Abstract
Healthcare providers who use controlling or coercive strategies may compel short-term enactment of HIV and sexually transmitted infection prevention behaviors but may inadvertently undermine their client’s motivation to maintain those behaviors in the absence of external pressure. Autonomous motivation refers to the self-emanating and self-determined drive for engaging in health behaviors. It is associated with long-term maintenance of health behaviors. We used structural equation modeling to investigate whether autonomy support was associated with increased odds of therapeutic serum levels of pre-exposure prophylaxis, through a pathway that satisfies basic psychological needs for autonomous self-regulation and competence regarding pre-exposure prophylaxis use. We also investigated whether autonomy support was associated with decreased odds of condomless anal intercourse via the same psychological needs-satisfaction pathway of autonomous self-regulation and competence regarding condom use. We tested these two theorized pathways using secondary data from a longitudinal sample of Black men who have sex with men from across three cities in the US (N = 226). Data from the sample fit the theorized models regarding the pathways by which autonomy support leads to the presence of therapeutic PrEP levels in serum (χ2 = 0.56; RMSEA = 0.04; CFI = .99, TLI = 0.98) and how it also leads to decreased odds of condomless anal intercourse (χ2 = 0.58; RMSEA = 0.03; CFI = 0.99; TLI = 0.98). These findings provide scientific evidence for the utility of self-determination theory as a model to guide intervention approaches to optimize the implementation and impact of PrEP for Black men who have sex with men.
Keywords: HIV prevention , Self-determination theory, Multi-level intervention, Black MSM, Multicomponent intervention, Path analysis, Autonomy support, PrEP, Disparities, Sexually transmitted infection, HIV, HIV Prevention Trials Network, HPTN, Structural equation modeling Condom use
Introduction
Black men who have sex with men (MSM) are overrepresented in HIV incidence in the United States (US) [1–5]. The current lifetime risk of acquiring HIV among Black MSM is 50% [6] with some estimates that upwards of 60% of Black MSM could acquire HIV by the time they are 40 years old [7]. The advent of HIV pre-exposure prophylaxis (PrEP) has helped to significantly curb rates of new infections among MSM; however, those important gains have been observed almost exclusively among White MSM [1]. While the prevention effectiveness of HIV PrEP is now firmly established, it remains problematic that PrEP’s public health implementation has not had a robust prevention impact for Black MSM.
A bedrock focus of HIV prevention for Black MSM has been the targeting of individual-level sexual behavioral risk. This continues to be an important focus given that HIV transmission for Black MSM still occurs primarily through sexual contact between individuals relying on either condom use or circulating serum chemoprophylaxis or anti-retroviral (ARV) treatment-induced HIV viral load suppression to break the chain of transmission. Nonetheless, there is evidence that these prevention strategies are significantly influenced by provider behavior and communication in healthcare settings about the prevention of HIV and other sexually transmitted infections (STI). Findings from multiple studies have identified that social biases among healthcare providers, including sexual stigma, HIV stigma, anti-Black racism, and homonegativity, influence how they interpret the relevance and viability of prevention options for their patients [8–12]. At one extreme, these biases can lead providers to withhold disclosing, discussing, and facilitating access to the full range of prevention options that are available to Black MSM, including promotion of PrEP and condom use [8, 9, 13]. On the other extreme, these biases can lead providers to apply powerful pressure, such as scare tactics, shaming, or threats of punitive consequences, that coerce individuals to “agree” to adopt a target behavior [14–16]. In both instances, these healthcare provider practices undermine the individual autonomy of their patients. While it may succeed at compelling the initial adoption of a prevention behavior, it comes at the expense of the individual’s psychological well-being [17] and is less likely to result in long-term maintenance of the behavior during a person’s period of risk vulnerability [16].
Self-determination theory (SDT) is a social psychological theory of human motivation and behavior that considers the influences of social environments [18, 19]. Social environments, such as healthcare settings, are dynamic and can influence the health behaviors (e.g., medication adherence and condom use) of Black MSM. SDT also considers the constituent members of those healthcare environments (e.g., healthcare workers) to be key influencers whose own attitudes and behaviors affect the overall social climate of the healthcare setting and may directly influence the behavior of clients with whom they come into contact. According to SDT, successful behavior change is optimized in social environments that support three basic human psychological needs: autonomy, competence, and relatedness [20]. Autonomy is the need to be free to act on one’s own volition, which includes the potential for incorporating external input from key influencers in social environments [20, 21]. Competence is the need to feel that one is capable of accomplishing the steps involved in reaching a behavioral goal [20]. Lastly, relatedness is the need to feel genuine connection with people who are involved in one’s decisional process [20].
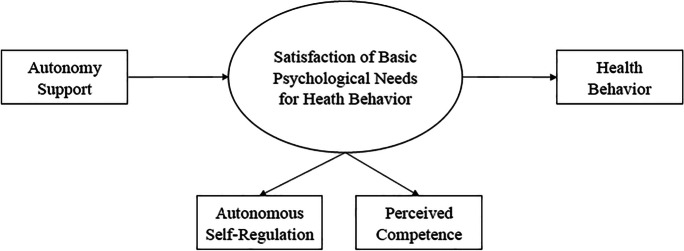
SDT has a long history of use across a wide range of health domains [22–24]. SDT specifies a causal pathway (see Fig. 1) in which individuals who receive greater autonomy support will experience greater autonomous self-regulation (i.e., autonomous motivation) and an increased sense of capability to accomplish (i.e., perceived competence) a specific behavioral goal [25–27]. Furthermore, when these two basic psychological needs are satisfied, the individual will then be more likely to achieve and maintain the behavioral goal [25–28]. A meta-analysis of 73 studies that used experimental designs to test SDT-based interventions found that they consistently produced post-intervention increases in perceived autonomy, competence, and relatedness as well small-to-medium effects in clinical outcomes [24]. Another meta-analysis of 56 SDT-based intervention studies also found they produced small, yet significant, effects on health outcomes; the interventions’ effects were mediated by autonomous self-regulation and perceived competence [23]. However, the use of SDT in HIV research is sparse by comparison [29–33], with even fewer studies applying SDT to HIV/STI prevention [34, 35]. It is important to understand the utility of SDT in predicting condom use and therapeutic (i.e., protective) levels of PrEP use in Black MSM in the US as well as the potential mechanisms by which those associations occur.
