Abstract
The majority of high school–aged adolescents obtain less than the recommended amount of sleep per night, in part because of imposed early school start times. Utilizing a naturalistic design, the present study evaluated changes in objective measurements of sleep, light, and physical activity before (baseline) and during the first wave of the COVID-19 pandemic (during COVID-19) in a group of US adolescents. Sixteen adolescents (aged 15.9 ± 1.2 years, 68.8% female) wore an actigraphy monitor for 7 consecutive days during an in-person week of school before the pandemic (October 2018-February 2020) and again during the pandemic when instruction was performed virtually (May 2020). Delayed weekday sleep onset times of 1.66 ± 1.33 h (p < 0.001) and increased sleep duration of 1 ± 0.87 h (p < 0.001) were observed during COVID-19 compared with baseline. Average lux was significantly higher during COVID-19 compared with baseline (p < 0.001). Weekday physical activity parameters were not altered during COVID-19 compared with baseline, except for a delay in the midpoint of the least active 5 h (p value = 0.044). This analysis provides insight into how introducing flexibility into the traditional school schedule might influence sleep in adolescents.
Keywords: COVID-19, school start times, sleep, adolescents, circadian rhythms
A biologically induced slowed build-up of homeostatic sleep drive (sleep pressure) across the day and a physiological delay in circadian rhythms leads to late sleep onset in adolescence (Crowley et al., 2018). Combined with today’s academic and psychosocial demands, imposed early school start times, and daily profile of electronics use and light exposure, nearly 80% of high school–age adolescents obtain less than the recommended 8 to 10 h sleep per night (Paruthi et al., 2016; Crowley et al., 2018; Centers for Disease Control and Prevention, 2019). Insufficient sleep and circadian misalignment are associated with numerous negative outcomes, including mental health problems, obesity and dysregulated metabolism, learning difficulties, and poor academic achievement (Crowley et al., 2018).
Adolescents were profoundly affected in all aspects of daily life by the school closures and stay-at-home orders implemented in the United States in spring 2020 to mitigate the spread of COVID-19. Since the onset of these health measures, evidence is emerging of subsequent changes in daily routines and lifestyle activities in youth (Bates et al., 2020). Adolescents showed patterns of delayed bed and wake times, longer sleep duration, and less daytime sleepiness during COVID-19 per subjective self- and parent reports (Gruber et al., 2020; Becker et al., 2021; Bruni et al., 2021; Lavigne-Cerván et al., 2021; Illingworth et al., 2022). However, these studies are limited to primarily retrospective reports prior to COVID-19 and lack of objective assessment.
A later sleep-wake schedule may be more in line with adolescents’ circadian rhythms (Crowley et al., 2018). Indeed, multiple studies have confirmed the benefits of later high school start times (allowing for later wake time) on adolescent sleep and well-being (Owens et al., 2017; Meltzer et al., 2021b; Biller et al., 2022). Yet, going to bed and sleeping in later limits opportunities for participation in physical activity and for obtaining morning light exposure which serves to synchronize and entrain circadian rhythms (Youngstedt et al., 2016; Bates et al., 2020). Furthermore, high levels of light exposure from electronics, particularly late at night, may further delay sleep timing and the circadian system (Wams et al., 2017; Hisler et al., 2020). Lack of daily structure, increased electronics use, and spending less time outside were all associated with an irregular sleep-wake schedule and greater delay in self-reported sleep times during COVID-19 in adolescents (Amran, 2022). To our knowledge, despite the relationship and importance to sleep, light and activity levels have not been systematically evaluated in adolescents during COVID-19.
Utilizing a naturalistic design, we evaluated changes in the objective measurement of sleep, light, and activity before and during the COVID-19 pandemic in adolescents. Without the restrictions of early start times and the structure of traditional schooling, we hypothesized that adolescents would have longer, later, and less variable sleep, increased and later light exposure, and decreased physical activity during COVID-19 compared with prior.
Materials and Methods
Participants from a study examining insulin sensitivity and sleep in adolescents (ClinicalTrials.gov: NCT03500458) were invited to participate in an additional study week during COVID-19 stay-at-home orders. Inclusion criteria for the primary study included habitually sedentary (<3 h of reported physical activity per week) high school students 14 to 19 years of age with < 7 h sleep on school nights without a diagnosis of a sleep disorder or regular use of medications affecting sleep. The study was approved by the Colorado Multiple Institutional Review Board, and participants and guardians who previously consented for the primary study provided verbal consent/assent for the optional COVID-19 week. Participants completed 7 consecutive days of at-home monitoring at two time points: the baseline week took place prior to COVID-19, between October 2018 and February 2020, and the COVID-19 week occurred in May 2020. Participants were required to be attending traditional, in-person high school prior to COVID-19, and all study participation took place during the academic year while school was in session. Efforts were made to avoid data collection during daylight saving weeks and over school breaks; one study participant participated at the end of daylight savings at baseline. Actigraphy devices were delivered to families and returned by mail. During both study weeks, participants were asked to maintain their current, typical schedule.
Outcome Measures
Participants wore a Spectrum Plus actigraphy monitor (Philips Respironics, Bend, OR) on their nondominant wrist for 7 consecutive days. Participants were asked to press the event marker button on the watch at the time that they attempted sleep and again upon awakening, and concurrent sleep diaries were completed daily to facilitate scoring. Data were scored using proprietary software (Actiware Version 6, Philips Respironics, Pittsburgh, PA) and standard scoring rules (Ancoli-Israel et al., 2015).
