Abstract
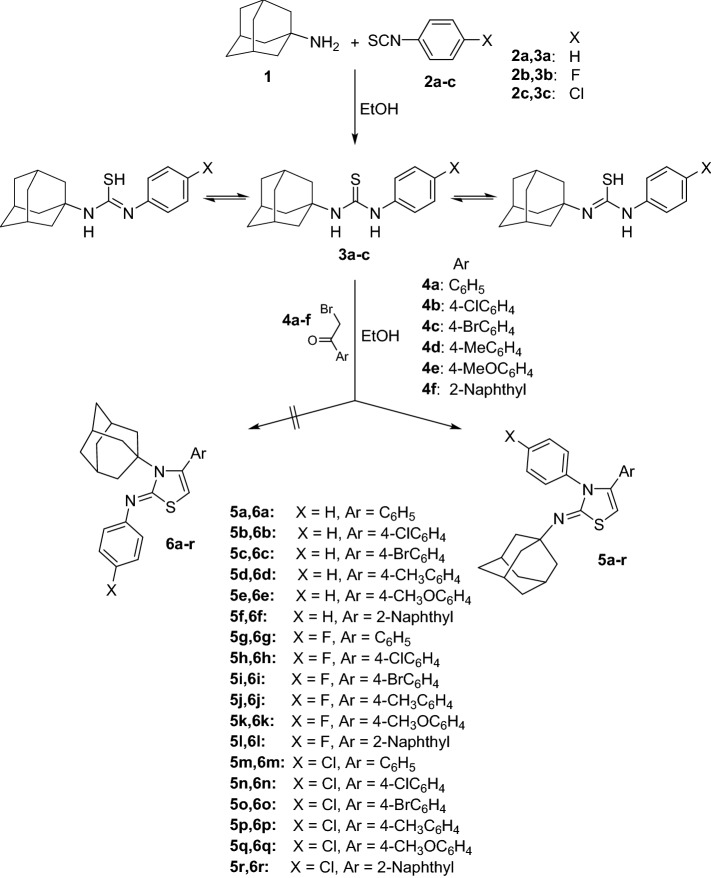
A series of (Z)-N-(adamantan-1-yl)-3,4-diarylthiazol-2(3H)-imines (5a-r) was synthesized via condensation of 1-(adamantan-1-yl)-3-arylthioureas (3a-c) with various aryl bromomethyl ketones (4a-f). The structures of the synthesized compounds were characterized by 1H NMR, 13C NMR and by X-ray crystallography. The in vitro inhibitory activities of the synthesized compounds were assessed against a panel of Gram-positive and Gram-negative bacteria, and pathogenic fungi. Compounds 5c, 5g, 5l, 5m, and 5q displayed potent broad-spectrum antibacterial activity, while compounds 5a and 5o showed activity against the tested Gram-positive bacteria. Compounds 5b, 5l and 5q displayed potent antifungal activity against Candida albicans. In addition, the synthesized compounds were evaluated for anti-proliferative activity towards five human tumor cell lines. The optimal anti-proliferative activity was attained by compounds 5e and 5k which showed potent inhibitory activity against all the tested cell lines. Molecular docking analysis reveals that compounds 5e and 5k can occupy the positions of NAD cofactor and the histone deacetylase inhibitor EX527 at the active site of SIRT1 enzyme.
Subject terms: Cancer, Computational biology and bioinformatics, Drug discovery, Microbiology, Chemistry
Introduction
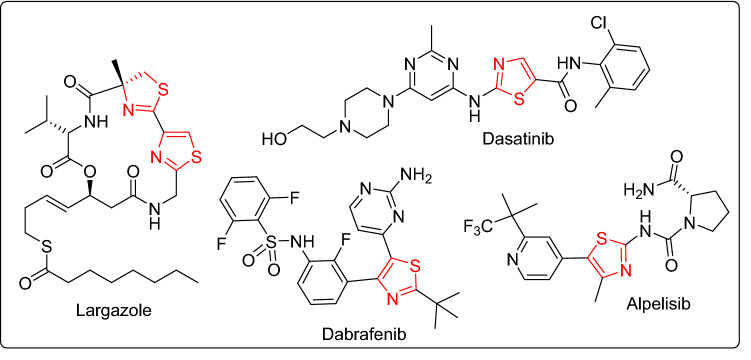
Adamantane cage is an interesting core of several drugs possessing diverse pharmacological properties1–4. The chemotherapeutic effectiveness of adamantane-based compounds was early explored after the discovery of amantadine5,6 and rimantadine7 as efficient medications for the control of Influenza A viral infections. Tromantadine was also developed as potent antiviral drug for the treatment of skin infection caused by herpes simplex virus8. Adamantane cage constitutes an important moiety of currently employed anticancer drugs. Adaphostin is a tyrosine kinase inhibitor that displays anti-proliferative activity in leukemia, non-small cell lung-cancer, and prostate cancer9. Adarotene, a IκB kinase-β inhibitor, was also developed as potent anticancer drug for the treatment of lymphoma, leukemia and prostate cancer10. The adamantane-based synthetic retinoid CD437 was also reported as a promising anticancer agent acting by inhibition of DNA polymerase11, while Opaganib is a recently approved adamantane-based anticancer drug for the treatment of advanced solid tumors12. Opaganib induces its anticancer activity via inhibition of sphingosine kinase (SK). Recently, opaganib proved to be a promising candidate for the treatment of severe COVID-19 pneumonia13. Other adamantane-based derivatives have demonstrated potent activity against pathogenic bacteria, mycobacteria and fungi. The adamantane-diamine hybrid derivative SQ109 was discovered for treatment of drug-resistant tuberculosis14, while the structurally-related dipiperidine analogue SQ609 was developed as a lead compound with potent action against Mycobacterium tuberculosis15 (Fig. 1).
Figure 1.
Adamantane-based chemotherapeutic drugs and drug candidates.
On the other hand, thiazole heterocycle represents the bioactive core of numerous drugs16–18. Several thiazole-based compounds were found to be effective chemotherapeutic agents with anticancer19,20, antifungal21–23, antibacterial24,25, antiviral26 and anti-leishmanial27 activities. The thiazole-based natural product largazole was discovered as potent anticancer drug acting via inhibition of histone deacetylases (HDACs)28,29. Dasatinib30, dabrafenib31 and alpelisib32 are currently-used drugs for the treatment of acute lymphoblastic leukemia, advanced melanoma and breast cancer, respectively (Fig. 2). Besides, the thiazole-based natural products patellamide A33, ixabepilone34 and epothilones35 are clinically useful for the treatment of resistant prostate cancer, metastatic breast cancer, ovarian and rectal cancers.
Figure 2.
Thiazole-based anticancer drugs.
In light of the previously reported observations and as a continuation of an ongoing interest in the chemotherapeutic activities of adamantane-based derivatives36–38, we report herein the synthesis, characterization, and biological evaluation of a series of adamantane-linked thiazole derivatives as potential antibacterial, antifungal and anti-proliferative agents.
Results and discussion
Chemical synthesis
Adamantan-1-amine 1 was allowed to react with phenyl isothiocyanate 2a, 4-fluorophenyl isothiocyanate 2b and 4-chlorophenyl isothiocyanate 2c via heating in ethanol under reflux for 4 h to yield the corresponding 1-(adamantan-1-yl)-3-arylthiourea derivatives 3a-c in good yields39–41. The thiourea derivatives 3a-c were subsequently reacted with different aryl bromomethyl ketones 4a-f via prolonged heating in ethanol followed by addition of sodium acetate to yield the corresponding (Z)-N-(adamantan-1-yl)-3,4-diarylthiazol-2(3H)-imine derivatives 5a-r rather than the isomeric (Z)-3-(adamantan-1-yl)-4,N-diarylthiazol-2(3H)-imines 6a-r. The structures of the diarylthiazol-2(3H)-imines 5a-r were assigned based on 1H NMR, 13C NMR and elemental analysis. Single crystal X-ray analysis of compounds 5d and 5f. was also performed to assign their configuration and to exclude the formation of compounds 6a-r (Fig. 3). The 1H NMR spectra of compounds 5a-r showed the adamantane protons (15H) as three singlets (condensed multiplets) at δ 1.65–1.76 (6H), 1.84–1.99 (6H) and 2.06–2.17 (3H) ppm. The thiazole CH protons were shown as distinguished singlets at δ 5.78–6.04 ppm. Meanwhile, the 13C NMR spectra of compounds 5a-r showed the adamantane carbons as four characteristic peaks at δ 29.64–30.12, 36.60–37.08, 40.63–41.17 and 53.49–54.19 ppm. In addition, the C5, C4 and C2 carbons of the thiazole ring were shown at δ 96.11–99.09, 148.47–150.28 and 158.89–160.08 ppm, respectively. The aromatic protons and carbons and their substituents were properly shown in their 1H NMR and 13C NMR spectra (see “Materials and methods” section).
Figure 3.
Synthetic outline of the target compounds 5a-r.
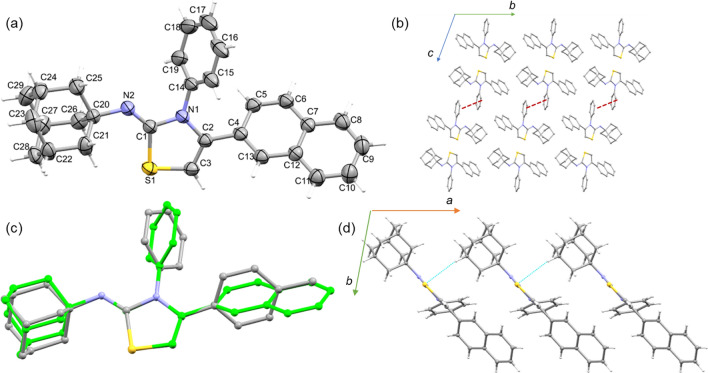
The molecular and crystal structures of compounds 5d and 5f. were determined by single crystal X-ray analysis to explore the configuration of compounds 5a-r and to exclude the formation of compounds 6a-r. The crystal data and refinement parameters of the compounds are summarized in Table S1.
Compound 5d crystallizes in the monoclinic system with the space group I2/a. The asymmetric unit contains one molecule of the compound as cyclohexane solvate (Fig. 4a). The atoms C27 and C30 of the cyclohexane molecule lie on two-fold axis. The six-membered rings of the adamantane moiety exhibit a chair conformation, whereas a cyclohexane solvent adopts twist-boat conformation. The central thiazole ring is oriented at an angle of 47.30° with the mean plane of the p-tolyl group, whereas the same thiazole unit makes 53.66° angle with the phenyl ring attached at the third position of the thiazole ring. In the solid state, molecules of 5d and cyclohexane solvent observed in the crystal lattice forming in a columnar fashion (Fig. 4b) as observed in many adamantane derivatives. No classical hydrogen bonds observed in the crystal structure. However, a weak intermolecular C–H…π (involving the tolyl proton, H6 and centroid of the thiazole ring; C6–H6…Cg1 = 149°; H6…Cg1 = 2.72 Å, C6…Cg1 = 3.572(3); symmetry element: − x, y + 1/2, − z + 1/2).
