Abstract
Objectives
Nursing home (NH) residents have been significantly affected by the coronavirus disease 2019 (COVID-19) pandemic. Studies addressing the immune responses induced by COVID-19 vaccines in NH residents have documented a good postvaccination antibody response and the beneficial effect of a third booster vaccine dose. Less is known about vaccine-induced activation of cell-mediated immune response in frail older individuals in the long term. The aim of the present study is to monitor messenger RNA SARS-CoV-2 vaccine-induced T-cell responses in a sample of Italian NH residents who received primary vaccine series and a third booster dose and to assess the interaction between T-cell responses and humoral immunity.
Design
Longitudinal cohort study.
Setting and Participants
Thirty-four residents vaccinated with BNT162b2 messenger RNA SARS-CoV-2 vaccine between February and April 2021 and who received a third BNT162b2 booster dose between October and November 2021 were assessed for vaccine-induced immunity 6 (prebooster) and 12 (postbooster) months after the first BNT162b2 vaccine dose.
Methods
Pre- and postbooster cell-mediated immunity was assessed by intracellular cytokine staining of peripheral blood mononuclear cells stimulated in vitro with peptides covering the immunodominant sequence of SARS-CoV-2 spike protein. The simultaneous production of interferon-γ, tumor necrosis factor-α, and interleukin-2 was measured. Humoral immunity was assessed in parallel by measuring serum concentration of antitrimeric spike IgG antibodies.
Results
Before the booster vaccination, 31 out of 34 NH residents had a positive cell-mediated immunity response to spike. Postbooster, 28 out of 34 had a positive response. Residents without a previous history of SARS-CoV-2 infection, who had a lower response prior the booster administration, showed a greater increase of T-cell responses after the vaccine booster dose. Humoral and cell-mediated immunity were, in part, correlated but only before booster vaccine administration.
Conclusions and Implications
The administration of the booster vaccine dose restored spike-specific T-cell responses in SARS-CoV-2 naïve residents who responded poorly to the first immunization, while a previous SARS-CoV-2 infection had an impact on the magnitude of vaccine-induced cell-mediated immunity at earlier time points. Our findings imply the need for a continuous monitoring of the immune status of frail NH residents to adapt future SARS-CoV-2 vaccination strategies.
Keywords: SARS-CoV-2, COVID-19 vaccines, nursing homes, cell-mediated immunity, vaccine booster
Older adults have been significantly affected by the coronavirus disease 2019 (COVID-19) pandemic.1 Among them, nursing home (NH) residents who often present with a high burden of comorbidities and clinical complexities suffered the greatest impact of the pandemic.2, 3, 4, 5, 6 Epidemiologic data indicate that during the first pandemic wave, up to 50% of deaths from COVID-19 may have occurred within NH facilities.7 For this reason, SARS-CoV-2 vaccination of NH residents has been a priority in most countries including Italy to reduce the risk of COVID-19-related morbidity and mortality.8
Several studies have been performed to determine the quality and duration of immune responses induced by SARS-CoV-2 vaccines in NH residents. As a general feature, a good early postvaccination antibody response followed by a decline over time and the beneficial effect of a third booster vaccine dose has been documented.9, 10, 11, 12, 13, 14, 15, 16 Less is known about vaccine-induced activation of T-cell mediated immune response in frail older persons and about its interaction with humoral response. T-cell immunity plays a central role in the control of SARS-CoV-2 and increasing evidence now supports a potential role in both preventing initial infection and, more importantly, limiting the extent of disease following infection.17 , 18 Data collected so far have highlighted an impaired T-cell response in older persons compared with younger adults after two vaccine doses, with a greater response in previously infected older subjects,19, 20, 21, 22 and the immune-potentiating effect of a third booster dose in previously unresponsive older adults.23
The aim of the present study is to monitor, over a 12-month period, messenger RNA (mRNA) SARS-CoV-2 vaccine-induced T-cell responses in a sample of Italian NH residents who received the primary mRNA vaccine series and a booster dose and to assess the interaction between T-cell responses and humoral immunity.
Methods
Study Design
The present study has been conducted in the framework of the GeroCOVID VAX project, aimed at investigating effects of anti-SARS-CoV-2 vaccine use in NH residents in Italy.24 Fifty-one NH residents belonging to the GeroCOVID VAX cohort, were selected for assessment of T-cell mediated immunity and followed up longitudinally to 1 year after the administration of the first vaccine dose.
