Abstract
Background:
The United States Centers for Disease Control and Prevention recommended a third dose or booster of the Pfizer-BioNTech Comirnaty (BNT162b2) COVID-19 mRNA vaccine in September 2021 for high-risk individuals. Pregnant and high-risk lactating women were encouraged to receive the booster to obtain potential prolonged protection for themselves and their infants.
Research Aim:
To investigate the ability of the booster vaccine to increase IgA and IgG antibodies specific to the receptor-binding domain of the SARS-CoV-2 spike protein in human milk compared to levels pre-booster.
Methods:
This was a prospective one-group study with a pretest-posttest design. Six of 12 participants were recruited prospectively. Participants were instructed to collect ≥ 2 ounces of milk in the morning at 30 days and 1-day pre-booster, and 7, 14, 21, 30, 45, and 60 days post-booster. Levels of IgA and IgG antibodies specific to the receptor-binding domain of the SARS-CoV-2 spike protein were quantified in human milk via an ELISA assay.
Results:
We found a significant increase in anti-receptor-binding domain-specific IgA and IgG antibodies in human milk 1–2 weeks after the Pfizer-BioNTech booster and at the study endpoint (45- and 60-days post-booster)
Conclusions:
This suggests that the booster vaccination enhances SARS-CoV-2 specific immunity in human milk, which may be protective for infants.
Keywords: booster, breastfeeding, Covid-19, IgA and IgG Antibodies in Human Milk, immunology, lactation, passive immunity, SARS-CoV-2, United States, vaccination
Key Messages.
To date, researchers have not investigated COVID-19 immunity in human milk following the third dose (i.e., booster) of the Pfizer-BioNTech Comirnaty vaccine.
In participants (N = 12), both IgA and IgG levels specific to SARS-COV-2 RBD in human milk were significantly higher 7 days post-booster versus pre-booster.
Antibodies to SARS-COV-2 RBD were detectable in blood ≥ 60 days post-Pfizer-BioNTech booster.
This suggests that the booster vaccination enhances SARS-CoV-2 specific immunity in human milk, which may be protective for infants.
Background
As of August 2022, over 92 million cases of coronavirus disease of 2019 (COVID-19) were confirmed in the United States, and over 1 million deaths (United States Centers for Disease Prevention and Control [CDC], 2020). The American Academy of Pediatrics (2022) reported that children represented 19% of the cumulative COVID-19 cases and 3.2% of the total hospitalizations for COVID-19 in the United States. While children aged 6 months and older were eligible as of late June 2022 for the Pfizer-BioNTech Comirnaty vaccination, those under 6 months of age remain ineligible and at risk for infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19. Vaccination of pregnant or lactating mothers can protect infants from diseases e.g., pertussis and the flu (De Schutter et al., 2015; Zaman et al., 2008). Previously, researchers have reported elevated levels of SARS-CoV-2-specific IgA and IgG in milk from lactating women following the first and second doses of the Pfizer-BioNTech vaccine (Baird et al., 2021; Gray et al., 2021; Low et al., 2021; Perl et al., 2021; Valcarce et al., 2021). Therefore, in the absence of FDA-approved COVID-19 vaccinations for infants under 6 months of age, maternal vaccination against COVID-19 may offer passive immunity to infants, similarly to other vaccines typically administered to pregnant or lactating women (e.g., Tdap and Influenza). However, to our knowledge, no one has assessed the antibody response in human milk > 2 months beyond the initial two-dose vaccination series for COVID-19.
In September 2021, the CDC (2021a) recommended a third dose or booster of the Pfizer-BioNTech Comirnaty (BNT162b2) COVID-19 mRNA vaccine for people aged 65 and older, adults with underlying health conditions, and frontline workers. Pregnant and lactating women in these groups were advised to consider the booster for possible prolonged protection for themselves and their infants. We sought to address whether the booster changed the levels of anti-SARS-CoV-2 antibodies in human milk. We investigated the ability of the booster vaccine to increase IgA and IgG antibodies specific to the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein in human milk compared to levels pre-booster. We hypothesized that the booster would increase SARS-CoV-2 RBD-specific immunoglobulins in human milk.
Methods
Research Design
This was a prospective one-group study with a pretest-posttest design. The study compared the levels of human milk antibodies after the third dose of the Pfizer-BioNTech (BNT162b2) vaccine to the levels present ≥ 6 months following the second dose of the Pfizer-BioNTech (BNT162b2) vaccine. This study design allowed us to observe the effect of the booster vaccine on antibodies in human milk, which was the outcome of interest. The study was approved by the college’s institutional review board (IRB# 1817816-1) in November 2021.
