Abstract
Background
Physical activity (PA) is associated with improved health-related quality of life (HRQoL) among women with breast cancer; however, uncertainty remains regarding PA types and dose (frequency, duration, intensity) and various HRQoL measures. A systematic review and meta-analysis of randomized controlled trials was conducted to clarify whether specific types and doses of physical activity was related to global and specific domains of HRQoL, as part of the Global Cancer Update Programme, formerly known as the World Cancer Research Fund–American Institute for Cancer Research Continuous Update Project.
Methods
PubMed and CENTRAL databases were searched up to August 31, 2019. Weighted mean differences (WMDs) in HRQoL scores were estimated using random effects models. An independent expert panel graded the evidence.
Results
A total of 79 randomized controlled trials (14 554 breast cancer patients) were included. PA interventions resulted in higher global HRQoL as measured by the Functional Assessment of Cancer Therapy–Breast (WMD = 5.94, 95% confidence intervals [CI] = 2.64 to 9.24; I2 = 59%, n = 12), Functional Assessment of Cancer Therapy–General (WMD = 4.53, 95% CI = 1.94 to 7.13; I2 = 72%, n = 18), and European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–C30 (WMD = 6.78, 95% CI = 2.61 to 10.95; I2 = 76.3%, n = 17). The likelihood of causality was considered probable that PA improves HRQoL in breast cancer survivors. Effects were weaker for physical function and mental and emotional health. Evidence regarding dose and type of PA remains insufficient for firm conclusions.
Conclusion
PA results in improved global HRQoL in breast cancer survivors with weaker effects observed for physical function and mental and emotional health. Additional research is needed to define the impact of types and doses of activity on various domains of HRQoL.
Since 1949, the World Health Organization has noted that health is “a state of complete physical, mental, and social well-being and not merely the absence of disease” (1). In ensuing years, health-related quality of life (HRQoL) emerged as a relevant outcome, and among populations with cancer, it is a powerful predictor of mortality and morbidity (2,3). Thus, integrating the assessment of HRQoL into cancer clinical trials is critical and may provide greater insight into relevant outcomes beyond tumor response and survival (4).
Lifestyle factors, such as physical activity, are hypothesized to influence HRQoL in breast cancer patients. Several randomized controlled trials (RCTs) have reported beneficial effects of physical activity during or after adjuvant therapy on HRQoL after breast cancer (5-8) and other cancers (9,10) with little evidence of adverse effects. The benefits include improvement in specific symptoms such as fatigue (5,6), secondary lymphedema (11), physical function (12,13), emotional function and mental health (14,15), and global HRQoL (13,15,16). However, the available trials have often had small sample sizes and have in some cases failed to show clinically meaningful effects. In addition, different instruments that measure various aspects of HRQoL have been employed to measure quality of life (QoL) components, which could contribute toward heterogeneity in results between studies. For instance, the Functional Assessment of Cancer Therapy–General (FACT-G) and European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) have greater emphasis on cancer-related symptoms and less on physical functioning compared with the Short Form Health Survey (SF-36) (17). Clarifying whether the impact of physical activity varies by the instrument used to measure HRQoL could be important. In addition, various types of physical activity interventions have been tested across different studies including aerobic exercise, resistance exercise, aerobic and resistance exercise combined, walking, yoga, stretching, Tai chi, and Qigong. Additional research clarifying whether physical activity overall or specific types and combinations of physical activity are particularly beneficial for improving HRQoL in breast cancer patients is necessary to develop better physical activity recommendations for this patient group. Therefore, we conducted a systematic literature review and meta-analysis of physical activity and HRQoL as part of the Global Cancer Update Programme (CUP Global), previously known as the World Cancer Research Fund–American Institute for Cancer Research (WCRF-AICR) Continuous Update Project (https://www.wcrf.org/int/continuous-update-project). The aims of this review and meta-analysis were to assess the existing evidence on whether physical activity improves global as well as physical, emotional, and mental domains of HRQoL measured by several common instruments; whether specific types of physical activity are particularly effective; and whether other aspects of interventions, such as the timing of the intervention (during or after primary treatment) or mode of intervention (group-based, individual-based, mixed), affected the results.
Methods
Search Strategy
PubMed and CENTRAL databases were searched up to August 31, 2019, for RCTs of physical activity and HRQoL among female breast cancer survivors. The search strategy is described in detail in the Supplementary Materials (Supplementary Text 1 and 2, available online) and included search terms for diet and body fatness as part of a larger ongoing project in the CUP Global. The database searches were supplemented by hand searches of the reference lists of the included studies and previous systematic reviews and meta-analyses on the topic. A peer-reviewed protocol was prepared before the review was conducted and is available online (18). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline for the reporting of systematic reviews and meta-analyses was followed (19).
Study Selection
We included peer-reviewed RCTs of physical activity and exercise before, during, or after primary treatment (defined as any surgical treatment and/or [neo]adjuvant therapy, including chemotherapy, radiotherapy, and/or hormonal therapy, in the acute phase of cancer diagnosis and treatment, but excluding extended use of hormonal therapy that may last years after diagnosis) and HRQoL overall and its main functional domains (physical, emotional, or mental) measured using validated tools to assess HRQoL, among women who had been diagnosed with breast cancer as first cancer during adulthood, at any stage of diagnosis. For the current analysis, exclusion criteria were 1) nonrandomized clinical trials, quasi-randomized trials, 1-arm pre-posttest design studies, population-level studies, cohort studies, cross-sectional studies, case-control studies, retrospective studies; 2) studies in which the comparison group is not from the same study population; 3) studies of exposures other than physical activity; 4) multimodal or combined interventions where the effect of the physical activity intervention could not be separated from other interventions; 5) HRQoL outcomes not reported; 6) comments, reviews, news, and conference abstracts; 7) studies with mixed cancer sites where breast cancer was not evaluated separately; and 8) studies with less than 20 participants.
Data Extraction
The following information was extracted: author; publication year; title; study design and type (parallel, factorial, crossover, other); study name; number of study centers; study period; country; study participant characteristics (age, race and ethnicity); menopausal status at cancer diagnosis; body mass index; smoking status; comorbidities (eg, hypertension, cardiovascular disease, and diabetes mellitus); other specific characteristics (BRCA1 and BRCA2 mutation carriers); disease characteristics (ductal carcinoma in situ, invasive, local, regional, distant, or metastatic breast cancer; tumor, node, and metastases classification; grade; other stage; molecular characteristics); year of breast cancer diagnosis; time since breast cancer diagnosis; breast cancer treatment (surgery, radiation, chemotherapy, hormone therapy) and modality (neoadjuvant, adjuvant, palliative); interventions (modality, frequency, intensity, duration of intervention); number of participants allocated to each arm; randomization method; allocation method; method for assessment of HRQoL; means (per arm) or mean differences (between arms); standard deviations or standard errors; confidence intervals (CIs); P values; and analysis (intention to treat or per protocol).
Risk of Bias Assessment
Risk of bias was assessed using the Revised Cochrane Risk-of-Bias tool for Randomized trials (20). The tool assesses the risk of bias of RCTs based on several signaling questions on 1) bias arising from the random assignment process, 2) bias because of deviations from intended interventions, 3) bias because of missing outcome data, 4) bias in measurement of the outcome, and 5) bias in selection of the reported result. The HRQoL assessment was considered unlikely to be influenced by knowledge of intervention received when the patient-reported HRQoL measures showed an effect in the same direction (or null effect) as the objective physical or cognitive performance measures in the studies. For each domain, risk of bias was graded as 1) low, 2) with some concerns, or 3) high, and an overall assessment across items was made as well.
Evidence Grading
An independent WCRF-AICR expert panel graded the evidence by its strength and likelihood of causality into strong (subgrades evaluating likelihood of causality: convincing or probable) or limited (subgrades evaluating likelihood of causality: limited suggestive or limited no conclusion) level. Predefined grading criteria were used to assess study design; risk of bias; the quantity, consistency, magnitude, and precision of the effect estimates; existence of a dose response; and the generalizability and mechanistic plausibility of the results (Supplementary Table 2, available online).
Statistical Methods
Inverse variance DerSimonian-Laird random effects models were used to calculate summary weighted mean differences (WMDs), weighted mean change differences (WMCDs), and standardized mean differences (SMDs) (95% CIs) for participants who were randomly assigned to physical activity interventions compared with those randomly assigned to a control group (21). We were interested in the effect of assignment to intervention (the intention-to-treat effect) but used the results from the per-protocol analysis if this was the only analysis conducted in the studies. Final HRQoL and change from baseline measures were analyzed separately because the baseline values may be different in the intervention and control groups. Separate analyses were conducted for each HRQoL instrument, including the cancer-specific measures EORTC QLQ-C30, FACT-G, with its breast cancer-specific subscale (FACT-B) or fatigue subscale (Functional Assessment of Chronic Illness Therapy–Fatigue [FACIT-F]), because of different constructs in their scales and subscales. A list of items included in the instruments is shown in Table 1, and effect sizes of minimum clinical importance by HRQoL scale are shown in Supplementary Table 1 (available online). Exceptions were made when the instruments were similar and could be combined (eg, the generic measures Medical Outcomes Study–36-item short-form [MOS SF-36] and RAND SF-36 for the analysis of general health perceptions). The meta-analysis included analyses of global HRQoL scores (FACT-B, FACT-G, EORTC QLQ-C30), general health perceptions (MOS SF-36, RAND SF-36), physical well-being (FACIT-F, FACT-B, FACT-G), physical functioning (EORTC QLQ C30, MOS SF-36, RAND SF-36), physical component summary score (MOS SF-36), emotional well-being functioning (FACIT-F, FACT-B, FACT-G), emotional functioning (EORTC QLQ-C30), mental health score (MOS SF-36, RAND SF-36), and mental component summary score (MOS SF-36). Across all instruments, WMDs, WMCDs, and SMDs statistically significant results above 0 indicate beneficial effects of the physical activity intervention, whereas statistically significant results below 0 indicate adverse effects. The main analysis was performed on the HRQoL measures from the final assessment (maximal follow-up). These values and their measures of variability such as standard deviations, confidence intervals, and number of participants per group were required to calculate the effect estimates. Missing data were imputed when possible following standard approaches (22). We did not use an external estimate of standard deviation or a correlation coefficient to impute missing standard deviations for the mean changes, to avoid making unjustifiable assumptions. When there was more than 1 exercise group in the RCT and when the raw data per group (eg, means, standard deviations, and number of participants) were available, we pooled the means from each arm using standardized formulas (22). If only the effect sizes were available, we pooled those using a fixed effects model before including the study in the overall analysis. For studies with a crossover design, we used the data from the start of the intervention until the time point where the second (delayed intervention) group started the crossover, so the second (delayed intervention) group served as a control group as they had not yet received the intervention (equivalent to a parallel design trial). Heterogeneity between studies was evaluated using Q and I2 statistics (23). Subgroup and meta-regression analyses were conducted to investigate potential sources of heterogeneity and were stratified by type of exercise (aerobic and resistance exercise, aerobic exercise, resistance exercise, yoga, others), frequency (1-3 or >3 days per week), duration per session (<60 or ≥60 minutes per session), and total duration (<120, 120-180, or ≥180 minutes per week), as well as timing (during or after primary adjuvant treatment), mode of intervention (group based, individual based, mixed), and type of control group (attention control, others such as usual care, waiting list controls). Other subgroups by study or patient characteristics were generally small in numbers and not analyzed. Sensitivity analyses were conducted by estimating mean differences at postintervention or follow-up immediately after intervention (minimum follow-up) and by calculating SMDs as the same HRQoL assessment tools may differ slightly between the versions. Small study effects, such as publication bias, were assessed using Egger regression asymmetry test (24) and by visual inspection of the funnel plots, when there were 10 or more studies in the analysis. All statistical tests were 2-sided. The statistical analyses were conducted using the software package Stata, version 13.0 (StataCorp, TX, USA).
