical impact of HPD is well described and important for patient care.
In a retrospective study of 218 patients enrolled in Phase I clinical trials, the authors used TGR before ICI treatment and compared it with TGR while on ICI therapy to better understand the relationship between TGR, anatomical variables, and OS outcomes. This study defined HPD as a twofold or more increase in TGR from pre-ICI to on-ICI scans and progressive disease (PD) on the first evaluation by Response Evaluation Criteria In Solid Tumors (RECIST). In this cohort, 9% of patients were found to have HPD with a median increase in TGR of 20.7-fold (range, 2.0–141.3). Interestingly, the patients who were determined to have HPD had fewer new lesions at the first evaluation than their non-HPD counterparts (33% vs 84%, P=0.0019). This result should not be overinterpreted in the context of a retrospective study because: (i) TGR was calculated based on the target lesions only and patients who showed a rapid growth rate in new lesions were not evaluated for HPD; and (ii) the authors did not include 18 patients who had clinical progression before being evaluated. A significant difference was noted in patients aged over 65 years having more HPD (19% vs 5%, P=0.018) but the authors caution the effect of a limited sample size although the difference was significant. The association between HPD status and OS was also alarming when compared with the cohorts who were defined to have complete or partial responses (CR-PR), stable disease (SD), or PD. Inevitably patients with SD, PD, or HPD fared worse than their CR-PR counterparts, but those with HPD had a markedly higher likelihood of death [hazard ratio (HR) 25.94; 95% confidence limits (CI) 5.57–120.74; P=0.000033) [1]. These findings suggest not only that HPD is a serious adverse effect of ICI, but also that it confers a poorer outcome and is significantly detrimental to survival.
In 182 patients who were enrolled in early-phase clinical trials across a breadth of tumor types [head and neck squamous cell carcinoma (HNSCC), gynecological, lung, gastrointestinal (GI), genitourinary (GU), melanoma, sarcoma, endocrine, and breast], there was a subset of patients found to have HPD. This study also defined HPD as a twofold or more increase in TGR from pre-ICI to on-ICI scans and PD on the first evaluation by RECIST 1.1 criteria. Of these patients, 80% had received single-agent ICI. In this cohort of various tumor types, 7% of patients were identified as having HPD and it was more likely in females than in males (P=0.01). Having HPD was also significantly associated with a shorter PFS of 1.6 months versus 2.8 months (HR 3.7; 95% CI, 2.0–7.1; P<0.001) [4]. Thus, this is another example of HPD resulting from ICI use and, importantly, shows that it is not tumor type specific and may occur across a variety of histologies.
Further analysis of 214 patients in Phase I trials with multiple tumor types found 15% (33/214) of patients to be considered to have HPD based on the criteria used (TTF <2 months and a minimum increase in target lesions of 10 mm plus either an increase of ≥40% in the target tumor burden or of ≥20% with additional new lesions being present). However, pretreatment scans were not necessary for inclusion in this study. The study found that the median OS for patients with HPD was 4.8 months (95% CI, 3.4–7.3) versus 8.7 months (95% CI, 6.3–10.2) in patients without HPD (HR 1.87; 95% CI, 1.1–3.3; P=0.03) [5]. The conclusion of this study, which was validated in multiple other studies, was that patients with HPD have poorer outcomes. In a piggyback study, the authors further found no difference between the TGR pre-ICI between patients with HPD and patients with non-HPD (P=0.15), but found that TGR on-ICI was significantly higher in patients with HPD (P<0.001) [6], again supporting the hypothesis that ICIs have a crucial role in the development of HPD.
A retrospective study analyzing patients with non-small-cell lung cancer (NSCLC) receiving ICI reviewed imaging from 220 patients and found 17% (37/220) to have HPD based on volume-based growth kinetics. This study also found that patients with HPD had both significantly lower median PFS and median OS. Compared with patients without HPD, the PFS of patients with HPD was 1.2 months versus 4.1 months (P<0.000) [7]. This study further implicates the role of ICIs in developing HPD and an association with poorer outcomes.
In the largest retrospective study to date, which included 406 patients with NSCLC, the authors mandated in their inclusion criteria that patients had to have had a minimum of two computerized tomography (CT) scans before ICI treatment and one CT scan during ICI. The authors calculated TGR by using the percentage increase in tumor volume, based on summing the largest diameters of the target lesion, per month [8]. They subsequently reported the difference between TGR pre-and post-ICI to validate their findings. Furthermore, HPD was defined as a difference in TGR >50% and PD on first evaluation. With these criteria, 13.8% (56/406) of patients treated with ICIs were identified to have HPD compared with only 5.1% of those treated with chemotherapy. This study additionally found a significant positive association between developing HPD and having more than two metastatic sites before the initiation of ICI (P=0.006) [3]. The importance of the presence of pre-ICI CT scans is noteworthy because an objective analysis of TGR before the initiation of ICI is not possible. Thus, this study further supports the relationship between ICI and HPD.
