Abstract
Background
Female thyroid cancer survivors are more likely to have a higher risk of breast cancer compared to the general population, and the underlying causes are yet to be understood. The potential role of I-131 treatment on this association remains controversial.
Methods
We pooled individual data of women who were treated for differentiated thyroid cancer from 1934 to 2005 in France, Italy and Sweden. Standardized incidence ratios (SIRs) for breast cancer were estimated by comparison with age, sex and calendar-year expected values of the general population in each country. We estimated breast cancer risk in relation to I-131 treatment using time-dependent Poisson models.
Results
Of 8475 women (mean age at diagnosis: 45 years, range 2–90 years), 335 were diagnosed with breast cancer [SIR = 1.52, 95% confidence interval (CI): 1.36–1.69] during a median follow-up time of 12.7 years since diagnosis. Overall, breast cancer risk did not differ between women treated or not with I-131 (relative risk=1.07, 95% CI 0.84–1.35). However, breast cancer risk increased with increasing cumulative I-131 activity, without significant departure from linearity (excess relative risk per 100 mCi=17%, 95% CI: 2% to 38%). The higher risk associated with a cumulative I-131 activity of ≥100 mCi and ≥400 mCi was translated into 4 (95% CI −4 to 13) and 42 (95% CI −8 to 93) excess breast cancer cases per 10,000 person-years, respectively.
Conclusions
An elevated risk was observed for the highest cumulative administered activity (>=400 mCi), and a significant dose-dependent association was observed among thyroid cancer survivors who were treated with I-131. However, overall, I-131 treatment might only explain partly the increase in breast cancer risk among female thyroid cancer survivors.
Subject terms: Risk factors, Epidemiology, Breast cancer
Introduction
Thyroid cancer is the most common endocrine cancer [1], with an increasing observed incidence over the last decades, particularly among women [2, 3]. Most cases are differentiated thyroid cancers (DTC), including papillary and follicular thyroid cancers, peaking at the age of 45–54 [2, 3]. Treatment for DTC consists of thyroidectomy with or without a single administration of I-131 to ablate remnants and repeated administrations of I-131 in case of neck recurrence or distant metastases. Most thyroid cancers have a good prognosis with a 10-year overall survival exceeding 90% [4]. With a long-life expectancy, a major concern is thus long-term adverse outcomes. Previous studies have shown a bidirectional relationship between thyroid and breast cancers [5], in which thyroid cancer survivors could have a 1.25-fold higher rate of developing breast cancer compared to the general population [6]. This increase cannot be explained by surveillance bias alone [5–7]. There is a need of identifying factors that contribute to the increased breast cancer risk among thyroid cancer survivors.
As the female breast tissue is one of the most radiosensitive organs [8], I-131 treatment could be a factor constituting the elevated breast cancer risk after a thyroid cancer diagnosis, along with genetic susceptibility, or shared hormonal and environmental factors [7, 9]. Although ecological data from the Chernobyl radiation accident previously suggested such an association [10, 11], results on the exposure to medical I-131 treatment have been conflicting. Some studies reported a higher breast cancer risk among I-131-treated thyroid cancer survivors [9, 12, 13] while others showed no association or even a lower risk compared to non-I-131-treated patients [14–18]. In addition, the limited number of cases and short follow-up time in some studies [12, 15, 16], the lack of a non-I-131-treated comparison group [19] and the unavailability of details on I-131 activities in some others [17, 19, 20] also hamper the interpretation of the association between I-131 treatment and breast cancer risk among thyroid cancer survivors [21].
In a pooled analysis of three large cohorts in Europe, we previously reported a higher breast cancer risk among thyroid cancer survivors compared to the general population, which was unlikely to be associated with I-131 treatment [22]. However, the risk related to I-131 treatment was estimated based on a limited number of cases. The present paper aimed to update results on breast cancer incidence in these cohorts with new patients included and a longer follow-up, and to evaluate the dose-response relationship with cumulative I-131 activities.
Methods
Population
We combined data from three European cohorts (The concerted action FI4P-CT98–0078) for patients with histologically confirmed papillary or follicular thyroid cancer diagnosis as the first primary cancer. These cohorts have been described in detail elsewhere [22–25]. Briefly, the Swedish cohort included all patients treated for thyroid cancer between 1950 and 1983 in six university hospitals. The Italian cohort consists of patients diagnosed with or treated for thyroid cancer from 1958 to 1996 at the General Hospital in Busto Arsizio, Italy. The French cohort included patients treated for thyroid cancer from 1934 to 2005 at four hospitals. Compared to the previous pooled analysis [22], the current study included 2202 and 92 new patients from the French and Italian cohorts, respectively, who were initially treated during the period 1995–2005. The population from the Swedish cohort remained as previously included. We extended the follow-up time up to 7, 11 and 20 years for the Swedish, Italian, and French cohorts, respectively.
We excluded patients with external radiotherapy prior to thyroid cancer diagnosis (n = 80), any malignancy in the 2 years after thyroid cancer diagnosis (n = 273), less than 2 years of follow-up (n = 543) or patients who were diagnosed with thyroid cancer at the age of ≥95 (n = 1). Finally, our study population included 8475 women (Fig. 1).
