Abstract
Objective:
Studies have observed variable associations of prior contact sport participation with subjective and objective measures of cognitive function. This study directly investigated the association between subjective self-report and objective performance-based cognition among former collegiate football players, as well as its relationship to self-reported concussion history.
Method:
Former collegiate football players (N=57; mean age = 37.9 years [SD=1.49]) retired from sport 15-years prior were enrolled. Linear regression models examined associations between subjective cognition (Quality of Life in Neurological Disorders Cognitive Functioning-Short Form), and performance on a neuropsychological battery. Domain specific (executive function) metrics of subjective (Behavior Rating Inventory of Executive Function-Adult) and objective cognition were also exclusively examined. Associations between self-reported concussion history with subjective and objective measures were tested. Potential influential factors (sleep quality and distress) were included as covariates.
Results:
Subjective cognition was not significantly associated with any objective measures of cognitive functioning (p’s>.05). Greater self-reported concussion history was inversely associated with subjective cognition (B=−2.49, p=.004), but not objective performance-based cognition (p’s>.05). Distress was significantly related to all metrics of subjective cognition (p’s<.001) as well as performance on delayed recall and verbal fluency (p’s<.05). Sleep quality was only significantly related to timed visuospatial sequencing (p=.033).
Conclusions:
Reliance on self-reported measures of cognitive functioning alone is insufficient when assessing cognition in former contact sport athletes. Assessment of other factors known to influence subjective cognitive complaints should also be examined in determining the presence of cognitive deficits.
Keywords: brain concussion, cognition, athletes, contact sport participation, concussion, TBI cognitive functioning
Introduction
The potential long-term adverse outcomes among contact sport athletes remains an important area of research, particularly among those with greater exposure to repetitive head impacts or concussion. Studies of former contact sport athletes have found contact sport participation to be a potential risk factor for adverse long-term outcomes, such as cognitive impairment (Alosco et al., 2018; Brett et al., 2021; Guskiewicz et al., 2005; Guskiewicz et al., 2003a; Manley et al., 2017), although not all studies have observed this association (Deshpande et al., 2017; Willer et al., 2018). Self-report (subjective) and performance-based (objective) measures of cognitive functioning are often used to assess potential long-term outcomes associated with contact sport participation among former athletes. In studies utilizing both subjective and objective methods of assessment, the association between prior contact sport exposure and cognitive function have not always aligned. For example, in a study examining the effects of age of first exposure to football, Alosco et al. (2018) reported a significant association between age of first exposure to football and self-reported cognitive difficulties (dysfunction ratings on the Behavior Rating Inventory of Executive Function-Adult Version), but not to objective cognitive performance (Brief Test of Adult Cognition by Telephone).
The discrepancy between subjective self-report and objective performance is seen throughout the literature including the general population and various clinical subpopulations. For example, a systematic review of cognitive function following chemotherapy found that across 45 studies that examined the association between self-report and performance-based cognition, only 14 studies found weak associations, while 31 studies showed a lack of association (Bray et al., 2018). A similar discrepancy between subjective and objective cognition has been observed across various clinical populations, such as Parkinson’s disease (Purri et al., 2020; Siciliano et al., 2021), epilepsy (Hall et al., 2009), and multiple sclerosis (Beier et al., 2015; van Geest et al., 2017). While this discrepancy can be due to a number of non-cognitive related factors (e.g., greater subjective cognitive difficulties associated with greater levels of psychiatric symptoms; (Nelson et al., 2021; Srisurapanont et al., 2017; Stillman et al., 2019), it has also been suggested that subjectively endorsed cognitive difficulties may reflect more subtle cognitive changes which objective measures are not able to detect due to insufficient sensitivity (Burmester et al., 2016). As such, inclusion of subjective and objective cognitive assessment is vital when investigating long-term outcomes among older former contact sport athletes.
Contact sport athletes are at risk for sustaining a concussion (Chun et al., 2021; O’Connor et al., 2017), which has been reported as being inversely associated with subjective and objective cognitive functioning in older adults (Brett et al., 2021; Gallo et al., 2020; Manley et al., 2017). In younger, active athletes, greater difficulties with subjectively rated cognition, but not objective performance-based cognitive function, have been reported among those with a greater history of concussion (Brett et al., 2019). The agreement between two methods of evaluating cognition and their association with concussion history among former athletes at early midlife several years removed from sport is extremely limited and studies are needed to investigate this critical intermediary time point in the life span. Furthermore, several factors that have been observed in former contact sport athletes such as poorer sleep quality and higher distress are known to distinctly influence subjective ratings of cognition (Schwenk et al., 2007), while not necessarily affecting performance-based cognitive function (Nelson et al., 2021; Nicolazzo et al., 2021; Srisurapanont et al., 2017; Stillman et al., 2019; Zavecz et al., 2020). Given the influence of these factors on subjectively rated cognition, their consideration as potential confounds may enhance the level of agreement between self-report subjective and performance-based objective cognitive abilities.
Executive dysfunction has been reported as a common corollary of cumulative concussion and participation in contact sports (Goswami et al., 2016; Katz et al., 2021; Montenigro et al., 2017; Terpstra et al., 2019), indicating that this domain is particularly important when investigating long-term outcomes in former contact sport athletes. While most studies have observed weak to no relationships between subjective and objective cognition using global measures of subjective cognition, theoretically, comparing domain specific measures could yield more precise agreement between subjective and objective aspects of cognition. Given the pertinent nature of executive dysfunction within the subpopulation and the potential to improve our understanding of the agreement between subjective and objective cognition through domain specific measures, there is a vital need to investigate the association between subjective and objective executive functioning among former contact sport athletes.
While inconsistencies in associations between contact sport history with subjective and objective cognitive function have been observed, no studies to date have directly investigated the relationship between subjectively rated cognitive function and performance on objectively measured cognitive function among former contact sport athletes several years removed from sport. Doing so would further elucidate the potential long-term cognitive outcomes in individuals with a history of contact sport participation and prior concussions. Therefore, the aims of the current study were to: 1) investigate the association between general subjective cognition and various domains of objective performance-based cognitive functioning in former athletes; 2) assess the association between domain-specific subjective and objective performance-based cognitive functioning (i.e., executive functioning); and 3) investigate associations of self-reported concussion history with subjective and objective cognitive functioning. Based on prior literature, we hypothesize that: 1) there will be a weak association between subjective and objective functioning and this relationship would be strengthened when controlling for subjectively rated sleep quality and general distress; 2) there will be greater concordance between subjective executive functioning and performance on objective executive functioning measures; and 3) both subjectively rated cognition and objective cognitive performance would have an association with self-reported concussion history.
Materials and Methods
Participants and Procedure
This study was approved by an Institutional Review Board and all participants provided written informed consent prior to study activities. Former collegiate athletes, retired for approximately 15-years who had previously participated in Part I of the study, which included an online health survey (Kerr et al., 2018a) were recruited from various National Collegiate Athletic Association (NCAA) member institutions. Recruitment for the initial online health survey (Part I) involved contacting athletic directors, sports information directors, alumni representatives, and athletic trainers of universities who participated in the original NCAA concussion Study (1999–2001) (Guskiewicz et al., 2003b) to obtain contact information of former collegiate players (Part I response rate for those with valid contact information was 23.4%) (Kerr et al., 2018a). Potential participants who completed the online health survey were screened for eligibility by a research coordinator based on inclusion/exclusion criteria. Inclusion criteria included at least one year of collegiate football participation. Exclusion criteria included history of psychotic disorder with active symptoms and any contraindications to study procedures (e.g., contraindications to neuroimaging, unable to travel). Participants travelled to and completed in-person assessments at either of the two study sites (Medical College of Wisconsin or University of North Carolina at Chapel Hill).
Measures
Subjective Cognitive Functioning
General Cognitive Functioning.
To assess self-reported perceived difficulties in general cognitive abilities, participants completed the Quality of Life in Neurological Disorders (Neuro-QoL) Cognitive Functioning-Short Form (National Institute of Neurological Disorders and Stroke Version 2.0., 2015). Using a Likert scale (1–5), participants answered 8 questions on cognitive functioning over the past 7 days and currently. Scores on the Neuro-QoL were converted to normative-based T-scores (M = 50, SD = 10). Higher T-scores on this measure represent better self-rated cognitive functioning.
Domain-specific (Executive Functioning) Cognitive Functioning.
The Behavior Rating Inventory of Executive Function-Adult (BRIEF-A) is a 75-item self-report questionnaire that assesses various aspects of executive functioning (Roth et al., 2005). Within the BRIEF-A, the Behavioral Regulation (BRI) and Metacognition (MI) summary indices were of primary interest. Scores on the BRIEF-A were also converted to T-scores using the normative data provided in the measure manual. Higher scores indicate greater dysfunction within that area of executive functioning.
