Abstract
To evaluate the role of putative group A streptococcal virulence factors in the initiation of skin infections, we compared the adherence of a wild-type M49-protein skin-associated strain to that of a series of 16 isogenic mutants created by insertional inactivation of virulence genes. None of the mutants, including the M-protein-deficient (emm mutant) strain, displayed reduced adherence to early-passage cultured human keratinocytes, but adherence of the mutant lacking hyaluronic acid capsule expression (has mutant) was increased 13-fold. In contrast, elimination of capsule expression in M2-, M3-, and M18-protein has mutants increased adherence only slightly (1.3- to 2.3-fold) compared to their respective wild-type strains. A mutant with inactivation of both emm and has displayed high-level adherence (34.9 ± 4.1%) equal to that of the has mutant strain (40.7 + 8.0%), confirming the lack of involvement of M49 protein in attachment. Moreover, adherence of the M49-protein-deficient (emm mutant) and wild-type strains was increased to the same level (57 and 55%, respectively) following enzymatic digestion of their hyaluronic acid capsule. Adherence of mutants lacking oligopeptide permease (Opp) expression was increased 3.8- to 5.5-fold, in association with decreased cell-associated hyaluronic acid capsule. Finally, soluble CD46 failed to inhibit adherence of M49- and M52-serotype skin strains. We conclude that (i) bacterial M protein and keratinocyte CD46 do not mediate adherence of M49 skin-associated Streptococcus pyogenes to epidermal keratinocytes, (ii) hyaluronic acid capsule impedes the interaction of bacterial adhesins with keratinocyte receptors, (iii) modulation of capsule expression may be important in the pathogenesis of skin infections, and (iv) the molecular interactions in attachment of skin strains of S. pyogenes to keratinocytes are unique and remain unidentified.
Streptococcus pyogenes (group A streptococcus) is unsurpassed among bacterial pathogens in its ability to cause a variety of skin infections ranging from self-limited superficial impetigo to fulminant life-threatening, soft-tissue destruction and necrotizing fasciitis (26–28). Increased global incidence of severe, invasive disease due to S. pyogenes over the past decade, with the skin serving as the portal of entry in more than half of the cases, has highlighted our need to understand the molecular pathogenesis of skin infections (66). Pathogenic mechanisms of streptococcal skin infections are almost entirely unknown, however, since efforts have focused on the interaction of streptococci with mucosal epithelium, and in vitro models which emulate human skin disease (23) have not been available.
An initial step in group A streptococcal skin infection appears to involve adherence of the bacteria via its adhesin(s) to host cell receptor(s) (25). The type of adhesin utilized for specific binding may vary depending on the streptococcal strain and type of host cell and tissue involved. M protein is the most well-documented virulence factor of S. pyogenes. Based on studies with a genetically engineered M6 protein isolate from the pharynx, it was proposed that the C-repeat domain of M protein mediated adherence to the CD46 molecule on keratinocytes (53, 54, 56). Adherence of the M6-protein strain, however, was greater to undifferentiated than differentiated keratinocytes (53). This is opposite to what one would predict based on the histopathology of impetigo (23), which shows localization of the infection to highly differentiated subcorneal keratinocytes. The complexity of bacterium-host interactions was further illustrated when various M proteins were expressed in an isogenic group A streptococcal background and their adherence to transformed human keratinocytes was compared (8). Pharyngeal-tissue-associated M6- and M18-protein strains bound via distinct cellular receptors, and CD46 played a role in attachment of only an M3-protein strain. It appears that mechanisms for the attachment of strains trophic for respiratory epithelium may differ from pathogenic mechanisms of skin infection (25), highlighting the need for utilizing appropriate model experimental systems.
We have developed techniques utilizing cultured, early-passage human keratinocytes to examine extracellular adherence and intracellular invasion by group A streptococcus (24, 25). S. pyogenes adhered to keratinocytes in an inoculum- and time-dependent manner suggestive of a receptor-mediated process (25). In vitro adherence of an impetigo strain of S. pyogenes was specifically promoted by keratinocyte differentiation, as is observed in lesions of impetigo (23). Presumably, host cell receptors for adherence were most highly expressed on these differentiated keratinocytes. Neither a pharyngeal strain of S. pyogenes nor a nonpathogenic isolate of Streptococcus gordonii adhered preferentially to differentiated keratinocytes, suggesting that the molecules involved in pathogenesis are tissue specific and that the adhesins for attachment of impetigo strains of S. pyogenes to keratinocytes are unique. Thus, adherence in our experimental system simulates impetigo and provides an in vitro model for investigating the pathogenesis of cutaneous infections. We have utilized this in vitro experimental model in conjunction with genetic manipulation of skin-derived and non-skin-associated strains of S. pyogenes to examine the role of putative group A streptococcal virulence factors in attachment to keratinocytes to initiate skin infections.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Strains of S. pyogenes were grown in Todd-Hewitt broth (THB) (Difco Laboratories, Detroit, Mich.) or on Todd-Hewitt agar (1.5% agar) (THA) (Difco) at 37°C in a 5% CO2–20% O2 atmosphere. The parent strains of S. pyogenes used for producing insertional mutants were M49-serotype strain CS101, originally provided and characterized by Patrick Cleary and colleagues at the University of Minnesota (30), and serotype M2-protein strain T2/44/RB4/119, obtained from Dwight Johnson, World Health Organization (WHO) Streptococcal Reference Laboratory, Minneapolis, Minn. M1 (strain 950771)- and M12 (strain 950333)-serotype clinically invasive, mucoid strains also were obtained from the WHO Streptococcal Reference Laboratory. The M3 (isolated from a patient with necrotizing fasciitis)- and M18 (from the pharynx of a patient with rheumatic fever)-serotype wild-type and capsule-deficient (has mutant) strains were provided by Cameron Ashbaugh at the Channing Laboratory, Boston, Mass. (4, 71). S. pyogenes 3732, provided by Susan K. Hollingshead, University of Alabama-Birmingham, is an M-protein 52 serotype isolated from a lesion of impetigo. All recombinant strains of S. pyogenes, except the double-knockout emm has mutant M49-protein strain, were grown on selective THA containing 60 mg of spectinomycin per liter, well above the MIC of spectinomycin (10 mg/liter) for the wild-type M49-protein strain CS101 (58). The recombinant M49-protein emm has mutant was grown on selective THA containing 60 mg of spectinomycin (to select for recombinant sequences from pSF152 which insertionally inactivated emm) and 150 mg of kanamycin (to select for recombinant sequences from pFW13 which insertionally inactivated has) per liter. The host for recombinant plasmids was Escherichia coli DH5α, which was grown at 37°C in an ambient atmosphere in Luria-Bertani broth (Sigma Chemical Co., St. Louis, Mo.) or on Luria-Bertani agar supplemented with 100 mg of spectinomycin per liter for selection of pSF-based plasmids. The bacterial growth rate was determined by measuring the optical density at 600 nm (OD600) at time points which spanned the lag, logarithmic, and stationary growth phases.
Plasmids.
Plasmid pSF152 is a suicide vector that does not replicate autonomously in S. pyogenes. It contains a pUC19-derived origin of replication, a pUC18-derived multiple cloning site, and a spectinomycin resistance gene (aad9) which is active in both gram-positive and gram-negative bacteria. This plasmid and its derivatives (60) were used to generate the M49- and M2-protein mutants (14, 48, 58–59, 61, 64). Plasmid pFW13 (60) containing a kanamycin resistance gene (aacA-aphD) was used to generate the double-knockout mutant with inactivation of has in strain M49-protein emm mutant.
