Abstract
Background
Recent reports suggest that direct oral anticoagulants (DOAC) may induce different anticoagulant and profibrinolytic responses. We performed a head-to-head comparison of the changes in thrombin generation (TG) parameters and tissue plasminogen activator (t-PA)-induced clot lysis produced by different DOAC.
Material and methods
We tested 137 plasma samples from patients with non-valvular atrial fibrillation (n=72) and venous thromboembolism (n=65) under treatment with apixaban (n=38), edoxaban (n=29), rivaroxaban (n=39), or dabigatran (n=31). TG was evaluated by a fluorometric assay and fibrinolysis by measuring the lysis time of clots exposed to 40 ng/mL t-PA.
Results
Trough-to-peak changes of TG parameters, along with correlation analysis, showed that all DOAC prolonged the lag-time in a concentration-dependent fashion. As for the other parameters, anti-factor Xa drugs markedly reduced the thrombin peak and velocity index but had little (rivaroxaban) or no effect on endogenous thrombin potential (ETP); dabigatran, instead, reduced ETP, weakly decreased thrombin peak and did not influence the velocity index, as also inferred from the changes in TG values after neutralisation of dabigatran with idarucizumab. Concerning the profibrinolytic effect of DOAC, intergroup comparison showed that the clot lysis time of dabigatran samples was significantly shorter than that of the apixaban and rivaroxaban samples, at both C-Trough and C-Peak. Moreover, a significant correlation between trough-to-peak changes in drug level and clot lysis time was only observed in the dabigatran group (rho=0.53). Finally, after DOAC removal by DOAC-stop, only dabigatran samples showed a significant increase in lysis time.
Discussion
Our data show that dabigatran inhibits TG in a different way than anti-Xa DOAC; moreover, under our conditions, only dabigatran displayed profibrinolytic activity, most likely because of its distinctive effect on the TG curve.
Keywords: anticoagulants, coagulation, fibrinolysis
INTRODUCTION
The new direct oral anticoagulants (DOAC) are rapidly replacing vitamin K antagonists for the treatment/prevention of venous thromboembolism (VTE) and the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation (NVAF)1. There are currently four DOAC approved by the European Medicines Agency for the treatment of VTE and NVAF: the thrombin inhibitor dabigatran and the anti-Xa inhibitors apixaban, edoxaban and rivaroxaban2. Contrary to vitamin K antagonist therapy, DOAC treatment does not require regular laboratory controls. However, there are situations, such as surgery, invasive procedures, bleeding or thrombotic complications and others, in which assays of DOAC are required1. Recommended methods for the assay of DOAC are clotting or chromogenic tests that measure anti-thrombin or anti-Xa activity of the drug after challenging the plasma sample with fixed amounts of purified enzymes1. These assays, while appropriate to determine the plasma concentration of each DOAC, do not provide accurate information about the actual anticoagulant effect of the drugs, which is greatly influenced by the individual procoagulant potential. For these reasons, alternative methods have been used to assess the efficacy of DOAC, such as the thrombin generation (TG) assay and thromboelastography or thromboelastometry. Rigano et al.3, using the TG assay, observed a remarkable interindividual variability in TG inhibition among plasma samples of healthy subjects spiked with fixed amounts of DOAC. Allegedly, such a variability may be even more pronounced in patients with VTE or NVAF, who are generally old and frequently have comorbidities and additional risk factors that may heavily affect the haemostatic balance and, consequently, the anticoagulant response to DOAC treatment. A typical TG assay evaluates the time course of thrombin formation and decay in plasma challenged with picomolar concentrations of tissue factor (TF), so mimicking the physiological clotting process4. The TG curve is described by several parameters, which provide different information, and which may be variably affected by anticoagulants depending on their mechanism of action. Several reports indicate that TG is a suitable test to measure the anticoagulant activity of DOAC3,5–7. However, there are some inconsistencies as to the effect of each DOAC on the various TG parameters, particularly as far as dabigatran is concerned5,6,8,9. Indeed, dabigatran inhibits the alpha2-macroglobulin complex used as an internal calibrator in the TG assay, giving rise to artificially increased values8. Thus, when dealing with samples from patients treated with dabigatran, the accuracy of TG data will depend on the strategies used to avoid this artifact. Moreover, to our knowledge, only one study has compared all major DOAC5, most others being focussed on one or two DOAC.
Another important aspect to be considered is the possible profibrinolytic effect of anticoagulants. In fact, thrombin inhibits the fibrinolytic process through multiple mechanisms10–12 and thus drugs inhibiting TG are expected to promote fibrinolysis. However, while in vitro studies show that all tested DOAC hasten the lysis of clots challenged with a plasminogen activator13–15, ex vivo experiments on plasma from patients treated with DOAC gave conflicting results16–19. Our study was undertaken to compare the anticoagulant and profibrinolytic effects of dabigatran, apixaban, rivaroxaban and edoxaban in patients with NVAF and VTE, using TG and a well-established clot lysis assay. The anticoagulant and profibrinolytic responses to DOAC were evaluated by the difference between C-Trough and C-Peak values, by the correlation between drug level and the studied variables and by determining the effect of DOAC removal or neutralisation.
