Abstract
We have investigated the synergistic interactions of a naturally occurring peptidoglycan fragment (muramyl peptide) and bacterial endotoxin in the induction of inflammatory processes within respiratory epithelial cells, at the levels of both signal transduction events and ultimate cellular metabolic effects. The source of the muramyl peptide is Bordetella pertussis, the causative agent of the respiratory disease pertussis. During log-phase growth, B. pertussis releases the muramyl peptide tracheal cytotoxin (TCT), which has the structure N - acetylglucosaminyl - 1,6 - anhydro - N - acetylmuramyl - (l) - alanyl - γ - (d) - glutamyl - meso - diaminopimelyl - (d) - alanine, equivalent to a monomeric subunit of gram-negative bacterial peptidoglycan. When applied to hamster trachea epithelial (HTE) cells, TCT and endotoxin were found to be highly synergistic in the induction of interleukin-1α (IL-1α), type II (inducible) nitric oxide synthase (iNOS), nitric oxide production, and inhibition of DNA synthesis. Neither molecule alone significantly triggered these responses. The serine/threonine protein kinase inhibitor H7 blocked induction of both IL-1α and iNOS. More selective inhibitors of protein kinase C, cyclic AMP-dependent protein kinase, and cyclic GMP-dependent protein kinase were not capable of blocking the effects of TCT and endotoxin, suggesting that the H7-inhibited component in this pathway is not among the commonly described kinase targets of H7. Treatment of HTE cells with exogenous IL-1 reproduced the induction of iNOS and DNA synthesis inhibition caused by TCT and endotoxin. H7 was not capable of interfering with effects caused by exogenous IL-1, implying that the H7-sensitive step in the pathway is upstream of IL-1 protein production. Similar assays with the phorbol ester phorbol myristate acetate indicate that it could effectively synergize with endotoxin but not with TCT, suggesting that TCT and endotoxin induce different signal transduction events that combine synergistically. The synergy observed with TCT and endotoxin in epithelial cells is significantly different from their interaction with other cell types, revealing a unique inflammatory response by epithelial cells to these natural bacterial products.
Bacterial cell wall components are widely recognized as inflammatory mediators, especially in leukocytes. The most broadly studied inflammatory bacterial molecules are endotoxin (most notably Escherichia coli lipopolysaccharide [LPS]) and muramyl peptides. These molecules, alone or in combination, have been observed to stimulate production of tumor necrosis factor alpha, interleukin-1α (IL-1α), IL-1β, IL-6, and nitric oxide synthase (NOS), among numerous other effects. However, there are two major features that distinguish the present study from most others involving these bacterial components.
First, the majority of work involving peptidoglycan fragments has employed N-acetylmuramyl-l-alanyl-d-isoglutamine (muramyl dipeptide [MDP]), the minimum active fragment of peptidoglycan adjuvants and the prototypical representative of the muramyl peptide family. While this synthetic muramyl peptide is an effective replacement for peptidoglycan in immune stimulation, it is not clear that it accurately represents the effects of naturally occurring peptidoglycan fragments in other functions. Indeed, in the hamster trachea epithelial (HTE) cell system described here, MDP is completely ineffective (35). Major differences between MDP and natural peptidoglycan fragments have been noted by others examining somnogenic activity (29, 31) or cytokine production (11).
Furthermore, while most studies of LPS and peptidoglycan fragments have used monocytes or macrophages, we have studied the effects of these components in an epithelial cell system. Mucosal epithelium is the first site of colonization for many bacterial infections and represents an important point of nonspecific immunity. There has been little investigation of the effects of natural bacterial products on epithelial cells, particularly in combination.
The disease pertussis (or whooping cough) provides a useful, albeit extreme, model for epithelial activation by natural bacterial cell wall components. The gram-negative bacterium Bordetella pertussis, the causative agent of the disease, releases a small glycopeptide toxin called tracheal cytotoxin (TCT) (17). The structure of TCT is N-acetylglucosaminyl-1,6-anhydro-N-acetylmuramyl - (l) - alanyl - γ - (d) - glutamyl - meso - diaminopimelyl-(d)-alanine, a monomeric subunit of bacterial peptidoglycan (7). E. coli, and presumably most gram-negative bacteria, produce this glycopeptide in the course of remodeling their peptidoglycan during growth, through the action of a transglycosylase (25). This product is typically not detectable in the culture supernatant of growing organisms but is instead efficiently recycled back into the cytoplasm for reincorporation into the peptidoglycan biosynthesis pathway (18, 42). However, unlike most other gram-negative bacteria, B. pertussis releases a large amount of this glycopeptide into the culture supernatant. This toxin was isolated from B. pertussis culture supernatant based on its ability to reproduce the pattern of epithelial cytopathology observed in the pertussis syndrome. When applied to explanted hamster tracheal tissue, TCT causes specific destruction of the ciliated cell population, leaving an intact epithelium denuded of cilia (17). We have determined that the induction of type II NOS (inducible NOS [iNOS]) is the key mechanism by which TCT causes damage: prevention of nitric oxide (NO·) production by specific inhibitors completely abrogates the damage caused by TCT to tracheal tissue (21). We believe that an essential mediator in the induction of iNOS is intracellular IL-1α, a known inducer of iNOS. The production of this cytokine is induced rapidly in tracheal tissue exposed to TCT, but it is found only in cellular lysates and not secreted into the medium (22).
While TCT does trigger pertussis-like ciliated cell destruction in explanted tracheal tissue, the source of NO· has been shown to be the cartilage cells, which are likely not the physiological source in a B. pertussis infection. We have recently reported that the combination of TCT with bacterial endotoxin is necessary to induce iNOS within the epithelium of explanted tracheal tissue, precisely reproducing the cellular responses to B. pertussis. Neither TCT alone nor endotoxin alone induces significant NO· production within the epithelium (12).
In addition to the tissue-based assay, we previously developed a more quantitative assay based on a homogeneous, nonciliated, nontransformed, proliferating epithelial cell population prepared from hamster trachea epithelium (16). These HTE cells reflect the same mechanism of toxin action as does intact tissue: intracellular IL-1α is rapidly induced, followed by the induction of iNOS and the production of NO· (21, 22). Unlike the target ciliated cells of the epithelium, these cells are not destroyed per se but instead are inhibited in DNA synthesis (17). Just as observed for the epithelium of explanted tissue, toxicity in the HTE cell model requires the combination of TCT with bacterial endotoxin (20, 22). While TCT alone or endotoxin alone cause little effect in these cells, TCT plus endotoxin induce substantial NO· production and inhibit DNA synthesis. Thus, the HTE cell model system accurately mimics the signaling requirements for epithelial activation observed in explanted tracheal tissue.