In another large retrospective study of 155 patients with a variety of tumor types [melanoma, NSCLC, HNSCC, cutaneous SCC, renal cell carcinoma (RCC), and colorectal carcinoma (CRC)], specific genomic aberrations were identified in association with HPD. This study defined HPD as >50% increase in tumor burden compared with pre-ICI with a <2-month TTF and doubling of pace progression. The study reported that 4% (6/155) of patients were found to have HPD. In the six patients with MDM2/4 amplifications, four met the criteria for HPD and had increases in progression rate (range 2.3–42.3-fold). In the ten patients with mutations in EGFR, two had HPD and subsequent progression rate increased (range, 35.7–41.7-fold). The authors conducted a bootstrap analysis and found significant correlations between MDM2/4 and EGFR alterations in patients with HPD (P=0.001 and P=0.014, respectively) [2]. This study offers new insights into the role of specific genomic alterations that may drive or predispose certain individuals to developing HPD.
Further validating these data, a review of a molecular database including 696 patients analyzed the pre-ICI next-generation sequencing (NGS) of tumors from patients who had HPD defined as a >50% increase in tumor size compared with pre-ICI, progression on first evaluation, and greater than a twofold increase in TGR. Although infrequently found, five (0.72%) patients (NSCLC, esophageal adenocarcinoma and SCC, and RCC) had HPD and, of these five, four had NGS results with the most common amplifications in MDM2/4 (50%) and EGFR (25%). In the entire database, MDM2/4 amplifications were present in 4% of patients (N=26), and EGFR amplifications were seen in 4% of patients (N=26) as well as 11q13 amplifications in 4% of patients (N=25). In total, ten patients with these alterations received ICIs; the subsequent incidence of HPD was 66%, 50%, and 43% in patients with MDM2/4, EGFR, and 11q13 amplifications, respectively [9]. The fact that these findings were similar to those from other studies from other institutions should prompt researchers to continue their efforts to identify prognostic biomarkers to better identify which patients may develop HPD.
A study of 34 patients with recurrent or metastatic HNSCC treated with ICI used a ratio ≥2 of the rate of tumor growth pre-ICI compared with the rate on-ICI (TGKR) to define HPD. The study found ten patients (29%) to have HPD and showed that regional recurrence was significantly associated with HPD (P=0.008). This study also found that HPD was associated with a lower PFS of 2.5 months vs. 3.4 months (P=0.003) [10]. Although the finding of HPD was higher than that reported for other tumor types, this does not negate the findings or significance of the study. The reported number of patients with HPD is most likely due to using TGKR as the criterion and supports the importance of identifying universal criteria to define HPD. Additionally, this study serves as evidence supporting the function of ICIs in the evolution of HPD and that HPD confers poorer outcomes for patients.
Researchers analyzed 263 patients to evaluate the occurrence of HPD in patients with recurrent and/or advanced NSCLC treated with programmed cell death (ligand)-1 [PD-(L)1] inhibitors. This study used TGK, TGR, and TTF to categorize HPD and, based on these definitions, reported 20.9% (N=55), 20.5% (N=54), and 37.3% (N=98), respectively of patients in the cohort to have HPD. Patients who met the criteria for TGK and TGR of HPD had a significantly lower PFS (HR, 4.619; 95% CI, 2.868–7.440) and OS (HR, 5.079; 95% CI, 3.136–8.226) compared with patients without HPD [11]. Additionally, the authors found a lower ratio of CCR7-negative CD45RA-negative T cells to total CD8+ T cells and higher proportion of TIGIT-negative T cells to PD-1-positive CD8+ T cells in patients with HPD and poorer survival [11]. The findings of all of the discussed studies are detailed in Table 1.
Table 1.