Setting and Relevant Context
The study was conducted using human milk samples from participants across the United States. Lactating mothers in academia/education and healthcare were targeted because these groups were approved earlier than the general public for the booster and would better align with the timeline of this study. All participants were eligible to receive the booster between September and November 2021.
In the United States, the American Academy of Pediatrics (AAP) recommends that infants be fed human milk exclusively for the first 6 months of life, followed by an introduction of solid foods with the continuation of human milk (Meek & Noble, 2022). In June 2022, the AAP expanded its guidelines; they now suggest that infants continue to receive human milk up to 2 years of age, which is in accordance with guidelines from the World Health Organization (WHO, 2021). Despite these recommendations, only 25.8% of infants are exclusively breastfed through 6 months in the United States (CDC, 2021b).
There are significant challenges to breastfeeding in the United States since there is no federally-mandated paid parental leave. However, some mothers may be eligible for 12 weeks of unpaid maternity leave through the federal Family and Medical Leave Act, depending on their employer and how long they have been employed. Many mothers cannot take unpaid maternity leave due to the financial burden it places on the family (Sriraman & Kellams, 2016). Additionally, only 51% of employers in the United States report having an on-site lactation room (CDC, 2021b). The lack of these facilities at a place of employment causes further challenges for those who wish to continue feeding their infant human milk while returning to the workplace.
There are also disparities in breastfeeding rates in the United States across different races, incomes, and ages (CDC, 2021b). For example, only 19.3% of mothers under age 20 are breastfeeding when the infant is 6 months of age. In comparison, 33.8% of mothers ages 20–29 and 48.5% of mothers ≥ 30 years of age are breastfeeding when the infant is 6 months of age.
Sample
Six of the 12 participants were recruited prospectively. The additional six participants were recruited approximately 1 month into the 90-day timeline for the study but were eligible because they had dated and frozen aliquots of milk from the earlier required time points in the study. Participants’ samples were represented across the majority of time points for analysis of both RBD-specific IgA and IgG antibodies, except 30 days pre-booster (Day -30) and 90 days post-booster (Day 90) (Table 1). Those two time points were considered optional milk collection dates for the participants. Two participants could not provide milk samples from 1 day pre-booster (Day -1), so their samples from 30 days pre-booster (Day -30) were used for the analysis instead. Four participants voluntarily provided milk samples at 90 days post-booster (Day 90) to further extend the study timeline. Participants were included in the study if they could provide at least one pre-booster milk sample (Day -30 or Day -1) and a post-booster milk sample on Day 60. All twelve participants satisfied the aforementioned sample criteria.
Table 1.
Participants Represented at Each Time Point in the Study (N = 12).
| Time (Day) |
IgA Analysis n (%) |
IgG Analysis n (%) |
|---|---|---|
| -30 | 8 (67) | 8 (67) |
| -1 | 10 (83) | 10 (83) |
| 7 | 7 (58) | 9 (75) |
| 14 | 10 (83) | 10 (83) |
| 21 | 9 (75) | 9 (75) |
| 30 | 11 (92) | 11 (92) |
| 45 | 11 (92) | 11 (92) |
| 60 | 12 (100) | 12 (100) |
| 90 | 4 (25) | 4 (25) |
Note. IgA = Immunoglobulin A; IgG = Immunoglobulin G.
To be eligible for this study, participants needed to be lactating, must have received the standard two-dose Pfizer-BioNTech vaccination series at least 6-months prior, and provided written informed consent. Participants were excluded if they had a known diagnosis or suspected infection for COVID-19 and were currently pregnant. Inclusion criteria were determined via an initial demographic and health questionnaire completed online by the participants at enrollment (see online Supplementary Material). Participants were not compensated for their involvement in this study. The sample size (N = 12) was adequate to detect a difference in antibody concentration of 4 units/ml with greater than 80% power for IgA and a difference in 40 units/ml with greater than 80% power for IgG.
