Abstract
Background: Social network strategies (SNS) assumes that people in the same social share similar HIV risk. Methods: This study evaluated SNS to promote HIV testing of young men who have sex with men (YMSM) and transgender women (YTGW) aged 15–24 years. “Recruiters” referred their ‘network members’ (NMs) to clinic. NMs were provided HIV testing. Proportions of first-time HIV testers and number of NMs were analyzed. Results: Between April 2021 to March 2022, 83 recruiters referred 202 NMs. Median age of NMs was 19 years (IQR 17-20), 62% were YMSM. One-hundred-and-twenty-four NMs (61%) were first-time HIV testers. YTGW recruited more NMs per recruiter (5.4 vs 1.4, p = 0.002). HIV prevalence was 3.0% (95% CI 1.1-6.4). Thirty-one-point-three percent of NMs at HIV risk initiated oral HIV preexposure prophylaxis. Conclusions: SNS is a good strategy to reach adolescents at risk of HIV infection. More than half of NMs were first-time HIV testers.
Keywords: HIV testing, adolescents, men who have sex with men (MSM), HIV prevention, HIV treatment
Introduction
The Joint United Nations Program on HIV/AIDS (UNAIDS) has set goals to end AIDS by 2030 using 95–95-95 for treatment: 95% of people living with HIV knowing their HIV status; 95% of people who know their status on treatment; and 95% of people on treatment with suppressed viral loads.1 The HIV epidemic in the Asia-Pacific region is concentrated in young men who have sex with men (YMSM) and young transgender women (YTGW).2,3 In 2020, 53% of new HIV infections were in men who have sex with men (MSM), and of these, half occurred in young people aged 15 to 24 years.2,3 HIV testing coverage, defined as receipt of a test in the last 12 months, is less than 50% among MSM and TGW compared to targets of over 90% by UNAIDS.2 One of the key focuses of Thailand's 2017–2030 National AIDS Strategy is increasing HIV testing coverage for key populations including that in MSM, and transgender women (TGW).2,4,5
The World Health Organization (WHO) has recommended social network-based HIV testing to approach key populations as part of the HIV prevention package of care since 2019.6 Social network strategies (SNS) are effective in increasing new HIV diagnoses, have high acceptability for HIV partner services and are feasible for implementation across multiple income settings.6 The premise of SNS utilizes peers to reach and recruit their network members into HIV services. An advantage to this is that recruiters with different behavioral risks and HIV statuses are likely to have access to a wide variety of HIV at-risk populations.7 SNS has been used across a range of populations including MSM, transgender people, and use has been associated with a reduction of HIV incidence, increased testing uptake, improved participant retention and reduction of HIV sexual risk behaviors8,9
Young key populations are vulnerable to HIV infection yet have limited ability to navigate complicated segmented medical services. Barriers include stigma regarding their sexual debut and gender identity-related stigma.10,11 Differentiated care for adolescents with a rapid, friendly, and integrated one-stop model will facilitate adolescents in accessing services.8,12 Although SNS has not been widely studied in adolescents and young adults, several peer-led HIV educational initiatives have been implemented as reported from Canadian young adult showed a reduction of disparities in knowledge on HIV prevention and treatment in those who had attended peer-led education sessions.9 A study conducted adolescents living with HIV in Zimbabwe found increased medication adherence and improved self-esteem in adolescent-centered services.13
The benefits of trained peer leaders in the context of SNS use of HIV service engagement could have multiple applications, including demand creation, extending care to underserved at-risk young populations, support of adolescents to HIV testing and services.6 HIV testing services are a crucial first step in facilitating entry to the double-cascade of HIV prevention and treatment services.14,15 SNS is a potentially good fit intervention to implement in HIV prevention operations for this age group.16 This study aimed to evaluate the effectiveness of using SNS to increase HIV testing uptake in YMSM and YTGW and subsequently link them to an adolescent-focused integrated HIV care service cascade.