Residents were vaccinated with the Pfizer BNT162b2 mRNA SARS-CoV-2 vaccine between February and April 2021. Following recommendations issued by the Italian Ministry of Health, residents who experienced SARS-CoV-2 infection in the 6 months before vaccination received a single vaccine dose, while other residents were administered a second dose 1 month after the first one. A booster vaccination with lower dose BNT162b2 was administered to NH residents between October and November 2021 (7 to 9 months from the first dose). Blood samples were collected after primary vaccine series (6 months after first vaccine dose – prebooster) and after booster dose (12 months after first vaccination – postbooster). SARS-CoV-2 spike-specific IgG and spike-specific T-cell responses were assessed concomitantly pre- and postbooster. A diagram of the study design is shown in Supplementary Figure 1.
Supplementary Fig. 1.
Study design. Immune response to vaccination was assessed in a sample of 34 NH residents 6 and 12 months after the first SARS-CoV-2 vaccination (T6 and T12). A booster SARS-CoV-2 vaccine dose was administered to NH residents after blood sampling at T6. Mean follow-up time between booster dose and T12 assessment was 5 months.
Demographic and clinical data were collected in a dedicated electronic form and included sex, age, prior SARS-CoV-2 infection, comorbidities, and time of vaccination.
SARS-CoV-2 IgG Immunoassays
Blood samples (5 mL) were collected in Serum Separator Tubes (BD Diagnostic Systems) and centrifuged at room temperature at 1600 rpm for 10 minutes. Serum aliquots were transferred to 2 mL polypropylene, screw cap cryo tubes (Nunc, Thermofisher Scientific) and immediately frozen at -20°C. Sera were evaluated by the DiaSorin Liaison SARS-CoV-2 trimeric spike IgG assay (DiaSorin), a 2-step chemiluminescence immunoassay for the detection of IgG antibodies against the spike protein of SARS-CoV-2 in its trimeric native form. The assay was performed on the LIAISON XL chemiluminescence analyzer. The analyzer automatically calculates SARS-CoV-2 trimeric spike IgG antibody concentrations expressed as binding antibody units (BAU/mL) and grades the results. The assay range is up to 2080 BAU/mL. According to manufacturer instructions, values of ≥33.8 BAU/mL were interpreted as positive. If the results were above the assay range, samples were automatically diluted 1/20 and testing was repeated.
Peripheral Blood Mononuclear Cell Isolation
Whole blood (5 mL) was collected from patients in sodium heparin Vacutainer tubes (BD Biosciences). Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficoll-Paque PLUS (GE Healthcare), washed twice with cold wash buffer, and resuspended at 2.0–2.5 × 106 cells/mL in R-10 medium (RPMI 1640 supplemented with 10% fetal bovine serum, sodium pyruvate, penicillin, streptomycin, and nonessential amino acids (all from Sigma-Aldrich). Freshly isolated PBMCs were then frozen in 90% fetal bovine serum and 10% dimethyl sulfoxide and stored in −196°C liquid nitrogen for later experiments.
T-Cell Stimulation Assay and Cytokine Detection
A total of 2 × 106 PBMCs were cultured for 20 hours in R-10 medium at 37°C with 5% CO2 in FACS tubes with 0.6 nmol of a pool of peptides encompassing the immunodominant sequence domain of SARS-CoV-2 spike protein (Peptivator, Miltenyi, Bergisch Gladbach). Unstimulated PBMCs were used as negative control. As a positive control, the nonspecific superantigen staphylococcal enterotoxin B was added at 100 ng/mL (Sigma-Aldrich). Brefeldin-A (Sigma-Aldrich) was added during the last 18 hours of incubation, at 10 μg/mL, to inhibit cellular secretion. After overnight stimulation, PBMCs were stained with Live/Dead Fixable Violet Dead Cell Stain Kit (Thermo Fisher Scientific) to exclude dead cells from the analyses. Cells were washed twice with FACS buffer and then fixed with BD Cytofix/Cytoperm buffer (BD Biosciences) for 20 minutes at 4°C. Following fixation and permeabilization, cells were washed twice with 1× BD Perm/Wash buffer and stained with a predetermined optimal concentration of fluorochrome-conjugated Abs: anti-CD3-APC-H7, anti-interleukin (IL)-2-FITC, anti-tumor necrosis factor (TNF)-α-PE-Cy7 (all from BD Biosciences), anti-interferon (IFN)-γ-PerCP-Cy5.5 (Biolegend), anti-CD8-APC (eBiosciences, Thermo Fisher Scientific). Cells were fixed in 200 μL of 1× PBS/formaldehyde (2% v/v), acquired by flow cytometry in a Gallios flow cytometer and then analyzed using Kaluza software (Beckman Coulter). This gating strategy allowed to identify CD8+ (CD3+/CD8+) and CD4+ (CD3+/CD8-) T cells. Stained samples were acquired with a standard stopping gate set at 50,000 CD3+ T cells. Frequencies of cytokine producing cells were calculated after subtraction of cytokine positive cells in the relative negative control tube (ie, unstimulated sample). A frequency above 0.04% was considered a positive cell-mediated immunity (CMI) response.