Measurement
Anti-RBD-specific IgA and IgG levels were assessed via enzyme-linked immunosorbent assay (ELISA; Ancell Corp.). First, 96-well plates were coated for 2 hr with 100 µl/well of 4 µg/ml purified RBD-His spike protein (Ancell Corp.). The plates were then aspirated, and 300 µl/well of blocking buffer (Tris buffered saline/glycine-01% BSA-10% glycerol 0.04% sodium azide, pH = 7.45) was added for 1 hr at room temperature. Human milk specimens were diluted 1:5, and blood serum was diluted 1:100 for IgG analysis and 1:50 for IgA analysis and incubated on the plates for 1 hr at room temperature with shaking. Plates were washed twice with 300 µl/well Tris-buffered saline (TBS)/glycine, 0.1% BSA, 0.1% pluronic acid, pH = 7.49. Monoclonal mouse anti-human IgG-HRP (ICO-97; 0.8 µg/ml) and mouse anti-human IgA-HRP (Hisa43; 2 µg/ml) antibodies (100 µl/well; Ancell Corp.) were added to capture the binding signal, and the plates were incubated at room temperature with shaking for 1 hr. Plates were washed three times with 300 µl/well TBS. TMB H2O2 substrate (Ancell Corp.) was used for detection at 450 nm on a Biotek Powerwave X plate reader. All human milk and blood samples were run in triplicate. The negative control for this assay was a human milk sample from July 2019. A positive control and standard curve were generated by serially diluting blood serum obtained from a COVID-19 positive patient in May 2020 into the 2019 negative control human milk sample in duplicate. IgA and IgG levels were converted to units/ml.
Data Collection
The study milk samples were collected in 2021–2022. Twelve participants provided a total of 87 milk samples. The negative control was human milk from July 2019. Changes in participants’ health status were monitored with a post-study survey (see supplementary material). Participants were instructed to collect ≥ 2 oz of milk in the morning at 30 days and 1 day pre-booster, and 7, 14, 21, 30, 45, and 60 days post-booster.
Samples were immediately stored at -20 °C until analyzed. Upon receipt in the lab, each sample containing 2 oz or more of human milk was thawed in a 37 °C water bath, aliquoted into a 50 ml conical tube, and centrifuged for 25 min at 872 x g (2000 rpm) and 4 °C on a Thermo Scientific Sorvall Legend XTR centrifuge. After centrifugation, a 25 ml serological pipette was used to separate the aqueous layer on the bottom of the tube from the fat layer on the top. The aqueous layer was transferred to a new 15 ml conical tube and stored at -20 °C until the ELISA assay was performed.
Participants used an alcohol swab and a finger-prick lancet device to collect up to 200 µl of blood into a Becton Dickinson (BD) microtainer in January 2022 (≥ 60 days post-booster). The blood was allowed to clot for 20 min at room temperature and then promptly stored at 4 °C. Upon receipt in the lab, the blood was centrifuged at 9391 x g (10,000 rpm) for 10 min at room temperature on an Eppendorf 5424 centrifuge. The serum layer was collected and stored at -20 °C until the ELISA assay was performed. Individual data were confidentially maintained, assigned a random participant code, and stored on a secure institutional server.
Data Analysis
To describe the characteristics of the study sample, we used mean and standard deviations for the continuous variables and frequencies and percentages for the categorical variables. Data were analyzed with GraphPad Prism 7. IgA and IgG levels were reported as units/ml. Data were expressed as median values with upper and lower limits, or mean values and 95% CIs. A Kruskal-Wallis H test with Dunn’s multiple comparisons test was used to determine differences between the mean ranks of IgA and IgG pre-booster (Day -1) to all other time points. The same tests were also used to determine differences between the mean ranks of IgA and IgG levels within each time point. The significance threshold was p < .05.
Results
Characteristics of the Participants
All participants were White, not Hispanic or Latino, or of Spanish origin. Five participants had gestational diabetes during their recent pregnancy, two participants had hypertension during their recent pregnancy, and two reported using antibiotics within the past 6 months. Participants were a mean (SD) age of 35.45 (4.17) years and infants had a mean age of 3.58 (1.8) months at the time of the booster (Table 2). The mean (SD) time between the second dose of the vaccine and the booster was 7.01 (0.62) months (Table 2). Vaccine-related adverse events were reported by 91.67% of participants after the booster, with injection site soreness the most frequent event (75%; Table 3). At ≥ 60 days post-booster, 58.33% of participants had RBD-specific IgA antibodies, and 100% had RBD-specific IgG antibodies in their blood (Table 3).
Table 2.
Participant Characteristics (N = 12).
| Characteristic | M (SD) |
|---|---|
| Maternal Characteristics | |
| Maternal Age, years | 35.45 (4.17) |
| Weight, kg | 83.50 (23.57) |
| Height, m | 1.654 (0.075) |
| Pregnancy | |
| Gravidity | 2.75 (1.42) |
| Birth week | 39.30 (0.74) |
| First vaccine during pregnancy | |
| Pregnancy week at first dose | 22.02 (7.21) |
| Pregnancy week at second dose | 25.02 (7.31) |
| Infant age (months) when participant received booster | 3.58 (1.8) |
| First vaccine after pregnancy | |
| Infant age (months) when participant received booster | 10.84 (0.64) |
| Time between 2nd vaccine dose and booster (months) | 7.01 (0.62) |
Note. Missing values: First vaccine during pregnancy = 4; first vaccine after pregnancy = 8.