Conventional DNA techniques.
Chromosomal DNA was prepared from S. pyogenes by methods of Caparon et al. (11), modified to include polysaccharide removal by using 1.1% (vol/vol) hexadecyltrimethyl-ammonium bromide (cTAB) (Sigma) after proteinase K digestion. Plasmid DNA was prepared from S. pyogenes and E. coli by using DEAE column chromatography (Qiagen, Inc., Santa Clarita, Calif.); S. pyogenes was lysed with group B streptococcal protoplast buffer (13) prior to DNA isolation.
Plasmid DNA was introduced into S. pyogenes by electroporation. Bacteria were made competent by growth overnight in THB with 5% yeast extract (THY) and 20 mM glycine. The overnight culture was diluted 1:20 in THY containing 20 mM glycine and grown to an OD600 of 0.2. Bacteria were washed and resuspended in ice-cold elpo-medium containing 272 mM glucose and 1 mM MgCl2 at pH 6.5. For electroporation, 5 μg of plasmid DNA in 1× TE (10 mM Tris, 1 mM EDTA; pH 8) was added to 150 μl of the cell suspension on ice and electroporated in a 0.2-cm cuvette at 1.75 kV, 400 Ω, and 25 μF in a Bio-Rad electroporator with a pulse controller. The suspension was placed on ice for 3 min, diluted with 10 ml of prewarmed THY, and incubated for 2 h at 37°C. Bacteria were pelleted, resuspended in 400 μl THY, and plated on selective media.
DNA mutagenesis.
Mutant strains were generated by insertional inactivation of genes encoding putative group A streptococcal virulence factors as described previously (Table 1) (43, 58, 60, 63). Briefly, isogenic mutants of the M49-protein impetigo strain CS101 or M2-serotype strain T2/44/RB4/119 were derived from single crossover events between the targeted gene and recombinant intragenic sequences of DNA on pSF152-derivative plasmids containing an antibiotic-resistance marker, leading to insertional inactivation of the targeted gene. All mutants were nonpolar, except the has (44), opp (59), and cia mutants, since these genes are part of operons. Double crossover allelic replacement mutagenesis was used to generate the mutants with inactivation of the oppA (59) or sdaD (61) genes, as described previously. To generate the M49-protein double-knockout emm has mutant, the has gene was insertionally inactivated by introducing plasmid pFW13 into the M49-protein emm mutant chromosome. Mutations were confirmed using Southern blot hybridization with genomic DNA digested with restriction enzymes in at least two different ways and probed with elements directed to the inserted sequence (i.e., antibiotic-resistance gene), as described previously (58). In addition, confirmatory PCR was done by using primers specific for sequences immediately upstream of the inserted fragment (i.e., on the streptococcal genome) and in the antibiotic-resistance gene of the integrated plasmid (58). Oligonucleotide primers were designed with the aid of OLIGO 5.0 software (National Bioscience, Plymouth, Minn.). The primers used for PCR confirmation of the introduction of the has insert on plasmid pFW13 into the emm mutant M49 strain were hasFOR (GATATCTATCTTGATTTCTCTAA), hasREV (CCTCTTATAAATTTCTTTTC), emmFOR (AAAGAGCTATACGACCAAATC), and emmREV (AGCTTAGTTTTCTTCTTTGCG). The PCR products (has insert predicted size of 3,335 bp; emm insert predicted size of 3,582 bp) were resolved by agarose gel electrophoresis (58).
TABLE 1.
S. pyogenes M49 and M2 mutants with knockouts in genes encoding putative virulence factors for pathogenesis of skin infections
| Gene | Protein productd | Source or reference (protein type) |
|---|---|---|
| mgaa | trans-Acting activator of virR regulon transcription | 58 (M49) |
| mrpa | IgG-binding protein; ability to bind IgG correlates with ability to survive in human blood and to cause invasive infections when injected subcutaneously in mice | 58 (M49), 37 (M2) |
| emma | M and M-like proteins; prevent complement-mediated phagocytosis | 58 (M49), 37 (M2) |
| enna | IgA-binding protein | 58 (M49) |
| scpa | C5a peptidase; delays influx of neutrophils into a nidus of infection | 58 (M49) |
| orfXa | Potential surface protein of unknown function | 62 (M49) |
| hasb | Hyaluronic acid capsule; antiphagocytic, associated with severe, invasive infections | 44 (M49), 29 (M2), 4 (M3), 46 (M18) |
| hasb emm | See has and emm | This study (M49) |
| speB | Streptococcal cysteine protease; associated with severe, invasive disease | 31 (M49) |
| oppA to oppFb | Oligopeptide permease protein (Opp) complex; postulated to be involved in adherence | 59 (M49) |
| oppAb | Membrane-associated substrate-binding lipoprotein of the Opp complex | 59 (M49) |
| oppD/Fb | Inner-membrane-associated ATPases of the Opp complex | 59 (M49) |
| ska | Streptokinase; activates plasminogen, resulting in unregulated generation of plasmin, which has cell surface enzymatic activity | 14 (M49) |
| sdaD | Extracellular DNase; expression of anti-DNase antibodies occurs during infections | 61 (M49) |
| rof | Negative regulator of genes encoding a collagen-binding protein and protein F2, which binds fibronectin | 63 (M49) |
| ciab | Two-component sensor regulator involved in cell wall synthesis | 43 (M49)c |
| lrp | Regulatory element involved in starvation control | (M49)c |
Component of vir regulon.
Polar mutants.
Derivation of mutant (unpublished).
IgG, immunoglobulin G; IgA, immunoglobulin A.
Measurement of cell-associated streptococcal hyaluronic acid.
A modified protocol of the Stains-All assay (6, 35, 71) was utilized to determine the amount of hyaluronic acid capsule of strains of S. pyogenes. To isolate hyaluronic acid capsule, 10 ml of THB was inoculated with 10 to 20 colonies of S. pyogenes and grown overnight (<16 h). Aliquots of the overnight culture were inoculated into 10 ml of fresh THB until an OD600 of 0.05 was reached. The resulting suspension was grown to an OD600 of 0.2 to 0.4, pelleted, washed with 10 ml of 10 mM Tris (pH 7.5), and vortexed for 10 s. Bacteria were pelleted, the supernatant was discarded, and the cell pellet was resuspended in 1.5 ml of H2O and vortexed for 10 s. This washing sequence was repeated, and the cell pellet was resuspended in H2O to a final volume of 1.5 ml. From this final suspension, the number of CFU per milliliter was determined by plating dilutions of duplicate 20 μl aliquots of the suspension on THA. Hyaluronic acid capsule was extracted by adding 1.5 ml of CHCl3 and vortexing for 60 s. Cells remained at room temperature for 1 h and then were pelleted, and 500 μl of the aqueous phase was removed.
