Abstract
Glucose utilization by visceral adipose tissue (VAT) reflects inflammatory activity, which also promotes tumor growth and carcinogenesis. The effect of metabolically active VAT on survival outcomes in breast cancer is unknown. We investigated survival outcomes in patients with breast cancer based on the standardized uptake value (SUV) of VAT (SUVmean-VAT) using 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT). A total of 148 patients with breast cancer were divided into high- and low groups according to their SUVmean-VAT and SUVmax-tumor. Clinical characteristics and survival outcomes were compared between the groups. High SUVmean-VAT was associated with poor recurrence-free survival (RFS; hazard ratio [HR], 2.754; 95% confidence interval [CI], 1.090–6.958, p = 0.032) and distant metastasis-free survival (DMFS; HR, 3.500; 95% CI, 1.224–10.01, p = 0.019). Multivariate analysis showed that high SUVmean-VAT was a significant factor for poor RFS and poor DMFS (p = 0.023 and 0.039, respectively). High SUVmax-tumor was significantly associated with short RFS (p = 0.0388). Tumors with a high SUV tended to have a short DMFS, although the difference was not significant (p = 0.0718). Our findings showed that upregulated glucose metabolism in the VAT measured using 18F-FDG PET/CT may be a prognostic biomarker for adverse outcomes in breast cancer.
Subject terms: Breast cancer, Breast cancer
Introduction
Breast cancer is a leading cause of cancer-related death in women worldwide1. Despite advances in early cancer detection and the multimodal treatment approach, systemic relapse of breast cancer still occurs. Recent studies have reported the independent association between tumor relapse and elevation of serum inflammatory biomarkers, including C-reactive protein (CRP), interleukin-6, and serum amyloid A; this suggests that systemic inflammation affects breast cancer recurrence2. The inflammatory compartment of the tumor microenvironment is deeply involved in breast cancer progression and metastasis3, which includes tumor-associated macrophages, matrix metalloproteinase, sphingosine 1-phosphate (S1P), and CRP4–6. Particularly, S1P and CRP promote breast cancer metastasis by influencing tumor cell invasion7.
18Fluorodeoxyglucose (18F-FDG) uptake by positron emission tomography (PET)/computed tomography (CT) is higher in areas with high metabolic activities, such as inflammatory reaction sites and tumors. Pahk et al. showed that 18F-FDG uptake in visceral adipose tissue (VAT) was positively correlated with carotid artery inflammation and the severity of coronary artery disease in humans8. Furthermore, they reported a correlation between the relative metabolic activity of VAT, estimated as the maximum standardized uptake value (SUVmax) divided by the SUVmax of subcutaneous adipose tissue (SAT), and frequent lymph node metastasis in luminal breast cancer in postmenopausal patients9. Based on previous study results8,9, we speculated that an increased SUV of VAT (SUVmean-VAT) reflects the systemic inflammatory status, which can be related to the clinical outcome of breast cancer. In this study, we aimed to measure the SUVmean-VAT and maximum SUV of the tumors (SUVmax-tumor) in patients with breast cancer to analyze their clinical implications.
Results
Baseline characteristics based on the 18F-FDG uptake of VAT
In total, 922 patients with breast cancer were screended for this study. We excluded 211 patients with de novo stage IV breast cancer, ductal carcinoma in situ alone, and etc (Figure 1). In addition, 563 patients had no PET-CT exam and thus were excluded. Data from 148 patients were analyzed in this study. Figure 1 shows the patient flow diagram. The median age of the study population was 49 years (range: 26–87 years). The average SUVmax-tumor for all the patients was 4.49 ± 3.67, and the median value was 3.30 (Supplementary Fig. 1a). The SUVmean-VAT was 0.42 ± 0.11, and the median value was 0.41 (Supplementary Fig. 1b). We used the median SUV value to determine the cut-off points for SUVmean-VAT and SUVmax-tumor as 0.41 and 3.30, respectively. According to the cutoff value, 73 (49.3%) and 75 (50.7%) patients were assigned to the high- and low SUVmean-VAT groups, respectively. No clinicopathological differences were observed between the high- and low SUVmean-VAT groups (Table 1).
Figure 1.
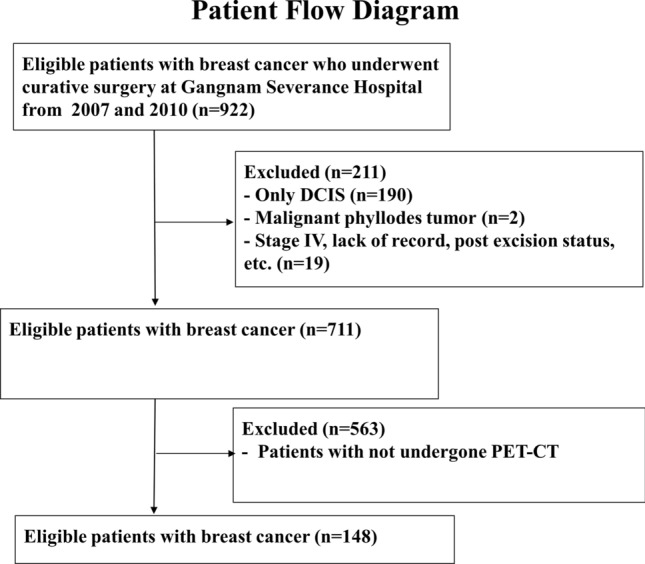
Patient flow diagram showing the study design. DCIS: ductal carcinoma in situ, PET/CT: positron emission tomography/computed tomography.