MATERIALS AND METHODS
Patients’ samples
We examined plasma samples from 137 patients with NVAF (n=72) or VTE (n=65), collected at the Haemostasis and Thrombosis Centre of the Hospital Institutes of Cremona, Italy. Patients received dabigatran etexilate (Pradaxa® [Boehringer Ingelheim Italia, Milan, Italy], n=31), apixaban (Eliquis® [Bristol-Myers Squibb, Rome, Italy], n=38), edoxaban (Lixiana® [Daiichi Sankyo Italia, Rome, Italy], n=29) or rivaroxaban (Xarelto® [Bayer, Milan, Italy], n=39) according to the prescription criteria of AIFA, the Italian Drug Agency. All patients were on DOAC treatment for at least 1 month (range, 1–84 months). Exclusion criteria were alcohol abuse, infections, and cancer. Venous blood was collected into 0.109 M citrate using Becton Dickinson coagulation tubes (BD, Franklin Lakes, NJ, USA). Two samples were collected in the same morning, one just before drug intake (C-Trough sample) and one 2 h after drug intake (C-Peak sample). Blood was immediately centrifuged at 2,000 g for 20 min at room temperature, and the resulting plasma was partly used for the DOAC assay and partly stored at −80°C. Samples were then shipped in dry ice to the university of Bari where they were kept at −80°C until analysis. After thawing, samples were tested for TG and clot lysis and the remaining plasma was aliquoted and refrozen for further experiments. Dabigatran concentration in patient’s plasma was measured by diluted thrombin time (STA-thrombin, Diagnostica Stago, Asnieres, France) as described elsewhere20. Rivaroxaban, apixaban and edoxaban concentrations were measured by a chromogenic anti-Xa assay (STA®-Liquid Anti-Xa, Diagnostica Stago). Reference values were obtained by testing healthy blood donors (n=26) in parallel to the patients’ samples. The study conformed to the Declaration of Helsinki and informed written consent was obtained from each patient. The study protocol was approved by the institutional Ethic Committee.
Reagents
The following reagents were purchased from the indicated sources: single-chain recombinant tissue plasminogen activator (t-PA) and idarucizumab (Praxbind®) from Boehringer Ingelheim GmbH (Florence, Italy); human thromboplastin (Recombiplastin) and calcium chloride from Werfen (Milan, Italy); fluorogenic thrombin substrate Z-Gly-Gly-Arg-7-amino-4-methylcoumarin (ZGGR-AMC) and thrombin calibrator from Diagnostica Stago (Asnieres, France). Small unilamellar phospholipid vesicles, composed of 20% phosphatidylserine, 40% phosphatidylcholine and 40% phosphatidylethanolamine (Avanti Polar Lipids, Alabaster, AL, USA), were prepared by sonication. DOAC-stop minitabs were a gift from Haematex Research (Sydney, Australia). Due to the small volumes of plasma available, DOAC removal with DOAC-stop was carried out as follows. A minitab of DOAC-stop was added to 100 μL of Hepes buffer (final volume of suspension = 150 μL) and vigorously mixed on a vortex. Then, 10 μL of the suspension were added to 100 μL test plasma in an Eppendorf tube and incubated for 5 min at room temperature under continuous mixing on a rotating plate. Afterwards, the sample was centrifuged for 2 min at 2,000 g, and the supernatant was collected and immediately tested. As controls, we used the same plasma (100 μL) to which 6.7 μL Hepes buffer were added (which corresponds to the fluid volume of the DOAC-stop suspension), and then processed in the same way as the DOAC-stop treated plasma. Preliminary control experiments showed that our modified procedure of DOAC removal resulted in complete disappearance of anti-Xa and anti-IIa activity from the C-Peak samples, selected among patients with the highest drug concentration (n=3 per group, data not shown).
Thrombin generation assay
TG was evaluated by a fluorogenic assay according to Hemker et al.4, with minor modifications. In brief, 40 μL plasma, 2.5 μL thromboplastin (1:1,000, final dilution, corresponding to approximately 6 pM TF), 2.5 μL phospholipids (5 μg/mL, final concentration) and 5 μL buffer were added to round-bottomed 96-well plates (Immulon 2HB; Dynex Technologies). The reaction was started by the addition of a 10 μL mixture containing 100 mM CaCl2 and 2.5 mM ZGGR-AMC (Fluca reagent; Thrombinoscope BV). Measurements were taken every 20 s in a FluoroScan Ascent fluorometer (Thermo Scientific, Dreieich, Germany), and data were analysed using Thrombinoscope software. To correct for inner-filter effects and substrate consumption, each measurement was calibrated against the fluorescence curve obtained with the same plasma to which a fixed amount of thrombin-α2-macroglobulin complex (thrombin calibrator) had been added. The TG parameters evaluated in this study were lag-time, thrombin peak, endogenous thrombin potential (ETP) and velocity index. All samples and calibrators were run in duplicate. For dabigatran samples, the internal calibrator was supplemented with idarucizumab (1 mg/mL plasma) to prevent the inhibition of thrombin-α2-macroglobulin complex by dabigatran. The validity of this approach was supported by ad hoc experiments performed on plasma spiked with dabigatran (see the Online Supplementary Content).
Plasma clot lysis assay
The lysis of plasma clots exposed to exogenous t-PA was studied with a turbidimetric assay13 modified as follows. Forty microlitres of plasma, 2.5 μL thromboplastin (1:1000, final dilution), 2.5 μL t-PA (40 ng/mL, final concentration), 5 μL phospholipids (5 μg/mL, final concentration) were added to microplate wells, after which the clotting reaction was started with 10 μL CaCl2 (16.6 mM, final concentration). The plate was incubated at 37°C, and the changes in optical density at 405 nm were measured every minute in a microplate reader (Multiskan FC; Thermo Fisher Scientific, Waltham, MA, USA). Clotting time was defined as the time to the midpoint of the clear to maximum turbidity transition whereas clot lysis time was defined as the interval between the clotting time and the midpoint of the maximum turbidity to clear transition.
Statistical analysis
Data are presented as median and interquartile range, number or percent. Differences among groups were assessed by the Kruskal-Wallis test followed by post-hoc pairwise comparison according to Conover (continuous variables) or by the chi-squared test (categorical variables). Differences between C-Trough and C-Peak samples were assessed by the Wilcoxon test. Correlations were assessed by Spearman’s rank analysis. Statistical analyses were performed by MedCalc® (Mariakerke, Belgium). Concerning the study power, we calculated that 20 patients per group were needed to detect a 15% paired difference in the study’s variables between C-Peak and C-Trough samples (β = 0.8; α = 0.05), assuming a standard deviation of the difference equal to 150% of the difference.
