Abstract
Background
Transfusion-related acute lung injury (TRALI) is a rare but potentially fatal transfusion reaction. An effective haemovigilance programme is important in implementing successful and targeted risk reduction strategies. We aim to provide a summary of TRALI cases referred for investigation in Queensland (QLD) Australia from 1999 to 2019, describing the epidemiological and laboratory features of local TRALI cases.
Materials and methods
A retrospective audit evaluated all cases reported to the QLD Australian Red Cross Lifeblood over the 20-year study period. Cases were categorised according to the 2004 Canadian consensus criteria.
Results
Of the 91 cases referred for investigation, expert review confirmed 30 of TRALI and 18 of possible TRALI. A total of 238 donors and 110 blood products were assessed in confirmed cases. TRALI affected patients of all ages. Most patients had underlying haematological malignancies (25%), surgery (15%) or liver disease (13%). TRALI incidence was measured at 1 in 130,000 per issued product in QLD. Red cells were transfused in 32 cases, platelets in 18 and plasma products in 21, with 16 cases involving multiple products. Following laboratory assessment, 23% of cases had findings supportive of antibody mediated TRALI and 21% as likely non-antibody mediated. Possible TRALI was identified in 37.5% of cases of which 25% were antibody mediated and 12.5% non-antibody mediated. Nine (18.5%) cases were uncategorised due to insufficient immunologic investigations.
Discussion
Rates of TRALI incidence measured are lower than those seen in many international studies. A reduction in confirmed cases has been noted over recent years, supporting the implementation of risk-reduction strategies. We report a relatively higher proportion of non-antibody mediated TRALI and possible TRALI cases in more recent years, suggesting the need to further understand the role of product age and biological risk modifiers.
Keywords: transfusion-related acute lung injury (TRALI), haemovigilance, blood transfusion, adverse effects
INTRODUCTION
Transfusion-related acute lung injury (TRALI) is a serious and potentially fatal transfusion complication. The 2004 TRALI Canadian consensus criteria was adopted for use by both the International Society of Blood Transfusion (ISBT) and Australian Red Cross Lifeblood (Lifeblood)1. This criterion proposes separate case definitions for TRALI and possible TRALI dependent on the presence of clinical criteria and alternate risk factors for acute lung injury (ALI). Recently, there has been a consensus redefinition2; however, this has yet to be widely implemented and the Canadian consensus criteria remains the most used classification system.
The pathogenesis of TRALI has been described as a two-hit model3. This suggests that the first “hit’ relates to predisposing recipient factors (e.g. sepsis) which contribute to the adhesion of primed neutrophils to the endobronchial vascular endothelium. The second “hit” is a consequence of the transfusion and results in activation of the neutrophils, causing endothelial damage3. TRALI is commonly differentiated into antibody mediated and non-antibody mediated depending on the second hit4. Non-antibody mediated TRALI may be related to biological response modifiers (BRMs) within the transfused product5. BRMs have been found to accumulate during blood product storage including in red blood cells leukocyte depleted (RBC) and platelets6. Antibody mediated TRALI is considered the result of transfusing human neutrophil antigen (HNA) or human leucocyte antigen (HLA) antibodies that match a cognate recipient antigen7.
With a decline in infectious complications, TRALI became one of the leading causes of transfusion-related mortality8, though it has now been surpassed by transfusion-associated cardiac overload (TACO)9. Estimates of TRALI incidence vary markedly between 0.08 to 15% per patient transfused and 0.0002–1.12% per transfused product4,10–15; however, TRALI is likely under-diagnosed and under-reported especially in critically ill patients16. TRALI has a mortality rate estimated between 5–25%4,17, but can be higher among critically ill and surgical patients, with a rate of 47% reported in intensive care patients18. Clinical management of TRALI is supportive and consequently, efforts to reduce TRALI have focussed on preventative strategies19. As HLA and HNA antibodies are found most frequently in multiparous women20, TRALI risk-reduction has focused on reducing patient exposure to plasma from female donors19.
In Australia, the state of Queensland (QLD) has a population of approximately 5 million people21. Lifeblood is responsible for the collection and distribution of blood products Australia-wide22. About 135,000 units of RBC, 36,000 units of plasma and 31,000 units of platelets were distributed to QLD health services in 2020. To mitigate TRALI risk, male predominant plasma was introduced in Australia in July 2007, and made up 100% of clinical plasma by 201223. TRALI attributed to apheresis platelets was minimised by moving to a plateletpheresis panel comprised of only male donors by July 201624, and reducing plasma content in apheresis platelets by resuspension in platelet additive solution (PAS) in March 201925. Lifeblood produces buffy coat derived pooled platelets and apheresis platelets, both of which are resuspended in PAS. This reduces the amount of plasma in platelet concentrates from 100% to approximately 40% in apheresis platelets and 30% in pooled platelets.
Despite decades of risk-reduction strategies, a comprehensive longitudinal review of cases reported to Lifeblood QLD has not been performed. We provide a summary of TRALI cases reported to Lifeblood QLD from 1999 to 2019 to describe the epidemiological and laboratory features of TRALI locally.
MATERIALS AND METHODS
Data collection and review
In Australia, suspected TRALI cases are reported to Lifeblood for further investigations and donor management. This study accessed all cases of suspected TRALI referred to Lifeblood QLD between March 1999 and December 2019. The 2004 Canadian consensus definition was applied26 and cases were categorised as either “TRALI”, “possible TRALI”, “not TRALI” or “insufficient data” based on three independent reviewer assessments. Each patient and all associated donors were comprehensively reviewed, including historical records of clinical findings and interventions obtained from referring clinicians at the time of reporting the case.
TRALI clinical characteristics
Severity grades for cases were defined using criteria adopted by the French Haemovigilance Network12:
Mild: spontaneous recovery or supplemental oxygen via face mask.
Severe: requiring non-invasive ventilation or transfer to an intensive care unit.
Life-threatening: requiring invasive mechanical ventilation with or without additional therapy e.g. vasopressors.
Death: where there was a temporal relationship between death and TRALI.
TRALI incidence
TRALI incidence was calculated from 2006–2019, as total issued blood product numbers were only available for this timeframe. Incidence rates were computed by dividing the number of TRALI and possible TRALI cases by the total number of blood products issued. Calculations did not account for blood products discarded post-issue.
The number of cases before and after 2012 were measured by TRALI subtype (antibody mediated/non-antibody mediated) to assess for changes in the proportions of TRALI cases.
Donor and recipient antibody testing
Assessing the presence of donor leucocyte antibodies and compatibility testing is a critical part of the TRALI investigative process27. TRALI is a clinical diagnosis and absence of antibodies does not reject or reduce the possibility of TRALI; however, the results guide the management of implicated donors and blood products. As part of Lifeblood practice, all involved donors are temporarily deferred, and available associated products quarantined while laboratory investigations conclude. If donor leucocyte antibodies are confirmed, the donor may be permanently deferred from future clinical donations, depending on antibody type and clinical significance27.