Fig. 1. Flowchart of the study.
Flowchart describing the exclusions in the three European cohorts (France, Italy and Sweden).
We retrieved information on thyroid cancer diagnosis, treatment modalities (surgery, external radiotherapy, and internal radiotherapy with I-131), all administration dates and administered activities from the medical records of each hospital. I-131 activities for diagnostic purposes were not systematically recorded in all centers and when available, the information on whether I-131 administration was for diagnostic or therapeutic purposes was not recorded. Therefore, we considered activities of ≥10 mCi (0.37 GBq) as therapeutic I-131 and lower activities as diagnostic I-131. We used data on therapeutic I-131 to conduct our main analyses and data on both diagnostic and therapeutic I-131 in a sensitivity analysis. We also reconstructed doses incidentally delivered by I-131 administration and external radiotherapy (Supplementary Method 1).
Invasive subsequent cancer cases and deaths after 2 years of thyroid cancer diagnosis were ascertained with medical records in the French and Italian cohorts, and with national cancer and death registries in the Swedish cohort. Follow-up time started on the date of thyroid cancer diagnosis and ended on the date of any second cancer diagnosis (except non-melanoma skin cancer), death, last visit to the treatment center, or the end of the study period (December 31, 2004, December 31, 2008, and December 31, 2014 for the Swedish, Italian, and French cohorts, respectively), whichever occurred first. We censored the follow-up at age 95 years because beyond that age, second cancer records are likely to be inaccurate (n = 31), and at the start date of external radiotherapy, if any, in the Italian cohort because of the unavailability of technical parameters needed for the dose calculation (n = 14).
Statistical analysis
We computed age-standardized incidence ratios (SIRs-, the ratio of observed to expected number of breast cancer cases) and 95% confidence intervals (CIs), assuming a Poisson distribution of the observed cases of breast cancer. Expected numbers of cases were calculated using sex-, age- and calendar-year-specific incident rates to the appropriate person-years at risk in each country. The reference rates for the French, Italian, and Swedish cohorts were from the estimations of cancer incidence in France (period 1980–2012) [26], the registry of Varese, Lombardy (data availability starting since 1978) [27], and the Swedish national cancer registry (data availability starting since 1970) [28], respectively. In the French cohort, we used the registry of Varese as the reference rates for 178 patients who came from Italy. To compute the expected number of breast cancer cases before the availability of the reference sources, we considered the rate from the nearest available period of time for each country. We also stratified SIRs according to age at thyroid cancer diagnosis (<30/30–40/40–50/≥50 years of age), year at thyroid cancer diagnosis (≤1960/1960–1980/>1980), and follow-up time (≤10/10–20/>20 years).
The use of I-131 treatment (yes/no) and cumulative activity (no I-131 treatment/<40/40–100/100–200/200–400/≥400 mCi) were analyzed as time-dependent variables. We assumed ten years as the shortest time needed for the development and detection of breast cancer after I-131 treatment or external radiotherapy (hereafter, minimal latency time) [29–31]. Accordingly, the relative risk (RR) of subsequent breast cancer at a given calendar period and attained age was modeled as a function of the expected number of breast cancer from the reference rates, and of the cumulative activity of I-131 treatment administered ten years or more before. We further adjusted for country, age at thyroid cancer diagnosis and cumulative dose of external radiotherapy, except where stated otherwise.
We assessed the dose–response association and a possible threshold dose for breast cancer risk. The absolute excess risk (AER) was calculated as the observed minus expected number of neoplasms, divided by the person-years at risk and multiplied by 10,000. We also estimated excess relative risks (ERRs) per 100 mCi, and evaluated possible departures from linearity for the shape of dose-response models for therapeutic I-131 cumulative activity by comparing models with linear terms, linear-quadratic terms, and linear-exponential terms. We evaluated possible linear threshold models which specify a linear relationship starting at a threshold activity (i.e., an activity below which there is no radiation effect) (Supplementary Method 2) [32]. Possible effect modifications by external radiotherapy, age/year at diagnosis and follow-up time were evaluated by testing the statistical significance of an interaction term between I-131 treatment and the studied covariate (likelihood-ratio χ² tests). A single administration of I-131 treatment is often considered for adjuvant therapy, and can be repeated in case of neck recurrence or distant metastases, and the current recommended activities in a single administration of I-131 is <200 mCi [33]. Therefore, we stratified the risk estimates according to the number of administered activity (1/>1) and the maximum activity in a single administration (200/≥200 mCi).
Several sensitivity analyses were conducted. We computed risk estimates incorporating both I-131 activities for diagnostic and therapeutic purposes. We censored women after 10 years of external radiotherapy. As data on thyroid cancer diagnosis and treatments could be erroneous in the early years, we excluded women diagnosed with thyroid cancer before 1960. We evaluated the association between breast cancer risk and I-131 estimated cumulative absorbed doses among women aged >15 years at thyroid cancer diagnosis (Supplementary Method 1). Because I-131-treated women could be different from women without I-131 treatment in terms of indications, and lost to follow-up, we conducted several analyses to further understand to which extent this could bias the risk estimates: First, we considered lost to follow-up as our primary outcome (instead of breast cancer diagnoses). Second, we applied inverse probability weighting (IPW) accounting for the probability of receiving I-131 treatment, external radiotherapy and of lost to follow-up (Supplementary Method 3).