Methods
Study Location and Participants
This study was conducted at “Buddy CU Clinic”, a comprehensive adolescent-focused HIV clinic. The clinic is a one-stop service providing the full cascade of HIV and sexual transmitted infection (STI) diagnosis, treatment, and prevention. Clients aged 15–24 years were linked to our clinic for HIV testing by passive recruitment methods including online advertisements and provider-initiated HIV testing for clients presenting with symptomatic sexually transmitted diseases. Social network strategies were used to recruit potential study participants. Eligibility criteria for recruiters included those 1) aged 15–24 years 2) assigned male gender at birth and self-defined gender identity of MSM or TGW 3) Living with HIV, or at risk of HIV, using Pre-exposure prophylaxis (PrEP), or at-risk of HIV coming for regular HIV testing 4) good rapport with peers, knowledgeable on HIV, comfortable discussing HIV testing with peers, and willing to become a recruiter. Eligibility criteria for network members (NMs) included those 1) aged 15–24 years 2) assigned male gender at birth and self-defined gender identity of MSM or TGW 3) self-reported oral and/or anal sex with a man in the past 12 months 4) at risk of HIV infection, defined as having more than one sex partner and inconsistent condom (defined as less than 100% condom use) in the preceding 6 months 5) Self-reported history of no HIV testing within 3 months prior to study entry.
The study protocol allowed participants aged 15 to less than 18 years of age to give consent to participate in the study without requiring parental consent for HIV testing. The Thai Medical Council permits youth aged 13 years and above to access to HIV testing and reproductive health services without requiring parental consent, as these services directly benefit adolescents.
The protocol of this study was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University for ethics approval. (IRB No.904/63). All patients provided written informed consent prior to enrollment in the study.
Social Network Strategy Procedures
The study design consisted of 4 implementation phases of SNS
Phase 1: Recruiter Enlistment and Engagement
Recruiter enlistment: Our staff invited current clients and NMs who personally knew and were comfortable reaching out to HIV at-risk peers from their social or sexual networks. There were 2 rounds of recruiter enrollments. The first group included 46 participants from existing clients in the clinic; 7 youth living with HIV, 28 youth taking PrEP, and 11 youth receiving regular HIV testing. The second round included subsequent NMs who showed interest in and received training to become recruiters.
Recruiter engagement: All the recruiters attended 30–60 min of training sessions provided by our staff on HIV knowledge, usage of HIV antibody self-tests, usage of HIV prevention packages including PrEP and condoms, and coaching on SNS implementation-related skills, such as approaching NMs, through role play and discussion.
Phase 2: Recruitment of Network Members
Recruiters contacted, engaged with and invited peers with potential to become their NMs who were at risk of HIV acquisition to our adolescent clinic for HIV testing. Methods used to reach NMs and conversations between recruiter and their NMs varied by their relationships but all discussed the importance of HIV testing. NMs who agreed to testing were given a recruiter's code and the telephone number of their recruiter to visit our adolescent clinic for HIV testing. Recruiters received 3 USD compensation for successfully recruiting an eligible NMs who agreed to get HIV testing.
Phase 3: HIV Testing
NMs received HIV pretest counselling and were offered a choice of HIV self-testing, either oral fluid-based (OraQuickTM) or blood-based (INSTITM) testing. They performed HIV self-testing with support from clinic counsellors. During Thailand's peak period of COVID transmission between August to November 2021, NMs seeking HIV testing contacted our adolescent clinic to make appointments and HIV self-tests were sent to them via post. They received pre-test HIV counselling, supervision on performing their HIV self-tests and also post-test counselling. Confirmatory HIV antibody testing was performed on venous blood sampling using three standard assays, the Elecsys® (Roche Diagnostics Ltd, Rotkreuz, Switzerland) (p24Ag and anti-HIV test), immunochromatography, and gel particle agglutination. Syphilis testing was performed using the electrochemiluminescence immunoassay analyzer (ECLIA) and chemiluminescent magnetic microparticle immunoassay (CMIA). The non-treponemal assays used was the rapid plasma regain (RPR) test, with titers above 1:1 considered reactive, defining syphilis infection. Syphilis infection diagnoses were divided into stages of infection, primary, secondary, tertiary and latent infection. HIV and syphilis testing was reimbursable under Thailand's National HIV/AIDS program and therefore free of charge to participants.17 Ten USD was given to NMs as compensation for travel to the clinic.
Phase 4: Linkage to Care
Participants who had positive HIV antibody tests were evaluated for clinical signs and/or symptoms of opportunistic infections and offered same day antiretroviral treatment (ART), DTG-based regimens in accordance with Thai National Guidelines 2021.17 Syphilis was treated with 1–3 doses of benzathine penicillin intramuscularly in accordance with their disease stage per CDC Treatment guidelines.18 HIV prevention services include risk reduction counselling, condoms, initiation of oral PrEP in eligible participants according to the Thai National guidelines 2021. Those with multiple sexual partners and inconsistent condom use confirmed to be HIV negative with ongoing infection risk were initiated on PrEP.17 NMs expressing interest and knew their high-risk peers were enlisted as potential recruiters and invited to recruiter training. The cycle of engagement and linkage of adolescents at HIV risk was then repeated.