Objective Cognitive Testing
Performance-based measures of cognitive functioning included the Trail Making Test A and B (TMT-A and TMT-B; (Reitan & Wolfson, 1985), Symbol Digit Modalities Test (SDMT; (Smith, 1973), Hopkins Verbal Learning Test-Revised (HVLT-R; indices of interest were Total Immediate and Delayed Recall; (Brandt & Benedict, 2001), and Verbal Fluency (F-A-S; (Strauss et al., 2006). Due to the fact that Neuro-QoL T-scores are not standardized based on age, raw score performances, as opposed to standardized scores derived from age-based normative data, were included as outcomes of interest for performance-based measures. This ultimately allowed for uniformity of models across subjective and objective cognitive measures (inclusion of age and education as covariates). For most measures, higher raw score indicates a better performance, except for TMT-A&B where higher scores (i.e., slower completion times) represent worse performance. For domain-specific comparisons measures of executive functioning included: F-A-S and TMT-B (Lezak et al., 2012). To examine the rate of lower scores (low average or worse) on objective measures, a cutoff of ≤ 1 SD below the mean based on normative data was used. T-score were calculated for HVLT-R based on normative data provided in the manual. Z-scores were calculated based on age and education corrected standard scores for TMT (Tombaugh, 2004), F-A-S (Tombaugh et al., 1999), and SDMT (Sheridan et al., 2006).
Covariates of Cognition
Age and estimated premorbid ability (Wechsler Test of Adult Reading (WTAR) Word Reading raw score) were included as covariates in all models. Given the previously reported influence of subjectively rated sleep quality and distress with subjective and performance-based cognition (Hromas et al., 2021; Nelson et al., 2021; Nicolazzo et al., 2021; Srisurapanont et al., 2017; Stillman et al., 2019; Zavecz et al., 2020), these factors were tested within the model as secondary covariates (see below). The Brief Symptom Inventory-18 (BSI-18) is measure of general distress and includes a Global Severity Index (GSI) that is comprised of three subscales representing different dimensions of psychological symptomology (somatization, depression, anxiety). The 18-items are rated on a scale of 0 (not at all) through 4 (very much) based on the degree in which they are affected by the listed item (Derogatis, 2001). Raw scores were converted to T-scores, with higher scores representing greater levels of current general distress. The Pittsburgh Sleep Quality Index (PSQI) is a measure of quality based on respondent’s retrospective recall of sleep quality and disturbance over the previous 1-month (Smyth, 1999). Based on items reflecting various aspects of sleep quality (e.g., sleep latency, sleep disturbances, daytime dysfunction), a total PSQI score is calculated and considered as an overall estimate of sleep quality.
Self-reported Concussion History
Respondents were asked to report the number of concussions that they believed they had sustained during participation in sports, including before and at the high school and collegiate levels, and also report the number of non-sports-related concussions (e.g., from motor vehicle accident, falls, or violence). To aid in their classification of concussions, participants were provided with the following definition: Concussions are defined as occurring typically but not necessarily from a blow to the head, followed by a variety of symptoms that may include any of the following: headache, dizziness, loss of balance, blurred vision, “seeing stars,” feeling in a fog or slowed down, memory problems, poor concentration, nausea, throwing up, and loss of consciousness (Kerr et al., 2018b). Given that prior studies have demonstrated a dose response relationship with prior concussion and outcomes of interest (Guskiewicz et al., 2005; Walton et al., 2021a), total lifetime self-reported concussion history was discretized into four categories (0–1, 2–4, 5–7, 8+) based on the distribution of the sample, which was the most parsimonious way to test the linear association of cumulative self-reported concussion history with outcomes while trying to balance distribution across the sample (0–1 prior concussion = 38.6%; 2–4 prior concussions = 17.5%; 5–7 prior concussions = 21.1%, 8+ prior concussions = 22.8%).
Statistical Analyses
Statistical analyses were performed using SPSS version 27 using an a priori alpha at the 0.05 level. For aim 1, general linear regression models (GLMs) were fit to examine the association between subjective cognitive functioning and objective cognitive performance, using subjective general cognitive functioning (i.e., Neuro-QoL) as the independent variable. Models were fit first with primary covariates (age and WTAR Word Reading raw score) and then with secondary covariates (psychological distress and sleep quality) added to the model in order to assess adjusted effect estimates. For aim 2, GLMs were fit to examine the association between domain specific (executive functioning) subjectively related cognition and objective cognitive performance, using the BRIEF-A index scores (MI and BRI) as separate independent variables. Similar to general cognition, models were fit without and then with secondary covariates (psychological distress and sleep quality). Objective measures of executive functioning as dependent variables included F-A-S and TMT-B. For aim 3, to compare association of self-reported concussion history with subjectively rated cognition and performance-based executive function, GLMs were fit to examine the association of self-reported concussion history (independent ordinal variable) with indices of subjective and performance-based objective cognition.
Given that associations between cumulative head impact exposure via contact sport participation and study outcomes of interest have been reported (Brett et al., 2021; Montenigro et al., 2017), sensitivity analyses were performed to ensure that the observed relationships between self-reported concussion history and indices of subjective and performance-based objective cognition were not primarily driven by head impact exposure history (i.e., GLMs were performed with total years of contact sport exposure as covariates within the models).
Results
Demographics
Of the 58 former collegiate football players enrolled, one participant from the parent study was excluded due to failure to complete subjective measures of cognition (N = 57; Table 1). Performance and symptom validity were examined where possible. In this sample, there were no individuals with suspected noncredible performance using the embedded cutoffs TMT (Ashendorf et al., 2017; Iverson et al., 2002) and HVLT (Sawyer et al., 2017). Symptom validity was examined in accordance with the BRIEF-A manual (Roth et al., 2005). One participant scored above the cutoff on the Infrequency validity scale. Sensitivity analyses showed that exclusion of this participant did not meaningfully influence the associations between subjective and objective measures of cognition (differences in Pearson’s r’s ≤ .06).
Table 1.
Demographic, sport, and medical history (N=57)
| M ± SD / n (%) | |
|---|---|
|
| |
| Age | 38.0 ± 1.48 |
| Race | |
| White/Non-Hispanic | 48 (84.2%) |
| African American | 6 (10.5%) |
| Biracial | 3 (5.3%) |
| WTAR SS | 112.1 ± 7.4 |
| NIH Toolbox Picture Vocabulary | 113.6 ± 9.9 |
| Education | |
| Bachelor’s degree | 33 (57.9%) |
| Graduate / Professional Degree | 24 (42.1%) |
| Years of Football Participation a | 12.4 ± 4.1 |
| Low Exposure (0 – 5 years) | 0 (0.0%) |
| Substantial Exposure (>5 years) | 27 (47.4%) |
| Extensive Exposure (>11 years) | 31 (52.6%) |
| NCAA Level of Play | |
| Division I | 41 (71.9%) |
| Division III | 16 (28.1%) |
| Concussion History | |
| 0 to 1 | 22 (38.6%) |
| 2 to 4 | 10 (17.5%) |
| 5 to 7 | 12 (21.1%) |
| 8+ | 13 (22.8%) |
| Self-Reported Mental Health and Neurodevelopmental History | |
| Anxiety | 8 (13.8%) |
| Depression | 7 (12.1%) |
| Attention-deficit/hyperactivity disorder | 3 (5.2%) |
| Learning disability/disorder | 1 (1.7%) |
| Self-Reported Medical History | |
| Migraine | 3 (5.3%) |
| Hypertension | 8 (14.0%) |
| High Cholesterol | 9 (15.8%) |
| Osteoarthritis | 7 (12.3%) |
| Rheumatoid Arthritis | 1 (1.8%) |
| Sleep Apnea | 6 (10.3%) |
| Chronic Obstructive Pulmonary Disease; Coronary Artery Disease; Diabetes Mellitus-II; Emphysema; Myocardial Infarction; Kidney Disease; Liver Disease; Stroke/Cerebrovascular Accident; Inflammatory Bowel Disease | 0 (0.0%) |
Note. M = Mean; NCAA = National Collegiate Athletic Association; SD = Standard Deviation; WTAR SS = Wechsler Test of Adult Reading Standard Score.
The sample age was a mean of 37.9 years (SD = 1.49) and predominately identified as White, non-Hispanic (83.9%). Athletes reported an average of 12 years (SD = 3.14) of football participation and retired from sport approximately 15 years prior to study participation. Performance on objective measures of cognitive functioning and subjective self-report ratings are shown in Table 2. On a group level, players performed in the average range across all measures. Additionally, consistent with the general population, players reported their overall cognitive functioning and executive functioning were within the average range.
Table 2.