Table 1.
List of items in the global, physical, and emotional domains of the health-related quality of life instruments
| Instrument | FACT-G | EORTC QLQ-C30 | SF-36 (MOS/RAND) | |
|---|---|---|---|---|
| Domain | Version 4.0 | Version 3.0 | 36 items, 8 domains | |
| 27 items, 4 domains | 30 items, 9 domains, 6 single items | 3-, 5-, and 6-point Likert rating scale | ||
| 5-point Likert-rating scale | 4- and 7-point Likert rating scale | Score range for each domain 0-100 | ||
| Total score range 0-108 | Score range for each domain 0-100 | Higher scores—better QoL | ||
| Higher scores—better QoL | Higher scores—better QoL | |||
| Physical well-being and function | Physical well-being | Physical functioning | Physical functioning | Physical component scale |
| (7 items, score range 0-28) | (5 items, score range 0-100) | (10 items, score range 0-100) | (4 scales, 21 items, score range 0-100) | |
| Symptoms: | Strenuous activities | Vigorous activities | ||
| Have nausea | Short walk | Moderate activities | Physical functioning | |
| Have pain | Long walk | Lifting or carrying groceries | Physical role limitations | |
| Bothered by side effects of treatment | Stay in bed or chair during the day | Climbing several flights of stairs | Pain | |
| Help with eating, dressing, washing yourself, or using toilet | Climbing 1 flight of stairs | General health | ||
| Impact: | Bending, kneeling, or stooping | |||
| Trouble meeting the needs of family | Walking more than 1 mile | |||
| Lack of energy | Walking several blocks | |||
| Feel ill | Walking 1 block | |||
| Spend time in bed | Bathing or dressing | |||
| Emotional well-being, function and mental health | Emotional well-being | Emotional functioning | Mental health and emotional well-being scale | Mental component scale |
| (6 items, score range 0-24) | (4 items, score range 0-100) | (5 items, score range 0-100) | (4 scales, 14 items, score range 0-100) | |
| Feel sad | Felt tense | Been nervous | Mental health | |
| Coping with illness | Worry | Felt down in the dumps | Emotional role limitations | |
| Losing hope in the fight against illness | Felt irritable | Felt calm and peaceful | Vitality | |
| Feel nervous | Felt depressed | Felt downhearted and blue | Social functioning | |
| Worry about dying | Been happy | |||
| Worry that condition will get worse | ||||
| Global, total score, and general health | Total FACT-G: sum of all items (physical, emotional, functional,a and social and familyb well-being) (27 items, score range 0-108) Total FACT-B: sum of all items in FACT-G and -Bc (37 items, score range 0-148) Total FACT-B-4: sum of all items in FACT-G and B-4d (41 items, score range 0-148) |
Global QoL (2 items, score range 0-100) Self-rated overall health Self-rated overall QoL |
General health (5 items, score range 0-100): Self-perceived health in general More easily get sick than others Healthy as others Expect health to get worse Excellent health |
|
FACT-G: functional well-being (7 items, score range 0-28): able to work and is fulfilling; enjoy life and enjoy things for fun; accepted illness; sleep well; content with quality of life. EORTC QLQ-C30 = European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–C30; FACT-B = Functional Assessment of Cancer Therapy–General and Breast cancer; FACT-B+4 = Functional Assessment of Cancer Therapy for Breast Cancer + 4; FACT-G = Functional Assessment of Cancer Therapy–General; QoL = quality of life; SF-36 (MOS/RAND) = Short Form-36 (Medical Outcomes Study/RAND).
FACT-G: Social and family well-being (7 items, score range 0-28): feel close to friends; get emotional support from family; get support from friends; family accepted illness; satisfied with family communication about illness; feel close to partner; satisfied with sex life.
FACT-B: Breast cancer subscale (10 items, score range 0-40): short of breath; self-conscious about the way I dress; have swollen or tender arm(s); feel sexually attractive; bothered by hair loss; worry that other family members might get breast cancer; worry about the effect of stress on illness; bothered by weight change; able to feel like a woman; experience pain in body.
FACT-B-4: Breast cancer subscale plus arm subscale (4 items): movement is painful; have poor movements; feels numb; have stiffness; is/are swollen or tender.
Results
Study Selection and Study Characteristics
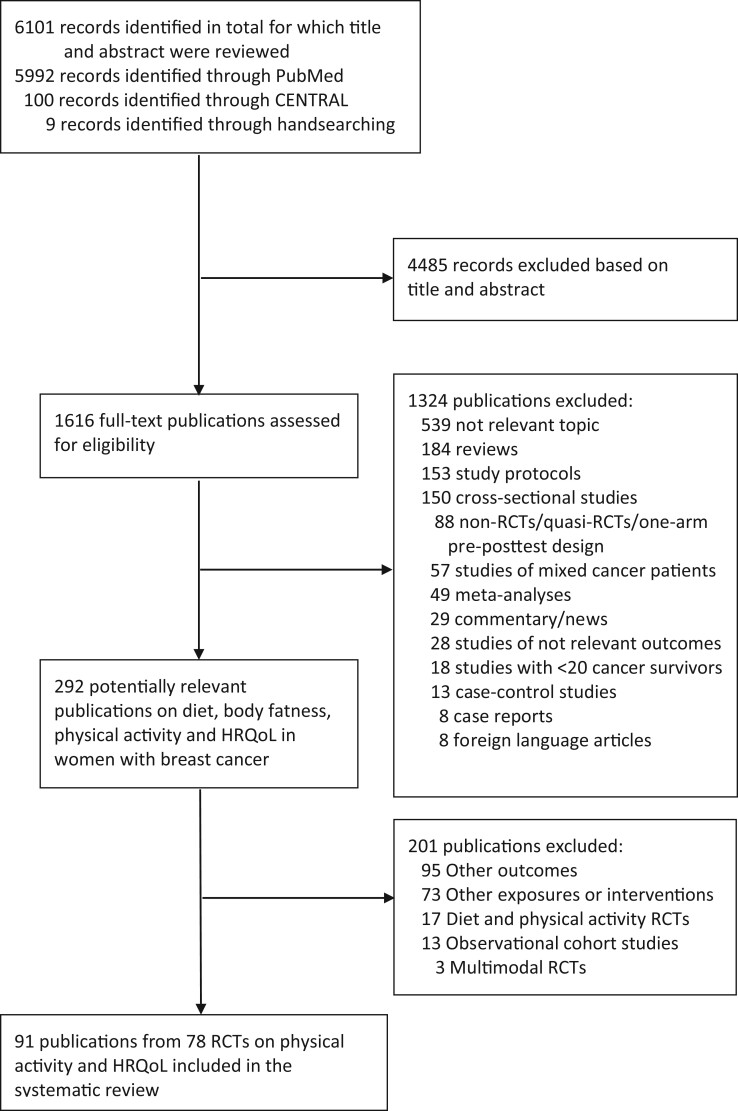
From a total of 6101 records that were identified by the search (PubMed, CENTRAL, and handsearching), a total of 79 RCTs (92 publications) (12-16,25-111) were included in the systematic review (Figure 1; Supplementary Table 3, available online). Of these, 59 publications were included in the meta-analyses (12-16,25,27,31,33-38,43-47,49-52,54,56,58-60,64-67,69,74,75,79,81,83-86,88,91-97,99-102,104-107,109,110). The number of participants in the RCTs ranged from 20 to 573, with a median of 64 participants. Two of the included trials had a crossover design that compared immediate with delayed exercise after completing adjuvant therapy (12,49), whereas the remaining studies were of a parallel design, comparing the effects of physical activity or exercise with a control group.
Figure 1.
Flow chart of study selection. HRQoL = health-related quality of life; RCT = randomized controlled trial.
Ten studies conducted per-protocol analyses (27,48,49,66,73,81,91,97,102,108), and the remaining studies conducted intention-to-treat analyses. A total of 20 publications were from a pilot or feasibility study (28,32,35,44-50,53,57,62,74,84,89,93,94,98,103). Three pilot studies (4 publications) (32,35,93,94) were followed by a separate complete trial that published results later (13,34,82,83,90,92), and the pilot studies and the trials were included as populations were not overlapping. A total of 35 publications (13-15,26-29,31,34,35,38-42,44,45,48,62,65,73,74,80,85,88-94,99,102,103,110) were from North America, 33 were from Europe (16,25,32,33,37,43,46,47,52,57,58,63,66,68-70,76-79,81-83,86,87,95-98,101,104,105,107), 9 were from Australia or New Zealand (12,30,36,53,59-61,71,72,84), 8 were from East Asia or Southeast Asia (49-51,64,67,75,109,111), 1 was from India (106), 1 from Iran (100), and 4 from Turkey (54-56,108).