RESULTS
Patients’ characteristics
The main characteristics of the patients, grouped according to the type of DOAC administered, are summarised in Table I. There were no statistical differences among groups as to age, gender, type of disease (NVAF or VTE), smoking habit, body mass index, comorbidities, and antiplatelet therapy (p>0.1 for all). The median trough-to-peak increase of drug concentration was approximatively 2-fold for apixaban and dabigatran, and 6–9-fold for edoxaban and rivaroxaban. Consistent with previous studies21, DOAC levels displayed great inter-individual variability.
Table I.
Patients’ characteristics
| Characteristics | Dabigatran (n=31) | Apixaban (n=38) | Edoxaban (n=29) | Rivaroxaban (n=39) |
|---|---|---|---|---|
| Age (years) | 69 [49.8 – 78] | 75,5 [71 – 82] | 78 [70 – 84] | 74 [68 – 79] |
| Weight (kg) | 75 [60–99] | 81 [69–85] | 73 [63–82] | 74 [62–83] |
| Body mass index | 25.7 [23.2–32.3] | 27.1 [24.5–30.5] | 25.9 [23.4–28.4] | 25.8 [23.5–27.8] |
| Male/females (n/n) | 19/12 | 24/14 | 11/18 | 18/21 |
| NVAF/VTE (n/n) | 15/16 | 19/19 | 19/10 | 19/20 |
| Daily dose of DOAC (n) | 2×150 mg (25) 2×110 mg (6) |
2×5 mg (29) 2×2,5 mg (9) |
60 mg (23) 30 mg (6) |
20 mg (31) 15 mg (8) |
| Smokers (%) | 3.2 | 0 | 0 | 0 |
| Diabetes (%) | 3.2 | 18.4 | 13.8 | 5.1 |
| Hypertension (%) | 54.8 | 57.9 | 48.3 | 56.4 |
| Antiplatelet therapy (%) | 6.5 | 13.2 | 13.8 | 23.1 |
| Drug concentration at C-Trough (ng/mL) * | 63 [45 – 119] | 94 [56 – 130] | 29 [21 – 44] | 34 [28 – 44] |
| Drug concentration at C-Peak (ng/mL) * | 152 [91 – 230] | 172 [130 – 288] | 266 [180 – 347] | 205 [131 – 274] |
Data are presented as median [interquartile range], number or %.
, There were no differences in drug levels between patients taking high or low doses of direct oral anticoagulants.
NVAF: non-valvular atrial fibrillation; VTE: venous thromboembolism; DOAC: direct oral anticoagulant.
Because there were no differences between NVAF and VTE patients in any studied variables (not shown), the data from the two groups were pooled.
Effect of direct oral anticoagulants on thrombin generation
The influence of each DOAC on the various TG parameters is illustrated in Figure 1A (graphs a–d). As expected, C-Peak samples showed a greater inhibitory effect on TG than did C-Trough samples, as indicated by longer lag-time and lower thrombin peak and velocity index. Concerning ETP, a significant difference between C-Peak and C-Trough was only seen in dabigatran and rivaroxaban samples. The most striking differences between dabigatran and anti-Xa DOAC was the latters’ ability to inhibit thrombin peak (Figure 1Ab) and velocity index (Figure 1Ad). Indeed, for both parameters, the reduction observed with anti-Xa drugs was much greater than that induced by dabigatran and the difference was evident even when the C-Peak dabigatran samples were compared to C-Trough anti-Xa samples. Almost the opposite was observed for ETP, in which case the inhibitory effect of dabigatran was more pronounced than that of anti-Xa drugs (Figure 1Ac).
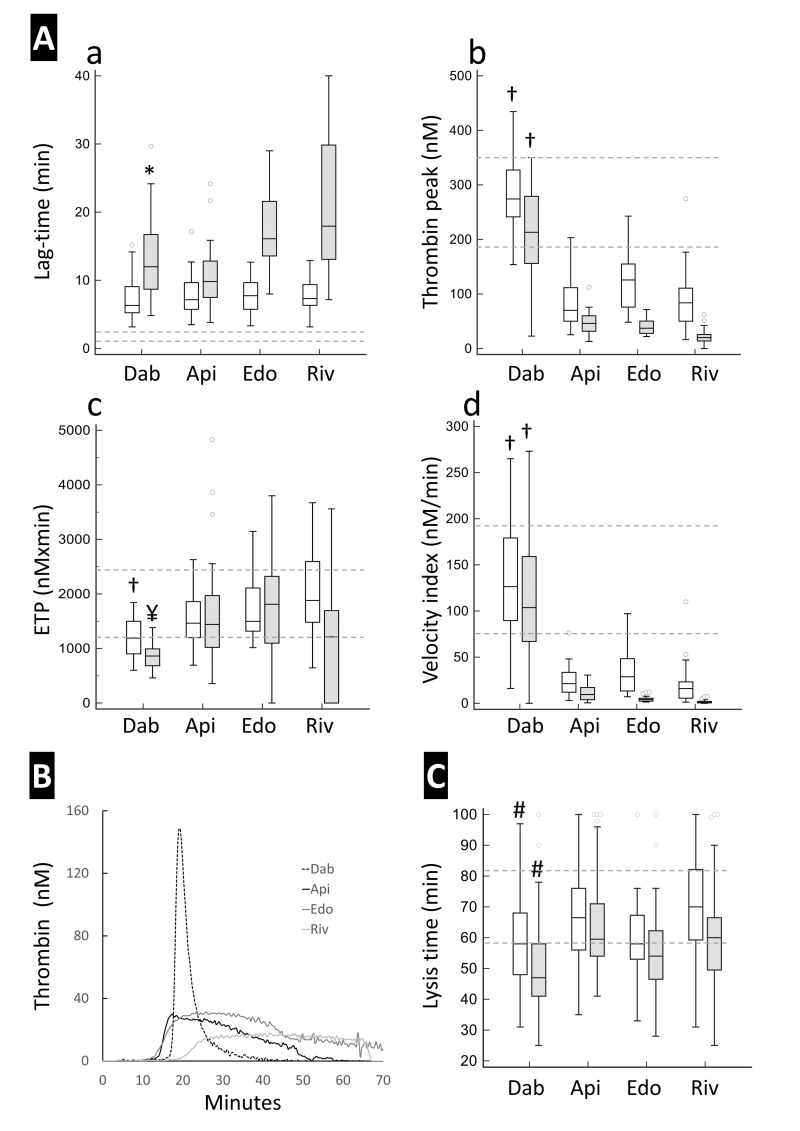
Figure 1. Thrombin generation and clot lysis of plasma samples from patients under treatment with direct oral anticoagulants.
