Abstract
Salmonella enterica serovar Typhi strain CVD 908-htrA is a live attenuated strain which may be useful as an improved oral typhoid vaccine and as a vector for cloned genes of other pathogens. We conducted a phase 2 trial in which 80 healthy adults received one of two dosage levels of CVD 908-htrA in a double-blind, placebo-controlled, crossover study. There were no differences in the rates of side effects among volunteers who received high-dose vaccine (4.5 × 108 CFU), lower-dose vaccine (5 × 107 CFU), or placebo in the 21 days after vaccination, although recipients of high-dose vaccine (8%) had more frequent diarrhea than placebo recipients (0%) in the first 7 days. Seventy-seven percent and 46% of recipients of high- and lower-dose vaccines, respectively, briefly excreted vaccine organisms in their stools. All blood cultures were negative. Antibody-secreting cells producing antilipopolysaccharide (LPS) immunoglobulin A (IgA) were detected in 100 and 92% of recipients of high- and lower-dose vaccines, respectively. Almost half the volunteers developed serum anti-LPS IgG. Lymphocyte proliferation and gamma interferon production against serovar Typhi antigens occurred in a significant proportion of vaccinees. This phase 2 study supports the further development of CVD 908-htrA as a single-dose vaccine against typhoid fever and as a possible live vector for oral delivery of other vaccine antigens.
Attenuated Salmonella enterica serovar Typhi oral vaccine Ty21a (7) and parenteral purified Vi polysaccharide vaccine (1, 13) have replaced parenteral killed whole-cell vaccine as the recommended prophylaxis against typhoid fever. However, both of these vaccines have disadvantages. The Vi vaccine is T-cell independent and so does not stimulate helper T cells that could enhance and broaden the immune response and elicit immunologic memory. Ty21a requires three or four doses for optimal immunogenicity.
A single-dose, oral serovar Typhi vaccine strain is highly desirable. Moreover, such a strain would also be a promising vector for the delivery of heterologous cloned antigens (2, 6, 8, 9, 22, 25). One strategy for attenuating salmonellae has been to introduce defined deletions into the genes encoding enzymes of the aromatic amino acid biosynthesis pathway, thereby rendering the bacteria auxotrophic for para-aminobenzoic acid (PABA) and dihydroxybenzoate (DHB) (10). These are substrates that the organism cannot scavenge in sufficient quantities in mammalian tissues to sustain growth. Such aro deletion mutants of S. enterica serovar Typhimurium are safe and immunogenic as live oral vaccines in mice and cattle (4, 10, 12, 19). Analogous auxotrophic mutants of serovar Typhi have been prepared as typhoid vaccines and vaccine vectors for humans.
In recent studies, vaccine strain CVD 908, a derivative of wild-type strain Ty2 harboring deletion mutations in aroC and in aroD, has been evaluated with adult volunteers. CVD 908 was well tolerated and highly immunogenic when given to volunteers in phase 1 studies after having been freshly harvested from solid agar plates and washed (20, 21, 23, 24). However, CVD 908 was frequently detected in the blood cultures of volunteers who received higher doses. Although these volunteers were afebrile and the vaccine bacteremia was transient and self-limited, vaccine bacteremia was deemed undesirable.
The htrA locus, which encodes a heat shock protein in Salmonella, was chosen to further attenuate CVD 908. When this gene is deleted in serovar Typhimurium or Typhi, the resulting mutant is less virulent because of an impaired ability to survive and/or replicate in host tissues (11, 17). In vitro, ΔhtrA mutants of serovar Typhimurium are more susceptible to oxidative stress than the wild type, suggesting that the mutants may be less able to withstand oxidative killing within macrophages. Nonetheless, ΔhtrA mutants of serovar Typhimurium conferred on orally vaccinated mice a high level of protection against a lethal challenge with wild-type serovar Typhimurium (5). When given to 22 volunteers in a phase 1 study, serovar Typhi strain CVD 908-htrA freshly harvested from agar plates was generally well tolerated at doses of 5 × 107 to 5 × 109 CFU (26). No vaccine bacteremias were observed, and CVD 908-htrA retained significant immunogenicity (26). The purpose of this study was to conduct the initial phase 2 safety and immunogenicity study of CVD 908-htrA, formulated as a lyophilate, with U.S. outpatient volunteers.
MATERIALS AND METHODS
Eighty healthy adult volunteers, recruited from the Baltimore-Washington community, the University of Maryland, Baltimore campus, and the University of Maryland, College Park campus, participated in a randomized, placebo-controlled, double-blind crossover study. The inclusion criteria for participation were as follows: healthy men and women 18 to 40 years old; normal medical history and vital signs; and normal complete blood count and serum creatinine, glucose, and hepatic transaminase levels. Exclusion criteria were as follows: history of typhoid fever or typhoid vaccination; work as a commercial food handler or health care worker involved in patient care; use of antibiotics in the 7 days before immunization; a household contact who was under age 2 and/or who was immunocompromised, pregnant, or employed as a commercial food handler; allergy to ciprofloxacin or trimethoprim-sulfamethoxazole; and failure to pass a written examination. The protocol was reviewed by the Institutional Review Boards, University of Maryland, Baltimore and College Park, and the vaccine strain and protocol are described in BB-IND 7096 of the U.S. Food and Drug Administration. Following appropriate screening, detailed explanation of the protocol, and obtaining of informed consent, outpatient volunteers were randomized to receive with buffer a single oral dose of (i) high-dose CVD 908-htrA (4.5 × 108 CFU) (n = 20); (ii) lower-dose CVD 908-htrA (5 × 107 CFU) (n = 20); (iii) placebo preparation 1 (n = 20); or (iv) placebo preparation 2 (n = 20). The placebo preparations were identical and consisted of buffer solution alone. Subjects were randomized in blocks of four, whereby one subject initially received high-dose CVD 908-htrA, one received lower-dose CVD 908-htrA, one received placebo 1, and one received placebo 2, by use of the Microsoft Excel random-number function RAND.