Data Collection and Statistical Analysis
Data relating to recruiters and NM participants including demographic data eg, age, gender, income, education, sexual history (eg, number of sexual partners, condom usage), HIV testing history and current HIV status was collected by physicians through face-to-face interviews.
Categorical variables were presented with absolute numbers and percentages, and continuous variables with medians and interquartile range (IQR) or means and 95% confidence interval (CI). Chi-square test, Z-test for proportions and t-tests for means were performed with an alpha-value of 0.05 to determine statistically significant differences, as appropriate. Effectiveness of SNS was measured by the proportion of first-time testers among participants enrolled, defined as the number of first-time NM testers divided by total number of NMs enrolled. SNS is considered effective when the proportions exceed 50%. Stata/SE 13.0 was used for data analyses.
Network mapping was performed using three steps, firstly preparing data using the Structured Query Language (MySQL), a language program use to create, modify and extract data from a relational database. Secondly, data was analyzed using a general-purpose scripting language toward web development Personal Home Page version 8 (PHP8) and finally, used HIGHCHARTS that utilized a Javascript library to create network mapping. The recruiter's telephone number and a 4-digit pin code was used by each NM to register to the clinic. Visual analysis of network mapping was analyzed to inform the gender of recruiter and their NMs, degree and type of clustering of HIV status of NMs including HIV positive, HIV negative at risk and HIV negative.
Results
From April 2021 to March 2022, a total of 83 recruiters were enrolled (46 in round one, and 37 in round 2) (Table 1). Median (IQR) age was 20 (18-21) years, 72.3% were YMSM, eight (10%) were youth living with HIV. All had behaviorally acquired HIV infection, two (25%) had disclosed their status in the community, none of them belonged to peer support groups. Sixty-five point one percent were youth taking PrEP and 25.3% were youth attending for regular HIV testing. A total of 205 NMs were recruited, all except three were eligible (98.5%). Reasons for ineligibility included never having had oral or anal sex (n = 2) and HIV testing within 3 months (n = 1). Finally, 202 NMs were recruited, 89% of the NMs performed HIV self-tests in-clinic and 11% online, all with staff supervision.
Table 1.
Characteristics of Recruiters for Social Network Strategy HIV Testing.
| Recruiters | Total (n = 83) | First round recruiters (n = 46) | NM to recruiter (n = 37) | p-value |
|---|---|---|---|---|
| Age (years), median (IQR) | 20 (18-21) | 20 (18-22) | 19 (18-21) | − |
| Age, n (%) | ||||
| 15–19 years | 41(49.4%) | 20 (43.5%) | 21 (57.8%) | 0.23a |
| 20–24 years | 42 (50.6%) | 26 (56.5%) | 16 (43.2%) | |
| Gender identity, n (%) | ||||
| MSM | 60 (72.3%) | 38 (82.6%) | 22 (59.5%) | 0.02a |
| TGW | 23 (27.7%) | 8 (17.4%) | 15 (40.5%) | |
| Education, n(%) | ||||
| Under university degree | 48 (57.8%) | 26 (56.5%) | 22 (59.4%) | 0.79a |
| University degree or higher | 35 (42.1%) | 20 (43.4%) | 15 (40.5%) | |
| Employment status, n (%) | ||||
| No | 38 (45.7%) | 23 (50.0%) | 15 (40.5%) | 0.39a |
| Yes | 45 (54.2%) | 23 (50.0%) | 22 (59.4%) | |
| HIV status, n (%) | ||||
| HIV positive | 8 (9.6%) | 7 (15.2%) | 1 (2.7%) | 0.05b |
| HIV negative taking PrEP | 59 (71.1%) | 28 (60.9%) | 31 (86.1%) | 0.02b |
| HIV negative received regular HIV test | 16 (19.3%) | 11 (29.9%) | 5 (13.5%) | 0.23b |
MSM = Men who have sex with men, TGW = Transgender women, PrEP = Pre-exposure prophylaxis.
Chi-squre test, b z-test for proportion.
