Abstract
Borrelia burgdorferi spirochetes that do not cause arthritis or carditis were developed and used to investigate Lyme disease pathogenesis. A clonal isolate of B. burgdorferi N40 (cN40), which induces disease in C3H/HeN (C3H) mice, was repeatedly passaged in vitro to generate nonpathogenic spirochetes. The passage 75 isolate (N40-75) was infectious for C3H mice but did not cause arthritis or carditis, and spirochetes were at low levels or absent in the joints or hearts, respectively. N40-75 could, however, cause disease in severe combined immunodeficient (SCID) mice, suggesting that the response in immunocompetent mice prevented effective spirochete dissemination and the subsequent development of arthritis and carditis. Administration of immune sera at 4 days after spirochete challenge aborted N40-75, but not cN40, infection in SCID mice. A B. burgdorferi genomic expression library was differentially probed with sera from cN40- and N40-75-infected mice, to identify genes that may not be effectively expressed by N40-75 in vivo. N40-75 was defective in the up-regulation of several genes that are preferentially expressed during mammalian infection, including dbpAB, bba64, and genes that map to the cp32 family of plasmids. These data suggest that adaptation and gene expression may be required for B. burgdorferi to effectively colonize the host, evade humoral responses, and cause disease.
Borrelia burgdorferi, the causative agent of Lyme disease, is the most common vector-borne pathogen in the United States (15). B. burgdorferi-infected mice develop arthritis and carditis, thereby partially mimicking the human illness (3, 5, 8, 10, 19, 43). The pathogenesis of murine Lyme borreliosis is multifactorial. The severity of disease has been associated with the number of B. burgdorferi in the affected organs (57), the virulence of specific spirochete isolates (12, 32, 40), and the host immune response to this organism (2, 11, 33, 35, 37, 53, 56). The severity of murine Lyme arthritis is genotype dependent. For example, C3H/HeN (C3H) mice develop more severe arthritis and have greater numbers of spirochetes in the joints than BALB/c mice (5, 36, 44).
B. burgdorferi spirochetes adapt to different environments during their life cycle in Ixodes scapularis ticks and the reservoir host. Within engorging ticks, the B. burgdorferi protein expression dramatically changes, with the synthesis of OspC and down-regulation of OspA (21, 46). These changes are likely to occur in response to the incoming blood meal, temperature shifts, and alterations in spirochete density (21, 42, 49). In the mammalian host, B. burgdorferi must evade host antibodies that arise in response to infection, and selective gene expression and recombination events may facilitate spirochete survival (21, 58–60). Several genes, including eppA, ospE/F paralogues (erps), bbk32, bbk50, and the decorin-binding protein gene dbpA, are not expressed, or are expressed at low levels, by B. burgdorferi cultured in laboratory medium but are apparently up-regulated during infection (1, 16, 25, 51, 54). The function of most of these conditionally expressed gene products is not known. OspA may be one of several plasminogen-binding proteins (28), DbpA adheres to decorin (30), and BBK32 mediates the attachment of B. burgdorferi to fibronectin (41).
The examination of spirochetes continuously cultured in vitro has enhanced our understanding of B. burgdorferi pathogenesis. The sequential passage of uncloned B. burgdorferi in Barbour-Stoenner-Kelly (BSK) II medium results in selection of noninfectious subpopulations with variable plasmid and protein profiles (4, 34, 39, 45, 47). This suggests that specific genes are required for infection. A correlation between plasmid content, or specific B. burgdorferi genes, and infectivity has more recently been demonstrated using cloned spirochetes. Xu and colleagues have shown that specific plasmids are associated with the infectivity of B. burgdorferi B31 (55). Zhang and associates (58) used subtractive hybridization to identify a plasmid-encoded gene family, designated the vmp-like sequence locus (vls), which undergo antigenic variation by promiscuous recombination. This gene family is present in an infectious, clonal isolate of B. burgdorferi B31 but not in clonal in vitro-passaged organisms, suggesting that it is necessary for spirochete infectivity (58).
A clonal isolate of B. burgdorferi N40 (cN40) which causes severe arthritis and carditis in C3H mice has now been passaged in BSK II medium. Spirochetes passaged 75 times in vitro, designated N40-75, were investigated for infectivity and pathogenicity and compared to the parental isolate. The lack of arthritis and carditis was then correlated to a defect in differential N40-75 gene expression in vivo, resulting in an inability of N40-75 to rapidly adapt to the murine B. burgdorferi-specific antibody response.
MATERIALS AND METHODS
Mice.
C3H, C57BL/6 (B6), C3H severe combined immunodeficient (C3H-scid), and C.B-17-scid mice were purchased from the Frederick Cancer Research Center, Frederick, Md. Mice were kept in filter-framed cages and euthanized with CO2.
B. burgdorferi and infections.
cN40 is a clonal isolate of B. burgdorferi N40 that is infectious and pathogenic in mice (9). Spirochetes were grown in BSK II medium at 33°C. To obtain derivatives of cN40, B. burgdorferi spirochetes were serially passaged in BSK II medium every 3 to 5 days by inoculating 50 μl of grown cultures into 7 ml of fresh medium. Clones of N40-75 were obtained by subsurface culture in solid BSK medium, as reported previously (58, 60).
Every 5 to 10 passages, spirochetes were assessed for infectivity and the ability to induce carditis and arthritis in 3- to 6-week-old C3H mice. Individual mice in groups of five were inoculated with 104 or 107 spirochetes in the midline of the back by intradermal injection (26). The animals were necropsied at 21 and 60 days, time points that represent the peak of acute disease and the regression phase of inflammation (10), respectively. Mice were assessed for infection by culturing specimens of the blood, urinary bladder, and skin (at the inoculation site) in BSK II medium at 33°C (24). Cultures were read after 14 days, a time period sufficient to allow a single spirochete to grow to stationary phase.
Quantitation of spirochetes in vitro.
Quantitation of the spirochetes grown in vitro was performed as follows. Cultures were spun down and washed twice with phosphate-buffered saline (PBS). The spirochetes were resuspended in PBS, and the absorbance at 600 nm was determined. The absorbance at 600 nm was shown in parallel experiments to correlate with the number of spirochetes, as counted by dark-field microscopy, in a linear manner. One unit of absorbance at 600 nm was determined to equal 1.9 × 109 spirochetes/ml.