Objective functioning and subjective self-report (N=57)
| Measure | Raw Score M ± SD | Standardized Score M ± SD |
|---|---|---|
|
| ||
| Objective Cognitive Functioning | ||
| HVLT-R Immediate Recall | 27.33 ± 3.31 | T = 47.93 ± 7.93 |
| HVLT-R Delayed Recall | 9.74 ± 1.79 | T = 47.93 ± 8.59 |
| F-A-S | 44.16 ± 9.80 | Z = −0.05 ± 0.88 |
| SDMT | 56.79 ± 8.29 | Z = 0.43 ± 0.93 |
| TMT-A | 22.05 ± 6.53 | Z = -0.64 ± 0.65 |
| TMT-B | 47.11 ± 13.87 | Z = −0.69 ± 0.84 |
| Subjective Cognitive Functioning | ||
| Neuro-QoL Cognition | — | T = 51.64 ± 7.94 |
| BRIEF-A MI | — | T = 50.11 ± 11.00 |
| BRIEF-A BRI | — | T = 49.11 ± 10.18 |
| Other Functioning | ||
| BSI-18 GSI | — | T = 49.28 ± 8.90 |
| PSQI | 5.67 ± 3.08 | — |
Note. BRI = Behavioral Regulation Index; BRIEF-A = Behavior Rating Inventory of Executive Function – Adult; BSI-18 GSI= Brief Symptom Inventory-18 Global Severity Index; F-A-S = Verbal Fluency; HVLT-R = Hopkins Verbal Learning Test-Revised; M = Mean; MI = Metacognition Index; Neuro-QoL Cognition = Quality of Life in Neurological Disorders Cognitive Functioning Short-form; PSQI = Pittsburgh Sleep Quality Index; SD = Standard deviation; SDMT = Symbol Digit Modalities Test; T = T-score (M = 50; SD = 10); TMT = Trail Making Test; Z = Z-score (M = 0; SD = 1).
Subjective and objective cognitive functioning
Subjectively rated cognitive function (i.e., Neuro-QoL Cognitive Function) was not significantly associated with any performance-based objective measures of cognition (See Table 3 and Figure 1). Domain-specific subjective executive functioning across two indices was not related to objective performance on either measure of executive functioning (i.e., F-A-S or TMT-B; See Table 3 and Figure 2). Effect estimates were approximately comparable across all general and domain specific analyses. In other words, the weak relationships between subjective and performance-based cognition were not improved by examining corresponding domain specific abilities. The addition of secondary covariates (psychological distress and sleep quality) did not improve the strength of the association between subjective and objective measures of executive function (Table 3).
Table 3.
Linear regression models of objective functioning and subjective self-report (N=57)
| Neuro-QoL Cognition | BRIEF-A MI | BRIEF-A BRI | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Objective Cognitive Functioning | B | (95% CI) | β | p | R 2 | Β | (95% CI) | β | p | R 2 | Β | (95% CI) | β | p | R 2 |
|
| |||||||||||||||
| HVLT-R Immediate Recall a | .10 | [−.01, .21] | .24 | .084 | .12 | — | — | — | — | — | — | — | — | — | — |
| HVLT-R Immediate Recall b | .06 | [−.09, .20] | .13 | .446 | .13 | — | — | — | — | — | — | — | — | — | — |
| HVLT-R Delayed Recall a | .05 | [−.00, .11] | .23 | .094 | .09 | — | — | — | — | — | — | — | — | — | — |
| HVLT-R Delayed Recall b | .02 | [−.06, .09] | .07 | .702 | .19 | — | — | — | — | — | — | — | — | — | — |
| SDMT a | −.16 | [−.44, .12] | −.15 | .261 | .11 | — | — | — | — | — | — | — | — | — | — |
| SDMT b | −.22 | [−.59, .15] | −.21 | .237 | .11 | — | — | — | — | — | — | — | — | — | — |
| TMT-A a | −.02 | [−.25, .21] | −.02 | .872 | .04 | — | — | — | — | — | — | — | — | — | — |
| TMT-A b | −.07 | [−.36, .22] | −.08 | .650 | .12 | — | — | — | — | — | — | — | — | — | — |
| TMT-B a | .29 | [−.15, .73] | .17 | .186 | .23 | −.13 | [−.45, .19] | −.10 | .418 | .21 | −.02 | [−.37, .34] | −.01 | .923 | .20 |
| TMT-B b | .46 | [−.11, 1.03] | .26 | .113 | .24 | −.24 | [−.70, .22] | −.19 | .307 | .22 | −.00 | [−.53, .52] | −.00 | .988 | .20 |
| F-A-S a | .05 | [−.29, .38] | .04 | .781 | .12 | −.14 | [−.38, .09] | −.16 | .230 | .14 | −.06 | [−.32, .21] | −.06 | .666 | .12 |
| F-A-S b | −.03 | [−.45, .40] | −.02 | .899 | .15 | −.15 | [−.49, .18] | −.17 | .363 | .17 | .07 | [−.31, .45] | .07 | .726 | .16 |
Note. 95% CI = unstandardized beta 95% confidence interval; B = unstandardized beta estimate; BRI = Behavioral Regulation Index; BRIEF-A = Behavior Rating Inventory of Executive Function – Adult; BSI-18 GSI= Brief Symptom Inventory-18 Global Severity Index; F-A-S = Verbal Fluency; HVLT-R = Hopkins Verbal Learning Test-Revised; MI = Metacognition Index; Neuro-QoL Cognition = Quality of Life in Neurological Disorders Cognitive Functioning Short-form; p = p-value; PSQI = Pittsburgh Sleep Quality Index; R2 = r-squared (coefficient of determination); SDMT = Symbol Digit Modalities Test; TMT = Trail Making Test; β = standardized beta estimate.
= model includes primary covariates (age and Wechsler Test of Adult Reading raw score).
= model includes primary and secondary covariates of sleep quality (PSQI) and psychological distress (BSI-GSI).
Figure 1.

Scatter plots with regression lines of subjective and objective cognition (N=57). The X-axis represents normative-based T-scores (M = 50, SD = 10) on the Quality of Life in Neurological Disorders (Neuro-QoL) Cognitive Functioning-Short. Higher T-scores on this measure represent better self-rated cognitive functioning. The Y-axes represent T-scores and Z-scores on performance-based measures of cognitive functioning including the Hopkins Verbal Learning Test-Revised (HVLT-R; Total Immediate and Delayed Recall), Verbal Fluency (F-A-S), and the Trail Making Test A and B (TMT-A and TMT-B). Higher T-scores and Z-scores (M=0, SD=1) on measures represent better performance/cognitive ability in that domain.
Figure 2.
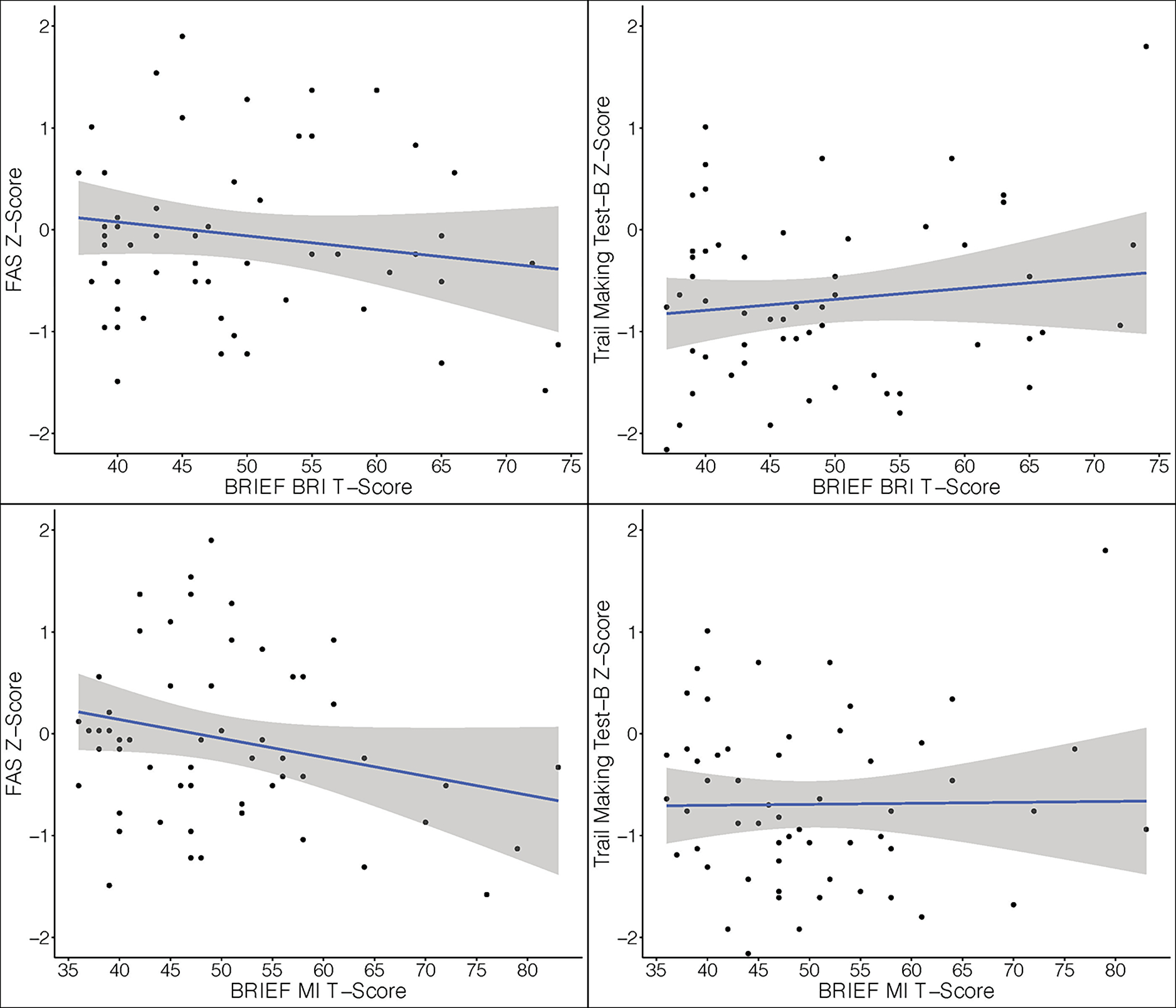
Scatter plots with regression lines of domain specific (executive function) subjective and objective cognition (N=57). The X-axis represents normative-based T-scores (M = 50, SD = 10) on the Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A) the Behavioral Regulation (BRI) and Metacognition (MI) summary indices. Higher scores on indicate greater dysfunction within that area of executive functioning. The Y-axes represent Z-scores (M=0, SD=1) on performance-based measures of executive functioning including Verbal Fluency (F-A-S) and the Trail Making Test B (TMT-B). Higher Z-scores represent better performance/cognitive ability in that domain.