Recruitment Activities and Social Mapping
Fifty-six recruiters (67%) recruited at least 1 NM, with a mean of 5 weeks (range 0-37) following training. These proportions were similar in those who started out as recruiters (67.4%) versus those who started as NMs and switched to being recruiters (67.6%) (p = 0.9). The averages number of NMs per recruiters stratified by recruiter's characteristics are shown in Table 2. The overall recruitment mean was 2.4 (95%CI 1.3-3.6) NMs per recruiter. The younger age group (15-19 years) recruited more NMs per recruiter than the young adult group (20-24 years) (2.8 vs 2.0, p = 0.05). The recruitment mean among TGW was significantly higher than MSM (5.4 vs 1.4, p = 0.002). Recruiters living with HIV tended to have lower recruitment means compared to HIV negative recruiters (0.6 vs 2.6, p = 0.31). Additionally, the number of NMs recruited per recruiter was not statistically significant different between recruiters with differing education levels when comparing those at university level versus those at lower level of education (2.1 vs 2.6, p = 0.70). The recruited mean tended to be higher among recruiters with self-earned income, which could be a proxy of being more able to accomplish assigned tasks, but was not statistically significant (3.0 vs 1.7, p = 0.22).
Table 2.
Characteristics of Network Members Stratified by Recruiters’ Characteristics.
| Characteristic of recruiters | Recruiter | Network members (n = 202) | First time tester network members (n = 124) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Per recruiter mean (95% CI) | P-valuea | No. | Per recruiter Mean (95% CI) | P valuea | ||||
| Recruiter group, n (%) | |||||||||
| Recruiter | 46 | 108 | 2.4 (0.5, 4.2) | 0.87 | 54 | 1.2 (0.1, 2.2) | 0.35 | ||
| NM to recruiter | 37 | 94 | 2.5 (1.2, 3.9) | 70 | 1.9 (0.7, 3.0) | ||||
| Age, n (%) | |||||||||
| 15–19 years | 41 | 116 | 2.8 (0.8, 4.9) | 0.05 | 76 | 1.8 (0.6, 3.2) | 0.35 | ||
| 20–24 years | 42 | 86 | 2.0 (0.9, 3.2) | 48 | 1.1 (0.3, 2.0) | ||||
| Gender, n (%) | |||||||||
| MSM | 60 | 81 | 1.4 (0.9, 1.8) | 0.002 | 46 | 0.8 (0.4, 1.1) | 0.002 | ||
| TGW | 23 | 121 | 5.3 (1.3, 9.2) | 78 | 3.4 (0.8, 6.0) | ||||
| Recruiter's HIV status, n (%) | |||||||||
| Positive | 8 | 5 | 0.6 (0, 1.2) | 0.31 | 3 | 0.4 (0, 1.0) | 0.34 | ||
| Negative | 75 | 197 | 2.6 (1.4, 3.9) | 121 | 1.6 (0.8, 2.4) | ||||
| At high risk for HIV infection among HIV negative (n = 75) | |||||||||
| No | 16 | 18 | 1.1 (0, 2.6) | 0.22 | 11 | 0.7 (0, 1.8) | 0.25 | ||
| Yes | 59 | 179 | 3.0 (1.5, 4.6) | 110 | 1.9 (0.8, 2.9) | ||||
p-value for t-test for means.
MSM = Men who have sex with men, TGW = Transgender women.
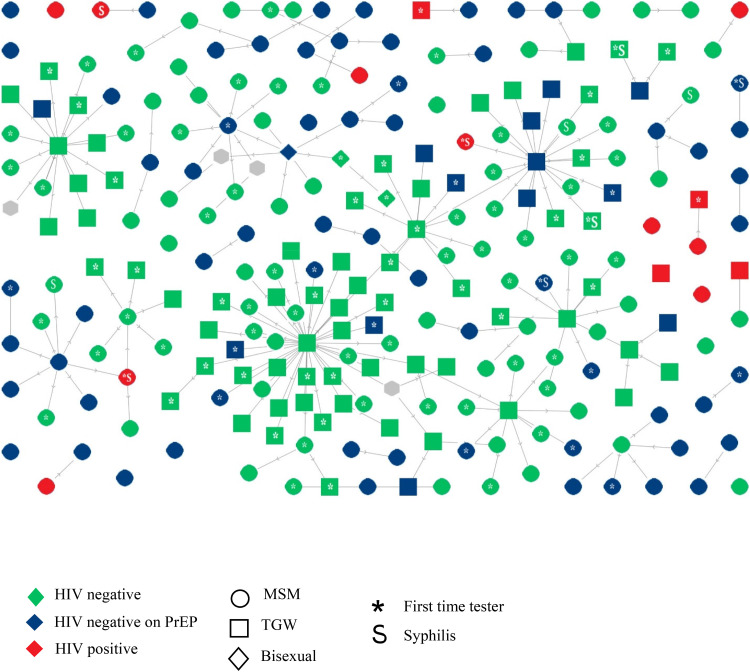
The overall mean of first-time testers recruited was 1.5 (95% CI, 0.7-2.3) and TGW recruited more first time HIV testers, a mean of 3.4 NMs per recruiter compared to 0.8 among MSM (p = 0.002). Recruitment patterns, generated by social mapping, are shown in Figure 1. Relatively large recruitment chains: defined as recruiters referring more than 4 NMs were generated by 11 recruiters. One TGW recruiter referred 40 NMs, with 11 of these NMs going on to become recruiters.