Fig. 1.
Theoretical model of hypothesized effect pathway of autonomy support on health behavior
The purpose of the study was to test whether healthcare provider autonomy support (encompassing autonomy support, competence support and relatedness support) was positively associated with HIV prevention behaviors in a sample of Black MSM and then to determine if the associations were explained by the satisfaction of their basic psychological needs for autonomous self-regulation and perceived competence. We hypothesized that autonomy support would lead to therapeutic serum levels of PrEP by satisfying the basic psychological needs for autonomous self-regulation and perceived competence regarding PrEP use. We also hypothesized a mechanistic pathway wherein autonomy support would lead to decreased condomless anal intercourse (CAI) by satisfying basic psychological needs for autonomous self-regulation and perceived competence regarding condom use.
Methods
Participants
We used data collected as part of the HIV Prevention Trials Network (HPTN) 073 study, a non-randomized open-label study examining PrEP initiation, use, and adherence among Black MSM (N = 226) recruited from Chapel Hill, NC; Los Angeles, CA; and Washington, DC [35]. Participants were followed every 3 months for 52 weeks. Inclusion criteria for HPTN 073 were as follows: (1) age 18 or older, (2) cis-gender man, (3) HIV-negative, (4) reported having sex with men, (5) self-reported behavioral or clinical indicators of high risk for potential HIV exposure in the previous six months [35], (6) willing to provide contact information, and (7) meeting clinical safety criteria for PrEP use.
Procedures
Detailed procedures for HPTN 073 are published elsewhere [35]. All study sites implemented client-centered care coordination (referred to as C4™). C4™ is an SDT-based approach to facilitating HIV/STI prevention behaviors [36]. Research site staff responsible for providing behavioral counseling and care coordination to study participants received a 3-day training on techniques to promote autonomous motivation for behavior change. Staff were also trained on the use of integrative anti-racism [37] as a lens to view and understand the myriad socio-structural challenges that may impede participants’ progress towards their behavioral goals [36, 38, 39]. PrEP use was not required for study participation. The study received Institutional Review Board approval from all participating sites. Data were collected between 2013 and 2015.
Survey Measures
Survey data were collected using audio-computer-assisted self-interview (ACASI) at baseline and at 4-, 8-, 12-, 26-, 39-, and 52-week follow-up study visits. Data were collected on SDT constructs and sexual health behaviors.
Self-Determination Theory Constructs
Autonomy support
The 15-item health care climate questionnaire (HCCQ) was used to assess the degree to which participants perceived that their basic motivational needs for autonomy were supported by their healthcare providers [25, 27, 28]. The scale demonstrated reliability and validity in previous HIV research studies [32, 34] and demonstrated excellent internal consistency reliability in the current study (α = 0.96).
Autonomous motivation
The 15-item self-regulation questionnaire (SRQ) was used to assess the relative autonomy of the reasons motivating participants’ PrEP and condom use [40]. The Treatment SRQ (TSRQ) was adapted to the specific target behavior as either PrEP use or condom use. The TSRQ has been adapted to assess autonomous motivation for a variety of health behaviors across multiple studies, including HIV treatment adherence [32] and glycemic control [41]. Autonomous motivation was assessed separately for PrEP use and condom use. The PrEP use SRQ (α = 0.95) and Condom Use SRQ (α = 0.95) demonstrated excellent internal consistency reliability in the current study.
Perceived competence
The 4-item Perceived Competence Scale (PCS) scale was used to assess the degree to which participants felt competent to accomplish the steps necessary for PrEP use and for condom use [42, 43]. Perceived competence was assessed separately for PrEP and condom use. The PCS has been adapted to assess perceived competence for a variety of health behaviors across multiple studies [32, 44]. The PCS-PrEP use (α = 0.84) and PCS-Condom Use (α = 0.80) both demonstrated good internal consistency reliability in the current study.
Sexual Health Behavior
Condomless anal intercourse
Using ACASI, participants self-reported their frequency of engagement in insertive and receptive CAI with main and casual male partners over the previous 3 months. Engagement in any CAI was dichotomized into as either ≥ 1 instance of CAI in the past 3 months or no CAI in the past 3 months [45]. We used the measure of CAI taken at the 52-week study visit.
Clinical Outcome
Therapeutic Serum Levels of PrEP
We opted to use the broader clinical variable of “therapeutic serum levels of PrEP” in lieu of the behavioral variable “adherence” because adherence is an active behavioral phenomenon that would not apply to individuals who had not yet opted to initiate PrEP. PrEP levels were assessed at 26 weeks. We define therapeutic level as meeting the 90% sensitivity threshold for ≥ 4 oral doses per week of combination emtricitabine (FTC)/tenofovir disoproxil fumarate (TDF) in either a blood plasma or peripheral blood monocyte cell (PMBC) sample [46]. This threshold was measured by concentrations of tenofovir diphosphate (TFV; a byproduct of TDF metabolism) and FTC: ≥ 4.2 ng/mL for TFV and ≥ 4.6 ng/mL for FTC in plasma; 9.9 fmol/106 for TFV diphosphate and 0.4 fmol/106 for FTC triphosphate in PBMCs [47, 48]. Levels at or above either of these thresholds were coded as therapeutic and those below the thresholds were coded as not having therapeutic serum levels of PrEP [35]. Individuals who did not initiate PrEP during the study period (n = 48) and those who initiated PrEP but not provide a blood sample (n = 17) were also coded as not having therapeutic levels of PrEP.
Data Analysis
All enrolled participants (N = 226) were included in the analysis. We used this approach because our interest was in understanding the mechanisms of autonomy support in the entire sample, which included people who engage in the antecedent behaviors (condom use, PrEP use) and those who did not. In other words, we neither excluded people who did not use condoms nor people who did not initiate PrEP, lest our analyses be biased towards those already exhibiting HIV/STI prevention-oriented behaviors. Preliminary data analyses included the examination of normality, alpha-level (α) reliabilities and descriptive statistics. We calculated descriptive statistics to convey the distribution of these constructs within the sample. Multicollinearity was also examined, to ensure that all variables were within the designated parameter ranges for variance inflation factor (< 10) and tolerance (> 0.2). Proportions were used to summarize dichotomized (yes/no) variables: CAI and therapeutic PrEP levels. We also examined correlations between study variables.