Panel A shows the thrombin generation parameters, namely lag-time (a), thrombin peak (b), endogenous thrombin potential (c) and velocity index (d) at C-Trough (empty boxes) and C-Peak (grey boxes). Panel B illustrates representative thrombin generation curves of samples at C-Peak drug levels. Panel C reports the lysis time at C-Trough (empty boxes) and C-Peak (grey boxes) as assessed by the clot lysis turbidimetric assay. Dotted lines represent the reference values recorded in samples from healthy donors (n=26) tested in parallel. Values at C-Peak were statistically different from those at C-Trough (by Wilcoxon’s test) in all groups for all parameters except for endogenous thrombin potential in the apixaban and edoxaban groups and for lysis time in the apixaban group (not shown in the figure). †: statistically different from all Xabans; *: statistically different from edoxaban and rivaroxaban; ¥: statistically different from apixaban and edoxaban; #: statistically different from apixaban and rivaroxaban by Kruskal Wallis and post-hoc pairwise analysis according to Conover.
ETP: endogenous thrombin potential; Dab: dabigatran; Api: apixaban; Edo: edoxaban; Riv: rivaroxaban.
Typical TG curves are shown in Figure 1B. As can be seen, in dabigatran samples thrombin formation started after a prolonged lag-time, increased rapidly, and reached a rather high peak, after which it quickly decayed. In anti-Xa samples, instead, the rise in thrombin activity was slower, peak thrombin levels were clearly lower than in dabigatran samples, and thrombin decay occurred slowly resulting in a greater ETP. A similar difference in the shape of TG curves between dabigatran and anti-Xa DOAC was observed in selected C-Peak samples when clotting was triggered by 20 pM TF (Online Supplementary Content, Figure S2).
By rank correlation analysis, the only parameter of the calibrated automated thrombogram (CAT) that correlated well with the plasma concentration of all DOAC was lag-time (Table II). Thrombin peak and velocity index were strongly correlated with anti-Xa drug levels but not with dabigatran levels, which showed a rather weak correlation with thrombin peak and no correlation at all with velocity index. ETP, instead, showed a strong correlation with dabigatran concentration, a significant but weaker correlation with rivaroxaban levels and no correlation with apixaban and edoxaban levels.
Table II.
Correlations between direct oral anticoagulant concentration and calibrated automated thrombogram parameters
| Drug concentration | DOAC | Lag-time | Peak | ETP | Velocity | |
|
| ||||||
| Dabigatran (n=62) | rho | 0.681 | −0.373 | −0.627 | −0.181 | |
| p | <0.0001 | 0.0076 | <0.0001 | 0.21 | ||
|
| ||||||
| Apixaban (n=76) | rho | 0.790 | −0.741 | −0.149 | −0.631 | |
| p | <0.0001 | <0.0001 | 0.21 | <0.0001 | ||
|
| ||||||
| Edoxaban (n=58) | rho | 0.843 | −0.921 | −0.126 | −0.931 | |
| p | <0.0001 | <0.0001 | 0.40 | <0.0001 | ||
|
| ||||||
| Rivaroxaban (n=78) | rho | 0.732 | −0.773 | −0.506 | −0.777 | |
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
Rank correlation analysis was performed by combining C-Trough and C-Peak samples. ETP: endogenous thrombin potential; rho, Spearman’s correlation coefficient; DOAC: direct oral anticoagulant.
Effect of direct oral anticoagulants on clot lysis
The profibrinolytic activity of DOAC treatment was investigated by measuring the lysis time of clots exposed to exogenous t-PA (Figure 1C). The lysis time at peak drug level was significantly shorter than that at trough in all DOAC groups but apixaban. Intergroup comparison revealed that dabigatran samples displayed significantly shorter lysis time than apixaban and rivaroxaban samples, at both trough and peak drug concentrations.
Spearman’s analysis showed a borderline correlation between clot lysis time and dabigatran levels (p=0.053) and no correlation between lysis time and the levels of anti-Xa drugs (Table III). However, if we considered the differences between C-Peak and C-Trough values, instead of the absolute values, the correlation between the delta drug concentration and delta lysis time became much stronger in the dabigatran group (rho –0.525, p=0.003) but remained insignificant in the Xaban groups (Table III).
Table III.
Correlation between drug concentration and lysis time
| Drug Concentration | DOAC | Lysis time | Δ Concentration | Δ Lysis time | ||
|
|
|
|||||
| Dabigatran (n=62) | rho | −0.251 | rho | −0.525 | ||
| p | 0.053 | p | 0.003 | |||
|
|
|
|||||
| Apixaban (n=76) | rho | −0.032 | rho | −0.061 | ||
| p | 0.79 | p | 0.72 | |||
|
|
|
|||||
| Edoxaban (n=58) | rho | −0.153 | rho | −0.054 | ||
| p | 0.25 | p | 0.78 | |||
|
|
|
|||||
| Rivaroxaban (n=78) | rho | −0.180 | rho | −0.212 | ||
| p | 0.115 | p | 0.196 | |||
Correlations (rho) were calculated between drug concentration and lysis time by combining C-Trough and C-Peak samples (left), and between peak-trough differences (Δ) in drug concentration and lysis time (right), in which case the number of pairs is halved. DOAC: direct oral anticoagulant.