On day 28, there was a crossover in which all volunteers received another preparation. Volunteers who ingested CVD 908-htrA vaccine on day 0 now received placebo; volunteers who received placebo on day 0 now received CVD 908-htrA vaccine in either a high or a lower dose.
Clinical and bacteriologic surveillance.
The volunteers kept a daily diary of symptoms for 21 days after each ingestion of vaccine or placebo, including daily oral temperature determinations measured in the evening. On days 1 to 5 after each dose of vaccine or placebo, stools were cultured to detect excretion of the vaccine strain. These times were chosen based on previous studies of CVD 908-htrA in which shedding was detected only on days 0, 1, and 2 (26). Stools were inoculated directly onto supplemented Salmonella-Shigella agar and into gram-negative enrichment broth supplemented with PABA and DHB. After overnight incubation at 37°C, subcultures were made on Salmonella-Shigella agar supplemented with PABA and DHB. Suspicious colonies were transferred to triple sugar iron slants, and confirmation was made by agglutination with serovar Typhi O, H, and Vi antisera. Quantitative culturing was performed with whole stool specimens.
Blood culturing to detect vaccine organisms was done on days 1, 3, 5, 7, 9, and 11 after each dose of vaccine or placebo or if fever occurred. Whole blood was inoculated into Septi-chek broth (Becton Dickinson) for culturing.
ASC measurements.
Before and on days 7, 28, and 35 after ingestion of the first dose of vaccine or placebo, peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation over lymphocyte separation medium (Organon-Teknika, Durham, N.C.) to measure class-specific antibody-secreting cells (ASC) by an ELISPOT assay (24); cells secreting antibody to serovar Typhi lipopolysaccharide (LPS) O and H antigens were sought. A significant rise in ASC was defined as the mean + 3 standard deviations (SD) of baseline values on day 7 or 28.
Serum antibody measurements.
Blood was collected before immunization and on days 7, 28, 35, and 56 after the first ingestion of vaccine or placebo to measure serum immunoglobulin G (IgG), IgM, and IgA antibodies to serovar Typhi LPS O, H, and Vi antigens by an enzyme-linked immunosorbent assay (ELISA) (14, 24). For anti-LPS IgG responses, sera were tested after a single 1:100 dilution in phosphate-buffered saline (PBS); a change in the optical density between pre- and postvaccination specimens of at least 0.2 was considered seroconversion. As a secondary analysis, anti-LPS O antigen IgG responses were also measured by an ELISA with serial dilutions of serum beginning at 1:50 and, as an endpoint, the highest dilution in which the optical density was ≥0.20 (determined as the mean plus 3 standard deviations of a negative control population). A fourfold rise in titer between prevaccination and peak postvaccination samples was considered a positive response.
Cell-mediated immune responses.
PBMC were isolated from blood obtained before and 14, 28, 42, and/or 56 days after the first ingestion of vaccine or placebo by density gradient centrifugation over lymphocyte separation medium, resuspended in medium (AIM-V containing 50 μg of gentamicin per ml), and used immediately for measuring proliferative responses and gamma interferon (IFN-γ) production in response to serovar Typhi antigens as previously described (21, 26).
(i) Antigen preparations.
Serovar Typhi flagella (STF) were purified from the rough serovar Typhi strain Ty2R by a bulk shearing method at the Center for Vaccine Development as previously described (21, 26). This preparation was further purified over a column of polymyxin B followed by ENDX-B15, an LPS removal column (Associates of Cape Cod, Inc., Woods Hole, Mass.). This STF preparation formed a single strong precipitate band with a rabbit anti-Ty2R flagellum antiserum in an Ouchterlony gel, confirming that the protein in the purified preparation was STF. The purity of the 10-mg/ml stock of STF was determined using sodium dodecyl sulfate–10 to 15% polyacrylamide gel electrophoresis–Tris acetate minigels. The STF preparation used in these studies consisted of a single flagellin band of ∼55 kDa in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Less than 24 pg of LPS per ml was present in the purified STF preparation, as determined by using chromogenic Limulus amebocyte lysate kits (Associates of Cape Cod; level of sensitivity, 24 pg/ml).
Particulate serovar Typhi consisted of heat-phenol–killed whole-cell Ty2 bacteria (typhoid vaccine from Wyeth Laboratories, Marietta, Pa.). Bovine serum albumin (BSA; fraction V; Sigma) was used as a control protein in these studies. Phytohemagglutinin (PHA; HA-17; Wellcome Diagnostics, Beckenham, United Kingdom) was used to confirm the ability of PBMC obtained from immunized volunteers to proliferate in response to mitogenic stimulation.
(ii) Measurement of lymphoproliferative responses.
A standard lymphocyte proliferation assay was used to examine the responses to whole-cell serovar Typhi particles, purified STF, BSA, or PHA (21, 26). Briefly, 1.5 × 105 PBMC were added in triplicate to the wells of 96-well plates containing AIM-V medium. Antigens or mitogen was added to the following final concentrations: STF, 2 μg/ml; BSA, 2 μg/ml; and PHA, 2 μg/ml. Whole-cell bacteria were added at 2 × 105 particles per well in a final volume of 200 μl. The final volume was 200 μl/well. Cells were cultured for 2 days (for PHA) or 6 days (for antigens) at 37°C in 5% CO2, and 1 μCi of tritiated thymidine (3H-TdR) per well was added. Plates were harvested 20 h after the addition of 3H-TdR in a Wallac (Gaithersburg, Md.) cell harvester, and incorporated 3H-TdR (reported as counts per minute) was measured on a Wallac Trilux Microbeta counter. Net counts per minute were calculated by subtracting the 3H-TdR incorporation of cells in the absence of antigens from the 3H-TdR incorporation in antigen-containing cultures for that same day and subject. A positive lymphoproliferative response was defined as a difference (P, <0.01; one-tailed t test) in mean net counts per minute between triplicate pre- and postvaccination samples stimulated with each antigen (i.e., STF, whole-cell particulate antigen, or BSA).