Figure 4.
(a) ORTEP plot of compound 5d and a cyclohexane solvent in the crystalline lattice (symmetry element (a) − x + 1/2, y, − z). The displacement ellipsoid is drawn at the 50% probability level along atom-labelling scheme and (b) columnar packing mode of compound 5d along the crystallographic ac plane. The lattice cyclohexane solvent is shown in red. All H atoms have been omitted for the sake of clarity.
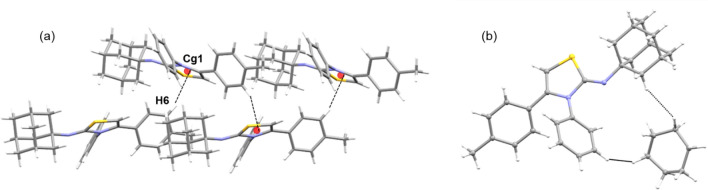
This interaction links the molecules into a chain that runs parallel to the crystallographic b axis (Fig. 5a). The cyclohexane solvent establishes a pair of weak H…H contacts with the protons of the adamantane and phenyl moieties (Fig. 5b).
Figure 5.
(a) Molecular chain formed by a weak C–H…π interaction in the solid state of compound and (b) cyclohexane…adamantane/phenyl interactions in compound 5d.
Compound 5f. crystallizes in the triclinic system with the P-1 space group. The asymmetric unit contains one molecule (Fig. 6a). This compound has a naphthalene ring instead of the p-tolyl position in compound 5d. In compound 5f., the central thiazole ring is oriented at an angle of 42.03° with the mean plane of the naphthalene ring. However, the mean plane of the phenyl ring makes 60.25° with the thiazole core.
Figure 6.
(a) ORTEP representation of compound 5f. with atom-labelling scheme, (b) crystal packing of compound 5f. projected along bc plane and the π-stacking interaction between phenyl rings is shown as red dashed lines, (c) structural superposition of compounds 5d and 5f. (cyclohexane solvent has been omitted for clarity) and (d) molecular chain is generated via a weak intermolecular C–H…S interaction.
The six-membered rings of the adamantane cage exhibits chair conformation as observed in related structures. In the crystalline state, the molecules pack in a columnar fashion (Fig. 6b). Figure 6c shows the structural overlay between 5d and 5f. structures and one can see from this figure that the central thiazole and adamantane cage overlap well and the aryl rings show twist orientations. The crystal structure of 5f. is stabilized primarily by a weak π-stacking interaction formed between phenyl rings in adjacent columns (centroid–centroid = 3.848 (2), symmetry element: 1-x, 1-y, 1-z). In addition, a weak C–H…S interaction (C27-H27…S1 = 161°, H27…S1 = 3.02 Å and C27…S1 = 3.959 Å; symmetry element: x-1, y, z) links the neighboring molecules into a chain that runs parallel to the crystallographic a axis as shown in Fig. 6d.
In vitro antibacterial and antifungal activities
The in vitro antibacterial and antifungal activity of newly designed compounds 5a-r was assessed against a panel of standard pathogenic strains of the Institute of Fermentation of Osaka (IFO) namely; Staphylococcus aureus IFO 3060 (SA), Bacillus subtilis IFO 3007 (BS), Micrococcus luteus IFO 3232 (ML) (Gram-positive bacteria), Escherichia coli IFO 3301 (EC), Pseudomonas aeruginosa IFO 3448 (PA) (Gram-negative bacteria ) and the pathogenic fungi Candida albicans IFO 0583 (CA), Aspergillus oryzae IFO 4177 (AO) and Aspergillus niger IFO 4414 (AN). The initial screening was carried out using the semi-quantitative agar disc-diffusion method using Müller-Hinton agar medium42.
The results of the initial antimicrobial screening of compounds 5a-r (200 µg/disc); the antibacterial antibiotics Ampicillin trihydrate, Ciprofloxacin (100 µg/disc) and the antifungal drug Fluconazole (100 µg/disc) are shown in Table 1. The results showed varying degrees of inhibition against the tested microorganisms. In general, potent antibacterial activity was displayed by compounds 5a, 5c, 5e, 5g, 5h, 6j, 5l, 5m, 5n, 5o, 5p and 5q, which produced growth inhibition zones ≥ 18 mm against one or more of the tested bacterial strains. Meanwhile, compounds 5b, 5d, 5f., 5i, 5k and 5r displayed moderate antibacterial activity (growth inhibition zones 14–17 mm). In addition, the Gram-positive bacteria Staphylococcus aureus, Bacillus subtilis and to a lesser extent Micrococcus luteus are considered the most sensitive among the tested bacterial strains. The inhibitory activity against the tested Gram-negative bacteria was generally lower than the Gram-positive bacteria; compounds 5c, 5g, 5l, 5m and 5q showed potent inhibitory activity against Escherichia coli, while only compounds 5c, 5l and 5m were strongly active against Pseudomonas aeruginosa. The optimum antibacterial activity was attained by compounds 5c, 5g, 5l, 5m and 5q which displayed potent broad spectrum antibacterial activity.
Table 1.
Antibacterial and antifungal activities of compounds 5a-r (200 μg/8 mm disc), the broad-spectrum antibacterial drugs Ampicillin trihydrate, Ciprofloxacin and the antifungal drug Fluconazole (100 μg/8 mm disc) against the Gram-positive bacteria Staphylococcus aureus IFO 3060 (SA), Bacillus subtilis IFO 3007 (BS) and Micrococcus luteus IFO 3232 (ML), the Gram-negative bacteria Escherichia coli IFO 3301 (EC) and Pseudomonas aeruginosa IFO 3448 (PA) and the pathogenic fungi Candida albicans IFO 0583 (CA), Aspergillus oryzae IFO 4177 (AO) and Aspergillus niger IFO 4414 (AN).

| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comp. No | X | Ar | Diameter of Growth Inhibition Zone (mm)* | |||||||
| SA | BS | ML | EC | PA | CA | AO | AN | |||
| 5a | H | C6H5 | 25 | 26 | 20 | 12 | - | - | - | - |
| 5b | H | 4-ClC6H4 | 14 | 17 | 10 | - | - | 18 | - | - |
| 5c | H | 4-BrC6H4 | 28 | 23 | 24 | 25 | 19 | 10 | - | - |
| 5d | H | 4-CH3C6H4 | 17 | 14 | 15 | 14 | 12 | - | - | - |
| 5e | H | 4-CH3OC6H4 | 18 | 15 | 15 | - | - | - | - | - |
| 5f. | H | 2-Naphthyl | 14 | 15 | 12 | 11 | 10 | - | - | - |
| 5g | F | C6H5 | 21 | 17 | 21 | 21 | 15 | - | 13 | - |
| 5h | F | 4-ClC6H4 | 18 | 15 | 17 | 16 | 14 | 12 | 10 | - |
| 5i | F | 4-BrC6H4 | 12 | 14 | 12 | 12 | 11 | - | - | - |
| 5j | F | 4-CH3C6H4 | 18 | 17 | 15 | 15 | 12 | 14 | - | - |
| 5k | F | 4-CH3OC6H4 | 14 | 14 | 12 | 14 | 10 | 17 | - | - |
| 5l | F | 2-Naphthyl | 25 | 28 | 22 | 22 | 18 | 18 | - | - |
| 5m | Cl | C6H5 | 22 | 20 | 18 | 26 | 20 | - | - | - |
| 5n | Cl | 4-ClC6H4 | 18 | 17 | 15 | 15 | 12 | - | - | - |
| 5o | Cl | 4-BrC6H4 | 26 | 22 | 18 | 14 | 12 | - | - | - |
| 5p | Cl | 4-CH3C6H4 | 18 | 15 | 10 | 12 | - | - | - | - |
| 5q | Cl | 4-CH3OC6H4 | 24 | 22 | 22 | 18 | 15 | 19 | 14 | - |
| 5r | Cl | 2-Naphthyl | 14 | 10 | 12 | - | - | - | - | |
| Ampicillin trihydrate | 28 | 30 | 25 | 24 | 22 | NT | NT | NT | ||
| Ciprofloxacin | 34 | 38 | 32 | 38 | 36 | NT | NT | NT | ||
| Fluconazole | NT | NT | NT | NT | NT | 21 | 22 | 24 | ||
NT not tested.
*-Inactive (inhibition zone < 10 mm).
Significant values are in bold.
The antifungal activity of the compounds 5a-r was generally lower than their antibacterial activity. Compounds 5b, 5l and 5q displayed high activity against the yeast-like pathogenic fungus Candida albicans, while compounds 5j and 5k were moderately active and compounds 5c and 5h exhibited marginal activity (growth inhibition zones 10–13 mm). Compounds 5g, 5h and 5q were almost weakly against Aspergillus oryzae, and all the compounds were totally inactive against Aspergillus niger.
In general, the antibacterial and antifungal potency and spectrum are greatly influenced by the nature of the aryl groups at positions 3 and 4 of the core thiazole nucleus. In the 3-phenyl series 5a-f, the 3,4-diphenyl analogue 5a displayed potent antibacterial activity against the tested Gram-positive bacteria and lacked activity against the tested Gram-negative bacteria and pathogenic fungi. Replacement of the 4-phenyl group with 4-(4-chlorophenyl) substituent (compound 5b) deteriorated the antibacterial activity and greatly enhanced the activity against Candida albicans. Meanwhile, the 4-(p-tolyl) 5d and 4-(2-naphthyl) 5f. analogues retained moderate activity against the tested Gram-positive bacteria, with only marginal activity against the tested Gram-negative bacteria, and no antifungal activity. In addition, the 4-(4-methoxyphenyl) derivative 5e displayed good activity against Staphylococcus aureus and moderate activity against Bacillus subtilis and Micrococcus luteus with no activity against the tested Gram-negative bacteria and fungi. The optimum antibacterial activity was attained in the 4-bromophenyl analogue 5c which displayed excellent broad-spectrum antibacterial activity.
In the 3-(4-fluorophenyl) series 5g-l, the 4-phenyl, 4-(4-chlorophenyl) and 4-(p-tolyl) derivatives 5g, 5h and 5j showed marked broad-spectrum antibacterial activity and weak antifungal activity against Candida albicans and/or Aspergillus oryzae. Unlike the antimicrobial activity in the 3-phenyl derivatives 5a-f, the antibacterial activity of the 4-bromophenyl analogue 5i was found inferior compared to the majority of the 3-(4-fluorophenyl) derivatives 5g-l, and with no antifungal activity. Meanwhile, the 4-(p-tolyl) 5j and 4-(4-methoxyphenyl) 5k analogues showed almost moderate or weak antibacterial activity and moderate activity against Candida albicans. In contrary to the antimicrobial activity of the 3-phenyl derivatives 5a-f, the 4-(2-naphthyl) analogue 5l displayed excellent broad-spectrum antibacterial activity in addition to potent antifungal activity against Candida albicans.