In this study, we more completely characterize the synergy between TCT and endotoxin in respiratory epithelial cells. We further desired to study the early signal transduction events induced by these two molecules and to establish the level at which the two signals combine in a synergistic manner. Our results indicate that TCT and endotoxin act in a highly synergistic manner in all examined downstream effects: induction of IL-1α mRNA, iNOS protein, nitric oxide production, and inhibition of DNA synthesis. The synergy between TCT and endotoxin occurs before induction of IL-1α, but there is evidence of differential activities of the two molecules early in the signaling process. Our observations also imply that the signal transduction pathway stimulated by TCT and endotoxin includes activation of a previously uncharacterized serine/threonine protein kinase. The high degree of synergy between TCT and endotoxin represents an important difference between the responses of epithelial cells and monocytes/macrophages to natural peptidoglycan fragments.
MATERIALS AND METHODS
Materials.
TCT was purified from B. pertussis culture supernatant as previously described (6). Murine IL-1β (ca. 50 U/ng) was a gift from David Chaplin, Washington University. W7, HA1004, calphostin C, and A3 were obtained from Calbiochem (San Diego, Calif.). Both B. pertussis lipooligosaccharide (LOS) and E. coli LPS were used, as noted for individual experiments. These molecules have comparable biological activities and are biochemically very similar, differing primarily in that B. pertussis LOS has a much shorter O-polysaccharide chain (5). We detected no difference in activity in our assays between B. pertussis LOS and E. coli LPS, based on equivalent endotoxin activity units. Endotoxin activity was determined by the Limulus amoebocyte lysate assay (Whittaker Bioproducts, Walkersville, Md.) and is expressed in endotoxin units per milliliter. Fetal bovine serum (FBS) was obtained from HyClone Laboratories (Logan, Utah) and was dialyzed before use. Purified B. pertussis LOS (approximately 3.0 endotoxin units [eU]/ng) was from List Biological Laboratories (Campbell, Calif.); LPS from E. coli strain O26:B6 (approximately 1 eU/ng) was from Sigma (St. Louis, Mo.). Phorbol 12-myristate 13-acetate (PMA), H7 [1-(5-isoquinolinylsulfonyl)-2-methylpiperazine], and trifluoperazine were from Sigma, as were all other chemicals not otherwise noted.
Cell culture.
HTE cells, a homogenous nontransformed dividing cell population, were derived from hamster tracheal tissue as previously described (16). They were cultured in F-12 medium [Life Technologies (Gibco BRL), Grand Island, N.Y.] supplemented with 10% FBS and antibiotics in a humidified 5% CO2 atmosphere at 37°C. RAW264.7 murine macrophage-like cells were cultured in Dulbecco modified Eagle medium (with 4.5 g of d-glucose and 110 mg of pyruvate per liter) (Life Technologies) supplemented with 10 mM HEPES (United States Biochemical, Amersham Life Sciences, Arlington Heights, Ill.), 10% FBS, and antibiotics.
Nitrite assay.
Nitric oxide production was assessed by measurement of the concentration in the culture medium of nitrite, a stable breakdown product of nitric oxide. Nitrite accumulation was assayed by mixing 100 μl of culture medium with an equal amount of Griess reagent (19). The assay product was quantitated by measuring absorbance at 562 nm with a microplate reader and by comparison against a standard nitrite curve prepared in culture medium.
Toxicity assay.
Biological response to TCT-endotoxin and inhibitors was assessed by measuring inhibition of DNA synthesis by HTE cells as previously described (36). Briefly, HTE cells were synchronized in their growth cycle by culturing in minimum essential medium (Life Technologies) supplemented with 2.5% FBS. After 24 h, this medium was removed by aspiration, and TCT-endotoxin samples were added to the cells in serum-free minimum essential medium. After 4 h, [3H]thymidine (ICN Pharmaceuticals, Costa Mesa, Calif.) was added directly to the samples along with serum to promote resumption of cell growth. After 26 h, DNA synthesis was assessed by scintillation counting of trichloroacetic acid-precipitable material, and NO· production was assessed by the nitrite assay described above.
Since kinase inhibitors have the potential of causing general cellular toxicity, their effects were assessed using a modified HTE cell toxicity assay: the inhibitor was included with the TCT-endotoxin sample in serum-free medium; after 4 h, the sample and inhibitor were removed and replaced with [3H]thymidine in serum-containing medium. DNA synthesis and nitrite accumulation were assessed as usual. For trials with calphostin C, the samples were exposed to a fluorescent light placed inside the incubator, because this drug is reported to be activated by light (4).
Bioassay for IL-1 activity.
IL-1 activity in HTE cell lysates was assessed by the method of Hill et al. (24), with minor modifications. HTE cells treated as described above (“Toxicity assay”) were lysed by two −80°C freeze-thaw cycles in 50 μl of fresh medium (RPMI 1640 with 10% FBS). This lysate plus 50 μl of fresh medium rinse was transferred to wells containing 105 RINm5F cells (a rat insulinoma cell line) in 50 μl of fresh medium and were incubated at 37°C (95% air–5% CO2 atmosphere). After 24 h of incubation, 50 μl of the RINm5F culture supernatant was combined with an equal volume of Griess reagent (see “Nitrite assay” above) to measure nitrite produced by the RINm5F cells as a selective response to IL-1 in the HTE lysates. IL-1 determinations were calculated by comparing these samples to a standard curve of nitrite production by RINm5F cells exposed to purified IL-1α (0 to 100 pg/ml).
RNase protection assay (RPA).
An approximately 300-bp segment of hamster IL-1α was amplified by PCR from HTE cell genomic DNA using the primers 5′-CGGAATTCTCATCACAGGTAGTGAGACC-3′ (EcoRI-sense) and 5′-GCGGATCCGGTAAACATTCATTTAGAATT-3′ (BamHI-antisense). These primers were expected to hybridize within a single exon, based on the genomic map of human IL-1α. A PCR product of the expected size was partially sequenced to verify its identity as hamster IL-1α, which showed slight deviations from the corresponding human, murine, and rat sequences. The amplified product was digested with EcoRI and BamHI, ligated into pBluescript SK+ (Stratagene Cloning Systems, La Jolla, Calif.), and transformed into E. coli HB101 by electroporation, all according to standard molecular biology techniques (2), resulting in the cloned plasmid pTF03.