Effect of removal/neutralisation of direct oral anticoagulants on the drugs’ activities
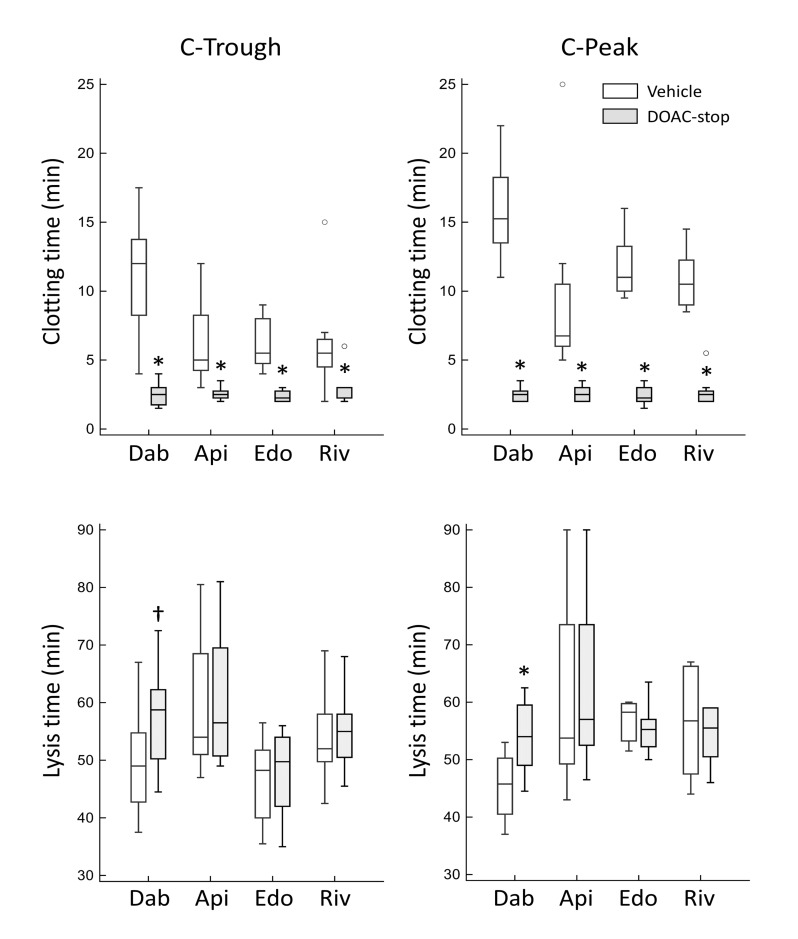
To assess the anticoagulant and profibrinolytic activities of DOAC directly, we evaluated the effect of DOAC-stop treatment, which was previously shown to efficiently remove all types of DOAC22. For this purpose, we used the microplate assay because it allowed us to evaluate both the anticoagulant and profibrinolytic activities of DOAC. As shown in Figure 2 (upper panels), DOAC-stop treatment caused a marked and significant shortening of clotting time in all groups (n=8 per group) and the difference between C-Trough and C-Peak samples was no longer visible. However, at variance with these findings, a significant prolongation of lysis time after DOAC-stop treatment was only seen in the dabigatran group, in both C-Trough and C-Peak samples (Figure 2, lower panels). Under our conditions, addition of DOAC-stop to normal plasma samples caused negligible changes in clotting and fibrinolysis times (2.0±0.35 vs 1.94±0.30 min and 46.1±4.7 min vs 47.6±4.7 min, respectively, n=8).
Figure 2. Effect of DOAC-stop (grey boxes) on clotting (upper panels) and lysis time (lower panels) of plasma from patients under treatment with direct oral anticoagulants.
The experiments were performed using the clot lysis turbidimetric assay, which also allows evaluation of the clotting time (i.e. the time to reach the midpoint of the clear to maximum turbidity transition). C-Trough and C-Peak samples (n=8 for both) are shown in the left and right panels, respectively. *: p=0.008; †: p=0.016 vs corresponding controls (empty boxes) by Wilcoxon’s test. Please note that, due to the limited volumes of plasma, C-Peak and C-Trough samples were obtained from different patients. Dab: dabigatran; Api: apixaban; Edo: edoxaban; Riv: rivaroxaban.
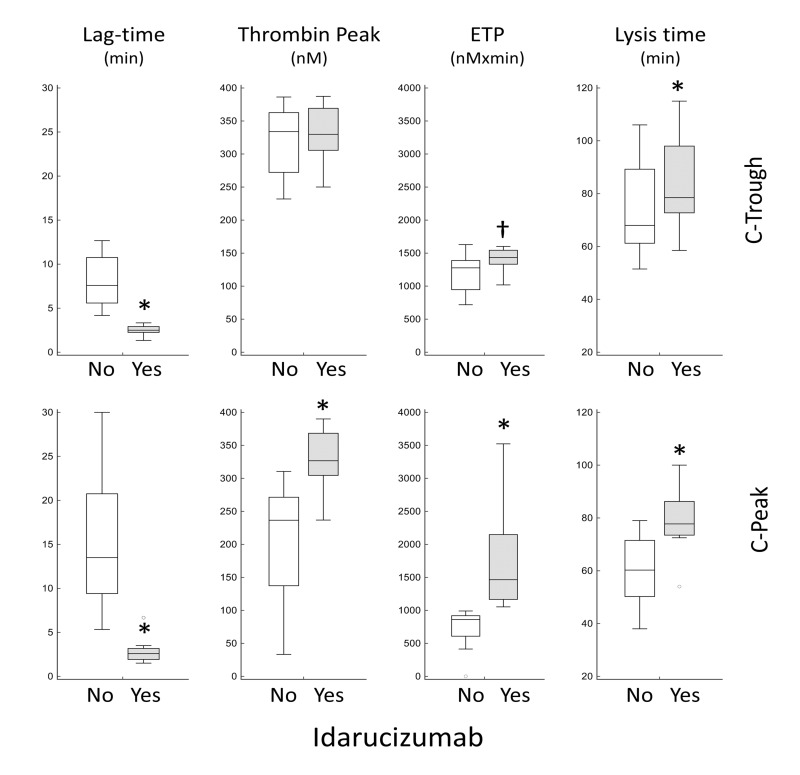
In another set of experiments, we investigated how the addition of idarucizumab to test plasma influences TG parameters and lysis time of dabigatran samples (n=8, Figure 3). Consistent with the results in Table II, dabigatran neutralisation caused a significant shortening of lag-time and a significant increase of ETP in both C-Trough and C-Peak samples. As for the other parameters, idarucizumab increased thrombin peak in C-Peak samples (Figure 3) and had no effect on velocity index (not shown). Moreover, in agreement with the DOAC-stop data, neutralisation of dabigatran was associated with a prolongation of lysis time in both C-Trough and C-Peak samples (Figure 3).
