Abstract
Each year, hundreds of millions of individuals are affected by respiratory disease leading to approximately 4 million deaths. Most respiratory pathologies involve substantially dysregulated immune processes that either fail to resolve the underlying process or actively exacerbate the disease. Therefore, clinicians have long considered immune-modulating corticosteroids (CSs), particularly glucocorticoids (GCs), as a critical tool for management of a wide spectrum of respiratory conditions. However, the complex interplay between effectiveness, risks and side effects can lead to different results, depending on the disease in consideration. In this comprehensive review, we present a summary of the bench and the bedside evidence regarding GC treatment in a spectrum of respiratory illnesses. We first describe here the experimental evidence of GC effects in the distal airways and/or parenchyma, both in vitro and in disease-specific animal studies, then we evaluate the recent clinical evidence regarding GC treatment in over 20 respiratory pathologies. Overall, CS remain a critical tool in the management of respiratory illness, but their benefits are dependent on the underlying pathology and should be weighed against patient-specific risks.
Keywords: anti-inflammatory agents, respiratory system, glucocorticoids, lung diseases, critical care
Introduction
Glucocorticoids (GCs) are a class of endogenous or synthetic steroid hormones that exhibit potent anti-inflammatory effects by regulating expression of inflammation-related genes. The GCs also influence metabolism, homeostasis, development and cognition.1 Synthetic GCs include dexamethasone, methylprednisolone, prednisolone, hydrocortisone, cortisone, and betamethasone.2 Natural GCs are produced by the adrenal gland and released into the systemic circulation. Natural and synthetic GCs both diffuse from the bloodstream to the cellular cytoplasm, where they bind GC receptors and form a protein complex with glucocorticoid receptor α (GRα). This GC-receptor-chaperone complex shuttles to the nucleus where it can bind DNA, promoters, transcription factors and other regions to regulate transcription of inflammation-related genes.3 4 The GCs can suppress the transcription of inflammatory genes such as STAT and NF-κB and increase the transcription of anti-inflammatory genes such as the NF-κB inhibitor IκBα, interleukin (IL)-10; IL-12, and other immune-controlling genes. They have become the backbone of the management of many acute and chronic respiratory illnesses, with an excellent safety profile and ample evidence for their effectiveness at suppressing pathologic inflammation.5 6
Mechanisms of action
General molecular mechanisms
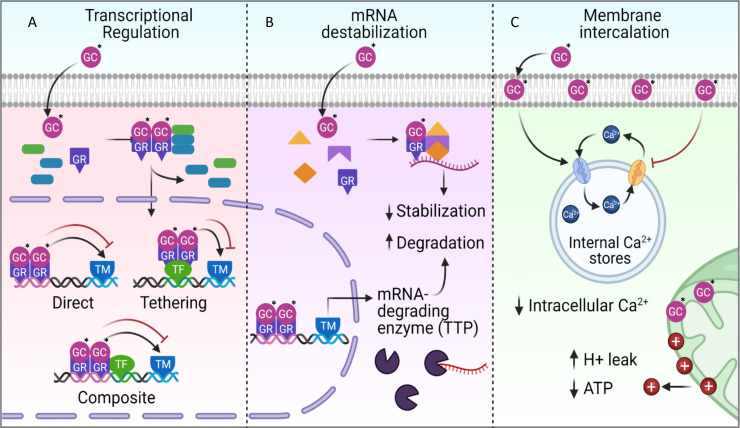
The GCs modulate transcription and translation of inflammation-related genes through a myriad of molecular interactions with the cellular membrane and its DNA, RNA and proteins (figure 1). GCs are known for their ability to complex with GRα and its chaperone proteins in the cytoplasm. This complex then shuttles GCs into the nucleus where it may interact with DNA in three primary ways to ultimately modulate the transcription machinery and influence the transcription of inflammation-related genes.3 7 First, the GR-GC complex may directly bind to accessible DNA to influence transcription. This is termed direct binding. Second, the GR-GC complex may bind a transcription factor that in turn binds DNA. This is termed tethering. Finally, the GR-GC complex may bind DNA directly, while also associating with a DNA-bound transcription factor. This is termed composite binding (figure 1A). All three of these DNA binding mechanisms are capable of promoting or downregulating transcription. Indeed, many canonical proinflammatory genes are downregulated (NF-κB, AP1, STAT, C/EBP, and NFAT inflammatory pathways), but GCs are also known to upregulate anti-inflammatory genes including TLR signaling inhibitors (DUSP1, MAPK1, IRAK3) and NF-κB inhibitors (IκBα, GILZ).3
Figure 1.
Molecular mechanisms of glucocorticoid (GC) action. (A) GCs are most well known for their effects on DNA transcription of inflammation-related genes. GCs in the cytoplasm associate with chaperone proteins and glucocorticoid receptor α (GR). This complex translocates to the nucleus to bind promoter regions of proinflammatory and anti-inflammatory genes. The GR-GC complex can promote or inhibit gene expression through directly binding the DNA promoter region (Direct); by binding to a transcription factor (Tethering); or by binding both the DNA and the transcription factor (Composite). (B) GCs can also interrupt inflammatory cascades by intercepting mRNA transcripts for proinflammatory genes such as NF-κB. GCs complex with GR and other chaperones to form a protein complex that binds to mRNA causing it to lose stability and degrade.8 9 GR-GC protein complexes also promote the transcription of the mRNA-degrading enzyme TTP, thereby increasing the degradation of cytoplasmic mRNA. (C) GCs induce immediate, non-genomic changes by intercalating in the cell membrane’s lipophilic interior. The intercalating GCs promote a reduction in intracellular calcium and ATP levels (* marks the GC).
GCs can also exert substantial non-genomic effects outside the nucleus through interaction with cellular membranes, cytoplasmic proteins, and mRNA (figure 1B). Interestingly, it was recently shown that GCs can destabilize cytoplasmic mRNA, resulting in the downregulated translation of proinflammatory mRNA. This mechanism involves the complexing of cytoplasmic GC with GR and chaperone proteins that then directly bind to mRNA and induce its degradation.8–10 GCs also modulate the expression of the mRNA-degrading protein tristetaprolin (TTP in figure 1B), which can increase degradation of proinflammatory genes in the cytoplasm. Many proinflammatory mediators are controlled by this mechanism including tumor necrosis factor-α (TNF-α), inteferon-β (IFN-β), interleukin (IL)-1α, IL-1β, IL-6, iNOS, and COX2.11–13
Additionally, GCs can exert virtually instantaneous effects on cellular energy metabolism, agonist-induced Ca2+ mobilization and reactive oxygen species (ROS) production. These effects are proposed to result from the intercalation of lipophilic GCs in cellular and mitochondrial membranes that induce complex signaling cascades14–16 (figure 1C). In GC-treated bronchial epithelial cells, for example, ATP consumption is reduced and Ca2+ is inhibited from cycling through intracellular stores into the cytoplasm.15 These rapid transcription-independent responses likely influence substantially the overall effects of GCs.
The GC effects greatly depend on the variant of GC, GR and cell phenotype.1 3 17 There are many types of GCs with specific molecular properties such as lipophilicity and binding affinity that influence their effects. Also, many variants of GR-a are possible through alternative splicing and post-translational modification. In kind, many genomic regulations are possible depending on the cell phenotype. Different cells may have variable accessibility of GC complex binding sites on chromatin that are regulated by epigenetic histone modifications, histone loops and other mechanisms.1 As a result, GC effects are cell, tissue and patient specific. A more comprehensive discussion of GC mechanisms can be found in the articles by Newton and Cruz-Topete and Cidlowski.3 6
Cellular mechanisms in the pulmonary microenvironment
GCs induce functional changes to each cell type in the distal airways and alveoli. In vitro studies of isolated cell types have identified both positive and negative physiologic effects in the distal lung microenvironment (figure 2). GCs enhance epithelial barrier function,18 19 reduce inflammatory cell infiltration,20–22 and suppress production of proinflammatory cytokines.17 However, GCs also appear to induce epithelial apoptosis,23 24 reduce airway cell proliferation,25 26 and suppress type I to type II pneumocyte transdifferentiation27 for the coverage of desquamated regions. These effects in aggregate may interfere with tissue regeneration after acute lung injury. Indeed, it is well known that delay dermal wound healing, although this is hypothesized to be an effect of suppressed fibroblast proliferation.28 However, recent clinical evidence does suggest that GCs have a net positive effect in acute lung injury.29
Figure 2.
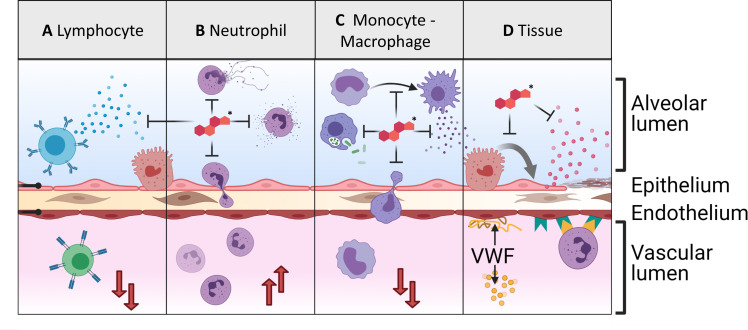
Glucocorticoid mechanism and cellular effects. (A) Glucocorticoids (GCs) inhibit lymphocytes’ production and release of inflammatory cytokines and reduce circulating lymphocyte counts by redirecting circulating lymphocytes to the lymphoid organs. (B) GCs inhibit inflammatory neutrophil behaviors such as degranulation, NETosis, and recruitment. They also increase circulating neutrophil counts by enhancing the maturation of neutrophils in the bone marrow. (C) GCs inhibit inflammatory macrophage and monocyte behaviors including the activation of monocytes into macrophages; the phagocytosis of bacteria; the release of cytokines by activated macrophages; and the recruitment of monocytes to inflamed areas. (D) GCs inhibit the transdifferentiation of alveolar type II pneumocytes into type I to cover damaged tissue. They also inhibit the release of proinflammatory cytokines. However, they also appear to increase the release of von Willebrand factor (VWF) and the expression of endothelial adhesion proteins (* marks the GC).
GCs also regulate immune and endothelial cells to suppress and promote inflammation. In vitro, GCs appear to enhance leukocyte adhesion to the endothelium by upregulating adhesion markers.30–32 They also increase the production of procoagulant factors including tissue factor and von Willebrand factor (VWF).30–33 Conversely, GCs inhibit neutrophil recruitment by suppressing the production of chemokines in the tissue and by resident immune cells. Finally, GCs suppress proliferation of lymphocytes, perhaps contributing to GC-induced lymphopenia.5 33 GC effects on fibroproliferation are contradictory and appear situation dependent.28 34–36
However, many of the effects observed in monocultures do not appear to translate to physiologic effect in vivo. For instance, there is little evidence of procoagulant effects in human subjects due to GCs despite ample evidence in vitro.37 Additionally, despite evidence that GCs induce enhanced endothelial expression of adhesion factors, neutrophil infiltration is substantially reduced at sites of inflammation following GC administration.22 Finally, while GCs appear to inhibit antibacterial capabilities of immune cells in vitro, there is little clinical evidence for increased risk of nosocomial infection in patients receiving GC treatment for acute respiratory failure, although opportunistic infections are possible with long-term treatment.38–40 Generally, while in vitro evidence can provide motivation for animal and human studies, it is limited in its ability to provide clinically useful insight independently (table 1).
Table 1.
Cell-specific GC effects
| Cell type | Effects |
| Global | ↓ Proinflammatory and chemotactic factors17
~ Coagulopathy37 ↓ Wound healing28 ↑ Infection risk (long term)40 |
| Lymphocyte | ↑ Apoptosis3
↓ Proliferation33 ↓ Circulating counts5 |
| Neutrophil | ↓ Recruitment21
,
22
↓ Inflammatory genes22 ↓ Apoptosis275 ↑ Circulating counts22 |
| Monocyte, macrophage | ↓ ROS, inflammasome20
↓ Shift M1,21 M2276 ↓ Chemotactic factors43 ↓ Adhesion, recruitment, accumulation5 , 43 ↓ Efferocytosis, phagocytosis43 ↓ Circulating counts5 |
| Alveolar and airway epithelium | ↑ Barrier integrity18
,
19
,
277–279
↑ SPA, SPD280 , 281 ↑ Type II maturation282 ↓ MUC5AC283 ↓ Type I>type II27 , 282 ↑ Apoptosis23 ↓ Proliferation, repair24–26 |
| Fibroblast | ↑ Contractility35
↓ Collagen deposition28 ~ Proliferation28 34 |
| Endothelium | ↓ NOS/vasoconstriction15
↓ Angiogenesis284 ↑ VWF, TF, ICAM, VCAM30–32 ↑ Neutrophil adhesion30–32 |
GC, glucocorticoid; ROS, reactive oxygen species; VWF, von Willebrand factor.
GCs in pulmonary pathology
Granulomatous and allergic inflammation
Granulomatous lesions are organized aggregates of primarily monocytic leukocytes and their derivatives, along with lymphocytes, multinucleated giant cells, epithelioid cells, and fibroblasts.41 Lesions form in response to foreign bodies, certain pathogens, smoke or toxin exposure, or as a consequence of allergic hypersensitivity. Granulomatous inflammation appears in the lung parenchyma, airways and/or lymphoid organs depending on etiology and generally responds well to corticosteroids (CSs).42 This is unsurprising given that GCs exhibit potent effects on the primary actors of granulomatous inflammation. Significantly for the clearance of granuloma tissue, GCs increase macrophages’ phagocytosis capacity to engulf apoptotic cells.43 GCs also reduce the proliferation and migration of inflammatory cells into granulomas by inhibiting monocyte and macrophage signaling of proinflammatory mediators, including IL-1β, IL-6, IL-12, TNFα, and GM-CSF, and downregulate the expression of chemokines like IL-8, RANTES, and MCP-1. Finally, GCs stimulate macrophages to produce anti-inflammatory mediators including Annexin-1, IL-10, and CD163.43
Pulmonary sarcoidosis
Several studies have suggested clinical benefits of CSs in pulmonary sarcoidosis. Randomized controlled trials (RCTs) exploring their efficacy were primarily conducted several decades ago, comprising heterogeneous populations, dosing, duration of therapy, and clinical follow-up.44–48 Regardless, overall outcomes generally support short-term improvements in symptoms, chest radiography, and pulmonary function. In a Cochrane review incorporating these studies, data analysis particularly favored treatment in those with parenchymal disease (stage II or III) with mean difference improvements in percent predicted of vital capacity and diffusion capacity increasing by 4.2% (CI 0.4% to 7.9%) and 5.7% (CI 1.0% to 10.5%), respectively.49
While evidence supports short-term improvements, data are lacking regarding CS treatment’s effect on long-term outcomes or modulating the natural progression of pulmonary sarcoidosis. Earlier RCTs demonstrated no persisting benefit on long-term follow-up after initial treatment with oral CSs for 3–24 months. However, certain key factors limit interpretation such as the inclusion of patients without parenchymal disease (stage I) or treating patients up-front without an observational period during which time many patients show partial or complete resolution. A 5-year longitudinal follow-up study of 149 patients with newly diagnosed parenchymal sarcoidosis aimed to address this gap.50 In this study, patients were initially observed for a 6-month period after which those who had persisting radiographic abnormalities on chest radiographs (39%) were allocated to receive oral CSs (regardless of symptoms) for 18 months or continued observation (with selective treatment if symptoms developed). Patients in the treated group experienced mild and comparative symptomatic and radiographic improvement with an average adjusted increase in vital capacity by 9% at the end of 5 years. Notably, the untreated group also tended toward higher fibrotic scores, although not reaching statistical significance. Similar results were reported in another RCT wherein patients with stage II and III disease were randomized to receive oral prednisone for 3 months followed by 15 months of inhaled CSs or placebo.51 After 5 years, patients that received immediate treatment had significant improvements in forced vital capacity (FVC) and diffusing capacity of the lungs for carbon monoxide (DLCO) and less subsequent requirements for CSs. Although CSs appear to have a marginal benefit for early treatment in parenchymal disease, clinical heterogeneity of disease and difficulty in selecting for patients with a tendency for progressive, refractory, or fibrotic phenotypes limit definitive interpretation.
The optimal dose and duration of therapy are unknown, so treatment guidelines make no specific recommendations. However, the typical initiating dosage varies from 20 mg daily (0.3 mg/kg/day) for those with indolent progressive symptoms, 40 mg daily (0.6 mg/kg/day) for those with rapidly progressive disease, and 80–100 mg daily for those with acute respiratory failure.52–54 This dose is usually maintained for 4–6 weeks after which steroids are slowly tapered over a 12 month period by 5–10 mg every 4–12 weeks once symptomatic, physiologic, or radiographic parameters improve. Recurrences appear in up to 50%–60% of patients as the dosage is reduced or once CSs are discontinued altogether. Monotherapy with inhaled CSs have also been explored as alternative therapy, although results appear conflicting. Clinical benefits have been reported in some patients, but evidence for clear and objective improvement is lacking.55–58
Acute hypersensitivity pneumonitis
The role of CSs has been primarily evaluated in farmer’s and bird fancier’s lung, although the data are fairly limited.59–62 In one randomized control study in patients presenting with farmer’s lung comparing 8 weeks of prednisolone (n=20) to placebo (n=16), prednisolone treatment was associated with an improvement in forced expiratory volume in 1 s (FEV1), FVC, and DLCO at 1 month. Thereafter, these differences diminished and no disparities in pulmonary function were noted at 1 and 5 years.60 Another study reported similar initial benefits without significant long term differences in symptoms or pulmonary function with 4 weeks, 12 weeks, or no therapy.59 These findings suggest that although CSs do not improve long-term outcomes, they provide short-term relief in those with severe or persistent symptoms.
Chronic hypersensitivity pneumonitis (cHP)
Although unrecognized and untreated acute episodes may evolve into cHP, many patients have no acute episodes and present with progressive and chronic respiratory insufficiency over several weeks to months resulting from persistent, low level antigen exposure.63 These patients can be further classified into either a non-fibrotic or fibrotic disease pattern. The latter is associated with reduced survival, especially accompanied by usual interstitial pneumonia (UIP)-like histology.64 Antigen avoidance remains key to management. Patients lacking an identifiable inciting antigen have increased mortality and adverse outcomes.65 For those with progressive symptoms, CSs have been the primary immunosuppressive therapy for decades. Despite this, no randomized trials or observational studies have evaluated CS efficacy in cHP. However, the lymphocytic disease process suggests steroid responsive disease process and positive studies in acute HP support its use.66–69
CS efficacy in non-fibrotic (nfHP) and fibrotic (fHP) phenotypes of HP is now an important distinction that is predictive of treatment responsiveness and long-term outcomes.64 Only one observational study to date has evaluated the role of CSs in those with nfHP and fHP.70 In this report of 202 patients, the nfHP cohort (n=93) treated with CSs experienced a monthly improvement in FVC of 0.84% compared with a 0.35% monthly decline prior to initiation of treatment. A non-significant trend toward increased DLCO was also noted. Conversely, patients with fHP (n=109) continued to experience a decline in both FVC and DLCO irrespective of CS dose or duration of therapy. Those treated with CSs trended toward worse survival in comparison to untreated patients with fHP. These results distinctly vary from another study that evaluated outcomes in patients with cHP treated with prednisone, azathioprine, or mycophenolate mofetil.71 The cohort treated with prednisone alone experienced a FVC decline of 10.8% (±2.7%) over 36 months. Notably, this analysis did not distinguish patients based on a predominant non-fibrotic or fibrotic phenotype despite 85% of patients having ground glass opacities on high-resolution CT and 51% having honeycombing. As a result, there is a possibility that the magnitude of decline may have been driven by a proportion of patients with a predominant fibrotic phenotype. These outcomes in pulmonary function were similar to the mycophenolate only group, but in those receiving prednisone and subsequently commenced on mycophenolate or azathioprine, the slope of monthly decline in FVC was significantly reduced (−0.7% vs −0.2%) along with a reduction in treatment associated adverse events.
Overall, these data suggest that corticosteroids have greater utility in treating patients with a predominant non-fibrotic phenotype which is in line with acute HP. In patients with fibrotic HP, CSs do not seem to reduce decline in pulmonary function and could be associated with increased mortality. In all patients with cHP irrespective of phenotype, prednisone monotherapy may be inferior to mycophenolate or azathioprine in reducing the rate of decline in pulmonary function.
GPA and MPA
Historically, no RCTs have evaluated corticosteroid monotherapy for the treatment of granulomatous polyangiitis (GPA) and microscopic polyangiitis (MPA). Initial, observational and anecdotal experiences in acute episodes of vasculitis described very high rates of relapses and mortality, prolonging survival by only several months.72–74 Further disease phenotype characterizations, the description of ANCA and the introduction of combination therapy including cyclophosphamide transformed the therapeutic landscape, with significantly improved remission and mortality.72 75–77 Resultantly, CSs are now predominantly used as adjunctive therapy with additional immunosuppressants such as cyclophosphamide, rituximab, azathioprine, or methotrexate in induction and maintenance phases of therapy.
For patients with organ or life-threatening disease, commonly used induction regimens consist of combination therapy with either cyclophosphamide or rituximab in addition to high-dose oral CS therapy of 1 mg/kg/day prednisone maintained for at least 1 month slowly tapered over several months to a lower maintenance dose between 5 and 10 mg/day.75 78–81 High-dose pulse intravenous steroids (ie, methylprednisolone 7–15 mg/kg up to 1000 mg/day for 1–3 days) are generally used in cases of alveolar hemorrhage, rapidly progressive glomerulonephritis, optic neuritis, or mononeuritis multiplex. In the landmark RAVE trial that demonstrated non-inferiority of rituximab to cyclophosphamide (in addition to CS), and which included patients with pulmonary manifestations in 50% of patients, this approach resulted in similar rates of remission at the end of 6 months (64% vs 53%).79 In the 25% with alveolar hemorrhage (none of whom required ventilatory support), 57% of those with rituximab (vs 41%) achieved the study endpoint. Similarly, in the absence of organ or life-threatening generalized disease, studies evaluating the use of cyclophosphamide and methotrexate used an initial oral prednisone or prednisolone dose of 1 mg/kg/day.75 82
