Abstract
Background: Meal timing resets circadian clocks in peripheral tissues, such as the liver, in seven days without affecting the phase of the central clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Anterior hypothalamus plays an essential role in energy metabolism, circadian rhythm, and stress response. However, it remains to be elucidated whether and how anterior hypothalamus adapts its circadian rhythms to meal timing.
Methods: Here, we applied transcriptomics to profile rhythmic transcripts in the anterior hypothalamus of nocturnal female mice subjected to day- (DRF) or night (NRF)-time restricted feeding for seven days.
Results: This global profiling identified 128 and 3,518 rhythmic transcripts in DRF and NRF, respectively. NRF entrained diurnal rhythms among 990 biological processes, including ‘Electron transport chain’ and ‘Hippo signaling’ that reached peak time in the late sleep and late active phase, respectively. By contrast, DRF entrained only 20 rhythmic pathways, including ‘Cellular amino acid catabolic process’, all of which were restricted to the late active phase. The rhythmic transcripts found in both DRF and NRF tissues were largely resistant to phase entrainment by meal timing, which were matched to the action of the circadian clock. Remarkably, DRF for 36 days partially reversed the circadian clock compared to NRF.
Conclusions: Collectively, our work generates a useful dataset to explore anterior hypothalamic circadian biology and sheds light on potential rhythmic processes influenced by meal timing in the brain (www.circametdb.org.cn).
Keywords: Circadian rhythm, time-restricted feeding, entrainment, anterior hypothalamus, transcriptomics, Hippo signaling, oxidative phosphorylation, amino acid degradation
Introduction
Meal timing resets circadian clocks in peripheral tissues (peripheral clocks), particularly in the liver. 1 – 3 Day time-restricted feeding (DRF) in nocturnal rodents is misaligned with daily rhythms generated by the circadian clock. DRF reverses the liver clock within seven days in mice without altering the central clock located in the suprachiasmatic nucleus (SCN). 4 , 5 DRF contributes to increased adiposity and glucose intolerance when combined with high-fat diet feeding. 6 – 9 Conversely, night time-restricted feeding (NRF), is known to protect against metabolic diseases, such as obesity, insulin resistance, and fatty liver in rodents. 10 , 11 Nevertheless, both DRF and NRF increase longevity in mice, when compared to arrhythmic feeding. 12
Emerging evidence shows that peripheral clocks entrain to DRF in a tissue-specific manner. 13 , 14 Peripheral clocks in the heart and kidneys exhibit resistance to phase entrainment by DRF in female mice, so do the diurnal transcriptomes in these organs. 14 This effect is also present in male mice. 13 Of note, the liver clock seems to condition circadian rhythms in transcriptome in distant organs such as the lungs and adipose tissue. 13 The tissue specificity of phase entrainment to DRF lends support to the proposed network organization or hierarchy of peripheral clocks. 15 – 17
Anterior hypothalamus is a center for circadian rhythm biogenesis, thermal regulation, endocrine functions, and energy metabolism. It includes the medial preoptic nucleus, supraoptic nucleus, SCN, anterior hypothalamic nucleus, and the paraventricular nucleus of the hypothalamus (PVH). While the SCN generates circadian rhythms, the PVH orchestrates circadian rhythms of metabolism and endocrine factors. 18 In addition, the PVH is a hub for nutrient sensing and receives neural signaling from the hunger neurons located in the posterior hypothalamus. 19 , 20 The SCN and PVH host cellular circadian clocks, and the former projects to the latter to output circadian signals. 21 Perceivably, the SCN clock is coupled to the light/dark cycle, whereas the PVH clock integrates circadian signals from both light and food. Genome-wide transcript profiling studies have been done in the SCN or the hypothalamus for rhythmic transcripts in constant darkness. 22 , 23 It remains to be elucidated whether and how anterior hypothalamus adapts its circadian biology to meal timing in mice.
To explore the regulation of anterior hypothalamic circadian biology by time-restricted feeding, we took a systems approach to profile rhythmic transcripts in this tissue from NRF and DRF female mice. We determined rhythmicity features of diurnal transcripts as well as the category and distribution of the enriched rhythmic pathways. This is followed by examining phase entrainment of the circadian clock, rhythmic transcripts and pathways found in both NRF and DRF tissues. Lastly, we compared the profiles of clock genes in the typical seven-day time-restricted feeding regimen to those in the 36-day long-term regimen in order to elucidate the dynamics of the circadian clock. In summary, we have defined circadian signatures of anterior hypothalamus in time-restricted feeding and generated a useful resource to explore the physiology and pathophysiology associated with different types of time-restricted feeding.