In the 3-(4-chlorophenyl) series 5m-r, the optimum antibacterial activity was shown by the 4-phenyl 5m and 4-(4-methoxyphenyl) 5q derivatives which almost displayed potent broad-spectrum antibacterial activity, while the 4-(4-bromophenyl) derivative 5o retained potent and almost weak activity against the tested Gram-positive and Gram-negative bacteria, respectively. The antibacterial activity of the 4-(4-chlorophenyl) 5n and 4-(p-tolyl) 5p derivatives was found similar, but to lower extent, to the activity of the 4-(4-bromophenyl) derivative 5o. The 4-(2-naphthyl) analogue 5r only retained moderate or marginal activity against the tested Gram-positive bacteria. Potent and moderate antifungal activity against Candida albicans and Aspergillus oryzae, respectively, was displayed by the 4-(4-methoxyphenyl) 5q derivative.
Taken together, the broad spectrum antibacterial activity was exhibited by monohalogenated derivatives rather than the dihalogenated ones. This is illustrated by the activity 5c, 5l, 5m and 5q. The same results were clearly demonstrated by the antifungal activity of compounds 5b, 5l and 5q. Upon comparing the antibacterial activity of the two isomeric structures 5b and 5m, it has been discovered that the halogen substitution pattern is more beneficial at the N-3 position than the C-4 position.
The minimal inhibitory concentrations (MIC) and the minimal biocidal concentrations (MBC, bactericidal and fungicidal) for seven of the most active compounds (5a, 5c, 5g, 5l, 5m, 5o and 5q) against the same microorganisms used in the primary screening were carried out using the micro-dilution susceptibility method in Müller-Hinton Broth43–45. The MIC and MBC values of compounds 5a, 5c, 5g, 5l, 5m, 5o, 5q, Ampicillin trihydrate, Ciprofloxacin and Fluconazole are depicted in Table 2. The MIC of the tested compounds were in accordance with the results obtained in the primary screening. According to the antimicrobial standards, an agent is usually considered as bactericidal or fungicidal if the MBC/MIC ratio is not more than 446. The MBC/MIC ratio for the tested compounds (Table 2) were found to be around 2. According to the MBC/MIC values of the tested compounds, it could be concluded that the (Z)-N-(adamantan-1-yl)-3,4-diarylthiazol-2(3H)-imine derivative 5a, 5c, 5g, 5l, 5m, 5o and 5q could be considered as potential antibacterial candidates for further studies. Though these compounds are of inferior activity compared to the reference drugs, they have displayed MBC/MIC ratio equivalent to that of the references.
Table 2.
The Minimal Inhibitory Concentrations (MIC) and Minimal Biocidal Concentrations (MBC, bactericidal and fungicidal) of compounds 5a, 5c, 5g, 5l, 5m, 5o and 5q in comparison with the broad spectrum antibacterial drugs Ampicillin trihydrate, Ciprofloxacin and the antifungal drug Fluconazole against the Gram-positive bacteria Staphylococcus aureus IFO 3060 (SA), Bacillus subtilis IFO 3007 (BS) and Micrococcus luteus IFO 3232 (ML), the Gram-negative bacteria Escherichia coli IFO 3301 (EC) and Pseudomonas aeruginosa IFO 3448 (PA) and the pathogenic fungus Candida albicans IFO 0583 (CA).
| Comp. No | MIC/MBC (μg/mL)* | |||||
|---|---|---|---|---|---|---|
| SA | BS | ML | EC | PA | CA | |
| 5a | 3.25/7.20 | 3.5/8.25 | 7.0/15.5 | ND/ND | ND/ND | ND/ND |
| 5c | 2.0/5.20 | 4.0/7.50 | 3.0/6.20 | 6.5/11.50 | 10.25/ND | ND/ND |
| 5g | 6.50/10.25 | ND/ND | 6.50/12.0 | 8.0/16.20 | ND/ND | ND/ND |
| 5l | 3.25/8.50 | 2.25/5.0 | 4.0/9.50 | 7.20/15.50 | ND/ND | 8.50/16.20 |
| 5m | 6.0/12.50 | 8.50/15.50 | ND/ND | 4.25/8.75 | 8.50/ND | ND/ND |
| 5o | 3.20/8.25 | 3.75/8.0 | ND/ND | ND/ND | ND/ND | ND/ND |
| 5q | 3.50/8.20 | 3.75/7.50 | 4.20/7.80 | ND/ND | ND/ND | 7.25/13.50 |
| Ampicillin | 2.0/3.5 | 1.0/3.0 | 2.5/4.0 | ND/ND | ND/ND | ND/ND |
| Ciprofloxacin | 0.75/1.50 | 0.50/1.0 | 1.0/2.0 | 0.25/1.0 | ND/ND | ND/ND |
| Fluconazole | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | 6.25/12.0 |
ND not determined.
*Data shown are the mean values of three experiments.
The adamantane-linked thiazoles 5a, 5c, 5l and 5o which displayed the best antibacterial activity against Gram-positive bacteria were selected for further assessment of their bacterial biofilm inhibitory activity using the crystal violet staining method47. Bacterial biofilms are clusters of bacteria that are attached to a surface and/or to each other and embedded in a self-produced matrix. The biofilm matrix mainly consists of proteins and polysaccharides. In addition to the protection offered by the matrix, bacteria in biofilms can employ several survival strategies to evade the host defense systems48,49. The minimal biofilm inhibitory concentrations (IC50) of compounds 5a, 5c, 5l, 5o and the broad-spectrum antibiotic Erythromycin towards the Gram-positive bacteria Staphylococcus aureus and Micrococcus luteus are shown in Table 3. The IC50 (the lowest concentrations of tested compounds that exhibited 50% inhibition on the biofilm formation) ranged from 1.6 to 2.5 and 3.4 to 7.4 μg/mL against Staphylococcus aureus and Micrococcus luteus, respectively.
Table 3.
Anti-biofilm activity (IC50, μg/mL) of compounds 5a, 5c, 5l, 5o and the broad-spectrum antibiotic Erythromycin against Staphylococcus aureus IFO 3060 (SA) and Micrococcus luteus IFO 3232 (ML).
| Comp. No | Anti-biofilm activity (μg/mL)* | |
|---|---|---|
| SA | ML | |
| 5a | 2.4 ± 0.12 | 4.8 ± 0.20 |
| 5c | 1.6 ± 0.25 | 6.5 ± 0.25 |
| 5l | 2.5 ± 0.15 | 3.4 ± 0.15 |
| 5o | 2.0 ± 0.15 | 7.4 ± 0.21 |
| Erythromycin | 0.45 ± 0.15 | 0.62 ± 0.21 |
*Results are the mean values of three experiments ± SD.
In vitro anti-proliferative activity
The in vitro anti-proliferative activity of the adamantane-linked thiazoles 5a-r was assessed against five human tumor cell lines namely; PC-3 (human prostate cancer), HCT-116 (human colorectal cancer), HepG-2 (human hepatocellular cancer), HeLa (human cervical cancer) and MCF-7 (human breast cancer) using the 3-[4,5-dimethylthiazoyl-2-yl]-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay50. The obtained results, together with that of the anticancer drug doxorubicin, are shown in Table 4.
Table 4.
In vitro anti-proliferative activity of compounds 5a-r and the anticancer drug Doxorubicin toward human prostate cancer (PC-3), colorectal carcinoma (HCT-116), hepatocellular carcinoma (HepG-2), epithelioid cervical carcinoma (HeLa) and mammary gland breast cancer (MCF-7) cell lines.
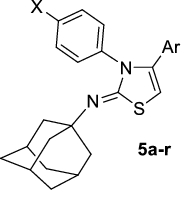
| |||||||
|---|---|---|---|---|---|---|---|
| Comp. No | X | Ar | IC50 (µM)a | ||||
| PC-3 | HCT-116 | HepG-2 | HeLa | MCF-7 | |||
| 5a | H | C6H5 | 67.26 ± 3.6 | 59.23 ± 3.3 | 48.32 ± 2.9 | 41.81 ± 2.4 | 51.26 ± 2.9 |
| 5b | H | 4-ClC6H4 | 87.82 ± 4.1 | 85.51 ± 4.4 | 65.29 ± 3.4 | 71.48 ± 3.7 | 78.62 ± 3.9 |
| 5c | H | 4-BrC6H4 | 78.97 ± 3.9 | 80.25 ± 4.2 | 56.43 ± 3.2 | 83.86 ± 4.0 | 72.31 ± 3.7 |
| 5d | H | 4-CH3C6H4 | 53.19 ± 2.9 | 58.63 ± 3.2 | 38.24 ± 2.5 | 49.83 ± 2.7 | 45.66 ± 2.6 |
| 5e | H | 4-CH3OC6H4 | 12.85 ± 1.0 | 11.96 ± 0.9 | 16.30 ± 1.4 | 9.87 ± 0.7 | 6.90 ± 0.4 |
| 5f. | H | 2-Naphthyl | > 100 | 94.26 ± 4.8 | 81.62 ± 4.2 | > 100 | 86.49 ± 4.4 |
| 5g | F | C6H5 | 16.73 ± 1.3 | 28.27 ± 2.1 | 20.87 ± 1.6 | 30.67 ± 2.1 | 19.64 ± 1.4 |
| 5h | F | 4-ClC6H4 | 23.56 ± 1.9 | 39.74 ± 2.5 | 22.07 ± 1.8 | 18.11 ± 1.5 | 32.78 ± 2.2 |
| 5i | F | 4-BrC6H4 | > 100 | > 100 | 88.57 ± 4.5 | > 100 | 91.18 ± 4.8 |
| 5j | F | 4-CH3C6H4 | 90.04 ± 4.6 | 73.86 ± 3.9 | 70.28 ± 3.8 | 52.53 ± 2.9 | 66.28 ± 3.5 |
| 5k | F | 4-CH3OC6H4 | 9.71 ± 0.8 | 6.47 ± 0.5 | 11.27 ± 0.9 | 7.09 ± 0.5 | 3.51 ± 0.2 |
| 5l | F | 2-Naphthyl | > 100 | 89.05 ± 4.5 | 77.21 ± 4.0 | 93.62 ± 4.7 | 84.03 ± 4.3 |
| 5m | Cl | C6H5 | 33.59 ± 2.2 | 36.42 ± 2.3 | 29.53 ± 2.2 | 21.59 ± 1.8 | 26.04 ± 1.9 |
| 5n | Cl | 4-ClC6H4 | 40.70 ± 2.4 | 46.11 ± 2.6 | 31.98 ± 2.3 | 25.65 ± 2.0 | 39.21 ± 2.5 |
| 5o | Cl | 4-BrC6H4 | 61.39 ± 3.4 | 24.50 ± 1.8 | 47.38 ± 2.8 | 15.28 ± 1.2 | 13.72 ± 1.2 |
| 5p | Cl | 4-CH3C6H4 | 55.86 ± 3.1 | 62.79 ± 3.5 | 43.65 ± 2.7 | 37.26 ± 2.3 | 57.39 ± 3.2 |
| 5q | Cl | 4-CH3OC6H4 | 42.08 ± 2.6 | 17.58 ± 1.4 | 35.18 ± 2.5 | 10.49 ± 0.9 | 8.53 ± 0.7 |
| 5r | Cl | 2-Naphthyl | 75.45 ± 3.8 | 68.35 ± 3.7 | 51.79 ± 3.1 | 56.40 ± 3.3 | 63.54 ± 3.4 |
| Doxorubicin | 8.87 ± 0.6 | 5.23 ± 0.3 | 4.50 ± 0.2 | 5.57 ± 0.4 | 4.17 ± 0.2 | ||
aIC50 Values presented as the mean ± SD of three separate determinations.