Figure 3. Effect of idarucizumab (1 mg/mL) on thrombin generation parameters, determined by a calibrated automated thrombogram (CAT) assay, and lysis time, determined by a turbidimetric assay, of dabigatran samples at C-Trough (upper panels, n=8) and C-Peak (lower panels, n=8).
Differences between samples with (grey boxes) and wihout (empty boxes) idarucizumab were assessed by Wilcoxon’s test (*: p=0.008; †: p=0.023). Idarucizumab had no effect on CAT velocity index (not shown in figure). Please note that, due to the limited volumes of plasma, C-Peak and C-Trough samples were obtained from different patients.
DISCUSSION
The main results of our study can be summarised as follows: (i) the DOAC targeting factor Xa influence the TG curve in a different way as compared to the thrombin-inhibitor dabigatran; and (ii) under our experimental conditions, only dabigatran samples showed a clear-cut increase in plasma fibrinolytic capacity (reduction in fibrinolytic resistance), a finding most likely related to the way dabigatran modifies the TG curve.
Concerning the TG parameters, all DOAC prolonged lag-time in a concentration-dependent manner, which agrees with findings of previous studies3,5–9. However, once thrombin formation has begun, the trajectory of thrombin activity differs remarkably between anti-IIa and anti-Xa DOAC. In particular, the rate of thrombin formation and the height of the thrombin peak were clearly greater in dabigatran samples than in anti-Xa samples (Figure 1B). Moreover, thrombin activity decayed rather quickly in dabigatran samples and very slowly in Xaban samples. Consequently, dabigatran had a different influence on the CAT parameters compared to anti-Xa drugs. In fact, besides lag-time, the only parameter that showed a good correlation with dabigatran concentration was ETP, since thrombin peak and velocity index displayed weaker or no correlation. In contrast, the levels of anti-Xa drugs were significantly correlated with lag-time, thrombin peak and velocity index. Concerning ETP, we found a significant, although relatively weak correlation with rivaroxaban but not with apixaban and edoxaban levels. All in all, our results, while confirming previous data on the good correlation between DOAC levels and lag-time3,5–9, question other findings, particularly as far as the influence of dabigatran on peak thrombin and ETP is concerned. In fact, some investigators reported that dabigatran treatment does not influence either ETP or thrombin peak5,6–8 whereas others observed a paradoxical increase of both parameters9. At variance with these findings, we show that: (i) dabigatran levels correlate significantly with ETP and peak thrombin; (ii) ETP and thrombin peak values were significantly lower in C-Peak than in C-Trough samples; and (iii) the addition of idarucizumab increased ETP (in both C-Peak and C-Trough samples) and thrombin peak (in C-Peak samples). These observations provide compelling evidence that dabigatran treatment, besides delaying thrombin formation (lag-time), reduces the total amount of the enzyme. The reasons for the discrepancy between our and others’ findings are unknown and could be related to the sample studied (patients, healthy volunteers) and/or the assay conditions, particularly with regard to the nature and composition of the internal calibrator.
Concerning the effect of DOAC on clot lysis, we provide further evidence suggesting that dabigatran is superior to anti-Xa drugs as a profibrinolytic agent. First, clot lysis times in the dabigatran group were shorter than in the anti-Xa groups, and the difference was statistically significant compared to the apixaban and rivaroxaban groups. Second, a significant correlation between drug concentration and clot lysis time was only observed in the dabigatran group. In particular, if we considered the peak-to-trough difference in drug concentration and clot lysis, in order to estimate the individual fibrinolytic changes induced by the rise in DOAC level, the correlation between delta drug level and delta clot lysis time was highly significant in the dabigatran group but very poor in the other groups. Finally, and more importantly, the addition of DOAC-stop, which efficiently abolishes the anticoagulant activity of all DOAC, prolonged the lysis time only in the dabigatran group, even in C-Trough samples. This observation is strengthened by the fact that neutralisation of dabigatran by idarucizumab also caused a significant prolongation of lysis time in both C-Peak and C-Trough dabigatran samples. In the light of these findings, the small but significant shortening of lysis time at C-Peak in edoxaban and rivaroxaban groups might be due to the circadian rhythm of several fibrinolytic factors, particularly plasminogen activator inhibitor-1, which is known to decrease from early to late morning23.