Statistical Analyses
The Wilcoxon test was used to compare median values of cytokine positive T cells pre- and postbooster. Given the differences in vaccination strategy, analyses of T-cell mediated immune response were performed not only in the whole sample, but also after stratification by history of former COVID-19. The Mann-Whitney test was used to compare median values of cytokine positive T cells of previous SARS-CoV-2 infection and COVID-19 naïve residents pre- and postbooster. The McNemar tests were used to investigate the change in percentage of cytokine positive T cells before and after the booster vaccine dose. Given the nonnormal distribution of SARS-CoV-2 trimeric spike IgG antibody concentrations, analyses were performed using log-transformed values and the distribution of IgG levels pre- and postbooster was expressed as geometric mean titer (GMT). The Student t test was used to compare antibody titer values between non-low CMI responders, producing 0‒1 cytokine upon spike stimulation and high CMI responders, producing 2‒3 cytokines upon spike stimulation. Correlation analyses were performed using Spearman rank correlation coefficient. Statistical analysis was carried out with STATA Software v 17.0 (Stata Corporation) and Prism 8.1 (GraphPad Software). P value of < .05 was considered statistically significant. Data plotted in logarithmic scales were expressed as median. T-cell data have been calculated as background subtracted data.
Ethical Approval
The study was approved by the Italian National Ethical Committee with the permission number 264/2021 (January 26, 2021).
Results
Study Sample
The initial study sample consisted of 51 residents. During the 12 months follow-up period 10 persons dropped out of the study due to death (n = 5), discharge (n = 3), or transfer to another facility (n = 2). Two persons were excluded from the study due to ongoing SARS-CoV-2 infection at time of 12-month blood sampling. Samples from 5 persons could not be analyzed due to scarce PBMCs yield from whole blood samples. Hence, the final study sample consisted of 34 persons. Demographic and clinical characteristics of the study sample are shown in Table 1 . Among the 24 residents with prior SARS-CoV-2 infection, 21 (87.5%) received a single vaccine dose and 3 (12.5%) 2 doses. Mean follow-up time between booster dose and postbooster assessment was 5 months.
Table 1.