Over the 20-year period there were several improvements in laboratory assays used; however, testing for HLA and HNA donor antibodies remained a constant. If HLA or HNA antibodies were detected, a virtual crossmatch was done. HLA and HNA genotyping were performed on the recipients’ samples. HLA typing and antibody testing was earlier performed by the Victorian (V) Transplantation and Immunogenetics Service (TIS) laboratory of Lifeblood using sequence-specific oligonucleotide (SSO) for HLA typing and antibody screening by complement dependent microlymphocytotoxicity (LCT) assay. HLA antibody screening by LCT was replaced by solid phase immunoassay (Luminex LABScreen) from 2006.
The Queensland (Q) TIS laboratory implemented HLA antibody testing in 2014 and HLA genotyping in 2018 using the same methods as VTIS. Granulocyte antibody testing was performed by granulocyte immunofluorescence test (GIFT), granulocyte agglutination test (GAT) and the monoclonal antibody immobilisation of granulocyte antigen assay (MAIGA) as required28. HNA genotyping was performed using validated in-house methods; Real time Taqman polymerase chain reaction (PCR) and an allele specific PCR using sequence specific primers (PCR-SSP)28.
Case definitions for antibody mediated and non-antibody mediated TRALI were based on those used by Ozier et al.29. A case was classified as “antibody mediated” if an antibody to a cognate recipient antigen was detected, a positive granulocyte crossmatch was observed, or if an antibody to a ubiquitous antigen was identified. Cases where no antibodies were found, or where an identified antibody did not meet the above criteria were labelled as “non-antibody mediated”. Unlike Ozier et al., cases were labelled “uncategorised” rather than “non-antibody mediated” if there were insufficient immunologic investigations including genotyping/phenotyping.
In reverse TRALI, patient antibodies react with cognate antigens on transfused cells30. Where possible, we also tested for recipient HNA and HLA antibodies using the methods described above, although this did not occur in all cases.
Product characteristics
The blood products associated with each referred TRALI case were assessed. Details collected included blood product type, number of units transfused and product age. Adjusted age was calculated by dividing the age of the transfused product with the product’s total shelf life. This was used to assess the influence of blood product storage duration on TRALI incidence.
The platelet product type was assessed. This included platelet-rich plasma (PRP) platelets, buffy coat derived pooled platelets and apheresis platelets. Pooled platelet processing was introduced in July 2002, though PRP platelets continued to be produced until June 2007. Therefore, there was a mixed inventory of the two types from July 2002 to June 2007. Leucodepletion (RBC and platelets) was introduced in October 2008.
Statistical analysis
Fisher’s exact tests or chi-squared tests were used to compare incidence data. Unpaired t-tests were used to analyse continuous variables. Results were considered significant if the two-tailed p-value was ≤0.05.
RESULTS
Overall description (1999–2019)
A total of 91 cases were referred to Lifeblood QLD over the 20-year study period (Online Supplementary Figure S1). Thirty cases were categorised as TRALI and 18 as possible TRALI. Of the remaining 43 cases, 33 were categorised as not TRALI, nine had insufficient information for categorisation, and one was withdrawn from investigation.
Patient and clinical characteristics
The patient’s clinical condition can contribute to the development of TRALI4. Therefore, the clinical characteristics of TRALI and possible TRALI patients were reviewed (Table I) and found to be similar. TRALI was seen in all ages and affected genders similarly. Incidence was highest in persons aged 41–50 years and referrals were equal among metropolitan and regional hospitals. Most patients had underlying haematological and non-haematological malignancies, surgery and liver disease. For cases of possible TRALI, the most common ALI risk factors included shock (28%), pneumonia (28%) and sepsis (22%).
Table I.
Demographics and clinical characteristics of TRALI and possible TRALI cases between 1999 and 2019
| Characteristics | TRALI | Possible TRALI | All categories | |
|---|---|---|---|---|
|
| ||||
| Number | 30 | 18 | 48 | |
|
| ||||
| Sex | • Males | 15 (50.0%) | 11 (61.1%) | 26 (54.2%) |
| • Females | 15 (50.0%) | 7 (38.9%) | 22 (45.8%) | |
| • Sex ratio M/F | 1.0 | 1.6 | 1.2 | |
|
| ||||
| Age (years) | • Mean | 47.1 | 48.5 | 47.6 |
| • Median | 46.5 | 47.5 | 47.5 | |
| • Range | 4–84 | 2–87 | 2–87 | |
|
| ||||
| Hospital Location | • Metropolitan | 16 (53.3%) | 8 (44.4%) | 24 (50.0%) |
| • Rural/Regional | 14 (46.7%) | 10 (55.5%) | 24 (50.0%) | |
|
| ||||
| Fatalities | 0 (0%) | 2 (11.1%) | 2 (4.2%) a | |
|
| ||||
| Primary clinical condition | • Haematological malignancy | 8 (26.7%) | 4 (22.2%) | 12 (25.0%) |
| • Surgery | 2 (6.7%) | 5 (27.8%) | 7 (14.6%) | |
| • Liver disease | 4 (13.3%) | 2 (11.1%) | 6 (12.5%) | |
| • Non-haematological malignancy | 4 (13.3%) | 1 (5.6%) | 5 (10.4%) | |
| • Aplasia/MDS | 3 (10.0%) | 1 (5.6%) | 4 (8.3%) | |
| • Trauma | 3 (10.0%) | 1 (5.6%) | 4 (8.3% | |
| • Immunological disorder | 2 (6.7%) | 0 (0.0%) | 2 (4.2%) | |
| • Other | 4 (13.3%) | 4 (22.2%) | 8 (16.7%) | |
|
| ||||
| ALI risk factors | • Shock | 5 (27.8%) | ||
| • Pneumonia | 5 (27.8%) | |||
| • Sepsis | 4 (22.2%) | |||
| • Cardiopulmonary bypass | 3 (16.7%) | |||
| • Multi-trauma | 1 (5.6%) | |||
|
| ||||
| Timing of onset (hr) | • <1 hr post | 26 (86.7%) | 10 (55.6%) | 36 (75.0%) |
| • 1–3 hrs post | 2 (6.7%) | 4 (22.2%) | 6 (12.5%) | |
| • 3–6 hrs post | 2 (6.7%) | 4 (22.2%) | 6 (12.5%) | |
|
| ||||
| Clinical features | • Evidence of hypoxia and/or respiratory distress b | 30 (100%) | 18 (100%) | 48 (100%) |
| • Chestradiological findings | 30 (100%) | 18 (100%) | 48 (100%) | |
|
| ||||
| Admitted to ICU c | 6 (20.0%) | 6 (33.3%) | 12 (25.0%) | |
|
| ||||
| Treatment | • Oxygen | 26 (86.7%) | 17 (94.4%) | 43 (89.6%) |
| • Furosemide | 13 (43.3%) | 6 (33.3%) | 19 (39.6%) | |
| • Respiratory support | 9 (30.0%) | 7 (38.9%) | 16 (33.3%) | |
| • Steroids | 7 (23.3%) | 2 (11.1%) | 9 (18.8%) | |
| • Other | 6 (20.0%) | 6 (33.3%) | 12 (25.0%) | |
| • Treatment not stated | 1 (3.3%) | 1 (5.6%) | 2 (4.2%) | |
|
| ||||
| Severity | • Mild | 15 (50.0%) | 4 (22.2%) | 19 (39.6%) |
| • Severe | 5 (16.7%) | 4 (22.2%) | 9 (18.8%) | |
| • Life threatening/Death | 10 (33.3%) | 10 (55.6%) | 20 (41.7%) | |
excludes fatality events unrelated to TRALI;
Hypoxia presenting with either low oxygen saturations or need for oxygen/ntilator support;
Admitted to ICU at time of reporting.