Hyperprogressive Disease (HPD): Results of Published Studiesa
| Setting of study | Number of patients included | Tumor type | HPD inclusion criteria | ICI used | Total no. of patient found to have HPD (%) | Significant associations | Survival outcomes | Refs |
|---|---|---|---|---|---|---|---|---|
| Early-phase trials | 182 | HNSCC, gyn, lung, GI, GU, melanoma, sarcoma, endocrine, breast | ≥Twofold increase in TGR from pre-ICI to on-ICI scans and PD | Not reported | 7% | Not reported | Median PFS significantly shorter in HPD | [24] |
| Phase I trials | 218 | Various tumor types | Two-fold increase in TGR from pre-ICI to on-ICI scans and PD | PD-(L)1 inhibitors | 9% | Median increase in TGR of 20.7-fold | Not reported | [1] |
| Phase I trials | 214 | Various tumor types | TTF <2 mo + minimum increase in TL 10 mm + either increase ≥40% in TL burden or increase ≥20% with additional new lesions | Not reported | 15% | Not reported | Median OS significantly shorter in HPD | [5] |
| Phase II trial (case series) | 3 | Adult T cell leukemia-lymphoma | Not reported | Nivolumab | 100% | HTLV-1 levels increased 63.0- and 2.4-fold | Not reported | [21] |
| Retrospective | 220 | NSCLC | Volume-based growth kinetics | Not reported | 17% | Not reported | Median PFS significantly shorter in HPD | [7] |
| Retrospective | 406 | NSCLC | Difference in TGR >50% and PD on first evaluation | Nivolumab, pembrolizumab, atezolizumab, durvalumab | 13.8% | >Two mets at baseline significantly associated with HPD | Not reported | [3] |
| Retrospective | 155 | Melanoma, NSCLC, HNSCC, cutaneous SCC, RCC, CRC | >50% increase in tumor burden compared with pre-ICI with <2 mo TTF, twofold increase progression rate | CTLA-4, PD-(L) 1, investigational agents | 4% | MDM2/4 and EGFR significantly associated with HPD development | Not reported | [2] |
| Retrospective (case series) | 5 | NSCLC, esophageal adenocarcinoma and SCC, RCC | >Twofold increase in TGR, >50% increase in tumor size compared with pre-ICI, PD on first evaluation | Pembrolizumab, nivolumab | 100% | MDM2/4, EGFR, and 11q13 amplifications detected in patients with HPD | Not reported | [9] |
| Retrospective | 34 | R/M HNSCC | TGKR≥2 | PD-(L)1 inhibitors | 29% | Not reported | Median PFS significantly shorter, RR significantly associated with HPD | [10] |
| Retrospective | 263 | R/M NSCLC | TGK defined as change in sum of longest diameters (SLD) of target lesions according to RECIST 1.1 criteria per month; TGR defined as log-scale calibrated change in sum of volumes of target lesions according to RECIST 1.1 criteria per month; TTF <2 mo | PD-(L)1 inhibitors | TGK: 20.9%; TGR: 20.5%; TTF: 37.3% | Lower CCR7-CD45RA− T cell:CD8+ T cell ratio; higher TIGIT+ T cell: PD-1+CD8+ T cell ratio | Poorer survival rate in patients with HPD | [11] |
Abbreviations: CTLA-4, cytotoxic T-lymphocyte associated protein-4; gyn, gynecologic; mets, metastatic sites; mo, months; PD-(L)1, programmed cell death (ligand) 1; R/M, recurrent or metastatic; RR, regional recurrence; TL, target lesion.
Are these Radiological Definitions Exclusive to ICIs?
TGR and TGKR [12] were developed many years ago with the advent of targeted therapies. The first description of TGR was made based on a Phase I population by Gomez-Roca et al. [13]. Here, 73 patients treated in Phase I clinical trials were evaluated with this method. An increased in TGR was observed in 20 (38%) of the 53 patients considered as nonprogressive at week 12 according to RECIST, and 12 out of 23 (53%) patients were classified as progressive according to RECIST with a decrease in TGR. The authors discussed the use of growth rate measured during the experimental period when it was significantly correlated with the evaluation of response according to RECIST criteria. However, the paper does not refer to the cut-off points used for HPD. The first attempt to combine TGR and RECIST was made by Ferté et al. [8], again in patients treated in Phase I clinical trials: 201 patients were assessed for TGR and RECIST, and 26% (N=51) were classified as PD, of which 35% showed an increase in TGR. In this cohort, an increase in the TGR did not correlate with the appearance of new lesions; in fact, 20 out of the 28 patients (71%) progressing with new lesions at the first tumor evaluation experienced simultaneously a decrease in TGR. Although the authors did not specify the TGR of each patient, in Figure 2 of [8] there were at least five patients with PD and TGR ≥2. Thus, it would be interesting to determine the cut-off point for HPD in this population.
