Abstract
Objectives
Many countries are administering a third dose of some coronavirus disease 2019 (COVID-19) vaccines, but the evaluation of vaccine-induced immunity after boosting in East Asia is insufficient. This study aimed to evaluate anti-spike immunoglobulin G [IgG(S)] titers after the third BNT162b2 vaccination.
Methods
The dynamics of IgG(S) titers were assessed two months following the third BNT162b2 vaccination in 52 participants. All participants received the primary series of vaccination with BNT162b2 and received the third dose eight months after the second vaccination. Associations among the IgG(S) titer, baseline characteristics, and adverse reactions were also evaluated.
Results
The geometric mean titer of IgG(S) one month after the third vaccination was 17,400 AU/ml, which increased by approximately 30 times that immediately before the third vaccination. The rate of IgG(S) titer decline was significantly slower after the third vaccination (35.7%) than after the second vaccination (59.1%). The IgG(S) titer was significantly negatively associated with age (r = −0.31). Participants who had a headache at the time of vaccination showed significantly higher IgG(S) titers than those without a headache.
Conclusions
The IgG(S) titer induced by primary immunization with BNT162b2 waned over time. The third dose of BNT162b2 substantially increased the IgG(S) titer, with a slower rate of decline.
Keywords: COVID-19, BNT162b2, Anti-spike IgG antibody
COVID-19; BNT162b2; Anti-spike IgG antibody.
1. Introduction
Although vaccines against coronavirus disease 2019 (COVID-19), including messenger RNA (mRNA) vaccines, were highly effective in preventing COVID-19 at the initiation of vaccination [1, 2], it has become increasingly difficult to prevent the spread of COVID-19 with the first series of vaccines as mutant variants have emerged. On November 26, 2021, the World Health Organization named the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) B.1.1.529 variant omicron and classified it as a variant of concern based on a rapid increase in the number of confirmed cases of SARS-CoV-2 infection with this variant in South Africa [3, 4]. The omicron variant has increased transmissibility and immune evasion even after natural infection and vaccination because it has a large number of mutations, including multiple mutations in the receptor-binding domain of the spike protein [5]. In addition, early laboratory data indicate that the neutralizing antibody response to the omicron strain is substantially reduced compared to that to the original strain or the delta variant in vaccinated individuals [6, 7, 8, 9]. COVID-19 vaccines are highly effective against both symptomatic and severe diseases caused by the original strain and the alpha variant [1, 2]. Although waning of anti-spike immunoglobulin G (IgG) titers and protection has been observed with time after vaccination, especially for the delta variant, booster (third) doses provide rapid and robust increases in anti-spike IgG titers and protection against both mild and severe diseases [10, 11, 12].
Based on these findings, many countries have commenced administering a booster (third) vaccine dose to curb the pandemic [10, 11, 12, 13]. In addition, in December 2021, Israel became the first country in the world to administer a fourth dose [14]. Booster doses of both BNT162b2 and mRNA-1273 have been approved in Japan since December 2021 for those over 12 years old [15]. However, there are few reports regarding the postbooster anti-spike IgG antibody response and adverse reactions in East Asian populations; therefore, we aimed to evaluate the post-booster anti-spike IgG titers and adverse reactions in the Japanese population.
2. Materials and methods
2.1. Study participants and design
Study participants were recruited from among health care workers at Haradoi Hospital, a mixed-care hospital in Fukuoka [16, 17]. Of the 485 health care workers in this hospital, 52 (10.7%) participated in this long-term prospective study. Most of the study participants were nurses, and approximately 85% were women. All three vaccines administered to the participants were the BNT162b2 mRNA COVID-19 vaccine (Comirnaty®: Pfizer/BioNTech). All participants were offered the first, second, and third doses of the vaccine in March, April, and December 2021. All participants provided written informed consent prior to enrollment. This study was carried out in accordance with the principles of the Declaration of Helsinki, as revised in 2008, and approved by the Haradoi Hospital institutional ethics review committee prior to data collection (approval No. 2020-08).