Maintenance therapy following 3–6 months of induction consists of methotrexate, azathioprine, or mycophenolate in addition to low-dose corticosteroids with the goal of preventing disease relapse and minimizing cumulative CS exposure. Although there are no standardized protocols or strong evidence for long-term use, a meta-analysis of 13 RCTs and observational studies suggested that early withdrawal of CSs was associated with higher rates of disease relapse.83 Only 14% of patients receiving CSs (vs 43% of controls) experienced at least one relapse. While these findings suggest that CSs play an important role in maintaining disease remission, interpretation of these results may be limited due to heterogeneity in treatment regimens. This question remains to be answered in RCTs.
Eosinophilic granulomatosis with polyangiitis (EGPA)
Similar to GPA and MPA, CSs are used in conjunction with additional immunosuppressive therapy in the presence of poor prognostic factors (Five Factor Score (FFS)≥1) or life-threatening or organ-threatening disease such as alveolar hemorrhage or cardiac, gastrointestinal, central nervous system, or renal (glomerulonephritis) involvement.84 85 In the absence of these factors, single-agent anti-inflammatory monotherapy is often successful. For example, in the only trial (n=72) evaluating the efficacy of systemic CSs alone without poor prognostic factors (FFS)=0) and in which 67% of patients had pulmonary infiltrates, 93% achieved clinical remission (absence of active vasculitis for 3 months) with initial high-dose prednisone monotherapy alone (1 mg/kg/day for 3 weeks, tapered to minimal effective dosage).86 Five patients failed to respond and 25 (total 42%) had relapsed symptoms following tapering or termination of CSs requiring randomization to receive adjuvant azathioprine or cyclophosphamide. While relapses and long-term CS use is not uncommon and predominantly driven by difficult to control asthma, alveolar manifestations appear to respond well to therapy with only four patients found to have new pulmonary infiltrates—a phenomenon that has been reported elsewhere.87 88
Although alveolar hemorrhage is infrequently encountered, it remains a life-threatening and under-recognized complication in patients with EGPA. Data evaluating outcomes in this subset of patients are sparse and only described in case reports. As a result, current understanding of optimal therapy and long-term outcomes is limited and largely extrapolated from anecdotal reports and experiences gathered from alveolar hemorrhage in GPA and MPA. Current EGPA Task Force Consensus Guidelines recommend using combination therapy with high-dose systemic CSs and cyclophosphamide followed by maintenance therapy with azathioprine or methotrexate for patients with alveolar hemorrhage.84
Asthma
Historically, oral cortisone was first introduced as routine therapy for chronic bronchial asthma in the 1950s.89 90 Although effectively used as mainstay therapy for the subsequent two decades, adverse effects of chronic systemic therapy paved the development and routine application of inhaled corticosteroid (ICS) therapy. The first of these was beclomethasone, with initial randomized studies in the 1970s confirming the efficacy of inhaled monotherapy by demonstrating improvement in symptom control, lung function (FEV1), and dose reduction of chronic oral CSs.91–94 Since, several inhaled CS of varying potency have been developed and used as monotherapy and in combination with short-acting and long-acting beta agonists (SABA, LABA), long-acting muscarinic antagonists (LAMA), leukotriene inhibitors, and biologics. Cumulative evidence has solidified these initially noted benefits; in addition, it has demonstrated improved quality of life, reduced rates of acute exacerbation, and providing a protective effect against severe exacerbations.95 96 For example, in a Cochrane analysis of 68 studies comprising 11,104 subjects, monotherapy with inhaled fluticasone propionate in patients with mild and moderate disease was associated with a dose-dependent increase in FEV1 (0.13–0.45 L), morning peak expiratory flow (27–47 L/min), symptom scores, reduction in rescue beta-2 agonist use (reduction between 1.2 and 2.2 puffs/day), and reduction in the number of patients dependent on systemic therapy.97 Large population-based studies have also suggested a mortality benefit for maintenance therapy in patients with persistent disease. In an analysis of a cohort comprising 30,569 individuals from Saskatchewan, Canada, the rate ratio of death from asthma exceeded 2.5 for patients who received no ICS therapy and decreased to 0.25 in patients that used ICS consistently (12 cannisters per year). It was estimated that with each additional cannister of ICS used in the previous year, there was a 21% reduction in the rate of death.98 Underlying these clinical benefits, the use of ICS therapy, even in short durations, has consistently shown to effectively reduce airway inflammation and chronic airway remodeling particularly in patients with an atopic and eosinophilic phenotypes.95 99–101 In summary, existing data strongly support the use of ICS as mainstay therapy in the treatment of persistent asthma and as such, comprises the backbone of treatment recommendations set forth by Global Initiative for Asthma (GINA) guidelines.102
While the role of systemic CSs as part of maintenance therapy for severe persistent disease has diminished with the introduction of biologics-based therapy, oral CSs play a vital role in the management of acute exacerbations. In outpatients treated for mild to moderate exacerbations presenting to the emergency room, short courses equivalent to 40–60 mg/day for 5–7 days are associated with a reduction in symptom severity, SABA usage, and probability of subsequent exacerbations requiring additional therapy or further healthcare utilization.103–106 While the data in regard to the optimal dosage and duration of therapy for those treated for severe exacerbations leading to hospital admission are not as robust, expert opinion has often advocated for higher dose requirements, particularly in patients with respiratory failure requiring intensive care unit (ICU) admission (ie, methylprednisolone 60–80 mg every 6–8 hours).107 108
In summary, CSs are the mainstay of therapy in asthma, especially in the eosinophilic pheno-endotypes, while in more severe or non-eosinophilic asthma, CS may in fact fail to suppress the neutrophilic inflammation and may even promote neutrophil survival.
Chronic obstructive pulmonary disease (COPD)
In vitro studies show that airway inflammation in COPD is generally unresponsive to CS, and that drugs such as beta-2 adrenergic agonists, macrolides and theophylline may increase the CS sensitivity, yet these observations have not had major implications on the current standard of care.109 110
In vivo studies found that dose–response relationships and long-term (>3-year duration) safety of ICS therapy in COPD are still unclear and require further investigation.111 As such, because the effects of ICS in COPD could be modulated by concomitant use of long-acting bronchodilators, these combinations are discussed separately.
ICS monotherapy
Most studies found inconclusive evidence of benefit in COPD, as ICS monotherapy does not change FEV1 decline or general mortality over time.112 In the TORCH study,113 a trend toward higher mortality was noted in the fluticasone propionate alone arm versus those on placebo or on salmeterol plus fluticasone propionate combination. However, in the SUMMIT trial,114 the increase in mortality was non-observed in patients with COPD treated with fluticasone furoate; furthermore, in moderate COPD, the groups on fluticasone furoate alone or fluticasone furoate plus vilanterol had slower declines in FEV1 versus placebo or vilanterol alone.
ICS in combination therapy with long-acting bronchodilators
ICSs are frequently prescribed for patients with COPD in combination with inhaled LAMA and LABA therapy. Long-term randomized control trials evaluating the use of ICS monotherapy in patients with COPD have demonstrated varying effects on clinical endpoints, however, have failed to demonstrate a modifying effect on lung function.115–118 Similarly, two meta-analyses, including one large Cochrane review of 55 RCTs have confirmed a lack of benefit of ICS therapy in attenuating the rate of decline in FEV1 (mean difference 5.80 mL/year with ICS vs placebo, 95% CI –0.28 to 11.88) in 2333 participants.112 119 In addition, in this Cochrane analysis, long-term use was not associated with a mortality benefit, although there were additional benefits in secondary outcomes, including a reduction in both the rate of annual exacerbation (−0.26; 95% CI,−0.37 to −0.14) and the rate of decline in the quality of life, as measured by St. George’s Respiratory Questionnaire (SGRQ; mean difference −1.22 units/year, 95% CI −1.83 to −0.60).112
In the subset of patients with moderate to very severe COPD and history of exacerbations, LAMA and/or ICS combination therapy with LABA appears to be more effective than either component alone. A meta-analysis evaluating 14 RCTs comparing ICS/LABA to LABA monotherapy found a reduction in annual exacerbation rates (rate ratio 0.76, 95% CI 0.68 to 0.84) in 9921 participants, and an improvement in SGRQ (1.58 to 2.69 units lower), dyspnea, symptoms, and rescue inhaler use without a difference in mortality.120 Likewise, triple therapy (ie, ICS, LAMA and LABA combination) is associated with similar clinical benefits, in addition to providing a mortality benefit in this population. This was first demonstrated in the IMPACT trial, which evaluated the role of triple therapy to ICS/LBA and LAMA/LABA.121 All-cause mortality was significantly lower in those treated with triple therapy than LAMA/LABA (HR 0.58, 95% CI 0.38 to 0.88) in addition to reducing the annual rate of severe exacerbation resulting in hospitalization (rate ratio 0.66, 95% CI 0.56 to 0.78).122 Further, in the ETHOS trial that evaluated the role of triple therapy at two doses of budesonide (160 and 320 µg budesonide), a reduction in mortality was only noted in patients treated with higher dose budesonide triple therapy compared with LABA/LAMA (HR 0.54; 95% CI 0.34 to 0.87).123
Despite the benefits attributable to ICS, their widespread use has been limited by a potential undesired increased risk of developing pneumonia, especially in those with severe COPD.124 The first major study that brought to light such a relationship was the TORCH study,113 and numerous others have added evidence in support of this observation.124
Current Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines advocate for use of ICS therapy in patients that have factors that are predictive of a therapeutic response.32 Evidence suggests that ICS therapy has little effect in patients with peripheral blood eosinophil counts of <100 cells/µL, with incremental benefits noted in those with higher counts.32 125–127 Blood eosinophil counts ≥300 cells/µL have been suggested to distinguish patients that may have the greatest probability of benefiting with ICS therapy.32 Moreover, a beneficial therapeutic response has been shown for patients with high exacerbation risk (≥2 exacerbations and/or 1 hospitalization within the previous year).32 125–127
Systemic CSs are frequently used in the management of acute exacerbations of COPD. Data support a reduction in recovery time, improvement in FEV1, risk of relapse, treatment failure, and length of hospitalization.128–130 Shorter durations of 5 approximately days have been advocated after the REDUCE trial demonstrated non-inferiority compared with previously accepted 14-day courses.32 131
In summary, regular treatment with ICS may increase the risk of pneumonia, especially in those with severe COPD; ICS combined with a LABA is more effective than either individual component in improving lung function and health status, and in reducing the exacerbation rate in moderate to very severe COPD; and triple therapy with inhaled ICS/LABA/LAMA improves lung function, health status and symptoms, and reduces exacerbations compared with dual or LAMA monotherapy; long-term use of oral CS has numerous side effects and questionable benefits.124
Diffuse alveolar inflammation/infection
Acute, severe inflammation of the alveo-capillary tissue, termed acute lung injury (ALI), describes the condition of the alveoli wherein there is induce a rapid efflux of neutrophils and their mediators; activation of resident macrophages and the tissue barrier itself, leading to loss of alveo-capillary barrier function, and an efflux of proteinaceous fluid in the airspace.132 This is caused by a range of etiologies ranging from acute exposure, infection, trauma or septic shock to chemical exposure, drowning, or pneumonia. In severe ALI, heightened presence and activation of inflammatory cells results in an overexpression of inflammatory mediators that can cause breakdown of the epithelial-endothelial barrier, resulting in fulminant respiratory failure, termed acute respiratory distress syndrome (ARDS).133
Glucocorticoids have shown promise at resolving ALI in experimental studies. In animal models, GCs attenuate diffuse alveolar inflammation and reduce the risk of progression from ALI to ARDS.134 135 In vitro studies indicate that neutrophilic inflammation is tempered by GCs.136 Additionally, although GCs induce maturation of neutrophils from the bone marrow resulting in peripheral neutrophilia, they also tightly regulate the migration of neutrophils to sites of inflammation. GCs prevent neutrophil accumulation in tissues by downregulating L, P and E-selectin from the cells’ surface, reducing neutrophil attachment to the endothelium and therefore preventing extravasation. GCs also reduce endothelial expression of selectin ligands including ICAM and VCAM. Finally, GCs reduce the activation of neutrophils by tamping proinflammatory cytokines and reduce superoxide release and ROS levels.22 It is well known that GCs inhibit the mRNA transcription of proinflammatory genes, which may be significant to reducing neutrophilic inflammation due to evidence that lung-transmigrated neutrophils exhibit a rapid burst of inflammatory transcription on arrival in the airspace.137 However, GCs have generally failed to show efficacy in ALI/ARDS in human clinical studies.138 Indeed, the influence of GCs in infection-induced acute injury appears pathogen-dependent. In murine studies, GCs did not improve lung injury pathology score of H5N1-infected mice but GCs improved the score of H1N1 pandemic influenza-infected mice139–141 (online supplemental table S1). Similarly, GCs improved lung injury score for mice infected with SARS-CoV-2 and not fungal pneumonia models142–145 (online supplemental table S1).
jim-2021-002161supp002.pdf (39.6KB, pdf)
COVID-19
While viral load clears within 3–5 days of the initial infection, the serious sequelae of COVID-19 emerge days later when viral titers are low or undetectable, suggesting that pathophysiologic mechanisms are linked to the dysregulated immunity following infection.146 147 As such, there is great interest in immunomodulatory therapies,148 149 and CSs have been evaluated in several case series, studies and trials.150 151 Initial results from case studies were negative, prompting the WHO to recommend against CS therapy in COVID-19.152 These guidelines were reversed afterward, in July 2020, when the RECOVERY Collaborative Group published positive results in its preliminary report on the open-label, controlled trial of dexamethasone for COVID-19.153 154 They reported that in 2104 patients receiving dexamethasone versus 4321 in the usual care group, dexamethasone reduced mortality among patients on invasive mechanical ventilation (rate ratio, 0.64; 95% CI, 0.51 to 0.81) and among those receiving oxygen without invasive mechanical ventilation (rate ratio, 0.82; 95% CI 0.72 to 0.94).154 Due to these findings, similar trials of GCs for COVID-19155–157 were halted, and international clinical practice guidelines were modified to support the use of GCs in moderate to severe COVID-19.151 158 While the most attention has been focused on dexamethasone since the influential RECOVERY trial, similar studies using methylprednisolone29 159 160 and hydrocortisone155 157 have also suggested benefit in COVID-19, although the quality of evidence for these studies is low to moderate, as the studies were either halted prematurely or were observational.
As evidence continues to emerge, GCs have become a first-line treatment in moderate to severe COVID-19. Indeed, a recent systematic review and meta-analysis by Cano et al 161 that evaluated 73 studies on 21,350 patients with COVID-19 concluded that CSs provide mortality benefit in severely ill patients (OR 0.65; 95% CI 0.51 to 0.83, p=0.0006).
Influenza
CSs as adjunct therapy to neuraminidase inhibitors were primarily used during the Influenza A/H1N1 pandemic of 2009, with some epidemiologic studies estimating its use in 18%–53% of critically ill patients requiring mechanical ventilation.162–164 Unfortunately and despite their widespread use, several meta-analyses have raised the issue of a potentially augmented risk of death with or without the use of concomitant neuraminidase inhibitors and irrespective of timing and dosage of CS therapy (relative risk (RR) or equivalent OR of 1.53–4.22).66–68 165 This is also associated with an increased risk of nosocomial infection (RR 1.98–3.15),66 67 165 rate of ICU admission,165 higher proportion of patients requiring mechanical ventilation,165 and increased ICU length of stay.66 To our knowledge, there are no RCTs that have assessed the benefits of CSs in viral influenza. Data incorporated into these analyses are heterogeneous and almost exclusively based on case-control or cohort studies, of which none proved unequivocally favorable effects. Overall, this is in stark contrast with data applicable to community acquired pneumonia of general etiologies (bacterial and mixed), and from animal models, which have shown improvement in histopathologic scores and survival rates.69 141
Pneumocystis jirovecii
Classically known as an opportunistic infection in HIV individuals, Pneumocystis jirovecii (previously called P. carinii) pneumonia (PCP or PJP) is increasingly recognized as posing a substantial risk to transplant recipients, patients with malignancies, and in those receiving chronic immunosuppression or cytotoxic therapy. Affected individuals can present with a wide spectrum of disease severity, ranging from an insidious onset of dyspnea in HIV-infected individuals, to fulminant respiratory failure in HIV-uninfected patients, with mortality approaching 50%–80% in this population.166 167
CSs used in a 21-day tapered regimen have been used as established adjuvant therapy for patients with HIV and moderate to severe PJP pneumonia, which is defined by (1) a partial pressure of oxygen <70 mm Hg on room air or (2) an alveolar-arterial (A-a) gradient of ≥35 mm Hg.168 169 Presumptively, the anti-inflammatory benefits may mitigate an enhanced inflammatory response and subsequent clinical deterioration known to occur within 3–5 days in response to microbial death caused by the initiation of anti-Pneumocystis-specific therapy.168 170 Earlier RCTs evaluating the use of adjuvant CS therapy in HIV-associated PJP showed significant reductions in the mortality rate at 1 month (RR 0.21–0.48)171–173 and need for mechanical ventilation (RR 0.24–0.35).171 173 Furthermore, aggregate data in a Cochrane review with three additional RCTs170 174 175 mirrored these favorable benefits with an overall reduction in the relative risk of death of 44% (RR 0.56) at 1 month and 41% (RR 0.59) at 3–4 months.176 This subsequently translated into a number needed to treat to prevent 1 death in 9 patients not on highly active antiretroviral therapy and 1 in 23 in those on it, along with a robust reduction in the need for mechanical ventilation at 1 month by 62% (RR 0.38).176
Despite clear-cut benefits in the HIV-infected population, the role of adjunctive CSs in HIV-uninfected patients remains controversial. Current evidence is weak and predominantly based on retrospective cohort studies, with a large majority failing to demonstrate favorable effects on mortality and need for mechanical ventilation.177–182 In a study of 323 HIV-negative patients with PJP, early CS therapy (within 48 hours of diagnosis) was not associated with any survival benefit, length of hospital stay, admission to the ICU, and need for mechanical ventilation by 1 month or physiologic improvement in respiratory Sequential Organ Failure Assessment (SOFA) score at day 5 following initiation of CS and pneumocystis-specific therapy.181 Conversely, in a larger study on 1299 participants, CSs were associated with improved 60-day mortality in patients with severe disease, as defined by a PaO2 ≤60 mm Hg HR 0.71) but not in those with moderate disease.183
Meta-analyses have also failed to demonstrate a survival benefit, suggesting in fact an increased risk of death (OR 1.37, CI 1.07 to 1.75).184 185 This analysis185 also revealed a survival benefit in patients with respiratory failure (OR 0.63), although the definition of respiratory failure was relatively heterogeneous among included studies, and a majority of cases (55%) originated from the study conducted by Inoue and colleagues, which defined respiratory failure as PaO2 ≤60 mm Hg.186
Community-acquired pneumonia (CAP)
CSs have been proposed as adjunctive therapy for the treatment of CAP for many years. Several animal models have demonstrated decline in circulating and pulmonary proinflammatory cytokine levels and reduction in histopathologic severity scores with adjunctive CS therapy.187–190 Similar reductions have been noted in prospective cohort studies for patients presenting with CAP and septic shock.191–193