(iii) Measurement of IFN-γ by chemiluminescence ELISAs.
PBMC were incubated in 24-well plates with purified STF or BSA at the concentrations indicated above. Supernatants were collected after 3 days of incubation and kept at −70°C until analyzed. Chemiluminescence ELISAs were performed as previously described (18). Briefly, 96-well black opaque plates were coated with anti–IFN-γ monoclonal antibodies (PharMingen, San Diego, Calif.) diluted in 0.1 M sodium carbonate buffer (pH 9.6) and incubated overnight. Plates were subsequently washed and blocked with PBS containing 3% BSA (PBS-BSA). After the plates were washed again, serial twofold dilutions of supernatants and recombinant human IFN-γ (standard) (PharMingen) were incubated in duplicate wells overnight at 4°C. Biotinylated anti–IFN-γ monoclonal antibodies (PharMingen) were added, and plates were incubated for 1 h at 37°C. Wells were then washed, and avidin-peroxidase diluted 1:400 in PBS-BSA was added for 1 h at 37°C. Chemiluminescence ELISA reagent was used as a substrate. Chemiluminescence (relative light units per second) was measured on a 1450 Microbeta Trilux plate reader (Wallac, Turku, Finland). The concentration of IFN-γ in each sample was calculated by interpolation on the standard curves. The limit of sensitivity was 4 pg/ml. Net IFN-γ production levels (in picograms per milliliter) were calculated by subtracting the IFN-γ produced by PBMC in the absence of antigens from the IFN-γ produced in antigen-containing cultures for that same day and subject. A positive IFN-γ response was defined as a difference of more than 125 pg/ml in the levels of net IFN-γ production between pre- and postvaccination PBMC stimulated with each antigen (i.e., STF or BSA).
Preparation and administration of vaccine.
The vaccine was prepared by Evans Medical Limited (Medeva Group), Speke, United Kingdom. The organisms were grown in nutrient broth, washed three times, and lyophilized. The formulation for the high dose consisted of two glass vials each containing approximately 2.2 × 108 viable organisms of lyophilized vaccine to be resuspended in buffer solution (see below). The formulation for the lower dose consisted of a single vial containing 5 × 107 viable organisms to which buffer solution was added. Viable counts for the vaccine vials were determined before and after the trial, and the dose levels were confirmed.
Volunteers fasted for 90 min before and after vaccination. Two grams of sodium bicarbonate (Humco Laboratory, Texarkana, Tex.) was added to 150 ml of distilled water (buffer solution). Each volunteer ingested 120 ml of buffer solution. The remaining 30 ml was given as a vaccine solution as follows. Each vial of lyophilized vaccine was suspended in 5 ml of buffer solution. The vial was then rinsed with an additional 5 ml of buffer solution to ensure that the entire contents were removed. The suspended vaccine and rinse solutions were added to the remaining buffer in a small medicine cup to comprise a 30-ml vaccine-buffer mix, which the volunteer ingested.
Placebo consisted of sodium bicarbonate solution alone, given as 120 ml followed 1 min later by 30 ml in a small medicine cup.
Definitions.
Diarrhea was defined as the passage of three liquid stools within a 24-h period. A liquid stool was defined as one that does not hold its shape in the toilet; a formed stool holds its shape in the toilet. Fever was defined as an oral temperature of ≥38.2°C (100.8°F). For subjective symptoms, a symptom of grade 1 severity was defined as one that was mild or hardly noticed; a symptom of grade 2 severity was defined as one that was bothersome, but some activities could be continued; and a symptom of grade 3 severity was defined as one which interfered with all activities or sleep.
Analytic strategy.
For reactogenicity, the primary endpoint variables were incidence of fever, headache, malaise, diarrhea, loss of appetite, nausea, vomiting, or cramps after ingestion of vaccine at either dose or placebo during the 21-day observation period. The null hypothesis was that the incidence of individual symptoms among volunteers who ingested vaccine would be no higher than that among volunteers who ingested placebo. It was assumed that there were no crossover effects on rates of reactogenicity, so that period 1 and period 2 results were analyzed together. Hence, the groups receiving high- and lower-dose vaccines were compared independently to the combined groups receiving placebo by Fisher's exact tests evaluated at the 5% level. A Bonferroni correction for multiple comparisons in these analyses was not applied, representing a conservative approach in detecting vaccine reactogenicity.
Seroconversion rates were compared by Fisher's exact tests. Significant differences in the magnitude of proliferative responses before and after immunization were detected using one-tailed Wilcoxon tests. The significance of differences in the magnitude of IFN-γ production before and after immunization was examined using the Wilcoxon signed-rank test.
The correlations among the measures of immune response were further examined using a maximum-likelihood factor analysis, a method used to simplify the complex relationships among several measured variables. Included in this analysis were significant rise in ASC producing IgA, IgG, and IgM anti-H antigen; rise in ASC producing IgA, IgG, and IgM anti-LPS; rise in serum IgA, IgG, and IgM anti-H antigen; rise in serum IgA, IgG, and IgM anti-LPS; rise in serum IgA, IgG, and IgM anti-Vi antigen; proliferation on day 28 in response to STF; and IFN-γ production on day 28 in response to STF. Resulting factors were rotated orthogonally (varimax) to simplify their interpretation. Associations between measured variables and underlying factors were considered strong if the standardized regression coefficients were ≥30%.
RESULTS
Study participants.
Eighty adults, 48 (60%) men and 32 (40%) women, with a mean age of 27 years (range, 18 to 40 years), participated in the trial. Seventy-six doses of placebo, 39 high-dose vaccines, and 39 lower-dose vaccines were administered during the course of the crossover study. Six volunteers did not cross over after the first dose of vaccine or placebo for a variety of reasons. Two volunteers (one high-dose vaccinee and one placebo recipient) were excluded from the crossover because of noncompliance with follow-up visits; one volunteer (who had received placebo) was excluded from the crossover because he had taken a course of antibiotics for a sexually transmitted disease; one volunteer, who had received lower-dose vaccine, withdrew consent; one volunteer, who had received lower-dose vaccine, left town because of family illness in another state; and one volunteer, who had received high-dose vaccine, was excluded because of syncope 25 days after vaccination. This volunteer underwent a thorough inpatient evaluation, and his syncope was ultimately attributed to a vasovagal reaction unrelated to vaccine because of the timing of the episode 25 days after vaccination and the nature of his symptoms.