Analyses were performed using SAS software and the EPICURE AMFIT software package [34]. 95% confidence intervals (CIs) were estimated with maximum likelihood methods. When lower bounds could not be estimated, results from Wald estimation were calculated.
Results
In the study population, 8475 (100%) women were treated with surgery, 5292 (62%) with I-131 treatment and 970 (11.4%) with external radiotherapy (Table 1 and Supplementary Table 1). I-131-treated patients received a median cumulative activity of 100 mCi (range 10–1597 mCi) (Table 1). Compared to women not treated with I-131, I-131-treated women were unlikely to have a longer follow-up or to receive external radiotherapy (Supplementary Tables 2 and 3).
Table 1.
Characteristics of the pooled cohort.
| France (N = 5469) | Italya (N = 1551) | Sweden (N = 1455) | Pooled cohort (N = 8475) | |
|---|---|---|---|---|
| Year of treatment, year, median (min–max) | 1993 (1934–2005) | 1988 (1958–1996) | 1965 (1950–1983) | 1989 (1934–2005) |
| Age at thyroid cancer diagnosis, year, mean (min–max) | 44 (2–90) | 44 (5–81) | 49 (5–90) | 44.5 (2–90) |
| Follow-up time, year, median (min–max) | 12 (2–66.5) | 11 (2–37) | 24 (2–55) | 12.7 (2.0–66.5) |
| Breast cancer cases, n (%) | 202 (3.7) | 38 (2.5) | 95 (6.5) | 335 (4.0) |
| Time to breast cancer, year, median (min–max) | 12 (2–55) | 12 (2–35) | 25 (2–46) | 14.1 (2.0–55.2) |
| Treatment of thyroid cancer by ionising radiation | ||||
| External radiotherapy, n (%) | 430 (7.9) | – | 540 (37) | 970 (11.4) |
| Time to the first administration since thyroid cancer diagnosis, year, median (IQR) | 0.2 (0.1–1.5) | – | 0 (0–0) | 0 (0–0.3) |
| I-131 therapy, n (%) | 3403 (62) | 1307 (84) | 582 (40) | 5292 (62.4) |
| Time since thyroid cancer diagnosis, median (IQR) | 0.2 (0.1–0.4) | 0.2 (0.1–0.3) | 0 (0–0) | 0.1 (0–0.2) |
| Number of therapeutic I-131 activity, median (min–max) | 1 (1–14) | 1 (1–15) | 1 (1–10) | 1 (1–15) |
| Cumulative activity of therapeutic I-131, mCi, median (min–max) | 100 (10–1597) | 100 (25–1491) | 75 (10–1330) | 100 (10–1597) |
| Both I-131 treatment and external radiotherapy | 326 (6.0) | – | 199 (13.7) | 525 (6.2) |
| Cumulative radiation dose delivered to the breasts | ||||
| I-131 therapy, mGy, median (min–max)b | 247 (25–3942) | 247 (61–3680) | 185 (25–3283) | 247 (25–3942) |
| External radiotherapy, mGy, median (min–max) | 1299 (10–43,480) | – | 272 (1–46,595) | 566 (1–46,595) |
| Imputed dosimetry for external radiotherapy, n (%) | 61 (14) | 0 | 406 (75) | 467 (48) |
aPatients with external radiotherapy were excluded at inclusion or censored at the start date of external radiotherapy.
bPatients aged >15 years at thyroid cancer diagnosis.
During a median follow-up of 12.7 years, 335 women developed breast cancer, i.e. 1.52 (95% CI 1.36–1.69) times more than the expected rates from the general population (Supplementary Table 3). This ratio did not substantially vary among the individual cohorts (P-Cochran’s test = 0.16), but decreased with age at thyroid cancer diagnosis, and increased with follow-up and calendar year at thyroid cancer diagnosis (P-trends < 0.001).