Despite evidence for favorable pathophysiologic responses attributed to CS therapy, effects on mortality remain controversial. With the exception of two studies (Confalonieri et al,194 Nafae et al 195) that demonstrated a positive effect on mortality, most RCTs did not replicate these results in patients with severe CAP.194–204 Results from pooled meta analyses remained controversial for patients treated for severe CAP, however, have demonstrated no effect on mortality in those with non-severe CAP.205–209 The presence of differences can primarily be attributed to variability in study methodology and likely to poorly defined study populations due to heterogeneity in CS type, dosing, treatment duration, and criteria for defining CAP severity among individual RCTs. Irrespective of these potential confounding factors, CSs have demonstrated beneficial effects in improving time to clinical stability, reducing hospital length of stay, need for mechanical ventilation, and progression to acute respiratory distress syndrome, without increasing the risk for gastrointestinal bleeding or contributing to treatment failure.194–196 201 202 205–209 These results mirror data currently available for stress-dose CS therapy in patients with refractory septic shock.153 210 At the same time, one should remember that CS use in the intensive care setting could be associated with critical illness polyneuropathy, critical illness myopathy and/or delirium.
Fibrotic inflammation
Fibrotic inflammation is a dysregulated response to tissue injury that results in uncontrolled deposition of extracellular matrix (ECM) that interrupts tissue function. In the distal airways and alveoli, such fibrotic inflammation can result from acute or chronic exposure, infection, or trauma. These insults produce inflammation that activates fibroblasts to proliferate and produce ECM components. The resultant fibrotic pathologies are varied with a common theme of disruption of normal tissue structure by fibrotic processes.211–213 Despite the fact that inflammation seems to drive early fibroproliferation through the signaling of cytokines such as IL-1β and IL-6, CS treatment has proven ineffective in most fibrotic lung diseases.214 Further, animal models of pulmonary fibrosis are limited, slowing the progress toward antifibrotic therapeutics. The most common model is bleomycin injury that does not recapitulate the non-resolving IPF-associated fibroproliferation. Future studies of anti-fibrotic therapeutics are aimed at microphysiological systems incorporating human cells and tissues.215
Usual interstitial pneumonia UIP)
Almost universally, the presence of UIP, especially in IPF, is accompanied by a progressive clinical course resulting in chronic respiratory failure with poor long-term prognosis and response to several immunosuppressive therapies, including CSs. Hallmark histologic characteristics include a heterogeneous appearance of fibroblastic foci (both geographically and temporally), composed of dense collagen, fibroblasts, and myofibroblasts alternating with areas of normal lung parenchyma in a predominantly peripheral and subpleural distribution. Interstitial inflammatory infiltrates comprised of lymphocytes and plasma cells are generally mild and considered a non-dominant feature, highlighting support behind poor responses to immunosuppressive treatments.216
Idiopathic pulmonary fibrosis (IPF)
For many years, CSs were considered to be the backbone of conventional therapy based on retrospective observational studies that suggested a mild clinical benefit in approximately 15%–30% of patients.217–219 Notably, patients that responded to treatment were found to be younger and with a cellular-appearing biopsy,217 which is inconsistent with the typical demographic profile for IPF and current pathologic understanding and definition of UIP. As a result, it seems likely that those that responded favorably may have done so if they had steroid responsive histologic patterns such as desquamative interstitial pneumonia (DIP) or non-specific interstitial pneumonia (NSIP), which were not clearly delineated as separate entities until mid-1990s.220 221 Additional factors that limit interpretation of these results included heterogeneous definitions of treatment response and lack of objective, validated endpoints.
As a result, owing to a potential benefit in a disorder with a poor prognosis, no RCTs directly explored GC use versus placebo until the late 1980s, when CSs were used in the control arms of several trials evaluating both immunosuppressive (cyclophosphamide, azathioprine, colchicine) and non-immunosuppressive (N-acetylcysteine, D-penicillamine) adjunctive therapies.221–226 None of these studies demonstrated a clinically meaningful symptomatic, physiological, or survival advantage from any combination of therapy. Two trials evaluating (1) high-dose prednisolone versus a combination of low-dose prednisolone and cyclophosphamide223 and (2) high-dose prednisone versus colchicine221 demonstrated a trend toward a decline in pulmonary function and shortened survival, while a third and more recent study (PANTHER) evaluating the use of combination N-acetylcysteine to prednisone and azathioprine revealed an increased risk of hospitalization and death.225
Finally, one retrospective cohort study evaluating outcomes in patients that were either treated with or without CSs prior to a presentation of an acute exacerbation of IPF (AE-IPF), suggested that those treated with CSs had significantly adverse outcomes, with a 25% survival rate (HR 3.54). Survivors were also noted to have worse 1-year survival rates, although these comparative cohorts included small number of patients.227 Current treatment practices have shifted away from routine use of general immunosuppressive agents to targeted anti-fibrotic therapy, although robust data in support for effective long-term outcomes are still lacking.228–230
A small minority of patients with IPF can experience acute and rapid deterioration in lung function (acute exacerbation, AE-IPF) characterized by increased areas of ground glass on CT correlating with acute or organizing diffuse alveolar damage (DAD) or less commonly, organizing pneumonia.231 High-dose steroids (prednisone 1 mg/kg/day to methylprednisolone 1 g/day) have been suggested by international guidelines; however, this recommendation is weak and based on anecdotal evidence from uncontrolled retrospective cohort studies.231–235 Despite treatment with high-dose steroids, AEs-IPF carry significant morbidity and mortality. One study evaluating outcomes of patients with exacerbations of interstitial pneumonias admitted to the hospital revealed that those presenting with an AE-IPF were found to have an overall 90-day mortality of 69%.234 In another cohort of 25 patients admitted to the ICU with 84% needing mechanical ventilation, 24 (96%) died. All patients received high-dose CSs, with eight also receiving additional immunosuppressive therapy with cyclophosphamide.232
Connective tissue disease–usual interstitial pneumonia (CTD-UIP)
CSs are frequently used for management of extrapulmonary manifestations; however, definitive evidence supporting use in CTD-UIP is lacking although there is justification in using immunosuppression to control underlying autoimmunity in mitigating further decline of lung function. To date, no RCTs have been conducted, while several studies do not differentiate UIP from non-UIP subtypes which makes interpretation of the results difficult. Current management practices are now incorporating the use of antifibrotics such as nintedanib and pirfenidone particularly with experiences extrapolated from patients with IPF-UIP and the INBUILD and SENSCIS trials.236
While no RCTs have evaluated the utility of CSs in the treatment of COP, several case series have supported the use of CSs as effective treatment in controlling disease activity.237–243 From pooled data comprising of 12 case series and approximately 160 patients with histologically confirmed COP, treatment with CSs was associated with a complete response (generally with resolution of presenting symptoms and pulmonary opacities without leaving significant physiologic or imaging sequalae) in 59.4% of patients while a partial response was noted in 26.9%. Of the remaining 20%, only 6% had a fatal outcome.244 245
The optimal dosage of CSs is unknown; however, most experiences and collaborative practice guidelines propose initiating high-dose prednisone equivalent to 0.75–1 mg/kg/day.237 238 241 244–246 Although the duration of therapy is also uncertain, the initial dose is typically maintained for 1–3 months with a gradual taper advocated over a 6–12 month period during which time frequent disease relapses are known to occur, particularly as steroids are discontinued or dose reduced under 20 mg/day.238 239 241 In one of the largest and well-described series of 48 patients, 42% (n=20) that were treated with high-dose CSs had complete recovery without disease relapse—typically classified by the reappearance of new infiltrates with compatible clinical features. Of the remaining 58% (n=28), 15 (31%) had one relapse, whereas 13 (27%) experienced two or more relapses with 5 patients (10%) experiencing four or more. The majority of relapses occurred within the first year of diagnosis with the probability of a relapse-free course being 65% at 6 months, 49% at 1 year, 32% at 2 years, and 16% at 4 years after initial diagnosis.238 These data have been similarly described elsewhere in the literature, although with some variability. For example, in a larger series originating from China of 73 patients with CS-treated COP with a similar treatment protocol, 31.5% (n=23) developed relapses, with only 3 having two or more.241
Respiratory bronchiolitis–interstitial lung disease (RB-ILD)
CS therapy’s role in management remains controversial and with variable results. For example, in a series of 12 patients of whom 11 were treated with CSs, initial improvement was observed in 6 (54%); however, sustained benefit as defined by ATS/ERS criteria at the end of the follow-up period was notable in only 2 (18%). Approximately two-thirds were still smoking.247 In another study, 43% (9) of patients treated with steroids, demonstrated improvement in the extent of centrilobular nodules and ground glass opacities on follow-up CT.248 Conversely, in the largest study reporting characteristics of 25 cases of RB-ILD, 15 (60%) patients were treated with oral prednisone. Overall symptomatic improvement was noted in only 2 patients (13%), whereas a significant number (10, 67%) reported symptomatic worsening with 8 experiencing a decline in pulmonary function. Sixty-four per cent of patients in this series were successful in quitting smoking.249 To our knowledge, data regarding dosing, duration, and criteria for initiation of CS therapy have not been clearly defined in any reported studies nor have any RCTs directly evaluating the role of corticosteroids. As a result, significant treatment heterogeneity may exist and contribute to the variability in the reported data and our current understanding of this rare disorder.
Desquamative interstitial pneumonia (DIP)
The largest study to date is a prospective longitudinal study of 40 patients with biopsy-proven DIP who were followed up over a 24-year period and were not treated at presentation.250 In this study, 22% recovered spontaneously (15% had complete remission), 15% remained unchanged, while 62% had progressive disease necessitating treatment with long-term systemic CSs (30–60 mg prednisone tapered to 20 mg/day for at least 6 months). Among the 26 patients that were treated for a mean period of 3.1 (±2.8) years, 61.5% improved, 11.5% remained unchanged, and 27% worsened based on longitudinal changes in clinical, physiologic, and radiographic data. Notably, the extent of fibrosis on diagnostic histopathology correlated with favorable treatment outcomes in those with mild or moderate fibrosis, whereas none of the patients with severe fibrosis improved—a finding supported in another clinicopathologic analysis.251 On a radiological standpoint, two studies comprising 19 patients demonstrated that CS therapy resulted in improvement in areas of ground glass attenuation in more than half the cases on follow-up chest CT, while areas of cystic and fibrotic changes were generally left unaffected.252 253 Several other series have echoed utility of long-term therapy with high-dose CSs. Recent composite data from almost 200 patients included in eight series and several individual cases revealed that 57% improved, 22% remained stable, and 20% worsened with CS therapy.254 While the lack of RCTs do raise a question whether this benefit is due to smoking or exposure limitation, corticosteroid treatment, or the natural course of disease, CSs do remain a reasonable therapeutic option for those with progressive disease. The lack of RCTs raises the question whether this benefit is due to smoking cessation, exposure limitation, CS treatment or the natural course of disease; however, several series have added in support of long-term therapy in those with progressive therapy. Indeed, recent composite data from almost 200 patients included in eight series and several individual cases revealed that 57% improved, 22% remained stable, and 20% worsened with CS therapy.254
Non-specific interstitial pneumonia (NSIP)
Due to the lack of robust prospective data and clinical heterogeneity of this subtype, the overall impact of treatment has been difficult to evaluate; however, in comparison to other idiopathic interstitial pneumonia (IIP)s, CSs have favorable disease-modifying effects. Current practices are largely based on clinical experience and extrapolated from retrospective studies. In those with idiopathic NSIP, these studies comprise varying populations of cellular and fibrosing phenotypes along with variable and loosely defined treatment regimens or meaningful clinical endpoints which limit interpretation. However, universally, CS treatment was associated with symptomatic, physiologic, and radiographic improvement in a majority of included patients.255–259 These effects have also been observed in those with NSIP associated with a variety of connective tissue disorders in which CSs comprise backbone therapy in treating both lung and systemic disease.
The optimal dose and duration of therapy is unknown and are often variable based on individual practice experiences. In the absence of respiratory failure, which is generally treated with pulse dose steroids, a reasonable initiating dose of prednisone at 0.5–1 mg/kg of ideal body weight (up to 60 mg/day) is maintained over a 1-month period.258–260 Thereafter, higher doses comprising 30–40 mg/day are maintained for an additional 1–2 months after which a gradual taper over several months to low dose prednisone can be implemented in those improving or stabilized disease.258 259 In patient’s that are unable to be tapered from higher doses of prednisone, those with frequent relapses, or those with contraindications to long-term CS therapy, additional steroid-sparing immunosuppressants can be supplemented.
Acute eosinophilic pneumonia (AEP)
While reports of successful remission with smoking cessation and exposure limitation without therapy have not been infrequently reported in those with mild disease, a significant portion of patients present with acute respiratory failure with progressive disease often requiring mechanical ventilation and treatment with GCs.261–265 The utility of CSs has not been evaluated in RCTs, but several reports and series have demonstrated efficacy as mainstay therapy.261 262 264 265 The largest is a series comprised of 137 young military personnel in the Korean Army, 93% of whom were treated with high-dose CSs in a protocolized manner.262 Those that were admitted with respiratory failure (58%, 3 requiring mechanical ventilation) as defined by P/F ratio ≤300 and/or tachypnea (respiration rate >30 breaths/min) were treated with intravenous methylprednisolone 60 mg every 6 hours for 3 days prior to transitioning to high-dose oral prednisolone tapered over either 2 or 4 weeks. Reported outcomes were favorable with all patients experiencing improvement in all symptoms within a median of 7 days with defervescence occurring within 48 hours and quick reversal of respiratory failure. All patients were discharged with complete resolution of symptoms and radiographic abnormalities with only one patient experiencing a relapse after resuming smoking. No significant differences in clinical outcomes were appreciable in patients that received the shorter 2-week course. In another series of 22 patients comprising a greater population of those requiring mechanical ventilation (8 intubation, 6 NIPPV), 16 were treated with CSs for a mean of 89 days. All patients irrespective of therapy were discharged from the hospital with most being followed at a mean of 12.7 months. All but one had normalized chest radiographs with a mean delay of 27 days. No relapses occurred and all patients receiving pulmonary function testing had no abnormalities in measured FEV1 and FVC.261 These clinical outcomes (resolution of symptoms including respiratory failure, normalization of chest radiographs, lack of frequent recurrence, and minimal residual abnormalities on pulmonary function testing) have been consistently reported among other studies.264 265
In summary, CSs are effective in the treatment of AEP. Several series have consistently suggested favorable outcomes in relation to quick resolution of symptoms (including respiratory failure), normalization of chest radiographs, lack of frequent recurrence, and minimal residual abnormalities on pulmonary function. To date, the optimal dosage and length of therapy is not known. In general, treatment with high-dose intravenous steroids (60–125 mg every 6 hours) is reserved to patients with severe hypoxemia and those requiring mechanical ventilation, whereas in the absence of respiratory failure, high-dose oral CSs (40–60 mg daily) or supportive care in those with mild disease is reasonable approach. A 2-week regimen appears to be effective; however, in those presenting with peripheral eosinophilia (>500 cells/µL) at presentation, early cessation on clinical stabilization has been advocated by some authors.264 Notably, patients in this cohort had milder disease compared with patients that did not have peripheral eosinophilia at presentation with only a small minority meeting criteria for respiratory failure.
Chronic eosinophilic pneumonia (CEP)
The efficacy of CSs in treating CEP has been universally accepted and described in the literature, although no formal management guidelines exist. Responses are often dramatic with most patients experiencing symptomatic and radiographic improvement in as little as 48 hours and 1 week, respectively, after institution at an initial dose of 0.5–1.0 mg/kg/day.266–272 Subsequent disease activity is usually well controlled on maintenance therapy; however, a substantial portion of patients require prolonged treatment with low-dose CSs given the high frequency of relapses (50%–80%) on cessation or tapering of therapy.273 In the largest series of 133 patients, 64% required oral prednisolone for more than 1 year with 37% requiring therapy for more than 3 years.268 Despite the high frequency of relapses, favorable response are often noted with resumption or increased dose adjustments without a substantial impact on disease-related outcomes.266–272 Long-term studies have also demonstrated improvement in restrictive abnormalities on pulmonary function testing as parenchymal abnormalities recover. Interestingly, obstructive defects which are not uncommon at time of diagnosis increase in frequency at follow-up highlighting the prevalence of asthma and role of eosinophils in the pathogenesis and modulation of bronchial obstruction in this condition.268 271
To date, only one randomized trial in CEP has been conducted.272 In this open-labeled, parallel group study, 55 patients with CEP were treated with an initial dose of 0.5 mg/kg/day and tapered over either 3 or 6 months. All patients responded to initial treatment and no significant differences were noted in either rates or median times to relapse (182 days vs 211 days) in either group at the end of a 2-year observation period. These results coupled with favorable outcomes and responses to retreatment, suggest that a shorter treatment duration may be more suitable to mitigate the consequences of extended corticosteroid therapy. ICSs are frequently prescribed in patients with CEP and coexisting asthma. Their utility has been explored to facilitate dose reduction of oral CSs in several retrospective studies, although data have been conflicting. Successful reports have been described267 269 but not consistently reflected or replicated in others. A small series evaluating the role of high-dose inhaled beclomethasone monotherapy did not demonstrate any efficacy in controlling disease activity.274
Acute respiratory distress syndrome (ARDS)
ARDS is in part the end-result of an innate immune cell-mediated inflammatory response that causes damage to the alveoli and the surround structures in response to a direct injury. It has long been hypothesized that treatment with CS may be beneficial in patients with ARDS, regardless of the etiology. A more recent systematic review and meta-analysis is the first to support this hypothesis, indicating that CS may reduce mortality and the duration of mechanical ventilation in all patients with ARDS. Furthermore, corticosteroids likely cause few side effects, except for an increase in hyperglycemia. This effect on mortality appears to be consistent across COVID-19 ARDS (regardless of strict ARDS criterion) and non-COVID-19 patients, and between corticosteroid type, timing and dose, although a longer duration of therapy may be more beneficial compared with a shorter course. Given the consistency of the results between ARDS etiology, this analysis supports the hypothesis that CSs should be considered in all patients with ARDS, assuming no contraindications.
Conclusions
GCs are powerful immunosuppressants that work by modulating the transcription and translation of inflammation-related genes. They have revolutionized the standard of care for many inflammatory conditions, but their effects in the distal lungs depend on the type of inflammatory pathology and the individual patient (table 2). Results from clinical studies across several diseases suggest that GCs are effective at reducing granulomatous inflammation. Evidence is emerging that diffuse alveolar damage can be reduced or prevented with early GC treatment during acute lung injury in certain cases and on a pathogen-dependent basis. However, few to no studies have shown benefit in reducing fibrosis-driven inflammatory pathologies like IPF and ARDS-induced fibroproliferation. Results from these studies are, however, difficult to compare directly due to inconsistencies in study design, patient population, and outcome measures. Further investigation is required to elucidate the optimal dosing method, dosing strategy, drug choice, and timing of intervention in most pulmonary pathologies that might benefit from GC intervention.
Table 2.
Summary: corticosteroids in lung disease
| Benefit? | Indication | Effect | Evidence strength | Unanswered questions |