Methods
Animals
Animal use was approved by the Laboratory Animal Welfare and Ethics Committee of the Third Military Medical University, China (No. AMUWEC20201106, approval date: 2020/04/15). All experiments conform to the relevant regulatory standards of the institution. All efforts were made to ameliorate harm to animals such as daily monitoring of food and water availability and routine husbandry. This study is reported in line with the ARRIVE guidelines. 41
Special pathogen-free (SPF) mice were purchased from Hunan SJT Laboratory Animal Co. and housed in a SPF barrier facility. C57BL/6J female mice at seven weeks of age were group housed and entrained to a 12h light:12h dark cycle with free access to normal chow diet and water during acclimation for one week. Animals were randomly assigned in a 1:1 ratio to time-restricted feeding groups and fed for seven or 36 days as previously described. 24 The body weight statistics were balanced between groups before subjecting to time-restricted feeding so that there was no significant difference in body weight between experimental groups. DRF permits access to food from zeitgeber time 0 (ZT0, light-on time) to ZT12 (light-off time), and NRF allows food access from ZT12 to ZT0. At the end of time-restricted feeding, mice were euthanized by cervical dislocation, and subjected to tissue collection every 4 h for a complete 24-h cycle. Refer to doi:10.1016/j.xpro.2021.100701 for step-by-step procedures. Anterior hypothalamus was dissected, snap frozen in liquid nitrogen and stored in -80°C (n = 4 per group per time-point).
RNA extraction and sequencing
Total RNA from mouse anterior hypothalamus was extracted by TRIzol method (Invitrogen). RNA integrity (RIN > 7), purity and concentration were assessed and all samples passed the quality control. A total of 200 ng total RNA per sample was subjected to mRNA purification by poly-T oligo magnetic beads and the 150-bp pair-end RNA sequencing in the MGISEQ2000 (BGISEQ-500, RRID:SCR_017979) platform for a minimal coverage of 43.1 million reads by BGI (Shenzhen, China) as described. 14 The RNA sequencing unit was blinded of the grouping information. Raw reads were cleaned via SOAPnuke (RRID:SCR_015025) (v1.5.2), mapped to the reference genome (GRCm38.p6) by HISAT2 (RRID:SCR_015530) (v2.0.4), aligned to the reference coding gene set by Bowtie (RRID:SCR_005476) (v2.2.5), and quantified using RSEM (RRID:SCR_013027) (v1.2.12). Raw counts were normalized by trimmed mean of M-values by edgeR (RRID:SCR_012802) (v3.30.3) and converted to Wagner’s transcripts per million (TPM). No sample was excluded (Pearson correlation coefficient per time point per group > 0.8). Transcripts with a TPM < 1 in more than 20% of samples were filtered. Data were then analyzed by MetaCycle (v1.2.0). 25 The cutoff p-value for a rhythmic transcript was set to BH.Q-value < 0.05.
Phase set enrichment analysis
Pathway enrichment analysis was performed on rhythmic genes as described 14 , 26 (Source file: c5.bp.v7.1.symbols.gmt from https://www.gsea-msigdb.org/gsea/msigdb). Mouse gene nomenclature was converted to human gene nomenclature via “Human and Mouse Homology Classes with Sequence information” (curated by http://www.informatics.jax.org). Domain was set from 0 to 24, indicating the minimal and maximal period length is 0 and 24 h, respectively. Minimal and maximal items per set are 10 and 10000, respectively. Kuiper’s test FDR q-value < 0.05 is considered as statistical significance.
Gene expression analysis
Tissue RNA was isolated using Eastep Super Total RNA Extraction Kit (Promega). Complementary DNA (cDNA) was synthesized using the GoScript Reverse Transcription Mix (Promega). cDNA was amplified and analyzed using iTaq universal SYBR Green Supermix (Bio-Rad) and the Bio-Rad CFX96 Real-Time PCR Detection System (Bio-Rad). PCR protocol: DNA denaturation at 95°C for 3 min; denaturation at 95°C for 10 sec; annealing/extension and plate read at 60°C for 30 sec; 40 cycles of quantitative PCR. Results were normalized to u36B4. Period circadian protein homolog 2 (Per2)-forward (F), ATGCTCGCCATCCACAAGA; Per2-reverse (R), GCGGAATCGAATGGGAGAAT; Nuclear receptor subfamily 1 group D member 1 (Nr1d1)-F, TACATTGGCTCTAGTGGCTCC; Nr1d1-R, CAGTAGGTGATGGTGGGAAGTA; D site-binding protein (Dbp)-F, CGTGGAGGTGCTAATGACCTTT; Dbp-R, CATGGCCTGGAATGCTTGA; u36B4-F, AGATGCAGCAGATCCGCAT; u36B4-R, GTTCTTGCCCATCAGCACC. Experiments were repeated twice with similar results.
Statistics
Statistical analysis was conducted in R Project for Statistical Computing (RRID:SCR_001905) (v 4.0.2) and RStudio (RRID:SCR_000432) (v 1.2.5033). BH.Q-value < 0.05 (MetaCycle, v1.2.0), Kuiper test FDR q < 0.05 (phase set enrichment analysis), or p < 0.05 (Bonferroni test or circacompare, v0.1.0 27 ) was considered to be statistically significant. Heatmap of expression profile was visualized by R package: pheatmap (RRID:SCR_016418) (v1.0.12) with row-wise scaling and gene-specific clustering by Euclidean correlation.