Sleep-Wake
The following variables were derived separately for all recording days and averaged over weekdays (Sunday-Thursday nights) and weekends (Friday and Saturday nights): sleep onset, sleep offset, sleep midpoint, total sleep duration (difference between sleep onset and offset minus wake after sleep onset), and sleep efficiency. In addition, social jetlag (difference between weekend and weekday sleep midpoints; Mathew et al., 2019) and sleep regularity (intraindividual standard deviations of sleep onset, offset, midpoint, and duration) were calculated.
Light
The Actiwatch Spectrum calculates white light illuminance (lux) on a minute-by-minute basis by integrating the input from separate red-, green-, and blue-colored light sensors. The light data were summarized as average lux values over 24 h, during the average estimated waking window, and during the period 2 h before average bedtime, and as a percentage of time at > 1000 lux and < 100 lux, indicative of likely exposure to outdoor and indoor light, respectively (Bhandary et al., 2021).
Activity
The Actiwatch Spectrum estimates physical activity levels using accelerometer counts, with higher values indicating more activity. Changes in physical activity patterns over 24 h were evaluated from the accelerometer count data using the nonparametric methods first described by Witting et al. (1990) and more recently reviewed by Gonçalves et al. (2015). Briefly, these methods attempt to estimate the stability and variability of the 24 h rest-activity cycle across days of measurement. The ‘nparACT’ package (Blume et al., 2016) for R Core Team (R Development Core Team, 2020) was used to derive the following variables:
Inter-daily Stability (IS): An estimate of stability of the 24 h rest-activity cycle across days, where a value of 1 = perfect stability.
Intra-daily Variability (IV): An estimate of the fragmentation of the 24 h rest-activity cycle where a value of 0 = a perfect sine wave with no fragmentation and 2 = no apparent pattern in the 24 h rest-activity cycle.
The L5 indicates the period of time with the lowest 5 h of activity (in accelerometer counts).
The M10 indicates the period of time with the highest 10 h of activity (in accelerometer counts).
The relative amplitude (RA) is the ratio of the M10 and L5 average accelerometer counts. Higher values indicate more robust 24 h rest-activity patterns.
Questionnaires
Participants self-reported demographic information at baseline. Chronotype was assessed at baseline with the Morningness-eveningness Scale for Children (MESC), a validated 10-item self-report multiple-choice measure (Carskadon et al., 1993). Scores range from 10 to 42 with higher scores indicating more morning preference and lower scores indicating more evening preference. Using cut-off points based on the 25th to 75th percentiles, individuals with scores of 10 to 23 were categorized as evening-type, individuals with scores of 24 to 27 were categorized as intermediate-type, and individuals with scores of 28 to 40 were categorized as morning-type (Díaz-Morales and Gutiérrez Sorroche, 2008). At the COVID-19 assessment, participants responded to questions derived for the purpose of the current study asking them to report if they spent more, less, or the same amount of time engaged in behaviors including electronics/technology, physical activity, time outside, social interactions, and schoolwork. They also completed the PROMIS Anxiety and Depression short form measures at both baseline and during COVID-19 to assess anxiety and depression symptoms over the past week (Irwin et al., 2012). Raw scores are summed and converted to T-scores, with scores of 55 and below described as “within normal limits,” 55 to 60 “mild,” 60 to 70 “moderate,” and >70 “severe” (Kaat et al., 2019).
Analyses
All analyses were stratified by weekday and weekend. Comparisons of each outcome (sleep, light, and activity) from baseline to the period during COVID-19 outcome were evaluated using paired sample t tests or Wilcoxon signed-rank test. Shapiro-Wilk tests were used to determine the normality of outcomes and those identified as non-normal (Shapiro-Wilk p value < 0.05) were compared using the nonparametric Wilcoxon signed-rank test. Data are presented as mean ± standard deviation (SD) regardless of the test used. To account for multiple comparisons, p values were adjusted using the Holm–Bonferroni method. The p values were adjusted within each type of outcome (sleep, light, and activity) for weekdays and weekends separately.
We hypothesized that the MESC score (evening, intermediate, or morning chronotype) might explain any observed changes in sleep given that the participants may have had more flexibility in their daily schedules during the COVID-19 week. To assess this, linear mixed models were fit to predict sleep onset, sleep offset, sleep duration, and sleep efficiency based on the day of the week, study time point (baseline, during COVID-19), and chronotype. In addition, interactions were included for night-by-study time point, and study time point-by-chronotype, with this time-by-chronotype interaction being of particular interest in addressing our hypothesis. The linear mixed models accounted for within-subject correlations using an AR(1) covariance structure and a random intercept by subject. All models were fit separately for weekdays and weekends. The estimated difference between baseline and COVID-19 was calculated from the linear mixed models for each chronotype at each study time point. For each model, a Type 3 Test of Fixed Effects for the time-by-chronotype interaction was used to directly address if the chronotype explains the observed changes in sleep between study time points. The p values from the Type 3 Tests of Fixed Effects were adjusted for multiple comparisons using the Holm–Bonferroni method. The p values were adjusted for weekdays and weekends separately.
Linear mixed models were fit using SAS Software (SAS Institute Inc., Cary, NC, USA), while all other analyses were conducted in the R computing environment (R Development Core Team, 2020). Results with an adjusted p value < 0.05 were considered statistically significant.
Sensitivity Analyses
This study included one subject who had a set school start time during COVID-19. A sensitivity analysis was performed to assess if the inclusion of this subject altered the results of the statistical analyses. In addition, one subject’s baseline data were collected during time change at the end of daylight savings. A separate sensitivity analysis was performed to assess the impact of the inclusion of this subject in analyses. For each of these sensitivity analyses, all statistical analyses were performed with the exclusion of the associated subject, and results were assessed to determine if the results changed statistical or clinical significance when the subject was excluded. The exclusion of these participants did not change the statistical results appreciably; thus, both participants were retained for subsequent analyses.