|
Sarcoidosis | Short-term improved symptoms, chest imaging and pulmonary function | Short term: Strong Long term: Weak |
Long-term efficacy; dosage and duration |

|
Pulmonary tuberculosis | No benefit | Strong | TB-infected ALI patients |

|
Pneumcystis jirovecii | In HIV-infected individuals, reduction in mortality rate and need for mech vent at 1 month | Strong | Role in non-HIV infected individuals |

|
Influenza | No benefit, possible harm: increased risk of nosocomial infection, rate of ICU admit, and req. for mech vent | Moderate | No RCT on viral influenza with CS |

|
Community acquired pneumonia | Improved time to clinical stability, reduced hospital length of stay and req. for mech vent, reduced progression to ARDS | Moderate | |

|
Acute hypersensitivity pneumonitis | Improved FEV1, FVC, DLCO at 1 month that diminishes at 1 and 5 years | Weak | |

|
Chronic hypersensitivity pneumonitis | No benefit in fibrotic phenotype | Weak | |

|
Acute eosinophilic pneumonia | Resolution of symptoms including respiratory failure, normalization of chest radiographs, lack of frequent recurrence, and minimal residual abnormalities on pulmonary function testing | Strong | |

|
Chronic eosinophilic pneumonia | Complete response; relapse on cessation; improvement in restrictive abnormalities on pulmonary function | Strong | CEP with coexisting asthma |

|
Desquamative interstitial pneumonia | Effective in mild/moderately fibrotic cases | Moderate | Confounding with smoking cessation |

|
Microscopic polyangiitis and granulomatosis with polyangiitis | In combination with cyclophosphamide, improved remission and mortality outcomes | Moderate | Evidence for long-term use |

|
Asthma | Improved quality of life, decreased rate of acute exacerbations, and providing a protective effect against severe exacerbations | Strong | |

|
Chronic obstructive pulmonary disease | Controversial efficacy; more effective in combination with LABA and/or LAMA and in eosinophilic patients | Strong | |

|
Eosinophilic granulomatosis with polyangiitis (Churg Strauss Syndrome) | Clinical remission in patients without poor prognostic factors | Moderate | Dosage and duration; use in alveolar hemorrhage |

|
COVID-19 | Reduced risk of death in severe COVID-19 induced ARDS | Strong | Combination therapies, use in non-life-threatening COVID-19 |

|
Seasonal and pandemic influenza | Increased risk of death, nosocomial infection, rate of ICU admit, mech vent | Weak | Missing RCT for viral influenza GCs |

|
Pneumocystis jirovecii | Reduced risk of death, vent dependence | Strong (HIV) Weak (non-HIV) |
|

|
Community acquired pneumonia | Improving time to clinical stability, reducing hospital length of stay, need for mechanical ventilation, and progression to acute respiratory distress syndrome, | Moderate | Controversial effect on mortality |

|
Usual interstitial pneumonia | No benefit | Weak | |

|
Idiopathic pulmonary fibrosis | Possible harm—reduced survival | Strong | Effect of GCs in acute exacerbation of IPF |

|
Connective tissue disease–UIP | Regularly used but weak evidence | Weak | Rare—only case studies, no differentiation between IPF-UIP and CTD-UIP |

|
Cryptogenic organizing pneumonia | Complete response (generally with resolution of presenting symptoms and pulmonary opacities without leaving significant physiologic or imaging sequalae) | Moderate | Dosage and duration unknown |

|
Respiratory bronchiolitis–Interstitial lung disease | Decline in pulmonary function possible | Weak | Lack of studies—rare condition |