Our first step in the main analysis was to conduct a confirmatory factor analysis to test two measurement models. The model fit was assessed using the model chi square, the root mean square error of approximation (RMSEA), the comparative ft index (CFI), and Tucker-Lewis index (TLI). Once adequate fit was determined, we performed structural equation modeling (SEM) using M-plus version 8.3 [49]. We examined two separate models. In the first model, we investigated whether autonomy support was associated with therapeutic serum levels of PrEP and if association was mediated by the satisfaction of basic psychological needs regarding PrEP use. In the second model, we investigated if the satisfaction of basic psychological needs regarding condom use mediated an association between autonomy support and CAI. The mean-and-variance adjusted weighted least squares (WLMV) estimator was used, as this is the preferred when the dependent variables are categorical and when data are not normally distributed (Kline, 2015). Standardized beta coefficients and p values were included.
Results
Selected demographic characteristics of the sample are summarized in Table 1. African Americans were the ethnic group that composed the largest proportion (86%) of the sample. The remaining 14% were distributed across African, Afro‐Caribbean, and Afro‐Latino ethnicities. The mean age was 26 years (SD = 8.6) with 40% of the sample younger than 25 years old. Participants reported an average of 4.4 (SD = 8.8) sex partners in the 6 months prior to enrollment. One‐quarter (25%) had a high school diploma or less and 48% reported an annual income of less than $20,000. Detailed descriptions of participant characteristics are included in previous reports [35, 45]. A summary of SDT constructs is presented in Table 2. The mean scales scores were higher than the mid-point (3.5) for all SDT constructs. The correlation matrix (Table 3) shows statistically significant associations among all SDT constructs.
Table 1.
Demographics
| Demographics of participant enrolled in the study (N = 226) | Frequency (%) |
|---|---|
| Age | |
| < 25 | 91 (40%) |
| ≥ 25 | 135 (60%) |
| Ethnicity | |
| Black non-Hispanic (e.g., African American, Afro-Caribbean) | 204 (90%) |
| Black Hispanic | 17 (8%) |
| Other | 5 (2%) |
| Education | |
| High school or less | 56 (25%) |
| Some college or vocation school | 93 (41%) |
| Two-year college or greater | 77 (34%) |
| Annual income | |
| < $20,000 | 108 (48%) |
| $20,000 to $40,000 | 55 (25%) |
| ≥ $40,000 | 60 (27%) |
| No response | 3 (1%) |
| Health Insurance (current) | 155 (69%) |
| CAI with HIV + or unknown causal male partner | |
| No | 127 (57%) |
| Yes | 98 (43%) |
| STI prevalence at baseline | |
| No | 194 (86%) |
| Yes | 32 (14%) |
CAI, condomless anal intercourse; HIV, human immunodeficiency virus; STI, sexually transmitted infection
Table 2.
Variable descriptive statistics and factors loadings in confirmatory factor analysis
| Variable | Range | %Yes | M (SD) | Factor loading |
|---|---|---|---|---|
| Therapeutic serum levels of PrEP | 0–1 | 21 | NA | NA |
| Condomless anal intercourse | 0–1 | 55 | NA | NA |
| Autonomy support | NA | 5.38 (0.93) | NA | |
| Basic psychological needs satisfaction—PrEP Use (latent) | ||||
| PrEP use autonomous self-regulation | 5.69 (1.27) | |||
| Because I feel that I want to take responsibility for my own health | 1–7 | 5.60 (1.77) | 0.69*** | |
| Because I personally believe it is the best thing for my health | 1–7 | 5.56 (1.74) | 0.69** | |
| Because I have carefully thought about it and believe it is very important for many aspects of my life | 1–7 | 5.58 (1.72) | 0.55* | |
| Because it is an important choice I really want to make | 1–7 | 5.33(1.86) | 0.73*** | |
| Because it is consistent with my life goals | 1–7 | 6.26(1.35) | 0.78*** | |
| Because it is very important for being as healthy as possible | 1–7 | 5.79(1.68) | 0.79*** | |
| Perceived competence for PrEP use | 5.78 (1.50) | |||
| I feel confident in my ability to use PrEP daily, as recommended | 1–7 | 5.74 (1.71) | 0.79*** | |
| I am capable now of handling using PrEP daily | 1–7 | 5.84 (1.68) | 0.90*** | |
| I am able to do what it takes to ensure that I use PrEP every day | 1–7 | 5.79 (1.65) | 0.94*** | |
| I feel able to meet the challenge of using PrEP every day | 1–7 | 5.76 (0.167) | 0.85*** | |
| Basic psychological needs satisfaction—Condom Use (latent) | ||||
| Condom use autonomous self-regulation | 5.79 (1.25) | |||
| Because I would feel guilty or ashamed of myself if I did not | 1–7 | 5.58 (1.72) | 0.74*** | |
| Because I personally believe it is the best thing for my health | 1–7 | 5.95 (1.58) | 0.75*** | |
| Because I have carefully thought about it and believe it is very important for many aspects of my life | 1–7 | 5.87 (1.64) | 0.45** | |
| Because I would feel bad about myself if I did not | 1–7 | 5.74 (1.66) | 0.77*** | |
| Because it is consistent with my life goals | 1–7 | 5.65 (1.75) | 0.75** | |
| Because it is very important for being as healthy as possible | 1–7 | 6.01 (1.52) | 0.69** | |
| Perceived competence for condom use | 5.50 (1.50) | |||
| I feel confident in my ability to use condoms every time I have sex | 1–7 | 5.44 (1.78) | 0.87*** | |
| I am capable now of using condoms every time I have sex | 1–7 | 5.58 (1.75) | 0.90*** | |
| I am able to do what it takes to ensure that I use condoms whenever I have sex | 1–7 | 5.55 (1.76) | 0.87*** | |
| I feel able to meet the challenge of using condoms every time I have sex | 1–7 | 5.76 (1.67) | 0.78*** | |
The values for each SDT construct reflect the mean across all study visits starting with baseline for autonomous motivation variables and at week 4 for the remaining variables. PrEP, pre-exposure prophylaxis; SDT, self-determination theory
Table 3.