Results
Sixteen participants completed procedures at both baseline and COVID-19 follow-up weeks. All participants had ≥ 4 weekdays and ≥ 2 weekend days of actigraphy data at both baseline and during COVID-19, with the exception of one participant that did not have any baseline weekend date. This participant was excluded from all weekend analyses except for linear mixed models which allow for missing data. Participants were on average 15.9 ± 1.2 years old at baseline, 68.8% female, 87.5% White, and 25.0% Hispanic/Latino. Fifty percent of participants were classified as evening chronotype, while 18.8% and 31.3% were classified as intermediate and morning chronotype, respectively. During the COVID-19 week, all participants reported participation in online learning due to in-person school closures. Prior to COVID-19, reported school start time ranged from 0730 to 0830 h. Only 1 participant (6.3%) had a set start time for online learning during COVID-19, which was reportedly consistent with their baseline school start time (0830 h); the remaining participants reported that learning was conducted according to their own schedule. Participants reported doing their schoolwork primarily in the afternoon (1200 -1700 h; 47%) during COVID-19, while most participants completed schoolwork in the evening (after 1800 h; 53%) at baseline. The majority of participants (67%) reported spending less time on schoolwork during COVID-19 compared with baseline.
Sixty percent of participants endorsed spending less time in social interactions with peers, 53% spent more time outside, and approximately half the sample reported spending more time engaged in physical activity during COVID-19 compared with baseline. Time spent using technology and electronics increased for 93% of participants during COVID-19 compared with baseline. Ratings of anxiety and depression symptoms remained on average in the normal to mild range at both baseline (mean PROMIS depression t-scores = 53.3 ± 10.9; mean PROMIS anxiety t scores = 50.5 ± 11.1) and COVID-19 (mean PROMIS depression t scores = 54.6 ± 9.8; mean PROMIS anxiety t scores = 46.9 ± 11.4) with no significant change between timepoints (all p values > 0.05). No participants reported COVID-19-related symptoms or a prior diagnosis of COVID-19 while participating in the study.
Change in Sleep
Actigraphy-estimated sleep measures at baseline and during COVID-19 are presented in Table 1 and Figure 1. Participants obtained on average 1 more hour of sleep per night on weekdays during COVID-19 compared with baseline (p < 0.001), while weekend sleep duration during COVID-19 was not significantly different from baseline (p = 0.268). At baseline, 10.6% of all recorded sleep episodes were greater than 8 h in duration, compared with 24.8% of all recorded sleep episodes during the COVID-19 week (χ2 test p = 0.008). Bedtime, waketime, and the midpoint of sleep were each significantly delayed on both weekdays and weekends during COVID-19 compared with baseline (all p values < 0.05). The largest delay was observed in weekday waketime which occurred nearly 3 h later during COVID-19 than baseline. Sleep efficiency decreased modestly by −3.75% (p < 0.001) during the weekdays but was not significantly changed during the weekends (p = 0.268). Social jetlag (defined here as the difference in weekday and weekend sleep midpoints; Mathew et al., 2019) was not statistically different between time points (p = 0.058). Similarly, sleep regularity was also not significantly different during COVID-19 compared with baseline (all p values > 0.05; see Supplemental Table 1).
Table 1.
Weekday and weekend sleep parameters assessed with wrist actigraphy in N = 16 teenagers before the COVID-19 pandemic and during the initial stay-at-home and safer-at-home phase of the pandemic in Colorado. Data are shown as mean ± SD.
| Baseline | During COVID-19 | Δ | Adjusted p value | |
|---|---|---|---|---|
| Weekday recordings | ||||
| Time of sleep onset | 00:01 ± 00:59 | 01:41 ± 01:26 | 1.66 ± 1.13 | <0.001 |
| Time of sleep offset | 06:30 ± 00:35 | 09:23 ± 01:19 | 2.88 ± 0.94 | <0.001 |
| Time of sleep midpoint | 03:16 ± 00:45 | 05:32 ± 01:18 | 2.27 ± 0.9 | <0.001 |
| Sleep duration (h) | 5.93 ± 0.41 | 6.93 ± 0.86 | 1 ± 0.87 | <0.001 |
| Sleep efficiency (%) | 87.9 ± 4.46 | 84.15 ± 3.51 | -3.75 ± 3.1 | <0.001 |
| Social jetlag (h)* | 0.92 ± 1.5 | 0.46 ± 0.89 | -0.46 ± 1.81 | 0.058 |
| Weekend recordings | ||||
| Time of sleep onset | 00:27 ± 01:08 | 02:07 ± 01:52 | 1.66 ± 1.79 | 0.01 |
| Time of sleep offset | 08:30 ± 01:12 | 09:40 ± 01:17 | 1.15 ± 1.21 | 0.01 |
| Time of sleep midpoint | 04:28 ± 00:59 | 05:53 ± 01:25 | 1.43 ± 1.38 | 0.007 |
| Sleep duration (h) | 7.25 ± 1.11 | 6.76 ± 1.36 | -0.49 ± 1.2 | 0.268 |
| Sleep efficiency (%) | 84.54 ± 4.51 | 82.33 ± 5.65 | -2.21 ± 6.12 | 0.268 |
Data are shown as mean ± standard deviation. Change represents difference in activity variables from baseline to during COVID-19 and p values correspond to paired-sample t-tests or Wilcoxon signed-rank tests (indicated by *), adjusted for multiple comparisons using the Holm–Bonferroni method.