|
Non-specific interstitial pneumonia | Benefit to symptoms and radiographic movement | Weak | Optimal dosage |
ARDS, acute respiratory distresss syndrome; CEP, chronic eosinophilic pneumonia; CS, corticosteroid; CTD-IUP, connective tissue disease–usual interstitial pneumonia; DLCO, diffusing capacity of the lungs for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GC, glucocorticoid; ICU, intensive care unit; IPF, idiopathic pulmonary fibrosis; RCT, randomized controlled trial; TB, tuberculosis.
jim-2021-002161supp001.pdf (39.6KB, pdf)
Footnotes
Contributors: OCL and DAA conceived the manuscript. HV and DAA wrote the manuscript. OCL, DAA, and HV critically edited the manuscript. All authors approved for publication. HV and DAA contributed equally to this manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: Octavian Ioachimescu is an Associate Editor for the Journal of Investigative Medicine.
Provenance and peer review: Commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Ramamoorthy S, Cidlowski JA. Corticosteroids: mechanisms of action in health and disease. Rheum Dis Clin North Am 2016;42:15–31. 10.1016/j.rdc.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ericson-Neilsen W, Kaye AD. Steroids: pharmacology, complications, and practice delivery issues. Ochsner J 2014;14:203–7. [PMC free article] [PubMed] [Google Scholar]
- 3. Cruz-Topete D, Cidlowski JA. Glucocorticoids: Molecular Mechanisms of Action. In: Riccardi C, Levi-Schaffer F, Tiligada E, eds. Immunopharmacology and inflammation. Cham: Springer International Publishing, 2018: 249–66. [Google Scholar]
- 4. Kan M, Himes BE. Insights into glucocorticoid responses derived from omics studies. Pharmacol Ther 2021;218:107674. 10.1016/j.pharmthera.2020.107674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parrillo JE, Fauci AS. Mechanisms of glucocorticoid action on immune processes. Annu Rev Pharmacol Toxicol 1979;19:179–201. 10.1146/annurev.pa.19.040179.001143 [DOI] [PubMed] [Google Scholar]
- 6. Newton R, Leigh R, Giembycz MA. Pharmacological strategies for improving the efficacy and therapeutic ratio of glucocorticoids in inflammatory lung diseases. Pharmacol Ther 2010;125:286–327. 10.1016/j.pharmthera.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 7. Cain DW, Cidlowski JA. Specificity and sensitivity of glucocorticoid signaling in health and disease. Best Pract Res Clin Endocrinol Metab 2015;29:545–56. 10.1016/j.beem.2015.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park OH, Do E, Kim YK. A new function of glucocorticoid receptor: regulation of mRNA stability. BMB Rep 2015;48:367–8. 10.5483/bmbrep.2015.48.7.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barnes PJ. Corticosteroid effects on cell signalling. Eur Respir J 2006;27:413–26. 10.1183/09031936.06.00125404 [DOI] [PubMed] [Google Scholar]
- 10. Cho H, Park OH, Park J, et al. Glucocorticoid receptor interacts with PNRC2 in a ligand-dependent manner to recruit Upf1 for rapid mRNA degradation. Proc Natl Acad Sci U S A 2015;112:E1540–9. 10.1073/pnas.1409612112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ronchetti S, Migliorati G, Bruscoli S, et al. Defining the role of glucocorticoids in inflammation. Clin Sci 2018;132:1529–43. 10.1042/CS20171505 [DOI] [PubMed] [Google Scholar]
- 12. Stellato C. Post-transcriptional and nongenomic effects of glucocorticoids. Proc Am Thorac Soc 2004;1:255–63. 10.1513/pats.200402-015MS [DOI] [PubMed] [Google Scholar]
- 13. Clark AR. Anti-Inflammatory functions of glucocorticoid-induced genes. Mol Cell Endocrinol 2007;275:79–97. 10.1016/j.mce.2007.04.013 [DOI] [PubMed] [Google Scholar]
- 14. Song I-H, Buttgereit F. Non-genomic glucocorticoid effects to provide the basis for new drug developments. Mol Cell Endocrinol 2006;246:142–6. 10.1016/j.mce.2005.11.012 [DOI] [PubMed] [Google Scholar]
- 15. Panettieri RA, Schaafsma D, Amrani Y, et al. Non-genomic effects of glucocorticoids: an updated view. Trends Pharmacol Sci 2019;40:38–49. 10.1016/j.tips.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buttgereit F, Scheffold A. Rapid glucocorticoid effects on immune cells. Steroids 2002;67:529–34. 10.1016/s0039-128x(01)00171-4 [DOI] [PubMed] [Google Scholar]
- 17. Brattsand R, Linden M. Cytokine modulation by glucocorticoids: mechanisms and actions in cellular studies. Aliment Pharmacol Ther 1996;10 Suppl 2:81–90. 10.1046/j.1365-2036.1996.22164025.x [DOI] [PubMed] [Google Scholar]
- 18. Metz JK, Wiegand B, Schnur S, et al. Modulating the barrier function of human alveolar epithelial (hAELVi) cell monolayers as a model of inflammation. Altern Lab Anim 2020;48:252–67. 10.1177/0261192920983015 [DOI] [PubMed] [Google Scholar]
- 19. Kielgast F, Schmidt H, Braubach P, et al. Glucocorticoids regulate tight junction permeability of lung epithelia by modulating claudin 8. Am J Respir Cell Mol Biol 2016;54:707–17. 10.1165/rcmb.2015-0071OC [DOI] [PubMed] [Google Scholar]
- 20. Yang J-W, Mao B, Tao R-J, et al. Corticosteroids alleviate lipopolysaccharide-induced inflammation and lung injury via inhibiting NLRP3-inflammasome activation. J Cell Mol Med 2020;24:12716–25. 10.1111/jcmm.15849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xie Y, Tolmeijer S, Oskam JM, et al. Glucocorticoids inhibit macrophage differentiation towards a pro-inflammatory phenotype upon wounding without affecting their migration. Dis Model Mech 2019;12. 10.1242/dmm.037887. [Epub ahead of print: 30 05 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ronchetti S, Ricci E, Migliorati G, et al. How glucocorticoids affect the neutrophil life. Int J Mol Sci 2018;19:4090. 10.3390/ijms19124090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. White SR, Dorscheid DR. Corticosteroid-Induced apoptosis of airway epithelium: a potential mechanism for chronic airway epithelial damage in asthma. Chest 2002;122:278S–84. 10.1378/chest.122.6_suppl.278s [DOI] [PubMed] [Google Scholar]
- 24. Dorscheid DR, Patchell BJ, Estrada O, et al. Effects of corticosteroid-induced apoptosis on airway epithelial wound closure in vitro. Am J Physiol Lung Cell Mol Physiol 2006;291:L794–801. 10.1152/ajplung.00322.2005 [DOI] [PubMed] [Google Scholar]
- 25. Wadsworth SJ, Nijmeh HS, Hall IP. Glucocorticoids increase repair potential in a novel in vitro human airway epithelial wounding model. J Clin Immunol 2006;26:376–87. 10.1007/s10875-006-9029-z [DOI] [PubMed] [Google Scholar]
- 26. Liu J, Zhang M, Niu C, et al. Dexamethasone inhibits repair of human airway epithelial cells mediated by glucocorticoid-induced leucine zipper (GILZ). PLoS One 2013;8:e60705. 10.1371/journal.pone.0060705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Castaldi A, Horie M, Rieger ME, et al. Genome-wide integration of microRNA and transcriptomic profiles of differentiating human alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 2020;319:L173–84. 10.1152/ajplung.00519.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Durant S, Duval D, Homo-Delarche F. Factors involved in the control of fibroblast proliferation by glucocorticoids: a review. Endocr Rev 1986;7:254–69. 10.1210/edrv-7-3-254 [DOI] [PubMed] [Google Scholar]
- 29. Salton F, Confalonieri P, Meduri GU, et al. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. Open Forum Infect Dis 2020;7:ofaa421. 10.1093/ofid/ofaa421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burke-Gaffney A, Hellewell PG. Regulation of ICAM-1 by dexamethasone in a human vascular endothelial cell line EAhy926. Am J Physiol Cell Physiol 1996;270:C552–61. 10.1152/ajpcell.1996.270.2.C552 [DOI] [PubMed] [Google Scholar]
- 31. Kerachian MA, Cournoyer D, Harvey EJ, et al. Effect of high-dose dexamethasone on endothelial haemostatic gene expression and neutrophil adhesion. J Steroid Biochem Mol Biol 2009;116:127–33. 10.1016/j.jsbmb.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 32. Matsuda A, Orihara K, Fukuda S, et al. Corticosteroid enhances TNF-alpha-mediated leukocyte adhesion to pulmonary microvascular endothelial cells. Allergy 2008;63:1610–6. 10.1111/j.1398-9995.2008.01775.x [DOI] [PubMed] [Google Scholar]
- 33. Gillis S, Crabtree GR, Smith KA. Glucocorticoid-induced inhibition of T cell growth factor production. I. The effect on mitogen-induced lymphocyte proliferation. J Immunol 1979;123:1624–31. [PubMed] [Google Scholar]
- 34. Warshamana GS, Martinez S, Lasky JA, et al. Dexamethasone activates expression of the PDGF-alpha receptor and induces lung fibroblast proliferation. Am J Physiol 1998;274:L499–507. 10.1152/ajplung.1998.274.4.L499 [DOI] [PubMed] [Google Scholar]
- 35. Miki H, Mio T, Nagai S, et al. Glucocorticoid-induced contractility and F-actin content of human lung fibroblasts in three-dimensional culture. Am J Physiol Lung Cell Mol Physiol 2000;278:L13–18. 10.1152/ajplung.2000.278.1.L13 [DOI] [PubMed] [Google Scholar]
- 36. Meduri GU, Belenchia JM, Estes RJ, et al. Fibroproliferative phase of ARDS. clinical findings and effects of corticosteroids. Chest 1991;100:943–52. 10.1378/chest.100.4.943 [DOI] [PubMed] [Google Scholar]
- 37. van Zaane B, Nur E, Squizzato A, et al. Systematic review on the effect of glucocorticoid use on procoagulant, anti-coagulant and fibrinolytic factors. J Thromb Haemost 2010;8:2483–93. 10.1111/j.1538-7836.2010.04034.x [DOI] [PubMed] [Google Scholar]
- 38. Zayed Y, Barbarawi M, Ismail E, et al. Use of glucocorticoids in patients with acute respiratory distress syndrome: a meta-analysis and trial sequential analysis. J Intensive Care 2020;8:1–10. 10.1186/s40560-020-00464-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meduri GU, Bridges L, Shih M-C, et al. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients' data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med 2016;42:829–40. 10.1007/s00134-015-4095-4 [DOI] [PubMed] [Google Scholar]
- 40. Agustí C, Rañó A, Filella X, et al. Pulmonary infiltrates in patients receiving long-term glucocorticoid treatment: etiology, prognostic factors, and associated inflammatory response. Chest 2003;123:488–98. 10.1378/chest.123.2.488 [DOI] [PubMed] [Google Scholar]
- 41. Stern MH, Dreizen S, Mackler BF, et al. Quantitative analysis of cellular composition of human periapical granuloma. J Endod 1981;7:117–22. 10.1016/S0099-2399(81)80125-2 [DOI] [PubMed] [Google Scholar]
- 42. Hirsh BC, Johnson WC. Concepts of granulomatous inflammation. Int J Dermatol 1984;23:90–100. 10.1111/j.1365-4362.1984.tb05679.x [DOI] [PubMed] [Google Scholar]
- 43. Ehrchen JM, Roth J, Barczyk-Kahlert K. More than suppression: glucocorticoid action on monocytes and macrophages. Front Immunol 2019;10:2028. 10.3389/fimmu.2019.02028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis 1973;107:609–14. 10.1164/arrd.1973.107.4.609 [DOI] [PubMed] [Google Scholar]
- 45. James DG, Carstairs LS, Trowell J. Report of a controlled therapeutic trial. Lancet 1967;2:526–8. 10.1016/s0140-6736(67)90493-x [DOI] [PubMed] [Google Scholar]
- 46. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish pulmonary sarcoidosis Study Group. Chest 1999;116:424–31. 10.1378/chest.116.2.424 [DOI] [PubMed] [Google Scholar]
- 47. Selroos O, Sellergren TL. Corticosteroid therapy of pulmonary sarcoidosis. A prospective evaluation of alternate day and daily dosage in stage II disease. Scand J Respir Dis 1979;60:215–21. [PubMed] [Google Scholar]
- 48. Zaki MH, Lyons HA, Leilop L, et al. Corticosteroid therapy in sarcoidosis. A five-year, controlled follow-up study. N Y State J Med 1987;87:496–9. [PubMed] [Google Scholar]
- 49. Paramothayan NS, Lasserson TJ, Jones PW. Corticosteroids for pulmonary sarcoidosis. Cochrane Database Syst Rev 2005;2005:CD001114. 10.1002/14651858.CD001114.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gibson GJ, Prescott RJ, Muers MF, et al. British Thoracic Society sarcoidosis study: effects of long term corticosteroid treatment. Thorax 1996;51:238–47. 10.1136/thx.51.3.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pietinalho A, Tukiainen P, Haahtela T, et al. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest 2002;121:24–31. 10.1378/chest.121.1.24 [DOI] [PubMed] [Google Scholar]
- 52. Sabbagh F, Gibbs C, Efferen LS. Pulmonary sarcoidosis and the acute respiratory distress syndrome (ARDS). Thorax 2002;57:655–6. 10.1136/thorax.57.7.655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chirakalwasan N, Dallal MM. Pulmonary sarcoidosis presenting with acute respiratory failure. South Med J 2005;98:382–4. 10.1097/01.SMJ.0000154317.31912.E1 [DOI] [PubMed] [Google Scholar]
- 54. Cremers JP, Drent M, Bast A, et al. Multinational evidence-based world association of sarcoidosis and other granulomatous disorders recommendations for the use of methotrexate in sarcoidosis: integrating systematic literature research and expert opinion of sarcoidologists worldwide. Curr Opin Pulm Med 2013;19:545–61. 10.1097/MCP.0b013e3283642a7a [DOI] [PubMed] [Google Scholar]
- 55. Alberts C, van der Mark TW, Jansen HM. Inhaled budesonide in pulmonary sarcoidosis: a double-blind, placebo-controlled study. Dutch Study Group on pulmonary sarcoidosis. Eur Respir J 1995;8:682–8. [PubMed] [Google Scholar]
- 56. Zych D, Pawlicka L, Zielinski J. Inhaled budesonide vs prednisone in the maintenance treatment of pulmonary sarcoidosis. Sarcoidosis 1993;10:56–61. [PubMed] [Google Scholar]
- 57. du Bois RM, Greenhalgh PM, Southcott AM, et al. Randomized trial of inhaled fluticasone propionate in chronic stable pulmonary sarcoidosis: a pilot study. Eur Respir J 1999;13:1345–50. 10.1183/09031936.99.13613519 [DOI] [PubMed] [Google Scholar]
- 58. Milman N, Graudal N, Grode G, et al. No effect of high-dose inhaled steroids in pulmonary sarcoidosis: a double-blind, placebo-controlled study. J Intern Med 1994;236:285–90. 10.1111/j.1365-2796.1994.tb00798.x [DOI] [PubMed] [Google Scholar]
- 59. Mönkäre S. Influence of corticosteroid treatment on the course of farmer's lung. Eur J Respir Dis 1983;64:283–93. [PubMed] [Google Scholar]
- 60. Kokkarinen JI, Tukiainen HO, Terho EO. Effect of corticosteroid treatment on the recovery of pulmonary function in farmer's lung. Am Rev Respir Dis 1992;145:3–5. 10.1164/ajrccm/145.1.3 [DOI] [PubMed] [Google Scholar]
- 61. Kokkarinen JI, Tukiainen HO, Terho EO. Recovery of pulmonary function in farmer's lung. A five-year follow-up study. Am Rev Respir Dis 1993;147:793–6. 10.1164/ajrccm/147.4.793 [DOI] [PubMed] [Google Scholar]
- 62. Cormier Y, Desmeule M. Treatment of hypersensitivity pneumonitis: contact avoidance versus corticosteroid treatment. Canadian Respiratory Journal 1994;1:643515. [Google Scholar]
- 63. Selman M, Pardo A, King TE. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med 2012;186:314–24. 10.1164/rccm.201203-0513CI [DOI] [PubMed] [Google Scholar]
- 64. Raghu G, Remy-Jardin M, Ryerson CJ, et al. Diagnosis of hypersensitivity pneumonitis in adults. An official ATS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2020;202:e36–69. 10.1164/rccm.202005-2032ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fernández Pérez ER, Swigris JJ, Forssén AV, et al. Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest 2013;144:1644–51. 10.1378/chest.12-2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ni Y-N, Chen G, Sun J, et al. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care 2019;23:99. 10.1186/s13054-019-2395-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhou Y, Fu X, Liu X, et al. Use of corticosteroids in influenza-associated acute respiratory distress syndrome and severe pneumonia: a systemic review and meta-analysis. Sci Rep 2020;10:3044. 10.1038/s41598-020-59732-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang Y, Sun W, Svendsen ER, et al. Do corticosteroids reduce the mortality of influenza A (H1N1) infection? A meta-analysis. Crit Care 2015;19:46. 10.1186/s13054-015-0764-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ottolini M, Blanco J, Porter D, et al. Combination anti-inflammatory and antiviral therapy of influenza in a cotton rat model. Pediatr Pulmonol 2003;36:290–4. 10.1002/ppul.10320 [DOI] [PubMed] [Google Scholar]