Symptoms were seen within an hour of transfusion onset in 86.7% of TRALI cases and 55.6% of possible TRALI cases.
All patients presented with recorded evidence of hypoxia, respiratory distress and supportive chest radiograph findings. The severity of symptoms differed between patients; ranging from needing supplemental oxygen to mechanical ventilation. All cases where treatment was recorded had supplemental oxygen, in conjunction with additional respiratory support, diuretics (e.g. furosemide) and corticosteroids in many cases.
Two fatalities were observed, therefore the mortality rate of TRALI/possible TRALI patients in QLD was 4.2%. These fatalities were possible TRALI cases and categorised as non-antibody mediated. Plasma was implicated in one case, while multiple products were involved in the other. In terms of clinical characteristics, the two patients were similarly aged (46 vs 47 years) but had differing clinical conditions (liver disease vs haematological malignancy).
TRALI incidence
TRALI is associated with all blood products; however, typically incidence is higher in high plasma-containing products (e.g. plasma and platelets)13,15,29,31. Therefore, the risk of TRALI and possible TRALI per blood product issued was calculated (Table II). Considering 4,174,356 units were issued from 2006–2019, the risk of TRALI was calculated at 1 in 130,000 per any issued product. The risk of TRALI was highest in plasma products, calculated at 1 in 13,000 units compared to 1 in 42,000 and 1 in 21,000 units for RBC and platelet products respectively. The different platelet types were all implicated, though cases involving pooled platelets were most common (10 cases), followed by PRP (6 cases) and apheresis platelets (4 cases). Buffy coat derived pooled platelets in Australia includes donations from female donors. Mixed sex pools accounted for 80% of cases involving pooled platelets, compared to 20% from all male pools.
Table II.
Risk of TRALI per product issued, in QLD from 2006 to 2019
| Blood Product | All | Red cells | Platelets | Plasmaa |
|---|---|---|---|---|
| No. of units issued | 4,174,356 | 2,068,022 | 344,288 | 616,656 |
| Number of units associated with TRALI cases b | ||||
| TRALI (19 cases) c | 66 | 27 | 11 | 28 |
| Possible TRALI (13 cases) | 47 | 22 | 6 | 19 |
| Total (32 cases) | 113 | 49 | 17 | 47 |
| Risk of blood product being associated with a case | ||||
| TRALI (19 cases) | 1 in 220,000 | 1 in 76,000 | 1 in 33,000 | 1 in 22,000 |
| Possible TRALI (13 cases) | 1 in 320,000 | 1 in 94,000 | 1 in 57,000 | 1 in 32,000 |
| Total (32 cases) | 1 in 130,000 | 1 in 42,000 | 1 in 21,000 | 1 in 13,000 |
Includes FFP and cryoprecipitate.
Some cases had multiple products associated.
Includes only cases from 2006–2019, as issued blood product numbers were only available for this timeframe.
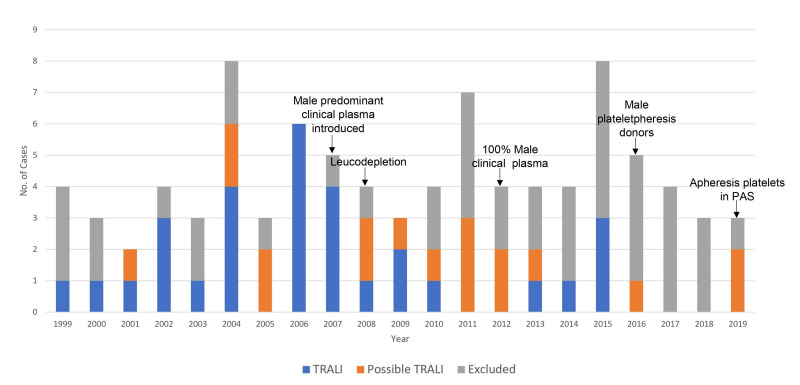
Various risk-reduction strategies were implemented during the study period (Figure 1), including the use of plasma exclusively from male donors in 2012 and male plateletpheresis donors in 2016. There was a notable decline in TRALI and possible TRALI cases following the implementation of these strategies, with only five TRALI and four possible TRALI cases observed since 2012. There were no confirmed cases of TRALI between 2016 and 2018.
Figure 1.
Number of cases referred for TRALI investigations to Lifeblood QLD from 1999–2019 and display of case categorisation by year. Shows key risk reduction strategies implemented in QLD during study period by year
Donor antibody testing
Antibody investigations are important for donor management and reducing the risk of TRALI27. The 48 TRALI/possible TRALI patients received blood products from 238 donors, with a mean of 4.9 donors per case (median 4, range 1–17). The male to female ratio of donors was 131: 107 (55%: 45%) (Table III). In 10 cases, only male donors were implicated. In 11 cases, only female donors were associated and this was only noted prior to 2011.
Table III.