Results
Global transcript profiling of anterior hypothalamus from time-restricted fed female mice
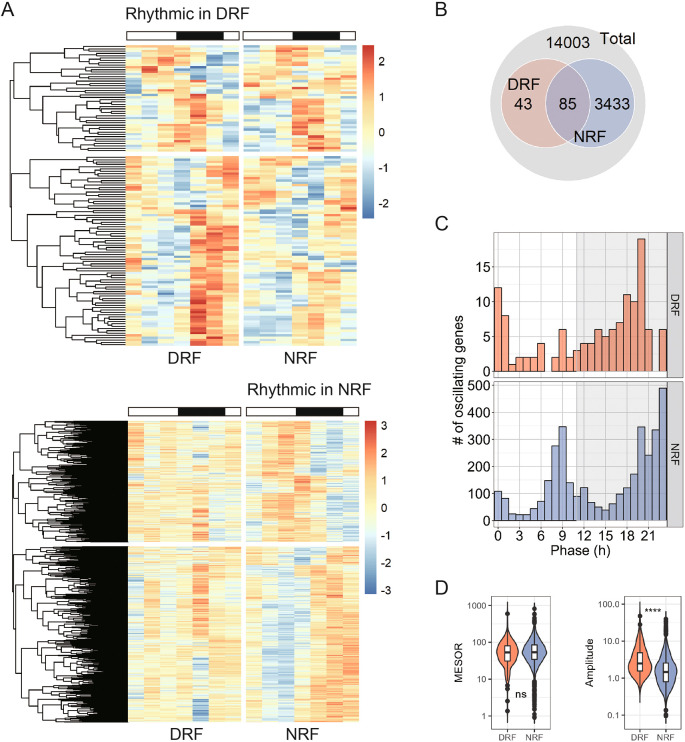
To characterize whether and how diurnal rhythms in the anterior hypothalamus entrain to feeding rhythm, we performed RNA sequencing on samples dissected from nine week-old female C57BL/6J mice that had been subjected to either DRF or NRF for seven days. Seven days of DRF is sufficient to entrain peripheral clocks in female mice. 14 Samples were collected every four hours for a complete diurnal cycle and assigned four biological replicates per time point. This is a commonly used study design used in global transcript profiling experiment. RNA sequencing reached a minimal depth of 43.1 million reads, which follows the guideline for genome-scale analysis of circadian rhythms. 28 We applied an BH-adjusted p-value (meta2d_BH.Q) of 0.05 to filter rhythmic transcripts and acquired 128 and 3,518 rhythmic transcripts in DRF and NRF tissues, respectively ( Figure 1A). 41 Notably, DRF significantly decreased the number of rhythmic transcripts in anterior hypothalamus, compared to NRF. There were 83 rhythmic transcripts that are shared by DRF and NRF tissues ( Figure 1B). As shown in Figure 1C, DRF rhythmic transcripts were clustered in the late active period (ZT18-ZT0), whereas NRF rhythmic transcripts exhibited a bimodal distribution that clustered in the pre-dawn hours (ZT23) and the late sleep phase (ZT9). Averagely speaking, rhythmic transcripts in DRF tissue exhibited a higher amplitude than those in NRF without any significant difference in the basal expression levels (midline estimating statistic of rhythm, MESOR) ( Figure 1D).
Figure 1. Global transcript profiling of anterior hypothalamus from time-restricted fed female mice.
(A) Heatmap showing 24-h expression profiles of rhythmic transcripts in the anterior hypothalamus. Samples were dissected from time-restricted fed nine-week female mice every four hours for a complete daily cycle (n = 4 mice per time point). (B) Interaction of rhythmic transcripts between day time-restricted feeding (DRF) and night time-restricted feeding (NRF). (C) Distribution of the phase. (D) Midline estimating statistic of rhythm (MESOR) and amplitude of rhythmic transcripts. Data are presented as boxplots. Mann-Whitney test; ns, not significant, ****p < 0.0001.
Rhythmic pathways entrained by NRF in the anterior hypothalamus
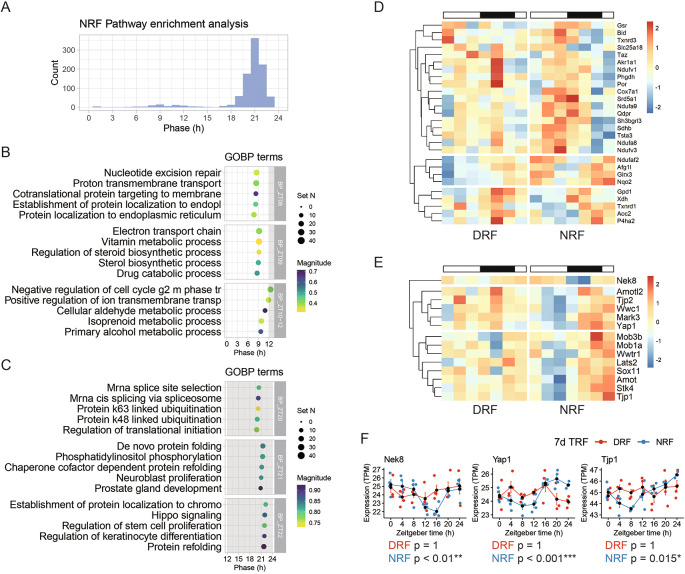
Next, we performed pathway analysis among rhythmic transcripts found in NRF tissue. As shown in Figure 2A, 990 pathways were significantly enriched and distributed towards the late active phase (ZT21) and the late sleep phase (ZT9). We examined these pathways by hour and found that ‘cotranslational protein targeting to membrane’ (ZT8), ‘electron transport chain’ (ZT9), ‘sterol biosynthetic process’ (ZT9), ‘primary alcohol metabolic process’ (ZT10), and ‘negative regulation of cell cycle g2 m phase tr’ (cell cycle G2-M phase transition) (ZT12) ( Figure 2B). Compared to the late sleep phase, the late active phase is a rush hour for hundreds of rhythmic biological pathways, which are represented by ‘mRNA cis-splicing via spliceosome’ (ZT20), ‘regulation of translational initiation’ (ZT20), ‘protein k63 linked ubiquitination’ (ZT20), ‘protein k48 linked ubiquitination’ (ZT20), ‘de novo protein folding’ (ZT21), ‘phosphatidylinositol phosphorylation’ (ZT21), and ‘Hippo signaling’ (ZT22) ( Figure 2C).