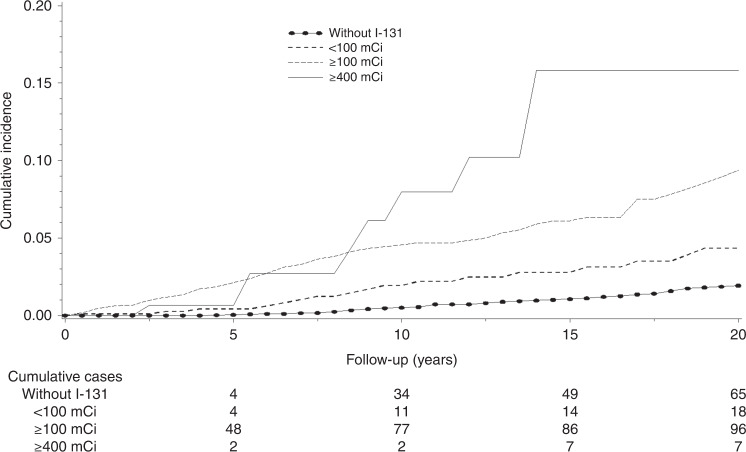
The 10-year cumulative breast cancer incidences were 4.1% (95% CI: 3.3–5.1%) and 0.5% (95% CI: 0.4–0.7%) among women treated with and without I-131 treatment, respectively (P-log-rank test <0.01) (Fig. 2). Overall, we found no significant association between I-131 treatment and subsequent breast cancer risk (RR = 1.07, 95% CI: 0.84–1.35, AER per 10,000 person-years = 0.8, 95% CI: −4.9–6.4). We found no evidence of departure from linearity in the shape of dose-response models for I-131 activities. There was a statistically significant increased risk with increasing I-131 activity (ERR per 100 mCi=17%, 95% CI: 2–38%, ERR per 100 mGy = 5%, 95% CI: 0–14%). The highest relative risk was observed among women who received a cumulative I-131 activity of ≥400 mCi (RR = 2.41, 95%CI: 1.13–3.52) (Table 2). We estimated that among women who received a cumulative I-131 activity of ≥100 mCi and of ≥400 mCi, the risk associated with the exposure to I-131 was translated into 4 (95% CI −4 to 13) and 42 (95% CI: −8 to 93) excess breast cancer cases per 10,000 person-years, respectively. Examining deviances to estimate the threshold dose, we found the minimum deviance of the linear threshold models at 80 mCi with the upper limit of 95% CI at 184 mCi (Supplementary Fig. 1).
Fig. 2.
Cumulative breast cancer incidence among thyroid cancer survivors by cumulative activities of I-131.
Table 2.
Breast cancer risk associated with therapeutic I-131 (considering a ten-year minimal latency time).
| Pooled cohort | ||||
|---|---|---|---|---|
| BC cases/person-years | RRa (95% CI) | AERb | ||
| Therapeutic I-131 activity | ||||
| No | 234/85,715 | 1 | ||
| Yes | 101/27,685 | 1.07 (0.84–1.35) | ||
| P-heterogeneity | >0.5 | |||
| Cumulative activity of therapeutic I-131 (mCi) | ||||
| No I-131 treatment | 234/85,715 | 1 | ||
| <40 | 4/2316 | 0.49 (0.15–1.15) | ||
| 40–100 | 16/6499 | 0.77 (0.44–1.25) | ||
| 100–200 | 53/14,029 | 1.10 (0.80–1.47) | ||
| 200–400 | 19/3731 | 1.55 (0.92–2.44) | ||
| ≥400 | 9/1112 | 2.41 (1.13–4.52) | 42 (−8–93)c | |
| P-heterogeneity | 0.039 | |||
| P-trend | 0.028 | |||
| ERR per 100 mCia | 0.17 (0.02–0.38) | |||
| ERR per 100 mCi among women who received I-131 treatmenta | 0.30 (0.08–0.64) | |||
| Cumulative radiation dose of I-131 therapy (mGy)d | ||||
| No I-131 treatment | 232/83,162 | 1 | ||
| <100 | 5/2644 | 0.54 (0.19–1.18) | ||
| 100–250 | 52/16,637 | 0.91 (0.67–1.22) | ||
| 250–500 | 20/4682 | 1.26 (0.76–1.95) | ||
| 500–1000 | 16/2,043 | 2.34 (1.33–3.81) | 37 (4–82) | |
| ≥1000 | 3/697 | 1.20 (0.29–3.18) | ||
| P-heterogeneity | 0.033 | |||
| P-trend | 0.094 | |||
| ERR per 100 mGya,d | 0.05 (0.00–0.14) | |||
| ERR per 100 mGy among women who received I-131 treatmenta,d | 0.10 (0.01–0.24) | |||
AER absolute excess risk per 10,000 person-years, BC breast cancer, CI confidence interval, ERR excess relative risk, RR relative risk.
aAdjusted for the country, age at diagnosis, and dose of external radiotherapy delivered to the breast in the background risks
bAER are shown only when the corresponding RRs were statistically significant at P < 0.05.
cThe lower bounds could not be estimated with maximum likelihood methods, AER calculated with Wald estimation was shown
dAnalysis conducted among women aged >15 years at thyroid cancer diagnosis.
The risk estimates remained consistent among women with a maximum activity <200 mCi in a single administration. Stratification by the number of I-131 administrations had little influence on the risk estimates, except an increased risk among women who received a single administration of 200–400 mCi (RR = 2.45, 95% CI 1.10–4.67), based on a few cases (Table 3). We found neither significant modifying effects of other factors (Supplementary Table 6), nor substantial difference between the main analyses and the sensitivity analyses. Analysis accounting for IPW even showed a stronger effect of I-131 treatment (Supplementary Table 7).
Table 3.
