Abstract
This study aimed to evaluate the demographic and clinical characteristics, treatments and outcomes of concomitant acute myocardial infarction (AMI) and acute intracranial hemorrhage (ICH). All patients diagnosed with concomitant AMI and acute ICH admitted to our institution were included retrospectively. The patient demographics, clinical characteristics, neuroimaging and treatment approaches were analyzed, and the outcomes of interest included disability as defined by the modified Rankin Scale (mRS) score and all-cause mortality within 1 year of follow-up. Of a total of 4972 patients with AMI, 8 patients (0.2%) with concomitant acute ICH were recruited for the study, including ST-segment elevation myocardial infarction (STEMI, 5 cases) and non-STEMI (3 cases). New-onset acute ICH in 4 of the 5 patients (80%) occurred within 24 hours after the AMI event, and all these patients had a sudden decrease in the level of consciousness, with an average decrease of 4.6 on the Glasgow Coma Scale. All 5 out of 8 patients had irregular shapes and uncommon sites of hematoma presentation documented on CT scans. Unfortunately, 2 patients died from a progression of ICH within 1 week, and 2 of the 6 survivors had poor functional outcomes (mRS ≥3) at the 1-year follow-up. Concomitant acute ICH and AMI are rare complications displaying unique iconography. Acute ICH caused serious prejudice in AMI with higher mortality and poor functional outcomes, and cardiac catheterization without the administration of antithrombotic or antiplatelet agents was feasible for patients who had unstable hemodynamics or STEMI.
Keywords: myocardial infarction, stroke
WHAT IS ALREADY KNOWN ON THIS TOPIC
Acute myocardial infarction (AMI) and acute intracranial hemorrhage (ICH) require contradictory therapies.
Previous studies have shown that multiple secondary cardiac pathological changes are common in patients with acute ICH and subarachnoid hemorrhage, and acute ICH is also a rare but catastrophic complication after AMI.
Little is known about the clinical characteristics, potential pathogenesis, therapeutic experience and outcomes of concomitant AMI and ICH.
WHAT THIS STUDY ADDS
In our study, the incidence of concomitant AMI and acute ICH was 0.2%. The study found that patients suffering from concomitant AMI and acute ICH commonly presented with neurological impairment with sudden disorders of consciousness and lobar hemorrhage with irregular shapes commonly documented on CT scans.
Most of the new-onset ICH in patients with AMI occurred within 24 hours after AMI, and half of patients who concomitantly suffered from AMI and acute ICH had a poor prognosis (modified Rankin Scale ≥3), especially in patients with primary ICH.
In clinical practice, the decision on whether to rescue the brain or the heart first should be based on the hemodynamic stability of the patient in this condition, and cardiac catheterization without the administration of antithrombotic or antiplatelet agents is feasible for patients who have unstable hemodynamics or ST-segment elevation myocardial infarction after acute ICH.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
In clinical practice, the research results could provide reference for diagnosis and therapy of concomitant AMI and acute ICH.
Introduction
Acute myocardial infarction (AMI) and acute intracranial hemorrhage (ICH) are leading causes of mortality worldwide.1 2 Studies have shown that multiple secondary cardiac pathological changes are common in patients with acute ICH and subarachnoid hemorrhage (SAH),3 including arrhythmias, myocardial necrosis, electrocardiogram (ECG) changes, pulmonary edema, and acute left heart failure (HF).4 On the other hand, ICH can be a rare but catastrophic complication after AMI, regardless of the usage of percutaneous coronary interventions (PCIs) over thrombolytic therapy for early revascularization.5 Previous studies showed that ICH occurred in approximately 0.35% of patients within 1 year after discharge for AMI.6 However, reports of concomitant AMI and acute ICH are limited, and the very low incidence makes it difficult to assess the clinical characteristics, potential pathogenesis and outcomes of this condition. Therefore, we conducted this study to evaluate the clinical characteristics, potential pathogenesis and 1-year outcomes of patients with concomitant AMI and acute ICH.
Materials and methods
Study population
The study population consisted of patients who met the diagnostic criteria for AMI and had hemorrhagic lesions confirmed by brain CT from January 2015 to July 2020 at The First Affiliated Hospital of Xi’an Jiaotong University. We excluded patients who were <18 years old, and we also excluded patients who lacked clinical data and were unwilling to participate in this study.