Figure 2. Rhythmic pathways entrained by NRF in the anterior hypothalamus.
(A) Phase distribution of enriched rhythmic pathways based on the anterior hypothalamic diurnal transcriptome. (B) Representative rhythmic pathways that peak around zeitgeber time 9 h (ZT9). (C) Representative rhythmic pathways that peak around ZT21. (D) Diurnal expression profiles of the rhythmic genes involved in electron transport chain. (E) Diurnal expression profiles of the rhythmic genes involved in Hippo signaling. (F) Representative rhythmic transcripts related to Hippo signaling. Data are presented as mean ± SEM (n = 4 mice per time point for seven time points). MetaCycle method, *BH adjusted p-value < 0.05, **p < 0.01, ***p < 0.001. NRF, night time-restricted feeding; DRF, day time-restricted feeding; GOBP, gene ontology biological processes; Nek8, NIMA related kinase 8; Yap1, Yes-associated protein 1; Tjp1, Tight junction protein 1.
Previously, microarray-based transcriptome profiling of the SCN has shown that the oxidative phosphorylation pathway exhibited circadian rhythm in ad libitum feeding and peaked in the late subjective night. 23 Examination of the electron transport chain pathway revealed that transcripts related to three respiratory complexes (CI, CII, and CIV) are rhythmic in the NRF anterior hypothalamus and peak in the late active phase ( Figure 2D). For example, these transcripts include NADH dehydrogenase [ubiquinone] flavoprotein 1, mitochondrial ( Ndufv1) (CI), NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 9, mitochondrial ( Ndufa9) (CI), Succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial ( Sdhb) (CII), and Cox7a1 (CIV).
Hippo signaling is essential in organ size control through inhibiting cell proliferation and regulating apoptosis. 29 We found that key components from the constituents and regulators of Hippo signaling ( e.g., Transcriptional coactivator YAP1 ( Yap1), Serine/threonine-protein kinase LATS2 ( Lats2) and Wwc1) ( Figure 2E). As shown in Figure 2F, representative transcripts encoding Serine/threonine-protein kinase Nek8 ( Nek8), Yap1, and Tjp1 exhibited robust daily rhythm in NRF but lost the rhythmicity upon DRF.
Rhythmic pathways entrained by DRF in the anterior hypothalamus
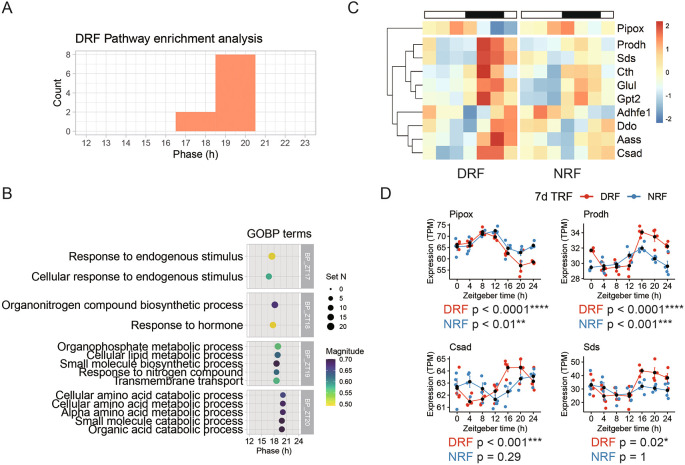
As shown in Figure 1B, the number of rhythmic transcripts was reduced notably by DRF in the tissue, compared to NRF. Pathway analysis of the 128 rhythmic transcripts revealed 20 enriched rhythmic pathways with peak hours ranging from ZT17 to ZT20 ( Figure 3A). These DRF rhythmic pathways include ‘response to endogenous stimuli’ (ZT17), ‘response to hormone’ (ZT18), ‘organophosphate metabolic process’ (ZT19), and ‘cellular amino acid metabolic process’ (ZT20) ( Figure 3B). With respect to the magnitude of enrichment, amino acid metabolism is highly scored. Hierarchical analysis identified two clusters of rhythmic transcripts involved in cellular amino acid metabolism ( Figure 3C).