HLA/HNA antibody investigations in donors associated with TRALI cases between 1999–2019
| Investigations | All donors | Male | Female |
|---|---|---|---|
| Antibody screen | (n=238 donors) | (n=131 donors) | (n=107 donors) |
| HLA/HNA antibodies detected | 76 (31.9%) | 30 (12.6%) | 46 (19.3%) |
| HLA/HNA antibodies not detected | 158 (66.4%) | 98 (41.2%) | 61 (25.6%) |
| Not tested | 4 (1.7%) | 3 (1.3%) | 1 (0.4%) |
| Antibody specificity donors) | (n=76 | (n=30 donors) | (n=46 donors) |
| HNA only | 18 (23.7%) | 8 (10.5%) | 10 (13.2%) |
| HLA class I only | 22 (28.5%) | 13 (17.1%) | 9 (11.8%) |
| HLA class II only | 16 (21.1%) | 7 (9.2%) | 9 (11.8%) |
| Multiple types (HLA class I, II and/or HNA) | 20 (26.3%) | 2 (2.6%) | 18 (23.7%) |
| Donor outcomes | (n=238 donors) | (n=131 donors) | (n=107 donors) |
| Donor deferred | 117 (49.2%) | 52 (21.8%) | 65 (27.3%)a |
| Donor cleared | 121 (50.8%) | 79 (33.2%) | 42 (17.6%)a |
p-value <0.05 vs male donors.
Of the 238 donors, 76 (32%) had a positive HLA/HNA antibody screen, with a male to female ratio of 30: 46 (Table III). HLA antibodies were most commonly detected. Many donors had more than one antibody type, and this was more common in female donors. Parity information was available for 73 out of 107 female donors and 57 (78%) had a previous history of pregnancy. Female donors of increased parity had a greater incidence of HLA antibodies. Testing was not completed for one female donor. Following investigations, 117 (49.2%) donors were permanently deferred from blood donation (Table III).
Antibody mediated and non-antibody mediated TRALI/possible TRALI
Detection of donor antibodies does not necessarily implicate them as the causative agent in a TRALI case as the recipient may not express the cognate antigen and not all cases are associated with antibodies32. Therefore, we used similar methods to Ozier et al.29 to distinguish between antibody mediated and non-antibody mediated cases. HLA/HNA antibodies were detected in 75% of cases (Table IV), of which 48% had laboratory findings supportive of antibody mediated TRALI and possible TRALI. Excluding the nine uncategorised cases resulted in antibody mediated TRALI/possible TRALI accounting for 59% of categorised cases compared to 41% of non-antibody mediated TRALI/possible TRALI categorised cases.
Table IV.
HLA/HNA antibody investigations in TRALI cases between 1999–2019
| Investigations | All | Antibody mediated TRALI | Non-antibody mediated TRALI | Antibody mediated possible TRALI | Non-antibody mediated possible TRALI | Uncategorised TRALIa | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Number of cases [cases (% of all cases)] | 48 | 11 (22.9%) | 10 (20.8%) | 12 (25.0%) | 6 (12.5%) | 9 (18.8%) | |
|
| |||||||
| HLA/HNA antibody screen [cases (% of cases for column)] | Antibodies detected | 36 (75.0%) | 11 (100%) | 1 (10.0%) | 12 (100%) | 3 (50.0%) | 9 (100%) |
| • Concordant | 23 (63.9%) | 11 (100%) | 0 (0%) | 12 (100%) | 0 (0%) | N/A | |
| • Non-concordant | 4 (11.1%) | 0 (0%) | 1 (10.0%) | 0 (0%) | 3 (50.0%) | N/A | |
| No antibodies detected | 12 (25.0%) | 0 (0%) | 9 (90.0%) | 0 (0%) | 3 (50.0%) | 0 (0%) | |
|
| |||||||
| Concordant antibody specificity [cases (% of cases for column)] | HLA Class I only | 4 (8.3%) | 1 (9.1%) | 0 (0%) | 3 (27.3%) | 0 (0%) | N/A |
| HLA Class II only | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A | |
| HNA only | 1 (2.1%) | 1 (9.1%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A | |
| Multiple specificitiesb | 18 (37.5%) | 9 (81.2%) | 0 (0%) | 9 (75.0%) | 0 (0%) | N/A | |
| HLA class I +II | 4 (8.3%) | 2 (18.2%) | 0 (0%) | 2 (16.7%) | 0 (0%) | N/A | |
| HLA class I + HNA | 3 (6.3%) | 0 (0%) | 0 (0%) | 3 (25.0%) | 0 (0%) | N/A | |
| HLA class II + HNA | 4 (8.3%) | 3 (27.2%) | 0 (0%) | 1 (8.3% | 0 (0%) | N/A | |
| HLA class I/II + HNA | 7 (14.6%) | 4 (36.4%) | 0 (0%) | 3 (25.0%) | 0 (0%) | N/A | |
|
| |||||||
| Non-concordant antibody specificity [cases (% of cases for column)] | HLA Class I only | 3 (6.3%) | 0 (0%) | 1 (10.0%) | 0 (0%) | 2 (33.3%) | N/A |
| HLA Class II only | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A | |
| HNA only | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A | |
| Multiple specificities | 1 (2.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (16.7%) | N/A | |
| HLA class + II | 1 (2.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (16.7%) | N/A | |
Uncategorised TRALI cases lacked donor testing to define concordance, and in these cases antibody detection was as follows: only HLA class I in 1 (11.1%) case, only HLA class II antibodies in 3 (33.3%) cases, only HNA antibodies in 2 (22.2%) cases and multiple specificities in 3 (33.3%) cases.
Some cases had more than one antibody type.
Of the antibody types, HLA class I antibodies were most commonly detected (45.8%), followed by HLA class II antibodies (33.3%) either in isolation or in combination with other antibody types. In cases of antibody mediated TRALI, multiple antibody specificities were common, of which HLA class II antibodies were most observed (81.8%) in combination with other types. There was a notable decrease in proportions of antibody mediated TRALI/possible TRALI over the study period (Online Supplementary Figure S2), with non-antibody mediated presentations being more frequent after 2012 (8 vs 3 cases, p=0.03).
The majority of cases involved only RBC transfusions (37.5%), though many were associated with multiple products (33.3%) (Table V). We observed a higher proportion of non-antibody mediated TRALI cases were associated with only RBC units (Table V). Pooled platelets were the most common platelet product type involved in cases. Of the cases involving pooled platelets, 70% were involved in antibody mediated TRALI/possible TRALI, 20% in non-antibody mediated TRALI/possible TRALI and 10% in uncategorised cases (data not shown).
Table V.