As previously noted, on a group level participants performed in the average range across all cognitive measures and subjectively rated cognitive function also fell within the average range. Table 4 shows the proportion of the sample with scores falling in the abnormal range (± 1 SD depending on the direction indicating worse performance). On objective measures, 4–40% of scores were below the cutoff. TMT-B had the highest proportion of individuals with scores in the abnormal range. On subjective measures, 9–19% of ratings were suggestive of higher levels of cognitive symptom endorsement (± 1 SD depending on the direction indicating worse performance). One participant endorsed cognitive symptoms/difficulties ≥ 1.5 SD above the mean on the Neuro-QoL Cognitive Function measure. Nine participants endorsed executive function symptoms/difficulties (either on the MI, BRI, or both) that fell ≥ 1.5 SD above the mean. Table 4 shows the relationships between abnormal scores on objective measures and subjective rating scales.
Table 4.
Proportion of scores equal to or exceeding one standard deviation on objective and subjective measures (N=57)
| Neuro-QoL Cognition | BRIEF-A MI | BRIEF-A BRI | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Sample (N = 57) | WNL | Low | WNL | Low | WNL | Low | ||
| Objective Cognitive Functioning | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
|
| ||||||||
| HVLT-R Immediate Recall a | WNL | 45 (78.9) | 42 (93.3) | 3 (6.7) | — | — | — | — |
| Low | 12 (21.0) | 10 (83.3) | 2 (16.7) | — | — | — | — | |
| HVLT- R Delayed Recall a | WNL | 43 (75.4) | 42 (97.7) | 1 (2.3) | — | — | — | — |
| Low | 14 (24.6) | 10 (71.4) | 4 (28.6) | — | — | — | — | |
| SDMT a | WNL | 55 (96.5) | 50 (90.9) | 5 (9.1) | — | — | — | — |
| Low | 2 (3.5) | 2 (100) | 0 (0) | — | — | — | — | |
| TMT-A a | WNL | 38 (66.7) | 34 (89.5) | 4 (10.5) | — | — | — | — |
| Low | 19 (33.3) | 18 (94.7) | 1 (5.3) | — | — | — | — | |
| TMT-B a | WNL | 34 (59.6) | 30 (88.2) | 4 (11.8) | 27 (79.4) | 7 (20.6) | 27 (79.4) | 7 (20.6) |
| Low | 23 (40.4) | 22 (95.7) | 5 (4.3) | 21 (91.3) | 2 (8.7) | 19 (82.6) | 4 (17.4) | |
| F-A-S a | WNL | 50 (87.7) | 48 (96.0) | 2 (4.0) | 44 (88.0) | 6 (12.0) | 42 (84.0) | 8 (16.0) |
| Low | 7 (12.3) | 4 (57.1) | 3 (42.9) | 4 (57.1) | 3 (42.9) | 4 (57.1) | 3 (42.9) | |
| Subjective Cognitive Functioning | ||||||||
| Neuro-QoL Cognition a | WNL | 52 (91.2) | — | — | — | — | — | — |
| Low | 5 (8.8) | — | — | — | — | — | — | |
| BRIEF-MI b | WNL | 48 (84.2) | 46 (95.8) | 2 (4.2) | — | — | — | — |
| Low | 9 (15.8) | 6 (66.7) | 3 (33.3) | — | — | — | — | |
| BRIEF-BRI b | WNL | 46 (80.7) | 44 (95.7) | 2 (4.3) | — | — | — | — |
| Low | 11 (19.3) | 8 (72.7) | 3 (27.3) | — | — | — | — | |
Note. BRI = Behavioral Regulation Index; BRIEF-A = Behavior Rating Inventory of Executive Function – Adult; BSI-18 GSI= Brief Symptom Inventory-18 Global Severity Index; F-A-S = Verbal Fluency; HVLT-R = Hopkins Verbal Learning Test-Revised; MI = Metacognition Index; Neuro-QoL Cognition = Quality of Life in Neurological Disorders Cognitive Functioning Short-form; SDMT = Symbol Digit Modalities Test; TMT = Trail Making Test; WNL = within normal limits (± 1 SD).
= Low defined as ≤1 SD of the mean.
= Low defined as ≥1 SD of mean.
Self-reported concussion history with subjective and objective cognitive functioning
Greater self-reported concussion history was significantly associated with subjectively rated general cognition (unstandardized beta [95% CI] = −2.22 [−3.88, −.57], p = .009). Specifically, individuals with total lifetime self-reported concussion history between 0–1 reported the highest/best general cognitive functioning (NeuroQoL mean T = 54.88; SD = 6.78) with a general increase in self-reported cognitive symptoms with greater history of self-reported concussions (2–4 concussions NeuroQoL mean T = 51.58, SD = 7.66; 5–7 concussions NeuroQoL mean T = 50.42, SD = 9.21; 8+ concussions NeuroQoL mean T = 47.35, SD = 7.19). Conversely, statistically significant associations were not observed between self-reported concussion history and objective performance-based indices of cognitive function (Table 5). The addition of secondary covariates of general distress and sleep quality rating attenuated the relationship between self-reported concussion history and subjective cognitive functioning (i.e., subjective cognition was no longer significantly associated with concussion and weakened effect estimates; unstandardized beta [95% CI] = −1.31 [−2.79, .17], p = .081). Sensitivity analyses indicated that the results reported were robust and there were no meaningful changes when total years of contact sport exposure was included within the models.
Table 5.