Histopathology of murine Lyme disease.
Formalin-fixed, paraffin-embedded hearts and joints (both knees and tibiotarsi) were examined microscopically for evidence of disease (23). Arthritis and carditis were assessed as previously described (3, 5). All histopathologic assessments were made in a blinded fashion.
In vivo serum treatments.
Relative susceptibility of cN40 and N40-75 spirochetes to immune serum in the murine host was studied in C3H-scid mice. Mice were administered 50 μl of immune sera at the time of spirochete inoculation and 4 and 8 days after infection (7, 20). Immune sera were obtained from immunocompetent C3H mice that had been infected with cN40 or N40-75 for 21 days. C3H-scid mice were necropsied 14 days after challenge and assessed for infection.
DNA extraction and PCR.
Cultured B. burgdorferi DNA was obtained by proteinase K digestion (100 μg/ml, overnight at 55°C) followed by phenol-chloroform-isoamyl alcohol (25:24:1) extraction and ethanol precipitation. PCR was performed on DNA samples using primers for flaB and p39 (chromosomal targets), ospA (lp54 plasmid), erpT (lp28-1), bbk50 (lp36), ospD (lp38), ospC (cp26) and erps (cp32 family of plasmids [52]).
Detection and quantitation of B. burgdorferi in infected tissues.
DNA was extracted from ears, joints, and hearts of cN40- and N40-75-infected mice, using the QIAamp tissue kit (Qiagen Inc., Chatsworth, Calif.). Quantitation of B. burgdorferi was performed by PCR of twofold serial dilutions of the purified DNA. Primers corresponded to the flaB gene. The number of spirochetes was calculated per milligram of DNA, using the following formula: reciprocal of the last dilution with a positive PCR × 200/milligram of DNA used in the first dilution. The sensitivity of the assay was determined to be approximately 200 spirochetes in parallel experiments, and it did not depend on the source of the DNA (not shown).
Immunoscreening of a genomic expression library.
An N40 genomic expression library in lambda ZAP II (51) was differentially screened using immune sera from mice infected with cN40 or N40-75 for 21 days. Duplicate nitrocellulose filters were obtained for each plate, which contained approximately 104 plaques. Duplicate filters were screened with cN40- and N40-75-immune sera, and cN40-specific reactive plaques were rescued for further screening. Potential specific clones were subjected to three rounds of screening. Filters were blocked for 2 h using PBS with 3% bovine serum albumin (PBS-BSA) and incubated for 1 h with sera (1:100 dilution). Membrane filters were then washed three times using PBS with 0.05% Tween 20 and incubated for 30 min with goat anti-mouse immunoglobulin G in PBS-BSA (1:1,000 dilution), conjugated to alkaline phosphatase (Sigma Chemical Co., St. Louis, Mo.). After washing, the filters were incubated with tetramethylbenzidine substrate (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.). Positive clones were recovered in SM buffer with chloroform. After three rounds of screening, positive clones were further tested for possible reactivity with low titers of antibodies using N40-75-immune sera at a dilution of 1:50. Clones 2, 10, and 16 were then excised and sequenced using standard protocols. DNA homology comparisons were performed using BLAST search and the B. burgdorferi genome database (27).
RNA purification and RT-PCR.
RNA was extracted from the spleens of cN40- or N40-75-infected C3H mice by the guanidinium thiocyanate method (17), using a Micro RNA isolation kit (Stratagene, La Jolla, Calif.). To check for DNA contamination, RNA samples were subjected to PCR without further processing, using 100 ng of RNA and primers corresponding to flaB, ospA, bba64 (27), dbpA, dbpB, bba65 (27), bba66 (27), p21, erpD, gene-1, and gene-2. Forty cycles were performed, with denaturation (94°C, 1 min), annealing (55°C, 1 min), and extension (72°C, 1 min) steps. If necessary, the samples were treated with DNase (Boehringer Mannheim, Indianapolis, Ind.) for 2 h at 37°C, extracted with phenol-chloroform-isoamyl alcohol (25:24:1), and ethanol precipitated. Aliquots of 5 to 10 μg were then used to obtain cDNA with a reverse transcription (RT)-PCR kit (Stratagene) utilizing random primers. PCR were then performed using the same primers and conditions and 2 to 5 μl of the cDNA samples.
Primers.
Sequences of the primers used are shown in Table 1.
TABLE 1.