Figure 3. Rhythmic pathways entrained by DRF in the anterior hypothalamus.
(A) Phase distribution of enriched rhythmic pathways based on the anterior hypothalamic diurnal transcriptome. (B) Representative rhythmic pathways that peak around zeitgeber time 18.5 h (ZT18.5). (C) Diurnal expression profiles of the rhythmic genes involved in cellular amino acid metabolism. (D) Representative rhythmic transcripts related to cellular amino acid metabolism. Data are presented as mean ± SEM (n = 4 mice per time point for seven time points). MetaCycle method, *BH adjusted p-value < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. NRF, night time-restricted feeding ; DRF, day time-restricted feeding; GOBP, gene ontology biological processes; Pipox, Pipecolic acid and sarcosine oxidase; Prodh, Proline dehydrogenase 1; Csad, Cysteine sulfinic acid decarboxylase; Sds, Serine dehydrastase.
Pipox transcript encoding pipecolic acid and sarcosine oxidase, an essential enzyme in lysine degradation-forms one cluster by itself, which peaked in the late sleep phase ( Figure 3D). Proline dehydrogenase 1 ( Prodh) transcript encodes the first enzyme in proline degradation. Cysteine sulfinic acid decarboxylase ( Csad) is an enzyme in cysteine and methionine degradation. Serine dehydrastase ( Sds) catalyzes serine and threonine degradation. As shown in Figure 3D, transcripts of Prodh, Csad, and Sds exhibited robust diurnal oscillation in anterior hypothalamus of DRF mice. In particular, Csad and Sds did not oscillate in NRF. Thus, transcript signatures suggest that DRF entrains diurnal rhythms of cellular amino acid degradation.
Food entrainment of the circadian clock in the anterior hypothalamus
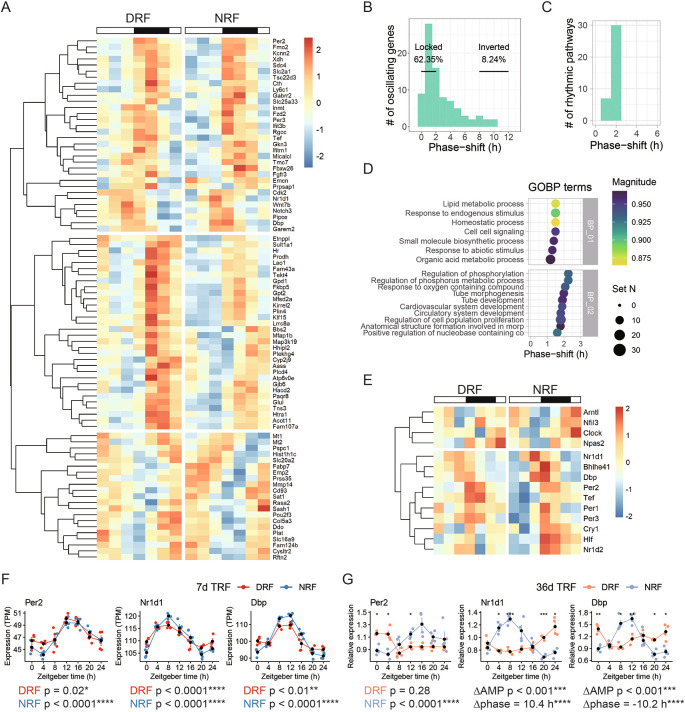
After examining circadian signatures of the anterior hypothalamus in each time-restricted feeding regimen, we determined the features associated with rhythmic transcripts found in both NRF and DRF tissues. Hierarchical analysis segregated the 83 dual-cycling transcripts into three clusters ( Figure 4A). DRF reserved only 8.24% of rhythmic transcripts for more than 8 h compared to NRF, whereas more than 60% of the rhythmic transcripts remained phase-locked to the light/dark cycle ( Figure 4B). This is consistent with the results of pathway analysis. In fact, none of the 37 enriched rhythmic pathways were shifted in phase for more than 2 h by DRF compared to NRF ( Figure 4C). Representative enriched pathways include ‘lipid metabolic process’ (phase-shift 1 h), ‘regulation of phosphorylation’ (phase-shift 2 h), and ‘circulatory system development’ (phase shift 2 h) ( Figure 4D).
Figure 4. Food entrainment of the circadian clock in the anterior hypothalamus.
(A) Hierarchical clustering analysis of rhythmic transcripts found in both DRF and NRF anterior hypothalamus. (B-C) Distribution of the phase-shift between DRF and NRF among (B) rhythmic transcripts or (C) enriched rhythmic pathways. (D) Representative enriched pathways based on shared rhythmic transcripts between DRF and NRF. (E) Hierarchical clustering analysis of the circadian clock genes. (F-G) Diurnal expression of selected clock genes in the anterior hypothalamus from female mice subjected to DRF or NRF for (F) seven days or (G) 36 days. Data are presented as mean ± SEM (n = 4 mice per time point). MetaCycle, *BH adjusted p-value < 0.05; Circacompare method, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. NRF, night time-restricted feeding ; DRF, day time-restricted feeding; GOBP, gene ontology biological processes; Per2, Period circadian protein homolog 2; Nr1d1, Nuclear receptor subfamily 1 group D member 1; Dbp, D site-binding protein.