Table 1.
Clinical characteristics in relation to the 18F-FDG uptake of visceral adipose tissue.
| SUV-High, n = 73 (%) | SUV-Low, n = 75 (%) | p value | |
|---|---|---|---|
| Age (year, mean ± SD) | 50.90 ± 11.22 | 49.08 ± 8.30 | 0.264 |
| BMI (mean ± SD) | 23.19 ± 3.49 | 23.50 ± 3.11 | 0.571 |
| SUVmax-tumor | 4.50 ± 3.28 | 4.47 ± 4.04 | 0.960 |
| Serum glucose (mg/dL, mean ± SD) | 99.80 ± 14.18 | 103.24 ± 20.80 | 0.257 |
| DM | 0.513 | ||
| No | 71 (97.3) | 72 (96.0) | |
| Yes | 2 (2.7) | 3 (4.0) | |
| ER | 0.211 | ||
| Positive | 47 (64.4) | 41 (54.7) | |
| Negative | 23 (31.5) | 31 (41.3) | |
| Missing | 3 (4.1) | 3 (4) | |
| PR | 0.721 | ||
| Positive | 40 (54.8) | 39 (52) | |
| Negative | 30 (41.1) | 33 (44) | |
| Missing | 3 (4.1) | 3 (4) | |
| HER2 | 0.657 | ||
| Positive | 17 (23.3) | 21 (28) | |
| Negative | 48 (65.8) | 50 (66.7) | |
| Missing | 8 (11.0) | 4 (5.3) | |
| HG | 0.942 | ||
| I, II | 39 (53.4) | 38 (50.7) | |
| III | 26 (35.6) | 26 (34.7) | |
| Missing | 8 (11.0) | 11 (14.7) | |
| Tumor size | 0.168 | ||
| ≤ 2 cm | 48 (65.8) | 41 (54.7) | |
| > 2 cm | 25 (34.2) | 34 (45.3) | |
| Lymph node metastasis | 0.414 | ||
| Negative | 47 (64.4) | 53 (70.7) | |
| Positive | 26 (35.6) | 22 (29.3) | |
| AJCC stage* | 0.943 | ||
| I | 35 (47.9) | 36 (48) | |
| II | 30 (41.1) | 32 (42.7) | |
| III | 8 (11.0) | 7 (9.3) | |
| Lymphovascular invasion | 0.628 | ||
| Negative | 12 (16.4) | 14 (18.7) | |
| Positive | 52 (71.2) | 49 (65.3) | |
| Missing | 9 (12.3) | 12 (16) | |
| Chemotherapy | 0.765 | ||
| Done | 45 (61.6) | 48 (64) | |
| Not done | 25 (34.2) | 24 (32) | |
| Missing | 3 (4.1) | 3 (4) | |
| Radiotherapy | 0.203 | ||
| Done | 33 (45.2) | 24 (32) | |
| Not done | 37 (50.7) | 42 (56) | |
| Missing | 3 (4.1) | 9 (12) | |
| Endocrine therapy | 0.446 | ||
| Done | 49 (67.1) | 44 (58.7) | |
| Not done | 23 (31.5) | 27 (36) | |
| Missing | 1 (1.4) | 4 (5.3) |
SD Standard deviation; BMI Body mass index; DM Diabetes mellitus; ER Estrogen receptor; PR Progesterone receptor; HER2, Human epidermal growth factor receptor 2; HG Histologic grade.
*AJCC stage was performed based on 8th edition.
Prognostic significance of 18F-FDG uptake of VAT
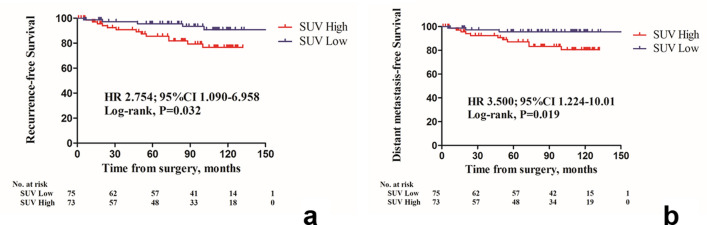
By the median follow-up duration of 95.5 months, recurrence and distant metastases had occurred in 18 and 14 patients, respectively. The median follow-up durations of the low SUVmax-tumor group and high SUVmax-tumor group were 96 months (range, 6–152 months) and 94 months (range, 1–132 months) for each. In the low SUVmax-tumor group, three patients had metastases in the liver, mediastinal lymph node, and contralateral axillary lymph node, respectively. In the high SUVmax-tumor group, 11 metastatic events that had occurred (including duplicates) at the following sites: bone (n = 4), lung (n = 2), cervical lymph node (n = 2), mediastinal lymph node (n = 2), liver (n = 1), pleura (n = 1), and intraperitoneal lymph node (n = 1). The high SUVmean-VAT group had significantly shorter recurrence-free survival (RFS) and distant metastasis-free survival (DMFS) (Fig. 2a; hazard ratio [HR], 2.754; 95% confidence interval [CI], 1.090–6.958; p = 0.032; Fig. 2b; HR, 3.500; 95% CI, 1.224–10.01; p = 0.019, respectively—compared using the log-rank test].