Figure 1.
Social mapping showing recruitment pattern of social network strategy recruitment for HIV test among adolescent and young adult.
Network Members and Recruitment Effectiveness
Of the 202 NMs recruited, the median (IQR) age was 19 (17-21) years, median (IQR) age of sexual debut was 16 (15-17) years, and 62% were self-identified as YMSM. The majority had multiple lifetime sex partners (85.6%), with a median (IQR) of 5 (3-10) sex partners. Eighty-one percent of NMs reported inconsistent condom use. Six (3.0%) tested HIV positive. There were 137 (67.8%) NMs although testing HIV negative at high risk for HIV infection as they had multiple partners and used condom inconsistently (Table 3). Among the 202 NMs, 124 (61.4%, 95% CI, 54.3-68.1) were first time HIV testers. Recruiters who were previously NMs were able to recruit a higher proportion of participants who were first-time HIV testers (74.5%; 95% CI, 65.6-83.3) when compared to new recruiters (50.0%; 95% CI, 40.6-59.4) (p < 0.001).
Table 3.
Characteristics of Network Member Stratified by Type of Recruiters.
| Characteristics | No. of Network member | Recruiters (n = 83) | p-value | |
|---|---|---|---|---|
| Recruiters (n = 46) | NM to recruiter (n = 37) | |||
| 202 | 108 | 94 | - | |
| Age, n (%) | ||||
| 15–19 years | 112 (55.4%) | 67 (62.0%) | 45 (47.9%) | 0.04a |
| 20–24 years | 90 (44.6%) | 41 (38.0%) | 49 (52.1%) | |
| Gender, n (%) | ||||
| MSM | 125 (61.9%) | 63 (58.3%) | 62 (66.0%) | 0.26a |
| TGW | 77 (38.1%) | 45 (41.7%) | 32 (34.0%) | |
| Inconsistent condom use, n (%) | 164 (81.2%) | 88 (81.5%) | 76 (80.8%) | 0.90b |
| HIV positive, n (%) | 6 (3.0%) | 4 (3.7%) | 2 (2.2%) | 0.50b |
| HIV negative at risk, n (%) | 137(67.8%) | 71 (65.1%) | 66 (71.0%) | 0.67b |
| Linkage to initiate PrEP (n = 137), n (%) | 43/137 (31.4%) | 25/71 (35.2%) | 18/66 (27.3%) | 0.42b |
| Syphilis infection among those tested (n = 164), n (%) | 8/164 (4.9%) | 4/91 (4.4%) | 4/73 (5.5%) | 0.75b |
MSM = Men who have sex with men, TGW = Transgender women, PrEP = Pre-exposure prophylaxis.
High risk of HIV acquisition defined as had multiple sex partners and inconsistent condom use.
HIV infection defined as having a positive HIV antibody test via HIV self testing and confirmatory testing with venous blood sampling.
Syphilis infection defined as non treponemal assay, rapid plasma regain (RPR) titers above 1:1 is reactive.
Chi-squre test, b z-test for proportion.
Linkage to Care: Treatment and Prevention Cascade
Of the total 202 NMs, 6 tested positive for HIV, (HIV prevalence 3.0%, 95%CI, 1.1-6.4). Four were first-time testers. Four were YMSM, mean age of sexual debut was 15 years (range 14-18), all reported ≥8 life-time sex partners. Mean baseline CD4 lymphocyte count was 306 cells/mm3 (range 154-447). Five participants initiated same day ART with dolutegravir once daily regimens. One participant had miliary pulmonary tuberculosis infection, was started on anti-tuberculous treatment and then initiated on ART 2 weeks later. Three participants had syphilis co-infection (1 secondary syphilis and 2 late latent syphilis).