Correlation between study variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. Autonomy support | –- | 0.50** | 0.49** | 0.53** | 0.48** | 0.10 | 0.03 |
| 2. PrEP use autonomous self-regulation | –- | 0.52** | 0.75** | 0.44** | 0.01 | − 0.01 | |
| 3. Perceived competence for PrEP | –- | 0.48** | 0.34** | − 0.03 | − 0.02 | ||
| 4. Condom use autonomous self-regulation | –- | 0.55** | 0.001 | − 0.05 | |||
| 5. Perceived competence for condom use | –- | 0.03 | 0.01 | ||||
| 6. Condomless anal intercourse | –- | − 0.05 | |||||
| 7. Therapeutic serum levels of PrEP | –- |
Measurement Models
For the mediators, latent factors were formed using items from the scales that measured those individual constructs. The latent factor basic psychological needs satisfaction for PrEP (BNS PrEP Use) was formed using the PrEP Use Self-Regulation Questionnaire and the Perceived Competence Scale for PrEP use. The latent factor basic psychological needs satisfaction for condom use (BNS Condom Use) was formed using the Condom Use Self-Regulation Questionnaire and the Perceived Competence Scale for Condom Use. Factor loadings on the latent construct for BNS PrEP Use ranged from 0.55 to 0.94. The model for BNS PrEP Use provided a good model fit χ2(52) = 93.87, p = 0.05, root mean square error of approximation = 0.04, CFI: 0.98, TLI:0.98. Factor loadings on the BNS Condom Use ranged from 0.45 to 0.90. The measurement model for BNS Condom Use also provided a good model fit, χ2(33) = 127.68, p = 0.05, root mean square error of approximation = 0.05, CFI: 0.98, TLI: 0.97.
Structural Equation model
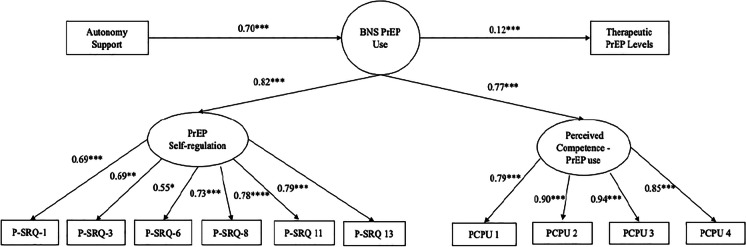
The SEM data on the therapeutic PrEP level outcome is displayed in Fig. 2 and includes standardized betas and p-values. The model examined the direct and indirect associations between autonomy support, BNS PrEP Use, and therapeutic serum level of PrEP. The hypothesized model demonstrated a good fit with the data as shown by the indices in Table 4. The variable BNS PrEP Use explained 52% of the variance in therapeutic serum levels of PrEP. There was a significant direct path from autonomy support to BNS PrEP Use (β = 0.69, p < 0.001). There was a significant direct path from BNS PrEP Use to therapeutic serum levels of PrEP (β = 0.12, p < 0.001). Autonomy support was also indirectly associated with therapeutic serum levels of PrEP (β = 0.03, p = 0.03).
Fig. 2.
Structural equation model: effect of autonomy support and basic psychological needs satisfaction for PrEP on therapeutic PrEP levels (N = 226). Note: BNS PrEP Use—basic psychological needs satisfaction for PrEP use. PrEP use autonomous self-regulation was measured at every study visit, including the baseline visit. Autonomy support and perceived competence for PrEP use were measured at every study visit starting at week 4. Standardized betas are reported.
Table 4.
Goodness of fit indices for autonomy support to therapeutic PrEP levels and condomless anal intercourse
| Discrepancy | ||||||
|---|---|---|---|---|---|---|
| Model | χ2 | df | p | RMSEA | CFI | TLI |
| 1. Autonomy support to therapeutic serum levels of PrEP | 93 | 52 | 0.56 | 0.04 | 0.99 | 0.98 |
| 2. Autonomy support to condomless anal intercourse | 79 | 33 | 0.58 | 0.03 | 0.99 | 0.98 |
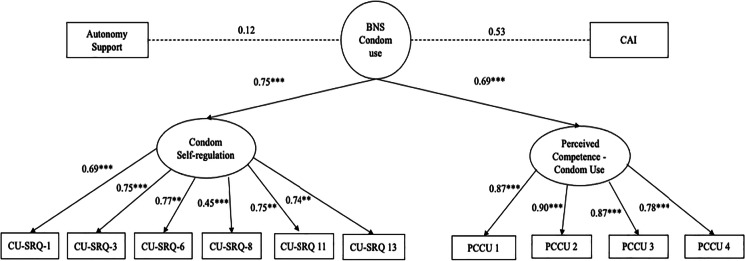
The SEM results on the CAI outcomes are displayed in Fig. 3, including standardized betas and p-values. The model examined the direct and indirect associations between autonomy support, BNS Condom Use, and CAI. The hypothesized model also demonstrated a good fit the study data (Table 4). BNS Condom Use explained 61% of the variance in CAI. In this model, there was not a direct path from autonomy support to BNS Condom Use. There was also not a direct path from BNS Condom Use to CAI. Autonomy support was indirectly associated with CAI (β = 0.05, p = 0.01).
Fig. 3.
Structural equation model: effect of autonomy support and basic psychological needs satisfaction for condom use on CAI (N = 226). Note: BNS Condom Use—basic psychological needs satisfaction needs for condom use. CAI—condomless anal intercourse. Condom use autonomous self-regulation was measured at every study visit, including the baseline visit. Autonomy support and perceived competence for condom use were measured at every study visit starting with week 4. CAI was measured at week 52. Standardized betas are reported.
Discussion
The purpose of this study was to use structural equation modeling to test our hypotheses that autonomy support was associated with HIV prevention behavior indicators among Black MSM and that these associations were explained by the satisfaction of their basic psychological needs for autonomous self-regulation and perceived competence. Both theorized models were good fits with the data in this study. The first pathway explained 52% of the variance in therapeutic serum levels of PrEP at 26 weeks post-enrollment in HPTN 073. The second pathway explained 61% of the variance in CAI at 52 weeks. Based on our review of the literature, this is among the first reports to examine the associations between autonomy support, basic psychological needs satisfaction, and condom use [34] and is the first known report documenting the associations of autonomy support, basic psychological needs satisfaction, and therapeutic serums levels of PrEP. This is also the only known study, to date, that empirically tested the utility of SDT for the design of intervention strategies to optimize the prevention impact of clinical HIV/STI prevention tools such as condom use and PrEP. The effect of autonomy support has been examined for a wide variety of health outcomes across a variety of populations [21, 22, 24, 50, 51]. Our findings are also supported by a recent random effects meta-analysis of 135 effect sizes from 67 studies [52]. The results indicated interventions that increased autonomous motivation had medium effect on health behavior change (d+ = 0.47; 95%CI = 0.44, 0.83) and that increasing perceived competence had small-to-moderate effect on behavior change (d+ = 0.34; 95% CI = 0.22, 0.47) [52]. Moreover, health behavior changes effects were very small in interventions that did not have significant increases in autonomous motivation or perceived competence [52]. The results reported here indicate that autonomy support also affects sexual health outcomes among Black MSM, which is a critically significant discovery given the underrepresentation of PrEP usage in Black MSM, their overrepresentation in HIV incidence in the US, and the urgent need to identify viable implementation strategies for increasing PrEP coverage in this high priority population.