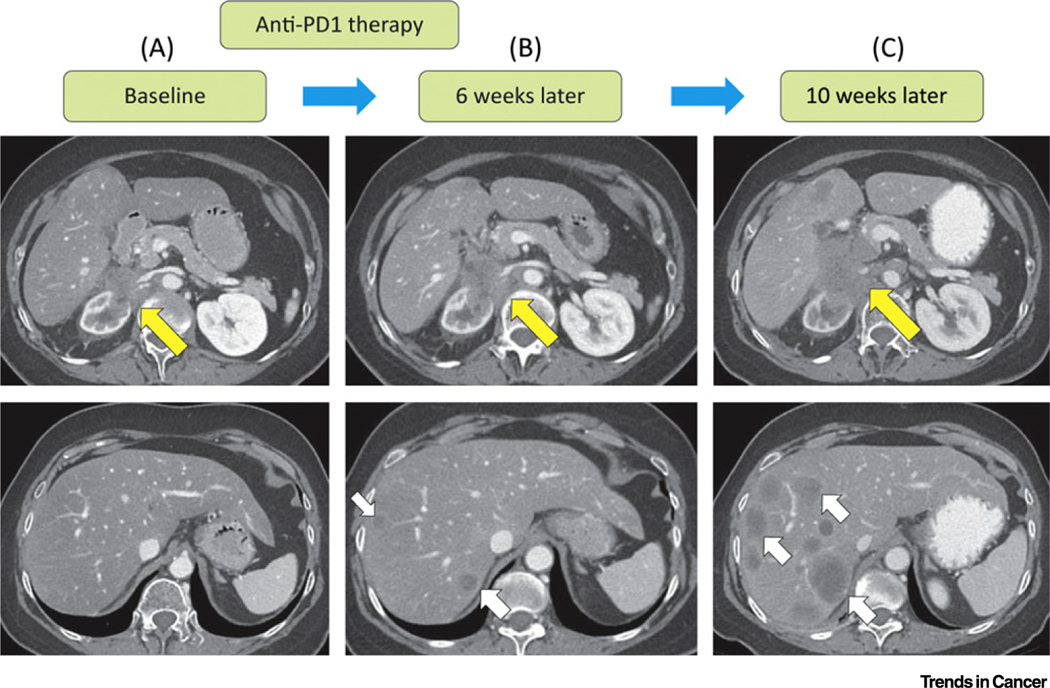
Figure 2. Case Study of a Patient’s Scans Exhibiting Hyperprogression.
A case study of a female in her 60s with renal cell carcinoma. The tumor showed significant progression with nivolumab. (A) A baseline scan before treatment with nivolumab demonstrated an infiltrative retroperitoneal mass involving her right kidney and renal vessels. (B) A restaging scan done 6 weeks after nivolumab therapy demonstrated an increase in the retroperitoneal mass and new hepatic metastases. (C) A follow-up scan done 10 weeks after initiation of nivolumab demonstrated an extensive increase in size of the retroperitoneal mass and a significant increase in size and number of new hepatic metastases, confirming disease progression. Abbreviation: PD-1, programmed cell death 1.
TGR was evaluated in patients with metastatic renal cell carcinoma treated in the Treatment Approaches in Renal Cancer Global Evaluation Trial (TARGET) (sorafenib vs placebo) and Renal Cell cancer treatment with Oral RAD001 given Daily (RECORD) (everolimus vs placebo) Phase III trials. Higher TGR (first cycle) was associated with worse PFS (HR 3.61; 95% CI 2.45–5.34) and worse OS (HR 4.69; 95% CI 1.54–14.39), independently from the Motzer score and from the treatment arm in the entire TARGET cohort [14]. Recently, in a control cohort with patients with NSCLC treated with chemotherapy, Ferrara et al. described a HPD rate of 14.2% (three of 21) (TGR >50%) in patients with PD as best response [3].
Finally, Matos et al. analyzed 180 patients treated in Phase I clinical trials with targeted agents for HPD using TGR (>2) and RECIST [15]. Overall, 48 patients had PD as the best response and were evaluable for both criteria. The authors observed an HPD rate of 33% (12 out of 48 patients ) and 26% (ten out of 48 patients), respectively, with no difference in overall survival between the HPD group versus non-HPD progressors [15]. Although HPD criteria can be met in patients on targeted agents, the lack of survival impact (which differs from internal and external cohorts exposed to ICIs) suggests that it is not a relevant clinical finding.
Potential Pathological Mechanisms for Hyperprogressive Disease
Various groups have hypothesized mechanisms for HPD, including modulation of subpopulations of immunosuppressive cells and differing responses to antibody domains. There has also been evidence involving deleterious transcription factors. These mechanisms may act independently, in concert, or may be complementary.
A study of 187 patients with NSCLC found 39 patients to have HPD by meeting the following inclusion criteria: (i) TTF <2 months; (ii) increase of ≥50% of target lesions; (iii) at least two new lesions in any previously involved organ; (iv) spread of disease to a different organ; and (v) clinical deterioration with a decline in performance status ≥2 points on the Eastern Cooperative Oncology Group scale within the first 2 months of treatment [16]. Additionally, this study only included patients who had at least two cycles of IO and consequent HPD. All of the patients with HPD were found to have tumor infiltration by M2-like epithelioid macrophages. NSCLC tumor tissue from patients was transplanted in athymic nude or severe combined immunodeficient (SCID) mice [patient-derived xenografts (PDX)] that were subsequently treated with nivolumab or nivolumab F(ab)2 fragments, the latter lacking the Fc region of the antibodies. The authors found that both subsets of mice treated with nivolumab had a massive increase in the number of intratumoral macrophages and, consequently, HPD. Furthermore, this study found that tumor-associated macrophages (TAM) have the ability to express PD-1 and blocking it maintains the TAM anti-cancer effect [16,17]. However, in mice treated with nivolumab F(ab)2 fragments, there was no substantial tumor growth or HPD. Another experiment carried out by these authors assessed the role of EGFR on HPD in PDX SCID mice. In contrast to control mice with wildtype EGFR that responded to nivolumab, those mice with mutated versions of EGFR had significant increases in TGR and cancer cell dissemination (P=0.0286). To further support the hypothesis that the Fc portion of the antibody causes HPD, mice that had mutated EGFR PDX were treated with nivolumab F(ab)2 fragments, but showed no evidence of HPD or of cancer cell dissemination [16]. Thus, this study provides insight into the complexity of the tumor microenvironment and the interplay between various moieties.