There were 8 NMs with positive syphilis serologic tests. Prevalence of syphilis infection was 4.9% (95% CI 2.1-9.4). There were 7 cases of late latent syphilis and one case of secondary syphilis presenting with rash at their arms, palms and soles. All were prescribed benzathine penicillin G. Five patients who had syphilis infection were at high risk of HIV acquisition and 4 started oral daily PrEP.
Of the 137 (67.8%) HIV negative NMs defined to be at risk for HIV infection, all were provided HIV prevention services, including sex education, condom use and education about STI prevention and 43 (31.3%) were initiated on PrEP. The remaining received appointments for HIV testing in the next 6 months.
Discussion
This study demonstrated the effectiveness of SNS implementation among YMSM and YTGW aged 15–24 years with a mean of 2.4 clients per recruiter. Moreover, two-third of the clients were first time HIV testers. In this population, HIV and syphilis prevalence was 3.0% and 4.8%. respectively, After HIV testing, participants were linked to antiretroviral treatment or HIV preexposure prophylaxis services as appropriate.
This study found 67% of recruiters referred at least one network members with a mean of 2.4 clients per recruiter. This is similar to previous reports among MSM adults living with HIV in the USA, where the median age was 35 years (range 18-63). In this study, 51% of recruiters referred at least one peer with a median of 2 clients per recruiter19 Another study in high-risk American young women showed a recruitment mean of 2 NMs.20 Even though or study was conducted during the COVID-19 pandemic, our recruiters were able to reach the effectiveness level set at 50% for first time testers. Furthermore, the second round of recruiters, who switched from being network member themselves, were able to reach more first-time testers than the first round of recruiters (74.5% vs 50.0%). This showed the benefit of the SNS approach; new circles of targeted NMs were expanded by newer generation recruiters.
Recruiters with different characteristics had their own strengths. Characteristics of recruiters who recruited more average number of NMs per recruiters were younger (15-19 years). This may be explained by several reasons, including cognitive and behavioral development in this age group being heavily influenced by peers.21 In this study we found YTGW recruiters were able to refer many more NMs compared to YMSM. This may be explained by their ability to empower and support each other in accessing HIV services; to overcome barriers of social stigma, social discrimination.22,23 In our study recruiters living with HIV referred less clients than recruiters who did not have HIV (0.62 vs 2.63 client/recruiter). This was similarly seen in previous study done across 7 US cities that found recruiters living with HIV tended to refer clients for HIV testing less than those who were HIV negative at risk (network index 6.2 vs 9.4).7 This may be explained by internalized stigma, and inadvertent disclosure of their own HIV status to peers and fears on the confidentiality of their test results.24,25
The HIV prevalence of NMs in this project was 3.0%, a similar figure to that seen in a previous study in our clinic in 2019, conducted among YMSM and YTGW aged 15–19 years looking at performance HIV self-testing. This study found an HIV prevalence of 2.2%, possibly lower due to the younger age of participants.26 This prevalence was similar to a previous study in young Thai male army conscripts in 2018, of which HIV prevalence among MSM was 4.0%.27 Similarly, HIV prevalence rate among males aged 15–24 years in a Thai National AIDS Program (NAP) surveillance was at 2.87%.2 This suggests SNS was able to reach a population with a similar prevalence to known HIV at risk groups.
A strength of this study is that it was conducted at “Buddy CU Clinic”, an established adolescent-friendly clinic offering a one-stop double cascade of HIV services, including HIV testing with HIV self-testing, same-day ART, and same day PrEP. This approach has been shown in previous studies to be associated with higher uptake of both antiretroviral therapy and PrEP.28,29 SNS allowed us to enroll an initial 46 clients in the clinic and trained them to be a recruiters, and subsequently one-fifth of network members converted to become recruiters themselves. Secondly, SNS strategies continued HIV testing service during COVID-19 pandemic restrictions where clinic visit were provided by appointment only. The training that recruiters received empowered them to be able to refer peers to get HIV testing and prevention or treatment services. This was an invaluable benefit of SNS. Our study also had several limitations. This study focused only two key population groups, YMSM and YTGW so may not be generalizable to other key populations, such as people who inject drugs. Secondly, the study was designed as an implementation study with no direct comparisons with other recruitment strategies and no specific data variables were collected to formally calculate the cost-effectiveness of this strategy, which should be done in further studies. Thirdly, HIV and STD services are free of charge under Thailand's HIV National Program, however, in this study we provided travel compensation to adolescents, which may have created more engagement service users than what would usually be seen in generally available HIV services.