For capsule determination via the Stains-All assay, duplicate samples (10 to 75 μl, depending on the mucoid phenotype of the strain of S. pyogenes) of the aqueous phase were placed in 12-by-75-mm glass test tubes, and H2O was added to bring the volume to 100 μl. Standards were prepared containing 0.5, 1, 2, and 3 μg of hyaluronic acid (Sigma). Fresh Stains-All reagent (50 ml of formamide, 50 ml of distilled and deionized H2O, 16 μl of acetic acid, and 20 mg of Stains-All reagent [Sigma]) was prepared, and 1 ml was added to each sample. After vortexing for 2 s, spectrophotometric absorbance at 640 nm was read immediately. The amount of hyaluronic acid capsule was determined by interpolation from the hyaluronic acid standard curve and was expressed as femtograms per CFU.
In vitro bactericidal assay.
Resistance of mutant strains of S. pyogenes to phagocytosis in whole blood was performed by a modification of the Lancefield bactericidal assay (42, 73). A 6-ml suspension of an overnight culture in serum broth (THB plus 20% fetal calf serum) was pelleted and resuspended in 0.8 ml of serum broth. Aliquots of bacterial suspension were added to 6 ml of fresh serum broth until an OD560 of 0.15 was reached. The resulting suspension was incubated at 37°C for 90 min and then diluted with THB to obtain 100 to 200 CFU in a 100-μl suspension. The 100-μl bacterial suspension was mixed with 300 μl of freshly drawn human blood anticoagulated with 8 to 10 U of heparin per ml in a 15-ml polypropylene tube. After incubation for 3 h at 37°C with gentle end-to-end rocking, 100 μl of the mixture and dilutions of 10−1 and 10−2 were plated on THA. The growth index was calculated as the CFU in the sample after a 3-h rotation divided by the CFU inoculated into the sample at the beginning of the test. For positive control strains that possess M proteins and M-like proteins (i.e., emm, mrp, enn) and thus are resistant to phagocytosis (18, 40, 51, 55, 64), a minimum growth index of 32 is required for reliable testing.
Assays for bacterial adherence to keratinocytes.
Radiolabeling of S. pyogenes and keratinocyte culture and differentiation were performed as described previously (24, 25). Briefly, S. pyogenes in log-phase growth was l-[3H]leucine radiolabeled, and nonspecific bacterial sites for adherence were blocked prior to adherence assays by preincubation with 0.25% gelatin (Difco) in Hank's balanced salt solution (HBSS) (Gibco BRL, Grand Island, N.Y.) for 30 min at 4°C. For experiments measuring the effect of CD46 (soluble membrane cofactor protein) on adherence, radiolabeled, nonspecifically blocked M52- or M49-protein wild-type strains of S. pyogenes also were pretreated with CD46 (2 to 40 μg/ml) for 30 min. To test the role of bacterial cysteine protease in the adherence of the opp mutants, 28 mM trans-epoxysuccinyl-l-leucylamido-3-methyl-butane (E-64; Sigma), a specific inhibitor of cysteine protease, was added to nonspecifically blocked bacterial suspensions at 37°C for 30 min prior to initiating adherence. For some experiments, hyaluronic acid capsule was removed enzymatically prior to adherence assays by incubation for 30 min in THB at 37°C containing 10 μg of bovine testicular hyaluronidase (Sigma) per ml. After pretreatment, the bacteria were washed and resuspended at a concentration of 5 × 107 to 1 × 108 CFU/ml in HBSS containing 0.25% gelatin and the corresponding pretreatment concentration of CD46, E-64, or hyaluronidase for use in adherence assays.
Keratinocytes were cultured from neonatal foreskins and seeded in keratinocyte growth medium (Clonetics Corp., San Diego, Calif.) into six-well tissue culture plates (Corning Glass Works, Corning, N.Y.) (25). Cultured keratinocytes at 80% of confluence were induced to terminally differentiate by adjusting the extracellular calcium concentration to 1.0 mM for 2 days prior to adherence assays. Nonspecific sites for adherence on keratinocytes were blocked by exposure to 0.25% gelatin in HBSS for 2 h at 4°C. Keratinocytes also were exposed to 2 to 40 μg of CD46 for 30 min prior to experiments measuring the effect of CD46 on adherence.
To initiate adherence, wells were aspirated to dryness, and 1.5 ml of bacterial suspension was added to each well. The multiplicities of infection ranged from approximately 50 to 100 bacteria per keratinocyte. Unless indicated otherwise, adherence occurred for 4 h at 4°C to prevent confounding of the data by internalization of bacteria (25), with no centrifugation of bacteria against the keratinocytes. For experiments employing hyaluronidase digestion of the capsule, adherence assays were performed at 37°C to maintain hyaluronidase activity. To terminate adherence, nonspecifically attached bacteria were removed by washing and vortexing. For assays performed at 4°C, keratinocytes were solubilized, and aliquots were removed for scintillation counting as a measure of adherent bacteria. Counts per minute (cpm) in wells without keratinocytes (background control) were subtracted from the cpm in wells containing keratinocytes (bound) to correct for nonspecific adherence. The percent adherence was calculated as follows: (cpm bound − background)/(cpm added − cpm ambient) × 100. For assays performed at 37°C, total cell-associated bacteria were quantified by dislodging keratinocytes from the tissue culture plate by incubation with 0.25% trypsin (Gibco) at 37°C for 5 min, followed by 0.025% Triton X-100 (Sigma). The number of viable, cell-associated bacteria was determined by plating aliquots of the final suspension on THA. CFU recovered from triplicate wells without keratinocytes was subtracted from the CFU from triplicate wells containing keratinocytes. To determine the denominator for calculating the percent adherence at 37°C, accounting for bacterial growth during the adherence assay, the CFU in the supernatant after vortexing the plate was added to the CFU associated with the keratinocytes in triplicate wells. All experiments were repeated at least three times. Data represent the average of all experiments (Table 2) or, for figures, a representative experiment is presented.
TABLE 2.
Adherence of S. pyogenes mutants with knockouts in genes encoding putative virulence factors for skin infections
| Virulence factor mutant | Adherence of mutants relative to wild-type strain (M49, M2, M3, M18)a |
|---|---|
| mga | 1.3 |
| mrp | 1.8 |
| emm | 1.4, 1.0 |
| enn | 1.0 |
| scpA | 2.1 |
| orfX | 1.6 |
| has | 13.0, 2.3, 1.3, 2.0 |
| speB | 1.3 |
| oppA to oppF | 3.9 |
| oppA | 3.8 |
| oppD/F | 5.5 |
| ska | 1.7 |
| rof | 0.9 |
| cia | 1.0 |
| lrp | 1.2 |
| sdaD | 1.2 |
Adherence of mutant/adherence of wild-type strain. Values are the mean of at least two experiments.
RESULTS
Adherence of S. pyogenes mutants with knockout of putative virulence factors.
A conclusive method for identifying virulence factors in pathogenesis is through analysis of isogenic mutants that differ from the wild-type strain by a single defined mutation. We hypothesized that if a given gene product acts as an adhesin, then the lack of expression of the gene in a mutant strain would lead to decreased adherence compared to the wild-type strain. None of the mutants tested had decreased adherence (Table 2), suggesting that the putative virulence factors we examined, including M49 and M2 proteins, do not act directly as adhesins for binding of S. pyogenes to keratinocytes.
Role of hyaluronic acid capsule.