The first model we tested theorized that healthcare provider autonomy support was associated with increased odds of therapeutic serum levels of PrEP through a pathway of basic psychological needs satisfaction for PrEP use. To our knowledge, this is the first published study to examine this mediating pathway on serum levels of HIV anti-retroviral medication for PrEP (clinical outcome). In a previous study, researchers examined this pathway in sample of people living with HIV. In that study, autonomous motivation and perceived competence mediated the association between healthcare provider autonomy support and adherence to ARV treatment [32]. Although the model demonstrated that autonomy support was associated with increased ARV adherence for treatment, it was not conducted with individuals using ARVs for PrEP given the study population of focus. It also did not examine the impact of the autonomy support on HIV viral suppression, which the current state of the science indicates is a key health status indicator that contributes to HIV prevention efforts. This existing gap identifies an opportunity for additional research to understand the effect of healthcare provider autonomy support on HIV viral suppression, especially as HIV-status neutral interventions and research designs are embraced by prevention scientists. [1]
The second model tested theorized that autonomy support was associated with decreased odds of CAI through a pathway of basic psychological needs satisfaction for condom use. These results are consistent with previous studies that investigated associations between healthcare provider autonomy support and sexual behavior outcomes. For example, healthcare provider autonomy support was associated with condom use for anal (AOR = 3.29, p < 0.01) and vaginal (AOR = 1.8, p < 0.01) sex among 137 MSM sampled from across three cities in Ghana [34]. These important findings did not include a test of the mediating pathway between autonomy support and condom use. Our results build on these findings by explicating a theoretically derived pathway through which this association occurs. In this model, the path from BNS Condom Use and CAI was not significant. This may be due to generally low occurrences of CAI in the study sample, especially among people who declined to use PrEP [45] such that there may not have been sufficient variation to detect an association between perceived competence and CAI. It is also important to note that the parent study [35] from which this secondary analysis is drawn involved the implementation of the C4™ intervention [36] to all study participants, regardless of whether they initiated or persisted in PrEP usage. It is plausible that the C4™ intervention may have suppressed rates of CAI across the sample, which may not have occurred in a purely observational study. We are unable test this explanation because the parent study did not include a control group.
There are several important limitations of this study. First, the study used a non-probability sample and findings cannot be generalized to all Black MSM in the US. Second, the non-significant paths in the structural model were retained in favor of demonstrating our overall test of the SDT theory pathways, both of which fit the study data. This also prevented biasing the impact of the other variables on therapeutic serum levels of PrEP and on CAI. Although this analysis is based on longitudinal data that supports the validity of claims regarding the predictive utility of the SDT, the study was also conducted in the context of an intervention. Given that HPTN 073 was a demonstration project that did not include a comparison group, it is not possible for us to control for any potential intervention effects on the observed pathways. Nevertheless, the available evidence is compelling that the C4™ model can be a useful tool for jurisdictions in their efforts to scale up PrEP use and optimize HIV prevention. Broader scale implementation of C4™ can help to accelerate progress towards the goals of Ending the HIV Epidemic (EHE) in the US by 2020 by bringing the intervention to scale, especially in jurisdictions that have been identified as high-priority communities in the federal EHE plan—while still generating additional evidence to advance scientific understanding of its impact on HIV prevention efforts. Last, this study was conducted in 2013–2017; however, these findings remain relevant given the continued low rates of PrEP use among Black MSM and the need for culturally responsive interventions to help ensure that current and newer biomedical discoveries, such as long-acting injectable antivirals for PrEP [53], will have equitable implementation impact on the epidemic in Black MSM. These limitations notwithstanding, the results of this study provide important evidence regarding the mechanism of autonomy support’s influence on sexual health behaviors and clinical outcomes among Black MSM.
In conclusion, SDT is a useful theory for the development of HIV/STI prevention interventions. Its social psychological orientation makes it a good fit for interventions with Black MSM because SDT accounts for the influence of actions of the healthcare team on the motivational and behavioral antecedents of HIV and STIs among Black MSM. Future research using SDT for HIV/STI prevention can be applied to interventions approaches with other key priority populations in the US epidemic, including Black women [54]—inclusive of cisgender and transgender women. Given the relationship between behavior change and autonomous motivation, motivational interviewing (MI) may be an initial approach to developing SDT-guided interventions. MI is an evidence-based counseling intervention that implores individuals to act on their motivations [55, 56]. It is well-established that peer- and provider-led MI programs have have reduced the risks of HIV exposure among HIV-vulnerable populations [55–57]. However, MI does not account for the other psychological factors influencing health behaviors, implicating the need to apply SDT principles to MI therapeutics [58–60]. MI interventions aligned with SDT principles have resulted in larger effects on behavioral and clinical outcomes [58–60]. Thus, SDT provides a theoretical framework that clinicians and counselors can use to inform their MI techniques and practices [60]. SDT can also be applied to novel structural approaches to HIV prevention such as HIV testing in emergency departments [61, 62] and HIV self-testing [63–68] combined with community-based initiation of PrEP or HIV treatment. SDT has a long history of application in health domains and its utility for HIV/STI prevention can spur new intervention approaches to help accelerate progress towards ending the HIV epidemic in the US.