Risk of Bias of the Studies
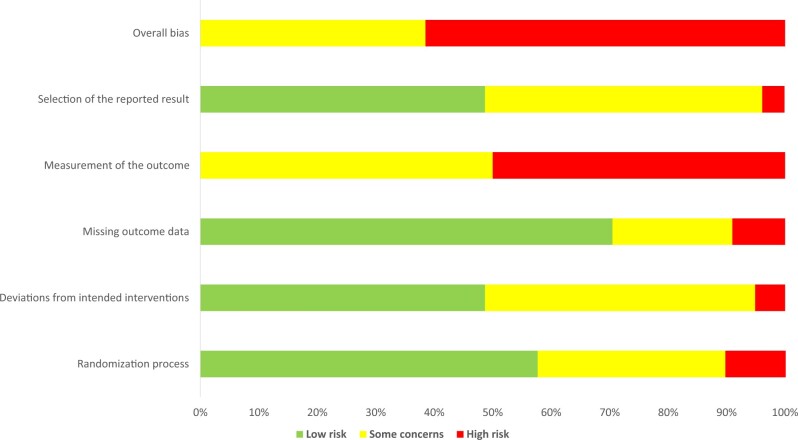
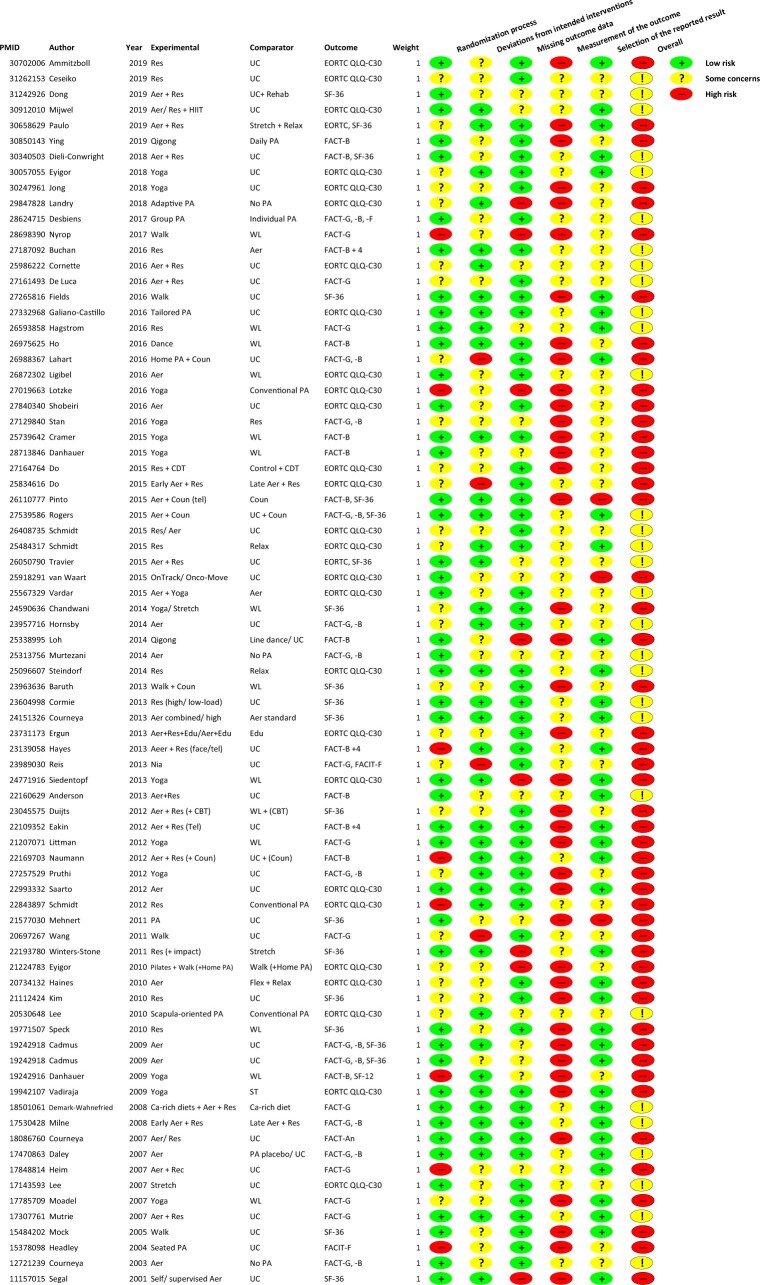
Figures 2 and 3 and Supplementary Table 4 (available online) show the summary of the risk of bias assessment across studies and for each RCT investigating the effects of physical activity on HRQoL. Half of the studies were of high risk of bias because the perceived HRQoL outcomes were self-reported by the participants and there were no objective measures to corroborate these patients’ reported outcomes, which could have been influenced by their knowledge of being in the physical activity intervention or not. Because physical activity is generally recognized as beneficial for health, this would likely result in more favorable responses to the outcome assessment and make physical activity appear more beneficial. For the other risk-of-bias domains, approximately 50%-70% were assessed as low risk: 57.7% in randomization process (selection bias); 48.7% in deviations from intended interventions; 70.5% in missing outcome data; and 48.7% in selection of the reported result. The other studies were primarily of some concern (20.5%-50%) or at high risk (3.8%-10.3%) of bias.
Figure 2.
Summary of the risk of bias assessment across studies.
Figure 3.
Summary of the risk of bias assessment for the individual studies. Aer = aerobic; Ca-rich diet = calcium-rich diet; CBT = cognitive behavioral therapy; CDT = complex decongestive therapy; Coun = counseling; Edu = education; EORTC QLQ-C30 = European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30; face = face-to-face intervention; FACT-An = Functional Assessment of Cancer Therapy–Anemia; FACT-B = Functional Assessment of Cancer Therapy–General and Breast cancer; FACT-B+4 = Functional Assessment of Cancer Therapy for Breast Cancer B + 4; FACT-Cog = Functional Assessment of Cancer Therapy–Cognitive; FACT-ES = Functional Assessment of Cancer Therapy–Endocrine Symptoms; FACT-F = Functional Assessment of Cancer Therapy–Fatigue; FACT-G = Functional Assessment of Cancer Therapy–General; FACIT-F = Functional Assessment of Chronic Illness Therapy–Fatigue; HIIT = high-intensity interval training; HRQoL = health-related quality of life; PA = physical activity; Rehab = rehabilitation; Relax = relaxation; Res = resistance; SF-12 = Short Form 12; SF-36 = Short Form-36; ST = supportive therapy; tel = over-the-telephone intervention; UC = usual care; WL = wait list.
Global HRQoL
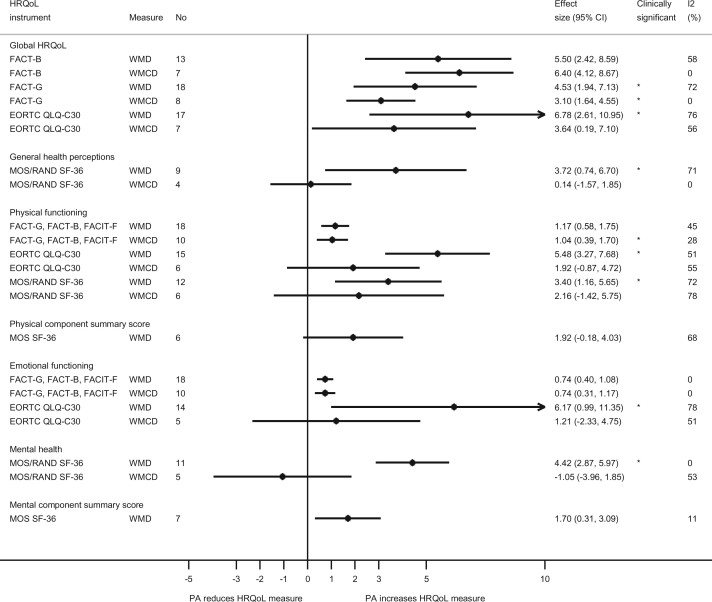
Physical activity interventions vs control resulted in higher global HRQoL as measured by FACT-B [WMD = 5.50, 95% CI = 2.42 to 8.59; I2 = 58%, Pheterogeneity = .004, n = 13] studies (12,13,15,16,27,38,43,45,64,65,69,75,88) and WMCD = 6.40, 95% CI = 4.12 to 8.67; I2 = 0%, Pheterogeneity = .49, n = 7 (13,16,38,44,69,81,84)], FACT-G [WMD = 4.53, 95% CI = 1.94 to 7.13; I2 = 72%, Pheterogeneity < .001, n = 18 (12-15,31,38,43,46,47,59,65,69,74,83,85,91,109) and WMCD = 3.10, 95% CI = 1.64 to 4.55; I2 = 0%, Pheterogeneity = .58, n = 8 (31,38,69,81,83,85,93)], and EORTC QLQ-C30 [WMD = 6.78, 95% CI = 2.61 to 10.95; I2 = 76.3%, Pheterogeneity < .001, n = 17 (25,33,37,49,50,54,56,58,60,66,79,86,96,97,100,101,104) and WMCD = 3.64, 95% CI = 0.19 to 7.10; I2 = 54.7%, Pheterogeneity = .03, n = 8 (37,50,60,79,95,96,105)] (Figure 4; Supplementry Figures 1-6).
