Abstract
Objective
This study assessed the transfer of patients from paediatric cardiac to adult congenital heart disease (ACHD) services in England and the factors impacting on this process.
Methods
This retrospective cohort study used a population-based linked data set (LAUNCHES QI data set: ‘Linking Audit and National datasets in Congenital Heart Services for Quality Improvement’) including all patients born between 1987 and 2000, recorded as having a congenital heart disease (CHD) procedure in childhood. Hospital Episode Statistics data identified transfer from paediatric to ACHD services between the ages of 16 and 22 years.
Results
Overall, 63.8% of a cohort of 10 298 patients transferred by their 22nd birthday. The estimated probability of transfer by age 22 was 96.5% (95% CI 95.3 to 97.7), 86.7% (95% CI 85.6 to 87.9) and 41.0% (95% CI 39.4 to 42.6) for severe, moderate and mild CHD, respectively. 166 patients (1.6%) died between 16 and 22 years; 42 of these (0.4%) died after age 16 but prior to transfer. Multivariable ORs in the moderate and severe CHD groups up to age 20 showed significantly lower likelihood of transfer among female patients (0.87, 95% CI 0.78 to 0.97), those with missing ethnicity data (0.31, 95% CI 0.18 to 0.52), those from deprived areas (0.84, 95% CI 0.72 to 0.98) and those with moderate (compared with severe) CHD (0.30, 95% CI 0.26 to 0.35). The odds of transfer were lower for the horizontal compared with the vertical care model (0.44, 95% CI 0.27 to 0.72). Patients who did not transfer had a lower probability of a further National Congenital Heart Disease Audit procedure between ages 20 and 30 compared with those who did transfer: 12.3% (95% CI 5.1 to 19.6) vs 32.5% (95% CI 28.7 to 36.3).
Conclusions
Majority of patients with moderate or severe CHD in England transfer to adult services. Patients who do not transfer undergo fewer elective CHD procedures over the following decade.
Keywords: Epidemiology; Health Care Economics and Organizations; Quality of Health Care; Heart Defects, Congenital
WHAT IS ALREADY KNOWN ON THIS TOPIC
Previous studies have reported high rates of loss to follow-up at the point of transfer to adult congenital heart disease (ACHD) services.
Gaps in care are associated with worse outcomes.
WHAT THIS STUDY ADDS
This study demonstrates that transfer from paediatric to ACHD services in England for patients with more complex congenital heart disease is highly effective, with a stepwise reduction in transfer rates in moderately complex and mildly complex patients.
The study demonstrates clear differences in practice between centres with a vertical and a horizontal model of delivering care.
Patients who do not transfer undergo fewer interventional or surgical procedures during the following decade.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The UK model of specialised service provision with ACHD services closely affiliated with paediatric cardiology centres facilitates transfer of moderately and severely complex patients.
Horizontal and vertical model centres clearly have different transfer policies, with more patients from horizontal models (stand-alone paediatric hospitals) transferring later and failing to ultimately transfer at all.
More work is required to understand the value of ongoing care in adulthood for patients with simple lesions.
Barriers to transfer for ethnic minorities and those from deprived areas should be further assessed and addressed.
Introduction
Survival after paediatric cardiac surgery and catheter interventions for congenital heart disease (CHD) in the UK is excellent and the vast majority of children undergoing treatment for even complex anatomy now reach adulthood.1 2 Because these patients are at increased risk of late cardiac complications, including arrhythmia, pulmonary hypertension, heart failure, endocarditis and premature death,3–7 long-term follow-up in adult congenital heart disease (ACHD) services is recommended.8 Patients lost to specialist ACHD follow-up have an increased risk of premature death and do not benefit from standard interventions designed to optimise cardiac function and longevity.9 10
Between the 1960s and 1980s paediatric cardiac surgery was provided in many small units across England, before becoming more concentrated in a smaller number of higher volume centres. Many of the current designated ACHD programmes have developed in conjunction with those centres, but until the late 1990s the relatively small number of patients with complex ACHD and the scarcity of expertise in ACHD meant that services were fragmented. Often patients were referred to general adult cardiology services, and as many as 30% of patients were lost to follow-up at the point of transfer.11 A formal structure for healthcare services for patients with ACHD is now well established in the UK, supported by the publication of the National Service Standards and Specifications in 2016.12 An entire section is dedicated to transition, including standards for a structured transition programme beginning at age 12, with transfer from paediatric to specialised ACHD care from age 16.
This study used the LAUNCHES QI (‘Linking Audit and National datasets in Congenital Heart Services for Quality Improvement’) data set13 to examine the transfer of patients from paediatric to adult congenital heart services in England.