- 70. De Sadeleer LJ, Hermans F, De Dycker E, et al. Effects of corticosteroid treatment and antigen avoidance in a large hypersensitivity pneumonitis cohort: a single-centre cohort study. J Clin Med 2018;8. 10.3390/jcm8010014. [Epub ahead of print: 21 12 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Adegunsoye A, Oldham JM, Fernández Pérez ER, et al. Outcomes of immunosuppressive therapy in chronic hypersensitivity pneumonitis. ERJ Open Res 2017;3:00016–2017. 10.1183/23120541.00016-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992;116:488–98. 10.7326/0003-4819-116-6-488 [DOI] [PubMed] [Google Scholar]
- 73. Walton EW. Giant-Cell granuloma of the respiratory tract (Wegener's granulomatosis). Br Med J 1958;2:265–70. 10.1136/bmj.2.5091.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nachman PH, Hogan SL, Jennette JC, et al. Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996;7:33–9. 10.1681/ASN.V7133 [DOI] [PubMed] [Google Scholar]
- 75. Fauci AS, Haynes BF, Katz P, et al. Wegener's granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med 1983;98:76–85. 10.7326/0003-4819-98-1-76 [DOI] [PubMed] [Google Scholar]
- 76. de Groot K, Harper L, Jayne DRW, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 2009;150:670–80. 10.7326/0003-4819-150-10-200905190-00004 [DOI] [PubMed] [Google Scholar]
- 77. Adu D, Pall A, Luqmani RA, et al. Controlled trial of pulse versus continuous prednisolone and cyclophosphamide in the treatment of systemic vasculitis. QJM 1997;90:401–9. 10.1093/qjmed/90.6.401 [DOI] [PubMed] [Google Scholar]
- 78. Frankel SK, Schwarz MI. The pulmonary vasculitides. Am J Respir Crit Care Med 2012;186:216–24. 10.1164/rccm.201203-0539CI [DOI] [PubMed] [Google Scholar]
- 79. Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010;363:221–32. 10.1056/NEJMoa0909905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Novack SN, Pearson CM. Cyclophosphamide therapy in Wegener's granulomatosis. N Engl J Med 1971;284:938–42. 10.1056/NEJM197104292841703 [DOI] [PubMed] [Google Scholar]
- 81. Mukhtyar C, Guillevin L, Cid MC, et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis 2009;68:310–7. 10.1136/ard.2008.088096 [DOI] [PubMed] [Google Scholar]
- 82. Faurschou M, Westman K, Rasmussen N, et al. Brief report: long-term outcome of a randomized clinical trial comparing methotrexate to cyclophosphamide for remission induction in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2012;64:3472–7. 10.1002/art.34547 [DOI] [PubMed] [Google Scholar]
- 83. Walsh M, Merkel PA, Mahr A, et al. Effects of duration of glucocorticoid therapy on relapse rate in antineutrophil cytoplasmic antibody-associated vasculitis: a meta-analysis. Arthritis Care Res 2010;62:1166–73. 10.1002/acr.20176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Groh M, Pagnoux C, Baldini C, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) (EGPA) consensus Task force recommendations for evaluation and management. Eur J Intern Med 2015;26:545–53. 10.1016/j.ejim.2015.04.022 [DOI] [PubMed] [Google Scholar]
- 85. Guillevin L, Pagnoux C, Seror R, et al. The five-factor score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French vasculitis Study Group (FVSG) cohort. Medicine 2011;90:19–27. 10.1097/MD.0b013e318205a4c6 [DOI] [PubMed] [Google Scholar]
- 86. Ribi C, Cohen P, Pagnoux C, et al. Treatment of Churg-Strauss syndrome without poor-prognosis factors: a multicenter, prospective, randomized, open-label study of seventy-two patients. Arthritis Rheum 2008;58:586–94. 10.1002/art.23198 [DOI] [PubMed] [Google Scholar]
- 87. Guillevin L, Cohen P, Gayraud M, et al. Churg-Strauss syndrome. clinical study and long-term follow-up of 96 patients. Medicine 1999;78:26–37. 10.1097/00005792-199901000-00003 [DOI] [PubMed] [Google Scholar]
- 88. Cohen P, Pagnoux C, Mahr A, et al. Churg-Strauss syndrome with poor-prognosis factors: a prospective multicenter trial comparing glucocorticoids and six or twelve cyclophosphamide pulses in forty-eight patients. Arthritis Rheum 2007;57:686–93. 10.1002/art.22679 [DOI] [PubMed] [Google Scholar]
- 89. Controlled trial of effects of cortisone acetate in chronic asthma; report to the medical Research Council by the Subcommittee on clinical trials in asthma. Lancet 1956;271:798–803. [PubMed] [Google Scholar]
- 90. Crompton G. A brief history of inhaled asthma therapy over the last fifty years. Prim Care Respir J 2006;15:326–31. 10.1016/j.pcrj.2006.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gaddie J, Reid IW, Skinner C, et al. Aerosol beclomethasone dipropionate in chronic bronchial asthma. Lancet 1973;1:691–3. 10.1016/s0140-6736(73)91479-7 [DOI] [PubMed] [Google Scholar]
- 92. Lal S, Harris DM, Bhalla KK, et al. Comparison of beclomethasone dipropionate aerosol and prednisolone in reversible airways obstruction. Br Med J 1972;3:314–7. 10.1136/bmj.3.5822.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hodson ME, Batten JC, Clarke SW, et al. Beclomethasone dipropionate aerosol in asthma. transfer of steroid-dependent asthmatic patients from oral prednisone to beclomethasone dipropionate aerosol. Am Rev Respir Dis 1974;110:403–8. 10.1164/arrd.1974.110.4.403 [DOI] [PubMed] [Google Scholar]
- 94. Cameron SJ, Cooper EJ, Crompton GK, et al. Substitution of beclomethasone aerosol for oral prednisolone in the treatment of chronic asthma. Br Med J 1973;4:205–7. 10.1136/bmj.4.5886.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Covar RA. Pivotal efficacy trials of inhaled corticosteroids in asthma. Annals of Allergy, Asthma & Immunology 2016;117:582–8. 10.1016/j.anai.2016.07.035 [DOI] [PubMed] [Google Scholar]
- 96. Pauwels RA, Pedersen S, Busse WW, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet 2003;361:1071–6. 10.1016/S0140-6736(03)12891-7 [DOI] [PubMed] [Google Scholar]
- 97. Adams NP, Bestall JC, Lasserson TJ. Inhaled fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database Syst Rev 2005;2:Cd003135. 10.1002/14651858.CD003135.pub2 [DOI] [PubMed] [Google Scholar]
- 98. Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000;343:332–6. 10.1056/NEJM200008033430504 [DOI] [PubMed] [Google Scholar]
- 99. Olivieri D, Chetta A, Del Donno M, Donno MD, et al. Effect of short-term treatment with low-dose inhaled fluticasone propionate on airway inflammation and remodeling in mild asthma: a placebo-controlled study. Am J Respir Crit Care Med 1997;155:1864–71. 10.1164/ajrccm.155.6.9196087 [DOI] [PubMed] [Google Scholar]
- 100. Booth H, Richmond I, Ward C, et al. Effect of high dose inhaled fluticasone propionate on airway inflammation in asthma. Am J Respir Crit Care Med 1995;152:45–52. 10.1164/ajrccm.152.1.7599861 [DOI] [PubMed] [Google Scholar]
- 101. Trigg CJ, Manolitsas ND, Wang J, et al. Placebo-controlled immunopathologic study of four months of inhaled corticosteroids in asthma. Am J Respir Crit Care Med 1994;150:17–22. 10.1164/ajrccm.150.1.8025745 [DOI] [PubMed] [Google Scholar]
- 102. Reddel HK, Bacharier LB, Bateman ED, et al. Global initiative for asthma strategy 2021: Executive summary and rationale for key changes. J Allergy Clin Immunol Pract 2022;10:S1–18. 10.1016/j.jaip.2021.10.001 [DOI] [PubMed] [Google Scholar]
- 103. Fiel SB, Swartz MA, Glanz K, et al. Efficacy of short-term corticosteroid therapy in outpatient treatment of acute bronchial asthma. Am J Med 1983;75:259–62. 10.1016/0002-9343(83)91202-0 [DOI] [PubMed] [Google Scholar]
- 104. Chapman KR, Verbeek PR, White JG, et al. Effect of a short course of prednisone in the prevention of early relapse after the emergency room treatment of acute asthma. N Engl J Med 1991;324:788–94. 10.1056/NEJM199103213241202 [DOI] [PubMed] [Google Scholar]
- 105. Rowe BH, Spooner CH, Ducharme FM, et al. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev 2007;3:Cd000195. 10.1002/14651858.CD000195.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev 2016;2016:Cd011801. 10.1002/14651858.CD011801.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. McFadden ER. Acute severe asthma. Am J Respir Crit Care Med 2003;168:740–59. 10.1164/rccm.200208-902SO [DOI] [PubMed] [Google Scholar]
- 108. Leatherman J. Mechanical ventilation for severe asthma. Chest 2015;147:1671–80. 10.1378/chest.14-1733 [DOI] [PubMed] [Google Scholar]
- 109. Barnes PJ. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat Rev Drug Discov 2013;12:543–59. 10.1038/nrd4025 [DOI] [PubMed] [Google Scholar]
- 110. Boardman C, Chachi L, Gavrila A, et al. Mechanisms of glucocorticoid action and insensitivity in airways disease. Pulm Pharmacol Ther 2014;29:129–43. 10.1016/j.pupt.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 111. Bateman ED, Chapman KR, Singh D, et al. Aclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multicentre, randomised studies (ACLIFORM and augment). Respir Res 2015;16:92. 10.1186/s12931-015-0250-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yang IA, Clarke MS, Sim EHA, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012;2016. 10.1002/14651858.CD002991.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Calverley PMA, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775–89. 10.1056/NEJMoa063070 [DOI] [PubMed] [Google Scholar]
- 114. Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet 2016;387:1817–26. 10.1016/S0140-6736(16)30069-1 [DOI] [PubMed] [Google Scholar]
- 115. Lung Health Study Research Group, Wise R, Connett J, et al. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med 2000;343:1902–9. 10.1056/NEJM200012283432601 [DOI] [PubMed] [Google Scholar]
- 116. Pauwels RA, Löfdahl CG, Laitinen LA, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society study on chronic obstructive pulmonary disease. N Engl J Med 1999;340:1948–53. 10.1056/NEJM199906243402503 [DOI] [PubMed] [Google Scholar]
- 117. Vestbo J, Sørensen T, Lange P, et al. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 1999;353:1819–23. 10.1016/s0140-6736(98)10019-3 [DOI] [PubMed] [Google Scholar]
- 118. Burge PS, Calverley PM, Jones PW, et al. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000;320:1297–303. 10.1136/bmj.320.7245.1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Highland KB, Strange C, Heffner JE. Long-term effects of inhaled corticosteroids on FEV1 in patients with chronic obstructive pulmonary disease. A meta-analysis. Ann Intern Med 2003;138:969–73. 10.7326/0003-4819-138-12-200306170-00008 [DOI] [PubMed] [Google Scholar]
- 120. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012;2012:Cd006829. 10.1002/14651858.CD006829.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bourdin A, Criner G, Devouassoux G, et al. Informing the pathway of COPD treatment (impact trial) Single-Inhaler triple therapy (fluticasone Furoate/Umeclidinium/Vilanterol) versus fluticasone furoate/vilanterol and umeclidinium/vilanterol in patients with COPD: analysis of the Western Europe and North America regions. Chronic Obstr Pulm Dis 2021;8. 10.15326/jcopdf.2020.0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med 2018;378:1671–80. 10.1056/NEJMoa1713901 [DOI] [PubMed] [Google Scholar]
- 123. Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in Moderate-to-Very-Severe COPD. N Engl J Med 2020;383:35–48. 10.1056/NEJMoa1916046 [DOI] [PubMed] [Google Scholar]
- 124. Global Initiative for Chronic Obstructive Lung Disease . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Available: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf2020
- 125. Bafadhel M, Peterson S, De Blas MA, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med 2018;6:117–26. 10.1016/S2213-2600(18)30006-7 [DOI] [PubMed] [Google Scholar]
- 126. Siddiqui SH, Guasconi A, Vestbo J, et al. Blood eosinophils: a biomarker of response to Extrafine Beclomethasone/Formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015;192:523–5. 10.1164/rccm.201502-0235LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med 2015;3:435–42. 10.1016/S2213-2600(15)00106-X [DOI] [PubMed] [Google Scholar]
- 128. Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999;354:456–60. 10.1016/s0140-6736(98)11326-0 [DOI] [PubMed] [Google Scholar]
- 129. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 1999;340:1941–7. 10.1056/NEJM199906243402502 [DOI] [PubMed] [Google Scholar]
- 130. Thompson WH, Nielson CP, Carvalho P, et al. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 1996;154:407–12. 10.1164/ajrccm.154.2.8756814 [DOI] [PubMed] [Google Scholar]
- 131. Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the reduce randomized clinical trial. JAMA 2013;309:2223–31. 10.1001/jama.2013.5023 [DOI] [PubMed] [Google Scholar]
- 132. Fowler AA, Hyers TM, Fisher BJ, et al. The adult respiratory distress syndrome. cell populations and soluble mediators in the air spaces of patients at high risk. Am Rev Respir Dis 1987;136:1225–31. 10.1164/ajrccm/136.5.1225 [DOI] [PubMed] [Google Scholar]
- 133. Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers 2019;5:18. 10.1038/s41572-019-0069-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Tu G-W, Shi Y, Zheng Y-J, G-w T, Y-j Z, et al. Glucocorticoid attenuates acute lung injury through induction of type 2 macrophage. J Transl Med 2017;15:181. 10.1186/s12967-017-1284-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Vettorazzi S, Bode C, Dejager L, et al. Glucocorticoids limit acute lung inflammation in concert with inflammatory stimuli by induction of SPHK1. Nat Commun 2015;6:7796. 10.1038/ncomms8796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kosutova P, Mikolka P, Balentova S, et al. Intravenous dexamethasone attenuated inflammation and influenced apoptosis of lung cells in an experimental model of acute lung injury. Physiol Res 2016;65:S663–72. 10.33549/physiolres.933531 [DOI] [PubMed] [Google Scholar]
- 137. Margaroli C, Moncada-Giraldo D, Gulick DA, et al. Transcriptional firing represses bactericidal activity in cystic fibrosis airway neutrophils. Cell Rep Med 2021;2:100239. 10.1016/j.xcrm.2021.100239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Peter JV, John P, Graham PL, Preeta G, Petra L, et al. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ 2008;336:1006-9. 10.1136/bmj.39537.939039.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Salomon R, Hoffmann E, Webster RG. Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection. Proc Natl Acad Sci U S A 2007;104:12479–81. 10.1073/pnas.0705289104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Xu T, Qiao J, Zhao L, et al. Effect of dexamethasone on acute respiratory distress syndrome induced by the H5N1 virus in mice. Eur Respir J 2009;33:852–60. 10.1183/09031936.00130507 [DOI] [PubMed] [Google Scholar]
- 141. Li C, Yang P, Zhang Y, et al. Corticosteroid treatment ameliorates acute lung injury induced by 2009 swine origin influenza A (H1N1) virus in mice. PLoS One 2012;7:e44110. 10.1371/journal.pone.0044110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Jakab Ágnes, Mogavero S, Förster TM, et al. Effects of the glucocorticoid betamethasone on the interaction of Candida albicans with human epithelial cells. Microbiology 2016;162:2116–25. 10.1099/mic.0.000383 [DOI] [PubMed] [Google Scholar]