Bold values indicate p < 0.05.
Figure 1.
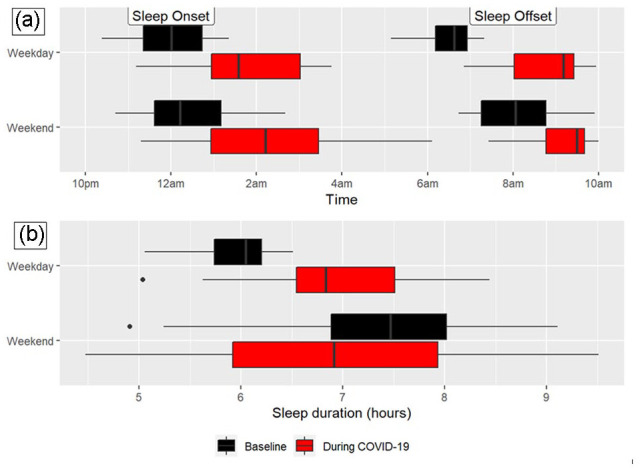
Sleep timing (a) and duration (b) at baseline and during the COVID-19 pandemic on weekdays and weekends. Sleep onset and offset were significantly delayed on both weekdays and weekends during the COVID-19 week compared with baseline. Sleep duration on weekdays significantly increased during COVID-19 compared with baseline, while weekday sleep duration was not significantly different between time points.
Change in Sleep by Chronotype
Estimated changes in sleep onset, sleep offset, sleep duration, and sleep efficiency from baseline to during COVID-19 by chronotype from linear mixed models are presented in Supplemental Table 2. Although the estimated sleep outcome changed between baseline and COVID-19 for each chronotype, none of the time-by-chronotype interactions were statistically significant (all p values > 0.05), indicating that MESC chronotype did not significantly impact the observed changes in these sleep outcomes from baseline to during COVID-19.
Change in Light
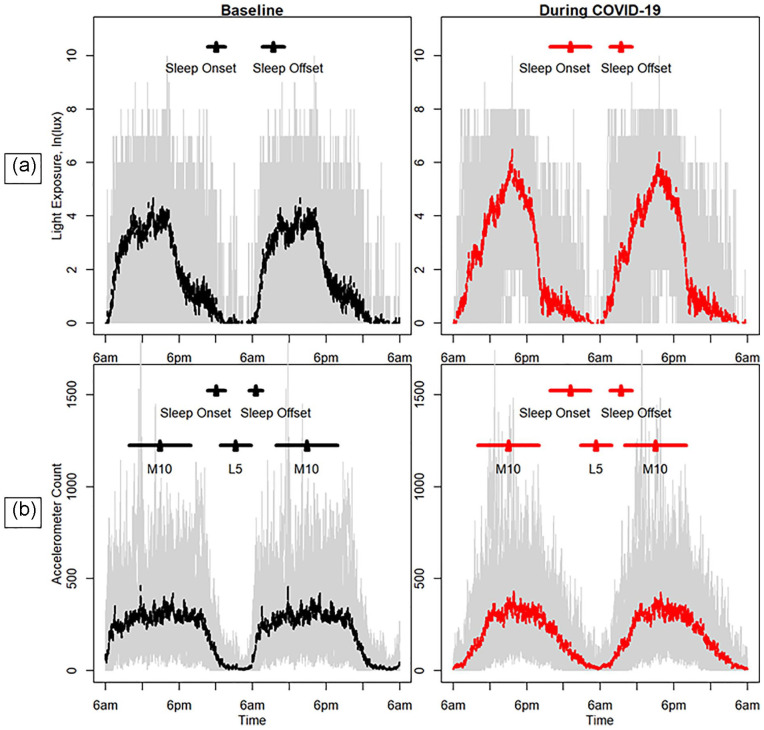
Light variables are presented in Table 2 and Figure 2. Weekday average lux over 24 h and while awake were significantly higher during COVID-19 compared with baseline (p = 0.001 for both outcomes), with participants more than doubling average lux levels during COVID-19. Weekday average light exposure in the 2 h prior to bedtime (i.e., evening light) was also significantly higher during COVID-19 compared with baseline (p value = .007). In addition, the percentage of time spent at > 1000 lux was significantly higher and the percentage of time at < 100 lux was significantly lower during COVID-19 compared with baseline (p values = 0.007 for both outcomes).
Table 2.
Weekday and weekend light levels were assessed with wrist actigraphy in N = 16 teenagers before the COVID-19 pandemic and during the initial stay-at-home and safer-at-home phase of the pandemic in Colorado.