The main objective of this study was to evaluate the dynamics of anti-spike IgG titers, and the anti-spike IgG titers were measured nine times: before the first vaccination; three weeks after the first vaccination (just before the second vaccination); one, two, four, and six months after the second vaccination; before the third vaccination (approximately eight months after the second vaccination); and one and two months after the third vaccination. The secondary objective of this study was to assess the safety of the BNT162b2 mRNA COVID-19 vaccine by blood tests and interviews to solicit information about adverse reactions during vaccination. Participants provided information on their height, weight, smoking habits (current, past, or never), drinking habits (daily, often, or never), allergies, medical history, and medication use; whether they had experienced adverse reactions to the second and third vaccinations (fever, fatigue, headache, and swelling of axillary lymph nodes); and whether they had needed antipyretics.
2.2. Laboratory measurements
Levels of anti-spike IgG were quantified using a SARS-CoV-2 IgG II Quant assay (Abbott Diagnostics, Chicago, IL, USA) [18]. Participants underwent blood testing to quantitatively assess anti-spike IgG nine times (before the first vaccination; just before the second vaccination; one, two, four, and six months after the second vaccination; just before the third vaccination; and one and two months after the third vaccination). The results of anti-spike IgG quantification are expressed as arbitrary units per milliliter (AU/ml) (positive threshold: 50 AU/ml). We also performed qualitative tests for IgG/immunoglobulin M (IgM) antibodies against the SARS-CoV-2 nucleocapsid protein (positive thresholds: 1.40 index [S/C] for anti-nucleocapsid IgG and 1.00 index [S/C] for anti-nucleocapsid IgM) for all participants to exclude the effects of SARS-CoV-2 infection. Participants also had blood tests for total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GTP), and serum creatinine levels using standard enzymatic methods. The estimated glomerular filtration rate was calculated using the following equation: 194 × serum creatinine−1.094 × age−0.287 (× 0.739 [if female]).
2.3. Statistical analysis
Data are expressed as median values with 25th and 75th percentile values for continuous variables. The geometric mean titers (GMTs) of anti-spike IgG were calculated. Categorical variables are reported as frequencies and percentages. The Mann–Whitney U test was used to compare two groups, and the Kruskal–Wallis test was used to compare three groups. The Tukey–Kramer method was used for each two-group comparison among the three groups. Anti-spike IgG levels, with adjustment for age and sex, were determined by the least means square method. Paired t test was performed to evaluate differences in anti-spike IgG titers across timepoints, using log-transformed IgG titers. McNemar's test was used to evaluate differences between adverse reactions at the second and third vaccinations. The Wilcoxon signed-rank test was used to evaluate differences in laboratory data before and after the vaccinations. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). A P value less than 0.05 was considered to indicate statistical significance.
3. Results
3.1. Baseline characteristics of the participants
Table 1 shows the baseline characteristics of the 52 participants of this study. The median age was 40.5 years, 84.6% were women, and the median body mass index (BMI) was 20.9 kg/m2. There were no current smokers, 13.5% were daily alcohol drinkers, and 17.3% had an allergy. Among the study participants, 25 (48.1%) had comorbidities. One patient had rheumatoid arthritis but did not take corticosteroids or immunosuppressants. Another had a history of colorectal cancer without any evidence of recurrence at the enrollment of this study. Before the first vaccination, the titers of anti-spike IgG and the anti-nucleocapsid IgG and IgM of all 52 participants were below the positive threshold, indicating that no one had contracted COVID-19 before participating in this study.
Table 1.
Baseline characteristics of 52 participants†.
| Demographics | |
| Age – years | 40.5 [28.5, 49.5] |
| Sex – no. (%) | |
| Female | 44 (84.6) |
| Male | 8 (15.4) |
| Body mass index‡ – kg/m2 | 20.9 [18.7, 22.9] |
| Smoking habit – no. (current/past/never) | 0/6/46 |
| Alcohol drinking habit – no. (daily/often/never) | 7/30/15 |
| Allergies – no. (%) | 9 (17.3) |
| Comorbidities | |
| Number of comorbidities – no.. | 0 [0, 1] |
| Hypertension – no. (%) | 6 (11.5) |
| Diabetes – no. (%) | 3 (5.8) |
| Dyslipidemia – no. (%) | 5 (9.6) |
| Hyperuricemia – no. (%) | 1 (1.9) |
| Coronary heart disease – no. (%) | 0 (0.0) |
| Arrhythmia – no. (%) | 3 (5.8) |
| Stroke – no. (%) | 0 (0.0) |
| Lung disease – no. (%) | 4 (7.7) |
| Thyroid disease – no. (%) | 1 (1.9) |
| Atopic dermatitis – no. (%) | 8 (15.4) |
| Autoimmune disease – no. (%) | 1 (1.9) |
| Cancer – no. (%) | 1 (1.9) |
Continuous variables are presented as median [1st quartile, 3rd quartile], and categorical variables are presented as number (%).