Data source
In this single-center retrospective analysis, the data were derived from the Biobank of The First Affiliated Hospital of Xi’an Jiaotong University and then validated by a medical record review. We collected the following patient data: demographics, including age and sex; common risk factors, including hypertension, diabetes, high cholesterol, drinking and smoking; medical history, including history of myocardial infarction (MI), atrial fibrillation, HF, stroke/transient ischemic attack, chronic renal disease, chronic lung disease, vascular disease and anemia; and data related to acute ICH, including main symptoms, signs, severity of neurological functions, consciousness and neuroimaging. The Glasgow Coma Scale (GCS) score and the National Institutes of Health Stroke Scale (NIHSS) score were evaluated within 24 hours after ICH. The hematoma volume was defined according to the ABC/2 system,7 and the location of the hemorrhage was named based on the cerebral location of the hematoma. The laboratory values at diagnosis were abstracted by a standardized protocol from the electronic medical record system of the hospital. The treatment methods were also collected, and these included PCI and the medications used (anticoagulant drugs, antiplatelet drugs).
Study outcomes
Patients were prospectively followed. The primary endpoint of this study was patient disability assessed by the modified Rankin Scale (mRS) score at the 1-year follow-up, and a poor outcome was defined as a mRS ≥3.8 The secondary endpoint was defined as all-cause mortality. The follow-up time was 1 year after discharge, and the follow-up mainly included outpatient visits and telephone interviews.
Results
Clinical characteristics
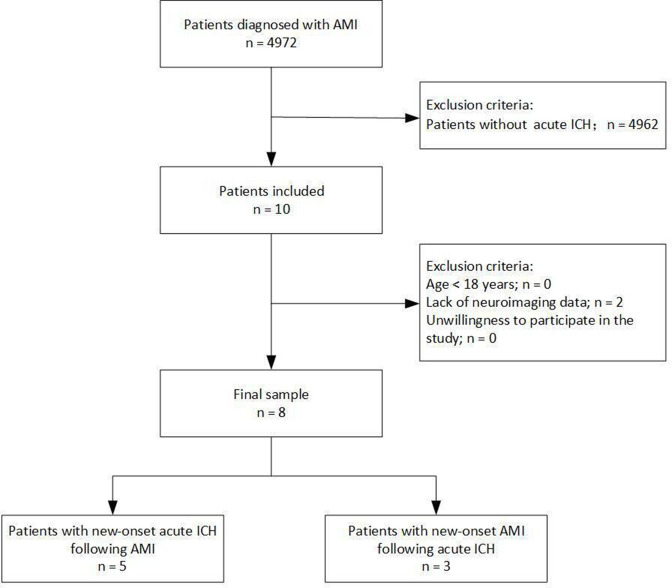
Between January 2015 and July 2020, a total of 4972 patients with AMI were initially assessed in the study, of which 8 patients (0.2%) had concomitant AMI and acute ICH (figure 1). Five men and 3 women were included in the study. Their average age ranged from 39 to 82 years (62.9±14.8 years). Of the 8 patients, new-onset acute ICH developed in 5 patients with AMI, while new-onset AMI developed in 3 patients with acute ICH. Some traditional risk factors, including smoking (n=4), hypertension (n=5) and diabetes mellitus (n=2), were observed. A significant decrease in blood pressure was observed 12–24 hours before the onset of ICH in 3 of these 5 patients with primary AMI, and 2 patients even endured the administration of continuous dopamine to obtain normal perfusion. Based on laboratory indicators, none of the patients developed coagulation dysfunction or dyslipidemia. The levels of platelets were increased in the blood tests of 2 patients with new-onset AMI following ICH, which could indicate a prothrombotic state in these patients (table 1). Among these patients, 5 underwent ST-segment elevation myocardial infarction (STEMI), and 3 underwent non-STEMI (table 2).
Figure 1.
Study protocol. Patient selection and flow of the current study. AMI, acute myocardial infarction; ICH, intracranial hemorrhage.
Table 1.