Table 3.
Participants’ Reported or Measured Outcomes (N = 12).
| Outcome | n (%) |
|---|---|
| Booster vaccine adverse reactions | 11 (92) |
| Injection site soreness | 9 (75) |
| Injection site rash | 1 (8) |
| Injection site swelling | 3 (25) |
| Injection site redness | 4 (33) |
| Headache | 2 (17) |
| Muscle or body aches | 4 (33) |
| Joint pain | 3 (25) |
| Fatigue or tiredness | 4 (33) |
| Fever | 3 (25) |
| Chills | 5 (42) |
| Syncope | 1 (8) |
| IgA positive blood serum at day ≥60 | 7 (58) |
| IgG positive blood serum at day ≥60 | 12 (100) |
Note. IgA = Immunoglobulin A; IgG = Immunoglobulin G.
RBD-Specific IgG and IgA Levels
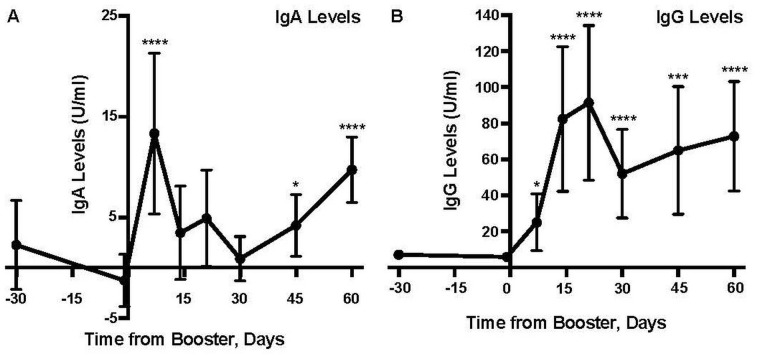
A Kruskal-Wallis H test (p < .0001) followed by a Dunn’s multiple comparisons test indicated that the median levels of RBD-specific IgG antibodies in human milk were significantly higher than the median levels of RBD-specific IgA antibodies across all time points in this study except Day 7 (Table 4). A Kruskal-Wallis H test showed that there was a statistically significant difference (p < .0001) in RBD-specific IgG antibodies between the pre-booster Day -1 and all six post-booster time points (Table 4 and Figure 1). A post hoc Dunn’s multiple comparisons test indicated that the median for the pre-booster Day -1 (Mdn = 5.548) was significantly lower (p < .05) than post-booster Day 7 (Mdn = 9.058), Day 14 (Mdn = 22.945), Day 21 (Mdn = 15.461), Day 30 (Mdn = 14.419) Day 45 (Mdn = 12.801), and day 60 (Mdn = 28.243). RBD-specific IgG antibodies in milk significantly increased by Days 7 and 14 (Table 4 and Figure 1); On Day 7 post-booster, 33.33% of participants had an increased level compared to pre-booster (Day -1). By Day 14 post-booster, 80% of participants had an increased level compared to pre-booster (Day -1). At 21, 45, and 60 days post-booster, respectively, 66.67%, 63.64%, and 75% of participants displayed significantly increased RBD-specific IgG antibodies.
Table 4.
Comparisons of the Levels of Anti-RBD-Specific IgA and IgG in Participants’ Milk Following the Vaccine Booster (N = 12).