Concussion history with objective cognitive functioning and subjective self-report (N=57)
| Concussion History | BSI-GSI | PSQI Total Score | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| B | 95% CI | β | p | R 2 | Β | 95% CI | β | p | Β | 95% CI | β | p | |
|
|
|||||||||||||
| Objective Cognitive Functioning | |||||||||||||
| HVLT-R Immediate Recall a | −.41 | [−1.14, .32] | −.15 | .267 | .09 | — | — | — | — | — | — | — | — |
| HVLT-R Immediate Recall b | −.27 | [−1.06, .52] | −.10 | .494 | .13 c | −.08 | [−.20, .03] | −.22 | .158 | −.01 | [−.35, .33] | −.01 | .948 |
| HVLT-R Delayed Recall a | −.25 | [−.64, .15] | −.17 | .220 | .07 | — | — | — | — | — | — | — | — |
| HVLT-R Delayed Recall b | −.26 | [−.67, .15] | −.18 | .260 | .21 c | −.09 | [−.17, −.03] | −.43 | .005 * | .15 | [−.03, .32] | .25 | .097 |
| SDMT a | −.75 | [−2.56, 1.07] | −.11 | .414 | .10 | — | — | — | — | — | — | — | — |
| SDMT b | −1.04 | [−1.04, .30] | −.15 | .302 | .11 c | .02 | [−.27, .31] | .02 | .885 | .26 | [−.60, 1.12] | .10 | .542 |
| TMT-A a | .01 | [−1.46, 1.49] | .00 | .985 | .04 | — | — | — | — | — | — | — | — |
| TMT-A b | .57 | [−.99, 2.13] | .11 | .466 | .13 c | .11 | [−.12, .34] | .15 | .327 | −.73 | [−1.41, −.06] | −.35 | .033 * |
| TMT-B a | .83 | [−2.02, 3.68] | .07 | .562 | .21 | — | — | — | — | — | — | — | — |
| TMT-B b | 1.00 | [−2.16, 4.16] | .09 | .529 | .21 c | −.04 | [−.50, .43] | −.02 | .878 | −.12 | [−1.48, 1.24] | −.03 | .864 |
| F-A-S a | .91 | [−1.20, 3.02] | .11 | .389 | .13 | — | — | — | — | — | — | — | — |
| F-A-S b | .79 | [−1.51, 3.09] | .10 | .493 | .16 c | −.24 | [−1.41, .17] | −.21 | .165 | .49 | [−.50, 1.47] | .15 | .329 |
| Subjective Cognitive Functioning | |||||||||||||
| Neuro-QoL Cognition a | −2.22 | [−3.88, −.57] | −.34 | .009 * | .19 | — | — | — | — | — | — | — | — |
| Neuro-QoL Cognition b | −1.31 | [−2.79, .17] | −.20 | .081 | .47 c | −.49 | [−.70, −.27] | −.55 | <.001 * | −.17 | [−.81, .47] | −.07 | .593 |
| BRIEF-A MI a | 1.13 | [−1.31, 3.57] | .12 | .358 | .08 | — | — | — | — | — | — | — | — |
| BRIEF-A MI b | −.47 | [−2.38, 1.44] | −.05 | .624 | .54 c | .87 | [.59, 1.15] | .70 | <.001 * | .27 | [−.55, 1.09] | .08 | .508 |
| BRIEF-A BRI a | 1.74 | [−.42, 3.91] | .21 | .112 | .15 | — | — | — | — | — | — | — | — |
| BRIEF-A BRI b | .69 | [−1.01, 2.39] | .08 | .423 | .57 c | .83 | [.59, 1.08] | .73 | <.001 * | −.21 | [−.94, .52] | −.06 | .564 |
Note. 95% CI = unstandardized beta 95% confidence interval; B = unstandardized beta estimate; BRI = Behavioral Regulation Index; BRIEF-A = Behavior Rating Inventory of Executive Function – Adult; BSI-18 GSI= Brief Symptom Inventory-18 Global Severity Index; F-A-S = Verbal Fluency; HVLT-R = Hopkins Verbal Learning Test-Revised; MI = Metacognition Index; Neuro-QoL Cognition = Quality of Life in Neurological Disorders Cognitive Functioning Short-form; p = p-value; PSQI = Pittsburgh Sleep Quality Index; R2 = r-squared (coefficient of determination); SDMT = Symbol Digit Modalities Test; TMT = Trail Making Test; β = standardized beta estimate.
= model includes primary covariates (age and Wechsler Test of Adult Reading raw score).
= model includes primary and secondary covariates of sleep quality (PSQI) and psychological distress (BSI-GSI).
= R2 for the full model with Concussion History, primary covariates, and secondary covariates.
p < .05.
Discussion
Consistent with previous findings in the general population and across various clinical populations, subjectively rated cognition was not robustly associated with objective performance-based cognition in this sample of former collegiate football players several years removed from sport. A history of more self-reported concussions was associated with greater subjectively endorsed cognitive symptoms, but not objectively measured performance. Taken together, the current results suggest that subjectively rated cognition may not accurately reflect objective abilities at early midlife, particularly within those experiencing distress; and lower scores on subjectively rated cognitive abilities, not lower objective performance, are more likely among those with a greater history of self-reported concussions.
Subjective cognition in clinical populations and confounding factors
In our sample of former athletes several years removed from college play, we did not observe an association between subjective cognitive functioning and broad objective cognitive performance, nor between domain-specific abilities. These results add to the extant literature reporting a similar discrepancy between subjective and objective cognitive performance across a variety of populations. For example, a considerable proportion of older adults report subjective cognitive impairment despite no clear evidence of such impairment on objective neuropsychological assessment (e.g., (Reid et al., 2012; Steinberg et al., 2013). Similarly, subjective cognitive complaints without objective evidence of cognitive impairment are also prevalent in patients with Parkinson’s disease (Purri et al., 2020; Siciliano et al., 2021), multiple sclerosis (Beier et al., 2015; van Geest et al., 2017), epilepsy (Feldman et al., 2018), and history of chemotherapy (Bray et al., 2018; Hutchinson et al., 2012). The current findings provide evidence of comparable discrepancy between subjectively rated cognition and performance-based cognitive abilities in former collegiate contact sport athletes (e.g., football players) with a range of reported concussion histories.
Consistent with previous research, we found that subjective cognitive functioning was strongly correlated with psychological distress. Prior studies robustly suggest that influential factors such as distress and sleep disturbance maintain stronger independent relationships with subjective cognitive functioning than objective cognitive performance (Bray et al., 2018; Hromas et al., 2021; Reid et al., 2012; Stillman et al., 2019). For example, Hromas et al., (2021) found that more affective distress in patients with mild traumatic brain injury was associated with higher reported cognitive symptoms in the context of normatively average performance on neuropsychological measures. Similar to the current results, other researchers have found that inclusion of factors such as mood symptoms attenuate the relationship between subjective memory complaints and objective memory performance (Reid et al., 2012; Stillman et al., 2019). Taken together, these results suggest that reliance on self-reported measures of cognitive functioning alone are insufficient when assessing functional status in former contact sport athletes and that assessment of other factors known to influence subjective cognitive complaints should also be examined. Further, it is important to note that while concussion history was statistically associated with greater endorsement of cognitive-related symptoms, the clinical significance of this is unclear, as the highest concussion history group subjectively rated their cognition as being in the average range at the group level (8+ concussion group NeuroQoL Cognitive Function mean T-score = 47.35).
Subjective cognitive symptoms, potential risk for future decline, and alternative possibilities
It is possible that subjective cognitive difficulties in the absence of objective cognitive decline may reflect individuals’ insight into more subtle changes not yet detected by objective cognitive assessments due to insufficient test sensitivity (Burmester et al., 2016). Among older adults, report of subjective cognitive difficulties has been correlated with neurobiological markers of neurodegeneration (e.g., hippocampal volume, amyloid burden; (Dauphinot et al., 2020; Ebenau et al., 2020) and risk factors for neurodegenerative changes (e.g., decreased white matter integrity; (Ohlhauser et al., 2019; van Rooden et al., 2018). Additionally, several meta-analyses have demonstrated that subjective cognitive difficulties may be predictive of future cognitive decline, even in the absence of objective cognitive impairment on traditional cognitive measures (Burmester et al., 2016; Mitchell et al., 2014; Pike et al., 2021). Within the current sample and other validation cohorts of former contact sport athletes, further work is needed to investigate the association between subjective cognitive functioning, the above-mentioned neurobiological measures, and objective cognitive changes over time.
While it is possible that the low agreement between subjective and objective cognitive measures reflects a risk factor or prodromal process for future decline, subjective cognitive symptoms lack specificity and endorsement could reflect a range of various etiologies, such as sleep difficulties (Towns et al., 2015) and psychiatric symptoms (Roberts et al., 2020; Sabatini et al., 2021), as evidenced in the current study. Cognitive symptoms can also be influenced by a number of other factors, such as pain (Schwenk et al., 2007), current life-related stressors (Stenfors et al., 2013), or fatigue (Rasouli et al., 2019), and may not necessarily represent changes in cognitive function or risk for future decline. Negative expectations associated with history of concussion, known as ‘diagnosis threat,’ also contains the potential to influence cognitive outcomes (Iverson et al., 2010; Suhr & Gunstad, 2005). Consistent with this notion, higher levels of concern regarding the long-term effects of concussion on memory abilities, thinking skills, and risk of developing chronic traumatic encephalopathy was associated with a personal greater history of concussion in a cohort of former professional football players (Walton et al., 2021b). Within the same study, those with higher levels of concern across the three aforementioned areas endorsed greater levels of subjective cognitive difficulties, though objective cognition was not assessed.
Clinical implications and practice
Based on the discordance between subjective and objective cognition in the current study, the assessment of cognitive function in former athletes with a range of self-reported concussion history requires a multifaceted approach. The current results add to mounting evidence that subjective cognitive impairment alone is insufficient for a diagnosis of a neurocognitive disorder given the weak relationship between self-report and objective test data. Others have demonstrated that reliance solely on self-reported cognitive decline in older adults may contribute to misdiagnosis of neurocognitive disorders (Edmonds et al., 2014). Therefore, a comprehensive evaluation involving both subjective and objective performance-based data is optimal. Although a discrepancy may be observed, inclusion of both subjective and objective measures may provide useful information when assessing potential long-term outcomes associated with concussion history in former athletes. For example, given the association between subjective cognitive decline and future longitudinal decline in some older adults, those endorsing subjective cognitive difficulties may require additional monitoring and structured follow-up.