Significant values are in bold.
Compounds 5a-r displayed variable degrees of inhibitory activity against the tested cancer cell lines. In general, compounds 5e, 5g, 5h, 5k, 5o and 5q displayed potent anti-proliferative activity (IC50 < 25 μg/mL) against at least three of the tested cancer cell lines, compounds 5a, 5d and 5n showed moderate activity (IC50 > 25 to 50 μg/mL), compounds 5b, 5c, 5j and 5r showed weak activity (IC50 > 50 to 75 μg/mL) while compounds 5f., 5i and 5l were inactive (IC50 > 75 μg/mL). In addition, the optimal anti-proliferative activity was attained by compounds 5e and 5k which showed excellent activity against all the tested cell lines.
The anti-proliferative potency was found to be mainly dependent on the nature of the aryl groups at positions 3 and 4 of the N-(adamantan-1-yl)-thiazol-2(3H)-imine core. Within the 3-phenyl derivatives 5a-f, the 3,4-diphenyl analogue 5a exhibited moderate activity against HepG-2 and HeLa cell lines with weak activity against PC-3, HCT-116 and MCF-7 cell lines. Replacement of the 4-phenyl group with 4-(4-chlorophenyl), 4-(4-bromophenyl) or 4-(p-tolyl) substituents greatly deteriorated the anti-proliferative activity against PC-3 and HCT-116 cell lines with almost weak or marginal activity against HepG-2, HeLa and MCF-7 cell lines. The 4-(4-methoxyphenyl) substituent (compound 5e) is optimistic for anti-proliferative activity against all the tested cell lines. In contrary, a 4-(2-naphthyl) substituent (compound 5f.) dramatically declined the anti-proliferative activity.
In the 3-(4-fluorophenyl) derivatives 5g-l, the 4-phenyl analogue 5g displayed potent activity against PC-3, HepG-2 and MCF-7 cell lines and moderate activity against HCT-116 and HeLa cell lines, while the 4-(4-chlorophenyl) analogue 5h exhibited high activity against PC-3, HepG-2 and HeLa cell lines and moderate activity against HCT-116 and MCF-7 cell lines. In addition, the 4-(4-bromophenyl), 4-(p-tolyl) and 4-(2-naphthyl) derivatives 5i, 5j and 5l were almost inactive against all the tested cell lines. The 4-(4-methoxyphenyl) analogue 5k was found to the most active among the 3-(4-fluorophenyl) derivatives 5g-l which resembles the anti-proliferative activity of Doxorubicin.
The anti-proliferative activity of the 3-(4-chlorophenyl) derivatives 5m-r was rather lower than the 3-(4-fluorophenyl) derivatives 5g-l. the 4-phenyl analogue 5m displayed potent activity against HeLa cell lines and moderate activity against PC-3, HCT-116, HepG-2 and MCF-7 cell lines, while the 4-(4-chlorophenyl) analogue 5n showed moderate activity against all the tested cell lines. In contrary to the activity of the 3-phenyl and 3-(4-fluorophenyl) derivatives (5c and 5i), the 4-(4-bromophenyl) analogue 5o showed potent activity against HCT-116, HeLa and MCF-7 cell lines, moderate activity against HepG-2 and week activity against PC-3 cell lines. As in case of the 3-phenyl and 3-(4-fluorophenyl) derivatives (5e and 5k). the anti-proliferative activity of the 4-(4-methoxyphenyl) analogue 5q was found to the most active among the 3-(4-chlorophenyl) derivatives 5m-r as it in displayed potent activity against HCT-116, HeLa and MCF-7 cell lines, moderate activity against HepG-2 and week activity against PC-3 cell lines.
In view of the available results, it can be concluded that the presence of a 4-methoxyphenyl group at C-4 of the thiazole ring is optimal for the anti-proliferative activity, regardless of the nature of the C-3 substitution. Other C-4 substitutions diminish such activity, especially the 2-naphthyl group that impaired activity against all tested cell lines.
Prediction of activity spectra and molecular docking studies
The online PASS (Prediction of Activity Spectra) structure–activity relationship tool51 was utilized to predict a suitable target of the designed (Z)-N-(adamantan-1-yl)-3,4-diarylthiazol-2(3H)-imine compounds 5a-r. The PASS analysis results indicated that the predicted highest probability of biological activity (Pa) was for histone deacetylase (HDAC) SIRT1 enzyme (Table S2). The PASS prediction results are supported by the reported HDAC inhibitory activity of various adamantane52–54 and thiazole28,29,55–59 derivatives.
HDACs are capable of catalyzing the removal of N-acetyl group from acetylated lysine residues in histones or some non-histone proteins. The overexpression of HDACs has been linked to a variety of solid and hematological malignancies, neurological disorders, inflammatory diseases and metabolic disorders60–62.
As shown in Table 4, compounds 5e and 5k showed excellent anti-proliferative activity against all the tested cell lines. In order to understand the bioactivity of these compounds, we carried out molecular docking against the SIRT1 enzyme. Before docking of 5e and 5k with SIRT1, we carried out a positive control docking simulation for the NAD cofactor and EX527 inhibitor63 (PDB ID: 4I5) with SIRT1. The result showed that there is an excellent agreement between experimental conformations and the predicted poses of NAD cofactor and EX527 (Fig. 7). The binding affinity for the NAD ligand was calculated to be − 13.2 kcal mol−1 and − 11.0 kcal mol−1 for EX527. The same docking parameters were used for compounds 5e and 5k and the results suggest that both compounds showed similar binding affinities (5e: − 7.0 and 5k: − 7.3 kcal mol−1).
Figure 7.
Experimental conformations (yellow) and predicted poses (magenta) of NAD cofactor and EX527 inhibitor are in stick representation at the active site of SIRT1. The top ranked predicted pose of 5e (green) and 5k (blue) at the active site is also shown.
As can be seen from Fig. 7, compounds 5e and 5k bound in a similar manner at the active site of the SIRT1. Moreover, the adamantane cage of these compounds occupies the position of the cycloheptanecarboxamide moiety of the nanomolar inhibitor EX52764. The five-membered ring overlaps with the position of the 5-chlorophenyl of EX527. The methoxyphenyl and the substituted phenyl moieties of 5e and 5k are closer to the position of the nicotinamide and pentose sugar moieties of the NAD cofactor. The predicted pose of 5e and 5k clearly suggest that they could act as potential inhibitors.
As shown in Fig. 8, the interacting residues of the active site of SIRT1 are identical for both compounds 5e and 5k because the binding poses of 5e and 5k are similar. The adamantane moiety establishes hydrophobic interactions with Ala 262, Ile 270, Asn 346, Ile 347 and Asp 348. The substituted phenyl also participated in the hydrophobic interaction with Gln 345 and Val 445. It is interesting to note that His 363 establishes π-stacking interaction with the substituted phenyl ring and the methoxy phenyl ring in compounds 5e and 5k. The active site residue Phe 414 has participated in a hydrophobic interaction with the methoxy phenyl ring.
Figure 8.
Interactions between SIRT1 (a) compound 5e and (b) compound 5k. Small spheres represent the centroid of the respective rings.
Conclusions
Eighteen new adamantane-linked thiazole derivatives were synthesized and their structures were confirmed by 1H NMR, 13C NMR data in addition to single crystal X-ray crystallography. The in vitro antibacterial, antifungal activities of the synthesized compounds were assessed against a panel of pathogenic Gram-positive, Gram-negative bacteria and fungi. Compounds 5c, 5g, 5l, 5m, and 5q displayed potent broad-spectrum antibacterial activity and compounds 5a and 5o showed potent activity against the tested Gram-positive bacteria, while compounds 5b, 5l and 5q displayed potent antifungal activity against Candida albicans. The anti-proliferative activity of the synthesized compounds was evaluated towards five human tumor cell lines. Compounds 5e and 5k showed potent inhibitory activity against all the tested cell lines. Molecular docking simulation analysis demonstrated that the potent compounds 5e and 5k bind to the active site of the SIRT1 enzyme, occupying the positions of the NAD cofactor and the known histone deacetylase inhibitor EX527. The adamantane moiety of these compounds establishes extensive hydrophobic interactions with the active site residues in addition to other interactions.
Materials and methods
Melting points (℃, uncorrected) were measured in open glass capillaries using Stuart SMP30 electro-thermal melting point apparatus. NMR spectra were obtained on a Bruker 400 Avance III at 400.20 MHz for 1H and 100.63 MHz for 13C; and Jeol ECA 500 III at 500.16 MHz for 1H and 125.77 MHz for 13C using CDCl3 as solvent. The chemical shifts are expressed in δ (ppm) downfield from tetramethylsilane (TMS) as internal standard; coupling constants (J) are pressed in Hz. The splitting patterns were designated as: s (singlet), d (doublet), t (triplet), q (quartet) and m (multiplet). Monitoring the reactions and checking the purity of the final products were carried out by thin layer chromatography (TLC) using silica gel pre-coated aluminum sheets (60 F254, Merck), petroleum ether/ethyl acetate (8:2) as eluent and visualization with ultraviolet light (UV) at 365 and 254 nm. Compounds 3a-c were prepared via condensation of adamantan-1-amine 1 with the corresponding aryl isothiocyanate according to the reported procedures39–41. Elemental analyses (C, H, N & S) were in agreement with the proposed structures within ± 0.3% of the theoretical values (Table S3). The standard experimental methods for evaluation of the antimicrobial and anti-proliferative activities42–45,47,50 are presented in the supplementary data. The reference drugs Ampicillin trihydrate (CAS 7177-48-2), Ciprofloxacin (CAS: 85721-33-1), Fluconazole (CAS: 86386-73-4), Erythromycin (CAS: 114-07-8) and Doxorubicin (CAS 23214-92-8) were purchased from Sigma-Aldrich Chemie GmbH, Germany.