Primers used
| Primer (reference) | Sequence |
|---|---|
| flaB | 5′-AGA ATT ACT TCA AAG GCT-3′ |
| 5′-TGT AGT TGT AAC ATT AAC-3′ | |
| ospA | 5′-GGT CAA ACC ACA CTT GAA GTT-3′ |
| 5′-GTC AGT GTC ATT AAG TTC-3′ | |
| erpT | 5′-TTC ACA AGT AGC ACA ACA TGC TCC-3′ |
| 5′-GCT ATC ACC ACC AAT TTC AAG TCC-3′ | |
| bbk50 | 5′-CAG CTT CTT CTA AAG AGG TAG AAG C-3′ |
| 5′-TAA ATC GCA ACA AGC TTT AGC-3′ | |
| ospD | 5′-TAT CTT GTG TTC ATG ATA AAC-3′ |
| 5′-ATC CAA TGT CTC TTT GAC TG-3′ | |
| ospC | 5′-GCC GTG AAA GAA GTT GAG ACC TTA-3′ |
| 5′-TAA GAT TGT CCA GAC CAA GCA CTG-3′ | |
| erps | |
| E(13) (52) | 5′-ATG TTT ATT ATT TGT GCT GTT T-3′ |
| E (310) (52) | 5′-AAA CTA GTT TTA AAT GAT CC-3′ |
| dbpA | 5′-GCT ACT GTA GTA GCT CGC A-3′ |
| 5′-TTG AAT CGT CCT CTA AGG-3′ | |
| dbpB | 5′-CTG GAA ACA GTG GTC AAT TC-3′ |
| 5′-TCT CTC CAC CAT TTT CAA T-3′ | |
| bba64 | 5′-TTC CTT CGT TGA AAT TTA AAG ATG-3′ |
| 5′-TGA TTT CTT GCT CTC TGG AAG TC-3′ | |
| bba65 | 5′-AGC GGA TCT AAT TGA CAA GC-3′ |
| 5′-TCC ATG TTG ATT GCT AAA TC-3′ | |
| bba66 | 5′-GAT TGT TTT TAT TTT CTT GCA CG-3′ |
| 5′-TGA AAC GGC TTG AAT AGG TAT TC-3′ | |
| p21 | 5′-TTC TAA CTT CTA TTT TAA ATT-3′ |
| 5′-AAT AAT GAG TTA AAA GTT AAG CAA-3′ | |
| erpD | 5′-GCG ATG ATC CTA ATG GCA-3′ |
| 5′-CCA AGT TGT ACG TAT AGA GCT-3′ | |
| gene-1 | 5′-AAC AAG CTC TAT TTA ACT AGT C-3′ |
| 5′-GAT TCT TAC TGG TGA AAA G-3′ | |
| gene-2 | 5′-GCA TTT AAG CAA TAT TCT TAG-3′ |
| 5′-TCA CTT GAG CCC TTT GCT ATT-3′ |
RESULTS
B. burgdorferi N40 loses pathogenicity, but not infectivity, upon in vitro passage.
A clonal isolate of B. burgdorferi N40 (cN40) that is highly infectious and pathogenic in C3H mice was continuously passaged in vitro to determine whether the spirochete would lose the ability to infect mice or cause disease. Despite being passaged 75 times in vitro, N40 retained its capacity to infect C3H mice, as demonstrated by recovery of the spirochete from selected organs at 2 to 3 weeks following inoculation with 104 organisms (Table 2). B. burgdorferi started to lose pathogenicity (the ability to cause arthritis and carditis) after 22 passages in vitro (Table 2). At passage 75, the spirochetes no longer elicited joint or cardiac inflammation in immunocompetent mice, regardless of their genetic (C3H or B6) background or dose (up to 107) of inoculum (Table 2). Arthritis and carditis were also not evident when N40-75-infected animals were examined for evidence of inflammation after 60 days (Table 2), indicating that the appearance of disease was not merely delayed.
TABLE 2.
Loss of B. burgdorferi pathogenicity with in vitro passage in immunocompetent micea
| Mouse | Isolate | Day of sacrifice | Infection (no. of specimens positive/no. examined)
|
Arthritis (no. of mice with arthritis/no. examined) | Carditis (no. of mice with carditis/no. examined) | ||
|---|---|---|---|---|---|---|---|
| Blood | Inoculation site | Bladder | |||||
| C3H | cN40 | 21 | 1/5 | 4/5 | 5/5 | 5/5 | 5/5 |
| p22 | 21 | 2/5 | 2/5 | 5/5 | 3/5 | 3/5 | |
| p51 | 21 | 0/5 | 2/3 | 4/5 | 0/5 | 1/5 | |
| N40-75 | 21 | 1/5 | 4/5 | 3/5 | 0/5 | 0/5 | |
| N40-75 | 60 | 0/5 | 4/5 | 4/4 | 0/5 | 0/5 | |
| N40-75 (107) | 21 | 0/5 | 3/4 | 2/3 | 0/5 | 0/5 | |
| B6 | cN40 | 21 | 0/5 | 5/5 | 5/5 | 0/5 | 5/5 |
| N40-75 | 21 | 0/5 | 3/5 | 4/5 | 0/5 | 0/5 | |
C3H and B6 mice were infected with cN40 or its derivatives (p22, p51, and N40-75) obtained by in vitro culturing. Mice were infected with 104 spirochetes, unless otherwise indicated, and necropsied at 21 or 60 days after infection. Arthritis and carditis were assessed in a blinded fashion.
Characterization of N40-75.
To understand why N40-75 was not pathogenic, we compared N40-75 and cN40 for antigenic and genetic differences. The protein profiles of cultured cN40 and N40-75 were similar except for a lower level of OspA/B and increased level of OspC expression by N40-75 (Fig. 1A). Nevertheless, the level of expression of OspA/B varied among different clonal N40-75 spirochetes (Fig. 1A). Moreover, the analysis of N40 and N40-75 extracts by Western blotting using rabbit cN40 hyperimmune sera showed no differences between the two passages (Fig. 1B). Western blot analysis of cN40 and N40-75 with cN40- and N40-75-infected mouse sera revealed some differences in the proteins recognized by both sera (Fig. 1C). The differences were more notable in the range of 19 to 25 kDa, as well as 30 to 32 kDa (Fig. 1C, arrows), indicating that infection with both passages induced differentiated antibody responses.
FIG. 1.
cN40 and N40-75 do not differ substantially when grown in vitro. cN40, N40-75, and several N40-75 clones were grown in vitro to stationary phase and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis to compare protein contents (A). Western blot analysis with cN40 rabbit hyperimmune sera was also performed to test the presence of OspA, -B, and -C in both passages (B). The immune response to cN40 and N40-75 was also tested by Western blotting (C), by using 21-day-infected mouse sera with cN40 and N40-75.
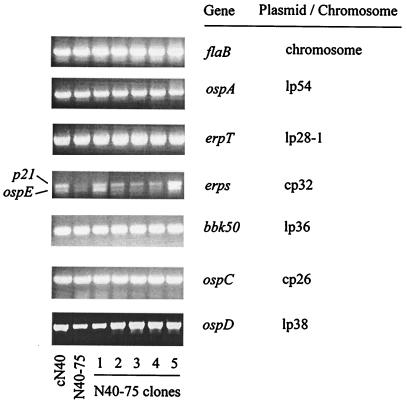
DNA PCR targeted at genes expressed in the linear chromosome, as well as several plasmids, indicated the presence of these genes both in cN40 and N40-75 (Fig. 2), as well as several N40-75 clones (Fig. 2). The analysis of the amplification products obtained with cN40 and N40-75 DNA when using primers E(13) and E(310) (52) indicated the presence of ospE and p21, but no other erps, in both passages (not shown), in agreement with the fact that only these two genes have been reported to be sequenced from this isolate (N40) (52). The presence of erpT by PCR (Fig. 2) and the vls locus by Southern blotting (not shown) in both spirochetes indicated that plasmid lp28-1 had not been lost during in vitro passage. These data indicate that no readily apparent loss of DNA could be associated with the loss of pathogenicity resulting from in vitro passage.