Methods
Data set
Information on patients with CHD and their utilisation of healthcare services in England and Wales is not available in a single data set. Since April 2000, the main source of information on outcomes following therapeutic congenital cardiovascular procedures in the UK has been the mandatory National Congenital Heart Disease Audit (NCHDA).14 As part of the LAUNCHES QI project, a combined data set for understanding patient journeys across care systems was built to explore variation across services and identify priorities for quality improvement.13 The NCHDA was linked with national validated registries: ‘PICANet’ (Paediatric Intensive Care Audit Network) 15 and ‘ICNARC-CMP’ (Intensive Care National Audit and Research Centre-Case Mix Programme) 16; death registrations from Office for National Statistics (ONS); and Hospital Episode Statistics (HES) for routine National Health Service data on hospital admissions, accident and emergency attendances, and outpatient appointments in England.17 Using the LAUNCHES QI data set, this retrospective study examines transfer from paediatric services to ACHD services in a large cohort of patients and the factors affecting successful transfer.
Patient selection
From the LAUNCHES QI data set, 10 298 patients born between 1 April 1987 and 31 March 2000 aged over 16 years at the time of data collection were studied (figure 1).
Figure 1.
Inclusion and exclusion criteria. HES, Hospital Episode Statistics; NCHDA, National Congenital Heart Disease Audit; NHS, National Health Service.
Baseline characteristics were determined, including birth cohort (two groups: those born in 1987/1988–1993/1994 and those born in 1995/1996–1999/2000), sex, ethnicity and deprivation quintile. Complexity classification (mild, moderate, severe) in accordance with the current European Society of Cardiology (ESC) guidelines was assigned to each patient using the NCHDA diagnostic and procedural categories and the HES International Classification of Diseases Version 10 (ICD-10) diagnostic codes (see online supplemental material and tables S4-S6).18 Patients were grouped by whether their paediatric cardiology centre before age 16 employed a horizontal (paediatric services at a separate children’s hospital with affiliated ACHD service at a different hospital site) or a vertical (paediatric cardiac services and ACHD services within the same hospital site) model of care. Deprived (Q1, Q2) or non-deprived (Q3, Q4, Q5) status was assigned according to postcode-derived Index of Multiple Deprivation.19 Further details are given in online supplemental tables S2 and S3.
heartjnl-2022-321085supp001.pdf (535.3KB, pdf)
Main outcome measures
The primary outcome (evidence that transfer from paediatric to ACHD services had occurred) was assigned when the patient was seen in cardiology outpatients or admitted electively as a cardiology inpatient in a recognised UK specialist ACHD centre or a recognised affiliated outreach centre before their 22nd birthday.11
Many children with mild lesions are purposefully discharged during childhood as they are not considered to require lifelong ongoing follow-up. Those with mild lesions referred on for adult follow-up may only need to be seen every 4–5 years, so we determined that data collection from ages 16–22 should capture the overwhelming majority of patients. However, all patients with moderate or severely complex conditions would be expected to be seen at least every 2 years with transfer to specialist adult services primarily at ages 16–19 years.8 We therefore studied a subgroup of patients with moderate or severe disease (n=5824) up to their 20th birthday to minimise the effects of right-censoring of available data and purposeful discharge in the mildly complex group.
Death after age 16 but before transfer was a competing risk to transfer. Life status was ascertained using the ONS mortality registry; patients with missing life status (no linkage to ONS) were censored at last known visit.
We explored factors which may affect transfer, including birth cohort, sex, ethnicity, deprivation and paediatric model of care.
Finally, we examined whether failure to transfer was associated with increased mortality or differences in further procedures between ages 20 and 30.
Statistical analyses
Patient characteristics and outcomes are first described using counts and percentages. Conditional probability functions (CPFs) were fitted to estimate probability of transfer subject to being alive.20 CPF differences between groups were assessed using Pepe-Mori tests for all pairwise comparisons.21 CPFs are expressed as average (%, 95% CI).
Single variable and multivariable logistic regressions were used to explore factors potentially affecting transfer, including birth cohort, age at transfer, sex, ethnicity, diagnostic complexity, socioeconomic deprivation and service model for severe and moderate complexity patients. Kaplan-Meier and CPFs were used to estimate the probability of death and reintervention, respectively, between ages 20 and 30 by transfer status at age 20.
Results
The characteristics of the patients are shown in table 1.
Table 1.