| Time (Day) | IgA Analysis |
IgG Analysis |
Group Comparison |
||||
|---|---|---|---|---|---|---|---|
| Mdn | UL, LL | p | Mdn | UL, LL | p | p | |
| -30 | -0.954 | 36.28, -10.08 | 0.9926 | 6.281 | 12.85, 4.16 | >0.9999 | 0.0394 |
| -1 | -2.433 | 20.74, -10.32 | - | 5.548 | 9.46, 2.68 | - | 0.0004 |
| 7 | 9.069 | 55.74, -1.32 | <0.0001 | 9.058 | 143.02, 4.56 | 0.0380 | >0.9999 |
| 14 | -1.600 | 49.36, -7.00 | 0.9659 | 22.945 | 294.35, 3.15 | <0.0001 | <0.0001 |
| 21 | -1.117 | 41.03, -11.02 | 0.3060 | 15.461 | 294.35, 3.93 | <0.0001 | <0.0001 |
| 30 | -1.471 | 18.48, -8.80 | 0.7981 | 14.419 | 235.67, 3.83 | <0.0001 | <0.0001 |
| 45 | 0.795 | 26.91, -7.60 | 0.0479 | 12.801 | 296.85, 3.07 | 0.0003 | <0.0001 |
| 60 | 7.915 | 32.81, -7.44 | <0.0001 | 28.243 | 294.35, 3.31 | <0.0001 | 0.0252 |
Note. Median IgA and IgG levels are reported in units/ml (U/ml). Negative values indicate that the standardized sample value was lower than the negative control milk value. The p values reported within each IgA and IgG analysis column were determined via a Dunn’s multiple comparisons test where the dataset from each time was compared to the dataset from Day -1. The p values reported in the last column are from a Dunn’s multiple comparisons test evaluating differences between IgA and IgG levels at each time point. Missing values: Day -30 = 4, Day -1 = 2, Day 7 = 5 for the IgA analysis and 3 for the IgG analysis, Day 14 = 2, Day 21 = 3, Day 30 = 1, and Day 45 = 1. IgA = Immunoglobulin A; IgG = Immunoglobulin G; Mdn = median; UL = upper limit; LL = lower limit.
Figure 1.
Levels of Anti-RBD-Specific IgA and IgG in Human Milk Following the Pfizer-BioNTech Booster Vaccine (N = 12).
Note. IgA = Immunoglobulin A; IgG = Immunoglobulin G; ELISA = enzyme-linked immunosorbent assay; CI = confidence interval. Each time point is compared to the pre-booster levels of (A) IgA and (B) IgG at Day -1. Each participant’s sample was run in triplicate in one ELISA. Table 1 indicates the number of participants represented at each time point. Data points represent means; error bars, 95% CIs. All data points have error bars present.
*p < .05.
**p < .01.
***p ≤ .001.
****p ≤ .0001.
A Kruskal-Wallis H test showed that there was a statistically significant difference (p < .0001) in RBD-specific IgA antibodies between the pre-booster Day -1 and three post-booster time points (Days 7, 45, and 60; Table 4 and Figure 1). A post hoc Dunn’s multiple comparisons test indicated that the RBD-specific IgA antibodies in milk were significantly increased by Day 7 (Mdn =9 .069), Day 45 (Mdn = 0.795), and Day 60 (Mdn = 7.915) compared to pre-booster (Day -1; Mdn = -2.433; Table 4 and Figure 1). On post-booster Day 7, 71.43% of participants had an increased level compared to pre-booster (Day -1). On post-booster Days 45 and 60, 45.45% and 41.67% of participants had significantly increased RBD-specific IgA antibodies, respectively.
On an individual level, 9 of the 12 participants (75%) had a significant increase (p < .05) in RBD-specific IgA levels at one or more time points post-booster compared to pre-booster, whereas all 12 (100%) had a significant increase in RBD-specific IgG levels at two or more time points post-booster compared to pre-booster (individual data not shown). Additionally, of the four participants who provided a milk sample at 90 days post-booster, 3/4 (75%) had significantly elevated RBD-specific IgA antibodies (mean 15.24 U/ml) and RBD-specific IgG antibodies (mean 55.67 U/ml) at Day 90 compared to pre-booster (Day -1; data not shown).
Discussion
We found significant increase in RBD-specific IgA and IgG antibodies in human milk following the third dose (booster) of the Pfizer-BioNTech Comirnaty vaccination was found. Our results similar to those reported previously by researchers investigating the tetanus-diphtheria-acellular pertussis vaccination (Abu Raya et al., 2014), which demonstrated that vaccine-specific antibodies were detected in human milk after intramuscular immunization. Other researchers have reported high RBD-specific IgG antibodies in human milk following the standard two-dose vaccination against SARS-CoV-2 (Baird et al., 2021; Gray et al., 2021; Low et al., 2021; Perl et al., 2021; Young et al., 2022), and we observed a similar IgG-dominant effect post-booster. This could be due to the administration method of the vaccination—intramuscular injection may induce a more robust IgG response than other routes, which would favor mucosal (IgA) immunity. While secretory IgA is the dominant immunoglobulin in human milk (Goldman & Goldblum, 1989), IgG in human milk may be important for protection against viral infections (e.g., RSV and HIV; Fouda et al., 2011; Mazur et al., 2019). These results and those of previous researchers (Baird et al., 2021; Gray et al., 2021; Low et al., 2021; Perl et al., 2021; Young et al., 2022) suggest a role for IgG in human milk for infant immunity to SARS-CoV-2.