Figure 2.
Kaplan–Meier survival curves of (a) recurrence-free survival (RFS) and (b) distant metastasis-free survival (DMFS) according to the 18F-FDG uptake of visceral adipose tissue (VAT). Patients with high SUVmean-VAT showed a poorer RFS and DMFS, (A, hazard ratio 2.754, 95% confidence interval [CI] 1.090–6.958, p = 0.032; B, HR 3.500, 95% CI 1.224–10.01, p = 0.019, respectively).
Regarding RFS, significant risk factors for RFS were lymph node metastasis, advanced American Joint Committee on Cancer (AJCC) stage, no endocrine therapy, and high SUVmean-VAT (Table 2; HR, 2.928; 95% CI, 1.043–8.215; p = 0.041). Among these, high SUVmean-VAT was a significant independent risk factor for shorter RFS in the multivariate analysis (Table 2; HR, 3.361; 95% CI, 1.181–9.566; p = 0.023).
Table 2.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for recurrence-free survival (RFS).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HRs (95% CIs) | p value | HRs (95% CIs) | p value | |
| Age (continuous) | 1.007 (0.957–1.059) | 0.785 | N/A | |
| BMI (continuous) | 1.047 (0.920–1.191) | 0.490 | N/A | |
| ER | 0.239 | N/A | ||
| Negative | 1 | |||
| Positive | 0.574 (0.228–1.446) | |||
| PR | 0.102 | N/A | ||
| Negative | 1 | |||
| Positive | 0.454 (0.176–1.171) | |||
| HER2 | 0.704 | N/A | ||
| Negative | 1 | |||
| Positive | 0.806 (0.265–2.450) | |||
| HG | 0.864 | N/A | ||
| I, II | 1 | |||
| III | 1.044 (0.637–1.711) | |||
| Tumor size | 0.228 | N/A | ||
| ≤ 2 cm | 1 | |||
| > 2 cm | 1.766 (0.701–4.450) | |||
| Lymph node metastasis | 0.037 | 0.597 | ||
| Negative | 1 | 1 | ||
| Positive | 2.689 (1.061–6.815) | 1.404 (0.398–4.954) | ||
| AJCC stage | 0.040 | 0.079 | ||
| I | 1 | 1 | ||
| II | 3.745 (1.192–11.764) | 3.197 (1.015–10.072) | ||
| III | 3.534 (0.791–15.798) | 3.337 (0.745–14.935) | ||
| Lymphovascular invasion | 0.820 | N/A | ||
| Negative | 1 | |||
| Positive | 0.864 (0.246–3.039) | |||
| SUVmean-VAT | 0.041 | 0.023 | ||
| Low | 1 | 1 | ||
| High | 2.928 (1.043–8.215) | 3.361 (1.181–9.566) | ||
| Chemotherapy | 0.543 | N/A | ||
| Not done | 1 | |||
| Done | 0.745 (0.289–1.922) | |||
| Radiotherapy | 0.969 | N/A | ||
| Not done | 1 | |||
| Done | 0.982 (0.387–2.491) | |||
| Endocrine therapy | 0.021 | 0.014 | ||
| Not done | 1 | 1 | ||
| Done | 0.329 (0.127–0.848) | 0.300 (0.115–0.786) | ||
BMI Body mass index; ER Estrogen receptor; PR Progesterone receptor; HER2 Human epidermal growth factor receptor 2; HG Histologic grade; VAT Visceral adipose tissue; N/A Not assessed.
High SUVmean-VAT and advanced AJCC were significant risk factors for DMFS in the univariate and multivariate analyses (Table 3).
Table 3.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for distant metastasis-free survival (DMFS).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HRs (95% CIs) | p value | HRs (95% CIs) | p value | |
| Age (continuous) | 1.024 (0.968–1.082) | 0.408 | N/A | |
| BMI | 1.005 (0.861–1.173) | 0.949 | N/A | |
| ER | 0.119 | N/A | ||
| Negative | 1 | |||
| Positive | 0.569 (0.200–1.624) | |||
| PR | 0.098 | N/A | ||
| Negative | 1 | |||
| Positive | 0.397 (0.133–1.184) | |||
| HER2 | 0.836 | N/A | ||
| Negative | 1 | |||
| Positive | 1.131 (0.355–3.605) | |||
| HG | 0.994 | N/A | ||
| I, II | 1 | |||
| III | 0.998 (0.562–1.772) | |||
| Tumor size | 0.119 | N/A | ||
| ≤ 2 cm | 1 | |||
| > 2 cm | 2.320 (0.804–6.688) | |||
| Lymph node metastasis | 0.052 | N/A | ||
| Negative | 1 | |||
| Positive | 2.859 (0.992–8.241) | |||
| AJCC stage | 0.015 | 0.019 | ||
| I | 1 | 1 | ||
| II | 6.024 (1.301–27.886) | 5.810 (1.255–26.900) | ||
| III | 7.125 (1.190–42.650) | 6.631 (1.106–39.734) | ||
| Lymphovascular invasion | 0.693 | N/A | ||
| Negative | 1 | |||
| Positive | 1.301 (0.352–4.815) | |||
| SUVmean-VAT | 0.031 | 0.039 | ||
| Low | 1 | 1 | ||
| High | 4.066 (1.134–14.580) | 3.846 (1.072–13.795) | ||
| Chemotherapy | 0.392 | N/A | ||
| Not done | 1 | |||
| Done | 0.630 (0.218–1.815) | |||
| Radiotherapy | 0.915 | N/A | ||
| Not done | 1 | |||
| Done | 0.944 (0.327–2.723) | |||
| Endocrine therapy | 0.082 | N/A | ||
| Not done | 1 | |||
| Done | 0.390 (0.135–1.125) | |||
BMI Body mass index; ER Estrogen receptor; PR Progesterone receptor; HER2 Human epidermal growth factor receptor 2; HG Histologic grade; VAT Visceral adipose tissue; N/A Not assessed.