One possible explanation for the different behaviour of dabigatran and anti-Xa DOAC as profibrinolytic agents may be related to the way they influence TG. One major mechanism by which anticoagulants stimulate fibrinolysis is through the inhibition of thrombin-induced activation of thrombin activatable fibrinolysis inhibitor (TAFI)24. Activated TAFI, in turn, inhibits fibrinolysis with a threshold mechanism, meaning that fibrin degradation will be halted as long as the concentration of activated TAFI is above the threshold level25. In dabigatran samples, the rapid formation and disappearance of thrombin means that TAFI activation takes place in a narrow time window and the inhibition of fibrinolysis is short-lived because of the instability of activated TAFI12. In samples containing the anti-Xa DOAC, thrombin activity decays at a much slower pace and, although thrombin levels are small, low grade TAFI activation may take place for a longer period. Given that the concentration of activated TAFI required to inhibit fibrinolysis is very low26, this might be enough to delay the start of fibrin degradation compared to that in dabigatran samples.
One limitation of our study is that we lacked a proper control group. The data obtained from healthy donors, which we used to establish the reference values, cannot be compared to those deriving from patients, who are sick and significantly older. Nevertheless, the comparison among the different treatment groups, the differences between C-Trough and C-Peak samples along with the data from DOAC-stop and idarucizumab experiments provide sufficient information to appreciate the major differences between anti-Xa and anti-IIa drugs and their effects on coagulation and fibrinolytic processes.
CONCLUSION
Our study adds to previous reports on the effect of DOAC on TG and fibrinolysis. The data presented here show that dabigatran inhibits TG in a different way from anti-Xa DOAC and that the difference is probably behind the greater profibrinolytic activity of dabigatran. The availability of reliable assays to measure the antithrombotic activity of DOAC may have clinical implications. Previous studies have shown that thrombotic as well as bleeding complications occurred in patients with DOAC levels in the “on-therapy” range27–30, meaning that the assay of drug levels may not be enough to identify patients at risk. Global assays that measure the actual anticoagulant and profibrinolytic activities of any given DOAC concentration may provide a tailored picture of a patient’s response to treatment and help to identify those at higher risk of thrombotic or bleeding complications. Prospective clinical studies are warranted to assess the meaning of each CAT parameter in the stratification of risk in patients treated with different DOAC. The greater profibrinolytic activity of dabigatran observed in our patients may represent a double-edged sword as it may improve efficacy but may also affect safety by increasing the bleeding risk. Unfortunately, no head-to-head trials directly comparing the drugs against each other have been performed and the available information stemming from indirect comparisons by network meta-analysis does not allow any definitive conclusion to be drawn about the relative efficacy and safety of the different DOAC31–34.
Supplementary Information
Footnotes
FUNDING
The study was partially supported by a grant (to MC) from “Aldo Moro” University, Bari.
AUTHORSHIP CONTRIBUTIONS
MC designed and supervised the study and wrote the manuscript. LD, AV, FM and TCA performed the research. CD and OP collected the samples, supervised the clinical study management, and performed some assays. ST designed the study and critically revised the manuscript. All Authors approved the final version of the manuscript.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Douxfils J, Ageno W, Samama CM, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost. 2018;16:209–19. doi: 10.1111/jth.13912. [DOI] [PubMed] [Google Scholar]
- 2.Chan N, Sobieraj-Teague M, Eikelboom JW. Direct oral anticoagulants: evidence and unresolved issues. Lancet. 2020;396:1767–76. doi: 10.1016/S0140-6736(20)32439-9. [DOI] [PubMed] [Google Scholar]
- 3.Rigano J, Ng C, Nandurkar H, Ho P. Thrombin generation estimates the anticoagulation effect of direct oral anticoagulants with significant interindividual variability observed. Blood Coagul Fibrinolysis. 2018;29:148–54. doi: 10.1097/MBC.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 4.Hemker HC, Al Dieri R, De Smedt E, Béguin S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb Haemost. 2006;96:553–61. [PubMed] [Google Scholar]
- 5.Pfrepper C, Metze M, Siegemund A, et al. Direct oral anticoagulant plasma levels and thrombin generation on ST Genesia system. Res Pract Thromb Haemost. 2020;4:619–27. doi: 10.1002/rth2.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Artang R, Anderson M, Riley P, Nielsen JD. Assessment of the effect of direct oral anticoagulants dabigatran, rivaroxaban, and apixaban in healthy male volunteers using a thrombin generation assay. Res Pract Thromb Haemost. 2017;1:194–201. doi: 10.1002/rth2.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tripodi A, Padovan L, Veena C, et al. How the direct oral anticoagulant apixaban affects thrombin generation parameters. Thromb Res. 2015;135:1186–90. doi: 10.1016/j.thromres.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 8.Bloemen S, Zwaveling S, Douxfils J, et al. The anticoagulant effect of dabigatran is reflected in the lag time and time-to-peak, but not in the endogenous thrombin potential or peak, of thrombin generation. Thromb Res. 2018;171:160–6. doi: 10.1016/j.thromres.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Helin TA, Lemponen M, Hjemdahl P, et al. From laboratory to clinical practice: dabigatran effects on thrombin generation and coagulation in patient samples. Thromb Res. 2015;136:154–60. doi: 10.1016/j.thromres.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 10.Undas A, Ariens RA. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler Thromb Vasc Biol. 2011;31:e88–99. doi: 10.1161/ATVBAHA.111.230631. [DOI] [PubMed] [Google Scholar]