Breast cancer risk associated with therapeutic I-131 stratified by the number of administered activities and the maximum activity in a single administration to the breast.
| I-131 cumulative activity (mCi) | 10-year latency | |
|---|---|---|
| BC cases | RR (95% CI) | |
| No I-131 treatment | 234 | 1 |
| Number of administered activity | ||
| 1 | ||
| <40 | 4 | 0.51 (0.16–1.20) |
| 40–100 | 16 | 0.80 (0.46–1.30) |
| 100–200 | 49 | 1.09 (0.79–1.48) |
| 200–400 | 8 | 2.45 (1.10–4.67) |
| ≥400 | 0 | – |
| >1 | ||
| <40 | 0 | – |
| 40–100 | 0 | – |
| 100–200 | 4 | 1.10 (0.34–2.62) |
| 200–400 | 11 | 1.22 (0.62–2.15) |
| ≥400 | 9 | 2.57 (1.20–4.84) |
| The maximum activity in a single administration (mCi) | ||
| Maximum activity <200 mCi | ||
| <40 | 4 | 0.49 (0.15–1.15) |
| 40–100 | 16 | 0.77 (0.44–1.25) |
| 100–200 | 53 | 1.10 (0.80–1.47) |
| 200–400 | 8 | 1.10 (0.49–2.11) |
| ≥400 | 2 | 3.23 (0.53–10.37) |
| Maximum activity ≥200 mCi | ||
| 200–400 | 11 | 2.18 (1.11–3.83) |
| ≥400 | 7 | 2.24 (0.94–4.48) |
BC breast cancer, CI confidence interval, RR relative risk.
Discussion
We found that female thyroid cancer survivors had a 1.5-fold higher breast cancer risk compared to the general population. There was no significant association between breast cancer risk and exposure to I-131 treatment overall, suggesting that I-131 treatment could not fully explain the higher breast cancer risk among thyroid cancer survivors. However, accounting for a 10-year minimal latency time, we found a linear dose–response relationship between I-131 cumulative activities and breast cancer risk with a significant ERR per 100 mCi of 17% (ERR per 100 mGy of 5%). Among women with a cumulative I-131 activity of ≥100 mCi and ≥400 mCi (based on a limited number of cases), 4 and 42 excess breast cancer cases, respectively, could occur for every 10,000 person-years. When considering a linear threshold model, estimate of a threshold activity was 80 mCi with an upper 95% confidence bound of <200 mCi.
Exposure to ionizing radiation has been demonstrated to increase the lifetime risk of female breast cancer [29]. However, the ionizing radiation-related estimated risks varied considerably across medically, occupationally, and environmentally exposed populations. A recent study on breast cancer mortality associated with I-131 treatment for hyperthyroidism with individualized dosimetry estimated an increased risk of 12% per 100 mGy of absorbed dose to the breast [35]. Our increased risk of 5% per 100 mGy of I-131 absorbed dose to the breasts was 2–4-fold higher than the risk associated with external radiation therapy among cancer survivors (ERR/100 mGy varied from 0.01 to 0.03) [36], but of the same magnitude as the risk estimates reported from the US Radiologic Technologists Study (ERR/100 mGy = 0.07) [37], the Life Span Study of Atomic Bomb Survivors (ERR/100 mGy varied from 0.09 to 0.11) [38, 39], and the Techa River Incidence Cohort (ERR/100 mGy: 0.19) [40] (Supplementary Method 4). The discrepancy could possibly be due to differences in dose rate, dose ranges, age at exposure, information quality, background risks and statistical variability or uncertainties. Hypotheses and approximations used for the dose reconstruction might also contribute to the differences. For I-131 dosimetry, we could not go further than a standard phantom and reference dose coefficients, i.e., S-values, because we did not have detailed individualized data on the anatomy of each subject. Our dosimetry, therefore, could not account for anatomy-specific variations in dose distributions or breast sub-volume dose heterogeneity, which might also play a role.
Our findings support that increasing cumulative activity of I-131 was associated with an increased breast cancer risk among female thyroid cancer survivors. However, a few previous studies found no higher risks related to I-131 cumulative activities up to >4.4 GBq or 150 mCi, after adjusting for confounders [12, 15, 16]. Given the long latency time of radiation-induced breast cancers (a minimal latency time of 10–15 years [29, 31] and of 5–10 years [35, 41] after external and internal exposures to radiation, respectively), breast cancer risk associated with I-131 treatment was probably underestimated in studies with short follow-up times [12, 15, 16]. Of note, in the previous pooled study, we found a linear relationship between I-131 treatment and all solid cancer risk, and the risk increased particularly among patients who received a cumulative activity of 400 mCi [22].
In our study, the lack of an effect modification by age at thyroid cancer diagnosis (Supplementary Table 6) could be explained by a large proportion of patients diagnosed in adulthood (80% of patients were aged ≥30 years at thyroid cancer diagnosis). Previous studies have shown that individuals exposed to ionizing radiation are the most radiosensitive at early ages, mostly before the age of 30 [13, 29, 39], and the highest risks are found around menarche [39]. In this study, the limited number of women aged before 30 years at thyroid cancer diagnosis limited our ability to detect an effect modification in this setting. Due to the limited number of 7 breast cancer cases among women exposed to ≥1 Gy of external radiotherapy after 10 years of latency, we were not able to obtain reliable risk estimates related to the use of external radiotherapy and investigate the interaction between internal–external radiation.