Acknowledgements
Overall support for the HIV Prevention Trials Network (HPTN) is provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068619 (HPTN Leadership and Operations Center), UM1AI068617 (HPTN Statistical and Data Management Center), and UM1AI068613 (HPTN Laboratory Center). Additional support was provided by the National Institute on Drug Abuse and the National Institute of Mental Health, of the National Institutes of Health, US Department of Health and Human Services. This was also made possible by core services and support provided by the Yale University Center for Interdisciplinary Research on AIDS (P30 MH062294) and the Yale Research Education Institute for Diverse Scholars (R25 MH087271).
Declarations
Disclaimer
The first author was neither involved in the recruitment of study participants nor in obtaining their informed consent. C4TM is a trademark of tuliptree systems, LLC. The first author is a shareholder and officer of tuliptree systems, LLC.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mayer KH, Nelson LE, Hightow-Weidman L, et al. The persistent and evolving HIV epidemic in American men who have sex with men. Lancet. 2021;397(10279):1116–1126. doi: 10.1016/S0140-6736(21)00321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millett GA, Peterson JL, Flores SA, et al. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, and USA: a meta-analysis. Lancet. 2012;380(9839):341–348. doi: 10.1016/S0140-6736(12)60899-X. [DOI] [PubMed] [Google Scholar]
- 3.CDC. Diagnoses of HIV infection in the United States and dependent areas, 2020. HIV Surveillance Report 2020. 2022;33. Available online at https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html
- 4.Mayer KH, Wang L, Koblin B, et al. Concomitant socioeconomic, behavioral, and biological factors associated with the disproportionate HIV infection burden among black men who have sex with men in 6 U.S. cities. PLoS ONE. 2014;9(1):e87298. doi: 10.1371/journal.pone.0087298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan PS, Rosenberg ES, Sanchez TH, et al. Explaining racial disparities in HIV incidence in black and white men who have sex with men in Atlanta, GA: a prospective observational cohort study. Ann Epidemiol. 2015;25(6):445–454. doi: 10.1016/j.annepidem.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hess KL, Hu X, Lansky A, Mermin J, Hall HI. Lifetime risk of a diagnosis of HIV infection in the United States. Ann Epidemiol. 2017;27(4):238–243. doi: 10.1016/j.annepidem.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthews DD, Herrick AL, Coulter RW, et al. Running backwards: consequences of current HIV incidence rates for the next generation of black MSM in the United States. AIDS Behav. 2016;20(1):7–16. doi: 10.1007/s10461-015-1158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calabrese SK, Earnshaw VA, Krakower DS, et al. A closer look at racism and heterosexism in medical students’ clinical decision-making related to HIV pre-exposure prophylaxis (PrEP): implications for PrEP education. AIDS Behav. 2018;22(4):1122–1138. doi: 10.1007/s10461-017-1979-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calabrese SK, Earnshaw VA, Underhill K, et al. Prevention paradox: medical students are less inclined to prescribe HIV pre-exposure prophylaxis for patients in highest need. J Int AIDS Soc. 2018;21(6):e25147. doi: 10.1002/jia2.25147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Ryn M, Burgess DJ, Dovidio JF, et al. The impact of racism on clinician cognition, behavior, and clinical decision making. Du Bois Rev. 2011;8(1):199–218. doi: 10.1017/S1742058X11000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks RA, Nieto O, Landrian A, Fehrenbacher A, Cabral A. Experiences of pre-exposure prophylaxis (PrEP)-related stigma among black MSM PrEP users in Los Angeles. J Urban Health: Bull N Y Acad Med. 2020;97(5):679–691. doi: 10.1007/s11524-019-00371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quinn K, Bowleg L, Dickson-Gomez J. “The fear of being Black plus the fear of being gay”: the effects of intersectional stigma on PrEP use among young Black gay, bisexual, and other men who have sex with men. Soc Sci Med. 2019;232:86–93. doi: 10.1016/j.socscimed.2019.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinn K, Dickson-Gomez J, Zarwell M, Pearson B, Lewis M. “A gay man and a doctor are just like, a recipe for destruction”: how racism and homonegativity in healthcare settings influence PrEP uptake among young Black MSM. AIDS Behav. 2019;23(7):1951–1963. doi: 10.1007/s10461-018-2375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elder JP, Ayala GX, Harris S. Theories and intervention approaches to health-behavior change in primary care. Am J Prev Med. 1999;17(4):275–284. doi: 10.1016/S0749-3797(99)00094-X. [DOI] [PubMed] [Google Scholar]
- 15.Werth R. The construction and stewardship of responsible yet precarious subjects: punitive ideology, rehabolitation and ‘tough love’ among parole personnel. Punishment Soc. 2013;15(3):219–246. doi: 10.1177/1462474513481720. [DOI] [Google Scholar]
- 16.Denning P. Harm reduction therapy with families and friends of people with drug problems. J Clin Psychol. 2010;66(2):164–174. doi: 10.1002/jclp.20671. [DOI] [PubMed] [Google Scholar]
- 17.Sheldon KM. The self-determination theory perspective on positive mental health across cultures. World Psychiatr: Off J World Psychiatr Assoc. 2012;11(2):101–102. doi: 10.1016/j.wpsyc.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self-determination of behavior. Psychol Inq. 2000;11(4):227–268. doi: 10.1207/S15327965PLI1104_01. [DOI] [Google Scholar]
- 19.Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. 2000;55(1):68–78. doi: 10.1037/0003-066X.55.1.68. [DOI] [PubMed] [Google Scholar]
- 20.Ryan RM, Patrick H, Deci EL, Williams GC. Facilitating health behaviour change and its maintenance: interventions based on self-determination theory. Eur Health Psychol. 2008;10(1):2–5. [Google Scholar]
- 21.Chirkov V, Ryan RM, Kim Y, Kaplan U. Differentiating autonomy from individualism and independence: a self-determination theory perspective on internalization of cultural orientations and well-being. J Pers Soc Psychol. 2003;84(1):97–110. doi: 10.1037/0022-3514.84.1.97. [DOI] [PubMed] [Google Scholar]
- 22.Ng JYY, Ntoumanis N, Thogersen-Ntoumani C, et al. Self-determination theory applied to health contexts: a meta-analysis. Perspect Psychol Sci. 2012;7(4):325–340. doi: 10.1177/1745691612447309. [DOI] [PubMed] [Google Scholar]
- 23.Sheeran P, Wright CE, Avishai A, et al. Self-determination theory interventions for health behavior change: meta-analysis and meta-analytic structural equation modeling of randomized controlled trials. J Consult Clin Psychol. 2020;88(8):726–737. doi: 10.1037/ccp0000501. [DOI] [PubMed] [Google Scholar]