Body mass index was calculated using the following equation: body weight (kg)/height (m)/height (m).
3.2. Dynamics of anti-spike IgG titers
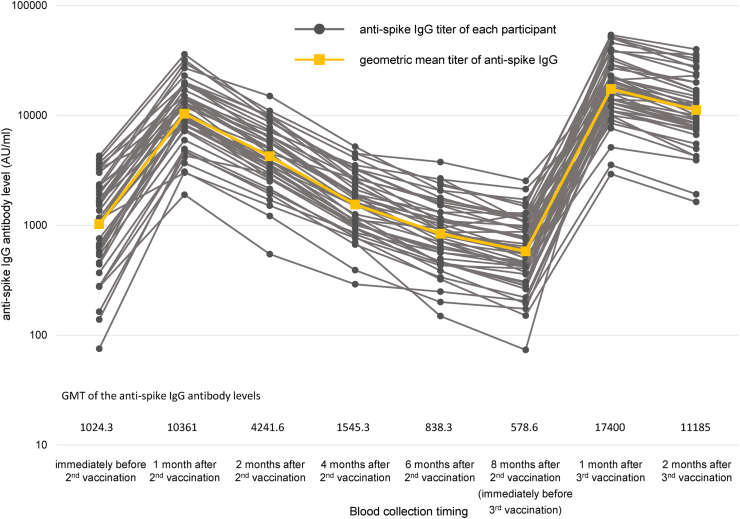
The dynamics of the anti-spike IgG titers are shown in Figure 1. Black circles and gray lines indicate anti-spike IgG titers and their dynamics in each participant, while orange squares and lines represent the GMTs of anti-spike IgG and their dynamics in all study participants. One participant was excluded from the result for two months after the third vaccination because the anti-nucleocapsid IgM was positive at two months after the third vaccination. After the first vaccination, all participants had an anti-spike IgG level ≥50 AU/ml, and the GMT three weeks after the first vaccination (just before the second vaccination) was 1024.3 AU/ml. The GMT of anti-spike IgG increased 10-fold to 10361 AU/ml one month after the second vaccination. The anti-spike IgG titer peaked at one month after the second vaccination and continued to decrease until eight months after the vaccination (just before the third vaccination) as 4241.6, 1545.3, 838.3, and 578.6 AU/ml, at two, four, six, and eight months after the second vaccination, respectively. The GMT of anti-spike IgG at eight months after the second vaccination represented an average decrease rate of 93.5% from one month after the second vaccination. One month after the third vaccination, the GMT of anti-spike IgG titer was 17400 AU/ml, which increased 30-fold compared to that just before the third vaccination and 1.67-fold compared to that one month after the second vaccination. Similar to those after the second vaccination, the anti-spike IgG levels declined at two months compared to those at one month after the third vaccination, with a GMT of anti-spike IgG of 11185 AU/ml. However, the average rate of decline was 35.7%, a significantly slower pace of decline than the 59.1% rate of decline after the second vaccination (P < 0.01). The anti-spike IgG titers showed statistically significant differences at each timepoint (all P < 0.05).
Figure 1.
Dynamics of anti-spike IgG titers. The dynamics of the anti-spike IgG titer of each participant (black circles and gray lines) and geometric mean titer of anti-spike IgG (orange squares and lines) are shown. Compared to that at one month after the second vaccination, the geometric mean titer of anti-spike IgG before the third vaccination decreased by approximately one-tenth. One month after the third vaccination, the geometric mean titer of anti-spike IgG increased 30-fold before the third vaccination and 1.7-fold one month after the second vaccination.