These findings and the importance of a comprehensive evaluation have clinical relevance when assessing potential long-term cognitive sequalae in former athletes, particularly those with a history of multiple concussions and exposure to repetitive head impacts. Studies have reported greater subjective cognitive difficulties among older former professional football players with a greater history of concussion, though objective performance-based cognition was not assessed (Brett et al., 2021; Roberts et al., 2020; Walton et al., 2021a). The same studies also observed greater levels of subjective cognitive difficulties in the presence of other confounding factors, such as distress, psychosocial difficulties and health (e.g., pain, sleep apnea), all of which have been observed among former contact sport athletes at higher levels (collegiate and professional; (Brett et al., 2021; Esopenko et al., 2017; Hind et al., 2020; Roberts et al., 2020). As such, a comprehensive clinical evaluation should include assessment of modifiable factors (e.g., depression, anxiety) that may be contributing to an individual’s subjective cognitive concerns, alongside objective performance-based cognition. In alignment with this notion, the importance of performance-based testing for the detection of objective cognitive decline has been emphasized within the recently developed research diagnostic criteria for traumatic encephalopathy syndrome (Katz et al., 2021) in which neuropsychological assessment (a minimum of an objective screening tool such as the Mini-Mental State Examination) is required for the determination of documented cognitive impairment.
Limitations
The current study highlights the importance of a multimodal assessment; however, it is not without limitations. Notably, the current sample of former athletes were predominately White males with estimated premorbid intellectual abilities in the high average range based on the sample mean performance on a single word reading task. Additional studies involving greater racial and ethnic diversity, female athletes, and a wider range of educational backgrounds are needed to further explore factors that may influence between- and within-group differences in subjective and objective cognitive outcomes (Taylor et al., 2018; Weuve et al., 2018). Moreover, this sample was composed of former football players at the collegiate level. Therefore, findings from the current study may not be generalized players who only played at lower levels. Concussion history was self-reported and not able to be corroborated via medical records. Due to the retrospective nature of inquiring about concussion history, acute injury characteristics were not incorporated into the current analyses. Greater consistency of reported concussion history has been observed among those who are younger in age and at lower levels of play (Kerr et al., 2012; Wojtowicz, 2017), and the current sample is in the mid-range for both factors. Factors such as worsening physical and mental health functioning have been observed as influencing consistency in concussion history recall (Kerr et al., 2012). The current sample was relatively healthy in the context of medical history/diagnoses and future studies involving samples with greater medical complexity should seek to investigate the way in which medical comorbidities may influences the association between subjective and objective cognition. Lastly, examination of executive functioning was limited to the measures administered within the study and other aspects of executive functioning (e.g., inhibition, decision making, etc.) may relate differentially to executive-based cognitive symptom endorsement.
Conclusions
Taken together, these results suggest that reliance on self-reported measures of cognitive functioning alone is insufficient when assessing cognition in former collegiate football players and that assessment of other factors known to influence subjective cognitive complaints should be examined to determine the presence of cognitive deficits. Future studies are required to examine the longitudinal trajectories among those endorsing subjective cognitive difficulties in the absence of performance-based deficits, as well as the way in which confounding factors (e.g., distress and concussion history) influence these trajectories over time.
Acknowledgments
We are grateful for the participation of the athletes, without whom this research would not be possible. The authors would also like to thank Robyn Furger (Department of Neurosurgery at the Medical College of Wisconsin), Amy Nader (Department of Neurosurgery at the Medical College of Wisconsin), Candice Goerger (Center for the Study of Retired Athletes at the University of North Carolina at Chapel Hill), and Leah Thomas (Center for the Study of Retired Athletes at the University of North Carolina at Chapel Hill) for their study coordination and management efforts.
Conflicts of interest/disclosures: BLB reports grants from the National Institute on Aging and National Institute of Neurological Disorders and Stroke. KMG reports compensation from National Collegiate Athletic Association for other services and grants from National Football League (via subaward from Boston Children’s Hospital). MAM acknowledges researching funding from the National Institutes of Health, U.S. Department of Defense, Centers for Disease Control and Prevention, National Collegiate Association and National Football League (via subaward from Boston Children’s Hospital). WBB provides expert witness testimony in legal cases involving concussion and CTE. ZYK reports grants from National Institutes of Health; grants from Centers for Disease Control and Prevention; and grants from National Football League. All other authors (AMB and SRW) report no COI. This study was funded by the National Collegiate Athletic Association (NCAA; grant number NA), Medical College of Wisconsin (grant number NA), University of North Carolina at Chapel Hill (grant number NA), the National Institutes of Health National Institute on Aging (BLB, grant number K23AG073528–01).
References
- Alosco ML, Mez J, Tripodis Y, Kiernan PT, Abdolmohammadi B, Murphy L, Kowall NW, Stein TD, Huber BR, Goldstein LE, Cantu RC, Katz DI, Chaisson CE, Martin B, Solomon TM, McClean MD, Daneshvar DH, Nowinski CJ, Stern RA, & McKee AC (2018). Age of first exposure to tackle football and chronic traumatic encephalopathy. Annals of Neurology, 83(5), 886–901. 10.1002/ana.25245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashendorf L, Clark EL, & Sugarman MA (2017). Performance validity and processing speed in a va polytrauma sample. The Clinical Neuropsychologist, 31(5), 857–866. 10.1080/13854046.2017.1285961 [DOI] [PubMed] [Google Scholar]
- Beier M, Amtmann D, & Ehde DM (2015). Beyond depression: Predictors of self-reported cognitive function in adults living with ms. Rehabilitation Psychology, 60(3), 254–262. 10.1037/rep0000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, & Benedict RHB (2001). Hopkins verbal learning test--revised: Professional manual. Psychological Assessment Resources. [Google Scholar]
- Bray VJ, Dhillon HM, & Vardy JL (2018). Systematic review of self-reported cognitive function in cancer patients following chemotherapy treatment. Journal of Cancer Survivorship, 12(4), 537–559. 10.1007/s11764-018-0692-x [DOI] [PubMed] [Google Scholar]
- Brett BL, Huber DL, Wild A, Nelson LD, & McCrea MA (2019). Age of first exposure to american football and behavioral, cognitive, psychological, and physical outcomes in high school and collegiate football players. Sports Health, 11(4), 332–342. 10.1177/1941738119849076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett BL, Walton SR, Kerr ZY, Nelson LD, Chandran A, Defreese JD, Echemendia RJ, Guskiewicz KM, Meehan Iii WP, & McCrea MA (2021). Distinct latent profiles based on neurobehavioural, physical and psychosocial functioning of former national football league (nfl) players: An nfl-long study. Journal of Neurology, Neurosurgery & Psychiatry, 92(3), 282. 10.1136/jnnp-2020-324244 [DOI] [PubMed] [Google Scholar]
- Burmester B, Leathem J, & Merrick P (2016). Subjective cognitive complaints and objective cognitive function in aging: A systematic review and meta-analysis of recent cross-sectional findings. Neuropsychology Review, 26(4), 376–393. 10.1007/s11065-016-9332-2 [DOI] [PubMed] [Google Scholar]
- Chun BJ, Furutani T, Oshiro R, Young C, Prentiss G, & Murata N (2021). Concussion epidemiology in youth sports: Sports study of a statewide high school sports program. Sports Health, 13(1), 18–24. 10.1177/1941738120932570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphinot V, Bouteloup V, Mangin JF, Vellas B, Pasquier F, Blanc F, Hanon O, Gabelle A, Annweiler C, David R, Planche V, Godefroy O, Rivasseau-Jonveaux T, Chupin M, Fischer C, Chene G, Dufouil C, & Krolak-Salmon P (2020). Subjective cognitive and non-cognitive complaints and brain mri biomarkers in the memento cohort. Alzheimers Dement (Amst), 12(1), e12051. 10.1002/dad2.12051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR (2001). Brief symptom inventory (bsi)-18: Administration, scoring and procedures manual [Manual]. NCS Pearson. [Google Scholar]