Glyceraldehyde phosphate dehydrogenase (GAPDH) was used as an internal standard in some assays. In a similar manner as for IL-1α, a small segment of hamster GAPDH was amplified using the primers 5′-CGGGATCCGTGAACGGATTTGGCCGTATTGG-3′ (BamHI-sense) and 5′-GGGGTACCAGCCTTCTCCATGGTGGTGAAGACG-3′ (KpnI-antisense). This fragment was cloned into the BamHI and KpnI sites of pBluescript SK+, resulting in pTF08.
The RPA was performed essentially according to established protocols (2), with the following specific details. To prepare the antisense IL-1α riboprobe, pTF03 was linearized with EcoRI, and RNA was transcribed using T3 RNA polymerase in the presence of [α-32P]CTP (10 mCi/ml, 400 Ci/mmol; Amersham) and unlabeled CTP in a ratio determined to produce a probe with a calculated specific activity of ∼40 × 103 dpm/fmol. The GAPDH riboprobe was prepared similarly, except that the plasmid was linearized with HindIII, and RNA was transcribed with T7 RNA polymerase with a [α-32P]CTP/CTP ratio which resulted in a probe activity of approximately 2 × 103 dpm/fmol. Total RNA was prepared from treated HTE cells by using Ultraspec RNA reagent (Biotecx Laboratories, Houston, Tex.) according to the manufacturer's directions. The probe (∼5 fmol/sample) was hybridized with 10 to 20 μg of sample RNA at 42°C for 12 to 18 h. The RNase digestion mixture contained RNase A (10 μg/ml) and RNase T1 (100 U/ml) (Boehringer Mannheim, Indianapolis, Ind.). Carrier yeast tRNA was included in the proteinase K mix. Protected fragments were separated on 5% polyacrylamide gels. Dried gels were exposed to X-ray film (Amersham) or exposed to an imaging screen to permit quantitation using a storage phosphor imaging system (Bio-Rad Laboratories, Hercules, Calif.).
RESULTS
Synergistic action of TCT and endotoxin.
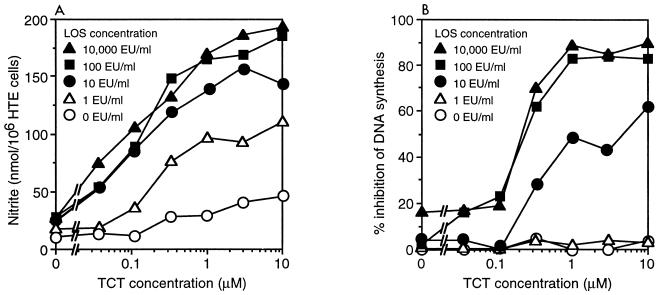
The toxic effect of TCT and endotoxin on HTE cells was assessed by measuring the degree of DNA synthesis inhibition and the production of NO·. NO· has been shown to be an essential mediator of this inhibition, in that blockage of NO· production with selective iNOS inhibitors completely abrogates the effects of TCT and endotoxin on DNA synthesis (21). Synchronized HTE cells were exposed to TCT and B. pertussis LOS for 4 h in serum-free medium. Serum was then supplied to the cells along with [3H]thymidine, and after 26 h the cells were assayed for thymidine incorporation and for the production of nitrite, a stable oxidation product of NO·. As shown in Fig. 1, cells were exposed to a titration of TCT concentrations in combination with various amounts of LOS. Note that neither high levels of TCT without LOS nor high levels of LOS without TCT were capable of eliciting substantial effects. However, in the presence of LOS, there was an obvious synergistic interaction with TCT in both the production of NO· and the inhibition of DNA synthesis.
FIG. 1.
Synergy of TCT and B. pertussis LOS. HTE cells were treated with the indicated concentrations of B. pertussis LOS in combination with various amounts of TCT. After 30 h, nitrite accumulation (A) and DNA synthesis inhibition (B) were assessed. Note that the leftmost point of each curve represents the small effects of LPS alone. Each data point is the mean of triplicate samples; standard deviation of each point was <10% of the mean.
With regard to NO· production, 100 eU of endotoxin per ml was sufficient to result in maximal activity in combination with TCT, while 10 eU/ml was suboptimal (Fig. 1A). For TCT, the maximal activity appeared to be at 3 μM in combination with endotoxin; increasing concentrations of TCT beyond 10 μM resulted in very little increase in nitrite accumulation (data not shown). There appears to be a relatively smooth nitrite response to the doses of both TCT and endotoxin up to their optimal concentrations.
With regard to inhibition of DNA synthesis, the increasing levels of endotoxin and TCT revealed a threshold effect, as depicted in Fig. 1B. Endotoxin at 1 eU/ml was completely ineffective even at high concentrations of TCT, while at 10 eU/ml there was a substantial inhibition of DNA synthesis at the upper concentrations of TCT. This is interesting because endotoxin at 1 eU/ml combined with the upper doses of TCT resulted in measurable nitrite accumulation and yet had no effect on DNA synthesis. In a similar manner, 0.1 μM TCT in combination with 10 or 100 eU of endotoxin per ml had very little effect on DNA synthesis, even though at these concentrations there was notable nitrite accumulation. These results suggest that there is some threshold for NO· production below which there is no effect on DNA synthesis. Such threshold effects have been observed in other NO·-dependent cytostatic responses (3). It is important to recognize that under physiological conditions, NO· has a half-life of only seconds (14), but the assay for accumulated nitrite is an indication of the total NO· production over time. It is reasonable to assume that NO· production must reach a substantial flux of production to achieve an inhibitory concentration.
Experiments performed with E. coli LPS revealed essentially identical results, with the same optimal levels of TCT and endotoxin (data not shown).
Synergistic induction of IL-1α by TCT/endotoxin.
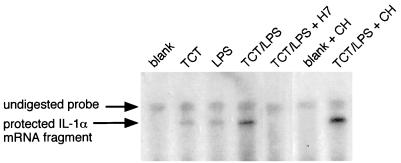
As we have described previously, TCT plus endotoxin can induce IL-1α production in HTE cells, and IL-1 is a strong inducer of iNOS activity in these cells (21), as it is in many other systems (9). Since it is likely that IL-1α is a key intermediate in the toxicity pathway, we wished to characterize the synergistic induction of IL-1α by TCT plus endotoxin and to place this step in the pathway relative to other signaling events. To obtain an early and sensitive indication of IL-1α induction, we used an RPA to assess the level of IL-1α mRNA (Fig. 2). While exposure to TCT alone or LPS alone induced a small amount of IL-1α mRNA, the two signals combined had a synergistic effect. To assess the dependence of this induction on the synthesis of new protein, cycloheximide was included. Cycloheximide did not induce IL-1α mRNA by itself, and it did not inhibit the induction by TCT plus LPS; in fact, it seems that cycloheximide may enhance the level of IL-1α mRNA, in agreement with previous observations (28, 48).