Prognostic significance of the 18F-FDG uptake of tumor
The patients were divided into high (n = 73, 49.3%) and low (n = 75, 50.7%) SUVmax-tumor groups. The high SUVmax-tumor group had the following aggressive features: estrogen receptor (ER)-negative, progesterone (PR)-negative, higher histologic grade (HG), larger tumor size, advanced AJCC stage, and no endocrine treatment (Supplementary Table 1).
Kaplan–Meier survival plots for RFS and DFMS were drawn to compare the high- and low SUVmax-tumor groups (Supplementary Fig. 2). The high SUVmax-tumor group had a significantly inferior RFS (Supplementary Fig. 2a; HR, 2.760; 95% CI, 1.092–6.974; p = 0.0388); however, there was no difference in DMFS between the two groups in relation to the SUVmax-tumor (Supplementary Fig. 2b; HR, 2.625; 95% CI, 0.918–7.509; p = 0.0718).
High SUVmax-tumor was a significant risk factor for shorter RFS in the univariate analysis (Supplementary Table 2; HR, 2.934; 95% CI, 1.046–8.232, p = 0.041). The high SUVmax-tumor group tended to have a shorter DMFS; however, the difference was not significant (Supplementary Table 2; HR, 2.772; 95% CI, 0.869–8.841; p = 0.085).
Discussion
In this study, we measured both SUVmean-VAT and SUVmax-tumor using PET/CT in patients with breast cancer and found that high SUVmean-VAT was significantly associated with tumor recurrence. Our results suggest that activated visceral fat metabolism may be a potential clinical target for breast cancer treatment by controlling dysmetabolism and systemic inflammation.
Understanding where and how cellular energy consumption occurs in humans in vivo is the key to understanding cancer metabolism. Obesity and related metabolic complications are considered to have a clinical impact; this has triggered intense research to elucidate the comprehensive molecular-level mechanisms10,11. Glucose utilization in VAT is different from that in SAT in terms of inflammatory mediators, gene expression, and cell morphology12–15. Christen et al. found that VAT has a higher glucose uptake and different metabolic activity than non-visceral adipose tissue using 18F-FDG PET imaging16. Visceral adiposity is known to increase cancer risk17,18, and is thought to be a surrogate parameter for cancer incidence19,20. Strategies for fat remodeling (adipose tissue browning) and lifestyle interventions have been suggested20,21. These results illustrate that VAT might be involved in cancer metabolism and act as a biomarker, which is in line with our results of VAT being a significant prognostic marker in breast cancer.
In this study, there was no significant difference in clinicopathological characteristics between the high- and low SUVmean-VAT groups. Both RFS and DMFS were significantly inferior in the high SUVmean-VAT group (p = 0.032 and 0.019, respectively). Regarding overall survival, only five patients died during the study period, which was too small for an appropriate statistical analysis. However, there was a trend of increased death events in the high SUVmean-VAT group than in the low SUVmean-VAT group (n = 4 vs. n = 1), although the difference was not significant (p = 0.149). In the Cox proportional hazard model, SUVmean-VAT was found to be an independent predictor of shorter RFS and DMFS, even more significant than pathologic parameters, including tumor size and lymphovascular invasion (LVI).
To the best of our knowledge, this is the first study to report the prognostic impact of VAT metabolism measured using 18F-FDG PET/CT in patients with breast cancer. There was a clear association between high SUVmean-VAT and survival outcomes in patients with breast cancer. PET/CT is a preoperative non-invasive assessment modality that can evaluate the whole body during a single examination and provides additional information such as tumor metabolism, systemic inflammatory status, cancer diagnosis, and staging.
Many studies have investigated the effects of adipocyte biology on cytokine production22–25. Adipokines, secreted by adipocytes, have been receiving attention for their role in cancer metabolism26. Regarding VAT, a special compartment of adipocytes, its upregulated activity has been a point of focus in several studies: VAT activity was related to myocardial infarction8. It also reflected the inflammatory status of the carotid artery in metabolic syndrome27,28. The predictive role of VAT activity has been assessed by preoperative 18F-FDG PET/CT in gastric, colorectal, and thyroid cancers29–31. These studies reported that dysfunctional adipocytes in VAT secrete inflammatory adipokines that induce an inflammation-sustaining microenvironment, which in turn promotes carcinogenesis and tumor cell growth. Hence, the metabolic activity of VAT may be a surrogate marker for tumor progression, and glucose metabolism could be measured using 18F-FDG PET/CT.