General procedure for the synthesis of (Z)-N-(adamantan-1-yl)-3,4-diarylthiazol-2(3H)-imines (5a-r)
A mixture of the appropriate 1-(adamantan-1-yl)-3-arylthiourea 3a-c (2.0 mmol) and the appropriate aryl bromomethyl ketone 4a-f (2.0 mmol), in ethanol (10 mL), was heated under reflux for 24 h. On cooling, sodium acetate (1.0 g) and crushed ice (100 g) were added and the mixture was stirred for 30 min. and allowed to stand at room temperature for 3 h. The precipitated crude product was filtered, washed with water, dried and crystallized from EtOH/CHCl3 or EtOH to yield the target compounds (5a-r) as fine colorless crystals.
(Z)-N-(Adamantan-1-yl)-3,4-diphenylthiazol-2(3H)-imine 5a. Yield: 78%, m.p.: 198–200 °C (EtOH/CHCl3), Mol. Formula (Mol. Wt.): C25H26N2S (386.56). 1H NMR (500.16 MHz): δ 1.68 (s, 6H, Adamantane-H), 1.89 (s, 6H, Adamantane-H), 2.09 (s, 3H, Adamantane-H), 5.88 (s, 1H, Thiazole-H), 7.05–7.07 (m, 2H, Ar–H), 7.11–7.23 (m, 8H, Ar–H). 13C NMR (125.77 MHz): δ 29.64, 36.60, 40.63, 53.49 (Adamantane-C), 96.97 (Thiazole C-5), 126.02, 127.60, 127.77, 127.90, 129.05, 132.52, 138.84, 139.08 (Ar–C), 148.98 (Thiazole C-4), 160.02 (Thiazole C-2).
(Z)-N-(Adamantan-1-yl)-4-(4-chlorophenyl)-3-phenylthiazol-2(3H)-imine 5b. Yield: 62%, m.p.: 176–178 °C (EtOH/CHCl3), Mol. Formula (Mol. Wt.): C25H25ClN2S (421.0). 1H NMR (400.20 MHz): δ 1.68 (s, 6H, Adamantane-H), 1.88 (s, 6H, Adamantane-H), 2.08 (s, 3H, Adamantane-H), 5.89 (s, 1H, Thiazole-H), 6.98 (d, 2H, Ar–H, J = 8.4 Hz), 7.10–7.18 (m, 5H, Ar–H), 7.22–7.28 (m, 2H, Ar–H). 13C NMR (100.63 MHz): δ 29.83, 36.77, 40.82, 53.90 (Adamantane-C), 98.14 (Thiazole C-5), 123.99, 124.59, 126.65, 128.28, 128.45, 129.21, 133.82, 137.98 (Ar–C), 149.30 (Thiazole C-4), 159.34 (Thiazole C-2).
(Z)-N-(Adamantan-1-yl)-4-(4-bromophenyl)-3-phenylthiazol-2(3H)-imine 5c. Yield: 55%, m.p.: 179–181 °C (EtOH/CHCl3), Mol. Formula (Mol. Wt.): C25H25BrN2S (465.45). 1H NMR (400.20 MHz): δ 1.68 (s, 6H, Adamantane-H), 1.88 (s, 6H, Adamantane-H), 2.08 (s, 3H, Adamantane-H), 5.89 (s, 1H, Thiazole-H), 6.93 (d, 2H, Ar–H, J = 8.4 Hz), 7.10–7.18 (m, 3H, Ar–H), 7.22–7.30 (m, 4H, Ar–H). 13C NMR (100.63 MHz): δ 29.78, 36.65, 40.75, 54.19 (Adamantane-C), 99.09 (Thiazole C-5), 120.69, 122.28, 127.12, 128.57, 129.12, 129.58, 131.44, 138.22 (Ar–C), 148.90 (Thiazole C-4), 159.97 (Thiazole C-2).
(Z)-N-(Adamantan-1-yl)-3-phenyl-4-(p-tolyl)thiazol-2(3H)-imine 5d. Yield: 88%, m.p.: 158–160 °C (EtOH), Mol. Formula (Mol. Wt.): C26H28N2S (400.58). 1H NMR (400.20 MHz): δ 1.70 (s, 6H, Adamantane-H), 1.92 (s, 6H, Adamantane-H), 2.11 (s, 3H, Adamantane-H), 2.28 (s, 3H, CH3), 5.85 (s, 1H, Thiazole-H), 6.97–6.99 (m, 4H, Ar–H), 7.14–7.17 (m, 3H, Ar–H), 7.22–7.28 (m, 2H, Ar–H). 13C NMR (100.63 MHz): δ 21.29 (CH3), 29.89, 36.83, 40.86, 53.78 (Adamantane-C), 96.56 (Thiazole C-5), 123.90, 126.27, 127.98, 128.89, 129.36, 130.0, 137.78, 139.19 (Ar–C), 149.42 (Thiazole C-4), 160.08 (Thiazole C-2).
(Z)-N-(Adamantan-1-yl)-4-(4-methoxyphenyl)-3-phenylthiazol-2(3H)-imine 5e. Yield: 85%, m.p.: 156–158 °C (EtOH/CHCl3), Mol. Formula (Mol. Wt.): C26H28N2OS (416.58). 1H NMR (400.20 MHz): δ 1.67 (s, 6H, Adamantane-H), 1.89 (s, 6H, Adamantane-H), 2.08 (s, 3H, Adamantane-H), 3.73 (s, 3H, OCH3), 5.78 (s, 1H, Thiazole-H), 6.67 (d, 2H, Ar–H, J = 8.8 Hz), 6.97 (d, 2H, Ar–H, J = 8.8 Hz), 7.11–7.15 (m, 3H, Ar–H), 7.20–7.26 (m, 2H, Ar–H). 13C NMR (100.63 MHz): δ 29.87, 36.82, 40.86, 53.80 (Adamantane-C), 55.22 (OCH3), 96.13 (Thiazole C-5), 113.56, 125.12, 126.38, 128.10, 129.39, 138.88, 139.25, 157.71 (Ar–C), 149.77 (Thiazole C-4), 159.23 (Thiazole C-2).
(Z)-N-(Adamantan-1-yl)-4-(naphthalen-2-yl)-3-phenylthiazol-2(3H)-imine 5f. Yield: 80%, m.p.: 177–179 °C (EtOH/CHCl3), Mol. Formula (Mol. Wt.): C29H28N2S (436.62). 1H NMR (400.20 MHz): δ 1.76 (s, 6H, Adamantane-H), 1.99 (s, 6H, Adamantane-H), 2.17 (s, 3H, Adamantane-H), 6.04 (s, 1H, Thiazole-H), 7.08–7.26 (m, 6H, Ar–H), 7.47- 7.50 (m, 2H, Ar–H), 7.59 (d, 1H, Ar–H, J = 8.4 Hz), 7.74- 7.78 (m, 3H, Ar–H). 13C NMR (100.63 MHz): δ 29.85, 36.78, 40.84, 53.94 (Adamantane-C), 98.21 (Thiazole C-5), 125.55, 126.38, 126.42, 126.51, 127.15, 127.56, 127.64, 128.08, 128.19, 129.21, 132.66, 133.02, 139.03, 139.18 (Ar–C), 150.02 (Thiazole C-4), 159.02 (Thiazole C-2).
(Z)-N-(Adamantan-1-yl)-3-(4-fluorophenyl)-4-phenylthiazol-2(3H)-imine 5g. Yield: 90%, m.p.: 193–195 °C (EtOH/CHCl3), Mol. Formula (Mol. Wt.): C25H25FN2S (404.55). 1H NMR (500.16 MHz): δ 1.65 (s, 6H, Adamantane-H), 1.85 (s, 6H, Adamantane-H), 2.06 (s, 3H, Adamantane-H), 5.85 (s, 1H, Thiazole-H), 6.86–6.89 (m, 2H, Ar–H), 7.03–7.08 (m, 4H, Ar–H), 7.15–7.17 (m, 3H, Ar–H). 13C NMR (125.77 MHz): δ 29.88, 36.84, 40.93, 53.77 (Adamantane-C), 97.24 (Thiazole C-5), 114.86, 128.02, 128.29, 130.76, 132.54, 135.25, 138.90, 161.73 (Ar–C), 149.27 (Thiazole C-4), 159.78 (Thiazole C-2).
(Z)-N-(Adamantan-1-yl)-4-(4-chlorophenyl)-3-(4-fluorophenyl)thiazol-2(3H)-imine 5h. Yield: 92%, m.p.: 194–196 °C (EtOH/CHCl3), Mol. Formula (Mol. Wt.): C25H24ClFN2S (438.99). 1H NMR (500.16 MHz): δ 1.66 (s, 6H, Adamantane-H), 1.85 (s, 6H, Adamantane-H), 2.07 (s, 3H, Adamantane-H), 5.87 (s, 1H, Thiazole-H), 6.89–6.93 (m, 2H, Ar–H), 6.96–6.99 (m, 2H, Ar–H), 7.04–7.07 (m, 2H, Ar–H), 7.14–7.16 (m, 2H, Ar–H). 13C NMR (125.77 MHz): δ 29.84, 36.76, 40.88, 53.99 (Adamantane-C), 98.20 (Thiazole C-5), 115.19, 128.62, 128.80, 129.11, 130.74, 134.09, 137.87, 161.95 (Ar–C), 149.76 (Thiazole C-4), 159.99 (Thiazole C-2).
(Z)-N-(Adamantan-1-yl)-4-(4-bromophenyl)-3-(4-fluorophenyl)thiazol-2(3H)-imine 5i. Yield: 84%, m.p.: 221–223 °C (EtOH/CHCl3), Mol. Formula (Mol. Wt.): C25H24BrFN2S (483.44). 1H NMR (400.20 MHz): δ 1.66 (s, 6H, Adamantane-H), 1.85 (s, 6H, Adamantane-H), 2.07 (s, 3H, Adamantane-H), 5.88 (s, 1H, Thiazole-H), 6.89–6.95 (m, 4H, Ar–H), 7.04–7.08 (m, 2H, Ar–H), 7.29 (d, 2H, Ar–H, J = 8.4 Hz). 13C NMR (100.63 MHz): δ 29.85, 36.81, 40.93, 53.80 (Adamantane-C), 97.90 (Thiazole C-5), 115.03, 122.14, 129.46, 130.71, 131.44, 135.04, 137.73, 162.04 (Ar–C), 148.72 (Thiazole C-4), 159.60 (Thiazole C-2).