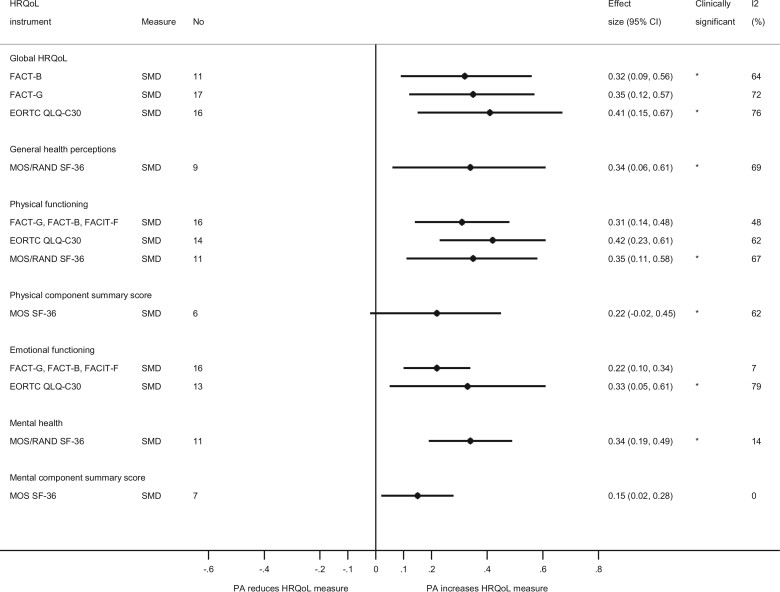
Figure 4.
Summary weighted mean differences and weighted mean change differences (95% confidence intervals) for physical activity and different domains of health-related quality of life. Effects that were considered clinically significant are marked with *. CI = confidence interval; EORTC QLQ-C30 = European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30; ES = effect size; FACT-B = Functional Assessment of Cancer Therapy–General and breast cancer; FACT-G = Functional Assessment of Cancer Therapy–General; FACIT-F = Functional Assessment of Chronic Illness Therapy–Fatigue; HRQoL = health-related quality of life; MOS/RAND SF-36 = Medical Outcomes Study and RAND Short Form-36; PA = physical activity; WMCD = weighted mean change difference; WMD = weighted mean difference.
General Health Perceptions
Physical activity interventions vs control resulted in higher general health perception scores as measured by the MOS SF-36 and RAND SF-36 on WMDs [WMD = 3.72, 95% CI = 0.74 to 6.70; I2 = 71%, Pheterogeneity = .001, n = 9 (15,31,34,36,51,67,86,92)] but not for WMCDs [WMCD = 0.14, 95% CI = -1.57 to 1.85; I2 = 0%, Pheterogeneity = .93, n = 4 (31,99,105)] (Figure 4; Supplementary Figures 7 and 8, available online).
Physical Functioning
Physical activity interventions vs control resulted in higher physical functioning as measured by FACIT-F, FACT-B, or FACT-G [WMD = 1.17, 95% CI = 0.58 to 1.75; I2 = 44.7%, Pheterogeneity = .02, n = 18 (12-16,31,38,43,45,59,65,69,74,75,83,85,91) and WMCD = 1.04, 95% CI = 0.39 to 1.70; I2 = 28%, Pheterogeneity = .18, n = 10 (16,31,38,69,81,83-85,93)], EORTC QLQ-C30 [WMD = 5.48, 95% CI = 3.27 to 7.68; I2 = 50.4%, Pheterogeneity = .001, n = 16 (25,33,49,50,58,60,66,79,86,96,97,100,104,106,107) although not for WMCD, which was 1.92, 95% CI = −0.87 to 4.72; I2 = 55%, Pheterogeneity = .05, n = 6 (50,60,79,95,96,105)], and MOS SF-36 and RAND SF-36 [WMD = 3.40, 95% CI = 1.16 to 5.65; I2 = 72%, Pheterogeneity < .001, n = 12 (15,31,34,36,51,52,67,86,88,92,110) but not for WMCD, which was 2.16, 95% CI = −1.42 to 5.75; I2 = 78%, Pheterogeneity < .001, n = 6 (31,52,99,105,110)] (Figure 4; Supplementary Figure 9-14, available online).
Physical Component Summary Score
Physical activity intervention vs control resulted in no difference in physical component summary score as measured by MOS SF-36 [WMD = 1.92, 95% CI = -0.18 to 4.03; I2=68%, Pheterogeneity = .01, n = 6 (15,34,36,88,92,102)] (Figure 4; Supplementary Figure 15, available online).
Emotional Functioning
Physical activity intervention vs control resulted in higher emotional functioning as measured by FACIT-F, FACT-B, or FACT-G [WMD = 0.74, 95% CI = 0.40 to 1.08; I2 = 0%, Pheterogeneity = .47, n = 18 (12-16,31,38,43,45,59,65,69,74,75,83,85,91) and WMCD = 0.74, 95% CI = 0.31 to 1.17; I2 = 0%, Pheterogeneity = .81, n = 10 (16,31,38,69,81,83-85,94)] and EORTC QLQ-C30 (WMD = 6.17, 95% CI = 0.99 to 11.35; I2 = 77.8%, Pheterogeneity < .001, n = 14 (25,33,49,50,58,60,66,79,86,96,97,100,104,106) although not for WMCD, which showed 1.21, 95% CI = −2.33 to 4.75; I2 = 51%, Pheterogeneity = .09, n = 5 (60,79,95,96,105)] (Figure 4; Supplementary Figure 16-19, available online).
Mental Health Score
Physical activity intervention vs control resulted in higher mental health score as measured by MOS and RAND SF-36 [WMD = 4.42, 95% CI = 2.87 to 5.97; I2 = 0%, Pheterogeneity = .75, n = 11 (15,31,35,36,51,52,67,86,88,92) but no effect was observed for WMCDs with -1.05, 95% CI = -3.96 to 1.85; I2 = 52.8%, Pheterogeneity = .07, n = 5 (31,52,99,105)] (Figure 4; Supplementary Figures 20 and 21, available online).
Mental Component Summary Score
Physical activity intervention vs control resulted in higher mental component summary score as measured by MOS SF-36 [WMD = 1.70, 95% CI = 0.31 to 3.09; I2 = 11%, Pheterogeneity = .35, n = 7 (15,34,36,52,88,92,102)] (Figure 4; Supplementary Figure 22, available online).
Sensitivity Analyses, Subgroup Analyses, and Publication Bias
Additional analyses were conducted using SMDs, and results were largely consistent with the overall analysis for WMDs (Figure 5). Subgroup and sensitivity analyses were conducted stratified by minimum duration of follow-up, type of physical activity, frequency, duration per session, total duration, intervention time frame, mode of intervention, and type of control (Supplementary Tables 9-20 and Supplementary Figures 23-110, available online). In general, analyses using minimum duration of follow-up showed relatively stronger effects than the main analysis that used the maximum follow-up. Stronger effects were observed for combined aerobic and resistance exercise, and intermediate effects were observed for aerobic exercise and resistance exercise separately, however, some exceptions to this pattern were observed. When using meta-regression analyses, there was a suggestion of between-subgroup heterogeneity (P = .05) with a slightly stronger effect observed between aerobic and resistance exercise combined vs aerobic only and WMDs in physical functioning (FACT-G, FACT-B, FACIT-F) (Supplementary Table 12, available online) and statistically significant between-subgroup heterogeneity (P = .006) with stronger effects for aerobic and resistance exercise combined vs aerobic exercise only for general health perceptions (MOS and RAND SF-36) (Supplementary Table 11, available online). Subgroup analyses stratified by frequency, duration per session, and total duration showed little evidence of heterogeneity between subgroups with meta-regression analyses (Supplementary Tables 9-20, available online). Some indication of stronger point estimates was also observed for group-based interventions compared with individual-based interventions, and for interventions after vs during primary adjuvant treatment in several, although not all, analyses, however, the tests for heterogeneity between subgroups were not statistically significant.
Figure 5.
Summary standardized mean differences (95% confidence intervals) for physical activity and different domains of health-related quality of life. Effects that were considered clinically significant are marked with *. CI = confidence interval; EORTC QLQ-C30 = European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30; ES = effect size; FACT-B = Functional Assessment of Cancer Therapy–General and breast cancer; FACT-G = Functional Assessment of Cancer Therapy–General; FACIT-F = Functional Assessment of Chronic Illness Therapy–Fatigue; HRQoL = health-related quality of life; MOS/RAND SF-36 = Medical Outcomes Study and RAND Short Form-36; PA = physical activity; SMD = standardized mean difference.
There was no indication of publication bias across analyses, except for the analysis on physical functioning as measured by the MOS SF-36 and RAND SF-36, where there was indication of publication bias with Egger test (P = .09) (Supplementary Figures 111-121, available online).
Adverse Events
Of the 59 studies that provided information on adverse events, 2 (13,27) reported serious intervention-related adverse events (pelvic stress fracture and foot stress fracture); 20 studies (28,29,38,41,48,53,60,65,66,68-72,81,88,96,103,107,109) reported minor adverse events such as musculoskeletal injuries, chest or muscle pain, dizziness, and treatment-related side effects; and 37 studies (15,30-33,36,37,39,45-47,50,51,54,55,57,59,62,64,67,73-75,78,80,82,84,85,91,99,100,104-106,110,111) reported no adverse events in the intervention and/or control groups (Supplementary Table 21, available online).
Grading of Evidence
Evidence was graded according to the predefined CUP Global grading criteria shown in Supplementary Table 2 (available online). There was evidence from multiple RCTs, some observed heterogeneity (but studies were generally consistent regarding the direction of effect), and no indication of publication bias (except for 1 analysis). The strength of the effects was, in general, small to trivial (1 exception was Global HRQoL EORTC QLQ-C30 for which the effect was of medium strength in the analysis of SMDs), but the results were considered clinically significant (small to medium effect or reached minimum important difference) in half of the 34 analyses conducted (Figures 4 and 5; Supplementary Tables 5 and 6, available online), and results were similar in sensitivity analyses using minimum follow-up (Supplementary Tables 7 and 8, available online). Details of the cutoff values that were considered clinically significant for the different tools can be found in Supplementary Table 1 (available online). There was limited evidence for a dose-response effect (Supplementary Tables 9-20, available online). Plausible mechanistic pathways through which physical activity may improve HRQoL include physical activity being associated with improved self-efficacy, which in turn is associated with improvements in health status indicators that are associated with better global QoL (112). Overall, the expert panel judged that there was strong evidence (ie, probable causality) that interventions for increasing physical activity result in improved HRQoL in breast cancer survivors. There was insufficient evidence to draw conclusions on specific domains of QoL or the types and doses of activity for QoL.