Obesity and obesity-driven metabolic dysfunction in postmenopausal women are risk factors for breast cancer32. A recent study of 173 postmenopausal patients with breast cancer showed that the SUV ratio of the VAT and SAT correlated significantly with that of axillary lymph node metastasis9. This is partially in line with our finding of the association between high SUVmean-VAT and the biologically aggressive behavior of breast cancer. White adipose tissue, which comprises most VAT, has an increased number of mast cells33 and macrophages34 that secrete pro-inflammatory cytokines. The systemic release of pro-inflammatory cytokines induces chronic low-grade inflammation, which results in the production of reactive oxygen species and enhancement of tumor promotion35. A previous study found that VAT, but not SAT, was independently associated with upregulated serum inflammatory markers including white blood cell count and high-sensitivity CRP36. Furthermore, VAT is more metabolically active and has a larger number of inflammatory and immune cells, which confers its greater glucose uptake capacity than SAT37. Thus, SUVmean-VAT may be a non-invasive marker of systemic inflammation and a prognostic indicator in patients with breast cancer.
This study has several limitations. First, this study had a retrospective design, a small sample size, and was a single-center study. Although significant prognoses were observed, in-depth analyses of the patients’ characteristics (e.g., post-menopause) or characteristics of the molecular subgroups were not performed. Second, we included all available breast cancers for analysis regardless of the molecular subtype, which might have resulted in an uneven distribution of patients in each molecular subgroup and selection bias that could skew the statistics. Third, SUVmean-VAT, the main parameter analyzed in this study, had a very narrow range of values; this implies that SUVmean-VAT might be vulnerable to changes in other variables during PET/CT procedures, such as scanning, imaging protocols, radiopharmaceutical dose, and image acquisition time after 18F-FDG injection. These variables should be controlled precisely for an accurate SUV acquisition37,38. Nonetheless, SUV is affected by multiple factors, such as plasma glucose and insulin levels; thus, inter-subject and intra-subject variability could also exist. Further validation of these findings in prospective large multicenter studies and other types of malignancies is required. However, despite these limitations, we observed a significant correlation between SUVmean-VAT and patient prognosis in breast cancer.
In conclusion, the increased glucose metabolism of VAT assessed using 18F-FDG PET/CT was significantly associated with RFS and DMFS in patients with breast cancer, which might also reflect pro-tumor systemic inflammation. Upregulated SUVmean-VAT levels in patients with breast cancer could be a predictor of tumor recurrence.
Methods
Patients
This was a retrospective study. Patients with invasive breast cancer (stages I–III; age ≥ 20 years at the time of surgery) who were treated between January 2007 and December 2010 at Gangnam Severance Hospital according to standard protocols were included in the study. The patients were followed up till December 31, 2020. We collected the patients’ clinical information from their electronic medical records, including data on age, body mass index (BMI), treatment modalities (chemotherapy, radiotherapy, and endocrine therapy), and survival outcomes including recurrence and metastasis. RFS was defined as the interval from the date of primary surgery to the date of any breast cancer recurrence (locoregional and/or distant metastasis), death due to any cause, or last follow-up. DMFS was defined as the interval from primary curative surgery to the first breast cancer-derived distant metastasis, death due to any cause, or end of follow-up. Data of patients who did not exhibit relevant events were censored at the end of the follow-up period. The patients’ pathological parameters were collected from pathologic reports of resected specimens. These included tumor size, HG, LVI, lymph node metastasis, and status of ER, PR, and human epidermal growth factor receptor 2. The anatomical tumor (T), node (N), and metastasis (M) (TNM) stages were classified according to the AJCC 8th edition39. The histologic grade of the tumor was determined according to the modified Scarf–Bloomer–Richardson grading system40. Patients who met the following criteria were excluded:
Only ductal carcinoma in situ
Stage IV breast cancer
Other malignancies (except for thyroid cancer)
Unavailable electronic medical records
Unavailable detailed PET/CT information
Other inflammatory diseases (including autoimmune diseases and infections)
Organ failure
PET/CT image from the referral hospital with different protocols
The patients were categorized into high and low groups according to their median SUVmean-VAT and SUVmax-tumor (high SUVmean-VAT /low SUVmean-VAT and high SUVmax-tumor/low SUVmax-tumor groups).
This study was approved by the Institutional Review Board (IRB) of Gangnam Severance Hospital (Local IRB Number: 3-2021-0074) in Seoul, Republic of Korea. The IRB waived the requirement for informed consent due to the retrospective study design.