Patient characteristics
| All (n) | All (%) | Severe and moderate (n) | Severe and moderate (%) | |
| All | 10 298 | 5820 | ||
| Birth cohort | ||||
| Born between 1987/1988 and 1993/1994 (7 years) | 3293 | 32.0 | 1979 | 34.0 |
| Born between 1994/1995 and 1999/2000 (6 years) | 7005 | 68.0 | 3841 | 66.0 |
| Sex | ||||
| Male | 5435 | 52.8 | 3389 | 58.2 |
| Female | 4863 | 47.2 | 2431 | 41.8 |
| Ethnicity | ||||
| White | 8590 | 83.4 | 4803 | 82.5 |
| Non-white | 1536 | 14.9 | 953 | 16.4 |
| Black | 332 | 3.2 | 201 | 3.5 |
| Asian | 897 | 8.7 | 561 | 9.6 |
| Other | 307 | 3.0 | 191 | 3.3 |
| Missing | 172 | 1.7 | 64 | 1.1 |
| Area of residence deprivation | ||||
| Deprived area | 4431 | 43.0 | 2496 | 42.9 |
| IMD Q1 (most deprived) | 2391 | 23.2 | 1310 | 22.5 |
| IMD Q2 | 2040 | 19.8 | 1186 | 20.4 |
| Non-deprived area | 5867 | 57.0 | 3324 | 57.1 |
| IMD Q3 | 1954 | 19.0 | 1158 | 19.9 |
| IMD Q4 | 1931 | 18.8 | 1094 | 18.8 |
| IMD Q5 (least deprived) | 1982 | 19.2 | 1072 | 18.4 |
| Model of care | ||||
| Vertical model (same site adults and children) | 6040 | 58.7 | 3368 | 57.9 |
| Horizontal model (different site adults and children) | 4258 | 41.3 | 2452 | 42.1 |
| Complexity score* | ||||
| Severe | 1454 | 14.1 | 1454 | 25.0 |
| Moderate | 4366 | 42.4 | 4366 | 75.0 |
| Mild | 4478 | 43.5 | ||
*Complexity score: severe includes (repaired/unrepaired) double outlet ventricle, functionally univentricular heart (with or without Fontan palliation), interrupted aortic arch, pulmonary atresia (all types), common arterial trunk (truncus arteriosus), heterotaxy syndromes, cyanotic congenital heart disease (unoperated/palliated) and transposition of great arteries (except post arterial switch); moderate includes anomalous pulmonary venous connections, atrioventricular septal defects, coarctation of aorta, repaired tetralogy of Fallot, repaired transposition of great arteries with arterial switch, severe pulmonary valvar disease, aortic subvalvar/supravalvar stenosis and Ebstein anomaly; mild includes isolated unrepaired small septal defects, repaired large septal defects, isolated mild aortic, and pulmonary and mitral valvar disease.
IMD, Index of Multiple Deprivation; Q, quintile.
The outcomes for the whole cohort (N=10 298) are shown in table 2 and figure 2.
Table 2.
Outcomes of 10 298 patients at their 22nd birthday, overall and by complexity group
| n | Transfer to ACHD services n (row %) |
Death without transfer n (row %) |
Not transferred to ACHD services (alive) n (row %) |
Outcome censored before age 22 n (row %) |
Estimated probability of transfer at age 22 % (95% CI) |
|
| All patients | 10 298 | 6567 (63.8) | 42 (0.4) | 1402 (13.6) | 2287 (22.2) | 68.3 (67.3 to 69.3) |
| Complexity | ||||||
| Severe | 1454 | 1329 (91.4) | 12 (0.8) | 19 (1.3) | 94 (6.5) | 96.5 (95.3 to 97.7) |
| Moderate | 4366 | 3573 (81.8) | 15 (0.3) | 264 (6.0) | 514 (11.8) | 86.7 (85.6 to 87.9) |
| Mild | 4478 | 1665 (37.2) | 15 (0.3) | 1119 (25.0) | 1679 (37.5) | 41.0 (39.4 to 42.6) |
The estimated probabilities (conditional probability function) of transfer are conditional on survival of patients and take into account the mortality and censoring of patients.
ACHD, adult congenital heart disease.
Figure 2.
Whole cohort estimated probability of transfer if alive. Overall estimate (left) and by complexity (right) over the follow-up period between the 16th and 22nd birthdays. The estimated probabilities conditional on survival of patients take into account the mortality and right-censoring of patients. Note all complexity conditional probability functions were significantly different pairwise (Pepe-Mori test p<0.001).
Of the whole cohort, 63.8% transferred to ACHD services by their 22nd birthday. Of the patients, 166 (1.6%) died between 16 and 22 years; 42 of these (0.4%) died after the age of 16 but prior to transfer. The rates of transfer are determined by complexity. In 22.2% (n=2287) of the whole cohort, there were insufficient years of follow-up in the data set to ascertain their status by their 22nd birthday, but they had not died or had been transferred at the point of censoring.
The estimated probability of transfer by the 22nd birthday (calculated to take account of competing risk of death and right-censoring of data) was 68.3% (95% CI 67.3 to 69.3) for the whole cohort, 96.5% (95% CI 95.3 to 97.7) in the severely complex group, 86.7% (95% CI 85.6 to 87.9) in the moderate group and only 41.0% (95% CI 39.4 to 42.6) in the mild complexity group.
Moderate and severe patients
Transfer and estimated probability (CPF) of transfer by age 20 for the moderate and severe cohort overall and according to our predetermined factors are shown in table 3 and online supplemental figure S2.
Table 3.