3.3. Systemic adverse reactions and laboratory finding changes
Table 2 shows the differences in systemic adverse reactions after the second and third vaccinations. After both the second and third vaccinations, approximately half of the participants experienced fever; more participants had a fever of 38 °C or higher after the third vaccination than after the second vaccination, but there was no significant difference. The proportion experiencing fatigue also did not differ between the second and third vaccinations, with approximately 60% experiencing fatigue at either time. On the other hand, headache and axillary lymphadenopathy were more common after the third vaccination. After the third vaccination, headache occurred twice, and the rate of axillary lymphadenopathy was seven times higher than that after the second vaccination.
Table 2.
Comparison of adverse reactions at the 2nd and 3rd vaccinations.
| 2nd vaccination | 3rd vaccination | P value† | |
|---|---|---|---|
| Fever – no. (%) | 25 (48.1) | 23 (45.1) | 0.80 |
| Highest fever degree – no. (%) | |||
| 37.0–37.4 °C | 6 (11.5) | 6 (11.5) | 0.50 |
| 37.5–37.9 °C | 11 (21.2) | 6 (11.5) | |
| ≥38.0 °C | 8 (15.4) | 11 (21.2) | |
| Fatigue – no. (%) | 35 (67.3) | 31 (59.6) | 0.39 |
| Headache – no. (%) | 11 (21.2) | 21 (40.4) | 0.02 |
| Swelling of axillary lymph nodes – no. (%) | 3 (5.8) | 21 (40.4) | <0.01 |
The McNemar test was performed for comparisons of discordant pairs.
Table 3 shows the changes in laboratory findings before and after each vaccination. In this study, we measured total bilirubin, AST, ALT, γ-GTP, and serum creatinine levels along with anti-spike IgG levels. Estimated glomerular filtration rates were calculated using the Cockcroft–Gault formula. Blood test results before the first vaccination were within normal limits for most of the study participants. Blood test values hardly changed before and after each vaccination. Values of ALT and γ-GTP levels decreased by 1 IU/ml after the first vaccination, but we do not consider these changes to be of clinical significance.
Table 3.
Comparison of the laboratory data before and after vaccination.†
| Before vaccination | After vaccination‡ | Change of value | P value§ | |
|---|---|---|---|---|
| 1st vaccination | ||||
| Total bilirubin – mg/dl | 0.6 [0.5, 0.9] | 0.6 [0.4, 0.8] | 0.0 [−0.2, 0.0] | 0.15 |
| Aspartate aminotransferase – IU/l | 20 [16, 23] | 19 [17, 21] | 0.0 [−1.5, 1.0] | 0.57 |
| Alanine aminotransferase – IU/l | 15 [12, 20] | 14 [11, 19] | −1 [−3, 1] | 0.046 |
| γ-Glutamyl transpeptidase – IU/l | 16 [14, 25] | 16 [13, 28] | −1 [−3, 0] | 0.01 |
| Serum creatinine – mg/dl | 0.63 [0.57, 0.70] | 0.62 [0.56, 0.69] | 0.00 [−0.03, 0.05] | 0.46 |
| eGFR‖ – ml/min/m2 | 89.2 [78.6, 99.9] | 91.3 [76.0, 97.4] | 0.0 [−6.3, 5.1] | 0.57 |
| 2nd vaccination | ||||
| Total bilirubin – mg/dl | 0.6 [0.4, 0.8] | 0.6 [0.5, 0.8] | 0.0 [−0.1, 0.1] | 0.25 |
| Aspartate aminotransferase – IU/l | 19 [17, 21] | 18 [16, 21] | 0 [−2, 1] | 0.43 |
| Alanine aminotransferase – IU/l | 14 [11, 19] | 14 [11, 19] | 0 [−2, 2] | 0.94 |
| γ-Glutamyl transpeptidase – IU/l | 16 [13, 28] | 17 [13, 28] | 0 [−1, 1] | 0.93 |
| Serum creatinine – mg/dl | 0.62 [0.56, 0.69] | 0.61 [0.55, 0.68] | 0.00 [−0.05, 0.02] | 0.41 |
| eGFR‖ – ml/min/m2 | 91.3 [76.0, 97.4] | 90.4 [74.7, 101.1] | 0.0 [−3.5, 6.1] | 0.43 |
| 3rd vaccination | ||||
| Total bilirubin – mg/dl | 0.6 [0.5, 0.8] | 0.6 [0.5, 0.8] | 0.0 [−0.1, 0.1] | 0.46 |
| Aspartate aminotransferase – IU/l | 18 [16, 21] | 19 [17, 22] | 0 [−2, 2] | 0.30 |
| Alanine aminotransferase – IU/l | 14 [11, 19] | 16 [11, 21] | 0 [−1, 2] | 0.47 |