FIG. 2.
DNA contents of cN40 and N40-75, as well as of several N40-75 clones. DNA PCR for several genes was performed to detect any possible loss of DNA as a result of in vitro passage. The plasmids in which the amplified genes are present are indicated.
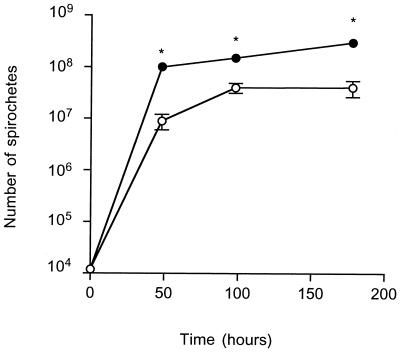
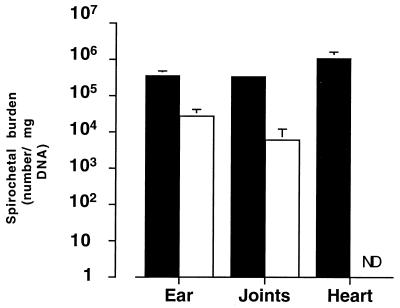
To determine whether pathogenicity was related to spirochete growth, cN40 and N40-75 multiplication rates in vitro and bacterial burden in vivo were examined. When equal numbers of cN40 and N40-75 (1.4 × 104) were incubated in BSK II medium, N40-75 grew more rapidly than cN40 (Fig. 3). Differences were evident as early as 48 h (doubling times of 5.2 and 3.7 h for cN40 and N40-75, respectively) and continued throughout the culture period (t test, P < 0.05). Once stationary-phase growth had been reached, the density of N40-75 was greater than that of cN40 (Fig. 3). In contrast, N40-75 was less abundant than cN40 during infection of immunocompetent mice. Two weeks after inoculation into mice, the number of N40-75 in the skin (ear tissues) was significantly (P < 0.05) less than the number of cN40 (average ± standard error [SE] per milligram of DNA, 27,000 ± 15,000 for N40-75 and 360,000 ± 120,000 for cN40 [Fig. 4]). The number of N40-75 spirochetes in the joints of the infected mice was extremely low (6,000 ± 6,000) compared with cN40 (340,000 ± 88,000) (Fig. 4). N40-75 could not be detected by PCR in the heart tissues, whereas large numbers of cN40 (1,100,000 ± 500,000) were apparent (Fig. 4).
FIG. 3.
N40-75 grows faster than cN40 in vitro. Equal amounts of cN40 and N40-75 were grown in BSK II medium and analyzed at different time points over 1 week. Data represent the average of five individual experiments ±SE. An asterisk indicates significant difference between cN40 and N40-75 at that particular time point (t test, P < 0.05).
FIG. 4.
N40-75 does not effectively disseminate in the murine host. Ear, joint, and heart tissue was used to quantitate B. burgdorferi by DNA PCR using flaB targets in mice infected for 3 weeks. Data represent the average ± SE of five individual mice. ■, cN40; □, N40-75.
N40-75 causes arthritis and carditis in SCID mice.
Pathogenicity and bacterial burden in five cN40- and five N40-75-infected C3H-scid mice were then examined to assess whether spirochete growth was influenced by the B. burgdorferi-specific response. All of the N40-75-infected SCID mice developed arthritis and carditis that was indistinguishable from cN40-induced disease. Moreover, the severity of the inflammation was comparable in cN40- and N40-75-infected SCID mice (not shown). Quantitative PCR revealed similar number of spirochetes in ear (N40-75, 200,000 ± 10,000; cN40, 400,000 ± 200,000), joint (N40-75, 50,000 ± 10,000; cN40, 60,000 ± 20,000), or heart (N40-75, 180,000 ± 110,000; cN40, 70,000 ± 60,000) tissues in N40-75- and cN40-infected SCID mice (Fig. 5).
FIG. 5.
N40-75 disseminates efficiently in SCID mice. cN40 and N40-75 were quantitated in ears, joints, and hearts of C.B-17-scid mice after 2 weeks of infection as for Fig. 3. ■, cN40; □, N40-75. Data represent five individual mice.
N40-75 does not evade immune clearance as rapidly as cN40.
The SCID mouse studies suggest that the inability of N40-75 to efficiently colonize murine tissues may be related to the host immune response. Previous reports showed that B. burgdorferi-specific antibodies protect against infection (6, 7). Passive transfer experiments also demonstrated that B. burgdorferi spirochetes become resistant to immune clearance within 4 to 5 days following syringe inoculation (7, 20). We therefore determined whether B. burgdorferi-specific humoral responses were responsible for the decreased number of spirochetes in N40-75-infected immunocompetent mice. Antisera from B. burgdorferi-infected C3H mice were administered to cN40- or N40-75-infected SCID mice. As expected (20), administration of immune sera at the time of challenge with cN40 resulted in protection of the mice (Table 3). The protective effect of immune sera was not apparent at 4 days after cN40 inoculation (20), suggesting that syringe-administered cN40 spirochetes adapt to their new environment by this interval (Table 3). In contrast, when N40-75-infected SCID mice were administered immune sera from B. burgdorferi-infected immunocompetent mice, protection against spirochetal challenge was evident when the sera were administered at 4 days after infection (Table 3). Sera from either cN40- or N40-75-infected immunocompetent mice were equally protective (Table 3), suggesting that the protective antibody specificities in the two sera were similar. At day 8 of infection, immune sera were no longer protective (Table 3). These data suggest that N40-75 requires a longer period of time than cN40 to adapt and evade antibodies present in sera from B. burgdorferi-infected immunocompetent mice.
TABLE 3.