The weak response in phase entrainment is matched with the response of the circadian clock. Hierarchical analysis segregated the core clock genes into two clusters. One cluster of genes, e.g., Brain-and-muscle ANRT-like 1 ( Bmal1/Arntl) and Circadian locomotor output cycles protein kaput ( Clock), reached the peak time in the dawn under NRF and damped under DRF ( Figure 4E). The other cluster of genes, e.g., Per1, Per2, Per3, Nr1d1 and Nr1d2, reached the peak time in the dusk in both DRF and NRF ( Figure 4E). As expected, representative transcripts encoding Per2, Nr1d1, and Dbp, was not shifted in phase by DRF ( Figure 4F). After an extension of the time from seven days to 36 days, Per2 rhythm damped in DRF ( Figure 4G). Remarkably, 36 days of DRF significantly inverted the phase and reduced the amplitude of Nr1d1 and Dbp in the anterior hypothalamus compared to NRF ( Figure 4G).
Discussion/conclusions
In this study, we applied an unbiased approach to characterize circadian signatures of the anterior hypothalamus in time-restricted fed female mice. We found that DRF, the feeding rhythm that is mis-aligned with the circadian clock, decreased the number of rhythmic transcripts from 3,518 to only 128 compared to NRF within seven days. Pathway analysis identified synchronized rhythms among 990 and 20 biological processes in NRF and DRF, respectively. Typical NRF active pathways include ‘electron transport chain’ (peak time ZT9) and ‘Hippo signaling’ (ZT22). Meanwhile, DRF entrained robust diurnal oscillation among 43 transcripts including those related to ‘cellular amino acid catabolic process’ (ZT20). At the transcriptomic level, only 8.24% rhythmic transcripts were reversed in phase by DRF compared to NRF. This is matched with the resistance of the anterior hypothalamic clock to DRF. However, long-term DRF inverted the phase in some clock genes, such as Nr1d1 and Dbp.
Global profiling of rhythmic transcripts and metabolites have been conducted in a few brain regions particularly including the SCN. In constant darkness, 365 and 642 rhythmic transcripts were identified in the SCN and hypothalamus, respectively. 22 , 23 High-fat diet feeding increases the number of rhythmic metabolites in the SCN but not in the prefrontal cortex. 30 Our transcriptome profiling of anterior hypothalamus uncovered 3,518 rhythmic transcripts in NRF, which suggests that synchronized rhythms from the light and food may markedly increase the prevalence of diurnal rhythms in the hypothalamus.
Anterior hypothalamus is composed of different hub neural regions, such as the preoptic area, SCN and PVH. Our study identified distinct sets of circadian signatures in this tissue under different regimens of time-restricted feeding. Compared to previous studies elucidating the landscape of circadian rhythms in the SCN, hunger neurons, or the hypothalamus, 22 , 23 , 31 NRF entrains diurnal rhythms among a similar set of biological pathways, including oxidative phosphorylation and intracellular trafficking among organelles. By contrast, DRF almost ablates the diurnal rhythms in anterior hypothalamus. This effect may come as a trade-off of two reverse rhythms originating from the light/dark cycles to the SCN and from the feed/fast cycle to the PVH, respectively. Cellular amino acid metabolism is a key circadian signature associated with DRF, which implies the robust daily activity and signaling related to amino acids in anterior hypothalamus. This is supported by a recent study reporting the role of tryptophan metabolism in the entrainment of the brain clock after integrating signals from the light and food. 32
The PVH is emerging as a critical peripheral oscillator on top of its well-recognized role as a hub for nutrient sensing and energy homeostasis. 19 , 20 It outputs circadian rhythm of energy expenditure, the rhythmicity of which is crucial for body weight homeostasis. 33 PVH also mediates the circadian tone from the SCN to orchestrate daily rhythm of plasma glucose. 34 Recently, it has been shown that corticotrophin-releasing hormone-producing neurons in the PVH receive circadian inputs from the SCN and drive daily release of glucocorticoids. 35 Previously, olfactory bulb and cerebellum are proposed as food entrainable peripheral oscillators and involved in food anticipatory behavior induced by DRF. 36 – 38 In our study, the anterior hypothalamus is partially food-entrainable as indicated by transcript rhythms of Nr1d1 and Dbp after a 36-day DRF. It could be due to the actions in the non-SCN brain area within the anterior hypothalamus, or the SCN. It has been shown recently that time-restricted feeding near the light-on time modulates the SCN functions and impacts thermal homeostasis, locomotor activity and wakefulness. 39 , 40 These results suggest that brain regions within the anterior hypothalamus may entrain to the feeding rhythm after a long DRF.
In summary, we have determined circadian signatures of the anterior hypothalamus in time-restricted fed mice. The accessibility of the global transcript profiling data in CircaMetDB 42 , 43 would provide a useful resource to study the physiological significance and the entrainment of circadian rhythms in anterior hypothalamus.