| Baseline | During COVID-19 | Δ | Adjusted p value | |
|---|---|---|---|---|
| Weekday recordings | ||||
| 24 h average, lux* | 85.17 ± 59.53 | 234.29 ± 175.35 | 149.12 ± 173.1 | 0.001 |
| Waking average, lux* | 109.48 ± 76.44 | 343.48 ± 303.83 | 234 ± 295.28 | 0.001 |
| Average 2 h prior to sleep, lux | 86.02 ± 64.69 | 237.9 ± 167.97 | 151.88 ± 168.97 | 0.007 |
| Percent of time > 1000 lux* | 1.37 ± 1.26 | 7.37 ± 7.56 | 5.99 ± 7.51 | 0.007 |
| Percent of time < 100 lux | 83.95 ± 10.16 | 72.58 ± 12.25 | –11.37 ± 11.89 | 0.007 |
| Weekend recordings | ||||
| 24 h average, lux | 87.59 ± 60.8 | 240.25 ± 179.82 | 152.66 ± 178.57 | 0.015 |
| Waking average, lux* | 112.4 ± 78.2 | 352.41 ± 312.32 | 240.01 ± 304.63 | 0.003 |
| Average 2 h prior to sleep, lux | 77.51 ± 58.4 | 248.28 ± 198.01 | 170.77 ± 208.13 | 0.015 |
| Percent of time > 1000 lux* | 1.43 ± 1.28 | 7.63 ± 7.75 | 6.2 ± 7.73 | 0.013 |
| Percent of time < 100 lux* | 83.69 ± 10.46 | 72.36 ± 12.65 | –11.33 ± 12.31 | 0.007 |
Data are shown as mean ± standard deviation. Change represents the difference in the light variables from baseline to during COVID-19 and p values correspond to paired-sample t tests or Wilcoxon signed-rank tests (indicated by *), adjusted for multiple comparisons using the Holm–Bonferroni method.
Bold values indicate p < 0.05.
Figure 2.
Weekday light (ln lux; a) and daily accelerometer counts (b) at baseline and during the COVID-19 pandemic. The standard deviation in the average local time of sleep onset and offset are shown by the black and red horizontal bars. In addition, for accelerometry, horizontal lines are included to indicate the standard deviation in the local time of the lowest level of activity over a 5-h period (L5) and the highest level of activity over a 10-h period (M10). The gray background indicates the range (minimum and maximum) of the observed light exposures or accelerometer counts. Color version of the figure is available online.
Change in Activity
Physical activity variables are presented in Table 3 and Figure 2. The weekday midpoint of the least active 5 h was significantly delayed by approximately 2 h during COVID-19 compared with baseline (p value = 0.044). There were no other significant differences in the actigraphy-estimated activity outcomes between baseline and the COVID-19 week for weekdays or weekends.
Table 3.
Weekday and weekend physical activity variables assessed with wrist actigraphy in N = 16 teenagers before the COVID-19 pandemic and during the initial stay-at-home and safer-at-home phase of the pandemic in Colorado.
| Baseline | During COVID-19 | Δ | Adjusted p value | |
|---|---|---|---|---|
| Weekday recordings | ||||
| Interdaily stability | 0.62 ± 0.1 | 0.51 ± 0.15 | –0.11 ± 0.19 | 0.147 |
| Intradaily variability | 0.82 ± 0.24 | 0.75 ± 0.22 | –0.07 ± 0.18 | 0.388 |
| Relative amplitude* | 0.93 ± 0.05 | 0.84 ± 0.14 | –0.08 ± 0.15 | 0.147 |
| Avg. counts, least active 5 h (L5)* | 11.66 ± 6.78 | 23.7 ± 19.95 | 12.04 ± 20.33 | 0.147 |
| Avg. counts, most active 10 h (M10)* | 317.56 ± 81.28 | 307.31 ± 125.3 | –10.25 ± 103.86 | 0.807 |
| Mid-point time of the L5* | 0610 ± 0744 h | 0823 ± 0732 h | 0213 ± 0902 h | 0.044 |
| Mid-point time of the M10 | 1447 ± 0242 h | 1502 ± 0220 h | 0016 ± 0229 h | 0.807 |
| Weekend recordings | ||||
| Interdaily stability | 0.67 ± 0.14 | 0.76 ± 0.12 | 0.09 ± 0.2 | 0.488 |
| Intradaily variability | 0.57 ± 0.15 | 0.64 ± 0.25 | 0.07 ± 0.29 | 0.671 |
| Relative amplitude* | 0.94 ± 0.03 | 0.92 ± 0.05 | –0.02 ± 0.04 | 0.500 |
| Avg. counts, least active 5 h (L5) | 9.81 ± 4.01 | 13.84 ± 8.9 | 4.03 ± 6.29 | 0.158 |
| Avg. counts, most active 10 h (M10)* | 359.89 ± 145.28 | 361.39 ± 153.35 | 1.5 ± 155.77 | 0.762 |
| Mid-point time of the L5* | 0515 ± 0553 h | 0523 ± 0142 h | 0008 ± 0648 h | 0.106 |
| Mid-point time of the M10* | 1509 ± 0213 h | 1626 ± 0141 h | 0117 ± 0250 h | 0.488 |
Data are shown as mean ± standard deviation. Change represents the difference in activity variables from baseline to during COVID-19 and p values correspond to paired-sample t tests or Wilcoxon signed-rank tests (indicated by *), adjusted for multiple comparisons using the Holm–Bonferroni method.
Bold values indicate p < 0.05.
Discussion
The current analysis of changes in objectively assessed sleep, light, and activity from before and during the first wave of the COVID-19 pandemic found that adolescents delayed sleep times, increased sleep duration, and received more light exposure during COVID-19 compared with baseline. With the majority of participants free from the constraints of an early school start time, adolescents obtained longer sleep duration during the COVID-19 pandemic, notably by sleeping later in the mornings on school days. This equates to an additional 5 h of sleep over the course of a school week. Sleep efficiency decreased modestly on weekdays during COVID-19, but the clinical significance of this change is unclear. Moreover, bedtimes and waketimes shifted later on both weekdays and weekends, with the most significant delay in weekday waketime of ~3 h. These findings are similar to naturalistic studies of U.S. and Brazilian adolescents that self-reported a delay in bed and waketimes of 1-1.5 and 1.5 to 2 h, respectively, and increased sleep duration during COVID-19 compared with assessments that took place prior to COVID-19 (Becker et al., 2021; Genta et al., 2021). In a sample of college students assessed via sleep log before and during COVID-19, weekday time in bed increased, weekday sleep timing delayed, and social jetlag was reduced during COVID-19 (Wright et al., 2020). In contrast, social jetlag did not significantly change in our sample.