Conclusions
This study found that social network strategies are feasible and effective in reaching adolescent and young adult key populations to establish HIV services. This model may be considered for expansion to other settings in Thailand to reach adolescents, in order to achieve national HIV prevention program targets with the ultimate goal of ending the AIDS epidemic by 2030.
Acknowledgements
The authors would like to acknowledge Dr Rangsima Lolekha and Dr Supattra Rungmaitree for their suggestion before submission. Ms.Chomnad Manopaiboon and Ms.Farida Langkafah from Thailand MOPH and U.S. CDC Collaboration (TUC) for their technical and IT support. We would also like to thank Ms. Sapphire Cartledge for English editing and Mr Pathomchai Amornrattanapaijit for administrative support for this study.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Ratchadapisek Sompoch Endowment Fund (2021) under Telehealth Cluster, Chulalongkorn University, the Chulalongkorn University Community Engagement Fund, the Center of Excellence in Transgender Health, Chulalongkorn University, the Thailand MOPH-US.CDC Collaboration (TUC) and the Collaborative Initiative for Paediatric HIV Education and Research (CIPHER) Grant Programme - International AIDS Society (CIPHER).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ Contributions: NP was responsible for writing the initial version of the manuscript. SK conducted the data analysis. WNS and SK revised later versions of the manuscript. NP, AP, PW, JM and SK performed recruitment and appointment of participants and followed up for data collection. TP supervised the overall study operations, manuscript writing, and content. All authors have read and approved the final version of this manuscript.
ORCID iDs: Nantika Paiboon https://orcid.org/0000-0003-3950-5383
Wipaporn Natalie Songtaweesin https://orcid.org/0000-0003-3440-732X
Surinda Kawichai https://orcid.org/0000-0003-0189-4125
References
- 1.UNAIDS. UNAIDS DATA. 2021. https://www.unaids.org/sites/default/files/media_asset/JC3032_AIDS_Data_book_2021_En.pdf. (2021, accessed 19 April 2021)
- 2.HIV and AIDS Data Hub for Asia-Pacific Review in slides Thailand. (Last updated: September 2021). https://www.aidsdatahub.org/resource/thailand-country-slides. (2021, accessed 1 April 2021).
- 3.Seekaew P, Pengnonyang S, Jantarapakde J, et al. Characteristics and HIV epidemiologic profiles of men who have sex with men and transgender women in key population-led test and treat cohorts in Thailand. PLoS One. 2018;13(8):e0203294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holtz TH, Wimonsate W, Mock PA, et al. Why we need pre-exposure prophylaxis: Incident HIV and syphilis among men, and transgender women, who have sex with men, Bangkok, Thailand, 2005–2015. Int J STD AIDS. 2019;30(5):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Committee for the Prevention and Response to AIDS. Thailand National Strategy to End AIDS 2017–2030. Bureau of AIDS, TB, and STIs, Department of Disease Control, Ministry of Public Health; 2017. [Google Scholar]
- 6.World Health Organization. WHO recommends social network-based HIV testing approaches for key populations as part of partner services package: policy brief. World Health Organization; 2019. [Google Scholar]
- 7.Kimbrough LW, Fisher HE, Jones KT, Johnson W, Thadiparthi S, Dooley S. Accessing social networks with high rates of undiagnosed HIV infection: The social networks demonstration project. Am J Public Health. 2009;99(6):1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reif LK, McNairy ML, Lamb MR, Fayorsey R, Elul B. Youth-friendly services and differentiated models of care are needed to improve outcomes for young people living with HIV. Curr Opin HIV AIDS. 2018;13(3):249–256. [DOI] [PubMed] [Google Scholar]
- 9.Closson K, Chown S, Armstrong HL, et al. HIV Leadership programming attendance is associated with PrEP and PEP awareness among young, gay, bisexual, and other men who have sex with men in Vancouver, Canada. BMC Public Health. 2019;19(1):429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UNAIDS. Prevention gap report. https://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf (2016, accessed 1 April 2021).