- 143. Clemons KV, Schwartz JA, Stevens DA. Therapeutic and toxicologic studies in a murine model of invasive pulmonary aspergillosis. Med Mycol 2011;49:834–47. 10.3109/13693786.2011.577822 [DOI] [PubMed] [Google Scholar]
- 144. Ye Z-W, Yuan S, Chan JF-W, et al. Beneficial effect of combinational methylprednisolone and remdesivir in hamster model of SARS-CoV-2 infection. Emerg Microbes Infect 2021;10:291–304. 10.1080/22221751.2021.1885998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Rocha SM, Fagre AC, Latham AS. A novel glucocorticoid and androgen receptor modulator reduces viral entry and innate immune inflammatory responses in the Syrian hamster model of SARS-CoV-2 infection. Front Immunol 2021;13. 10.3389/fimmu.2022.811430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Polidoro RB, Hagan RS, de Santis Santiago R, et al. Overview: systemic inflammatory response derived from lung injury caused by SARS-CoV-2 infection explains severe outcomes in COVID-19. Front Immunol 2020;11:1626. 10.3389/fimmu.2020.01626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020;181:e1039:1036–45. 10.1016/j.cell.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hue S, Beldi-Ferchiou A, Bendib I. Uncontrolled innate and impaired adaptive immune responses in patients with COVID-19 ARDS. American Journal of Respiratory and Critical Care Medicine 2020. 10.1164/rccm.202005-1885OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Tay MZ, Poh CM, Rénia L, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020;20:363–74. 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wang Y, Lu X, Li Y, Chen H, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med 2020;201:1430-1434. 10.1164/rccm.202003-0736LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Matthay MA, Thompson BT. Dexamethasone in hospitalised patients with COVID-19: addressing uncertainties. Lancet Respir Med 2020;8:1170–2. 10.1016/S2213-2600(20)30503-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Jv P, Vos JS, Hoekstra EM. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Critical Care 2020;24:1–22. 10.1186/s13054-020-03400-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med 2008;358:111–24. 10.1056/NEJMoa071366 [DOI] [PubMed] [Google Scholar]
- 154. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693–704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Dequin P-F, Heming N, Meziani F, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA 2020;324:1298–306. 10.1001/jama.2020.16761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and Ventilator-Free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the Codex randomized clinical trial. JAMA 2020;324:1307–16. 10.1001/jama.2020.17021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA 2020;324:1317–29. 10.1001/jama.2020.17022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Agarwal A, Rochwerg B, Lamontagne F, Siemieniuk R, Agoritsas T, et al. A living who guideline on drugs for covid-19. BMJ 2020;370:m3379. 10.1136/bmj.m3379 [DOI] [PubMed] [Google Scholar]
- 159. Kory P, Meduri GU, Iglesias J, et al. Clinical and Scientific Rationale for the "MATH+" Hospital Treatment Protocol for COVID-19. J Intensive Care Med 2021;36:135–56. 10.1177/0885066620973585 [DOI] [PubMed] [Google Scholar]
- 160. Corral-Gudino L, Bahamonde A, Arnaiz-Revillas F. Methylprednisolone in adults hospitalized with COVID-19 pneumonia. Wiener klinische Wochenschrift, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Cano EJ, Fonseca Fuentes X, Corsini Campioli C, et al. Impact of corticosteroids in coronavirus disease 2019 outcomes: systematic review and meta-analysis. Chest 2021;159:1019–40. 10.1016/j.chest.2020.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009;302:1872–9. 10.1001/jama.2009.1496 [DOI] [PubMed] [Google Scholar]
- 163. ANZIC Influenza Investigators, Webb SAR, Pettilä V, et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med 2009;361:1925–34. 10.1056/NEJMoa0908481 [DOI] [PubMed] [Google Scholar]
- 164. Ríos FG, Estenssoro E, Villarejo F, et al. Lung function and organ dysfunctions in 178 patients requiring mechanical ventilation during the 2009 influenza A (H1N1) pandemic. Crit Care 2011;15:R201. 10.1186/cc10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Lansbury L, Rodrigo C, Leonardi-Bee J, et al. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev 2019;2:Cd010406. 10.1002/14651858.CD010406.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Carmona EM, Limper AH. Update on the diagnosis and treatment of Pneumocystis pneumonia. Ther Adv Respir Dis 2011;5:41–59. 10.1177/1753465810380102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Festic E, Gajic O, Limper AH, et al. Acute respiratory failure due to pneumocystis pneumonia in patients without human immunodeficiency virus infection: outcome and associated features. Chest 2005;128:573–9. 10.1378/chest.128.2.573 [DOI] [PubMed] [Google Scholar]
- 168. National Institutes of Health-University of California Expert Panel for Corticosteroids as Adjunctive Therapy for Pneumocystis Pneumonia . Consensus statement on the use of corticosteroids as adjunctive therapy for pneumocystis pneumonia in the acquired immunodeficiency syndrome. N Engl J Med 1990;323:1500–4. 10.1056/NEJM199011223232131 [DOI] [PubMed] [Google Scholar]
- 169. Kaplan JE, Benson C, Holmes KK. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of health, and the HIV medicine association of the infectious diseases Society of America. MMWR Recomm Rep 2009;58:quiz CE201-204:1–207. [PubMed] [Google Scholar]
- 170. Montaner JS, Lawson LM, Levitt N, et al. Corticosteroids prevent early deterioration in patients with moderately severe Pneumocystis carinii pneumonia and the acquired immunodeficiency syndrome (AIDS). Ann Intern Med 1990;113:14–20. 10.7326/0003-4819-113-1-14 [DOI] [PubMed] [Google Scholar]
- 171. Bozzette SA, Sattler FR, Chiu J, et al. A controlled trial of early adjunctive treatment with corticosteroids for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. California Collaborative treatment group. N Engl J Med 1990;323:1451–7. 10.1056/NEJM199011223232104 [DOI] [PubMed] [Google Scholar]
- 172. Gagnon S, Boota AM, Fischl MA, et al. Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A double-blind, placebo-controlled trial. N Engl J Med 1990;323:1444–50. 10.1056/NEJM199011223232103 [DOI] [PubMed] [Google Scholar]
- 173. Nielsen TL, Eeftinck Schattenkerk JK, Jensen BN, et al. Adjunctive corticosteroid therapy for Pneumocystis carinii pneumonia in AIDS: a randomized European multicenter open label study. J Acquir Immune Defic Syndr 1992;5:726–31. [PubMed] [Google Scholar]
- 174. Clement M, Edison R, Turner J. Corticosteroids as adjunctive therapy in severe Pneumocystis carinii pneumonia: a prospective placebo-controlled trial. Am Rev Respir Dis 1989;139:A250. [Google Scholar]
- 175. Walmsley S, Levinton C, Brunton J, et al. A multicenter randomized double-blind placebo-controlled trial of adjunctive corticosteroids in the treatment of Pneumocystis carinii pneumonia complicating the acquired immune deficiency syndrome. J Acquir Immune Defic Syndr Hum Retrovirol 1995;8:348–57. 10.1097/00042560-199504000-00005 [DOI] [PubMed] [Google Scholar]
- 176. Ewald H, Raatz H, Boscacci R, et al. Adjunctive corticosteroids for Pneumocystis jiroveci pneumonia in patients with HIV infection. Cochrane Database Syst Rev 2015;2015:Cd006150. 10.1002/14651858.CD006150.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Delclaux C, Zahar JR, Amraoui G, et al. Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneumonia in non-human immunodeficiency virus-infected patients: retrospective study of 31 patients. Clin Infect Dis 1999;29:670–2. 10.1086/598651 [DOI] [PubMed] [Google Scholar]
- 178. Moon SM, Kim T, Sung H, et al. Outcomes of moderate-to-severe Pneumocystis pneumonia treated with adjunctive steroid in non-HIV-infected patients. Antimicrob Agents Chemother 2011;55:4613–8. 10.1128/AAC.00669-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Pareja JG, Garland R, Koziel H. Use of adjunctive corticosteroids in severe adult non-HIV Pneumocystis carinii pneumonia. Chest 1998;113:1215–24. 10.1378/chest.113.5.1215 [DOI] [PubMed] [Google Scholar]
- 180. Roblot F, Le Moal G, Godet C, et al. Pneumocystis carinii pneumonia in patients with hematologic malignancies: a descriptive study. J Infect 2003;47:19–27. 10.1016/s0163-4453(03)00038-0 [DOI] [PubMed] [Google Scholar]
- 181. Wieruszewski PM, Barreto JN, Frazee E, et al. Early corticosteroids for Pneumocystis pneumonia in adults without HIV are not associated with better outcome. Chest 2018;154:636–44. 10.1016/j.chest.2018.04.026 [DOI] [PubMed] [Google Scholar]
- 182. Lemiale V, Debrumetz A, Delannoy A, et al. Adjunctive steroid in HIV-negative patients with severe Pneumocystis pneumonia. Respir Res 2013;14:87. 10.1186/1465-9921-14-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Inoue N, Fushimi K. Adjunctive corticosteroids decreased the risk of mortality of non-HIV Pneumocystis pneumonia. Int J Infect Dis 2019;79:109–15. 10.1016/j.ijid.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 184. Injean P, Eells SJ, Wu H, et al. A systematic review and meta-analysis of the data behind current recommendations for corticosteroids in non-HIV-related PCP: knowing when you are on shaky foundations. Transplant Direct 2017;3:e137. 10.1097/TXD.0000000000000642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Ding L, Huang H, Wang H, et al. Adjunctive corticosteroids may be associated with better outcome for non-HIV Pneumocystis pneumonia with respiratory failure: a systemic review and meta-analysis of observational studies. Ann Intensive Care 2020;10:34. 10.1186/s13613-020-00649-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Inoue D, Marumo S, Ishii H, et al. Secondary pulmonary alveolar proteinosis during corticosteroid therapy for organising pneumonia associated with myelodysplastic syndrome. BMJ Case Rep 2019;12. 10.1136/bcr-2019-231055. [Epub ahead of print: 18 Sep 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Sibila O, Luna CM, Agustí C, et al. Effects of glucocorticoids in ventilated piglets with severe pneumonia. Eur Respir J 2008;32:1037–46. 10.1183/09031936.00009208 [DOI] [PubMed] [Google Scholar]
- 188. Li Y, Cui X, Li X, et al. Risk of death does not alter the efficacy of hydrocortisone therapy in a mouse E. coli pneumonia model: risk and corticosteroids in sepsis. Intensive Care Med 2008;34:568–77. 10.1007/s00134-007-0921-7 [DOI] [PubMed] [Google Scholar]
- 189. Hirao S, Wada H, Nakagaki K, et al. Inflammation provoked by Mycoplasma pneumoniae extract: implications for combination treatment with clarithromycin and dexamethasone. FEMS Immunol Med Microbiol 2011;62:182–9. 10.1111/j.1574-695X.2011.00799.x [DOI] [PubMed] [Google Scholar]
- 190. Hicks CW, Sweeney DA, Danner RL, et al. Beneficial effects of stress-dose corticosteroid therapy in canines depend on the severity of staphylococcal pneumonia. Intensive Care Med 2012;38:2063–71. 10.1007/s00134-012-2735-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Endeman H, Meijvis SCA, Rijkers GT, et al. Systemic cytokine response in patients with community-acquired pneumonia. Eur Respir J 2011;37:1431–8. 10.1183/09031936.00074410 [DOI] [PubMed] [Google Scholar]
- 192. Bentzer P, Fjell C, Walley KR, et al. Plasma cytokine levels predict response to corticosteroids in septic shock. Intensive Care Med 2016;42:1970–9. 10.1007/s00134-016-4338-z [DOI] [PubMed] [Google Scholar]
- 193. Oppert M, Schindler R, Husung C, et al. Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Crit Care Med 2005;33:2457–64. 10.1097/01.ccm.0000186370.78639.23 [DOI] [PubMed] [Google Scholar]
- 194. Confalonieri M, Urbino R, Potena A, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med 2005;171:242–8. 10.1164/rccm.200406-808OC [DOI] [PubMed] [Google Scholar]
- 195. Nafae RM, Ragab MI, Amany FM, et al. Adjuvant role of corticosteroids in the treatment of community-acquired pneumonia. Egypt J Chest Dis Tuberc 2013;62:439–45. 10.1016/j.ejcdt.2013.03.009 [DOI] [Google Scholar]
- 196. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2015;385:1511–8. 10.1016/S0140-6736(14)62447-8 [DOI] [PubMed] [Google Scholar]
- 197. El-Ghamrawy AH, Shokeir MH, A.A E. Effects of low-dose hydrocortisone in ICU patients with severe community-acquired pneumonia. Egypt J Chest Dis Tuberc 2006;55:91–9. [Google Scholar]
- 198. Fernández-Serrano S, Dorca J, Garcia-Vidal C, et al. Effect of corticosteroids on the clinical course of community-acquired pneumonia: a randomized controlled trial. Crit Care 2011;15:R96. 10.1186/cc10103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Marik P, Kraus P, Sribante J, et al. Hydrocortisone and tumor necrosis factor in severe community-acquired pneumonia. A randomized controlled study. Chest 1993;104:389–92. 10.1378/chest.104.2.389 [DOI] [PubMed] [Google Scholar]
- 200. McHardy VU, Schonell ME. Ampicillin dosage and use of prednisolone in treatment of pneumonia: co-operative controlled trial. Br Med J 1972;4:569–73. 10.1136/bmj.4.5840.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Meijvis SCA, Hardeman H, Remmelts HHF, et al. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. Lancet 2011;377:2023–30. 10.1016/S0140-6736(11)60607-7 [DOI] [PubMed] [Google Scholar]
- 202. Mikami K, Suzuki M, Kitagawa H, et al. Efficacy of corticosteroids in the treatment of community-acquired pneumonia requiring hospitalization. Lung 2007;185:249–55. 10.1007/s00408-007-9020-3 [DOI] [PubMed] [Google Scholar]
- 203. Snijders D, Daniels JMA, de Graaff CS, et al. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med 2010;181:975–82. 10.1164/rccm.200905-0808OC [DOI] [PubMed] [Google Scholar]
- 204. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015;313:677–86. 10.1001/jama.2015.88 [DOI] [PubMed] [Google Scholar]
- 205. Bi J, Yang J, Wang Y, et al. Efficacy and safety of adjunctive corticosteroids therapy for severe community-acquired pneumonia in adults: an updated systematic review and meta-analysis. PLoS One 2016;11:e0165942. 10.1371/journal.pone.0165942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Horita N, Otsuka T, Haranaga S, et al. Adjunctive systemic corticosteroids for hospitalized community-acquired pneumonia: systematic review and meta-analysis 2015 update. Sci Rep 2015;5:14061. 10.1038/srep14061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Siemieniuk RAC, Guyatt GH. Corticosteroids in the treatment of community-acquired pneumonia: an evidence summary. Pol Arch Med Wewn 2015;125:570–5. 10.20452/pamw.2971 [DOI] [PubMed] [Google Scholar]
- 208. Stern A, Skalsky K, Avni T, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev 2017;12:CD007720. 10.1002/14651858.CD007720.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Wan Y-D, Sun T-W, Liu Z-Q, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest 2016;149:209–19. 10.1378/chest.15-1733 [DOI] [PubMed] [Google Scholar]
- 210. Venkatesh B, Finfer S, Cohen J, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med 2018;378:797–808. 10.1056/NEJMoa1705835 [DOI] [PubMed] [Google Scholar]
- 211. Meduri GU, Chinn AJ, Leeper KV, et al. Corticosteroid rescue treatment of progressive fibroproliferation in late ARDS. patterns of response and predictors of outcome. Chest 1994;105:1516–27. 10.1378/chest.105.5.1516 [DOI] [PubMed] [Google Scholar]
- 212. Meduri GU, Eltorky MA. Understanding ARDS-associated fibroproliferation. Intensive Care Med 2015;41:517–20. 10.1007/s00134-014-3613-0 [DOI] [PubMed] [Google Scholar]