One potential contributor to these changes in sleep includes greater flexibility of schedules associated with online learning and lack of commute to school resulting in greater opportunity for more sleep. Indeed, a cross-sectional study of 6- to 12-grade students in the United States found that instruction type was significantly associated with timing and duration of sleep during COVID-19 such that bed and waketimes were latest and sleep opportunity was longer on nights when students had online/asynchronous learning compared with online/synchronous, and in-person schooling (Meltzer et al., 2021a). This is consistent with studies completed prior to the pandemic that have shown that delaying high school start times by 50-70 min allows students to obtain ~40-45 min more weeknight sleep compared with high school students before the change in start times (Meltzer et al., 2021b) and compared with students at schools that maintained an early school start time of 0730 h (Widome et al., 2020).
Although sleep duration increased and the percentage of adolescents that obtained more than 0800 h of sleep more than doubled during COVID-19 in our sample, approximately 75% of adolescents still obtained insufficient sleep during COVID-19. These findings highlight that adolescent sleep is modifiable but that additional strategies beyond flexibility in schedule may be needed to improve sleep health and specifically sleep duration to recommended levels for age. While there was no significant effect of chronotype on the observed changes in sleep variables in the current study, other studies suggest strategies to improve sleep health individualized depending on chronotype and other characteristics may be needed (Gradisar et al., 2011; Blake et al., 2019).
In the current study, adolescents had greater light exposure during COVID-19 over the 24-h period, while awake, and in the 2 h prior to sleep, as well as increased the percentage of time > 1000 lux, which may reflect greater exposure to sunlight or electronics. These findings should be considered within the limitations of the current data such that there was a longer daylight period during the COVID-19 data collection period in May compared to participants who completed their baseline assessment in the fall or winter. Notably, only roughly half of the participants reported spending more time outside, while nearly all participants endorsed spending more time using electronics during COVID-19 compared with baseline. In a sample of youth attending a morning or afternoon-shift school schedule, evening electronics use was correlated with later bedtimes and shorter time in bed regardless of school start time (Arrona-Palacios, 2017). Thus, future studies should include measurement of daily electronics usage to evaluate its role in sleep timing. In a study conducted prior to the pandemic, actigraphy-measured light exposure and dim light melatonin onset (DLMO) were compared between 14 young adults with delayed sleep and 14 matched controls and found that the delayed sleep group had relatively greater exposure to white and blue light 2 h after DLMO, a circadian time with maximal phase-delay effect (Van der Maren et al., 2018). A limitation of the current study is that we were not able to evaluate melatonin; consideration of the circadian phase in adolescents is important for future studies.
We did not find a significant change in objective activity parameters from baseline to COVID-19, except for a delay in the weekday midpoint of the least active 5 h. This may be due to the similarly observed delay in sleep times. These findings are in contrast to a study of school-age children in Israel who wore Actiwatch and demonstrated a significant decrease in time engaged in moderate-to-vigorous physical activity during COVID-19 compared with before COVID-19 (Guo et al., 2021). This difference may be in part due to age, as physical activity decreases throughout adolescence, primarily replaced by sedentary activity (Kandola et al., 2020). Moreover, inclusion criteria for the current parent study required participants to have a typical low level of physical activity (< 3 h of reported physical activity per week), although this is broadly representative of the adolescent population (Guthold et al., 2020). Notably, participants in the current sample were evenly split in their subjective report of engaging in more and less physical activity during COVID-19 compared with baseline.
The current findings should be considered within the limitations of the study, including a small sample size. Our sample included adolescents that obtained insufficient sleep during the academic year prior to COVID-19 and thus may not be generalizable to all youth. Potentially important variables, such as daily patterns of electronics usage, specific virtual school schedules, individual and family stressors, and other factors that may have impacted sleep behaviors were not assessed in the present study. Using the Actiwatch to derive estimates of physical activity is not ideal and may have missed changes in sedentary behaviors which may be more accurately measured by other devices. Similarly, the Actiwatch has been found to underestimate light levels compared with gold standard measures (Howell et al., 2021). In addition, the validity of actigraphy devices in estimating certain sleep parameters is inconsistent, with the devices tending to underestimate onset latency in adolescents (Meltzer et al., 2015). Finally, intraindividual standard deviations were used to estimate sleep regularity, but future analysis using the Sleep Regularity Index is recommended (Phillips et al., 2017). However, strengths of the study include objective measurement of sleep, activity, and light both before and during COVID-19. Although the study was performed in the early months of the pandemic when restrictions to mitigate the spread of disease and safety concerns limited research activities, the current analysis is a significant contribution to the existing COVID-19 literature that primarily relies on subjective measurement and retrospective comparisons.
Unintended effects of the lifestyle changes during the COVID-19 pandemic, such as the shift to online learning, may have provided adolescents the opportunity to obtain longer and later sleep, although the majority of adolescents still obtained insufficient sleep during COVID-19. These findings may inform parents and policy makers as they consider shifting high schools schedules to start no earlier than 0830 h, in line with recommendations (Adolescent Sleep Working Group et al., 2014; Ziporyn et al., 2022). Continued efforts by clinicians to promote healthy sleep habits and inform adolescents of the potential benefits of obtaining healthy sleep remain important. Finally, additional research to better understand the impact of these changed sleep behaviors on daytime functioning, academic performance, and health outcomes is particularly urgent as schools return to in-person learning and plan for future academic years.