Adherence of the skin-associated M49-protein strain of S. pyogenes was increased 13-fold following elimination of hyaluronic acid capsule production by insertional inactivation of has (Table 2). Greater adherence of the has mutant strain was observed at 4°C (Table 2) as well as 37°C (data not shown). This presumably was due to greater exposure of functional adhesins on the surface of the capsule-deficient mutant, leading to more ready attachment to keratinocyte receptors. Although the has mutant bacteria adhered in a uniform distribution to cultured keratinocytes, they readily aggregated in culture media. The enhanced ability of the has mutant M49-protein strain to adhere to keratinocytes could not be attributed to a differential in growth, since the assays were performed at 4°C under nonproliferating conditions, and, furthermore, the growth rates of the has mutant and wild-type M49 strains in liquid culture were equivalent (data not shown). Adherence of the M2, M3, and M18 protein strains of S. pyogenes was increased only slightly (one- to twofold) in the respective has mutants (Table 2), suggesting that there was no or only slight impedance of adherence of these strains by the capsule. The amount of cell-associated hyaluronic acid, as determined by the Stains-All assay, was equivalent for the M18 (121 ± 46 fg/CFU)- and M49 (101 ± 34 fg/CFU)- protein strains but was somewhat less for wild-type M3 protein (68 ± 53 fg/CFU). Adherence, however, was approximately the same for each of the mucoid, wild-type M3 (8 to 12%)-, M18 (8 to 15%)-, and M49 (1 to 10%)-protein strains. Therefore, the greater increase in adherence of the M49-protein has mutant compared to the M3- and M18-protein has mutant strains, relative to their respective wild-type strains, could not be attributed to greater capsule production by the wild-type M49-protein strain.
The decreased adherence of mucoid, clinically invasive isolates of M1 (18%)- and M12 (37%)-protein group A streptococci compared to the low-capsule-producing M52-protein skin-associated strain 3732 (49%) further supported the notion that hyaluronic acid capsule sterically hindered attachment to keratinocytes. Alternatively, the attachment of the mucoid, invasive strains may have been less avid, enabling the bacteria to be washed from the keratinocytes more easily.
Role of M protein.
Since the presence of hyaluronic acid capsule might sterically hinder interaction of M protein with its keratinocyte receptor, we created a mutant with knockout of both the emm and has genes in M49-protein strain CS101 and compared its adherence to the mutant with knockout of capsule production (has). We hypothesized that if M protein does not act as an adhesin, then adherence of the double emm has knockout mutant would be the same as for the has mutant.
Integration of intragenic sequences of hasA on plasmid pFW13 into the chromosome of the M49-protein emm mutant strain by homologous recombination led to inactivation of has and lack of production of hyaluronic acid capsule. The genotype of the mutant with knockout of both has and emm was confirmed by PCR, demonstrating the presence of both the has (3,335 bp) and the emm (3,582 bp) inserts in the chromosome. The absence of detectable hyaluronic acid capsule using a modified Stains-All assay (data not shown) and the markedly increased susceptibility to phagocytosis in whole human blood confirmed the expected phenotype; the growth indices of the wild-type and emm, has, and emm has mutant strains were 497, 154, 137, and 14.5, respectively. These differences in survival in human blood could not be attributed to differences in growth potential in human serum (data not shown).
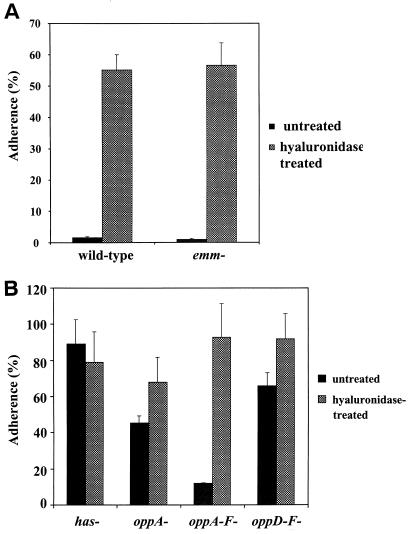
Adherence of the M49-protein emm has mutant (34.9 ± 4.1%) was the same as that for the has mutant (40.7 ± 8.0%). Moreover, after hyaluronidase digestion of the capsule, adherence of the M49-protein emm mutant and wild-type strains was increased to the same level (Fig. 1A), confirming that adherence was not mediated by M protein.
FIG. 1.
Adherence of wild-type and emm mutant strains (A) and has and opp mutant strains (B) of M49-protein S. pyogenes to cultured human keratinocytes at 37°C with (hatched bars) or without (shaded bars) enzymatic digestion of the bacterial hyaluronic acid capsule achieved by incubation with 10 μg of hyaluronidase per ml for 30 min prior to and during the adherence assay. Keratinocytes were isolated from neonatal human foreskins and grown to confluence in six-well tissue culture plates. To initiate adherence, keratinocyte cultures were inoculated with 108 CFU of l-[3H]leucine-radiolabeled S. pyogenes per ml. To calculate the percent adherence, the total CFU remaining in triplicate six-well keratinocyte cultures after washing and vortexing away the nonadherent bacteria minus the adherent CFU in wells lacking keratinocytes was divided by the total CFU in the supernatant plus those on the monolayer (in the absence of washing and vortexing) at the end of 3 h of incubation. Error bars represent the standard deviation.
Role of oligopeptide permease (Opp).
Inactivation of the genes encoding the membrane-associated substrate-binding lipoprotein (oppA), the inner membrane ATPases (oppD/F) or expression of all genes of the Opp operon (oppA to oppF) in the M49-protein strain resulted in increased (3.8- to 5.5-fold) adherence compared to the wild-type strain (Table 2). The opp mutants readily formed a tight pellet upon centrifugation, a result similar to that observed with strains lacking hyaluronic acid capsule. This led us to evaluate the amount of cell-associated capsule of the opp mutants in log-phase growth, since the decreased capsule might explain their increased adherence. Each of the opp mutants had a decreased cell-associated hyaluronic acid capsule compared to the wild-type strain as follows: M49-protein wild type, 95 ± 41 fg/CFU; oppA, 65 ± 35 fg/CFU; oppD/F, 48 ± 25 fg/CFU; and oppA to oppF, 60 ± 16 fg/CFU. Furthermore, adherence of the opp mutants after enzymatic digestion of their hyaluronic acid capsule was equivalent to that of the has mutant strain (Fig. 1B), suggesting that their adherence characteristics reflect alterations in the amount of capsule and not the expression of fundamentally different receptors. Since inactivation of the Opp complex in M49-protein strain CS101 S. pyogenes was associated with decreased, but detectable, cysteine protease activity (59), we tested whether total inhibition of cysteine protease activity with the specific inhibitor E-64 would further impact adherence. We found no effect of cysteine protease inhibition on the adherence of the wild-type strain or the opp mutants (data not shown). Similarly, insertional inactivation of speB did not impact adherence (Table 2). Taken together, these data suggest that the effect of opp mutant inactivation on adherence was due, at least in part, to decreased amounts of hyaluronic acid capsule and was independent of cysteine protease activity.
Role of keratinocyte CD46.