(Z)-N-(Adamantan-1-yl)-3-(4-fluorophenyl)-4-(p-tolyl)thiazol-2(3H)-imine 5j. Yield: 64%, m.p.: 144–146 °C (EtOH), Mol. Formula (Mol. Wt.): C26H27FN2S (418.57). 1H NMR (400.20 MHz): δ 1.69 (s, 6H, Adamantane-H), 1.89 (s, 6H, Adamantane-H), 2.10 (s, 3H, Adamantane-H), 2.29 (s, 3H, CH3), 5.84 (s, 1H, Thiazole-H), 6.90–7.01 (m, 6H, Ar–H), 7.09–7.12 (m, 2H, Ar–H). 13C NMR (100.63 MHz): δ 21.23 (CH3), 29.82, 36.76, 40.85, 53.78 (Adamantane-C), 96.68 (Thiazole C-5), 114.84, 127.95, 128.94, 130.77, 135.16, 137.94, 138.95, 161.96 (Ar–C), 149.56 (Thiazole C-4), 159.52 (Thiazole C-2).
(Z)-N-(Adamantan-1-yl)-3-(4-fluorophenyl)-4-(4-methoxyphenyl)thiazol-2(3H)-imine 5k. Yield: 94%, m.p.: 179–181 °C (EtOH/CHCl3), Mol. Formula (Mol. Wt.): C26H27FN2OS (434.57). 1H NMR (400.20 MHz): δ 1.68 (s, 6H, Adamantane-H), 1.87 (s, 6H, Adamantane-H), 2.08 (s, 3H, Adamantane-H), 3.74 (s, 3H, OCH3), 5.78 (s, 1H, Thiazole-H), 6.69 (d, 2H, Ar–H, J = 8.8 Hz), 6.88–6.98 (m, 4H, Ar–H), 7.06–7.10 (m, 2H, Ar–H). 13C NMR (100.63 MHz): δ 29.86, 36.80, 40.90, 53.80 (Adamantane-C, 55.24 (OCH3), 96.11 (Thiazole C-5), 113.67, 114.88, 124.85, 129.44, 130.85, 135.15, 138.68, 161.99 (Ar–C), 149.76 (Thiazole C-4), 159.55 (Thiazole C-2).
(Z)-N-(Adamantan-1-yl)-3-(4-fluorophenyl)-4-(naphthalen-2-yl)thiazol-2(3H)-imine 5l. Yield: 60%, m.p.: 155–157 °C (EtOH/CHCl3), Mol. Formula (Mol. Wt.): C29H27FN2S (454.61). 1H NMR (500.16 MHz): δ 1.68–1.69 (m, 6H, Adamantane-H), 1.89–1.00 (m, 6H, Adamantane-H), 2.09 (s, 3H, Adamantane-H), 5.98 (s, 1H, Thiazole-H), 6.85–6.89 (m, 2H, Ar–H), 7.01–7.02 (m, 1H, Ar–H), 7.11–7.15 (m, 2H, Ar–H), 7.43–7.46 (m, 2H, Ar–H), 7.57 (d, 1H, Ar–H, J = 8.5hz), 7.68 (s, 1H, Ar–H), 7.71–7.75 (m, 2H, Ar–H). 13C NMR (125.77 MHz): δ 29.86, 36.78, 40.85, 54.04 (Adamantane-C), 98.18 (Thiazole C-5), 115.10, 125.58, 126.58, 127.37, 127.74, 128.14, 129.79, 130.77, 130.84, 132.77, 133.06, 134.91, 139.13, 161.88 (Ar–C), 150.28 (Thiazole C-4), 159.93 (Thiazole C-2).
(Z)-N-(Adamantan-1-yl)-3-(4-chlorophenyl)-4-phenylthiazol-2(3H)-imine 5m. Yield: 76%, m.p.: 190–192 °C (EtOH/CHCl3), Mol. Formula (Mol. Wt.): C25H25ClN2S (421.0). 1H NMR (500.16 MHz): δ 1.66–1.68 (m, 6H, Adamantane-H), 1.86–1.87 (m, 6H, Adamantane-H), 2.08 (s, 3H, Adamantane-H), 5.87 (s, 1H, Thiazole-H), 7.04–7.07 (m, 4H, Ar–H), 7.15–7.20 (m, 5H, Ar–H). 13C NMR (125.77 MHz): δ 30.12, 37.08, 41.17, 54.08 (Adamantane-C), 97.97 (Thiazole C-5), 128.25, 128.34, 128.44, 128.64, 130.67, 132.74, 138.09, 138.87 (Ar–C), 149.10 (Thiazole C-4), 159.09 (Thiazole C-2).
(Z)-N-(Adamantan-1-yl)-3,4-bis(4-chlorophenyl)thiazol-2(3H)-imine 5n. Yield: 77%, m.p.: 197–199 °C (EtOH/CHCl3), Mol. Formula (Mol. Wt.): C25H24Cl2N2S (455.44). 1H NMR (400.20 MHz): δ 1.66 (s, 6H, Adamantane-H), 1.85 (s, 6H, Adamantane-H), 2.06 (s, 3H, Adamantane-H), 5.88 (s, 1H, Thiazole-H), 6.97 (d, 2H, Ar–H, J = 8.4 Hz), 7.03 (d, 2H, Ar–H, J = 8.8 Hz), 7.15–7.20 (m, 4H, Ar–H). 13C NMR (100.63 MHz): δ 29.81, 36.74, 40.86, 53.99 (Adamantane-C), 98.48 (Thiazole C-5), 128.45, 128.68, 129.18, 130.34, 134.08, 137.54 (Ar–C), 148.55 (Thiazole C-4), 159.02 (Thiazole C-2).
(Z)-N-(Adamantan-1-yl)-4-(4-bromophenyl)-3-(4-chlorophenyl)thiazol-2(3H)-imine 5o. Yield: 82%, m.p.: 210–212 °C (EtOH/CHCl3), Mol. Formula (Mol. Wt.): C25H24BrClN2S (499.90). 1H NMR (400.20 MHz): δ 1.66 (s, 6H, Adamantane-H), 1.84 (s, 6H, Adamantane-H), 2.07 (s, 3H, Adamantane-H), 5.89 (s, 1H, Thiazole-H), 6.91 (d, 2H, Ar–H, J = 8.0 Hz), 7.03 (d, 2H, Ar–H, J = 8.4 Hz), 7.18 (d, 2H, Ar–H, J = 8.4 Hz), 7.30 (d, 2H, Ar–H, J = 8.0 Hz). 13C NMR (100.63 MHz): δ 29.81, 36.75, 40.88, 53.93 (Adamantane-C), 98.46 (Thiazole C-5), 122.24, 128.42, 129.40, 130.33, 131.61, 137.53 (Ar–C), 148.47 (Thiazole C-4), 159.07 (Thiazole C-2).
(Z)-N-(Adamantan-1-yl)-3-(4-chlorophenyl)-4-(p-tolyl)thiazol-2(3H)-imine 5p. Yield: 90%, m.p.: 188–190 °C (EtOH), Mol. Formula (Mol. Wt.): C26H27ClN2S (435.03). 1H NMR (500.16 MHz): δ 1.65–1.67 (m, 6H, Adamantane-H), 1.85–1.86 (m, 6H, Adamantane-H), 2.07 (s, 3H, Adamantane-H), 2.28 (s, 3H, CH3), 5.82 (s, 1H, Thiazole-H), 6.92 (d, 2H, Ar–H, J = 8.0 Hz), 6.98 (d, 2H, Ar–H, J = 8.0 Hz), 7.04–7.06 (m, 2H, Ar–H), 7.15–7.17 (m, 2H, Ar–H). 13C NMR (125.77 MHz): δ 21.33 (CH3), 29.87, 36.83, 40.91, 53.80 (Adamantane-C), 97.03 (Thiazole C-5), 127.90, 128.18, 129.09, 129.50, 130.47, 131.25, 138.02, 138.69 (Ar–C), 149.0 (Thiazole C-4), 158.89 (Thiazole C-2).
(Z)-N-(Adamantan-1-yl)-3-(4-chlorophenyl)-4-(4-methoxyphenyl)thiazol-2(3H)-imine 5q. Yield: 82%, m.p.: 160–162 °C (EtOH/CHCl3), Mol. Formula (Mol. Wt.): C26H27ClN2OS (451.03). 1H NMR (500.16 MHz): δ 1.65–1.67 (m, 6H, Adamantane-H), 1.85–1.86 (m, 6H, Adamantane-H), 2.07 (s, 3H, Adamantane-H), 3.75 (s, 3H, OCH3), 5.78 (s, 1H, Thiazole-H), 6.70 (d, 2H, Ar–H, J = 9.0 Hz), 6.95 (d, 2H, Ar–H, J = 9.0 Hz), 7.04 (d, 2H, Ar–H, J = 9.0 Hz), 7.16 (d, 2H, Ar–H, J = 8.5hz). 13C NMR (125.77 MHz): δ 29.85, 36.80, 40.86, 53.84 (Adamantane-C), 55.30 (OCH3), 96.47 (Thiazole C-5), 113.76, 124.84, 128.23, 129.39, 130.53, 131.71, 137.78, 138.44 (Ar–C), 149.43 (Thiazole C-4), 159.36 (Thiazole C-2).
(Z)-N-(Adamantan-1-yl)-3-(4-chlorophenyl)-4-(naphthalen-2-yl)thiazol-2(3H)-imine 5r. Yield: 89%, m.p.: 177–179 °C (EtOH/CHCl3), Mol. Formula (Mol. Wt.): C29H27ClN2S (471.06). 1H NMR (500.16 MHz): δ 1.68 (s, 6H, Adamantane-H), 1.88–1.89 (m, 6H, Adamantane-H), 2.09 (s, 3H, Adamantane-H), 5.99 (s, 1H, Thiazole-H), 6.99–7.02 (m, 1H, Ar–H), 7.10–7.15 (m, 4H, Ar–H), 7.44–7.48 (m, 2H, Ar–H), 7.58 (d, 1H, Ar–H, J = 9.0 Hz), 7.69 (s, 1H, Ar–H), 7.71–7.75 (m, 2H, Ar–H). 13C NMR (125.77 MHz): δ 29.87, 36.83, 40.93, 53.90 (Adamantane-C), 98.34 (Thiazole C-5), 125.49, 126.59, 127.11, 127.76, 127.89, 128.13, 128.28, 130.05, 130.37, 131.72, 132.77, 133.12, 137.79, 138.71 (Ar–C), 148.94 (Thiazole C-4), 158.96 (Thiazole C-2).
Preparation of the single crystal of compounds 5d and 5f
Analytically-pure samples of compounds 5d and 5f. (50 mg) were dissolved in 15mL cyclohexane/CHCl3 and EtOH/CHCl3 (1:1, v/v), respectively. Slow evaporation of the solutions at room temperature yielded the single crystals as colorless prisms.