18F-FDG PET/CT acquisition
All the patients fasted for at least 6 h and had blood glucose levels below 140 mg/dL before 18F-FDG (dose: 5.5 MBq/kg of body weight) was intravenously administered, and 18F-FDG PET/CT was performed using a PET/CT scanner (Biograph 40 TruePoint, Siemens Healthcare Solutions USA, Inc., Knoxville, TN). Whole-body low-dose CT images were obtained for attenuation correction using automatic dose modulation against the standard reference values of 40 mA and 120 kV. The CT images were acquired with a 3 mm slice thickness and up to 4640 projections per 360° rotations. The scanned images were reconstructed onto 512 × 512-pixel CT image matrices (0.98 mm × 0.98 mm × 3.0 mm) using the B30f. kernel. PET data were acquired from the skull base to the proximal thigh for 3 min per bed position in the three-dimensional mode. PET images were reconstructed using the ordered subset expectation maximization algorithm (OSEM) using two iterations and 21 subsets. The matrix size and thickness of the reconstructed PET images were 128 × 128 and 5 mm, respectively. Automated corrections such as normalization, correction for random coincidences, correction for scattered radiation, and correction for dead time were applied. Maximum intensity projection (MIP), cross-sectional views, and fusion images were generated and reviewed. Cross-calibration between PET and the dose calibrator was performed monthly.
PET/CT image analysis and data acquisition of SUV
All PET/CT images were evaluated by experienced nuclear medicine physicians, who were blinded to the participant’s clinical data. Decisions concerning analyses of the PET/CT datasets were reached by consensus. Image analysis was performed using the MIM software (MIM, 6.8.10, Cleveland, OH, USA). For semi-quantitative evaluation, the SUV was calculated by measuring the tumor absorption of 18F-FDG in the region of interest (ROI) as follows:
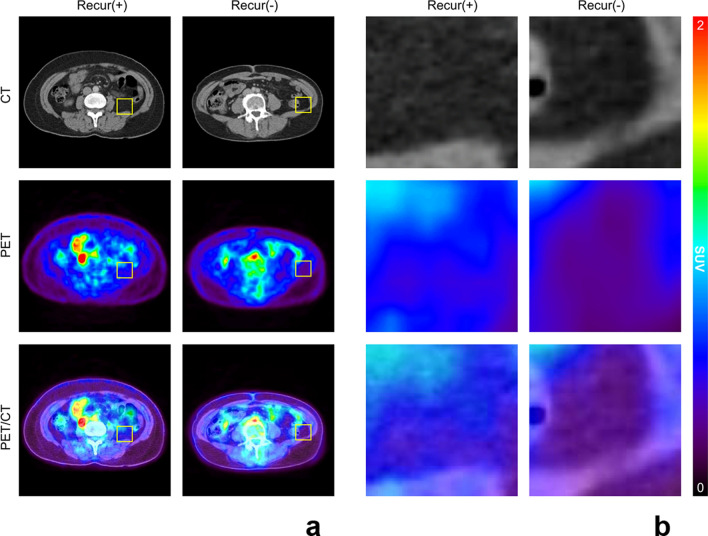
A volume of interest (VOI) was manually drawn over the primary breast cancer lesion on the PET/CT images, and the SUVmax-tumor was measured. The image analysis was performed to assess the metabolic activity of VAT. The VAT regions were recognized based on predefined Hounsfield units (range: − 110 to − 70 HU41, which corresponds to adipose tissue densities in humans) from the corresponding CT images, as described previously9,16,27,42. There are several methods to measure the SUV for VAT. The method of measuring the SUV in multiple consecutive planes and calculating the average was widely used in previous studies and can reduce the bias or noise that occurs when only one ROI is selected. Regarding the evaluation of VAT uptake, two-dimensional ROIs were placed on three consecutive slices of the abdominal VAT area. Furthermore, ROIs were placed away from the intestines, kidneys, vessels, and/or muscles, to exclude the inclusion of physiological 18F-FDG uptake in these areas9,27,30,42. We obtained the mean SUV from consecutive ROIs. Both intra—and interobserver correlation analyses in SUV measurements demonstrated excellent reliability (correlation coefficient > 0.9). The differences in the glucose uptake of VAT according to breast cancer recurrence on PET-CT are shown in Fig. 3.
Figure 3.
Representative images of (a) 18F-FDG uptake of visceral adipose tissue (VAT) according to the breast cancer recurrence status and (b) their corresponding magnified views. CT: computed tomography, PET: positron emission tomography, Recur: Recurrence.
The interval time between PET/CT acquisition and curative surgery was 3.79 ± 5.01 days in 145 patients, excluding 4 patients who received neoadjuvant chemotherapy.
Statistical analysis
Continuous variables between the two groups were compared using Student’s t-test or Mann–Whitney U test. Categorical variables were compared using the chi-square or Fisher’s exact test. Survival curves were obtained using the Kaplan–Meier method, and two-group comparisons were performed using the log-rank test. Univariate and multivariate Cox proportional hazard models were used to identify the factors associated with survival outcomes (RFS and DMFS). Variables that were significant in the univariate analysis were included in the multivariate Cox proportional hazards models. Statistical analyses were performed using SPSS version 24 (SPSS Inc., Chicago, IL, USA). The threshold for statistical significance was set at p < 0.05, with a 95% confidence interval (CI) not including 1.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The protocol was approved by the Institutional Review Board (Local IRB Number: 3-2021-0074) of Gangnam Severance Hospital. The need for informed consent was waived by the IRB due to the retrospective study design.