| γ-Glutamyl transpeptidase – IU/l | 15 [13, 20] | 16 [14, 23] | 0 [−1, 2] | 0.10 |
| Serum creatinine – mg/dl | 0.62 [0.57, 0.69] | 0.62 [0.55, 0.70] | 0.00 [−0.04, 0.03] | 0.50 |
| eGFR‖ – ml/min/m2 | 90.4 [80.8, 98.1] | 89.2 [74.8, 101.3] | 0.0 [−3.8, 6.9] | 0.44 |
eGFR, estimated glomerular filtration rate.
Data are shown as the median [1st quartile, 3rd quartile].
Blood samples were collected three weeks after the 1st vaccination (just before the 2nd vaccination) and one month after the 2nd and 3rd vaccinations.
P values were calculated using the Wilcoxon signed-rank sum test.
The parameter was calculated using the following equation: 194 ∗ serum creatinine−1.094 ∗ age−0.287 (∗ 0.739 [if female]).
3.4. Factors influencing the anti-spike IgG titer after the third vaccination
We examined factors affecting the anti-spike IgG titer, such as age, BMI, sex, habits, comorbidities, and systemic adverse reactions to the vaccination. As we previously reported, age was the most substantial factor affecting the anti-spike IgG titer induced by vaccination, and there was a significantly negative association between age and the anti-spike IgG titer one month after the third vaccination (r = −0.31, P = 0.02). However, this association disappeared two months after the third vaccination (r = −0.22, P = 0.13). BMI, sex, smoking habits, and any comorbidities did not correlate with anti-spike IgG titers either one or two months after the third vaccination. Although we previously reported that daily alcohol drinkers had significantly lower anti-spike IgG titers, there was no significant association between alcohol drinking habits and anti-spike IgG titers after the third vaccination in this study. Regarding systemic adverse reactions, fever and fatigue did not correlate with anti-spike IgG titers; however, those who experienced headache or axillary lymphadenopathy tended to have higher anti-spike IgG titers after the third vaccination. Using antipyretics also did not affect the anti-spike IgG titer. After adjustment for age, only those who experienced a headache at the third vaccination had significantly higher anti-spike IgG titers one month after the third vaccination (29760 vs. 22182 AU/ml, respectively, P = 0.04).
We also examined the associations between anti-spike IgG titers before and after the third vaccination. The participants were divided into two groups based on anti-spike IgG titer one month after the second vaccination. Participants with anti-spike IgG titers below 10,000 AU/ml were grouped into the lower group, and those with anti-spike IgG titers greater than 10,000 AU/ml were grouped into the higher group. As expected, the higher group always had significantly higher anti-spike IgG titers than the lower group after the second vaccination. Although the GMT of anti-spike IgG in the higher group was approximately three times that in the lower group one month after the second vaccination (17,458 vs. 6148.8 AU/ml), the difference just before the third vaccination (eight months after the second vaccination) was approximately 1.7 times (732.1 vs. 438.1 AU/ml), indicating that the difference between the two groups had narrowed. One month after the third vaccination, the anti-spike IgG titer of both groups increased by a factor of approximately 30; the GMTs of the higher group and the lower group were 22,067 and 11851 AU/ml, respectively. Compared with that one month after the second vaccination, the GMT of the higher group increased by a factor of 1.15, but that of the lower group increased by a factor of 1.87, indicating that the third vaccination might be more effective for the lower group than for the higher group.