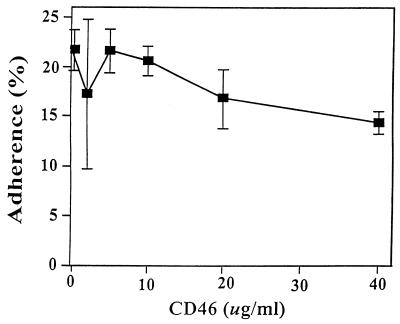
CD46 on human keratinocytes can act as a cellular receptor for some strains of S. pyogenes (8, 54), and soluble CD46 binds directly to M protein to act as a competitive inhibitor of adherence (54). Soluble CD46 did not interfere with adherence of M52-protein strain 3732 to keratinocytes (Fig. 2), nor did 10 and 20 μg of CD46 per ml inhibit adherence of the M49-protein has mutant strain (data not shown). These data suggested that keratinocyte CD46 was not a principal receptor for these skin strains of group A streptococcus.
FIG. 2.
Effect of soluble CD46 (membrane cofactor protein) on the adherence of M52-protein S. pyogenes to cultured keratinocytes. Bacteria and keratinocytes were preincubated with CD46 for 30 min, and CD46 was present throughout the adherence assay.
DISCUSSION
We have utilized our previously described in vitro experimental model system (15), which simulates superficial skin infections such as impetigo, to examine the role of putative group A streptococcal virulence factors in the pathogenesis of skin infections. Our knowledge of the pathogenesis of skin infections is based to date almost solely on extrapolation from experiments with respiratory epithelium and/or respiratory-associated strains of S. pyogenes. These data may not be applicable to skin infections, since epidermal and mucosal keratinocytes exist in markedly different environments and produce distinct arrays of surface-expressed molecules (25). Moreover, strains of S. pyogenes that have a propensity to cause pyogenic skin infections differ from those that infect respiratory epithelia in a number of important traits (2, 9, 10, 31, 57, 70).
S. pyogenes produces a variety of virulence factors that may be postulated to play a role in the pathogenesis of skin infections. Some of these factors are expressed on the bacterial surface, while others are extracellular products (Table 1). We chose to examine the impact of these factors on bacterial adherence to keratinocytes, since attachment to keratinocytes may be an initiating event in infection of the skin. None of the putative virulence factors we examined was an adhesin or impacted the expression of adhesins for cultured human keratinocytes, as none of the M49-protein mutants with knockouts of genes encoding the wild-type virulence factors had decreased adherence compared to the wild-type strain. Similarly, knockout of M2-protein expression did not alter adherence compared to the M2-protein wild-type strain. Our data contrast with those of Caparon and coworkers, who suggested that the C-repeat domain of M6-protein mediated adherence to the C46 molecule on keratinocytes (53, 54, 56), since an M6-protein-deficient mutant displayed reduced adherence to human epidermal keratinocytes (53, 65). Because M6-protein strains of S. pyogenes typically infect respiratory epithelia rather than the skin, however, the relevance of M6 protein in the pathogenesis of skin infections is questionable. Adherence of a respiratory strain of S. pyogenes to keratinocytes was not promoted by keratinocyte differentiation (25, 53), in contrast with the greater adherence of skin-associated S. pyogenes to differentiated keratinocytes seen histopathologically in impetigo (23) and in our in vitro model (25). The role of M protein in attachment appears to be highly variable, depending on the M-protein serotype and the host tissue involved (8, 12, 17–19, 33, 36, 40, 52, 65, 69). We now demonstrate that neither streptococcal M protein nor keratinocyte CD46 was involved in attachment of M49 skin-associated S. pyogenes to keratinocytes. Lack of involvement of M protein in adherence to keratinocytes and, presumably, in the initiation of skin infections was demonstrated by showing equivalent adherence of (i) M49-protein wild-type and emm mutant strains, with or without removal of cell-associated hyaluronic acid by hyaluronidase treatment; (ii) M2-protein wild-type and emm mutant strains; and (iii) M49 protein has and emm has mutant strains. These data support the concept that M protein is not a universal adhesin but mediates attachment of only certain M-type proteins to epithelial cells from specific tissues (65). Although CD46 on an immortalized keratinocyte line was shown to be a cellular receptor for the C-repeat domain of M6 protein (53, 54, 56), recent evidence has suggested that the C-repeat domain may not be directly involved in M-protein-mediated adherence (8, 32) and that CD46 may act as a primary receptor for some (M3) but not other (M6 and M18) M proteins (8). It appears that CD46 is not a primary keratinocyte receptor for attachment of M49 protein.
Hyaluronic acid capsule expression proved to be an important modulating factor in the adherence of M49-protein S. pyogenes to keratinocytes. Lack of hyaluronic acid capsule expression resulted in markedly increased adherence of the M49-protein has mutant strain regardless of the presence (wild-type [emm+]) or absence (emm mutant) of M protein, as has been reported previously for M18 (65)- and M24 (18)-protein strains. In contrast, knockout of capsule expression in M2-, M3-, and M18-protein has mutants only slightly (1.3- to 2.3-fold) increased adherence compared to their respective wild-type strains, a finding also in agreement with previous findings with an M18-protein strain (7). These data suggest that the hyaluronic acid capsule of either M2-, M3-, M18-, or M49-protein S. pyogenes was not a ligand for keratinocytes, in contrast to the proposal that hyaluronic acid capsule is a universal adhesin for group A streptococcal attachment to epithelial cells (17, 65). To the contrary, it appears that the capsule sterically inhibited interaction of unidentified cell wall components or surface proteins of skin-associated group A streptococci with keratinocyte receptors (5, 65). Since elimination of hyaluronic acid capsule expression promoted adherence of the skin-associated M49-protein strain to a greater degree than the M2-, M3-, or M18-protein strains, it appears that (i) the M49-protein strain had more surface-exposed adhesins; (ii) once exposed, the adhesins on the M49-protein strain were capable of binding more avidly; and/or (iii) there were fewer molecules on the surface interfering with adherence of the M49-protein strain.
The concept that hyaluronic acid capsule inhibited adherence was further supported by the finding that increased adherence of the opp mutants was associated with decreased amounts of cell-associated hyaluronic acid, independent of cysteine protease activity. Bacterial Opps are membrane-associated transporter lipoprotein complexes involved in oligopeptide uptake and adherence to substrates, other bacteria, or eukaryotic cells. In addition, Opps may play a regulatory role in cell wall metabolism and the development of competence (3). Unlike our finding, mutations in Opp genes in Streptococcus pneumoniae and S. gordonii were associated with decreased adherence to eucaryotic cells or substrates, either due directly to loss of expression of a peptide in the membrane-associated complex which can act as an adhesin or by loss of other adhesins whose expression is regulated by Opp (21, 34, 37, 38, 49, 50). Mutation in either oppA or oppD/F of M49 protein S. pyogenes CS101 did not affect adherence to a variety of eucaryotic cells or substrates, although adherence to keratinocytes was not examined at that time (59). These mutants, however, did show markedly reduced production of extracellular cysteine protease (SpeB) (59), the principal extracellular protease and an important group A streptococcal virulence factor (47). Cysteine protease has been shown to be an important virulence factor for development of invasive group A streptococcal skin infections in some studies (47) but not in others (4). It appears to play little role in initial attachment to keratinocytes to initiate superficial skin infections since biochemical inhibition or insertional inactivation of cysteine protease did not impact adherence. Although our data suggest that the increased adherence of the opp mutants may be due to decreased amounts of cell-associated hyaluronic acid capsule, increased adherence of the opp mutants also could be due, in part, to altered Opp-mediated regulatory activity resulting in altered expression of adhesins.