FIG. 2.
TCT-LPS induction of IL-1α mRNA. HTE cells were exposed to TCT (3.2 μM), LPS (100 eU/ml), cycloheximide (CH; 10 μM), and H7 (100 μM) in serum-free medium as indicated. After 4 h, total RNA was prepared and subjected to RPA analysis for IL-1α. (A small remnant of undigested probe remains in all samples due to incomplete degradation of the DNA template used to transcribe the labeled RNA probe.) The protected IL-1α fragment runs slightly lower than the probe, due to noncomplementary vector sequence present at one end of the probe.
As previously described, IL-1α protein accumulates intracellularly in HTE cells treated with TCT plus LPS and can be detected by a bioassay of freeze-thaw lysates of treated cells (22). We repeated these observations (using a different IL-1 bioassay), and in addition tested TCT alone and B. pertussis LOS alone. The production of IL-1α bioactivity demonstrated the same synergy between TCT and endotoxin as seen in the RPA; among three representative experiments, the combined signal of TCT (3 μM) plus LOS (100 eU/ml) yielded IL-1 bioactivity at least 5.5-fold greater than the response to either signal alone. (IL-1 values from these individual experiments were as follows: experiment 1, LOS = 0.306, TCT = 0.273, TCT/LOS = 2.083; experiment 2, LOS = 0, TCT = 0.550, TCT/LOS = 3.025; experiment 3, LOS = 0.022, TCT = 0, TCT/LOS = 3.364.)
Effects of the serine/threonine kinase inhibitor H7.
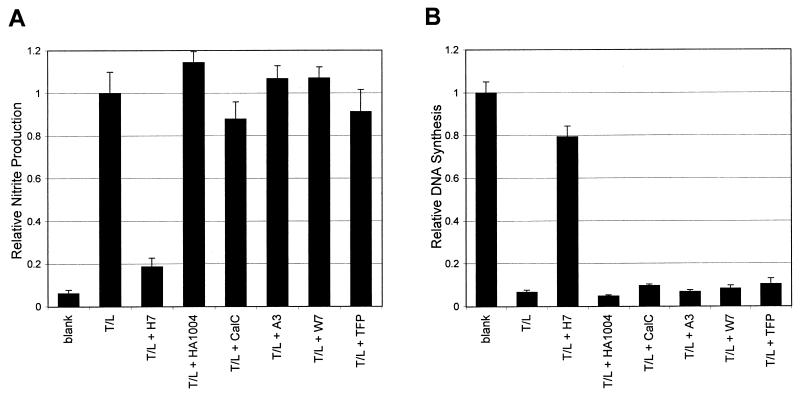
Many endotoxin effects, as well as other processes that induce IL-1, have been shown to involve protein phosphorylation. To determine the potential involvement of protein kinases in the signal transduction pathways activated by TCT and endotoxin, we examined the effects of protein kinase inhibitors selective for serine/threonine kinases. Since kinase inhibitors have the potential of causing general cellular toxicity, their effects were assessed using a modified HTE cell toxicity assay. Using DNA synthesis as a measure of the inhibitors' general toxicity, each inhibitor was titrated to find the maximum concentration at which it was not inhibitory to the growth of the HTE cells; this maximal level is reflected in the concentration listed for each inhibitor in Fig. 3.
FIG. 3.
Effects of serine/threonine kinase inhibitors on TCT-LPS-induced DNA synthesis inhibition and nitrite production. HTE cells were treated with TCT (3.2 μM) and LPS (100 eU/ml) (T/L) plus the kinase inhibitors H7 (320 μM), HA1004 (1 mM), calphostin C (CalC; 32 nM), A3 (32 μM), W7 (10 μM), and trifluoperazine (TFP; 10 μM). Inhibitor effects were assayed using the modified HTE cell toxicity assay as described in Materials and Methods, and DNA synthesis and nitrite production were assessed. In the case of nitrite production (A), the results shown are scaled relative to TCT-LPS-treated cells; in the case of DNA synthesis (B), the results shown are scaled relative to untreated cells (blank). The concentration chosen for each inhibitor was the highest tested concentration that did not cause significant toxicity by itself (<15% DNA synthesis inhibition relative to untreated cells [data not shown]). Essentially identical results were obtained for each inhibitor in at least three separate experiments. Similar results were observed in experiments in which the cells were pretreated with the inhibitors for 30 min prior to the addition of TCT and LPS (data not shown). Evaluation of the data by t test demonstrated that the nitrite and DNA synthesis effects caused by TCT-LPS plus H7 were significantly different than those caused by TCT-LPS (P < 0.001).
As depicted in Fig. 3A, we found that kinase inhibitor H7 was capable of blocking the production of NO· induced by TCT plus LPS almost completely. As shown in Fig. 3B, H7 also prevented the toxicity (i.e., the inhibition of DNA synthesis) caused by TCT plus LPS (also see Fig. 5). The drug by itself had little inhibitory effect on the HTE cells, as assessed by DNA synthesis, at concentrations up to 1 mM (data not shown).
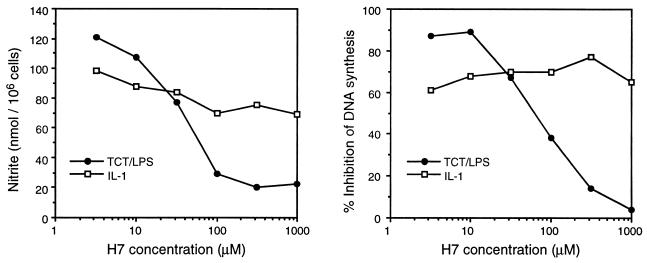
FIG. 5.
H7 inability to block exogenous IL-1 toxicity. HTE cells were treated with TCT (1 μM), LPS (316 ng/ml), or recombinant murine IL-1β (100 ng/ml), along with various doses of H7, in a modified HTE cell toxicity assay. Each data point is the mean of triplicate samples; standard deviation of each point was <10% of the mean.