Molecular Modelling and molecular docking analysis
The structures of 5e and 5k were drawn using ChemSketch Freeware (www.acdlabs.com) and these structures were subsequently optimized with the B3LYP/6-31G level of theory using the Gaussian 09 program65. The optimized structures were used for molecular docking simulations. The crystal structure of SIRT1 catalytic domain bound to NAD and an EX527 analog was retrieved from the protein data bank (PDB ID: 4I5I). The molecular docking simulation against SIRT1 was performed using the AutoDock Vina program66 available in PyRX-0.8 virtual tools67. The chain A of 4I5I and the ligands 5e and 5k were prepared and used for docking calculation. The active site grid was constructed based on the position of NAD ligand. To check the predictive power of the AutoDock Vina program, we also performed docking for the cocrystallized ligand NAD. The experimental and predicted poses were very similar. The top ranked binding poses were considered for protein–ligand interaction analysis using PLIP web server68.
Supplementary Information
Author contributions
A.A.El-E. and M.B.El-A. designed the study and supervised the chemical synthesis. E.T.W. synthesized characterized the compounds. M.S.M.A. and S.G.-G. performed the X-ray data collection and the resolution of the crystal structure. E.E.H. conceived the antimicrobial activity studies. S.T. conducted the molecular docking analysis. All authors contributed in the preparation of the manuscript, discussed the contents and approved the submission.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
The crystallographic data for the structures of compounds 5d (CCDC #: 2,155,510) and 5f. (CCDC #: 2,155,511) could be obtained free of charge from the Cambridge Crystallographic Data Centre (www.ccdc.cam.ac.uk/data_request/cif).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-25390-0.
References
- 1.Spilovska K, Zemek F, Korabecny J, Nepovimova E, Soukup O, Windisch M, Kuca K. Adamantane-a lead structure for drugs in clinical practice. Curr. Med. Chem. 2016;23:3245–3266. doi: 10.2174/0929867323666160525114026. [DOI] [PubMed] [Google Scholar]
- 2.Wanka L, Iqbal K, Schreiner PR. The lipophilic bullet hits the targets: Medicinal chemistry of adamantane derivatives. Chem. Rev. 2013;113:3516–3604. doi: 10.1021/cr100264t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Obando D, Liao V, Lifa T, Codd R. The many faces of the adamantyl group in drug design. Eur. J. Med. Chem. 2011;46:1949–1963. doi: 10.1016/j.ejmech.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 4.Lamoureux G, Artavia G. Use of the adamantane structure in medicinal chemistry. Curr. Med. Chem. 2010;17:2967–2978. doi: 10.2174/092986710792065027. [DOI] [PubMed] [Google Scholar]
- 5.Davies WL, Grunnert RR, Haff RF, McGahen JW, Neumeyer EM, Paulshock M, Watts JC, Wood TR, Hermann EC, Hoffmann CE. Antiviral activity of 1-adamantamine (amantadine) Science. 1964;144:862–863. doi: 10.1126/science.144.3620.862. [DOI] [PubMed] [Google Scholar]
- 6.Wendel HA, Snyder MT, Pell S. Trial of amantadine in epidemic influenza. Clin. Pharmacol. Therap. 1966;7:38–43. doi: 10.1002/cpt19667138. [DOI] [PubMed] [Google Scholar]
- 7.Wingfield WL, Pollack D, Grunert RR. Treatment of influenza. The therapeutic efficacy of rimantadine HCl in a naturally occurring influenza A2 respiratory illness in man. N. Engl. J. Med. 1969;281:579–584. doi: 10.1056/NEJM196909112811102. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal KS, Sokol MS, Ingram RL, Subramanian R, Fort RC. Tromantadine: Inhibitor of early and late events in herpes simplex virus replication. Antimicrob. Agents Chemother. 1982;22:1031–1036. doi: 10.1128/AAC.22.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long J, Manchandia T, Ban K, Gao S, Miller C, Chandra J. Adaphostin cytoxicity in glioblastoma cells is ROS-dependent and is accompanied by upregulation of heme oxygenase-1. Cancer Chemother. Pharmacol. 2007;59:527–735. doi: 10.1007/s00280-006-0295-5. [DOI] [PubMed] [Google Scholar]
- 10.Lorenzo P, Alvarez R, Ortiz MA, Alvarez S, Piedrafita FJ, de Lera AR. Inhibition of IκB kinase-β and anticancer activities of novel chalcone adamantyl arotinoids. J. Med. Chem. 2008;51:5431–5440. doi: 10.1021/jm800285f. [DOI] [PubMed] [Google Scholar]
- 11.Han T, Goralski M, Capota E, Padrick SB, Kim J, Xie Y, Nijhawan D. The antitumor toxin CD437 is a direct inhibitor of DNA polymerase α. Nat. Chem. Biol. 2016;12:511–515. doi: 10.1038/nchembio.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai L, Smith CD, Foroozesh M, Miele L, Qin Z. The sphingosine kinase 2 inhibitor ABC294640 displays anti-non-small cell lung cancer activities in vitro and in vivo. Int. J. Cancer. 2018;142:2153–2162. doi: 10.1002/ijc.31234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurd R, Ben-Chetrit E, Karameh H, Bar-Meir M. Compassionate use of opaganib for patients with severe COVID-19. J. Emerg. Dis. Virol. 2020;5:3. doi: 10.16966/2473-1846.159. [DOI] [Google Scholar]
- 14.Protopopova M, Hanrahan C, Nikonenko B, Samala R, Chen P, Gearhart J, Einck L, Nacy CA. Identification of a new antitubercular drug candidate, SQ109, from a combinatorial library of 1,2-ethylenediamines. J. Antimicrob. Chemother. 2005;56:968–974. doi: 10.1093/jac/dki319. [DOI] [PubMed] [Google Scholar]
- 15.Bogatcheva E, Hanrahan C, Chen P, Gearhart J, Sacksteder K, Einck L, Nacy C, Protopopova M. Discovery of dipiperidines as new antitubercular agents. Bioorg. Med. Chem. Lett. 2010;20:201–205. doi: 10.1016/j.bmcl.2009.10.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leoni A, Locatelli A, Morigi R, Rambaldi M. Novel thiazole derivatives: A patent review (2008–2012. Part 2) Expert Opin. Ther. Pat. 2022;24:759–777. doi: 10.1517/13543776.2014.910196. [DOI] [PubMed] [Google Scholar]
- 17.Ayati A, Emami S, Asadipour A, Shafiee A, Foroumadi A. Recent applications of 1,3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem. 2015;97:699–718. doi: 10.1016/j.ejmech.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Petrou A, Fesatidou M, Geronikaki A. Thiazole ring-a biologically active scaffold. Molecules. 2021;26:3166. doi: 10.3390/molecules26113166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Siqueira LRP, de Moraes GPAT, de Lima FLP, de Melo RMJB, Leite ACL. Multi-target compounds acting in cancer progression: Focus on thiosemicarbazone, thiazole and thiazolidinone analogues. Eur. J. Med. Chem. 2019;170:237–260. doi: 10.1016/j.ejmech.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Sharma PC, Bansal KK, Sharma A, Sharma D, Deep A. Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur. J. Med. Chem. 2020;188:112016. doi: 10.1016/j.ejmech.2019.112016. [DOI] [PubMed] [Google Scholar]
- 21.de Sá NP, Lino CI, Fonseca NC, Borelli BM, Ramos JP, Souza-Fagundes EM, Rosa CA, Santos DA, de Oliveira RB, Johann S. Thiazole compounds with activity against Cryptococcus gattii and Cryptococcus neoformans in vitro. Eur. J. Med. Chem. 2015;102:233–242. doi: 10.1016/j.ejmech.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 22.Maillard LT, Bertout S, Quinonéro O, Akalin G, Turan-Zitouni G, Fulcrand P, Demirci F, Martinez J, Masurier N. Synthesis and anti-Candida activity of novel 2-hydrazino-1,3-thiazole derivatives. Bioorg. Med. Chem. Lett. 2012;23:1803–1807. doi: 10.1016/j.bmcl.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 23.Borelli C, Schaller M, Niewerth M, Nocker K, Baasner B, Berg D, Tiemann R, Tietjen K, Fugmann B, Lang-Fugmann S, Korting HC. Modes of action of the new arylguanidine abafungin beyond interference with ergosterol biosynthesis and in vitro activity against medically important fungi. Chemotherapy. 2008;54:245–259. doi: 10.1159/000142334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy GM, Garcia JR, Reddy VH, de Andrade AM, Camilo A, Jr, Ribeiro RAP, de Lazaro SR. Synthesis, antimicrobial activity and advances in structure-activity relationships (SARs) of novel tri-substituted thiazole derivatives. Eur. J. Med. Chem. 2016;123:508–513. doi: 10.1016/j.ejmech.2016.07.062. [DOI] [PubMed] [Google Scholar]
- 25.Mohammad M, Cushman M, Seleem MN. Antibacterial evaluation of synthetic thiazole compounds in vitro and in vivo in a methicillin-resistant staphylococcus aureus (MRSA) skin infection mouse model. PLoS ONE. 2015;10:0142321. doi: 10.1371/journal.pone.0142321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh IP, Gupta S, Kumar S. Thiazole compounds as antiviral agents: An update. Med. Chem. 2020;16:4–23. doi: 10.2174/1573406415666190614101253. [DOI] [PubMed] [Google Scholar]
- 27.Brito CCB, da Silva HVC, Brondani DJ, de Faria AR, Ximenes RM, da Silva IM, de Albuquerque JFC, Castilho MS. Synthesis and biological evaluation of thiazole derivatives as LbSOD inhibitors. J. Enzyme Inhib. Med. Chem. 2019;34:333–342. doi: 10.1080/14756366.2018.1550752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Salvador LA, Byeon S, Ying Y, Kwan JC, Law BK, Hong J, Luesch H. Anticolon cancer activity of largazole, a marine-derived tunable histone deacetylase inhibitor. J. Pharmacol. Exp. Ther. 2010;335:351–361. doi: 10.1124/jpet.110.172387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Awadhi FH, Salvador-Reyes LA, Elsadek LA, Ratnayake R, Chen QY, Luesch H. Largazole is a brain-penetrant class I HDAC inhibitor with extended applicability to glioblastoma and CNS diseases. ACS Chem. Neurosci. 2020;11:1937–1943. doi: 10.1021/acschemneuro.0c00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brattås MK, Reikvam H, Tvedt THA, Bruserud Ø. Dasatinib as an investigational drug for the treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia in adults. Expert Opin. Investig. Drugs. 2019;28:411–420. doi: 10.1080/13543784.2019.1597052. [DOI] [PubMed] [Google Scholar]
- 31.Trinh VA, Davis JE, Anderson JE, Kim KB. Dabrafenib therapy for advanced melanoma. Ann. Pharmacother. 2014;48:519–529. doi: 10.1177/1060028013513009. [DOI] [PubMed] [Google Scholar]
- 32.Copur MS. Alpelisib to treat breast cancer. Drugs Today. 2020;56:357–363. doi: 10.1358/dot.2020.56.6.3137526. [DOI] [PubMed] [Google Scholar]
- 33.Rashid MA, Gustafson KR, Cardellina JH, Boyd MRPF. A new cytotoxic cyclic peptide from the colonial ascidian Lissoclinum patella. J. Nat. Prod. 1995;58:594–597. doi: 10.1021/np50118a020. [DOI] [PubMed] [Google Scholar]
- 34.Ibrahim NK. Ixabepilone: Overview of effectiveness, safety, and tolerability in metastatic breast cancer. Front. Oncol. 2021;11:617874. doi: 10.3389/fonc.2021.617874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pronzato P. New therapeutic options for chemotherapy-resistant metastatic breast cancer: The epothilones. Drugs. 2008;68:139–146. doi: 10.2165/00003495-200868020-00001. [DOI] [PubMed] [Google Scholar]
- 36.El-Emam AA, Al-Deeb OA, Al-Omar M, Lehmann J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorg. Med. Chem. 2004;12:5107–5113. doi: 10.1016/j.bmc.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 37.El-Emam AA, Al-Tamimi AMS, Al-Omar MA, Alrashood KA, Habib EE. Synthesis and antimicrobial activity of novel 5-(1-adamantyl)-2-aminomethyl-4-substituted-1,2,4-triazoline-3-thiones. Eur. J. Med. Chem. 2013;68:96–102. doi: 10.1016/j.ejmech.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 38.Hassan HM, Al-Wahaibi LH, Shehatou GS, El-Emam AA. Adamantane-linked isothiourea derivatives suppress the growth of experimental hepatocellular carcinoma via inhibition of TLR4-MyD88-NF-κB signaling. Am. J. Cancer Res. 2021;11:350–369. [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Wahaibi LH, Ghabbour HA, Mostafa GAE, Almutairi MS, El-Emam AA. Crystal structure of 1-(adamantan-1-yl)-3-phenylthiourea, C17H22N2S. Z. Krist. New Crystal Struct. 2016;231:593–595. doi: 10.1515/ncrs-2015-0205. [DOI] [Google Scholar]
- 40.Demirtaş G, Dege N, Al-Shehri MM, El-Emam AA, El-Brollosy NR, Büyükgüngör O. 1-(Adamantan-1-yl)-3-(4-fluorpphenyl)thiourea. Acta Crystallogr. 2012;E68:o1523. doi: 10.1107/S1600536812017515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Omary FAM, Ghabbour HA, AlRabiah H, Al-Abdullah ES, El-Emam AA. Crystal structure of 1-(adamantan-1-yl)-3-(4-chlorophenyl)thiourea, C17H21ClN2S. Z. Krist. New Crystal Struct. 2017;232:707–709. doi: 10.1515/ncrs-2015-0241. [DOI] [Google Scholar]
- 42.Murray, P. R., Baron, E. J., Pfaller, M. A., Tenover, F. C. & Yolken, R. H. in: Wood, G. L. & Washington, J. A. (Eds.). Manual of Clinical Microbiology, Am. Soc. Microbiol., Washington D. C., 1995.