- 11.Chikwari CD, Dringus S, Ferrand RA. Barriers to, and emerging strategies for, HIV testing among adolescents in sub-Saharan Africa. Curr Opin HIV AIDS. 2018;13(3):257–264. [DOI] [PubMed] [Google Scholar]
- 12.Pike C, Celum C, Bekker L. Adolescent healthcare: I’m Lovin’ it. 2020. 2020;21(1). [DOI] [PMC free article] [PubMed]
- 13.Bernays S, Tshuma M, Willis N, et al. Scaling up peer-led community-based differentiated support for adolescents living with HIV: Keeping the needs of youth peer supporters in mind to sustain success. J Int AIDS Soc. 2020;23(Suppl 5):e25570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vannakit R, Janyam S, Linjongrat D, et al. Give the community the tools and they will help finish the job: Key population-led health services for ending AIDS in Thailand. J Int AIDS Soc. 2020;23(6):e25535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meehan SA, Draper HR, Burger R, Beyers N. What drives ‘first-time testers’ to test for HIV at community-based HIV testing services? Public Health Action. 2017;7(4):304–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desrosiers A, Betancourt T, Kergoat Y, Servilli C, Say L, Kobeissi L. A systematic review of sexual and reproductive health interventions for young people in humanitarian and lower-and-middle-income country settings. BMC Public Health. 2020;20(1):666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiboolsanti S, Kiertiburanakul S, Putcharoen O, Lolekha R, Sukkul A. Thailand National Guidelines on HIV/AIDS Diagnosis, Treatment and Prevention 2020. Department of Disease control, Ministry of Public Health; 2020, [cited 2022 Jan 03]. Available from: http://www.thaiaidssociety.org/index.php?option=com_content&view=article&id=79&Itemid=86 [Google Scholar]
- 18.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golden MR, Gift TL, Brewer DD, et al. Peer referral for HIV case-finding among men who have sex with men. AIDS. 2006;20(15):1961–1968. [DOI] [PubMed] [Google Scholar]
- 20.Boyer CB, Hightow-Weidman L, Bethel J, et al. An assessment of the feasibility and acceptability of a friendship-based social network recruitment strategy to screen at-risk African American and Hispanic/Latina young women for HIV infection. JAMA Pediatr. 2013;167(3):289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holland-Hall C, Burstein G. Chapter 110 adolescence medicine. In: Kliegman RM, ed. Nelson Textbook of pediatrics, 20th edition. Elsevier; 2016:926–936.e1. [Google Scholar]
- 22.Bockting W, MacCrate C, Israel H, Mantell JE, Remien RH. Engagement and retention in HIV care for transgender women: Perspectives of medical and social service providers in New York city. AIDS Patient Care STDS. 2020;34(1):16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang F, Janamnuaysook R, Boyd MA, Phanuphak N, Tucker JD. Key populations and power: People-centred social innovation in Asian HIV services. Lancet HIV. 2020;7(1):e69–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kingori C, Reece M, Obeng S, et al. Impact of internalized stigma on HIV prevention behaviors among HIV-infected individuals seeking HIV care in Kenya. AIDS Patient Care STDS. 2012;26(12):761–768. [DOI] [PubMed] [Google Scholar]
- 25.Donnelly LR, Bailey L, Jessani A, Postnikoff J, Kerston P, Brondani M. Stigma experiences in marginalized people living with HIV seeking health services and resources in Canada. J Assoc Nurses AIDS Care. 2016;27(6):768–783. [DOI] [PubMed] [Google Scholar]
- 26.Phongphiew P, Songtaweesin W, Phiphatkhunarnon P, et al. Acceptability of blood-based HIV self-testing among adolescents aged 15–19 years at risk of HIV acquisition in Bangkok. The Asia-Pacific AIDS and Co-Infections Conference 2020; Virtual2020. [DOI] [PubMed]
- 27.Jose JED, Sakboonyarat B, Mungthin M, Nelson KE, Rangsin R. Rising prevalence of HIV infection and associated risk factors among young Thai men in 2018. Sci Rep. 2021;11(1):7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ongwandee S, Lertpiriyasuwat C, Khawcharoenporn T, et al. Implementation of a test, treat, and prevent HIV program among men who have sex with men and transgender women in Thailand, 2015–2016. PLoS One. 2018;13(7):e0201171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray KR, Dulli LS, Ridgeway K, et al. Improving retention in HIV care among adolescents and adults in low- and middle-income countries: A systematic review of the literature. PLoS One. 2017;12(9):e0184879. [DOI] [PMC free article] [PubMed] [Google Scholar]