Discussion
The current systematic review and meta-analysis of 79 RCTs including 14 554 women diagnosed with breast cancer found small, but statistically significant positive effects of physical activity intervention on global HRQoL and physical functioning as measured by FACT-B, FACT-G, EORTC QLQ-C30, and the SF-36 and on the general health perceptions scale of SF-36. Effects were weaker for mental and emotional health. Although the effect sizes were, in general, small, in half of the analyses the results were nevertheless considered clinically significant. There was little evidence of serious adverse events across studies, although some studies reported minor adverse events. There was strong evidence that interventions for increasing physical activity improved QoL in breast cancer survivors with a level of causality that was judged as probable. There was insufficient evidence to draw conclusions regarding specific domains of QoL or the types and doses of activity for QoL.
Our review suggests that the positive effect of physical activity on QoL is more likely to be observed when the intervention is initiated after primary adjuvant treatment rather than during treatment when measured by the FACT (but not by the EORTC QLQ-C30), but more research is needed regarding this question. A possible explanation is that women undergoing cancer treatment may be affected by side effects related to breast cancer treatment including fatigue, pain, and peripheral neuropathy (113), which could interfere with the adherence to physical activity interventions during treatment and attenuate any impact on QoL. Also, the EORTC QLQ-C30 is designed to assess QoL related to chronic treatment side effects (114), thus it may be less suitable for capturing short-term QoL leading to an underestimation and increased variability of the underlying effect when considered after treatment. Our results are supported by 2 Cochrane Systematic Reviews focusing on exercise interventions during (115) and after treatment (7).
We also observed somewhat greater effects in group-based interventions compared with individual-based or mixed interventions, however, the evidence base was limited by the low number of studies and limited number of participants. Although the current review was structured to capture the distinct effect of physical activity disentangled from other types of interventions such as those that were multimodal, we cannot exclude the possibility that other aspects of social support may interplay with the effect of physical activity in group-based interventions. This hypothesis—that group-based interventions may have additional postulated benefits via indirect effects involving social support and group dynamics like comradeship of breast cancer survivors—has been previously examined (116,117) and showed no difference in results when compared with individual-based interventions. However, the possibility that the design of the group-based interventions may not have involved sufficient social interaction to show a difference in results could not be excluded.
With respect to specific types of physical activity, a combined aerobic and resistance intervention seemed to result in higher QoL improvement compared with other types of activity, although the number of RCTs in each subgroup was low. The effects of resistance-only and aerobic-only interventions were generally lower in magnitude. Including walking interventions in the aerobic exercise group as a sensitivity analysis did not materially change the results. The effects of yoga in all QoL scales and subscales were either statistically nonsignificant or not clinically meaningful. A previous Cochrane review for yoga also showed only small, short-term benefits compared with no exercise on overall QoL (6). Few studies were published on other types of physical activity, such as dancing, stretching, Nia, Tai chi or Qigong. Although some of the included studies focused primarily on activities that may be more pertinent to specific treatment goals and outcomes that were not considered in the current review (eg, resistance training for lymphedema or walking for aromatase inhibitor–associated arthralgia) (30,36,50,57,67,85), these studies were few and are unlikely to have had a major impact on the overall findings. Further studies are needed to clarify to what extent specific types of activity are particularly beneficial for various QoL outcomes. Future work within the CUP Global will include reviewing the impact of other exposures, such as diet, nutrition, and body weight, on QoL outcomes. This will enable WCRF International and the national entities (AICR, WCRF UK, Wereld Kancer Onderzoek Fonds) to expand their recommendations for improving QoL outcomes in this group.
In this comprehensive systematic review, we summarized the available evidence from RCTs regarding the impact of physical activity on QoL in women with breast cancer. The structure of the research question allowed for identifying and disentangling the effect of physical activity on QoL. We specifically focused on the independent effect of physical activity regimens; evidence from multimodal interventions that included physical activity were not evaluated as the component-specific effects could not be estimated. Furthermore, the considerable number of included studies allowed for a comprehensive evaluation of several validated tools of QoL measurement and their subscales. Although different tools for QoL assessment emphasize different aspects of QoL [eg, FACT-G and EORTC QLQ-C30 place more emphasis on symptoms and less on physical functioning compared with SF-36 (17)], results were similar when different tools for QoL assessment were considered. This might suggest that physical activity could have a beneficial impact across a range of QoL components. Other published reviews further support the beneficial effects of any type of exercise (118) or of specific types of exercise (119) on QoL in women with breast cancer, and an individual-participant meta-analysis of 34 RCTs (70% of participants were breast cancer survivors) also found a small but significant effect of exercise on QoL and physical function (z score = 0.15, 95% CI = 0.10 to 0.20, and z score = 0.18, 95% CI = 0.13 to 0.23, respectively) (120).
Despite the large evidence base, most of the identified studies were relatively small (the majority of the RCTs had less than 100 participants), and several studies may therefore individually have had insufficient power to detect significant effects. When change scores between recruitment and end of follow-up were considered, less pronounced effects were observed at more distal time points. One potential explanation could be that baseline values were accounted for in the mean change scores. Furthermore, the weighted mean change score may be a more appropriate measure of effect compared with the mean difference of final scores, because more than 70% of the included trials had less than 100 participants and the baseline values were not always balanced between groups. Other explanations for this discrepancy could be that the analysis of WMCDs only included about half or less the number of studies that were included in the analysis of WMDs, providing less statistical power to detect relations, or it could be because of response shift, which involves changes in internal standards, values, and the conceptualization of QoL related to a breast cancer diagnosis (121).
The accumulated evidence from RCTs had a high risk of bias overall, based on the Cochrane Revised Cochrane Risk-of-Bias tool for Randomized trials, but this can be mostly attributed to potential biases in measurement of the outcome as only patient-reported outcomes were assessed and the participants could not be blinded to the intervention. The other bias domains showed substantially lower risk of bias. Despite being self-reported, the tools assessed in this review have been validated and were also relevant to the population of interest, with some, such as the FACT-B, being specifically tailored to breast cancer survivors (122). A varying degree of heterogeneity across the analyses was observed, which could potentially limit the generalizability of the results from some of the analyses. Although we did perform subgroup and sensitivity analyses based on several prespecified factors, such as type of physical activity, frequency, duration, mode of intervention, time frame of intervention, type of control, and on the studies’ minimum follow-up, other potentially relevant modifiers such as tumor stage at diagnosis, age, or comorbidities could not be evaluated. However, across the numerous analyses that were conducted, the majority of the studies showed either a beneficial effect or no significant relation, and there were few studies that showed estimates in the direction of an adverse effect. Some studies may have been missed from our review, however, the literature search was comprehensive and supplemented by hand searches of relevant reviews and should have identified the majority of the relevant studies. In general, we did not find evidence of publication bias by visually inspecting the funnel plots or based on Egger regression test, but the number of studies included in some of the meta-analyses was relatively small. The only exception was for SF-36 physical function score where there was evidence of an inflated summary estimate because of small study effects.
There is strong evidence that interventions for increasing physical activity result in improved QoL in breast cancer survivors with a level of causality that was judged as probable. There was insufficient evidence to draw conclusions regarding different domains of QoL (eg, physical, global, emotional functioning) or for specific types, combinations, or doses of activity. It is therefore recommended that breast cancer survivors should be encouraged to be physically active. In the absence of strong evidence for more specific recommendations unique to breast cancer survivors, they should follow the general population national guidelines for physical activity, under the guidance of their health-care team. Additional research should focus on larger, well-conducted studies on the relationship between dose of physical activity (including frequency, duration, and intensity) and type of physical activity and QoL.
Funding
This work was funded by the World Cancer Research Fund network of charities (American Institute for Cancer Research [AICR]; World Cancer Research Fund [UK]; Wereld Kanker Onderzoek Fonds [WKOF]). Support was provided by the American Cancer Society (CRP-19-175-06-COUN) to Wendy Demark-Wahnefried, and Anne McTiernan was supported by the Breast Cancer Research Foundation grants BCRF-20-107 and BCRF-21-107.
Notes
Role of the funder: The funders of this study had no role in the decisions about the design and conduct of the study; collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript, or the decision to submit the manuscript for publication.
Disclosures: Dagfinn Aune, Georgios Markozannes, Rita Vieira, Katia Balducci, Margarita Cariolou, Konstantinos K Tsilidis, and Doris S.M. Chan are supported by the World Cancer Research Fund network of charities. Teresa Norat, Leila Abar, and Neesha Nanu were previously supported by the World Cancer Research Fund network of charities. All authors have no conflict of interest.
Author contributions: Conceptualization: TN, DSMC, KKT. Data curation: YOA, DA, GM, DSMC. Formal analysis: DA, GM. Funding acquisition: TN, DSMC, KKT. Investigation: DSMC, NN, LA, KB, MC, RV, DA, GM. Methodology: TN, DSMC, KKT, GV, AMT, SC, WDW, VL, GM, DCG. Project administration: DSMC, KKT, KA, NGT, HC, DK, DMGG, PM, MW. Software: YOA. Supervision: KKT, DSMC, GV, WDW, AMT, SKC, ELG, EK, AAJ, MJG, VL, ER, DCG. Writing—original draft: DA, GM. Writing—review & editing: DA, GM, LA, KB, MC, NN, RV, YOA, DCG, SKC, ELG, MJG, AJJ, EK, VL, AMT, ER, KA, NTB, HC, DK, DMGG, PM, MW, GV, WDW, TN, KKT, DSMC.
Acknowledgements: We thank Dr Martijn Bours (Maastricht University) for his expert opinion on the review protocol. We also acknowledge the input of Isobel Bandurek and Susannah Brown as past CUP Secretariat members.