Outcomes of 5820 severe and moderate patients on their 20th birthday, overall and by group characteristics
| n | Transfer to ACHD services n (row %) |
Death without transfer n (row %) |
Not transferred to ACHD services (alive) n (row %) |
Outcome censored before age 20 n (row %) |
Estimated probability of transfer at age 20 % (95% CI) |
|
| All severe and moderate complexity | 5820 | 4747 (81.6) | 26 (0.4) | 611 (10.5) | 436 (7.5) | 84.7 (83.7 to 85.7) |
| Complexity | ||||||
| Severe | 1454 | 1303 (89.6) | 12 (0.8) | 67 (4.6) | 72 (5.0) | 93.5 (92.1 to 94.9) |
| Moderate | 4366 | 3444 (78.9) | 14 (0.3) | 545 (12.5) | 364 (8.3) | 81.7 (80.5 to 83.0) |
| Birth cohort | ||||||
| Born between 1987/1988 and 1993/1994 | 1979 | 1685 (85.1) | 15 (0.8) | 279 (14.1) | 0 (0) | 85.8 (84.3 to 87.3) |
| Born between 1995/1996 and 1999/2000 | 3841 | 3062 (79.7) | 11 (0.3) | 332 (8.6) | 436 (11.4) | 83.9 (82.5 to 85.2) |
| Sex | ||||||
| Male | 3389 | 2799 (82.6) | 19 (0.6) | 338 (10.0) | 233 (6.9) | 85.7 (84.5 to 87.0) |
| Female | 2431 | 1948 (80.1) | 7 (0.3) | 273 (11.2) | 203 (8.4) | 83.1 (81.5 to 84.7) |
| Ethnicity | ||||||
| White | 4803 | 3975 (82.8) | 20 (0.4) | 463 (9.6) | 345 (7.2) | 85.9 (84.8 to 86.9) |
| Non-white | 953 | 731 (76.7) | 6 (0.6) | 130 (13.6) | 86 (9.0) | 79.9 (77.2 to 82.6) |
| Missing | 64 | 41 (64.1) | 0 (0) | 18 (28.1) | 5 (7.8) | 65.9 (53.8 to 77.9) |
| Area or residence deprivation | ||||||
| Deprived area | 2496 | 1977 (79.2) | 11 (0.4) | 294 (11.8) | 214 (8.6) | 82.5 (80.9 to 84.1) |
| Non-deprived area | 3324 | 2770 (83.3) | 15 (0.5) | 317 (9.5) | 222 (6.7) | 86.2 (85.0 to 87.5) |
| Model of care* | ||||||
| Vertical model | 3368 | 2959 (87.9) | 10 (0.3) | 249 (7.4) | 150 (4.5) | 89.3 (88.2 to 90.4) |
| Horizontal model | 2452 | 1788 (72.9) | 16 (0.7) | 362 (14.8) | 286 (11.7) | 78.3 (76.5 to 80.1) |
The estimated probabilities (conditional probability function) of transfer are conditional on survival of patients and take into account the mortality and censoring of patients.
*Model of care: vertical if paediatric cardiac services and ACHD services are within the same hospital site; horizontal if paediatric services are in a dedicated children’s hospital with an affiliated ACHD service at a different hospital site. Details in online supplemental material.
ACHD, adult congenital heart disease.
Of the moderate and severely complex patients (n=5820), 81.6% (n=4747) were known to have transferred to adult services, 0.4% (n=26) died without transfer occurring, 10.5% (n=611) were known to be alive but had not transferred, and 436 (7.5%) did not have enough years of data to fully assess outcome on their 20th birthday. The estimated probability of transfer in the group as a whole at age 20 was 84.7% (95% CI 83.7 to 85.7).
Single variable and multivariable ORs (95% CI) are shown in table 4. In the multivariable model, moderate complexity (rather than severe) was the factor most likely to determine non-transfer (OR=0.30 (95% CI 0.26 to 0.35), p<0.001), followed by missing ethnicity (OR=0.31 (95% CI 0.18 to 0.52), p<0.001), horizontal model of care (OR=0.44 (95% CI 0.27 to 0.71), p=0.001), deprived area (OR=0.84 (95% CI 0.72 to 0.98), p=0.023) and female sex (OR=0.87 (95% CI 0.78 to 0.98), p=0.014).
Table 4.
OR for transfer to ACHD services of severe and moderate patients between age 16 and their 20th birthday, adjusting for covariates one at a time (single variable OR) or together (multivariable OR)
| Single variable OR (95% CI) |
Multivariable OR (95% CI) |
|
| Birth cohort | ||
| Born between 1987/1988 and 1993/1994 | 1.11 (0.87 to 1.40) | |
| Born between 1994/1995 and 1997/1998 (REF) | 1.00 | |
| Sex | ||
| Male (REF) | 1.00 | 1.00 |
| Female | 0.85** (0.77 to 0.94) | 0.87* (0.78 to 0.97) |
| Ethnicity | ||
| White (REF) | 1.00 | 1.00 |
| Non-white | 0.63* (0.40 to 1.00) | 0.68 (0.46 to 1.01) |
| Missing | 0.29*** (0.17 to 0.51) | 0.31*** (0.18 to 0.52) |
| Area of residence deprivation | ||
| Non-deprived area (REF) | 1.00 | 1.00 |
| Deprived area | 0.75*** (0.65 to 0.85) | 0.84* (0.72 to 0.98) |
| Complexity | ||
| Severe (REF) | 1.00 | 1.00 |
| Moderate | 0.33*** (0.28 to 0.38) | 0.30*** (0.26 to 0.35) |
| Model of care | ||
| Vertical (same site) model (REF) | 1.00 | 1.00 |
| Horizontal (not same site) model | 0.45** (0.26 to 0.75) | 0.44*** (0.27 to 0.71) |
The sample consists of 4036 moderate and severe complexity patients born before 1998/1999 (data covering all of their ages between 16 and 20) and alive at age 20 (2 patients were excluded to allow clustering SEs by last centre as child; see online supplemental material): 3425 were transferred to adult services and 611 were not. The multivariable model includes only factors that were significant in the single variable analysis.