Safety.
There were no statistically significant differences in the incidence of side effects among recipients of placebo, high-dose vaccine, or lower-dose vaccine in the first 21 days after vaccination (Table 1). Fever was observed in 3% of placebo recipients, 3% of lower-dose vaccinees, and none of the high-dose vaccinees. Diarrhea, a symptom observed in a small number of vaccinees in an uncontrolled, phase 1 study (26), occurred in 3 (4%) of 76 placebo recipients, 1 (3%) of 39 lower-dose vaccine recipients (P, 0.58; Fisher's exact test), and 4 (10%) of 39 high-dose vaccine recipients (P, 0.18; Fisher's exact test). The number of liquid stools was not different between recipients of either dose of vaccine and recipients of placebo. Of the five vaccinees who had diarrhea, only two had loose stools when their stool cultures were positive; in both cases, this event occurred within the first 24 h after vaccination. In the other three vaccinees, diarrhea occurred on days 7, 12, and 17 after vaccination. There were no differences in the maximum reported intensity of symptoms among recipients of vaccine or placebo (Fisher's exact tests) (data not shown).
TABLE 1.
Incidence of side effects observed during the 21 days after ingestion of high-dose vaccine, lower-dose vaccine, or buffer placebo
| Symptom or sign | No. (%) of recipients in the following group:
|
P valuea for placebo vs the following vaccine:
|
|||
|---|---|---|---|---|---|
| Placebo | Vaccine
|
Lower dose | High dose | ||
| Lower dose | High dose | ||||
| Malaise | 22 (29) | 10 (26) | 12 (31) | 0.83 | 0.83 |
| Anorexia | 13 (17) | 6 (15) | 9 (23) | 1.00 | 0.46 |
| Headache | 30 (39) | 13 (33) | 18 (46) | 0.55 | 0.55 |
| Cramps | 18 (24) | 13 (33) | 12 (31) | 0.28 | 0.50 |
| Nausea | 15 (20) | 8 (21) | 5 (13) | 1.00 | 0.44 |
| Vomiting | 3 (4) | 3 (8) | 1 (3) | 0.33 | 0.58 |
| Fever | 2 (3) | 1 (3) | 0 (0) | 1.00 | 0.55 |
| Diarrhea | 3 (4) | 1 (3) | 4 (10) | 0.58 | 0.18 |
Determined with one-tailed Fisher's exact test.
As a secondary analysis, the incidence of symptoms in the first 7 days after vaccination was examined. Recipients of high-dose vaccine had more frequent diarrhea (3 of 39; 8%) than recipients of placebo (0 of 76) in the first 7 days (P, 0.04; Fisher's exact test). No recipient of lower-dose vaccine had diarrhea in the first 7 days. Recipients of high-dose vaccine (21%) reported loss of appetite more frequently than did recipients of placebo (7%) in the first 7 days (P, 0.03; Fisher's exact test). There were no significant differences in the incidence of other symptoms during this period or in the incidence of any symptoms between lower-dose vaccinees and placebo recipients in the first 7 days.
Microbiology.
After ingestion of the lower dose of vaccine, 46% of volunteers shed the vaccine strain in their stools for a mean of 1.8 days (range, 0.6 to 3.8 days) (Table 2). Only 15% of lower-dose vaccinees shed vaccine for more than 2 days, and only 3% of this group shed vaccine for more than 3 days. Volunteers who received lower-dose vaccine excreted a maximum of 2.8 × 106 CFU/g of stool. Among recipients of high-dose vaccine, 77% shed vaccine for a mean of 2.3 days (range, 0.8 to 4.2 days), with maximum shedding of 8 × 106 CFU/g. Thirty-three percent of high-dose vaccinees excreted vaccine for more than 2 days, and 13% excreted vaccine for more than 3 days. No blood culture obtained at any time point was positive. No stool culture obtained from a placebo recipient was positive.
TABLE 2.
Recovery of live oral typhoid vaccine CVD 908-htrA in stool and blood cultures after ingestion of high-dose vaccine, lower-dose vaccine, or buffer placebo
| Group | No. (%) of positive cultures
|
Geometric mean (range) peak shedding in stool, in CFU/ga | Mean (range) duration of shedding, in daysb | |
|---|---|---|---|---|
| Stool | Blood | |||
| Placebo | 0 (0) | 0 (0) | 0 | Not applicable |
| Vaccine | ||||
| Lower dose | 18 (46) | 0 (0) | 38 (0–2.8 × 106) | 1.8 (0.6–3.8) |
| High dose | 30 (77) | 0 (0) | 5.6 × 103 (0–8.0 × 106) | 2.3 (0.8–4.2) |
For all volunteers.
For volunteers who shed vaccine in stools.
ASC responses.
ASC producing anti-LPS IgA, a measure of priming of the mucosal immune system, were detected in 100% of recipients of high-dose vaccine and 92% of recipients of lower-dose vaccine (Table 3). Recipients of the high dose of CVD 908-htrA had a geometric mean of 189 anti-LPS IgA ASC per 106 PBMC. Vigorous IgM and IgG ASC responses were also detected among vaccinees (Table 3). ASC producing anti-H antigen IgA occurred in 79 and 73% of high- and lower-dose vaccine recipients, respectively (Table 3).
TABLE 3.