- Deshpande SK, Hasegawa RB, Rabinowitz AR, Whyte J, Roan CL, Tabatabaei A, Baiocchi M, Karlawish JH, Master CL, & Small DS (2017). Association of playing high school football with cognition and mental health later in life. JAMA Neurology, 74(8), 909–918. 10.1001/jamaneurol.2017.1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebenau JL, Timmers T, Wesselman LMP, Verberk IMW, Verfaillie SCJ, Slot RER, van Harten AC, Teunissen CE, Barkhof F, van den Bosch KA, van Leeuwenstijn M, Tomassen J, Braber AD, Visser PJ, Prins ND, Sikkes SAM, Scheltens P, van Berckel BNM, & van der Flier WM (2020). Atn classification and clinical progression in subjective cognitive decline: The science project. Neurology, 95(1), e46–e58. 10.1212/WNL.0000000000009724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, & Bondi MW (2014). Subjective cognitive complaints contribute to misdiagnosis of mild cognitive impairment. Journal of the International Neuropsychological Society, 20(8), 836–847. 10.1017/S135561771400068X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esopenko C, Chow TW, Tartaglia MC, Bacopulos A, Kumar P, Binns MA, Kennedy JL, Müller DJ, & Levine B (2017). Cognitive and psychosocial function in retired professional hockey players. Journal of Neurology, Neurosurgery & Psychiatry, 88(6), 512. 10.1136/jnnp-2016-315260 [DOI] [PubMed] [Google Scholar]
- Feldman L, Lapin B, Busch RM, & Bautista JF (2018). Evaluating subjective cognitive impairment in the adult epilepsy clinic: Effects of depression, number of antiepileptic medications, and seizure frequency. Epilepsy & Behavior, 81, 18–24. 10.1016/j.yebeh.2017.10.011 [DOI] [PubMed] [Google Scholar]
- Gallo V, Motley K, Kemp SPT, Mian S, Patel T, James L, Pearce N, & McElvenny D (2020). Concussion and long-term cognitive impairment among professional or elite sport-persons: A systematic review. Journal of Neurology, Neurosurgery & Psychiatry, 91(5), 455. 10.1136/jnnp-2019-321170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami R, Dufort P, Tartaglia MC, Green RE, Crawley A, Tator CH, Wennberg R, Mikulis DJ, Keightley M, & Davis KD (2016). Frontotemporal correlates of impulsivity and machine learning in retired professional athletes with a history of multiple concussions. Brain Structure & Function, 221(4), 1911–1925. 10.1007/s00429-015-1012-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, & Jordan BD (2005). Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery, 57(4), 719–726. 10.1227/01.NEU.0000175725.75780.DD [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, Onate JA, & Kelly JP (2003a). Cumulative effects associated with recurrent concussion in collegiate football players: The ncaa concussion study. JAMA, 290(19), 2549–2555. 10.1001/jama.290.19.2549 [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, Onate JA, & Kelly JP (2003b). Cumulative effects associated with recurrent concussion in collegiate football playersthe ncaa concussion study. JAMA, 290(19), 2549–2555. 10.1001/jama.290.19.2549 [DOI] [PubMed] [Google Scholar]
- Hall KE, Isaac CL, & Harris P (2009). Memory complaints in epilepsy: An accurate reflection of memory impairment or an indicator of poor adjustment? A review of the literature. Clinical Psychology Review, 29(4), 354–367. 10.1016/j.cpr.2009.03.001 [DOI] [PubMed] [Google Scholar]
- Hind K, Konerth N, Entwistle I, Theadom A, Lewis G, King D, Chazot P, & Hume P (2020). Cumulative sport-related injuries and longer term impact in retired male elite- and amateur-level rugby code athletes and non-contact athletes: A retrospective study. Sports medicine (Auckland, N.Z.), 50(11), 2051–2061. 10.1007/s40279-020-01310-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromas GA, Houck ZM, Asken BM, Svingos AM, Greif SM, Heaton SC, Jaffee MS, & Bauer RM (2021). Making a difference: Affective distress explains discrepancy between objective and subjective cognitive functioning after mild traumatic brain injury. The Journal of Head Trauma Rehabilitation, 36(3), 186–195. 10.1097/htr.0000000000000618 [DOI] [PubMed] [Google Scholar]
- Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, & Wilson C (2012). Objective and subjective cognitive impairment following chemotherapy for cancer: A systematic review. Cancer Treatment Reviews, 38(7), 926–934. 10.1016/j.ctrv.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Iverson GL, Lange RT, Brooks BL, & Rennison VLA (2010). “Good old days” bias following mild traumatic brain injury. Clinical Neuropsychologist, 24(1), 17–37, Article Pii 915817610. 10.1080/13854040903190797 [DOI] [PubMed] [Google Scholar]
- Iverson GL, Lange RT, Green P, & Franzen MD (2002). Detecting exaggeration and malingering with the trail making test. The Clinical Neuropsychologist, 16(3), 398–406. 10.1076/clin.16.3.398.13861 [DOI] [PubMed] [Google Scholar]
- Katz DI, Bernick C, Dodick DW, Mez J, Mariani ML, Adler CH, Alosco ML, Balcer LJ, Banks SJ, Barr WB, Brody DL, Cantu RC, Dams O, Connor K, Geda YE, Jordan BD, McAllister TW, Peskind ER, Petersen RC, Wethe JV, Zafonte RD, Foley ÉM, Babcock DJ, Koroshetz WJ, Tripodis Y, McKee AC, Shenton ME, Cummings JL, Reiman EM, & Stern RA (2021). National institute of neurological disorders and stroke consensus diagnostic criteria for traumatic encephalopathy syndrome. Neurology, 96(18), 848. 10.1212/WNL.0000000000011850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr ZY, Thomas LC, Simon JE, McCrea M, & Guskiewicz KM (2018a). Association between history of multiple concussions and health outcomes among former college football players: 15-year follow-up from the ncaa concussion study (1999–2001). The American Journal of Sports Medicine, 46(7), 1733–1741. 10.1177/0363546518765121 [DOI] [PubMed] [Google Scholar]
- Kerr ZY, Wilkerson GB, Caswell SV, Currie DW, Pierpoint LA, Wasserman EB, Knowles SB, Dompier TP, Comstock RD, & Marshall SW (2018b). The first decade of web-based sports injury surveillance: Descriptive epidemiology of injuries in united states high school football (2005–2006 through 2013–2014) and national collegiate athletic association football (2004–2005 through 2013–2014). Journal of Athletic Training, 53(8), 738–751. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6188086/pdf/i1062-6050-53-8-738.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GJ, Do C, & Suhr JA (2021). Effects of personal dementia exposure on subjective memory concerns and dementia worry. Aging, Neuropsychology, and Cognition, 28(6), 855–870. 10.1080/13825585.2020.1836119 [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, & Tranel D (2012). Neuropsychological assessment, 5th ed. Oxford University Press. [Google Scholar]
- Manley G, Gardner AJ, Schneider KJ, Guskiewicz KM, Bailes J, Cantu RC, Castellani RJ, Turner M, Jordan BD, Randolph C, Dvořák J, Hayden KA, Tator CH, McCrory P, & Iverson GL (2017). A systematic review of potential long-term effects of sport-related concussion. British Journal of Sports Medicine, 51(12), 969–977. 10.1136/bjsports-2017-097791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, & Stubbs B (2014). Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis [ 10.1111/acps.12336]. Acta Psychiatrica Scandinavica, 130(6), 439–451. 10.1111/acps.12336 [DOI] [PubMed] [Google Scholar]
- Montenigro PH, Alosco ML, Martin BM, Daneshvar DH, Mez J, Chaisson CE, Nowinski CJ, Au R, McKee AC, Cantu RC, McClean MD, Stern RA, & Tripodis Y (2017). Cumulative head impact exposure predicts later-life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. Journal of Neurotrauma, 34(2), 328–340. 10.1089/neu.2016.4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A, Gavelin HM, Boraxbekk C-J, Eskilsson T, Josefsson M, Slunga Järvholm L, & Neely AS (2021). Subjective cognitive complaints in patients with stress-related exhaustion disorder: A cross sectional study. BMC Psychology, 9(1), 84. 10.1186/s40359-021-00576-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolazzo J, Xu K, Lavale A, Buckley R, Yassi N, Hamilton GS, Maruff P, Baril A-A, Lim YY, & Pase MP (2021). Sleep symptomatology is associated with greater subjective cognitive concerns: Findings from the community-based healthy brain project. Sleep, 44(9). 10.1093/sleep/zsab097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor KL, Baker MM, Dalton SL, Dompier TP, Broglio SP, & Kerr ZY (2017). Epidemiology of sport-related concussions in high school athletes: National athletic treatment, injury and outcomes network (nation), 2011–2012 through 2013–2014. Journal of Athletic Training, 52(3), 175–185. 10.4085/1062-6050-52.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlhauser L, Parker AF, Smart CM, Gawryluk JR, & Alzheimer’s Disease Neuroimaging I (2019). White matter and its relationship with cognition in subjective cognitive decline. Alzheimers Dement (Amst), 11, 28–35. 10.1016/j.dadm.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike KE, Cavuoto MG, Li L, Wright BJ, & Kinsella GJ (2021). Subjective cognitive decline: Level of risk for future dementia and mild cognitive impairment, a meta-analysis of longitudinal studies. Neuropsychology Review. 10.1007/s11065-021-09522-3 [DOI] [PubMed] [Google Scholar]