***P≤0.001, **P<0.01, *P<0.05.
ACHD, adult congenital heart disease; REF, reference.
Model of care
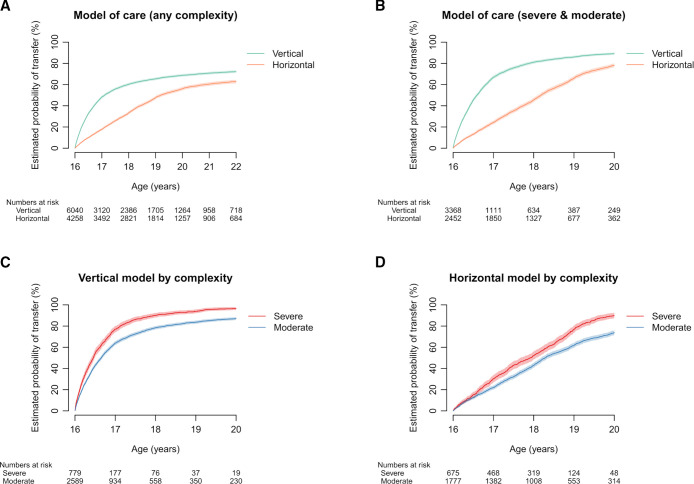
The multivariable analysis demonstrates that model of care is an important factor in determining transfer of moderate and severe patients. The estimated probability of transfer in the whole cohort at age 22 was 68.8% (95% CI 67.6 to 67.0) in the vertical model and 56.1% (95% CI 54.5 to 57.7) in the horizontal model. In the moderate/severe subgroup, the estimated probability of transfer at age 20 was 89.3% (95% CI 88.2 to 90.4) in the vertical model and 78.3% (95% CI 76.5 to 80.1) in the horizontal model (see table 3). The timing and rate of transfer by model are shown in figure 3.
Figure 3.
Outcomes by model of care and complexity. (A) Whole cohort by model of care. (B) Severe and moderate complexity by model of care. (C) Severe and moderate patients in the vertical model of care. (D) Severe and moderate patients in the horizontal model of care. The estimated probabilities conditional on survival of patients take into account the mortality and censoring of patients. For each subfigure, the pairs of conditional probability functions were significantly different (Pepe-Mori test p<0.001).
Transfer occurs significantly earlier in patients in a vertical model than in a horizontal model. Transfer by complexity in each model is shown in figure 3C, D, demonstrating that the timing of transfer is mostly determined by model of care rather than by complexity of the patient.
Patients who have not transferred by age 20
Of the 611 patients in the severe/moderate cohort who had not transferred by age 20 (table 3), 155 (25.4%) subsequently transferred between ages 20 and 22. Of these, 107 (69.0%) were from horizontal centres and 129 (83.2%) were of moderate rather than severe complexity.
Of the 283 patients in the severe/moderate cohort who were known to have not transferred by age 22 (table 2), 57.6% were from horizontal centres despite these patients only making up 42.1% of the overall severe/moderate cohort, demonstrating a shortfall in transfer for patients from horizontal centres even up to age 22. For patients between ages 16 and 22, 26 of 283 were only seen in cardiology at paediatric centres, and a further 89 patients had either an inpatient or outpatient episode in general adult cardiology. Of the remaining 168 patients, it was not possible to identify whether they were sent any cardiac appointments (and failed to attend) or were never sent appointments.
Outcomes in relation to transfer status
Despite complex CHD, the probability of death in both groups remained very low and was not impacted by transfer status: 2.4% (95% CI 0.8 to 4.0) vs 3.9% (95% CI 3.1 to 4.8) (figure 4A). Patients transferred by age 20 had significantly higher probability of undergoing a further NCHDA procedure between ages 20 and 30: 12.3% (95% CI 5.1 to 19.6) vs 32.5% (95% CI 28.7 to 36.3) (figure 4B).
Figure 4.
(A) Kaplan-Meier average (%, 95% CI) survival curves by transfer status at age 20. The sample is a subgroup of 4038 severe and moderate patients alive at age 20 and still followed by the data set (born before 1998/1999). (B) Cumulative probability functions of undergoing a further NCHDA procedure between the ages of 20 and 30 by transfer status at age 20. The sample for B is a subgroup of 3391 severe and moderate patients alive at age 20 and still followed by the NCHDA data set (born before 1997/1998). The two conditional probability functions were significantly different (Pepe-Mori test p<0.001). NCHDA, National Congenital Heart Disease Audit.