Products associated with referred TRALI cases in Queensland, 1999–2019
| Products | All cases (n=48) | Antibody mediated TRALI (n=11) | Non-antibody mediated TRALI (n=10) | Antibody mediated possible TRALI (n=12) | Non-antibody mediated possible TRALI (n=6) | Uncategorised TRALI (n=9) |
|---|---|---|---|---|---|---|
| Red cells only | 18 (37.5%) | 1 (9.1%) | 7 (70.0%)a | 3 (33.3%) | 3 (50.0%) | 4 (44.4%) |
| Plasma products only | 10 (20.8%) | 4 (36.4%) | 1 (10.0%) | 1 (8.3%) | 2 (33.3%) | 2 (22.2%) |
| Platelets only | 4 (8.3%) | 2 (18.2%) | 0 (0%) | 2 (16.7%) | 0 (0%) | 0 (0%) |
| Multiple products | 16 (33.3%) | 4 (36.4%) | 2 (20.0%) | 6 (50.0%) | 1 (16.7%) | 3 (33.3%) |
| Red cell cases | 32 | 4 | 9 | 8 | 4 | 7 |
| Units | 80 | 11 | 18 | 28 | 8 | 15 |
| Mean age (days) | 19.3 | 16.2 | 16.7 | 17.7 | 18.0 | 28.0 |
| Mean adjusted age | 0.46 | 0.36 | 0.40 | 0.43 | 0.43 | 0.67 |
| Platelets cases | 18 | 5 | 2 | 8 | 1 | 2 |
| Units | 26 | 4 | 2 | 9 | 2 | 2 |
| Mean age (days) | 3.5 | 3.7 | 4.5 | 3.5 | 3.0 | 3.0 |
| Mean adjusted age | 0.70 | 0.74 | 0.9 | 0.67 | 0.60 | 0.6 |
| Plasma cases | 21 | 8 | 2 | 5 | 2 | 4 |
| Units | 75 | 25 | 3 | 22 | 5 | 20 |
| Mean age (days) | 43.6 | 24.5 | 130 | 63.6 | 29.2 | 37.1 |
| Mean adjusted age | 0.12 | 0.09 | 0.36 | 0.17 | 0.08 | 0.1 |
p-value <0.05 vs antibody mediated TRALI.
Blood product storage is associated with BRM accumulation that is thought to precipitate non-antibody mediated TRALI33. Accordingly, various laboratory and animal models have demonstrated non-antibody mediated TRALI following transfusion with fractions of date-of-expiry blood products6. Therefore, we examined the storage duration of blood products associated with cases (Table V). The mean age of transfused RBC, platelets and plasma was 19.3, 3.7 and 43.6 days respectively. There was no significant difference in the mean storage duration of transfused RBC or platelets between non-antibody mediated and antibody mediated TRALI cases or between non-antibody mediated and antibody mediated possible TRALI cases (Table V).
DISCUSSION
This was the first longitudinal review of TRALI cases reported in QLD, Australia. It covered a 20-year period with changes in clinical practice, investigation policies, and HLA/HNA testing platforms as well as the introduction of risk-reduction strategies. We observed a low incidence of TRALI and possible TRALI in QLD, with a decrease in cases following the implementation of risk-reduction strategies. Proportions of antibody mediated and non-antibody mediated TRALI/possible TRALI were similar, though non-antibody mediated cases were comparatively higher after 2012.
The cohort of patients included in this review were referred to Lifeblood prior to publication of the 2019 re-definition2. Therefore, our study used the 2004 Canadian consensus definition for TRALI as this was the definition applied in Australia at the time of these cases. Furthermore, this remains the definition currently in use in Australia and is still widely used worldwide34–37. Its use in this study also allowed for comparison with previous reports of TRALI cases4,29. Nonetheless, the 2019 re-definition is likely to be increasingly adopted around the world in the coming years as validation studies are completed37, 38 and in this context its implementation in Australia is under consideration34. Due to the recognition of under-reporting17, Lifeblood has a low threshold for initiating TRALI investigations upon referral of an adverse reaction. The reporting of suspected TRALI cases to Lifeblood does not influence clinical management and referrals are predominately considered from a donor management perspective. In the cases categorised as “not TRALI”, either the required clinical criteria were not met, or it became apparent following discussion with clinicians that an alternative transfusion reaction or non-transfusion complication caused the presentation.
TRALI mortality in QLD was estimated as 4.2%. This was comparable to the 5–25% reported elsewhere4,15,17,29,39–41, considering that lower rates in this range are more common4,29,39–41. Possible TRALI might be associated with higher mortality and severity than TRALI due to underlying ALI risk factors42. The two fatalities that we observed were both possible TRALI cases, with no fatalities observed in the TRALI group.
TRALI incidence in QLD was estimated as 1 in 130,000 (0.0008%) per issued product. Reported TRALI incidence rates vary widely. Our finding was comparable with reports ranging from 0.0002 to 0.03%10,11,13,15, and aligned with rates from passive reporting systems11–13,15. TRALI is thought to be grossly under-diagnosed and under-reported especially among the critically ill16, which may also be the case in our study. It is considered that since ALI is so common among the critically unwell, TRALI is rarely recognised16. Several cases included in this study were managed with interventions that are not indicated in TRALI management, including the use of diuretics and corticosteroids. While this clinical management may have been tailored to the clinical features seen at the time of the reaction (e.g. to exclude fluid overload), this might reflect a lack of understanding of TRALI. We noted a reduction in the number of confirmed TRALI cases over the study period; however, TRALI referrals were consistent and this may reflect increased clinical consideration.
Reporting of haemovigilance data in Australia is voluntary and based on state-based haemovigilance programs. Until 2013, the QLD haemovigilance system was centralised but now haemovigilance data validation occurs at the hospital and health services level. Passive reporting systems such as this underestimate incidence rates when compared with active systems43–46. Likewise prospective studies that review transfused patients14,46, and prospective observational studies16 report higher incidence rates. TRALI incidence also varies between patient groups, with higher rates amongst those who are critically unwell or have specific clinical risk factors4,43,47, including haematological malignancies and surgical patients13–15,29. The patient groups in our cohort reflected those reported in most studies12,13,15,29,41; however, certain at-risk groups (e.g. cardiopulmonary bypass patients), were under-reported. TRALI incidence was highest in patients aged 41–50 years, which was younger than the average age of patients receiving RBC products in Australia (65–84 years)23. Similar numbers of TRALI referrals were from metropolitan and regional QLD hospitals, despite metropolitan hospitals being issued more units of blood. Considering this, TRALI rates were comparatively low in QLD tertiary centres. This may relate to differences in reporting practices among different sites. Variations in reporting practices can be observed between hospitals in addition to variations from year-to-year48.
Risk-reduction strategies, such as male predominant/exclusive plasma, male-only plateletpheresis panels and reducing the volume of plasma in blood products, all address antibody mediated TRALI6. Accordingly, QLD TRALI incidence decreased with the introduction of risk-reduction measures. For example, following the introduction of a male-only plateletpheresis panel in 2015, we observed only three cases of possible TRALI. Similar findings have been reported elsewhere10,15,19,43. Differences in when risk-reduction strategies were implemented in different jurisdictions could account for variations in reported rates of TRALI49. Considering the risk-reduction strategies implemented by Lifeblood, our findings may align better with recent studies12.