- 213. Meduri GU. Pulmonary fibroproliferation and death in patients with late ARDS. Chest 1995;107:5–6. 10.1378/chest.107.1.5 [DOI] [PubMed] [Google Scholar]
- 214. Olman MA, White KE, Ware LB, et al. Pulmonary edema fluid from patients with early lung injury stimulates fibroblast proliferation through IL-1 beta-induced IL-6 expression. J Immunol 2004;172:2668–77. 10.4049/jimmunol.172.4.2668 [DOI] [PubMed] [Google Scholar]
- 215. Yamanishi C, Robinson S, Takayama S. Biofabrication of phenotypic pulmonary fibrosis assays. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018;198:e44–68. 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 217. Turner-Warwick M, Burrows B, Johnson A. Cryptogenic fibrosing alveolitis: response to corticosteroid treatment and its effect on survival. Thorax 1980;35:593–9. 10.1136/thx.35.8.593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Rudd RM, Haslam PL, Turner-Warwick M. Cryptogenic fibrosing alveolitis. American Review of Respiratory Disease 1981;124:1–8. 10.1164/arrd.1981.124.1.1 [DOI] [PubMed] [Google Scholar]
- 219. Stack BH, Choo-Kang YF, Heard BE. The prognosis of cryptogenic fibrosing alveolitis. Thorax 1972;27:535–42. 10.1136/thx.27.5.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Katzenstein AL, Fiorelli RF. Nonspecific interstitial pneumonia/fibrosis. histologic features and clinical significance. Am J Surg Pathol 1994;18:136–47. [PubMed] [Google Scholar]
- 221. Douglas WW, Ryu JAYH, Swensen SJ, et al. Colchicine versus prednisone in the treatment of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998;158:220–5. 10.1164/ajrccm.158.1.9709089 [DOI] [PubMed] [Google Scholar]
- 222. Raghu G, Depaso WJ, Cain K, et al. Azathioprine combined with prednisone in the treatment of idiopathic pulmonary fibrosis: a prospective double-blind, randomized, placebo-controlled clinical trial. Am Rev Respir Dis 1991;144:291–6. 10.1164/ajrccm/144.2.291 [DOI] [PubMed] [Google Scholar]
- 223. Johnson MA, Kwan S, Snell NJ, et al. Randomised controlled trial comparing prednisolone alone with cyclophosphamide and low dose prednisolone in combination in cryptogenic fibrosing alveolitis. Thorax 1989;44:280–8. 10.1136/thx.44.4.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Demedts M, Behr J, Buhl R, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 2005;353:2229–42. 10.1056/NEJMoa042976 [DOI] [PubMed] [Google Scholar]
- 225. Prednisone A. And N-acetylcysteine for pulmonary fibrosis. New England Journal of Medicine 2012;366:1968–77. 10.1056/NEJMoa1113354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Selman M, Carrillo G, Salas J, et al. Colchicine, D-penicillamine, and prednisone in the treatment of idiopathic pulmonary fibrosis: a controlled clinical trial. Chest 1998;114:507–12. 10.1378/chest.114.2.507 [DOI] [PubMed] [Google Scholar]
- 227. Papiris SA, Kagouridis K, Kolilekas L, et al. Survival in idiopathic pulmonary fibrosis acute exacerbations: the non-steroid approach. BMC Pulm Med 2015;15:162. 10.1186/s12890-015-0146-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083–92. 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- 229. Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071–82. 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- 230. Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (capacity): two randomised trials. Lancet 2011;377:1760–9. 10.1016/S0140-6736(11)60405-4 [DOI] [PubMed] [Google Scholar]
- 231. Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Al-Hameed FM, Sharma S. Outcome of patients admitted to the intensive care unit for acute exacerbation of idiopathic pulmonary fibrosis. Can Respir J 2004;11:117–22. 10.1155/2004/379723 [DOI] [PubMed] [Google Scholar]
- 233. Suzuki H, Sekine Y, Yoshida S, et al. Risk of acute exacerbation of interstitial pneumonia after pulmonary resection for lung cancer in patients with idiopathic pulmonary fibrosis based on preoperative high-resolution computed tomography. Surg Today 2011;41:914–21. 10.1007/s00595-010-4384-z [DOI] [PubMed] [Google Scholar]
- 234. Tachikawa R, Tomii K, Ueda H, et al. Clinical features and outcome of acute exacerbation of interstitial pneumonia: collagen vascular diseases-related versus idiopathic. Respiration 2012;83:20–7. 10.1159/000329893 [DOI] [PubMed] [Google Scholar]
- 235. Akira M, Hamada H, Sakatani M, et al. CT findings during phase of accelerated deterioration in patients with idiopathic pulmonary fibrosis. AJR Am J Roentgenol 1997;168:79–83. 10.2214/ajr.168.1.8976924 [DOI] [PubMed] [Google Scholar]
- 236. Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med 2019;381:1718–27. 10.1056/NEJMoa1908681 [DOI] [PubMed] [Google Scholar]
- 237. Epler GR, Colby TV, McLoud TC, et al. Bronchiolitis obliterans organizing pneumonia. N Engl J Med 1985;312:152–8. 10.1056/NEJM198501173120304 [DOI] [PubMed] [Google Scholar]
- 238. Lazor R, Vandevenne A, Pelletier A, et al. Cryptogenic organizing pneumonia. characteristics of relapses in a series of 48 patients. The Groupe d'Etudes et de Recherche sur les Maladles "Orphelines" Pulmonaires (GERM"O"P). Am J Respir Crit Care Med 2000;162:571–7. 10.1164/ajrccm.162.2.9909015 [DOI] [PubMed] [Google Scholar]
- 239. Niksarlıoğlu EY, Özkan GZ, Bakan ND, et al. Cryptogenic organizing pneumonia: clinical and radiological features, treatment outcomes of 17 patients, and review of the literature. Turk J Med Sci 2016;46:1712–8. 10.3906/sag-1508-114 [DOI] [PubMed] [Google Scholar]
- 240. Oymak FS, Demirbaş HM, Mavili E, et al. Bronchiolitis obliterans organizing pneumonia. Clinical and roentgenological features in 26 cases. Respiration 2005;72:254–62. 10.1159/000085366 [DOI] [PubMed] [Google Scholar]
- 241. Zhou Y, Wang L, Huang M, et al. A long-term retrospective study of patients with biopsy-proven cryptogenic organizing pneumonia. Chron Respir Dis 2019;16:1479973119853829. 10.1177/1479973119853829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Barroso E, Hernandez L, Gil J, et al. Idiopathic organizing pneumonia: a relapsing disease. 19 years of experience in a hospital setting. Respiration 2007;74:624–31. 10.1159/000103240 [DOI] [PubMed] [Google Scholar]
- 243. Izumi T, Kitaichi M, Nishimura K, et al. Bronchiolitis obliterans organizing pneumonia. clinical features and differential diagnosis. Chest 1992;102:715–9. 10.1378/chest.102.3.715 [DOI] [PubMed] [Google Scholar]
- 244. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 2008;63 Suppl 5:v1–58. 10.1136/thx.2008.101691 [DOI] [PubMed] [Google Scholar]
- 245. Cordier JF. Organising pneumonia. Thorax 2000;55:318–28. 10.1136/thorax.55.4.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Epler GR. Bronchiolitis obliterans organizing pneumonia. Arch Intern Med 2001;161:158–64. 10.1001/archinte.161.2.158 [DOI] [PubMed] [Google Scholar]
- 247. Ryu JH, Myers JL, Capizzi SA, et al. Desquamative interstitial pneumonia and respiratory bronchiolitis-associated interstitial lung disease. Chest 2005;127:178–84. 10.1378/chest.127.1.178 [DOI] [PubMed] [Google Scholar]
- 248. Park JS, Brown KK, Tuder RM, et al. Respiratory bronchiolitis-associated interstitial lung disease: radiologic features with clinical and pathologic correlation. J Comput Assist Tomogr 2002;26:13–20. 10.1097/00004728-200201000-00003 [DOI] [PubMed] [Google Scholar]
- 249. Portnoy J, Veraldi KL, Schwarz MI, et al. Respiratory bronchiolitis-interstitial lung disease: long-term outcome. Chest 2007;131:664–71. 10.1378/chest.06-1885 [DOI] [PubMed] [Google Scholar]
- 250. Carrington CB, Gaensler EA, Coutu RE, et al. Natural history and treated course of usual and desquamative interstitial pneumonia. N Engl J Med 1978;298:801–9. 10.1056/NEJM197804132981501 [DOI] [PubMed] [Google Scholar]
- 251. Tubbs RR, Benjamin SP, Reich NE, et al. Desquamative interstitial pneumonitis. cellular phase of fibrosing alveolitis. Chest 1977;72:159–65. 10.1378/chest.72.2.159 [DOI] [PubMed] [Google Scholar]
- 252. Akira M, Yamamoto S, Hara H, et al. Serial computed tomographic evaluation in desquamative interstitial pneumonia. Thorax 1997;52:333–7. 10.1136/thx.52.4.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Hartman TE, Primack SL, Kang EY, et al. Disease progression in usual interstitial pneumonia compared with desquamative interstitial pneumonia. assessment with serial CT. Chest 1996;110:378–82. 10.1378/chest.110.2.378 [DOI] [PubMed] [Google Scholar]
- 254. Hellemons ME, Moor CC, von der Thüsen J, et al. Desquamative interstitial pneumonia: a systematic review of its features and outcomes. Eur Respir Rev 2020;29:190181. 10.1183/16000617.0181-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Nagai S, Kitaichi M, Itoh H, et al. Idiopathic nonspecific interstitial pneumonia/fibrosis: comparison with idiopathic pulmonary fibrosis and BOOP. Eur Respir J 1998;12:1010–9. 10.1183/09031936.98.12051010 [DOI] [PubMed] [Google Scholar]
- 256. Cottin V, Donsbeck AV, Revel D, et al. Nonspecific interstitial pneumonia. individualization of a clinicopathologic entity in a series of 12 patients. Am J Respir Crit Care Med 1998;158:1286–93. 10.1164/ajrccm.158.4.9802119 [DOI] [PubMed] [Google Scholar]
- 257. Fujita J, Yamadori I, Suemitsu I, et al. Clinical features of non-specific interstitial pneumonia. Respir Med 1999;93:113–8. 10.1016/s0954-6111(99)90300-1 [DOI] [PubMed] [Google Scholar]
- 258. Flaherty KR, Toews GB, Travis WD, et al. Clinical significance of histological classification of idiopathic interstitial pneumonia. Eur Respir J 2002;19:275–83. 10.1183/09031936.02.00182002 [DOI] [PubMed] [Google Scholar]
- 259. Park IN, Jegal Y, Kim DS, et al. Clinical course and lung function change of idiopathic nonspecific interstitial pneumonia. Eur Respir J 2009;33:68–76. 10.1183/09031936.00158507 [DOI] [PubMed] [Google Scholar]
- 260. Hauber H-P, Bittmann I, Kirsten D. [Non-specific interstitial pneumonia (NSIP)]. Pneumologie 2011;65:477–83. 10.1055/s-0030-1256284 [DOI] [PubMed] [Google Scholar]
- 261. Philit F, Etienne-Mastroïanni B, Parrot A, et al. Idiopathic acute eosinophilic pneumonia: a study of 22 patients. Am J Respir Crit Care Med 2002;166:1235–9. 10.1164/rccm.2112056 [DOI] [PubMed] [Google Scholar]
- 262. Rhee CK, Min KH, Yim NY, et al. Clinical characteristics and corticosteroid treatment of acute eosinophilic pneumonia. Eur Respir J 2013;41:402–9. 10.1183/09031936.00221811 [DOI] [PubMed] [Google Scholar]
- 263. Ogawa H, Fujimura M, Matsuda T, et al. Transient wheeze. eosinophilic bronchobronchiolitis in acute eosinophilic pneumonia. Chest 1993;104:493–6. 10.1378/chest.104.2.493 [DOI] [PubMed] [Google Scholar]
- 264. Jhun BW, Kim SJ, Kim K, et al. Outcomes of rapid corticosteroid tapering in acute eosinophilic pneumonia patients with initial eosinophilia. Respirology 2015;20:1241–7. 10.1111/resp.12639 [DOI] [PubMed] [Google Scholar]
- 265. Shorr AF, Scoville SL, Cersovsky SB, et al. Acute eosinophilic pneumonia among US military personnel deployed in or near Iraq. JAMA 2004;292:2997–3005. 10.1001/jama.292.24.2997 [DOI] [PubMed] [Google Scholar]
- 266. Carrington CB, Addington WW, Goff AM, et al. Chronic eosinophilic pneumonia. N Engl J Med 1969;280:787–98. 10.1056/NEJM196904102801501 [DOI] [PubMed] [Google Scholar]
- 267. Marchand E, Reynaud-Gaubert M, Lauque D, et al. Idiopathic chronic eosinophilic pneumonia. A clinical and follow-up study of 62 cases. The Groupe d'Etudes et de Recherche sur les Maladies "Orphelines" Pulmonaires (GERM"O"P). Medicine 1998;77:299–312. 10.1097/00005792-199809000-00001 [DOI] [PubMed] [Google Scholar]
- 268. Suzuki Y, Oyama Y, Hozumi H, et al. Persistent impairment on spirometry in chronic eosinophilic pneumonia: a longitudinal observation study (Shizuoka-CEP study). Ann Allergy Asthma Immunol 2017;119:e422:422–8. 10.1016/j.anai.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 269. Naughton M, Fahy J, FitzGerald MX. Chronic eosinophilic pneumonia. A long-term follow-up of 12 patients. Chest 1993;103:162–5. 10.1378/chest.103.1.162 [DOI] [PubMed] [Google Scholar]
- 270. Ishiguro T, Takayanagi N, Uozumi R, et al. The long-term clinical course of chronic eosinophilic pneumonia. Intern Med 2016;55:2373–7. 10.2169/internalmedicine.55.6765 [DOI] [PubMed] [Google Scholar]
- 271. Durieu J, Wallaert B, Tonnel AB. Long-term follow-up of pulmonary function in chronic eosinophilic pneumonia. Groupe d'Etude en Pathologie Interstitielle de la Société de Pathologie Thoracique Du Nord. Eur Respir J 1997;10:286–91. 10.1183/09031936.97.10020286 [DOI] [PubMed] [Google Scholar]
- 272. Oyama Y, Fujisawa T, Hashimoto D, et al. Efficacy of short-term prednisolone treatment in patients with chronic eosinophilic pneumonia. Eur Respir J 2015;45:1624–31. 10.1183/09031936.00199614 [DOI] [PubMed] [Google Scholar]
- 273. Suzuki Y, Suda T. Eosinophilic pneumonia: a review of the previous literature, causes, diagnosis, and management. Allergol Int 2019;68:413–9. 10.1016/j.alit.2019.05.006 [DOI] [PubMed] [Google Scholar]
- 274. Minakuchi M, Niimi A, Matsumoto H, et al. Chronic eosinophilic pneumonia: treatment with inhaled corticosteroids. Respiration 2003;70:362–6. 10.1159/000072898 [DOI] [PubMed] [Google Scholar]
- 275. Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J Immunol 1995;154:4719–25. [PubMed] [Google Scholar]
- 276. Drasler B, Karakocak BB, Tankus EB, et al. An inflamed human alveolar model for testing the efficiency of anti-inflammatory drugs in vitro. Front Bioeng Biotechnol 2020;8:987. 10.3389/fbioe.2020.00987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Salter M, Biggadike K, Matthews JL, et al. Pharmacological properties of the enhanced-affinity glucocorticoid fluticasone furoate in vitro and in an in vivo model of respiratory inflammatory disease. Am J Physiol Lung Cell Mol Physiol 2007;293:L660–7. 10.1152/ajplung.00108.2007 [DOI] [PubMed] [Google Scholar]
- 278. Carayol N, Campbell A, Vachier I, et al. Modulation of cadherin and catenins expression by tumor necrosis factor-alpha and dexamethasone in human bronchial epithelial cells. Am J Respir Cell Mol Biol 2002;26:341–7. 10.1165/ajrcmb.26.3.4684 [DOI] [PubMed] [Google Scholar]
- 279. Stellato C. Glucocorticoid actions on airway epithelial responses in immunity: functional outcomes and molecular targets. J Allergy Clin Immunol 2007;120:1247–63. 10.1016/j.jaci.2007.10.041 [DOI] [PubMed] [Google Scholar]
- 280. Schleimer RP. Glucocorticoids suppress inflammation but spare innate immune responses in airway epithelium. Proc Am Thorac Soc 2004;1:222–30. 10.1513/pats.200402-018MS [DOI] [PubMed] [Google Scholar]
- 281. Schleimer RP, Schulman ES, MacGlashan DW, et al. Effects of dexamethasone on mediator release from human lung fragments and purified human lung mast cells. J Clin Invest 1983;71:1830–5. 10.1172/jci110938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Gonzales LW, Guttentag SH, Wade KC, et al. Differentiation of human pulmonary type II cells in vitro by glucocorticoid plus cAMP. Am J Physiol Lung Cell Mol Physiol 2002;283:L940–51. 10.1152/ajplung.00127.2002 [DOI] [PubMed] [Google Scholar]
- 283. Chen Y, Nickola TJ, DiFronzo NL, et al. Dexamethasone-mediated repression of MUC5AC gene expression in human lung epithelial cells. Am J Respir Cell Mol Biol 2006;34:338–47. 10.1165/rcmb.2005-0176OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Logie JJ, Ali S, Marshall KM, et al. Glucocorticoid-mediated inhibition of angiogenic changes in human endothelial cells is not caused by reductions in cell proliferation or migration. PLoS One 2010;5:e14476. 10.1371/journal.pone.0014476 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jim-2021-002161supp002.pdf (39.6KB, pdf)
jim-2021-002161supp001.pdf (39.6KB, pdf)