Previously researchers (Pace et al., 2021; Young et al., 2022) reported that the IgA and IgG antibodies induced in human milk following COVID-19 infection could neutralize SARS-CoV-2 in vitro. Therefore, the results from previous neutralization assays imply that the immunoglobulins induced in human milk following the parent’s third dose of the vaccination could offer the infant enhanced protection against SARS-CoV-2. The mother would also have increased protection against SARS-CoV-2 from the systemic neutralizing antibodies induced following the Comirnaty booster vaccination (Yu et al., 2022).
Limitations
The sample size in this study was small. At the time of recruitment for this study, the CDC had authorized the third dose of the Pfizer-BioNTech vaccination for high-risk individuals or frontline workers, for example, healthcare workers, first responders, or educators. Therefore, the study population was skewed and mainly included participants who were healthcare workers or educators and were of an older average age (35.5 years) than the typical age at first birth (26.9 years). Participants were recruited via social media posts in academic or local mom groups. This recruitment strategy may have been a source of potential bias as it could have excluded participants who lacked the time and resources to engage in social media. The study population should be expanded to include participants from broader ranges of races and ethnicities and age ranges more representative of the average child-bearing population.
No functional assays were performed to assess the protective activity of the immunoglobulins from human milk following the third dose of the vaccine. However, one researcher has determined that (serum) antibodies induced following the third dose of the Comirnaty vaccine could neutralize the parental WA1/2020 strain of SARS-CoV-2 and the variant BA.1 and BA.2 Omicron strains. The neutralizing antibody titers had increased substantially after the third dose of the vaccine compared to the titers measured after the initial two doses of the vaccine (Yu et al., 2022). Future investigations should use in vitro neutralization assays to determine whether the SARS-CoV-2-specific antibodies induced in human milk post-booster are protective and capable of neutralizing the virus.
The ELISA assay in this study used mouse anti-human IgA-HRP (Hisa43) antibody to capture the binding signal of anti-RBD-specific IgA antibodies. The anti-human IgA-HRP (Hisa43) antibody is specific to the CH3 domain of the Fc region of IgA and recognizes secretory IgA, the most dominant form of IgA in human milk (Biewenga et al., 1986, 1991). A capture antibody specific to the secretory component could be utilized as a more precise method for measuring the secretory form of the anti-RBD-specific IgA antibodies.
This study did not assess immunoglobulins following a booster with the Moderna vaccine or a mix-and-match (Pfizer-BioNTech, Moderna, or Johnson & Johnson) approach to Doses 1, 2, and 3. It also did not assess the level of immunoglobulins in human milk following a fourth dose of the Comirnaty vaccine. Future research is needed to assess optimal vaccination strategies (such as the mix-and-match approach) for providing the highest levels of antibodies against SARS-CoV-2 in human milk.
Participants were not tested for SARS-CoV-2 via real-time reverse transcriptase polymerase chain reaction (RT-PCR) as part of this study; therefore, it is difficult to determine whether elevated antibody levels could also be attributed to infection (either symptomatic or asymptomatic). However, no participants reported any known exposures to COVID-19 or any positive at-home or RT-PCR test results throughout the study. Additionally, this study concluded in early January 2022, so most milk sample collection occurred before the emergence of the highly transmissible Omicron BA.1 and BA.2 variants of SARS-CoV-2 in the United States. Future researchers could quantify the levels of immunoglobulins in human milk specific to the SARS-CoV-2 nucleocapsid (N) protein as a way to detect immunity to past infections (since N antigens are not present in the currently available COVID-19 vaccinations).
Conclusions
The recent clinical trial which tested the Pfizer-BioNTech vaccination in pregnant women assessed safety and efficacy but did not directly quantify immunoglobulin levels (Dagan et al., 2021). Healthcare providers and mothers have limited information regarding the ability of the COVID-19 vaccination to induce long-term immunity in human milk. Our research suggests administering the third dose of the vaccination ≥6 months after the standard two-dose Pfizer-BioNTech vaccination increases SARS-CoV-2 RBD-specific IgA and IgG antibodies in human milk. This may be a source of passive and protective immunity for infants. As of early August 2022, less than half of the adult population (48.2%) that is booster-eligible in the United States has received their booster vaccine (CDC, 2020). Pregnant or lactating women who are booster-eligible may wish to consider the booster vaccination for potential protection for their infants.