Data availability
Reporting guidelines and underlying data
Mendeley Data: F1000Research-125368, Zhou, Chen. https://doi.org/10.17632/h4bvgm2z6s.1. 41
This project contains the following underlying data:
-
-
Raw data from RT-qPCR experiment, associated with Figure 4G
-
-
ARRIVE checklist – The ARRIVE Essential 10
-
-
Gene expression profile.csv
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The accession numbers for the global profiling dataset:
Gene Expression Omnibus: Global circadian transcript profile of mouse anterior hypothalamus entrained by inverted feeding. Accession number GSE150958; https://identifiers.org/geo:GSE150958. 42
China National GeneBank DataBase: Global circadian transcript profile of mouse anterior hypothalamus entrained by inverted feeding. Accession number CNP0001601; https://db.cngb.org/search/project/CNP0001601/. 43
Funding Statement
This work was supported by National Natural Science Foundation of China (92057109).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Reinke H, Asher G: Crosstalk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol. 2019 Apr 11;20(4):227–241. 10.1038/s41580-018-0096-9 Reference Source [DOI] [PubMed] [Google Scholar]
- 2. Guan D, Lazar MA: Interconnections between circadian clocks and metabolism. J. Clin. Invest. 2021 Aug 2;131(15):e148278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li MD: Clock-modulated checkpoints in time-restricted eating. Trends Mol. Med. 2022 Jan;28(1):25–35. 10.1016/j.molmed.2021.10.006 [DOI] [PubMed] [Google Scholar]
- 4. Stokkan KA, Yamazaki S, Tei H, et al. : Entrainment of the Circadian Clock in the Liver by Feeding. Science (1979). 2001 Jan 19;291(5503):490–493. Reference Source [DOI] [PubMed] [Google Scholar]
- 5. Damiola F, Minli N, Preitner N, et al. : Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14(23):2950–2961. 10.1101/gad.183500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yasumoto Y, Hashimoto C, Nakao R, et al. : Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice. Metabolism. 2016;65(5):714–727. 10.1016/j.metabol.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 7. Arble DM, Vitaterna MH, Turek FW: Rhythmic leptin is required for weight gain from circadian desynchronized feeding in the mouse. PLoS One. 2011;6(9):e25079. 10.1371/journal.pone.0025079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arble DM, Bass J, Laposky AD, et al. : Circadian timing of food intake contributes to weight gain. Obesity. 2009;17(11):2100–2102. 10.1038/oby.2009.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kolbe I, Leinweber B, Brandenburger M, et al. : Circadian clock network desynchrony promotes weight gain and alters glucose homeostasis in mice. Mol Metab. 2019;30(October):140–151. 10.1016/j.molmet.2019.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petersen MC, Gallop MR, Flores Ramos S, et al. : Complex Physiology and Clinical Implications of Time-restricted Eating. Physiol. Rev. 2022 Jul 14:9–25. Reference Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manoogian EN, Chow LS, Taub PR, et al. : Time-restricted eating for the prevention and management of metabolic diseases. Endocr. Rev. 2021 Sep 22;43:405–436. 10.1210/endrev/bnab027/6371193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Acosta-Rodríguez V, Rijo-Ferreira F, Izumo M, et al. : Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science (1979). 2022 Jun 10;376(6598):1192–1202. 10.1126/science.abk0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manella G, Sabath E, Aviram R, et al. : The liver-clock coordinates rhythmicity of peripheral tissues in response to feeding. Nat. Metab. 2021 Jun 31;3(6):829–842. 10.1038/s42255-021-00395-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xin H, Deng F, Zhou M, et al. : A multi-tissue multi-omics analysis reveals distinct kinetics in entrainment of diurnal transcriptomes by inverted feeding. iScience. 2021 Apr 23;24(4):102335. 10.1016/j.isci.2021.102335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allada R, Bass J: Circadian Mechanisms in Medicine. Longo DL, editor. N. Engl. J. Med. 2021 Feb 11;384(6):550–561. 10.1056/NEJMra1802337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koronowski KB, Sassone-Corsi P: Communicating clocks shape circadian homeostasis. Science (1979). 2021 Feb 12;371(6530):eabd0951. 10.1126/science.abd0951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang Z, Shui G, Li MD: Time to eat reveals the hierarchy of peripheral clocks. Trends Cell Biol. 2021 Nov 14;31(11):869–872. 10.1016/j.tcb.2021.08.003 Reference Source [DOI] [PubMed] [Google Scholar]
- 18. Li MD, Xin H, Yuan Y, et al. : Circadian Clock-Controlled Checkpoints in the Pathogenesis of Complex Disease. Front. Genet. 2021 Sep 7;12(September):721231. 10.3389/fgene.2021.721231/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morton GJ, Meek TH, Schwartz MW: Neurobiology of food intake in health and disease. Nat. Rev. Neurosci. 2014;15(6):367–378. 10.1038/nrn3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Myers MG, Affinati AH, Richardson N, Schwartz MW: Central nervous system regulation of organismal energy and glucose homeostasis. Nat. Metab. 2021 Jun 1;3(6):737–50. 10.1038/s42255-021-00408-5 [DOI] [PubMed] [Google Scholar]