- 24.Ntoumanis N, Ng JYY, Prestwich A, et al. A meta-analysis of self-determination theory-informed intervention studies in the health domain: effects on motivation, health behavior, physical, and psychological health. Health Psychol Rev. 2021;15(2):214–244. doi: 10.1080/17437199.2020.1718529. [DOI] [PubMed] [Google Scholar]
- 25.Williams GC, Rodin GC, Ryan RM, Grolnick WS, Deci EL. Autonomous regulation and long-term medication adherence in adult outpatients. Health Psychol. 1998;17(3):269–276. doi: 10.1037/0278-6133.17.3.269. [DOI] [PubMed] [Google Scholar]
- 26.Williams GC, Niemiec CP, Patrick H, Ryan RM, Deci EL. The importance of supporting autonomy and perceived competence in facilitating long-term tobacco abstinence. Ann Behav Med: Publ Soc Behav Med. 2009;37(3):315–324. doi: 10.1007/s12160-009-9090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams GC, McGregor HA, Sharp DS, et al. A self-determination multiple risk intervention trials to improve smokers health. J Gen Intern Med. 2006;21:1288–1294. doi: 10.1111/j.1525-1497.2006.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams GC, Cox EM, Kouides R, Deci EL. Presenting the facts about smoking to adolescents: effects of an autonomy-supportive style. Arch Pediatr Adolesc Med. 1999;153(9):959–964. doi: 10.1001/archpedi.153.9.959. [DOI] [PubMed] [Google Scholar]
- 29.Hogan A, Catley D, Goggin K, Evangeli M. Coding client language in motivational interviewing for HIV medication adherence using self-determination theory. Int J Behav Med. 2019;26(2):230–235. doi: 10.1007/s12529-018-09766-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toth M, Messer LC, Quinlivan EB. Barriers to HIV care for women of color living in the Southeastern US are associated with physical symptoms, social environment, and self-determination. AIDS Patient Care STDS. 2013;27(11):613–620. doi: 10.1089/apc.2013.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quinlivan EB, Messer LC, Adimora AA, et al. Experiences with HIV testing, entry, and engagement in care by HIV-infected women of color, and the need for autonomy, competency, and relatedness. AIDS Patient Care STDS. 2013;27(7):408–415. doi: 10.1089/apc.2012.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennedy S, Goggin K, Nollen N. Adherence to HIV medications: utility of the theory of self-determination. Cogn Ther Res. 2004;28:611–628. doi: 10.1023/B:COTR.0000045568.95219.e2. [DOI] [Google Scholar]
- 33.Igreja I, Zuroff DC, Koestner R, Saltaris C, Brouillette M, Lalonde R. Applying self-determination theory to the prediction of distress and well-being in gay men with HIV and AIDS. J Appl Soc Psychol. 2000;30(4):686–706. doi: 10.1111/j.1559-1816.2000.tb02819.x. [DOI] [Google Scholar]
- 34.Nelson LE, Wilton L, Agyarko-Poku T, et al. Predictors of condom use among peer social networks of men who have sex with men in Ghana, West Africa. PLoS One. 2015;10(1):e0115504. doi: 10.1371/journal.pone.0115504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wheeler DP, Fields SD, Beauchamp G, et al. Pre-exposure prophylaxis initiation and adherence among Black men who have sex with men (MSM) in three US cities: results from the HPTN 073 study. J Int AIDS Soc. 2019;22(2):e25223. doi: 10.1002/jia2.25223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson LE, Wilton L, Williams GC et al. Client-centered care coordination for HIV/STI prevention: a theoretical, conceptual and methodological overview—HIV Prevention Trials Network (HPTN) 073. Sex Res Soc Policy. 2022. Published online. 10.1007/s13178-022-00687-x
- 37.Sefa-Dei GJ. Integrative anti-racism: intersection of race, class and gender. Race Gend Class. 1995;2(3):11–30. [Google Scholar]
- 38.Sefa-Dei GJ. The denial of difference: reframing anti-racist praxis. Race Ehthn Educ. 1999;2:17–38. doi: 10.1080/1361332990020103. [DOI] [Google Scholar]
- 39.Nelson LE, Walker JJ, DuBois SN, Giwa S. Your blues ain't like mine: considering integrative antiracism in HIV prevention research with black men who have sex with men in Canada and the United States. Nurs Inq. 2014;21(4):270–282. doi: 10.1111/nin.12055. [DOI] [PubMed] [Google Scholar]
- 40.Levesque CS, Williams GC, Elliot D, Pickering MA, Bodenhamer B, Finley PJ. Validating the theoretical structure of the Treatment Self-Regulation Questionnaire (TSRQ) across three different health behaviors. Health Educ Res. 2007;22(5):691–702. doi: 10.1093/her/cyl148. [DOI] [PubMed] [Google Scholar]
- 41.Williams GC, McGregor HA, Zeldman A, Freedman ZR, Deci EL. Testing a self-determination theory process model for promoting glycemic control through diabetes self-management. Health Psychol. 2004;23(1):58–66. doi: 10.1037/0278-6133.23.1.58. [DOI] [PubMed] [Google Scholar]
- 42.Williams GC, McGregor HA, King D, Nelson CC, Glasgow RE. Variation in perceived competence, glycemic control and patient satisfaction: relationsip to autonomy support from physicians. Patient Educ Couns. 2005;57:39–45. doi: 10.1016/j.pec.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Williams GC, Deci EL. Internalization of biopsychosocial values by medical students: a test of self-determination theory. J Pers Soc Psychol. 1996;70(4):767–779. doi: 10.1037/0022-3514.70.4.767. [DOI] [PubMed] [Google Scholar]
- 44.Williams GC, Patrick H, Niemiec CP, et al. Reducing the health risks of diabetes: how self-determination theory may help improve medication adherence and quality of life. Diabetes Educ. 2009;35(3):484–492. doi: 10.1177/0145721709333856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hightow-Weidman LB, Magnus M, Beauchamp G, et al. Incidence and correlates of sexually transmitted infections among Black men who have sex with men participating in the HIV Prevention Trials Network 073 Preexposure Prophylaxis Study. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2019;69(9):1597–1604. doi: 10.1093/cid/ciy1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Clarke W, Marzinke MA, et al. Evaluation of a multidrug assay for monitoring adherence to a regimen for HIV preexposure prophylaxis in a clinical study, HIV Prevention Trials Network 073. Antimicrob Agents Chemother. 2017;61(7):e0243–16. [DOI] [PMC free article] [PubMed]
- 48.Hendrix CW, Andrade A, Bumpus NN, et al. Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066) AIDS Res Hum Retroviruses. 2016;32(1):32–43. doi: 10.1089/aid.2015.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muthén LK, Muthén BO. Statistical analysis with latent variables: user’s guide. 7th ed. Los Angeles, CA: Muthén & Muthén; 2010.