Supplemental Material
Supplemental material, sj-pdf-1-jhl-10.1177_08903344221134631 for Increase in SARS-CoV-2 RBD-Specific IgA and IgG Antibodies in Human Milk From Lactating Women Following the COVID-19 Booster Vaccination by Andrea M. Henle in Journal of Human Lactation
Acknowledgments
We thank Joan Donner and Paul Everson (Ancell Corp.) for technical assistance with the ELISA assays. We thank Qinzi Ji (Carthage College) for assistance processing the milk samples. We thank Crystal Nelson (Save the Milk) for recommendations and guidance on shipping milk samples. No one was compensated for their contributions.
Footnotes
Author contribution(s): Andrea M. Henle: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Andrea M. Henle  https://orcid.org/0000-0002-7383-7616
https://orcid.org/0000-0002-7383-7616
Supplemental Material: Supplementary material may be found in the “Supplemental Material” tab in the online version of this article.
References
- Abu Raya B., Srugo I., Kessel A., Peterman M., Bader D., Peri R., Ashtamker N., Gonen R., Bamberger E. (2014). The induction of breast milk pertussis specific antibodies following gestational tetanus-diphtheria-acellular pertussis vaccination. Vaccine, 32(43), 5632–5637. 10.1016/j.vaccine.2014.08.006 [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics. (2022). Children and COVID-19: State-level data report. http://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/
- Baird J. K., Jensen S. M., Urba W. J., Fox B. A., Baird J. R. (2021). SARS-CoV-2 antibodies detected in mother’s milk post-vaccination. Journal of Human Lactation, 37(3), 492–498. 10.1177/08903344211030168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biewenga J., Faber A., Pronk J. C., Haaijman J. J. (1986). Production and characterization of pepsin fragments of human IgA1 to determine domain-specificity of monoclonal anti-IgA antibodies. Immunology, 59(1), 153–158. 10.1177/000456329102800311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biewenga J., Stoop A. E., Baker H. E., Swart S. J., Nauta J. J. P., van Kamp G. J., van der Baan S. (1991). Nasal secretions from patients with polyps and healthy individuals, collected with a new aspiration system: Evaluation of total protein and immunoglobulin concentrations. Annals of Clinical Biochemistry, 28(3), 260–266. 10.1177/000456329102800311 [DOI] [PubMed] [Google Scholar]
- Dagan N., Barda N., Biron-Shental T., Makov-Assif M., Key C., Kohane I. S., Hernán M. A., Lipsitch M., Hernandez-Diaz S., Reis B. Y., Balicer R. D. (2021). Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nature Medicine, 27(10), 1693–1695. 10.1038/s41591-021-01490-8 [DOI] [PubMed] [Google Scholar]
- De Schutter S., Maertens K., Baerts L., De Meester I., Van Damme P., Leuridan E. (2015). Quantification of vaccine-induced antipertussis toxin secretory IgA antibodies in breast milk: Comparison of different vaccination strategies in women. The Pediatric Infectious Disease Journal, 34(6), e149–152. 10.1097/INF.0000000000000675 [DOI] [PubMed] [Google Scholar]
- Fouda G. G., Yates N. L., Pollara J., Shen X., Overman G. R., Mahlokozera T., Wilks A. B., Kang H. H., Salazar-Gonzalez J. F., Salazar M. G., Kalilani L., Meshnick S. R., Hahn B. H., Shaw G. M., Lovingood R. V., Denny T. N., Haynes B., Letvin N. L., Ferrari G., . . . Center for HIV/AIDS Vaccine Immunology. (2011). HIV-specific functional antibody responses in breast milk mirror those in plasma and are primarily mediated by IgG antibodies. Journal of Virology, 85(18), 9555–9567. 10.1128/JVI.05174-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman A. S., Goldblum R. M. (1989). Immunoglobulins in human milk. In Atkinson S. A., Lonngerdal B., Proteins and non-protein nitrogen in human milk. 43–51. CRC Press. 10.1201/9780367812805 [DOI] [Google Scholar]