TRALI occurs with most blood products50, but high plasma volume products, including FFP and some platelet products (e.g. PRP and apheresis platelets), are most frequently implicated13,15,29,31. We observed cases related to RBC, platelets, FFP, and cryoprecipitate. RBC were most involved, particularly in non-antibody mediated TRALI. However, comparatively more RBC units were issued, and once adjusted for this they had the lowest relative risk of TRALI. Similar findings were noted in France41. We observed that pooled platelets were more frequently implicated compared to other platelet products. This differs from other studies where platelet products with higher plasma volumes were more commonly associated29. However, residual plasma volume as small as 10–20 mL can contain leukocyte antibodies and cause TRALI49.
Laboratory investigations are important for blood donor management27, and as seen elsewhere15, have evolved over time. In QLD, testing for HLA and HNA donor antibodies was performed throughout the entire study period, while antigen-antibody concordance by HLA/HNA genotyping of recipients was introduced after 2004. The reported frequency of antibodies in TRALI varies widely, with HNA antibodies in 0.0 to 33.3% of cases, and HLA antibodies in 11.4 to 67% of cases13, 51–53. HLA class II antibodies (either in isolation or in combination with other antibodies) were detected in at least one donor for 82% of antibody mediated TRALI cases in QLD. This corresponded with other reports that HLA class II antibodies are the most frequently detected antibody type in antibody mediated TRALI13,15,29,51,52.
Historically, antibodies are associated with up to 80% of TRALI cases7,54. In QLD, we observed only 23% of antibody mediated TRALI cases. This increased to 48% when including antibody mediated possible TRALI. Notably, nine cases (19%) did not have HLA/HNA genotyping performed. This meant that antibody-antigen concordance could not be assessed, and we reported these cases as “uncategorised TRALI”. This might have contributed to our lower antibody mediated TRALI rate, as some studies consider cases as antibody mediated if positive leucocyte antibodies are present irrespective of antigen/antibody compatibility7,54. However, a mechanism for how non-concordant antibodies might cause TRALI is unexplained, and studies that took a similar approach to ours reported similar rates of antibody mediated TRALI from 20 to 58%12,13,29. Excluding the nine uncategorised TRALI cases from our analyses resulted in antibody mediated TRALI/possible TRALI accounting for 59.0% of cases.
Cases in which antibodies are not detected can be referred to as non-antibody mediated TRALI52. In QLD, non-antibody mediated TRALI comprised 20.8% of cases, which increased to 33.3% when including non-antibody mediated possible TRALI. When uncategorised TRALI cases were excluded, these rates increased to 25.6% and 41.0% respectively. These rates were similar to those reported in other retrospective studies12,29,54. For instance, reports from the French and German haemovigilance networks reported non-antibody mediated TRALI in 26% and 25% of cases respectively12,54. Experimental models have demonstrated non-antibody mediated TRALI development following exposure to date-of-expiry RBC or platelet units, and to specific BRMs6. Therefore it seems likely that non-antibody mediated TRALI is dependent upon BRMs that accumulate in RBC and platelet units during routine storage6. However, the average age of RBCs transfused for TRALI cases in our study was 19.3 days with no difference in RBC storage time between antibody mediated and non-antibody mediated TRALI. Similarly, we observed no difference in platelet storage duration between antibody mediated and non-antibody mediated TRALI cases. We note that clinical evidence for an association between storage duration and TRALI is limited and many clinical studies have similarly failed to show an association between blood product age and TRALI risk18,43. It is also recognised that differences in storage variables can be partially donor dependent55 and information about “good” and “poor” storers was unknown in this study. Furthermore, reverse TRALI may account for some of the cases in this cohort which were identified as non-antibody mediated. Leukoreduction had been considered to reduce the risk of reverse TRALI; however, recent case reports suggest it is still a concern30. Laboratory experiments and a case report provided evidence that soluble antigens released during blood component processing could induce reverse TRALI when transfused to pre-immunised recipients56. The continued possibility of reverse TRALI highlights the importance of testing for recipient neutrophil and HLA antibodies. While we completed this in many of the investigations, it was not always possible, and this highlights an area for improvement in the future.
CONCLUSIONS
TRALI incidence in QLD was comparatively lower than other studies. Risk-reduction strategies have decreased the incidence of antibody mediated TRALI/possible TRALI, resulting in a higher proportion of non-antibody mediated TRALI/possible TRALI cases in recent years. Further investigations into blood product age and the role of BRMs is needed to inform the development of additional TRALI risk-reduction strategies.
Supplementary Information
ACKNOWLEDGEMENTS
Australian governments fund the Australian Red Cross Lifeblood (Lifeblood) to provide blood, blood products and services to the Australian community.
We gratefully acknowledge the staff in QLD TIS and VTIS laboratories and Grant Mraz (VTIS) for their assistance which has been integral to this paper.
Footnotes
AUTHORSHIP CONTRIBUTIONS
FS, SB, JPT, NG and AS were involved in extracting and interpreting the data. SB, JPT and FS were involved in reviewing the referred cases. AS and FS were involved in preparing the initial manuscript. All Authors were involved in preparing the final manuscript and approved the submitted version.
The Authors declare no conflicts of interest.
REFERENCES
- 1.International Society of Blood Transfusion (ISBT) EN 2011 ISBT Proposed standard definitions for surveillance of non-infectious adverse transfusion reactions. [Accessed on 30/05/2022]. Available at: https://www.isbtweb.org/resource/en-2011-isbt-proposed-standard-definitions-for-surveillance-of-non-infectiou-sadverse-transfusion-reactions.html.
- 2.Vlaar A, Toy P, Fung M, Looney MR, Juffermans NP, Bux J, et al. A consensus redefinition of transfusion-related acute lung injury. Transfusion. 2019;59:2465–2476. doi: 10.1111/trf.15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silliman C. The two-event model of transfusion-related acute lung injury. Crit Care Med. 2006;34:s125–2131. doi: 10.1097/01.CCM.0000214292.62276.8E. [DOI] [PubMed] [Google Scholar]
- 4.Vlaar A, Juffermans N. Transfusion-related acute lung injury: a clinical review. Lancet. 2013;382:984–994. doi: 10.1016/S0140-6736(12)62197-7. [DOI] [PubMed] [Google Scholar]
- 5.Roubinian N. TACO and TRALI: biology, risk factors and prevention strategies. Hematology Am Soc Hematol Educ Program. 2018;1:585–594. doi: 10.1182/asheducation-2018.1.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fung YL, Tung JP. Non-antibody mediated transfusion-related acute lung injury an enigma. Ann Blood. 2019;4:7. doi: 10.21037/aob.2019.03.02. [DOI] [Google Scholar]
- 7.Middelburg R, Stein D, Briët E, Van der Bom J. The role of donor antibodies in the pathogenesis of transfusion-related acute lung injury: a systemic review. Transfusion. 2008;48:2167–2176. doi: 10.1111/j.1537-2995.2008.01810.x. [DOI] [PubMed] [Google Scholar]
- 8.Stainsby D, Jones H, Asher D, Atterbury C, Boncinelli A, Brant L, et al. Serious Hazards of Transfusion: A decade of hemovigilance in the UK. Transfus Med Rev. 2006;20:273–282. doi: 10.1016/j.tmrv.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 9.FDA. Fatalities reported to Food and Drug Administration (FDA) following blood collection and transfusion; annual summary for fiscal year 2017. 2019. [Accessed 18/04/2021]. Available from: www.fda.gov/media/124796/downloads.