H7 is widely regarded as an inhibitor of protein kinase C (PKC), but it is also effective at similar concentrations against cyclic AMP (PKA)- and cyclic GMP (PKG)-dependent protein kinases (23). Therefore, we used a panel of inhibitors possessing greater selectivity for these kinases in an attempt to define the actual target of H7 in this system (Fig. 3). The kinase inhibitors A3 and HA1004, which are more selective for PKA and PKG than for PKC (1, 26), were completely ineffective in blocking the NO· and DNA synthesis inhibition induced by TCT plus LPS. Somewhat surprisingly, the inhibitor calphostin C, which is very selective for PKC (30), was also incapable of blocking the effects of TCT plus LPS. As a positive control for the activity of calphostin C in this system, we observed that it was capable of inhibiting PMA-stimulated effects (Fig. 4). Furthermore, inhibitors of Ca2+/calmodulin-dependent kinase, W7 (27) and trifluoperazine (44), also did not modulate the effects of TCT and LPS.
FIG. 4.
Synergy of PMA with LPS. HTE cells were exposed to TCT (1 μM), LPS (100 eU/ml), and PMA (100 nM) in a modified HTE cell toxicity assay as described in Materials and Methods. Some samples included H7 (100 μM) or calphostin C (cal C; 32 nM).
To establish the position of the H7-sensitive step within the signaling pathway of TCT plus LPS, we examined its effects on IL-1α induction. Using RPA as described above to examine the IL-1α mRNA levels, we found that H7 completely blocked the IL-1α induction caused by TCT plus LPS (Fig. 2). The small amount of IL-1α mRNA induced by TCT alone or LPS alone (as shown in Fig. 2) was also blocked by H7 (not shown). These results indicate that the H7-sensitive step lies upstream of IL-1α induction.
Differential signaling effects by TCT and endotoxin.
Even though the evidence from the PKC inhibitor calphostin C suggested that PKC was not involved in TCT-endotoxin signal transduction, we examined the effects of direct kinase activation in the HTE cell system with the phorbol ester PMA, which strongly activates PKC (39). Using PMA, we obtained results that imply that TCT and endotoxin induce separate signaling pathways.
As shown in Fig. 4, PMA had only a small effect on the production of NO· by HTE cells, comparable to that caused by TCT alone or LPS alone. PMA also had no effect on DNA synthesis (data not shown). When PMA was used in combination with the toxins, though, differential effects were observed. The combination of PMA with LPS induced a high level of NO·, equivalent to that induced by TCT plus LPS (P = 0.76 in t-test analysis). However, the combination of PMA with TCT induced very little NO·, approximately the sum of their individual effects. Thus, direct activation of PKC by PMA can provide a signal that effectively synergizes with LPS, comparable to the synergy of TCT with LPS. However, PMA cannot synergize with TCT. This observation is an indication that the signals induced by TCT are, at least partially, distinct from those induced by endotoxin. TCT must have some unique effects, which can be mimicked by PMA stimulation, that are necessary for the synergistic interaction observed with endotoxin.
The nitrite induction by PMA plus LPS also provided a positive control for the two kinase inhibitors used above, which are known to be active against PKC. H7 and calphostin C both inhibited the PMA-LPS induction of NO·, presumably by blocking PMA-stimulated PKC as expected (Fig. 4). This is in contrast to the situation with TCT-LPS, in which H7 can inhibit the induction of NO· by TCT-LPS, but calphostin C cannot (Fig. 3).
H7 and the response to exogenous IL-1.
To evaluate whether H7 was affecting only early signal transduction steps in the toxicity pathway, we bypassed the H7 blockage by triggering a downstream effector. As we have previously shown, exogenously added IL-1 can reproduce the effects of TCT plus endotoxin in HTE cells (22). Therefore, we treated HTE cells with murine IL-1β in the presence of H7. As seen in Fig. 5, while again H7 was able to inhibit both nitrite production and DNA synthesis inhibition induced by TCT plus LPS, H7 was ineffective in inhibiting the nitrite production and DNA synthesis inhibition induced by exogenous IL-1. This result provides evidence that there are no H7-sensitive steps downstream of the activity induced by IL-1. The experiment also demonstrates that exogenously added IL-1 is not activating the same early responses as TCT-endotoxin, in accordance with the concept of IL-1 working in an autocrine role to stimulate the induction of intracellular IL-1α through a pathway which is not sensitive to H7.
DISCUSSION
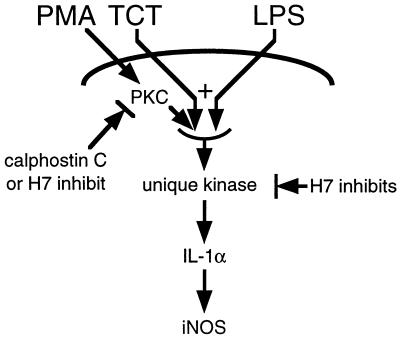
We have shown that two products of B. pertussis, TCT and endotoxin, can function in a synergistic manner to trigger inflammatory responses within respiratory epithelial cells. TCT and endotoxin were seen to be synergistic in the induction of IL-1α mRNA and protein, production of NO·, and inhibition of DNA synthesis. While the synergy between TCT and endotoxin occurs at a relatively early step, the activity of the molecules was separable: TCT activity could be mimicked by activation of PKC, but the activity of endotoxin could not be replaced by PKC activation. Our observations also suggest that the signal transduction pathway utilized by TCT and endotoxin includes activation of an uncharacterized serine/threonine protein kinase. Figure 6 depicts a signaling model that is consistent with our findings.
FIG. 6.
Model of TCT and endotoxin signaling. TCT and LPS each act through cell surface receptors to induce differential signaling events, which combine synergistically to activate a unique H7-sensitive kinase. Direct activation of PKC by PMA can produce a signal that mimics TCT, in that it can synergize with LPS in the same manner as does TCT. However, TCT and LPS signaling pathways do not proceed via PKC, since the PKC-specific inhibitor calphostin C does not block effects of TCT and LPS but does block effects of PMA and LPS. The signaling pathway induces IL-1α, causing induction of iNOS and the production of NO·, which leads to cytostasis. Note that while the PMA-stimulated pathway may not proceed through the same kinase as TCT and LPS, the depiction is consistent with our data.
This work has also resulted in further correlative evidence for the involvement of IL-1α as a necessary intermediate in the toxicity pathway. Previously we reported that production of intracellular IL-1α is induced soon after treatment with TCT and endotoxin and that exogenously added IL-1 is capable of reproducing the effects of TCT and endotoxin on respiratory cells and explanted tissue (22). In this report we conclude that the synergy between TCT and endotoxin occurs at a point upstream of IL-1α transcriptional activation. Furthermore, we observed that the kinase inhibitor H7, which blocked the downstream effects of TCT and endotoxin, also blocked the induction of IL-1α. In all situations, the level of IL-1α induction was correlated to the level of iNOS induction and ultimate toxicity in HTE cells. These observations strongly support the idea that IL-1α is an essential mediator of TCT-endotoxin toxicity.