Supplementary Information
Abbreviations
- CRP
C-reactive protein
- SIP
Sphingosine 1-phosphate
- 18F-FDG
18Fluorodeoxyglucose
- PET
Positron emission tomography
- CT
Computed tomography
- VAT
Visceral adipose tissue
- SAT
Subcutaneous adipose tissue
- SUVmax
Maximum standardized uptake value
- BMI
Body mass index
- HG
Histologic grade
- ER
Estrogen receptor
- PR
Progesterone receptor
- HER2
Human epidermal growth factor receptor 2
- LVI
Lymphovascular invasion
- TNM
Tumor, node, metastasis
- AJCC
American joint committee on cancer
- IRB
Institutional review board
- OSEM
Ordered subset expectation maximization algorithm
- MIP
Maximum intensity projection
- ROI
Region of interest
- VOI
Volume of interest
- RFS
Recurrence-free survival
- DMFS
Distant metastasis-free survival
- HR
Hazard ratio
- CI
Confidence interval
Author contributions
Y.J.C. and C.I.Y. had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. These quthors equally contributed to this work. Conceptualization, H.J.K., Y.J.C. and C.I.Y.; Data curation, H.J.K., D.K., S.J.B., S.G.A., Y.L., Y.J.C. and C.I.Y.; Funding acquisition, H.J.K., Y.J.C.; Investigation, H.J.K., Y.J.C. and C.I.Y.; Methodology, H.J.K., Y.J.C. and C.I.Y.; Resources, S.G.A. and J.J.; Formal analysis, C.I.Y. and C.J.Y.; Supervision, J.J., W.C.P., Y.H.R., Y.L. and T.J.J.; Writing-original draft, H.J.K., Y.J.C. and C.I.Y. All authors have given consent for publication.
Funding
This research was funded by the Basic Science Research Program of the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology [Grant No. NRF-2017R1D1A1B0303438814], the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIT) [Grant No. NRF-2021R1G1A1093596), and faculty research grant from the Yonsei University College of Medicine [Grant No. 6-2021-0159, 6-2022-0123]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
All data generated or analyzed during this study are included in this research article and the supplementary information files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yoon Jin Cha, Email: yooncha@yuhs.ac.
Chang Ik Yoon, Email: fayn03@catholic.ac.kr.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-25540-4.
References
- 1.Jemal A, et al. Cancer statistics, 2007. CA Cancer J. Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.McAndrew NP, et al. Effects of systemic inflammation on relapse in early breast cancer. NPJ Breast Cancer. 2021;7:7. doi: 10.1038/s41523-020-00212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ham M, Moon A. Inflammatory and microenvironmental factors involved in breast cancer progression. Arch. Pharmacal. Res. 2013;36:1419–1431. doi: 10.1007/s12272-013-0271-7. [DOI] [PubMed] [Google Scholar]
- 4.Edsparr K, Basse PH, Goldfarb RH, Albertsson P. Matrix metalloproteinases in cytotoxic lymphocytes impact on tumour infiltration and immunomodulation. Cancer Microenviron. Off. J. Int. Cancer Microenviron. Soc. 2011;4:351–360. doi: 10.1007/s12307-010-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long JS, et al. Sphingosine kinase 1 induces tolerance to human epidermal growth factor receptor 2 and prevents formation of a migratory phenotype in response to sphingosine 1-phosphate in estrogen receptor-positive breast cancer cells. Mol. Cell. Biol. 2010;30:3827–3841. doi: 10.1128/MCB.01133-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim ES, et al. Inflammatory lipid sphingosine-1-phosphate upregulates C-reactive protein via C/EBPbeta and potentiates breast cancer progression. Oncogene. 2014;33:3583–3593. doi: 10.1038/onc.2013.319. [DOI] [PubMed] [Google Scholar]
- 8.Pahk K, Kim EJ, Joung C, Seo HS, Kim S. Association of glucose uptake of visceral fat and acute myocardial infarction: A pilot (18)F-FDG PET/CT study. Cardiovasc. Diabetol. 2020;19:145. doi: 10.1186/s12933-020-01115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pahk K, Joung C, Kim S. Visceral fat metabolic activity evaluated by preoperative (18)F-FDG PET/CT significantly affects axillary lymph node metastasis in postmenopausal luminal breast cancer. Sci. Rep. 2020;10:1348. doi: 10.1038/s41598-020-57937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atawia RT, Bunch KL, Toque HA, Caldwell RB, Caldwell RW. Mechanisms of obesity-induced metabolic and vascular dysfunctions. Front. Biosci. (Landmark Ed) 2019;24:890–934. doi: 10.2741/4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung UJ, Choi MS. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014;15:6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perrini S, et al. Fat depot-related differences in gene expression, adiponectin secretion, and insulin action and signalling in human adipocytes differentiated in vitro from precursor stromal cells. Diabetologia. 2008;51:155–164. doi: 10.1007/s00125-007-0841-7. [DOI] [PubMed] [Google Scholar]
- 13.Virtanen KA, et al. Increased fat mass compensates for insulin resistance in abdominal obesity and type 2 diabetes: A positron-emitting tomography study. Diabetes. 2005;54:2720–2726. doi: 10.2337/diabetes.54.9.2720. [DOI] [PubMed] [Google Scholar]
- 14.Virtanen KA, et al. Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J. Clin. Endocrinol. Metab. 2002;87:3902–3910. doi: 10.1210/jcem.87.8.8761. [DOI] [PubMed] [Google Scholar]