Although the increased breast cancer risk among women with a high cumulative activity of I-131 is biologically plausible and in line with epidemiological studies on (external) radiation exposure, our results could also be due to, at least partly, confounding by indications, selection bias due to lost to follow-up, and surveillance bias. Thyroid cancer survivors who received a high cumulative activity of I-131 could have worse prognostic factors and a higher probability of cancer recurrence [33, 42], which require further management, possibly leading to a more intensive follow-up and a more intensive screening strategy than women without the treatment or with lower cumulative activities. However, to date, no specific breast cancer screening program has been recommended for thyroid cancer survivors. Analyses considering a long latency time of 10 years after the exposure of I-131 also minimized the potential surveillance bias. Restricting analyses to the Swedish population which has a complete, passive (non-selected) follow-up for all individuals through the national registries yielded similar risk estimates (ERR = 24%, 95% CI −10 to 85%). Surprisingly, the results from sensitivity analyses which considered lost to follow-up as an outcome or used IPW (to neutralize the differences caused by a possible indication and/or selection bias) suggested that the risk could have been underestimated among women with the highest cumulative activities of I-131.
The current study has major strengths, including large population size, with a confirmed thyroid cancer diagnosis. The pooled cohort also includes detailed information on administration dates, and activities for I-131 treatment and external radiotherapy. We were able to use both administered activities and the estimate of absorbed doses of I-131 treatment, which enabled us to yield risk estimates more precisely and compare results with previous studies. We also had an internal comparison group of thyroid cancer patients who did not receive I-131 treatment, which minimize indication bias.
We acknowledge several limitations. Since the possible effects of I-131 are considered to be modest and can be subject to long latency times, the follow-up could not be long enough to account for all effects. The number of cases among patients treated at low (e.g., <100 mCi) and high (>400 mCi) dose of I-131 treatment were limited, and risk estimations among these populations should be interpreted with caution. Details on cancer stage, grade, and breast cancer form (unilateral or bilateral) were unavailable. Lack of information on exposure to radiological examinations (e.g., chest X-rays, bone X-rays, CT-scans), environmental and occupational radiation, genetic characteristics, history of benign breast disease, and relevant confounders (e.g., obesity, hormonal factors) requires caution when interpreting the results. We were not able to estimate reliably absorbed doses from I-131 administrations for women aged ≤15 years at thyroid cancer diagnosis, and the risk estimates related to I-131 absorbed doses might not be generalized to this population. Finally, we could not obtain information on diagnostic I-131 administrations or estimate doses to the external radiotherapy for the whole population.
In conclusion, we found a slightly higher breast cancer risk among women treated for thyroid cancer compared to the general population, which could be partly attributable to I-131 treatment, especially among women who received a high cumulative activity of I-131. Based on a limited number of cases, the estimated attributable risk related to exposure to a cumulative activity of I-131 of ≥100 mCi and ≥400 mCi could translate into 4 and 42 excess breast cancer cases per 10,000 person-years, respectively.
Supplementary Information
Acknowledgements
We would like to thank Dr. Carine Corone for her contribution on the setting up of the study at the Rene Huguenin Center.
Author contributions
Study conception and design: FDV, CR, TTVT, MS, MD, PH, SL and ID. Statistical analysis: TTVT, CR, FDV and NJ. writing of the original draft: TTVT. Interpretation of the results: FDV, TTVT, CR, NJ and MS. All authors revised the paper and approved the final version.
Funding
Thi-Van-Trinh TRAN received a doctoral grant from the Paris Sud-Paris Saclay University. Michel Henry-Amar is supported by the French National League Against Cancer (LNC). This study was supported by grants from the European Commission (Concerted action no. FI4P-CT98-0078, DG 12-WSMN), and European Community’s Seventh Framework Program (EURATOM) contract Fission-269553 (EpiRadBio), and by the Epidemiology Commission of Electricity of France (EDF). Role of funders: The funders do not have any role in the conception and the analysis of the study nor in the writing or the article.
Data availability
The data underlying this paper will be shared upon reasonable request to the corresponding author.
Code availability
The code used to generate results that are reported in the paper will be shared on reasonable request to the corresponding author.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The French cohort’s protocol has been approved by the French National Agency regulating Data Protection (CNIL), and consent was obtained from the study participants. The Swedish cohort obtained approval from the Swedish data inspection board. Because the sole aim of the Italian cohort is to evaluate the safety of radio-iodine treatment administered by medical doctors of this hospital, without any other specific contact with patients, no special authorization has been needed for this population. The pooled cohort study contains only pseudonymized individual data.
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thi-Van-Trinh Tran, Email: Thivantrinh.tran@gustaveroussy.fr.
Carole Rubino, Email: Carole.rubino@gustaveroussy.fr.
Florent de Vathaire, Email: Florent.DEVATHAIRE@gustaveroussy.fr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-01982-5.
References
- 1.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: cancer today. Lyon, France: International Agency for Research on Cancer, https://gco.iarc.fr/today. Accessed 5 January 2020.