4. Discussion
The following three findings emerged from this prospective observational study. First, anti-spike IgG titers increased considerably after the third vaccination, and the GMTs of anti-spike IgG after the third vaccination were higher than those after the second vaccination. In addition, the rate of decline in anti-spike IgG titers after the third vaccination was slower than that after the second vaccination, suggesting that the vaccine-induced anti-spike IgG titer may be maintained for a more extended period. Second, low anti-spike IgG titers after the second vaccination correlated with a greater rate of increase after the third vaccination. As a result, the GMT of anti-spike IgG of study participants increased, with smaller differences between participants. Our results suggest that the third dose might be necessary to obtain sufficient immunogenicity with mRNA vaccines. Finally, no severe adverse reactions were observed for the series of vaccinations, including the third dose, indicating that the BNT162b2 vaccine was well tolerated.
In December 2021, Israel became the first country in the world to apply a fourth vaccination dose. A study of health care workers in Israel who received a fourth dose of BNT162b2 or mRNA-1273 four months after the third dose in a series of three BNT162b2 doses showed that IgG titers increased 3 to 4 times before the fourth dose [14]. However, the IgG titers after the fourth vaccination were just slightly higher than those after the third vaccination. The results of this study suggest that there may be an upper limit to the immunity induced by the mRNA vaccines and that the third vaccination may have achieved maximal immunogenicity. In our study, anti-spike IgG titers increased with the third vaccination by a factor of 3.0 compared to those before the third vaccination and by a factor of 1.7 compared to those after the second vaccination. In addition, participants with low pre-boost anti-spike IgG titers had a higher rate of increase after the third vaccination. A study of 61 healthy subjects also reported that low antibody titers before the third vaccination were associated with a higher rate of increase in antibody titers after the third vaccination [19]. Along with previous studies [19, 20, 21], our findings also suggest that the third vaccination restored vaccine effectiveness against SARS-CoV-2-induced disease, but mRNA vaccine immunogenicity may have an upper limit.
Vaccination is thought to be the most effective method of preventing infectious diseases. Since mRNA vaccines were used for the first time in humans, how immunogenic they are and how many doses are required to provide adequate immunity are unclear. And data from East Asia is insufficient to elucidate these questions. A study of 129 healthcare workers in Greece, including previously infected participants, showed that the third dose of BNT162b2 six months after the primary series of BNT162b2 vaccination increased anti-spike IgG titer to 20231 AU/ml from 437 AU/ml [22], similar to our result. Another study of 90 healthcare workers in Indonesia reported that a booster dose mRNA-1273 vaccine increased anti-spike IgG titer 700-fold compared to that six months after primary vaccination with CoronaVac [21]. The result suggests that mRNA vaccines might elicit an antibody response regardless of previous types of vaccine. The efficacy of a booster dose BNT162b2 vaccine among the elderly was also reported [19]. The third dose of BNT162b2 increased anti-spike IgG titer to 25468 AU/ml, 57.8-fold compared to pre-boost IgG titer among 97 elderly Israelis. No significant association was found between IgG titers and comorbidities or age. However, the median age of the participants was 70 years. Further studies on more elderly populations are also needed. In our previous report [16], the GMT of anti-spike IgG declined to approximately 40% from one month to two months after the second vaccination. In the current study, we found that the rate of decline of the GMT became significantly slower after the third vaccination. Our results suggest that mRNA vaccines, similar to inactivated vaccines such as the hepatitis B vaccine, require booster vaccinations to induce adequate immunity.
Fever, headache, fatigue, and pain at the injection site were the most commonly reported adverse reactions to COVID-19 vaccines, and overall, most adverse reactions were mild and short-lived [23, 24]. Although very rare, there have been reports of serious adverse reactions. The following four serious adverse reactions to certain types of COVID-19 vaccination have been found: anaphylaxis, thrombosis with thrombocytopenia syndrome, myocarditis and pericarditis, and Guillain–Barré syndrome [25, 26, 27]. There are also some reports of death after COVID-19 vaccination. According to the Vaccine Adverse Event Reporting System in the United States, preliminary death rates after the COVID-19 vaccination were 0.0024% [28]. Along with these previous reports, BNT162b2 appeared to be well tolerated in this study. Even at the third dose, blood tests showed that hepatic enzyme and serum creatinine levels did not change. Regarding systemic reactions, the rates of fever and fatigue did not differ between the second and third vaccinations. Although headache and axillary lymphadenopathy were more frequently observed with the third vaccination than with the second vaccination, no severe adverse reactions were observed.