- 15.Westergren H, Danielsson A, Nystrom FH, Stralfors P. Glucose transport is equally sensitive to insulin stimulation, but basal and insulin-stimulated transport is higher, in human omental compared with subcutaneous adipocytes. Metab. Clin. Exp. 2005;54:781–785. doi: 10.1016/j.metabol.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Christen T, et al. Increased glucose uptake in visceral versus subcutaneous adipose tissue revealed by PET imaging. JACC Cardiovasc. Imaging. 2010;3:843–851. doi: 10.1016/j.jcmg.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 18.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 19.Okamura T, et al. Visceral adiposity index is a predictor of incident colorectal cancer: A population-based longitudinal study. BMJ Open Gastroenterol. 2020;7:e00400. doi: 10.1136/bmjgast-2020-000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crudele L, Piccinin E, Moschetta A. Visceral adiposity and cancer: Role in pathogenesis and prognosis. Nutrients. 2021;13:2101. doi: 10.3390/nu13062101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurylowicz A, Puzianowska-Kuznicka M. Induction of adipose tissue browning as a strategy to combat obesity. Int. J. Mol. Sci. 2020;21:6241. doi: 10.3390/ijms21176241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 23.Fuster JJ, Ouchi N, Gokce N, Walsh K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ. Res. 2016;118:1786–1807. doi: 10.1161/Circresaha.115.306885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horakova D, et al. Total and high-molecular-weight adiponectin levels and prediction of insulin resistance. Endokrynol. Pol. 2018;69:375–380. doi: 10.5603/EP.a2018.0035. [DOI] [PubMed] [Google Scholar]
- 25.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 26.Choi J, Cha YJ, Koo JS. Adipocyte biology in breast cancer: From silent bystander to active facilitator. Prog. Lipid Res. 2018;69:11–20. doi: 10.1016/j.plipres.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Bucerius J, et al. Impact of bariatric surgery on carotid artery inflammation and the metabolic activity in different adipose tissues. Medicine (Baltimore) 2015;94:e725. doi: 10.1097/MD.0000000000000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tahara N, et al. Vascular inflammation evaluated by 18F-fluorodeoxyglucose positron emission tomography is associated with the metabolic syndrome. J. Am. Coll. Cardiol. 2007;49:356a–356a. doi: 10.1016/j.jacc.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 29.Lee JW, et al. Significance of CT attenuation and F-18 fluorodeoxyglucose uptake of visceral adipose tissue for predicting survival in gastric cancer patients after curative surgical resection. Gastric Cancer. 2020;23:273–284. doi: 10.1007/s10120-019-01001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pahk K, Choi S, Kim S. Functional visceral fat activity evaluated by preoperative F-18 FDG PET/CT predicts regional lymph node metastasis in differentiated thyroid cancer. Clinical Endocrinology. 2018;88:963–968. doi: 10.1111/cen.13604. [DOI] [PubMed] [Google Scholar]
- 31.Pahk K, Rhee S, Kim S, Choe JG. Predictive role of functional visceral fat activity assessed by preoperative F-18 FDG PET/CT for regional lymph node or distant metastasis in patients with colorectal cancer. PLoS ONE. 2016;11:e0148776. doi: 10.1371/journal.pone.0148776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park B, Kim S, Kim H, Cha C, Chung MS. Associations between obesity, metabolic health, and the risk of breast cancer in East Asian women. Br. J. Cancer. 2021;125:1718–1725. doi: 10.1038/s41416-021-01540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018;282:121–150. doi: 10.1111/imr.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cancello R, et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55:1554–1561. doi: 10.2337/db06-0133. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan, N. J. Interleukin-6 in the breast tumor microenvironment. In Breast Cancer—Focusing Tumor Microenvironment, Stem Cells and Metastasis (ed. M Gunduz), 165–175 (2011).
- 36.Yu JY, Choi WJ, Lee HS, Lee JW. Relationship between inflammatory markers and visceral obesity in obese and overweight Korean adults: An observational study. Medicine (Baltimore) 2019;98:e14740. doi: 10.1097/MD.0000000000014740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibrahim MM. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 38.Thie JA. Understanding the standardized uptake value, its methods, and implications for usage. J. Nucl. Med. 2004;45:1431–1434. [PubMed] [Google Scholar]
- 39.Amin, M. B. E. S. B. G. F. L. A. J. C. o. C. AJCC cancer staging manual (2017).
- 40.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 41.Yoshizumi T, et al. Abdominal fat: Standardized technique for measurement at CT. Radiology. 1999;211:283–286. doi: 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]
- 42.Vanfleteren LEGW, et al. A possible link between increased metabolic activity of fat tissue and aortic wall inflammation in subjects with COPD. A retrospective 18F-FDG-PET/CT pilot study. Respir. Med. 2014;108:883–890. doi: 10.1016/j.rmed.2014.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this research article and the supplementary information files.