In the case of iNOS induction driven by exogenous IL-1, it is likely that intracellular IL-1α is still the key mediator, being induced in an autocrine fashion by exogenous IL-1 through a separate pathway that is not inhibited by H7. Indeed, we noted that exogenous IL-1 induced IL-1α mRNA in HTE cells (data not shown), in agreement with observations in other systems (33). It is interesting to speculate how such an autocrine activation could play a role in the pertussis syndrome. As the ciliated cells are shed from the epithelium, it is possible that ruptured ciliated cells release a significant amount of IL-1, thus activating neighboring cells within the epithelium. While IL-1β must be processed by IL-1-converting enzyme to become biologically active, that is not the case for IL-1α (13). Thus, release of IL-1α via the rupture of extruded cells may be an important mechanism for spreading and prolonging the damage to the tracheal epithelium caused by a B. pertussis infection.
The serine/threonine protein kinase inhibitor H7 was able to block almost completely the toxic effects of TCT and endotoxin. The H7-sensitive link in the signaling pathway is upstream of IL-1α induction, since H7 blocked IL-1α mRNA accumulation. However, the target of the inhibitor in the signaling pathway of TCT-endotoxin is not known. While PKC is a common target of H7, experiments using a more selective PKC inhibitor demonstrated that PKC is not involved in TCT-endotoxin-mediated effects and that it is not the relevant target of H7 in this system. Inhibitors of other protein kinases for which H7 is also known to have inhibitory effects (PKA and PKG) failed to block toxicity. It is important to note that the ineffective kinase inhibitors we used may have failed to block toxicity for a variety of reasons; except for calphostin C, we did not demonstrate that the inhibitors were capable of blocking their putative targets in the HTE cells. However, the concentrations tested were all above the levels reported to be effective in other systems. Thus, the effects produced by TCT and endotoxin may be mediated by an uncharacterized H7-sensitive kinase, which is downstream of the synergistic combination of TCT and endotoxin signals.
There are reports in other systems of uncommon (i.e., not PKC, PKA, PKG, or Ca2+/calmodulin-dependent kinase) H7-sensitive steps in the signal transduction pathways leading to the activation of primary response genes. The autocrine induction of IL-1α depends on an H7-sensitive kinase that is distinct from PKC or PKA (34). The activation of junB by IL-6 was shown to be dependent on an uncharacterized H7-sensitive kinase (37, 38). In this case, later workers identified the H7-sensitive kinase as potentially being p90rsk (50). Finally, in studies of TCT [termed “G(Anh)MTetra”] in human monocytes, the induction of IL-1β, IL-6, and granulocyte colony-stimulating factor is inhibited by H7 but not by H8 (PKA inhibitor) or genistein (a protein tyrosine kinase inhibitor) (11). The conclusion from this work is that the H7-sensitive kinase is PKC, but no direct evidence was provided to implicate PKC versus another H7-sensitive kinase.
Synergy between endotoxin and muramyl peptides has been noted in other systems, but the activation of epithelial cells that we have described is unique. Most muramyl peptide-endotoxin studies have used MDP in monocytes/macrophages (Mφ). For example, pretreatment of animals with MDP enhances the level of LPS-stimulated tumor necrosis factor alpha and tumorcidal activity, as well as enhancing the lethal effects of LPS (40, 41); similar synergy is seen in isolated macrophages (8, 32). Other mediators produced in Mφ by the synergistic combination of MDP and LPS include IL-1 (32), IL-6 (32, 45), and nitric oxide (3). However, a major caveat in comparing these results in Mφ to our observations in epithelial cells is that we know that MDP is inactive in both explanted hamster tracheal tissue and HTE cells (35). Thus, there seems to be a difference in structural requirements for response to muramyl peptides between Mφ and epithelial cells.
There are also a number of studies that have examined the effects of peptidoglycan preparations from gram-positive organisms upon Mφ. These peptidoglycan preparations induce the secretion of IL-1 and IL-6 from murine and human macrophages (15, 46, 49) and also induce NO· production (43). However, the agonists in these Mφ studies are polymeric peptidoglycan chains (average molecular weight [MW] of 125,000) or entire insoluble cell walls, while TCT is a single low-MW species (MW = 921) which is a monomeric component of the peptidoglycan polymer. Furthermore, extensive structure-function studies of the peptide unit of TCT have shown that the side chain functional groups of the diaminopimelic acid are critical for epithelial activity (36). However, in the gram-positive peptidoglycan used for the Mφ studies, lysine typically takes the place of the diaminopimelic acid found in TCT; a TCT analog peptide containing such a gram-positive substitution is inactive in our respiratory epithelial cell system (35). It could be imagined that the two molecules might be recognized by their similar saccharide structures; however, we have demonstrated that the disaccharide portion of TCT is completely dispensable for activity in respiratory tissue and HTE cells (35). Therefore, it is unlikely that gram-positive peptidoglycan and TCT activate cells by interacting with the same recognition site.
Finally, there are some studies of Mφ activation by natural, low-MW peptidoglycan fragments like TCT, but they are distinguished primarily by the lack of TCT-endotoxin synergy which we observe in epithelial cells (10, 11). For example, both TCT alone and LPS alone induce IL-1β and IL-6 in human monocytes, but in those cells the effects of TCT plus LPS are less than the sum of the individual effects (11), unlike the synergy observed in our epithelial cell system. In the murine macrophage-like cell line RAW264.7, TCT alone is not capable of inducing NO·, nor does it augment the NO· induced by suboptimal doses of LPS in this cell line (data not shown). Taken together, these observations indicate that epithelial cells and Mφ may recognize different structural features within peptidoglycan and that epithelial cells may have a more strict requirement for dual activation by peptidoglycan and endotoxin.
The synergistic activation of epithelial cells described in this study by a natural muramyl peptide and endotoxin may represent a unique and important mode of nonspecific mucosal immunity. Most gram-negative bacteria contain monomeric peptidoglycan pieces comparable to TCT, which are released in small amounts (18). Furthermore, processing by macrophages of phagocytosed bacteria results in production of TCT and similar molecules (but not MDP) (47). The presence of endotoxin would also be expected during a bacterial infection. These factors may combine synergistically to activate a localized antibacterial nitric oxide response from epithelial cells. It is possible that some bacteria like B. pertussis have evolved to take advantage of this system by releasing a large amount of peptidoglycan pieces, thus overstimulating the defense system to autotoxic levels. In the case of pertussis, the resulting ciliated cell damage compromises the natural airway clearance mechanism, and the corresponding coughing reaction becomes an efficient means for disease transmission.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI22243 and HL56419 (to W.E.G.) and AI07172 (to Washington University).