Clinical materials in 8 patients with concomitant AMI and acute ICH
| Case | Age (years) and gender | Traditional risk factors | PCI after AMI | Blood tests | Coagulation functions | Plasma lipid |
| 1 | Female in her 70s | Hypertension | No | PLT 161 HB 100 |
PT 15.20 APTT 36.10 TT 17.30 INR 1.22 FIB 3.03 D-dimer 7.50 |
Cholesterol 3.79 Triglyceride 0.80 HDL 1.21 LDL 1.33 |
| 2 | Male in his 60s | Hypertension, diabetes mellitus, a history of AIS, smoking | Yes | PLT 109 HB 136 |
PT 12.60 APTT 40.70 TT 16.70 INR 0.96 FIB 3.41 D-dimer 1.17 |
Cholesterol 3.68 Triglyceride 0.87 HDL 1.21 LDL 2.30 |
| 3 | Male in his 50s | Smoking | Yes | PLT 596 HB 119 |
PT 12.80 APTT 35.30 TT 18.30 INR 0.96 FIB 2.16 D-dimer 0.25 |
Cholesterol 5.59 Triglyceride 2.68 HDL 0.92 LDL 2.04 |
| 4 | Male in his 60s | Hypertension, smoking | Yes | PLT 173 HB 136 |
PT 14.60 INR 1.16 |
Cholesterol 3.63 Triglyceride 0.68 HDL 0.94 LDL 2.27 |
| 5 | Male in his 40s | Smoking | No | PLT 215 HB 150 |
PT 13.20 APTT 34.40 TT 14.50 INR 1.02 FIB 3.22 D-dimer 0.00 |
Cholesterol 3.93 Triglyceride 1.49 HDL 0.97 LDL 2.24 |
| 6 | Female in her 60s | Hypertension, diabetes mellitus, a history of AIS | No | PLT 411 HB 119 |
PT 13.80 APTT 36.10 TT 15.80 INR 1.04 FIB 3.42 D-dimer 1.93 |
Cholesterol 3.83 Triglyceride 1.49 HDL 1.11 LDL 2.28 |
| 7 | Male in his 70s | No | No | PLT 400 HB 85 |
PT 15.70 APTT 36.90 TT 16.20 INR 1.22 FIB 7.19 D-dimer 5.92 |
Cholesterol 3.87 |
| 8 | Female in her 80s | Hypertension, a history of AMI | No | PLT 103 HB 111 |
PT 13.40 APTT 36.50 TT 24.00 INR 1.04 FIB 4.96 D-dimer 1.66 |
Cholesterol 3.35 Triglyceride 1.95 HDL1.09 LDL 2.49 |
Case 1–5, patients with new-onset ICH following AMI; case 6–8, patients with new-onset AMI following ICH.
PLT=125~350×109/L; HB=115~150 g/L; PT=11~14 s; APTT=28~43.5 s; TT=14~21 s; D-dimer=<0.5 mg/L; INR=0.94~1.30; FIB=2~4 g/L; cholesterol=3.10~5.69 mmol/L; triglyceride=0.56~1.47 mmol/L; HDL=1.16~1.42 mmol/L; LDL=2.07~3.10 mmol/L.
AIS, acute ischemic stroke; AMI, acute myocardial infarction; APTT, activated partial thromboplastin time; FIB, fibrinogen; HB, hemoglobin; HDL, high-density lipoprotein; ICH, intracerebral hemorrhage; INR, international normalized ratio; LDL, low-density lipoprotein; PCI, percutaneous coronary intervention; PLT, platelets; PT, prothrombin time; TT, thrombin time.
Table 2.