Disclaimer: The views expressed in this review are the opinions of the authors. They may differ from those in future updates of the evidence related to food, nutrition, physical activity, and quality of life in breast cancer survivors. Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Prior presentations: These results have been presented as an abstract at the National Cancer Research Institute Cancer Conference in November 2021 in London, United Kingdom.
Supplementary Material
Contributor Information
Dagfinn Aune, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK; Department of Nutrition, Bjørknes University College, Oslo, Norway; Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway; Unit of Cardiovascular and Nutritional Epidemiology, Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden.
Georgios Markozannes, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK; Department of Hygiene and Epidemiology, University of Ioannina School of Medicine, Ioannina, Greece.
Leila Abar, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK.
Katia Balducci, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK.
Margarita Cariolou, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK.
Neesha Nanu, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK.
Rita Vieira, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK.
Yusuf O Anifowoshe, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK.
Darren C Greenwood, Leeds Institute for Data Analytics, Faculty of Medicine and Health, University of Leeds, Leeds, UK.
Steven K Clinton, The Comprehensive Cancer Center, The Ohio State University, Columbus, OH, USA; Division of Medical Oncology, Department of Internal Medicine, The Ohio State University, Columbus, OH, USA.
Edward L Giovannucci, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Harvard Medical School, Brigham and Women’s Hospital, Boston, MA, USA.
Marc J Gunter, Section of Nutrition and Metabolism, International Agency for Research on Cancer-World Health Organization, Lyon, France.
Alan Jackson, National Institute for Health Research, Southampton Biomedical Research Centre, Southampton, UK.
Ellen Kampman, Division of Human Nutrition and Health, Wageningen University and Research, Wageningen, the Netherlands.
Vivien Lund, World Cancer Research Fund International, London, UK.
Anne McTiernan, Division of Public Health Sciences, Program in Epidemiology, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; School of Public Health, Department of Epidemiology, University of Washington, Seattle, WA, USA; School of Medicine, Department of Medicine (Geriatrics), University of Washington, Seattle, WA, USA.
Elio Riboli, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK.
Kate Allen, World Cancer Research Fund International, London, UK.
Nigel T Brockton, American Institute for Cancer Research, Arlington, VA, USA.
Helen Croker, World Cancer Research Fund International, London, UK.
Daphne Katsikioti, World Cancer Research Fund International, London, UK.
Deirdre McGinley-Gieser, World Cancer Research Fund International, London, UK.
Panagiota Mitrou, World Cancer Research Fund International, London, UK.
Martin Wiseman, World Cancer Research Fund International, London, UK.
Galina Velikova, School of Medicine, Faculty of Medicine and Health, University of Leeds, Leeds, UK.
Wendy Demark-Wahnefried, O’Neal Comprehensive Cancer Center, University of Alabama at Birmingham, AL, USA.
Teresa Norat, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK.
Konstantinos K Tsilidis, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK; Department of Hygiene and Epidemiology, University of Ioannina School of Medicine, Ioannina, Greece.
Doris S M Chan, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK.
Data Availability
The data underlying this article are available in the article itself and in its online Supplementary Material.
References
- 1. World Health Organization. WHO Definition of Health. Preamble to the Constitution of the World Health Organization as adopted by the International Health Conference. New York, 19-22 June, 1946; signed on 22 July 1946 by the representatives of 61 States and entered into force on 7 April 1948. https://www.who.int/about/governance/constitution#:~:text=Health%20is%20a%20state%20of,belief%2C%20economic%20or%20social%20condition. Accessed November 11, 2022.
- 2. DeSalvo KB, Bloser N, Reynolds K, He J, Muntner P.. Mortality prediction with a single general self-rated health question. A meta-analysis. J Gen Intern Med. 2006;21(3):267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Epplein M, Zheng Y, Zheng W, et al. Quality of life after breast cancer diagnosis and survival. J Clin Oncol. 2011;29(4):406-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Mara AM, Denicoff AM.. Health related quality of life in NCI-sponsored cancer treatment trials. Semin Oncol Nurs. 2010;26(1):68-78. [DOI] [PubMed] [Google Scholar]
- 5. Lipsett A, Barrett S, Haruna F, Mustian K, O’Donovan A.. The impact of exercise during adjuvant radiotherapy for breast cancer on fatigue and quality of life: a systematic review and meta-analysis. Breast. 2017;32:144-155. [DOI] [PubMed] [Google Scholar]
- 6. Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ.. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev. 2017;1:CD010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lahart IM, Metsios GS, Nevill AM, Carmichael AR.. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst Rev. 2018;1:CD011292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soares Falcetta F, de Araújo Vianna Träsel H, et al. Effects of physical exercise after treatment of early breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2018;170(3):455-476. [DOI] [PubMed] [Google Scholar]
- 9. Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH.. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87-100. [DOI] [PubMed] [Google Scholar]
- 10. Mishra SI, Scherer RW, Geigle PM.. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;2012:CD007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baumann FT, Reike A, Hallek M, Wiskemann J, Reimer V.. Does exercise have a preventive effect on secondary lymphedema in breast cancer patients following local treatment–a systematic review. Breast Care (Basel). 2018;13(5):380-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Milne HM, Wallman KE, Gordon S, Courneya KS.. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2008;108(2):279-288. [DOI] [PubMed] [Google Scholar]
- 13. Rogers LQ, Courneya KS, Anton PM, et al. Effects of the BEAT Cancer physical activity behavior change intervention on physical activity, aerobic fitness, and quality of life in breast cancer survivors: a multicenter randomized controlled trial. Breast Cancer Res Treat. 2015;149(1):109-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moadel AB, Shah C, Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. J Clin Oncol. 2007;25(28):4387-4395. [DOI] [PubMed] [Google Scholar]
- 15. Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial. Breast Cancer Res. 2018;20(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cramer H, Rabsilber S, Lauche R, Kummel S, Dobos G.. Yoga and meditation for menopausal symptoms in breast cancer survivors-a randomized controlled trial. Cancer. 2015;121(13):2175-2184. [DOI] [PubMed] [Google Scholar]
- 17. Ferrans CE. Differences in what quality-of-life instruments measure. J Natl Cancer Inst Monogr. 2007;2007(37):22-26. [DOI] [PubMed] [Google Scholar]
- 18. Imperial College Continuous Update Project team. Protocol for the data collection and systematic literature reviews on the role of diet, body fatness and physical activity on health-related quality of life after diagnosis of breast cancer. https://www.imperial.ac.uk/school-public-health/epidemiology-and-biostatistics/research/cancer-and-nutritional-epidemiology/global-cancer-update-programme/. Accessed November 01, 2022.
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG; for the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 21. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JP, Green S. Chapter 7: Selecting studies and collecting data. In: Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Updated March 2011. https://handbook-5-1.cochrane.org/front_page.htm Accessed August 18, 2020.
- 23. Higgins JP, Thompson SG.. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. [DOI] [PubMed] [Google Scholar]
- 24. Egger M, Davey SG, Schneider M, Minder C.. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ammitzboll G, Kristina Kjær T, Johansen C, et al. Effect of progressive resistance training on health-related quality of life in the first year after breast cancer surgery–results from a randomized controlled trial. Acta Oncol. 2019;58(5):665-672. [DOI] [PubMed] [Google Scholar]
- 26. An KY, Morielli AR, Kang DW, et al. Effects of exercise dose and type during breast cancer chemotherapy on longer-term patient-reported outcomes and health-related fitness: a randomized controlled trial. Int J Cancer. 2020;146(1):150-160. [DOI] [PubMed] [Google Scholar]
- 27. Anderson RT, Kimmick GG, McCoy TP, et al. A randomized trial of exercise on well-being and function following breast cancer surgery: the RESTORE trial. J Cancer Surviv. 2012;6(2):172-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baruth M, Wilcox S, Der AC, Heiney S.. Effects of home-based walking on quality of life and fatigue outcomes in early stage breast cancer survivors: a 12-week pilot study. J Phys Act Health. 2015;12(suppl 1):S110-S118. [DOI] [PubMed] [Google Scholar]
- 29. Brown JC, Schmitz KH.. Weight lifting and physical function among survivors of breast cancer: a post hoc analysis of a randomized controlled trial. J Clin Oncol. 2015;33(19):2184-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buchan J, Janda M, Box R, Schmitz K, Hayes S.. A randomized trial on the effect of exercise mode on breast cancer-related lymphedema. Med Sci Sports Exerc. 2016;48(10):1866-1874. [DOI] [PubMed] [Google Scholar]
- 31. Cadmus LA, Salovey P, Yu H, Chung G, Kasl S, Irwin ML.. Exercise and quality of life during and after treatment for breast cancer: results of two randomized controlled trials. Psychooncology. 2009;18(4):343-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Campbell A, Mutrie N, White F, McGuire F, Kearney N.. A pilot study of a supervised group exercise programme as a rehabilitation treatment for women with breast cancer receiving adjuvant treatment. Eur J Oncol Nurs. 2005;9(1):56-63. [DOI] [PubMed] [Google Scholar]
- 33. Cešeiko R, Eglītis J, Srebnijs A, et al. The impact of maximal strength training on quality of life among women with breast cancer undergoing treatment. Exp Oncol. 2019;41(2):166-172. [DOI] [PubMed] [Google Scholar]