Another interesting potential mechanism for HPD is the impact of the relationship between senescent CD4+ T cells (Tsens) and PD-(L)1 expression. In a study of 45 patients with NSCLC treated with either nivolumab, pembrolizumab, or atezolizumab who had baseline low levels of Tsens (<57.7%), a significant increase in TGR after the initial cycle of an ICI (P=0.006) was recorded. Patients who experienced an increase in Tsens of 12.4% (95% CI, 6.2–18.5; P<0.0001) also had HPD. By contrast, patients who experienced a decrease in Tsens of 14.4% (95% CI, 8–21; P<0.0001) experienced tumor regression with ICI [18]. Although the role of Tsens is not fully understood, this study provides evidence that they have a significant role in tumor biology.
Another study analyzed changes in the amount of Ki67-positive effector regulatory T cells in patients with advanced gastric cancer who subsequently went on to develop HPD compared with the cohort that did not develop HPD. This study reported that, in patients with HPD, the Ki67-positive effector regulatory T cell:CD8+ T cell ratio did not significantly change after treatment with an PD-1 inhibitor; by contrast, this ratio decreased significantly in patients without HPD. Furthermore, the authors found that the amount of Ki67-positive effector regulatory T cells in tumor-infiltrating lymphocytes (TILs) increased significantly compared with patient without HPD, in whom these were reduced post treatment [19].
Results from a T cell non-Hodgkin lymphoma (T-NHL) mouse model showed that homogenously or heterogeneously deleted PDCD1 T cells led to aggressive T-NHL. In general when the receptor binding sites of T cells are bound with a ligand, PD-1 is upregulated and acts as a safety net against autoimmunity [20]. This study found that, in some lymphomas, PD-1 serves as a tumor suppressor. Consequently, inhibiting this protein results in significant tumor growth. The authors used chimeric ITK-SYK proteins, which are specific to T cell lymphoma, to validate the finding that PDCD1 deletion causes profound proliferation. In mice treated with ICI that were deficient in PDCD1, there was an immediate ITK-SYK positive T cell expansion. However, when ITK-SYK proteins were transplanted in cells with normal PDCD1 copies, the lymphomatous T cells were unable to proliferate. This is a relevant topic in the era of NGS of tumor tissues and circulating tumor DNA (ctDNA) and also raises the important question of the impact of patient T cell genomics.
Another mouse model study found that cells expressing human T cell leukemia virus type 1 bZIP factor (HBZ) also expressed high levels of PD-1; however, the immunosuppressive effect of PD-1 was null. In these cells, HBZ acted to impede the inhibitory effect of PD-1 signaling and hijacked it for massive CD4+ T cell expansion in a positive feedback loop. Furthermore, HBZ acted to upregulate PD-1 by impacting its inhibitory pathway, preventing ICI treatment from having any relevant effect [21]. This study further implicates the PD-1 pathway in HPD and its significance in identifying patients who may develop HPD.
In a Phase II trial of nivolumab in patients with adult T cell leukemia-lymphoma (ATLL) with increased tumor mutation burden (TMB) and high levels of PD-L1, the first three patients in the study showed prolific progression of disease after a single dose. At baseline, two of the patients had slowly progressing disease over the course of months and the other had stable disease (SD), all with laboratory values within normal limits. Furthermore, with the exception of skin tumors, the patients had negative positron-emission tomography and CT scans. After one dose of nivolumab, all three patients developed marked leukocytosis, hypercalcemia, renal insufficiency, and increases in lactate dehydrogenase (LDH). In two of the patients, measurements of HTLV-1, the causative virus of ATLL, increased by a factor of 63.0 and 2.4, respectively, and these patients also had 24% and 30% atypical lymphocytes, respectively and hyperbilirubinemia. Thus, these three patients were immediately taken off the trial and two received salvage chemotherapy and one received radiotherapy to skin and splenic lesions [22]. This case series is an example of the potential effect that ICI can have on HPD and further supports the previously described mechanism of the effect of HBZ in HTLV-1 infected cells treated with ICI.
A recent study evaluated the genomic and immunologic landscapes between pre- and post-treatment samples of two patients whose tumors showed HPD after ICI treatment [23]. Interestingly, somatic mutations were seen in tumor suppressor genes, such as TSC2 and VHL, in addition to transcriptional upregulation of oncogenic pathways, including IGF-1, ERK/MAPK, PI3K/AKT, and TGF-β, in post-therapy HPD tumors compared with pretherapy tumors [23]. Moreover, post-therapy HPD samples exhibited reduced immunogenicity. Intriguingly, they also showed an increase in the ILC3 subset of the innate lymphocyte system after anti-PD-1 immunotherapy [23].