ECG and myocardial markers in 8 patients with concomitant AMI and acute ICH
| Case | Age (years) and gender | ECG | Serum cardiac markers | NT-proBNP | |
| Type of AMI | Parameters | ||||
| 1 | Female in her 70s | STEMI | P 71 bpm P-R atrial fibrillation QRS 84 ms QT/QTc 393/428 ms |
CK 364 CK-MB 27 TnT-hs 1.69 |
3067.0 |
| 2 | Male in his 60s | NSTEMI | P 87 bpm P-R 180 ms QRS 90 ms QT/QTc 372/457 ms |
CK 4355 CK-MB 428 TnT-hs 1.13 |
8020.0 |
| 3 | Male in his 50s | STEMI | P 59 bpm P-R 154 ms QRS 84 ms QT/QTc 430/439 ms |
CK 66 CK-MB 3 hs-TnI 491 |
614.0 |
| 4 | Male in his 60s | STEMI | P 88 bpm P-R 237 ms QRS 92 ms QT/QTc 390/473 ms |
CK 7997 CK-MB 1040 |
5232.0 |
| 5 | Male in his 40s | STEMI | P 58 bpm P-R 146 ms QRS 119 ms QT/QTc 469/465 ms |
CK 784 CK-MB 39 TnT-hs 1.95 |
1010.0 |
| 6 | Female in her 60s | STEMI | P 110 bpm P-R 172 ms QRS 92 ms QT/QTc 302/367 ms |
CK 392 CK-MB 35 TnT-hs 0.633 |
288.4 |
| 7 | Male in his 70s | NSTEMI | P 167 bpm P-R 106 ms QRS 78 ms QT/QTc 332/423 ms |
CK 1364 CK-MB 77 TnT-hs 3.04 hs-TnI >50 000.0 |
19 015.0 |
| 8 | Female in her 80s | NSTEMI | P 73 bpm P-R 152 ms QRS 86 ms QT/QTc 438/463 ms |
MB 151.5 CK-MB 1.90 TnT-hs 0.022 hs-TnI 38.78 |
437.0 |
CK=50~310 U/L; CK-MB=0~24 U/L; hs-TnI=0~26.20 pg/mL; TnT-hs=0~0.014 ng/mL; NT-proBNP=0~125 pg/mL.
AMI, acute myocardial infarction; bpm, beats per minute; CK, creatine kinase; CK-MB, creatine kinase isoenzyme MB; hs-TnI, hypersensitivity cardiac troponin I; ICH, intracranial hemorrhage; NSTEMI, non-ST-segment elevation myocardial infarction; NT-proBNP, N-terminal pro-brain natriuretic peptide; STEMI, ST-segment elevation myocardial infarction; TnT-hs, hypersensitivity cardiac troponin T.
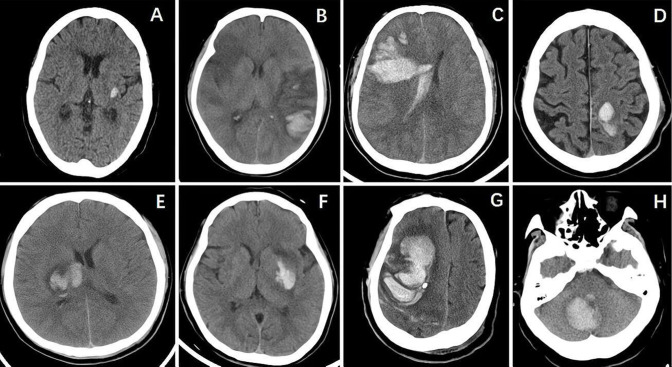
The symptoms of acute ICH in these 8 patients included the following acute clinical presentations: sudden headache, dizziness, disturbance of consciousness, sudden seizure, hemiparesis and unclear speech. Clinical deterioration, in addition to sudden onset of a decreased level of consciousness, was a prominent clinical symptom in all 5 patients with primary AMIs compared with patients with new-onset AMI following acute ICH, with an average decrease of 4.6 on the GCS. Three patients were in a coma and were in the range of 3–6 on the GCS, and 5 patients were not in a coma and were in the range of 1–14 on the NIHSS. In 4 of 5 patients, new-onset ICH occurred within 24 hours after the occurrence of AMI. Hematomas with irregular shapes were documented on CT scans in 5 of 8 patients with ICH (figure 2), and the average hematoma volume was 18.0 mL (ranging from 1 to 46 mL). We also found that hematomas developed in uncommon sites in 5 of the 8 patients, and SAH and lobar hemorrhages were found in 4 patients. Among 8 patients’ CT scans, significant edemas surrounding hemorrhages could be observed in 5 patients, and the spot sign could be observed in 6 of 8 patients. All hematomas displayed mixed density, with an average difference in CT value (maximum value–minimum value) of 49.6±5.7 Hounsfield unit.
Figure 2.