Demographic and Clinical Characteristics of the Study Sample
| Whole Sample (N = 34) | Previous COVID-19 (n = 24) | Naïve (n = 10) | P Value | |
|---|---|---|---|---|
| Age | ||||
| Mean | 74.3 | 72.3 | 79.3 | .58 |
| SD | 9.1 | 1.6 | 3.1 | |
| Sex | ||||
| Female | 22 (65%) | 15 | 7 | .68 |
| Male | 12 (35%) | 9 | 3 | |
| Comorbidities | ||||
| Dementia | 21 (62%) | 13 | 8 | .16 |
| Arterial Hypertension | 12 (35%) | 8 | 4 | .71 |
| Chronic obstructive pulmonary disease | 8 (23%) | 6 | 2 | .75 |
| Cardiomyopathy ischemic | 7 (21%) | 3 | 4 | .07 |
| Peripheral arterial disease | 7 (21%) | 6 | 1 | .32 |
| Diabetes | 4 (12%) | 3 | 1 | .84 |
| Obesity | 4 (12%) | 3 | 1 | .84 |
| Cardiac failure | 2 (6%) | 1 | 1 | .51 |
| Stroke | 2 (6%) | 1 | 1 | .51 |
| Chronic renal Failure | 1 (3%) | 1 | 0 | .51 |
SARS-CoV-2 S-Specific T-Cell Response
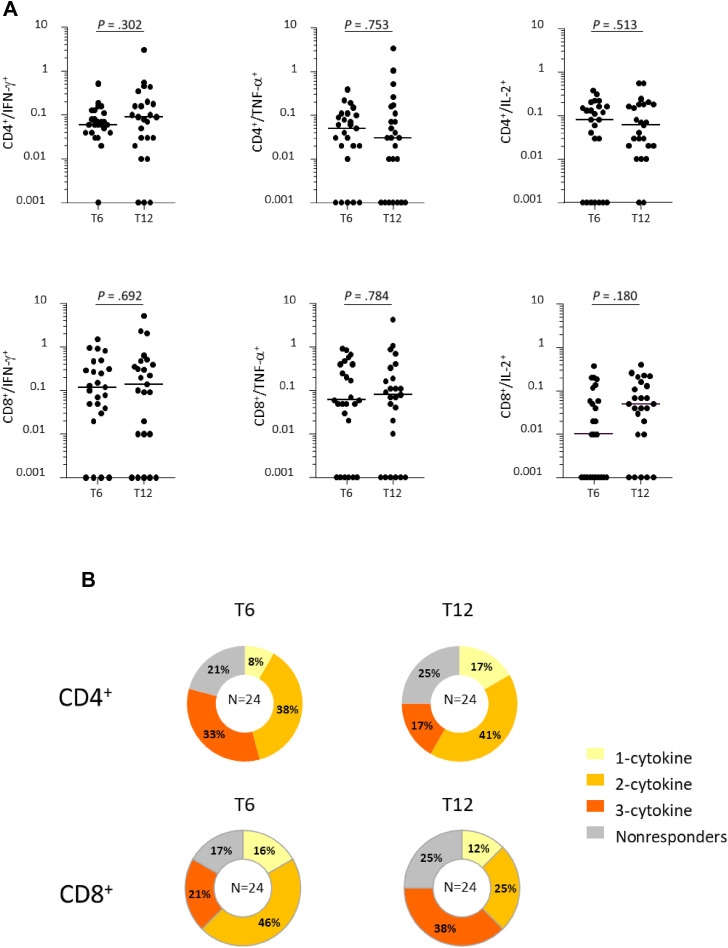
Six months after vaccination (prebooster) most of 34 NH residents displayed a T-cell mediated immunity to spike, indeed, 31 subjects (91.2%) had a positive response to spike antigenic stimulation in the CD4+ or in the CD8+ compartment to at least 1 of the cytokines analyzed. More in details, 75% of the residents had CD4+ T cells and 65% of them had CD8+ T cells producing at least 1 cytokine. At postbooster assessment, 28 residents (82.3%) had a positive response to spike antigenic stimulation in the CD4+ or in the CD8+ compartment to at least 1 of the cytokines analyzed. The magnitude of T-cell response showed no significant variation compared with prebooster assessment (Figure 1 , A). In the CD8+ compartment, an increase was observed in the median frequencies of cytokine positive cells compared with prebooster, but this difference only reached a borderline significance for IL-2 (P = .066). Polyfunctional CD4+ and CD8+ T cells, simultaneously producing more than 1 cytokine, were detected in stimulated PBMCs from NH residents pre- and postbooster (Figure 1, B). A slight decrease in the percentage of subjects with polyfunctional CD4+ T cells after booster administration compared with prebooster assessment was noticed, while subjects with polyfunctional CD8+ T cells were increased (Figure 1, B).
Fig. 1.
T-cell mediated immune response in NH residents 6 months (pre-booster, T6) and 12 months (postbooster, T12) after first immunization. (A) Frequencies of CD4 and CD8 T cells producing IFN-γ, TNF-α, and IL-2 in response to in vitro stimulation with spike. (B) Percentages of persons with nonresponding CD4+ and CD8+ T cells or producing 1 to 3 cytokines pre- and postbooster. Differences between median frequencies of cytokine positive T cells at T6 and T12 were calculated by the Wilcoxon test.