REFERENCES
- 1.Asano T, Hidaka H. Vasodilatory action of HA1004 [N-(2-guanidinoethyl)-5-isoquinolinesulfonamide], a novel calcium antagonist with no effect on cardiac function. J Pharmacol Exp Ther. 1984;231:141–145. [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1987. [Google Scholar]
- 3.Barratt G M, Raddassi K, Petit J-F, Tenu J-P. MDP and LPS act synergistically to induce arginine-dependent cytostatic activity in rat alveolar macrophages. Int J Immunopharmacol. 1991;13:159–165. doi: 10.1016/0192-0561(91)90094-n. [DOI] [PubMed] [Google Scholar]
- 4.Bruns R F, Miller F D, Merriman R L, Howbert J J, Heath W F, Kobayashi E, Takahashi I, Tamaoki T, Nakano H. Inhibition of protein kinase C by calphostin C is light-dependent. Biochem Biophys Res Commun. 1991;176:288–293. doi: 10.1016/0006-291x(91)90922-t. [DOI] [PubMed] [Google Scholar]
- 5.Chaby R, Caroff M. Lipopolysaccharides of Bordetella pertussis endotoxin. In: Wardlaw A C, Parton R, editors. Pathogenesis and immunity in pertussis. Chichester, England: John Wiley & Sons; 1988. pp. 247–271. [Google Scholar]
- 6.Cookson B T, Cho H-L, Herwaldt L A, Goldman W E. Biological activities and chemical composition of purified tracheal cytotoxin of Bordetella pertussis. Infect Immun. 1989;57:2223–2229. doi: 10.1128/iai.57.7.2223-2229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cookson B T, Tyler A N, Goldman W E. Primary structure of the peptidoglycan-derived tracheal cytotoxin of Bordetella pertussis. Biochemistry. 1989;28:1744–1749. doi: 10.1021/bi00430a048. [DOI] [PubMed] [Google Scholar]
- 8.Daemen T, Veninga A, Regts J, Scherphof G L. Maintenance of tumoricidal activity and susceptibility to reactivation of subpopulations of rat liver macrophages. J Immunother. 1991;100:200–206. doi: 10.1097/00002371-199106000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Dinarello C A. Inflammatory cytokines: interleukin-1 and tumor necrosis factor as effector molecules in autoimmune diseases. Curr Opin Immunol. 1991;3:941–948. doi: 10.1016/s0952-7915(05)80018-4. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello C A, Krueger J M. Induction of interleukin 1 by synthetic and naturally occurring muramyl peptides. Fed Proc. 1986;45:2545–2548. [PubMed] [Google Scholar]
- 11.Dokter W H A, Dijkstra A J, Koopmans S B, Mulder A B, Stulp B K, Keck W, Halie M R, Vellenga E. G(Anh)MTetra, a natural bacterial cell wall breakdown product, induces interleukin-1β and interleukin-6 expression in human monocytes. J Biol Chem. 1994;269:4201–4206. [PubMed] [Google Scholar]
- 12.Flak T A, Goldman W E. Signaling and cellular specificity of airway nitric oxide production in pertussis. Cell Microbiol. 1999;1:51–60. doi: 10.1046/j.1462-5822.1999.00004.x. [DOI] [PubMed] [Google Scholar]
- 13.Fuhlbrigge R C, Fine S M, Unanue E, Chaplin D D. Expression of membrane interleukin 1 by fibroblasts transfected with murine pro-interleukin 1 alpha cDNA. Proc Natl Acad Sci USA. 1988;85:5649–5653. doi: 10.1073/pnas.85.15.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaston B, Drazen J M, Loscalzo J, Stamler J S. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med. 1994;149:538–551. doi: 10.1164/ajrccm.149.2.7508323. [DOI] [PubMed] [Google Scholar]
- 15.Gold M R, Miller C L, Mishell R I. Soluble non-cross-linked peptidoglycan polymers stimulate monocyte-macrophage inflammatory functions. Infect Immun. 1985;49:731–741. doi: 10.1128/iai.49.3.731-741.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman W E, Baseman J B. Selective isolation and culture of a proliferating epithelial cell population from the hamster trachea. In Vitro. 1980;16:313–319. doi: 10.1007/BF02618337. [DOI] [PubMed] [Google Scholar]
- 17.Goldman W E, Klapper D G, Baseman J B. Detection, isolation, and analysis of a released Bordetella pertussis product toxic to cultured tracheal cells. Infect Immun. 1982;36:782–794. doi: 10.1128/iai.36.2.782-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodell E W, Schwarz U. Release of cell wall peptides into culture medium by exponentially growing Escherichia coli. J Bacteriol. 1985;162:391–397. doi: 10.1128/jb.162.1.391-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 20.Heiss L N. Ph.D. dissertation. St. Louis, Mo: Washington University; 1993. [Google Scholar]
- 21.Heiss L N, Lancaster J R, Jr, Corbett J A, Goldman W E. Epithelial autotoxicity of nitric oxide: role in the respiratory cytopathology of pertussis. Proc Natl Acad Sci USA. 1994;91:267–270. doi: 10.1073/pnas.91.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heiss L N, Moser S A, Unanue E R, Goldman W E. Interleukin-1 is linked to the respiratory epithelial cytopathology of pertussis. Infect Immun. 1993;61:3123–3128. doi: 10.1128/iai.61.8.3123-3128.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hidaka H, Inagaki M, Kawamoto S, Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984;23:5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- 24.Hill J R, Corbett J A, Baldwin A C, McDaniel M L. Nitric oxide production by the rat insulinoma cell line, RINm5F, is specific for IL-1: a spectrophotometric IL-1 bioassay. Anal Biochem. 1996;236:14–19. doi: 10.1006/abio.1996.0125. [DOI] [PubMed] [Google Scholar]
- 25.Höltje J V, Mirelman D, Sharon N, Schwarz U. Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975;124:1067–1076. doi: 10.1128/jb.124.3.1067-1076.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inagaki M, Kawamoto S, Itoh H, Saitoh M, Hagiwara M, Takahashi J, Hidaka H. Naphthalenesulfonamides as calmodulin antagonists and protein kinase inhibitors. Mol Pharmacol. 1986;29:577–581. [PubMed] [Google Scholar]