Supplemental Material
Supplemental material, sj-docx-1-jbr-10.1177_07487304221123455 for A Naturalistic Actigraphic Assessment of Changes in Adolescent Sleep, Light Exposure, and Activity Before and During COVID-19 by Corey A. Rynders, Anne E. Bowen, Emily Cooper, John T. Brinton, Janine Higgins, Kristen J. Nadeau, Kenneth P. Wright Jr. and Stacey L. Simon in Journal of Biological Rhythms
Acknowledgments
This study was supported by funding from K23 DK117021 (S.L.S.), K01 DK113063 (C.A.R.), UL1 TR002535 (Colorado CTSA).
Supplementary material is available for this article online.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: K.P.W. reports during the conduct of the study being a consultant to/and/or receiving personal fees from Circadian Therapeutics, Inc., Circadian Biotherapies, Inc., Philips, Inc. outside the submitted work. All other authors declare that there are no conflicts of interest.
ORCID iD: Stacey L. Simon  https://orcid.org/0000-0003-4755-8151
https://orcid.org/0000-0003-4755-8151
References
- Adolescent Sleep Working Group, Committee on Adolescence, and Council on School Health (2014) School start times for adolescents. Pediatrics 134:642-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amran MS. (2022) Psychosocial risk factors associated with mental health of adolescents amidst the COVID-19 pandemic outbreak. Int J Soc Psychiatry 68:6-8. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Martin JL, Blackwell T, Buenaver L, Liu L, Meltzer LJ, Sadeh A, Spira AP, Taylor DJ. (2015) The SBSM guide to actigraphy monitoring: clinical and research applications. Behav Sleep Med 13:S4-S38. [DOI] [PubMed] [Google Scholar]
- Arrona-Palacios A. (2017) High and low use of electronic media during nighttime before going to sleep: a comparative study between adolescents attending a morning or afternoon school shift. J Adolesc 61:152-163. [DOI] [PubMed] [Google Scholar]
- Bates LC, Zieff G, Stanford K, Moore JB, Kerr ZY, Hanson ED, Barone Gibbs B, Kline CE, Stoner L. (2020) COVID-19 impact on behaviors across the 24-hour day in children and adolescents: physical activity, sedentary behavior, and sleep. Children (Basel) 7:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker SP, Dvorsky MR, Breaux R, Cusick CN, Taylor KP, Langberg JM. (2021) Prospective examination of adolescent sleep patterns and behaviors before and during COVID-19. Sleep 44:zsab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandary SK, Dhakal R, Sanghavi V, Verkicharla PK. (2021) Ambient light level varies with different locations and environmental conditions: potential to impact myopia. Plos One 16:e0254027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller AM, Molenda C, Zerbini G, Roenneberg T, Winnebeck EC. (2022) Sleep improvements on days with later school starts persist after 1 year in a flexible start system. Sci Rep 12:2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake MJ, Latham MD, Blake LM, Allen NB. (2019) Adolescent-sleep-intervention research: current state and future directions. Curr Dir Psychol Sci 28:475-482. [Google Scholar]
- Blume C, Santhi N, Schabus M. (2016) nparACT’ package for R: a free software tool for the non-parametric analysis of actigraphy data. MethodsX 3:430-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni O, Malorgio E, Doria M, Finotti E, Spruyt K, Melegari MG, Villa MP, Ferri R. (2021) Changes in sleep patterns and disturbances in children and adolescents in Italy during the Covid-19 outbreak. Sleep Med 91:166-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. (1993) Association between puberty and delayed phase preference. Sleep 16:258-262. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2019) Youth risk behavior survey data. https://www.cdc.gov/healthyyouth/data/yrbs/index.htm
- Crowley SJ, Wolfson AR, Tarokh L, Carskadon MA. (2018) An update on adolescent sleep: new evidence informing the perfect storm model. J Adolesc 67:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Morales JF, Gutiérrez Sorroche M. (2008) Morningness-eveningness in adolescents. Span J Psychol 11:201-206. [DOI] [PubMed] [Google Scholar]
- Genta FD, Rodrigues Neto GB, Sunfeld JPV, Porto JF, Xavier AD, Moreno CRC, Lorenzi-Filho G, Genta PR. (2021) COVID-19 pandemic impact on sleep habits, chronotype, and health-related quality of life among high school students: a longitudinal study. J Clin Sleep Med 17:1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves BS, Adamowicz T, Louzada FM, Moreno CR, Araujo JF. (2015) A fresh look at the use of nonparametric analysis in actimetry. Sleep Med Rev 20:84-91. [DOI] [PubMed] [Google Scholar]
- Gradisar M, Dohnt H, Gardner G, Paine S, Starkey K, Menne A, Slater A, Wright H, Hudson JL, Weaver E, et al. (2011) A randomized controlled trial of cognitive-behavior therapy plus bright light therapy for adolescent delayed sleep phase disorder. Sleep 34:1671-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber R, Saha S, Somerville G, Boursier J, Wise MS. (2020) The impact of COVID-19 related school shutdown on sleep in adolescents: a natural experiment. Sleep Med 76:33-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YF, Liao MQ, Cai WL, Yu XX, Li SN, Ke XY, Tan SX, Luo ZY, Cui YF, Wang Q, et al. (2021) Physical activity, screen exposure and sleep among students during the pandemic of COVID-19. Sci Rep 11:8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthold R, Stevens GA, Riley LM, Bull FC. (2020) Global trends in insufficient physical activity among adolescents: a pooled analysis of 298 population-based surveys with 1·6 million participants. Lancet Child Adolesc Health 4:23-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisler GC, Hasler BP, Franzen PL, Clark DB, Twenge JM. (2020) Screen media use and sleep disturbance symptom severity in children. Sleep Health 6:731-742. [DOI] [PubMed] [Google Scholar]