- Purri R, Brennan L, Rick J, Xie SX, Deck BL, Chahine LM, Dahodwala N, Chen-Plotkin A, Duda JE, Morley JF, Akhtar RS, Trojanowski JQ, Siderowf A, & Weintraub D (2020). Subjective cognitive complaint in parkinson’s disease patients with normal cognition: Canary in the coal mine? Movement Disorders, 35(9), 1618–1625. 10.1002/mds.28115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasouli O, Gotaas ME, Stensdotter A-K, Skovlund E, Landrø NI, Dåstøl P, & Fors EA (2019). Neuropsychological dysfunction in chronic fatigue syndrome and the relation between objective and subjective findings. Neuropsychology, 33(5), 658. [DOI] [PubMed] [Google Scholar]
- Reid M, Parkinson L, Gibson R, Schofield P, D’Este C, Attia J, Tavener M, & Byles J (2012). Memory complaint questionnaire performed poorly as screening tool: Validation against psychometric tests and affective measures. Journal of Clinical Epidemiology, 65(2), 199–205. 10.1016/j.jclinepi.2011.06.006 [DOI] [PubMed] [Google Scholar]
- Reitan RM, & Wolfson D (1985). The halstead–reitan neuropsycholgical test battery: Therapy and clinical interpretation. Neuropsycholoigical Press. [Google Scholar]
- Roberts AL, Zafonte RD, Speizer FE, Baggish A, Taylor HA, Nadler L, & Weisskopf MG (2020). Modifiable risk factors for poor cognitive function in former american-style football players: Findings from the harvard football players health study. Journal of Neurotrauma, 38(2), 189–195. 10.1089/neu.2020.7070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R, Isquith P, & Gioia G (2005). Behavior rating inventory of executive function - adult version (brief-a) (Vol. 20). [Google Scholar]
- Sabatini S, Woods RT, Ukoumunne OC, Ballard C, Collins R, & Clare L (2021). Associations of subjective cognitive and memory decline with depression, anxiety, and two-year change in objectively-assessed global cognition and memory. Aging, Neuropsychology, and Cognition, 1–27. 10.1080/13825585.2021.1923634 [DOI] [PubMed] [Google Scholar]
- Sawyer RJ, Testa SM, & Dux M (2017). Embedded performance validity tests within the hopkins verbal learning test – revised and the brief visuospatial memory test – revised. The Clinical Neuropsychologist, 31(1), 207–218. 10.1080/13854046.2016.1245787 [DOI] [PubMed] [Google Scholar]
- Schwenk TL, Gorenflo DW, Dopp RR, & Hipple E (2007). Depression and pain in retired professional football players. Medicine and Science in Sports and Exercise, 39(4). https://journals.lww.com/acsm-msse/Fulltext/2007/04000/Depression_and_Pain_in_Retired_Professional.4.aspx [DOI] [PubMed] [Google Scholar]
- Sheridan LK, Fitzgerald HE, Adams KM, Nigg JT, Martel MM, Puttler LI, Wong MM, & Zucker RA (2006). Normative symbol digit modalities test performance in a community-based sample. Archives of Clinical Neuropsychology, 21(1), 23–28. 10.1016/j.acn.2005.07.003 [DOI] [PubMed] [Google Scholar]
- Siciliano M, Trojano L, De Micco R, Sant’Elia V, Giordano A, Russo A, Passamonti L, Tedeschi G, Chiorri C, & Tessitore A (2021). Correlates of the discrepancy between objective and subjective cognitive functioning in non-demented patients with parkinson’s disease. Journal of Neurology. 10.1007/s00415-021-10519-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A (1973). Symbol digit modalities test. Western Psychological Services Los Angeles. [Record #11670 is using a reference type undefined in this output style.] [Google Scholar]
- Srisurapanont M, Suttajit S, Eurviriyanukul K, & Varnado P (2017). Discrepancy between objective and subjective cognition in adults with major depressive disorder. Scientific Reports, 7(1), 3901. 10.1038/s41598-017-04353-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg SI, Negash S, Sammel MD, Bogner H, Harel BT, Livney MG, McCoubrey H, Wolk DA, Kling MA, & Arnold SE (2013). Subjective memory complaints, cognitive performance, and psychological factors in healthy older adults. American Journal of Alzheimer’s Disease & Other Dementias®, 28(8), 776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenfors CUD, Marklund P, Magnusson Hanson LL, Theorell T, & Nilsson L-G (2013). Subjective cognitive complaints and the role of executive cognitive functioning in the working population: A case-control study. PloS One, 8(12), e83351. 10.1371/journal.pone.0083351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman AM, Madigan N, Torres K, Swan N, & Alexander MP (2019). Subjective cognitive complaints in concussion. Journal of Neurotrauma, 37(2), 305–311. 10.1089/neu.2018.5925 [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, & Spreen O (2006). A compendium of neuropsychological tests: Administration, norms, and commentary. American Chemical Society. [Google Scholar]
- Suhr JA, & Gunstad J (2005). Further exploration of the effect of “diagnosis threat” on cognitive performance in individuals with mild head injury. Journal of the International Neuropsychological Society, 11(1), 23–29. 10.1017/S1355617705050010 [DOI] [PubMed] [Google Scholar]
- Taylor CA, Bouldin ED, & McGuire LC (2018). Subjective cognitive decline among adults aged ≥45 years - united states, 2015–2016. MMWR. Morbidity and mortality weekly report, 67(27), 753–757. 10.15585/mmwr.mm6727a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra AR, Vasquez BP, Colella B, Tartaglia MC, Tator CH, Mikulis D, Davis KD, Wennberg R, & Green REA (2019). Comprehensive neuropsychiatric and cognitive characterization of former professional football players: Implications for neurorehabilitation [ 10.3389/fneur.2019.00712]. Frontiers in Neurology, 10, 712. 10.3389/fneur.2019.00712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN (2004). Trail making test a and b: Normative data stratified by age and education. Archives of Clinical Neuropsychology, 19(2), 203–214. 10.1016/S0887-6177(03)00039-8 [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J, & Rees L (1999). Normative data stratified by age and education for two measures of verbal fluency: Fas and animal naming. Archives of Clinical Neuropsychology, 14(2), 167–177. 10.1016/S0887-6177(97)00095-4 [DOI] [PubMed] [Google Scholar]
- Towns SJ, Silva MA, & Belanger HG (2015). Subjective sleep quality and postconcussion symptoms following mild traumatic brain injury. Brain Injury, 29(11), 1337–1341. 10.3109/02699052.2015.1045030?needAccess=true [DOI] [PubMed] [Google Scholar]
- van Geest Q, Westerik B, Van der Werf Y, Geurts J, & Hulst H (2017). The role of sleep on cognition and functional connectivity in patients with multiple sclerosis. Journal of Neurology, 264(1), 72–80. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5225184/pdf/415_2016_Article_8318.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooden S, van den Berg-Huysmans AA, Croll PH, Labadie G, Hayes JM, Viviano R, van der Grond J, Rombouts S, & Damoiseaux JS (2018). Subjective cognitive decline is associated with greater white matter hyperintensity volume. J Alzheimers Dis, 66(3), 1283–1294. 10.3233/JAD-180285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton SR, Kerr ZY, Brett BL, Chandran A, DeFreese JD, Smith-Ryan AE, Stoner L, Echemendia RJ, McCrea M, Meehan IIIWP, & Guskiewicz KM (2021a). Health-promoting behaviours and concussion history are associated with cognitive function, mood-related symptoms and emotional–behavioural dyscontrol in former nfl players: An nfl-long study. British Journal of Sports Medicine, 55(12), 683. 10.1136/bjsports-2020-103400 [DOI] [PubMed] [Google Scholar]
- Walton SR, Kerr ZY, Mannix R, Brett BL, Chandran A, DeFreese JD, McCrea MA, Guskiewicz KM, Meehan WP, & Echemendia RJ (2021b). Subjective concerns regarding the effects of sport-related concussion on long-term brain health among former nfl players: An nfl-long study. Sports Medicine. 10.1007/s40279-021-01589-5 [DOI] [PubMed] [Google Scholar]
- Weuve J, Barnes LL, Mendes de Leon CF, Rajan KB, Beck T, Aggarwal NT, Hebert LE, Bennett DA, Wilson RS, & Evans DA (2018). Cognitive aging in black and white americans: Cognition, cognitive decline, and incidence of alzheimer disease dementia. Epidemiology (Cambridge, Mass.), 29(1), 151–159. 10.1097/EDE.0000000000000747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer BS, Zivadinov R, Haider MN, Miecznikowski JC, & Leddy JJ (2018). A preliminary study of early-onset dementia of former professional football and hockey players. The Journal of Head Trauma Rehabilitation, 33(5), E1–E8. 10.1097/HTR.0000000000000421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavecz Z, Nagy T, Galkó A, Nemeth D, & Janacsek K (2020). The relationship between subjective sleep quality and cognitive performance in healthy young adults: Evidence from three empirical studies. Scientific Reports, 10(1), 4855. 10.1038/s41598-020-61627-6 [DOI] [PMC free article] [PubMed] [Google Scholar]