- 43.Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 44.Andrews JM. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001;48:5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 45.Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing: Approved standards M100-S27, Wayne, PA, 2018.
- 46.French GL. Bactericidal agents in the treatment of MRSA infections-the potential role of daptomycin. J. Antimicrob. Chemother. 2006;58:1107–1117. doi: 10.1093/jac/dkl393. [DOI] [PubMed] [Google Scholar]
- 47.Song YJ, Yu HH, Kim YJ, Lee NK, Paik HD. Anti-biofilm activity of grapefruit seed extract against Staphylococcus aureus and E. coli. J. Microbiol. Biotechnol. 2019;29:1177–1183. doi: 10.1041/jmb.1905.05022. [DOI] [PubMed] [Google Scholar]
- 48.Vestby LK, Grønseth T, Simm R, Nesse LL. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics. 2020;9:59. doi: 10.3390/antibiotics9020059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 50.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 51.Lagunin A, Stepanchikova A, Filimonov D, Poroikov V. PASS: Prediction of activity spectra for biologically active substances. Bioinformatics. 2000;16:747–748. doi: 10.1093/bioinformatics/16.8.747. [DOI] [PubMed] [Google Scholar]
- 52.Gopalan B, Ponpandian T, Kachhadia V, Bharathimohan K, Vignesh R, Sivasudar V, Narayanan S, Mandar B, Praveen R, Saranya N, Rajagopal S, Rajagopal S. Discovery of adamantane based highly potent HDAC inhibitors. Bioorg. Med. Chem. Lett. 2013;23:2532–2637. doi: 10.1016/j.bmcl.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Nepali K, Hsu TI, Hsieh CM, Lo WL, Lai MJ, Hsu KC, Lin TE, Chuang JY, Liou JP. Pragmatic recruitment of memantine as the capping group for the design of HDAC inhibitors: A preliminary attempt to unravel the enigma of glioblastoma. Eur. J. Med. Chem. 2021;217:113338. doi: 10.1016/j.ejmech.2021.113338. [DOI] [PubMed] [Google Scholar]
- 54.Cincinelli R, Musso L, Giannini G, Zuco V, De Cesare M, Zunino F, Dallavalle S. Influence of the adamantyl moiety on the activity of biphenylacrylohydroxamic acid-based HDAC inhibitors. Eur. J. Med. Chem. 2014;79:251–259. doi: 10.1016/j.ejmech.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 55.Zhang SW, Gong CJ, Su MB, Chen F, He T, Zhang YM, Shen QQ, Su Y, Ding J, Li J, Chen Y, Nan FJ. Synthesis and in vitro and in vivo biological evaluation of tissue-specific bisthiazole histone deacetylase (HDAC) inhibitors. J. Med. Chem. 2020;63:804–815. doi: 10.1021/acs.jmedchem.9b01792. [DOI] [PubMed] [Google Scholar]
- 56.Tilekar K, Hess JD, Upadhyay N, Bianco AL, Schweipert M, Laghezza A, Loiodice F, Meyer-Almes FJ, Aguilera RJ, Lavecchia A, Ramaa CS. Thiazolidinedione, "magic bullets" simultaneously targeting PPARγ and HDACs: Design, synthesis, and investigations of their in vitro and in vivo antitumor effects. J. Med. Chem. 2021;64:6949–6971. doi: 10.1021/acs.jmedchem.1c00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Upadhyay N, Tilekar K, Jänsch N, Schweipert M, Hess JD, Macias HL, Mrowka P, Aguilera RJ, Choe JY, Meyer-Almes FJ, Ramaa CS. Discovery of novel N-substituted thiazolidinediones (TZDs) as HDAC8 inhibitors: In-silico studies, synthesis, and biological evaluation. Bioorg. Chem. 2020;100:103934. doi: 10.1016/j.bioorg.2020.103934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Secci D, Carradori S, Bizzarri B, Bolasco A, Ballario P, Patramani Z, Fragapane P, Vernarecci S, Canzonetta C, Filetici P. Synthesis of a novel series of thiazole-based histone acetyltransferase inhibitors. Bioorg. Med. Chem. 2014;22:1680–1689. doi: 10.1016/j.bmc.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 59.Yang F, Peng S, Li Y, Su L, Peng Y, Wu J, Chen H, Liu M, Yi Z, Chen Y. A hybrid of thiazolidinone with the hydroxamate scaffold for developing novel histone deacetylase inhibitors with antitumor activities. Org. Biomol. Chem. 2016;14:1727–1735. doi: 10.1039/c5ob02250a. [DOI] [PubMed] [Google Scholar]
- 60.Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat. Rev. Drug Discov. 2014;13:673–791. doi: 10.1038/nrd4360. [DOI] [PubMed] [Google Scholar]
- 61.Zhang XH, Qin-Ma WHP, Khamis MY, Li YH, Ma LY, Liu HM. A review of progress in histone deacetylase 6 inhibitors research: Structural specificity and functional diversity. J. Med. Chem. 2021;64:1362–1391. doi: 10.1021/acs.jmedchem.0c01782. [DOI] [PubMed] [Google Scholar]
- 62.Ho TCS, Chan AHY, Ganesan A. Thirty years of HDAC inhibitors: 2020 insight and hindsight. J. Med. Chem. 2020;63:12460–12484. doi: 10.1021/acs.jmedchem.0c00830. [DOI] [PubMed] [Google Scholar]
- 63.Gertz M, Fischer F, Nguyen GTT, Lakshminarasimhan M, Schutkowski M, Weyand M, Steegborn C. Ex-527 inhibits Sirtuins by exploiting their unique NAD+-dependent deacetylation mechanism. Proc. Natl. Acad. Sci. USA. 2013;110:E2772–E2781. doi: 10.1073/pnas.1303628110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao X, Allison D, Condon B, Zhang F, Gheyi T, Zhang A, Ashok S, Russell M, MacEwan I, Qian Y, Jamison JA, Luz JG. The 2.5 Å crystal structure of the SIRT1 catalytic domain bound to nicotinamide adenine dinucleotide (NAD+) and an indole (EX527 analogue) reveals a novel mechanism of histone deacetylase inhibition. J. Med. Chem. 2013;56:963–969. doi: 10.1021/jm301431y. [DOI] [PubMed] [Google Scholar]
- 65.Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X., Hratchian, H. P., Izmaylov, A. F., Bloino, J., Zheng, G., Sonnenberg, J. L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J. A. Jr., Peralta, J. E., Ogliaro, F., Bearpark, M. J., Heyd, J., Brothers, E. N., Kudin, K. N., Staroverov, V. N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A. P., Burant, J. C., Iyengar, S. S., Tomasi, J., Cossi, M., Rega, N., Millam, N. J., Klene, M., Knox, J. E., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Martin, R. L., Morokuma, K., Zakrzewski, V. G., Voth, G. A., Salvador, P., Dannenberg, J. J., Dapprich, S., Daniels, A. D., Farkas, Ö., Foresman, J. B., Ortiz, J. V., Cioslowski, J., Fox, D. J. Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, USA, 2013.
- 66.Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dallakyan S, Olson AJ. Small-molecule library screening by docking with PyRx. Methods Mol. Biol. 2015;1263:243–250. doi: 10.1007/978-1-4939-2269-7_19. [DOI] [PubMed] [Google Scholar]
- 68.Adasme MF, Linnemann KL, Bolz SN, Kaiser F, Salentin S, Haupt VJ, Schroeder M. PLIP 2021: Expanding the scope of the protein-ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021;49:W530–W534. doi: 10.1093/nar/gkab294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The crystallographic data for the structures of compounds 5d (CCDC #: 2,155,510) and 5f. (CCDC #: 2,155,511) could be obtained free of charge from the Cambridge Crystallographic Data Centre (www.ccdc.cam.ac.uk/data_request/cif).