- Gray K. J., Bordt E. A., Atyeo C., Deriso E., Akinwunmi B., Young N., Baez A. M., Shook L. L., Cvrk D., James K., De Guzman R., Brigida S., Diouf K., Goldfarb I., Bebell L. M., Yonker L. M., Fasano A., Rabi S. A., Elovitz M. A., . . . Edlow A. G. (2021). Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. American Journal of Obstetrics and Gynecology, 225(3), 303.e1–303.e17. 10.1016/j.ajog.2021.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low J. M., Gu Y., Ng M. S. F., Amin Z., Lee L. Y., Ng Y. P. M., Shunmuganathan B. D., Niu Y., Gupta R., Tambyah P. A., MacAry P. A., Wang L. W., Zhong Y. (2021). Codominant IgG and IgA expression with minimal vaccine mRNA in milk of BNT162b2 vaccinees. NPJ Vaccines, 6, 105. 10.1038/s41541-021-00370-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur N. I., Horsley N. M., Englund J. A., Nederend M., Magaret A., Kumar A., Jacobino S. R., de Haan C. A. M., Khatry S. K., LeClerq S. C., Steinhoff M. C., Tielsch J. M., Katz J., Graham B. S., Bont L. J., Leusen J. H. W., Chu H. Y. (2019). Breast Milk prefusion F Immunoglobulin G as a correlate of protection against respiratory syncytial virus acute respiratory illness. The Journal of Infectious Diseases, 219(1), 59–67. 10.1093/infdis/jiy477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek J. Y., Noble L. (2022). Breastfeeding and the use of human milk. 150(1): e2022057988. 10.1542/peds.2022-057988 [DOI] [PubMed] [Google Scholar]
- Pace R. M., Williams J. E., Järvinen K. M., Belfort M. B., Pace C. D. W., Lackey K. A., Gogel A. C., Nguyen-Contant P., Kanagaiah P., Fitzgerald T., Ferri R., Young B., Rosen-Carole C., Diaz N., Meehan C. L., Caffé B., Sangster M. Y., Topham D., McGuire M. A., . . . McGuire M. K. (2021). Characterization of SARS-CoV-2 RNA, antibodies, and neutralizing capacity in milk produced by women with COVID-19. MBio, 12(1), e03192–20. 10.1128/mBio.03192-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl S. H., Uzan-Yulzari A., Klainer H., Asiskovich L., Youngster M., Rinott E., Youngster I. (2021). SARS-CoV-2-specific antibodies in breast milk after COVID-19 vaccination of breastfeeding women. JAMA, 325(19), 2013–2014. 10.1001/jama.2021.5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriraman N. K., Kellams A. (2016). Breastfeeding: What are the barriers? Why women struggle to achieve their goals. Journal of Women’s Health, 25(7), 714–722. 10.1089/jwh.2014.5059 [DOI] [PubMed] [Google Scholar]
- United States Centers for Disease Control and Prevention. (2020). COVID data tracker. https://covid.cdc.gov/covid-data-tracker
- United States Centers for Disease Control and Prevention. (2021. a) Coronavirus disease 2019. https://www.cdc.gov/media/releases/2021/p0924-booster-recommendations-.html
- United States Centers for Disease Control and Prevention. (2021. b). Facts about nationwide breastfeeding goals. https://www.cdc.gov/breastfeeding/data/facts.html
- Valcarce V., Stafford L. S., Neu J., Cacho N., Parker L., Mueller M., Burchfield D. J., Li N., Larkin J. (2021). Detection of SARS-CoV-2-specific IgA in the human milk of COVID-19 vaccinated lactating health care workers. Breastfeeding Medicine, 16(12), 1004–1009. 10.1089/bfm.2021.0122 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2021). Infant and young child feeding. https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding
- Young B. E., Seppo A. E., Diaz N., Rosen-Carole C., Nowak-Wegrzyn A., Cruz Vasquez J. M., Ferri-Huerta R., Nguyen-Contant P., Fitzgerald T., Sangster M. Y., Topham D. J., Järvinen K. M. (2022). Association of human milk antibody induction, persistence, and neutralizing capacity with SARS-CoV-2 infection vs. mRNA vaccination. JAMA Pediatrics, 176(2), 159–168. 10.1001/jamapediatrics.2021.4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Collier A.-R. Y., Rowe M., Mardas F., Ventura J. D., Wan H., Miller J., Powers O., Chung B., Siamatu M., Hachmann N. P., Surve N., Nampanya F., Chandrashekar A., Barouch D. H. (2022). Neutralization of the SARS-CoV-2 Omicron BA.1 and BA.2 variants. The New England Journal of Medicine, 386(16), 1579–1580. 10.1056/NEJMc2201849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman K., Roy E., Arifeen S. E., Rahman M., Raqib R., Wilson E., Omer S. B., Shahid N. S., Breiman R. F., Breiman R. E., Steinhoff M. C. (2008). Effectiveness of maternal influenza immunization in mothers and infants. The New England Journal of Medicine, 359(15), 1555–1564. 10.1056/NEJMoa0708630 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jhl-10.1177_08903344221134631 for Increase in SARS-CoV-2 RBD-Specific IgA and IgG Antibodies in Human Milk From Lactating Women Following the COVID-19 Booster Vaccination by Andrea M. Henle in Journal of Human Lactation