Hyaluronic acid capsule is antiphagocytic (22, 46, 51, 71, 72), and its expression is necessary for development of invasive soft tissue infection in a murine model of necrotizing fasciitis (4) and is associated epidemiologically with invasive disease in humans (29, 41). Both M protein (18, 40, 51, 55, 64) and hyaluronic acid capsule (22, 46, 51, 71, 72) contribute to resistance to phagocytosis in whole blood. The presence of both traits in the wild-type strain provided optimal protection from phagocytosis, although expression of either M protein (has mutant strain) or hyaluronic acid capsule (emm mutant strain) was sufficient for multiplication in whole blood. Protection from phagocytosis was nearly eliminated by inactivation of both traits in the emm has mutant, suggesting that expression of these factors is crucial for survival in human blood.
Evidence is accumulating that the level of capsule expression may be critical for pathogenesis of skin infections. Capsule expression varies under different conditions, apparently enabling the organism to adapt to environmental niches encountered during human infection (1, 16, 39, 44, 45). Perhaps downregulated capsule expression in bacteria on the skin surface favors their attachment and survival, enabling them to form a nidus of infection. We found that unencapsulated bacteria tended to aggregate, which might play a protective role by minimizing surface exposure and oxygen uptake, thereby reducing O2 metabolism and resultant oxidative damage to catalase-negative group A streptococci (15). Since capsule is not expressed during the stationary phase but, rather, deposits are shed from the surface (20, 67, 68), this might favor adherence. Irreversible, avid attachment (32), and/or the inability of the bacteria to subsequently upregulate capsule expression and thereby avoid phagocytosis might explain the containment of infection in the superficial epidermis observed in impetigo. On the other hand, reversible attachment to keratinocytes, followed by high-level expression of capsule during the log-phase growth of acute purulent infection, as has been observed for strains causing invasive disease (41), might enable the bacteria to avoid phagocytosis while it utilizes other virulent factors to penetrate host tissues to cause invasive disease.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant P30 HD28834 through the University of Washington Child Health Research Center and a Clinical Career Development Award from the Dermatology Foundation and Pfizer Pharmaceutical Co., Inc.
CytoMed, Inc., kindly provided soluble CD46.
REFERENCES
- 1.Alberti S, Ashbaugh C D, Wessels M R. Structure of the has operon promoter and regulation of hyaluronic acid capsule expression in group A Streptococcus. Mol Microbiol. 1998;28:343–353. doi: 10.1046/j.1365-2958.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- 2.Alkan M, Ofek I, Beachey E H. Adherence of pharyngeal and skin strains of group A streptococci to human skin and oral epithelial cells. Infect Immun. 1977;18:555–557. doi: 10.1128/iai.18.2.555-557.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alloing G, Martin B, Granadel C, Claverys J P. Development of competence in Streptococcus pneumoniae: pheromone autoinduction and control of quorum sensing by the oligopeptide permease. Mol Microbiol. 1998;29:75–83. doi: 10.1046/j.1365-2958.1998.00904.x. [DOI] [PubMed] [Google Scholar]
- 4.Ashbaugh C D, Warren H B, Carey V J, Wessels M R. Molecular analysis of the role of group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. J Clin Investig. 1998;102:550–560. doi: 10.1172/JCI3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartelt M A, Duncan J L. Adherence of group A streptococci to human epithelial cells. Infect Immun. 1978;20:200–208. doi: 10.1128/iai.20.1.200-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benchetrit L C, Pahuja S L, Gray E D, Edstrom R D. A sensitive method for the assay of hyaluronidase activity. Anal Biochem. 1977;79:431–437. doi: 10.1016/0003-2697(77)90418-3. [DOI] [PubMed] [Google Scholar]
- 7.Bennett-Wood V R, Carapetis J R, Robins-Browne R M. Ability of clinical isolates of group A streptococci to adhere to and invade Hep-2 epithelial cells. J Med Microbiol. 1998;47:899–906. doi: 10.1099/00222615-47-10-899. [DOI] [PubMed] [Google Scholar]
- 8.Berkower C, Ravins M, Moses A E, Hanski E. Expression of different group A streptococcal M proteins in an isogenic background demonstrates diversity in adherence to and invasion of eukaryotic cells. Mol Microbiol. 1999;31:1463–1475. doi: 10.1046/j.1365-2958.1999.01289.x. [DOI] [PubMed] [Google Scholar]
- 9.Bessen D, Fischetti V. A human IgG receptor of group A streptococci is associated with tissue site infection and streptococcal class. J Infect Dis. 1990;161:747–754. doi: 10.1093/infdis/161.4.747. [DOI] [PubMed] [Google Scholar]
- 10.Bessen D E, Sotir C M, Readdy T L, Hollingshead S K. Genetic correlates of throat and skin isolates of group A streptococci. J Infect Dis. 1996;173:896–900. doi: 10.1093/infdis/173.4.896. [DOI] [PubMed] [Google Scholar]
- 11.Caparon M G, Scott J R. Genetic manipulation of streptococci. Methods Enzymol. 1991;204:556–586. doi: 10.1016/0076-6879(91)04028-m. [DOI] [PubMed] [Google Scholar]
- 12.Caparon M G, Stephens D S, Olsen A, Scott J R. Role of M protein in adherence of group A streptococci. Infect Immun. 1991;59:1811–1817. doi: 10.1128/iai.59.5.1811-1817.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaffin D O, Rubens C E. Blue/white screening of recombinant plasmids in gram-positive bacteria by interruption of alkaline phosphatase gene (phoZ) expression. Gene. 1998;219:91–99. doi: 10.1016/s0378-1119(98)00396-5. [DOI] [PubMed] [Google Scholar]
- 14.Christner R, Li Z, Raeder R, Podbielski A, Boyle M D P. Identification of key gene products required for acquisition of plasmin-like enzymatic activity by group A streptococci. J Infect Dis. 1997;175:1115–1120. doi: 10.1086/516450. [DOI] [PubMed] [Google Scholar]
- 15.Cleary P P, Larkin A. Hyaluronic acid capsule: strategy for oxygen resistance in group A streptococci. J Bacteriol. 1979;140:1090–1097. doi: 10.1128/jb.140.3.1090-1097.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cleary P P, McLandsborough L, Ikeda L, Cue D, Krawczak J, Lam H. High-frequency intracellular infection and erythrogenic toxin A expression undergo phase variation in M1 group A streptococci. Mol Microbiol. 1998;28:151–167. doi: 10.1046/j.1365-2958.1998.00786.x. [DOI] [PubMed] [Google Scholar]
- 17.Courtney H S, Ofek I, Hasty D L. M protein mediated adhesion of M type 24 Streptococcus pyogenes stimulates release of interleukin-6 by Hep-2 tissue culture cells. FEMS Microbiol Lett. 1997;151:65–70. doi: 10.1016/s0378-1097(97)00139-0. [DOI] [PubMed] [Google Scholar]