- 11.Sakata Y, Aoki N. Significance of cross-linking of alpha 2-plasmin inhibitor to fibrin in inhibition of fibrinolysis and in hemostasis. J Clin Invest. 1982;69:536–42. doi: 10.1172/JCI110479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foley JH, Kim PY, Mutch NJ, Gils A. Insights into thrombin activatable fibrinolysis inhibitor function and regulation. J Thromb Haemost. 2013;11:306–15. doi: 10.1111/jth.12216. [DOI] [PubMed] [Google Scholar]
- 13.Ammollo CT, Semeraro F, Incampo F, et al. Dabigatran enhances clot susceptibility to fibrinolysis by mechanisms dependent on and independent of thrombin-activatable fibrinolysis inhibitor. J Thromb Haemost. 2010;8:790–8. doi: 10.1111/j.1538-7836.2010.03739.x. [DOI] [PubMed] [Google Scholar]
- 14.Varin R, Mirshahi S, Mirshahi P, et al. Whole blood clots are more resistant to lysis than plasma clots - greater efficacy of rivaroxaban. Thromb Res. 2013;131:e100–9. doi: 10.1016/j.thromres.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 15.Morishima Y, Honda Y. A direct oral anticoagulant edoxaban accelerated fibrinolysis via enhancement of plasmin generation in human plasma: dependent on thrombin-activatable fibrinolysis inhibitor. J Thromb Thrombolysis. 2019;48:103–10. doi: 10.1007/s11239-019-01851-8. [DOI] [PubMed] [Google Scholar]
- 16.Semeraro F, Incampo F, Ammollo CT, et al. Dabigatran but not rivaroxaban or apixaban treatment decreases fibrinolytic resistance in patients with atrial fibrillation. Thromb Res. 2016;138:22–9. doi: 10.1016/j.thromres.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 17.Janion-Sadowska A, Chrapek M, Konieczyńska M, Undas A. Plasma fibrin clot properties in the G20210A prothrombin mutation carriers following venous thromboembolism: the effect of rivaroxaban. Thromb Haemost. 2017;117:1739–49. doi: 10.1160/TH17-01-0060. [DOI] [PubMed] [Google Scholar]
- 18.Königsbrügge O, Weigel G, Quehenberger P, et al. Plasma clot formation and clot lysis to compare effects of different anticoagulation treatments on hemostasis in patients with atrial fibrillation. Clin Exp Med. 2018;18:325–36. doi: 10.1007/s10238-018-0490-9. [DOI] [PubMed] [Google Scholar]
- 19.Bavalia R, Abdoellakhan R, Brinkman HJM, et al. Emergencies on direct oral anticoagulants: Management, outcomes, and laboratory effects of prothrombin complex concentrate. J Thromb Haemost. 2018;16:2276–88. doi: 10.1002/rth2.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate - a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116–27. doi: 10.1160/TH09-11-0758. [DOI] [PubMed] [Google Scholar]
- 21.Denny NDR, Keighley L, Siganporia Z, et al. A level-headed approach to measuring direct oral anticoagulants: a 2-year retrospective analysis of DOAC levels from a tertiary UK centre. Int J Lab Hematol. 2019;41:200–7. doi: 10.1111/ijlh.12944. [DOI] [PubMed] [Google Scholar]
- 22.Exner T, Michalopoulos N, Pearce J, et al. Simple method for removing DOACs from plasma samples. Thromb Res. 2018;163:117–22. doi: 10.1016/j.thromres.2018.01.047. [DOI] [PubMed] [Google Scholar]
- 23.Carmona P, Mendez N, Ili CG, Brebi P. The role of clock genes in fibrinolysis regulation: circadian disturbance and its effect on fibrinolytic activity. Front Physiol. 2020;11:129. doi: 10.3389/fphys.2020.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colucci M, Semeraro N. Thrombin activatable fibrinolysis inhibitor: at the nexus of fibrinolysis and inflammation. Thromb Res. 2012;129:314–9. doi: 10.1016/j.thromres.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 25.Leurs J, Nerme V, Sim Y, Hendriks D. Carboxypeptidase U (TAFIa) prevents lysis from proceeding into the propagation phase through a threshold-dependent mechanism. J Thromb Haemost. 2004;2:416–23. doi: 10.1111/j.1538-7836.2004.00605.x. [DOI] [PubMed] [Google Scholar]
- 26.Bajzar L, Morser J, Nesheim M. TAFI, or plasma procarboxypeptidase B, couples the coagulation and fibrinolytic cascades through the thrombin-thrombomodulin complex. J Biol Chem. 1996;271:16603–8. doi: 10.1074/jbc.271.28.16603. [DOI] [PubMed] [Google Scholar]
- 27.Ten Cate H, Olie RH, Ten Cate-Hoek AJ, Henskens YMC. Direct oral anticoagulants: when to consider laboratory testing? Int J Lab Hematol. 2018;40(Suppl 1):30–3. doi: 10.1111/ijlh.12816. [DOI] [PubMed] [Google Scholar]
- 28.Testa S, Paoletti O, Legnani C, et al. Low drug levels and thrombotic complications in high-risk atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2018;16:842–8. doi: 10.1111/jth.14001. [DOI] [PubMed] [Google Scholar]
- 29.Sennesael AL, Larock AS, Douxfils J, et al. Rivaroxaban plasma levels in patients admitted for bleeding events: insights from a prospective study. Thromb J. 2018;16:28. doi: 10.1186/s12959-018-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albaladejo P, Bonhomme F, Blais N, et al. French Working Group on Perioperative, Management of direct oral anticoagulants in patients undergoing elective surgeries and invasive procedures: updated guidelines from the French Working Group on Perioperative Hemostasis (GIHP) Anaesth Crit Care Pain Med. 2017;36:73–6. doi: 10.1016/j.accpm.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Lip GY, Larsen TB, Skjøth F, Rasmussen LH. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2012;60:738–46. doi: 10.1016/j.jacc.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Morimoto T, Crawford B, Wada K, Ueda S. Comparative efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation: a network meta-analysis with the adjustment for the possible bias from open label studies. J Cardiol. 2015;66:466–74. doi: 10.1016/j.jjcc.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Li G, Zeng J, Zhang J, Thabane L. Comparative effects between direct oral anticoagulants for acute venous thromboembolism: indirect comparison from randomized controlled trials. Front Med. 2020;7:280. doi: 10.3389/fmed.2020.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang L, Brooks MM, Glynn NW, et al. Real-world direct comparison of the effectiveness and safety of apixaban, dabigatran, rivaroxaban, and warfarin in Medicare beneficiaries with atrial fibrillation. Am J Cardiol. 2020;126:29–36. doi: 10.1016/j.amjcard.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.