ASC responses after ingestion of high-dose vaccine, lower-dose vaccine, or buffer placebo
| Antibody | No. (%) of recipients responding to:
|
Geometric mean no. of cells/106 PBMC in response to:
|
||||
|---|---|---|---|---|---|---|
| Placebo | Vaccine
|
Placebo | Vaccine
|
|||
| Lower dose | High dose | Lower dose | High dose | |||
| Anti-LPS IgA | 10 (28) | 34 (92) | 34 (100) | <1 | 62 | 189 |
| Anti-LPS IgG | 1 (3) | 30 (81) | 32 (94) | <1 | 22 | 42 |
| Anti-LPS IgM | 1 (3) | 33 (89) | 33 (97) | <1 | 43 | 116 |
| Anti-H antigen IgA | 4 (11) | 27 (73) | 27 (79) | <1 | 7 | 11 |
| Anti-H antigen IgG | 0 (0) | 11 (30) | 17 (50) | <1 | 3 | 5 |
| Anti-H antigen IgM | 3 (8) | 25 (68) | 28 (82) | <1 | 6 | 14 |
Serum antibody measurements.
Serum anti-LPS IgG responses measured by the single-dilution method were detected in 49% of high-dose vaccinees, 51% of lower-dose vaccinees, and 8% of placebo recipients. Serum anti-LPS IgG responses measured by the serial dilution method occurred in 49 and 46% of high- and lower-dose vaccine recipients, with geometric mean titers after immunization of 197 and 207, respectively, and in no placebo recipients (Table 4). Notably, none of the volunteers developed a rise in serum anti-Vi antigen IgG antibodies.
TABLE 4.
Serum antibody responses after ingestion of high-dose vaccine, lower-dose vaccine, or buffer placebo
| Antibody | No. (%) of recipients responding to:
|
Geometric mean peak reciprocal titer in response to:
|
||||
|---|---|---|---|---|---|---|
| Placebo | Vaccine
|
Placebo | Vaccine
|
|||
| Lower dose | High dose | Lower dose | High dose | |||
| Anti-LPS IgA | 7 (18) | 23 (59) | 30 (77) | 38 | 168 | 262 |
| Anti-LPS IgGa | 0 (0) | 18 (46) | 19 (49) | 60 | 207 | 197 |
| Anti-LPS IgM | 5 (13) | 16 (41) | 27 (69) | 47 | 113 | 190 |
| Anti-H antigen IgA | 0 (0) | 13 (33) | 26 (67) | 22 | 44 | 80 |
| Anti-H antigen IgG | 1 (3) | 11 (28) | 16 (41) | 91 | 134 | 182 |
| Anti-H antigen IgM | 4 (10) | 9 (23) | 13 (33) | 25 | 45 | 38 |
| Anti-Vi antigen IgA | 0 (0) | 3 (8) | 3 (8) | 15 | 20 | 20 |
| Anti-Vi antigen IgG | 0 (0) | 0 (0) | 0 (0) | 14 | 15 | 16 |
| Anti-Vi antigen IgM | 0 (0) | 1 (3) | 0 (0) | 12 | 13 | 13 |
Anti-LPS IgG data were from a secondary analysis (see the text).
CMI responses.
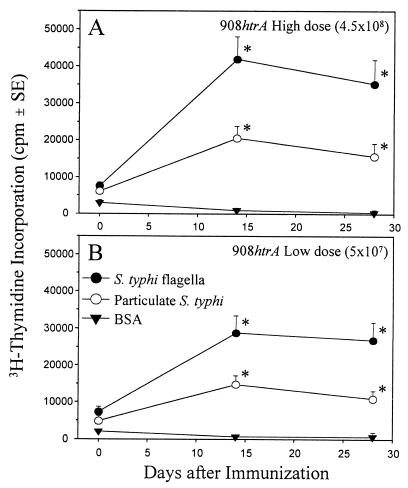
Significant increases (P, <0.01) in lymphoproliferative responses to STF by PBMC obtained 14 days following immunization occurred in 63% of volunteers given the high-dose vaccine, in 44% of volunteers given the lower-dose vaccine, and in 8% of volunteers who ingested buffer (Fig. 1). Moreover, significant increases (P, <0.01) in lymphoproliferative responses to particulate whole-cell serovar Typhi were observed in 44% of volunteers given the high-dose vaccine, in 41% of volunteers given the lower-dose vaccine, and in 8% of volunteers who ingested buffer. Proliferative responses to STF were dose dependent, since significantly higher levels of thymidine incorporation were observed in the high-dose vaccine group than in the lower-dose vaccine group (P, 0.03; one-tailed Wilcoxon test). No significant differences in proliferative responses to BSA were observed in PBMC obtained before and after immunization. These responses among vaccinees persisted at day 28. Responses among placebo recipients could not be interpreted at day 28 because significant proliferative responses to BSA (a control antigen) were measured on day 28.
FIG. 1.
Proliferative responses in volunteers following ingestion of 4.5 × 108 CFU of live oral typhoid vaccine CVD 908-htrA (high dose) (A) or 5 × 107 CFU of CVD 908-htrA (lower dose) (B). PBMC obtained from the volunteers before and 14 or 28 days after immunization were evaluated for lymphoproliferative responses to STF, particulate whole-cell serovar Typhi, or BSA (control) as described in Materials and Methods. Results are expressed as mean net counts per minute ± standard errors (SE) for all volunteers in each group. The asterisks denote P values of <0.01, as determined by a one-tailed paired t test of postimmunization versus preimmunization values.
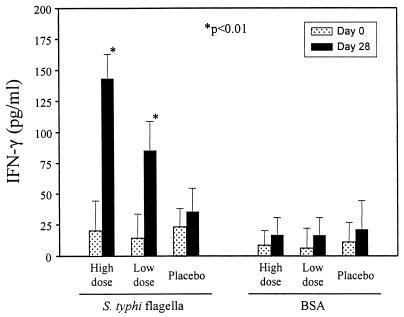
Significant increases (P, <0.01) in IFN-γ production in cultures containing PBMC obtained following immunization and incubated in the presence of STF were observed in both the high- and lower-dose vaccine groups (Fig. 2). Increases in IFN-γ production by PBMC obtained 28 days after immunization occurred in 30% of volunteers given high-dose vaccine, in 18% of volunteers given lower-dose vaccine, and in 6% of volunteers who ingested buffer. No significant differences in IFN-γ production in response to BSA were observed in PBMC obtained before and after immunization (Fig. 2).