CT images showing cerebral hemorrhage of 8 patients. (A) Case 1—CT of the brain revealed an elliptical hemorrhagic lesion at the left external capsule. (B) Case 2—CT of the brain revealed irregular hemorrhagic lesions involving the left occipital lobe, accompanied by hypodense lesions involving the right frontal lobe, right temporal lobe and left temporoparietal lobe concomitantly. (C) Case 3—CT of the brain revealed irregular hemorrhagic lesions involving the right frontal lobe and temporoparietal lobe and compressing the right ventricle. (D) Case 4—CT of the brain revealed elliptical hemorrhagic lesions involving the left parietal lobe. (E) Case 5—CT of the brain revealed irregular hemorrhagic lesions at the right basal ganglia and thalamus compressing the right ventricle. (F) Case 6—CT of the brain showed that an irregular hemorrhagic lesion had developed at the left basal ganglia. (G) Case 7—CT of the brain revealed irregular hemorrhagic lesions involving the right frontal lobe and temporoparietal lobe. (H) Case 8—CT of the brain revealed elliptical hemorrhagic lesions in the right cerebellum.
One-year outcomes
Antiplatelet and anticoagulation therapies were immediately discontinued in 4 of 5 patients with AMIs when new-onset ICH was found, and these patients did not have cardiac deterioration after AMI or another ischemic stroke during hospitalization. Of the 8 patients, 2 patients (25.0%) died from ICH progression within 1 week, and these patients included 1 patient with AMI who continued to take antiplatelet and anticoagulation therapies after new-onset ICH and 1 patient with new-onset AMI following ICH. The average length of stay was 15.4±12.3 days (ranging from 5 to 36 days). Among 6 patients who survived at 1 year, 2 of the 6 survivors had poor outcomes (mRS ≥3), and both of them had new-onset AMI following acute ICH. The median mRS was 2.5 (table 3).
Table 3.
Clinical data of ICH in 8 patients with concomitant AMI and acute ICH
| Case | Age (years) and gender | Time from MI/ICH to ICH/MI | Main clinical presentations | Shape, site and volume of ICH on brain CT | NIHSS or GCS (scale) | Length of stay (days) | mRS |
| 1 | Female in her 70s | <24 hours | Transient delirium, dysarthria | Elliptical hematoma at left external capsule (1 mL) | NIHSS 1 GCS 14 |
9 | 0 |
| 2 | Male in his 60s | <24 hours | Sudden deep coma | Irregular hematoma at left occipital lobe (16 mL); SAH | GCS 3 | 6 | 6 |
| 3 | Male in his 50s | 4 days | Headache, superficial coma | Irregular hematoma at right frontal lobe, temporoparietal lobe (35 mL); SAH | GCS 6 | 32 | 1 |
| 4 | Male in his 60s | <24 hours | Stupor state, dysarthria | Elliptical hematoma at left parietal lobe (5 mL); SAH | NIHSS 6 GCS 15 |
5 | 1 |
| 5 | Male in his 40s | <24 hours | Stupor state, headache, dizziness | Irregular hematoma at right basal ganglia, thalamus (10 mL); SAH | NIHSS 8 GCS 14 |
5 | 2 |
| 6 | Female in her 60s | <24 hours | Hemiparesis, dysarthria, sudden seizure | Irregular hematoma at left basal ganglia (7 mL) | NIHSS 6 GCS 12 |
18 | 3 |
| 7 | Male in his 70s | 24–48 hours | Hemiparesis, deep coma after AMI | Irregular hematoma at right frontal lobe, parietal lobe and temporoparietal lobe (46 mL) | GCS 3 | 12 | 6 |
| 8 | Female in her 80s | 6 days | Headache, dizziness, vomiting, stupor state | Elliptical hematoma at right cerebellum (24 mL) | NIHSS 10 GCS 12 |
36 | 4 |
Patients who were in coma were assessed only by GCS.
AMI, acute myocardial infarction; GCS, Glasgow Coma Scale; ICH, intracranial hemorrhage; MI, myocardial infarction; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; SAH, subarachnoid hemorrhage.