Effect of immune sera on N40-75- or cN40-infected SCID micea
| Spirochete challenge | Immune serum | Day of serum administration | Infection (no. of mice infected/no. examined)
|
||
|---|---|---|---|---|---|
| Blood | Inoculation site | Bladder | |||
| cN40 | NMS | 0 | 1/5 | 5/5 | 5/5 |
| cN40 immune | 0 | 0/5 | 0/5 | 0/5 | |
| 4 | 2/5 | 4/5 | 5/5 | ||
| 8 | 1/4 | 4/4 | 4/4 | ||
| N40-75 immune | 0 | 0/5 | 0/5 | 0/5 | |
| 4 | 0/5 | 3/5 | 3/4 | ||
| N40-75 | NMS | 0 | 2/5 | 3/5 | 5/5 |
| cN40 immune | 0 | 0/5 | 0/5 | 0/5 | |
| 4 | 0/5 | 0/5 | 0/5 | ||
| 8 | 1/4 | 4/4 | 3/4 | ||
| N40-75 immune | 0 | 0/5 | 0/5 | 0/5 | |
| 4 | 0/5 | 0/5 | 0/5 | ||
C3H-scid mice were inoculated with 104 spirochetes and then treated with immune sera from B. burgdorferi-infected mice. Immune sera were administered within 1 h of spirochete inoculation (day 0) or 4 or 8 days after inoculation. Immune sera from immunocompetent C3H mice that had been infected with cN40 or N40-75 for 21 days were used. NMS, normal mouse serum (control).
N40-75 is deficient in in vivo gene expression.
After entering the mammalian host, several B. burgdorferi genes are differentially expressed (21). We examined whether the delayed ability of N40-75 to host adapt was associated with alterations in the up- or down-regulation of in vivo-expressed genes or genes coding for antigens recognized by antibodies in immune sera. To identify antigens selectively recognized by sera from cN40-infected mice, a genomic expression library was differentially screened with immune sera from cN40- and N40-75-infected mice (51). Immune sera from B. burgdorferi-infected mice contain antibodies to antigens synthesized during infection. If the antigenic profiles of N40-75 and cN40 are different during infection, then the antibodies raised during infection may differ. Using this strategy, we isolated three clones that were recognized by cN40- but not N40-75-infected murine sera (Table 4). Clone 2 had an insert of 2,570 bp, containing the genes bba64 (a p35 homologue [29] in the published B31 genome sequence [27]), bba65, and bba66 (yet another p35 homologue) (Table 4). Clone 10 had an insert of 2,032 bp and included dbpA (incomplete) and dbpB (bba24 and bba25, respectively), which form an operon (22, 31). It also contained three small open reading frames (bba26, bba27, and bba28) (27) and the incomplete sequence for bba29. Clone 16 had an insert of 3,360 bp and contained sequences related to the ospEF gene family: p21 (51), erpD (13, 50), and two unknown genes (Table 4). Both clones 2 and 10 mapped within the linear plasmid of 54 kb, while clone 16 contained sequences not present in the published B31 sequence (27). The presence of p21 and ErpD suggests that this clone may belong to the cp32 family of plasmids.
TABLE 4.
cN40-specific clones selected by immunoscreening of an N40 genomic library with cN40- and N40-75-immune seraa
N40-75 does not express bba64, bba65, bba66, and dbpAB.
To directly determine whether these specific genes, which were identified by differential immunoscreening, were expressed by cN40 but not N40-75 during infection, we performed RT-PCR analysis of spleens from cN40- and N40-75-infected mice. dbpAB, bba64, bba65, bba66, erpD, and gene-2 mRNA could be readily detected in spleens of cN40-infected mice at 2 weeks of infection (Fig. 6). In contrast, N40-75-infected spleens lacked mRNA for these genes. As expected, the flaB gene (control) could be detected in cN40- or N40-75-infected animals, indicating that the spirochetes were present in these tissues. Neither cN40 or N40-75 expressed detectable levels of p21 mRNA (Fig. 6), consistent with previous reports for this time period of infection (18). Finally, gene-1 mRNA was lacking in cN40 or N40-75 upon infection during 2 weeks (Fig. 6).
FIG. 6.
N40-75 does not up-regulate in vivo genes. cN40- and N40-75-infected mouse spleens were used to extract total RNA, and RT-PCR for B. burgdorferi dbpA, dbpB, bba64, bba65, bba66, p21, erpD, gene-1, and gene-2 was performed. As a control, flaB was used to identify spirochetes in spleens of the infected animals. N40-75 DNA was used as a positive control, demonstrating the presence of the genes in high-passage N40.
DISCUSSION
We characterized the pathogenicity of derivatives of a clonal population of B. burgdorferi cN40. Previous researchers have shown that B. burgdorferi passaged in vitro rapidly loses infectivity (40, 58). Our results differ in that cloned N40 spirochetes remained infectious in mice for up to 75 passages, suggesting that the infectivity of cN40 is relatively stable compared with other spirochetes passaged in vitro. Alternatively, infectivity could be maintained becaused short subculture intervals were used. It may be that prolonged growth under stationary-phase conditions leads to plasmid loss and more rapid reduction in infectivity. Indeed, both cN40 and N40-75 contained the plasmid expressing vls genes, supporting the contention that vls genes are important for infectivity and are not lost by cN40 spirochetes. Although N40-75 is infectious in C3H mice, these spirochetes do not cause arthritis or carditis in immunocompetent mice. The lack of disease in N40-75-infected mice correlated with a reduction, or lack, of spirochetal DNA in the joints and hearts. N40-75 could be readily cultured, or amplified by PCR, from the bladder, skin, and spleen, suggesting that the reduction in pathogenesis is due to an inability of N40-75 to effectively colonize specific tissues in sufficient numbers to cause disease. Lack of pathogenicity of N40-75 cannot be explained by assuming that the majority of cells in the uncloned N40-75 population are not infectious, since infection of mice with 104 spirochetes rendered culture positive organs that were not significantly different between cN40- and N40-75-infected animals (Table 2). Moreover, infection with 1,000 times more N40-75 spirochetes (107) did not change the nonpathogenic phenotype of high-passaged N40, arguing against this explanation. This reasoning is supported by the obtention and characterization at the DNA level by PCR of several clones of N40-75, which showed no loss of different pieces of DNA tested. These clones were infectious in C3H mice, but they did not cause arthritis or carditis.