- 2.Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12:646–53. doi: 10.1038/nrendo.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M, Dal Maso L, Vaccarella S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. 2020;8:468–70. doi: 10.1016/S2213-8587(20)30115-7. [DOI] [PubMed] [Google Scholar]
- 4.Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973-1991. Cancer. 1997;79:564–73. doi: 10.1002/(SICI)1097-0142(19970201)79:3<564::AID-CNCR20>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Reiners C, Schneider R, Platonova T, Fridman M, Malzahn U, Mäder U, et al. Breast cancer after treatment of differentiated thyroid cancer with radioiodine in young females: what we know and how to investigate open questions. review of the literature and results of a multi-registry survey. Front Endocrinol. 2020;11:381. doi: 10.3389/fendo.2020.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subramanian S, Goldstein DP, Parlea L, Thabane L, Ezzat S, Ibrahim-Zada I, et al. Second primary malignancy risk in thyroid cancer survivors: a systematic review and meta-analysis. Thyroid. 2007;17:1277–88. doi: 10.1089/thy.2007.0171. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen SM, White MG, Hong S, Aschebrook-Kilfoy B, Kaplan EL, Angelos P, et al. The breast-thyroid cancer link: a systematic review and meta-analysis. Cancer Epidemiol, Biomark Prev. 2016;25:231–8. doi: 10.1158/1055-9965.EPI-15-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Council NR. Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2. Washington, DC: The National Academies Press; 2006. [PubMed]
- 9.Clement SC, Peeters RP, Ronckers CM, Links TP, van den Heuvel-Eibrink MM, Nieveen van Dijkum EJ, et al. Intermediate and long-term adverse effects of radioiodine therapy for differentiated thyroid carcinoma-a systematic review. Cancer Treat Rev. 2015;41:925–34. doi: 10.1016/j.ctrv.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Pukkala E, Kesminiene A, Poliakov S, Ryzhov A, Drozdovitch V, Kovgan L, et al. Breast cancer in Belarus and Ukraine after the Chernobyl accident. Int J Cancer. 2006;119:651–8. doi: 10.1002/ijc.21885. [DOI] [PubMed] [Google Scholar]
- 11.Rivkind N, Stepanenko V, Belukha I, Guenthoer J, Kopecky KJ, Kulikov S, et al. Female breast cancer risk in Bryansk Oblast, Russia, following prolonged low dose rate exposure to radiation from the Chernobyl power station accident. Int J Epidemiol. 2020;49:448–56. doi: 10.1093/ije/dyz214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin CY, Lin CL, Huang WS, Kao CH. Risk of breast cancer in patients with thyroid cancer receiving or not receiving 131i treatment: a nationwide population-based cohort study. J Nucl Med. 2016;57:685–90. doi: 10.2967/jnumed.115.164830. [DOI] [PubMed] [Google Scholar]
- 13.Pasqual E, Schonfeld S, Morton LM, Villoing D, Lee C, Berrington de Gonzalez A, et al. Association between radioactive iodine treatment for pediatric and young adulthood differentiated thyroid cancer and risk of second primary malignancies. J Clin Oncol. 2022;40:1439–49. doi: 10.1200/JCO.21.01841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berthe E, Henry-Amar M, Michels J-J, Rame J-P, Berthet P, Babin E, et al. Risk of second primary cancer following differentiated thyroid cancer. Eur J Nucl Med Mol imaging. 2004;31:685–91. doi: 10.1007/s00259-003-1448-y. [DOI] [PubMed] [Google Scholar]
- 15.Ahn HY, Min HS, Yeo Y, Ma SH, Hwang Y, An JH, et al. Radioactive iodine therapy did not significantly increase the incidence and recurrence of subsequent breast cancer. J Clin Endocrinol Metab. 2015;100:3486–93. doi: 10.1210/JC.2014-2896. [DOI] [PubMed] [Google Scholar]
- 16.Teng CJ, Hu YW, Chen SC, Yeh CM, Chiang HL, Chen TJ, et al. Use of radioactive iodine for thyroid cancer and risk of second primary malignancy: a nationwide population-based study. J Natl Cancer Inst. 2016;108:djv314. [DOI] [PubMed]
- 17.Brown AP, Chen J, Hitchcock YJ, Szabo A, Shrieve DC, Tward JD. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab. 2008;93:504–15. doi: 10.1210/jc.2007-1154. [DOI] [PubMed] [Google Scholar]
- 18.Kim C, Bi X, Pan D, Chen Y, Carling T, Ma S, et al. The risk of second cancers after diagnosis of primary thyroid cancer is elevated in thyroid microcarcinomas. Thyroid. 2013;23:575–82. doi: 10.1089/thy.2011.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verkooijen RB, Smit JW, Romijn JA, Stokkel MP. The incidence of second primary tumors in thyroid cancer patients is increased, but not related to treatment of thyroid cancer. Eur J Endocrinol. 2006;155:801–6. doi: 10.1530/eje.1.02300. [DOI] [PubMed] [Google Scholar]
- 20.Iyer NG, Morris LG, Tuttle RM, Shaha AR, Ganly I. Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer. 2011;117:4439–46. doi: 10.1002/cncr.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu CY, Saeed O, Goldberg AS, Farooq S, Fazelzad R, Goldstein DP, et al. A systematic review and meta-analysis of subsequent malignant neoplasm risk after radioactive iodine treatment of thyroid cancer. Thyroid. 2018;28:1662–73. doi: 10.1089/thy.2018.0244. [DOI] [PubMed] [Google Scholar]