Discussion
Concomitant AMI and acute ICH are uncommon but devastating with poor prognosis. Our study showed that (1) 0.2% of AMI cases are complicated by concomitant acute ICH mostly presenting as lobar hemorrhage with irregular shapes commonly documented on CT scans, (2) a sudden decreased level of consciousness was a prominent clinical symptom in patients with new-onset acute ICH after AMI, (3) most of the new-onset ICH in patients with AMIs occurred within 24 hours after AMI, and (4) half of patients who concomitantly suffered from AMI and acute ICH had a poor prognosis (mRS ≥3), especially in patients with primary ICH.
Our study showed that most acute ICH events generally occurred within 24 hours after AMI, similar to previous studies.9 10 Alqahtani et al 5 demonstrated that there was no significant difference in secondary acute ICH between patients with AMI with or without cardiac catheterization. The possible mechanisms underlying the development of new-onset ICH in patients with primary AMI are explained below. First, hemodynamic instability as a result of AMI could increase the risk of acute ICH, and previous studies have revealed that severe hypotension following AMI can lead to hemorrhagic stroke.11 12 In our study, 3 of 5 patients with new-onset ICH after AMI developed prolonged hypotension, which may pose a risk for the development of acute ICH. Second, the mainstay of treatment for AMI is fibrinolytic and/or antiplatelet medications, which also increase the risk of ICH. Additional possible mechanisms that could increase the risk of ICH include some incidental cerebrovascular diseases, such as cerebral amyloid angiopathy and vascular malformations, which usually lead to irregular shapes and uncommon locations of hematomas.13 In this study, hematomas with uncommon locations and irregular shapes were observed in a large percentage of these patients. This indicated that some rare cerebrovascular causes exclusive to a few traditional risk factors may have been responsible for new-onset ICH.13 Furthermore, significant edemas surrounding hemorrhages and the spot sign could be observed in a large percentage of these patients, which are known to be associated with hematoma expansion and a poor prognosis.14 15
Cardiac involvement is common in patients who have acute central nervous system damage, which presents with elevated concentrations of cardiac biochemical markers, abnormal ECG, and abnormal left ventricular function.3 According to previous research, 0.3% of patients with hemorrhagic stroke develop MI,16 and the possible mechanisms underlying the development of new-onset AMI in patients with primary ICH are as follows: first, excessive sympathetic nervous system stimulation as a result of injury in the insular cortex17 can cause cardiac abnormalities, which can lead to intracellular calcium overload and reversible myocyte dysfunction, resulting in biochemical marker increases, ECG changes and contractile dysfunction due to the release of catecholamines.18 19 Second, primary coronary artery disease, sharing similar risk factors with ICH, occasionally deteriorates. Third, some iatrogenic factors play indispensable roles in cardiac involvement. High hematocrit and increased plasma osmotic pressure worsen the hypercoagulable state due to diuresis and dehydration therapy. Two patients with primary ICH in our study had increased platelet levels secondary to dehydration, and this mechanism is considered one of the possible predisposing causes of AMI.
Due to its rarity, concomitant AMI and ICH pose significant challenges and dilemmas for physicians. First, these two conditions make a definitive diagnosis difficult due to the decreased mentation and aphasia of the patients. Additionally, the two conditions require contradictory therapies. Therapy for AMI usually requires a PCI based on full-dose parenteral anticoagulation plus dual antiplatelet therapy, which could worsen the hematoma. The decision of whether AMI or ICH should be treated first is difficult, and emergency therapy for either of these conditions could worsen the other. In view of a previous study on ‘cardiocerebral infarction’,16 we suggest that facilitating the decision on whether to rescue the brain or the heart first should be based on the hemodynamic stability of the patient, and close cardiac monitoring should be adopted for these patients. Obagi and Schoenfeld20 previously reported that a young man who underwent PCI without the administration of antithrombotic or antiplatelet agents after concurrent cardiac arrest and ICH had a good outcome, which indicates that ICH may not be an absolute contraindication for PCI. This provides a new treatment algorithm in which cardiac catheterization without the administration of antithrombotic or antiplatelet agents is feasible for patients who have unstable hemodynamics or STEMI after acute ICH. Moreover, the discontinuation of antithrombotic therapy is controversial in patients with AMI after acute ICH, and it is currently unknown when to restart these therapies again if they are discontinued. In our study, 4 out of 5 patients with AMI stopped taking antithrombotic therapy, including 3 patients who underwent PCI after new-onset ICH, and 2 of these 3 patients had a poor outcome. None of these patients suffered AMI deterioration or another ischemic stroke during hospitalization.