Controversy
Given that all of the evidence for HPD is retrospective, with no specific biomarker revealed, researchers contend that HPD could still be the ‘natural history of disease’ [24], unresponsive and/or resistant to immunotherapy progressing when patients are unresponsive to therapy (Figure 2). The genomic and immunologic markers reported for HPD are from only a few patients and could just be a red herring, tumor heterogeneity, or additional accumulation of genomic events in response to cancer. Thus, understanding the phenomenon of HPD is less than straightforward and prompts several questions. From both studies presented as well as our anecdotal experiences, we believe that HPD is a valid outcome of ICI [23]. Another question is how can one differentiate HPD from ICI versus traditional chemotherapy versus targeted therapy? This again is difficult to answer. There are sufficient data to suggest that HPD is associated with worse outcomes regardless of the inciting agent [3,5,7,10,11,25]. A plausible way to be able to discern the intrinsic differences of ICI versus chemotherapy-induced HPD in the future may come from trials that deem patients not fit for chemotherapy and use ICI in a frontline setting with treatment-naïve patients. Comparing HPD respectively from groups who exclusively received chemotherapy versus ICI could provide greater insight into these differences, but again with questionable clinical implications.
Concluding Remarks
HPD is a provocative phenomenon in the era of ICI (Figure 3) [26]. There continues to be an increasing amount of retrospective evidence that shows that it is caused by ICI. HPD has been reported across multiple tumor types. The tumor microenvironment along with varying T cell subtypes has been postulated to have an intricate role, as have different proteomic domains within the antibody complex [Fc-F(ab)2]. Continued efforts to identify which patients may be afflicted by HPD after initiation of ICI is vital. The use of ICI in the face of various cancers has led to profound clinical benefits for many patients, but for some it has been devastating. Thus, universal criteria are needed to define HPD to prospective identify patients along with predictive likelihood factors to identify these patients (see Outstanding Questions).
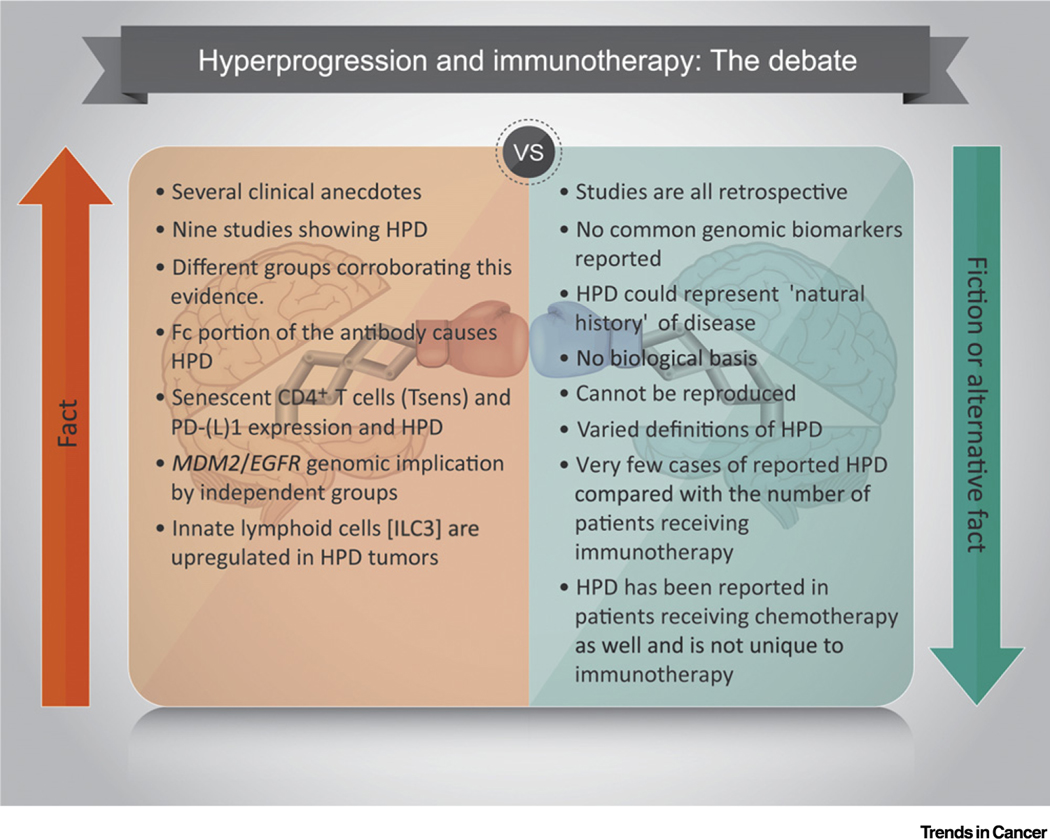
Figure 3. Hyperprogressive Disease (HPD) and Immunotherapy: Quite the Debate.