Limitations of this study should be noted. Because the anti-spike IgG titer measured in this study was against the original strain of SARS-CoV-2, it is difficult to estimate the vaccine efficacy against the omicron variant. The observed anti-spike IgG titer might be estimated to be lower against the omicron variant. Moreover, we did not assess cell-mediated immunity. However, a higher anti-spike IgG titer is still considered protective against SARS-CoV-2 infection, including that by the omicron variant. Therefore, our results suggest that the third vaccination might restore vaccine effectiveness against SARS-CoV-2 infection. In addition, the number of participants was small, and the participants were young and healthy. Additional researches with more participants and a more comprehensive age range are necessary to validate our findings. Finally, we could not assess the effect of the third dose of the vaccine on COVID-19 prevention because there was only one participant possibly infected with SARS-CoV-2, and there was no control group. Further analyses with nationwide surveys are necessary to confirm the efficacy of the additional COVID-19 vaccination.
In conclusion, the third dose of BNT162b2 vaccination successfully increased the anti-spike IgG titer, and the efficacy of vaccination might be maintained longer because the rate of decline of anti-spike IgG titers is slower than that after the second vaccination.
Declarations
Author contribution statement
Hiroaki Ikezaki: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Hideyuki Nomura; Nobuyuki Shimono: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The data that has been used is confidential.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank Ms. Ryoko Nakashima for managing the dataset and Drs. Kahori Miyoshi, Yuichi Hara, Jun Hayashi, and Hiroshi Hara for scientific advice.
References
- 1.Pritchard E., Matthews P.C., Stoesser N., Eyre D.W., Gethings O., Vihta K.D., et al. Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat. Med. 2021;27:1370–1378. doi: 10.1038/s41591-021-01410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paris C., Perrin S., Hamonic S., Bourget B., Roué C., Brassard O., et al. Effectiveness of mRNA-BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 vaccines against COVID-19 in healthcare workers: an observational study using surveillance data. Clin. Microbiol. Infect. 2021;27 doi: 10.1016/j.cmi.2021.06.043. 1699.e5–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Update on Omicron. https://www.who.int/news/item/28-11-2021-update-on-omicron. [accessed April 6, 2022].
- 4.Viana R., Moyo S., Amoako D.G., Tegally H., Scheepers C., Althaus C.L., et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022;603:679–686. doi: 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Centre for Disease Prevention and Control Implications of the further emergence and spread of the SARSCoV-2 B.1.1.529 variant of concern (Omicron) for the EU/EEA – first update. 2021. https://www.ecdc.europa.eu/sites/default/files/documents/threat-assessment-covid-19-emergence-sars-cov-2-variant-omicron-december-2021.pdf [accessed April 6, 2022]
- 6.Cele S., Jackson L., Khoury D.S., Khan K., Moyo-Gwete T., Tegally H., et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602:654–656. doi: 10.1038/s41586-021-04387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt F., Muecksch F., Weisblum Y., Silva J.D., Bednarski E., Cho A., et al. Plasma neutralization of the SARS-CoV-2 omicron variant. N. Engl. J. Med. 2022;386:599–601. doi: 10.1056/NEJMc2119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L., Iketani S., Guo Y., Chan J.F.W., Wang M., Liu L., et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602:676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- 9.Karim S.S.A., Karim Q.A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398:2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barda N., Dagan N., Cohen C., Hernán M.A., Lipsitch M., Kohane I.S., et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., et al. Protection of BNT162b2 vaccine booster against covid-19 in Israel. N. Engl. J. Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews N., Stowe N., Kirsebom F., Toffa S., Rickeard T., Gallagher E., et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N. Engl. J. Med. 2022 doi: 10.1056/NEJMoa2119451. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patalon T., Gazit S., Pitzer V.E., Prunas O., Warren J.L., Weinberger D.M. Odds of testing positive for SARS-CoV-2 following receipt of 3 vs 2 doses of the BNT162b2 mRNA vaccine. JAMA Intern. Med. 2022;182:179–184. doi: 10.1001/jamainternmed.2021.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regev-Yochay G., Gonen T., Gilboa M., Mandelboim M., Indenbaum V., Amit S., et al. Efficacy of a fourth dose of Covid-19 mRNA vaccine against omicron. N. Engl. J. Med. 2022 doi: 10.1056/NEJMc2202542. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministry of Health, Labour and Welfare. COVID-19 vaccine booster shots (3rd dose). https://www.mhlw.go.jp/stf/covid-19/booster.html. [accessed April 6, 2022].