Despite the low incidence of patients with concomitant AMI and ICH in our study, this clinical scenario still had a substantial negative impact on short-term survival and long-term prognosis. In this study, 2 patients (25.0%) died within 1 week, and 2 patients (25.0%) were disabled with an mRS ≥3 at the 1-year follow-up. In addition, concomitant AMI and ICH led to an increase in the hospitalization time. According to our study, there was a correlation between the site and volume of cerebral hemorrhage and the prognosis for patients with concomitant AMI and acute ICH because hematomas with large volumes and vital locations always lead to a poor prognosis. Previous studies have previously shown that a low GCS was associated with a poor recovery,21 and our study has similar results. However, the sample size in our study was relatively small that might generate uncertain results. To break out of this conundrum, we attempted to add extend statistics through using clustering algorithm to predict the outcomes more truly. But based on the data now, it is not enough to establish a statistical model to increase samples, and the research is needed to be proceeded to obtain a larger sample size that can reflect the true clinical situation.
Limitations
Several limitations of this study should be acknowledged. First, this was a retrospective analysis with a relatively small sample size conducted in a single center, and the low frequency of concomitant AMI and acute ICH cases may make it difficult to reflect the true clinical scenario, and we fail to establish a model to increase samples. Second, CT examinations were not routinely performed to detect cerebral abnormalities in patients with AMI; thus, the occurrence of subclinical ICH may have been underestimated. Furthermore, silent myocardial infarctions could have been underestimated due to the lack of continuous ECG monitoring (sometimes ECG is unnecessary in clinical practice) and evaluation.
Conclusion
Concomitant acute ICH and AMI are a rare complication displaying unique iconography. In our study, acute ICH caused serious prejudice in AMI with higher mortality and poor functional outcomes, and cardiac catheterization without the administration of antithrombotic or antiplatelet agents was feasible for patients who had unstable hemodynamics or STEMI. The decision on how to manage these patients still poses tremendous challenges and dilemmas, and further efforts are still required to develop an optimum treatment algorithm to improve the prognosis of these patients.
Acknowledgments
We would like to thank all participating patients, physicians and nurses.
Footnotes
XC, MW and MT contributed equally.
Contributors: FG and CS conceived the idea and design of the study. HL, XC, ZJ and MT collected data from the biobank. GL and XC analyzed the data. XC, MW and MT drafted the manuscript. FG, CS and XY revised the final version of the manuscript and supervised the study. All authors have read and approved the final version of the manuscript for publication. FG is the guarantor.
Funding: This study was supported by the Shaanxi Provincial Key R&D Program General Project (2022SF-058).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by the Ethics Committee of The First Affiliated Hospital of Xi’an Jiaotong University (No. XJTU1AF2021LSK116). Participants gave informed consent to participate in the study before taking part.
References
- 1. Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Heart disease and stroke Statistics-2016 update: a report from the American heart association. Circulation 2016;133:E38–60. 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 2. GBD 2013 Mortality and Causes of Death Collaborators . Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet 2015;385:117–71. 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jespersen CM, Fischer Hansen J, Hansen JF. Myocardial stress in patients with acute cerebrovascular events. Cardiology 2008;110:123–8. 10.1159/000110491 [DOI] [PubMed] [Google Scholar]
- 4. Mierzewska-Schmidt M, Gawecka A. Neurogenic stunned myocardium - do we consider this diagnosis in patients with acute central nervous system injury and acute heart failure? Anaesthesiol Intensive Ther 2015;47:175–80. 10.5603/AIT.2015.0017 [DOI] [PubMed] [Google Scholar]