- Howell CM, McCullough SJ, Doyle L, Murphy MH, Saunders KJ. (2021) Reliability and validity of the Actiwatch and Clouclip for measuring illumination in real-world conditions. Ophthalmic Physiol Opt 41:1048-1059. [DOI] [PubMed] [Google Scholar]
- Illingworth G, Mansfield KL, Espie CA, Fazel M, Waite F. (2022) Sleep in the time of COVID-19: findings from 17000 school-aged children and adolescents in the UK during the first national lockdown. Sleep Adv 3:zpab021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DE, Gross HE, Stucky BD, Thissen D, DeWitt EM, Lai JS, Amtmann D, Khastou L, Varni JW, DeWalt DA. (2012) Development of six PROMIS pediatrics proxy-report item banks. Health Qual Life Outcomes 10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaat AJ, Kallen MA, Nowinski CJ, Sterling SA, Westbrook SR, Peters JT. (2019) PROMIS® Pediatric Depressive Symptoms as a Harmonized Score Metric. J Pediatr Psychol 45:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandola A, Lewis G, Osborn DPJ, Stubbs B, Hayes JF. (2020) Depressive symptoms and objectively measured physical activity and sedentary behaviour throughout adolescence: a prospective cohort study. Lancet Psychiat 7:262-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne-Cerván R, Costa-López B, Juárez-Ruiz de, Mier R, Real-Fernández M, Sánchez-Muñoz de, León M, Navarro-Soria I. (2021) Consequences of COVID-19 confinement on anxiety, sleep and executive functions of children and adolescents in Spain. Front Psychol 12:565516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew GM, Li X, Hale L, Chang AM. (2019) Sleep duration and social jetlag are independently associated with anxious symptoms in adolescents. Chronobiol Int 36:461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer LJ, Saletin JM, Honaker SM, Owens JA, Seixas A, Wahlstrom KL, Wolfson AR, Wong P, Carskadon MA. (2021. a) COVID-19 instructional approaches (in-person, online, hybrid), school start times, and sleep in over 5,000 U.S. adolescents. Sleep 44:zsab180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer LJ, Wahlstrom KL, Plog AE, Strand MJ. (2021. b) Changing school start times: impact on sleep in primary and secondary school students. Sleep 44:zsab048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer LJ, Walsh CM, Peightal AA. (2015) Comparison of actigraphy immobility rules with polysomnographic sleep onset latency in children and adolescents. Sleep Breath 19:1415-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens JA, Dearth-Wesley T, Herman AN, Oakes JM, Whitaker RC. (2017) A quasi-experimental study of the impact of school start time changes on adolescent sleep. Sleep Health 3:437-443. [DOI] [PubMed] [Google Scholar]
- Paruthi S, Brooks LJ, D’Ambrosio C, Hall WA, Kotagal S, Lloyd RM, Malow BA, Maski K, Nichols C, Quan SF, et al. (2016) Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med 12:785-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AJK, Clerx WM, O’Brien CS, Sano A, Barger LK, Picard RW, Lockley SW, Klerman EB, Czeisler CA. (2017) Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci Rep 7:3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team (2020) R: A Language and Environment for Statistical Computing. Vienna (Austria): R Foundation for Statistical Computing. [Google Scholar]
- Van der Maren S, Moderie C, Duclos C, Paquet J, Daneault V, Dumont M. (2018) Daily profiles of light exposure and evening use of light-emitting devices in young adults complaining of a delayed sleep schedule. J Biol Rhythms 33:192-202. [DOI] [PubMed] [Google Scholar]
- Wams EJ, Woelders T, Marring I, van Rosmalen L, Beersma DGM, Gordijn MCM, Hut RA. (2017) Linking light exposure and subsequent sleep: a field polysomnography study in humans. Sleep 40:zsx165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widome R, Berger AT, Iber C, Wahlstrom K, Laska MN, Kilian G, Redline S, Erickson DJ. (2020) Association of delaying school start time with sleep duration, timing, and quality among adolescents. JAMA Pediatr 174:697-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. (1990) Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry 27:563-572. [DOI] [PubMed] [Google Scholar]
- Wright KP, Jr, Linton SK, Withrow D, Casiraghi L, Lanza SM, Iglesia H, Vetter C, Depner CM. (2020) Sleep in university students prior to and during COVID-19 Stay-at-home orders. Curr Biol 30:R797-R798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstedt SD, Kline CE, Elliott JA, Zielinski MR, Devlin TM, Moore TA. (2016) Circadian phase-shifting effects of bright light, exercise, and bright light + exercise. J Circadian Rhythms 14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziporyn TD, Owens JA, Wahlstrom KL, Wolfson AR, Troxel WM, Saletin JM, Rubens SL, Pelayo R, Payne PA, Hale L, et al. (2022) Adolescent sleep health and school start times: setting the research agenda for California and beyond. A research summit summary. Sleep Health 8:11-22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jbr-10.1177_07487304221123455 for A Naturalistic Actigraphic Assessment of Changes in Adolescent Sleep, Light Exposure, and Activity Before and During COVID-19 by Corey A. Rynders, Anne E. Bowen, Emily Cooper, John T. Brinton, Janine Higgins, Kristen J. Nadeau, Kenneth P. Wright Jr. and Stacey L. Simon in Journal of Biological Rhythms