- 18.Courtney H S, Bronze M S, Dale J B, Hasty D L. Analysis of the role of M24 protein in group A streptococcal adhesion and colonization by use of Ω-interposon mutagenesis. Infect Immun. 1994;62:4868–4873. doi: 10.1128/iai.62.11.4868-4873.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courtney H S, Liu S, Dale J B, Hasty D L. Conversion of M serotype 24 of Streptococcus pyogenes to M serotypes 5 and 18: effect on resistance to phagocytosis and adhesion to host cells. Infect Immun. 1997;65:2472–2474. doi: 10.1128/iai.65.6.2472-2474.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crater D L, van de Rijn I. Hyaluronic acid synthesis operon (has) expression in group A streptococci. J Biol Chem. 1995;270:18452–18458. doi: 10.1074/jbc.270.31.18452. [DOI] [PubMed] [Google Scholar]
- 21.Cundell D R, Pearce B J, Naughton A M, Masure H R. Peptide permeases from Streptococcus pneumoniae affect adherence to eucaryotic cells. Infect Immun. 1995;63:2493–2498. doi: 10.1128/iai.63.7.2493-2498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dale J B, Washburn R G, Marques M B, Wessels M R. Hyaluronate capsule and surface M protein in resistance to phagocytosis of group A streptococci. Infect Immun. 1996;64:1495–1501. doi: 10.1128/iai.64.5.1495-1501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darmstadt G L, Lane A T. Impetigo: an overview. Pediatr Dermatol. 1994;4:293–303. doi: 10.1111/j.1525-1470.1994.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 24.Darmstadt G L, Fleckman P, Rubens C E. TNF-α and IL-1α decrease the adherence of Streptococcus pyogenes to cultured keratinocytes. J Infect Dis. 1999;180:1718–1721. doi: 10.1086/315066. [DOI] [PubMed] [Google Scholar]
- 25.Darmstadt G L, Fleckman P, Jonas M, Chi E, Rubens C E. Differentiation of cultured keratinocytes promotes adherence of Streptococcus pyogenes. J Clin Investig. 1998;101:128–136. doi: 10.1172/JCI680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darmstadt G L. Superficial skin infections, p. 476–485; subcutaneous tissue infections and abscesses. In: Long S S, Prober C G, Pickering L K, editors. Principles and practice of pediatric infectious diseases. New York, N.Y: Churchill Livingstone; 1996. pp. 507–517. [Google Scholar]
- 27.Darmstadt G L. Oral antibiotics for treatment of uncomplicated skin infections in children. Pediatr Infect Dis J. 1997;16:227–240. doi: 10.1097/00006454-199702000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Darmstadt G L. Staphylococcal and streptococcal skin infections. In: Harahap M, editor. Diagnosis and treatment of skin infections. Oxford, England: Blackwell Scientific, Ltd.; 1997. pp. 7–115. [Google Scholar]
- 29.Ferrieri P. Microbiological features of current virulent strains of group A streptococci. Pediatr Infect Dis J. 1991;10:S20–S24. doi: 10.1097/00006454-199110001-00005. [DOI] [PubMed] [Google Scholar]
- 30.Haanes E J, Cleary P P. Identification of a divergent M protein gene and an M protein-related gene family in Streptococcus pyogenes serotype M49. J Bacteriol. 1989;171:6397–6408. doi: 10.1128/jb.171.12.6397-6408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haanes E J, Heath D G, Cleary P P. Architecture of the vir regulons of group A streptococci parallels opacity factor phenotype and M protein class. J Bacteriol. 1992;174:4967–4976. doi: 10.1128/jb.174.15.4967-4976.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasty D L, Ofek I, Courtney H S, Doyle R J. Multiple adhesins of streptococci. Infect Immun. 1992;60:2147–2152. doi: 10.1128/iai.60.6.2147-2152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollingshead S K, Simecka J W, Michalek S M. Role of M protein in pharyngeal colonization by group A streptococci in rats. Infect Immun. 1993;61:2277–2283. doi: 10.1128/iai.61.6.2277-2283.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmes A R, McNab R, Jenkinson H F. Candida albicans binding to the oral bacterium Streptococcus gordonii involves multiple adhesin-receptor interactions. Infect Immun. 1996;64:4680–4685. doi: 10.1128/iai.64.11.4680-4685.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hotez P, Cappello M, Hawdon J, Beckers C, Sakanari J. Hyaluronidases of the gastrointestinal invasive nematodes Ancylostoma caninum and Anisakis simplex: possible functions in the pathogenesis of human zoonoses. J Infect Dis. 1994;170:918–926. doi: 10.1093/infdis/170.4.918. [DOI] [PubMed] [Google Scholar]
- 36.Husmann L K, Yung D L, Hollingshead S K, Scott J R. Role of putative virulence factors of Streptococcus pyogenes in mouse models of long-term throat colonization and pneumonia. Infect Immun. 1997;65:1422–1430. doi: 10.1128/iai.65.4.1422-1430.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkinson H F, Easingwood R A. Insertional inactivation of the gene encoding a 76-kilodalton cell surface polypeptide in Streptococcus gordonii Challis has a pleiotropic effect on cell surface composition and properties. Infect Immun. 1990;58:3689–3697. doi: 10.1128/iai.58.11.3689-3697.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenkinson H F. Adherence, coaggregation, and hydrophobicity of Streptococcus gordonii associated with expression of cell-surface lipoproteins. Infect Immun. 1992;60:1225–1228. doi: 10.1128/iai.60.3.1225-1228.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji Y, McLandsborough L, Kondagunta A, Cleary P P. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect Immun. 1996;64:503–510. doi: 10.1128/iai.64.2.503-510.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji Y, Schnitzler N, DeMaster E, Cleary P. Impact of M49, Mrp, Enn, and C5a peptidase proteins on colonization of the mouse oral mucosa by Streptococcus pyogenes. Infect Immun. 1998;66:5399–5405. doi: 10.1128/iai.66.11.5399-5405.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson D R, Stevens D L, Kaplan E L. Epidemiological analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever, or uncomplicated pharyngitis. J Infect Dis. 1992;166:374–382. doi: 10.1093/infdis/166.2.374. [DOI] [PubMed] [Google Scholar]
- 42.Lancefield R C. Differentiation of group A streptococci with a common R antigen into three serological types, with special reference to the bactericidal test. J Exp Med. 1957;106:525–544. doi: 10.1084/jem.106.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leonard B A B, Podbielski A. Emerging density-dependent control systems in gram-positive cocci. In: Dunny G M, Winans S C, editors. Cell-cell signaling in bacteria. Washington, D.C.: American Society for Microbiology; 1999. pp. 315–331. [Google Scholar]
- 44.Leonard B A B, Woischnik M, Podbielski A. Production of stabilized virulence factor-negative variants by group A streptococci during stationary phase. Infect Immun. 1998;66:3841–3847. doi: 10.1128/iai.66.8.3841-3847.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levin J C, Wessels M R. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A streptococcus. Mol Microbiol. 1998;30:209–219. doi: 10.1046/j.1365-2958.1998.01057.x. [DOI] [PubMed] [Google Scholar]
- 46.Liu S, Courtney H S, Bessen D E, Hasty D L, Dale J B. M protein expression is not required for resistance to phagocytosis of type 18 group A streptococci. Adv Exp Med Biol. 1997;418:725–727. doi: 10.1007/978-1-4899-1825-3_170. [DOI] [PubMed] [Google Scholar]