The analysis of the proteins profiles of cN40 and N40-75 indicated differences in OspA/B and OspC. OspA/B appeared to be down-regulated in in vitro-grown N40-75, although the analysis of both passages by Western blotting using cN40-hyperimmune sera revealed that both contained the proteins. Moreover, the analysis of N40-75 clones revealed variability in the amount of OspA/B expressed. In contrast, OspC was up-regulated in high-passage N40. The down-regulation in vivo of ospAB and the up-regulation of ospC gene expression after syringe inoculation have been demonstrated (18, 38). Therefore, these differences are not likely to be related with the lack of N40-75 pathogenicity.
In order to survive and efficiently colonize its hosts, B. burgdorferi must adapt to different environments during its life cycle. It is well known that when infected I. scapularis engorge, the spirochete protein expression alters before migrating to the tick salivary glands and entering the vertebrate host (4, 21, 45, 47, 55). These changes include the up-regulation of ospC and the down-regulation of ospA (21, 46). The adaptation to invade the mammalian host is associated with changes in several genes, including bbk32, bbk50, ospEF homologues, and dbpA/B. There is a time period (around 4 days after syringe inoculation) in which cN40 is no longer vulnerable to the passive administration of infected mouse sera (7, 20). The process of adaptation and immune evasion probably involves changes in gene expression or recombination events in genes like vls (60). Zhang and collaborators have demonstrated that recombination of the vls gene occurs in vivo, after experimental infection of mice and as soon as 4 days after the initiation of the infection (60). The evasion of the B. burgdorferi-specific humoral responses is probably due to a combination of selective gene expression and recombination, although the relative contribution of each remains to be elucidated.
These data provide evidence that long-term-cultured B. burgdorferi spirochetes are more susceptible to host immunity. Susceptibility of N40-75-infected SCID mice to the development of arthritis and carditis suggests that efficient colonization and dissemination in the host involves immune evasion. Passive transfer of immune sera to N40-75-infected SCID mice at 8 days following challenge did not influence the establishment of infection, indicating the N40-75 can adapt within this interval. The delayed adaptation period contribute to the lower number of N40-75 in immunocompetent mice.
Spirochete genes selectively expressed during infection are likely to be important for the maintenance of B. burgdorferi in the mammalian host and, ultimately, the completion of the vector-host-vector cycle. Initial characterization of antibody responses arising against cN40 and N40-75 in vivo (Fig. 1C) indicated that cN40-immune sera reacted with a wider range of proteins. Our immunoscreening procedure resulted in the detection of three clones that represented genes expressed exclusively by cN40 and not N40-75 upon infection. These expression units included genes that are preferentially expressed in the vertebrate host, including dbpAB (14) and ospEF homologues (21). Our results also showed that these genes were not merely lost by N40-75, since we could readily amplify these sequences when using N40-75 DNA. Moreover, DNA subtraction techniques indicated that a loss of DNA contents had not occurred during in vitro passage (data not shown). Interestingly, other researchers have reported a low level of expression of both DbpA and -B in high (>30)-passage B. burgdorferi (30, 31), consistent with our findings for N40-75. Clone 2 contained three genes that could have expressed antigens that were only recognized by the immune sera from cN40-infected mice. The three genes, bba64, bba65, and bba66, were expressed exclusively by cN40 in infected mice. Clone 16 contained four genes: p21 (18), erpD, and two unknown genes. Of them, p21 was not detected in cN40- or N40-75-infected mice, consistent with a later time point expression during infection (18), erpD and gene-2 were specifically expressed by cN40 at 2 weeks of infection.
The exact functions of most of the in vivo genes, as well as the signals that trigger the initiation of their expression once B. burgdorferi enters the mammalian host, remain unknown. Among the factors that could trigger changes in gene expression or recombination events, temperature has been the most extensively studied. Stevenson and collaborators (48, 49) have examined the effect of temperature shifting on the expression of ospC (49) and the erps (48). The temporal expression of p21, an ospE homologue, cannot be explained solely by temperature alterations, since this gene is not expressed until 21 days after initiation of the infection (18). Moreover, Zhang and collaborators have recently excluded temperature as the factor initiating recombination at the vls locus, speculating about the necessity of other host factors triggering this phenomenon (60). A detailed study of the changes in gene expression occurring after infection should provide insight into host factors that influence those changes.
Our results indicate that B. burgdorferi has to adapt to the mammalian host in order to be able to reach a certain number and induce inflammation. Our data also suggest that the adaptation process implies at least two phenomena: the evasion of immune responses arising during infection and the up-regulation of genes that are needed for new functions that need to be performed in the new environment. To comply with the first task, there are two possibilities that could explain our results: N40-75 has impaired the ability to induce recombination (i.e., at the vls locus), and/or the down-regulation of highly immunogenic genes that do not need to be expressed in the new environment is impaired in N40-75. The adaptation phenomenon would then be a complex process in which both immune evasion and up-regulation of certain genes need to be accomplished. This is true for immunocompetent mice, although our results with SCID mice suggest that immune evasion in the absence of an acquired immune response is not required for pathogenicity. However, until a good system is provided to study in vivo function of the genes obtained in the differential immunoscreening (i.e., a transformation system that works in infectious and pathogenic B. burgdorferi), no direct evidence will be obtained about the exact roles of these genes during infection.
In conclusion, we have generated a derivative of cN40, designated N40-75, which do not cause arthritis or carditis in immunocompetent mice. The inability of N40-75 to adapt to the mammalian host correlates with its inability to induce disease. This defect in differential gene expression results in an increase and prolonged susceptibility of N40-75 to the antibodies in immune sera and an inability of these N40-75 spirochetes to effectively colonize and disseminate within the murine host due to that immune-based vulnerability.
ACKNOWLEDGMENTS
This work was supported by grants AI-32947 and AR-45740 from the National Institutes of Health, by the Arthritis Foundation, and by the American Heart Association. E.F. is a recipient of a Burroughs Wellcome Clinical Scientist Award in Translational Research.