Effect of Previous SARS-CoV-2 Infection
To investigate the effect of a previous infection on the immune response to vaccination, we stratified the sample population based on a history of SARS-CoV-2 infection received before the vaccination. As shown in Figures 2 and 3 , it was evident that the previous infection promoted a superior T-cell response up to 6 months after vaccination. Prebooster median frequencies of CD4+ IFN-γ, CD8+ IFN-γ, and CD8+ TNF-α T cells were significantly lower in the naïve group (P = .0057, P = .0003, P = .0011; previous SARS-CoV-2 infection vs SARS-CoV-2 naïve residents; Wilcoxon test) and fewer persons displayed polyfunctional CD4+ and CD8+ T cells producing more than 1 cytokine simultaneously.
Fig. 2.
T-cell mediated immune response in NH residents with a history of SARS-CoV-2 infection 6 months (prebooster, T6) and 12 months (postbooster, T12) after first immunization. (A) Frequencies of CD4 and CD8 T cells producing IFN-γ, TNF-α, and IL-2 in response to spike. (B) Percentages of previously infected NH residents with nonresponding CD4+ and CD8+ T cells or producing 1 to 3 cytokines pre- and post-booster. Differences between median frequencies of cytokine positive T cells at T6 and T12 were calculated by the Wilcoxon test.
Fig. 3.
T-cell mediated immune response in SARS-CoV-2 naïve NH residents 6 months (prebooster, T6) and 12 months (postbooster, T12) after first immunization. (A) Frequencies of CD4 and CD8 T cells producing IFN-γ, TNF-α, and IL-2 in response to spike. (B) Percentages of SARS-CoV-2 naive NH residents with nonresponding CD4+ and CD8+ T cells or producing 1 to 3 cytokines pre- and postbooster. Differences between median frequencies of cytokine positive T cells at T6 and T12 were calculated by the Wilcoxon test.
Median frequencies of polyfunctional CD4+ and CD8+ T cells in the previous SARS-CoV-2 infection group at T12 showed minimal variations compared with prebooster (Figure 2, A). Importantly, the effect of the booster vaccine dose on T-cell responses was more evident in the naïve population. Indeed, the median frequencies of CD4+ IFN-γ T cells and CD8+ IFN-γ T cells significantly increased (Figure 3). Considering the frequencies of polyfunctional T cells, a shift to a more polarized CD8 response was observed in both groups (Figures 2 and 3), albeit the benefit of the booster dose on the polyfunctionality of T cells could not be statistically demonstrated, possibly because the number of studied residents is too small.
Correlation Between Humoral and Cell-Mediated Immune Response to Vaccination
The GMT of antitrimeric spike IgG, before the booster dose in the sample population was 892 BAU/mL. The postbooster GMT was markedly increased to 4397 BAU/mL. A significant positive correlation was found at prebooster assessment between serum levels of antitrimeric spike IgG and the percentage of CD4+ T cells producing IFN-γ, CD8+ T cells producing IFN-γ, and CD8+ T cells producing TNF-α (Supplementary Table 1). To analyze these data in more depth, the sample population was divided in 2 groups based on CD4+ and CD8+ T cell cytokine production: no/low CMI responders (0 or 1 cytokine produced) and CMI responders (2 or 3 cytokines produced). Confirming the correlation data (before booster administration), CMI responders had higher antibody levels than no/low CMI responders, reaching the statistical significance in the CD8+ compartment (Table 2 ). At the following time point (after booster administration), high antibody titers were measured both in non/low CMI responders and in CMI responders and no significant differences were found.
Table 2.
GMT of Anti-S IgG (BAU/mL) Before Booster Dose (T6) and 12 Months (T12) After First Dose of Vaccine
| Anti-S IgG in BAU/mL |
||||
|---|---|---|---|---|
| T6 |
T12 |
|||
| GMT | P Value | GMT | P Value | |
| CD4 | ||||
| Low or nonresponders | 496.50 | .047 | 4722.06 | .7953 |
| 2+ cytokines | 1282.50 | 4188.09 | ||
| CD8 | ||||
| Low or nonresponders | 496.21 | .0059 | 6124.18 | .1415 |
| 2+ cytokines | 1719.86 | 3165.29 | ||
Results of Student t-test for nonlow and high CMI responders.