- 27.Itoh H, Hidaka H. Direct interaction of calmodulin antagonists with Ca2+/calmodulin-dependent cyclic nucleotide phosphodiesterase. J Biochem. 1984;96:1721–1726. doi: 10.1093/oxfordjournals.jbchem.a135004. [DOI] [PubMed] [Google Scholar]
- 28.Jarrous N, Kaempfer R. Induction of human interleukin-1 gene expression by retinoic acid and its regulation at processing of precursor transcripts. J Biol Chem. 1994;269:23141–23149. [PubMed] [Google Scholar]
- 29.Johannsen L, Obal F, Jr, Kapas L, Kovalzon V, Krueger J M. Somnogenic activity of muramyl peptide-derived immune adjuvants. Int J Immunopharmacol. 1994;16:109–116. doi: 10.1016/0192-0561(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- 31.Krueger J M, Rosenthal R S, Martin S A, Walter J, Davenne D, Shoham S, Kubillus S L, Biemann K. Bacterial peptidoglycans as modulators of sleep. I. Anhydro forms of muramyl peptides enhance somnogenic potency. Brain Res. 1987;403:249–257. doi: 10.1016/0006-8993(87)90062-x. [DOI] [PubMed] [Google Scholar]
- 32.Le Contel C, Temime N, Charron D J, Parant M A. Modulation of lipopolysaccharide-induced cytokine gene expression in mouse bone marrow-derived macrophages by muramyl dipeptide. J Immunol. 1993;10:4541–4549. [PubMed] [Google Scholar]
- 33.Lee S, W, Morhenn V B, Ilnicka M, Eugui E M, Allison A C. Autocrine stimulation of interleukin-1 alpha and transforming growth factor alpha production in human keratinocytes and its antagonism by glucocorticoids. J Investig Dermatol. 1991;97:106–110. doi: 10.1111/1523-1747.ep12478503. [DOI] [PubMed] [Google Scholar]
- 34.Lee W Y, Butler A P, Locniskar M F, Fischer S M. Signal transduction pathway(s) involved in phorbol ester and autocrine induction of interleukin-1α mRNA in murine keratinocytes. J Biol Chem. 1994;269:17971–17980. [PubMed] [Google Scholar]
- 35.Luker K E, Collier J L, Kolodziej E W, Marshall G R, Goldman W E. Bordetella pertussis tracheal cytotoxin and other muramyl peptides: distinct structure-activity relationships for respiratory epithelial cytopathology. Proc Natl Acad Sci USA. 1993;90:2365–2369. doi: 10.1073/pnas.90.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luker K E, Tyler A N, Marshall G R, Goldman W E. Tracheal cytotoxin structural requirements for respiratory epithelial damage in pertussis. Mol Microbiol. 1995;16:733–743. doi: 10.1111/j.1365-2958.1995.tb02434.x. [DOI] [PubMed] [Google Scholar]
- 37.Nakajima K, Kusafuka T, Takeda T, Fujitani Y, Nakae K, Hirano T. Identification of a novel interleukin-6 response element containing an Ets-binding site and a CRE-like site in the junB promoter. Mol Cell Biol. 1993;13:3027–3041. doi: 10.1128/mcb.13.5.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakajima K, Wall R. Interleukin-6 signals activating junB and TIS11 gene transcription in a B-cell hybridoma. Mol Cell Biol. 1991;11:1409–1418. doi: 10.1128/mcb.11.3.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986;233:305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- 40.Parant M, Chedid L. Various aspects of synergism between endotoxin and MDPs. Adv Exp Med Biol. 1990;256:537–547. doi: 10.1007/978-1-4757-5140-6_47. [DOI] [PubMed] [Google Scholar]
- 41.Parant M, Parant F, Vinit M A, Jupin C, Noso Y, Chedid L. Priming effect of muramyl peptides for induction by lipopolysaccharide of tumor necrosis factor production in mice. J Leukoc Biol. 1990;47:164–169. doi: 10.1002/jlb.47.2.164. [DOI] [PubMed] [Google Scholar]
- 42.Park J T. Turnover and recycling of the murein sacculus in oligopeptide permease-negative strains of Escherichia coli: indirect evidence for an alternative permease system and for a monolayered sacculus. J Bacteriol. 1993;175:7–11. doi: 10.1128/jb.175.1.7-11.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pugin J, Heumann I D, Tomasz A, Kravchenko V V, Akamatsu Y, Nishijima M, Glauser M P, Tobias P S, Ulevitch R J. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 44.Roufogalis B D, Minocherhomjee A M, Al-Jobore A. Pharmacological antagonism of calmodulin. Can J Biochem Physiol. 1983;61:927–933. doi: 10.1139/o83-118. [DOI] [PubMed] [Google Scholar]
- 45.Shi F, Kurzman I D, MacEwen E G. In vitro and in vivo production of interleukin-6 induced by muramyl peptides and lipopolysaccharide in normal dogs. Cancer Biother. 1995;10:317–325. doi: 10.1089/cbr.1995.10.317. [DOI] [PubMed] [Google Scholar]
- 46.Vacheron F, Guenounou M, Nauciel C. Induction of interleukin 1 secretion by adjuvant-active peptidoglycans. Infect Immun. 1983;42:1049–1054. doi: 10.1128/iai.42.3.1049-1054.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vermeulen M W, Gray G R. Processing of Bacillus subtilis peptidoglycan by a mouse macrophage cell line. Infect Immun. 1984;46:476–483. doi: 10.1128/iai.46.2.476-483.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warner S J, Libby P. Human vascular smooth muscle cells. Target for and source of tumor necrosis factor. J Immunol. 1989;142:100–109. [PubMed] [Google Scholar]
- 49.Weidemann B, Brade H, Rietschel E T, Dziarski R, Bazil V, Kusumoto S, Flad H-D, Ulmer A J. Soluble peptidoglycan-induced monokine production can be blocked by anti-CD14 monoclonal antibodies and by lipid A partial structures. Infect Immun. 1994;62:4709–4715. doi: 10.1128/iai.62.11.4709-4715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin T, Yang Y C. Mitogen-activated protein kinases and ribosomal S6 protein kinases are involved in signaling pathways shared by interleukin-11, interleukin-6, leukemia inhibitory factor, and oncostatin M in mouse 3T3-L1 cells. J Biol Chem. 1994;269:3731–3738. [PubMed] [Google Scholar]