- 21. Mohawk JA, Green CB, Takahashi JS: Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012;35:445–462. 10.1146/annurev-neuro-060909-153128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang R, Lahens NF, Ballance HI, et al. : A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. U. S. A. 2014 Nov 11;111(45):16219–16224. 10.1073/pnas.1408886111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Panda S, Antoch MP, Miller BH, et al. : Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307–320. 10.1016/S0092-8674(02)00722-5 [DOI] [PubMed] [Google Scholar]
- 24. Xin H, Huang R, Zhou M, et al. : Protocol for setup and circadian analysis of inverted feeding in mice. STAR Protoc. 2021 Sep;2(3):100701. 10.1016/j.xpro.2021.100701 Reference Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu G, Anafi RC, Hughes ME, et al. : MetaCycle: An integrated R package to evaluate periodicity in large scale data. Bioinformatics. 2016;32(21):3351–3353. 10.1093/bioinformatics/btw405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang R, Podtelezhnikov AA, Hogenesch JB, et al. : Discovering Biology in Periodic Data through Phase Set Enrichment Analysis (PSEA). J. Biol. Rhythm. 2016;31(3):244–257. 10.1177/0748730416631895 [DOI] [PubMed] [Google Scholar]
- 27. Parsons R, Parsons R, Garner N, et al. : CircaCompare: A method to estimate and statistically support differences in mesor, amplitude and phase, between circadian rhythms. Bioinformatics. 2020;36(4):1208–1212. 10.1093/bioinformatics/btz730 [DOI] [PubMed] [Google Scholar]
- 28. Hughes ME, Abruzzi KC, Allada R, et al. : Guidelines for Genome-Scale Analysis of Biological Rhythms. J. Biol. Rhythm. 2017;32(5):380–393. 10.1177/0748730417728663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koo JH, Guan KL: Interplay between YAP/TAZ and Metabolism. Cell Metabolism. Cell Press. 2018;28:196–206. 10.1016/j.cmet.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 30. Dyar KA, Lutter D, Artati A, et al. : Atlas of Circadian Metabolism Reveals System-wide Coordination and Communication between Clocks. Cell. 2018;174(6):1571–1585.e11. 10.1016/j.cell.2018.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cedernaes J, Huang W, Ramsey KM, et al. : Transcriptional Basis for Rhythmic Control of Hunger and Metabolism within the AgRP Neuron. Cell Metab. 2019;29(5):1078–1091.e5. 10.1016/j.cmet.2019.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petrus P, Cervantes M, Samad M, et al. : Tryptophan metabolism is a physiological integrator regulating circadian rhythms. Mol Metab. 2022 Oct;64(July):101556. 10.1016/j.molmet.2022.101556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim ER, Xu Y, Cassidy RM, et al. : Paraventricular hypothalamus mediates diurnal rhythm of metabolism. Nat. Commun. 2020 Dec 30;11(1):3794. 10.1038/s41467-020-17578-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalsbeek A, Fleur S, Heijningen C, et al. : Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J. Neurosci. 2004 Sep 1;24(35):7604–7613. 10.1523/JNEUROSCI.5328-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jones JR, Chaturvedi S, Granados-Fuentes D, et al. : Circadian neurons in the paraventricular nucleus entrain and sustain daily rhythms in glucocorticoids. Nat. Commun. 2021 Dec 1;12(1):5763. 10.1038/s41467-021-25959-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pavlovski I, Evans JA, Mistlberger RE: Feeding Time Entrains the Olfactory Bulb Circadian Clock in Anosmic PER2::LUC Mice. Neuroscience. 2018;393:175–184. 10.1016/j.neuroscience.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 37. Granados-Fuentes D, Tseng A, Herzog ED: A circadian clock in the olfactory bulb controls olfactory responsivity. J. Neurosci. 2006;26(47):12219–12225. 10.1523/JNEUROSCI.3445-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mendoza J, Pévet P, Felder-Schmittbuhl MP, et al. : The cerebellum harbors a circadian oscillator involved in food anticipation. J. Neurosci. 2010;30(5):1894–1904. 10.1523/JNEUROSCI.5855-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Z, Zhai Q, Gu Y, et al. : Impaired function of the suprachiasmatic nucleus rescues the loss of body temperature homeostasis caused by time-restricted feeding. Sci Bull (Beijing). 2020 Aug;65(15):1268–1280. 10.1016/j.scib.2020.03.025 Reference Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhai Q, Zeng Y, Gu Y, et al. : Time-restricted feeding entrains long-term behavioral changes through the IGF2-KCC2 pathway. iScience. 2022;25(5):104267. 10.1016/j.isci.2022.104267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li M-D: “F1000Research-125368, Zhou, Chen”. Mendeley Data, V1. [Dataset]. 2022. 10.17632/h4bvgm2z6s.1 [DOI]
- 42. Li M-D: Global circadian transcript profile of mouse anterior hypothalamus entrained by inverted feeding. Gene Expression Omnibus [Dataset]. 2021. Reference Source
- 43. Li M-D: Global circadian transcript profile of mouse anterior hypothalamus entrained by inverted feeding. China National GeneBank DataBase [Dataset]. 2021. Reference Source