We thank Debbie Beck for technical assistance.
REFERENCES
- 1.Akins D R, Porcella S F, Popova T G, Shevchenko D, Baker S I, Li M, Norgard M V, Radolf J D. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homologue. Mol Microbiol. 1995;18:507–520. doi: 10.1111/j.1365-2958.1995.mmi_18030507.x. [DOI] [PubMed] [Google Scholar]
- 2.Anguita J, Persing D H, Rincon M, Barthold S W, Fikrig E. Effect of anti-interleukin 12 treatment on murine lyme borreliosis. J Clin Investig. 1996;97:1028–1034. doi: 10.1172/JCI118494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong A L, Barthold S W, Persing D H, Beck D S. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am J Trop Med Hyg. 1992;47:249–258. doi: 10.4269/ajtmh.1992.47.249. [DOI] [PubMed] [Google Scholar]
- 4.Barbour A G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J Clin Microbiol. 1988;26:475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold S W, Beck D S, Hansen G M, Terwilliger G A, Moody K D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 6.Barthold S W, Bockenstedt L K. Passive immunizing activity of sera from mice infected with Borrelia burgdorferi. Infect Immun. 1993;61:4696–4702. doi: 10.1128/iai.61.11.4696-4702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barthold S W, de Souza M, Feng S. Serum-mediated resolution of Lyme arthritis in mice. Lab Investig. 1996;74:57–67. [PubMed] [Google Scholar]
- 8.Barthold S W, de Souza M S, Janotka J L, Smith A L, Persing D H. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 9.Barthold S W, Fikrig E, Bockenstedt L K, Persing D H. Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect Immun. 1995;63:2255–2261. doi: 10.1128/iai.63.6.2255-2261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barthold S W, Persing D H, Armstrong A L, Peeples R A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 11.Brunet L R, Sellitto C, Spielman A, Telford S R I. Antibody response of the mouse reservoir of Borrelia burgdorferi in nature. Infect Immun. 1995;63:3030–3036. doi: 10.1128/iai.63.8.3030-3036.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll J A, Gherardini F C. Membrane protein variations associated with in vitro passage of Borrelia burgdorferi. Infect Immun. 1996;64:392–398. doi: 10.1128/iai.64.2.392-398.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casjens S, van Vugt R, Tilly K, Rosa P A, Stevenson B. Homology throughout the multiple 32-kilobase circular plasmids present in Lyme disease spirochetes. J Bacteriol. 1997;179:217–227. doi: 10.1128/jb.179.1.217-227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassat D R, Patel N K, Ulbrandt N D, Hanson M S. DbpA, but not OspA, is expressed by Borrelia burgdorferi during spirochetemia and is a target for protective antibodies. Infect Immun. 1998;66:5379–5387. doi: 10.1128/iai.66.11.5379-5387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Lyme disease—United States, 1996. Morbid Mortal Weekly Rep. 1997;46:531–535. [PubMed] [Google Scholar]
- 16.Champion C I, Blanco D R, Skare J T, Haake D A, Giladi M, Foley D, Miller J N, Lovett M A. A 9.0-kilobase-pair circular plasmid of Borrelia burgdorferi encodes an exported protein: evidence for expression only during infection. Infect Immun. 1994;62:2653–2661. doi: 10.1128/iai.62.7.2653-2661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 18.Das S, Barthold S W, Giles S S, Montgomery R R, Telford S R I, Fikrig E. Temporal pattern of Borrelia burgdorferi p21 expression in ticks and the mammalian host. J Clin Investig. 1997;99:987–995. doi: 10.1172/JCI119264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Defosse D L, Duray P H, Johnson R C. The NIH-3 immunodeficient mouse is a model for Lyme borreliosis myositis and carditis. Am J Pathol. 1992;141:3–10. [PMC free article] [PubMed] [Google Scholar]
- 20.de Silva A, Fikrig E, Hodzic E, Kantor F S, Telford S R I, Barthold S W. Immune evasion by tickborne and host-adapted Borrelia burgdorferi. J Infect Dis. 1998;177:395–400. doi: 10.1086/514200. [DOI] [PubMed] [Google Scholar]
- 21.de Silva A M, Fikrig E. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J Clin Investig. 1997;99:377–379. doi: 10.1172/JCI119169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng S, Hodzic E, Stevenson B, Barthold S W. Humoral immunity to Borrelia burgdorferi N40 decorin binding proteins during infection of laboratory mice. Infect Immun. 1998;66:2827–2835. doi: 10.1128/iai.66.6.2827-2835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fikrig E, Barthold S W, Flavell R A. OspA vaccination of mice with established Borrelia burgdorferi infection alters disease but not infection. Infect Immun. 1993;61:2553–2557. doi: 10.1128/iai.61.6.2553-2557.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fikrig E, Barthold S W, Kantor F S, Flavell R A. Long-term protection of mice from Lyme disease by vaccination with OspA. Infect Immun. 1992;60:773–777. doi: 10.1128/iai.60.3.773-777.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fikrig E, Barthold S W, Sun W, Feng W, Telford S R I, Flavell R A. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- 26.Fikrig E, Tao H, Kantor F S, Barthold S W, Flavell R A. Evasion of protective immunity by Borrelia burgdorferi by truncation of outer surface protein B. Proc Natl Acad Sci USA. 1993;90:4092–4096. doi: 10.1073/pnas.90.9.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Venter J C, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs H, Wallich R, Simon M M, Kramer M D. The outer surface protein A of the spirochete Borrelia burgdorferi is a plasmin(ogen) receptor. Proc Natl Acad Sci USA. 1994;91:12594–12598. doi: 10.1073/pnas.91.26.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilmore R D, Jr, Kappel K J, Johnson B J. Molecular characterization of a 35-kilodalton protein of Borrelia burgdorferi, an antigen of diagnostic importance in early Lyme disease. J Clin Microbiol. 1997;35:86–91. doi: 10.1128/jcm.35.1.86-91.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo B P, Norris S J, Rosenberg L C, Hook M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagman K E, Lahdenne P, Popova T G, Porcella S F, Akins D R, Radolf J D, Norgard M V. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect Immun. 1998;66:2674–2683. doi: 10.1128/iai.66.6.2674-2683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes C A, Engstrom S M, Coleman L A, Kodner C B, Johnson R C. Protective immunity is induced by a Borrelia burgdorferi mutant that lacks OspA and OspB. Infect Immun. 1993;61:5115–5122. doi: 10.1128/iai.61.12.5115-5122.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keane-Myers A, Nickell S P. Role of IL-4 and IFN-gamma in modulation of immunity to Borrelia burgdorferi in mice. J Immunol. 1995;155:2020–2028. [PubMed] [Google Scholar]