- 50.Chirkov VI, Ryan RM, Willness C. Cultural context and psychological needs in Canada and Brazil: testing a self-determination approach to the internalization of cultural practices, identity, and well-being. J Cross Cult Psychol. 2005;36:423–443. doi: 10.1177/0022022105275960. [DOI] [Google Scholar]
- 51.Gillison FB, Rouse P, Standage M, Sebire SJ, Ryan RM. A meta-analysis of techniques to promote motivation for health behaviour change from a self-determination theory perspective. Health Psychol Rev. 2019;13(1):110–130. doi: 10.1080/17437199.2018.1534071. [DOI] [PubMed] [Google Scholar]
- 52.Sheeran P, Wright CE, Avishai A, Villegas ME, Rothman AJ, Klein WMP. Does increasing autonomous motivation or perceived competence lead to health behavior change? A meta-analysis. Health Psychol. 2021;40(10):706–716. doi: 10.1037/hea0001111. [DOI] [PubMed] [Google Scholar]
- 53.Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med. 2021;385(7):595–608. doi: 10.1056/NEJMoa2101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heads AM, Hill MJ, Suchting R, Yammine L, Gilmore-Thomas A. Predictors of anticipated PrEP stigma among women with self-reported problematic substance use: implications for engaging women in the PrEP care continuum. Arch Sex Behav. 2021;50:2955–2964. doi: 10.1007/s10508-021-02031-7. [DOI] [PubMed] [Google Scholar]
- 55.Berg RC, Ross MW, Tikkanen R. The effectiveness of MI4MSM: how useful is motivational interviewing as an HIV risk prevention program for men who have sex with men? A systematic review. AIDS Educ Prevent: Off Publ Int Soc AIDS Educ. 2011;23(6):533–549. doi: 10.1521/aeap.2011.23.6.533. [DOI] [PubMed] [Google Scholar]
- 56.Outlaw AY, Naar-King S, Parsons JT, Green-Jones M, Janisse H, Secord E. Using motivational interviewing in HIV field outreach with young African American men who have sex with men: a randomized clinical trial. Am J Public Health. 2010;100(Suppl 1):S146–151. doi: 10.2105/AJPH.2009.166991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allicock M, Golin CE, Kaye L, Grodensky C, Blackman LT, Thibodeaux H. SafeTalk: training peers to deliver a motivational interviewing HIV prevention program. Health Promot Pract. 2017;18(3):410–417. doi: 10.1177/1524839916663486. [DOI] [PubMed] [Google Scholar]
- 58.Britton PC, Williams GC, Conner KR. Self-determination theory, motivational interviewing, and the treatment of clients with acute suicidal ideation. J Clin Psychol. 2008;64(1):52–66. doi: 10.1002/jclp.20430. [DOI] [PubMed] [Google Scholar]
- 59.Miller LS, Gramzow RH. A self-determination theory and motivational interviewing intervention to decrease racial/ethnic disparities in physical activity: rationale and design. BMC Public Health. 2016;16(1):768. doi: 10.1186/s12889-016-3413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patrick H, Williams GC. Self-determination theory: its application to health behavior and complementarity with motivational interviewing. Int J Behav Nutr Phys Act. 2012;9:18. doi: 10.1186/1479-5868-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hill MJ, Prater S, Bonnette A, Tinder A, McNeese M. An assessment of emergency nurses’ perspectives on nurse-driven human immunodeficiency virus testing in the emergency department. J Emerg Nurs. 2020;46(6):869–883. doi: 10.1016/j.jen.2020.05.020. [DOI] [PubMed] [Google Scholar]
- 62.Hill MJ, Cardenas-Turanzas M, Prater S, Campbell JW, McNeese M. Racial and sex disparities in HIV screening outcomes within emergency departments of Harris County, Texas. J Am Coll Emerg Physicians Open. 2020;1(4):476–483. doi: 10.1002/emp2.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mathews A, Farley S, Conserve DF, et al. “Meet people where they are”: a qualitative study of community barriers and facilitators to HIV testing and HIV self-testing among African Americans in urban and rural areas in North Carolina. BMC Public Health. 2020;20(1):494. doi: 10.1186/s12889-020-08582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mathews A, Conserve D, Mason H, Alston L, Rennie S, Tucker J. ‘Informed and empowered’: a mixed-methods study of crowdsourcing contests to promote uptake of HIV self-testing kits among African Americans. J Virus Erad. 2020;6(2):74–80. doi: 10.1016/S2055-6640(20)30020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lentz C, Iribarren S, Giguere R, et al. Broaching the topic of HIV self-testing with potential sexual partners among men and transgender women who have sex with men in New York and Puerto Rico. AIDS Behav. 2020;24(11):3033–3043. doi: 10.1007/s10461-020-02851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Conserve DF, Muessig KE, Maboko LL, et al. Mate Yako Afya Yako: formative research to develop the Tanzania HIV self-testing education and promotion (Tanzania STEP) project for men. PLoS One. 2018;13(8):e0202521. doi: 10.1371/journal.pone.0202521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conserve DF, Alemu D, Yamanis T, Maman S, Kajula L. “He told me to check my health”: a qualitative exploration of social network influence on men’s HIV testing behavior and HIV self-testing willingness in Tanzania. Am J Mens Health. 2018;12(5):1185–1196. doi: 10.1177/1557988318777674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abubakari GM, Turner D, Ni Z, et al. Community-based interventions as opportunities to increase HIV self-testing and linkage to care among men who have sex with men - lessons from Ghana, West Africa. Front Public Health. 2021;9:660256. doi: 10.3389/fpubh.2021.660256. [DOI] [PMC free article] [PubMed] [Google Scholar]