- 34. Chandwani KD, Perkins G, Nagendra HR, et al. Randomized, controlled trial of yoga in women with breast cancer undergoing radiotherapy. J Clin Oncol. 2014;32(10):1058-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chandwani KD, Thornton B, Perkins GH, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010;8(2):43-55. [PubMed] [Google Scholar]
- 36. Cormie P, Pumpa K, Galvao DA, et al. Is it safe and efficacious for women with lymphedema secondary to breast cancer to lift heavy weights during exercise: a randomised controlled trial. J Cancer Surviv. 2013;7(3):413-424. [DOI] [PubMed] [Google Scholar]
- 37. Cornette T, Vincent F, Mandigout S, et al. Effects of home-based exercise training on VO2 in breast cancer patients under adjuvant or neoadjuvant chemotherapy (SAPA): a randomized controlled trial. Eur J Phys Rehabil Med. 2016;52(2):223-232. [PubMed] [Google Scholar]
- 38. Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS.. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660-1668. [DOI] [PubMed] [Google Scholar]
- 39. Courneya KS, McKenzie DC, Mackey JR, et al. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst. 2013;105(23):1821-1832. [DOI] [PubMed] [Google Scholar]
- 40. Courneya KS, McKenzie DC, Mackey JR, et al. Moderators of the effects of exercise training in breast cancer patients receiving chemotherapy: a randomized controlled trial. Cancer. 2008;112(8):1845-1853. [DOI] [PubMed] [Google Scholar]
- 41. Courneya KS, Segal RJ, Gelmon K, et al. Six-month follow-up of patient-rated outcomes in a randomized controlled trial of exercise training during breast cancer chemotherapy. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2572-2578. [DOI] [PubMed] [Google Scholar]
- 42. Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25(28):4396-4404. [DOI] [PubMed] [Google Scholar]
- 43. Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A.. Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol. 2007;25(13):1713-1721. [DOI] [PubMed] [Google Scholar]
- 44. Danhauer SC, Griffin LP, Avis NE, et al. Feasibility of implementing a community-based randomized trial of yoga for women undergoing chemotherapy for breast cancer. J Community Support Oncol. 2015;13(4):139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Danhauer SC, Mihalko SL, Russell GB, et al. Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psychooncology. 2009;18(4):360-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. De Luca V, Minganti C, Borrione P, et al. Effects of concurrent aerobic and strength training on breast cancer survivors: a pilot study. Public Health. 2016;136:126-132. [DOI] [PubMed] [Google Scholar]
- 47. Demark-Wahnefried W, Case LD, Blackwell K, et al. Results of a diet/exercise feasibility trial to prevent adverse body composition change in breast cancer patients on adjuvant chemotherapy. Clin Breast Cancer. 2008;8(1):70-79. [DOI] [PubMed] [Google Scholar]
- 48. Desbiens C, Filion M, Brien MC, Hogue JC, Laflamme C, Lemieux J.. Impact of physical activity in group versus individual physical activity on fatigue in patients with breast cancer: a pilot study. Breast. 2017;35:8-13. [DOI] [PubMed] [Google Scholar]
- 49. Do J, Cho Y, Jeon J.. Effects of a 4-week multimodal rehabilitation program on quality of life, cardiopulmonary function, and fatigue in breast cancer patients. J Breast Cancer. 2015;18(1):87-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Do JH, Kim W, Cho YK, et al. Effects of resistance exercises and complex decongestive therapy on arm function and muscular strength in breast cancer related lymphedema. Lymphology. 2015;48(4):184-196. [PubMed] [Google Scholar]
- 51. Dong X, Yi X, Gao D, et al. The effects of the combined exercise intervention based on internet and social media software (CEIBISMS) on quality of life, muscle strength and cardiorespiratory capacity in Chinese postoperative breast cancer patients: a randomized controlled trial. Health Qual Life Outcomes. 2019;17(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Duijts SF, van BM, Oldenburg HS, et al. Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment-induced menopausal symptoms in patients with breast cancer: results of a randomized, controlled, multicenter trial. J Clin Oncol. 2012;30(33):4124-4133. [DOI] [PubMed] [Google Scholar]
- 53. Eakin EG, Lawler SP, Winkler EA, Hayes SC.. A randomized trial of a telephone-delivered exercise intervention for non-urban dwelling women newly diagnosed with breast cancer: exercise for health. Ann Behav Med. 2012;43(2):229-238. [DOI] [PubMed] [Google Scholar]
- 54. Ergun M, Eyigor S, Karaca B, Kisim A, Uslu R.. Effects of exercise on angiogenesis and apoptosis-related molecules, quality of life, fatigue and depression in breast cancer patients. Eur J Cancer Care (Engl). 2013;22(5):626-637. [DOI] [PubMed] [Google Scholar]
- 55. Eyigor S, Karapolat H, Yesil H, Uslu R, Durmaz B.. Effects of pilates exercises on functional capacity, flexibility, fatigue, depression and quality of life in female breast cancer patients: a randomized controlled study. Eur J Phys Rehabil Med. 2010;46(4):481-487. [PubMed] [Google Scholar]
- 56. Eyigor S, Uslu R, Apaydin S, Caramat I, Yesil H.. Can yoga have any effect on shoulder and arm pain and quality of life in patients with breast cancer? A randomized, controlled, single-blind trial. Complement Ther Clin Pract. 2018;32:40-45. [DOI] [PubMed] [Google Scholar]
- 57. Fields J, Richardson A, Hopkinson J, Fenlon D.. Nordic walking as an exercise intervention to reduce pain in women with aromatase inhibitor-associated arthralgia: a feasibility study. J Pain Symptom Manage. 2016;52(4):548-559. [DOI] [PubMed] [Google Scholar]
- 58. Galiano-Castillo N, Cantarero-Villanueva I, Fernandez-Lao C, et al. Telehealth system: a randomized controlled trial evaluating the impact of an internet-based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer. 2016;122(20):3166-3174. [DOI] [PubMed] [Google Scholar]
- 59. Hagstrom AD, Marshall PW, Lonsdale C, Cheema BS, Fiatarone Singh MA, Green S.. Resistance training improves fatigue and quality of life in previously sedentary breast cancer survivors: a randomised controlled trial. Eur J Cancer Care (Engl). 2016;25(5):784-794. [DOI] [PubMed] [Google Scholar]
- 60. Haines TP, Sinnamon P, Wetzig NG, et al. Multimodal exercise improves quality of life of women being treated for breast cancer, but at what cost? Randomized trial with economic evaluation. Breast Cancer Res Treat. 2010;124(1):163-175. [DOI] [PubMed] [Google Scholar]
- 61. Hayes SC, Rye S, Disipio T, et al. Exercise for health: a randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment-related side effects following breast cancer. Breast Cancer Res Treat. 2013;137(1):175-186. [DOI] [PubMed] [Google Scholar]
- 62. Headley JA, Ownby KK, John LD.. The effect of seated exercise on fatigue and quality of life in women with advanced breast cancer. Oncol Nurs Forum. 2004;31(5):977-983. [DOI] [PubMed] [Google Scholar]
- 63. Heim ME, Malsburg ML, Niklas A.. Randomized controlled trial of a structured training program in breast cancer patients with tumor-related chronic fatigue. Onkologie. 2007;30(8-9):429-434. [DOI] [PubMed] [Google Scholar]
- 64. Ho RT, Fong TC, Cheung IK, Yip PS, Luk MY.. Effects of a short-term dance movement therapy program on symptoms and stress in patients with breast cancer undergoing radiotherapy: a randomized, controlled, single-blind trial. J Pain Symptom Manage. 2016;51(5):824-831. [DOI] [PubMed] [Google Scholar]
- 65. Hornsby WE, Douglas PS, West MJ, et al. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol. 2014;53(1):65-74. [DOI] [PubMed] [Google Scholar]
- 66. Jong MC, Boers I, Schouten van der Velden AP, et al. A randomized study of yoga for fatigue and quality of life in women with breast cancer undergoing (neo) adjuvant chemotherapy. J Altern Complement Med. 2018;24(9-10):942-953. [DOI] [PubMed] [Google Scholar]
- 67. Kim DS, Sim YJ, Jeong HJ, Kim GC.. Effect of active resistive exercise on breast cancer-related lymphedema: a randomized controlled trial. Arch Phys Med Rehabil. 2010;91(12):1844-1848. [DOI] [PubMed] [Google Scholar]
- 68. Koch AK, Rabsilber S, Lauche R, et al. The effects of yoga and self-esteem on menopausal symptoms and quality of life in breast cancer survivors–a secondary analysis of a randomized controlled trial. Maturitas. 2017;105:95-99. [DOI] [PubMed] [Google Scholar]
- 69. Lahart IM, Metsios GS, Nevill AM, Kitas GD, Carmichael AR.. Randomised controlled trial of a home-based physical activity intervention in breast cancer survivors. BMC Cancer. 2016;16:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Landry S, Chasles G, Pointreau Y, Bourgeois H, Boyas S.. Influence of an adapted physical activity program on self-esteem and quality of life of breast cancer patients after mastectomy. Oncology. 2018;95(3):188-191. [DOI] [PubMed] [Google Scholar]
- 71. Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ.. Pectoral stretching program for women undergoing radiotherapy for breast cancer. Breast Cancer Res Treat. 2007;102(3):313-321. [DOI] [PubMed] [Google Scholar]
- 72. Lee SA, Kang JY, Kim YD, et al. Effects of a scapula-oriented shoulder exercise programme on upper limb dysfunction in breast cancer survivors: a randomized controlled pilot trial. Clin Rehabil. 2010;24(7):600-613. [DOI] [PubMed] [Google Scholar]
- 73. Ligibel JA, Giobbie-Hurder A, Shockro L, et al. Randomized trial of a physical activity intervention in women with metastatic breast cancer. Cancer. 2016;122(8):1169-1177. [DOI] [PubMed] [Google Scholar]
- 74. Littman AJ, Bertram LC, Ceballos R, et al. Randomized controlled pilot trial of yoga in overweight and obese breast cancer survivors: effects on quality of life and anthropometric measures. Support Care Cancer. 2012;20(2):267-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Loh SY, Lee SY, Murray L.. The Kuala Lumpur Qigong trial for women in the cancer survivorship phase–efficacy of a three-arm RCT to improve QOL. Asian Pac J Cancer Prev. 2014;15(19):8127-8134. [DOI] [PubMed] [Google Scholar]
- 76. Lotzke D, Wiedemann F, Rodrigues RD, et al. Iyengar-Yoga compared to exercise as a therapeutic intervention during (neo)adjuvant therapy in women with stage I-III breast cancer: health-related quality of life, mindfulness, spirituality, life satisfaction, and cancer-related fatigue. Evid Based Complement Alternat Med. 2016;2016:5931816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mehnert A, Veers S, Howaldt D, Braumann KM, Koch U, Schulz KH.. Effects of a physical exercise rehabilitation group program on anxiety, depression, body image, and health-related quality of life among breast cancer patients. Onkologie. 2011;34(5):248-253. [DOI] [PubMed] [Google Scholar]