Discussion
Lifelong specialist ACHD follow-up is appropriate for all but the least complex of congenital heart lesions detected in childhood, so as patients enter their teens the process of transition begins. Transition programmes for adolescent patients aim to reiterate the importance of long-term care and to empower patients to take ownership of their own healthcare decisions. Effective transition programmes improve the chance of transfer to adult care,9 which usually occurs at ages 16–18 depending on the individual needs and comorbidities of the patient. Rate of transfer is only one measurement of effectiveness and does not reflect other aspects of quality of a transition programme, which cannot be captured in routine data collection.
Our data demonstrate a very high rate of transfer to specialist ACHD services in England for patients with severe and moderate lesions, with an estimated probability of transfer of 96.5% for severely complex patients and 86.7% for moderately complex patients by their 22nd birthday. Only 1.3% of severely complex and 6.0% of moderately complex patients are identified as being lost to follow-up at this point, with small numbers of patients with unknown outcomes due to incompleteness of their timelines. Previous studies from Canada and the USA show higher proportions of patients being lost to follow-up.22 23 Despite these successes, overall, 10.5% of our moderate and complex patient cohort in England did not transfer to specialist adult congenital services by their 20th birthday, with very small numbers of patients continuing to transfer after the age of 20. Gaps in care and lack of regular specialist follow-up are likely to have a detrimental impact on long-term outcomes.24 It is important that patients in our cohort who do not transfer by age 20 undergo significantly fewer NCHDA procedures in the subsequent decade (figure 4B), suggesting they may be missing out on standard interventions offered relatively routinely to patients under active follow-up.
When we focus on those with moderate and severe complexity, various factors were found to be important for transfer. In our cohort women were slightly less likely to transfer than men and the reasons for this are unclear. Our cohort was unbalanced with regard to gender split at baseline, with more men than women. This gender imbalance in complex CHD is well described and the differences we see may merely reflect subtle differences in patient complexity not captured by our severity groupings.
Transition programmes develop over time, responding to local factors and the changing needs of patients, and as such we may expect to see an increase in effectiveness over time. However, we did not demonstrate any differences in the effectiveness of transfer between our two birth cohorts.
Social deprivation was a significant determinant of failure to transfer care in this study, as has been previously reported.12 24 While we did not demonstrate a difference between white and non-white groups, patients with ‘missing’ ethnicity data were less likely to transfer. It is likely that there is overlap and interaction between these two factors, as patients from ethnic minority communities are more likely to reside in areas of higher deprivation.25
How we organise care does appear to have a marked impact on both timing and eventual rate of transfer. In England there are two models: vertical model (care from infancy to death at the same institution) and horizontal model (where paediatric care and adult care are in two separate institutions). In our study, patients from a horizontal model were less likely to transfer to adult services by their 22nd birthday, regardless of complexity.
The optimal age for transfer to adult services for individual patients varies depending on their maturity, other health needs and patient preference, but most authors recommend transfer between 16 and 18 years.26 27 Later transfer may be appropriate in patients with complex needs remaining under the care of multiple paediatric specialties, but this may restrict autonomy of the young adult in relationships with both medical caregivers and parents and limit access to expert advice regarding sexual and reproductive health, more commonly the domain of adult practitioners. Later transfer may also pose difficulties in the event of acute admissions as access to inpatient facilities tends to be determined by age. Conversely, vertical model units transfer the majority of severe and moderately complex patients by age 17 and almost all by age 18. This approach may not necessarily be in the best interests of patients with complex needs, or low levels of maturity, and may reflect a lack of institutional flexibility in how care is best provided. These discussions aside, it remains more likely that patients from a horizontal model will be lost to follow-up at their 22nd birthday.
In our cohort, only 37% of patients with mild lesions, as defined by the ESC guidelines,18 were transferred to ACHD services by age 22. From this data set it cannot be determined if this low rate of transfer was due to clinically appropriate planned discharge or not. There is increasing evidence that unrepaired, and even repaired, mild lesions do carry an excess of cardiovascular and respiratory morbidity in later life,28 29 such that it could be argued all of these patients should stay under lifelong follow-up to facilitate access to specialist care and advice regarding non-cardiac surgery, future pregnancy, contraception, genetic risk and endocarditis. This is balanced against personal and healthcare costs of well patients receiving arguably unnecessary follow-up. Our data suggest that a large proportion of patients with mild lesions are discharged prior to adulthood, or are never transfer, and their needs and ways to meet these needs should be studied in more detail.
Only 166 patients (1.6%) died between the ages of 16 and 22 years, with 42 of those dying without transfer to ACHD. In contrast to historical cohorts, the life expectancy curves for patients born with CHD now much more closely mimic those of the general population.30 The extremely good prognosis for the vast majority of teenagers with CHD is another driver for timely transfer through to adult services in all patients so they can build and develop relationships with their adult team likely to be looking after them for many years to come.
Limitations
Our cohort consisted of patients undergoing a surgical or interventional cardiac procedure as a child. Patients with CHD who did not undergo a procedure were not included. However, the study was likely to capture almost all of those with moderate or severe disease who survived to adulthood.