- 47.Lukomski S, Montgomery C A, Rurangirwa J, Geske R S, Barrish J P, Adams G J, Musser J M. Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice. Infect Immun. 1999;67:1779–1788. doi: 10.1128/iai.67.4.1779-1788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lukomski S, Sreevatsan S, Amberg C, Reichardt W, Woischnik M, Podbielski A, Musser J M. Inactivation of Streptococcus pyogenes extracellular cysteine protease (SpeB) significantly decreases mouse lethality of serotype M3 and M49 strains. J Clin Investig. 1997;99:2574–2580. doi: 10.1172/JCI119445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McNab R, Holmes A R, Clarke J M, Tannock G W, Jenkinson H F. Cell surface polypeptide CshA mediates binding of Streptococcus gordonii to other oral bacteria and to immobilized fibronectin. Infect Immun. 1996;64:4204–4210. doi: 10.1128/iai.64.10.4204-4210.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McNab R, Jenkinson H F. Altered adherence properties of a Streptococcus gordonii hhpA (oligopeptide permease) mutant resulting from transcriptional effects on cshA adhesin gene expression. Microbiology. 1998;144:127–136. doi: 10.1099/00221287-144-1-127. [DOI] [PubMed] [Google Scholar]
- 51.Moses A E, Wessels M R, Zalcman K, Alberti S, Natanson-Yaron S, Menes T, Hanski E. Relative contributions of hyaluronic acid capsule and M protein to virulence in a mucoid strain of group A streptococcus. Infect Immun. 1997;65:64–71. doi: 10.1128/iai.65.1.64-71.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Natanson S, Sela S, Moses A E, Musser J M, Caparon M G, Hanski E. Distribution of fibronectin-binding proteins among group A streptococci of different M types. J Infect Dis. 1995;171:871–878. doi: 10.1093/infdis/171.4.871. [DOI] [PubMed] [Google Scholar]
- 53.Okada N, Pentland A P, Falk P, Caparon M G. M protein and protein F act as important determinants of cell-specific tropism of Streptococcus pyogenes in skin tissue. J Clin Investig. 1994;94:965–977. doi: 10.1172/JCI117463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okada N, Liszewski M K, Atkinson J P, Caparon M. Membrane cofactor protein (CD46) is a keratinocyte receptor for the M protein of the group A streptococcus. Proc Natl Acad Sci USA. 1995;92:2489–2493. doi: 10.1073/pnas.92.7.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perez-Casal J, Caparon M G, Scott J R. Introduction of the emm6 gene into an emm-depleted strain of Streptococcus pyogenes restores its ability to resist phagocytosis. Res Microbiol. 1992;143:549–558. doi: 10.1016/0923-2508(92)90112-2. [DOI] [PubMed] [Google Scholar]
- 56.Perez-Casal J, Okada N, Caparon M G, Scott J R. Role of the conserved C-repeat region of the M protein of Streptococcus pyogenes. Mol Microbiol. 1995;15:907–916. doi: 10.1111/j.1365-2958.1995.tb02360.x. [DOI] [PubMed] [Google Scholar]
- 57.Podbielski A. Three different types of organization of the vir regulon in group A streptococci. Mol Gen Genet. 1993;237:287–300. doi: 10.1007/BF00282810. [DOI] [PubMed] [Google Scholar]
- 58.Podbielski A, Flosdorff A, Weber-Heynemann J. The group A streptococcal vir49 gene controls expression of four structural vir regulon genes. Infect Immun. 1995;63:9–20. doi: 10.1128/iai.63.1.9-20.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Podbielski A, Pohl B, Woischnik M, Korner C, Schmidt K H, Rozdzinski E, Leonard B A B. Molecular characterization of group A streptococcal (GAS) oligopeptide permease (Opp) and its effect on cysteine protease production. Mol Microbiol. 1996;21:1087–1099. doi: 10.1046/j.1365-2958.1996.661421.x. [DOI] [PubMed] [Google Scholar]
- 60.Podbielski A, Spellerberg B, Woischnik M, Pohl B, Lutticken R. Novel series of plasmid vectors for gene inactivation and expression analysis in group A streptococci (GAS) Gene. 1996;177:137–147. doi: 10.1016/0378-1119(96)84178-3. [DOI] [PubMed] [Google Scholar]
- 61.Podbielski A, Zarges I, Flosdorff A, Weber-Heynemann J. Molecular characterization of a major serotype M49 group A streptococcal Dnase gene (sdaD) Infect Immun. 1996;64:5349–5356. doi: 10.1128/iai.64.12.5349-5356.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Podbielski A, Woischnik M, Pohl B, Schmidt K H. What is the size of the group A streptococcal vir regulon? The Mga regulator affects expression of secreted and surface virulence factors. Med Microbiol Immunol. 1996;185:171–181. doi: 10.1007/s004300050028. [DOI] [PubMed] [Google Scholar]
- 63.Podbielski A, Woischnik M, Leonard B A B, Schmidt K H. Characterization of nra, a global negative regulator gene in group A streptococci. Mol Microbiol. 1999;31:1051–1064. doi: 10.1046/j.1365-2958.1999.01241.x. [DOI] [PubMed] [Google Scholar]
- 64.Podbielski A, Schnitzler N, Beyhs P, Boyle M D P. M-related protein (Mrp) contributes to group A streptococcal resistance to phagocytosis by human granulocytes. Mol Microbiol. 1996;19:429–441. doi: 10.1046/j.1365-2958.1996.377910.x. [DOI] [PubMed] [Google Scholar]
- 65.Schrager H M, Alberti S, Cywes C, Dougherty G J, Wessels M R. Hyaluronic acid capsule modulates M-protein-mediated adherence and acts as a ligand for attachment of group A streptococcus to CD44 on human keratinocytes. J Clin Investig. 1998;101:1708–1716. doi: 10.1172/JCI2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stevens D L. The flesh-eating bacterium. What's next. J Infect Dis. 1999;179(Suppl. 2):S366–S374. doi: 10.1086/513851. [DOI] [PubMed] [Google Scholar]
- 67.van de Rijn I. Streptococcal hyaluronic acid: proposed mechanisms of degradation and loss of synthesis during stationary phase. J Bacteriol. 1983;156:1059–1065. doi: 10.1128/jb.156.3.1059-1065.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van de Rijn I, Bernish B, Crater D L. Analysis of hyaluronic acid capsule expression in group A streptococci. Adv Exp Med Biol. 1997;418:965–969. doi: 10.1007/978-1-4899-1825-3_227. [DOI] [PubMed] [Google Scholar]
- 69.Wang J R, Stinson M W. M-protein mediates streptococcal adhesion to Hep-2 cells. Infect Immun. 1994;62:442–448. doi: 10.1128/iai.62.2.442-448.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wannamaker L W. Differences between streptococcal infections of the skin and pharynx. N Engl J Med. 1970;282:78–85. doi: 10.1056/NEJM197001082820206. [DOI] [PubMed] [Google Scholar]
- 71.Wessels M R, Moses A E, Goldberg J B, DiCesare T J. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc Natl Acad Sci USA. 1991;88:8317–8321. doi: 10.1073/pnas.88.19.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wessels M R, Goldberg J B, Moses A E, DiCesare T J. Effects on virulence of mutations in a locus essential for hyaluronic acid capsule expression in group A streptococci. Infect Immun. 1994;62:433–441. doi: 10.1128/iai.62.2.433-441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.World Health Organization. Laboratory diagnosis of group A streptococcal infections. Document 81–95. Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]