FIG. 2.
IFN-γ production by volunteers following ingestion of 4.5 × 108 CFU of live oral typhoid vaccine CVD 908-htrA (high dose), 5 × 107 CFU of CVD 908-htrA (lower dose), or buffer (placebo). PBMC obtained from the volunteers before and 28 days after immunization were evaluated for IFN-γ production in response to STF or BSA (control) as described in Materials and Methods. Results are expressed as mean net picograms per milliliter ± standard errors for all volunteers in each group. The asterisks denote P values of <0.01, as determined by a one-tailed paired t test of postimmunization versus preimmunization values.
Correlations among measures of immune responses.
A two-factor model was fitted, as the likelihood ratio test indicated that no more than two factors were required (P, 0.21). The first two factors represented 38 and 19% of the variation among the 17 immunologic variables. The first rotated factor, accounting for 38% of the variation, was strongly associated with the following: rise in ASC producing anti-LPS IgA, IgG, and IgM; rise in serum anti-LPS IgA and IgG; rise in serum anti-H antigen serum IgA; and rise in serum anti-Vi antigen IgG. The second factor was strongly associated with a rise in ASC producing anti-H antigen IgA, IgG and IgM; a rise in ASC producing anti-LPS IgM; a rise in serum anti-LPS IgM; and a rise in serum anti-H antigen IgM. In contrast, lymphocyte proliferation and IFN-γ production in response to H antigen and a rise in anti-Vi antigen serum IgA and IgM were not correlated with either of these factors.
DISCUSSION
Salmonella serovar Typhi vaccine strain CVD 908-htrA meets many of the criteria for an improved oral typhoid vaccine. After a single oral dose, CVD 908-htrA stimulates vigorous mucosal, humoral, and cellular immune responses (20, 21) at levels that equal or surpass those measured after multiple doses of the currently licensed live oral strain Ty21a (3, 16). Moreover, CVD 908-htrA offers substantial potential advantages over the Vi polysaccharide vaccine, in that CVD 908-htrA elicits an array of immune responses and immunologic memory not elicited by the polysaccharide. It is notable that CVD 908-htrA does not stimulate serum anti-Vi antigen IgG antibodies. If single-dose CVD 908-htrA is efficacious in a wild-type challenge study and phase 3 field studies and protection is long lasting, this strain could replace the currently available oral vaccine in immunocompetent persons. Further tests of vaccine safety must be done with immunocompromised individuals. Preclinical studies further show that this vaccine strain has the added potential of serving as a vector for carrying cloned protective antigens of other pathogens (2, 6, 8).
This phase 2 crossover study confirms the clinical experience with CVD 908-htrA in phase 1 studies. One concern raised after the initial studies with the parent strain CVD 908 was the observation that many volunteers developed asymptomatic vaccine bacteremia in the week after vaccination (24). The current study corroborates previous observations (26) that the deletion of the htrA gene results in further attenuation, such that the vaccine cannot be detected in blood by routine surveillance culturing, yet the immunogenicity of the strain is not compromised.
Mild diarrhea was observed during the 14 days after vaccination in 2 (13%) of 15 inpatient vaccinees who received 5 × 108 to 5 × 109 CFU of vaccine in the phase 1 study (26) and in 5 (6%) of 78 outpatient vaccinees in the 21 days after receiving about 108 CFU of vaccine in this study. The pathogenesis of the loose stools is unclear. For the two volunteers who had diarrhea in the first 24 h after ingesting vaccine, the recovery of vaccine organisms from the stools suggests that the attenuated organism was in some way causing intestinal secretion that resulted in diarrhea. For the other three volunteers, whose diarrhea occurred later, when shedding had stopped (days 7 to 26), the mechanism by which the vaccine could have caused diarrhea is obscure. It is conceivable that small numbers of serovar Typhi organisms, below the level of sensitivity of the stool cultures, could have been present in the stools. The occurrence or severity of loose stools in children or infants in the United States or countries in which typhoid is endemic and who might receive CVD 908-htrA or a CVD 908-htrA vector vaccine will be evaluated in future studies.
The anti-LPS IgG seroconversion rate measured in this phase 2 study is somewhat lower than that observed in the previous phase 1 study (26). In the phase 1 study, 100% seroconversion with anti-LPS IgG antibodies was observed, while in the current study, 46 to 49% seroconversion to LPS was observed, depending on vaccine dose. Anti-H antigen serum IgG responses also occurred less frequently (41 and 28% in the high- and lower-dose groups, respectively) than previously observed. In the current study, CVD 908-htrA was prepared as a trial lot of lyophilate to be reconstituted immediately before ingestion, whereas in the previous study, vaccine consisted of organisms freshly harvested from agar plates before ingestion (26). Loss of protective immunogenicity of live bacterial vaccines after lyophilization has been previously described (15), and this observation may explain the apparently less vigorous humoral immune response to CVD 908-htrA formulated as a lyophilate than when given as freshly grown organisms.
Although the immune responses that correlate with protection against infection with serovar Typhi are currently unknown, the fact that serovar Typhi is an intracellular pathogen suggests that CMI responses (e.g., lymphoproliferation, IFN-γ production, and cytotoxic T-cell activity) are important mechanisms of protection against serovar Typhi infection. In fact, we have previously reported that immunization with attenuated strains of serovar Typhi elicits the appearance in circulation of CD8+, major histocompatibility complex class I-restricted, cytotoxic T-lymphocyte effector cells capable of killing autologous serovar Typhi-infected targets, as well as sensitized lymphocytes that proliferate and produce IFN-γ in response to stimulation with serovar Typhi antigens (20, 21). These observations suggest that CMI responses may play a crucial role in limiting the progression of typhoid infection. Induction of lymphoproliferative responses to serovar Typhi antigens was also demonstrated in volunteers who participated in the CVD 908-htrA phase 1 trial (26). In contrast to the results of the previous phase 1 trial (26), proliferative responses to STF in this trial were of greater magnitude than those observed following exposure to inactivated particulate whole-cell serovar Typhi (Fig. 1). The strong CMI responses in these volunteers confirm and extend the observation that, in a sizable proportion of volunteers, a single oral dose of CVD 908-htrA elicits the appearance in the circulation of sensitized lymphocytes that proliferate and produce IFN-γ in response to stimulation with serovar Typhi antigens. However, in contrast to the observation that the degree of induction of serum anti-LPS IgG antibodies appeared to be lower in the current study than in the phase 1 study (see above), CMI responses were of equal or greater magnitude. Therefore, it appears that CMI responses are not affected when a lyophilate, instead of freshly grown organisms, is used for oral immunization.