- 10.Vossoughi S, Gorlin J, Kessler D, Hillyer CD, Van Buren N, Jimenez A, et al. Ten years of TRALI mitigation: measuring our progress. Transfusion. 2019;59:2567–2574. doi: 10.1111/trf.15387. [DOI] [PubMed] [Google Scholar]
- 11.Karim F, Mansoori H, Rashid A, Moiz B. Reporting transfusion-related acute lung injury cases. Asian J Transfus Sci. 2020;14:126–130. doi: 10.4103/ajts.AJTS_152_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andreu G, Boudjedir K, Muller JY, Pouchol E, Ozier Y, Fevre G, et al. Analysis of transfusion-related acute lung injury and possible-transfusion-related acute lung injury reported to the French Hemovigiliance Network from 2007 to 2013. Transfus Med Rev. 2018;32:16–27. doi: 10.1016/j.tmrv.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 13.van Stein D, Beckers E, Sintnicolaas K, Porcelijn L, Danovic F, et al. Transfusion-related acute lung injury reports in the Netherlands: an observational study. Transfusion. 2010;50:213–220. doi: 10.1111/j.1537-2995.2009.02345.x. [DOI] [PubMed] [Google Scholar]
- 14.Silliman C, Boshkov L, Mehdizadehkashi Z, Elzi D, DIckey WO, et al. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood. 2003;101:454–462. doi: 10.1182/blood-2002-03-0958. [DOI] [PubMed] [Google Scholar]
- 15.Chapman C, Stainsby D, Jones H, Love E, Massey E, Win N, et al. Ten years of hemovigiliance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49:440–452. doi: 10.1111/j.1537-2995.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 16.Benson A, Moss M, Siliman C. Transfusion-Related Acute Lung Injury (TRALI): A clinical review with emphasis on the critically ill. Br J Haematol. 2009;147:431–443. doi: 10.1111/j.1365-2141.2009.07840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallis JP. Tranfusion-related acute lung injury (TRALI)’ under-diagnosed and under-reported. Br J Anaesth. 2003;90:573–576. doi: 10.1093/bja/aeg101. [DOI] [PubMed] [Google Scholar]
- 18.Vlaar A, Binnekade J, Prins D, van Stein D, Hofstra J, Schultz M, et al. Risk factors and outcome of transfusion-related acute lung injury in the critically ill: A nested case–control study. Crit Care Med. 2010;38:777–778. doi: 10.1097/CCM.0b013e3181cc4d4b. [DOI] [PubMed] [Google Scholar]
- 19.Fong H, Vekaria R, Buth K, Jackson D. Transfusion-Related Acute Lung Injury (TRALI) Risk Reduction Measures and The Impact on Preventing TRALI: Systemic Review and Meta- Analysis J Blood Disord Transfus. 2021:12. doi: 10.35248/2155-9864.20.12.450. [DOI] [Google Scholar]
- 20.Peters A, Van Stein D, Vlaar A. Antibody-mediated transfusion-related acute lung injury; from discovery to prevention. Br J Haematol. 2015;170:597–614. doi: 10.1111/bjh.13459. [DOI] [PubMed] [Google Scholar]
- 21.Queensland Government. Queensland Government Statistician’s Office: Queensland population counter. Australia: 2021. [Accessed on 18/04/2021]. Available at: https://www.qgso.qld.gov.au/ [Google Scholar]
- 22.Australian Red Cross Lifeblood. About us 2021. Available at: https://www.lifeblood.com.au/about. Accessed on v.
- 23.National Blood Authority Haemovigilance Advisory Committee. Australian Haemovigilance Report. Data for 2011-12 and 2012-13. 2013. [Accessed on 18/04/2021]. Available at: https://www.blood.gov.au/pubs/2015-haemovigilance/executive-summary/haemovigilance-data-2011-12-and-2012-13.html.
- 24.Australian Red Cross Lifeblood. Transfusion-related Acute Lung Injury (TRALI) 2021. [Accessed on 18/04/2021]. Available at: https://www.lifeblood.com.au/health-professionals/clinical-practice/adverse-events/TRALI.
- 25.Australian Red Cross Lifeblood. Information sheet: Introduction of apheresis platelets in platelet additive solution (PAS) and Triple Dose apheresis platelets. 2019. [Accessed on 30/05/2021]. Available at: https://transfusion.com.au/system/files/resource_library/customer_information_sheet_3265.pdf.
- 26.Kleinman S, Caulfield T, Chan P, et al. Toward an understanding of transfusion-related acute lung injury: a statement of a consensus panel. Transfusion. 2004;44:1774–1789. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 27.Fung YL, Goodison KA, Wong JK, Minchinton RM. Investigating transfusion-related acute lung injury (TRALI) Intern Med J. 2003;33:286–290. doi: 10.1046/j.1445-5994.2003.00352.x. [DOI] [PubMed] [Google Scholar]
- 28.Yuan Y, Dennington P, Daly J, Tung JP, Baidya S. A retrospective review of TRALI cases reported to the Australian Red Cross Lifeblood. Poster presented at: Blood 2021; 20–23 Sept 2021; Adelaide, Australia. 2021. [Accessed on 25/03/2022]. Available at: https://secure.tcc.co.nz/ei/images/BLOOD21/Abstract_Bookv3.pdf. [Google Scholar]
- 29.Ozier Y, Muller JY, Mertes PM, Renaudier P, Aguilon P, et al. Transfusion-related acute lung injury: reports to the French Hemovigilance Network 2007 through 2008. Transfusion. 2011;51:2102–2110. doi: 10.1111/j.1537-2995.2011.03073.x. [DOI] [PubMed] [Google Scholar]
- 30.Tung JP, Chiaretti S, Dean M, Sultana A, Reade M, Fung Y. Transfusion-related acute lung injury (TRALI): Potential pathways of development, strategies for prevention and treatment, and future research directions. Blood Rev. 2022 doi: 10.1016/j.blre.2021.100926. [DOI] [PubMed] [Google Scholar]
- 31.Eder AF, Herron R, Strupp A, Dy B, Notari EP, Chambers LA, et al. Transfusions-related acute lung injury surveillance (2003–2005) and the potential impact of the selective use of plasma from male donors in the Amercian Red Cross. Transfusion. 2007;47:599–607. doi: 10.1111/j.1537-2995.2007.01102.x. [DOI] [PubMed] [Google Scholar]
- 32.Fung YL. Transfusion-related acute lung injury investigation insights. ISBT Sci Ser. 2011;6:206–211. doi: 10.1046/j.1445-5994.2003.00352.x. [DOI] [Google Scholar]
- 33.Silliman C, Moore E, Kelher M, Khan S, Gellar L, Elzi D. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2011;51:2549–2554. doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan YDP, Daly J, Tung JP, Baidya S. The Impact of Revised Definitions for TACO and TRALI on Hemovigilance Reporting. Blood. 2021;138(Suppl 1):3252. doi: 10.1182/blood-2021-145294. [DOI] [Google Scholar]
- 35.Annual Report 2020. New Zealand: 2020. [Accessed on 30/05/2022]. NZBlood - New Zealand Blood Service [Internet] Available from: https://www.nzblood.co.nz/clinical-information/haemovigilance-programme/haemovigilance-annual-report-2012/ [Google Scholar]
- 36.SHOT - Serious Hazards of Transfusion [Internet] Definitions of current SHOT reporting categories & what to report. [Accessed on 30/05/2022]. Available from: https://www.shotuk.org/updated-shot-definitions-2022-now-available/
- 37.International Hemovigilance Network [Internet] Revised definitions for respiratory complications of blood transfusion. [Accessed on 30/05/2022]. Available from: https://www.ihn-org.com/revised-definitions-for-respiratory-complications-of-blood-transfusion/
- 38.Gupta A, Yan M. Transfusion-related acute lung injury (TRALI) [Accessed on 30/05/2022]. Available from: https://professionaleducation.blood.ca/en/transfusion/publications/transfusion-related-acute-lung-injury-trali#10.