Evidence supporting HPD from immunotherapy includes nine published studies showing HPD, multiple various groups across the world reporting this phenomenon with differing criteria, lab-based evidence to support the role of Fc antibodies, senescent T cells, as well as genomic data showing MDM2 and EGFR mutations from two independent groups. Limitations to the evidence for HPD include a lack of a common biomarker in all studies, all studies being retrospective, the possibility of the natural history of aggressive disease subtypes, a lack of defined biological mechanism, and multiple definitions used to define HPD. Abbreviation: PD-(L)1, programmed cell death (ligand) 1.
Outstanding Questions.
Can we establish consensus criteria to define HPD?
Can prospective studies prove or disprove the HPD phenomenon?
What are the genomic signatures of HPD?
What are the immunologic signatures of HPD?
What are the surrogate markers of HPD?
Can patients be prospectively identified as being at risk of HPD?
Is it possible to establish management algorithms post disease progression?
Highlights.
The phenomenon of hyperprogressive disease (HPD) is a provocative phenomenon in the era of immune checkpoint inhibitors (ICI).
Multiple groups report HPD in a variety of cancers treated with ICI and it to be associated with shorter progression free survival and overall survival.
The tumor microenvironment along with varying T cell subtypes have been postulated to have an intricate role in HPD, as well as different proteomic domains within the antibody complex [Fc-F(ab)2]. An increase in the ILC3 subset of the innate lymphocyte system has also been implicated.
Universal criteria, along with predictive likelihood factors, are needed to define HPD to identify patients, prospectively or otherwise.
Acknowledgments
Disclaimer Statement
I.M. has an ESMO Research fellowship - Translational focus 2019 sponsored by Roche. E.G. has received research grants from Novartis/Rochel is a principal or co-investigator on clinical trials run by Principia Biopharma Inc., Lilly, S.A, Novartis Pharmaceutica, S.A, Genentech Inc, Loxo Oncology Inc, F. Hoffmann La Roche Ltd, Symphogen A/S, Merck, Sharp & Dohme de España, S.A, Incyte Biosciences International, Pharma Mar, S.A.U, Kura Oncology Inc, Macrogenics Inc, Glycotope Gmbh, Pierre Fabre Medicament, Cellestia Biotech, Menarini Ricerche Spa, Blueprint Medicines Corporation, Beigene USA, Inc, Sierra Oncology, Inc, and Genmab B.V.; has received consultant honoraria from Roche/Genentech, F. Hoffmann/La Roche, Ellipses Pharma, Neomed Therapeutics1 Inc, Boehringer Ingelheim, Janssen Global Services, AstraZeneca, SeaGen, TFS, Alkermes; travel grants from Bristol-Mayers Squibb, Merck Sharp & Dohme, Menarini, Glycotope; and is on the speakers bureau of Bristol-Mayers Squibb, Merck Sharp & Dohme, Roche, and ThermoFisher. V.S. reports the following sources of research funding and/or grant support for clinical trials: Novartis, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, Pharmamar, D3, Pfizer, Multivir, Amgen, Abbvie, Alfa-sigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint Medicines, Loxo Oncology, Medimmune, Altum, Dragonfly Therapeutics, Takeda and Roche/Genentech, National Comprehensive Cancer Network, NCI-CTEP and UT MD Anderson Cancer Center; V.S. has received travel support from Novartis, Pharmamar, ASCO, ESMO, Helsinn, Incyte, and FDA and is on the consultancy/advisory boards of Helsinn, LOXO Oncology/Eli Lilly, R-Pharma US, INCYTE, Medimmune, and Novartis.