- 34.Kenefick K B, Lim L C, Alder J D, Schmitz J L, Czuprynski C J, Schell R F. Induction of interleukin-1 release by high- and low-passage isolates of Borrelia burgdorferi. J Infect Dis. 1993;167:1086–1092. doi: 10.1093/infdis/167.5.1086. [DOI] [PubMed] [Google Scholar]
- 35.Lengl-Janssen B, Strauss A F, Steere A C, Kamradt T. The T helper cell response in Lyme arthritis: differential recognition of Borrelia burgdorferi outer surface protein A in patients with treatment-resistant or treatment-responsive Lyme arthritis. J Exp Med. 1994;180:2069–2078. doi: 10.1084/jem.180.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma Y, Seiler K P, Eichwald E J, Weis J H, Teuscher C, Weis J J. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect Immun. 1998;66:161–168. doi: 10.1128/iai.66.1.161-168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matyniak J E, Reiner S L. T helper phenotype and genetic susceptibility in experimental Lyme disease. J Exp Med. 1995;181:1251–1254. doi: 10.1084/jem.181.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montgomery R R, Malawista S E, Feen K J, Bockenstedt L K. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–269. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moody K D, Barthold S W, Terwilliger G A. Lyme borreliosis in laboratory animals: effect of host species and in vitro passage of Borrelia burgdorferi. Am J Trop Med Hyg. 1990;43:87–92. doi: 10.4269/ajtmh.1990.43.87. [DOI] [PubMed] [Google Scholar]
- 40.Norris S J, Howell J K, Garza S A, Ferdows M S, Barbour A G. High- and low-infectivity phenotypes of clonal populations of in vitro-cultured Borrelia burgdorferi. Infect Immun. 1995;63:2206–2212. doi: 10.1128/iai.63.6.2206-2212.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Probert W S, Johnson B J B. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi B31. Mol Microbiol. 1998;30:1003–1016. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- 42.Ramamoorthy R, Phillip M T. Differential expression of Borrelia burgdorferi proteins during growth in vitro. Infect Immun. 1998;66:5119–5124. doi: 10.1128/iai.66.11.5119-5124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaible U E, Gay S, Museteanu C, Kramer M D, Zimmer G, Eichmann K, Museteanu U, Simon M M. Lyme borreliosis in the severe combined immunodeficiency (scid) mouse manifests predominantly in the joints, heart, and liver. Am J Pathol. 1990;137:811–820. [PMC free article] [PubMed] [Google Scholar]
- 44.Schaible U E, Kramer M D, Wallich R, Tran T, Simon M M. Experimental Borrelia burgdorferi infection in inbred mouse strains: antibody response and association of H-2 genes with resistance and susceptibility to development of arthritis. Eur J Immunol. 1991;21:2397–2405. doi: 10.1002/eji.1830211016. [DOI] [PubMed] [Google Scholar]
- 45.Schwan T G, Burgdorfer W, Garon C F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988;56:1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simpson W J, Garon C F, Schwan T G. Analysis of supercoiled circular plasmids in infectious and non-infectious Borrelia burgdorferi. Microb Pathog. 1990;8:109–118. doi: 10.1016/0882-4010(90)90075-2. [DOI] [PubMed] [Google Scholar]
- 48.Stevenson B, Bono J L, Schwan T G, Rosa P. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect Immun. 1998;66:2648–2654. doi: 10.1128/iai.66.6.2648-2654.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevenson B, Tilly K, Rosa P A. A family of genes located on four separate 32-kilobase circular plasmids in Borrelia burgdorferi B31. J Bacteriol. 1996;178:3508–3516. doi: 10.1128/jb.178.12.3508-3516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suk K, Das S, Sun W, Jwang B, Barthold S W, Flavell R A, Fikrig E. Borrelia burgdorferi genes selectively expressed in the infected host. Proc Natl Acad Sci USA. 1995;92:4269–4273. doi: 10.1073/pnas.92.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sung S Y, Lavoie C P, Carlyon J A, Marconi R T. Genetic divergence and evolutionary instability in ospE-related members of the upstream homology box gene family in Borrelia burgdorferi sensu lato complex isolates. Infect Immun. 1998;66:4656–4668. doi: 10.1128/iai.66.10.4656-4668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szczepanski A, Benach J L. Lyme borreliosis: host responses to Borrelia burgdorferi. Microbiol Rev. 1991;55:21–34. doi: 10.1128/mr.55.1.21-34.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallich R, Brenner C, Kramer M D, Simon M M. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Y, Kodner C, Coleman L, Johnson R C. Correlation of plasmids with infectivity of Borrelia burgdorferi sensu stricto type strain B31. Infect Immun. 1996;64:3870–3876. doi: 10.1128/iai.64.9.3870-3876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang L, Ma Y, Schoenfeld R, Griffiths M, Eichwald E, Araneo B, Weis J J. Evidence for B-lymphocyte mitogen activity in Borrelia burgdorferi-infected mice. Infect Immun. 1992;60:3033–3041. doi: 10.1128/iai.60.8.3033-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang L, Weis J H, Eichwald E, Kolbert C P, Persing D H, Weis J J. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang J R, Hardham J M, Barbour A G, Norris S J. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J R, Norris S J. Genetic variation of the Borrelia burgdorferi gene vlsE involves cassette-specific, segmental gene conversion. Infect Immun. 1998;66:3698–3704. doi: 10.1128/iai.66.8.3698-3704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J R, Norris S J. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect Immun. 1998;66:3689–3697. doi: 10.1128/iai.66.8.3689-3697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]