As in any similar study, the data set had limited granularity and was subject to the limitations of coding and hospital information systems throughout England.
Right-censoring of follow-up data for later births limited some data analyses, with not all patients reaching an event endpoint or age endpoint within the study time. Competing risks analysis (CPF estimation) was performed to minimise this impact.
Recording of ethnicity was incomplete, limiting our analyses into the impact of ethnicity.
There are likely to be other patients born during our study period who had procedures in childhood prior to the NCHDA being set up in the late 1990s, so those born between 1987 and 1997–2000 are likely to be under-represented.
Conclusion
Overall, transfer of severe and moderately complex congenital heart patients to specialist adult services in England is extremely effective. Future initiatives should focus on effective care planning for those at increased risk of loss to follow-up. These include transition programmes codesigned with partners from non-white groups and deprived areas to address barriers to transfer. Caregivers in both horizontal and vertical models should consider the demonstrated differences between models of care and whether changes should be made to their current programmes. Those in horizontal models should note evidence of lower numbers successfully transferring overall and further invest in robust links with their ACHD partners. Finally, careful thought should be given to the needs of those with minor lesions in whom there may be increased late morbidity.
Footnotes
Contributors: All authors planned the overall study design and analysis. FEP undertook the statistical analysis. RF used his coding expertise to allocate patients to complexity groupings, with clinical assistance from LS and KE. All authors were involved in the writing and approval of the final manuscript. KE is acting as guarantor.
Funding: This study was funded by The Health Foundation (grant #685009).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research as part of the LAUNCHES QI project.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. Data are subject to data agreements that do not allow third-party access.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study was approved by the North of Scotland Research Ethics Committee (trial #18/NS/0106).
References
- 1. Brown KL, Crowe S, Franklin R, et al. Trends in 30-day mortality rate and case mix for paediatric cardiac surgery in the UK between 2000 and 2010. Open Heart 2015;2:e000157. 10.1136/openhrt-2014-000157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raissadati A, Nieminen H, Jokinen E, et al. Progress in late results among pediatric cardiac surgery patients: a population-based 6-decade study with 98% follow-up. Circulation 2015;131:347–53. 10.1161/CIRCULATIONAHA.114.011190 [DOI] [PubMed] [Google Scholar]
- 3. Hernández-Madrid A, Paul T, Abrams D, et al. Arrhythmias in congenital heart disease: a position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working group on grown-up congenital heart disease, endorsed by HRS, PACES, APHRS, and SOLAECE. Europace 2018;20:1719–53. 10.1093/europace/eux380 [DOI] [PubMed] [Google Scholar]
- 4. Brida M, Gatzoulis MA. Pulmonary arterial hypertension in adult congenital heart disease. Heart 2018;104:1568–74. 10.1136/heartjnl-2017-312106 [DOI] [PubMed] [Google Scholar]
- 5. Leusveld EM, Kauling RM, Geenen LW, et al. Heart failure in congenital heart disease: management options and clinical challenges. Expert Rev Cardiovasc Ther 2020;18:503–16. 10.1080/14779072.2020.1797488 [DOI] [PubMed] [Google Scholar]
- 6. Vincent LL, Otto CM. Infective endocarditis: update on epidemiology, outcomes, and management. Curr Cardiol Rep 2018;20:86. 10.1007/s11886-018-1043-2 [DOI] [PubMed] [Google Scholar]
- 7. Yu C, Moore BM, Kotchetkova I, et al. Causes of death in a contemporary adult congenital heart disease cohort. Heart 2018;104:1678–82. 10.1136/heartjnl-2017-312777 [DOI] [PubMed] [Google Scholar]
- 8. Moons P, Bratt E-L, De Backer J, et al. Transition to adulthood and transfer to adult care of adolescents with congenital heart disease: a global consensus statement of the ESC Association of Cardiovascular Nursing and Allied Professions (ACNAP), the ESC Working Group on Adult Congenital Heart Disease (WG ACHD), the Association for European Paediatric and Congenital Cardiology (AEPC), the Pan-African Society of Cardiology (PASCAR), the Asia-Pacific Pediatric Cardiac Society (APPCS), the Inter-American Society of Cardiology (IASC), the Cardiac Society of Australia and New Zealand (CSANZ), the International Society for Adult Congenital Heart Disease (ISACHD), the World Heart Federation (WHF), the European Congenital Heart Disease Organisation (ECHDO), and the Global Alliance for Rheumatic and Congenital Hearts (Global ARCH). Eur Heart J 2021;42:4213–23. 10.1093/eurheartj/ehab388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wray J, Frigiola A, Bull C, et al. Loss to specialist follow-up in congenital heart disease; out of sight, out of mind. Heart 2013;99:485–90. 10.1136/heartjnl-2012-302831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mylotte D, Pilote L, Ionescu-Ittu R, et al. Specialized adult congenital heart disease care: the impact of policy on mortality. Circulation 2014;129:1804–12. 10.1161/CIRCULATIONAHA.113.005817 [DOI] [PubMed] [Google Scholar]