- 22.Rubino C, de Vathaire F, Dottorini ME, Hall P, Schvartz C, Couette JE, et al. Second primary malignancies in thyroid cancer patients. Br J Cancer. 2003;89:1638–44. doi: 10.1038/sj.bjc.6601319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall P, Holm LE, Lundell G, Bjelkengren G, Larsson LG, Lindberg S, et al. Cancer risks in thyroid cancer patients. Br J Cancer. 1991;64:159–63. doi: 10.1038/bjc.1991.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dottorini ME, Lomuscio G, Mazzucchelli L, Vignati A, Colombo L. Assessment of female fertility and carcinogenesis after iodine-131 therapy for differentiated thyroid carcinoma. J Nucl Med. 1995;36:21–7. [PubMed] [Google Scholar]
- 25.de Vathaire F, Schlumberger M, Delisle MJ, Francese C, Challeton C, de la Genardiére E, et al. Leukaemias and cancers following iodine-131 administration for thyroid cancer. Br J Cancer. 1997;75:734–9. doi: 10.1038/bjc.1997.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binder-Foucard F, Bossard N, Delafosse P, Belot A, Woronoff AS, Remontet L. Cancer incidence and mortality in France over the 1980–2012 period: solid tumors. Rev d'Épidémiologie et de Sté Publique. 2014;62:95–108. doi: 10.1016/j.respe.2013.11.073. [DOI] [PubMed] [Google Scholar]
- 27.Bray F, Ferlay J, Laversanne M, Brewster DH, Gombe Mbalawa C, Kohler B, et al. Cancer incidence in five continents: inclusion criteria, highlights from volume X and the global status of cancer registration. Int J Cancer. 2015;137:2060–71. doi: 10.1002/ijc.29670. [DOI] [PubMed] [Google Scholar]
- 28.National Board of Health and Welfare. Cancer incidence in Sweden, https://sdb.socialstyrelsen.se/if_can/val_eng.aspx Accessed 21 October.
- 29.Preston DL, Mattsson A, Holmberg E, Shore R, Hildreth NG, Boice JD., Jr Radiation effects on breast cancer risk: a pooled analysis of eight cohorts. Radiat Res. 2002;158:220–35. doi: 10.1667/0033-7587(2002)158[0220:REOBCR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Ronckers CM, Erdmann CA, Land CE. Radiation and breast cancer: a review of current evidence. Breast Cancer Res: BCR. 2005;7:21–32. doi: 10.1186/bcr970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Land CE, Tokunaga M, Koyama K, Soda M, Preston DL, Nishimori I, et al. Incidence of female breast cancer among atomic bomb survivors, Hiroshima and Nagasaki, 1950-1990. Radiat Res. 2003;160:707–17. doi: 10.1667/RR3082. [DOI] [PubMed] [Google Scholar]
- 32.Ulm K. A statistical method for assessing a threshold in epidemiological studies. Stat Med. 1991;10:341–9. doi: 10.1002/sim.4780100306. [DOI] [PubMed] [Google Scholar]
- 33.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preston D, Lubin J, Pierce D, McConney M. Epicure users guide. Seattle, WA: Hirosoft International Corporation, 1993;186..
- 35.Kitahara CM, Berrington de Gonzalez A, Bouville A, Brill AB, Doody MM, Melo DR, et al. Association of radioactive iodine treatment with cancer mortality in patients with hyperthyroidism. JAMA Intern Med. 2019;179:1034–42. doi: 10.1001/jamainternmed.2019.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berrington de Gonzalez A, Gilbert E, Curtis R, Inskip P, Kleinerman R, Morton L, et al. Second solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose-response relationship. Int J Radiat Oncol Biol Phys. 2013;86:224–33. doi: 10.1016/j.ijrobp.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preston DL, Kitahara CM, Freedman DM, Sigurdson AJ, Simon SL, Little MP, et al. Breast cancer risk and protracted low-to-moderate dose occupational radiation exposure in the US Radiologic Technologists Cohort, 1983-2008. Br J Cancer. 2016;115:1105–12. doi: 10.1038/bjc.2016.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, et al. Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 39.Brenner AV, Preston DL, Sakata R, Sugiyama H, de Gonzalez AB, French B, et al. Incidence of breast cancer in the life span study of atomic bomb survivors: 1958-2009. Radiat Res. 2018;190:433–44. doi: 10.1667/RR15015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis FG, Yu KL, Preston D, Epifanova S, Degteva M, Akleyev AV. Solid cancer incidence in the Techa river incidence cohort: 1956-2007. Radiat Res. 2015;184:56–65. doi: 10.1667/RR14023.1. [DOI] [PubMed] [Google Scholar]
- 41.Metso S, Auvinen A, Huhtala H, Salmi J, Oksala H, Jaatinen P. Increased cancer incidence after radioiodine treatment for hyperthyroidism. Cancer. 2007;109:1972–9. doi: 10.1002/cncr.22635. [DOI] [PubMed] [Google Scholar]
- 42.Luster M, Clarke SE, Dietlein M, Lassmann M, Lind P, Oyen WJ, et al. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol imaging. 2008;35:1941–59. doi: 10.1007/s00259-008-0883-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this paper will be shared upon reasonable request to the corresponding author.
The code used to generate results that are reported in the paper will be shared on reasonable request to the corresponding author.