Bold values are statistically significant.
Discussion
In the present study, we describe T-cell immune responses in a population of NH residents before and after the administration of the booster vaccine dose in a 12-month follow-up study. A robust and durable T-cell response to recall spike stimulation was measured pre-and post- the booster vaccination in most residents.
The immune response induced by COVID-19 vaccines in older people is significant, especially when considering immune impairments of this population, and showed to significantly prevent severe COVID-19.25, 26, 27 T-cell responses after 2 doses of BNT162b2 vaccine have been described in older people whose immunity was impaired.19 , 22 , 23 Accordingly, we found that 6 months after vaccination most NH residents had detectable cytokine-producing spike-specific T cells and that higher responses were in those residents with a previous history of SARS-CoV-2 infection.
A positive correlation between humoral and cellular immune response was found before booster vaccination. Lower anti-spike IgG levels were detected in no/low CMI responders, an observation similar to previously published data.28 Yet, we extended our observation up to 1-year after vaccination. The effect of the third booster dose, administered to NH residents between the 6- and 12-month blood withdrawals, on the magnitude of the humoral immune response was evident. Spike-specific IgG levels increased substantially, confirming our previous results in NH settings.16 A significant increase of spike-specific T-cell responses were observed for naïve SARS-CoV-2 residents but not for previously infected residents.
Overall, pre- and postbooster CMI data show that a previous SARS-CoV-2 infection represents a first immunizing event that built a T-cell memory pool efficiently expanded by the primary vaccination. Only 1 out of 24 previously infected residents did not show any CD4+ or CD8+ positive response at the prebooster assessment, while among naïve COVID-19 residents, 3 out of 10 were CD4+ and CD8+ nonresponders. Administration of the booster dose induced a marked increase of serum IgG levels, measured 12 months after the first vaccine shot, and a strong induction of cell-mediated immunity in low-responding NH residents. These findings confirm previous data of the effect of an additional vaccine dose in unresponsive older adults23 , 29 , 30 and show that repeated immunization might lead to a sustained T-cell immune response even in immune impaired frail older persons.
Conclusions and Implications
Although the study has some limitations, such as the limited number of participants and lack of a control group, it allows for important considerations. We show a robust CMI against the spike protein of SARS-CoV-2 in frail older NH residents 1 year after the first COVID-19 mRNA vaccination, an observation that adds to data on antibody induction.9, 10, 11
Our data regarding the effect of booster doses of mRNA vaccines to older NH residents show high levels of both the humoral and the cell-mediated components of the immune response 1 year after the immunization, in previously infected and in naïve subjects. However, how immunity induced by COVID-19 vaccines tailored against the ancestral spike protein translates into protection against SARS-CoV-2 variants in this population group needs to be assessed.
Acknowledgments
We gratefully acknowledge Iole Mosca for technical help and Matilde Bocci for administrative support.
Footnotes
Lucia Amici, BSN, Francesca Berardi, BSN, Riccardo Bernardi, MD, Mario Cardillo, BSN, Anila Cobani, MSN, Ida Confessore, BSN, Claudia Fiorucci, MD, Serena Guerriero, MSN, Liudmila Kountsevitch, MD, Vincenzo Leccese, MD, Federica Ruocco, BSN, Pasquale Sabino, MD, Antonio Sciarretta, MD, Deborah Spaccaferro, MD, Luciana Spinelli, MSN, Rita Ursino, MD, Romina Viotti, BSN, Roberta Granata, MD, Manuela Stefanelli, MD; Italian Hospital Group, Italy.
The GeroCovid Vax Study was funded by a grant from the Italian Medicines Agency.
The authors declare no conflicts of interest.
Contributor Information
the GeroCovid Vax CMI Study Group:
Lucia Amici, Francesca Berardi, Riccardo Bernardi, Mario Cardillo, Anila Cobani, Ida Confessore, Claudia Fiorucci, Serena Guerriero, Liudmila Kountsevitch, Vincenzo Leccese, Federica Ruocco, Pasquale Sabino, Antonio Sciarretta, Deborah Spaccaferro, Luciana Spinelli, Rita Ursino, Romina Viotti, Roberta Granata, and Manuela Stefanelli
Supplementary Data
Supplementary Table 1.