- 5. Alqahtani F, Aljohani S, Tarabishy A, et al. Incidence and outcomes of myocardial infarction in patients admitted with acute ischemic stroke. Stroke 2017;48:2931–8. 10.1161/STROKEAHA.117.018408 [DOI] [PubMed] [Google Scholar]
- 6. Graipe A, Binsell-Gerdin E, Söderström L, et al. Incidence, time trends, and predictors of intracranial hemorrhage during long-term follow-up after acute myocardial infarction. J Am Heart Assoc 2015;4:e002290. 10.1161/JAHA.115.002290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yuan R, Wang D, Liu M, et al. Long-term prognosis of spontaneous intracerebral hemorrhage on the Tibetan Plateau: a prospective cohort study at 2 hospitals. World Neurosurg 2016;93:6–10. 10.1016/j.wneu.2016.05.064 [DOI] [PubMed] [Google Scholar]
- 8. Poon MTC, Fonville AF, Al-Shahi Salman R. Long-Term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2014;85:660–7. 10.1136/jnnp-2013-306476 [DOI] [PubMed] [Google Scholar]
- 9. Gore JM, Granger CB, Simoons ML, et al. Stroke after thrombolysis. mortality and functional outcomes in the GUSTO-I trial. global use of strategies to open occluded coronary arteries. Circulation 1995;92:2811–8. 10.1161/01.cir.92.10.2811 [DOI] [PubMed] [Google Scholar]
- 10. Guptill JT, Mehta RH, Armstrong PW, et al. Stroke after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction: timing, characteristics, and clinical outcomes. Circ Cardiovasc Interv 2013;6:176–83. 10.1161/CIRCINTERVENTIONS.112.000159 [DOI] [PubMed] [Google Scholar]
- 11. Omar HR, Fathy A, Rashad R, et al. Concomitant acute right ventricular infarction and ischemic cerebrovascular stroke; possible explanations. Int Arch Med 2010;3:25. 10.1186/1755-7682-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yeo LLL, Andersson T, Yee KW, et al. Synchronous cardiocerebral infarction in the era of endovascular therapy: which to treat first? J Thromb Thrombolysis 2017;44:104–11. 10.1007/s11239-017-1484-2 [DOI] [PubMed] [Google Scholar]
- 13. Ruiz-Sandoval JL, Chiquete E, Gárate-Carrillo A, et al. Spontaneous intracerebral hemorrhage in Mexico: results from a multicenter nationwide hospital-based registry on cerebrovascular disease (RENAMEVASC). Rev Neurol 2011;53:705–12. [PubMed] [Google Scholar]
- 14. Blacquiere D, Demchuk AM, Al-Hazzaa M, et al. Intracerebral hematoma morphologic appearance on noncontrast computed tomography predicts significant hematoma expansion. Stroke 2015;46:3111–6. 10.1161/STROKEAHA.115.010566 [DOI] [PubMed] [Google Scholar]
- 15. Yu Z, Zheng J, Xu Z, et al. Accuracy of shape irregularity and density heterogeneity on noncontrast computed tomography for predicting hematoma expansion in spontaneous intracerebral hemorrhage: a systematic review and meta-analysis. World Neurosurg 2017;108:347–55. 10.1016/j.wneu.2017.09.022 [DOI] [PubMed] [Google Scholar]
- 16. Kijpaisalratana N, Chutinet A, Suwanwela NC. Hyperacute simultaneous cardiocerebral infarction: rescuing the brain or the heart first? Front Neurol 2017;8:664. 10.3389/fneur.2017.00664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laowattana S, Zeger SL, Lima JAC, et al. Left insular stroke is associated with adverse cardiac outcome. Neurology 2006;66:477–83. 10.1212/01.wnl.0000202684.29640.60 [DOI] [PubMed] [Google Scholar]
- 18. Mann DL, Kent RL, Parsons B, et al. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation 1992;85:790–804. 10.1161/01.CIR.85.2.790 [DOI] [PubMed] [Google Scholar]
- 19. Masuda T, Sato K, Yamamoto S-ichiro, et al. Sympathetic nervous activity and myocardial damage immediately after subarachnoid hemorrhage in a unique animal model. Stroke 2002;33:1671–6. 10.1161/01.STR.0000016327.74392.02 [DOI] [PubMed] [Google Scholar]
- 20. Obagi A, Schoenfeld M. Successful percutaneous balloon angioplasty in a patient presenting with STEMI and acute intracranial hemorrhage. Cureus 2021;13:e15166. 10.7759/cureus.15166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Faghih-Jouybari M, Taghi Raof M, Abdollahzade S, et al. Mortality and morbidity in patients with spontaneous intracerebral hemorrhage: a single-center experience. Current Journal of Neurology 2021;20:32–6. 10.18502/cjn.v20i1.6377 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.