Results from a field study with volunteers vaccinated with the Ty21a typhoid vaccine strain suggested a correlation between increased levels of antigen anti-LPS O serum IgG antibodies and protective efficacy (16). Based on that study, increased levels of IgG to serovar Typhi LPS have been proposed as a surrogate marker of protection, although serum antibodies to O antigen are not believed to be the main effector immune mechanism in protection against serovar Typhi infection. Since it is likely that both antibody and CMI responses play key roles in protection against serovar Typhi infection, it is of great importance to investigate whether there is a correlation between increased levels of O antigen IgG antibodies and CMI responses in individual volunteers. In the current study, we observed no correlation in the magnitude of the different immunological responses evaluated, i.e., antibody levels, ASC, lymphoproliferation, and IFN-γ production. These results, which are in agreement with our previous observations for CVD 908 and CVD 906 vaccinees (21), confirm that there is a wide variation among individuals in the predominance of the various components of the immune response elicited by vaccination with attenuated strains of serovar Typhi. Future clinical trials in which volunteers exhibiting a predominance of antibody or CMI responses are challenged with wild-type serovar Typhi should help establish the relative contributions of defined immunological responses that correlate with protection against serovar Typhi infection.
ACKNOWLEDGMENTS
We gratefully acknowledge the contributions of our volunteers and the effort of the staff of the Adult Clinical Studies Section, Center for Vaccine Development, including Kathleen Palmer, Catherine Black, Ron Grochowski, Brenda Berger, Theresa Mowry, Elizabeth Peddicord, and Elisa Sindall. We also acknowledge Susan DiLorenzo, Mardi Reymann, and Sofie Livio for expert technical assistance.
This study was supported in part by Peptide Therapeutics Group plc, by World Health Organization technical services agreement V267/1181/91, and by National Institute of Allergy and Infectious Diseases contract NO1-AI-45251 and grants RO1-AI-36525, RO1-AI-40297, and RO1-AI-29471.
REFERENCES
- 1.Acharya I L, Lowe C U, Thapa R, Gurubacharya V L, Shrestha M B, Cadoz M, Schulz D, Armand J, Bryla D A, Trollfors B, Cramton T, Schneerson R, Jobbins J B. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi. N Engl J Med. 1987;317:1101–1104. doi: 10.1056/NEJM198710293171801. [DOI] [PubMed] [Google Scholar]
- 2.Barry E M, Gomez-Duarte O, Chatfield S, Rappuoli R, Pizza M, Losonsky G, Galen J, Levine M M. Expression and immunogenicity of pertussis toxin S1 subunit-tetanus toxin fragment C fusions in Salmonella typhi vaccine strain CVD 908. Infect Immun. 1996;64:4172–4181. doi: 10.1128/iai.64.10.4172-4181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black R, Levine M M, Young C, Rooney J, Levine S, Clements M L, O'Donnell S, Hughes T, Germanier R, Chilean Typhoid Committee Immunogenicity of Ty21a attenuated Salmonella typhi given with sodium bicarbonate or in enteric-coated capsules. Dev Biol Stand. 1983;53:9–14. [PubMed] [Google Scholar]
- 4.Brown A, Hormaeche E C, Demarco de Hormaeche R, Winther M, Dougan G, Maskell D J, Stocker B A D. An attenuated aroA Salmonella typhimurium vaccine elicits humoral and cellular immunity to cloned β-galactosidose in mice. J Infect Dis. 1987;155:86–92. doi: 10.1093/infdis/155.1.86. [DOI] [PubMed] [Google Scholar]
- 5.Chatfield S N, Strahan K, Pickard D, Charles I G, Hormaeche C E, Dougan G. Evaluation of Salmonella typhimurium strains harbouring defined mutations in htrA and aroA in the murine salmonellosis model. Microb Pathog. 1992;12:145–151. doi: 10.1016/0882-4010(92)90117-7. [DOI] [PubMed] [Google Scholar]
- 6.Galen J E, Gomez-Duarte O G, Losonsky G A, Halpern J L, Lauderbaugh C S, Kaintuck S, Reymann M K, Levine M M. A murine model of intranasal immunization to assess the immunogenicity of attenuated Salmonella typhi live vector vaccines in stimulating serum antibody responses to expressed foreign antigens. Vaccine. 1997;15:700–708. doi: 10.1016/s0264-410x(96)00227-7. [DOI] [PubMed] [Google Scholar]
- 7.Germanier R, Fürer E. Isolation and characterization of gal E mutant Ty21a of Salmonella typhi: a candidate strain for a live oral typhoid vaccine. J Infect Dis. 1975;141:553–558. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- 8.Girón J A, Xu J-G, González R, Hone D, Kaper J B, Levine M M. Simultaneous expression of CFA/I and CS3 colonization factor antigens of enterotoxigenic Escherichia coli by ΔaroC, ΔaroD Salmonella typhi vaccine strain CVD 908. Vaccine. 1995;13:939–946. doi: 10.1016/0264-410x(95)00003-j. [DOI] [PubMed] [Google Scholar]
- 9.González R, Hone D, Noriega F R, Tacket C O, Davis J R, Losonsky G, Nataro J P, Hoffman S, Malik A, Nardin E, Sztein M B, Heppner D G, Fouts T R, Isibasi A, Levine M M. Salmonella typhi vaccine strain CVD 908 expressing the circumsporozoite protein of Plasmodium falciparum: strain construction and safety and immunogenicity in humans. J Infect Dis. 1994;169:927–931. doi: 10.1093/infdis/169.4.927. [DOI] [PubMed] [Google Scholar]