- 78. Mijwel S, Backman M, Bolam KA, et al. Adding high-intensity interval training to conventional training modalities: optimizing health-related outcomes during chemotherapy for breast cancer: the OptiTrain randomized controlled trial. Breast Cancer Res Treat. 2018;168(1):79-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mijwel S, Jervaeus A, Bolam KA, et al. High-intensity exercise during chemotherapy induces beneficial effects 12 months into breast cancer survivorship. J Cancer Surviv. 2019;13(2):244-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mock V, Frangakis C, Davidson NE, et al. Exercise manages fatigue during breast cancer treatment: a randomized controlled trial. Psychooncology. 2005;14(6):464-477. [DOI] [PubMed] [Google Scholar]
- 81. Murtezani A, Ibraimi Z, Bakalli A, Krasniqi S, Disha ED, Kurtishi I.. The effect of aerobic exercise on quality of life among breast cancer survivors: a randomized controlled trial. J Cancer Res Ther. 2014;10(3):658-664. [DOI] [PubMed] [Google Scholar]
- 82. Mutrie N, Campbell A, Barry S, et al. Five-year follow-up of participants in a randomised controlled trial showing benefits from exercise for breast cancer survivors during adjuvant treatment. Are there lasting effects? J Cancer Surviv. 2012;6(4):420-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mutrie N, Campbell AM, Whyte F, et al. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ. 2007;334(7592):517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Naumann F, Martin E, Philpott M, Smith C, Groff D, Battaglini C.. Can counseling add value to an exercise intervention for improving quality of life in breast cancer survivors? A feasibility study. J Support Oncol. 2012;10(5):188-194. [DOI] [PubMed] [Google Scholar]
- 85. Nyrop KA, Callahan LF, Cleveland RJ, Arbeeva LL, Hackney BS, Muss HB.. Randomized controlled trial of a home-based walking program to reduce moderate to severe aromatase inhibitor-associated arthralgia in breast cancer survivors. Oncologist. 2017;22(10):1238-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Paulo TRS, Rossi FE, Viezel J, et al. The impact of an exercise program on quality of life in older breast cancer survivors undergoing aromatase inhibitor therapy: a randomized controlled trial. Health Qual Life Outcomes. 2019;17(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Penttinen H, Utriainen M, Kellokumpu-Lehtinen PL, et al. Effectiveness of a 12-month exercise intervention on physical activity and quality of life of breast cancer survivors; five-year results of the BREX-study. In Vivo. 2019;33(3):881-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pinto B, Stein K, Dunsiger S.. Peer mentorship to promote physical activity among cancer survivors: effects on quality of life. Psychooncology. 2015;24(10):1295-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pruthi S, Stan DL, Jenkins SM, et al. A randomized controlled pilot study assessing feasibility and impact of yoga practice on quality of life, mood, and perceived stress in women with newly diagnosed breast cancer. Glob Adv Health Med. 2012;1(5):30-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ratcliff CG, Milbury K, Chandwani KD, et al. Examining mediators and moderators of yoga for women with breast cancer undergoing radiotherapy. Integr Cancer Ther. 2016;15(3):250-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Reis D, Walsh ME, Young-McCaughan S, Jones T.. Effects of Nia exercise in women receiving radiation therapy for breast cancer. Oncol Nurs Forum. 2013;40(5):E374-E381. [DOI] [PubMed] [Google Scholar]
- 92. Rogers LQ, Courneya KS, Carter SJ, et al. Effects of a multicomponent physical activity behavior change intervention on breast cancer survivor health status outcomes in a randomized controlled trial. Breast Cancer Res Treat. 2016;159(2):283-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rogers LQ, Hopkins-Price P, Vicari S, et al. Physical activity and health outcomes three months after completing a physical activity behavior change intervention: persistent and delayed effects. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1410-1418. [DOI] [PubMed] [Google Scholar]
- 94. Rogers LQ, Hopkins-Price P, Vicari S, et al. A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc. 2009;41(4):935-946. [DOI] [PubMed] [Google Scholar]
- 95. Saarto T, Penttinen HM, Sievanen H, et al. Effectiveness of a 12-month exercise program on physical performance and quality of life of breast cancer survivors. Anticancer Res. 2012;32(9):3875-3884. [PubMed] [Google Scholar]
- 96. Schmidt ME, Wiskemann J, Armbrust P, Schneeweiss A, Ulrich CM, Steindorf K.. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: a randomized controlled trial. Int J Cancer. 2015;137(2):471-480. [DOI] [PubMed] [Google Scholar]
- 97. Schmidt T, Weisser B, Durkop J, et al. Comparing endurance and resistance training with standard care during chemotherapy for patients with primary breast cancer. Anticancer Res. 2015;35(10):5623-5629. [PubMed] [Google Scholar]
- 98. Schmidt T, Weisser B, Jonat W, Baumann FT, Mundhenke C.. Gentle strength training in rehabilitation of breast cancer patients compared to conventional therapy. Anticancer Res. 2012;32(8):3229-3233. [PubMed] [Google Scholar]
- 99. Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19(3):657-665. [DOI] [PubMed] [Google Scholar]
- 100. Shobeiri F, Masoumi SZ, Nikravesh A, Heidari MR, Karami M.. The impact of aerobic exercise on quality of life in women with breast cancer: a randomized controlled trial. J Res Health Sci. 2016;16(3):127-132. [PMC free article] [PubMed] [Google Scholar]
- 101. Siedentopf F, Utz-Billing I, Gairing S, Schoenegg W, Kentenich H, Kollak I.. Yoga for patients with early breast cancer and its impact on quality of life–a randomized controlled trial. Geburtshilfe Frauenheilkd. 2013;73(4):311-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Speck RM, Gross CR, Hormes JM, et al. Changes in the body image and relationship scale following a one-year strength training trial for breast cancer survivors with or at risk for lymphedema. Breast Cancer Res Treat. 2010;121(2):421-430. [DOI] [PubMed] [Google Scholar]
- 103. Stan DL, Croghan KA, Croghan IT, et al. Randomized pilot trial of yoga versus strengthening exercises in breast cancer survivors with cancer-related fatigue. Support Care Cancer. 2016;24(9):4005-4015. [DOI] [PubMed] [Google Scholar]
- 104. Steindorf K, Schmidt ME, Klassen O, et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol. 2014;25(11):2237-2243. [DOI] [PubMed] [Google Scholar]
- 105. Travier N, Velthuis MJ, Steins Bisschop CN, et al. Effects of an 18-week exercise programme started early during breast cancer treatment: a randomised controlled trial. BMC Med. 2015;13:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Vadiraja HS, Rao MR, Nagarathna R, et al. Effects of yoga program on quality of life and affect in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Complement Ther Med. 2009;17(5-6):274-280. [DOI] [PubMed] [Google Scholar]
- 107. van Waart H, Stuiver MM, van Harten WH, et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol. 2015;33(17):1918-1927. [DOI] [PubMed] [Google Scholar]
- 108. Vardar Yagli N, Sener G, Arikan H, et al. Do yoga and aerobic exercise training have impact on functional capacity, fatigue, peripheral muscle strength, and quality of life in breast cancer survivors? Integr Cancer Ther. 2015;14:125-132. [DOI] [PubMed] [Google Scholar]
- 109. Wang YJ, Boehmke M, Wu YW, Dickerson SS, Fisher N.. Effects of a 6-week walking program on Taiwanese women newly diagnosed with early-stage breast cancer. Cancer Nurs. 2011;34(2):E1-E13. [DOI] [PubMed] [Google Scholar]
- 110. Winters-Stone KM, Dobek J, Bennett JA, Nail LM, Leo MC, Schwartz A.. The effect of resistance training on muscle strength and physical function in older, postmenopausal breast cancer survivors: a randomized controlled trial. J Cancer Surviv. 2012;6(2):189-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ying W, Min QW, Lei T, Na ZX, Li L, Jing L.. The health effects of Baduanjin exercise (a type of Qigong exercise) in breast cancer survivors: a randomized, controlled, single-blinded trial. Eur J Oncol Nurs. 2019;39:90-97. [DOI] [PubMed] [Google Scholar]
- 112. Phillips SM, McAuley E.. Physical activity and quality of life in breast cancer survivors: the role of self-efficacy and health status. Psychooncology. 2014;23(1):27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Binkley JM, Harris SR, Levangie PK, et al. Patient perspectives on breast cancer treatment side effects and the prospective surveillance model for physical rehabilitation for women with breast cancer. Cancer. 2012;118(suppl 8):2207-2216. [DOI] [PubMed] [Google Scholar]
- 114. van Leeuwen M, Husson O, Alberti P, et al. ; for the EORTC QLG. Understanding the quality of life (QOL) issues in survivors of cancer: towards the development of an EORTC QOL cancer survivorship questionnaire. Health Qual Life Outcomes. 2018;16(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Furmaniak AC, Menig M, Markes MH.. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2016;9(9):CD005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Floyd A, Moyer A.. Group vs. individual exercise interventions for women with breast cancer: a meta-analysis. Health Psychol Rev. 2009;4(1):22-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Leach HJ, Mama SK, Harden SM.. Group-based exercise interventions for increasing physical activity in cancer survivors: a systematic review of face-to-face randomized and non-randomized trials. Support Care Cancer. 2019;27(5):1601-1612. [DOI] [PubMed] [Google Scholar]
- 118. Juvet LK, Thune I, Elvsaas IKÃ, et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: a meta-analysis. Breast. 2017;33:166-177. [DOI] [PubMed] [Google Scholar]
- 119. Pinto-Carral A, Molina AJ, de Pedro Ã, Ayan C.. Pilates for women with breast cancer: a systematic review and meta-analysis. Complement Ther Med. 2018;41:130-140. [DOI] [PubMed] [Google Scholar]
- 120. Buffart LM, Kalter J, Sweegers MG, et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017;52:91-104. [DOI] [PubMed] [Google Scholar]
- 121. Sprangers MA, Schwartz CE.. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med. 1999;48(11):1507-1515. [DOI] [PubMed] [Google Scholar]
- 122. Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15(3):974-986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article itself and in its online Supplementary Material.