Correlation Between CD4/CD8 IFN-γ, IL-2, TFN-α, and SARS-CoV-2 Trimeric Spike IgG Antibody Concentrations
| Anti-S IgG in BAU/mL |
||||
|---|---|---|---|---|
| T6 |
T12 |
|||
| rs∗ | P Value | rs∗ | P Value | |
| CD4 IFN-γ | 0.36 | .036 | -0.07 | .70 |
| CD4 IL-2 | 0.21 | .24 | 0.03 | .86 |
| CD4 TFN- α | 0.04 | .80 | -0.10 | .58 |
| CD8 IFN-γ | 0.42 | .014 | -0.14 | .41 |
| CD8 IL-2 | 0.18 | .32 | 0.04 | .80 |
| CD8 TFN-α | 0.39 | .02 | -0.1 | .58 |
Bold values are statistically significant.
Spearman rank correlation coefficient.
References
- 1.Mueller A.L., McNamara M.S., Sinclair D.A. Why does COVID-19 disproportionately affect older people? Aging. 2020;12:9959–9981. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmieri L., Vanacore N., Donfrancesco C., et al. Clinical characteristics of hospitalized individuals dying with COVID-19 by Age Group in Italy. J Gerontol A Biol Sci Med Sci. 2020;75:1796–1800. doi: 10.1093/gerona/glaa146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heckman G.A., Kay K., Morrison A., et al. Proceedings from an International virtual townhall: reflecting on the COVID-19 pandemic: themes from long-term care. J Am Med Dir Assoc. 2021;22:1128–1132. doi: 10.1016/j.jamda.2021.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisman D.N., Bogoch I., Lapointe-Shaw L., McCready J., Tuite A.R. Risk factors associated with mortality among residents with coronavirus disease 2019 (COVID-19) in long-term care facilities in Ontario, Canada. JAMA Netw Open. 2020;3:e2015957. doi: 10.1001/jamanetworkopen.2020.15957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malara A., Noale M., Abbatecola A.M., et al. GeroCovid LTCFs Working Group Clinical features of SARS-CoV-2 infection in italian long-term care facilities: GeroCovid LTCFs Observational Study. J Am Med Dir Assoc. 2022;23:15–18. doi: 10.1016/j.jamda.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hägg S., Jylhävä J., Wanconnorsg Y., et al. Frailty, and comorbidity as prognostic factors for short-term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Dir Assoc. 2020;21:1555–1559. doi: 10.1016/j.jamda.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sepulveda E.R., Stall N.M., Sinha S.K. A comparison of COVID-19 mortality rates among long-term care residents in 12 OECD Countries. J Am Med Dir Assoc. 2020;21:1572–1574.e3. doi: 10.1016/j.jamda.2020.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guidelines of the Strategic Plan on COVID-19 vaccines approved by Parliament. Recommendations for the Organization of the Vaccination Campaign against SARS-CoV-2/COVID-19 and Vaccination Procedure. https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2020&codLeg=77981&parte=1&serie=null
- 9.Fedele G., Palmieri A., Malara A., et al. A third dose of mRNA COVID-19 vaccine significantly enhances Anti-SARS-CoV-2 spike igg response in nursing home residents in Italy. J Am Med Dir Assoc. 2022;23:1114–1115. doi: 10.1016/j.jamda.2022.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blain H., Tuaillon E., Gamon L., Pisoni A., Miot S., Picot M.C. Strong decay of SARS-CoV-2 spike antibodies after 2 BNT162b2 vaccine doses and high antibody response to a third dose in nursing home residents. J Am Med Dir Assoc. 2022;23:750–753. doi: 10.1016/j.jamda.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeulin H., Labat C., Duarte K., et al. Anti-spike IgG antibody kinetics following the second and third doses of BNT162b2 vaccine in nursing home residents. J Am Geriatr Soc. 2022;70:2552–2560. doi: 10.1111/jgs.17837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyer A.H., Noonan C., McElheron M., et al. Previous SARS-CoV-2 infection, age, and frailty are associated with 6-month vaccine-induced anti-spike antibody titer in nursing home residents. J Am Med Dir Assoc. 2022;23:434–439. doi: 10.1016/j.jamda.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salmerón Ríos S., Cortés Zamora E.B., Avendaño Céspedes A., et al. Immunogenicity after 6 months of BNT162b2 vaccination in frail or disabled nursing home residents: The COVID-A Study. J Am Geriatr Soc. 2022;70:650–658. doi: 10.1111/jgs.17620. [DOI] [PubMed] [Google Scholar]