- 11. Heery E, Sheehan AM, While AE, et al. Experiences and outcomes of transition from pediatric to adult health care services for young people with congenital heart disease: a systematic review. Congenit Heart Dis 2015;10:413–27. 10.1111/chd.12251 [DOI] [PubMed] [Google Scholar]
- 12. Congenital heart disease standards and specifications. Available: https://www.england.nhs.uk/wp-content/uploads/2018/08/Congenital-heart-disease-standards-and-specifications.pdf
- 13. Espuny Pujol F, Pagel C, Brown KL, et al. Linkage of national congenital heart disease audit data to Hospital, critical care and mortality national data sets to enable research focused on quality improvement. BMJ Open 2022;12:e057343. 10.1136/bmjopen-2021-057343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. NICOR | congenital heart disease in children and adults (congenital audit). Available: https://www.nicor.org.uk/national-cardiac-audit-programme/congenital-heart-disease-in-children-and-adults-congenital-audit/
- 15. Universities of Leeds & Leicester. PICANet – Paediatric Intensive Care Audit Network for the UK and Ireland. Available: https://www.picanet.org.uk
- 16. Harrison DA, Brady AR, Rowan K. Case mix, outcome and length of stay for admissions to adult, general critical care units in England, Wales and Northern Ireland: the Intensive Care National Audit & Research Centre Case Mix Programme Database. Crit Care 2004;9:S1. 10.1186/cc3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herbert A, Wijlaars L, Zylbersztejn A, et al. Data resource profile: hospital episode statistics admitted patient care (HES APC). Int J Epidemiol 2017;46:1093–1093i. 10.1093/ije/dyx015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baumgartner H, De Backer J, Babu-Narayan SV, et al. 2020 ESC guidelines for the management of adult congenital heart disease. Eur Heart J 2021;42:563–645. 10.1093/eurheartj/ehaa554 [DOI] [PubMed] [Google Scholar]
- 19. English indices of deprivation. Available: https://www.gov.uk/government/collections/english-indices-of-deprivation
- 20. Pintilie M. ‘Competing Risks: A Practical Perspective’. Wiley, 2006. ISBN: 978-0-470-87069-3. [Google Scholar]
- 21. Pepe MS, Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med 1993;12:737–51. 10.1002/sim.4780120803 [DOI] [PubMed] [Google Scholar]
- 22. Reid GJ, Irvine MJ, McCrindle BW, et al. Prevalence and correlates of successful transfer from pediatric to adult health care among a cohort of young adults with complex congenital heart defects. Pediatrics 2004;113:e197–205. 10.1542/peds.113.3.e197 [DOI] [PubMed] [Google Scholar]
- 23. Kollengode MS, Daniels CJ, Zaidi AN. Loss of follow-up in transition to adult CHD: a single-centre experience. Cardiol Young 2018;28:1001–8. 10.1017/S1047951118000690 [DOI] [PubMed] [Google Scholar]
- 24. Kempny A, Diller G-P, Dimopoulos K, et al. Determinants of outpatient clinic attendance amongst adults with congenital heart disease and outcome. Int J Cardiol 2016;203:245–50. 10.1016/j.ijcard.2015.10.081 [DOI] [PubMed] [Google Scholar]
- 25. Knowles RL, Ridout D, Crowe S, et al. Ethnic and socioeconomic variation in incidence of congenital heart defects. Arch Dis Child 2017;102:1–7. 10.1136/archdischild-2016-311143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yassaee A, Hale D, Armitage A, et al. The impact of age of transfer on outcomes in the transition from pediatric to adult health systems: a systematic review of reviews. J Adolesc Health 2019;64:709–20. 10.1016/j.jadohealth.2018.11.023 [DOI] [PubMed] [Google Scholar]
- 27. Moons P, Pinxten S, Dedroog D, et al. Expectations and experiences of adolescents with congenital heart disease on being transferred from pediatric cardiology to an adult congenital heart disease program. J Adolesc Health 2009;44:316–22. 10.1016/j.jadohealth.2008.11.007 [DOI] [PubMed] [Google Scholar]
- 28. Saha P, Potiny P, Rigdon J, et al. Substantial cardiovascular morbidity in adults with lower-complexity congenital heart disease. Circulation 2019;139:1889–99. 10.1161/CIRCULATIONAHA.118.037064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldberg JF. Long-term Follow-up of "Simple" Lesions--Atrial Septal Defect, Ventricular Septal Defect, and Coarctation of the Aorta. Congenit Heart Dis 2015;10:466–74. 10.1111/chd.12298 [DOI] [PubMed] [Google Scholar]
- 30. Khairy P, Ionescu-Ittu R, Mackie AS, et al. Changing mortality in congenital heart disease. J Am Coll Cardiol 2010;56:1149–57. 10.1016/j.jacc.2010.03.085 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2022-321085supp001.pdf (535.3KB, pdf)
Data Availability Statement
No data are available. Data are subject to data agreements that do not allow third-party access.