Glossary
- Complete response (CR)
disappearance of all target lesions
- Immune checkpoint inhibitors (ICI)
agents that block PD-(L)-1 on both tumor and T cells to allow for an immune response against cancer cells
- Overall survival (OS)
length of time from either the date of diagnosis or the start of treatment that a patient diagnosed with the disease remains alive
- Partial response (PR)
at least a 30% decrease in the sum of the longest diameter of target lesions, taking as reference the baseline sum longest diameter
- Progression-free survival (PFS)
length of time during and after the treatment that a patient lives with the disease but it does not get worse
- Progressive disease (PD)
at least a 20% increase in the sum of the longest diameter (LD) of target lesions, taking as reference the smallest sum LD recorded since the treatment started or the appearance of one or more new lesions
- Senescent CD4+ T cells (Tsens)
highly differentiated CD28− CD27− CD4 T cells
- Stable disease (SD)
neither a sufficient decrease to qualify for PR nor a sufficient increase to qualify for PD, taking as reference the smallest sum LD since the treatment started
References
- 1.Champiat S. et al. (2017) Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin. Cancer Res 23, 1920–1928 [DOI] [PubMed] [Google Scholar]
- 2.Kato S. et al. (2017) Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin. Cancer Res 23, 4242–4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara R. et al. (2018) Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 4, 1543–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanjanapan Y. et al. (2018) Hyperprogressive disease (HPD) in early-phase immunotherapy (IO) trials. J. Clin. Oncol 36, 3063 [Google Scholar]
- 5.Matos I. et al. (2018) Incidence and clinical implications of a new definition of hyperprogression (HPD) with immune checkpoint inhibitors (ICIs) in patients treated in phase 1 (Ph1) trials. J. Clin. Oncol 36, 3032 [Google Scholar]
- 6.Matos I. et al. (2018) Refining criteria of hyperprogression (HPD) with immune checkpoint inhibitors (ICIs) to improve clinical applicability. Ann. Oncol, mdy303.011 [Google Scholar]
- 7.Kim Y. et al. (2018) Hyperprogression after immunotherapy: clinical implication and genomic alterations in advanced non-small cell lung cancer patients (NSCLC). J. Clin. Oncol 36, 9075 [Google Scholar]
- 8.Ferté C. et al. (2014) Tumor growth rate is an early indicator of antitumor drug activity in phase I clinical trials. Clin. Cancer Res 20, 246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alqwasmi A. et al. (2017) 1140PDPredictive biomarkers for hyper–progression (HP) in response to immune checkpoint inhibitors (ICI) – analysis of somatic alterations (SAs). Ann. Oncol 28, mdx376.006 [Google Scholar]
- 10.Saada-Bouzid E. et al. (2017) Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann. Oncol 28, 1605–1611 [DOI] [PubMed] [Google Scholar]
- 11.Kim CG et al. (2019) Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann. Oncol 30, 1104–1113 [DOI] [PubMed] [Google Scholar]
- 12.Le Tourneau C. et al. (2012) Tumour growth kinetics assessment: added value to RECIST in cancer patients treated with molecularly targeted agents. Br. J. Cancer 106, 854–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez-Roca C. et al. (2011) Tumour growth rates and RECIST criteria in early drug development. Eur. J. Cancer 47, 2512–2516 [DOI] [PubMed] [Google Scholar]
- 14.Ferté C. et al. (2014) Tumor growth rate provides useful information to evaluate sorafenib and everolimus treatment in metastatic renal cell carcinoma patients: an integrated analysis of the TARGET and RECORD phase 3 trial data. Eur. Urol 65, 713–720 [DOI] [PubMed] [Google Scholar]
- 15.Matos I. et al. (2018) Immune prognostic index (IPI) and hyperprogressive disease (HPD) in patients (pts) exposed to targeted agents (TAs) in phase I trials (Ph1T): Can lessons from immune checkpoint inhibitors (ICIs) be translated to other scenarios? Ann. Oncol 29, mdy269.108 [Google Scholar]
- 16.Lo Russo G. et al. (2019) Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin. Cancer Res 25, 989–999 [DOI] [PubMed] [Google Scholar]
- 17.Dahan R. et al. (2015) FcgammaRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis. Cancer Cell 28, 285–295 [DOI] [PubMed] [Google Scholar]
- 18.Zuazo-Ibarra M. et al. (2018) Senescent CD4 T cells unequivocally identify primary resistance and risk of hyperprogression to PD-L1/PD-1 immune checkpoint blockade in lung cancer. BioRxiv. Published online May 11, 2019. 10.1101/320176 [DOI] [Google Scholar]
- 19.Kamada T. et al. (2019) PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl. Acad. Sci. U. S. A 116, 9999–10008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francisco LM et al. (2010) The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev 236, 219–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinosada H. et al. (2017) HTLV-1 bZIP factor enhances T-cell proliferation by impeding the suppressive signaling of co-inhibitory receptors. PLoS Pathog. 13, e1006120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratner L. et al. (2018) Rapid progression of adult T-cell leukemia-lymphoma after PD-1 inhibitor therapy. N. Engl. J. Med 378, 1947–1948 [DOI] [PubMed] [Google Scholar]
- 23.Xiong D. et al. (2018) Immunogenomic landscape contributes to hyperprogressive disease after anti-PD-1 immunotherapy for cancer. iScience 9, 258–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearson AT and Sweis RF (2019) Hyperprogression-immunotherapy-related phenomenon vs intrinsic natural history of cancer. JAMA Oncol. 5, 743. [DOI] [PubMed] [Google Scholar]
- 25.Kanjanapan Y. et al. (2019) Hyperprogressive disease in early-phase immunotherapy trials: clinical predictors and association with immune-related toxicities. Cancer 125, 1341–1349 [DOI] [PubMed] [Google Scholar]
- 26.Adashek JJ et al. (2019) Hyperprogression and immune checkpoint inhibitors: hype or progress? Oncologist. Published online November 20, 2019. 10.1634/theoncologist.2019-0636 [DOI] [PMC free article] [PubMed] [Google Scholar]