- 39.Moore S. Transfusion-related acute lung injury (TRALI): Clinical presentation, treatment, and prognosis. Crit Care Med. 2006;34(Suppl 5):S114–117. doi: 10.1097/01.CCM.0000214312.20718.3E. [DOI] [PubMed] [Google Scholar]
- 40.Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion. 1985;25:573–577. doi: 10.1046/j.1537-2995.1985.25686071434.x. [DOI] [PubMed] [Google Scholar]
- 41.Renaudier P, Schlanger S, Mai MP, Ounnoughene N, Breton P, Cheze S, et al. Epidemiology of transfusion related acute lung injury in France: preliminary results. Transfus Med Hemother. 2008;35:89–91. doi: 10.1159/000117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Looney M, Roubinian N, Ognjen G, Gropper M, Hubmayr R, Lowell C, et al. Prospective study on the clinical course and outcomes in transfusion-related acute lung injury. Crit Care Med. 2014;42:1676–1687. doi: 10.1097/CCM.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toy P, Gajic O, Bacchetti P, Looney M, Gropper M,RH, et al. Transfusion-related a cute lung injury: incidence and risk factors. Blood. 2012;(1–19):1757–1767. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahu A, Bajpai M. Determining the true incidence of acute transfusion reactions: Active surveillance at a specialised liver enter. Hematol Transfus Cell Ther. 2020;42:326–332. doi: 10.1016/j.htct.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raval JS, Mazepa MA, Russell SL, Immel CC, Whinna HC, Park YA. Passive reporting greatly underestimates the rate of transfusion-associated circulatory overload after platelet transfusion. Vox Sang. 2015;108:387–392. doi: 10.1111/vox.12234. [DOI] [PubMed] [Google Scholar]
- 46.Finlay H, Cassorla L, Feiner J, Toy P. Designing and testing a computer-based screening system for transfusion-related acute lung injury. Am J Clin Pathol. 2005;124:601–609. doi: 10.1309/1XKQKFF83CBU4D6H. [DOI] [PubMed] [Google Scholar]
- 47.Gajic O, Rana R, Winters J, Yilmaz M, Mendez J. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med. 2007;176:886–891. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiersum-Osselton J, Jong A, Zijlker-Jansen P, Watering L, Brand A, van der Bom J, et al. Variations between hospitals in rates of reporting transfusion reactions: is a high reporting rate an indicator of safer transfusion? Vox Sang. 2012;104:127–134. doi: 10.1111/j.1423-0410.2012.01642.x. [DOI] [PubMed] [Google Scholar]
- 49.Win N, Chapman C, Bowles K, Green A, Bradley S, Edmondson D, et al. How much residual plasma may cause TRALI? Transfus Med. 2008;18:276–280. doi: 10.1111/j.1365-3148.2008.00885.x. [DOI] [PubMed] [Google Scholar]
- 50.Toy P. TRALI - Definition, mechanisms, incidence and clinical relevance. Best Pract Res Clin Anaesthesiol. 2007;21:183–193. doi: 10.1016/j.bpa.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reil A, Keller-Stanislawski B, Gunay S, Bux J. Specificities of leucocyte alloantibodies in transfusion-related acute lung injury and results of leucocyte antibody screening of blood donors. Vox S ang. 2008;5:313–317. doi: 10.1111/j.1423-0410.2008.01092.x. [DOI] [PubMed] [Google Scholar]
- 52.Kopko P, Popovsky MA, MacKenzie MR, Paglieroni TG, Muto KN, Holland PV. HLA class II antibodies in transfusion-related acute lung injury. Transfusion. 2001;41:1244–1248. doi: 10.1046/j.1537-2995.2001.41101244.x. [DOI] [PubMed] [Google Scholar]
- 53.Zupanska B, Uhrynowska M, Michur H, Maslanka K, Zajko M. Transfusion-related acute lung injury and leucocyte-reacting antibodies. Vox Sang. 2007;93:70–77. doi: 10.1111/j.1423-0410.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- 54.Funk MB, Guenay S, Lohmann A, Henseler O, Heiden M, Hanschmann KMO, et al. Benefit of transfusion-related acute lung injury risk - minisation measures. German Haemovigiliance data 2006–2010. Vox Sang. 2012;102:317–323. doi: 10.1111/j.1423-0410.2011.01556.x. [DOI] [PubMed] [Google Scholar]
- 55.Bontekoe IMP, Hurk K, Verhoeven A, Korte D. Transfusion. Platelet storage performance is consistent by donor: a pilot study comparing “good” and “poor” storing platelets. Transfusion. 2017;57:2373–2380. doi: 10.1111/trf.14238. [DOI] [PubMed] [Google Scholar]
- 56.Bayat BNK, Bein G, Traum A, Burg-Roderfeld M, Sachs U. Transfusion of target antigens to preimmunized recipients: a new mechanism in transfusion-related a cute lung injury. Blood Advances. 2021;5(3):975–3985. doi: 10.1182/bloodadvances.2020003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.