- 10.Hoiseth S K, Stocker B A D. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 11.Johnson K, Charles I, Dougan G, Pickard D, O'Gaora P, Costa G, Ali T, Miller I, Hormaeche C. The role of a stress-response protein in Salmonella typhimurium virulence. Mol Microbiol. 1991;5:401–407. doi: 10.1111/j.1365-2958.1991.tb02122.x. [DOI] [PubMed] [Google Scholar]
- 12.Killar L M, Eisenstein T K. Immunity to Salmonella typhimurium infection in C3H/HeJ and C3H/HeNCrlBR mice: studies with an aromatic-dependent live S. typhimurium strain as a vaccine. Infect Immun. 1985;47:605–612. doi: 10.1128/iai.47.3.605-612.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klugman K P, Gilbertson I T, Koornof H J, Robbins J B, Schneerson R, Schulz D, Cadoz M, Armand J, Vaccination Advisory Committee Protective activity of Vi capsular polysaccharide vaccine against typhoid fever. Lancet. 1987;ii:1165–1169. doi: 10.1016/s0140-6736(87)91316-x. [DOI] [PubMed] [Google Scholar]
- 14.Levine M, Herrington D, Murphy J, Morris J, Losonsky G, Tall B, Lindberg A, Svenson S, Baqar S, Edwards M, Stocker B. Safety, infectivity, immunogenicity, and in vivo stability of two attenuated auxotrophic mutant strains of Salmonella typhi, 541Ty and 543Ty, as live oral vaccines in humans. J Clin Investig. 1987;79:888–902. doi: 10.1172/JCI112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine M M, DuPont H L, Hornick R B, Snyder M J, Woodward W, Gilman R H, Libonati J P. Attenuated streptomycin-dependent Salmonella typhi oral vaccine: potential deleterious effects of lyophilization. J Infect Dis. 1976;133:424–428. doi: 10.1093/infdis/133.4.424. [DOI] [PubMed] [Google Scholar]
- 16.Levine M M, Ferreccio C, Black R E, Tacket C O, Germanier R. Progress in vaccines against typhoid fever. Rev Infect Dis. 1989;2(Suppl. 3):S552–S567. doi: 10.1093/clinids/11.supplement_3.s552. [DOI] [PubMed] [Google Scholar]
- 17.Lowe D C, Savidge T C, Pickard D, Eckmann L, Kagnoff M F, Dougan G, Chatfield S N. Characterization of candidate live oral Salmonella typhi vaccine strains harboring defined mutations in aroA, aroC, and htrA. Infect Immun. 1999;67:700–707. doi: 10.1128/iai.67.2.700-707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasetti M F, Anderson R J, Noriega F R, Levine M M, Sztein M B. Attenuated ΔguaBA Salmonella typhi vaccine strain CVD 915 as a live vector utilizing prokaryotic or eukaryotic expression systems to deliver foreign antigens and elicit immune responses. Clin Immunol. 1999;92:76–89. doi: 10.1006/clim.1999.4733. [DOI] [PubMed] [Google Scholar]
- 19.Smith B P, Reina-Guerra M, Hoiseth S K, Stocker B A D, Habasha F, Johnson E, Merritt F. Aromatic-dependent Salmonella typhimurium as modified live vaccines for calves. Am J Vet Res. 1984;45:59–66. [PubMed] [Google Scholar]
- 20.Sztein M B, Tanner M K, Polotsky Y, Orenstein J M, Levine M M. Cytotoxic T lymphocytes after oral immunization with attenuated vaccine strains of Salmonella typhi in humans. J Immunol. 1995;155:3987–3993. [PubMed] [Google Scholar]
- 21.Sztein M B, Wasserman S S, Tacket C O, Edelman R, Hone D, Lindberg A A, Levine M M. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J Infect Dis. 1994;170:1508–1517. doi: 10.1093/infdis/170.6.1508. [DOI] [PubMed] [Google Scholar]
- 22.Tacket C O, Forrest B, Morona R, Attridge S R, LaBrooy J, Tall B D, Reymann M, Rowley D, Levine M M. Safety, immunogenicity, and efficacy against cholera challenge in humans of a typhoid-cholera hybrid vaccine derived from Salmonella typhi Ty21a. Infect Immun. 1990;58:1620–1627. doi: 10.1128/iai.58.6.1620-1627.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tacket C O, Hone D M, Curtiss III R, Kelly S M, Losonsky G, Guers L, Harris A, Edelman R, Levine M M. Comparison of the safety and immunogenicity of ΔaroC aroD and Δcya Δcrp Salmonella typhi strains in adult volunteers. Infect Immun. 1992;60:536–541. doi: 10.1128/iai.60.2.536-541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tacket C O, Hone D M, Losonsky G A, Guers L, Edelman R, Levine M M. Clinical acceptability and immunogenicity of CVD 908 Salmonella typhi vaccine strain. Vaccine. 1992;10:443–446. doi: 10.1016/0264-410x(92)90392-w. [DOI] [PubMed] [Google Scholar]
- 25.Tacket C O, Kelly S M, Schödel F, Losonsky G, Nataro J P, Edelman R, Levine M M, Curtiss R., III Safety and immunogenicity in humans of an attenuated Salmonella typhi vaccine vector strain expressing plasmid-encoded hepatitis B antigens stabilized by the Asd-balanced lethal vector system. Infect Immun. 1997;65:3381–3385. doi: 10.1128/iai.65.8.3381-3385.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tacket C O, Sztein M B, Losonsky G A, Wasserman S S, Nataro J P, Edelman R, Pickard D, Dougan G, Chatfield S N, Levine M M. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroD and immune response in humans. Infect Immun. 1997;65:452–456. doi: 10.1128/iai.65.2.452-456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]