- 16.Ikezaki H., Nomura H., Shimono N. Dynamics of anti-Spike IgG antibody level after the second BNT162b2 COVID-19 vaccination in health care workers. J. Infect. Chemother. 2022;28:802–805. doi: 10.1016/j.jiac.2022.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikezaki H., Hara Y., Hayashi J., Hara H., Nomura H., Shimono Y. Low IgG antibody production in the elderly Japanese population after full BNT162b2 vaccination. J. Hosp. Gen. Med. 2022;4:25–28. [Google Scholar]
- 18.Abbott Diagnostics. Architect SARS-COV-2 IgG II Quant Instructions for Use, H18566R01. Abbott Diagnostics, IL, USA.
- 19.Goel R.R., Painter M.M., Lundgreen K.A., Apostolidis S.A., Baxter A.E., Giles J.R., et al. Efficient recall of Omicron-reactive B cell memory after a third dose of SARS-CoV-2 mRNA vaccine. Cell. 2022;185:1875–1887. doi: 10.1016/j.cell.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eliakim-Raz N., Leibovici-Weisman Y., Stemmer A., Ness A., Awwad M., Ghantous N., et al. Antibody titers before and after a third dose of the SARS-CoV-2 BNT162b2 vaccine in adults aged ≥60 years. JAMA. 2021;326:2203–2204. doi: 10.1001/jama.2021.19885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cucunawangsih C., Wijaya R.S., Lugito N.P.H., Suriapranata I. Antibody response after a third dose mRNA-1273 vaccine among vaccinated healthcare workers with two doses of inactivated SARS-CoV-2 vaccine. Int. J. Infect. Dis. 2022;118:116–118. doi: 10.1016/j.ijid.2022.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kontopoulou K., Nakas C.T., Papazisis G. Significant increase in antibody titers after the 3rd booster dose of the Pfizer–BioNTech mRNA COVID-19 vaccine in healthcare workers in Greece. Vaccines. 2022;10:876. doi: 10.3390/vaccines10060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paran Y., Saiag E., Spitzer A., Angel Y., Yakubovsky M., Padova H., et al. Short-term safety of booster immunization with BNT162b2 mRNA COVID-19 vaccine in healthcare workers. Open Forum Infect. Dis. 2021;9:ofab656. doi: 10.1093/ofid/ofab656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.David S.S.B., Gez S.B., Rahamim-Cohen D., Shamir-Stein N., Lerner U., Zohar A.E. Immediate side effects of Comirnaty COVID-19 vaccine: a nationwide survey of vaccinated people in Israel, December 2020 to March 2021. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.13.2100540. pii=2100540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong K.M., Yang C.Y., Lin C.C., Lien W.C. Severe immune thrombocytopenia following COVID-19 vaccination (Moderna) and immune checkpoint inhibitor: a case report. Am. J. Emerg. Med. 2022 doi: 10.1016/j.ajem.2022.03.030. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogata S., Ishi Y., Asano K., Kobayashi E., Kubota S., Takahashi K., et al. Sensory ataxic Guillain-Barré Syndrome with dysgeusia after mRNA COVID-19 vaccination. Intern. Med. 2022 doi: 10.2169/internalmedicine.8967-21. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu R., Pan J., Zhang C., Sun X. Cardiovascular complications of COVID-19 vaccines. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.840929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaccine Adverse Event Reporting System. https://vaers.hhs.gov. [accessed April 6, 2022].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.