- 14.Chong Y., Tani N., Goto T., et al. Contrasting specific antibody response to BNT162b2 mRNA vaccination in SARS-CoV-2-naive and previously infected nursing home residents. J Infect. 2022;84:418–467. doi: 10.1016/j.jinf.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blain H., Tuaillon E., Gamon L., et al. Antibody response after one and two jabs of the BNT162b2 vaccine in nursing home residents: the CONsort-19 study. Allergy. 2022;77:271–281. doi: 10.1111/all.15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canaday D.H., Carias L., Oyebanji O.A., et al. Reduced BNT162b2 messenger RNA vaccine response in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-naive nursing home residents. Clin Infect Dis. 2021;73:2112–2115. doi: 10.1093/cid/ciab447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMahan K., Yu J., Mercado N.B., et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekine T., Perez-Potti A., Rivera-Ballesteros O., et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demaret J., Corroyer-Simovic B., Alidjinou E.K., et al. Impaired functional T-Cell response to SARS-CoV-2 after two doses of BNT162b2 mRNA vaccine in older people. Front Immunol. 2021;12:778679. doi: 10.3389/fimmu.2021.778679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collier D.A., Ferreira I.A.T.M., Kotagiri P., et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596:417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres I., Albert E., Giménez E., et al. B- and T-cell immune responses elicited by the Comirnaty® COVID-19 vaccine in nursing-home residents. Clin Microbiol Infect. 2021;27:1672–1677. doi: 10.1016/j.cmi.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tut G., Lancaster T., Sylla P., et al. Antibody and cellular immune responses following dual COVID-19 vaccination within infection-naive residents of long-term care facilities: an observational cohort study. Lancet Healthy Longev. 2022;3:e461–e469. doi: 10.1016/S2666-7568(22)00118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero-Olmedo A.J., Schulz A.R., Hochstätter S., et al. Induction of robust cellular and humoral immunity against SARS-CoV-2 after a third dose of BNT162b2 vaccine in previously unresponsive older adults. Nat Microbiol. 2022;7:195–199. doi: 10.1038/s41564-021-01046-z. [DOI] [PubMed] [Google Scholar]
- 24.Abbatecola AM, Incalzi RA, Malara A, et al. Monitoring COVID-19 vaccine use in Italian long term care centers: the GeroCovid VAX study. Vaccine. 2022;40:2324–2330. doi: 10.1016/j.vaccine.2022.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pawelec G., McElhaney J. Unanticipated efficacy of SARS-CoV-2 vaccination in older adults. Immun Ageing. 2021;18:7. doi: 10.1186/s12979-021-00219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pawelec G., Bronikowski A., Cunnane S.C., et al. The conundrum of human immune system “senescence”. Mech Ageing Dev. 2020;192:111357. doi: 10.1016/j.mad.2020.111357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mateos-Nozal J., Galán Montemayor J.C., Lores Torres S., et al. SARS-CoV-2 B 1.1.7 variant outbreak in a fully vaccinated nursing home—Madrid, June 2021. J Am Med Dir Assoc. 2021;22:2266–2268. doi: 10.1016/j.jamda.2021.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.San Román J., Candel F.J., Sanz J.C., et al. Humoral and cellular response after mRNA vaccination in nursing homes: influence of age and of history of COVID-19. Vaccines. 2022;10:383. doi: 10.3390/vaccines10030383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attias P., Azzaoui I., El Karoui K., et al. Immune responses after a third dose of mRNA vaccine differ in Virus-Naive versus SARS-CoV-2- recovered dialysis patients. Clin J Am Soc Nephrol. 2022;17:1008–1016. doi: 10.2215/CJN.00830122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giménez E., Albert E., Zulaica J., et al. SARS-CoV-2 adaptive immunity in nursing home residents following a third dose of the Comirnaty® COVID-19 vaccine. Clin Infect Dis. 2022:ciac223. doi: 10.1093/cid/ciac223. [DOI] [PMC free article] [PubMed] [Google Scholar]